Abstract
Mice are an invaluable resource for studying virus-induced disease. They are a small, genetically modifiable animal for which a large arsenal of genetic and immunologic tools is available for evaluation of pathogenesis and potential vaccines and therapeutics. SARS-CoV-2, the betacoronavirus responsible for the COVID-19 pandemic, does not naturally replicate in wild-type mice, due to structural differences between human and mouse ACE2, the primary receptor for SARS-CoV-2 entry into cells. However, several mouse strains have been developed that allow for SARS-CoV-2 replication and clinical disease. Two broad strategies have primarily been deployed for developing mouse strains susceptible to COVID-19-like disease: adding in the human ACE2 gene and adapting the virus to the mouse ACE2 receptor. Both approaches result in mice that develop several of the clinical and pathologic hallmarks of COVID-19, including acute respiratory distress syndrome and acute lung injury. In this review, we describe key acute pulmonary and extrapulmonary pathologic changes seen in COVID-19 patients that mouse models of SARS-CoV-2 infection ideally replicate, the essential development of mouse models for the study of Severe Acute Respiratory Syndrome and Middle Eastern Respiratory Syndrome and the basis of many of the models of COVID-19, and key clinical and pathologic features of currently available mouse models of SARS-CoV-2 infection.
Abbreviations: AAV, adeno-associated virus; ACE2, angiotensin-converting enzyme 2; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 19; DAD, diffuse alveolar damage; DPI, days postinfection; DPP4, dipeptidyl peptidase 4; hACE2, human angiotensin-converting enzyme 2; mAce2, mouse angiotensin-converting enzyme 2; MERS, Middle Eastern Respiratory Syndrome; MERS-CoV, Middle Eastern Respiratory Syndrome coronavirus; SARS, Severe Acute Respiratory Syndrome; SARS-CoV, Severe Acute Respiratory Syndrome coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome coronavirus 2
Coronaviruses are widespread and infect several different species. In humans, coronaviruses historically cause primarily mild respiratory diseases, such as the common cold. However, in 2003 a novel coronavirus emerged, Severe Acute Respiratory Syndrome coronavirus (SARS-CoV), which caused severe respiratory disease with high mortality.18 Since then, 2 other highly pathogenic coronaviruses from the same betacoronavirus genus have emerged: Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV) in 2012, and most recently, Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2).18,133,134 Spread of SARS-CoV-2, which causes coronavirus disease 2019 (COVID-19), has resulted in a once-in-a-lifetime pandemic, and as of May 2021, more than 160 million cases and more than 3.3 million deaths have been reported worldwide.123
Much like SARS-CoV, SARS-CoV-2 has 4 structural proteins—spike (S), envelope (E), membrane (M), and nucleocapsid (N)—and 8 accessory proteins. The S protein includes a receptor-binding domain, which is essential for the virus to bind to and subsequently infect a host cell.124 SARS-CoV and SARS-CoV-2 both use angiotensin-converting enzyme 2 (ACE2) as the primary receptor and transmembrane protease serine 2 as a cofactor,42,56,60 In contrast, the receptor for MERS-CoV is dipeptidyl peptidase 4 (DPP4).91 Because of amino acid changes in the S protein, SARS-CoV-2 binds ACE2 with a higher affinity than does SARS-CoV,124 which may explain the greater human infectivity of SARS-CoV-2.42,102 ACE2 is expressed throughout the body, allowing SARS-CoV-2 to potentially infect multiple organs, including the lung, heart, kidney, liver, intestines, and brain. Importantly, ACE2 is expressed on the apical surfaces of epithelial cells in these organs, permitting infection from direct viral contact with those cells.37 As its name suggests, ACE2 is a critical component of the renin–angiotensin cascade, limiting vasoconstriction and promoting vasodilation by converting angiotensin II to angiotensin 1–7. ACE2 in the lung is hypothesized to reduce lung inflammation; SARS-CoV and SARS-CoV-2 potentially could exacerbate lung inflammation by altering this pathway.98
A thorough understanding of disease pathogenesis and any potential vaccines or therapeutics for any emerging pathogen is facilitated by studying animal models. For example, the first FDA-approved treatment for COVID-19 was remdesivir. This drug initially was granted emergency-use authorization for COVID-19 patients in part because of demonstrated therapeutic efficacy against MERS-CoV infection in a mouse model.103 An ideal animal model of COVID-19 captures the wide spectrum of disease phenotypes attributed to this multifaceted disease, including organ-specific pathology and systemic changes such as hypercoagulation and cytokine storms. Animal models rarely capture every aspect of human disease, requiring the use of multiple models in order to replicate the numerous features of viral infection and disease and thereby build a comprehensive picture of what happens in human patients.
SARS-CoV-2 naturally infects numerous animal species, including mink on farms, big cats in zoos and sanctuaries, and domestic dogs and cats.28,36,68,72,81,87,104,107 Although mink are particularly vulnerable to severe SARS-CoV-2 disease and can transmit virus to humans, they are rarely used in research studies.68,81,87 Mice, hamsters, ferrets, and nonhuman primates are more widely used as experimental models of SARS-CoV-2 infection.68 Hamsters replicate human disease well, are small, and are widely available, making them an attractive choice.12,68,105 However, hamsters are less commonly used in research than are mice, and the availability of strains and reagents necessary for studying genetic and immune drivers of disease is limited for hamsters. Ferrets are frequently used as models for respiratory viruses, particularly influenza, primarily because they disperse and are susceptible to these viruses via airborne droplets, whereas rodents do not. However, ferrets do not develop severe symptoms or generate high virus titers in the lungs after SARS-CoV-2 infection.52,68,95,104 NHPs have similar immune responses to humans and are invaluable for safety studies for preclinical trials but are costly to use and require significant infrastructure to maintain studies.66,68,130 Mice provide a valuable model because they are widely available and relatively inexpensive, and a large arsenal of genetic and immunologic tools is available for mice. The use of mice to study SARS-CoV-2 infection provides the potential for a broader understanding of several different aspects of this complex disease.
Overview of SARS-CoV-2–induced Clinical Disease and Pathology in Humans
SARS-CoV-2 primarily causes respiratory disease, and like many other respiratory viruses, is predominantly transmitted via droplets and possibly aerosols.80,114 Initial symptoms of infection typically include fever, fatigue, malaise, and cough, with common progression to myalgia, headache, anosmia, ageusia, shortness of breath, and hospitalization.9,45,53,69,121,134 Less common symptoms include hemoptysis and diarrhea.45
Older persons and those with underlying health conditions, including heart disease and diabetes, are at a higher risk of developing severe symptoms such as acute respiratory distress syndrome (ARDS).14,27,31 SARS-CoV-2–associated ARDS is similar to other forms of ARDS, with increased respiratory rate and decreased oxygen saturation.8,24,30 Many patients with severe COVID-19 require mechanical ventilation, and ventilated patients have a significantly higher mortality rate than those that do not require ventilation.8,46,106 Men are more likely than women to be severely affected by SARS-CoV-2 infection, including a greater risk of ICU admission and death.8,14,29,46 Because ACE2 has higher expression in men than in women, this sex-related difference may be due to differences in ACE2 and transmembrane protease serine 2 expression.29 An alternative hypothesis is that sex-associated differences are due to different immune responses in men and women, given previous findings that women develop less severe disease than men after other viral infections.29
In addition to ARDS, SARS-CoV-2 infection often has systemic effects, including a hypercoagulable state characterized by elevated levels of serum d-dimer, fibrinogen, and clotting pathway factors that result in multiorgan thrombosis.2,9,25,33,38,67,69,78,79,101,108 SARS-CoV-2 infection also results in a proinflammatory state, and cytokine storms are believed to contribute to severe disease.62 Patients with severe COVID-19 have elevated serum proinflammatory cytokines, including IL6, C-C motif chemokine ligand 3, and TNF⟨, which can lead to shock and organ failure.11,82 Many mouse models of SARS-CoV-2 disease also show significant elevation of these proinflammatory cytokines, whereas other models show essential roles for interferon signaling in disease pathogenesis.22,32,34,39,47,55,94,112,113 This information suggests that excessive inflammation is a consistent feature of COVID-19 and likely contributes to its pathogenesis. Consequently, immune modulation has been tested as a potential therapeutic for patients with COVID-19. Combination treatment with baricitinib, a broad Janus kinase inhibitor, and the antiviral remdesivir has been shown to decrease the duration of illness and improve 15-d outcomes.50 In addition, intravenous corticosteroid treatment reduces the number of days that patients with ARDS require ventilation, suggesting that inhibiting inflammation reduces the severity of COVID-19.115 However, IL6 blockade did not reduce morbidity or mortality in a randomized, double-blind trial, indicating that the inflammatory response to SARS-CoV-2 is complex and requires further study.109
The lungs are considered the primary target organ for SARS-CoV-2-induced damage and pathology. On CT scans, patients with SARS-CoV-2 pneumonia display ground-glass opacities, fibrotic changes, and consolidation in their lungs.9,25,64,70,78,85,88,101 Abnormal CT scans have also been reported in asymptomatic persons who previously tested positive for SARS-CoV-2, indicating the SARS-CoV-2 infection can cause lung damage even in asymptomatic individuals.4,77
Postmortem pathologic examinations show that the lungs are grossly congested, with increased weight and discoloration and widespread distribution of lesions.9,25,70,78,101 Signs of chronicity, including consolidation, emphysematous changes, and fibrosis, are evident.9,25,78,101 Some of the key acute pathologic features of SARS-CoV-2 pneumonia include acute lung injury (ALI), microscopically manifesting as diffuse alveolar damage (DAD), with characteristic neutrophil infiltration, hyaline membrane formation or other fibrinous changes, and alveolar septal thickening.9,25,38,70,78,101 Vascular changes and systemic thrombotic disease have also been reported.67,78 These include pulmonary vasculitis, perivascular cuffing, hemorrhage, microthrombi and thrombi in lung parenchyma, and, in some cases, pulmonary embolism.9,25,33,38,67,70,78
SARS-CoV-2 replicates in the lung, specifically in epithelial cells of bronchi and alveoli. In particular, type II pneumocytes, which synthesize surfactant and serve as progenitor cells for damaged alveolar epithelium, have been identified as a cell type capable of productive viral infection.9,70,101 Pulmonary surfactant plays an important role in normal lung function by reducing surface tension, thus inhibiting alveolar and airway wall collapse, and regulating inflammation in the lung. Other pathologic features of COVID-19 in the lung include necrosis, cell sloughing, and reactive pneumocytes with type II pneumocyte hyperplasia.25,70,101 In response to active viral replication, inflammatory cells, including T lymphocytes, neutrophils, and macrophages, infiltrate the lung parenchyma.9,38,70,78,101 SARS-CoV-2 has also been identified in alveolar macrophages, resulting in multinucleated giant cells.70 However, SARS-CoV-2 infection of macrophages is considered abortive, without robust viral replication or spread.131
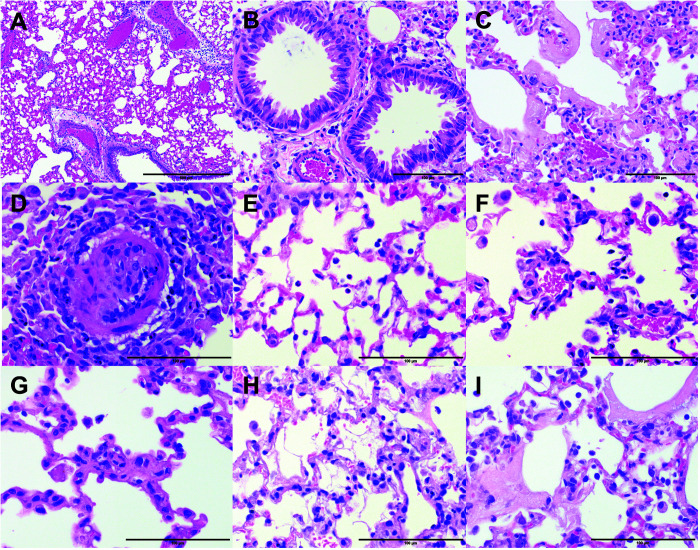
The gross and microscopic appearance of acute interstitial pneumonia is not unique to SARS-CoV-2 infection.70 Other viral pneumonias, as well as those due to bacterial and fungal infections and inhaled toxins, may cause these patterns, making positive testing or identification of SARS-CoV-2 essential to the diagnosis. However, the combination of DAD, ground-glass opacities on CT, and hyaline membrane formation has been widely documented in COVID-19, and therefore is thought to provide defining features of this disease.9,25,38,64,70,78,85,88,101 An animal model of COVID-19–like disease should allow virus replication in alveolar and bronchiolar epithelial cells and demonstrate cell sloughing. Further, an ideal mouse model should cause DAD, including its characteristic hyaline membranes. However, hyaline membranes or other features that require several days to develop may be difficult to replicate in rapidly progressive experimental infections. In particular, IACUC guidelines for humane endpoints may require euthanasia of mice before the formation of robust hyaline membranes. Nonetheless, hyaline membranes may be seen in severe infection in mouse models of both SARS-CoV-2 and MERS-CoV (Figure 1).59,132
Figure 1.
Histopathologic changes observed in lungs of mice infected with SARS-CoV-2-MA10. (A) Congestion, hyperemia, and perivascular edema. Magnification, 100×. (B) Bronchiolar epithelial damage and hyperplasia. Magnification, 400×. (C) Diffuse alveolar damage (DAD) with hyaline membrane formation. Magnification, 400×. (D) Degenerative change in a small-caliber vessel. Magnification, 600×. (E) Neutrophils and (F) macrophages in the alveolar space. Magnification, 600×. (G through I) Examples of lung specimens demonstrating features of pronounced DAD or acute lung injury (ALI) scores, such as (G) alveolar septal thickening, (H) proteinaceous debris in the alveolar space, and (I) cell death and hyaline membranes. Magnification, 600×. Hematoxylin and eosin stain; scale bars: 500 μ (A), 100 μ (B through I). The mouse studies were conducted under animal use protocols approved by the IACUC at the University of North Carolina at Chapel Hill.
Symptoms and pathology after SARS-CoV-2 infection often spread beyond the lungs. ACE2 is expressed on human digestive tract epithelium.9,61,125 On histologic analysis, viral proteins and viral RNA have been identified throughout the gastrointestinal tract, including the esophagus, stomach, small intestine (ileum and duodenum), large intestine, and rectum.9,61,125 No severe histopathologic manifestations have been documented in the gastrointestinal tract, although virus has been detected in feces, and some COVID-19 patients report nausea, vomiting, and diarrhea.9,14,53,61,125 Elevated liver markers, including ALT, AST, and bilirubin, all indicative of liver damage, have been reported in COVID-19 patients9,14,38,70,78,91,101,128 At autopsy, hepatomegaly, hepatic congestion, steatosis, cirrhosis, and infarction have been reported.9,14,38,70,78,91,101,128 These pathologic findings, however, may not be due to viral infection directly but rather may result from multiorgan failure or other underlying chronic conditions.9,70,78,101
Similar to changes in liver enzymes, BUN and creatinine levels are elevated in COVID-19 patients, indicative of kidney damage.14,91 ACE2 is expressed on epithelial cells within the kidney, and SARS-CoV-2 virions have been identified in the kidney by electron microscopy (EM), along with low levels of genomic RNA9,38,78,101,110 The main pathologic finding in the kidney is acute tubular injury or acute tubular necrosis with mild inflammation predominated by lymphocytes.9,38,78,101,110 The hypercoagulable state caused by SARS-CoV-2 infection also results in thrombi and erythrocyte aggregation without overt clotting in the kidney.38,78,110
ACE2 is expressed on cardiomyocytes and pericytes, and the virus has been detected in the cardiac tissues of COVID-19 patients.9,13,78,101 Cardiomegaly with right ventricular dilation, coronary artery disease, thrombosis, and acute myocardial ischemic damage are common autopsy findings, although they most likely reflect chronic heart disease rather than SARS-CoV-2 infection.9,25,38,78,101 However, higher viral loads in the lungs have been correlated with the most severe cardiac symptoms, suggesting that high SARS-CoV-2 loads could contribute to or exacerbate underlying heart disease.38 Furthermore, pericarditis has been reported in individuals without a history of heart disease, suggesting that SARS-CoV-2 infection and the subsequent immune responses can cause cardiac damage.38 In addition, cardiac fibrosis and necrotic myocytes have been observed, indicating damage to the heart that could affect function long term.23,70,78 These findings highlight the importance of cardiovascular disease as a preexisting condition that may contribute to SARS-CoV-2 pathogenesis.
Neurologic complications are a key extrapulmonary disease manifestation of SARS-CoV-2 infection. Anosmia (loss of sense of smell) and ageusia (loss of sense of taste) are common initial symptoms of SARS-CoV-2 infection, with up to 85% of symptomatic patients reporting olfactory dysfunction.53,69,90,108 Headache and dizziness are common neurologic symptoms in early SARS-CoV-2 infection, and encephalitis, meningitis, cognitive deficits, altered mental state, demyelination, ischemic and hemorrhagic strokes, and subarachnoid and brainstem hemorrhages have all been reported.6,7,9,14,40,41,51,53,69,108,127 However, these complications may be due to the systemic effects of SARS-CoV-2 infection rather than viral replication in the brain, because SARS-CoV-2 has only rarely been detected in brain parenchyma of COVID-19 patients, and brain inflammation is usually minimal.21,48,65,74,78,101 A correlation has been noted between severity of disease and an increase in neurologic symptoms, with approximately 84% of patients with SARS-CoV-2–associated ARDS also developing neurologic symptoms.40,41 Therefore, an ideal animal model for SARS-CoV-2 infection will demonstrate not only respiratory disease and pulmonary pathology, but also the systemic and extrapulmonary effects, resulting in a comprehensive representation of the disease.
Mouse Models of SARS-CoV and MERS-CoV: The Bases for Developing SARS-CoV-2 Mouse Models
Mice are incredibly useful as models of disease because of their ready availability, small size, and relatively low cost. Furthermore, because mice have been used as a model species to study many human diseases, reagents specific for working with mice and their tissues are widely available. Although SARS-CoV-2 can infect standard inbred mice, the virus does not replicate to high levels or cause severe disease like that seen in humans.1 However, many of the mouse models of COVID-19-like disease developed since the COVID-19 pandemic began used the same approaches and, in some cases, the same mouse strains, that were developed for studying infection with the betacoronaviruses SARS-CoV and MERS-CoV. Like SARS-CoV-2, SARS-CoV and MERS-CoV can infect mice but do not replicate well and consequently do not cause disease.15,17,98,133
Although the human ACE2 (hACE2) and mouse Ace2 (mAce2) homologs have the same physiology and function, they have important structural differences that prevent the S protein from binding with a high affinity to mAce2 in the same way that binds to hACE2.98,133 Similar issues of receptor homology may also prevent MERS-CoV from causing severe disease in mice.15 Alterations in either the host receptor (hACE2 or hDPP4 transgenic mice) or in the virus (mouse adaptation) are necessary to reproduce severe betacoronavirus diseases in mice.
Transgenic mouse model approaches.
Like SARS-CoV-2, SARS-CoV and MERS-CoV do not cause severe disease in mice as they do in humans.17,73 One group has been able to produce some facets of SARS-like disease, including lethal pneumonia, in an immunocompromised, aged STAT1−/− mouse line,111 and another group has reported virus replication and some respiratory disease in aged BALB/c mice infected with the Urbani strain of SARS.97 However, the majority of laboratory mouse strains are not susceptible to infection or disease induction by wild-type viruses. To address this issue, many groups have developed mice transgenic for the human SARS-CoV or MERS-CoV receptors, ACE2 and DPP4, respectively.3,73,84,89,93,103,116,126
The same general approach has been used to place hACE2 under the control of several different promoters in different transgenic mouse models. Disease severity has been found to be directly tied to hACE2 expression levels, with higher hACE2 expression resulting in severe and disseminated disease, and lower hACE2 expression limiting disease to the lungs.116 One group placed hACE2 under the control of a CAG promoter, but because CAG was not specifically expressed on a single cell type, hACE2 expression in this model was widespread rather than limited to epithelia.116 In another study, hACE2 was placed under the control of the keratin 18 (K18) promoter, thereby limiting hACE2 expression to epithelial cells.73 In K18-hACE2 mice, hACE2 expression occurs in lungs, small and large intestines, liver, kidney, brain, and bronchiolar epithelium, but not in alveolar epithelium, which is a key sites of SARS-CoV and SARS-CoV-2 infection and consequently pathology.9,70,99,101 K18-hACE2 mice develop severe lung disease, and the majority reach euthanasia criteria by 4 d postinfection (DPI). Brain viral loads are detectable after peak lung viral loads, and animals frequently show brain pathology.73 To better characterize these findings, one group backcrossed K18-hACE2 transgenic mice to wild-type C57BL/6 mice, reducing mortality and extending the course of infection, thereby allowing study of brain pathology at its peak.73 At euthanasia, limited inflammation was noted in the brain,73 but widespread neuronal death and actively infected neurons has been noted in other studies.84 The K18-hACE2 mouse model of SARS is therefore particularly useful for studying the effects of SARS-CoV on both the brain and the lung. Death due to CNS disease alone is very rare in humans infected with SARS-CoV, and therefore this model does not mimic the course of disease that occurs in the majority of human infections. In addition, because hACE2 expression is directed by a nonnative promotor, overexpression is common in various tissues and specific cell types and may not accurately replicate the natural infection pattern in SARS or COVID-19 patients.76,86 Regardless, some patients develop neurologic symptoms due to SARS-CoV and SARS-CoV-2 infection, and the model may be useful for studying that phenomenon.
Another study developed a transgenic mouse line with hACE2 under control of the mAce2 promoter, allowing hACE2 to be expressed in the same cells and to the same levels as mAce2.126 The majority of these mice recover from infection and do not have significant clinical findings. However, at necropsy, as in other hACE transgenic mice, classic SARS-CoV lung pathology is evident, including significant acute lung damage and proliferation of type II pneumocytes. In addition, virus and pathologic changes in extrapulmonary organs were identified, including damage of proximal tubule epithelium in the kidneys and cerebrovascular damage. Although this model captured more extrapulmonary disease phenotypes, lung disease was the major clinical feature.126
An important step forward in developing mouse models of betacoronaviruses has been the development of the adenovirus-transduced model, allowing transduction of the human receptor for viral entry into several different mouse strains to pinpoint drivers of pathology and study how different immune cells function in clearing the virus.129 However, adenovirus vectors are not transduced equally by all cells, resulting in patchy expression of the gene of interest, and adenovirus-transduced gene expression wanes over time, limiting the ability to study long-term effects. Another important consideration is that adenovirus vectors are highly immunogenic, which may confound pathologic findings.54 Nonetheless, these strategies have been used to develop a mouse model of MERS, which in turn has been used to inform the development of SARS-CoV-2. In one study, researchers placed hDDP4 under the CAGS promoter previously used for hACE2 mice, resulting in similarly disseminated virus and lethal disease.3 Another important advancement used for MERS research that contributed to the generation of current SARS-CoV-2 mouse models was the development of a knock-in line, replacing the mDDP4 gene with hDDP4.89 In addition, CRISPR/Cas9 technology was used to develop a hDDP4 knock-in mouse model of MERS; the virus was then further adapted to mice by passaging the virus in mice to better replicate lung pathology seen in humans, including hyaline membranes, alveolar edema, and lymphocytic perivascular cuffing.16 Because CRIPSR/Cas9 technology is relatively new and was not available when the first SARS-CoV mouse models were created, the development of the hDDP4 mouse for studying MERS provided the foundation for using CRISPR/Cas9 to generate similar SARS-CoV-2 mouse models.113
Mouse-adaptation approaches.
Mouse adaptation of a virus usually is achieved through serial passage, during which evolutionary pressures on the virus select for mutations that enhance the virus’s ability to replicate in mice.10 A strategy used to study MERS-CoV was a hDPP4 knock-in approach combined with mouse-adaptation to replicate MERS-CoV human pathology.57,58 Such models carry both the human receptor and a virus adapted to manifest specific pathologic lesions in the mouse. Nonetheless, using both a human receptor and mouse adaptation of a virus can be incredibly time-consuming. In addition, concerns arise regarding the receptor expression levels and cell specificity as compared with humans. Therefore, although the use of both strategies has benefits, mouse adaptation alone can circumvent these concerns, especially during the SARS-CoV-2 pandemic when rapid development of suitable mouse models is essential. One group passaged the Urbani strain of SARS-CoV in young BALB/c mice, resulting in virus replication and subsequent lung disease.96 The virus was also detected in extrapulmonary tissues, including the brain and liver, but no significant extrapulmonary pathology was reported.96 Another group further passaged this virus and determined that no single mutation was responsible for complete susceptibility and disease progression but that several mutations were working synergistically to enhance SARS-CoV pathology in mice.26 This outcome highlights the complex nature of viral infections and the difficulty of predicting the specific mutations that may increase pathogenicity. Another group replicated these findings by passaging the Urbani strain of SARS-CoV in their own lab.19 All of these models show interstitial pneumonia with pneumocyte necrosis and replicating virus in bronchiolar epithelium and alveolar pneumocytes.19,26,96
An important consideration when working with mouse-adapted viruses is that these strains are not clinical isolates. Mutations that arise during mouse adaptation may alter the virus such that it no longer behaves like human strains, which thus complicates the study of specific naturally arising viral variants. This caveat is particularly important as multiple variants of SARS-CoV-2 emerge.
Current Mouse Models of SARS-CoV-2 Pathogenesis
Mouse models of SARS-CoV-2 pathogenesis were developed quickly due to the rapid acceleration of the pandemic, urgent need for robust models to test potential therapeutics and vaccines, and extensive groundwork laid by previously developed mouse models for SARS-CoV and MERS-CoV. Because SARS-CoV and SARS-CoV-2 use the same receptor, many of the same transgenic hACE2 mouse models can be used to study both infection and pathogenesis. A major focus of current studies in mouse models is preclinical testing of therapeutics and vaccines. Convalescent plasma and prophylactic antibody treatments are effective at preventing disease in mice.39,112 In addition, several groups have determined that in mice the S protein, specifically the receptor-binding domain, is immunogenic and the most protective antigen for the development of future vaccines.22,34,55 Receptor-binding domain and S protein vaccines significantly reduce virus replication and prevent pathogenic manifestations.22,34,55 In addition, neutralizing antibodies develop after vaccination in mice, proving an effective protective immune response.34 However, the various mouse models are based on different development strategies and experimental designs, including mouse genetic background, virus strain, and inoculation dose. Consequently, the models display different degrees of disease severity and lung and extrapulmonary pathology, making direct comparisons challenging.
Viral vector models.
Similar to the model developed to study MERS-CoV,129 several groups have used adenoviral and adeno-associated virus (AAV) vectors to transduce expression of hACE2 into different mouse strains (Table 1).39,47,94,112 A significant benefit of using adenovirus and AAV vectors is that expression of the gene of interest can be transduced into any mouse genetic background. Currently successful transduction of hACE2 expression has been accomplished in both BALB/c and C57BL/6 mice.39,47,94,112 In addition, some groups have transduced hACE2 expression into various knock-out strains, allowing the study of a specific gene, pathway, or cell type in SARS-CoV-2 infection.47,112
Table 1.
Viral vector–induced hACE2 mouse models of COVID-19
| Transduction vector | Strain background | Mouse age | Infection dose | Virus strain |
|---|---|---|---|---|
| AdV-hACE239 | BALB/c | 9 wk | 105 FFU intranasally | USA_WA1/2020 |
| C57BL/6 | ||||
| AdV-hACE2 under CMV promoter112 | BALB/c | 6 to 10 wk | 105 PFU intranasally | CHN/IQTC01/2020 |
| C57BL/6 | ||||
| IFNAR−/− C57BL/6 | ||||
| USA_WA1/2020 | ||||
| STAT−/− C57BL/6 | ||||
| IFNγ−/− C57BL/6 | ||||
| AdV-hACE2 under CMV promoter94 | BALB/c | 6 wk | 106 PFU intranasally | USA_WA1/2020 |
| C57BL/6 | ||||
| AAV9-hACE247 | C57BL/6 | 6 to 12 wk | 106 PFU intranasally | USA_WA1/2020 |
| IFNAR1−/− C57BL/6 | ||||
| IRF3/7−/− C57BL/6 |
Adenoviral and AAV mouse models of SARS-CoV-2 infection have many similar clinical features. In all cases, peak virus loads are detected between 1 and 2 DPI, and virus is present in bronchiolar and alveolar epithelium.39,47,94,112 In addition, most mice clear virus between 5 to 7 DPI.47,86,112 Detection of virus outside of the lungs is variable, and no disease has been reported outside of the respiratory tract.39,47,94,112
Despite a lack of clinical features in most viral vector model mice, significant lung pathologic findings are reported consistently. Congestion and inflammation, consisting predominantly of neutrophils and macrophages, are the most common findings.39,47,94,112 Two groups have also reported fibrin deposits and occasional hyaline membranes later in infection.39,112 These groups were the only ones to note clinical signs. One of these groups reported approximately 10% weight loss throughout infection,39 while the other reported 20% weight loss in BALB/c mice and a smaller but significant amount of weight loss in C57BL/6 mice.112 These studies also reported additional clinical signs, including hunched posture, ruffled coat, and difficulty breathing in both strains.39,112
A few studies have transduced hACE2 transduced into several knockout strains to better understand the role of interferon signaling in SARS-CoV-2 pathogenesis.47,112 One study found that interferon receptor signaling is essential for virus clearance.112 However, another group found that although Ifnar1−/− mice do have fewer infiltrating monocytes based on flow cytometry and reduced expression of interferon-stimulated genes, viral clearance is not significantly accelerated.47 This effect is not caused by a difference in mouse background, because all knockout mice in both studies had a C57BL/6J background. However, the different results from these 2 studies may be due to the specific genes that were eliminated. Both groups used Ifnar1−/− mice, but one also used Stat1−/− and Ifng−/− mice,112 whereas the other used Irf3−/−/Irf7−/− mice.47 STAT1, IFNγ, and IRF3/7 exhibit considerable crosstalk, but each also has distinguishing signaling pathways.47,112 In addition, these groups used different vectors to express hACE2, with one using AdV-hACE2, which is highly immunogenic, and the other using the less immunogenic AAV9-hACE2.47,112,120 Transduction efficiency is tissue- and vector-specific;43 therefore variability in tissue distribution, transduction efficiency, and expression level may account for the differences noted.
Transgenic hACE2 mouse models.
Because both SARS-CoV and SARS-CoV-2 use the same receptor for entry into host cells, the K18-hACE2 transgenic mouse has also been used to study SARS-CoV-2 pathogenesis and to test potential therapies (Table 2). One study directly compared AdV-hACE2 and K18-hACE2 mice and found that overall the K18-hACE2 model produces more severe clinical symptoms and higher viral loads.94 Compared with AdV-hACE2 mice, K18-hACE2 mice demonstrate 2- to 3-log (102–103) higher viral load in the lungs, although viral replication in pneumocytes and macrophages occurs in both models. In addition, the K18-hACE2 mice lose a significant amount of body weight, become lethargic, scruffy, and hunched, and exhibit labored breathing and occasional mortality. In contrast, AdV-hACE2 mice do not show any clinical signs but develop worse lung pathology than K18-hACE2 mice. However, the main finding of lung inflammation in AdV-hACE2 mice suggests that the discordance may be due to an immune response to the adenovirus vector rather than to SARS-CoV-2 infection.94
Table 2.
Transgenic hACE2 mouse models of COVID-19
| Gene construct | Strain background | Mouse age | Infection dose | Virus strain |
|---|---|---|---|---|
| K18-hACE294 | C57BL/6 | 6 wk | 104 PFU intranasally | USA_WA1/2020 |
| K18-hACE232 | C57BL/6 | 9 wk | 2 × 104 PFU intranasally | USA_WA1/2020 |
| 2 × 103 PFU intranasally | ||||
| K18-hACE2132 | C57BL/6 | 7 to 9 wk | 103 PFU intranasally | USA_WA1/2019 |
| 104 PFU intranasally | ||||
| 105 PFU intranasally | ||||
| mAce2-hACE25 | ICR | 6 to 11 mo | 105 TCID50 intranasally | Wuhan/IVDC-HB-01/2020 |
| HFH4-hACE249 | C57BL/6 | 8 to 10 wk | 3 × 104 TCID50 intranasally | IVCAS 6.7512 |
| CRISPR/Cas9; target mAce2, insert hACE2113 | C57BL/6 | 4.5 wk; 30 wk | 4 × 105 PFU intranasally | Wuhan/AMMS01/2020 |
TCID50, median tissue culture infectious dose
Like the work just described, another group that studied K18-hACE2 mice found significant weight loss and labored breathing and identified virus in pneumocytes and alveolar macrophages, confirming that SARS-CoV-2 replicates in appropriate cell types in this model.32 The cited study found severe lung pathology, inflammatory cell infiltrates, consolidation, alveolar septal thickening, fibrin deposits, and edema in the majority of mice. In addition, fibrin thrombi and significant neutrophilic inflammation, with a few mice displaying pneumocyte hyperplasia, suggestive of significant damage. This discordance in lung pathology severity between the two K18-hACE2 studies most likely is due to a slightly higher viral load (104 PFU compared with 2 × 104 PFU) or potentially a difference in hACE2 expression.76,86 In either case, the K18-hACE2 mouse model replicates key features of lung pathology caused by SARS-CoV-2 in humans.32
SARS-CoV replicates well in the brain of K18-hACE2 mice and causes significant disease.73,84 Similarly, several studies of SARS-CoV-2 infection in K18-hACE2 mice report virus in the brain, albeit in the absence of any significant clinical signs of neurologic disease.32,94 Peak viral loads in the brains of K18-hACE2 mice infected with SARS-CoV-2 occurred several days after peaks in the lungs, which also occurs in SARS-CoV, suggesting that the virus infects the brain similarly for these 2 viruses.32,73,84,94 In situ hybridization showed that neurons are the main cell type infected in the brain by both SARS-CoV and SARS-CoV-2.32,84 One group identified some pathologic changes in the brains of these mice at later time points, including mononuclear perivascular immune cells, vasculitis, microgliosis, and astrogliosis, and in some cases, meningitis, although no clinical signs were reported.32 Considering the difficulty and subtlety of interpreting clinical signs of neurologic disease in mice, such effects may have gone unnoticed by the investigators. Importantly, one study has reported anosmia in K18-hACE2 mice.132 The researchers suggest that the anosmia is caused by damage by inflammation to olfactory neurons and neuroepithelium rather than by direct viral infection.132 However, others have hypothesized that anosmia is due to direct infection of these cells, suggesting that this model could be used to study neuroinvasion of SARS-CoV-2.90 Even though hACE2 is overexpressed in many tissues, including the brain, and is ectopically expressed in cells or regions of tissue that typically do not express ACE2, K18-hACE2 mice may be a useful model to study the neuropathogenesis of SARS-CoV-2 infection of the CNS and the potential long-term effects on behavior and neurologic health.76,86
Because K18-hACE2 transgenic mice develop significant extrapulmonary disease that is not always seen in people infected with SARS-CoV-2, other groups have developed transgenic mice with hACE2 under the control of more specific promoters to study SARS-CoV-2 lung pathology (Table 2).5,49,113 In HFH4-hACE2 transgenic mice, hACE2 is targeted to ciliated cells.49 The majority of these mice do not develop signs of disease, but a small subset shows continued weight loss, difficulty breathing, and neurologic symptoms. Virus is found in bronchiolar and alveolar epithelium, and all mice develop interstitial pneumonia with inflammatory cell infiltration and alveolar septal thickening. In mice that clear the infection, pneumonia begins to abate at 5 DPI. In the mice that eventually succumb to infection, virus levels in the lung remain high through 7 DPI. Consistent with these findings, these mice develop increasingly severe interstitial pneumonia with extensive inflammatory cell infiltration, congestion, edema, hyaline membranes, and necrosis. These mice also show a significant decrease in circulating lymphocyte count in comparison with mice that recover, consistent with lethal COVID-19 cases in humans.49,116
In addition to respiratory disease, some mice develop extrapulmonary infection, such as detectable viral RNA in the heart, with concomitant mild cardiac edema and necrotic cardiomyocytes.49 Further, only mice that develop severe SARS-CoV-2 disease have virus detectable in the brain and develop neurologic symptoms.49 This is consistent with the hypothesis that SARS-CoV—and likely SARS-CoV-2 as well—travels to the brain transneuronally.84 Transneuronal virus spread from the olfactory bulb neurons to neurons in the brain and then replication to detectable levels takes longer than it does for the virus to move into the lungs in droplets and subsequently infect pneumocytes. However, these mice were infected intranasally with superphysiologic concentrations of virus in relatively high volumes. Furthermore, this high concentration of virus introduced directly into the nasal cavity perhaps facilitates transneuronal spread from the olfactory bulb. Other hypotheses regarding neuroinvasion by SARS-CoV-2 include spread from the lungs to the brain via the hematogenous route or via infected immune cells trafficking to the brain.65
Similar to previous mouse models of SARS-CoV and MERS-CoV infection in mice, several groups have developed models of SARS-CoV-2 that place hACE2 under the control of the mouse Ace2 promoter or that completely replace mAce2 with hACE2 (Table 2).5,89,113,126 Mice with hACE2 under the control of the mAce2 promoter experience mild clinical disease, including ruffled fur and peak weight loss (approximately 8% of initial body weight) at 5 DPI.5 Virus is detected in the lungs, specifically in macrophages, alveolar, and bronchiolar epithelium, peaking between 1 and 3 DPI and decreasing from 5 to 7 DPI. At 3 DPI, mice develop interstitial pneumonia with alveolar septal thickening, edema, and inflammatory cell infiltrates that include lymphocytes, neutrophils, and macrophages. Pneumonia worsens over time; at 5 DPI, mice develop fibrin deposits, increased inflammation, and bronchiolar and alveolar epithelial cell necrosis and sloughing. Virus is transiently found in the gastrointestinal tract, although gastrointestinal pathology has not been reported.5
One group developed a CRISPR/Cas9 knock-in model, in which hACE2 completely replaces mAce2 (Table 2).113 Therefore, as in the previous model, hACE2 is under the control of the mouse Ace2 promoter;5 however, these mice have significantly less mAce2 expression. Both 4.5 wk old and 30-wk-old female mice have been infected to determine age-related effects on SARS-CoV-2 pathology.113 Whereas young mice do not show any clinical signs, old mice show significant weight loss (approximately 10% of their original body weight) by 3 DPI before recovering to their original body weight. Similar virus titers are detected in the lungs of both young and old mice. Both young and old mice develop interstitial pneumonia with inflammatory cell infiltrates, alveolar septal thickening, and some vascular degeneration, but aged mice develop worse interstitial pneumonia than young mice, with greater macrophage and neutrophil infiltration.113
Although significant disease outside of the lungs has not been reported in aged mice, viral RNA has been detected in their feces, as reported in adult humans.61,122,125 Virus was also detected in the brains of these mice, with the S protein of SARS-CoV-2 identified in neurons, astrocytes, and microglia.32,113 Although differences in the expression of the receptor were not reported, the different methods of generating hACE2 expression in these mice probably results in somewhat different levels or cellular expression of hACE2 and thus variability in types of cell infected. Monocytes and macrophages in the lungs can harbor virus,70 although whether this is a result of direct infection or because of phagocytosis is unclear. Considering the similarities between other tissue macrophages and microglia, microglia may also be susceptible to infection by SARS-CoV-2.
Mouse-adapted SARS-CoV-2 mouse models.
Mouse adaptation of viruses has been a successful way to study SARS-CoV and MERS-CoV.19,26,57,58,96 Consequently, several groups have adapted SARS-CoV-2 for mouse infectivity (Table 3). The primary barrier to using standard inbred mouse strains to study SARS-CoV-2 is the inability of clinical (human) SARS-CoV-2 isolates to bind to mAce2 for cell entry. Therefore, one group used reverse genetics to identify 2 residues in the SARS-CoV-2 S protein—Q498 and P499—that were predicted to prevent close binding with mAce2.22 By changing these residues from glutamine and proline to tyrosine and threonine, respectively, this mouse-adapted SARS-CoV-2 strain, named SARS-CoV-2-MA, was able to infect and replicate in mouse lungs. Evaluation of SARS-CoV-2-MA in both young and aged mice has revealed that whereas young mice do not develop any clinical signs of disease and can completely clear virus by 4 DPI, aged mice experience significant weight loss by 3 to 4 DPI and demonstrate slower virus clearance.22
Table 3.
Mouse-adapted SARS-CoV-2 mouse models of COVID-19
| Mouse-adapted virus | Adaptation process | Challenge strain background | Mouse age | Infection dose | Original virus strain |
|---|---|---|---|---|---|
| MA22 | Reverse genetics; Q498Y/P499T S protein | BALB/c | 10 wk; 1 y | 105 PFU intranasally | USA_WA1/2020 |
| MA1055 | 10 intranasal passages in 10-wk-old BALB/c | BALB/c | 10 wk; 1 y | 104 PFU intranasally | USA_WA1/2020 |
| C57BL/6 | 10 wk | ||||
| 103 PFU intranasally | |||||
| IFNR DKO | 10 wk | ||||
| MASCp634 | 6 intranasal passages in 9-mo-old BALB/c | BALB/c | 6 wk; 9 mo | 1.6 × 104 PFU intranasally | CHN/Beijing_IME-BJ05/2020 |
Lung function after infection with SARS-CoV-2-MA has been measured via whole-body plethysmography, specifically using Rpef and PenH. PenH is an indication of airway function, measuring resistance to inhalation and exhalation, whereas Rpef is measured by determining the ratio of peak expiratory flow and time to exhale. An increase in PenH and a decrease in Rpef indicates virus-induced lung damage.75 In young mice infected with SARS-CoV-2-MA, a minor decrease in lung function as measured by a decrease in Rpef and an increase in PenH was found. These lung function deficiencies are more pronounced in aged mice, consistent with the observation of more severe clinical signs in aged mice. On histologic examination, all mice, regardless of age, showed inflammatory cell infiltrates with congestion and epithelial cell damage. In aged mice, however, these changes were more severe, with occasional edema and rare hemorrhage. Extrapulmonary pathology or viral replication has not been reported.22 Overall, SARS-CoV-2-MA produces mild pathology, and severe lung pathology does not develop in mouse strains infected with this virus.22,75
To create a more pathogenic mouse-adapted SARS-CoV-2 strain, the same group passaged SARS-CoV-2-MA intranasally in young BALB/c mice.55 After 10 passages, young BALB/c mice consistently developed more severe disease after intranasal inoculation with the new pathogenic strain, named SARS-CoV-2-MA10, than with the original SARS-CoV-2-MA strain.22,55 Intranasal infection with different concentrations of virus caused dose-dependent severity of morbidity and mortality. At 104 PFU, intranasal infection with SARS-CoV-2-MA10 caused significant weight loss by 4 DPI (approximately 16% loss from initial weight) and a mortality rate of 15% to 20%. Virus was detected in airway epithelial cells and alveolar pneumocytes in alveoli, but in contrast to humans, was also found in secretory club cells. In surviving mice, recovery begins at 5 DPI, with infectious virus in the lungs dropping below detectable levels by 7 DPI.55
Grossly, lungs showed a variety of changes, including gray, brown, or maroon discoloration, with distribution ranging from single or multifocal patches centered on lung hilar regions to widespread dissemination across all lobes.55 Histopathologic examination of lungs from mice infected with 104 PFU SARS-CoV-2-MA10 identified a variety of lesions throughout the course of the infection, including inflammatory cell infiltrates, bronchiolar epithelial degeneration and necrosis (not shown), bronchiolar cell hyperplasia, alveolar degeneration and necrosis, and protein and fibrin exudates (Figure 1). Congestion, alveolar septal thickening, and occasional hyaline membranes were also reported.55 The presence of these pathologic features is critical, as they are key findings in humans infected with SARS-CoV-2.9,25,38,70,78,101 Infected mouse lungs have also been evaluated with both the DAD scoring system and the American Thoracic Society’s scoring system for ALI; ALI and consequently DAD are common in human autopsy cases of COVID-19,9,25,38,78,101 and the representative scoring systems provide a more quantifiable metric of disease severity for research purposes.71,100,103 Therefore, using both metrics is important to determine the pathologic severity of COVID-19 in mice in a way that reflects human disease. DAD and ALI scores are significantly increased in SARS-CoV-2-MA10–infected mice throughout the duration of infection, with no signs of improvement in either of these scores at the end of the study (7 DPI).55 Furthermore, lung function in mice has been tested by using whole-body plethysmography (WBP), and, consistent with previous work, Rpef and PenH are both altered during infection, although to a greater degree after infection with SARS-CoV-2-MA10 as compared with the original mouse-adapted SARS-CoV-2-MA virus.22,55
Neither pathologic changes nor viral proteins have been detected in extrapulmonary organs, including the brain, after SARS-CoV-2-MA10 infection, although viral RNA has been detected at low levels in the heart.55 However, the brain has been sampled only at 2 DPI, and given that virus peaks in the brain at later time points in other SARS-CoV and SARS-CoV-2 mouse models, neuroinvasion might have occurred after the time of evaluation.32,73,84,94
SARS-CoV-2-MA10 has also been evaluated in aged BALB/c mice. For these experiments, a PFU of 103 was used to minimize mortality before 7 DPI. With this lower infection dose, aged mice developed clinical signs that were similar to those of young mice, although aged mice continued to lose weight throughout the course of infection, and only 15% of mice survived to 7 DPI. In agreement with these findings, virus was not cleared in these mice and remained detectable throughout the 7-d experiment. In addition, lung histopathology was markedly worse in these mice, and they showed more prolonged lung dysfunction.55 This pattern replicates the age-dependent exacerbation of disease severity seen in older human COVID-19 patients.
Finally, young, age-matched C57BL/6 and BALB/c mice have been infected with 104 PFU of SARS-CoV-2-MA10.55 C57BL/6 mice developed less severe disease than did young BALB/c mice, losing approximately 10% of their body weight by 2 to 3 DPI, in comparison with 16% in young BALB/c mice. In addition, no mortality occurred in C57BL/6 mice, with all mice recovering by 7 DPI. C57BL/6 mice also had less congestion in their lungs and lower scores for ALI and DAD. Similar decreases in lung function were seen at 3 DPI as measured by WBP, but lung function was restored by 5 DPI in C57BL/6 mice, whereas young BALB/c mice still had impaired lung function at 7 DPI.55 These findings indicate that disease severity varies by mouse background strain and suggests that host genetics influence SARS-CoV-2 susceptibility.
Mouse adaptation of a clinical isolate of SARS-CoV-2 has also been achieved through passage in aged mice.34 After 6 passages, infection produced virus and pathologic changes in the lungs, but no clinical signs were reported, and similar to other studies, lung pathology was more severe in aged mice than in young mice despite similar viral loads.34,110 However, by 3 DPI, all mice, regardless of age, developed interstitial pneumonia, characterized by alveolar septal thickening, inflammatory cell infiltrates that included dendritic cells, macrophages, and T lymphocytes, and epithelial cell death as evidenced by cell sloughing. In addition, hemorrhage was reported in some severe cases. At 5 DPI, evidence of pneumonia resolution consistent with decreasing viral loads was reported.34
No extrapulmonary pathology has been observed in these mice, although virus was detected in several extrapulmonary tissues, including liver, heart, kidney, brain, and gastrointestinal tract.34 In addition, virus was found in feces at all timepoints measured through 7 DPI, with aged mice showing higher virus titers than did young mice. In the liver, heart, and intestines, virus titers were similar in young and aged mice, with peak virus load detected at 3 DPI. Although virus was also detected at early time points in the kidneys of young mice, virus was not detected in the kidneys of aged mice. In addition, virus was found in the brain, but the dynamics diverged in young and aged mice. In young mice, virus was found only at 5 DPI and was not detected at earlier or later time points.34 This late peak in viral loads in the brain is similar to that seen in several other mouse models of SARS-CoV and SARS-CoV-2.32,73,84,94 However, in aged mice, virus was detectable at 3 DPI and levels were steady until the end of the study at 7 DPI.34 This evidence highlights the importance of age on viral effects in the brain and the potential for increased neuropathology in older patients.
Humanized mice and SARS-CoV-2.
Several studies of SARS-CoV-2 infection in humanized mice are underway, with one study recently published.119 A previous report showed that ectopic human lung transplants can replicate MERS-CoV.118 One group developed a humanized mouse model of SARS-CoV-2 by injecting immune-deficient mice subcutaneously with human lung tissue.119At 2 DPI, the human lung tissue showed pathology similar to that of COVID-19 patients, with DAD, fibrin deposits, and pneumocyte damage. The study also tested EIDD-2801 as a potential pre- and postinfection therapeutic. When given prophylactically, EIDD-2801 was protective against robust infection, and after infection, EIDD-2801 significantly reduced viral loads.119
The immune responses and pathology found in humans would be better replicated by using the same ectopic human lung mouse in immunodeficient bone marrow-liver-thymus (BLT) mice with humanized bone marrow, liver, and thymus than by using a transgenic mouse or by using a mouse-adapted virus.92 In addition, pluripotent human stem cells could potentially be injected with human embryonic stem cells that are fated to become lungs, resulting in an immune-competent mouse with human lungs. Such mice have been developed but have yet to be used for studying SARS-CoV-2.83
Working with humanized mice has several drawbacks. They are incredibly expensive, time-consuming, and labor-intensive to develop. In addition, mice with transplanted tissues first must be immunocompromised, and they demonstrate high prevalence of graft-versus-host disease, organ rejection, and mortality in the development stage. However, working with humanized mice may result in improved understanding of SARS-CoV-2 pathogenesis that better reflects the disease seen in humans.
Summary and Conclusions
Mice are an essential resource for studying various human diseases. However, in the case of the highly pathogenic betacoronaviruses SARS-CoV, MERS-CoV, and SARS-CoV-2, wild-type mice are not an ideal model, because they do not replicate these viruses well and consequently do not develop severe disease.1,15,17,98,133 Several mouse strains have been developed to circumvent this issue. Many of the broad strategies used to develop these models had previously been used to develop mouse models of SARS and MERS, allowing rapid application to SARS-CoV-2 modeling.3,19,26,57,58,73,84,89,96,103,116,126,129 Currently available mouse models of SARS-CoV-2 infection presented here either induce expression of the human ACE2 receptor or adapt the virus to infect mice either by reverse genetics, serial passage, or a combination of the 2 methodologies. All of these models allow the virus to replicate to levels that cause disease, and each model has advantages and disadvantages for studying SARS-CoV-2 (Table 4).
Table 4.
Summary of available mouse models of SARS-CoV-2 pathogenesis
| Model | Clinical disease | Extrapulmonary disease | Pros | Cons | References |
|---|---|---|---|---|---|
| Viral vectors | Occasional weight loss Lung pathology |
Virus occasionally detected in liver, heart, kidney, GI, and brain No significant pathology |
Ability to express hACE2 in any background Ability to generate new hACE2 mice quickly Can use human virus isolates |
Adenovirus-induced immune responses Patchy gene transduction Expression time limited |
39, 47, 94, 112 |
| Transgenic hACE2 | Consistent clinical signs including weight loss Mortality Significant lung pathology |
Virus consistently detected in brain with occasional pathology Virus occasionally detected in GI tract and heart with no or mild pathology |
Develops consistent severe lung pathology Widely available Can use human virus isolates |
Significant mortality due to CNS disease that isn’t seen in humans hACE2 expression outside of what is seen in humans |
5, 32, 49, 94, 113, 132 |
| Mouse-adapted virus | Occasional weight loss Significant morbidity and mortality with MA10 infection Significant lung pathology |
Virus occasionally detected in liver, heart, kidney, GI tract, and brain No significant pathology |
Ability to use any mouse strain Replicates severe disease with MA10 No alterations to mice |
Cannot use human virus isolates Do not cause severe disease outside of passage mouse strain |
22, 34, 55 |
As the pandemic has progressed, more variants of SARS-CoV-2 have emerged. In particular, variants B.1.1.7, B.1.526, and B.1.351 contain mutations in the S protein, raising the question of whether vaccines will be effective against these strains.117 Vaccine breakthrough infections have been reported in persons infected with B.1.1.7 and B.1.526.35 Furthermore, monoclonal antibody therapy, a treatment that has been effective at reducing morbidity and mortality in patients infected with SARS-CoV-2, has not been effective in patients infected with variants B.1.1.7 and B.1.351. This situation is not wholly surprising, given that the monoclonal antibodies are directed toward specific residues of the S protein of the original SARS-CoV-2 strain.44 Consequently, studying the variants to better characterize vaccine breakthrough and disease caused by these variants is essential. Work evaluating these variants is already underway using mouse models, with one study showing that a bispecific antibody effectively prevents disease and virus escape mutations in mice.20 However, to study these variants in mice, either mice must be transgenic for hACE2 or induced with hACE220 or the variant must be adapted to mice while maintaining key mutations. Because the mutations are primarily in the S protein, this may be particularly challenging, as changes to the S protein occur during mouse adaptation to facilitate binding to mAce2.44,63,117
Although essential to the study of human disease pathogenesis, no animal model will replicate every aspect of human disease. With regard to SARS-CoV-2 pathogenesis, mice usually do not have the same underlying diseases and comorbidities, including cardiovascular disease, obesity, diabetes, and chronic lung disease, as do many patients with severe COVID-19;27 using mouse models to evaluate the effect of SARS-CoV-2 infection in the context of many of these underlying diseases and comorbidities is underway. In addition, sex-associated differences between male and female COVID-19 patients are less clear in mouse models. Differences in disease outcome related to sex have either not been mentioned or found in the mouse studies currently published,49 with the exception of one study in which female mice were reported to experience higher rates of morbidity and mortality than did male mice.32 The lack of reported sex differences in mice may be due to experimental factors such as small study numbers, or inability to specifically evaluate the effect of sex because only a single sex was used. Another possibility is that the sex disparities observed in infected humans are unique to primates. In addition, patients with chronic SARS-CoV-2–related ARDS who are on a ventilator will have very different lung pathophysiology and resulting histopathologic changes than will a mouse that died within a week of SARS-CoV-2 infection. Although many of the mouse models can replicate age-dependent severity as occurs in humans, the pathology seen in these models will not replicate every aspect of human pathology.
Current mouse models are a powerful tool to study acute viral infection and how SARS-CoV-2 infection causes disease in lung and extrapulmonary organs and can also be used to study chronic effects of SARS-CoV-2 infection, although no such studies have yet been published. Infecting mice that model other human diseases, such as heart disease, diabetes, and Alzheimer disease, with SARS-CoV-2 may be informative for understanding the complex interplay between the virus and chronic underlying diseases in humans. Although mouse models, like any animal model, cannot replicate every aspect of human disease, they nonetheless allow the scientific interrogation of critical pathways of SARS-CoV-2 pathogenesis and have already proven effective in identifying effective therapeutics and vaccines.
Acknowledgments
We thank Dr Mark Heise for providing tissue samples for representative images of SARS-CoV-2-MA10–induced lung pathology. This work was supported by NIH grants R01AI157253, U19AI100625, U01AI149644, and UL1TR002489, University of North Carolina at Chapel Hill School of Medicine ECBR_014, and North Carolina Policy Collaboratory. Additional support was provided by NIH grants T32AI007151 (to ACK) and K01OD026529 (to VKB). Histopathology services were performed by the Animal Histopathology & Laboratory Medicine Core at the University of North Carolina, which is supported in part by an NCI Center Core Support Grant (5P30CA016086-41) to the UNC Lineberger Comprehensive Cancer Center.
References
- 1. Abdel-Moneim AS, Abdelwhab EM. 2020. Evidence for SARS-CoV-2 infection of animal hosts. Pathogens 9: 1– 22. 10.3390/pathogens9070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adam EH, Zacharowski K, Miesbach W. 2020. A comprehensive assessment of the coagulation profile in critically ill COVID-19 patients. Thromb Res 194: 42– 44. 10.1016/j.thromres.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agrawal AS, Garron T, Tao X, Peng BH, Wakamiya M, Chan TS, Couch RB, Tseng CT. 2015. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol 89: 3659– 3670. 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ali R, Ghonimy M. 2020. Radiological findings spectrum of asymptomatic coronavirus (COVID-19) patients. EJRNM 51: 156. 10.1186/s43055-020-00266-3. [DOI] [Google Scholar]
- 5. Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, Qu Y, Li F, Lv Q, Wang W, Xue J, Gong S, Liu M, Wang G, Wang S, Song Z, Zhao L, Liu P, Zhao L, Ye F, Wang H, Zhou W, Shu N, Zhen W, Yu H, Zhang X, Guo L, Chen L, Wang C, Wang Y, Wang X, Xiao Y, Sun Q, Liu H, Zhu F, Ma C, Yan L, Yang M, Han J, Xu W, Tan W, Peng X, Jin Q, Wu G, Qin C. 2020. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583: 830– 833. 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 6. Benussi A, Pilotto A, Premi E, Libri I, Giunta M, Agosti C, Alberici A, Baldelli E, Benini M, Bocacina S, Brambilla L, Caratozzolo S, Cortinovis M, Costa A, Cotti Piccinelli S, Cottini E, Cristillo V, Delrio I, Filosto M, Gamba M, Gazzina S, Gilberti N, Gipponi S, Imarisio A, Invernizzi P, Leggio U, Leonardi M, Liberini P, Locateli M, Masciocchi S, Poli L, Rao R, Risi B, Rozzini L, Scalvini A, Schiano di Cola F, Spezi R, Vergani V, Volonghi I, Zoppi N, Borroni B, Magoni M, Pezzini A, Padovani A. 2020. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology 95: e910– e920. 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 7. Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jager HR, Losseff NA, Perry RJ, Shah S, Simister RJ, Turner D, Chandratheva A, Werring DJ. 2020. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 91: 889– 891. 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, Dongelmans DA, Hollmann MW, Horn J, Vlaar APJ, Schultz MJ, Neto AS, Paulus F, PRoVENT-COVID Collaborative Group . 2021. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicenter, observational cohort study. Lancet Respir Med 9: 139– 148. 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. 2020. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 396: 320– 332. 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown EG. 1990. Increased virulence of a mouse-adapted variant of influenza A/FM/1/47 virus is controlled by mutations in genome segments 4, 5, 7, and 8. J Virol 64: 4523– 4533. 10.1128/jvi.64.9.4523-4533.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao X. 2020. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20: 269– 270. 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai JP, Kok KH, Chu H, Chan KH, Sridhar S, Chen Z, Chen H, To KK, Yuen KY. 2020. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in a golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clin Infect Dis 71: 2428– 2446. 10.1093/cid/ciaa644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Li X, Chen M, Feng Y, Xiong C. 2020. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 116: 1097– 1100. 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507– 513. 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cockrell AS, Peck KM, Yount BL, Agnihothram SS, Scobey T, Curnes NR, Baric RS, Heise MT. 2014. Mouse dipeptidyl peptidase 4 is not a functional receptor for Middle East respiratory syndrome coronavirus infection. J Virol 88: 5195– 5199. 10.1128/JVI.03764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cockrell AS, Young BL, Scobey T, Jensen K, Douglas M, Beall A, Tang XC, Marasco WA, Heise MT, Baric RS. 2016. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol 2: 1– 11. 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coleman CM, Matthews KL, Goicochea L, Frieman MB. 2014. Wild-type and innate immune-deficient mice are not susceptible to the Middle East respiratory syndrome coronavirus. J Gen Virol 95: 408– 412. 10.1099/vir.0.060640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui J, Li F, Shi ZL. 2019. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17: 181– 192. 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Day CW, Baric R, Cai SX, Frieman M, Jumaki Y, Morrey JD, Smee DF, Barnard DL. 2009. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology 395: 210– 222. 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Gasparo R, Pedotti M, Simonelli L, Nickl P, Muecksch F, Cassaniti I, Percivalle E, Lorenzi JCC, Mazzola F, Magri D, Michalcikova T, Haviemik J, Honig V, Mrazkova B, Polakova N, Fortova A, Tureckova J, Iatsiuk V, Di Girolamo S, Palus M, Zudova D, Bednar P, Bukova I, Bianchini F, Mehn D, Nencka R, Strakova P, Pavlis O, Rozman J, Gioria S, Sammartino JC, Giardina F, Gaiarsa S, Pan-Hammarstrom Q, Barnes CO, Bjorkman PJ, Calzolai L, Piralla A, Baldanti F, Nussenzweig MC, Bieniasz PD, Hatziioannou T, Prochazka J, Sedlacek R, Robbiani DF, Ruzek D, Varani L. 2021. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice. Nature 593: 424– 428. 10.1038/s41586-021-03461-y. [DOI] [PubMed] [Google Scholar]
- 21. Deigendesch N, Sironi L, Kutza M, Wischnewski S, Fuchs V, Hench J, Frank A, Nienhold R, Mertz KD, Cathomas G, Matter MS, Siegmund M, Tolnay M, Schirmer L, Probstel AK, Tzankov A, Frank S. 2020. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol 140: 583– 586. 10.1007/s00401-020-02213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dinnon KH, 3rd, Leist SR, Schafer A, Edwards CE, Martinez DR, Montgomery SA, West A, Yount BL, Jr, Hou YJ, Adams LE, Gully KL, Brown AJ, Huang E, Bryant MB, Choong IC, Glenn JS, Gralinski LE, Sheahan TP, Baric RS. 2020. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586: 560– 566. 10.1038/s41586-020-2708-8. Correction. Nature 2021.590:E22. [DOI] [PubMed] [Google Scholar]
- 23. Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernandez M, Gea A, Arruti E, Aldecoa C, Martinez-Palli G, Martinez-Gonzalez MA, Slutsky AS, Villar J, COVID-Spanish ICU Network . 2020. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 46: 2200– 2211. 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernandez M, Gea A, Arruti E, Aldecoa C, Martinez-Palli G, Martinez-Gonzalez MA, Slutsky AS, Villar J, COVID-Spanish ICU Network . 2021. Correction to: Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 47: 144– 146. 10.1007/s00134-020-06251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Wander Heide RS. 2020. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 8: 681– 686. 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frieman M, Yount B, Agnihothram S, Page C, Donaldson E, Roberts A, Vogel L, Woodruff B, Scorpio D, Subbarao K, Baric RS. 2012. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J Virol 86: 884– 897. 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. 2020. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019-COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 69: 458– 464. 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaudreault NN, Trujillo JD, Carossino M, Meekins DA, Morozov I, Madden DW, Indran SV, Bold D, Balaraman V, Kwon T, Artiaga BL, Cool K, Garcia-Sastre A, Ma W, Wilson WC, Henningson J, Balasuriya UBR, Richt JA. 2020. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect 9: 2322– 2332. 10.1080/22221751.2020.1833687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. 2020. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ 11: 1– 13. 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gibson PG, Qin L, Puah SH. 2020. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 213: 54– 56.e1. 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gold JAW, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, Natarajan P, Morris SB, Fanfair RN, Rogers-Brown J, Bruce BB, Browning SD, Hernandez-Romieu AC, Furukawa NW, Kang M, Evans ME, Oosmanally N, Tobin-D’Angelo M, Drenzek C, Murphy DJ, Hollberg J, Blum JM, Jansen R, Wright DW, Sewell WM, 3rd, Owens JD, Lefkove B, Brown FW, Burton DC, Uyeki TM, Bialek SR, Jackson BR. 2020. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19-Georgia, March 2020. MMWR Morb Mortal Wkly Rep 69: 545– 550. 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Golden JW, Cline CR, Zeng X, Garrison AR, Carey BD, Mucker EM, White LE, Shamblin JD, Brocato RL, Liu J, Babka AM, Rauch HB, Smith JM, Hollidge BS, Fitzpatrick C, Badger CV, Hooper JW. 2020. Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. JCI Insight 5: 1– 14. 10.1172/jci.insight.142032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Griffin DO, Jensen A, Khan M, Chin J, Chin K, Saad J, Parnell R, Awwad C, Patel D. 2020. Pulmonary embolism and increased levels of d-dimer in patients with coronavirus disease. Emerg Infect Dis 26: 1941– 1943. 10.3201/eid2608.201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gu H, Chen Q, Yang G, He L, Fan H, Deng YQ, Wang Y, Teng Y, Zhao Z, Cui Y, Li Y, Li XF, Li J, Zhang NN, Yang X, Chen S, Guo Y, Zhao G, Wang X, Luo DY, Wang H, Yang X, Li Y, Han G, He Y, Zhou X, Geng S, Sheng X, Jiang S, Sun S, Qin CF, Zhou Y. 2020. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 369: 1603– 1607. 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, Schaefer-Babajew DJ, DaSilva J, Muecksch F, Gaebler C, Lifton R, Nussenzweig MC, Hatziioannou T, Bieniasz PD, Darnell RB. 2021. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 384: 2212– 2218. 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Halfmann PJ, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, Kinoshita N, Hattori SI, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Kawaoka Y. 2020. Transmission of SARS-CoV-2 in domestic cats. N Engl J Med 383: 592– 594. 10.1056/NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. 2004. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631– 637. 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdoirasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. 2020. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19; a post-mortem study. Lancet Microbe 1: e245– e253. 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hassan AO, Case JB, Winkler ES, Thackray LB, Kafai NM, Bailey AL, McCune BT, Fox JM, Chen RE, Aloussi WB, Turner JS, Schmitz AJ, Lei T, Hrihari S, Keeler S, Fremont SP, Greco DH, McCray S, Jr, Perlman PB, Holtzman S, Ellebedy MJ, Diamond AH MS. 2020. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 182: 744– 753.e4. 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. 2020. Neurologic features in severe SARS-Cov-2 infection. N Engl J Med 382: 2268– 2270. 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Helms J, Kremer S, Merdji H, Schenck M, Sevarac F, Clere-Jehl R, Studer A, Radosavljevic M, Kummerlen C, Monnier A, Boulay C, Fafi-Kramer S, Castelain V, Ohana M, Anheim M, Schneider F, Meziani F. 2020. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care 24: 1– 11. 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrier T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271– 280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsu JC, Di Pasquale G, Harunaga JS, Onodera T, Hoffman MP, Chiorini JA, Yamada KM. 2011. Viral gene transfer to developing mouse salivary glands. J Dent Res 91: 197– 202. 10.1177/0022034511429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu J, Peng P, Wang K, Fang L, Luo FY, Jin AS, Liu BZ, Tang N, Huang AL. 2021. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell Mol Immunol 18: 1061– 1063. 10.1038/s41423-021-00648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang D, Lian X, Song F, Ma H, Lian Z, Liang Y, Qin T, Chen W, Wang S. 2020. Clinical features of severe patients infected with 2019 novel coronavirus: a systematic review and meta-analysis. Ann Transl Med 8: 576. 10.21037/atm-20-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ioannou GN, Locke E, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Eastment MC, Dominitz JA, Fan VS. 2020. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 3: e2022310. 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Israelow B, Song E, Mao T, Lu P, Meir A, Liu F, Alfajaro MM, Wei J, Dong H, Homer RJ, Ring A, Wilen CB, Iwasaki A. 2020. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med 217: 1– 10. 10.1084/jem.20201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jäckel M, Berntgen X, Wengenmayer T, Bode C, Biever PM, Staudacher DL. 2020. Is delirium a specific complication of viral acute respiratory distress syndrome? Crit Care 24: 1– 4. 10.1186/s13054-020-03136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, Li Q, Zhang L, Zhu Y, Si HR, Wang Q, Min J, Wang X, Zhang W, Li B, Zhang HJ, Baric RS, Zhou P, Yang XL, Shi ZL. 2020. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182: 50– 58.e8. 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruis-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Leutkemeyer AF, Cohen Sh, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Goepfert P, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH. 2021. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med 384: 795– 807. 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, Kaya Y, Yildirim D, Tuzuner F, Yildirim MS, Ozluk E, Gucyetmez B, Karaarslan E, Koyluoglu I, Demirel Kaya HS, Mammadov O, Kisa O, Zdemir I, Afsar N, Citci Yalcinkaya B, Rasimoglu S, Guduk DE, Kedir Jima A, Ilksoz A, Ersoz V, Yonca Eren M, Celtik N, Arslan S, Korkmazer B, Dincer SS, Gulek E, Dikmen I, Yazici M, Unsal S, Ljama T, Demirel I, Ayyildiz A, Kesimci I, Bolsoy Deveci S, Tutuncu M, Kizilkilic O, Telci L, Zengin R, Dincer A, Akinci IO, Kocer N. 2020. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology 297: E232– E235. 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, Um J, Song MS, Jeong HW, Lai VD, Kim Y, Chin BS, Park JS, Chung KH, Foo SS, Poo H, Mo IP, Lee OJ, Webby Rj, Jung JU, Choi YK. 2020. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27: 704– 709.e2. 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjilali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. 2020. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277: 2251– 2261. 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, He TC. 2017. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis 4: 43– 63. 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leist SR, Dinnon KH, 3rd, Schafer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, Gully KL, Scobey T, Brown AJ, Sheahan TP, Moorman NJ, Boucher RC, Gralinski LE, Montgomery SA, Baric RS. 2020. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183: 1070– 1085.e12. 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Letko M, Marzi A, Munster V. 2020. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5: 562– 569. 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li K, Li Z, Wohlford-Lenane C, Meyerholz DK, Channappanavar R, An D, Perlman S, McCray PB, Jr, He B. 2020. Single-dose, intranasal immunization with recombinant parainfluenza virus 5 expressing Middle East Respiratory Syndrome Coronavirus (MERS-CoV) spike protein protects mice from fatal MERS- CoV infection. MBio 11: 1– 12. 10.1128/mBio.00554-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li K, McCray PB, Jr. 2019. Development of a mouse-adapted MERS coronavirus. Methods Mol Biol 2099: 161– 171. 10.1007/978-1-0716-0211-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li K, Wohlford-Lenane CL, Channappanavar R, Park JE, Earnest JT, Bair TB, Bates AM, Brogden KA, Flaherty HA, Gallagher T, Meyerholz DK, Perlman S, McCray PB, Jr. 2017. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci USA 114: E3119– E3128. 10.1073/pnas.1619109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li W, Moor MJ, Vasilleva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450– 454. 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. 2020. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 69: 997– 1001. 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 62. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. 2020. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55: 1– 10. 10.1016/j.ebiom.2020.102763. Corrected. EBioMedicine 2021. 66:103295. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Z, VanBlargan LA, Bloyet LM, Rothlauf PW, Chen RE, Stumpf S, Zhao H, Errico JM, Theel ES, Liebeskind MJ, Alford B, Buchser WJ, Ellebedy AH, Fremont DH, Diamond MS, Whelan SPJ. 2021. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe 29: 477– 488.e4. 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lomoro P, Verde F, Zerboni F, Simonetti I, Borghi C, Fachinetti C, Natalizi A, Martegani A. 2020. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: a single-center study and comprehensive radiologic literature review. Eur J Radiol Open 7: 1– 11. 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lou JJ, Movassaghi M, Gordy D, Olson MG, Zhang T, Kjurana MS, Chen Z, Perez-Rosendahl M, Tammachantha S, Singer EJ, Magaki SD, Vinters HV, Yong WH. 2021. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol 2: 1– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lu S, Zhao Y, Yu W, Yang Y, Gao J, Wang J, Kuang D, Yang M, Yang J, Ma C, Xu J, Qian X, Li H, Zhao S, Li J, Wang H, Long H, Zhou J, Luo F, Ding K, Wu D, Zhang Y, Dong Y, Liu Y, Zheng Y, Lin X, Jiao L, Zheng H, Dai Q, Sun Q, Hu Y, Ke C, Liu H, Peng X. 2020. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct Target Ther 5: 157. 10.1038/s41392-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. 2020. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 220: 1– 13. 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mahdy MAA, Younis W, Weaida Z. 2020. An Overview of SARS-CoV-2 and Animal Infection. Front Vet Sci 7: 1– 12. 10.3389/fvets.2020.596391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. 2020. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77: 683– 690. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, Goldsmith CS, Silva-Flannery L, Seixas JN, Reagan-Steiner S, Uyeki T, Denison A, Bhatnagar J, Shieh WJ, Zaki SR, COVID-Pathology Working Group. 2020. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis 26: 2005– 2015. 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MW, Slutsky AS, Kuebler WM, Acute Lung Injury in Animals Study Group . 2011. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44: 725– 738. 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McAloose D, Laverack M, Wang L, Killian ML, Caserta LC, Yuan F, Mitchell PK, Queen K, Mauldin MR, Cronk BD, Bartlett SL, Sykes JM, Zec S, Stokol T, Ingerman K, Delaney MA, Frederickson R, Ivancic M, Jenkins-Moore M, Mozingo K, Franzen K, Bergeson NH, Goodman L, Wang H, Fang Y, Olmstead C, McCann C, Thomas P, Goodrich E, Elvinger F, Smith DC, Tong S, Slavinski S, Calle PP, Terio K, Torchetti MK, Diel DG. 2020. From People to Panthera: Natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. MBio 11: 1– 13. 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McCray PB, Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. 2007. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81: 813– 821. 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brunink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rossler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Shulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Kortvelyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. 2021. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24: 168– 175. 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 75. Menachery VD, Gralinski LE, Baric RS, Ferris MT. 2015. New metrics for evaluating viral respiratory pathogenesis. PLoS One 10: e0131451. 10.1371/journal.pone.0131451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Menachery VD, Young VD, Jr, Sims AC, Debbink K, Agnihothram SS, Gralinski LE, Graham RL, Scobey T, Plant JA, Royal SR, Swanstrom J, Sheahan TP, Pickles RJ, Corti D, Randell SH, Lanzavecchia A, Marasco WA, Baric RS. 2016. SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci USA 113: 3048– 3053. 10.1073/pnas.1517719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Meng H, Xiong R, He R, Lin W, Hao B, Zhang L, Lu Z, Shen X, Fant T, Jiang W, Yang W, Li T, Chen J, Geng Q. 2020. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect 81: e33– e39. 10.1016/j.jinf.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Billi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. 2020. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 77: 198– 209. 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Miesbach W, Makris M. 2020. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost 26: 1– 7. 10.1177/1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mohanty SK, Satapathy A, Naidu MM, Mukhopadhyay S, Sharma S, Barton LM, Stroberg E, Duval EJ, Pradhan D, Tzankov A, Parwani AV. 2020. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19)—anatomic pathology perspective on current knowledge. Diagn Pathol 15: 1– 17. 10.1186/s13000-020-01017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Molenaar RJ, Vreman S, Hakze-van der Honig RW, Zwart R, de Rond J, Weesendorp E, Smit LAM, Koopmans M, Bouwstra R, Stegeman A, van der Poel WHM. 2020. Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovision vison). Vet Pathol 57: 653– 657. 10.1177/0300985820943535. [DOI] [PubMed] [Google Scholar]
- 82. Molnar Z, Bakker J. 2020. Attenuating hyperinflammation in COVID-19: A change in paradigm? J Crit Care 60: 334– 336. 10.1016/j.jcrc.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mori M, Furuhashi K, Danielsson JA, Hirata Y, Kakiuchi M, Lin CS, Ohta M, Riccio P, Takahashi Y, Xu X, Emala CW, Lu C, Nakauchi H, Cardoso WV. 2019. Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells. Nat Med 25: 1691– 1698. 10.1038/s41591-019-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. 2008. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82: 7264– 7275. 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ojha V, Mani A, Pandey NN, Sharma S, Kumar S. 2020. CT in coronavirus disease 2019 (COVID-19): a systematic review of chest CT findings in 4410 adult patients. Eur Radiol 30: 6129– 6138. 10.1007/s00330-020-06975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ostrowski LE, Hutchins JR, Zakel K, O’Neal WK. 2003. Targeting expression of a transgene to the airway surface epithelium using a ciliated cell-specific promoter. Mol Ther 8: 637– 645. 10.1016/S1525-0016. [DOI] [PubMed] [Google Scholar]
- 87. Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, van der Spek A, Tolsma P, Rietveld A, Brouwer M, Bouwmeester-Vincken N, Harders F, Hakze-van der Honing R, Wegdam-Blans MCA, Bouwstra RJ, GeurtsvanKessel C, van der Eijk AA, Velkers FC, Smit LAM, Stegeman A, van der Poel WHM, Koopmans MPG. 2021. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371: 172– 177. 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L. 2020. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 30: 3306– 3309. 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pascal KE, Coleman CM, Mujica AO, Kamat V, Badithe A, Fairhurst J, Hunt C, Strein J, Berrebi A, Sisk JM, Matthews KL, Babb R, Chen G, Lai KM, Huang TT, Olson W, Yancopoulos GD, Stahl N, Frieman MB, Kyratsous CA. 2015. Pre- and postexposure efficacy of fully human antibodies against spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci USA 112: 8738– 8743. 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Politi LS, Salsano E, Grimaldi M. 2020. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 77: 1028– 1029. 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 91. Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. 2020. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta 510: 475– 482. 10.1016/j.cca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pujhari S, Rasgon JL. 2020. Mice with humanized-lungs and immune system – an idealized model for COVID-19 and other respiratory illness. Virulence 11: 486– 488. 10.1080/21505594.2020.1763637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. 2013. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495: 251– 254. 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rathnasinghe R, Strohmeier S, Amanat F, Gillespie VL, Krammer F, Garcia-Sastre A, Coughlan L, Schotsaert M, Uccellini MB. 2020. Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect 9: 2433– 2445. 10.1080/22221751.2020.1838955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Richard M, Kok A, de Meulder D, Bestebroer TM, Lamers MM, Okba NMA, Fentener van Vlissingen M, Rockx B, Haagmans BL, Koopmans MPG, Fouchier RAM, Herfst S. 2020. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun 11: 1– 6. 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roberts A, Deming D, Paddock CD, Cheng A, Yount B, Vogel L, Herman BD, Sheahan T, Heise M, Genrich GL, Zaki SR, Baric R, Subbarao K. 2007. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog 3: 0023– 0037. 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Roberts A, Paddock C, Vogel L, Butler E, Zaki S, Subbarao K. 2005. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol 79: 5833– 5838. 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saponaro F, Rutigliano G, Sestito S, Bandini L, Storti B, Bizzarri R, Zucchi R. 2020. ACE2 in the era of SARS-CoV-2: controversies and novel perspectives. Front Mol Biosci 7: 1– 25. 10.3389/fmolb.2020.588618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schaefer IM, Padera RF, Solomon IH, Kanjilal S, Hammer MM, Hornick JL, Sholl LM. 2020. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol 33: 2104– 2114. 10.1038/s41379-020-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schmidt ME, Knudson CJ, Harwig SM, Pewe LL, Meyerholz DK, Langlois RA, Harty JT, Varga SM. 2018. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog 14: 1– 24. 10.1371/journal.ppat.1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N. 2020. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol 154: 190– 200. 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. 2020. Structural basis of receptor recognition by SARS-CoV-2. Nature 581: 221– 224. 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ, Montgomery SA, Hogg A, Baubsis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baris RS. 2020. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 11: 1– 14. 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368: 1016– 1020. 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, Kaewpreedee P, Perera RAPM, Poon LLM, Nicholls JM, Peiris M, Yen HL. 2020. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583: 834– 838. 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Signorelli C, Odone A. 2020. Age-specific COVID-19 case-fatality rate: no evidence of changes over time. Int J Public Health 65: 1435– 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sit THC, Brackman CJ, Ip SM, Tam KWS, Law PYT, To EMW, Yu VYT, Sims LD, Tsang DNC, Chu DKW, Perera RAPM, Poon LLM, Peiris M. 2020. Infection of dogs with SARS-CoV-2. Nature 586: 776– 778. 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. 2020. Neuropathological features of COVID-19. N Engl J Med 383: 989– 992. 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D’Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK, BACC Bay Tozilizumab Trial Investigators . 2020. Efficacy of Tocilizumab in patients hospitalized with COVID-19. N Engl J Med 383: 2333– 2344. 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. 2020. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219– 227. 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Subbarao K, Roberts A. 2006. Is there an ideal animal model for SARS? Trends Microbiol 14: 299– 303. 10.1016/j.tim.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sun J, Zhuang Z, Zheng J, Li K, Wong RL, Liu D, Huang J, He J, Zhu A, Zhao J, Li X, Xi Y, Chen R, Alshukairi AN, Chen Z, Zhang Z, Chen C, Huang X, Li F, Lai X, Chen D, Wen L, Zhuo J, Zhang Y, Wang Y, Huang S, Dai J, Shi Y, Zheng K, Leidinger MR, Chen J, Li Y, Zhong N, Meyerholz DK, McCray PB, Jr, Perlman S, Zhao J. 2020. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell 182: 734– 743.e5. 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sun SH, Chen Q, Gu HJ, Yang G, Wang YX, Huang XY, Liu SS, Zhang NN, Li XF, Ziong R, Guo Y, Deng YQ, Huang WJ, Liu Q, Liu QM, Shen YL, Zhou Y, Yang X, Zhao TY, Fan CF, Zhou YS, Qin CF, Wang YC. 2020. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 28: 124– 133.e4. 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. The Lancet Respiratory Medicine. 2020. COVID-19 transmission-up in the air. Lancet Respir Med 8: 1159. 10.1016/S2213-2600(20)30514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, O Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP, Coalition COVID-Brazil III Investigators. 2020. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 324: 1307– 1316. 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tseng CT, Huang C, Newman P, Wang N, Narayanan K, Watts DM, Makino S, Packard MM, Zaki SR, Chang TS, Peters CJ. 2007. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J Virol 81: 1162– 1173. 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. van Oosterhout C, Hall N, Ly H, Tyler KM. 2021. COVID-19 evolution during the pandemic – Implications of new SARS-CoV-2 variants on disease control and public health policies. Virulence 12: 507– 508. 10.1080/21505594.2021.1877066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wahl A, De C, Abad Fernandez M, Lenarcic EM, Xu Y, Cockrell AS, Cleary RA, Johnson CE, Schramm NJ, Rank LM, Mewsome IG, Vincent HA, Sanders W, Aguilera-Sandoval CR, Boone A, Hildebrand WH, Dayton PA, Baric RS, Pickles RJ, Braunstein M, Moorman JN, Goonetilleke N, Victor Garcia JV. 2019. Precision mouse models with expanded tropism for human pathogens. Nat Biotechnol 37: 1163– 1173. 10.1038/s41587-019-0225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wahl A, Gralinski LE, Johnson CE, Yao W, Kovarova M, Dinnon KH, 3rd, Liu H, Madden VJ, Kryztek HM, De C, White KK, Gully K, Schafer A, Zaman T, Leist SR, Grant PO, Blumling GR, Kolykhalov AA, Natchus MG, Askin FB, Painter G, Browne EP, Jones CD, Pickles RJ, Baric RS, Garcia JV. 2021. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 591: 451– 457. 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang AY, Peng PD, Ehrhardt A, Storm TA, Kay MA. 2004. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther 15: 405– 413. 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 121. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061– 1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. 2020. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323: 1843– 1844. 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. World Health Organization. [Internet] 2021. WHO coronavirus disease (COVID-19) dashboard. [Cited April 2021]. Available at: covid19.who.int
- 124. Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, Sheng J, Quan L, Xia Z, Tan W, Cheng G, Jiang T. 2020. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 27: 325– 328. 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. 2020. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158: 1831– 1833.e3. 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang XH, Deng W, Tong Z, Liu YX, Zhang LF, Zhu H, Gao H, Huang L, Liu YL, Ma CM, Xu YF, Ding MX, Deng HK, Qin C. 2007. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med 57: 450– 459. [PubMed] [Google Scholar]
- 127. Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, Fontanella MM. 2020. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 162: 1491– 1494. 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhang C, Shi L, Wang FS. 2020. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 5: 428– 430. 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ, Jr, Baric RS, Enjuanes L, Gallagher T, McCray PB, Jr, Perlman S. 2014. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA 111: 4970– 4975. 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zheng H, Li H, Guo L, Liang Y, Li J, Wang X, Hu Y, Wang L, Liao Y, Yang F, Li Y, Fan S, Li D, Cui P, Wang Q, Shi H, Chen Y, Yang Z, Yang J, Shen D, Cun W, Zhou X, Dong X, Wang Y, Chen Y, Dai Q, Jin W, He Z, Li Q, Liu L. 2020. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: a nonhuman primate model of COVID-19 progression. PLoS Pathog 16: e1008949. 10.1371/journal.ppat.1008949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zheng J, Wang Y, Li K, Meyerholz DK, Allamargot C, Perlman S. 2020. Severe Acute Respiratory Syndrome Coronavirus 2-induced immune activation and death of monocyte-derived human macrophages and dendritic cells. J Infect Dis 223: 785– 795. 10.1093/infdis/jiaa753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, Leidinger MR, Knudson CM, Meyerholz DK, McCray PB, Jr, Perlman S. 2021. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589: 603– 607. 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270– 273. 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating Research Team . 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727– 733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]