Abstract
Objective:
To evaluate the efficacy of an in-home 12-week physical therapy (PT) intervention that utilized a virtual reality (VR) gaming system to improve balance in individuals with traumatic brain injury (TBI).
Setting:
Home-based exercise program (HEP).
Participants:
Individuals (N=63; traditional HEP n=32; VR n=31) at least 1 year post-TBI, ambulating independently within the home, not currently receiving PT services.
Main Outcome Measures:
Primary: Community Balance and Mobility Scale (CB&M); Secondary: Balance Evaluation Systems Test (BESTest), Activities-Specific Balance Confidence Scale (ABC), Participation Assessment with Recombined Tools-Objective (PART-O).
Results:
No significant between-group differences were observed in the CB&M over the study duration (P=.9983) for individuals who received VR compared to those who received a HEP to address balance deficits after chronic TBI nor in any of the secondary outcomes: BESTest (P=.8822); ABC (P=.4343) and PART-O (P=.8822). However, both groups demonstrated significant improvements in CB&M and BESTest from baseline to 6, 12, and at 12 weeks follow-up (all P’s <.001). Regardless of treatment group, 52% of participants met or exceeded the minimal detectable change of 8 points on the CB&M at 24 weeks and 38% met or exceeded the minimal detectable change of 7.81 points on the BESTest.
Conclusion:
This study did not find that VR training was more beneficial than a traditional HEP for improving balance. However, individuals with chronic TBI in both treatment groups demonstrated improvements in balance in response to these interventions which were completed independently in the home environment.
Keywords: Balance, Evidence based medicine, Rehabilitation, Traumatic brain injuries, Virtual reality
Balance impairment is a common long-term deficit seen in individuals with traumatic brain injury (TBI),1,2 which can negatively impact physical function, independence, and quality of life; increase fall risk and subsequent injury3,4; and limit community participation.5 Despite rehabilitative efforts, balance deficits can persist in the chronic stages of TBI.6 Currently, there is limited evidence for treatment of impaired balance in chronic TBI.7
Typically, individuals with TBI receive written home exercise programs (HEPs) for continued balance training following formal physical therapy (PT). Reported adherence of using HEPs to prevent falls in adults is poor8 and there is limited research evaluating the efficacy and compliance associated with HEPs to manage balance impairments in adults with chronic TBI.
Virtual reality (VR) systems are computer-based applications that allow an individual to view a simulated environment and dynamically interact within this environment in real time.9,10 VR has been evaluated as an intervention to address balance deficits associated with multiple neurologic conditions,1,11-28 including TBI.29-31 Studies have shown that individuals with neurologic conditions who utilize VR have improved aspects of balance1,12-20,23-28,32,33 and some have also reported greater balance confidence using VR than traditional rehabilitation approaches.29,31
Although the evidence for the efficacy of VR in TBI rehabilitation remains limited,34 this area of research may offer an affordable approach for ongoing treatment outside of a structured insurance-reimbursed rehabilitation program. The purpose of this study was to assess the efficacy of an individually structured 12-week home VR-based intervention compared to a traditional HEP to improve balance in individuals with chronic balance deficits after TBI. We hypothesized that individuals who received VR-based intervention would demonstrate statistically significant improvements in balance, as measured by the Community Balance and Mobility Scale (CB&M), over those who received a traditional HEP.
Methods
Setting and participants
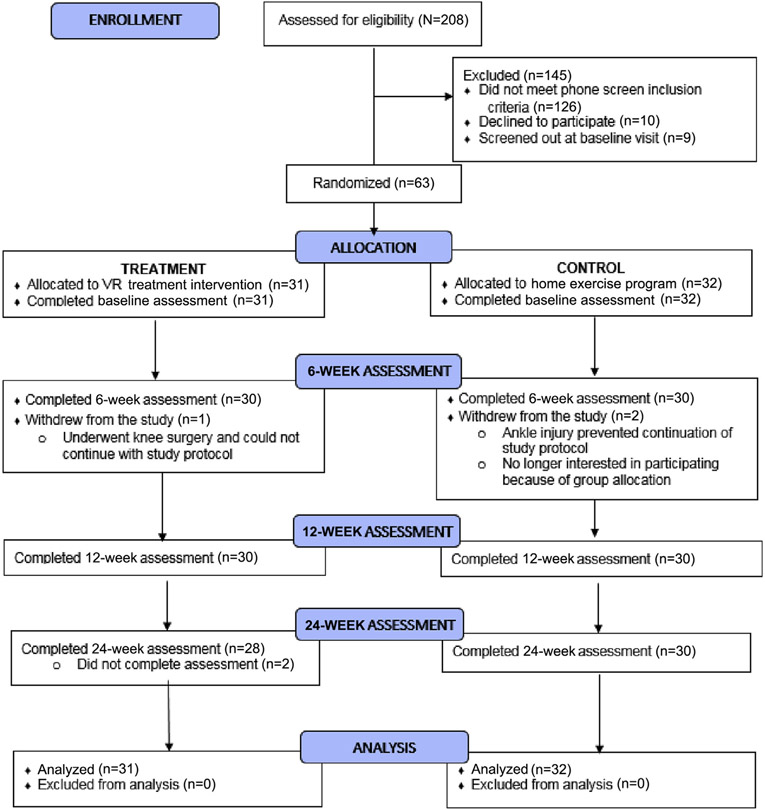
This study was approved by the institutional review board and was registered on clinicaltrials.gov (NCT01794585). Participants were recruited using mailings, posters in the hospital, and contact with local outpatient facilities who met the following criteria: 18-65 years old; at least 1-year post moderate to severe TBI; and currently living in the geographical area. Potential participants were then screened for additional criteria: able to ambulate independently in the home, no participation in skilled PT for the 3 previous months, and self-report of balance deficits. After passing screening criteria, individuals were consented into the study by the study coordinator prior to completing baseline testing. All testing (baseline, 6, 12, 24wk) was completed in a rehabilitation hospital by blinded PT assessors who underwent training and reliability testing on all measures. See figure 1 for subject recruitment and inclusion information.
Fig 1.
CONSORT diagram.
Outcomes
Community Balance and Mobility Scale
The CB&M is a standardized assessment for functional balance during community activities for individuals with TBI. It includes 13 activities scored from 0-96 points.35 It has excellent interrater and intrarater and test-retest reliability for the TBI population.35 Studies reported means and SDs for individuals with TBI in inpatient and outpatient settings ranging from 51.1-57.8 and 18.3-23.3, respectively.35,36 Based on this information, exercise categories were created using a mean of 54 and a SD of 21 points to establish difficulty levels for protocol prescription. Participants who scored more than 1 SD below the mean (CB&M<33) were prescribed the basic protocol; those who scored within 1 SD below the mean (33-54) were prescribed the intermediate protocol and participants who scored within 1 SD above the mean (55-75) were prescribed the advanced protocol. Individuals with scores more than 1 SD above the mean (>75) were excluded from the study.
Activities-Specific Balance Confidence Scale
The Activities-Specific Balance Confidence Scale (ABC) is a self-report measure of fear of falling during community activities. This 16-item measure is scored from 0 (no confidence) to 100 (complete confidence).37 It has excellent test-retest reliability and internal consistency, and adequate content validity.38 The ABC has been used in previous TBI research, and has been shown to be sensitive to treatment effects.29,36,39
Balance Evaluation Systems Test
The Balance Evaluation Systems Test (BESTest) is a standardized 36-item test with scores ranging from 0 (maximum impairment) to 108 (within normal limits). The test has 6 subscales, corresponding with Horak’s 6 balance systems40: Biomechanical Constraints, Stability Limits/Verticality, Anticipatory Postural Adjustments, Reactive Postural Responses, Sensory Orientation, and Stability in Gait. It is used in the Parkinson’s Disease and vestibular disorder populations showing high reliability and validity,41-43 but has not been commonly used in TBI.44
Participation Assessment with Recombined Tools-Objective
The Participation Assessment with Recombined Tools-Objective (PART-O) has 17 items designed to objectively measure community participation in individuals with TBI. Item scores range from 0 (never participate in these activities) to 5 (high participation in these activities). Higher scores indicate greater community participation. It has strong concurrent validity and adequate to excellent correlations with other participation and functional measures.45,46
Interventions
Participants were randomized to 1 of 2 treatment arms, traditional balance HEP or VR HEP. The focus of the balance programs in both the VR and traditional HEP groups was determined by the most impaired subscale of the BESTest. For example, when stability of gait was scored as the lowest BESTest subscale, Xbox Kinect games focusing primarily on dynamic standing activities such as single limb stance were included in the VR group exercise program. In parallel, dynamic activities including single limb stance were also included in the HEP for the traditional group who scored lowest on the stability of gait subscale of the BESTest. Exercise difficulty (basic, intermediate, and advanced) was determined by the total CB&M score. See supplemental fig S1 (available online only at http://www.archives-pmr.org/) for examples of the various exercises prescribed. Both groups were instructed to complete their program 3-4 times per week for 12 weeks, with each session lasting 30 minutes.
All participants were trained in their home by a PT who evaluated the safety of their home environment and made specific recommendations. The PT set up the gaming system for those in the VR arm. A second visit occurred within 1 week to confirm participant understanding of treatment program and offer additional safety recommendations. Following week 6 testing, exercise difficulty was updated based on CB&M stratification. All participants were required to complete an activity log documenting completion of daily sessions and a separate log documenting any adverse events.
Power and sample size calculations
An a priori sample size estimation using PASS 11a was based on detecting a moderate treatment group by time effect size of 0.5 with 80% power in a 2-arm design with 4 unequally spaced repeated measurements of the CB&M at a 5% significance level. An effect size of 0.5 corresponds to an approximate difference in change between groups of 10.25 points (SD=20.5), being larger than an 8-point difference suggested as clinical meaningful change by the CB&M authors.35 A minimum of 26 participants per treatment group were needed for this study, and a total of 66 participants were recruited to allow for attrition.
Statistical methods
Statistical analyses were conducted using SAS version 9.4b assuming a significance level of α=0.05, unless otherwise specified. Baseline demographic and injury characteristics were summarized by group and compared to assess for potential differences.
Data were analyzed as intent-to-treat, using all available data from all participants. Each outcome was analyzed using a repeated-measures linear mixed-effects model. All models included fixed effects for treatment group, assessment time, and the interaction between treatment group and time, as well as effects for age, time since injury, sex, and current living situation. For each model, the omnibus test of the treatment × time interaction effect was first tested to determine if the 2 treatment groups exhibited significantly different changes in the outcome variable over the 4 time points. If this interaction effect was significant (α=0.05), then post-hoc analyses were conducted to determine how the groups differed in their patterns of change from baseline. In particular, changes from baseline to week 6, 12, and 24 were compared between groups using a Bonferroni adjustment of α=0.05/3=0.0167 to control for multiple comparisons. Effect sizes were estimated to be the mean estimate (either the within-group change or the between-group difference in changes) divided by square root of the model based variance for each outcome at baseline. The average number of sessions completed per week from baseline to 6 weeks, 6 weeks to 12 weeks, and 12 weeks to 24 weeks was computed and compared between groups using t tests.
Results
Sample description
Table 1 shows the demographic and injury characteristics of the sample by treatment group, and the baseline cognitive measures are summarized in supplemental table S1 (available online only at http://www.archives-pmr.org/). The groups did not differ significantly on any demographic, injury, or baseline cognitive measures. Sample size assumptions were not met for statistical comparisons of education level between groups. No adverse events directly related to either intervention were reported.
Table 1.
Demographic and injury characteristics
| Characteristics |
VR (n=31) |
HEP (n=32) |
Comparison |
|---|---|---|---|
| Continuous Covariates | Mean ± SD | Mean ± SD | P Value |
| Age | 48.1±12.4 | 49.5±12.4 | .6528 |
| Time since Injury | 8.3±9.2 | 8.5±7.3 | .9405 |
| Categorical Covariates | n (%) | n (%) | P Value |
|---|---|---|---|
| Sex | .0697* | ||
| Male | 23 (74.2) | 16 (50.0) | |
| Female | 8 (25.8) | 16 (50.0) | |
| Race | .5131 | ||
| White | 29 (93.5) | 30 (93.8) | |
| Black | 0 (0.0) | 1 (3.1) | |
| Hispanic | 2 (6.5) | 1 (3.1) | |
| Education | –† | ||
| HS diploma | 3 (9.7) | 6 (18.8) | |
| Some college | 18 (58.1) | 10 (31.3) | |
| Bachelor’s degree | 9 (29.0) | 5 (15.6) | |
| Master’s or doctoral degree | 1 (3.2) | 11 (34.4) | |
| Employment | .4593 | ||
| Employed | 11 (35.5) | 6 (18.8) | |
| Unemployed | 10 (32.3) | 11 (34.4) | |
| Retired | 9 (29.0) | 14 (43.8) | |
| Other | 1 (3.2) | 1 (3.1) | |
| Marital status | .5208 | ||
| Married | 18 (58.1) | 16 (50.0) | |
| Not married | 13 (41.9) | 16 (50.0) | |
| Living with currently | .2782 | ||
| Alone | 6 (19.4) | 10 (31.3) | |
| Not alone | 25 (80.6) | 22 (68.8) | |
| Military service | .6149 | ||
| Yes | 3 (9.7) | 2 (6.3) | |
| No | 28 (90.3) | 30 (93.8) | |
| Mental health treatment | .3346 | ||
| Yes | 9 (29.0) | 13 (40.6) | |
| No | 22 (71.0) | 19 (59.4) | |
| Cause of injury | .0951 | ||
| Vehicular | 23 (74.2) | 19 (59.4) | |
| Violence | 0 (0.0) | 3 (9.4) | |
| Falls | 7 (22.6) | 5 (15.6) | |
| Sports | 1 (3.2) | 5 (15.6) |
Abbreviation: HS, high school.
Fisher exact test.
Chi-square test may not be valid due to low cell counts.
The unadjusted means and SD for each outcome are in supplemental table S2 (available online only at http://www.archives-pmr.org/). The estimated means from the repeated measures models, adjusted for covariates, are plotted in figure 2. The model based estimated changes from baseline to each endpoint (6, 12, 24wk) within each group, and the differences in changes between groups are summarized for each outcome in table 2.
Fig 2.
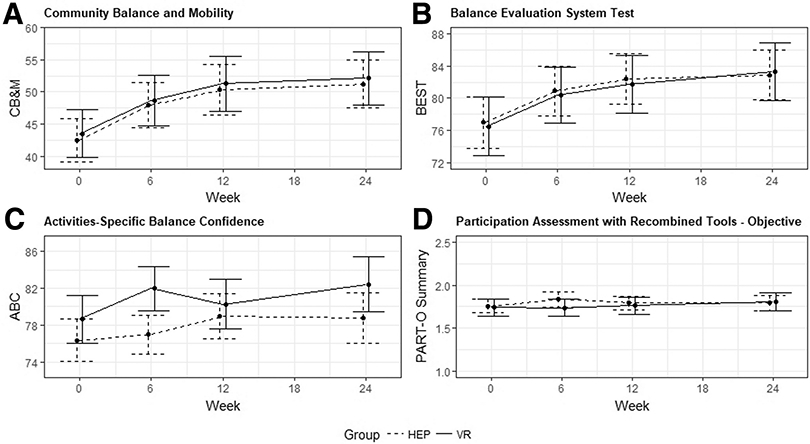
Adjusted mean outcome measure change.
Table 2.
Adjusted changes from baseline in balance and participation outcomes
| Treatment Group | Endpoint | Estimate | SE | 95% CI | P Value | ES | |
|---|---|---|---|---|---|---|---|
| CB&M | |||||||
| VR | Wk 6 | 5.19 | 1.31 | (2.57-7.81) | .0002 | * | 0.29 |
| HEP | Wk 6 | 5.49 | 1.31 | (2.87-8.11) | <.0001 | * | 0.31 |
| VR – HEP | Wk 6 | −0.30 | 1.85 | (−4.01 to 3.40) | .8716 | 0.02 | |
| VR | Wk 12 | 7.73 | 1.66 | (4.41-11.05) | <.0001 | * | 0.43 |
| HEP | Wk 12 | 7.87 | 1.66 | (4.55-11.19) | <.0001 | * | 0.44 |
| VR – HEP | Wk 12 | −0.14 | 2.35 | (−4.84 to 4.55) | .9522 | 0.01 | |
| VR | Wk 24 | 8.60 | 1.39 | (5.81-11.38) | <.0001 | * | 0.48 |
| HEP | Wk 24 | 8.73 | 1.37 | (5.99-11.48) | <.0001 | * | 0.49 |
| VR – HEP | Wk 24 | −0.14 | 1.95 | (−4.05 to 3.77) | .9438 | 0.01 | |
| ABC | |||||||
| VR | Wk 6 | 3.30 | 1.76 | (−0.23 to 6.82) | .0663 | 0.26 | |
| HEP | Wk 6 | 0.65 | 1.75 | (−2.86 to 4.16) | .7138 | 0.05 | |
| VR – HEP | Wk 6 | 2.65 | 2.49 | (−2.32 to 7.62) | .2910 | 0.21 | |
| VR | Wk 12 | 1.62 | 1.64 | (−1.66 to 4.90) | .3271 | 0.13 | |
| HEP | Wk 12 | 2.60 | 1.64 | (−0.67 to 5.88) | .1171 | 0.21 | |
| VR – HEP | Wk 12 | −0.98 | 2.32 | (−5.62 to 3.65) | .6723 | 0.08 | |
| VR | Wk 24 | 3.75 | 1.91 | (−0.08 to 7.57) | .0550 | 0.30 | |
| HEP | Wk 24 | 2.45 | 1.86 | (−1.28 to 6.18) | .1940 | 0.19 | |
| VR – HEP | Wk 24 | 1.30 | 2.67 | (−4.05 to 6.64) | .6292 | 0.10 | |
| BESTest | |||||||
| VR | Wk 6 | 3.90 | 1.31 | (1.28-6.52) | .0042 | * | 0.23 |
| HEP | Wk 6 | 3.89 | 1.31 | (1.27-6.51) | .0043 | * | 0.23 |
| VR – HEP | Wk 6 | 0.01 | 1.85 | (−3.70 to 3.71) | .9973 | 0.00 | |
| VR | Wk 12 | 5.27 | 1.69 | (1.89-8.65) | .0028 | * | 0.31 |
| HEP | Wk 12 | 5.36 | 1.69 | (1.99-8.74) | .0023 | * | 0.31 |
| VR – HEP | Wk 12 | −0.09 | 2.39 | (−4.87 to 4.68) | .9693 | 0.01 | |
| VR | Wk 24 | 6.80 | 1.44 | (3.92-9.68) | <.0001 | * | 0.40 |
| HEP | Wk 24 | 5.89 | 1.42 | (3.05-8.74) | .0001 | * | 0.34 |
| VR – HEP | Wk 24 | 0.91 | 2.02 | (−3.14 to 4.96) | .6558 | 0.05 | |
| PART-O Summary | |||||||
| VR | Wk 6 | 0.00 | 0.05 | (−0.11 to 0.10) | .9523 | 0.00 | |
| HEP | Wk 6 | 0.08 | 0.05 | (−0.03 to 0.19) | .1494 | 0.18 | |
| VR – HEP | Wk 6 | −0.08 | 0.08 | (−0.23 to 0.07) | .2867 | 0.18 | |
| VR | Wk 12 | 0.02 | 0.05 | (−0.09 to 0.13) | .7023 | 0.04 | |
| HEP | Wk 12 | 0.04 | 0.05 | (−0.07 to 0.14) | .4977 | 0.09 | |
| VR – HEP | Wk 12 | −0.02 | 0.08 | (−0.17 to 0.14) | .8341 | 0.04 | |
| VR | Wk 24 | 0.07 | 0.07 | (−0.08 to 0.21) | .3676 | 0.15 | |
| HEP | Wk 24 | 0.04 | 0.07 | (−0.11 to 0.18) | .6204 | 0.09 | |
| VR – HEP | Wk 24 | 0.03 | 0.10 | (−0.17 to 0.23) | .7645 | 0.07 |
NOTE. Statistically significant (α=0.0167) for comparison of changes between groups. Abbreviations: CI, confidence interval;ES, effect size;SE, standard error.
Statistically significant (α=0.05) for within-group changes.
Community Balance and Mobility Scale
There were no significant differences between groups in mean CB&M change over the study duration (treatment × time interaction P=.9983) after adjusting for covariates. Similarly, there were no significant differences in the changes over time between groups from baseline to each endpoint (P’s>.87). Between group effects sizes were near 0. However, both groups exhibited significant increases in mean CB&M from baseline to each endpoint. Regardless of group, CB&M increased on average about 5 units from baseline to 6 weeks, about 7 units from baseline to 12 weeks, and about 8 units from baseline to 24 weeks. Within-group effect sizes were 0.29-0.31 at 6 weeks, 0.43-0.44 at 12 weeks, and 0.48-0.49 at 24 weeks, all considered to be small (0.2) to moderate (0.5). Covariate effects in the adjusted model were not significant.
A minimal detectable change score of at least 8 units was used as suggested by the CB&M authors. Overall, 37% of subjects had a positive response to treatment at 6 weeks (40% VR, 33% HEP), 48% at 12 weeks (47% VR, 50% HEP), and 52% at 24 weeks (50% VR, 53% HEP). There were no between-group differences in response to treatment rates (P’s>.59).
Balance Evaluation System Test
Similar to CB&M, there were not significant differences between groups in mean BESTest changes over the study duration (interaction P=.8822), after adjusting for covariates, nor were there significant differences in the changes over time between groups from baseline to 6, 12, or 24 weeks (P’s>.65). Between-group effect sizes were near zero. Also similar to CB&M, both groups significantly increased in BESTest scores from baseline to 6, 12, and 24 weeks (approximately 4-7 units). Within-group effect sizes were small to moderate (0.23-0.40). Covariate effects in the adjusted model were not significant, except for a significant positive relationship between baseline age and BESTest scores (slope=0.38, P=.0394), such that younger age was associated with lower (worse) BESTest scores.
Using a minimal detectable change score of at least 7.81 units on the BESTest, 20% of subjects had a positive response to treatment at 6 weeks (23% VR, 17% HEP), 40% at 12 weeks (47% VR, 33% HEP) and 38% at 24 weeks (43% VR, 33% HEP). There were no between-group differences in response to treatment rates (P’s>.29).
ABC and PART-O
ABC and PART-O Summary showed no significant differences between treatment groups over the study duration (ABC P=.4343, PART-O Summary P=.4655). There were not significant within-group changes or between-group differences in changes from baseline to any endpoint (see table 2) for either outcome.
Dose and Compliance
Table 3 summarizes the mean number of sessions completed per week. Participants in the traditional HEP group reported a slightly higher average during the first 12 weeks and during 12 weeks of follow-up; however, no significant differences occurred between groups.
Table 3.
Average number of weekly sessions by group
| VR |
HEP |
Comparison |
|||
|---|---|---|---|---|---|
| Time Frame | n | Mean ± SD | n | Mean ± SD | P Value |
| Baseline-6 wk | 27 | 3.60±1.83 | 28 | 4.09±2.04 | .3525 |
| 6 wk-12 wk | 27 | 2.98±2.11 | 28 | 3.55±2.29 | .3446 |
| 12 wk-24 wk | 27 | 1.88±2.10 | 28 | 1.98±2.46 | .8650 |
Discussion
This study found no between-group differences in balance in individuals with chronic TBI who received VR in comparison to a traditional HEP. However, both treatment groups demonstrated statistically significant and similar improvements in balance over a 24-week period. This is remarkable given the chronicity of injury of this sample. The improvements in both groups may be related to the design of the interventions which targeted individual-specific balance impairments. This study was powered to show a difference and not equivalence between the 2 treatment arms. The power for the latter type of study design would require a much larger sample size and so this study is not powered to show that the 2 interventions are equivalent.
There were no statistical differences between groups in balance confidence during the intervention phase or the follow-up period. These findings are contrary to Thornton et al29 who reported that individuals 6 months post-TBI receiving VR training demonstrated greater balance confidence compared to a similar group receiving activity-based exercises. That study differed from this study as it did not analyze between-group statistical differences. Additionally, their participants were in the subacute phase of recovery, while these participants were at least 1 year post injury. Straudi et al30 evaluated VR training compared to balance platform training in individuals with chronic TBI and reported similar results to this study as both groups demonstrated within-group improvement on the CB&M without significant between-group difference.
No previously published studies evaluating the effects of VR training on community participation after TBI were found, and no significant improvements were found in this domain in response to either treatment in this study either. This intervention did not directly target community participation, and the follow-up period may have been too short to see changes in this domain. In regards to balance confidence, no significant improvements were noted in either group. Balance confidence did show an improvement at 6 weeks favoring the VR group (fig 2), but was not statistically significant and possibly due to the initial novelty of VR training.
Study limitations
There were limitations to this study. Although balance improvements are not expected in individuals with chronic TBI, no passive control group was available for comparison. This may have resulted in a halo effect as the blinded assessors were aware that both groups were receiving intervention, which may have introduced bias into their scoring. Dose was reported based on a self-report activity log. Previous studies suggest that dose and compliance may be an important factor for success in rehabilitation outcomes achieved in the home environment.5,47,48 Enjoyment associated with training type was not measured; it may be important to measure this in future studies as this may influence whether individuals continue training outside of a structured follow-up period. Sample sizes were too small to examine the relationship between covariates and response to treatment. Future investigations with larger sample size should focus on identifying characteristics of responders vs nonresponders to either intervention.
Conclusion
VR training was not more beneficial than a traditional HEP for improving balance in a cohort of individuals with chronic TBI. However, individuals in both treatment groups demonstrated improvements in balance in response to these interventions, suggesting that individuals with chronic TBI can show improvements in balance years after injury. Current health care limitations may place an artificial ceiling on balance recovery due to limited outpatient benefits. This study demonstrates that both interventions addressing balance impairments can be carried out safely and effectively in the home environment.
Supplementary Material
Acknowledgments
Supported by the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant no. 90DP0034). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this article do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the Federal Government.
List of abbreviations:
- ABC
Activities-Specific Balance Confidence Scale
- BESTest
Balance Evaluation Systems Test
- CB&M
Community Balance and Mobility Scale
- HEP
home exercise program
- PART-O
Participation Assessment with Recombined Tools-Objective
- PT
physical therapy
- TBI
traumatic brain injury
- VR
virtual reality
Footnotes
Suppliers
PASS, version 11; NCSS.
SAS, version 9.4; SAS Institute Inc.
Disclosures: none.
Clinical Trial Registration No.: NCT01794585.
References
- 1.Walker ML, Ringleb SI, Maihafer GC, et al. Virtual reality-enhanced partial body weight-supported treadmill training poststroke: feasibility and effectiveness in 6 subjects. Arch Phys Med Rehabil 2010;91:115–22. [DOI] [PubMed] [Google Scholar]
- 2.Black K, Zafonte R, Millis S, et al. Sitting balance following brain injury: does it predict outcome? Brain Inj 2000;14:141–52. [DOI] [PubMed] [Google Scholar]
- 3.Fu TS, Jing R, McFaull SR, Cusimano MD. Recent trends in hospitalization and in-hospital mortality associated with traumatic brain injury in Canada: a nationwide, population-based study. J Trauma Acute Care Surg 2015;79:449–54. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch KL, Buxton E, Hackney J, Lowers S. Balance, attention, and dual-task performance during walking after brain injury: associations with falls history. J Head Trauma Rehabil 2010;25:155–63. [DOI] [PubMed] [Google Scholar]
- 5.Winkler D, Unsworth C, Sloan S. Factors that lead to successful community integration following severe traumatic brain injury. J Head Trauma Rehabil 2006;21:8–21. [DOI] [PubMed] [Google Scholar]
- 6.Hillier SL, Sharpe MH, Metzer J. Outcomes 5 years post-traumatic brain injury (with further reference to neurophysical impairment and disability). Brain Inj 1997;11:661–75. [DOI] [PubMed] [Google Scholar]
- 7.Bland DC, Zampieri C, Damiano DL. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: a systematic review. Brain Inj 2011;25:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simek EM, McPhate L, Haines TP. Adherence to and efficacy of home exercise programs to prevent falls: a systematic review and meta-analysis of the impact of exercise program characteristics. Prev Med 2012;55:262–75. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo AA, Buckwalter JG. Virtual reality and cognitive assessment and rehabilitation: the state of the art. Stud Health Technol Inform 1997;44:123–45. [PubMed] [Google Scholar]
- 10.Zhang L, Abreu BC, Masel B, et al. Virtual reality in the assessment of selected cognitive function after brain injury. Am J Phys Med Rehabil 2001;80:597–604. quiz 605. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg M, Sherrington C, Killington M, et al. Video and computer-based interactive exercises are safe and improve task-specific balance in geriatric and neurological rehabilitation: a randomised trial. J Physiother 2016;62:20–8. [DOI] [PubMed] [Google Scholar]
- 12.Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2011;9:CD008349. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Hwang WH, Tsai YC, Liu FK, Hsieh LF, Chern JS. Improving balance skills in patients who had stroke through virtual reality treadmill training. Am J Phys Med Rehabil 2011;90:969–78. [DOI] [PubMed] [Google Scholar]
- 14.Cameirao MS, Badia SB, Oller ED, Verschure PF. Neurorehabilitation using the virtual reality based rehabilitation gaming system: methodology, design, psychometrics, usability and validation. J Neuroeng Rehabil 2010;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cikajlo I, Rudolf M, Goljar N, Burger H, Matjacic Z. Telerehabilitation using virtual reality task can improve balance in patients with stroke. Disabil Rehabil 2012;34:13–8. [DOI] [PubMed] [Google Scholar]
- 16.Flynn S, Palma P, Bender A. Feasibility of using the Sony PlayStation 2 gaming platform for an individual poststroke: a case report. J Neurol Phys Ther 2007;31:180–9. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Jang SH, Kim CS, Jung JH, You JH. Use of virtual reality to enhance balance and ambulation in chronic stroke: a double-blind, randomized controlled study. Am J Phys Med Rehabil 2009;88:693–701. [DOI] [PubMed] [Google Scholar]
- 18.Mirelman A, Patritti BL, Bonato P, Deutsch JE. Effects of virtual reality training on gait biomechanics of individuals post-stroke. Gait Posture 2010;31:433–7. [DOI] [PubMed] [Google Scholar]
- 19.Roy A, Forrester LW, Macko RF. Short-term ankle motor performance with ankle robotics training in chronic hemiparetic stroke. J Rehabil Res Dev 2011;48:417–29. [DOI] [PubMed] [Google Scholar]
- 20.Yang YR, Tsai MP, Chuang TY, Sung WH, Wang RY. Virtual reality-based training improves community ambulation in individuals with stroke: a randomized controlled trial. Gait Posture 2008;28:201–6. [DOI] [PubMed] [Google Scholar]
- 21.Kim N, Park Y, Lee BH. Effects of community-based virtual reality treadmill training on balance ability in patients with chronic stroke. J Phys Ther Sci 2015;27:655–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HY, Kim YL, Lee SM. Effects of virtual reality-based training and task-oriented training on balance performance in stroke patients. J Phys Ther Sci 2015;27:1883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang CY, Hwang WJ, Fang JJ, Sheu CF, Leong IF, Ma HI. Comparison of virtual reality versus physical reality on movement characteristics of persons with Parkinson’s disease: effects of moving targets. Arch Phys Med Rehabil 2011;92:1238–45. [DOI] [PubMed] [Google Scholar]
- 24.Suarez H, Geisinger D, Ferreira ED, et al. Balance in Parkinson’s disease patients changing the visual input. Braz J Otorhinolaryngol 2011;77:651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esculier JF, Vaudrin J, Beriault P, Gagnon K, Tremblay LE. Home-based balance training programme using Wii Fit with balance board for Parkinson’s disease: a pilot study. J Rehabil Med 2012;44:144–50. [DOI] [PubMed] [Google Scholar]
- 26.Bisson E, Contant B, Sveistrup H, Lajoie Y. Functional balance and dual-task reaction times in older adults are improved by virtual reality and biofeedback training. Cyberpsychol Behav 2007;10:16–23. [DOI] [PubMed] [Google Scholar]
- 27.Holden MK. Virtual environments for motor rehabilitation: review. Cyberpsychol Behav 2005;8:187–211. discussion 212-9. [DOI] [PubMed] [Google Scholar]
- 28.Kizony R, Raz L, Katz N, Weingarden H, Weiss PL. Video-capture virtual reality system for patients with paraplegic spinal cord injury. J Rehabil Res Dev 2005;42:595–608. [DOI] [PubMed] [Google Scholar]
- 29.Thornton M, Marshall S, McComas J, Finestone H, McCormick A, Sveistrup H. Benefits of activity and virtual reality based balance exercise programmes for adults with traumatic brain injury: perceptions of participants and their caregivers. Brain Inj 2005;19:989–1000. [DOI] [PubMed] [Google Scholar]
- 30.Straudi S, Severini G, Sabbagh Charabati A, et al. The effects of video game therapy on balance and attention in chronic ambulatory traumatic brain injury: an exploratory study. BMC Neurol 2017;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuthbert JP, Staniszewski K, Hays K, Gerber D, Natale A, O’Dell D. Virtual reality-based therapy for the treatment of balance deficits in patients receiving inpatient rehabilitation for traumatic brain injury. Brain Inj 2014;28:181–8. [DOI] [PubMed] [Google Scholar]
- 32.Adamovich SV, Fluet GG, Tunik E, Merians AS. Sensorimotor training in virtual reality: a review. NeuroRehabilitation 2009;25:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saposnik G, Levin M; Outcome Research Canada (SORCan) Working Group. Virtual reality in stroke rehabilitation: a meta-analysis and implications for clinicians. Stroke 2011;42:1380–6. [DOI] [PubMed] [Google Scholar]
- 34.Pietrzak E, Pullman S, McGuire A. Using virtual reality and videogames for traumatic brain injury rehabilitation: a structured literature review. Games Health J 2014;3:202–14. [DOI] [PubMed] [Google Scholar]
- 35.Howe JA, Inness EL, Venturini A, Williams JI, Verrier MC. The Community Balance and Mobility Scale–a balance measure for individuals with traumatic brain injury. Clin Rehabil 2006;20:885–95. [DOI] [PubMed] [Google Scholar]
- 36.Inness EL, Howe JA, Niechwiej-Szwedo E, Jaglal SB, McIlroy WE, Verrier MC. Measuring balance and mobility after traumatic brain injury: validation of the Community Balance and Mobility Scale (CB&M). Physiother Can 2011;63:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995;50A:M28–34. [DOI] [PubMed] [Google Scholar]
- 38.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1998;53:M287–94. [DOI] [PubMed] [Google Scholar]
- 39.Maskell F, Chiarelli P, Isles R. Dizziness after traumatic brain injury: overview and measurement in the clinical setting. Brain Inj 2006;20:293–305. [DOI] [PubMed] [Google Scholar]
- 40.Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther 2009;89:484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinsongkram B, Chaikeeree N, Saengsirisuwan V, Viriyatharakij N, Horak FB, Boonsinsukh R. Reliability and validity of the Balance Evaluation Systems Test (BESTest) in people with subacute stroke. Phys Ther 2014;94:1632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther 2011;91:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padgett PK, Jacobs JV, Kasser SL. Is the BESTest at its best? A suggested brief version based on interrater reliability, validity, internal consistency, and theoretical construct. Phys Ther 2012;92:1197–207. [DOI] [PubMed] [Google Scholar]
- 44.Peirone E, Goria PF, Anselmino A. A dual-task home-based rehabilitation programme for improving balance control in patients with acquired brain injury: a single-blind, randomized controlled pilot study. Clin Rehabil 2014;28:329–38. [DOI] [PubMed] [Google Scholar]
- 45.Malec JF, Whiteneck GG, Bogner JA. Another look at the PART-O using the Traumatic Brain Injury Model Systems national database: scoring to optimize psychometrics. Arch Phys Med Rehabil 2016;97:211–7. [DOI] [PubMed] [Google Scholar]
- 46.Whiteneck GG, Dijkers MP, Heinemann AW, et al. Development of the Participation Assessment with Recombined Tools—Objective for use after traumatic brain injury. Arch Phys Med Rehabil 2011;92:542–51. [DOI] [PubMed] [Google Scholar]
- 47.Liao HF, Liu YC, Liu WY, Lin YT. Effectiveness of loaded sit-to-stand resistance exercise for children with mild spastic diplegia: a randomized clinical trial. Arch Phys Med Rehabil 2007;88:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodd KJ, Taylor NF, Graham HK. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol 2003;45:652–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.