Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), rapidly spread across the world in late 2019, leading to a pandemic. While SARS-CoV-2 infections predominately affect the respiratory system, severe infections can lead to renal and cardiac injury and even death. Due to its highly transmissible nature and severe health implications, animal models of SARS-CoV-2 are critical to developing novel therapeutics and preventatives. Syrian hamsters (Mesocricetus auratus) are an ideal animal model of SARS-CoV-2 infections because they recapitulate many aspects of human infections. After inoculation with SARS-CoV-2, hamsters become moribund, lose weight, and show varying degrees of respiratory disease, lethargy, and ruffled fur. Histopathologically, their pulmonary lesions are consistent with human infections including interstitial to broncho-interstitial pneumonia, alveolar hemorrhage and edema, and granulocyte infiltration. Similar to humans, the duration of clinical signs and pulmonary pathology are short lived with rapid recovery by 14 d after infection. Immunocompromised hamsters develop more severe infections and mortality. Preclinical studies in hamsters have shown efficacy of therapeutics, including convalescent serum treatment, and preventatives, including vaccination, in limiting or preventing clinical disease. Although hamster studies have contributed greatly to our understanding of the pathogenesis and progression of disease after SARS-CoV-2 infection, additional studies are required to better characterize the effects of age, sex, and virus variants on clinical outcomes in hamsters. This review aims to describe key findings from studies of hamsters infected with SARS-CoV-2 and to highlight areas that need further investigation.
Abbreviations: ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; CT, computed tomography; dpi, days post inoculation; 18F-FDG, fluorine-18-fluorodeoxyglucose; 18F-FDS, fluorine-18-fluorodeoxysorbitol; GGO, ground glass opacity; IFNy, interferon gamma; IL, interleukin; IL2RG KO, interleukin 2 receptor gamma chain knockout; IN, intranasal; mo, months; OC, intraocular; pfu, plaque-forming units; RAG2 KO, recombination activating gene 2 knockout; SARS-CoV, severe acute respiratory syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TCID50, 50% tissue culture infective dose; TMPRSS2, transmembrane protease serine 2; TNF, tumor necrosis factor; wk, weeks
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel betacoronavirus that was first detected in Wuhan, China at the end of 2019.31 Coronavirus infections predominantly present with either respiratory or gastrointestinal manifestations, depending on the strain and host. While many coronavirus infections result in mild clinical symptoms, SARS-CoV-2 is highly pathogenic and poses significant health concerns.31,58,78 Although initial clinical signs are attributed to the respiratory system, severe infections result in systemic complications, such as acute cardiac and renal injury, secondary infections, and shock.31,58
SARS-CoV-2 relies on a structural surface spike glycoprotein to establish infection. The spike protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells to gain entry in a receptor-mediated fashion. This interaction facilitates both human-to-human transmission and cross-species infection.77 Species tropism is determined by the presence of ACE2 residues that recognize the SARS-CoV-2 spike protein. Animals permissive for SARS-CoV-2 infection include cats, ferrets, pigs, nonhuman primates, select genetically modified mice, and hamsters.5,7,23,37,67 Susceptible species can be both intermediate hosts and sources of infection of SARS-CoV-2 for humans.77 Rodents, such as mice and hamsters, are ideal models for the study of COVID-19 due to their small size, ready availability, low cost of care, SPF status, and in-depth characterization across a variety of translational models, including past and present betacoronavirus infections.60,61 Although transgenic mice expressing human ACE2 are susceptible to SARS-CoV-2 infection, Syrian hamsters (Mesocricetus auratus) naturally express ACE2 residues that recognize the SARS-CoV-2 spike protein.5,46,84 As such, Syrian hamsters are a valuable animal model for studying COVID-19.
Syrian hamsters, commonly referred to as golden hamsters, belong to the family Cricetidae and have a natural geographic range of arid southeast Europe and Asia Minor. Additional members of the Cricetidae family used in biomedical research include Chinese hamsters (Cricetulus griseus), European hamsters (Cricetus cricetus), Armenian hamsters (Cricetulus migratorius), and dwarf hamsters (Phodopus species). Unless otherwise noted, any mention of hamsters in this overview refers to Syrian hamsters. Laboratory hamsters primarily originated from one Syrian litter captured in 1930. Progeny of this litter were first imported into the United States in 1938.50 Outbred Syrian hamsters are widely available; recently developed transgenic hamsters are increasingly used in biomedical research and may provide unique insight into SARS-CoV-2 infections.22,44 Syrian hamsters have a rich history in biomedical research and can be used to model cancer and infectious, metabolic, cardiovascular, and respiratory diseases.50
Hamsters play an important role in SARS-CoV-2 studies. This is due, in part, to their susceptibility to the first described highly pathogenic coronavirus infection in the 21st century, severe acute respiratory syndrome (SARS-CoV). SARS-CoV emerged in late 2002 in Southern China. Although individuals in more than 20 countries contracted SARS-CoV, the spread was quickly contained, with the last reported case in July 2003.16,40 After experimental infection with SARS-CoV, hamsters developed high viral loads in the lungs and nasal turbinates.15,32,56,62,69 Pulmonary pathology included inflammation, cell necrosis, and consolidation without clinical signs of disease.61 Based on their susceptibility to SARS-CoV and natural expression of ACE2 capable of recognizing the SARS-CoV-2 spike protein, hamsters have been a preferred model of SARS-CoV-2. Hamster studies have replicated key aspects of SARS-CoV-2 infections in humans, including viral replication, transmission, and pathology. Furthermore, hamsters are a model organism for developing and testing novel preventions and therapeutics. However, using hamsters in biomedical research has several key limitations, including the lack of reagents, especially antibodies, suitable for use with hamster tissue and the relatively few established transgenic hamsters compared to mice. The purpose of this review is to describe key findings of hamster models of SARS-CoV-2 and to highlight gaps in our current understanding that will require further investigation.
Comparative Respiratory Anatomy
The respiratory tract of hamsters shares key anatomic characteristics to humans, making them desirable models for respiratory tract infections. While similarities exist, anatomic and physiologic differences are also present. For example, the human lung has 3 lobes on the right and 2 on the left, whereas hamsters have 5 lobes on the right and a single lobe on left, as do other rodents.6 Other notable anatomic differences in hamsters, as compared with humans, include the smaller lung volume, fewer ciliated epithelium and goblet cells in the nasal epithelium, subepithelial mucous glands limited to the trachea, and the rare neurosecretory cells and lymphoid tissue throughout the tracheobronchial tree.6,36,59 Physiologically, hamsters have a higher respiratory rate (33 to 127 breaths/minute), a smaller tidal volume (0.42 to 1.4 mL), and a smaller mean minute volume (30 to 42 mL) as compared with humans at 12 to 16 breath/minute, 500 mL, and 5 to 8 L, respectively.53,80 Despite these anatomic and physiologic differences, the respiratory tract of humans and hamsters have many similarities, including the branching patterns of airways, abundant Clara cells in bronchioles, and the distribution and type of blood vessels surrounding the lungs. The types of cells found in the upper and lower respiratory tracts also have many commonalities.36
Recent reports have identified the distribution of ACE2 in archived hamster tissues using immunohistochemical staining and quantitative PCR of ACE2 mRNA copies.72 ACE2 expression was found in hamster kidneys and portions of the gastrointestinal tract by both methodologies. While the lungs and tracheal epithelial cells were negative for ACE2 by immunohistochemical staining, trace quantities of ACE2 mRNA copies were present in these tissues.72 The lack of robust respiratory ACE2 expression reported in this study contrasts with reports describing ACE2 expression on human alveolar cells and transient secretory cells of the bronchus.26,48,71 This finding suggests that pathologic pulmonary changes in hamsters after SARS-CoV-2 infection may be independent of ACE2 expression.72 The study did not assess the nasal epithelium, which provides a logical route of entry when virus particles are administered into the nares.75 Furthermore, virus inoculum could be ingested after intranasal instillation, resulting in virus entry in the small intestines, which expresses ACE2. Additional studies describing ACE2 distribution patterns in hamsters of both sexes across various ages using multiple methodologies could help to determine the biologic significance of these findings. Prospective studies should determine the mechanism of SARS-CoV-2 infection in hamsters to expand upon our understanding of the model. Furthermore, results of studies involving SARS-CoV-2 infections of hamsters should be interpreted with consideration of the various anatomic and physiologic similarities and differences to humans.
Pathogenesis.
Table 1 summarizes key findings from all hamster studies of SARS-CoV-2 that had been published at the time this manuscript was prepared. Hamsters can be inoculated experimentally via intranasal instillation of infectious virus.14,15,32,56,62,65,69 Intraocular infection can mediate human transmission, but data are lacking to support this route in hamsters.81 One study used combined intranasal instillation of virus with topical ocular application; however, the study did not directly compare the combined routes of infection to intranasal or ocular inoculation alone.32 After infection, SARS-CoV-2 is readily transmitted to naïve hamsters via direct contact and aerosols.14,15,69 In contrast, transmission via fomites in soiled cages is not an efficient source of infection.69 When naïve hamsters are housed with experimentally infected index hamsters, naïve hamsters exposed by direct contact demonstrate infectious virus and SARS-CoV-2 RNA as early as 1 d after contact. Virus load and respiratory pathology are similar in index infected and contact infected hamsters.3,15 To demonstrate transmission via aerosols, infected hamsters were housed in wire bar cages adjacent to cages housing naïve animals.14 Positive virus titers and SARS-CoV-2 RNA in nasal washes from aerosol-exposed hamsters document aerosol transmission.14,69 These studies indicate that modes of transmission typical of human-to-human spread also occur in hamsters.
Table 1.
Summary of publications on COVID-19 infection in Syrian hamsters.
| Virus isolate and dose | Route | Age and sex | Outcomes | Reference |
|---|---|---|---|---|
| UT-NCGM02/ Human/2020/ Tokyo | IN + OC | 4 wk and 7–8 mo | More pronounced weight loss in hamsters inoculated with higher dose, exacerbated in older animals. Pulmonary inflammation, pneumonia and ground glass appearance on CTs demonstrated in infected hamsters. Severity of lung disease correlates to infectious dose. Neutralizing antibody response protected hamsters against reinfection and convalescent serum treatment reduced virus titer when subsequently infected. | 32 |
| High dose: 105.6 PFU Low dose: 103 PFU | Female | |||
| SARS-Related Coronavirus 2, Isolate USA-WA1/2020 | IN | 6–8 wk and >27 wk | Weight loss more pronounced in hamsters inoculated with higher dose with no difference between ages or sexes of hamsters. Moderate bronchopneumonia developed following infection. Prolonged viral persistence was demonstrated in IL2R KO hamsters. | 62 |
| High Dose: 105 TCID50Low Dose: 103 TCID50 | Male and female | |||
| BetaCoV/ Germany/ BavPat1/2020 | IN | 6 wk and 32–34 wk | Weight loss more pronounced in older hamsters while virus replication in respiratory tract was independent of age. Young animals demonstrated an earlier immune cell influx and rapid lung recovery by 14 dpi. | 56 |
| Dose: 105 PFU | Male and female | |||
| BetaCoV/Hong Kong/VM20001 061/2020 | IN | 4–5 wk | Weight loss occurred 6-7dpi. with a rebound to pre-inoculation weight by 14dpi. Viral agents were detected in nasal mucosa, bronchial epithelial cells and within consolidated lungs 2 and 5dpi. Transmission to naïve hamsters occurred via direct contact and aerosols, but not via soiled cages. All hamsters developed neutralizing antibodies. | 69 |
| Dose: 8 · 104 TCID50 | Male | |||
| SARS-CoV-2 isolated from a Hong Kong patient | IN | 6–10 wk | Non-contact transmission occurred in 66.7% of unprotected naïve hamsters. Greater lung pathology occurred in inoculated hamsters compared to non-contact infected hamsters. Surgical mask partitions between infected and naïve hamsters reduced transmission. Infection rates were lowest (16.7%) when surgical mask partition prevented droplet emissions from infected hamsters reaching naïve hamsters. | 14 |
| Dose: 105 PFU | Male and female | |||
| SARS-CoV-2 isolated from a Hong Kong patient | IN | 6–10 wk | Within the first 7 dpi, hamsters demonstrated lethargy, ruffled fur, hunched posture, tachypnea, weight loss, and lung pathology. Co-housing infected and naïve hamsters resulted in transmission to naïve animals. Immunoprophylaxis with convalescent serum reduced lung viral load but not pathology. | 15 |
| Dose: 105 PFU | Male and female | |||
| BetaCoV/ Germany/ BavPat1/2020 | IN | 6 wk | Screened non-self-reactive virus neutralizing antibodies for prophylactic or therapeutic treatment of infected hamsters. Pre and post inculation application of the CV07-209 neutralizing antibody protected against weight loss, lung pathology, and reduced viral loads. No mortality reported. | 39 |
| Dose: 105 PFU | Male and female | |||
| SARS-Related Coronavirus 2, Isolate USA-WA1/2020 | IN | 6–8 wk | Prophylactic and therapeutic treatment of infected hamsters with REGN-COV2, a neutralizing antibody cocktail (REGN10987 and REGN10933), limited weight loss, decreased lung viral titers, and reduced pneumonia. | 7 |
| Dose: 2.3 · 104 PFU | Male and female | |||
| Virus stock not described | IN | 8 wk | Prophylactic and therapeutic treated of infected hamsters with human monoclonal antibodies(C135-LS + C144-LS) effectively reduced lung viral loads. | 65 |
| Dose: 2.6 · 104 PFU | Sex not described | |||
| SARS-Related Coronavirus 2, Isolate USA-WA1/2020 | 10–12 wk | Weight loss more pronounced in hamsters inoculated with higher dose with mortality in a subset. Immunization with adenovirus serotype 26 vector-based vaccine against SARS-CoV-2 spike protein protected against weight loss and pneumonia, but not infection. No mortalities were reported in vaccinated hamsters. | 73 | |
| Low Dose: 5 · 104 TCID50 High Dose: 5 · 105 TCID50 | Male and female | |||
| SARS-Related Coronavirus 2, Isolate USA-WA1/2020 | 6–8 wk | Immunosuppressed hamsters, either RAG2 KO or by cyclophosphamide administration, developed more severe clinical signs including greater weight loss and viral loads. Infection in RAG2 KO hamsters resulted in mortality. Pretreatment with human neutralizing monoclonal antibody (Centi-F1) in immunosuppressed hamsters limited infection. | 12 | |
| Doses: 100; 1,000; 10,000; and 100,000 PFU | Male and female |
PFU, plaque forming units; TCID50, 50% tissue culture infective dose; IN, intranasal; OC, intraocular; wk, weeks; mo, months; dpi, days post inoculation; IL2R, interleukin-2 receptor; KO, knock out; RAG2, recombination activating gene 2.
In humans, the SARS-CoV-2 spike protein recognizes residues on ACE2 to gain entry into cells. Computational studies of ACE2 homology between humans and hamsters indicate that the hamster ACE2 also recognizes the binding domain of the SARS-CoV-2 spike protein.15,46,77 Furthermore, SARS-CoV-2 can infect a hamster kidney cell line (BHK cells) when ACE2 is expressed, but not in its absence.62 These studies suggest that the presence of ACE2 in hamsters mediates cellular infection. While ACE2 is present on cells in the human respiratory tract, available data do not confirm a similar ACE2 distribution in hamsters.26,48 ACE2 alone is not sufficient for cellular infection in humans.30 In vitro studies using human cell lines (293T and Vero cell lines) revealed that the spike protein of SARS-CoV-2 requires priming before cell entry.30 Once engaged with ACE2, a protease, transmembrane protease serine 2 (TMPRSS2), cleaves a portion of the spike protein, thereby permitting infection of host cells. Furthermore, inhibition of TMPRSS2 prevented viral entry.30 The role of proteases in hamster infections is currently unknown. Colocalization of TMPRSS2 and ACE2 in hamster tissues would help to explain viral entry in hamsters. Similarly, blockade of ACE2 or the generation of ACE2 KO hamsters would expand our understanding of the model.
SARS-CoV-2 replicates efficiently in the respiratory tract of hamsters. Nasal turbinates, trachea, and lung tissues display high viral titers, as assessed by real-time quantitative reverse transcription PCR or plaque assays, as early as 2 d post inoculation (dpi).14,15,32,39,56,62,65,69 Nasal washes or swabs and fecal samples also contained detectable viral RNA at 2 dpi.15,56,69 After a peak in viral load around 3 dpi, viral titers decrease in all tissues analyzed and are largely absent at 10 to 14 dpi, indicating rapid viral clearance.32,56,62 Additional organs with virus titers include the heart, liver, spleen, kidney, intestines, and brain. The presence of high viral loads in the respiratory tract of hamsters, combined with an acute course of infection followed by rapid viral clearance, closely mirrors SARS-CoV-2 infection in humans.
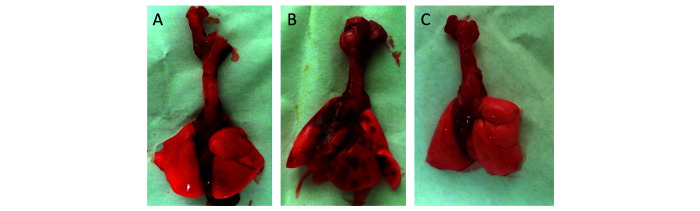
Hamsters demonstrate significant pathologic changes associated with SARS-CoV-2 infection, with pulmonary lesions being consistent with lesions described in humans.15,32,39,56,62,69 Grossly, multifocal to diffuse areas of lung consolidation are evident (Figure 1). Interstitial to broncho-interstitial pneumonia develops shortly after infection, with viral antigen-positive cells present in the alveoli (Figure 2).15,32,39,56,62,69 In one study, microcomputed tomographic imaging of infected hamsters revealed evidence of lung injury similar to lesions in humans including multilobular ground glass opacities and lung consolidation.32 Another group summarized the pulmonary histopathologic findings reported in SARS-CoV-2 animal models, including hamsters.25 Noteworthy pulmonary lesions shared among humans and hamsters include alveolar epithelial cell necrosis, alveolar hemorrhage and edema, and interstitial pneumonia with granulocyte infiltration and perivascular lymphocyte cuffing.1,2,9,15,25,69 By 10 to 14 dpi, pulmonary inflammation had resolved, and viral antigen was no longer detected.15,32,56,62 During the reparative stages, multinucleated epithelial cells and hyperplasia of alveolar epithelial cells were evident.9,15,25,32,56 Inconsistently reported findings in hamster models include endothelialitis, hyaline membrane formation, and intraalveolar fibrin, which can all be seen in severe or fatal human infections.1,9,15,39,56,62 Importantly, hamsters lack pulmonary microvascular thrombi and the extrapulmonary pathology seen in human infections.1,9,25 Reporting criteria for describing pneumonia in SARS-CoV-2 infections across animal models has been proposed.25 Consistent reporting of histopathology in hamsters is critical to increasing the translational potential of the model.
Figure 1.
Representative gross changes associated with SARS-CoV-2 infection in hamsters following inoculation with 105 TCID50 of SARS-CoV-2 USA-WA1/2020. A. Uninfected control lungs. B. 4 d post inoculation: All lung lobes contain randomly distributed, well demarcated dark red areas. C. 14 d post inoculation: Lung lobes are grossly unremarkable.
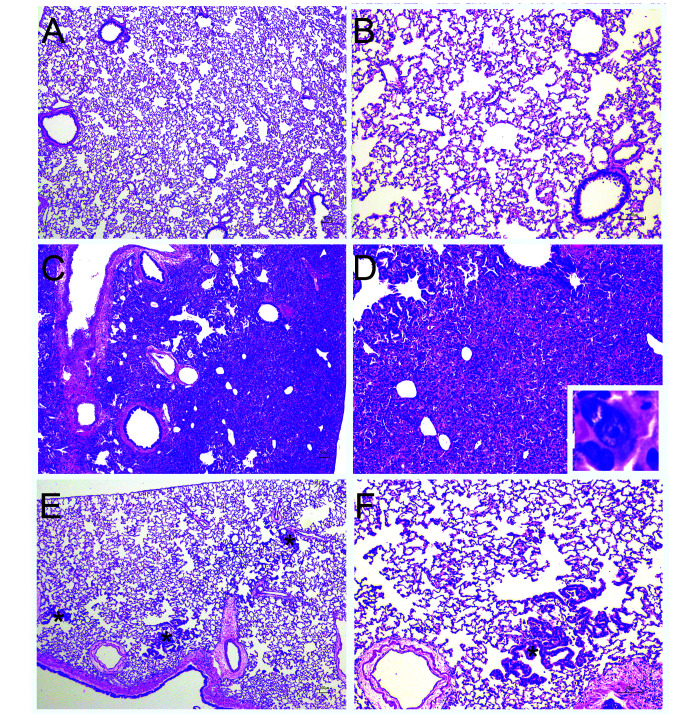
Figure 2.
Representative histopathologic changes associated with SARS-CoV-2 infection in hamsters after intranasl inoculation with 105 TCID50 of SARS-CoV-2 USA-WA1/2020. A-B. Representative uninfected control lungs. C-D. 7 d post inoculation. Lungs showed marked consolidation, with abundant type II pneumocyte hyperplasia including atypical and multinucleated cells, infiltration of macrophages, with fewer degenerate neutrophils and lymphocytes. Inset. Multinucleated atypical type II pneumocyte. E-F. 28 d post inoculation. Lungs were largely unremarkable, with scattered typical type II pnemocyte hyperplasia (*). A,C,E – 4· magnification, B, D, F – 10· magnification, H and E.
Clinical manifestations.
In humans, SARS-CoV-2 infections present with fatigue, fever, and respiratory symptoms that include coughing, increased respiratory rates, and dyspnea.31,58,78 Although hamsters demonstrate varying degrees of pneumonia, the literature indicates that clinical disease is mild and respiratory symptoms are often lacking.15,17,32,34,56,58,62,64,69,71 Weight loss is the most consistent finding across studies of SARS-CoV-2 infections in hamsters. Peak weight loss occurs 5 to 7 dpi with a gradual complete or partial weight recovery through 14 dpi.15,17,32,34,56,58,69,71 To date, 2 studies have investigated the effects of differing viral doses on weight loss in hamsters.32,34,62,64 Despite significant differences in study design, including virus stock, dose, and anesthesia used during inoculation, both studies demonstrated more pronounced weight loss in hamsters inoculated with higher doses of SARS-CoV-2. Monitoring changes in weight as compared with baseline values is a valuable indicator of the severity of infection, natural recovery, and response to treatment in SARS-CoV-2 infected hamsters.
Other clinical signs in hamsters after inoculation include lethargy, ruffled fur, hunched posture, and increased respiratory rates early in the course of infection.14,15,62 Body temperature does not significantly change between infected and mock-infected hamsters.56,62 Changes in respiratory rate, respiratory pattern, and the general appearance of hamsters varies greatly between studies and may be absent entirely in some studies, despite the presence of virus in analyzed tissues. Mortality is rarely reported in immunocompetent hamsters.73 Little work has been done to quantify changes in physiology, behavior, or clinical condition. A published clinical scoring system effectively demonstrated an increasing clinical score among aerosol exposed hamsters from 5 to 7 dpi post exposure (Figure 3).14 Clinical signs included in the scoring system include key components of established scoring systems in other rodent models.17,21,28 A growing body of literature in mice and other rodents demonstrates the assessment of behavioral characteristics to refine translational models of infectious disease.10,13,14,17,28,68 Clinical scoring systems often incorporate species-relevant behaviors, including nest-building, time spent grooming, time on running wheels, latency to consume highly valued food items, and a grimace scale.29,33,41,54 Additional behavioral and physiologic data may help further describe the course of infection in hamsters. Future studies should consider using a validated clinical scoring system to document subtle clinical, physiologic, and behavioral outcomes of SARS-CoV-2 infection.
Figure 3.

Clinical scoring system used to assess SARS-CoV-2 experimentally infected and aerosol contact exposed hamsters. Adapted from Chan and colleagues 2020.16
Age and sex differences.
Older people infected with SARS-CoV-2 have worse clinical disease and prognoses than do younger individuals.45 Hamsters, having a shorter natural life span, can be used to study disease in different age groups. At the time of this writing, 3 studies have compared the clinical manifestations, viral loads, and histopathologic changes of juvenile and mature hamsters.32,56,62 While age criteria vary between studies, young cohorts were defined as 4 to 8 wk of age and mature cohorts defined as 6 to 9 mo of age. Young hamsters displayed less significant weight loss or did not gain weight as rapidly as mock-infected controls, while mature hamsters demonstrated more pronounced weight loss than did the younger hamsters. One study did not find significant differences in percent weight loss between the young (4 to 6 wk old) and old (> 6 mo) cohort.62 Body weight loss does not seem to be affected by lung virus titers, which were not different between age groups across studies.32,56 However, evidence for age-dependent effects on lung histopathology is conflicting.32,56,62 One study reported a stronger immune cell influx into the lungs, including neutrophils, macrophages, and perivascular lymphocytic cuffing, as assessed by scoring lung-specific inflammation on histopathology, and more rapid recovery in young hamsters but pronounced alveolar and perivascular edema in aged hamsters.56 Furthermore, at 14 dpi, inflammation and lung damage persisted only in aged animals.56 A similar study found no difference in lung pathology or lung weights between young and aged hamsters.32 After finding no difference in lung viral loads between age groups, the authors only assessed lung pathology and microcomputed tomographic images in young hamsters.32 Conflicting findings regarding age-related differences in pulmonary pathology may be due to different study designs. Biologic and experimental differences among hamster studies include virus stock, viral challenge dose, anesthesia used during inoculation, and hamster source (see Figure 1 for synopsis). Hamsters have a total life span of 2 to 3 y.50 Age-related differences may be more apparent among hamsters beyond the first half of their life span. Future studies should characterize disease progression in even older hamsters. Current data suggest that hamsters may show age-related effects after SARS-CoV-2 infection that mirror findings in humans.
To date, only a few studies have compared lung pathology, viral loads, and clinical course of disease between male and female hamsters.19,56,62 While 2 studies did not find statistically significant sex related differences, one group found more severe disease in infected male hamsters.19,56,62 The lack of apparent difference between sexes in the first 2 studies may have been due to low sample sizes.56,62 In one of these studies, the percentage change in body weight after infection was less pronounced in female hamsters than in age-matched males.62 Despite potential trends, viral loads and infectious viral particles in female hamsters were not significantly lower than in age-matched male at 5dpi.62 Another group reported similar findings, with the percentage of body mass lost not significantly different in infected males and females, despite a potential trend.19 However, computed tomography imaging revealed more extensive pneumonia and a slower resolution of lung pathology in infected male hamsters.19 Female hamsters had a greater IgM, IgA, and IgG antibody response against the ACE2-spike receptor-binding domain and demonstrated a more robust virus neutralizing antibody response.19 These differences in immune responses after SARS-CoV-2 infection may contribute to a lower disease burden and more rapid recovery in female hamsters. Hamster cohorts in future studies should include both male and female age-matched hamsters to further characterize differences in disease progression and response after SARS-CoV-2 infection.
Immunology.
As an animal susceptible to clinical isolates of SARS-CoV-2, hamsters can provide important insights into the immunology of SARS-CoV-2 infections and vaccination. Despite limited hamster-specific reagents, multiple groups have studied hamster immune responses, including the cytokine/chemokine profile, infiltration of immune cells into the lungs, and the anti-SARS-CoV-2 antibody responses during hamster infections.8,15,18,42
Since the beginning of the COVID-19 pandemic, a major focus of study is on concentrations of cytokines such as interleukin (IL) 6, IL1↓, TNF↑, and IFNy in the plasma of COVID-19-infected patients.31,43,47 Multiple groups have also reported increases in cytokines in the serum, lungs, and nasal turbinates during SARS-CoV-2 infection of hamsters.8,15,18,42,57,83 Most human cytokine data in COVID-19 studies are from the peripheral blood; however, the hamster model allows the study of the cytokine profile at the site of infection, the respiratory system. In contrast to human patients in whom the exact time of infection can be unclear, the hamster model allows study of the kinetics of cytokine responses (Figure 4). In the nasal turbinates, cytokines and chemokines, specifically, IL6 IL1↓, TNF↑, CCL3, CCL5 and CXCL10, peak between 2 to 4 dpi and fall by 7 dpi.83 The expression of some of these cytokines/chemokines including IL1↓, IL6, TNF↑, and CXCL10 in the nasal turbinates at 4 dpi was confirmed in another study.18 The cytokine/chemokine profile and kinetics in the lungs have a similar time course, increasing at 2 dpi, peaking at around 4 dpi, and returning to baseline by around 7 dpi (Figure 4).8,15,18,42 Studies vary in the cytokines measured, and differences between studies may be due to differences in methodology, environment, subjects (age, sex and source of the hamsters used), and viral factors; however, all hamster studies found increased IL6 in the lung and/or nasal turbinates at 3 to 4 dpi.8,15,18,42 This finding is consistent with findings of increased IL6 in the serum and plasma of human COVID-19 patients.31,43,47 Furthermore, in human patients, impaired or dysregulated type 1 interferon responses are associated with severe COVID-19 (reviewed in83), and evidence indicates that Signal Transducer and Activator of Transcription (STAT)2-dependent interferon responses are necessary for viral control but also contribute to disease pathogenesis.8 Altogether, these findings indicate that the cytokine response in SARS-CoV-2-infected hamsters appears to model that of human COVID-19 patients.
Figure 4.
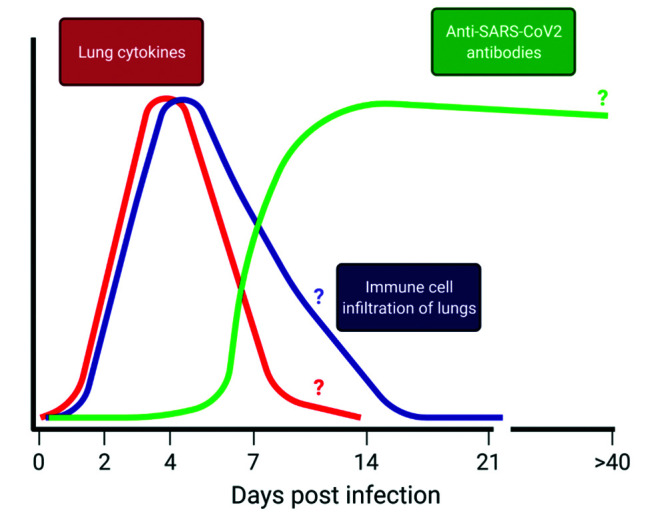
Immune response kinetics after intranasal infection of Syrian golden hamsters with SARS-CoV-2. After intranasal infection with SARS-CoV2, there is an early increase in cytokines/chemokines as well as immune cell infiltration of the lungs, peaking at around 4dpi. Cytokine/chemokine levels likely return to baseline around 7dpi, and immune cell infiltration likely resolves between 14 and 21dpi. Anti-SARS-CoV2 antibodies are detectable in serum starting at 5dpi, increasing rapidly until they plateau between 14 and 21dpi and likely begin to decrease thereafter. It is unknown for how long anti-SARS-CoV2 antibodies are detectable, with the furthest studied being detection at 43dpi. Question marks (?) indicate aspects of the curve that are not definitive in the available literature. Figure was generated in BioRender.
In addition to the cytokine profile in the lungs, the hamster model allows the analysis of immune cell infiltration into the respiratory system during SARS-CoV-2 infection, using methods such as hematoxylin and eosin (H and E) staining and immunohistochemistry. Multiple studies have reported the infiltration of innate immune cells, primarily neutrophils and monocytes/macrophages, into the lungs, especially the alveoli.11,15,20,44,56,57,62,73 A recent study detected interstitial and alveolar neutrophil and monocyte/macrophage infiltration at 2 dpi,56 and a second study reported neutrophil invasion of the mucosa and airway lumen at 3 dpi.62 The latter study also found that perivascular infiltrates of lymphocytes at 5 and 10 dpi.62 The immune cell infiltration appears to peak at 4 to 5 dpi and is comprised predominantly of monocyte/marcophages.12,15,20,73 Despite the lack of reagents to identify types of T cells in SARS-CoV-2 infected hamsters, they likely contribute to viral clearance and recovery, as hamsters without T and B cells, in contrast to their wild-type counterparts, die from intranasal SARS-CoV-2 infection by 1 dpi.12 Similarly, some COVID-19 patients show evidence of pulmonary lymphocyte and monocyte infiltration.9 In hamsters, pulmonary infiltration and inflammation resolves at around 14 dpi (Figure 4).11,15
Hamsters have also been used to study the kinetics of the anti-SARS-CoV-2 antibody response of IgG against both the spike protein of SARS-CoV-2 (antispike IgG) and neutralizing antibody (Figure 4).11,12,15,56,57 Antispike IgG was first detected at 5 dpi and increased for the duration of the study (14 dpi); another study also detected antispike IgG at 14 dpi.11,57 Other groups have detected neutralizing antibody at 7 and 14 dpi.15,57 Likewise, humans infected with SARS-CoV-2 produce antispike/spike-receptor binding domain IgG and neutralizing antibodies within a week after the onset of symptoms.38,70 Currently, the duration of production of these antibodies in hamsters is not known, although neutralizing antibodies have been detected up to 43 dpi.12 Future studies will be needed to determine the IgM and IgA antibody profile and the duration of the antibody responses.
Ultimately, insights into the immunology of SARS-CoV-2 infection have allowed the hamster model to be used in vaccine development studies.11,82 The use of hamsters in COVID-19 research is likely to increase as more immunologic reagents are developed. Understanding SARS-CoV-2 immunology in the hamster model will facilitate understanding of COVID-19 in humans.
Comparative bio-imaging of SARS-CoV-2-infected hamsters and humans.
Chest computed tomography (CT) is a widely available tool in developing and developed countries. It has been used as a noninvasive technology in patients with COVID-19 to monitor disease progression, severity, treatment response, as well as short- and long-term complications (for example, lung fibrosis).27,66 Multiple bilateral ground-glass opacities (GGO) and mixed GGO with consolidations are the hallmark findings in patients with COVID-19 (present in > 60% of cases).66 They can be detected as early as 3 to 5 d after symptom onset. In some patients, interlobular septal thickening, pleural effusion, pneumomediastinum, air bronchogram signs, reversed halo sign (also known as atoll sign), cavity air and lymphadenopathies can be present. When a single lung lobe is involved, the lower right lung tends to be most affected, while in the left lung, the upper lobe tends to be more frequently involved. The patterns of chest CT in COVID-19 differ with sex and age. One study reported that men older than 60 y have a peripheral distribution of these lesions.51 In comparison, men younger than 60 y are more likely to have an anterior distribution as compared with women of the same age.51 Moreover, women have significantly lower chest severity CT-score than do males, with males also having a higher risk of death.51
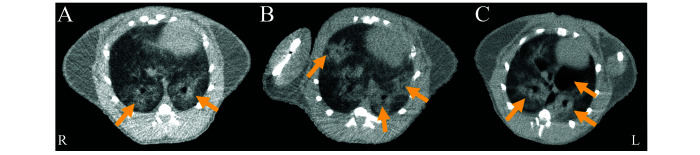
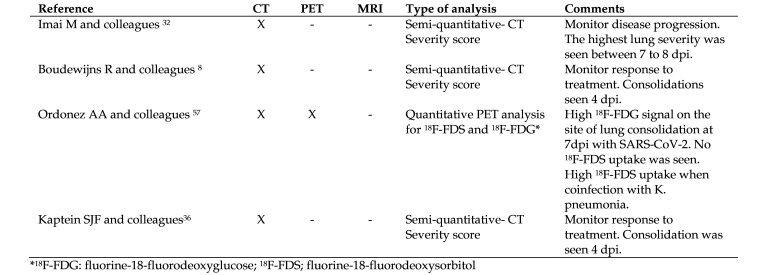
CT also serves as a noninvasive means to monitor disease progression and response to new therapeutics in animal models of SARS-CoV-2 (Figure 5).52 Similar to humans, GGO and consolidations have been reported in hamsters and can be detected as early as 4 d after intranasal infection with SARS-CoV-2.8,32 Representative chest CT images collected in our lab from 3 SARS-CoV-2-infected hamsters 7 d after intranasal infection are shown in Figure 5. In hamsters, lung damage is significantly higher in males than females and tends to be bilateral, multilobular, and peripheral.19,32,63 Hamsters can also develop spontaneous pneumomediastinum on day 4 to 7 after infection.24 However, the crazy-paving pattern, lymphadenopathies, and reversed halo sign have not been described in SARS-CoV-2 hamsters to date. A comparison of human and hamster lung pathology by chest CT is presented in Table 2. Currently, data are not available for hamsters using magnetic resonance or ultrasonography. However, one study described positron emission tomography (PET) imaging in SARS-CoV-2-infected hamsters.55 Fluorine-18-fluorodeoxyglucose (18F-FDG), a widely used marker to detect inflammation, had significant uptake in the GGO and consolidations at 7 d after intranasal infection.55 Conversely, fluorine-18-fluorodeoxysorbitol (18F-FDS), a specific tracer for Enterobacteriales (Gram-negative bacteria), showed no signal in the lung lesions.55 However,18 F-FDS signal was significantly higher on the pneumonic areas in hamsters coinfected with Klebsiella pneumoniae (an Enterobacteriales).55 Current literature descriptions of CT findings in the hamster model of SARS-CoV-2 are summarized in Figure 6.
Figure 5.
Representative chest CT from 3 hamsters 7 d after intranasal inoculation with 105 TCID50 of SARS-CoV-2 USA-WA1/2020. (A) Bilateral GGO at the level of the heart. (B) Bilateral, multilobular and peripheral GGO, consolidations, air bronchogram signs, and pneumonia. (C) Bilateral, multilobular and peripheral GGO and pneumomediastinum. Yellow arrows indicate the lung lesions.
Table 2.
Comparative radiologic findings between SARS-CoV-2-infected hamsters and humans.
| Radiologic findings | Humans 4,27,79 | Hamsters 8,32 |
|---|---|---|
| Ground glass opacities | +++ | +++ |
| Peripheral lesions | +++ | ++ |
| Multiple lesions | +++ | ++ |
| Bilateral | +++ | ++ |
| Consolidation | ++ | +++ |
| Interlobular septalthickening | ++ | — |
| Air Bronchogram | + | +* |
| Bronchial wall thickening | + | — |
| Tree-in-bud pattern | + | — |
| Air cavity | + | — |
| Pleural thickening orpleural effusion | ++ | — |
| Lymphadenopathy | + | — |
| Reversed halo sign | + | — |
| Pneumo-mediastinum | +26 | + |
+++ Higher than 60%; ++ between 20% to 60%; + Lower than 20%. - Not reported. *Unpublished data.
Figure 6.
Summary of imaging techniques in the hamster model of SARS-CoV-2.
Countermeasure testing.
Due to their small size and susceptibility to SARS-CoV-2 infection, hamsters are valuable for testing novel preventative and therapeutic treatments. Reducing the spread from infected individuals remains at the forefront of controlling the current pandemic. To assess the efficacy of surgical masks in reducing transmission of SARS-CoV-2, one group created unidirectional airflow from experimentally infected hamsters in wire bar caging toward adjacently housed naïve hamsters.14 Air was filtered through a surgical mask partition, either facing the infected or naïve hamsters and exposed naïve hamsters were assessed for infection. The surgical mask partition reduced transmission to naïve exposed hamsters, with the greatest reduction seen when the partition was positioned to mimic mask wearing by infected hamsters.14 Additional studies are required to determine the efficacy of the more widely used cloth face coverings. Nonetheless, this study provides evidence supporting the use of masks to prevent spread among close contact individuals. Once infected, safe and efficacious treatment options are needed to reduce morbidity and mortality, especially among high-risk patients.
Convalescent serum, which is collected from infected individuals that have mounted a neutralizing antibody response, shows promise in reducing disease severity in both hamsters and humans.15,32 When pretreated with convalescent sera collected from previously infected hamsters, subsequent infection of the treated hamsters resulted in lower nasal turbinate and lung viral loads at 14 dpi.15 Despite lower viral loads, hamsters receiving immunoprophylaxis showed no reduction in clinical signs or pulmonary histopathology.15 Intraperitoneal administration of convalescent serum at 1 or 2 dpi, which is a more clinically relevant scenario, found reductions in lung viral loads, and to a lesser extent in nasal turbinate viral loads.32 These findings suggest that pre- and postinfection treatment with convalescent serum can reduce viral replication and potentially improve patient outcomes. Because the use of convalescent serum depends on the availability of human donors, the production of human monoclonal antibodies (mAbs) that target the spike protein of SARS-CoV-2 is an area of great interest.
Human mAbs have shown great potential in treating SARS-CoV-2 infected individuals.75 Studies in hamsters demonstrate protection against infection, weight loss and lung pathology when antibodies are administered prophylactically or therapeutically.7,39,65 One study screened 598 human mAbs from SARS-CoV-2 infected individuals and found 40 that neutralized SARS-CoV-2.39 The most potent mAb, CV07-209, directly inhibited SARS-CoV-2 binding to ACE2 in vitro. Hamsters received CV07-209 either 24 h before or 2 h after infection. Both treatment groups demonstrated less weight loss, lower viral loads, less pulmonary edema, and failed to develop bronchitis as compared with mock-treated hamsters.39 Another study tested a combination mAb treatment in hamsters using 2 mAbs that targeted nonoverlapping regions of the SARS-CoV-2 spike protein, C135 and C144. When the combination of mAbs was administered 24 h before or 12 h after infection, lung viral loads were reduced compared with mock treated hamsters.65 Another study demonstrated the protective effects of REGN-COV-2, a human mAb combination therapy with FDA approval for SARS-CoV-2 treatment, when given prophylactically or therapeutically in hamsters.7 Weight loss was reduced in both treatment groups.7 When given prophylactically, REGN-COV-2 protected against pneumonia and effectively reduced lung viral RNA levels.7
In addition to testing novel therapeutics, hamsters are a key model for vaccine development. Eliciting host production of neutralizing antibodies to the spike protein of SARS-CoV-2 is the primary aim of vaccine development.35 At the time of preparing this overview, the FDA has authorized the emergency use of 3 SARS-CoV-2 vaccines.76 Additional vaccines containing mRNA fragments or adenovirus vectors of the SARS-CoV-2 spike protein are currently undergoing clinical trials.35 Hamsters provide a vital animal system for testing novel vaccines due to their natural expression of ACE2 capable of recognizing the SARS-CoV-2 spike protein.46 To date, only one study investigating the efficacy of SARS-CoV-2 vaccination in hamsters has been published, potentially representing their underuse in vaccine development.73 In this study, hamsters received an intramuscular injection of an adenovirus serotype 26 vector-based vaccine encoding a stabilized SARS-CoV-2 spike protein and were challenged 4 wk after immunization by administering a high dose of SAR-CoV-2 intranasally (5 · 105 50% tissue culture infective dose). Vaccinated hamsters had significantly less weight loss, lower lung viral loads, and less lung pathology and were protected against mortality, in comparison to mock-vaccinated hamsters. Furthermore, reduction in clinical disease correlated with vaccine induced antibody titers to the SARS-CoV-2 spike protein.73 Clinical trials assessing the efficacy of this adeno-virus vectored vaccine (Ad26-S.PP) are ongoing.35,73 Future preclinical vaccine studies should consider the use of hamsters as a model organism.
Immunocompromised and immunodeficient hamster models.
While immunocompetent hamsters exhibit mild symptoms after SARS-CoV-2 infection, hamsters with compromised adaptive immune responses develop a greater severity of disease.12,62 Immunosuppression in hamsters has been induced experimentally via genetic alterations and by administrating cyclophosphamide. Cyclophosphamide is an alkylating agent that suppresses T and B cell function, thereby suppressing the adaptive immune response. Cyclophosphamide-treated hamsters were previously used to study SARS-CoV infections and developed severe disease.64 In another study, SARS-CoV-2 infected hamsters received twice-weekly cyclophosphamide injections.12 A reduction in lymphocyte counts corresponded to persistent weight loss and viremia, lasting through the end of study at 35 dpi. Cyclophosphamide treated hamsters did not mount a neutralizing antibody response while receiving cyclophosphamide, and only a subset of hamsters mounted a response after cessation of cyclophosphamide treatment. Although spontaneous mortality did not occur, a subset of hamsters demonstrated cachexia and met the criteria for humane euthanasia prior to the end of study.12
Two types of transgenic hamsters have been used to study severe SARS-CoV-2 infections - interleukin 2 receptor ↖ chain knockout (IL2RG KO) and recombination activating gene 2 knockout (RAG2 KO) hamsters. IL2RG KO hamsters lack natural killer cells and have reduced numbers of T and B cells.44 RAG2 KO hamsters do not develop T or B cells.49 IL2RG KO hamsters became persistently infected, and SARS-CoV-2 infection in RAG2 KO hamsters was uniformly lethal with a median death time of 6 d.12,62 Respiratory signs were not observed in IL2RG KO hamsters and pulmonary histopathology was similar to infected immunocompetent hamsters included in the study.62 In contrast, RAG2 KO hamsters demonstrated more severe pulmonary pathology including edema and alveolar hemorrhage than did immunocompetent hamsters.12 These studies suggest that an adaptive immune response is critical in controlling SARS-CoV-2 infections in hamsters. Future efforts should assess the role of the innate immune response in SARS-CoV-2 infections.
Other hamster models of SARS-CoV-2.
While Syrian hamsters are the most widely used hamster in biomedical research, other hamster species are available. One group compared the susceptibility of 3 dwarf hamster species (Phodopus species) to SARS-CoV-2.74 Campbell’s dwarf hamsters (P. campbelli), Djungarian hamsters (P. sungorus), and Roborovski dwarf hamsters (P. roborovskii) were infected with 1 × 105 plaque-forming units (pfu) of SARS-CoV-2 and followed for 14 dpi.74 While Campbell’s dwarfs and Djungarian hamsters had no clinical signs of infection, Roborovski dwarf hamsters developed severe disease. Clinical signs of infection appeared within 3 dpi and included significant reduction in both body weight and body temperature, ruffled fur, lethargy, snuffling, and dyspnea. Pathologic changes in Roborovski hamsters mirrored severe infections in humans, including hyaline microthrombi and diffuse alveolar damage.74 Common histologic features of severe human infections include pulmonary hyaline membranes and microthrombi.1,9 To our knowledge, this is the only report of microthrombi in the lungs of SARS-CoV-2 infected hamsters.74 In this study, humane euthanasia was elected in terminally ill animals; as such, no mortalities were reported.74 ACE2 residues that interact with the SARS-CoV-2 spike proteins are largely conserved across the family Cricetidae.46,74 In silico analysis suggest that the ACE2 residues of Chinese hamsters (Cricetulus griseus) can recognize the spike protein of SARS-CoV-2, thereby permitting infection.46 In vivo experiments have yet to be performed with Chinese hamsters. These studies highlight the need to study SARS-CoV-2 in other hamster species. Although virus stock and dose are critical factors in disease progression, biologic variables including hamster age, sex, and species are also important considerations.
Conclusions
SARS-CoV-2, a highly pathogenic virus, is responsible for an ongoing pandemic. Preclinical studies in animals can help to characterize disease progression and test novel therapeutics and preventatives. Hamsters are a valuable small animal model of SARS-CoV-2 infection due to their susceptibility to infection and clinical course of the disease similar to human infection. After inoculation, hamsters show weight loss and detectable virus in respiratory tissue for up to 14 dpi.32,56,62,69 Although additional clinical signs vary between studies, this variation may be due to different experimental designs and hamster sources and ages. Transgenic Syrian hamsters and dwarf hamsters provide the opportunity to assess severe infections of SARS-CoV-2.12,62,74 Immunocompromised hamsters become chronically infected with SARS-CoV-2.12,62 Furthermore, immunocompetent hamsters mount a neutralizing antibody response while immunocompromised hamsters do not.12,14,15,32,56,69 Lung pathology in SARS-CoV-2 infected hamsters is consistent with lesions described in humans. Acutely, interstitial pneumonia, alveolar edema and hemorrhage and hyaline membrane formation develops, followed by alveolar hyperplasia during repair.15,25,32,39,56,62,69 Once infected, index hamsters are a source of infection for naïve conspecifics. Transmission occurs via direct contact and aerosols.14,15,69 Countermeasure studies in hamsters have documented transmission prevention, prophylaxis, and treatment options, many of which are used in clinical settings.7,14,15,32,39,65,75 Future studies should compare differing routes of infection, including intraocular and enteral inoculation, characterize viral entry into cells, and assess the effect of sex, age, and hamster species on disease progression to better characterize hamster models of SARS-CoV-2. This overview highlights the vital roles hamsters can play in SARS-CoV-2 translational research and suggests additional areas of investigation for future studies. Currently published studies vary greatly in experimental design, including virus stock, preparation, and viral dose. As these differences undoubtedly affect study outcomes, viral stocks and infective doses should be reported using accepted viral nomenclature. Hamster source, age, sex, and species, as well as environmental housing conditions, may complicate the generalization of results. All of these factors should be considered in future studies of SARS-CoV-2 studies in hamsters.
References
- 1. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. 2020. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120– 128. 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggrey AA, Srivastava K, Ture S, Field DJ, Morrell CN. 2013. Platelet induction of the acute-phase response is protective in murine experimental cerebral malaria. J Immunol 190: 4685– 4691. 10.4049/jimmunol.1202672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alcantara S, Reece J, Amarasena T, Rose RD, Manitta J, Amin J, Kent SJ. 2009. Thrombocytopenia is strongly associated with simian AIDS in pigtail macaques. J Acquir Immune Defic Syndr 51: 374– 379. 10.1097/QAI.0b013e3181a9cbcf. [DOI] [PubMed] [Google Scholar]
- 4. Awulachew E, Diriba K, Anja A, Getu E, Belayneh F. 2020. Computed tomography (CT) imaging features of patients with COVID-19: systematic review and meta-analysis. Radiol Res Pract 2020: 1– 8. 10.1155/2020/1023506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, Qu Y, Li F, Lv Q, Wang W, Xue J, Gong S, Liu M, Wang G, Wang S, Song Z, Zhao L, Liu P, Zhao L, Ye F, Wang H, Zhou W, Zhu N, Zhen W, Yu H, Zhang X, Guo L, Chen L, Wang C, Wang Y, Wang X, Xiao Y, Sun Q, Liu H, Zhu F, Ma C, Yan L, Yang M, Han J, Xu W, Tan W, Peng X, Jin Q, Wu G, Qin C. 2020. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583: 830– 833. 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 6. Barthold SW, Griffey SM, Percy DH. 2016. Hamster, p 173– 198. Chapter 3. In: Barthold SW, Griffey SM, Percy DH, editors. Pathology of laboratory rodents and rabbits, 4th ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 7. Baum A, Ajithdoss D, Copin R, Zhou A, Lanza K, Negron N, Ni M, Wei Y, Mohammadi K, Musser B, Atwal GS, Oyejide A, Goez-Gazi Y, Dutton J, Clemmons E, Staples HM, Bartley C, Klaffke B, Alfson K, Gazi M, Gonzalez O, Dick E, Jr, Carrion R, Jr, Pessaint L, Porto M, Cook A, Brown R, Ali V, Greenhouse J, Taylor T, Andersen H, Lewis MG, Stahl N, Murphy AJ, Yancopoulos GD, Kyratsous CA. 2020. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 370: 1110– 1115. 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boudewijns R, Thibaut HJ, Kaptein SJF, Li R, Vergote V, Seldeslachts L, Van Weyenbergh J, De Keyzer C, Bervoets L, Sharma S, Liesenborghs L, Ma J, Jansen S, Van Looveren D, Vercruysse T, Wang X, Jochmans D, Martens E, Roose K, De Vlieger D, Schepens B, Van Buyten T, Jacobs S, Liu Y, Marti-Carreras J, Vanmechelen B, Wawina-Bokalanga T, Delang L, Rocha-Pereira J, Coelmont L, Chiu W, Leyssen P, Heylen E, Schols D, Wang L, Close L, Matthijnssens J, Van Ranst M, Compernolle V, Schramm G, Van Laere K, Saelens X, Callewaert N, Opdenakker G, Maes P, Weynand B, Cawthorne C, Vande Velde G, Wang Z, Neyts J, Dallmeier K. 2020. STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat Commun 11: 1– 10. 10.1038/s41467-020-19684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. 2020. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 396:320–332. 10.1016/S0140-6736(20)31305-2. Erratum in Lancet 2020. 396: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Branche E, Simon AY, Sheets N, Kim K, Barker D, Nguyen A-VT, Sahota H, Young MP, Salgado R, Mamidi A, Viramontes KM, Carnelley T, Qiu H, Elong Ngono A, Regla-Nava JA, Susantono MX, Valls Cuevas JM, Kennedy K, Kodihalli S, Shresta S. 2019. Human polyclonal antibodies prevent lethal zika virus infection in mice. Sci Rep 9: 1– 12. 10.1038/s41598-019-46291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bricker TL, Darling TL, Hassan AO, Harastani HH, Soung A, Jiang X, Dai Y-N, Zhao H, Adams LJ, Holtzman MJ, Bailey AL, Case JB, Fremont DH, Klein R, Diamond MS, Boon ACM. 2020. A single intranasal or intramuscular immunization with chimpanzee adenovirus vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep 36: 1– 11. 10.1016/j.celrep.2021.109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brocato RL, Principe LM, Kim RK, Zeng X, Williams JA, Liu Y, Li R, Smith JM, Golden JW, Gangemi D, Youssef S, Wang Z, Glanville J, Hooper JW. 2020. Disruption of adaptive immunity enhances disease in SARS-CoV-2-infected Syrian hamsters. J Virol 94: e01683– e01620. 10.1128/JVI.01683-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brook B, Harbeson D, Amenyogbe N, Ben-Othman R, Kollmann TR, Aniba R. 2019. Robust health-score based survival prediction for a neonatal mouse model of polymicrobial sepsis. PLoS One 14: 1– 15. 10.1371/journal.pone.0218714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan JF, Yuan S, Zhang AJ, Poon VK, Chan CC, Lee AC, Fan Z, Li C, Liang R, Cao J, Tang K, Luo C, Cheng VC, Cai JP, Chu H, Chan KH, To KK, Sridhar S, Yuen KY. 2020. Surgical mask partition reduces the risk of noncontact transmission in a golden Syrian hamster model for Coronavirus disease 2019 (COVID-19). Clin Infect Dis 71: 2139– 2149. 10.1093/cid/ciaa644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai JP, Kok KH, Chu H, Chan KH, Sridhar S, Chen Z, Chen H, To KK, Yuen KY. 2020. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis 71: 2428– 2446. 10.1093/cid/ciaa325.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cherry JD. 2004. The chronology of the 2002–2003 SARS mini pandemic. Paediatr Respir Rev 5: 262– 269. 10.1016/j.prrv.2004.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collymore C, Kent L, Ahn SK, Xu W, Li M, Liu J, Turner PV, Banks EK. 2018. Humane endpoints for guinea pigs used for Mycobacterium tuberculosis vaccine research. Comp Med 68: 41– 47. [PMC free article] [PubMed] [Google Scholar]
- 18. de Melo GD, Lazarini F, Larrous F, Feige L, Kornobis E, Levallois S, Marchio A, Kergoat L, Hardy D, Cokelear T, Pineau P, Lecuit M, Lledo P-M, Changeux J-P, Bourhy H. 2021. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol Med 13: 1– 14. 10.15252/emmm.202114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhakal S, Ruiz-Bedoya CA, Zhou R, Creisher PS, Villano JS, Littlefield K, Ruelas Castillo J, Marinho P, Jedlicka AE, Ordonez AA, Bahr M, Majewska N, Betenbaugh MJ, Flavahan K, Mueller ARL, Looney MM, Quijada D, Mota F, Beck SE, Brockhurst J, Braxton AM, Castell N, Stover M, D'Alessio FR, Metcalf Pate KA, Karakousis PC, Mankowski JL, Pekosz A, Jain SK, Klein SL, Johns Hopkins. COVID-19 Hamster Study Group. 2021. Sex differences in lung imaging and SARS-CoV-2 antibody responses in a COVID-19 Golden Syrian hamster model. mBio 12: 1– 16. 10.1128/mBio.00974-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fagre AC, Manhard J, Adams R, Eckley M, Zhan S, Lewis J, Rocha SM, Woods C, Kuo K, Liao W, Li L, Corper A, Challa D, Mount E, Tumanut C, Tjalkens RB, Aboellail T, Fan X, Schountz T. 2020. A potent SARS-CoV-2 neutralizing human monoclonal antibody that reduces viral burden and disease severity in Syrian hamsters. Front Immunol 11: 1– 13. 10.3389/fimmu.2020.614256.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falkenberg MK, Teilmann AC, Henriksen T, Hau J, Poulsen HE, Abelson KS. 2019. Clinical, physiologic, and behavioral evaluation of permanently catheterized NMRI mice. J Am Assoc Lab Anim Sci 58: 380– 389. 10.30802/AALAS-JAALAS-18-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Z, Li W, Lee SR, Meng Q, Shi B, Bunch TD, White KL, Kong I-K, Wang Z. 2014. Efficient gene targeting in golden Syrian hamsters by the CRISPR/Cas9 system. PLoS One 9: 1– 9. 10.1371/journal.pone.0109755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, Gao H, Ge X, Kan B, Hu Y, Liu J, Cai F, Jiang D, Yin Y, Qin C, Li J, Gong X, Lou X, Shi W, Wu D, Zhang H, Zhu L, Deng W, Li Y, Lu J, Li C, Wang X, Yin W, Zhang Y, Qin C. 2020. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 369: 77– 81. 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gorospe L, Ayala-Carbonero A, Ureña-Vacas A, Fernández SF, Muñoz-Molina GM, Arrieta P, Almonacid-Sánchez C, Ramos-Sánchez A, Filigheddu E, Pérez-Fernández M. 2020. Spontaneous pneumomediastinum in patients with COVID-19: a case series of four patients. Arch Bronconeumol (Engl Ed) 56: 747– 763. 10.1016/j.arbres.2020.06.008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gruber AD, Osterrieder N, Bertzbach LD, Vladimirova D, Greuel S, Ihlow J, Horst D, Trimpert J, Dietert K. 2020. Standardization of reporting criteria for lung pathology in SARS-CoV-2-infected hamsters: What matters? Am J Respir Cell Mol Biol 63: 856– 859. 10.1165/rcmb.2020-0280LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. 2004. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631– 637. 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, Li Y, Cao Y, Gu J, Wu H. 2021. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 29: E177– E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herndon NL, Bandyopadhyay S, Hod EA, Prestia KA. 2016. Sustained-release buprenorphine improves postsurgical clinical condition but does not alter survival or cytokine levels in a murine model of polymicrobial sepsis. Comp Med 66: 455– 462. [PMC free article] [PubMed] [Google Scholar]
- 29. Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP. 2008. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J Am Assoc Lab Anim Sci 47: 25– 31. [PMC free article] [PubMed] [Google Scholar]
- 30. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271– 280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497– 506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, Watanabe T, Ujie M, Takahashi K, Ito M, Yamada S, Fan S, Chiba S, Kuroda M, Guan L, Takada K, Armbrust T, Balogh A, Furusawa Y, Okuda M, Ueki H, Yasuhara A, Sakai-Tagawa Y, Lopes TJS, Kiso M, Yamayoshi S, Kinoshita N, Ohmagari N, Hattori S-i, Takeda M, Mitsuya H, Krammer F, Suzuki T, Kawaoka Y. 2020. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA 117: 16587– 16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jirkof P. 2014. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 234: 139– 146. 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 34. Kaptein SJ, Jacobs S, Langendries L, Seldeslachts L, Ter Horst S, Liesenborghs L, Hens B, Vergote V, Heylen E, Barthelemy K. 2020. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2− infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci USA 117: 26955– 26965. 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaur SP, Gupta V. 2020. COVID-19 vaccine: A comprehensive status report. Virus Res 288: 1– 12. 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kennedy AR, Desrosiers A, Terzaghi M, Little JB. 1978. Morphometric and histological analysis of the lungs of Syrian golden hamsters. J Anat 125: 527– 553. [PMC free article] [PubMed] [Google Scholar]
- 37. Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, Um J, Song MS, Jeong HW, Lai VD, Kim Y, Chin BS, Park JS, Chung KH, Foo SS, Poo H, Mo IP, Lee OJ, Webby RJ, Jung JU, Choi YK. 2020. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27: 704– 709.e2. 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR, Benner SE, Littlefield K, Kumar S, Naik HM, Betenbaugh MJ, Shrestha R, Wu AA, Hughes RM, Burgess I, Caturegli P, Laeyendecker O, Quinn TC, Sullivan D, Shoham S, Redd AD, Bloch EM, Casadevall A, Tobian AA. 2020. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest 130: 6141– 6150. 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kreye J, Reincke SM, Kornau HC, Sánchez-Sendin E, Corman VM, Liu H, Yuan M, Wu NC, Zhu X, Lee CD, Trimpert J, Höltje M, Dietert K, Stöffler L, von Wardenburg N, van Hoof S, Homeyer MA, Hoffmann J, Abdelgawad A, Gruber AD, Bertzbach LD, Vladimirova D, Li LY, Barthel PC, Skriner K, Hocke AC, Hippenstiel S, Witzenrath M, Suttorp N, Kurth F, Franke C, Endres M, Schmitz D, Jeworowski LM, Richter A, Schmidt ML, Schwarz T, Müller MA, Drosten C, Wendisch D, Sander LE, Osterrieder N, Wilson IA, Prüss H. 2020. A Therapeutic Non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell 183: 1058– 1069.e19. 10.1016/j.cell.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling A-E, Humphrey CD, Shieh W-J, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang J-Y, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348: 1953– 1966. 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 41. Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7: 447– 449. 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 42. Lee AC, Zhang AJ, Chan JF, Li C, Fan Z, Liu F, Chen Y, Liang R, Sridhar S, Cai JP, Poon VK, Chan CC, To KK, Yuan S, Zhou J, Chu H, Yuen KY. 2020. Oral SARS-CoV-2 inoculation establishes subclinical respiratory infection with virus shedding in golden Syrian hamsters. Cell Rep Med 1: 1– 10. 10.1016/j.xcrm.2020.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, Hirayama AV, Mastroiani F, Turtle CJ, Harhay MO, Legrand M, Deutschman CS. 2020. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 8: 1233– 1244. 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li R, Ying B, Liu Y, Spencer JF, Miao J, Tollefson AE, Brien JD, Wang Y, Wold WSM, Wang Z, Toth K. 2020. Generation and characterization of an Il2rg knockout Syrian hamster model for XSCID and HAdV-C6 infection in immunocompromised patients. Dis Model Mech 13: 1– 34. 10.1242/dmm.044602.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Y, Mao B, Liang S, Yang J-W, Lu H-W, Chai Y-H, Wang L, Zhang L, Li Q-H, Zhao L, He Y, Gu X-L, Ji X-B, Li L, Jie Z-J, Li Q, Li X-Y, Lu H-Z, Zhang W-H, Song Y-L, Qu J-M, Xu J-F. Shanghai clinical treatment experts group for COVID-19. 2020. Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J 55: 1– 4. 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luan J, Lu Y, Jin X, Zhang L. 2020. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun 526: 165– 169. 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A, Yale IT, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. 2020. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584: 463– 469. 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R. 2020. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 39: 1– 15. 10.15252/embj.2020105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miao J, Ying B, Li R, Tollefson AE, Spencer JF, Wold WSM, Song S-H, Kong I-K, Toth K, Wang Y, Wang Z. 2018. Characterization of an N-Terminal non-core domain of RAG1 gene disrupted Syrian hamster model generated by CRISPR Cas9. Viruses 10: 1– 16. 10.3390/v10050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miedel EL, Hankenson FC. 2015. Biology and diseases of hamsters, p 209– 245. Chapter 5. In: Fox JG Anderson LC Otto GM Pritchett-Corning KR Whary MT editors. Laboratory animal medicine, 3rd ed. Boston (MA: ): Academic Press. [Google Scholar]
- 51. Moradi B, Ghanaati H, Kazemi MA, Gity M, Hashemi H, Davari-Tanha F, Chavoshi M, Rouzrokh P, Kolahdouzan K. 2020. Implications of sex difference in CT scan findings and outcome of patients with COVID-19 pneumonia. Radiol Cardiothorac Imaging 2: 1– 8. 10.1148/ryct.2020200248.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Muñoz-Fontela C, Dowling WE, Funnell SG, Gsell P-S, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M. 2020. Animal models for COVID-19. Nature 586: 509– 515. 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murray KA. 2012. Anatomy, physiology, and behavior. p 753– 763. Chapter 27. In: Suckow MA, Stevens KA, Wilson RP, editors. The laboratory rabbit, guinea pig, hamster, and other rodents. Boston (MA): Academic Press. [Google Scholar]
- 54. Oliver VL, Thurston SE, Lofgren JL. 2018. Using cageside measures to evaluate analgesic efficacy in Mice (Mus musculus) after surgery. J Am Assoc Lab Anim Sci 57: 186– 201. [PMC free article] [PubMed] [Google Scholar]
- 55. Ordonez AA, Wintaco LM, Mota F, Restrepo AF, Ruiz-Bedoya CA, Reyes CF, Uribe LG, Abhishek S, D’Alessio FR, Holt DP, Dannals RF, Rowe SP, Castillo VR, Pomper MG, Granados U, Jain SK. 2021. Imaging Enterobacterales infections in patients using pathogen-specific positron emission tomography. Sci Transl Med 13: eabe9805. 10.1126/scitranslmed.abe9805.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Osterrieder N, Bertzbach LD, Dietert K, Abdelgawad A, Vladimirova D, Kunec D, Hoffmann D, Beer M, Gruber AD, Trimpert J. 2020. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses 12: 1– 11. 10.3390/v12070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Port JR, Yinda CK, Owusu IO, Holbrook M, Fischer R, Bushmaker T, Avanzato VA, Schulz JE, Martens C, van Doremalen N, Clancy CS, Munster VJ. 2021. SARS-CoV-2 disease severity and transmission efficiency is increased for airborne compared to fomite exposure in Syrian hamsters. Nat Commun 12: 1– 15. 10.1038/s41467-021-25156-8. `/jrn [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ren L-L, Wang Y-M, Wu Z-Q, Xiang Z-C, Guo L, Xu T, Jiang Y-Z, Xiong Y, Li Y-J, Li X-W, Li H, Fan G-H, Gu X-Y, Xiao Y, Gao H, Xu J-Y, Yang F, Wang X-M, Wu C, Chen L, Liu Y-W, Liu B, Yang J, Wang X-R, Dong J, Li L, Huang C-L, Zhao J-P, Hu Y, Cheng Z-S, Liu L-L, Qian Z-H, Qin C, Jin Q, Cao B, Wang J-W. 2020. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 133: 1015– 1024. 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reznik GK. 1990. Comparative anatomy, physiology, and function of the upper respiratory tract. Environ Health Perspect 85: 171– 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roberts A, Lamirande EW, Vogel L, Jackson JP, Paddock CD, Guarner J, Zaki SR, Sheahan T, Baric R, Subbarao K. 2008. Animal models and vaccines for SARS-CoV infection. Virus Res 133: 20– 32. 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roberts A, Vogel L, Guarner J, Hayes N, Murphy B, Zaki S, Subbarao K. 2005. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J Virol 79: 503– 511. 10.1128/JVI.79.1.503-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rosenke K, Meade-White K, Letko M, Clancy C, Hansen F, Liu Y, Okumura A, Tang-Huau TL, Li R, Saturday G, Feldmann F, Scott D, Wang Z, Munster V, Jarvis MA, Feldmann H. 2020. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg Microbes Infect 9: 2673– 2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ruiz-Bedoya CA, Mota F, Ordonez AA, Foss CA, Singh AK, Praharaj M, Mahmud FJ, Ghayoor A, Flavahan K, De Jesus P, Bahr M, Dhakal S, Zhou R, Solis CV, Mulka KR, Bishai WR, Pekosz A, Mankowski JL, Villano J, Klein SL, Jain SK. 2021. 124I-Iodo-DPA-713 positron emission tomography in a hamster model of SARS-CoV-2 infection. Mol Imaging Biol Aug 23: 1– 9.doi: 10.1007/s11307-021-01638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schaecher SR, Stabenow J, Oberle C, Schriewer J, Buller RM, Sagartz JE, Pekosz A. 2008. An immunosuppressed Syrian golden hamster model for SARS-CoV infection. Virology 380: 312– 321. 10.1016/j.virol.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schäfer A, Muecksch F, Lorenzi JCC, Leist SR, Cipolla M, Bournazos S, Schmidt F, Maison RM, Gazumyan A, Martinez DR, Baric RS, Robbiani DF, Hatziioannou T, Ravetch JV, Bieniasz PD, Bowen RA, Nussenzweig MC, Sheahan TP. 2021. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J Exp Med 218: 1– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schalekamp S, Bleeker-Rovers CP, Beenen LF, Quarles van Ufford HM, Gietema HA, Stöger JL, Harris V, Reijers MH, Rahamat-Langendoen J, Korevaar DA, Smits LP, Korteweg C, van Rees Vellinga TFD, Vermaat M, Stassen PM, Scheper H, Winjnakker R, Borm FJ, Dofferhoff ASM, Prokop M. 2021. Chest CT in the emergency department for diagnosis of COVID-19 pneumonia: Dutch experience. Radiology 298: E98– E106. 10.1148/radiol.2020203465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 368: 1016– 1020. 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, Mele T. 2014. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes 7: 233. 10.1186/1756-0500-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sia SF, Yan L-M, Chin AWH, Fung K, Choy K-T, Wong AYL, Kaewpreedee P, Perera RAPM, Poon LLM, Nicholls JM, Peiris M, Yen H-L. 2020. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583: 834– 838. 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt CE, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, Lacorte JM, Yssel H, Parizot C, Dorgham K, Charneau P, Amoura Z, Gorochov G. 2020. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 13: 1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, Banovich NE, Barbry P, Brazma A, Collin J, Desai TJ, Duong TE, Eickelberg O, Falk C, Farzan M, Glass I, Gupta RK, Haniffa M, Horvath P, Hubner N, Hung D, Kaminski N, Krasnow M, Kropski JA, Kuhnemund M, Lako M, Lee H, Leroy S, Linnarson S, Lundeberg J, Meyer KB, Miao Z, Misharin AV, Nawijn MC, Nikolic MZ, Noseda M, Ordovas-Montanes J, Oudit GY, Pe’er D, Powell J, Quake S, Rajagopal J, Tata PR, Rawlins EL, Regev A, Reyfman PA, Rozenblatt-Rosen O, Saeb-Parsy K, Samakovlis C, Schiller HB, Schultze JL, Seibold MA, Seidman CE, Seidman JG, Shalek AK, Shepherd D, Spence J, Spira A, Sun X, Teichmann SA, Theis FJ, Tsankov AM, Vallier L, van den Berge M, Whitsett J, Xavier R, Xu Y, Zaragosi L-E, Zerti D, Zhang H, Zhang K, Rojas M, Figueiredo F, HCA Lung Biological Network . 2020. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26: 681– 687. 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Suresh V, Parida D, Minz AP, Sethi M, Sahoo BS, Senapati S. 2021. Tissue distribution of ACE2 protein in Syrian golden hamster (Mesocricetus auratus) and its possible implications in SARS-CoV-2 related studies. Front Pharmacol 11: 1– 11. 10.3389/fphar.2020.579330.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tostanoski LH, Wegmann F, Martinot AJ, Loos C, McMahan K, Mercado NB, Yu J, Chan CN, Bondoc S, Starke CE, Nekorchuk M, Busman-Sahay K, Piedra-Mora C, Wrijil LM, Ducat S, Custers J, Atyeo C, Fischinger S, Burke JS, Feldman J, Hauser BM, Caradonna TM, Bondzie EA, Dagotto G, Gebre MS, Jacob-Dolan C, Lin Z, Mahrokhian SH, Nampanya F, Nityanandam R, Pessaint L, Porto M, Ali V, Benetiene D, Tevi K, Andersen H, Lewis MG, Schmidt AG, Lauffenburger DA, Alter G, Estes JD, Schuitemaker H, Zahn R, Barouch DH. 2020. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat Med 26: 1694– 1700. 10.1038/s41591-020-1070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trimpert J, Vladimirova D, Dietert K, Abdelgawad A, Kunec D, Dökel S, Voss A, Gruber AD, Bertzbach LD, Osterrieder N. 2020. The Roborovski dwarf hamster Is a highly susceptible model for a rapid and fatal course of SARS-CoV-2 Infection. Cell Rep 33: 1– 9. 10.1016/j.celrep.2020.108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. US Food and Drug Administration. [Internet]. 2020. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. [Cited 01 March 2021]. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19
- 76. US Food and Drug Administration. [Internet]. 2020. FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. [Cited 01 March 2021]. Available at: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
- 77. Wan Y, Shang J, Graham R, Baric RS, Li F. 2020. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94: 1– 9. 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang W, Tang J, Wei F. 2020. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol 92: 441– 447. 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang X, Liu C, Hong L, Yuan C, Ding J, Jia Q, Sun G, Peng W, Sun Q. 2020. CT findings of patients infected with SARS-CoV-2. BMC Med Imaging 20: 1– 6. 10.1186/s12880-020-00471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. West JB. 2007. Ventilation: how gas gets to the alveoli, p 14– 20. Chapter 2. In: Duffy N, editor. Respiratory physiology: the essentials, 8th ed. Philadelphia (PA): Wolters Kluwer Health/Lippincott Williams and Wilkins. [Google Scholar]
- 81. Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. 2020. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 138: 575– 578. 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yahalom-Ronen Y, Tamir H, Melamed S, Politi B, Shifman O, Achdout H, Vitner EB, Israeli O, Milrot E, Stein D, Cohen-Gihon I, Lazar S, Gutman H, Glinert I, Cherry L, Vagima Y, Lazar S, Weiss S, Ben-Shmuel A, Avraham R, Puni R, Lupu E, Bar-David E, Sittner A, Erez N, Zichel R, Mamroud E, Mazor O, Levy H, Laskar O, Yitzhaki S, Shapira SC, Zvi A, Beth-Din A, Paran N, Israely T. 2020. A single dose of recombinant VSV-G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat Commun 11: 1– 13. 10.1038/s41467-020-20228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang AJ, Lee AC, Chu H, Chan JF, Fan Z, Li C, Liu F, Chen Y, Yuan S, Poon VK, Chan CC, Cai JP, Wu KL, Sridhar S, Chan YS, Yuen KY. 2020. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin Infect Dis 73: e503– e512. 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270– 273. 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]