Abstract
The endothelium forms a selective barrier between circulating blood or lymph and surrounding tissue. Endothelial cells play an essential role in vessel homeostasis, and identification of these cells is critical in vascular biology research. However, characteristics of endothelial cells differ depending on the location and type of blood or lymph vessel. Endothelial cell subsets are numerous and often identified using different flow cytometric markers, making immunophenotyping these cells complex. In part 1 of this two part review series, we present a comprehensive overview of markers for the flow cytometric identification and phenotyping of murine endothelial subsets. These subsets can be distinguished using a panel of cell surface and intracellular markers shared by all endothelial cells in combination with additional markers of specialized endothelial cell types. This review can be used to determine best markers for identifying and phenotyping desired murine endothelial cell subsets.
Keywords: Murine, endothelial cell, phenotyping, immunophenotyping, flow cytometry, endothelial cell subset
Introduction
Endothelial cell function and morphology are primarily determined by location. The plasticity of endothelial cells allows change of function and phenotype depending on the microenvironment. Proper identification and understanding of specific endothelial cell phenotypes grant valuable insight into tissue-specific physiology and pathology. As endothelial dysfunction is increasingly used as a diagnostic tool, the importance of accurately detecting targeted endothelial cell phenotypes is key to unlocking further endothelium-based medical diagnostics and research. Flow cytometric phenotyping of endothelial cells begins with selecting endothelial cell-specific markers. Combining pan-endothelial antigens with one or more dump channel markers allows for the gating of all endothelial cells that can be further analyzed and segregated into subsets. Commonly used pan-endothelial antigens and dump channels are listed in Table 1. Listed dump channel markers can be substituted for any other established cell-specific antigen.
Table 1.
Murine Pan-Endothelial Cell Markers/Probes and Dump Channels
| Pan-Endothelial Markers | References |
| Cell Surface | |
| CD31 (PECAM-1) | 36,37,38 |
| CD105 (Endoglin) | 23,24 |
| CD144 (VE-cad) | 39 |
| CD146 (P1H12, MCAM, MUC18, S-endo-1) | 40 |
| CD202b (Tie-2) | 38,41,42,43 |
| CD309 (VEGFR-2, KDR, Flk-1) | 38,44 |
| VEGFR-1 (Flk-1) | 29,45 |
| Tie-1 | 38,41,42 |
| Intracellular | |
| Dil-Ac-LDL Uptake | 46 |
| vWF | 25,26 |
| eNos | 47,48 |
| Dump Channels | |
| E-Cad (Epithelial Cells) | 49 |
| CD42 (Platelets) | 13 |
| CD45 (Hematopoietic Cells) | 33 |
| CD117 (c-kit) (Hematopoietic and Progenitor Cells) | 27,28,50 |
| CD133 (Stem Cells) | 30 |
| CD326 (EpCAM) (Epithelial Cells) | 51,49,39 |
Framed markers are used for both murine and human panels.
Organ-Specific Endothelial Phenotypes
Differentiation of the endothelium into specialized endothelial cells with organ-specific heterogeneity allows for further flow cytometric profiling. Lymphatic endothelial cells, for example, are functionally distinct from blood vessel endothelial cells and can be recognized by the expression of markers-such as CD90-absent from the blood endothelium in mice 1,2. Specific identifiable properties of specialized endothelial cells include loose junctions and lack of a basement membrane in the lymph, large and diaphragm-free fenestrae in the liver sinusoids, diaphragmed fenestrae in the renal peritubular capillaries, highly fenestrated islet capillaries in the pancreas, tight junctions forming a continuous barrier in the brain, and a lack of Weibel-Palade bodies in the alveolar capillaries 3,4,5,6. The unique characteristics of these endothelial cells enable the use of tissue-specific markers listed in Table 2 for identification of organ-specific phenotypes.
Table 2.
Murine Organ-specific Endothelial Cells
| Markers | ||
|---|---|---|
| Positive | Negative | |
| Lymphatic | CD90 1,2, LYVE-1 4, VEGFR-3 (Flt-4) 7, PROX-1 22 | vWF 25, PV-1 (PAL-E, PLVAP, MECA32) 52 |
| Liver Sinusoidal | LYVE-1 4,6 | PV-1 (PAL-E, PLVAP, MECA32) 34, PROX1* 16 |
| Renal Peritubular Capillary | PV-1 (PAL-E, PLVAP, MECA32) 33 | PDGFR-β 33, FSP-1 33 |
| Central Nervous System | CD133 32, Sca-1 32 | PV-1 (PAL-E, PLVAP, MECA32) * 53,54 |
| Spleen | LYVE-1 4, PV-1 (PAL-E, PLVAP, MECA32) 52 | |
| Pancreatic Islet | CD117 (c-kit) 32 | |
| Pulmonary | Sca-1 55 | |
| Alveolar Capillary | Sca-1 55 | vWF 26 |
Markers indicated with * are positive following injury. Markers in bold are the most commonly used to discriminate endothelial cells belonging to each listed district. Framed markers are used for both murine and human panels.
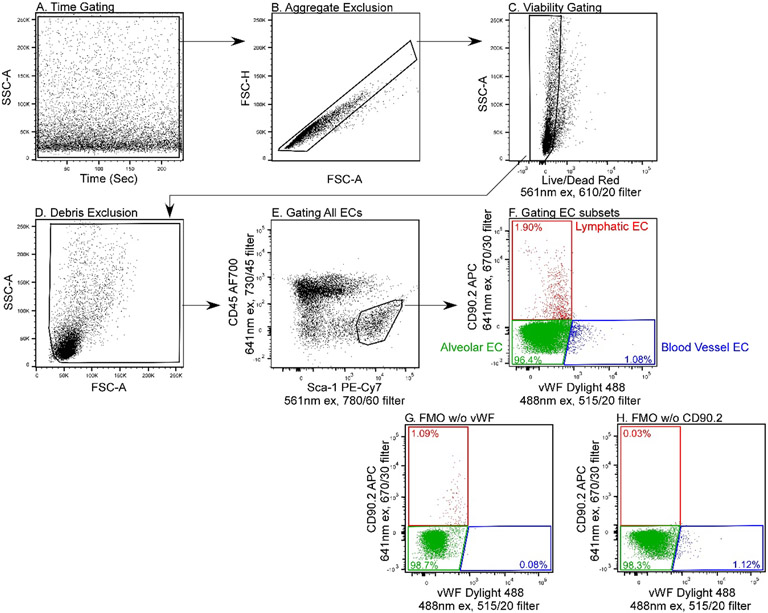
Characterization of described endothelial subsets should always follow a validated gating strategy. Figure 1 demonstrates an example gating strategy to identify three main murine pulmonary endothelial subsets. After excluding dead cells via a live/dead dye, an initial gate of CD45−/Sca-1+ selects all endothelial cells. The endothelial subsets can then be differentiated based on expression of CD90.2 and vWF. Alveolar capillary, lymphatic, and non-alveolar vascular endothelial cells are identified as CD90.2−/vWF−, CD90.2+/vWF−, and CD90.2−/vWF+, respectively.
Figure 1. Example Gating Strategy for Identification of Murine Pulmonary Endothelial Subsets.
Artifact exclusion included time gating to identify and exclude fluidic disturbances (A), removal of aggregates (B), selection of live cells based on uptake of a live/dead dye (C), and exclusion of debris based on low forward scatter (D). Unlike white blood cells, the FSC/SSC of endothelial cells is not characteristic. The best practice is to set the FSC threshold to acquire all cells and gate endothelial cells based on their specific antigen expression patterns. Pan-endothelial cells are selected as CD45−/Sca-1+ (E) then, based on expression of CD90.2 and Von Willebrand Factor lymphatic ECs (CD90.2+/vWF−), alveolar ECs (CD90.2−/vWF−), and non-alveolar ECs (CD90.2−/vWF+) were differentiated (F). Fluorescence minus one (FMO) controls containing all of the fluorophores in the panel except vWF (G) and all fluorophores except CD90.2 (H) were used to create the EC differentiation gates shown in plot F.
Endothelial Cell Subsets within Organs
Further specialization into various endothelial cell subsets can be identified using a combination of cell surface markers and intracellular markers. Combining constitutive endothelial markers with those listed in Table 3 permit endothelial cells to be segregated by vessel size, vessel type, and stemness.
Table 3.
Murine Endothelial cell phenotypes segregated by vessel size, vessel type, stemness, and inducibility
| Markers | |||
|---|---|---|---|
| Positive | Negative | ||
| Vessel Size | |||
| Large Vessel | CD157 56, Helix Pomatia Lectin (HPA) 57,58 | Griffonia Simplicifolia Lectin (GS-1) 57,58, Glycine Max Lectin (SBA) 57,58 | |
| Microvascular | Griffonia Simplicifolia Lectin (GS-1) 57,58, Glycine Max Lectin (SBA) 57,58 | CD157 56, Helix Pomatia Lectin (HPA) 57,58 | |
| Stemness | |||
| E-SP | CD34 59, CD157 60,56,61, CD200 60,56, Sca-1 59,62 | ||
| ECFC | CD117 (c-kit) 29, CD133 31 | PDGFR-β 33,63 | |
| Endothelial Stem Cell | CD157 60,56,61, CD200 60,56, Procr 64,65, Sca-1 64 | PDGFR-β 33,63 | |
| Lymph Node High Endothelial Venules (HEVs) | |||
| Mucosal | VEGFR-3 (Flt4) 7, MECA367 (MAdCAM-1) 8,9 | MECA-79 8,9 | |
| Peripheral Lymph Node | VEGFR-3 (Flt4) 7, MECA-79 8,9 | MECA367 (MAdCAM-1) 8,9 | |
| Other EC Subsets | |||
| Arterial | Ephrin β2 66,67 , Depp 68 | Ephrin β4 66,67 | |
| Venous | Ephrin β4 66,67,69,70 | Ephrin β2 66,67,69,70, Depp 68 | |
| Inducible Markers | CD54 (I-CAM) 14, CD62P (P-Selectin) 13, CD142 12,13 | ||
Markers in bold are the most commonly used to discriminate endothelial cells belonging to each listed district. Framed markers are used for both murine and human panels.
An identifiable subset of note is the high endothelial venule (HEV). HEVs are highly specialized post-capillary venule endothelial cells involved in lymphocyte trafficking 7. A combination of lymphatic endothelial markers with HEV-specific markers is used to phenotype HEVs, which can be further segregated into peripheral lymph HEVs and mucosal HEVs using markers MECA-79 and MECA-367 8,9,10,11.
Inducible Markers
Cytokine and chemokine activation of injured endothelium leads to phenotypic changes via the induction of endothelial activation markers. These upregulated molecules play a pivotal role in the inflammatory response mainly by promoting leukocyte recruitment. Upregulation of inducible markers like CD54 (I-CAM), CD62P (P-Selectin), and CD142 12 can be used to identify injured or dysfunctional endothelial cells 13,14,15. The inducible markers for endothelial cells are listed in Table 3.
Endothelial injury can also affect the specificity of PROX-1. The nuclear marker PROX-1, specific to the lymphatic endothelium, is found in murine liver sinusoidal endothelial cells following injury 16. PROX-1 is critically involved in the trans-differentiation of blood ECs into lymphatic vessels via inducing proliferation, migration and supporting survival. PROX-1 promotes upregulation of FGFR-3 and lymphatic EC markers such as and VEGFR-3 while downregulating blood EC markers 16,17,18,19,20,21,12,22.
Important Considerations
Just as the endothelium is diverse and highly specialized, certain flow cytometric markers have opposing or differential expression depending on the type of endothelial cell being analyzed. Specialized markers include CD117, CD133, PV-1, eNOS, and vWF. Both vWF and CD105, widely used pan-endothelial markers, are not expressed on the lymphatic endothelium 23,24,25. Expression of vWF is also absent from the alveolar endothelium and can be used to differentiate alveolar ECs from the rest of the pulmonary endothelium 26. The hematopoietic marker CD117 can be used as a dump channel in endothelial panels but is expressed on endothelial colony forming cells (ECFCs) and murine pancreatic islet endothelial cells 27,28,29. Endothelial expression of progenitor marker CD133 is limited to bone marrow-derived ECFCs and murine endothelial cells in the CNS 30,31,32. Expression of diaphragmed fenestrae marker PV-1 is lost as fetal liver sinusoidal and renal glomerular endothelial cells shed their diaphragms during maturation 33,34. It is also important to carefully verify negative markers as truly negative for desired phenotypes, especially if there is no overall consensus or if markers are only published by one group. Single-cell transcriptomics technology reveals more profound organ and site-specific heterogeneity among endothelial cells 35. Development of antibodies against the proteins defining these newly identified endothelial subsets will most definitely expand the flow cytometric endothelial phenotypes.
Acknowledgements
The authors thank the LRI Flow Cytometry Core technical staff for assistance with instrument QC and setup.
Literature Cited
- 1.Kretschmer S, Dethlefsen I, Hagner-Benes S, Marsh LM, Garn H and Konig P. Visualization of intrapulmonary lymph vessels in healthy and inflamed murine lung using CD90/Thy-1 as a marker. PLoS One. 2013;8:e55201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jurisic G, Iolyeva M, Proulx ST, Halin C and Detmar M. Thymus cell antigen 1 (Thy1, CD90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Exp Cell Res. 2010;316:2982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braet F and Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K and Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–56. [DOI] [PubMed] [Google Scholar]
- 5.Prevo R, Banerji S, Ferguson DJ, Clasper S and Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–30. [DOI] [PubMed] [Google Scholar]
- 6.Nonaka H, Tanaka M, Suzuki K and Miyajima A. Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Dev Dyn. 2007;236:2258–67. [DOI] [PubMed] [Google Scholar]
- 7.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M and Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streeter PR, Rouse BT and Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito K, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Kawamoto S, Okubo K and Miyasaka M. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and identification of a leucine-rich HEV glycoprotein as a HEV marker. J Immunol. 2002;168:1050–9. [DOI] [PubMed] [Google Scholar]
- 10.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–56. [DOI] [PubMed] [Google Scholar]
- 11.Hemmerich S, Butcher EC and Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M and Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emin MT, Sun L, Huertas A, Das S, Bhattacharya J and Bhattacharya S. Platelets induce endothelial tissue factor expression in a mouse model of acid-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, Johnson C, Fu H, Shan H, Du F, Hoffman NE, Yu D, Eguchi S, Madesh M, Koch WJ, Sun J, Jiang X, Wang H and Yang X. Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation. Arterioscler Thromb Vasc Biol. 2016;36:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. [DOI] [PubMed] [Google Scholar]
- 16.Meng F LYVE1 and PROX1 in the reconstruction of hepatic sinusoids after partial hepatectomy in mice. Folia Morphol (Warsz). 2017;76:239–245. [DOI] [PubMed] [Google Scholar]
- 17.Baxter SA, Cheung DY, Bocangel P, Kim HK, Herbert K, Douville JM, Jangamreddy JR, Zhang S, Eisenstat DD and Wigle JT. Regulation of the lymphatic endothelial cell cycle by the PROX1 homeodomain protein. Biochim Biophys Acta. 2011;1813:201–12. [DOI] [PubMed] [Google Scholar]
- 18.Falero-Perez J, Song YS, Zhao Y, Teixeira L, Sorenson CM and Sheibani N. Cyp1b1 expression impacts the angiogenic and inflammatory properties of liver sinusoidal endothelial cells. PLoS One. 2018;13:e0206756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono T, Kubo H, Shimazu C, Ueda Y, Takahashi M, Yanagi K, Fujita N, Tsuruo T, Wada H and Yamashita JK. Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arterioscler Thromb Vasc Biol. 2006;26:2070–6. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J and Hong YK. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood. 2009;113:1856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, Niwa H, Kubo H, Suda T and Miyazono K. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wigle JT and Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. [DOI] [PubMed] [Google Scholar]
- 23.Ge AZ and Butcher EC. Cloning and expression of a cDNA encoding mouse endoglin, an endothelial cell TGF-beta ligand. Gene. 1994;138:201–6. [DOI] [PubMed] [Google Scholar]
- 24.Kruse A, Hallmann R and Butcher EC. Specialized patterns of vascular differentiation antigens in the pregnant mouse uterus and the placenta. Biol Reprod. 1999;61:1393–401. [DOI] [PubMed] [Google Scholar]
- 25.Chen CY, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vicente A, Mericko P, Levy RJ, Makinen T, Oliver G and Kahn ML. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest. 2012;122:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asosingh K, Swaidani S, Aronica M and Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol. 2007;178:6482–94. [DOI] [PubMed] [Google Scholar]
- 27.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A and Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu FF, Jing X, Cui YG, Qian XQ, Mao YD, Liao LM and Liu JY. Isolation and characterization of side population cells in the postpartum murine endometrium. Reprod Sci. 2010;17:629–42. [DOI] [PubMed] [Google Scholar]
- 29.Fang S, Wei J, Pentinmikko N, Leinonen H and Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10:e1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O and Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiga J, Hoinoiu B, Stoichitoiu T, Dornean V, Nistor A, Barac S, Miclaus G, Ionac M, Paunescu V, Ursoniu S and Jiga LP. Induction of therapeutic neoangiogenesis using in vitro-generated endothelial colony-forming cells: an autologous transplantation model in rat. J Surg Res. 2013;181:359–68. [DOI] [PubMed] [Google Scholar]
- 32.Wylot B, Konarzewska K, Bugajski L, Piwocka K and Zawadzka M. Isolation of vascular endothelial cells from intact and injured murine brain cortex-technical issues and pitfalls in FACS analysis of the nervous tissue. Cytometry A. 2015;87:908–20. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Zhao H, Zhang Y, Tsatralis T, Cao Q, Wang Y, Wang Y, Wang YM, Alexander SI, Harris DC and Zheng G. Isolation and epithelial co-culture of mouse renal peritubular endothelial cells. BMC Cell Biol. 2014;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioannidou S, Deinhardt K, Miotla J, Bradley J, Cheung E, Samuelsson S, Ng YS and Shima DT. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc Natl Acad Sci U S A. 2006;103:16770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, Garcia-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, Karakach TK, Falkenberg KD, Parys M, Yin X, Vinckier S, Du Y, Fenton RA, Schoonjans L, Dewerchin M, Eelen G, Thienpont B, Lin L, Bolund L, Li X, Luo Y and Carmeliet P. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell. 2020;180:764–779 e20. [DOI] [PubMed] [Google Scholar]
- 36.Newman PJ. The role of PECAM-1 in vascular cell biology. Ann N Y Acad Sci. 1994;714:165–74. [DOI] [PubMed] [Google Scholar]
- 37.Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A and et al. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994;63:247–54. [PubMed] [Google Scholar]
- 38.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G and Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–31. [PubMed] [Google Scholar]
- 39.Nakano H, Nakano K and Cook DN. Isolation and Purification of Epithelial and Endothelial Cells from Mouse Lung. Methods Mol Biol. 2018;1799:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan H, Zhang C, Wang Z, Tu T, Duan H, Luo Y, Feng J, Liu F and Yan X. CD146 is required for VEGF-C-induced lymphatic sprouting during lymphangiogenesis. Sci Rep. 2017;7:7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato TN, Qin Y, Kozak CA and Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W and Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. [DOI] [PubMed] [Google Scholar]
- 43.Schnurch H and Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–68. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML and Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–98. [DOI] [PubMed] [Google Scholar]
- 45.Peters KG, De Vries C and Williams LT. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci U S A. 1993;90:8915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voyta JC, Via DP, Butterfield CE and Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moncada S, Palmer RM and Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 48.Lahdenranta J, Hagendoorn J, Padera TP, Hoshida T, Nelson G, Kashiwagi S, Jain RK and Fukumura D. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res. 2009;69:2801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trzpis M, McLaughlin PM, de Leij LM and Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q, Shen L, Huang W, Song Y, Xiao L, Xu W and Liu Y. Vasculogenesis of decidua side population cells of first-trimester pregnancy. Stem Cell Res Ther. 2013;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bantikassegn A, Song X and Politi K. Isolation of epithelial, endothelial, and immune cells from lungs of transgenic mice with oncogene-induced lung adenocarcinomas. Am J Respir Cell Mol Biol. 2015;52:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elgueta R, Tse D, Deharvengt SJ, Luciano MR, Carriere C, Noelle RJ and Stan RV. Endothelial Plasmalemma Vesicle-Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells. J Immunol. 2016;197:3970–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leenstra S, Das PK, Troost D, Bosch DA, Claessen N and Becker AE. PAL-E, monoclonal antibody with immunoreactivity for endothelium specific to brain tumours. Lancet. 1990;335:671. [DOI] [PubMed] [Google Scholar]
- 54.Hallmann R, Mayer DN, Berg EL, Broermann R and Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–32. [DOI] [PubMed] [Google Scholar]
- 55.Kotton DN, Summer RS, Sun X, Ma BY and Fine A. Stem cell antigen-1 expression in the pulmonary vascular endothelium. Am J Physiol Lung Cell Mol Physiol. 2003;284:L990–6. [DOI] [PubMed] [Google Scholar]
- 56.Wakabayashi T, Naito H, Suehiro JI, Lin Y, Kawaji H, Iba T, Kouno T, Ishikawa-Kato S, Furuno M, Takara K, Muramatsu F, Weizhen J, Kidoya H, Ishihara K, Hayashizaki Y, Nishida K, Yoder MC and Takakura N. CD157 Marks Tissue-Resident Endothelial Stem Cells with Homeostatic and Regenerative Properties. Cell Stem Cell. 2018;22:384–397 e6. [DOI] [PubMed] [Google Scholar]
- 57.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F and Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–51. [DOI] [PubMed] [Google Scholar]
- 58.Gebb S and Stevens T. On lung endothelial cell heterogeneity. Microvasc Res. 2004;68:1–12. [DOI] [PubMed] [Google Scholar]
- 59.Naito H, Wakabayashi T, Kidoya H, Muramatsu F, Takara K, Eino D, Yamane K, Iba T and Takakura N. Endothelial Side Population Cells Contribute to Tumor Angiogenesis and Antiangiogenic Drug Resistance. Cancer Res. 2016;76:3200–10. [DOI] [PubMed] [Google Scholar]
- 60.Takakura N Discovery of a Vascular Endothelial Stem Cell (VESC) Population Required for Vascular Regeneration and Tissue Maintenance. Circ J. 2018;83:12–17. [DOI] [PubMed] [Google Scholar]
- 61.Iba T, Naito H, Shimizu S, Rahmawati FN, Wakabayashi T and Takakura N. Isolation of tissue-resident endothelial stem cells and their use in regenerative medicine. Inflamm Regen. 2019;39:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang SX, Khachigian LM, Ahmadi Z, Yang M, Liu S and Chong BH. In vitro and in vivo proliferation, differentiation and migration of cardiac endothelial progenitor cells (SCA1+/CD31+ side-population cells). J Thromb Haemost. 2011;9:1628–37. [DOI] [PubMed] [Google Scholar]
- 63.Naito H, Kidoya H, Sakimoto S, Wakabayashi T and Takakura N. Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J. 2012;31:842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu QC, Song W, Wang D and Zeng YA. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 2016;26:1079–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu QC, Song W, Lai D and Zeng YA. A Novel Mammary Fat Pad Transplantation Technique to Visualize the Vessel Generation of Vascular Endothelial Stem Cells. J Vis Exp. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM and Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–60. [DOI] [PubMed] [Google Scholar]
- 67.Hamada K, Oike Y, Ito Y, Maekawa H, Miyata K, Shimomura T and Suda T. Distinct roles of ephrin-B2 forward and EphB4 reverse signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:190–7. [DOI] [PubMed] [Google Scholar]
- 68.Shin D and Anderson DJ. Isolation of arterial-specific genes by subtractive hybridization reveals molecular heterogeneity among arterial endothelial cells. Dev Dyn. 2005;233:1589–604. [DOI] [PubMed] [Google Scholar]
- 69.Wang HU, Chen ZF and Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. [DOI] [PubMed] [Google Scholar]
- 70.Gerety SS, Wang HU, Chen ZF and Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–14. [DOI] [PubMed] [Google Scholar]