Abstract
Background
Respiratory viruses are the leading cause of lower respiratory tract infection (LRTI) and hospitalisation in infants and young children. Respiratory syncytial virus (RSV) is the main infectious agent in this population. Palivizumab is administered intramuscularly every month during five months in the first RSV season to prevent serious RSV LRTI in children. Given its high cost, it is essential to know if palivizumab continues to be effective in preventing severe RSV disease in children.
Objectives
To assess the effects of palivizumab for preventing severe RSV infection in children.
Search methods
We searched CENTRAL, MEDLINE, three other databases and two trials registers to 14 October 2021, together with reference checking, citation searching and contact with study authors to identify additional studies. We searched Embase to October 2020, as we did not have access to this database for 2021.
Selection criteria
We included randomised controlled trials (RCTs), including cluster‐RCTs, comparing palivizumab given at a dose of 15 mg/kg once a month (maximum five doses) with placebo, no intervention or standard care in children 0 to 24 months of age from both genders, regardless of RSV infection history.
Data collection and analysis
We used Cochrane’s Screen4Me workflow to help assess the search results. Two review authors screened studies for selection, assessed risk of bias and extracted data. We used standard Cochrane methods. We used GRADE to assess the certainty of the evidence. The primary outcomes were hospitalisation due to RSV infection, all‐cause mortality and adverse events. Secondary outcomes were hospitalisation due to respiratory‐related illness, length of hospital stay, RSV infection, number of wheezing days, days of supplemental oxygen, intensive care unit length of stay and mechanical ventilation days.
Main results
We included five studies with a total of 3343 participants. All studies were parallel RCTs, assessing the effects of 15 mg/kg of palivizumab every month up to five months compared to placebo or no intervention in an outpatient setting, although one study also included hospitalised infants. Most of the included studies were conducted in children with a high risk of RSV infection due to comorbidities like bronchopulmonary dysplasia and congenital heart disease. The risk of bias of outcomes across all studies was similar and predominately low.
Palivizumab reduces hospitalisation due to RSV infection at two years' follow‐up (risk ratio (RR) 0.44, 95% confidence interval (CI) 0.30 to 0.64; 5 studies, 3343 participants; high certainty evidence). Based on 98 hospitalisations per 1000 participants in the placebo group, this corresponds to 43 (29 to 62) per 1000 participants in the palivizumab group. Palivizumab probably results in little to no difference in mortality at two years' follow‐up (RR 0.69, 95% CI 0.42 to 1.15; 5 studies, 3343 participants; moderate certainty evidence). Based on 23 deaths per 1000 participants in the placebo group, this corresponds to 16 (10 to 27) per 1000 participants in the palivizumab group. Palivizumab probably results in little to no difference in adverse events at 150 days' follow‐up (RR 1.09, 95% CI 0.85 to 1.39; 3 studies, 2831 participants; moderate certainty evidence). Based on 84 cases per 1000 participants in the placebo group, this corresponds to 91 (71 to 117) per 1000 participants in the palivizumab group. Palivizumab probably results in a slight reduction in hospitalisation due to respiratory‐related illness at two years' follow‐up (RR 0.78, 95% CI 0.62 to 0.97; 5 studies, 3343 participants; moderate certainty evidence). Palivizumab may result in a large reduction in RSV infection at two years' follow‐up (RR 0.33, 95% CI 0.20 to 0.55; 3 studies, 554 participants; low certainty evidence). Based on 195 cases of RSV infection per 1000 participants in the placebo group, this corresponds to 64 (39 to 107) per 1000 participants in the palivizumab group. Palivizumab also reduces the number of wheezing days at one year's follow‐up (RR 0.39, 95% CI 0.35 to 0.44; 1 study, 429 participants; high certainty evidence).
Authors' conclusions
The available evidence suggests that prophylaxis with palivizumab reduces hospitalisation due to RSV infection and results in little to no difference in mortality or adverse events. Moreover, palivizumab results in a slight reduction in hospitalisation due to respiratory‐related illness and may result in a large reduction in RSV infections. Palivizumab also reduces the number of wheezing days. These results may be applicable to children with a high risk of RSV infection due to comorbidities.
Further research is needed to establish the effect of palivizumab on children with other comorbidities known as risk factors for severe RSV disease (e.g. immune deficiencies) and other social determinants of the disease, including children living in low‐ and middle‐income countries, tropical regions, children lacking breastfeeding, living in poverty, or members of families in overcrowded situations.
Keywords: Child; Child, Preschool; Humans; Infant; Infant, Newborn; Hospitalization; Length of Stay; Palivizumab; Palivizumab/therapeutic use; Respiratory Syncytial Virus Infections; Respiratory Syncytial Virus Infections/prevention & control; Respiratory Syncytial Viruses
Plain language summary
Palivizumab for respiratory syncytial virus infection prevention in children
Review question
What are the effects (benefits and harms) of palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children?
Background
RSV is the main cause of acute respiratory infections in children, mainly during the first year of life, accounting for 33.1 million infections a year with an estimated 90.6% of these episodes occurring in low‐ and middle‐income countries. These infections may present with a runny nose, fever, cough, shortness of breath, wheezing, or difficulty feeding. They may result in hospitalisation, admission to an intensive care unit, and even death, in particular amongst infants aged less than two months, with an estimated hospitalisation rate of 1970 per 100,000 population and 59,600 deaths annually worldwide in children younger than five years old. They may also lead to long‐term complications such as recurrent wheezing and chronic lung problems.
Palivizumab, sold under the brand name Synagis, is a drug administered with an intramuscular injection every month up to five doses to prevent serious infections in children at high risk for severe disease.
Search date
The evidence is current to 14 October 2021.
Study characteristics
We included five studies with 3343 participants. All studies included a small number of participants, including children with a high risk of adverse outcomes if infected with RSV due to underlying health issues, such as premature birth or heart or pulmonary problems.
Study funding sources
Most studies did not specify their funding sources. One study was funded by Abbott Laboratories and by the Netherlands Organisation for Health Research and Development.
Key results
Palivizumab reduces hospitalisation due to RSV infection by 56%; based on 98 cases per 1000 participants in the placebo group, this corresponds to 43 per 1000 participants in the palivizumab group. Palivizumab probably results in little to no difference in mortality, and little to no difference in adverse events; based on 23 deaths per 1000 participants and 84 adverse events per 1000 participants in the placebo group, this corresponds to 16 deaths per 1000 participants and 81 adverse events per 1000 participants in the palivizumab group. Palivizumab probably results in a slight reduction in hospitalisation due to respiratory illness by 22% but may result in little to no difference in length of hospital stay. It may reduce RSV infection rate by 67% at two years’ follow‐up. Palivizumab also reduces the number of wheezing days by 61% but may result in little to no difference in days using oxygen, length of stay in the intensive care unit, or mechanical ventilation days.
Certainty of the evidence
The overall certainty of the evidence was moderate to high.
Summary of findings
Summary of findings 1. Palivizumab compared to placebo, no intervention or standard care for preventing respiratory syncytial virus (RSV) infection in children.
| Palivizumab compared to placebo, no intervention or standard care for preventing respiratory syncytial virus (RSV) infection in children | ||||||
|
Patient or population: children (0 to 24 months) Setting: inpatients and outpatients Intervention: palivizumab Comparison: placebo, no intervention or standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with palivizumab | |||||
|
Hospitalisation due to RSV infection Follow‐up: 2 years |
98 per 1000 |
43 per 1000
(29 to 62) |
RR 0.44
(0.30 to 0.64) |
3343 (5 RCTs) | ⨁⨁⨁⨁ HIGH | |
|
Mortality Follow‐up: 2 years |
23 per 1000 | 16 per 1000 (10 to 27) | RR 0.69 (0.42 to 1.15) | 3343 (5 RCTs) | ⨁⨁⨁◯ MODERATEa | |
|
Adverse events Follow‐up: 150 days |
84 per 1000 | 91 per 1000 (71 to 117) | RR 1.09 (0.85 to 1.39) | 2831 (3 RCTs) | ⨁⨁⨁◯ MODERATEb | |
|
Hospitalisation due to respiratory‐related illness Follow‐up: 2 years |
351 per 1000 | 274 per 1000 (218 to 340) | RR 0.78 (0.62 to 0.97) | 3343 (5 RCTs) | ⨁⨁⨁◯ MODERATEa | |
|
RSV infection Assessed with: incidence of laboratory‐confirmed RSV‐bronchiolitis Follow‐up: 2 years |
195 per 1000 | 64 per 1000 (39 to 107) | RR 0.33 (0.20 to 0.55) | 554 (3 RCTs) | ⨁⨁◯◯ LOWc | |
|
Number of wheezing days Assessed with: rates of wheezing per day Follow‐up: 12 months |
45 per 1000 | 18 per 1000 (16 to 19) | RR 0.39 (0.35 to 0.44) | 429 (1 RCT) | ⨁⨁⨁⨁ HIGH | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded 1 level due to imprecision. The 95% confidence interval includes appreciable benefit and little to no effect. bDowngraded 1 level due to imprecision. The 95% confidence interval includes appreciable benefit and harm. cDowngraded 2 levels due to few number of events and participants in both groups.
Background
Description of the condition
Respiratory viruses are the leading cause of lower respiratory tract infection (LRTI) and hospitalisation in infants and young children (Nair 2010; Nair 2011). Globally, respiratory syncytial virus (RSV) is the main infectious agent every year in this population (Nair 2010; Shi 2017). Over the past 15 years, there has been a significant reduction in child pneumonia mortality and morbidity due to the successful implementation of new vaccine schedules for Haemophilus influenzae and Streptococcus pneumoniae (Shi 2017). In this context of scale‐up in vaccines, the burden of RSV disease continues to increase (Shi 2017).
RSV, now reclassified as a member of the Pneumoviridae family and Orthopneumovirus genus (Amarasinghe 2019), was first identified in 1956 (Chanock 1957). Its viral genome, a single‐stranded and negative sensed ribonucleic acid (RNA), codes for 11 proteins (nine structural proteins and two non‐structural proteins) (Gálvez 2017; Hacking 2002). Transmission of the virus occurs mainly due to contact with contaminated secretions, including surfaces (where RSV can survive for several hours), hands, and direct contact with respiratory secretions. Inoculation develops in the upper respiratory tract, with subsequent infection of the respiratory epithelium, triggering the main infectious mechanisms to survive. Once infected, the incubation period may last four to five days (Lessler 2009).
The seasonality of RSV infection varies according to the location, with considerable fluctuations each year (Mullins 2003). Understanding RSV seasonality has become essential in order to determine the month in which to start immunoprophylaxis for vulnerable populations. In temperate climates, there is a clear affinity for colder seasons (Northern Hemisphere: November to April; Southern Hemisphere: April to September). However, in regions with tropical and subtropical climates, RSV may be present throughout the whole year, probably correlating with rainy seasons (Brady 2014; Hall 2009).
In the USA, RSV is one of the main causes of hospitalisation, in particular amongst infants aged less than two months, with an estimated hospitalisation rate of 1970 per 100,000 population (95% confidence interval (CI), 1787 to 2177) (Arriola 2019). Furthermore, RSV infected children in the USA, present a higher average of all‐cause cumulative hospitalisations rates, for at least five years after the initial infection (Simões 2020). However, the burden of the disease, the number of infected per total population, and the short‐ and long‐term complications are considerably lower in high‐income countries (HIC) (Arriola 2019; Shi 2017). In the Western Pacific Region, the incidence of RSV hospitalisation ranged between 4.9 and 30.9 per 1000 child‐years, varying according to age group (Pangesti 2019).
Globally, RSV accounts for 33.1 million (uncertainty range (UR) 21.6 to 50.3) LRTI a year in children younger than five years old, mainly during the first year of life (Shi 2017), with an estimate of 90.6% (30.0 million) of these episodes occurring in low‐ and middle‐income countries (LMIC) (Shi 2017). More than three million (UR 2.7 to 3.8) hospital admissions worldwide in young children are due to RSV LRTI, with the highest rate in children younger than six months old, in particular in neonates with 15.9 (95% CI 8.8 to 28.9) admissions per year in LMIC (Nair 2010; Shi 2017). Severe RSV LRTI (hypoxaemia) accounts for 1.0 million (UR 0.6 to 1.6) hospital admissions, and very severe LRTI (hospitalised LRTI with danger signs like cyanosis, difficulty in breastfeeding or drinking, vomiting, convulsions, lethargy, unconsciousness, head nodding or ICU admission/mechanical ventilation) for 0.6 million (UR 0.4 to 1) every year in LMIC, mostly in children younger than six months old (Shi 2017).
Regarding mortality, RSV is one of the leading causes of death in the paediatric population (Scheltema 2017; WHO 2018), with a worldwide annual estimate of 59,600 fatalities (UR 48,000 to 74,500) in children younger than five years old (Nair 2013). Most deaths occur in previously healthy children, in particular, in LMIC (Geoghegan 2017; Hall 2009; Hall 2013). In HIC, mortality cases are usually in older children who have a higher prevalence of comorbidities (7.0 years interquartile range (IQR) 3.6 to 16.8 and 70%, respectively) than in upper middle‐income countries (4 years IQR 2.0 to 10.0 and 47%) and LMIC (5 months IQR 2.3 to 11.0 and 28%) (Scheltema 2017). As expected, the case fatality rate is higher in children with comorbidities, in particular, those with chronic lung diseases, congenital heart disease, premature birth, Down's syndrome, and a diagnosis of sepsis or pneumothorax during hospitalisation (Geoghegan 2017; Lee 2016; Thorburn 2009; Welliver 2010). To add to this burden of disease of RSV LRTI, there is a considerable proportion of children who die at home without being hospitalised, in particular in LMIC, a reflection of limited access to hospital care (Caballero 2019; Shi 2017).
RSV infection has a significant economic burden on the health system, for example, in the USA, the total annual direct medical costs for all RSV infections in children younger than five years old is USD 652 million per year (USD 394 million from hospitalisations and USD 258 million from other medical encounters) (Paramore 2004). In LMIC, the cost of each RSV episode is less expensive, but due to the larger proportion of RSV infections, the economic burden may be more significant (Zhang 2016).
At an individual level, almost every child has been infected with the virus by the age of two years (Feldman 2015; Holberg 1991). Signs and symptoms range from an upper respiratory tract infection (nasal congestion, fever, cough, rhinorrhoea) or a LRTI (bronchiolitis or pneumonia) with difficulty breathing, wheezing, difficulty in feeding, or apnoea (Arms 2008; Domachowske 1999). Severity depends on the damage inflicted by the virus and the efficiency of the triggered immune response. Most children will only require ambulatory management, and only a few will need admission to a general ward (1% to 3%) Boyce 2000, or an ICU (less than 1%) (Bont 2016; Hervás 2012; Shay 2001). Children at higher risk for RSV life‐threatening disease include those with congenital heart disease, preterm birth, chronic lung disease, pulmonary hypertension and immunodeficiency (Damore 2008; Mansbach 2012; Purcell 2004). RSV infections in children, in particular those episodes requiring hospitalisation, are associated not only with acute complications but also with long‐term complications such as recurrent wheezing, impaired lung function and paediatric asthma (Esteban 2020; Fauroux 2017; Sigurs 2005; Singh 2007; Thomsen 2009).
Description of the intervention
Palivizumab is a humanised monoclonal antibody (mAb) against RSV fusion (F) glycoprotein, inhibiting RSV replication (Johnson 1997). It was first licenced under the name of Synagis in the USA. Trials were first conducted in 1996, published in 1998 with subsequent Food and Drug Administration (FDA) approval, proving safety and efficacy in highly vulnerable conditions (premature children born before 35 weeks and children with bronchopulmonary dysplasia) (IMpact‐RSV Study Group 1998). Following approval by the European Medicines Agency in 1999, the use of the drug became worldwide (SYNAGIS ® (palivizumab)).
The drug is administered intramuscularly on a monthly basis during the infant’s first RSV season (up to five doses at a dose of 15 mg/kg) to prevent serious RSV LRTI. In some cases, children with bronchopulmonary dysplasia or congenital heart disease receive a second season of the drug. Monoclonal antibodies promote a response that depends entirely from the given half‐life of the antibody in the host, without activating the immune system or inducing immunological memory, hence the requirement of monthly injection of palivizumab (Baxter 2007; Soto 2020).
Candidates for immunoprophylaxis with palivizumab have changed over the past 22 years, depending on countries' public health policy. Since 2014, the American Academy of Pediatrics Guidelines indications have become more restrictive (Brady 2014), with results still inconclusive regarding guidelines impact, however, with a worrying trend towards an increase in the number of RSV‐related hospitalisations (Capizzi 2017; Goldstein 2018; Krilov 2020; Zembles 2019). Nevertheless, at least 30% of individuals who receive palivizumab do not fit these recommendations, probably because palivizumab is the only available pharmacological prevention strategy against RSV (Trist 2018).
Since its first use, palivizumab has proved to be effective in several clinical trials in different settings (Anderson 2017; IMpact‐RSV Study Group 1998; Moore 2019). In a Cochrane Review from 2013, a significant effect was seen for palivizumab in preventing hospitalisations when compared to placebo (risk ratio 0.49, 95% CI 0.37 to 0.64) (Andabaka 2013). However, the cost of the medication remains high at USD 1416 per 100 mg. This is an important obstacle for its use, particularly in LMIC. This has led to a cost‐effectiveness analysis in different countries, settings, and conditions (Andabaka 2013). Although it has been proven cost‐effective in some countries (Mac 2019; Schmidt 2017), studies in Israel and Germany tend to disagree affirming that a substantial decrease in the cost (36.8% to 83.3%) is needed in order to be a cost‐effective strategy amongst vulnerable populations (Blanken 2013; Ginsberg 2018). As a consequence of its high economic burden, different studies have assessed whether a shorter course of palivizumab would provide the same efficacy and same RSV antibody levels than the regular five doses with inconclusive results (Claydon 2017; Claydon 2019; La Via 2013; Moore 2019; Robbie 2012).
Other pharmacologic strategies have been assessed since RSV was first identified in 1956, and several are now under investigation and development (Mejias 2015; Simões 2018; Tripp 2017). Palivizumab was also tested as an intravenous infusion during an acute RSV episode, but showed no benefit (Alansari 2019), nor did RSV immunoglobulin (Sanders 2019). In 2008, motavizumab, a mAb also used against RSV F glycoprotein, but with a 70‐fold increase in affinity compared to palivizumab was tested for preventing RSV LRTI (Fernández 2010). Although it proved to be more efficient than palivizumab in preventing RSV hospitalisations, it was not approved by the FDA due to dermatological side effects (Carbonell‐Estrany 2010). Other mAbs under investigation include human IgA antibody formats of palivizumab and motavizumab (Jacobino 2018), anti RSV G glycoprotein or N‐protein (Tripp 2017) and mAbs with a longer half‐life (Griffin 2020; Zhu 2017).
How the intervention might work
Palivizumab is a humanised monoclonal immunoglobulin G1, directed against an epitope of RSV surface glycoprotein F (Johnson 1997). When binding to it, palivizumab prevents the fusion of the viral particle and host cell membrane (avoiding the entry of the viral genome used for replication and transcription) and might also suppress the syncytia formation in respiratory epithelial cells (Soto 2020; Young 2002). In addition, palivizumab diminishes viral activity and cell‐to‐cell transmission, reducing RSV virulence and its risk of developing RSV LRTI (Collins 2011).
Why it is important to do this review
In 2013, a Cochrane Review assessed the role of palivizumab in preventing RSV LRTI (Andabaka 2013). Since then, many trials have continued evaluating its effectiveness and defining its usefulness in different subpopulations. A non‐Cochrane systematic review on the topic was published in 2014 with methodological limitations (Wegzyn 2014), such as the lack of risk of bias assessment. Given the high cost of the drug, it is essential to know if palivizumab continues to be effective in preventing severe RSV disease in high‐risk children. Meanwhile, the proportion of RSV infections continues to rise, especially in LMIC; despite the probable changing landscape regarding RSV interventions, palivizumab continues to be the only approved RSV‐related drug. Our main goal is to provide a high‐quality review of the evidence on the effects of palivizumab in preventing severe RSV infection in children.
Objectives
To assess the effects of palivizumab for preventing severe respiratory syncytial virus infection in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐RCTs. We did not include cross‐over RCTs as they are not relevant to the review question. We included studies reported as full text, those published as abstract only, and unpublished data where it was possible to establish eligibility for inclusion when data were limited. There were no language or publication restrictions.
Types of participants
We included children (0 to 24 months of age) of both genders, regardless of RSV infection history. We included children with immunodeficiency disorders. We excluded children with cystic fibrosis, as a related Cochrane Review has already been published on that topic (Robinson 2016).
Types of interventions
We included trials comparing palivizumab given intramuscularly or intravenously at a dose of 15 mg/kg once a month (maximum five doses) with placebo, no intervention or standard care alone (oxygen supplementation, bronchodilators, corticosteroids, intravenous fluids, etc). We included co‐interventions (e.g. corticosteroids) provided they were not part of the randomised treatment and were consistent across groups.
Types of outcome measures
The outcomes listed here were not eligibility criteria for this review but were outcomes of interest within the included studies.
Primary outcomes
Hospitalisation due to RSV infection: defined as the number of children hospitalised with laboratory‐confirmed infection.
Mortality: death due to all causes. We also reported the cause of deaths for each group (including deaths related to RSV infection).
Adverse events: defined as any unexpected or harmful occurrence in the participant, such as rash, pain in the injection site, fever, nausea, vomiting, diarrhoea, etc., reported in absolute numbers or proportions. We intended to report non‐serious and serious adverse events (including death, disability, life‐threatening events or those requiring hospitalisations) separately. Had there been multiple events reported within one participant, we would have reported this separately.
Secondary outcomes
Hospitalisation due to respiratory‐related illness: defined as the number of children needing admission to hospital for treatment of respiratory symptoms without alternative aetiology and with negative RSV antigen test or no test done.
Length of hospital stay: number of days in which a child has been hospitalised due to RSV infection or respiratory‐related illness.
RSV infection: incidence of laboratory‐confirmed RSV‐bronchiolitis.
Number of wheezing days: wheeze or bronchodilator medication use reported by parents.
Days of supplemental oxygen.
Intensive care unit length of stay.
Mechanical ventilation days.
For continuous outcomes only available in a subset of participants (length of hospital stay, number of wheezing days, days of supplemental oxygen, ICU length of stay, mechanical ventilation days) we presented the data as days per 100 randomised children following the guidance in Section 6.9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a).
Timing of outcome measurement
We considered outcomes measured up to and including 12 months after randomisation as short term and more than 12 months as long term. When multiple results were reported for each outcome, we included the longest follow‐up in each category.
Search methods for identification of studies
Electronic searches
We searched the following sources from the inception of each database to the date of search with no restrictions on the language of publication or publication status:
the Cochrane Central Register of Controlled Trials (CENTRAL) (2021, Issue 10) searched 14 October 2021;
MEDLINE (Ovid) from 1946 to 14 October 2021;
Embase (Elsevier.com) from 1947 to 15 October 2020;
Latin American and Caribbean Health Science Information database (LILACS) (BIREME) from 1982 to 14 October 2021;
CINAHL (Cumulative Index to Nursing and Allied Health Literature) from 1981 to 14 October 2021; and
Scopus from 1970 to 14 October 2021.
For detailed search strategies, see Appendix 1.
Searching other resources
We attempted to identify other potentially eligible studies or ancillary publications by searching the reference list of included studies, systematic reviews, meta‐analyses, and health technology assessment reports. We contacted experts in the field to identify additional unpublished materials. We searched the websites of relevant manufacturers for information on trials. We sought errata or retractions of the included studies. We contacted authors of the included studies to identify other unpublished studies. We searched for registered and ongoing trials in the following trial registers:
ClinicalTrials.gov (www.ClinicalTrials.gov) searched 14 October 2021; and
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch/) searched 14 October 2021.
For detailed search strategies, see Appendix 1.
Data collection and analysis
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as an RCT or as Not an RCT; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs, and if appropriate, Cochrane Crowd – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, visit the Screen4Me webpage on the Cochrane Information Specialist’s portal: https://community.cochrane.org/organizational‐info/resources/resources‐ groups/information‐specialists‐portal. More detailed information regarding evaluations of the Screen4Me components can also be found in the following publications: Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021;Thomas 2020.
Two review authors (LG, LS) independently screened the titles and abstracts of studies we identified as a result of the search for potential inclusion in the review.
We retrieved the full‐text study reports/publications deemed potentially eligible, and two review authors (LG, LS) independently screened the full texts and identified studies for inclusion and identified and recorded reasons for exclusion of the ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (JVAF) when required. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009). We used Covidence software for study selection (Covidence). We did not impose any language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. One review author (LG or LS) extracted study characteristics from the included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, RSV infection history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (LG, LS) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. Any disagreements were resolved by consensus or by involving a third review author (JVAF). One review author (LG) transferred data into Review Manager 5 software (Review Manager 2020). A second review author (JVAF) double‐checked that data had been entered correctly by comparing the data presented in the systematic review with the study reports. The second review author (JVAF) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (LG, LS) independently assessed risk of bias of the results of the main outcomes (those included in the Table 1, see below) in each study using a recently developed revision of the Cochrane risk of bias tool (RoB 2: a revised tool to assess the risk of bias in randomised trials) (Higgins 2019a; Sterne 2019). Any disagreements were resolved by discussion or by involving another review author (JVAF). We assessed risk of bias according to the following domains.
The randomisation process.
Deviations from intended interventions.
Missing outcome data.
Measurement of the outcome.
Selection of the reported results.
Answers to signalling questions and supporting information collectively led to a domain‐level judgement in the form of 'low risk', 'some concerns', or 'high risk' of bias. These domain‐level judgements informed an overall risk of bias judgement for the outcome. We considered the algorithm proposed judgements and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We also provided reasons for judgments that did not follow the algorithm. We summarised the risk of bias judgements across different studies for each of the domains listed. When judging the bias due to deviations from intended interventions, we focused the analyses on the effect of assignment to intervention (Higgins 2019a). We aimed to source published protocols for the assessment of selective reporting. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
We used the 22 August 2019 version of the RoB 2 Excel tool to manage the data supporting the answers to the signalling questions and risk of bias judgements (available at https://www.riskofbias.info/). All these data are publicly available as supplementary material in the Open Science Framework platform (osf.io/).
For cluster‐RCTs, we would have used the RoB 2 tool and added an additional domain specific to cluster RCTs from the archived version of the tool (Domain 1b ‐ ‘Bias arising from the timing of identification and recruitment of participants’; see https://www.riskofbias.info/) with its corresponding signalling questions, following the guidance in Section 23.1.2 and Table 23.1.a of the Cochrane Handbook (Higgins 2019c).
We made summary assessments of the risk of bias for each short‐ and long‐term result for each outcome (across domains) within and across studies (Higgins 2019a).
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We entered the outcome data for each study into data tables in Review Manager 5 to calculate the treatment effects (Review Manager 2020).
We analysed dichotomous data as risk ratios (RRs) and continuous data as mean difference (MD). We reported corresponding 95% confidence intervals (CIs). We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, that is if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
Unit of analysis issues
Where multiple trial arms were reported in a single trial, we included only the treatment arms relevant to the review topic. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same study, we would have followed the guidance in Section 6.2 of the Cochrane Handbook for Systematic Reviews of Interventions to avoid double‐counting (Higgins 2019b). Our preferred approach would have been to combine groups to create a single pair‐wise comparison. For cluster‐RCTs, we would have considered the cluster as the unit of analysis, not the individual participant, in order to avoid unit of analysis errors, as stated in Section 23.1.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b)
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only).
If numerical outcome data such as standard deviations or correlation coefficients were missing, and they could not be obtained from the authors, we calculated them from other available statistics such as P values according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b).
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity amongst the trials in each analysis. If we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis.
We used the rough guide to interpretation as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b), as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
We avoided the use of absolute cut‐off values but interpreted I² in relation to the size and direction of effects and strength of evidence for heterogeneity. We performed a random‐effects meta‐analysis, which accounts for between‐study heterogeneity.
Assessment of reporting biases
If there were more than 10 trials, we would create and examin a funnel plot to explore possible small‐study and publication biases. If searches identified trial protocols, clinical trial registrations or abstracts indicating the existence of unpublished studies, we attempted to determine the status of any unpublished studies through contact with the investigators.
We considered outcome reporting bias in our risk of bias assessments.
Data synthesis
We pooled data from studies judged to be clinically homogeneous using Review Manager 5 software (Review Manager 2020). If more than one study provided useable data in any single comparison, we performed a meta‐analysis. We used a random‐effects model, as this is usually a more conservative approach. We included all studies in the primary analysis and planned to explore the effect of bias in a sensitivity analysis (see Sensitivity analysis).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
High‐risk children (e.g. children with heart disease, respiratory diseases, premature children, children with low birth weight) versus average‐risk children.
Tropical regions versus non‐tropical regions.
High‐income countries versus low‐ and middle‐income countries
We were only able to carry out the subgroup analysis for high‐income countries versus low‐ and middle‐income countries, and only for the outcome hospitalisation due to respiratory‐related illness.
We used the Chi² test to test for subgroup interactions in Review Manager 5 (Review Manager 2020).
Sensitivity analysis
We had planned to carry out the following sensitivity analyses:
Repeated the analysis excluding unpublished studies (if there were any).
Repeated the analysis excluding studies at an overall high risk of bias.
Repeated the analysis excluding small studies (if there were any).
However, no studies fitted the criteria.
Summary of findings and assessment of the certainty of the evidence
We created Table 1 using the following outcomes:
hospitalisation due to RSV infection (long term);
mortality (long term);
adverse events (non‐serious/serious: long term);
hospitalisation due to respiratory‐related illness (long term);
RSV infection (long‐term); and
number of wheezing days (three to six years).
Two review authors (LG, LS) used the five GRADE considerations (overall RoB 2 judgement, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b) emplying GRADEproGDT software (GRADEpro GDT). Any disagreements were resolved by discussion or by involving another review author (JVAF). We assessed evidence certainty according to the GRADE criteria. We considered RCTs as high certainty evidence if the five factors above related to risk if bias (see Assessment of risk of bias in included studies) were not present to any serious degree, but downgraded the certainty to moderate, low or very low as needed. We downgraded the certainty of the evidence once if a GRADE consideration was serious, and twice if very serious. We justified all decisions to down‐ or upgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
For study details, see Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
For a detailed description of our screening process, see the study flow diagram (Figure 1).
1.

Flow diagram
The searches of the databases identified a total of 5046 search results of which 2787 records remained after deduplication. In assessing the studies, we used Cochrane’s Screen4Me workflow to help identify potential reports of randomised trials. The results of the Screen4Me assessment process are provided in Figure 2. We then assessed the remaining 142 records left after Screen4Me. Our searches of the trial registers identified a further 43 studies. Our screening of the reference lists of the included publications did not reveal any additional RCTs. We therefore had a total of 185 records of which 158 records were excluded based on title and abstract. We obtained the full texts of the remaining 27 records. We excluded 11 studies (see Characteristics of excluded studies). We added two records to Characteristics of studies awaiting classification. We did not identify any ongoing studies.
2.
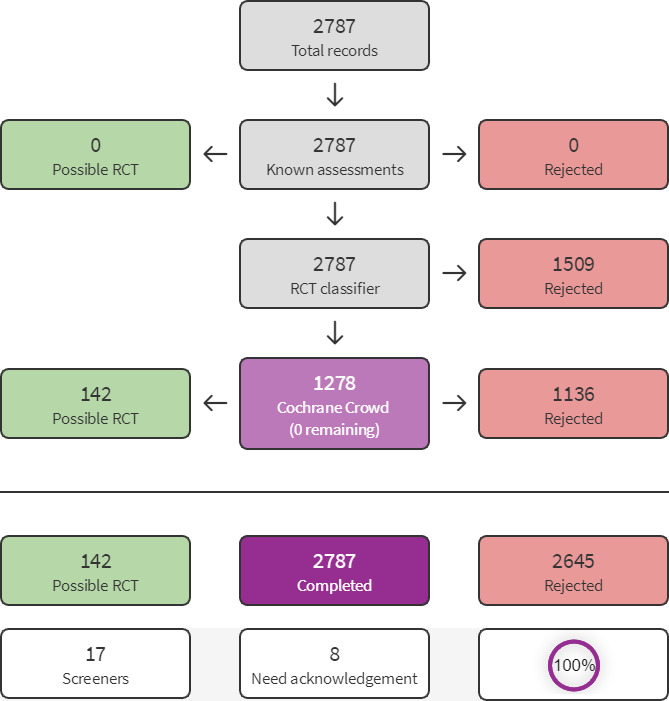
Screen4Me summary diagram
We finally included five studies reported in 14 references.
Included studies
We included five studies with a total 3343 participants (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998; Tavsu 2014).
Designs
All studies were parallel RCTs (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998; Tavsu 2014).
Sample sizes
The median sample size was 429 participants (interquartile range 83 to 1287). The largest sample size was 1502 participants (IMpact‐RSV Study Group 1998) and the smallest was 42 participants (Subramanian 1998).
Settings
All studies were conducted in an outpatient setting (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998; Tavsu 2014). One study also included neonatal ICU hospitalised infants (Tavsu 2014).
Four of the included studies were multicentre studies (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998). Two of these studies were also multinational studies (Feltes 2003; IMpact‐RSV Study Group 1998), conducted in the United States, Canada, Sweden, Germany, Poland, France and the United Kingdom. The other two multicentre studies were conducted in the United States, Subramanian 1998, and the Netherlands, Blanken 2013. One study was a single‐centre study conducted in Turkey (Tavsu 2014).
All studies were reported in the English language.
Participants
Three studies included infants 24 months of age or younger at the start of the RSV season with a gestational age of 35 weeks or less (Blanken 2013; IMpact‐RSV Study Group 1998; Subramanian 1998). One study included infants with a gestational age of 32 weeks or younger who were hospitalised in the neonatal ICU, infants 12 months of age or less at the beginning of the RSV season with a gestational age of 28 weeks, and infants born at 29 to 32 weeks of gestational age who were younger than six months old at the beginning of RSV season (Tavsu 2014). Two studies included children who had bronchopulmonary dysplasia (BPD) and were 24 months of age or younger (IMpact‐RSV Study Group 1998; Subramanian 1998). Finally, one study included children 24 months old or younger at the time of randomisation with documented haemodynamically significant congenital heart disease (CHD) determined by the investigator and had unoperated or partially corrected CHD (Feltes 2003).
Interventions
In all of the included trials, palivizumab was delivered intramuscularly, except for one study in which it was delivered intravenously (Subramanian 1998). All studies delivered 15 mg/kg doses. One study was a dose‐escalation study, testing three different doses (Subramanian 1998); only data for the recommended approved dose of 15 mg/kg were included in our analyses. Four studies compared palivizumab against placebo (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998), and one study compared palivizumab versus no intervention (Tavsu 2014).
Outcomes
All studies reported the effect of the intervention on hospitalisation due to RSV infection, all‐cause mortality, and hospitalisations due to respiratory‐related illness. Three studies reported adverse events (Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998). Three studies reported RSV infection (Blanken 2013; Subramanian 1998; Tavsu 2014). Two studies reported length of hospital stay, days of supplemental oxygen, intensive care unit length of stay, and mechanical ventilation days (Feltes 2003; IMpact‐RSV Study Group 1998). One study reported the number of wheezing days (Blanken 2013).
Funding sources
Most studies (four of five studies, 80%) did not specify their funding sources. Blanken 2013 was funded by Abbott Laboratories (Abbott International PLC (UK)) and the Netherlands Organisation for Health Research and Development, with no restrictions for publication of the research data.
Excluded studies
We excluded 11 reports after full‐text assessment. One report compared palivizumab against motavizumab (EUCTR2007‐002070‐61‐PL). Two reports were informative summaries with no authors declared and no original research data reported (Anonymous 1999; Anonymous 2004). One report was a narrative review (Driver 1999). Another report was an evidence synopsis (Ignacio 2013). The remaining six reports were observational studies (Johnson 1999; Koganesawa 2019; Naver 2002; Pin 2002; Rajakumar 2009; Tulloh 2011).
Risk of bias in included studies
The risk of bias assessments for each outcome, including all domain judgements and support for judgement, are provided in the risk of bias tables within the Characteristics of included studies section and at the side of all forest plots. To access the further detailed risk of bias assessment data, visit https://osf.io/26dns/ (DOI 10.17605/OSF.IO/26DNS).
Risk of bias was similar across most outcomes, judged as 'low'. The only exception was outcomes reported by one study (Tavsu 2014) which were judged as 'some concerns'. This trial did not adequately describe allocation concealment and, unlike the other trials, it compared palivizumab to 'no intervention', which raised concerns due to deviations from intended interventions, as this was not adequately described. This study reported hospitalisations (due to respiratory illness and RSV infection), mortality and RSV infection. However, this did not affect our GRADE judgement for these results considering the trial's relative contribution to the overall estimate.
Effects of interventions
See: Table 1
1. Palivizumab versus placebo or no intervention
Five studies with a total of 3343 participants were included in this comparison (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998; Tavsu 2014). See Table 1.
Primary outcomes
1.1. Hospitalisation due to RSV infection
Five studies with 3343 participants reported this outcome (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998; Tavsu 2014). Palivizumab reduces hospitalisation due to RSV infection compared to placebo or no intervention at two years' follow‐up (risk ratio (RR) 0.44, 95% confidence interval (CI) 0.30 to 0.64; I² = 23%; Analysis 1.1). We assessed the evidence for this outcome as of high certainty.
1.1. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 1: Hospitalisation due to RSV infection
1.2 Mortality
Five studies with 3343 participants reported this outcome (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998; Tavsu 2014). Palivizumab probably results in little to no difference in mortality compared to placebo or no intervention at two years' follow‐up (RR 0.69, 95% CI 0.42 to 1.15; I² = 0%; Analysis 1.2). We assessed the evidence for this outcome as of moderate certainty due to concerns about imprecision.
1.2. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 2: Mortality
1.3 Adverse events
Three studies with 2831 participants reported this outcome (Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998). Palivizumab probably results in little to no difference in adverse events compared to placebo or no intervention at 150 days' follow‐up (RR 1.09, 95% CI 0.85 to 1.39; I² = 0%; Analysis 1.3). We assessed the evidence for this outcome as of moderate certainty due to concerns about imprecision.
1.3. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 3: Adverse events
Secondary outcomes
1.4 Hospitalisation due to respiratory‐related illness
Five studies with 3343 participants reported this outcome (Blanken 2013; Feltes 2003; IMpact‐RSV Study Group 1998; Subramanian 1998; Tavsu 2014). Palivizumab probably results in a slight reduction in hospitalisation due to respiratory‐related illness compared to placebo or no intervention at two years' follow‐up (RR 0.78, 95% CI 0.62 to 0.97; I² = 45%; Analysis 1.4). We assessed the evidence for this outcome as of moderate certainty due to concerns about imprecision.
1.4. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 4: Hospitalisation due to respiratory‐related illness
1.5 Subgroup analysis: hospitalisation due to respiratory‐related illness
We found that palivizumab results in a higher reduction in hospitalisation due to respiratory‐related illness in lower‐ middle‐ime countries (LMIC) (RR 0.47, 95% CI 0.18 to 1.22) compared to HIC (RR 0.80, 95% CI 0.65 to 0.99) (test for subgroup differences: P = 0.28, I² = 14.0%; Analysis 1.5).
1.5. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 5: Subgroup analysis: hospitalisation due to respiratory‐related illness
1.6 Length of hospital stay
Two studies with 2789 participants reported this outcome (Feltes 2003; IMpact‐RSV Study Group 1998). Palivizumab may result in little to no difference in length of hospital stay compared to placebo or no intervention at 150 days' follow‐up (mean difference (MD) ‐42.24, 95% CI ‐84.77 to 0.29; I² = 64%; Analysis 1.6).
1.6. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 6: Length of hospital stay
1.7 RSV infection
Three studies with 554 participants reported this outcome (Blanken 2013; Subramanian 1998; Tavsu 2014). Palivizumab may result in a large reduction in RSV infection compared to placebo or no intervention at two years' follow‐up (RR 0.33, 95% CI 0.20 to 0.55; I² = 0%; Analysis 1.7). We assessed the evidence for this outcome as of low certainty due to serious concerns about imprecision.
1.7. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 7: RSV infection
1.8 Number of wheezing days
One study with 429 participants reported this outcome (Blanken 2013). Palivizumab reduces the daily rate of wheezing compared to placebo or no intervention at one year's follow‐up (RR 0.39, 95% CI 0.35 to 0.44; Analysis 1.8) We assessed the evidence for this outcome as of high certainty.
1.8. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 8: Number of wheezing days
1.9 Days of supplemental oxygen
Two studies with 2789 participants reported this outcome (Feltes 2003; IMpact‐RSV Study Group 1998). Palivizumab may result in little to no difference in days of supplemental oxygen compared to placebo or no intervention at 150 days' follow‐up (MD ‐36.85, 95% CI ‐85.19 to 11.49; I² = 59%; Analysis 1.9).
1.9. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 9: Days of supplemental oxygen
1.10 Intensive care unit length of stay
Two studies with 2789 participants reported this outcome (Feltes 2003; IMpact‐RSV Study Group 1998). Palivizumab may result in little to no difference in intensive care unit length of stay compared to placebo or no intervention at 150 days' follow‐up (MD ‐13.51, 95% CI ‐61.11 to 34.08; I² = 50%; Analysis 1.10).
1.10. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 10: Intensive care unit length of stay
1.11 Mechanical ventilation days
Two studies with 2789 participants reported this outcome (Feltes 2003; IMpact‐RSV Study Group 1998). Palivizumab may result in little to no difference in mechanical ventilation days compared to placebo or no intervention at 150 days' follow‐up (MD 5.78, 95% CI ‐10.37 to 21.92; I² = 0%; Analysis 1.11).
1.11. Analysis.

Comparison 1: Palivizumab versus placebo or no intervention, Outcome 11: Mechanical ventilation days
Discussion
Summary of main results
We included five studies with a total of 3343 participants assessing the effect of palivizumab compared to placebo or no intervention for preventing severe RSV infection in children. The certainty of the evidence for most outcomes was moderate to high. Palivizumab reduces hospitalisation due to RSV infection (high certainty evidence) and probably results in a slight reduction in hospitalisation due to respiratory‐related illness at two years' follow‐up (moderate certainty evidence). Palivizumab probably results in little to no difference in mortality and adverse events and may result in little to no difference in length of hospital stay, days of supplemental oxygen, length of stay in the ICU, and mechanical ventilation days at 150 days' follow‐up (low to moderate certainty evidence). Palivizumab may result in a large reduction in RSV infection at two years' follow‐up (low certainty evidence). Palivizumab reduces the number of wheezing days at one year's follow‐up (high certainty evidence).
Overall completeness and applicability of evidence
Participants in the included studies were similar to those that would be found in clinical practice, that is with a higher risk of severe RSV infection. The studies were conducted mainly in children and infants from six to 12 months old at the beginning of the RSV season. Two studies included participants with bronchopulmonary dysplasia and one study included participants with CHD, which might introduce a source of clinical heterogeneity. We deemed this not important for most outcomes. Most of the included studies delivered palivizumab intramuscularly, which is the current preferred route of administration (only one study delivered palivizumab intravenously).
Most studies reported all of the outcomes of interest in this review. We found heterogeneity in outcomes definitions amongst studies. Some outcomes reported hospitalisations under adverse events. Nevertheless, we were able to extract and analyse the available data accordingly.
Consistent with the 2013 Cochrane Review (Andabaka 2013), palivizumab reduced the risk for RSV‐related hospitalisation, presenting similar statistical results. This conclusion reinforces the safety and efficacy of its use in high‐risk children, in line with the different existing guidelines recommending its use (Brady 2014). Furthermore, these updated findings are of paramount significance in the context of the changing landscape of RSV preventive interventions, including a new single‐dose monoclonal antibody against RSV fusion protein (nirsevimab) that successfully diminished the incidence of RSV‐associated lower respiratory tract infection episodes and hospitalisations (Griffin 2020).
Interestingly, we found that palivizumab resulted in a higher reduction in hospitalisation due to respiratory‐related illness in LMIC than in HIC. This finding is aligned with the higher burden of RSV disease described in LMIC (Shi 2017). Moreover, a recent randomised clinical trial using a single intramuscular dose of RSV fusion protein nanoparticle vaccine in pregnant women showed a higher vaccine efficacy against RSV‐associated lower respiratory tract infection in LMIC than in HIC (Madhi 2020). The effect of palivizumab on mortality was downgraded due to imprecision, which may also be due to the fact that the included studies were not from LMIC, and the overall mortality was low.
Palivizumab also resulted in a reduction in the number of wheezing days during the first year of life, in line with similar probe studies assessing this outcome (Yoshihara 2013). Being able to prevent recurrent wheezing is of great impact in preschoolers since it is one of the most frequent chronic pathologies in that age range (Stein 1999). Despite these encouraging results, in two follow‐up studies, no differences in the diagnosis of asthma were seen at age six after the use of palivizumab (Mochizuki 2017; Scheltema 2018), although the effects of palivizumab on the subsequent diagnosis of asthma may be differ depending on the atopic status of the studied individuals (Simões 2010).
Quality of the evidence
The overall certainty of the evidence was moderate to high. Most outcomes reported in the studies were assessed as low risk of bias. A common problem found in several of the included studies was the incomplete reporting of dispersion measures that were not provided, leading us to calculate standard deviation converted from P values and thus making us less confident about the precision of the results. Some studies were very small and had few events which led to important imprecision. We were unable to assess publication bias due to the scarcity of studies per outcome.
Potential biases in the review process
We rearranged the mortality outcome to maximise the use of available data. We took precautions to avoid bias in this process by documenting all changes in the Differences between protocol and review section of the review. We found that several outcomes were reported in a subset of patients, such as hospitalised patients. As there were no data regarding all randomised participants in these cases, we decided to consider them as trials' exploratory analyses.
We followed the guidelines in Section 6.5 of the CochraneHandbook to obtain the standard deviation converted from P values (Higgins 2019c). We assumed a normal distribution of the available data. However, the probability of non‐normal distribution of data remains, as no precise evidence of normal distribution could be found in the trial reports.
Agreements and disagreements with other studies or reviews
A previous Cochrane Review incorporating most of the studies included in our review found that palivizumab prophylaxis was effective in reducing the frequency of hospitalisations due to RSV infection and reducing the incidence of severe lower respiratory tract RSV disease in children with chronic lung disease, congenital heart disease or those born prematurely (Andabaka 2013). However, the comparators considered in Andabaka 2013 differed from those in our review.
A previous non‐Cochrane systematic review included all of the studies in our review (Wegzyn 2014). Although our review did not include any new studies, we expanded the available knowledge related to the effect of palivizumab on different outcomes not considered in the previously mentioned review (Wegzyn 2014), such as the number of wheezing days. Furthermore, our review adds the risk of bias and certainty of the evidence assessments, which may be useful for decision‐makers in the clinical setting and may also guide the design of future research.
One review found similar results regarding all‐cause mortality and RSV hospitalisation amongst preterm infants at high risk (Checchia 2011); however, it included observational studies, both prospective and retrospective, and found mortality and hospitalisation rates lower than those found in our study. Another review found that prophylaxis with palivizumab reduced hospital admissions in preterm infants with or without chronic lung disease (Wang 2008). It also found that palivizumab reduced hospitalisation rate due to RSV amongst children with congenital heart disease. None of these non‐Cochrane systematic reviews were of high quality, and none of them incorporated GRADE methods in assessing the certainty of the evidence.
We found additional systematic reviews and health technology assessments concluding that palivizumab is effective in reducing hospital stay and risk of admission in children with congenital heart disease (Harris 2011), prematurely born infants, infants with lung complications, and infants from remote communities (Mac 2019), but did not provide value for money because of its exceptionally high cost (Harris 2011). In this regard, a previous Cochrane Review found inconsistencies in palivizumab's cost‐effectiveness, due to wide variations in incremental cost‐effectiveness ratios (ICER), arising from different mortality rates, time horizons, and stakeholders' perspectives used in economic models (Andabaka 2013).
No previous review reported results for the number of wheezing days. This is a major issue, given the spreading hypothesis and studies suggesting that RSV infection is a risk factor for recurrent wheezing amongst infants and young children (Schauer 2002).
Authors' conclusions
Implications for practice.
The available evidence suggests that prophylaxis with palivizumab reduces hospitalisation due to respiratory syncytial virus (RSV) infection and results in little to no difference in mortality or adverse events. Moreover, palivizumab results in a slight reduction in hospitalisation due to respiratory‐related illness and may result in a large reduction in severe RSV infections. Palivizumab also reduces the number of wheezing days. Despite our aim to determine the effect of palivizumab for preventing severe RSV infection in all children, no studies were found on healthy children without a higher risk for RSV life‐threatening disease or with immunodeficiency disorders, as all of the included studies were carried out in high‐risk populations.
Implications for research.
Further research is needed to establish the effect of palivizumab on children with other comorbidities known as risk factors for severe RSV disease (e.g. immune deficiencies) and other social determinants of the disease, including children living in low‐ and middle‐income countries, tropical regions, children lacking breastfeeding, living in poverty, or members of families in overcrowded situations. Further research must consider the cost of the intervention in relation to the potential benefits arising from extended use and the subsequent impact on equity.
History
Protocol first published: Issue 10, 2020
Risk of bias
Risk of bias for analysis 1.1 Hospitalisation due to RSV infection.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Blanken 2013 | Low risk of bias | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | Data were analysed in accordance with the pre‐specified plan and assessed based on pre‐specified domains and time‐points. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Feltes 2003 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| IMpact‐RSV Study Group 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Subramanian 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Tavsu 2014 | Some concerns | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Some concerns | Participants and carers were aware of the assigned intervention. There were no deviations from intended interventions. There is no information regarding the analyses used. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Some concerns | The study is judged to raise some concerns because of issues related to the randomisation process and awareness of assigned interventions. |
Risk of bias for analysis 1.2 Mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Blanken 2013 | Low risk of bias | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | Data were analysed in accordance with the pre‐specified plan and assessed based on pre‐specified domains and time‐points. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Feltes 2003 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| IMpact‐RSV Study Group 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Subramanian 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Tavsu 2014 | Some concerns | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Some concerns | Participants and carers were aware of the assigned intervention. There were no deviations from intended interventions. There is no information regarding the analyses used. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Some concerns | The study is judged to raise some concerns because of issues related to the randomisation process and awareness of assigned interventions. |
Risk of bias for analysis 1.3 Adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Feltes 2003 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| IMpact‐RSV Study Group 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Subramanian 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
Risk of bias for analysis 1.4 Hospitalisation due to respiratory‐related illness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Blanken 2013 | Low risk of bias | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | Data were analysed in accordance with the pre‐specified plan and assessed based on pre‐specified domains and time‐points. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Feltes 2003 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| IMpact‐RSV Study Group 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Subramanian 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Tavsu 2014 | Some concerns | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Some concerns | Participants and carers were aware of the assigned intervention. There were no deviations from intended interventions. There is no information regarding the analyses used. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Some concerns | The study is judged to raise some concerns because of issues related to the randomisation process and awareness of assigned interventions. |
Risk of bias for analysis 1.5 Subgroup analysis: hospitalisation due to respiratory‐related illness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.5.1 High‐income countries | ||||||||||||
| Blanken 2013 | Low risk of bias | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | Data were analysed in accordance with the pre‐specified plan and assessed based on pre‐specified domains and time‐points. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Feltes 2003 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| IMpact‐RSV Study Group 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Subramanian 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Subgroup 1.5.2 Low‐ and middle‐income countries | ||||||||||||
| Tavsu 2014 | Some concerns | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Some concerns | Participants and carers were aware of the assigned intervention. There were no deviations from intended interventions. There is no information regarding the analyses used. | Low risk of bias | Data were available for nearly all randomized participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Some concerns | The study is judged to raise some concerns because of issues related to the randomization process and awareness of assigned interventions. |
Risk of bias for analysis 1.7 RSV infection.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Blanken 2013 | Low risk of bias | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | Data were analysed in accordance with the pre‐specified plan and assessed based on pre‐specified domains and time‐points. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Subramanian 1998 | Low risk of bias | Randomisation and allocation were done centrally and there are no concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
| Tavsu 2014 | Some concerns | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Some concerns | Participants and carers were aware of the assigned intervention. There were no deviations from intended interventions. There is no information regarding the analyses used. | Low risk of bias | Data were available for nearly all randomised participants. | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | No pre‐specified analysis plan available, but the outcome is usually measured and analysed in the way presented in the trial. | Some concerns | The study is judged to raise some concerns because of issues related to the randomisation process and awareness of assigned interventions. |
Risk of bias for analysis 1.8 Number of wheezing days.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Blanken 2013 | Low risk of bias | The study does not adequately describe the randomisation process, although there are no serious concerns regarding baseline differences. | Low risk of bias | Participants and those involved in caring for participants were not aware of the assigned intervention. There are no concerns regarding the analysis used to estimate the effect of assignment to intervention. | Low risk of bias | There is a high proportion of missing data and authors performed no analyses to correct it due that most losses took place prior to commencing treatment (adequate post‐randomisation exclusions). | Low risk of bias | Method for outcome measurement was appropriate and equally applied between groups. Outcome assessors were blinded to intervention status. | Low risk of bias | Data were analysed in accordance with the pre‐specified plan and assessed based on pre‐specified domains and time‐points. | Low risk of bias | The study is judged to be at low risk of bias for all domains for this result. |
Acknowledgements
The Methods section of this review is based on a standard template developed by the Cochrane Airways Group and adapted by the Cochrane Acute Respiratory Infections Group. We wish to thank the following people for commenting on the draft of the protocol of this review: Theresa Wrangham, Lenny Krilov, Simon Nadel, Menelaos Konstantinidis, and Roderick Venekamp.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Susanna Wisniewski, Nikolaos Sideris, Basavaraj Poojar, Mohammad Aloulou, Ana‐Marija jubenković, Vighnesh Devulapalli, Richard Colling and Ahlam Jamal Hussain Alhemed.
We would also like to thank Matteo Bruschettini, who contacted us with Ms Styrmisdottir in the context of Cochrane's International Mobility Programme and provided clinical and methodological advice for the conduct of this review.
We would like to thank Kerry Dwan for her help in the analysis of the 'number of wheezing days' outcome.
We would also like to thank Howard Panitch and Simon Nadel for peer‐reviewing the draft of this review, the Statistical Editor Menelaos Konstantinidis, the Consumer Reviewers Dee Shneiderman and Theresa Wrangham, and Contact Editor Michelle Guppy.
Appendices
Appendix 1. Appendix 1. Search strategies
MEDLINE (Ovid)
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to October 14, 2021
1 Respiratory Syncytial Virus Infections/
2(respiratory syncytial virus* or rsv).tw.
3 respiratory syncytial viruses/ or respiratory syncytial virus, human/
4 exp Bronchiolitis/
5 bronchiolit*.tw.
6 exp Pneumonia/
7 (pneumon* or bronchopneumon* or pleuropneumon*).tw.
8 Respiratory Tract Infections/
9 lower respiratory infection*.tw.
10 (lower respiratory tract infection* or lrti).tw.
11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
12 Palivizumab/
13 palivizumab.tw,nm.
14 synagis.tw,nm.
15 medi‐493.tw,nm.
16 medi493.tw,nm.
17 "medi 493".tw,nm.
18 12 or 13 or 14 or 15 or 16 or 17
19 11 and 18
Embase (Elsevier)
#1. 'respiratory syncytial virus infection'/exp
#2. 'respiratory syncytial virus' OR rsv
#3. 'bronchiolitis'/exp
#4. bronchiolit*:ti,ab,kw
#5. 'pneumonia'/exp
#6. pneumon*:ti,ab,kw OR bronchopneumon*:ti,ab,kw OR pleuropneumon*:ti,ab,kw
#7. 'respiratory tract infection'/exp
#8. 'lower respiratory infection*':ti,ab,kw
#9. 'lower respiratory tract infection*':ti,ab,kw OR lrti:ti,ab,kw
#10. 'pneumovirus'/exp OR 'human respiratory syncytial virus'/exp
#11. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12. 'palivizumab'/exp
#13. palivizumab:ti,ab,kw
#14. synagis:ti,ab,kw
#15. 'medi‐493':ti,ab,kw
#16. 'medi493':ti,ab,kw
#17. 'medi 493':ab,kw
#18. #12 OR #13 OR #14 OR #15 OR #16 OR #17
#19. #11 AND #18
Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Respiratory Syncytial Virus Infections] explode all trees #2 ((respiratory syncytial virus* or rsv)):ti,ab,kw #3 MeSH descriptor: [Respiratory Syncytial Viruses] explode all trees #4 MeSH descriptor: [Respiratory Syncytial Virus, Human] explode all trees #5 MeSH descriptor: [Bronchiolitis] explode all trees #6 (bronchiolit*):ti,ab,kw #7 MeSH descriptor: [Bronchiolitis] explode all trees #8 ((pneumon* or bronchopneumon* or pleuropneumon*)):ti,ab,kw #9 MeSH descriptor: [Respiratory Tract Infections] explode all trees #10 ((lower respiratory infection*)):ti,ab,kw #11 ((lower respiratory tract infection* or lrti)):ti,ab,kw 3308 #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 MeSH descriptor: [Palivizumab] explode all trees #14 ((palivizumab)):ti,ab,kw #15 (synagis):ti,ab,kw #16 (medi‐493):ti,ab,kw #17 (medi493):ti,ab,kw #18 ("medi 493"):ti,ab,kw #19 #13 OR #14 OR #15 OR #16 OR #17 OR #18 #20 #12 AND #19
LILACS (BIREME)
( Respiratory Syncytial Virus Infections OR Infecciones por Virus Sincitial Respiratorio OR Infecções por Vírus Respiratório Sincicial OR Bronchiolitis OR bronquiolitis OR Bronquiolite OR Bronchopneumonia OR Bronconeumonía OR Broncopneumonia OR Respiratory Tract Infections OR Infecciones del Sistema Respiratorio OR Infecções Respiratórias ) AND (Palivizumab OR Synagis OR MEDI 493 OR MEDI‐493 OR MEDI493)
CINAHL
(respiratory syncytial virus infection (rsv) OR Bronchiolitis OR Pneumonia OR Respiratory Tract Infections OR lower respiratory infection) AND (Palivizumab OR synagis OR medi‐493 OR Medi 493 OR Medi493)
Scopus
TITLE‐ABS‐KEY ( ( "respiratory syncytial virus infection" OR bronchiolitis OR pneumonia OR "respiratory tract infections" OR "lower respiratory infection" ) AND ( palivizumab OR synagis OR medi‐493 OR medi 493 OR med493 ) )
ClinicalTrials.gov
Intervention: palivizumab OR synagis OR medi‐493 OR medi 493 OR med493
WHO ICTRP (Standard search)
palivizumab OR synagis OR medi‐493 OR medi 493 OR med493
Data and analyses
Comparison 1. Palivizumab versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Hospitalisation due to RSV infection | 5 | 3343 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.30, 0.64] |
| 1.2 Mortality | 5 | 3343 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.42, 1.15] |
| 1.3 Adverse events | 3 | 2831 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.85, 1.39] |
| 1.4 Hospitalisation due to respiratory‐related illness | 5 | 3343 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.97] |
| 1.5 Subgroup analysis: hospitalisation due to respiratory‐related illness | 5 | 3343 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.97] |
| 1.5.1 High‐income countries | 4 | 3260 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 0.99] |
| 1.5.2 Low‐ and middle‐income countries | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.22] |
| 1.6 Length of hospital stay | 2 | 2789 | Mean Difference (IV, Random, 95% CI) | ‐42.24 [‐84.77, 0.29] |
| 1.7 RSV infection | 3 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.55] |
| 1.8 Number of wheezing days | 1 | 429 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.35, 0.44] |
| 1.9 Days of supplemental oxygen | 2 | 2789 | Mean Difference (IV, Random, 95% CI) | ‐36.85 [‐85.19, 11.49] |
| 1.10 Intensive care unit length of stay | 2 | 2789 | Mean Difference (IV, Random, 95% CI) | ‐13.51 [‐61.11, 34.08] |
| 1.11 Mechanical ventilation days | 2 | 2789 | Mean Difference (IV, Random, 95% CI) | 5.78 [‐10.37, 21.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blanken 2013.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised study. Study dates: April 2008 through December 2010 Setting: hospital, multicentre, national Country: the Netherlands |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Total number of participants randomised: 429 Group 1: n = 214 palivizumab Male, n (%): 125 (58%) Birth weight, grams (95% CI): 2294 (1363 to 3325) Gestational age, weeks (95% CI) 34+3 (32+2‐35+6) Multiple birth, n (%): 38 (19%) Type of feeding, n (%): Breastfeeding and formula: 90 (44%) Breastfeeding: 59 (29%) Formula: 54 (27%) Maternal smoking during pregnancy, n (%) 32 (15%) Parental smoking, n (%): Mother (%) 33 (15%) Father (%) 57 (27%) Siblings (%) 82 (44%) Age mother, median (range): 31 (19 to 48) Age father, median (range): 34 (21 to 55) Atopy mother, n (%): 85 (40%) Physician diagnosis (mother) asthma, n (%): 22 (11%) Physician diagnosis (mother) hay fever, n (%): 48 (24%) Physician diagnosis (mother) eczema, n (%): 48 (24%) Atopy father, n (%): 73 (34%) Physician diagnosis (father) asthma, n (%): 27 (14%) Physician diagnosis (father) hay fever, n (%): 44 (22%) Physician diagnosis (father) eczema, n (%): 29 (15%) Household pets, n (%): 97 (48%) Daycare attendance, n (%): 103 (48%) Sibling attending daycare, n (%): 75 (37%) Doses palivizumab received, median(range): 4 (1 to 5) Group 2: n = 215 placebo Male, n (%): 94 (44%) Birth weight, grams (95% CI): 2289 (1385 to 3358) Gestational age, weeks (95% CI): 34 + 3 (32 + 3 to 35 + 6) Multiple births, n (%): 36 (18%) Type of feeding, n (%): Breastfeeding and formula: 107 (53%) Breastfeeding: 49 (24%) Formula: 46 (23%) Maternal smoking during pregnancy, n (%): 34 (16%) Parental smoking, n (%): Mother (%): 36 (17%) Father (%): 62 (29%) Siblings (%): 85 (45%) Age mother, median (range): 32 (18 to 44) Age father, median (range): 35 (22 to 52) Atopy mother, n (%): 72 (34%) Physician diagnosis (mother) asthma, n (%): 24 (12%) Physician diagnosis (mother) hay fever, n (%): 45 (23%) Physician diagnosis (mother) eczema, n (%): 30 (15%) Atopy father, n (%): 80 (37%) Physician diagnosis (father) asthma, n (%): 21 (11%) Physician diagnosis (father) hay fever, n(%): 52 (26%) Physician diagnosis (father) eczema, n (%): 27 (14%) Household pets, n (%): 98 (49%) Daycare attendance, n (%): 113 (53%) Sibling attending daycare, n (%): 79 (40%) Doses palivizumab received, median (range): 4 (2 to 5) |
|
| Interventions |
Group 1 (n = 214) palivizumab Interventions were intramuscular injections of palivizumab 15 mg/kg or placebo during 1 RSV season from 1 October or from discharge from the neonatal unit until 10 March. A minimum of 2 and a maximum of 5 injections were given. The RSV season was defined as running from 1 October through 31 March based on virological data obtained from the National Institute of Public Health and the Environment (RIVM). Group 2 (n = 215) placebo: placebo was intramuscular injection of physiological sodium chloride 0.9% solution. Treatment was started at the child's home from the first week of October or within 72 hours after discharge from the neonatal hospitalisation. Co‐interventions: All injections were administered at the child's home, and home visits ended after the last injection. |
|
| Outcomes |
Hospitalisation due to respiratory‐related illness How measured: children hospitalised with other respiratory tract illnesses (not RSV). Time points measured: 1 year Time points reported: 1 year Subgroups: none Group 1, n (%): 6 (2.8%) Group 2, n (%): 6 (2.8%) Mortality How measured: not reported Time points measured: throughout trial Time points reported: throughout trial Subgroups: none “There were no deaths” Hospitalisation due to RSV infection How measured: not reported Time points measured: 1 year Time points reported: 1 year Subgroups: none Group 1 (n = 214) palivizumab 1 year, n (%): 2 (0.9) Group 2 (n = 215) placebo 1 year, n (%): 11 (5.1) RSV infection How measured: parents were instructed to take a nasopharyngeal swab in case of the occurrence of respiratory symptoms with involvement of the upper or lower respiratory tract lasting more than 1 day. The swab was transported in a viral transport medium by regular mail to the laboratory and stored at −80 °C until PCR assays were performed. The presence of RSV RNA was determined by multiplex real‐time reverse‐transcriptase–PCR with the use of previously published primers and probes for RSV‐B21 and primers and probes for RSV‐A that were developed in‐house. Time points measured: 1 year Time points reported: 1 year Subgroups: none Group 1 (n = 214) palivizumab 1 year, n (%): 10 (4.7) Group 2 (n = 215) placebo 1 year, n (%): 30 (14.0) Number of wheezing days How measured: number of parent‐reported wheezing days in the first year of life. Parents recorded airway symptoms, doctor visits, and the use of airway drugs in a daily log until infant was 1 year of age. Instructions for completing the log were given during the first home visit, and compliance was checked at each subsequent home visit. Time points measured: 1 year Time points reported: 1 year Subgroups: none Group 1 (n = 214) palivizumab 1 year: Total log days (n): 53,075 Total symptoms days (n): 930 Incidence per day(%): 1.8 (Incidence of wheezing was calculated as the number of days with parent‐reported airway symptoms divided by the number of log days during follow‐up) P < 0.001 for all comparisons From Scheltema 2018: Current use of asthma medication at 3 years follow‐up: 14 (7.6%) (n = 184) Use of asthma medication in the past 12 months at 6 years follow up:18 (9·0%) (n = 199) Group 2 (n = 215) placebo 1 year: Total log days (n): 51,726 Total symptoms days (n): 2309 Incidence per day (%): 4.5 *Incidence of wheezing was calculated as the number of days with parent‐reported airway symptoms divided by the number of log days during follow‐up P < 0.001 for all comparisons From Scheltema 2018: Current use of asthma medication at 3 years follow‐up: 23 (12.2%) of 188 Use of asthma medication in the past 12 months at 6 years follow up:25 (12.8%) (n = 195) |
|
| Notes | Funding sources: Abbott International PLC (UK) ‐ no restrictions on publication of the research data. The 2 study groups were well‐balanced based on inclusion year, gestational age, and birth month. Birth weight, family history of atopy, presence of siblings, and other baseline characteristics were similar, except for sex (58% male infants in the RSV‐prevention group versus 44% in the placebo group). The study reported adverse events related to hospitalisations and deaths. We took account of this information in the relevant outcomes prespecified in the review. |
|
Feltes 2003.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial. Study dates: during 4 RSV seasons, 1998 until 2002. Randomisation from 1 November until 31 December each year between 1998 and 2001. Setting: multicentre, multinational, outpatient Country: the study was conducted at 76 centres in the United States (47), Canada (6), Sweden (3), Germany (4), Poland (6), France (4) and the United Kingdom (6). |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 1287 participants Group 1 (n = 639): palivizumab Age (mean ± SE): 6.8 ± 0.2 months Sex n (%): male: 349 (54.6), female: 290 (45.4) Race/ethnicity, n (%):
Multiple birth, n (%): 27 (4.2) Weight at entry (mean ± SE): 6.1 ± 0.1 kg Gestational age (mean ± SE): 38.5 ± 0.1 weeks RSV risk factors:
Characteristics of CHD at study entry:
Group 2 (n = 648): placebo Age (mean ± SE): 6.5 ± 0.2 months Sex n (%): male: 344 (53.1), female: 304 (46.9) Race/ethnicity, n (%):
Multiple births, n (%): 23 (3.5) Weight at entry (mean ± SE): 6.0 ± 0.1 kg Gestational age (mean ± SE): 38.5 ± 0.1 weeks RSV risk factors:
Characteristics of CHD at study entry:
|
|
| Interventions |
Group 1 (n = 639): palivizumab 15 mg/kg, intramuscular injection every 30 days for a total of 5 doses. Group 2 (n = 648): placebo (same formulation as palivizumab without antibody and with 0.02% Tween‐80 added), intramuscular injection every 30 days for a total of 5 doses. Co‐interventions: palivizumab and placebo were supplied as lyophillised product in coded vials that were reconstituted by the pharmacist with sterile water for injection (final concentration of palivizumab is 100 mg/mL) and dispensed in a syringe that did not identify the contents. |
|
| Outcomes |
Hospitalisation due to respiratory‐related illness How measured: number of children with cardiorespiratory hospitalisation, n (%) Time points measured: throughout the study Time points reported: throughout the study Group 1: 321 (50.2) Group 2: 359 (55.4) Mortality How measured: number of deaths (associated with RSV), n. Total number of deaths, n (%) Time points measured: throughout the study Time points reported: throughout the study Number of deaths (associated with RSV), n: Group 1: 2 Group 2: 4 Total number of deaths, n (%). Group 1: 21 (3.3) Group 2: 27 (4.2) ( P = 0.463) Adverse events How measured: reported as total number of adverse events, total number of children (n) with adverse event (%), total number of children (n) with related adverse event, total number of children (n) with serious adverse event (%) and total number of children (n) with related serious event (%). Treatment groups were compared for adverse events (COSTART coded terms) by evaluating the number of children in each group with at least 1 event by body system and the distribution of severity and relatedness of these events. Any adverse change from a child’s medical condition at entry was reported as an adverse event, graded for severity, and assessed by the blinded investigator as to potential relation to study drug. Serious adverse events were those that resulted in death; were life‐threatening; resulted in hospitalisation or prolonged hospitalisation; resulted in significant disability; or were another important medical event that required intervention to prevent 1 of the above outcomes. Time points measured: children were followed for 150 days from random assignment (30 days after the last scheduled study injection) for the occurrence of adverse events. Time points reported: children were followed for 150 days from random assignment (30 days after the last scheduled study injection) for the occurrence of adverse events. Total number of adverse events, n: Group 1: 4169 Group 2: 4518 Total number of children with adverse event, n (%): Group 1: 611 (95.6) Group 2: 625 (96.5) (P = 0.477) Total number of children with adverse event coding to cardiovascular system, n (%): Group 1: 286 (44.8) Group 2: 315 (48.6) (P = 0.180) Total number of children with adverse event coding to respiratory system, n (%): Group 1: 525 (82.2) Group 2: 547 (84.4) (P = 0.296) Total number of children with adverse event requiring medical intervention, n (%): Group 1: 588 (92.0) Group 2: 605 (93.4) (P = 0.392) Total number of children with related adverse event, n (%): Group 1: 46 (7.2) Group 2: 45 (6.9) (P = 0.914) Total number of children with related adverse event resulting in permanent discontinuation, n (%): Group 1: 0 (0.0) Group 2: 0 (0.0) Total number of children with serious adverse event, n (%): Group 1: 354 (55.4) Group 2: 409 (63.1) (P = 0.005) Total number of children with related serious event, n (%): Group 1: 0 (0.0) Group 2: 3 (0.5) (P = 0.249) Subgroups: Incidence of serious adverse of events, (%): Group 1: Cyanotic stratum: 59.9 “Other” stratum: 50.3 Group 2: Cyanotic stratum: 67.1 “Other” stratum: 58.7 Days of supplemental oxygen How measured: total RSV hospital days with increased oxygen requirement, n (total days per 100 children). Also reported as relative reduction (%). Time points measured: throughout the study. Time points reported: throughout the study. Group 1: 178 (27.9) Group 2: 658 (101.5) Reduction: 73 (P = 0.014) Intensive care unit length of stay How measured: total days of RSV‐associated intensive care, n (total days per 100 children). Also reported as relative reduction (%). Time points measured: throughout the study. Time points reported: throughout the study. Group 1: 101 (15.9) Group 2: 461 (71.2) Reduction: 78 (P = 0.080) Mechanical ventilation days How measured: total days of RSV‐associated mechanical ventilation, n (total days per 100 children). Also reported as relative reduction (%). Time points measured: throughout the study Time points reported: throughout the study Group 1: 42 (6.5) Group 2: 354 (54.7) Reduction: 41 (P = 0.224) Hospitalisation due to RSV infection How measured: incidence of RSV hospitalisation, n (%). Also reported as total days of RSV hospitalisation, D (total days per 100 children). In addition, reported as relative reduction of RSV hospitalisation rate, % and relative reduction of total days of RSV hospitalisation, %. Time points measured: throughout the study Time points reported: throughout the study Group 1: 34 (5.3), D: 367 (57.4) Group 2: 63 (9.7), D: 836 (129.0) Reduction (rate): 45 Reduction (days): 56 (P = 0.003) |
|
| Notes | Overall, 93.0% of children in the palivizumab group and 91.8% in the placebo group received all 5 planned injections; 95.6% in the palivizumab group and 95.5% in the placebo group completed the study. No child had study drug discontinued for a related adverse event. A total of 48 children died during the study: 21 (3.3%) in the palivizumab group and 27 (4.2%) in the placebo group. No deaths were attributed to study drug. Deaths associated with RSV infection occurred in 2 children in the palivizumab group and 4 children in the placebo group. | |
IMpact‐RSV Study Group 1998.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial, multicentre (139 sites), phase III trial. Study dates: randomisation from 15 November 1996 until 13 December 1996. Study executed during the 1996 to 1997 RSV season. Setting: multicentre, multinational, outpatient Country: the United States (110 centres), the United Kingdom (11 centres) and Canada (9 centres) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 1502 children. Group 1 (n = 500): placebo Age (mean ± SE): 6.0 ± 0.21 months Sex %: male: 56.8%, female:43.2% Race/ethnicity (%):
Birth weight (mean ± SE): 1.3 ± 0.02 kg Gestational age (mean ± SE):29 ± 0.14 weeks Proportion of children with gestational age ≤ 32 weeks (%): 83.4 Proportion of children with gestational age > 32 weeks (%): 16.6 Multiple birth (%): 27.4 Weight at entry (mean ± SE): 4.9 ± 0.1 kg Previous RSV (%): 1: 5.6 RSV neutralising antibody ≥ 1:200 (%): 5.6 Number of people in house (mean ± SE): 3.5 ± 0.07 Smoker in household (%):68.6 Child in daycare (%): 6.8 Family history of asthma (%): 35.2 Family history of hay fever (%): 29.6 Family history of eczema (%): 16.4 Premature children (no BPD), n (%): 234 (46.8) Children with BPD, n (%): 266 (53.2) Group 2 (n = 1002): palivizumab Age (years): mean age at study entry (months ± SE): 5.7 ± 0.15 Sex %: male: 56.9%, female: 43.1% Race/ethnicity (%):
Birth weight (mean ± SE): 1.3 ± 0.02 kg Gestational age (mean ± SE): 29 ± 0.10 weeks Proportion of children with gestational age ≤ 32 weeks (%): 83.8 Proportion of children with gestational age > 32 weeks (%): 16.2 Multiple birth (%): 31.7 Weight at entry (mean ± SE): 4.8 ± 0.1 kg Previous RSV (%): 3.8 RSV neutralising antibody ≥ 1:200 (%): 5.5 Number of people in house (mean ± SE): 3.5 ± 0.05 Smoker in household (%): 63.0 Child in daycare (%): 6.7 Family history of asthma (%): 36.1 Family history of hay fever (%): 28.6 Family history of eczema (%): 16.5 Premature children (no BPD), n (%): 506 (50.5) Children with BPD, n (%): 496 (49.5) |
|
| Interventions |
Group 1 (n = 500): placebo (same formulation as group 2 but without antibody and with 0.02% Tween‐80 added), intramuscular injection every 30 days, 5 doses. Group 2 (n = 1002): palivizumab 15 mg/kg (concentration 100 mg/mL), intramuscular injection every 30 days, 5 doses |
|
| Outcomes |
Hospitalisation due to respiratory‐related illness How measured: incidence of respiratory hospitalisations, %, and incidence of respiratory hospitalisations unrelated to RSV (%). Time points measured: throughout the study Time points reported: throughout the study Incidence of respiratory hospitalisations (%): Group 1: 22 Group 2: 16 (P = 0.008) Incidence of respiratory hospitalisations unrelated to RSV (%) Group 1: 14 Group 2: 13 (P = 0.470) Mortality How measured: number of deaths, n (%). (Cause of death not specified) Time points measured: throughout the study Time points reported: throughout the study Group 1: 5 (1.0) Group 2: 4 (0.4) Adverse events How measured: number of children (n) and proportion of children (%) reporting adverse events judged by the blinded investigator to be related to the study drug. Adverse events were assessed by the investigators with regard to severity (using a standard toxicity table modified from the Pediatric AIDS Clinical Trials Group) and potential relationship to the study drug. Treatment groups were compared for adverse events by evaluating the number of children in each group with at least 1 event by body system and the distribution of severity of these events. Time points measured: 150 days from randomisation (30 days after last scheduled injection). Time points reported: throughout the study period. Group 1: 50 (10) Group 2: 110 (11) Number of children reporting adverse events related to the injection site, n (%) Group 1: 9 (1.8) Group 2: 27 (2.7) Erythema, n (%): Group 1: 6 (1.2) Group 2: 14 (1.4) Pain, n (%): Group 1: 0 (0.0) Group 2: 6 (0.6) Induration/swelling, n (%): Group 1: 1 (0.2) Group 2: 6 (0.6) Bruising, n (%): Group 1: 2 (0.4) Group 2: 3 (0.3) Most frequently reported adverse events that were judged by the blinded investigator as potentially related to study drug, n (%). Fever: Group 1: 15 (3.0) Group 2: 28 (2.8) (P = 0.870) Nervousness: Group 1: 13 (2.6) Group 2: 25 (2.5) (P = 0.865) Injection site reaction: Group 1: 8 (1.6) Group 2: 23 (2.3) (P = 0.444) Diarrhoea: Group 1: 2 (0.4) Group 2: 10 (1.0) (P = 0.357) Rash: Group 1: 1 (0.2) Group 2: 9 (0.9) (P = 0.179) AST increased: Group 1: 3 (0.6) Group 2: 5 (0.5) (P = 0.726) Upper respiratory tract illness: Group 1: 2 (0.4) Group 2: 5 (0.5) (P = 1.000) Liver function abnormal (primarily elevations of both AST and ALT): Group 1: 1 (0.2) Group 2: 3 (0.3) (P = 1.000) ALT increased: Group 1: 2 (0.4) Group 2: 3 (0.3) (P = 0.670) Vomiting: Group 1: 2 (0.4) Group 2: 3 (0.3) (P = 0.670) Cough: Group 1: 1 (0.2) Group 2: 3 (0.3) (P = 1.000) Rhinitis: Group 1: 3 (0.6) Group 2: 3 (0.3) (P = 0.406) Children with mild or moderate elevations of AST (measured at baseline and before the fourth injection), n (%): Group 1: 8 (1.6) Group 2: 36 (3.6) Children with mild or moderate elevations of ALT (measured at baseline and before the fourth injection), n (%): Group 1: 10 (2.0) Group 2: 23 (2.3) Length of hospital stay How measured: total days of all hospitalisations per 100 children, total days of respiratory hospitalisations per 100 children and total days of respiratory hospitalisations unrelated to RSV per 100 children Time points measured: throughout the study. Time points reported: throughout the study. Total days of all hospitalisations per 100 children: Group 1: 242 Group 2: 191 (P = 0.005) Total days of respiratory hospitalisations per 100 children: Group 1: 180 Group 2: 124 (P = 0.004) Total days of respiratory hospitalisations unrelated to RSV per 100 children: Group 1: 118 Group 2: 88 (P = 0.369) Days of supplemental oxygen How measured: number of days in RSV hospitalisation with increased oxygen, n: Time points measured: throughout the study Time points reported: throughout the study Group 1: 50.6 Group 2: 30.3 (P < 0.001) Intensive care unit length of stay How measured: total days of RSV ICU admissions, n, and incidence of RSV ICU admissions (%) Time points measured: throughout the study Time points reported: throughout the study Group 1: 12.7 (3.0) Group 2: 13.3 (1.3) (n: P = 0.023, %: P = 0.026) Mechanical ventilation days How measured: total days, n, and incidence of mechanical ventilation (%) during RSV hospitalisation Time points measured: throughout the study Time points reported: throughout the study Group 1: 1.7 (0.2) Group 2: 8.4 (0.7) (n: P = 0.210, %: P = 0.280) Hospitalisation due to RSV infection How measured: total days of RSV hospitalisation per 100 children (D) and incidence of RSV hospitalisation, n (%). Also reported as reduction in hospitalisation as a result of RSV, % (95% CI). Time points measured: throughout the study Time points reported: throughout the study Total days of RSV hospitalisation per 100 children: Group 1: 62.6 Group 2: 36.4 (P < 0.001) Reduction: 55 (38 to 72) Incidence of RSV hospitalisation, n (%) Group 1: 53 (10.6) Group 2: 48 (4.8) (P < 0.001) Reduction: 55 (38 to 72) Subgroups: Number of RSV hospitalisations, n (%). Group 1: Premature (no BPD): 19 (8.1) BPD: 34 (12.8) Group 2: Premature (no BPD): 9 (1.8) BPD: 39 (7.9) Reduction in RSV hospitalisations, %: Infants > 5 kg: 51 Infants ≤ 5 kg: 57 Infants < 32 weeks gestational age: 47 Infants 32‐25 weeks gestational age: 80 |
|
| Notes | A total of 1486 (99%) children completed the protocol follow‐up (99% placebo, 99% palivizumab). Reasons for non‐completion included death, not judged as a result of palivizumab, (n = 7), withdrawal of consent (n = 4); or loss to follow‐up (n = 5) before day 150 and before any RSV hospitalisation. Overall, 94% of the placebo group and 92% of the palivizumab group received all 5 injections and more than 95% of both groups received at least 4 injections. Discontinuation of injections due to adverse events related to palivizumab was rare (0.3%). | |
Subramanian 1998.
| Study characteristics | ||
| Methods |
Study design: phase I/II multicentre (10 sites), randomised, double‐blind, placebo‐controlled, dose escalation trial. Study dates: randomisation from 27 November 1995 until 15 February 1996. Infusions completed by 19 April 1996. Setting: multicentre, national, outpatient. Country: the United States. |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 62 infants were randomised. 57 participants completed the study (received a study drug). Group 1 (n = 20): placebo. 0.9% saline intravenously every 30 days for up to 5 doses Age at study entry (mean ± SE): 5.0 ± 0.83 months Sex (M/F): not available. Relevant participant details:
Group 2 (n = 10): 3 mg/kg MEDI‐493 intravenously every 30 days for up to 5 doses Age at study entry (mean ± SE): 6.9 ± 1.28 months Sex (M/F): not available Relevant participant details:
Group 3 (n = 10): 10 mg/kg MEDI‐493 intravenously every 30 days for up to 5 doses Age at study entry (mean ± SE): 7.3 ± 2.01 months Sex (M/F): not available. Relevant participant details:
Group 4 (n = 22): 15 mg/kg MEDI‐493 intravenously every 30 days for up to 5 doses Age at study entry (mean ± SE): 8.1 ± 1.69 months Sex (M/F): not available Relevant participant details:
|
|
| Interventions |
Group 1 (n = 20): placebo. 0.9% saline intravenously every 30 days for up to 5 doses. Group 2 (n = 10): 3 mg/kg MEDI‐493 intravenously every 30 days for up to 5 doses. Group 3 (n = 10): 10 mg/kg MEDI‐493 intravenously every 30 days for up to 5 doses. Group 4 (n = 22): 15 mg/kg MEDI‐493 intravenously every 30 days for up to 5 doses. For groups 2, 3 and 4: MEDI‐493 was formulated in phosphate‐buffered saline without preservatives; 10 mg/mL, 10 mL/vial, pH 7.4. For administration MEDI‐493 was withdrawn from the vial using a syringe and 0.2‐μm pore size filter. The volume of study drug was calculated based on the child's weight (rounded to the nearest 0.1 kg) on the day of the visit. For groups 3 and 4: dosing was initiated at 3 mg/kg; escalations to 10 mg/kg and finally to 15 mg/kg were based on cumulative safety data from the preceding dosages. Co‐interventions: infusions were given in 2 to 5 min using standard intravenously infusion equipment. |
|
| Outcomes |
Hospitalisation due to respiratory‐related illness How measured: number of occurrences (O) and number of children hospitalised due to any respiratory infection, n (%). Time points measured: throughout the study. Time points reported: throughout the study. Group 1: (O: 4), 3 (15.0) Group 2: (O: 5), 5 (50.0) Group 3: (O: 1), 1 (10.0) Group 4: (O: 4), 3 (13.6) Mortality How measured: number of deaths, n (%) (not due to RSV infection) Time points measured: throughout the study Time points reported: throughout the study Group 1: 1 (5.0) (disseminated adenovirus infection) Group 2: 0 (0.0) Group 3: 0 (0.0) Group 4: 0 (0.0) Adverse events How measured: number of children that experienced adverse events judged as potentially related to study drug, n (%). Adverse events were graded for severity (mild, moderate, severe and life‐threatening) using a toxicity table (modified from the AIDS Clinical Trials Group paediatric toxicity tables). All adverse events were assessed by the blinded investigator as to potential relationship to study drug (none, remote, possible, probably or definitely related). Any adverse event graded as greater than moderate, as well as any fatal, life‐threatening or permanently disabling events and any inpatient hospitalisations, prolonged hospitalisations or overdoses were classified as serious adverse events. Adverse events were coded by body system using a standard COSTART adverse event dictionary. For analytical purposes the total numbers of infants in each dose group reporting 1 or more adverse events in each body system were compared. MEDI‐493 groups were compared with the pooled placebo group. Time points measured: adverse events were reported through 30 days after the last infusion visit. Time points reported: adverse events were reported through 30 days after the last infusion visit. Group 1: 3 (15) Group 2: 1 (10) Group 3: 0 (0) Group 4: 5 (23) Total number of adverse events judged potentially related to study drug reported by all participants: n = 14 Most frequent events reported by all participants, n: Fever: 3 Right upper lobe pneumonia: 2 Infusion site infiltration: 2 Increased transaminase values, n (%): Group 1: 1 (5) Group 2: 0 (0) Group 3: 1 (10) Group 4: 2 (9.1) RSV infection How measured: number of occurrences (O) and number of children with RSV respiratory illness, n (%). Measured with RSV antigen tests (and RSV cultures for some children). Time points measured: throughout the study. Time points reported: throughout the study. Group 1: (O: 4), 4 (20.0) Group 2: (O: 3), 3 (30.0) Group 3: (O: 1), 1 (10.0) Group 4: (O: 2), 2 (9.1) Hospitalisation due to RSV infection How measured: number of occurrences (O) and number of children with RSV respiratory illness hospitalisation, n (%). Time points measured: throughout the study. Time points reported: throughout the study. Group 1: (O: 2), 2 (10.0) Group 2: (O: 2), 2 (20.0) Group 3: (O: 0), 0 (0.0) Group 4: (O: 0), 0 (0.0) |
|
| Notes | 1 child was randomised to placebo but inadvertently received 15 mg/kg MEDI‐493 and was analysed as a 15 mg/kg MEDI‐493 patient. Overall 57 (91.9%) children completed the study. Reasons for premature termination included withdrawal of consent (4 children) and death (1 placebo patient). Only 4 children (6.5%) received fewer than 3 infusions (3 withdrawal of consent, 1 iv infiltration). No infants withdrew because of an adverse event. All of the study drug was administered in 96.2% of attempted infusions; incomplete infusions were the result of technical difficulties with the intravenous infusion. The intervention arms with a lower dose of palivizumab were not included in our analysis. | |
Tavsu 2014.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised study. Study dates: 2 RSV seasons (October to March, 2009 to 2010 and 2010 to 2011) Setting: inpatient, single centre, national Country: Turkey |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Total number of participants randomised: 83 Group 1: n = 41 palivizumab Gestational age, mean (SD): 29.4 (1.8) weeks Birth weight, mean (SD): 1317 (255) g Cesarean delivery, n (%): 35 (89.7) Female, n (%) 19 (48.7) Infants on mechanical ventilator, n (%): 30 (76.9) Respiratory distress syndrome, n (%): 30 (76.9) Days of hospitalisation, median (IQR): 35 (21 to 52) days Group 2: n = 42 no intervention Gestational age, mean (SD): 29.7 (1.6) weeks Birth weight, mean (SD): 1404 (270) g Cesarean delivery, n (%): 34 (82.9) Female, n (%): 24 (58.5) Infants on mechanical ventilator, n (%): 27 (65.9) Respiratory distress syndrome, n (%): 27 (65.9) Days of hospitalisation, median (IQR): 27 (19 to 46) days |
|
| Interventions |
Group 1 (n = 39) palivizumab: study group received prophylaxis with palivizumab for the prevention of RSV infection monthly for 1 season (15 mg/kg/dose, 5 doses between October and March). Group 2 (n = 41) no intervention: control group did not receive palivizumab prophylaxis. Co‐interventions: not reported. Both groups were followed up during 2 RSV seasons (2009 to 2010 and 2010 to 2011). |
|
| Outcomes |
Hospitalisation due to respiratory‐related illness How measured: infants were hospitalised if they had severe respiratory distress, intercostal retractions, cyanosis, and feeding intolerance with or without fever. In all hospitalised cases the test was performed within the first 48 hours of hospitalisation. If RSV was not present, the attack was denoted as non‐RSV infection. Time points measured: 1 and 2 years follow‐up Time points reported: 1 and 2 years follow‐up Subgroups: none Group 1 (n = 39) palivizumab 1‐year follow‐up, n (%): 1 (2.6) 2 year follow‐up, n (%): 5 (12.8) Group 2 (n = 41) no intervention 1‐year follow‐up, n (%): 2 (4.8) 2 year follow‐up, n (%): 1 (2.4) Mortality How measured: not reported Time points measured: 1 and 2 year follow‐up Time points reported: 1 and 2 year follow‐up Subgroups: none “No infants died during follow‐up.” Hospitalisation due to RSV infection How measured: Infants were hospitalised if they had severe respiratory distress, intercostal retractions, cyanosis, and feeding intolerance with or without fever. In all hospitalised cases the test was performed within the first 48 hours of hospitalisation. If RSV was not present, the attack was denoted as non‐RSV infection. Time points measured: 1 and 2 year follow‐up Time points reported: 1 and 2 year follow‐up Subgroups: none Group 1 (n = 39) palivizumab 1 year follow‐up, n (%): 0 2 year follow‐up, n(%): 0 Group 2 (n = 41) no intervention 1 year follow‐up, n (%): 10 (24.4) 2 year follow‐up, n (%): 10 (24.4) RSV infection How measured: Respi‐Strips (Coris BioConcept, Gembloux, Belgium) test was used for the detection of RSV (Coris BioConcept). For this purpose, a nasal swab was obtained and mixed in 0.5 mL of normal saline. Around 0.25 mL (8 drops) of this solution was mixed with 0.25 mL of test solution and 10 minutes later the test strip was inserted into the solution. A single line in the test strip was regarded as negative and 2 lines were regarded as positive for RSV. If the test was positive for RSV, but the infant had no symptom of respiratory illness, this infant was denoted as RSV (+) Time points measured: 1 year and 2 years follow‐up Time points reported: 1 year and 2 years follow‐up Subgroups: none Group 1 (n = 39) palivizumab 1 year follow‐up, n (%): 11 (28.2) LRTI due to RSV (1 year):9 (23.1) 2 years follow‐up, n (%): 14 (35.9) LRTI due to RSV (2 years): 6 (15.4) Group 2 (n = 41) no intervention 1‐year follow‐up, n (%): 24 (58.5) LRTI due to RSV (1 year): 22 (53.7) 2 years follow‐up, n (%): 22 (53.7) LRTI due to RSV (2 years): 20 (48.8) |
|
| Notes | A total of 41 infants were randomised to the study group and 42 infants to the control group. 2 infants from the study group and 1 infant from the control group dropped out of the study. | |
ALT: alanine aminotransferase AST: aspartate aminotransferase BPD: bronchopulmonary dysplasia BUN: blood urea nitrogen CHD: congenital heart disease CI: confidence interval COSTART: Coding Symbols for a Thesaurus of Adverse Reaction Terms FiO₂: fraction of inspired oxygen ICU: intensive care unit IgA: immunoglobulin A IgG: immunoglobulin G IgM: immunoglobulin M IQR: interquartile range LRI: lower respiratory infection LRTI: lower respiratory tract infection PCR: polymerase chain reaction RNA: ribonucleic acid RSV: respiratory syncytial virus SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1999 | Wrong study design. Commentary on palivizumab |
| Anonymous 2004 | No full text available: possibly a narrative review or comment. |
| Driver 1999 | Wrong study design. Narrative review of available evidence related to palivizumab |
| EUCTR2007‐002070‐61‐PL | Wrong comparator. Ongoing trial comparing palivizumab against motavizumab |
| Ignacio 2013 | Wrong study design. Commentary on Blanken 2013 |
| Johnson 1999 | Wrong study design. No full text available. Probably a narrative review or comment |
| Koganesawa 2019 | Wrong study design. Case series of patients with immunodeficiencies receiving palivizumab |
| Naver 2002 | Wrong study design. Commentary on palivizumab |
| Pin 2002 | Wrong study design. Narrative review on palivizumab |
| Rajakumar 2009 | Wrong study design. A retrospective descriptive study of children receiving palivizumab |
| Tulloh 2011 | Wrong study design. A cohort study of children who underwent cardiac surgery and where included previously in Feltes 2003 |
Characteristics of studies awaiting classification [ordered by study ID]
EUCTR 2018‐002980‐25‐IT.
| Methods | Parallel group, randomised, multicentre, open‐label study |
| Participants | Newborns until 6 months of age |
| Interventions | Palivizumab (dosage not reported) versus placebo |
| Outcomes |
Primary outcomes
Overall respiratory morbidity (composite primary endpoint), whose definition is:
‐ LRTIs by any pathogen (including, but not limited to, RSV) +
‐ recurrent wheezing (quantified as the presence of wheezing days and/or need for use of antiwheezing drugs) +/‐ asthma Secondary outcomes Difference of RSV‐related hospitalisation due to LRTI during the first RSV‐season (November 1st to March 31th) between Group A (treated with palivizumab) and Group B (not treated). Direct costs in terms of difference of health resources used by Group A and Group B respectively and related to: ‐ length of hospitalisations (days); ‐ mechanical ventilation and other support therapies (type, number, duration); ‐ ICU admission and length of stay (days); ‐ outpatient visits (type and number of specialistic visits); ‐ drugs (type, dosages and therapy duration); and ‐ diagnostic tests (type and number). Costs for resources used will be calculated using National Health Service tariffs. Indirect costs in terms of loss of working days for parents due to child illness. |
| Notes | Insufficient information on the trial record to determine inclusion as an ongoing trial. |
Lenney 1998.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. Possibly a secondary report of IMpact‐RSV Study Group 1998. |
ICU: intensive care unit LRTI: lower respiratory tract infection RSV: respiratory syncytial virus
Differences between protocol and review
We changed the review title to 'Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children' to fit the scope of the review, as suggested by peer reviewers.
We included one trial administrating palivizumab intravenously in order to maximize the use of available information (Subramanian 1998). We highlighted this in the results section.
Change in the outcomes
Mortality: we broadened this definition to "death due to all causes" as it would result in better use of the available information, considering that the trials had not systematically reported this information. Nevertheless, we also reported the cause of deaths for each group (including deaths related to RSV infection) when this information was available.
Length of hospital stay: based on input from the Cochrane Methods Support Unit this outcome was removed from the summary of findings table as it is reported in a subset of patients, which breaks randomisation and is considered a post hoc analysis in the trial that does not include all randomised participants and therefore does not comply with the analyses on the effect of assignment to intervention. We considered it an exploratory outcome and reported it accordingly.
We changed the order of the outcomes in the review. We changed the primary outcome to prevention of RSV hospitalisation and moved hospitalisation due to any respiratory infection to a secondary outcome, as suggested by peer reviewers.
Number of wheezing days: considering that the single included study providing datat for this outcome reported multiple analyses (including the total log days and the total symptoms days), we chose to display the relative reduction in the rate of daily wheezing to avoid any unit of analysis errors.
Search strategy
The search strategy was first performed on 15 October, 2020, and updated in October 2021. However, we did not update the Embase search strategy as we lacked access to the database this year, although the Embase records can be retrieved through CENTRAL.
Methods not implemented
We did not use the search filter for randomised controlled trials (Lefebvre 2019), since we used Screen4Me to identify trials.
Primary outcomes
We intended to report serious adverse events separately, however, the included studies reported the global incidence of adverse events. Nevertheless, we disaggregated some of these events (such as hospitalisation due to respiratory illness and overall mortality).
Subgroup analyses
We were not able to carry out two of our prespecified subgroup analyses (high‐risk children versus average‐risk children and tropical regions versus non‐tropical regions) as we found no studies with these characteristics. We were able to carry out the remaining subgroup analysis (high‐income countries versus low‐ and middle‐income countries) only for the outcome hospitalisation due to respiratory‐related illness.
Sensitivity analyses
We moved the specifications regarding sensitivity analysis from the Data synthesis section of the protocol to Sensitivity analysis section in the full review. However, we were unable to carry out any sensitivity analyses due to scarcity of data.
Contributions of authors
LG and JVAF: conceived, designed, and wrote the protocol and full review and performed all aspects of the data abstraction, analysis, risk of bias assessment and certainty of evidence ratings.
IE: drafted the Background section, provided input and literature for the Discussion section and drafted the full review.
LS: performed all aspects of the data abstraction, analysis and risk of bias assessment.
CMEL: designed and ran the electronic searches, and drafted the full review.
PR: reviewed critical content.
All authors read and approved the final draft of the review.
Sources of support
Internal sources
-
Instituto Universitario Hospital Italiano, Argentina
In‐kind support for the research team
External sources
No sources of support provided
Declarations of interest
LG: declared that they have no conflict of interest. LS: declared that they have no conflict of interest. PR: declared that they have no conflict of interest. CMEL: declared that they have no conflict of interest. IE: declared that they have no conflict of interest. JVAF: declared that they have no conflict of interest.
New
References
References to studies included in this review
Blanken 2013 {published and unpublished data}
- Blanken M, Rovers M, Molenaar J, Winkler-Seinstra P, Meijer A, Kimpen J. Respiratory syncytial virus prevention with Palivizumab is associated with a decrease in non-hospitalized RSV infections; 2013 May 4 – 7; Washington, D.C. In: Pediatric Academic Societies Annual Meeting. 2013.
- Blanken M, Rovers M, Sanders E, Bont L. Ethical considerations and rationale of the MAKI trial: a multicenter double-blind randomized placebo-controlled trial into the preventive effect of palivizumab on recurrent wheezing associated with respiratory syncytial virus infection in children with a gestational age of 33-35 weeks. Contemporary Clinical Trials 2012;33(6):1287-92. [DOI] [PubMed] [Google Scholar]
- Blanken MO, Frederix GW, Nibbelke EE, Hendrik K, Koffijberg H, Dutch RSV Neonatal Network. Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants. European Journal of Pediatrics 2018;177(1):133-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. New England Journal of Medicine 2013;368(19):1791-9. [DOI] [PubMed] [Google Scholar]
- Bont L, Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, et al. Causal relationship between respiratory syncytial virus infection and recurrent wheeze during the first year of life. American Journal of Respiratory and Critical Care Medicine 2013;187:A6127. [Google Scholar]
- Effect of palivizumab on respiratory syncytial virus-associated burden of disease – a randomized controlled trial. www.clinicaltrialsregister.eu/ctr-search/trial/2007-004105-10/NL (first received 2 June 2008).
- Man W, Scheltema N, Van Houten M, Nibbelke E, Achten N, Arp K, et al. Infant RSV prophylaxis, RSV infection, and nasopharyngeal microbiota at age six years. European Respiratory Journal 2019;54:OA4939. [Google Scholar]
- Man W, Scheltema N, Clerc M, Houten M, Nibbelke E, Achten N, et al. Infant respiratory syncytial virus prophylaxis and nasopharyngeal microbiota until 6 years of life: a subanalysis of the MAKI randomised controlled trial. Lancet Respiratory Medicine 2020;8(10):1022-31. [DOI] [PubMed] [Google Scholar]
- Scheltema NM, Nibbelke EE, Pouw J, Blanken MO, Rovers MM, Naaktgeboren CA, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respiratory Medicine 2018;6(4):257-64. [DOI] [PubMed] [Google Scholar]
- Xu C, Scheltema NM, Qi C, Vedder R, Klein LBC, Nibbelke EE, et al. Infant RSV infection changes nasal epithelial DNA methylation at 6 years of age. European Respiratory Journal 2019;54:PA4999. [Google Scholar]
Feltes 2003 {published data only}
- Feltes TF, Cabalka AK, Meissner C, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. Journal of Pediatrics 2003;143(4):532-40. [DOI] [PubMed] [Google Scholar]
IMpact‐RSV Study Group 1998 {published data only}
- Forbes M, Kumar V, Yogev R, Wu X, Robbie G, Ambrose C. Serum palivizumab level is associated with decreased severity of respiratory syncytial virus disease in high-risk infants. Human Vaccines & Immunotherapeutics 2014;10(10):2789-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102(3 Pt 1):531-7. [PubMed] [Google Scholar]
Subramanian 1998 {published data only}
- Subramanian K, Weisman L, Rhodes T, Ariagno R, Sanchez PJ, Steichen J, et al. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group. Pediatric Infectious Disease Journal 1998;17(2):110-5. [DOI] [PubMed] [Google Scholar]
Tavsu 2014 {published data only}
- Tavsu I, Gursoy T, Dirman S, Erbil N, Ovali F. Palivizumab prophylaxis: does It have any influence on the growth and development of the infants? American Journal of Perinatology 2014;31(8):667-71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1999 {published data only}
- Prevention of respiratory syncytial virus infection by monoclonal antibody. Deutsche Apotheker Zeitung 1999;139(28):36.
Anonymous 2004 {published data only}
- Palivizumab: new indication. Moderate reduction in hospitalisation rate. Prescrire International 2004;13(74):213-6. [PubMed]
Driver 1999 {published data only}
- Driver LC, Oertel MD. Synagis: an anti-RSV monoclonal antibody. Pediatric Nursing 1999;25(5):527-30. [PubMed] [Google Scholar]
EUCTR2007‐002070‐61‐PL {published data only}
- EUCTR2007-002070-61-PL. A study to evaluate the safety, tolerability, pharmacokinetics, and immunogenicity of MEDI-524, a humanized enhanced potency monoclonal antibody against respiratory syncytial virus (RSV), in children with hemodynamically significant congenital heart disease. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-002070-61-PL (first received 10 August 2007).
Ignacio 2013 {published data only}
- Ignacio L, Alfaleh K. Does RSV prophylaxis prevents future recurrent wheeze in preterm infants? Journal of Clinical Neonatology 2013;2(3):116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1999 {published data only}
- Johnson KE, Weisman LE. Palivizumab (Synagis®) in the prevention of lower respiratory tract infection in children due to respiratory syncytial virus (RSV). Today's Therapeutic Trends 1999;17(3):227-42. [Google Scholar]
Koganesawa 2019 {published data only}
- Koganesawa M, Ono T, Takamido S, Takagi T, Yamaoka D, Ujiie G, et al. Prophylactic palivizumab prevents respiratory syncytial virus infection in pediatric patients with immunodeficiencies. Pediatric Blood & Cancer 2019;66:S39. [Google Scholar]
Naver 2002 {published data only}
- Naver L, Eriksson M, Ewald U, Linde A, Lindroth M, Schollin J. Prophylaxis against RS virus infection [Profylax mot rs-virusinfektion.]. Läkartidningen 2002;99(3):170-1. [PubMed] [Google Scholar]
Pin 2002 {published data only}
- Pin I, Pilenko C, Bost M. Prevention of infection by respiratory syncytial virus (VRS) by SYNAGYS® (PALIVIZUMAB). Allergie et Immunologie 2002;34(10):371-4. [PubMed] [Google Scholar]
Rajakumar 2009 {published data only}
- Rajakumar D, Tan B, Bodani J, Zhu T, Janzen B, Bodani R et al. Children hospitalized with respiratory syncytial virus (RSV) infection after palivizumab prophylaxis in two large paediatric tertiary care centers. Acta Paediatrica 2009;98:214-5. [Google Scholar]
Tulloh 2011 {published data only}
- Tulloh R, Flanders L, Henderson J, Thompson R, Feltes T. Does RSV infection cause pulmonary hypertension in children undergoing cardiac surgery? Archives of Disease in Childhood 2011;96:A37-8. [Google Scholar]
References to studies awaiting assessment
EUCTR 2018‐002980‐25‐IT {published data only}
- EUCTR 2018-002980-25-IT. Randomized, multicenter, open-label study on PREvention of respiratory SEquelae of RSV bronchiolitis in preterm babies (PRESERV). www.clinicaltrialsregister.eu/ctr-search/trial/2018-002980-25/IT (first received 7 October 2020).
Lenney 1998 {published data only}
- Lenney W, Connor E. Humanised monoclonal antibody to respiratory syncytial virus (MEDI-493) significantly reduces the incidence of RSV hospitalisation in at-risk infants. European Respiratory Journal. Supplement 1998;12(Suppl 28):270S. [Google Scholar]
Additional references
Alansari 2019
- Alansari K, Toaimah FH, Almatar DH, El Tatawy LA, Davidson BL, Qusad M. Monoclonal antibody treatment of RSV bronchiolitis in young infants: a randomized trial. Pediatrics 2019;143(3):e20182308. [DOI: 10.1542/peds.2018-2308] [DOI] [PubMed] [Google Scholar]
Amarasinghe 2019
- Amarasinghe GK, Ayllón MA, Bào Y, Basler CF, Bavari S, Blasdell KR. Taxonomy of the order mononegavirales: update 2019. Archives of Virology 2019;164(7):1967-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andabaka 2013
- Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD006602. [DOI: 10.1002/14651858.CD006602.pub4] [DOI] [PubMed] [Google Scholar]
Anderson 2017
- Anderson EJ, Carosone-Link P, Yogev R, Yi J, Simões EA. Effectiveness of palivizumab in high-risk infants and children: a propensity score weighted regression analysis. Pediatric Infectious Disease Journal 2017;36(8):699-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arms 2008
- Arms JL, Ortega H, Reid S. Chronological and clinical characteristics of apnea associated with respiratory syncytial virus infection: a retrospective case series. Clinical Pediatrics 2008;47(9):953-8. [DOI] [PubMed] [Google Scholar]
Arriola 2019
- Arriola CS, Kim L, Langley G, Anderson EJ, Openo K, Martin AM, et al. Estimated burden of community-onset respiratory syncytial virus associated hospitalizations among children aged <2 years in the United States, 2014-15. Journal of the Pediatric Infectious Diseases Society 2019;9(5):587-95. [DOI: 10.1093/jpids/piz087] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baxter 2007
- Baxter D. Active and passive immunity, vaccine types, excipients and licensing. Occupational Medicine 2007;57(8):552-6. [DOI] [PubMed] [Google Scholar]
Bont 2016
- Bont L, Checchia PA, Fauroux B, Figueras-Aloy J, Manzoni P, Paes B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in Western countries. Infectious Diseases and Therapy 2016;5(3):271-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyce 2000
- Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. Journal of Pediatrics 2000;137(6):865-70. [DOI] [PubMed] [Google Scholar]
Brady 2014
- Brady MT, Byington CL, Davies HD, Edwards KM, Jackson MA, Maldonado YA, et al, on behalf of American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134(2):415-20. [DOI] [PubMed] [Google Scholar]
Caballero 2019
- Caballero MT, Bianchi AM, Nuño A, Ferretti AJ, Polack LM, Remondino I. Mortality associated with acute respiratory infections among children at home. Journal of Infectious Diseases 2019;219(3):358-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capizzi 2017
- Capizzi A, Silvestri M, Orsi A, Cutrera R, Rossi GA, Sacco O. The impact of the recent AAP changes in palivizumab authorization on RSV-induced bronchiolitis severity and incidence. Italian Journal of Pediatrics 2017;43(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbonell‐Estrany 2010
- Carbonell-Estrany X, Simões EA, Dagan R, Hall CB, Harris B, Hultquist M, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics 2010;125(1):e35-51. [DOI] [PubMed] [Google Scholar]
Chanock 1957
- Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. American Journal of Hygiene 1957;66(3):281-90. [DOI] [PubMed] [Google Scholar]
Checchia 2011
- Checchia PA, Nalysnyk L, Fernandes AW, Mahadevia PJ, Xu Y, Fahrbach K, et al. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: a systematic literature review and meta-analysis. Pediatric Critical Care Medicine 2011;12(5):580-8. [DOI] [PubMed] [Google Scholar]
Claydon 2017
- Claydon J, Sur A, Callejas A, Mihoko L, Eddie K, Richard T, et al. Respiratory syncytial virus-neutralizing serum antibody titers in infants following palivizumab prophylaxis with an abbreviated dosing regimen. PLOS ONE 2017;12(4):e0176152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Claydon 2019
- Claydon J, Popescu CR, Shaiba L, Christopherson C, Human D, Taylor R, et al. Outcomes related to respiratory syncytial virus with an abbreviated palivizumab regimen in children with congenital heart disease: a descriptive analysis. CMAJ Open 2019;7(1):E88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2011
- Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Research 2011;162(1-2):80-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 5 May 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Damore 2008
- Damore D, Mansbach JM, Clark S, Ramundo M, Camargo CA Jr. Prospective multicenter bronchiolitis study: predicting intensive care unit admissions. Academic Emergency Medicine 2008;15(10):887-94. [DOI] [PubMed] [Google Scholar]
Domachowske 1999
- Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clinical Microbiology Reviews 1999;12(2):298-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esteban 2020
- Esteban I, Stein RT, Polack FP. A durable relationship: respiratory syncytial virus bronchiolitis and asthma past their golden anniversary. Vaccines 2020;8(2):E201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fauroux 2017
- Fauroux B, Simões EA, Checchia PA, Paes B, Figueras-Aloy J, Manzoni P, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infectious Diseases and Therapy 2017;6(2):173-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman 2015
- Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. American Journal of Respiratory and Critical Care Medicine 2015;191(1):34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández 2010
- Fernández P, Trenholme A, Abarca K, Griffin MP, Hultquist M, Harris B, et al, on behalf of Motavizumab Study Group. A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatrics 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gálvez 2017
- Gálvez NM, Soto JA, Kalergis AM. New insights contributing to the development of effective vaccines and therapies to reduce the pathology caused by hRSV. International Journal of Molecular Sciences 2017;18(8):1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geoghegan 2017
- Geoghegan S, Erviti A, Caballero MT, Vallone F, Zanone SM, Losada JV, et al. Mortality due to respiratory syncytial virus. Burden and risk factors. American Journal of Respiratory and Critical Care Medicine 2017;195(1):96-103. [DOI] [PubMed] [Google Scholar]
Ginsberg 2018
- Ginsberg GM, Somekh E, Schlesinger Y. Should we use palivizumab immunoprophylaxis for infants against respiratory syncytial virus? - a cost-utility analysis. Israel Journal of Health Policy Research 2018;7(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 2018
- Goldstein M, Krilov LR, Fergie J, McLaurin KK, Wade SW, Diakun D, et al. Respiratory syncytial virus hospitalizations among U.S. preterm infants compared with term infants before and after the 2014 American Academy of Pediatrics Guidance on Immunoprophylaxis: 2012-2016. American Journal of Perinatology 2018;35(14):1433-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 13 October 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Griffin 2020
- Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. New England Journal of Medicine 2020;383(5):415-25. [DOI: 10.1056/NEJMoa1913556] [DOI] [PubMed] [Google Scholar]
Hacking 2002
- Hacking D, Hull J. Respiratory syncytial virus-viral biology and the host response. Journal of Infection 2002;45(1):18-24. [DOI] [PubMed] [Google Scholar]
Hall 2009
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. New England Journal of Medicine. 2009;360(6):588-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2013
- Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013;132(2):341-8. [DOI] [PubMed] [Google Scholar]
Harris 2011
- Harris KC, Anis AH, Crosby MC, Cender LM, Potts JE, Human DG. Economic evaluation of palivizumab in children with congenital heart disease: a Canadian perspective. Canadian Journal of Cardiology 2011;27(4):523.e11-5. [DOI] [PubMed] [Google Scholar]
Hervás 2012
- Hervás D, Reina J, Yañez A, Valle JM, Figuerola J, Hervás JA. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis. European Journal of Clinical Microbiology & Infectious Diseases 2012;31(8):1975-81. [DOI] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane 2019. Available from www.training.cochrane.org/handbook/archive/v6.
Higgins 2019b
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook/archive/v6. [DOI] [PMC free article] [PubMed]
Higgins 2019c
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook/archive/v6.
Holberg 1991
- Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. American Journal of Epidemiology 1991;133(11):1135-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobino 2018
- Jacobino SR, Nederend M, Reijneveld JF, Augustijn D, Jansen JH, Meeldijk J, et al. Reformatting palivizumab and motavizumab from IgG to human IgA impairs their efficacy against RSV infection in vitro and in vivo. mAbs 2018;10(3):453-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1997
- Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. Journal of Infectious Diseases 1997;176(5):1215-24. [DOI] [PubMed] [Google Scholar]
Krilov 2020
- Krilov LR, Fergie J, Goldstein M, Brannman L. Impact of the 2014 American Academy of Pediatrics Immunoprophylaxis Policy on the rate, severity, and cost of respiratory syncytial virus hospitalizations among preterm infants. American Journal of Perinatology 2020;37(2):174-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
La Via 2013
- La Via WV, Notario GF, Yu XQ, Sharma S, Noertersheuser PA, Robbie GJ. Three monthly doses of palivizumab are not adequate for 5-month protection: a population pharmacokinetic analysis. Pulmonary Pharmacology and Therapeutics 2013;26(6):666-71. [DOI] [PubMed] [Google Scholar]
Lee 2016
- Lee YI, Peng CC, Chiu NC, Huang DT, Huang FY, Chi H. Risk factors associated with death in patients with severe respiratory syncytial virus infection. Journal of Microbiology, Immunology, and Infection 2016;49(5):737-42. [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al, Cochrane Information Retrieval Methods Group. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Lessler 2009
- Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infectious Diseases 2009;9(5):291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mac 2019
- Mac S, Sumner A, Duchesne-Belanger S, Stirling R, Tunis M, Sander B. Cost-effectiveness of palivizumab for respiratory syncytial virus: a systematic review. Pediatrics 2019;143(5):e20184064. [DOI] [PubMed] [Google Scholar]
Madhi 2020
- Madhi S, Polack F, Piedra P, Munoz F, Trenholme A, Simões E, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. New England Journal of Medicine 2020;383(5):426-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansbach 2012
- Mansbach JM, Piedra PA, Stevenson MD, Sullivan AF, MARC-30 Investigators. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics 2012;130(3):e492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9:602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mejias 2015
- Mejias A, Ramilo O. New options in the treatment of respiratory syncytial virus disease. Journal of Infection 2015;71(Suppl 1):S80-7. [DOI] [PubMed] [Google Scholar]
Mochizuki 2017
- Mochizuki H, Kusuda S, Okada K, Yoshihara S, Furuya H, Simões E et al. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. American Journal of Respiratory and Critical Care Medicine 2017;196(1):29-38. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Version 2. PLOS Medicine 2009;339:b2535. [PMC free article] [PubMed] [Google Scholar]
Moore 2019
- Moore HC, Klerk N, Richmond PC, Fathima P, Xu R, Keil AD. Effectiveness of palivizumab against respiratory syncytial virus: cohort and case series analysis. Journal of Pediatrics 2019;214:121-7.e1. [DOI] [PubMed] [Google Scholar]
Mullins 2003
- Mullins JA, Lamonte AC, Bresee JS, Anderson LJ. Substantial variability in community respiratory syncytial virus season timing. Pediatric Infectious Disease Journal 2003;22(10):857-63. [DOI] [PubMed] [Google Scholar]
Nair 2010
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375(9725):1545-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2011
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011;378(9807):1917-30. [DOI] [PubMed] [Google Scholar]
Nair 2013
- Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013;381(9875):1380-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomized controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr A, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomized trials. Journal of Clinical Epidemiology 2021 Jan 18 [Epub ahead of print]. [DOI: 10.1016/j.jclinepi.2021.01.006] [DOI] [PubMed]
Pangesti 2019
- Pangesti KN, El Ghany MA, Kesson AM, Hill-Cawthorne GA. Respiratory syncytial virus in the Western Pacific Region: a systematic review and meta-analysis. Journal of Global Health 2019;9(2):020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paramore 2004
- Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. PharmacoEconomics 2004;22(5):275-84. [DOI] [PubMed] [Google Scholar]
Purcell 2004
- Purcell K, Fergie J. Driscoll Children's Hospital respiratory syncytial virus database: risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatric Infectious Disease Journal 2004;23(5):418-23. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Robbie 2012
- Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrobial Agents and Chemotherapy 2012;56(9):4927-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2016
- Robinson KA, Odelola OA, Saldanha IJ. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD007743. [DOI: 10.1002/14651858.CD007743.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanders 2019
- Sanders SL, Agwan S, Hassan M, Driel ML, Del Mar CB. Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD009417. [DOI: 10.1002/14651858.CD009417.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schauer 2002
- Schauer U, Hoffjan S, Bittscheidt J, Köchling A, Hemmis S, Bongartz S, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. European Respiratory Journal 2002;20(5):1277. [DOI] [PubMed] [Google Scholar]
Scheltema 2017
- Scheltema NM, Gentile A, Lucion F, Nokes DJ, Munywoki PK, Madhi SA, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet 2017;5(10):e984-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheltema 2018
- Scheltema NM, Nibbelke EE, Pouw J, Blanken MO, Rovers MM, Naaktgeboren CA, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respiratory Medicine 2018;6(4):257-64. [DOI] [PubMed] [Google Scholar]
Schmidt 2017
- Schmidt R, Majer I, García Román N, Rivas Basterra A, Grubb E, Medrano López C. Palivizumab in the prevention of severe respiratory syncytial virus infection in children with congenital heart disease; a novel cost-utility modeling study reflecting evidence-based clinical pathways in Spain. Health Economics Review 2017;7(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shay 2001
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. Journal of Infectious Diseases 2001;183(1):16-22. [DOI] [PubMed] [Google Scholar]
Shi 2017
- Shi T, McAllister DA, O'Brien KL, Simoes EA, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390(10098):946-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sigurs 2005
- Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. American Journal of Respiratory and Critical Care Medicine 2005;171(2):137-41. [DOI] [PubMed] [Google Scholar]
Simões 2010
- Simões EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR, for the Palivizumab Long-Term Respiratory Outcomes Study Group. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. Journal of Allergy and Clinical Immunology 2010;126(2):256-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simões 2018
- Simões EA, Bont L, Manzoni P, Fauroux B, Paes B, Figueras-Aloy J, et al. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infectious Diseases and Therapy 2018;7(1):87-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simões 2020
- Simões EA, Chirikov V, Botteman M, Kwon Y, Kuznik A. Long-term assessment of healthcare utilization 5 years after respiratory syncytial virus infection in US infants. Journal of Infectious Diseases 2020;221(8):1256-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 2007
- Singh AM, Moore PE, Gern JE, Lemanske RF Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. American Journal of Respiratory and Critical Care Medicine 2007;175(2):108-19. [DOI] [PubMed] [Google Scholar]
Soto 2020
- Soto JA, Gálvez NM, Pacheco GA, Bueno SM, Kalergis AM. Antibody development for preventing the human respiratory syncytial virus pathology. Molecular Medicine 2020;26(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 1999
- Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354(9178):541-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
SYNAGIS ® (palivizumab)
- SYNAGIS ® (palivizumab). www.ema.europa.eu/en/medicines/human/EPAR/synagis#overview-section (accessed 5 May 2020).
Thomas 2020
- Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020 Nov 7 [Epub ahead of print]. [DOI: 10.1016/j.jclinepi.2020.11.003] [DOI] [PMC free article] [PubMed]
Thomsen 2009
- Thomsen SF, Sluis S, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. American Journal of Respiratory and Critical Care Medicine 2009;179(12):1091-7. [DOI] [PubMed] [Google Scholar]
Thorburn 2009
- Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Archives of Disease in Childhood 2009;94(2):99-103. [DOI] [PubMed] [Google Scholar]
Tripp 2017
- Tripp RA, Power UF, Openshaw PJ, Kauvar LM. Respiratory syncytial virus: targeting the G protein provides a new approach for an old problem. Journal of Virology 2017;92(3):e01302-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trist 2018
- Trist S, Horsley E, Katf H, Tasker N, Mostaghim M. Improving the prescribing of palivizumab. Journal of Paediatrics and Child Health 2018;54(12):1353-6. [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang D, Cummins C, Bayliss S, Sandercock J, Burls A. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technology Assessment 2008;12:36. [DOI] [PubMed] [Google Scholar]
Wegzyn 2014
- Wegzyn C, Toh L, Notario G, Biguenet S, Unnebrink K, Park C, et al. Safety and effectiveness of palivizumab in children at high risk of serious disease due to respiratory syncytial virus infection: a systematic review. Infectious Diseases and Therapy 2014;3(2):133-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welliver 2010
- Welliver RC Sr, Checchia PA, Bauman JH, Fernandes AW, Mahadevia PJ, Hall CB. Fatality rates in published reports of RSV hospitalizations among high-risk and otherwise healthy children. Current Medical Research and Opinion 2010;26(9):2175-81. [DOI] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. World Health Statistics 2018: monitoring health for the SDGs. www.who.int/gho/publications/world_health_statistics/2018/en/.
Yoshihara 2013
- Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simões E. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics 2013;132(5):811. [DOI] [PubMed] [Google Scholar]
Young 2002
- Young J. Development of a potent respiratory syncytial virus-specific monoclonal antibody for the prevention of serious lower respiratory tract disease in infants. Respiratory Medicine 2002;96(Suppl 2):S31-5. [DOI] [PubMed] [Google Scholar]
Zembles 2019
- Zembles TN, Bushee GM, Willoughby RE. Impact of American Academy of Pediatrics palivizumab guidance for children ≥ 29 and < 35 weeks of gestational age. Journal of Pediatrics 2019;209:125-9. [DOI] [PubMed] [Google Scholar]
Zhang 2016
- Zhang S, Sammon PM, King I, Andrade AL, Toscano CM, Araujo SN, et al. Cost of management of severe pneumonia in young children: systematic analysis. Journal of Global Health 2016;6(1):010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2017
- Zhu Q, McLellan JS, Kallewaard NL, Ulbrandt ND, Palaszynski S, Zhang J, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Science Translational Medicine 2017;9(388):1-12. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Garegnani 2020
- Garegnani L, Roson Rodriguez P, Escobar Liquitay CM, Esteban I, Franco JV. Palivizumab for preventing respiratory syncytial virus (RSV) infection in children. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD013757. [DOI: 10.1002/14651858.CD013757] [DOI] [PMC free article] [PubMed] [Google Scholar]


