Abstract
The advent of nanotechnologies such as nanocarriers and nanotherapeutics has changed the treatment strategy and developed a more efficacious novel drug delivery system. Various drug delivery systems are focused on drug-targeting of brain cells. However, the manifestation of the brain barrier is the main hurdle for the effective delivery of chemotherapeutics, ultimately causing treatment failure of various drugs. To solve this problem, various nanocarrier-based drug delivery system has been developed for brain targeting. This review outlines nanocarrier-based composites for different brain diseases and highlights nanocarriers for drug targeting towards brain cells. It also summarizes the latest developments in nanocarrier-based delivery systems containing liposomal systems, dendrimers, polymeric micelles, polymeric nanocarriers, quantum dots (QDs), and gold nanoparticles. Besides, the optimal properties of nanocarriers and therapeutic implications for brain targeting have been extensively studied. Finally, the potential applications and research opportunities for nanocarriers in brain targeting are discussed.
Keywords: brain targeting, nanocarriers, blood–brain barrier, nanotherapeutics, in-vivo
Introduction
Despite the developments and advances of research, brain targeting is considered one of the challenging tasks. Among the most prominent central nervous system (CNS) disorders are brain tumor or glioma, Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, strokes, seizure or epilepsy, schizophrenia, migraine, traumatic brain injuries, cerebral palsy, CNS infection, and several psychological disorders including depression, anxiety, depression and many others.1 Multiple therapies including surgery, deep brain stimulation, intravenous (IV), oral and topical dosage forms, and rehabilitation therapies are currently available. However, conventional therapies have certain limitations that aid the drug entry into general blood circulation after passing different physiological barriers like the blood–brain barrier (BBB) as part of the apparent blood distribution volume.2 After that drug cargo reaches the brain to a lesser amount, exhibits limited therapeutic efficacy. In comparison, surgical approaches and brain implants are considered unsafe, short-term and highly invasive treatment approaches.3,4
One of the critical obstacles to therapeutics entry into the CNS is the BBB. The blood–brain barrier acts as a neuroprotective barrier by maintaining CNS homeostasis by displaying a sure sign of a higher metabolic rate. The brain is a challenging organ for medication administration since the BBB is the finest gatekeeper, shielding the CNS from external drugs. As a result, drug transport to the brain is problematic since many pharmaceuticals lack solubility, lipophilicity, and bioavailability, and the BBB can inhibit 98% of drugs. Because conventional medication therapies are inadequate, developing strategies to deliver therapeutic pharmaceuticals to the CNS safely and effectively is critical. Treatment failure in brain targeting is associated with a variety of difficult pharmaceutical issues, such as pharmacological or toxicity concerns, multiple drug resistance (MDR), the complex anatomical structure of delivery vehicles, and brain capillary endothelial cells (BCECs) that form the BBB.5,6
BCECs are classically considered a significant opportunity for brain drug targeting due to a wide-ranging network of receptors and transporters that enable the transport of essential components, including small solutes and large hydrophilic compounds (insulin, transferrin, etc.). The BBB also protects brain tissues and neural cells from pathogenic toxins and is selective for the transport of large size drug molecules via lacking fenestrations for drug uptake.7,8
It is evident from previous prestigious research that nanocarriers (NCs) targeting is the exceptional approach to treating brain diseases by overcoming the barriers, ie, the BBB. Drug delivery systems (DDS) based on nanocarriers has revolutionized therapeutic applications by improving the pharmacological and pharmacokinetic patterns of various drugs, allowing them to cross the BBB without disrupting its functionalization.9 Furthermore, DDS based on nanocarriers have some promising physicochemical and biological characteristics, including long blood circulation time, capacity to cross different barriers, cellular uptake, small size and large surface area, advanced pharmacokinetic features, ability to attach different molecules to their surface, and particular structural characteristics. Additionally, the utilization of nanocarriers led to the development of a highly effective regimen by increasing the therapeutic index and drug concentrations at the target site.10,11
Furthermore, the latest advances in nanotechnology have anticipated the development of novel nanotherapeutics. For example, by binding to an appropriate ligand, NCs can sustain and target drug cargo directly into the brain, thereby reducing peripheral toxicity. Furthermore, researchers have highlighted some exciting approaches to NCs to increase the drug residence time (DRT) by coupling them with preactivated and thiolated polymers to constrain the P-glycoprotein (P-gp) outflow efficiently. In this regard, various types of nanocarriers, including polymeric micelles, polymeric nanocarriers, dendrimers, liposomal systems (active-targeting, cationic, stimuli-sensitive conventional and long-circulating), gold nanoparticles, and quantum dots (QDs), were utilized for brain targeting via coupling with identified receptors to cross the BBB proficiently. Yet, one of the most intriguing mechanistic approaches to NCs is that NCs are endocytosed by endothelial cells after crossing the BBB, ultimately releasing the drug into the target cell.12
This review focused on different nanocarriers for drug targeting in the brain for various CNS-related disorders. We have highlighted the application of these NCs and their BBB pathology in brain diseases. In addition, the latest developments in nanocarrier-based delivery systems containing liposomal systems, dendrimers, polymeric micelles, polymeric nanocarriers, quantum dots, and gold nanoparticles (Au-NPs) are given in detail. Besides, the optimal properties of nanocarriers and therapeutic implications for brain targeting have been thoroughly discussed.
The Pathophysiology of Brain
In terms of its shape and number of nerve cells, the brain is the most complicated organ in the body, attributable to its branched and extended structure, complex interconnections, and scattering qualities.13 Specified cerebrovascular endothelial cells, astrocytes, neurons, and pericytes constitute the blood–brain barrier (BBB). Paracrine interactions between the brain’s endothelium and nearby glia are necessary for its optimal functioning, however. As a result of brain injuries, patients suffer from cognitive, motor, and sensory dysfunctions.14 Immediate and irreversible initial damage to the parenchyma triggers acute and irreversible primary damage to the brain, with secondary brain injuries occurring at a rather gradual rate, creating a window of opportunity for therapeutic approaches. The hallmarks of secondary brain damage include Wallerian degeneration of axons, mitochondrial malfunction, excitotoxicity, oxidative stress, and apoptotic cell death of neurons and glia. “Design to inspire action”.15 A brain injury, whether it is an ischemic stroke, a hemorrhagic stroke, or a traumatic brain injury, causes disruption of the BBB. Changes due to injuries in the BBB are linked to brain tissue loss and influence how neuroprotective drugs respond. Studies by Chodobski et al.16 Composed of specialised endothelial cells that line the blood–brain barrier (BBB), tight junction complexes combine to form the barrier, which acts as a physical barrier to paracellular transport and promotes high transendothelial electrical resistance (TEER) associated with the BBB. The results of.17
Paracellular Transport and Transcytosis
In terms of how the BBB functions, two different mechanisms must be considered: paracellular transport and transcellular transport. The CSF “sink” of the brain allows for a clearer description of intracranial mass and constitutive equilibrium.18 Since tight connections are found between the blood and the brain, paracellular transit is restricted.19 The movement of macromolecules from the apical to the basolateral plasma membrane is referred to as unidirectional transcytosis in polarised cells. Endocytosis, intracellular vesicular trafficking, and exocytosis are a few of the many steps on this pathway.20 It was due to the existence of specialised tight junctions that allowed the CNS barrier qualities to be maintained at low levels of transcytosis. Due to this revelation, it is now apparent that transcytosis suppression at the BBB is an active process, and genetic programmes particular to the CNS work to maintain this barrier.19 The transcytosis receptor is also present in all brain endothelial cells. It might be hypothesised that lower expression levels of certain receptors compared to transcytosis pathway inhibition could result from a reduced permeability to macromolecules across the blood–brain barrier.21
Extra Approaches
Li et al explained some additional opportunities including transporters and receptors, enzyme responsive system, tissue microenvironment-responsive nanomedicine, actively targeted nanomedicine and externally triggerable nanomedicine for developing smart functionalities.22
The Transporters and Receptors
BBB shuttles are expressed in the endothelium by several molecular transporters and receptors, including transferrin receptor (TfR) and glucose transporter type 1 (GLUT1). These attempts aimed to get the shuttles involved in improved brain targeting.23
Enzyme Responsive System
Cathepsins and MMPs are two enzymes that have been linked to disease progression and thus could act as a trigger. Poly[N-(2-hydroxypropyl) methacrylamide] GlyPheLeuGly-doxorubicin (DOX), a prodrug (synthetic polymer) conjugate originally developed by Kopeck et al, wherein the peptidyl linker of GPLG might be sliced to release doxorubicin through the use of cathepsins in the lysosome, is the first example of a clinically investigated enzyme responsive system.24
Tissue Microenvironment-Responsive Nanomedicine
In other circumstances, such as 2,3-dimethyl maleic amide, the chemical structure could be designed to be broken by tumour extracellular acidity to improve tissue penetration and cellular uptake.25
Actively Targeted Nanomedicine
The term “actively targeted nanomedicine” refers to nanomedicine that uses surface-decorated affinity ligands to engage receptors, allowing for extended tissue retention and higher cellular uptake using active nanomedicine, resulting in high bioavailability. In addition to small molecules and antibodies, peptides and aptamers are among the most commonly employed ligands.26
Externally Triggerable Nanomedicine
External energy, such as light, magnetic fields, and ultrasound, can be used to directly interact with nanomedicine-retained tissue as an alternative. Photocleavage/photoisomerization events, as well as photodynamic/photothermal effects, could be induced by light illumination, increasing the performance of nanomedicines. Tissue microenvironment-responsive nanomedicines, while exciting and promising, attain elegance through biological signals, which are typically heterogeneous.27
Various Types of Nanocarrier-Based Delivery Vehicles for Drug Targeting in Brain Tumors
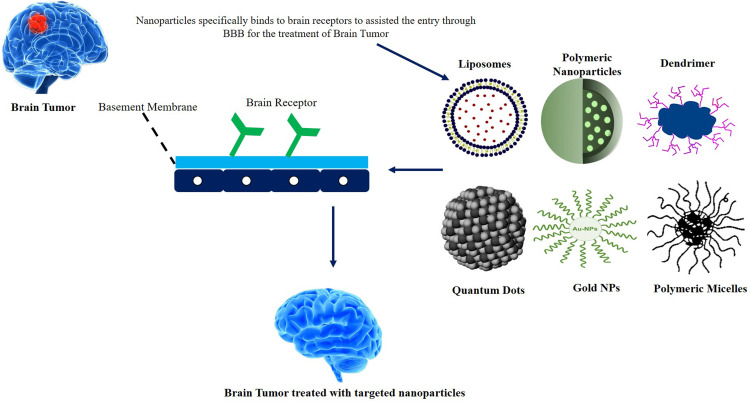
Recent advances in nanotechnology have significant effects on nanomedicine for biological applications.28 It helps develop emerging tools for diagnosis, treating, monitoring, and controlling biotechnological systems, facilitating the synthesis and manipulation of materials on the nanoscale. Nanomaterials are defined as a set of nanoscale, internal or surface-structured substances with any external dimension, approx. in size range 1 to 100 nm.29 Such nanostructured materials are a smart technique since they can infiltrate the blood–brain barrier because of their nanosized structure and the transportation of therapeutic compounds to their target location.30 Different nanomaterials, like polymeric micelles, polymeric nanocarriers, dendrimers, liposomal systems, quantum dots, and gold nanoparticles, were examined concerning potential drug delivery to the brain. Research outcomes of different nanocarriers and their indications have been presented in Table 1. The ability of NPs to overcome the restrictive nature of BBBs to drug molecules efficiently targets drugs to the brain.31 Low concentrations of pharmaceuticals, therapeutic complexes or medicines can be injected directly into the brain, than conventional doses of free medicinal goods, resulting in safe medicinal administration for therapeutic efficiency. Nanocarriers have far more specialized physicochemical characteristics compared to their parallel bulk materials including large surface area, high drug loading, the feasibility of incorporating hydrophilic, hydrophobic chemicals, and high stability. The qualities of the NPs rely on form and size, apart from their composition.32,33 To achieve monodispersed NPs for cell internalization, it is important to verify their shape and size and to minimize their accumulation.34,35
Table 1.
Various Novel Nanocarrier-Based System and Their Outcomes for Brain Targeting
| Nanocarrier-Based System | Targeting Agent | Indication | Outcomes | References |
|---|---|---|---|---|
| Liposomes | Doxorubicin (DOX) | Glioma | P1NS/TNC-FeLPs have shown GBM-specific cellular uptake and drug release profile. Developed NPs show a thermo-responsive transport, reduced tumor cell proliferation without affecting healthy brain cell function. Less toxic and greater drug accumulation in cancerous cells and long-term survival. |
[38] |
| Pegylated Liposomes | Doxorubicin | Brain tumor Regression |
[39] | |
| Dendrimers | Borneol and doxorubicin | Dual-functional glioma Targeting |
Improved area under the curve (AUC) and drug accumulation in brain tumors, prolonged half-life time and enhanced drug accumulation in glioma cells | [40] |
| Uptake mechanism of dendrimers into brain cells | Enhance permeation and uptake of polyether-copolyester (PEPE) dendrimers across the BBB | [41] | ||
| Polymeric Micelles | Dapoxetine (DPX) | Reduce DPX medication dependence | DPX micelles show improved bioavailability, brain delivery and efficacy across the BBB. | [42] |
| Polymeric nanoparticles | Curcumin | Alzheimer’s disease (AD) | Curcumin-loaded CS-BSA NPs penetrated the BBB, activated microglia, and expedited the phagocytosis of the Aβ peptide. Further, NPs showed promise in influencing macrophage polarisation in AD. | [43] |
| Anti-amyloid tibody | Alzheimer’s disease (AD) | Increased absorption and ability to permeate the BBB to target cerebrovascular amyloid formation. | [44] | |
| Gold nanoparticles (Au-NPs) | L-DOPA | Parkinson’s disease | Developed gold nanoparticles are readily absorbed by brain macrophages and cause no inflammation, effectively permeate the BBB, | [45] |
| Quantum dots (QDs) | Aromatic drugs paired α-COOH and NH2 groups | Brain tumor | Selective targeting and imaging to brain cancerous cells | [46] |
| Monoclonal antibodies (Ri7) | Brain endothelial delivery | Ri7-quantum dots complexed form has 4 times larger Vd in brain tissues, complicate endocytosis by brain capillary endothelial cells | [47] |
The potential for high biological and chemical stabilization of such NPs, the feasibility of the integration of hydrophilic and hydrophobic medicinal products, and the capability for different routes are even more attractive for healthcare purposes. NPs can also work by covalent conjugation with different ligands (such as proteins and aptamers) in certain tissues.36 The high volume-to-surface ratio of NPs allows many duplicates of a ligand to be linked and their binding affinity to be substantially enhanced via the multifunctional function. The greater surface-mass ratio of some NP applications other than conventional particles allows them to bind/conjugate, absorb, or transport other particles. In addition, two or more materials can be utilized or produced to improve their physical properties.37 The most popular nanocarriers and their penetration through BBB for brain targeting are reported in Figure 1 with their mechanism of targeting the brain.
Figure 1.
Novel nanocarriers and their penetration through BBB for brain targeting.
The Liposomes
Liposomes are sphere-shaped vesicles consisting of natural (Biodegradable) or synthetic bilayers of phospholipids and aqueous partitions.48 Because of the amphiphilic nature of phospholipids, these nanospheres form spontaneously.49 Depending on the technique of synthesis and post-formation processing, they are classified as unilamellar vesicles (ULVs) or multilamellar vesicles (MLVs). ULVs encapsulate an enormous aqueous core and are suitable for encapsulating drugs containing hydrophilic structure, but MLVs are better for encapsulating lipid-soluble pharmaceuticals.50 In general, MLVs have a larger entrapped volume than ULVs, while unilamellar liposomes with a hydrodynamic diameter of 250 nm and 2–3 lamellar bilayers release much faster than MLVs.51 They can intermingle with the cells of the tumor and use endocytosis to release drugs in the extracellular matrix. Liposomes can be targeted by passive or active mechanisms.52,53 While active targeting of tumors is not always more effective than passive targeting, targeting micrometastasis, vasculature, and blood tumors is advantageous. Polyethylene glycol (PEG) engineering and coating liposomes can increase biocompatibility, water-solubility, targeted drug delivery, controlled release, and half-life and decrease toxicity.54 The liposome surface can even be used to improve blood circulation and brain-focusing drug delivery through incorporating a broad range of macromolecules, like antibodies, peptides, aptamers, polymers, or polysaccharides. Presentation of the main liposomal medications and targeting agents that improve liposomal affinity and brain targeting is depicted in Figure 2.55 Liposomal formulations size has a significant impact on their half-life in the blood; liposomal nanostructures having a size up to 100 nm easily penetrate tumor cells, larger liposomes, on the other hand, have a shorter half-life due to better identification.56 For the past few years, liposomes have been widely used for nanomedicines to treat various cancers and neurological disorders.57,58 Two chemotherapeutics erlotinib and doxorubicin (DOX) were assembled in these produced liposomes to improve their translocation via the BBB to invasive glioblastoma tumors. Tf-Pen liposomes were encapsulated by Erlotinib and doxorubicin and significantly enhanced translocation (15%) through the BBB shown, resulting in tumor reversal in an in vitro brain tumor prototype. The in vitro study of hemocompatibility and cytotoxicity confirmed excellent biocompatibility, indicating acceptability for in vivo usage. Tf-Pen liposomes in the mouse brain were 3.3 and ~12 times higher than free drugs, loaded with erlotinib and doxorubicin. The nano-liposomal systems have also demonstrated improvised anticancer efficacy, associated with reverting about 90% of the tumor in the rat brain deprived of toxic effects.56,59
Figure 2.
Presentation of the main liposomal medications and targeting agents that improve liposomal affinity and brain targeting.
The potential for improving vitamin E’s therapeutic attributes has been dramatically enhanced by polyethylene glycolate (PEGylated) like D-tocopherol, PEG 1000 succinate or TPGS used in the pharma and food industry. Muthu et al have manufactured and used TPGS-packed liposomes for docetaxel encapsulation to develop and treat a brain tumor medicinal supply system.60 Liposomes loaded with coumarin-6 or docetaxel were prepared using a solvent injecting procedure, then described, and the cellular absorption and cytotoxicity with C6 glioma cells were assessed.61 The particle size was 126–191 nm in the range of TPGS-coated liposomes. After a 24-hour culture with C6 glioma cells, an IC50 of 31.04, 37.04, 7.70, and 5.93 g/mL was shown in the nude commercial Taxotere, PEG, and TPGS covered liposomes, respectively. The TPGS-capped nanoliposomes had higher advantages in vitro compared to PEG liposomes.
Paclitaxel is an antitumor drug directed by microtubules that shows potent activity against various tumors, including lung, ovary, brain tube, etc. However, owing to the deficiency of BBB penetration ability, the efficiency of the paclitaxel preparation available on the market is not adequate for glioma.62 Artemether also demonstrates strong cytotoxicity against several types of cancer cells by down-regulating VEGF production, hypoxia-inducible factor-1a, metalloproteins 9 matrices, and certain proteins implicated. Previously, drug translocation through the BBB, vasculogenic imitation brain channel destruction and stem cell eradication were considered functional nanotherapeutic systems.63 A new kind of liposomal system, loaded with paclitaxel and artemether was developed as an antitumor medicine and apoptosis regulator. The increased effectiveness for liposomes was linked to the destruction and induction of the Vasculogenic Channel (VM) mimics in brain cancer cells by inducing apoptotic enzymes and pro-apoptotic proteins while inhibiting anti-apoptotic protein factors.63
The Dendrimers
Dendrimers nanosized polymers of the highest order of ramification.64 Researchers have developed a broad range of dendrimers in recent times, and new types of dendrimers continue to be designed and prepared. Because of their well-organized three-dimensional architecture and extensive surface functions, these hyperbranched polymers are regarded as attractive drug carriers.65–67 Drug molecules can be attached or embedded in the interior emptiness of dendrimers on the surface groups. Different functional groups can effectively accommodate therapeutic molecules and drugs on the dendrimer surface.68–70
Nanosystems, particularly dendrimers, have been developed to prevent some of the limitations of various conventional drugs, including (i) low water solubility, (ii) a slight absorption, (iii) low targeted ability, (iv) strong affinity for plasma proteins, (v) speedy drug elimination, and (vi) low biodistribution affinity.71 To be considered a promising excipient, the dendrimer must cross the organism’s biological barriers. The dendrimer’s size, chemical composition, surface structure, and shape all influence its volume of distribution and cytotoxicity. Furthermore, these qualities enable us to comprehend how dendrimers are metabolised as well as the long-term influence of dendrimers at the cell level.72
Using nanocarrier-based DDS for example dendrimers, nanomedicine has shown great promise in treating many CNS diseases. These nanocarriers have demonstrated promising features in CNS drug administration, such as minimal toxicity and immunogenicity, as well as enhanced drug solubility, stability, and permeability. Dendrimers also have more efficient paracellular and transcellular transport across the BBB, making them suitable carriers for transporting medications to the brain that are insoluble in water.73
Katare et al examined the potential of PAMAM dendrimer for intranasal efficacy of the water-insoluble antipsychotic drug haloperidol to advance the delivery of water-insoluble drugs to the brain. They found that the dendrimer-based formulation boosted haloperidol’s aqueous solubility. A higher distribution of haloperidol in the brain and plasma was seen in the experimental formulation than in a placebo control.74
The most famous dendrimer synthesis molecule may be poly (amidoamine) of PAMAM. The central part of PAMAM is the diamine (usually ethylenediamine), which is responded to generation-0 PAMAM by methyl acrylate and then by an additional ethylenediamine. Subsequent reactions create generations of higher levels. Dendrimers have shown interparental or intraventricular injections, that PAMAMs dendrimer functionality dramatically affects the diffusion into the CNS tissues in vivo and penetrates the live neurons.75,76 Kannan et al demonstrated that polyamidoamine dendrimers were supplied systemically to locate newborn rabbits with cerebral palsy in activated microglia and astrocytes and provide possibilities as a means of conveying therapeutic messages for the treatment of neuroinflammatory disorders.77 Liu et al encapsulated a Fourth-generation PAMAM dendrimer BBB-penetrating nanocarrier system, incorporating angiopep-2 peptide and then combining a new peptide to enhance the effect of glioma targeting following penetration of the epidermal factor receptor (EGFR).78 The anticancer medicine doxorubicin (DOX) was then fed into the interior vacuums via non-covalent connections. In reaction to the tumor’s acidic environment, the dendrimer channel controls the release of integrated medicines and decreased the toxic effects in vivo and in vitro for normal tissues. In addition, the combination of peptides with the dendrimer carriers significantly improved the penetration of BBB and enhanced their antitumor activities following BBB crossing.79 In vivo testing reveal the enhanced permeability of the BBB and anti-glioma effects of DOX by the twofold functionality of the dendrimer nanocarriers.80
These studies confirm that modified dendrimers will be future drug nanocarriers able to enter BBB following transcytosis and reach the glioma location for targeted brain cancer treatment. The figure shows the easiest way to build an active, targeted drug delivery nanoparticle for glioma ligand-decorated, interconnected with PEG to enhance bioavailability.81 A Simple approach for ligand-decorated nanoparticles, linked to PEG for increased bioavailability for active, targeted medication in the glioma sector is shown in Figure 3.
Figure 3.
Scientific approach for ligand-decorated nanoparticles, linked to PEG for brain targeting.
While the use of these nanostructures as a pharmacological excipient provides significant advantages, the toxicity of dendrimers is critical to assess. Because these cell components are of the same dimension, the dendrimers interact with the cell membrane, nucleus, and proteins because of the size of the cell (1–100 nm). Moreover, dendrimers can complex certain metal ions for the hemoglobin’s biological action and renal function, such as iron and zinc. Dendrimer toxicity is mostly determined by the charge of the dendrimer’s surface. Pharmacokinetics and bioavailability influence polymer toxicity in vivo. As a result, biodistribution tests become essential for determining more cells and tissues that can store the medication, resulting in higher potential toxicity.82
Polymeric Micelles
Micelles are an intriguing family of amphiphilic spherical nanomaterial’s that form when amphiphilic molecules self-aggregate in water over a specific critical concentration (critical micelle concentration).83 Both hydrophilic and hydrophobic domains are present in micelles.84 The hydrophilic region of the molecules surrounds the shell of micelles, Though this hydrophobic zone captures the lipophilic bioactives, the lipophilic region forms the cores, where the hydrophobic bioactives are entrapped.85,86 These attractive nanocarriers carry large levels of chemotherapeutic agents for targeting ovarian cancers specific targets. Polymeric micelles are made by amphiphilic copolymers that create polymeric micelles in aqueous conditions, having hydrophilic layers on the outside and hydrophobic cores.87 Stability can be improved by crosslinking the shell and core chains. Polymeric micelles are made with additional adjustable properties, and these enable them to be sensitive to external stimuli such as pH, light, temperature, ultrasound, etc., resulting in a regulated release of the pharmaceuticals contained within the micelle.88
Yin et al formulated a delivery system of nano-drug consisting of doxorubicin (lactic-glycolic) acidlysoGM1 micelles, with a good percentage of encapsulation of this low-solubility drug (TSI) (total 61%). In vivo studies of mouse and zebrafish, this system might easily pass through the BBB and build up in the brain parenchyma using micropinocytosis and lysosomal pathways. Nanoformulation has shown excellent anti-glioma outcomes in rats, which shows its potential as an anti-glioma medicine.89
Shiraishi et al carried out the study in which gadolinium-micelles (Gd-micelles) was made as a contrasting agent for MRI. Later intravenous injection into a rat for approximately half an hour, ischemic hemisphere contrasted images have shown the BBB and its distribution area in the ischemic hemisphere.90
Sonali et al fabricated the docetaxel (DTX)-transferrin-loaded Vitamin E TPGS micelles to treat brain tumors. Solvent casting method was employed to formulate the micelles with and without transferrin conjugate. These synthesized conjugate micelles achieved over 80% OF encapsulation efficiency, 520 nm size, and continued drug release throughout 24 hrs. Increased solubility, permeability, and targeted drug delivery enable polypills to better deliver prescribed medications to the patients who need them. TPGS micelles were found to be a promising nanocarrier for brain therapy, resulting in more prolonged and more effective DTX brain targeting than non-targeted micelle formulations.91
In recent years, micelles have also been a leader in targeted treating brain tumors with drugs amongst nanocarriers. Because of its nano-dimensions, the phagocytic system is not easily identified, nor are its hydrophilic shells more permeable and retainable.92,93 Agarwal et al. The mechanism of bio-adhesive micelles charged with docetaxel has been hypothesized for brain tumor therapy. Chitosan has been combined with transferrin during micelle formation because of its exceptional bioadhesive properties to obtain synergistically assisted transcytosis through both the chitosan and transferrin receptors. The use of the Docetaxel encapsulated micelles in glioma cells of C6 was improved by this nano therapy approach and the effectiveness of the bio-adhesive micelle suggested to treat brain tumors was demonstrated. After a treatment of 48 hours, the targeted and non-targeted nano-micelles bioavailability was 4.08 and 2.89 times higher than that of pristine docetaxel.94
Polymeric Nanoparticles
Polymer NPs have a polymer core that usually has medication that is dispersed into the matrix between 60 and 200 nm in diameter.95 In addition to various drug delivery formulations, many drug delivery carriers have been employed. Several polymers have recently found use in the medical field and have gained traction in the bioactive agent release category.96 Several of these products are degraded in the body. Polylactides (PLA), polyglycolide (PGA). Poly lactic-co-glycolic acid (PLGA), polyanhydrides, polycyanoacrylates, and polycaprolactone are the most popular ones. Natural polymers, such as chitosan, are also used despite developing several synthetic and semi-synthetic polymers.97 Furthermore, it reported that in terms of increased drug delivery to the brain, these technologies have been offered as polymeric NPs. Mice were administered to the PLGA embedding drugs (isoniazid, rifampicin, ethambutol and pyrazinamide), maintaining high drug levels 5–8 days in plasma and 9 days in the brain, which is significantly longer than free drug.98 Five NP dosages (compared to 46 administrations of traditional, free medicines) results in undetectable germs in the meninges Mycobacterium tuberculosis-infected mice. Polybutylcyanoacrylate (PBCA) NPs successfully delivered neurons and neuronal cell lines to functional proteins.44,99
The recent tests focused on using poly(lactide-co-glycolic) acid as a material to synthesize nanoparticles for encapsulating therapeutic agents for Alzheimer’s disease and brain cancer.100 It has been demonstrated that polymer nanoparticles are more effective at penetrating the brain, reducing oxidative stress, inflammation, and plaque stress, improve the delivery of curcumin in Alzheimer’s disease treatment, and improve doxorubicin internalization in human glioma cells, leading to cytotoxic effects for cancer cells.101 An in vivo experiment involving the co-delivery of cisplatin and bolden, an antioxidant agent using the poly (lactide co-glycolic) nanocarriers, also achieve a successful target delivery for therapeutic applications in brain therapy.102 In addition, the use of the positive-charge polymers, poly (ethylene Imine), and poly (ethylene imine) copolymers were reported as vehicles for gene delivery (L-lysine). The backbone of the polymer was fixed to increase the cytocompatibility of L-glutathione (the ethylene imine) which also enhance the passage of the blood–brain barrier. Thus, the potential of nanoparticles was demonstrated based on poly (ethylene imine) for providing gene therapy genes for brain cancer. Another polymer for synthesizing nanoparticles within the brain is poly (allylamine).103 Kynurenic acid has been encapsulated into the core-shell structure during in vitro and in vivo experiments and has demonstrated neurological disorders’ neuroprotective and therapeutic potential. Other trials have focused on andrographolide in serum-albumin-based nanoparticles and poly-ethyl cyanoacrylate nanoparticles to manage neurodegenerative inflammation disease.104,105 The results showed that nanoparticles used for human serum albumin are slightly more porous.
In contrast, nanoparticles used for the in vitro experiment have reversibly affected the integrity of the monolayer cells.106 The development delivered docetaxel of an amphiphilic polymer-lipid nanoparticles treatment system for brain metastasis.107 Tests conducted in vivo have shown that the accumulation of nanoparticles on the tumor site has been inhibited with tumor growth and median survival increased compared to an equivalent dose of clinically used docetaxel solution formulation.108 Chitosan combined with L-valine was used as a vehicle to treat Alzheimer’s disease, a hydrophilic healing agent, for the supply of saxagliptin. In vivo studies, plasma stability in nanoparticles has been demonstrated to prevent the premature release and increase brain supply compared to the suspension of saxagliptin.109 Figure 4 shows the polymeric nanoparticles targeting tumor cells to treat brain cancer.
Figure 4.
Mechanism of polymeric nanoparticles for brain tumor.
The Gold Nanoparticles
Researchers are fascinated by gold nanoparticles for over a century and have extensively used these nanocarriers for biomedical and theranostics applications. Au-NPs are heavily utilized owing to multifunctional characteristics in imaging, therapeutics, and drug delivery systems.110,111 Some remarkable characteristics of AuNPs include tunable nanomaterial properties, for example, porosity or optical responsiveness, and the comparatively large surface area responsible for the conjugation of different targeting ligands. Other notable features include low toxicity, biocompatibility, high-X-ray absorption coefficient and high-atomic number, ease of synthesis, and cost-effectiveness.112,113 However, synthetic nanoparticle-based delivery systems, including AuNPs, show less selectivity towards targeting cells due to the lack of specific moieties that differentiate concerning targeted and non-targeted sites. To overcome these issues, cell-targeting ligands (antibodies, proteins, peptides, or aptamers) have been combined with AuNPs, consequently leads to the efficient delivery of AuNPs to the brain.114,115 In treating several CNS-related disorders, eg, brain cancer, Alzheimer’s, Parkinsonism’s, and efficient delivery of drug cargoes and biological therapeutics across blood–brain barrier surfaces, modifications of AuNPs are needed.116,117
Khongkow and colleagues reported a promising platform of AuNPs with brain-targeted exosomes to develop novel nanomaterials. It is considered as brain-targeted AuNPs synthesis with exosomes supposed to be a promising strategy for targeting moieties into the brain. Exosomes were derived from genetically engineered mammalian cells, and the surface modification of AuNPs (Figure 5) was performed for easy penetration into the brain.118
Figure 5.
Modified Au nanoparticles for improved BBB penetration with neuron-targeted exosome.
In another study, Gonzalez et al prepared L-DOPA-decorated Au-NPs termed multi-branched nanoflowers and investigated their brain targeting ability and efficiency to cross the BBB. The seed-mediated method was used to synthesize these nano vehicles (L-DOPA-AuNFs), catechols, a type of molecule, are used as a direct reducing-cum-capping agent. Results indicate that L-DOPA-AuNFs can cross the BBB and more efficiently internalize without causing inflammation by brain macrophages than other AuNFs functionalized with a non-targeting ligand. These findings indicate that L-DOPA-AuNFs is an efficient nanocarrier for delivering drug cargoes into the brain and acting as non-inflammatory BBB-penetrating nano vehicles.45
Over brain capillaries, surface transferrin receptors (TfR) are a popular strategy for brain-targeting. Johnsen et al reported TfR-targeted gold nanoparticles (AuNPs) and their transport through the BBB to enter the brain parenchyma. Valency and affinity of the AuNP-conjugated antibodies have a significant impact on the uptake capacity. Results indicate that monovalent ligands have a favorable impact on attaining TfR-targeted nanomedicines’ transcytosis through the BBB and remarkably improve uptake capacity. In contrast, antibodies with low and high reactivity induce an intermediate and low absorption of AuNPs into the brain, accordingly.119
The Quantum Dots
Quantum dots (QDs) have been used extensively as nanocarriers for brain targeting and neurological disorders in recent years. QDs are artificial semiconductor nanocarriers with a size range of 100 nm with excitons restricted in all three spatial dimensions. QDs were discovered in the 1980s by Alexie Ekimov, having fluorescence (20 times brighter) than ordinary fluorescent materials.120 Owing to their remarkable property, including large absorption spectra, high photobleaching and stability, they are considered ideal candidates for diagnosis, sensing, drug delivery and targeting applications. The emission spectra of QDs are adjustable from 450 to 1800 nm by varying the shape, size, and composition.121,122
QDs, both conjugated and single, can visualize different structures extending either from brain vasculature or towards single receptor molecules. Additionally, for the complete understanding of tumor development mechanism and development of novel methods for tumor treatment, these fluorescent nanocarriers can be easily detected by targeted to tumors can be detected by optical imaging. So, the surgeon uses a valuable strategy to detect and identify the brain tumor during biopsy and resection in real-time.123 Some examples of several well-known quantum dots are silver QDs,124 gold QDs,125 carbon QDs,126 selenium QDs,127 and silicon QDs.128 In addition, studies are also available on graphene-based nanocarriers for example reduced graphene and graphene oxide.129,130
Central nervous system (CNS) related disorders are characterized by a wide-ranging brain illness with various disabilities.131 A new paradigm for CNS-related disorders (Alzheimer’s, Parkinsonism) is provided by the nanocarriers approach.132 Several new Nanoparticles have been used for brain-targeted applications. Because of the potential for medicinal products throughout BBB, graphene quantum dots (GQDs) are among those carbon-based nanoparticles. Also, contribute to the administration of tumor-specific drugs.133
One of the primary causes of dementia is Alzheimer’s (neurodegenerative disease), which is triggered because of amyloid peptide accumulation in the brain.134,135 Therefore, agents that act by inhibiting the aggregation of amyloid are mainly used as treatment strategies for Alzheimer’s.136 Among these agents, GQDs are reported as a promising treatment for Alzheimer’s by inhibiting the aggregation of amyloid β peptides. Additionally, GQDs are also preferred as they protect from the cytotoxicity of peptides.137 Correspondingly, tramiprosate affinity towards amyloid β peptide and after binding produce an inhibitory effect on their aggregation. Covalently linkage of GQDs with tramiprosate was reported as one of the effective inhibitors of amyloid-β aggregation, consequently a synergistic effect produced by their combination in treating Alzheimer’s disease.138
Among the neurodegenerative disorders, the second prevalent disease is Parkinson’s disease. It was evident that its pathogenesis was linked with the transmission and accumulation of α-synuclein (α-syn) aggregates in the midbrain.139,140 To date, no anti-aggregation agents reported as fruitful for the treatment of the disease; however, GQDs have therapeutic powers and protect cells against α-synuclein toxicity. In animals, GQDs prevent α-synuclein fibrillization, and its spread between neurons also promotes their disaggregation. Recently, a research group studied the in vivo permeability of the BBB by using GQD–biotin for immunohistochemical analysis of the brain. According to the results, an enormous amount of GQD–biotin was identified in the CNS region, along with the cerebellum, the olfactory bulb, neocortex, and midbrain specifying the in vivo ability of GQDs to penetrate the BBB in vivo. These promising activities promise and BBB permeability GQDs are considered an effective therapy against neuronal disorders.141
In another study, dual amperometric and fluorescence-sensitive curcumin-graphene QDs were fabricated as DNA sensors for a variety of neurologic and vascular disorders. Dual GQDs-ITO transparent electrode used to sense APO e4, biomarker protein, responsible for Alzheimer’s disease. The developed system reveals high metrological presentations, for example, reproducibility, selectivity, repeatability, and long storage stability. All experiments were carried out by using human blood plasma (clinical fluids).142
Several new nanoparticles have been used for brain-targeted applications. Because of the potential for medicinal products throughout BBB, GQDs are among those carbon-based nanoparticles that also contribute to the administration of tumor-specific in vivo molecular and cellular imaging. However, their low blood–brain barrier permeability. Their low blood–brain barrier permeability, and poor stability, on the other hand, is a concern and severely limit their ability to enter, and following parenteral injection, they operate on their target locations in the CNS. To overcome these issues, Gao and collages created a brain imaging device, in which poly (ethylene glycol) poly (lactic acid) nanoparticles were coated with QDs and injected into the brain through the nasal route. The resultant nanoparticles are water-soluble, stable, and have good brain focus and picture characteristics with high payload capacity. Because the surface of the nanoparticles is available with PEG functional terminal categories, this nanoprobe enables conjugating different biological ligands with substantial potential for the creation of specialized imaging agents for diverse CNS.143
Despite the number of studies and research on QDs, they may be toxic due to ROS generation and toxic elements such as cadmium, selenium, tellurium, etc. As a result, various strategies for reducing QD toxicity have been developed, the most common of which are non-toxic materials and surface coatings with biocompatible molecules.
The Future Perspectives
Conventional therapies for brain targeting often remain unsuitable for penetrating the brain by crossing the BBB to accomplish targeting roles. However, with the advent of nanotechnology, it has become possible to actively target brain cells. In this review, various nanocarrier-based DDS such as polymeric micelles, polymeric nanocarriers, dendrimers, liposomal systems, quantum dots (QDs), and gold nanoparticles have been discussed to penetrate the BBB with promising applications. The use of novel nanocarriers, their flexible properties, and in vivo targeting for CNS disorders are potential findings of this review with novel discoveries. On the other hand, safety concerns are of utmost importance before discussing the clinical applications of these nanocarriers. To summarize, we reviewed the currently developed nanoplatforms for brain targeting and promising strategies for CNS-related disorders, including GBM, AD, and PD. Several studies have made fruitful progress in the last decade in finding biomarkers and developing nanomedicines designed to target biomarkers, and clinicians are about to overcome the current constraints that impede the clinical translation of CNS-targeting therapies. To highlight the remarkable capabilities of hybrid nanomedicines, several in vitro and in vivo experiments have been performed, which have resulted in successful clinical translation. These advancements in nanotechnology will enable the development of more advanced multifunctional nanomedicines for the synthesis and functionalization of biomarkers and nanomedicines, with these approaches resulting in a substantial improvement in the markers and nanomedicines that can be used to battle central nervous system diseases.
Acknowledgment
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-Track Path of Research Funding Program.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Agrawal M, Saraf S, Saraf S, et al. Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin Drug Deliv. 2018;15(6):589–617. [DOI] [PubMed] [Google Scholar]
- 2.Singh AV, Chandrasekar V, Janapareddy P, et al. Emerging Application of Nanorobotics and Artificial Intelligence To Cross the BBB: advances in Design, Controlled Maneuvering, and Targeting of the Barriers. ACS Chem Neurosci. 2021;1:448–455. [DOI] [PubMed] [Google Scholar]
- 3.Aderibigbe BA. In situ-based gels for nose to brain delivery for the treatment of neurological diseases. Pharmaceutics. 2018;10(2):40. doi: 10.3390/pharmaceutics10020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal M, Saraf S, Saraf S, et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J Controlled Release. 2020;24:12–85. [DOI] [PubMed] [Google Scholar]
- 5.Persano F, Batasheva S, Fakhrullina G, Gigli G, Leporatti S, Fakhrullin R. Recent advances in the design of inorganic and nano-clay particles for the treatment of brain disorders. J Mater Chem B. 2021;9(12):2756–2784. [DOI] [PubMed] [Google Scholar]
- 6.Bandopadhyay S, Manchanda S, Chandra A, Ali J, Deb PK. Overview of different carrier systems for advanced drug delivery. Drug Delivery Systems. 2020;179–233. [Google Scholar]
- 7.Jena L, McErlean E, McCarthy H. Delivery across the blood-brain barrier: nanomedicine for glioblastoma multiforme. Drug Deliv Transl Res. 2020;10(2):304–318. doi: 10.1007/s13346-019-00679-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Controlled Release. 2018;270:290–303. doi: 10.1016/j.jconrel.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 9.Ding S, Khan AI, Cai X, et al. Overcoming blood–brain barrier transport: advances in nanoparticle-based drug delivery strategies. Materials Today. 2020;37:112–125. doi: 10.1016/j.mattod.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Yang H, Yang W, et al. Nanoparticle-based diagnostic and therapeutic systems for brain tumors. J Mater Chem B. 2019;7(31):4734–4750. doi: 10.1039/C9TB00860H [DOI] [PubMed] [Google Scholar]
- 11.Zottel A, Videtič Paska A, Jovčevska I. Nanotechnology meets oncology: nanomaterials in brain cancer research, diagnosis and therapy. Materials. 2019;12(10):1588. doi: 10.3390/ma12101588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Carter D, Liu X, Tockary TA, et al. Targeting nanoparticles to the brain by exploiting the blood–brain barrier impermeability to selectively label the brain endothelium. Proce Nat Acad Sci. 2020;117(32):19141–19150. doi: 10.1073/pnas.2002016117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choquet D, Sainlos M, Sibarita J-B. Advanced imaging and labelling methods to decipher brain cell organization and function. Nat Rev Neurosci. 2021;22(4):237–255. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Yao K, Wang Y, et al. Brain-Targeted Dual Site-Selective Functionalized Poly (β-Amino Esters) Delivery Platform for Nerve Regeneration. Nano Lett. 2021;21(7):3007–3015. doi: 10.1021/acs.nanolett.1c00175 [DOI] [PubMed] [Google Scholar]
- 15.Ng SY, Lee AYW. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528. doi: 10.3389/fncel.2019.00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2(4):492–516. doi: 10.1007/s12975-011-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantrill CA, Skinner RA, Rothwell NJ, Penny JI. An immortalised astrocyte cell line maintains the in vivo phenotype of a primary porcine in vitro blood–brain barrier model. Brain Res. 2012;1479:17–30. doi: 10.1016/j.brainres.2012.08.031 [DOI] [PubMed] [Google Scholar]
- 18.Lam C, Hansen E, Janson C, Bryan A, Hubel A. The characterization of arachnoid cell transport II: paracellular transport and blood–cerebrospinal fluid barrier formation. Neuroscience. 2012;222:228–238. doi: 10.1016/j.neuroscience.2012.06.065 [DOI] [PubMed] [Google Scholar]
- 19.Ayloo S, Gu C. Transcytosis at the blood–brain barrier. Curr Opin Neurobiol. 2019;57:32–38. doi: 10.1016/j.conb.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W. Adsorptive-mediated brain delivery systems. Curr Pharm Biotechnol. 2012;13(12):2340–2348. doi: 10.2174/138920112803341851 [DOI] [PubMed] [Google Scholar]
- 21.Stewart PA. Endothelial vesicles in the blood–brain barrier: are they related to permeability? Cell Mol Neurobiol. 2000;20(2):149–163. doi: 10.1023/A:1007026504843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Kataoka K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: the next generation. J Am Chem Soc. 2020;143(2):538–559. doi: 10.1021/jacs.0c09029 [DOI] [PubMed] [Google Scholar]
- 23.Mizrahy S, Gutkin A, Decuzzi P, Peer D. Targeting central nervous system pathologies with nanomedicines. J Drug Target. 2019;27(5–6):542–554. doi: 10.1080/1061186X.2018.1533556 [DOI] [PubMed] [Google Scholar]
- 24.Kopeček J, Kopečková P. HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev. 2010;62(2):122–149. doi: 10.1016/j.addr.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Han Y, Chen Q, et al. Dual endogenous stimuli-responsive polyplex micelles as smart two-step delivery nanocarriers for deep tumor tissue penetration and combating drug resistance of cisplatin. J Mater Chem B. 2014;2(13):1813–1824. doi: 10.1039/C3TB21383H [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181(1):151–167. doi: 10.1016/j.cell.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 27.Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR. Smart nanostructures for cargo delivery: uncaging and activating by light. J Am Chem Soc. 2017;139(13):4584–4610. doi: 10.1021/jacs.6b08313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlawat J, Guillama Barroso G, Masoudi Asil S, et al. Nanocarriers as potential drug delivery candidates for overcoming the blood–brain barrier: challenges and possibilities. ACS Omega. 2020;5(22):12583–12595. doi: 10.1021/acsomega.0c01592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sengul AB, Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett. 2020;2:1–25. [Google Scholar]
- 30.Quader S, Kataoka K. Nanomaterial-enabled cancer therapy. Mol Therapy. 2017;25(7):1501–1513. doi: 10.1016/j.ymthe.2017.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain K. Nanobiotechnology-based drug delivery to the central nervous system. Neurodegenerative Dis. 2007;4(4):287–291. doi: 10.1159/000101884 [DOI] [PubMed] [Google Scholar]
- 32.Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Impact of nanoparticles on brain health: an up to date overview. J Clin Med. 2018;7(12):490. doi: 10.3390/jcm7120490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatoo MA, Naseem S, Arfat MY, Mahmood Dar A, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014;2014:4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasui T, Kaji N, Baba Y. Nanobiodevices for biomolecule analysis and imaging. Annu Rev Analytical Chem. 2013;6:83–96. doi: 10.1146/annurev-anchem-062012-092619 [DOI] [PubMed] [Google Scholar]
- 35.Frank D, Tyagi C, Tomar L, et al. Overview of the role of nanotechnological innovations in the detection and treatment of solid tumors. Int J Nanomedicine. 2014;9:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petkar KC, Chavhan SS, Agatonovik-Kustrin S, Sawant K. Nanostructured materials in drug and gene delivery: a review of the state of the art. Critical Rev Therapeutic Drug Carrier Sys. 2011;28(2). doi: 10.1615/CritRevTherDrugCarrierSyst.v28.i2.10 [DOI] [PubMed] [Google Scholar]
- 37.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent effects of RGD peptides obtained by nanoparticle display. J Med Chem. 2006;49(20):6087–6093. doi: 10.1021/jm060515m [DOI] [PubMed] [Google Scholar]
- 38.Shi D, Mi G, Shen Y, Webster TJ. Glioma-targeted dual functionalized thermosensitive Ferri-liposomes for drug delivery through an in vitro blood–brain barrier. Nanoscale. 2019;11(32):15057–15071. doi: 10.1039/C9NR03931G [DOI] [PubMed] [Google Scholar]
- 39.Soliman GM, Sharma R, Choi AO, et al. Tailoring the efficacy of nimodipine drug delivery using nanocarriers based on A2B miktoarm star polymers. Biomaterials. 2010;31(32):8382–8392. doi: 10.1016/j.biomaterials.2010.07.039 [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Li J, Han S, et al. A novel doxorubicin loaded folic acid conjugated PAMAM modified with borneol, a nature dual-functional product of reducing PAMAM toxicity and boosting BBB penetration. Eur J Pharmaceutical Sci. 2016;88:178–190. doi: 10.1016/j.ejps.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Liu C, Pang Z. Dendrimer-based drug delivery systems for brain targeting. Biomolecules. 2019;9(12):790. doi: 10.3390/biom9120790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abourehab MA, Ahmed OA, Balata GF, Almalki WH. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int J Nanomedicine. 2018;13:3679. doi: 10.2147/IJN.S168148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang R, Zheng Y, Wang Q, Zhao L. Curcumin-loaded chitosan–bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res Lett. 2018;13(1):1–9. doi: 10.1186/s11671-018-2759-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasadsri L, Kreuter J, Hattori H, Iwasaki T, George JM. Functional protein delivery into neurons using polymeric nanoparticles. J Biol Chem. 2009;284(11):6972–6981. doi: 10.1074/jbc.M805956200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Carter DA, Ong ZY, McGilvery CM, Dunlop IE, Dexter DT, Porter AE. L-DOPA functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles. Nanomedicine. 2019;15(1):1–11. doi: 10.1016/j.nano.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 46.Li S, Su W, Wu H, et al. Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nat Biomed Eng. 2020;4(7):704–716. doi: 10.1038/s41551-020-0540-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paris-Robidas S, Brouard D, Emond V, Parent M, Calon F. Internalization of targeted quantum dots by brain capillary endothelial cells in vivo. J Cerebral Blood Flow Metab. 2016;36(4):731–742. doi: 10.1177/0271678X15608201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chauhan SB, Gupta V. Recent advances in liposome. Res J Pharm Tech. 2020;13:2051–2056. [Google Scholar]
- 49.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 50.Li M, Du C, Guo N, et al. Composition design and medical application of liposomes. Eur J Med Chem. 2019;164:640–653. doi: 10.1016/j.ejmech.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 51.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632 [DOI] [PubMed] [Google Scholar]
- 52.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975. doi: 10.2147/IJN.S68861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. [DOI] [PubMed] [Google Scholar]
- 54.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297. [PMC free article] [PubMed] [Google Scholar]
- 55.Riaz MK, Riaz MA, Zhang X, et al. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: a review. Int J Mol Sci. 2018;19(1):195. doi: 10.3390/ijms19010195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lakkadwala S, Dos Santos Rodrigues B, Sun C, Singh J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J Controlled Release. 2019;307:247–260. doi: 10.1016/j.jconrel.2019.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, Vallejo-Cardona AA. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 2019;10(1):1–40. doi: 10.1186/s12645-019-0055-y [DOI] [Google Scholar]
- 58.Xing H, Hwang K, Lu Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics. 2016;6(9):1336. doi: 10.7150/thno.15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lakkadwala S, Singh J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf B Biointerfaces. 2019;173:27–35. doi: 10.1016/j.colsurfb.2018.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muthu MS, Kulkarni SA, Xiong J, Feng -S-S. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int J Pharm. 2011;421(2):332–340. doi: 10.1016/j.ijpharm.2011.09.045 [DOI] [PubMed] [Google Scholar]
- 61.Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly (ethylene glycol)-co-poly (lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Controlled Release. 2010;143(1):136–142. doi: 10.1016/j.jconrel.2009.12.020 [DOI] [PubMed] [Google Scholar]
- 62.Qiao Y, Wan J, Zhou L, et al. Stimuli‐responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(1):e1527. [DOI] [PubMed] [Google Scholar]
- 63.Postma T, Heimans J, Luykx S, et al. A Phase II study of paclitaxel in chemonaive patients with recurrent high-grade glioma. Ann Oncol. 2000;11(4):409–413. doi: 10.1023/A:1008376123066 [DOI] [PubMed] [Google Scholar]
- 64.Chis AA, Dobrea C, Morgovan C, et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules. 2020;25(17):3982. doi: 10.3390/molecules25173982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nanjwade BK, Bechra HM, Derkar GK, Manvi F, Nanjwade VK. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharmaceutical Sci. 2009;38(3):185–196. doi: 10.1016/j.ejps.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 66.Caminade A-M, Turrin C-O. Dendrimers for drug delivery. J Mater Chem B. 2014;2(26):4055–4066. doi: 10.1039/C4TB00171K [DOI] [PubMed] [Google Scholar]
- 67.Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139. doi: 10.4103/0975-7406.130965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noriega-Luna B, Godínez LA, Rodríguez FJ, Rodríguez A. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J Nanomater. 2014;2014. doi: 10.1155/2014/507273 [DOI] [Google Scholar]
- 69.Lloveras V, Vidal-Gancedo J. Polyphosphorhydrazone-Based Radical Dendrimers. Molecules. 2021;26(5):1230. doi: 10.3390/molecules26051230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen DH, Bach LG, Nguyen Tran D-H, et al. Partial surface modification of low generation polyamidoamine dendrimers: gaining insight into their potential for improved carboplatin delivery. Biomolecules. 2019;9(6):214. doi: 10.3390/biom9060214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rajani C, Borisa P, Karanwad T, et al. Cancer-targeted chemotherapy: emerging role of the folate anchored dendrimer as drug delivery nanocarrier. Pharmaceutical App Dendrimers. 2020;151–198. [Google Scholar]
- 72.Santos AICFd. Dendrimers as Pharmaceutical Excipients. Universidade de Coimbra; 2019. [Google Scholar]
- 73.Parajapati SK, Maurya SD, Das MK, Tilak VK, Verma KK, Dhakar RC. Potential application of dendrimers in drug delivery: a concise review and update. J Drug Delivery Therapeutics. 2016;6(2):71–88. doi: 10.22270/jddt.v6i2.1195 [DOI] [Google Scholar]
- 74.Katare YK, Daya RP, Sookram Gray C, et al. Brain targeting of a water insoluble antipsychotic drug haloperidol via the intranasal route using PAMAM dendrimer. Mol Pharm. 2015;12(9):3380–3388. doi: 10.1021/acs.molpharmaceut.5b00402 [DOI] [PubMed] [Google Scholar]
- 75.Dhanikula RS, Hammady T, Hildgen P. On the mechanism and dynamics of uptake and permeation of polyether-copolyester dendrimers across an in vitro blood–brain barrier model. J Pharm Sci. 2009;98(10):3748–3760. doi: 10.1002/jps.21669 [DOI] [PubMed] [Google Scholar]
- 76.Albertazzi L, Gherardini L, Brondi M, et al. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm. 2013;10(1):249–260. doi: 10.1021/mp300391v [DOI] [PubMed] [Google Scholar]
- 77.Kannan S, Dai H, Navath RS, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4(130):130ra46–ra46. doi: 10.1126/scitranslmed.3003162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu C, Zhao Z, Gao H, et al. Enhanced blood-brain-barrier penetrability and tumor-targeting efficiency by peptide-functionalized poly (amidoamine) dendrimer for the therapy of gliomas. Nanotheranostics. 2019;3(4):311. doi: 10.7150/ntno.38954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh AV, Maharjan R-S, Kanase A, et al. Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells. ACS Appl Mater Interfaces. 2020;13(1):1943–1955. doi: 10.1021/acsami.0c18470 [DOI] [PubMed] [Google Scholar]
- 80.Mariyam M, Ghosal K, Thomas S, Kalarikkal N, Latha MS. Dendrimers: general aspects, applications and structural exploitations as prodrug/drug-delivery vehicles in current medicine. Mini Rev Med Chem. 2018;18(5):439–457. doi: 10.2174/1389557517666170512095151 [DOI] [PubMed] [Google Scholar]
- 81.Muniswamy VJ, Raval N, Gondaliya P, Tambe V, Kalia K, Tekade RK. ‘Dendrimer-Cationized-Albumin’encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int J Pharm. 2019;555:77–99. doi: 10.1016/j.ijpharm.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 82.Santos A, Veiga F, Figueiras A. Dendrimers as pharmaceutical excipients: synthesis, properties, toxicity and biomedical applications. Materials. 2020;13(1):65. doi: 10.3390/ma13010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanafy NA, El-Kemary M, Leporatti S. Micelles structure development as a strategy to improve smart cancer therapy. Cancers. 2018;10(7):238. doi: 10.3390/cancers10070238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakaskar RR. General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J Drug Target. 2018;26(4):311–318. doi: 10.1080/1061186X.2017.1367006 [DOI] [PubMed] [Google Scholar]
- 85.Pantshwa JM, Kondiah PP, Choonara YE, Marimuthu T, Pillay V. Nanodrug Delivery Systems for the Treatment of Ovarian Cancer. Cancers. 2020;12(1):213. doi: 10.3390/cancers12010213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu F, Jiang F, Tang X, Wang B. N-octyl-N-arginine-chitosan micelles for gambogic acid intravenous delivery: characterization, cell uptake, pharmacokinetics, and biodistribution. Drug Dev Ind Pharm. 2018;44(4):615–623. doi: 10.1080/03639045.2017.1405973 [DOI] [PubMed] [Google Scholar]
- 87.Fathi M, Majidi S, Zangabad PS, Barar J, Erfan‐Niya H, Omidi Y. Chitosan‐based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med Res Rev. 2018;38(6):2110–2136. doi: 10.1002/med.21506 [DOI] [PubMed] [Google Scholar]
- 88.Yan L, Li X. Biodegradable stimuli-responsive polymeric micelles for treatment of malignancy. Curr Pharm Biotechnol. 2016;17(3):227–236. doi: 10.2174/138920101703160206142821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin Y, Wang J, Yang M, et al. Penetration of the blood–brain barrier and the anti-tumour effect of a novel PLGA-lysoGM1/DOX micelle drug delivery system. Nanoscale. 2020;12(5):2946–2960. doi: 10.1039/C9NR08741A [DOI] [PubMed] [Google Scholar]
- 90.Shiraishi K, Wang Z, Kokuryo D, Aoki I, Yokoyama M. A polymeric micelle magnetic resonance imaging (MRI) contrast agent reveals blood–brain barrier (BBB) permeability for macromolecules in cerebral ischemia-reperfusion injury. J Controlled Release. 2017;253:165–171. doi: 10.1016/j.jconrel.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 91.Sonali Agrawal P, Singh RP, Rajesh CV, et al. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats. Drug Deliv. 2016;23(5):1788–1798. doi: 10.3109/10717544.2015.1094681 [DOI] [PubMed] [Google Scholar]
- 92.Mochida Y, Cabral H, Kataoka K. Polymeric micelles for targeted tumor therapy of platinum anticancer drugs. Expert Opin Drug Deliv. 2017;14(12):1423–1438. doi: 10.1080/17425247.2017.1307338 [DOI] [PubMed] [Google Scholar]
- 93.Upponi JR, Jerajani K, Nagesha DK, et al. Polymeric micelles: theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials. 2018;170:26–36. doi: 10.1016/j.biomaterials.2018.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agrawal P, Singh RP, Sharma G, et al. Bioadhesive micelles of d-α-tocopherol polyethylene glycol succinate 1000: synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf B Biointerfaces. 2017;152:277–288. doi: 10.1016/j.colsurfb.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 95.Bhavna S, Ali M, Baboota S, et al. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev Ind Pharm. 2014;40(2):278–287. doi: 10.3109/03639045.2012.758130 [DOI] [PubMed] [Google Scholar]
- 96.Madan J, Pandey RS, Jain V, Katare OP, Chandra R, Katyal A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in glioblastoma cells. Nanomedicine. 2013;9(4):492–503. doi: 10.1016/j.nano.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 97.Zhang X, Chen G, Wen L, et al. Novel multiple agents loaded PLGA nanoparticles for brain delivery via inner ear administration: in vitro and in vivo evaluation. Eur J Pharmaceutical Sci. 2013;48(4–5):595–603. doi: 10.1016/j.ejps.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 98.Choonara YE, Pillay V, Ndesendo VM, et al. Polymeric emulsion and crosslink-mediated synthesis of super-stable nanoparticles as sustained-release anti-tuberculosis drug carriers. Colloids Surf B Biointerfaces. 2011;87(2):243–254. doi: 10.1016/j.colsurfb.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 99.Pandey R, Khuller G. Oral nanoparticle-based antituberculosis drug delivery to the brain in an experimental model. J Antimicrobial Chemotherapy. 2006;57(6):1146–1152. doi: 10.1093/jac/dkl128 [DOI] [PubMed] [Google Scholar]
- 100.Zhang -T-T, Li W, Meng G, Wang P, Liao W. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater Sci. 2016;4(2):219–229. doi: 10.1039/C5BM00383K [DOI] [PubMed] [Google Scholar]
- 101.Barbara R, Belletti D, Pederzoli F, et al. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int J Pharm. 2017;526(1–2):413–424. doi: 10.1016/j.ijpharm.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 102.Malinovskaya Y, Melnikov P, Baklaushev V, et al. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int J Pharm. 2017;524(1–2):77–90. doi: 10.1016/j.ijpharm.2017.03.049 [DOI] [PubMed] [Google Scholar]
- 103.Gao S, Tian H, Xing Z, et al. A non-viral suicide gene delivery system traversing the blood brain barrier for non-invasive glioma targeting treatment. J Controlled Release. 2016;243:357–369. doi: 10.1016/j.jconrel.2016.10.027 [DOI] [PubMed] [Google Scholar]
- 104.Mondal J, Patra M, Panigrahi AK, Khuda-Bukhsh AR. Boldine-loaded PLGA nanoparticles have improved efficiency of drug carriage and protective potential against Cisplatin-induced toxicity. J Ayurveda Integr Med. 2018;1:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varga N, Csapó E, Majláth Z, et al. Targeting of the kynurenic acid across the blood–brain barrier by core-shell nanoparticles. Eur J Pharmaceutical Sci. 2016;86:67–74. doi: 10.1016/j.ejps.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 106.Guccione C, Oufir M, Piazzini V, et al. Andrographolide-loaded nanoparticles for brain delivery: formulation, characterisation and in vitro permeability using hCMEC/D3 cell line. Eur J Pharmaceutics Biopharmaceutics. 2017;119:253–263. doi: 10.1016/j.ejpb.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 107.Englert C, Trützschler A-K, Raasch M, et al. Crossing the blood-brain barrier: glutathione-conjugated poly (ethylene imine) for gene delivery. J Controlled Release. 2016;241:1–14. doi: 10.1016/j.jconrel.2016.08.039 [DOI] [PubMed] [Google Scholar]
- 108.He C, Cai P, Li J, et al. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J Controlled Release. 2017;246:98–109. doi: 10.1016/j.jconrel.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 109.Fernandes J, Ghate MV, Mallik SB, Lewis SA. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int J Pharm. 2018;547(1–2):563–571. doi: 10.1016/j.ijpharm.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 110.Li W, Cao Z, Liu R, et al. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif Cells, Nanomed Biotechnol. 2019;47(1):4222–4233. doi: 10.1080/21691401.2019.1687501 [DOI] [PubMed] [Google Scholar]
- 111.Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. doi: 10.3390/ijms19071979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meola A, Rao J, Chaudhary N, Sharma M, Chang SD. Gold nanoparticles for brain tumor imaging: a systematic review. Front Neurol. 2018;9:328. doi: 10.3389/fneur.2018.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siddique S, Chow JC. Gold nanoparticles for drug delivery and cancer therapy. App Sci. 2020;10(11):3824. doi: 10.3390/app10113824 [DOI] [Google Scholar]
- 114.Gao Y, Li Y. Gold Nanostructures for Cancer Imaging and Therapy. Advances in Nanotheranostics I: Springer; 2016:53–101. [Google Scholar]
- 115.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Spherical Nucleic Acids. 2020;55–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morshed RA, Muroski ME, Dai Q, et al. Cell-penetrating peptide-modified gold nanoparticles for the delivery of doxorubicin to brain metastatic breast cancer. Mol Pharm. 2016;13(6):1843–1854. doi: 10.1021/acs.molpharmaceut.6b00004 [DOI] [PubMed] [Google Scholar]
- 117.Raliya R, Saha D, Chadha TS, Raman B, Biswas P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci Rep. 2017;7(1):1–8. doi: 10.1038/srep44718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khongkow M, Yata T, Boonrungsiman S, Ruktanonchai UR, Graham D, Namdee K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-44569-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnsen KB, Bak M, Kempen PJ, et al. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics. 2018;8(12):3416. doi: 10.7150/thno.25228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jain S, Park SB, Pillai SR, Ryan PL, Willard ST, Feugang JM. Applications of fluorescent quantum dots for reproductive medicine and disease detection. Unraveling the Safety Profile of Nanoscale Particles and Materials—From Biomedical to Environmental Applications. 2018.
- 121.Madni A, Batool A, Noreen S, et al. Novel nanoparticulate systems for lung cancer therapy: an updated review. J Drug Target. 2017;25(6):499–512. doi: 10.1080/1061186X.2017.1289540 [DOI] [PubMed] [Google Scholar]
- 122.Sharma A, Sharma R, Bhatia N, Kumari A. Review on Synthesis, Characterization and Applications of Silver Sulphide Quantum Dots. J Mater Sci Res Rev. 2021;42–58. [Google Scholar]
- 123.Cabral Filho PE, Cardoso AL, Pereira MI, et al. CdTe quantum dots as fluorescent probes to study transferrin receptors in glioblastoma cells. Biochimica et Biophysica Acta. 2016;1860(1):28–35. doi: 10.1016/j.bbagen.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 124.Sikorska K, Grądzka I, Sochanowicz B, et al. Diminished amyloid-β uptake by mouse microglia upon treatment with quantum dots, silver or cerium oxide nanoparticles: nanoparticles and amyloid-β uptake by microglia. Hum Exp Toxicol. 2020;39(2):147–158. doi: 10.1177/0960327119880586 [DOI] [PubMed] [Google Scholar]
- 125.Norouzi M. Gold nanoparticles in glioma theranostics. Pharmacol Res. 2020;156:104753. [DOI] [PubMed] [Google Scholar]
- 126.Zhou T, Huang Z, Wan F, Sun Y. Carbon quantum dots-stabilized Pickering emulsion to prepare NIR light-responsive PLGA drug delivery system. Mater Today Commu. 2020;23:100951. doi: 10.1016/j.mtcomm.2020.100951 [DOI] [Google Scholar]
- 127.Luo W, Wang Y, Lin F, et al. Selenium-Doped Carbon Quantum Dots Efficiently Ameliorate Secondary Spinal Cord Injury via Scavenging Reactive Oxygen Species. Int J Nanomedicine. 2020;15:10113. doi: 10.2147/IJN.S282985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chinnathambi S, Chen S, Ganesan S, Hanagata N. Silicon quantum dots for biological applications. Adv Healthcare Mater. 2014;3(1):10–29. doi: 10.1002/adhm.201300157 [DOI] [PubMed] [Google Scholar]
- 129.Madni A, Noreen S, Maqbool I, et al. Graphene-based nanocomposites: synthesis and their theranostic applications. J Drug Target. 2018;26(10):858–883. doi: 10.1080/1061186X.2018.1437920 [DOI] [PubMed] [Google Scholar]
- 130.Chen J, Yu Q, Fu W, et al. A highly sensitive amperometric glutamate oxidase microbiosensor based on a reduced graphene oxide/prussian blue nanocube/gold nanoparticle composite film-modified pt electrode. Sensors. 2020;20(10):2924. doi: 10.3390/s20102924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen W, Huang L, Tang Q, Wang S, Hu C, Zhang X. Central Nervous System Tuberculosis: challenge and Perspective. Radiol Infect Dis. 2020. doi: 10.1016/j.jrid.2020.07.005 [DOI] [Google Scholar]
- 132.Saeedi M, Eslamifar M, Khezri K, Dizaj SM. Applications of nanotechnology in drug delivery to the central nervous system. Biomed Pharmacother. 2019;111:666–675. doi: 10.1016/j.biopha.2018.12.133 [DOI] [PubMed] [Google Scholar]
- 133.Edis Z, Wang J, Waqas MK, Ijaz M, Ijaz M. Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. Int J Nanomedicine. 2021;16:1313. doi: 10.2147/IJN.S289443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):1–18. doi: 10.1186/s13024-019-0333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener. 2020;15(1):1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giorgetti S, Greco C, Tortora P, Aprile FA. Targeting amyloid aggregation: an overview of strategies and mechanisms. Int J Mol Sci. 2018;19(9):2677. doi: 10.3390/ijms19092677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y, Xu L-P, Dai W, Dong H, Wen Y, Zhang X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale. 2015;7(45):19060–19065. doi: 10.1039/C5NR06282A [DOI] [PubMed] [Google Scholar]
- 138.Liu Y, Xu L-P, Wang Q, Yang B, Zhang X. Synergistic inhibitory effect of GQDs–tramiprosate covalent binding on amyloid aggregation. ACS Chem Neurosci. 2017;9(4):817–823. doi: 10.1021/acschemneuro.7b00439 [DOI] [PubMed] [Google Scholar]
- 139.Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, Moratalla R. Modeling Parkinson’s disease with the alpha-synuclein protein. Front Pharmacol. 2020;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alegre-Abarrategui J, Brimblecombe KR, Roberts RF, et al. Selective vulnerability in α-synucleinopathies. Acta Neuropathol. 2019;138(5):681–704. doi: 10.1007/s00401-019-02010-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yoo JM. Graphene Quantum Dots Prevent Α-Synucleinopathy in Parkinson’s Disease. Studies on Graphene-Based Nanomaterials for Biomedical Applications: Springer; 2020:29–64. [Google Scholar]
- 142.Mars A, Hamami M, Bechnak L, Patra D, Raouafi N. Curcumin-graphene quantum dots for dual mode sensing platform: electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal Chim Acta. 2018;1036:141–146. doi: 10.1016/j.aca.2018.06.075 [DOI] [PubMed] [Google Scholar]
- 143.Gao X, Chen J, Chen J, Wu B, Chen H, Jiang X. Quantum dots bearing lectin-functionalized nanoparticles as a platform for in vivo brain imaging. Bioconjug Chem. 2008;19(11):2189–2195. doi: 10.1021/bc8002698 [DOI] [PubMed] [Google Scholar]