Abstract
Iron homeostasis is tightly regulated to balance the iron requirement for erythropoiesis and other vital cellular functions, while preventing cellular injury from iron excess. The liver hormone hepcidin is the master regulator of systemic iron balance by controlling the degradation and function of the sole known mammalian iron exporter ferroportin. Liver hepcidin expression is coordinately regulated by several signals that indicate the need for more or less iron, including plasma and tissue iron levels, inflammation, and erythropoietic drive. Most of these signals regulate hepcidin expression by modulating the activity of the bone morphogenetic protein (BMP)-SMAD pathway, which controls hepcidin transcription. Genetic disorders of iron overload and iron deficiency have identified several hepatocyte membrane proteins that play a critical role in mediating the BMP-SMAD and hepcidin regulatory response to iron. However, the precise molecular mechanisms by which serum and tissue iron levels are sensed to regulate BMP ligand production and promote the physical and/or functional interaction of these proteins to modulate SMAD signaling and hepcidin expression remain uncertain. This critical commentary will focus on the current understanding and key unanswered questions regarding how the liver senses iron levels to regulate BMP-SMAD signaling and thereby hepcidin expression to control systemic iron homeostasis.
Keywords: BMP, hepcidin, liver, iron sensing, hemochromatosis, anemia
Introduction
Iron is essential for all living organisms, but is toxic when in excess. The discovery of hepcidin as the regulator of systemic iron homeostasis provided a major breakthrough in our understanding of iron biology and pathobiology in health and disease. Hepcidin deficiency or excess has a pathogenic role in many clinical iron disorders, including the iron overload disorder hereditary hemochromatosis, iron loading anemias such as β-thalassemia, iron refractory iron deficiency anemia, and anemia of chronic diseases. It is well established that the main mechanism by which iron regulates hepcidin expression is through the bone morphogenetic protein (BMP)-small mothers against decapentaplegic (SMAD) pathway. Despite the substantial advances in our understanding of systemic iron homeostasis over the past two decades, the molecular mechanisms behind how the liver senses iron levels to regulate BMP-SMAD signaling and hepcidin expression are largely unknown.
This commentary will provide a brief overview of iron homeostasis and hepcidin regulation, and will focus on the current understanding of liver iron sensing and signaling mechanisms. We will discuss key unanswered questions about the molecular mechanisms of iron sensing and signaling that need to be addressed in order to improve our understanding of iron regulation in health and disease. These unanswered questions will provide opportunities for future studies, which may ultimately lead to the development of novel therapeutics for the improved clinical management of patients with iron disorders.
Hepcidin and iron homeostasis
Iron is an essential nutrient required for vital organismal processes including oxygen transport, intermediary metabolism, hormone synthesis, innate immunity, and growth. In biological systems, iron is predominantly found in the ferrous (+2) and ferric (+3) forms, often within prosthetic groups such as heme (for example in hemoglobin, myoglobin, and cytochromes) or iron-sulfur clusters, which are involved in redox and enzymatic activity. Iron functions as a co-factor in many chemical reactions in the body because of its ability to readily accept and donate electrons. An average adult human has 3-4 g of total body iron, more than half of which is contained in red blood cell hemoglobin (2-3 g), with the remaining 1 g stored in ferritin in the liver hepatocytes and macrophages (Figure 1A). Only a small portion of iron (2-4 mg) is present in plasma, mainly bound to the protein transferrin, which keeps iron in an unreactive state and distributes it to consuming tissues (Figure 1A). Most transferrin-bound iron is delivered to the bone marrow for incorporation into hemoglobin by red blood cell precursors, a process that requires 20-25 mg of iron daily (Figure 1A). In normal conditions, transferrin is only ~20-40% saturated with iron, but in iron overload conditions, plasma iron concentrations exceed the iron-carrying capacity of transferrin, and iron appears bound to low molecular weight molecules, known as non-transferrin bound iron (NTBI). NTBI is highly reactive and can readily generate reactive oxygen species such as hydroxyl radicals via the Fenton reaction. These reactive oxygen species damage DNA, proteins, and lipids, ultimately resulting in cellular dysfunction. The severity of cellular dysfunction is dependent on the rate of NTBI uptake and accumulation. Most circulating NTBI is cleared by the liver, but when the uptake capacity of the liver is exceeded, NTBI is also taken up by other organs, including the pancreas, heart, and kidney 1. In the liver, iron accumulation increases the risk of fibrosis, cirrhosis, and hepatocellular carcinoma 2. In extrahepatic tissues, excess iron deposits increase the risk of cardiomyopathy, heart failure, diabetes, and endocrine dysfunction 3,4. Since mammals cannot excrete iron as a compensatory mechanism to prevent iron overload, iron absorption is tightly regulated to provide enough iron for erythropoiesis while preventing cellular injury from iron toxicity.
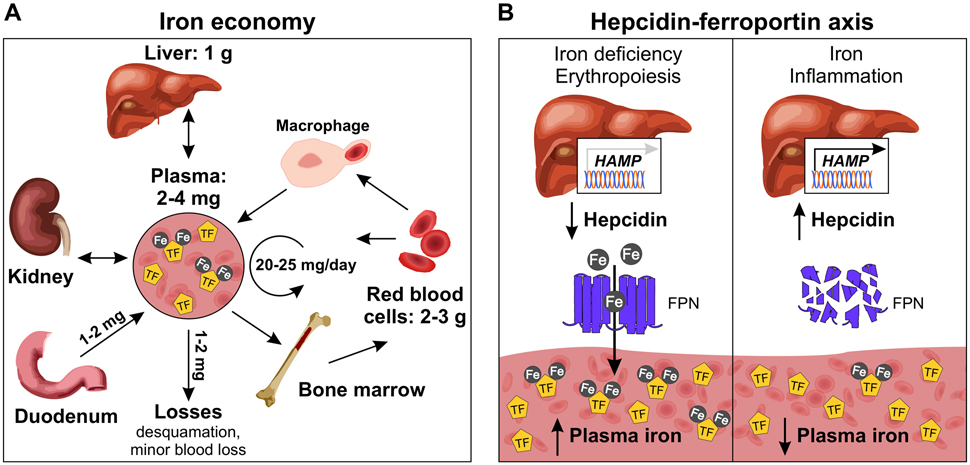
Figure 1. Systemic iron economy and hepcidin-ferroportin axis.
(A) The majority of body iron (3-4 g in adult humans) is contained in red blood cell hemoglobin or stored in ferritin in the liver hepatocytes and macrophages. Blood plasma contains only 2-4 mg of iron where it is normally bound to the protein transferrin (TF). Circulating iron is distributed to consuming tissues for utilization or storage. The major sources of iron export into plasma include release of recycled iron from macrophages that phagocytose old red blood cells, mobilization of stored iron from the liver, and absorption of dietary iron by enterocytes. Red blood cells and the kidney can also export iron into plasma. Minimal iron is lost daily through desquamation or minor blood loss. (B) Hepcidin is the master regulator of systemic iron homeostasis. Produced and secreted by liver hepatocytes, hepcidin acts by posttranslational regulation of its receptor and only known iron exporter ferroportin (FPN), which is expressed in all tissues that export iron into plasma. Hepcidin binds to FPN, blocks iron export, and triggers FPN degradation, resulting in iron sequestration in target cells and lower plasma iron levels. The major signals that regulate hepcidin are iron, erythropoietic drive, and inflammation. Iron deficiency and erythropoiesis suppress hepcidin, promoting iron export into plasma for red blood cell production. Hepcidin is induced by iron to prevent iron overload and is pathologically increased by inflammation to limit iron availability to pathogens.
Hepcidin (encoded by the gene HAMP) is the master regulator of plasma nonheme iron concentrations and tissue iron distribution. Hepcidin was first isolated in the early 2000s from human urine and blood ultrafiltrate and was named for its ability to inhibit microbial growth in vitro and for predominant expression in the liver 5-7. Hepcidin was ultimately linked to iron metabolism when its expression was found to be increased in iron-loaded mice 6, its overexpression caused severe iron deficiency anemia 8, and its deficiency caused the iron overload disorder hereditary hemochromatosis 9,10.
Hepcidin is a 25-amino acid peptide hormone that is produced and secreted by hepatocytes, the main parenchymal cells in the liver 5-7 (Figure 1B). Hepcidin controls iron homeostasis by inhibiting the major sources of iron export into plasma: absorption of dietary iron by enterocytes (1-2 mg/day, ~10% of dietary iron intake), release of recycled iron from macrophages that phagocytose senescent red blood cells (20-25 mg/day), and mobilization of stored iron from the liver (Figure 1) 11. Mature red blood cells can also export iron into plasma 12,13. Recent data also suggests a role for iron reclamation by the kidney in the systemic iron economy, particularly in the context of iron deficiency 14. Minimal amounts of iron are lost daily (1-2 mg/day) through desquamation and minor blood loss, which is compensated for by dietary absorption (Figure 1). Hepcidin exerts its effects through post-translational regulation of its receptor, the only known mammalian iron exporter ferroportin (FPN, encoded by SLC40A1). FPN is a 12-transmembrane-domain protein that is expressed in all tissues that export iron into plasma 15, and the rate of iron efflux is proportional to the amount of FPN. Hepcidin binds to FPN and triggers its ubiquitination 16 and degradation within lysosomes 17,18, resulting in iron sequestration in target cells and decreased iron export into plasma, lowering plasma iron levels (Figure 1B). Hepcidin also regulates FPN activity by binding to the central cavity and directly occluding iron export 18,19. In mice, FPN deficiency is embryonic lethal due to severe anemia, and has an essential role in placental iron transfer as lethality could be rescued by selective FPN inactivation in the embryo proper, but not the placenta 20. Furthermore, mutations in FPN that disrupt its normal activity cause hereditary iron disorders in humans 21.
Hepcidin is produced at a high rate 22 and exerts its effects rapidly, causing hypoferremia (low serum iron levels) within one hour and lasting for more than 48 hours in mice 23. Hepcidin is regulated at the level of transcription, and its expression is induced by iron loading, pathologically increased by inflammation and infection 24,25, and suppressed by erythropoietic drive 26, growth factors 27, and testosterone 28,29. Hepcidin regulation by iron is dependent on the liver’s ability to sense plasma and tissue iron levels. Although distinct pathways are involved in hepcidin regulation by plasma and tissue iron, both ultimately exert their effects by modulating the BMP-SMAD pathway.
BMP-SMAD signaling in hepcidin regulation
BMPs are growth factors that belong to the transforming growth factor-β (TGF-β) superfamily of ligands. More than 30 members of the TGF-β superfamily have been identified and characterized, and include BMPs, TGF-β proteins, activins, inhibins, nodals, and growth and differentiation factors (GDFs). BMPs were first discovered for their ability to induce ectopic bone formation 30-32 and are now well appreciated for having broader roles in many fundamental processes including embryonic growth and development, cell differentiation, and tissue homeostasis. More than twelve BMPs have been identified and are classified into subgroups based on structural similarities, consisting of BMP2/4, BMP5/6/7/8, BMP9/10, and BMP12/13/14 (also known as GDF5/6/7) groups. BMPs are synthesized as large (~400 amino acids) inactive precursors comprised of a secretion signal peptide, a prodomain (~250 amino acids) that aids in proper folding and dimerization, and a smaller ligand domain (~110-140 amino acids) 33. Inactive BMPs undergo proteolytic cleavage by members of the proprotein convertase family 34 to generate a biologically active dimeric ligand.
Members of the TGF-β superfamily exert their effects by binding to type I and type II receptors that consist of a short extracellular domain, a single transmembrane domain, and an intracellular domain with serine-threonine kinase activity. Seven type I receptors have been identified in mammals and are classified into three groups based on structural and functional similarities: activin A receptor-like type I (ACVRLI, also known as ALK1), activin A receptor (ACVR) type I (ACVR-I/ALK2), BMP receptor type (BMPR) IA (BMPR-IA/ALK3), activin A receptor type IB (ACVRIB/ALK4), TGF-β receptor I (TGFBRI/ALK5), BMPR-IB (also known as ALK6), and activin A receptor type IC (ACVRI/ALK7). Of the seven known receptors, ALK1, ALK2, ALK3, and ALK6 serve as the type I receptors for BMP ligands 35. Three type II receptors for BMP signaling have been identified in mammals: BMPR-II, ACVR-IIA, and ACVR-IIB. Whereas BMPR-II is specific for BMPs, ACVR-IIA and ACVR-IIB are shared by other members of the TGF-β family. Although BMPs are capable of binding to type I and type II receptors individually, their interaction with both receptors enhances binding affinity 36, and both receptors are required for signal transduction in a heterotetrameric complex. The serine-threonine kinases of type II receptors are constitutively active, and upon BMP ligand binding, phosphorylate the intracellular glycine-serine-rich domain of the type I receptors 35, which in turn activate intracellular signaling pathways.
In the canonical BMP signal transduction pathway, BMPs bind to type I and type II receptors to induce phosphorylation of receptor-regulated (R)-SMAD 1, 5, and 8 proteins, which reside in the cytoplasm. R-SMAD 1,5,8 proteins form a complex with common mediator SMAD4, translocate to the nucleus, and bind to BMP-response elements in promoter or enhancer regions of target genes to regulate transcription. Glycosylphosphatidylinositol (GPI)-anchored proteins of the repulsive guidance molecule (RGM) family act as coreceptors that bind directly to BMP ligands and enhance signal transduction 37-39. Three RGM proteins have been identified in humans: RGMa, RGMb (DRAGON), and RGMc (hemojuvelin). Inhibitory (I)-SMAD6 and I-SMAD7 proteins antagonize this pathway through multiple mechanisms. I-SMAD7 inhibits signaling by forming stable complexes with the activated type I receptor, which directly blocks the phosphorylation of R-SMADs and the formation of R-SMAD and co-SMAD complexes 40,41. I-SMAD6 inhibits signaling by competing for SMAD-4 binding, which disrupts R-SMAD-SMAD4 interactions 42,43. Furthermore, I-SMAD6 and I-SMAD7 exert their inhibitory effects in the nucleus to block transcription by interacting with transcriptional repressors or disrupting SMAD-DNA interactions 44,45. I-SMAD6 and I-SMAD7 also suppress signaling by interacting with other inhibitory proteins, such as E3 ligases SMURF1 and SMURF2, which targets the activated receptor and R-SMADs for degradation 46-48. I-SMAD6 effectively inhibits BMP signaling and weakly inhibits TGF-β/activin signaling, whereas I-SMAD7 effectively inhibits both pathways 49-51.
A critical functional role for the BMP-SMAD pathway in hepcidin regulation by iron is well-established and has been recently reviewed 52. BMP ligands robustly stimulate hepcidin transcription in liver cells via SMAD binding elements on the hepcidin promoter 53. Genetic ablation of any of multiple genes encoding BMP-SMAD pathway components in the liver, including BMP6 or BMP2 ligands, the BMP co-receptor hemojuvelin (HJV, also known as RGMc), BMP type I receptors ALK2 or ALK3, BMP type II receptors ACVRIIA and BMPR-II, SMAD1,5,8, or SMAD4 each lead to hemochromatosis, characterized by hepcidin deficiency and iron overload 54-64. Genetic mutations in HJV and the BMP6 prodomain have also been linked to iron overload in humans 61,65-68. By antagonizing the BMP-SMAD pathway, SMAD7 has been shown to function as an inhibitor of hepcidin expression in vitro 69, and its deficiency in mice causes elevated hepcidin and decreased iron stores 70. Importantly, BMP ligand expression and BMP-SMAD pathway activity in the liver is regulated by iron concordantly with hepcidin 57,71-74, and blocking BMP activity with neutralizing antibodies blocks hepcidin induction by dietary iron 57,73.
Iron disorders of dysregulated hepcidin production
Dysregulation of hepcidin production contributes to most disorders of iron metabolism. The mechanisms of hepcidin dysregulation in iron disorders and the development of therapeutic strategies was recently reviewed 75-78. Hepcidin deficiency and consequent dietary iron hyperabsorption cause iron overload in hereditary hemochromatosis 79 and contribute to iron-loading anemias including β-thalassemia and congenital chronic anemias 80,81. In these disorders, excess iron deposits in organs such as the liver, heart, pancreas and other endocrine glands leading to tissue injury and dysfunction, which are the leading cause of morbidity and mortality in these diseases 82. Hereditary hemochromatosis is caused by mutations in the genes encoding hepcidin (HAMP) itself, or other proteins involved in hepcidin regulation by iron, including homeostatic iron regulator (HFE), transferrin receptor 2 (TFR2), and HJV, which appear to exert their effects through functional interactions with the BMP-SMAD signaling pathway (discussed in more detail below; 39,61,79,83-91). β-thalassemia is an inherited hemoglobin disorder caused by mutations in the HBB gene encoding β-globin, which causes ineffective erythropoiesis as a result of decreased β-globin production. Ineffective erythropoiesis stimulates production of erythroferrone (encoded by ERFE) and other yet unidentified erythroid factor(s) which act to suppress hepcidin expression 92,93, facilitating excessive dietary iron absorption and ultimately iron overload. In patients with more severe forms of thalassemia, repeated blood transfusions (providing 200-250 mg iron/unit of blood) that are commonly used to alleviate anemia and maintain oxygen delivery to consuming tissues also contribute to iron overload 81. The mechanism by which ERFE suppresses hepcidin is by functioning as a ligand trap to sequester BMP ligands 94,95.
Currently, the most common treatment options for removing excess iron are phlebotomy (blood removal) and chelation therapy. However, these treatments are not always feasible, can be associated with significant adverse effects, and can be challenging for patients. Importantly, these treatments do not prevent further dietary iron absorption or target the underlying disease mechanisms. Identifying the genetic basis of iron disorders has identified hepcidin and the BMP6-SMAD pathway as potential therapeutic targets, which have demonstrated some efficacy in animal models. Exogenous administration of minihepcidins or BMP6 corrected the iron overload phenotype in mouse models of hemochromatosis 96-98. Similarly, exogenous treatment with minihepcidins 99,100 or thiazolidinone derivatives 101 was effective in correcting anemia and iron overload in mouse models of β-thalassemia. These studies are corroborated by genetic studies where transgenic overexpression of hepcidin 102,103 or loss of the negative hepcidin regulator transmembrane serine protease 6 (TMPRSS6) 104-106 prevented iron overload in mouse models of hemochromatosis and β-thalassemia.
In addition to hepcidin deficiency, pathological excess of hepcidin production also contributes to iron disorders. Abnormally high hepcidin expression contributes to iron-restricted anemias 8,107 by causing iron sequestration in body storage sites, impaired dietary iron absorption, and hypoferremia, thereby limiting iron availability for erythropoiesis. The causes of excess hepcidin in iron-restricted anemias vary and include pathological induction by chronic inflammation or genetic mutations in key players in the hepcidin signaling pathway.
Anemia of chronic disease, also known as anemia of inflammation, is a mild to moderately severe anemia that develops in the setting of infections and inflammatory diseases such as rheumatological disorders, malignancy, inflammatory bowel disease, and obesity 108. Anemia of chronic disease is characterized by low serum iron despite adequate iron stores. Onset of inflammation induces the production of inflammatory cytokines such as interleukin (IL) 6, which stimulates hepcidin transcription directly via a STAT3 binding element on the hepcidin promoter 109,110, thereby causing hypoferremia and iron-restricted erythropoiesis. The BMP-SMAD pathway has been proposed to crosstalk with the IL6-STAT3 pathway in hepcidin regulation by inflammation 53,56,111. Although the extent and mechanism of crosstalk has been a matter of debate 52, recent studies suggest that the predominant impact of BMP-SMAD signaling in this context is to control the basal setpoint of hepcidin 52,54,112. Inflammatory cytokines also contribute to anemia through other mechanisms 108,113-116. Treatments targeting inflammatory cytokines, including anti-IL6, anti-IL6 receptor, or anti-TNFα therapy, that have been used to treat patients with rheumatoid arthritis have been shown to be effective in alleviating anemia 117,118. However, the immunosuppressive properties of these therapies may also lead to adverse effects that may limit their use in some patients.
Iron-refractory iron-deficiency anemia (IRIDA) is an autosomal recessive disorder characterized by iron-deficiency anemia that is largely unresponsive to most iron treatment modalities. IRIDA is caused by mutations in TMPRSS6, which prevents hepcidin suppression in iron-deficient conditions, resulting in inappropriately high hepcidin activity, decreased dietary iron absorption, impaired iron release from body stores, iron-restricted erythropoiesis and anemia 119-121. TMPRSS6 is proposed to suppress hepcidin expression by cleaving HJV from the membrane surface to inhibit the BMP-SMAD signaling cascade 106. As patients with IRIDA are unresponsive to oral iron supplementation and only partially responsive to parenteral iron, the main treatment modality for IRIDA includes repeated intravenous iron infusions, and these strategies do not directly target the main disease mechanisms. For both IRIDA and anemia of inflammation, therapeutics targeting the BMP-SMAD-hepcidin pathway may provide an alternative therapeutic strategy to facilitate dietary iron absorption and mobilization of stored iron, ultimately correcting iron restriction. Indeed, modalities that target the BMP-SMAD-hepcidin pathway to lower hepcidin activity have already been shown to be effective in animal models of IRIDA 122, anemia of inflammation 122-126, and in phase-1 clinical trials in cancer patients with anemia 127.
Iron sensing and signaling: Current understanding and unanswered questions
Although the BMP-SMAD pathway is central to hepcidin regulation by most of its known signals and represents a potential therapeutic target for improved clinical management of patients with iron disorders, there are many outstanding questions that need to be answered to better understand how the BMP-SMAD pathway is modulated by iron to control hepcidin production. Answering these questions would not only provide improved understanding of iron homeostasis physiology and the pathophysiology of iron disorders, but also holds promise for developing new, more targeted therapies for iron disorders.
How does iron regulate the BMP-SMAD pathway to control hepcidin production? It is important to note that circulating plasma iron and tissue iron stores represent distinct signals to regulate hepcidin production 72,73. Indeed, acute iron administration by oral gavage or holo-transferrin that increases serum iron and transferrin saturation without increasing tissue iron levels, induces hepcidin. Additionally, chronic administration of a high iron diet continues to increase hepcidin expression in parallel to liver iron content even after serum iron and transferrin saturation have reached a maximal plateau. Moreover, switching mice from a high iron diet to a low iron diet, which normalizes serum iron and transferrin saturation but retains persistently elevated liver iron stores, leads to intermediate hepcidin levels that are increased above baseline, but lower than their peak levels.
How do plasma iron and tissue iron regulate BMP-SMAD signaling? Changes in liver iron content by feeding mice a low, adequate, or high iron diet concordantly regulate liver Bmp6 and Bmp2 expression, SMAD1,5,8 phosphorylation, and hepcidin production 57,71,73,74. Notably, Bmp6 mRNA levels are significantly correlated with liver iron content in these models 71,73. In contrast, isolated increases in serum transferrin saturation by oral iron gavage or holo-transferrin administration activate liver SMAD1,5,8 phosphorylation and induce hepcidin without impacting liver Bmp6 or Bmp2 expression 73,128. Together, these data suggest that tissue iron content in the liver regulates hepcidin production at least in part by regulating the production of BMP6 and BMP2 ligands, whereas plasma iron induces hepcidin by activating hepatocyte SMAD1,5,8 signaling downstream or independent of an increase in BMP2/6 ligand production.
Tissue iron sensing
How are tissue iron levels sensed by the liver to regulate BMP6 and BMP2 ligand production and thereby hepcidin? The recent identification of liver endothelial cells (LECs) as the source of BMP6 and BMP2 for hepcidin regulation suggests that these cells play a critical role. A functional role for LECs was first suggested by the findings that LECs express much higher levels of Bmp6 and Bmp2 mRNA compared to other liver cell populations 57-59,129-131. Importantly, mice with conditional deletion of Bmp6 and/or Bmp2 in endothelial cells exhibit hepcidin deficiency and hemochromatosis similar to global Bmp6-null mice, whereas mice with hepatocyte or macrophage-specific Bmp6 deletion do not 57-59. Although the Cre driver (Tek-Cre) employed by Canali et al. to generate the endothelial Bmp6 and Bmp2 knockout mice 57,58 is expected to delete Bmp6 and Bmp2 in all endothelial cells, Bmp6 and Bmp2 mRNA is regulated concordantly by dietary iron in parallel with changes liver iron only in LECs, whereas Bmp6 and Bmp2 mRNA levels are not concordantly regulated by iron in any other tissues studied 57-59,73,128,132.
Among the LECs, it has been proposed that the specific subset of endothelial cells within the liver sinusoid, termed liver sinusoidal endothelial cells or LSECs, are the primary cellular source of BMP2 and BMP6 ligands for hepcidin regulation. LSECs are specialized endothelial cells that line the sinusoidal capillary channels, representing the interface between blood and hepatocytes, and are the most abundant non-parenchymal cell population in the liver 133. LSECs differ from other vascular endothelial cells by the presence of pores or fenestrae that allow for nutrient exchange directly between the blood and liver parenchyma. The liver sinusoids have dual blood supply, receiving blood predominantly from the portal vein and to a lesser extent the hepatic artery 134. Since the portal vein delivers nutrients absorbed by the intestine, LSECs are one of the most proximal cell types exposed to circulating iron after dietary absorption, making them an attractive candidate for BMP ligand production. Notably, HJV is reported to be expressed in sinusoidal membrane of hepatocytes immediately adjacent to the LSEC membrane 58. Additionally, liver sections from mice injected with iron-rich holo-ferritin, which induces liver Bmp6 expression, show apparent sinusoidal distribution of Perl’s iron stain 131. Importantly, conditional knockout of Bmp2 in mice expressing Cre recombinase driven by a Stabilin-2 promoter, which is reported to be more specific for LSECs, also lead to hepcidin deficiency and hemochromatosis 59, suggesting an important functional role for LSECs in BMP ligand production; however, these data do not definitively rule out a contribution from other endothelial cells.
There are several important unanswered questions with respect to how tissue iron regulates BMP6 and BMP2 production in LECs. First, is the iron-mediated production of BMP6 and BMP2 limited to LSECs, or are BMP ligands produced in arterial and/or venous endothelial cells in the liver and/or other tissues? Even among LSECs, single cell RNA-sequencing (scRNA-seq) data suggests that periportal LSECs exhibit a distinct transcriptional profile compared with perivenous LSECs 135. Is BMP ligand expression restricted to a specific subset of LSECs or is it more widespread? Answers to these questions would benefit from additional scRNA-seq studies in models of iron overload and iron deficiency and improved methods for the isolation, identification, and culture of different LEC and LSEC populations.
Another important question is whether BMP6 and BMP2 production in LECs occurs in a cell autonomous fashion in response to LEC intracellular iron levels or whether intercellular communication with other cell types plays a role. Notably, intracellular iron loading does induce Bmp6 mRNA expression in isolated LECs in culture suggesting that intracellular iron loading is sufficient to induce Bmp6 in a cell-autonomous manner 136. Moreover, a stronger induction of hepatic Bmp6 was observed in iron-loaded ZRT/IRT-like protein (ZIP) 14 (Slc39a14)-deficient mice, where iron accumulates in non-parenchymal cells including LECs, rather than hepatocytes 137. Although this study does not rule out a contributory role for hepatocyte iron in Bmp6 induction by tissue iron loading, this study shows that hepatocyte iron loading is not required for Bmp6 induction in LECs.
Might there still be a contributory role for hepatocytes and/or other liver cell populations (e.g., liver macrophages/Kupffer cells) in tissue iron sensing? There is evidence that hepatocyte iron regulates expression of one of the plasma iron sensors, transferrin receptor 1 (TFR1), to contribute to hepcidin regulation, thereby linking the tissue iron sensing pathway with the plasma iron sensing pathway (described in more detail below; 138). It has also been proposed that the intracellular iron storage protein ferritin may also play a role. The composition of ferritin differs across cell types but is generally composed of heteromultimers of L and H subunits, which bind and oxidize iron to store it in an inert form 139. Ferritin production is induced by intracellular iron loading through the iron regulatory protein (IRP) system 140. Interestingly, ferritin is also secreted into the bloodstream, and circulating ferritin levels correlate with tissue iron stores 139. Although circulating ferritin is typically iron poor, it has been hypothesized that ferritin could serve as an iron carrier to transport iron between different cell populations at a local level 141. Interestingly, parenteral administration of ferritin in mice has been reported to deliver iron to LECs, leading to an increase LEC Bmp6 mRNA levels 131. These data suggest a potential model whereby increased intracellular iron levels in hepatocytes and/or Kupffer cells could induce ferritin production and secretion in the liver niche, which could then be taken up by LECs to regulate BMP6 production. This hypothesis awaits further validation.
Although LECs produce BMP6 and BMP2 in proportion to their intracellular iron content (Figure 2), the molecular mechanisms governing the iron signal(s) that induces BMP6 and BMP2 are largely unknown. An important remaining challenge is identifying which iron transporters are involved in controlling LEC iron flux and intracellular iron content, which ultimately drives BMP6 and BMP2 production and hepcidin regulation. LSECs are well positioned in the liver sinusoid to take up transferrin-bound iron from circulation and express TFR1 (encoded by TFRC), although transient changes in plasma iron do not stimulate Bmp6 expression within 4-8 hours 72,73. LSECs isolated from mice show inverse expression of Bmp6 and Tfrc with dietary iron depletion and loading 129, suggesting that TFR1 acts as a sensor of intracellular iron content. Transgenic mouse models that specifically ablate Tfrc in endothelial cells using the Cre/Lox system will provide insight into whether TFR1 controls LEC intracellular iron and participates in iron sensing. However, recombinase activity in commonly used pan endothelial Cre mice is generally observed in the hematopoietic compartment 142. This is disadvantageous in that it not only confounds the interpretation of the experiment, but loss of TFR1 in the hematopoietic compartment will also cause severe anemia 143,144. It will be essential to accurately determine recombination efficiency in not only the endothelium, but also hematopoietic cells in these experimental systems. The continued development of Cre models with specific expression in endothelial subpopulations such as LSECs will be a valuable resource for future studies of iron homeostasis.
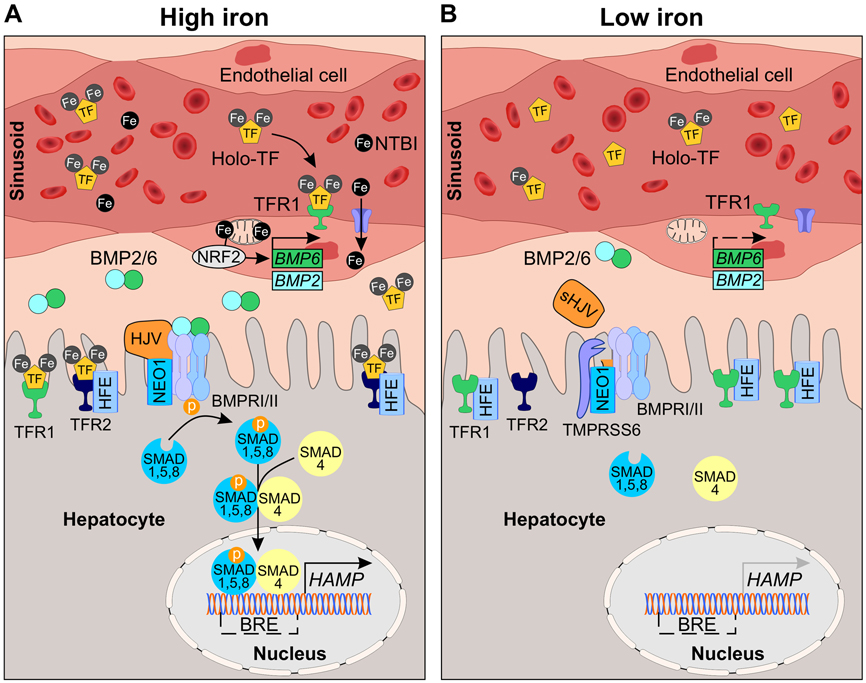
Figure 2. Current model of iron sensing and signaling in the liver.
Hepcidin production is regulated transcriptionally by iron, a process dependent on sensing of plasma and tissue iron levels. (A) In high iron conditions, plasma iron concentrations exceed the iron-binding capacity of transferrin (TF) and non-transferrin bound iron (NTBI) appears. Iron is taken up by endothelial cells through unknown mechanisms and stimulates BMP6 and to a lesser extent BMP2 expression. BMP6 transcription is partly controlled by nuclear factor erythroid 2-like 2 (NRF2) in response to iron-induced mitochondrial prooxidants. BMP2 and BMP6 bind hemojuvelin (HJV) and BMP type I and II receptors (ALK2, ALK3, ACVRIIA, BMPR-II; represented by BMPRI/II) on the hepatocyte membrane, forming a complex with neogenin (NEO1). The complex phosphorylates cytoplasmic SMAD1,5,8, which binds SMAD4, translocates to the nucleus, and binds to BMP-responsive elements (BRE) in the hepcidin (HAMP) promoter to induce transcription. Hepatocytes also directly sense plasma iron-TF levels by TFR1 and TFR2. Iron loading decreases TFR1 and increases TFR2 expression, favoring the displacement of HFE from TFR1 and the interaction with TFR2. HFE and TFR2 stimulate hepcidin expression via the SMAD pathway. (B) When iron availability is low, NTBI is absent and plasma iron-TF levels decrease. Low iron reduces the production of BMP ligands by endothelial cells. Sensing of low iron by hepatocytes increases TFR1 and decreases TFR2 expression, favoring the sequestration of HFE with TFR1. Iron deficiency increases expression of transmembrane serine protease 6 (TMPRSS6) which cleaves cell surface HJV, attenuating BMP signal transduction. Attenuated BMP signaling suppresses HAMP expression, promoting iron export into plasma.
In iron overload conditions, where the binding capacity of transferrin is overwhelmed and NTBI appears in circulation, LECs may also take up NTBI through the known NTBI transporters divalent metal-ion transporter 1 (DMT1), ZIP8 and ZIP14, or other yet unknown transporters (Figure 2). Any role of ZIP14 in LEC iron uptake is unlikely since induction of Bmp6 by iron is preserved in ZIP14-deficient mice 137. ZIP8 (encoded by SLC39A8) is an 8-transmembrane domain protein that transports zinc, manganese, iron, and cadmium 145-147. ZIP8 plays a physiologic role in gestational iron metabolism as ZIP8 hypomorph mice are embryonic lethal due to zinc and iron deficiency, and anemia 148. ZIP8 is reportedly expressed in vascular endothelial cells in the heart 149, testis 150, brain 151, and in HUVECs 149, where it functions to maintain zinc and manganese homeostasis. It is currently unknown whether ZIP8 is expressed in the liver endothelium and whether it functions to control iron or other ion homeostasis in LECs. DMT1 (encoded by SLC11A2) is metal-ion transporter with a broader substrate range including iron, zinc, manganese, cobalt, cadmium, nickel, and lead 152. DMT1 is centrally involved in dietary iron absorption and intracellular iron delivery 153. DMT1 is reportedly expressed in HUVECs, but its expression and contribution to iron homeostasis in LECs is unknown. It is also possible that LECs take up ferritin that is secreted by iron-loaded hepatocytes or macrophages into the perisinusoidal space as discussed above. TFR1, SCARA5, and TIM2 have all be reported to function as ferritin uptake receptors 154-156, but aside from TFR1, their expression in LECs has not been characterized. Finally, alterations in iron export by FPN could also influence LEC iron content and BMP ligand production 129. Systematic analysis of the expression of the various iron transporters and functional impact of their ablation in cultured endothelial cells and mouse models will provide important insight into the molecular mechanisms of iron uptake in LECs and their role in liver iron sensing.
Once inside LECs, how does iron regulate BMP6 and BMP2 expression? A recent study demonstrated a functional role for the transcription factor nuclear factor erythroid 2-like 2 (NRF2) in BMP6 induction by iron overload 136 (Figure 2). NRF2 (Nfe2l2) activity is increased in iron loaded LECs in parallel with Bmp6 mRNA expression, both in cell culture and mouse models. Moreover, pharmacologic induction of NRF2 activity induces Bmp6 expression in LEC cultures and mouse livers, whereas knockdown/knockout of Nrf2 blunts Bmp6 induction by iron. Iron-mediated activation of NRF2, and thereby Bmp6, in LECs occurs as a consequence of mitochondrial reactive oxygen species. It has been proposed that this system functions to protect against injury in the setting of toxic iron loading by activating protective antioxidant pathways and by inducing hepcidin to limit further iron accumulation.
Although the induction and role of NRF2 in the antioxidant response to iron excess is well appreciated 136,157, whether NRF2 has a functional role under normal or iron-deficient conditions is not as well described. It has been demonstrated that iron deficiency damages mitochondria 158 and activates NRF2 activity 159,160, but this is unlikely to be contributing to BMP6-hepcidin regulation in iron-deficient mice since liver Bmp6 mRNA is downregulated in this context 57,71. Additionally, even under iron overload conditions, there is persistent induction of both Bmp6 and hepcidin mRNA in Nrf2 knockout mice, suggesting the existence of other regulatory pathways 136. Moreover, manipulation of NRF2 expression and/or activity does not appear to regulate Bmp2 mRNA expression in LEC cultures or mouse livers suggesting that Bmp2 mRNA may be regulated by distinct mechanisms compared with Bmp6 136. Indeed, whereas Bmp6 mRNA is appropriately induced by iron overload in hemochromatosis mouse models, Bmp2 mRNA is not increased in Bmp6, Hjv, Tfr2, or Hfe-deficient mice despite iron overload 71,74,86,161,162. These data suggest that Bmp2 regulation by iron depends on BMP6 and hemochromatosis proteins, whereas Bmp6 regulation does not. Notably, the lack of validated antibodies to detect endogenous BMP6 protein expression has limited the ability to assess whether there is additional regulation of BMP production or activity by iron at the posttranscriptional level. Future studies would benefit from the development of improved antibodies and/or animal models expressing tagged BMP proteins to investigate these possibilities. Systematic and integrative analysis using unbiased multiomic screening of LECs with differing iron content may help identify upstream mediators of BMP6 and BMP2 regulation in iron deficient, replete, and loaded conditions.
Plasma iron sensing
Hepatocytes directly sense circulating iron that is bound to the iron carrier transferrin (TF) through transferrin receptors TFR1 and TFR2 on the hepatocyte membrane (Figure 2). TFR1 and TFR2 are type II transmembrane glycoproteins with high sequence similarity in the extracellular domain. The iron status of transferrin affects its binding affinity for TFR1 and TFR2, with higher affinity binding when TF is fully saturated with two iron atoms (holo-TF), compared with one or no iron atoms 163,164. Binding to holo-TF stabilizes TFR2 protein expression on the membrane surface by redirecting it from a degradative pathway to a recycling pathway 165,166. Holo-TF also inhibits the cleavage and release of TFR2 from the membrane surface 167. For TFR1, holo-transferrin binding leads to a displacement of HFE, which shares an overlapping binding site with TF on TFR1 168. The stabilization of TFR2 and displacement of HFE both contribute to stimulate hepcidin expression 169, a process which is impaired in patients with mutations in either of these genes, resulting in hereditary hemochromatosis 83-85.
HFE and TFR1 may also play a role in tissue iron sensing in hepatocytes (Figure 2). TFR1 is post-transcriptionally regulated by intracellular iron through the IRP system of RNA-binding proteins, which stabilize TFRC mRNA in limited iron conditions. Thus, in iron limited conditions, reductions in hepatocyte iron levels would increase TFR1 expression, while reductions in circulating holo-TF would decrease binding to TFR1. Both factors would lead to increased sequestration of HFE by TFR1, which would contribute to suppressing hepcidin expression. In contrast, iron loading conditions would increase hepatocyte iron, thereby reducing TFR1 expression, and increase holo-TF binding to the remaining TFR1. Both factors would increase the displacement of HFE from TFR1 to induce hepcidin expression. Support for this model is demonstrated in hepatocyte conditional Tfrc knockout mice, which display reduced hepatocyte iron and hypoferremia attributed to a combination of 1) reduced TF-bound iron uptake into hepatocytes and 2) inappropriately increased hepcidin expression as a consequence of unrestricted HFE activity 138.
Key unanswered questions in this area involve the molecular mechanisms by which TFR2 and HFE stimulate hepcidin expression. Co-immunoprecipitation and cell culture studies demonstrate that HFE and TFR2 can bind to each other and suggest that this interaction is required for hepcidin induction by holo-TF 170. However, interaction of the endogenous proteins at the membrane surface has yet to be demonstrated in vivo. Moreover, HFE overexpression induces hepcidin in Tfr2-deficient mice 171, suggesting that the interaction is not absolutely required for hepcidin induction in vivo. Both HFE and TFR2 are ultimately thought to activate hepcidin transcription through a functional interaction with the SMAD1,5,8 signaling pathway. Mutation or ablation of HFE or TFR2 leads to a reduction, whereas overexpression leads to an increase, in liver SMAD1,5,8 phosphorylation and BMP-SMAD1,5,8 target transcript expression in mice and/or humans 86-90,97. Co-immunoprecipitation studies using overexpressed proteins in cell culture systems have also demonstrated that HFE and TFR2 can interact with HJV 172. HFE has also been shown to interact with ALK3, which is reported to stabilize ALK3 by inhibiting its ubiquitination and proteasomal degradation 173. However, it remains unknown whether these interactions truly occur with endogenous proteins at the liver membrane surface in vivo.
Whereas HJV, HFE, and TFR2 are positive regulators of hepcidin in response to iron, TMPRSS6 is a negative regulator of hepcidin expression in response to iron deficiency 119-121 (Figure 2). TMPRSS6 expression increases with iron deficiency 130,174 and is proposed to act by cleaving HJV into an inactive form, thereby suppressing BMP-SMAD signal transduction 106. An important limitation of this proposed model is that the cleavage of HJV has only been documented in an overexpression system. The notion that HJV is a substrate of TMPRSS6 in vivo is supported by findings that mice lacking functional HJV and TMPRSS6 exhibit iron overload similar to Hjv-deficient mice 175. However, TMPRSS6 overexpression was still able to suppress hepcidin in Hjv knockout mice, suggesting that TMPRSS6 can regulate hepcidin through other mechanisms 176. TMPRSS6 has been suggested to cleave other components of the BMP-SMAD signaling transduction cascade, including ALK2, ALK3, ACVR-IIA, BMPR-II, HFE, and TFR2, in overexpression cell culture systems 176. However, mice lacking both Tmprss6 and Hfe or Tfr2 exhibited iron deficiency and microcytic anemia mimicking the phenotype of Tmprss6 knockout mice 105,177, suggesting that HFE and TFR2 do not have a key functional role as TMPRSS6 substrates in vivo. Mice lacking both Bmp6 and Tmprss6 had similar hepcidin levels to Bmp6 knockout mice, but iron overload was attenuated to some extent, suggesting that TMPRSS6 function was not entirely overlapping with the BMP6 signaling pathway 178. Interestingly, a recent study demonstrated that expression of protease dead TMPRSS6 in Tmprss6 knockout mice restored hepcidin expression, suggesting that the proteolytic activity of TMPRSS6 was not required for its hepcidin suppressive function 179. Future studies are needed to more clearly delineate the mechanism(s) of action of TMPRSS6 in hepcidin regulation.
Composition of the BMP ligand-receptor complex and contribution of accessory proteins to hepcidin regulation
Ultimately, both plasma iron and tissue iron converge to regulate the BMP-SMAD1,5,8 signaling pathway and thereby hepcidin transcription (Figure 2). Understanding the composition of the BMP ligand-receptor signaling complex and role of accessory proteins will be critical to understanding hepcidin regulation and systemic iron homeostasis.
BMPs are synthesized as inactive peptide precursors that undergo proteolytic processing by proprotein convertases to generate an active ligand dimer and prodomain fragments, which lack signaling activity but are essential for normal ligand processing 180,181. BMP ligands can signal as homodimeric or heterodimeric proteins, the latter having demonstrated higher activity than homodimers in several biological systems, possibly for their ability to bind with higher affinity to both type I and II receptors or assist in the assembly of heteromeric receptor complexes 182-184. Whether BMPs function primarily as homodimers or heterodimers in vivo is unclear, but there is increasing recognition of a biologic role for heterodimers 184-187. Notably, mice with dual deficiency in Bmp2 and Bmp6 in the endothelium have a similar iron loading phenotype compared to mice deficient in endothelial Bmp6 or Bmp2 alone 74. In contrast, dual deficiency of Bmp2 or Bmp6 in combination with other hemochromatosis proteins leads to an exacerbated hemochromatosis phenotype compared with single knockout animals 74,112,188. Moreover, neutralizing BMP antibodies further lower hepcidin expression and exacerbate iron overload in Bmp6 knockout mice 57. Together, these data suggest that BMP2 and BMP6 work together to regulate systemic iron balance, and it has been hypothesized that they predominantly function as a heterodimeric ligand. Moreover, these data suggest a non-redundant role for at least one other BMP ligand. The heterodimeric BMP2/6 ligand model may help explain why ALK3 has a more predominant role in hepcidin and iron homeostasis compared to ALK2 as demonstrated in knockout mice 62, since BMP2/6 heterodimeric ligands preferentially signal via ALK3, whereas homodimeric BMP6 and BMP2 preferentially signal via ALK2 and ALK3, respectively 95. This model may also explain the hepcidin inhibitory properties of ERFE, which binds and sequesters BMP2/6 heterodimeric ligands and class II BMP homodimers (BMP5, BMP6, and BMP7), but has a minimal inhibitory effect on BMP2 homodimers 94,95,189. Future studies will be needed to confirm whether BMP2 and BMP6 are in fact functioning as heterodimeric or homodimeric ligands, and which other BMP ligand(s) contribute to hepcidin and iron homeostasis regulation in vivo. Future studies will also be needed to better characterize BMP2 and BMP6 prodomains, proteolytic maturation, and the mechanisms of homodimer versus heterodimer formation, about which little is currently known. It is notable that several mutations in the BMP6 prodomain have been linked to unexplained iron overload in human patients 65-68. The functional consequences of these mutations on BMP6 homodimeric and BMP2/6 heterodimeric proteolytic maturation, dimerization, and function promises to provide important insights into why these mutations cause iron overload.
LEC-derived BMP2 and BMP6 act in a paracrine manner to induce hepcidin expression by binding to membrane-anchored HJV on neighboring hepatocytes 39,57-59. HJV is essential for iron homeostasis, since HJV mutations cause juvenile-onset (type II) hemochromatosis characterized by hepcidin deficiency and severe iron overload 61 similar to mutations in hepcidin (HAMP) itself 79. This is a more severe form of hemochromatosis than that caused by HFE or TFR2 mutations, which result in adult-onset form of the disease 83,84. Similar to other RGM-proteins, HJV acts as a coreceptor that binds directly to BMP ligands and enhances BMP signaling in cultured hepatocytes 39. HJV binds with high affinity to BMP2, 4, 5, 6, and 7, but has the highest affinity for class II BMP ligands (BMP5,6,7) 190 among the RGM proteins, consistent with the role of HJV as a coreceptor for BMP6-SMAD signaling and hepcidin expression. Notably, although HJV enhances BMP-SMAD signaling and hepcidin expression, it is not absolutely required. Indeed, BMP6 and BMP2 treatment still induces hepcidin in HJV-deficient or knockout hepatocyte cultures, albeit to a lesser extent than in the presence of HJV 39,162,191. Moreover, knockout of Bmp6 further suppresses hepcidin in Hjv knockout mice, suggesting that BMP6 can still induce hepcidin to some extent in the absence of HJV in vivo 112.
Additional key unanswered questions are: how does HJV facilitate BMP-SMAD signal transduction, and what is the complete nature of the BMP signaling complex that regulates hepcidin and iron homeostasis in vivo? Crystal structure data and cell-free binding assays demonstrate that HJV binds to BMP ligands at the same binding site as BMP type I receptors, and HJV and type I receptors compete for BMP ligand binding 192. However, cell culture assays clearly demonstrate an enhancing role for HJV in inducing BMP signal transduction rather than an inhibitory role that would be predicted by the binding studies 39. Notably, the HJV-BMP binding interaction is acid labile, whereas the type I receptor-BMP binding interaction is not 192. Healey et al 192 therefore proposed a model whereby HJV binds to BMP ligands on the cell surface, and upon endocytosis the acidic environment promotes displacement of HJV from the complex and replacement by type I receptors, leading to signal transduction. This model awaits functional validation.
BMP binding to HJV facilitates the formation of a signaling complex in the hepatocyte membrane consisting of BMP type I and type II receptors (Figure 2). Two type I receptors, ALK2 and ALK3, are required for optimal hepcidin expression since hepatocyte-specific deletion of either caused hepcidin deficiency and systemic iron overload, with loss of ALK3 causing a more severe phenotype 62. On the other hand, the two BMP type II receptors ACVR-IIA or BMPR-II have redundant roles in hepcidin expression, since altered hepcidin and iron homeostasis only occurred with deficiency of both 60. Co-immunoprecipitation studies have suggested that ALK3 forms ligand-independent homodimers and ligand-dependent heterodimers with ALK2 after exposure to BMP2 or BMP6, but ALK2 homodimers were not detected in either context 193. It has been proposed that hepcidin expression and regulation may depend on more than one type of signaling complex, including those containing ALK3 homodimers and ALK2/3 heterodimers 193. Previous work has demonstrated a requirement for heteromeric type I receptor complexes in some biologic contexts, including zebrafish dorsoventral patterning 184,187. Interestingly, in zebrafish dorsoventral patterning, although both type I receptors are required, only one type I receptor performs a signaling function 184. This raises the intriguing possibility that the BMP receptor complex could be comprised of one BMP type I receptor and HJV, rather than two BMP type I receptors. Future studies are needed to ascertain whether the BMP receptor complex that regulates hepcidin in vivo is comprised of homodimeric or heterodimeric type I receptors, whether the nature of the ligand (homodimeric versus heterodimeric) influences receptor complex composition, and whether and how the composition of the receptor complex influences the downstream signaling cascade.
The BMP signaling complex that regulates hepcidin also includes neogenin (NEO1, encoded by Neo1) (Figure 2). Neogenin is essential for hepcidin and iron homeostasis regulation since mice with a hepatocyte conditional knockout of Neo1 exhibit impaired liver BMP signaling, hepcidin deficiency, and iron overload 194,195. Moreover, the interaction of NEO1 with HJV is essential for optimal function since adenoviral expression of mutant forms of NEO1 or HJV that impair binding to each other failed to fully rescue the iron overload phenotype of the respective knockout mice 195,196. However, there was partial rescue with the HJV mutant that impairs NEO1 binding suggesting that some portion of HJV function is not reliant on its interaction with NEO1 195. Crystal structure and live-cell super-resolution fluorescence microscopy studies show a ternary interaction between BMP2, RGM, and NEO1, and suggest that BMP2 induces clustering of the RGM-NEO1 complex in the plasma membrane 192. The full mechanism of action of neogenin is still unclear, but it is hypothesized to function as a scaffolding protein that assists with BMP signaling complex formation and localization within the plasma membrane to facilitate signal transduction. A better understanding of the precise molecular mechanisms by which neogenin and HJV enhance BMP signal transduction to regulate hepcidin are important questions, not only for understanding iron homeostasis, but also for broader insights into BMP biology.
Crosstalk between BMP-SMAD signaling and mitogen-activated protein kinases (MAPK) signaling in hepcidin regulation
BMP-SMAD signaling may also integrate with other signaling pathways to regulate hepcidin expression, such as the MAPK signaling pathway. MAPKs comprise several protein kinases characterized into at least three families including extracellular signal-related protein kinase (ERK) 1/2, c-Jun N-terminal kinases (JNK) 1/2/3, and p38 mitogen-activated protein kinases. The MAPK pathway is known to be regulated by BMP signaling and can modulate SMAD signaling through multiple mechanisms 197. It has been reported that MAPK signaling may contribute to hepcidin regulation by TFR2 and HFE in response to holo-transferrin. Indeed, in primary hepatocytes and leukemic K562 cells, holo-transferrin treatment or TFR2 crosslinking enhanced ERK1/2 signaling 198,199, and treatment with an ERK1/2 inhibitor blunted hepcidin induction by holo-transferrin in primary hepatocytes 199. In liver carcinoma HepG2 cells, knockdown of HFE and TFR2 reduced ERK1/2 signaling, and holo-transferrin-mediated induction of ERK1/2 signaling was further increased by overexpression of TFR2 and HFE, but not the HFE mutant C282Y 200, which interferes with the ability of HFE to reach the cell surface 201,202. These cell culture studies are corroborated by reduced hepatic ERK1/2 expression in parallel with hepcidin in single Hfe, single Tfr2, and double Hfe/Tfr2 knockout mice 88. However, in wildtype mice, dietary iron loading and iron administration by gavage had no effect on hepatic ERK1/2 phosphorylation 73, which argues against a role for MAPK/ERK signaling in iron-dependent hepcidin regulation in vivo. Similarly, discordant effects of iron dextran injection on hepatic MAPK/ERK signaling were reported 203,204. Additionally, in vitro studies demonstrate differential effects of BMP ligands on ERK1/2 phosphorylation and ERK1/2 inhibition on hepcidin modulation by BMPs. One study demonstrated an induction of ERK1/2 phosphorylation by BMP2 and no evident effect of ERK1/2 inhibition on hepcidin induction by BMP2 in HepG2 cells 200. Another study reported that BMP2 suppressed ERK1/2 phosphorylation and that ERK1/2 inhibition had an additive effect to stimulate hepcidin expression in primary hepatocytes 205. Although the role of MAPK/ERK signaling in hepcidin regulation by iron remains controversial, MAPK/ERK signaling may function in hepcidin regulation by other signals, including growth factors and ineffective erythropoiesis 27,205. Further mechanistic studies and mouse models are warranted to fully understand any interactions of MAPK/ERK and BMP signaling in hepcidin regulation, and its significance in systemic iron homeostasis.
Limitations of in vitro models of iron sensing
Cell culture systems are a major tool used to elucidate molecular mechanisms in many biological systems. Despite their broad utility and ease of use, cell culture models pose a major challenge to studying the underlying mechanisms of iron sensing and signaling to regulate hepcidin.
Primary LSECs are notoriously difficult to identify, isolate, and maintain in culture for numerous reasons. The methods for isolation and purification of LSECs from mice was recently reviewed 206. Compared to centrifugal elutriation and selective adherence, magnetic separation based on surface antigen expression was the most efficient method for obtaining a highly pure population 206. However, obtaining a pure population of primary LSECs is especially challenging because they lack specific biochemical markers that can distinguish the LSEC subpopulation from other vascular endothelial cells in the liver. The gold standard for identification of LSECs is the presence of fenestrations by scanning electron microscopy, which distinguishes LSECs from other endothelial cells, but lacks utility for LSEC isolation. Furthermore, some markers are not only common to other endothelial cells, but also hematopoietic cells, increasing the likelihood of contamination. Common markers used commercially or marketed for LSEC isolation include cluster of differentiation (CD) 31, also known as platelet endothelial cell adhesion molecule (PECAM)-1, and CD146. CD31 is expressed on cell junctions of most endothelial cells, but its expression on LSECs remains controversial, whereas CD146 expression is also detected in smooth muscle cells and many tumors 207. Several studies report expression of CD31 in LSECS by immunohistochemistry in liver sections or in cultured cells after permeabilization, demonstrating that isolation can change the physiologic expression of CD31. Using electron microscopy, it was reported that CD31 is only detectable on the membrane after defenestration that occurs shortly after establishing cultures 208. Whether CD31 expression is maintained on the surface is an important factor for commonly used antibody-based isolations including fluorescent-activated and magnetic-activated cell sorting, in which the accessibility of the antigen is critical for successful isolation and subsequent culture. Furthermore, it is important to emphasize that isolation protocols utilizing CD31 and/or CD146 antibodies represent mixed populations of liver endothelial cells rather than the subpopulation from the sinusoid. In any case, freshly isolated cultures will need to be tested for their iron responsiveness before they can be established as a satisfactory model of iron sensing. One study reported commercially available primary liver endothelial cells induced BMP6 expression almost 2-fold in response to NTBI treatment using ferric ammonium citrate 136. Interestingly, these cells were isolated using CD31 immunomagnetic sorting, suggesting that isolating a broader population of endothelial cells is sufficient to recapitulate iron sensing in vitro. This finding awaits validation from independent researchers but presents exciting opportunities for future mechanistic studies.
To circumvent the issues with primary cell isolation, immortalized endothelial cell lines have been developed, and primary endothelial cells isolated from larger vessels including the umbilical vein and pulmonary artery are commercially available. A prior publication did report that iron induced BMP6 mRNA in a non-commercial immortalized human liver endothelial cell line (TMNK-1 cells) 136. However, to our knowledge, HUVECs do not induce BMP6 in response to cellular iron loading, limiting their use for the studies of iron sensing. It is currently unknown whether other commonly used endothelial cell lines recapitulate BMP6 induction by iron.
Similar to LSECs, primary hepatocytes and hepatocyte-derived cell lines are also problematic for studying the iron-dependent hepcidin regulation. Although it has been reported that freshly isolated hepatocytes can induce hepcidin in response to holo-transferrin, in an HJV- and BMP2/4-dependent manner, this only occurs when hepatocytes are freshly isolated and cultured for less than 48 hours 209. Many other studies have failed to demonstrate hepcidin induction by holo-transferrin or other forms of iron in primary hepatocytes or hepatocyte-derived cell lines 6,25. One limiting factor is that isolated hepatocytes lose 95% of hepcidin transcripts within the initial 8 hours of culture 209, along with many other transcripts, impeding their use for prolonged culture. Although isolated hepatocytes only appear to respond to iron under limited experimental conditions, hepatocyte-derived cell lines and primary hepatocytes do induce hepcidin when stimulated with BMP ligands and in response to inflammatory signals 25,39,210,211. It is possible that the iron sensing proteins are lost or become damaged in the isolation process. Importantly, it is also clear that intercellular communication from different liver cell populations is critical for hepcidin regulation by iron.
Given the importance of intercellular communication, modeling the interaction between hepatocytes and LSECs in hepcidin regulation using co-culture systems might constitute a better platform to study the underlying mechanisms. In 2-D co-culture systems, those containing a triculture of primary human hepatocytes, LSECs, and fibroblasts were shown to be the most effective at prolonging hepatocyte and LSEC function in culture 212, although whether this culturing system has any beneficial effect on maintaining iron sensing mechanisms was not tested. In parallel, 3-D organoids of the liver niche and microfluidic chip platforms have also been developed 213-215. Additional considerations for single culture and co-culture models include extracellular matrix, oxygen concentration, serum content, and addition of growth factors 212,216-218, which influence hepatocyte and LSEC differentiation. Thus, 2-D and 3-D co-culture and organoid systems remain to be tested for their utility in studying iron sensing but provide an attractive uncharted avenue for studies in hepatocyte-LSEC crosstalk. Ultimately, overcoming the limitations of in vitro models of iron homeostasis will be instrumental in future mechanistic studies to address the unanswered questions in liver iron sensing.
Conclusion
A key unanswered question in our understanding of iron homeostasis is how the liver senses plasma and tissue iron levels and transduces that signal to regulate hepcidin expression. Studies thus far have demonstrated the critical functional role for the BMP-SMAD pathway in this process, but many molecular details remain unknown. Understanding the precise molecular mechanisms by which plasma and tissue iron levels are sensed to regulate BMP-SMAD signaling transduction to control hepcidin production will provide improved understanding of how systemic iron homeostasis is maintained, and how iron balance is disrupted in diseases of hepcidin deficiency or excess, which may ultimately help to identify novel molecular targets to treat these diseases. Additionally, a better understanding of the upstream signals governing BMP ligand production by iron, the impact of BMP ligand processing on downstream signaling, and the nature of the BMP receptor signaling complex and its physical and/or functional interaction with other accessory molecules will aid in better understanding of BMP signal transduction that will have wider implications for many other fields where BMPs are important.
Grant support:
National Institutes of Health grants T32-DK007540 (to ALF), R01-DK087727 and R01-DK128068 (to JLB), and the Patricia and Scott Eston Massachusetts General Hospital Research Scholar Award (to JLB).
Footnotes
Conflict of interest disclosures
JLB has been a consultant for Disc Medicine, Incyte Corporation, and Alnylam Pharmaceuticals, and owns equity in Ferrumax Pharmaceuticals, a company focused on targeting RGM proteins (including hemojuvelin) and bone morphogenetic protein (BMP/TGF-beta) superfamily signaling as hepcidin modulating agents for the treatment of anemia and other iron disorders. Dr. Babitt’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. ALF declares no conflict of interest.
References
- 1.Wang CY, Knutson MD. Hepatocyte divalent metal-ion transporter-1 is dispensable for hepatic iron accumulation and non-transferrin-bound iron uptake in mice. Hepatology. August 2013;58(2):788–98. 10.1002/hep.26401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer. 2014;3(1):31–40. 10.1159/000343856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The Role of Iron in Diabetes and Its Complications. Diabetes Care. 2007;30(7):1926. 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 4.Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol. September 21 2010;56(13):1001–12. 10.1016/j.jacc.2010.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause A, Neitz S, Mägert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. September 1 2000;480(2-3):147–50. 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 6.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. March 16 2001;276(11):7811–9. 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 7.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. March 16 2001;276(11):7806–10. 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proceedings of the National Academy of Sciences. 2002;99(7):4596–4601. 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences. 2001;98(15):8780–8785. 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesbordes-Brion J-C, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108(4):1402–1405. 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 11.Drakesmith H, Nemeth E, Ganz T. Ironing out Ferroportin. Cell Metab. November 3 2015;22(5):777–87. 10.1016/j.cmet.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D-L, Wu J, Shah BN, et al. Erythrocytic ferroportin reduces intracellular iron accumulation, hemolysis, and malaria risk. Science. 2018;359(6383):1520–1523. 10.1126/science.aal2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D-L, Ghosh MC, Ollivierre H, Li Y, Rouault TA. Ferroportin deficiency in erythroid cells causes serum iron deficiency and promotes hemolysis due to oxidative stress. Blood. 2018;132(19):2078–2087. 10.1182/blood-2018-04-842997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Zheng X, Zhang J, et al. Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury. American Journal of Physiology-Renal Physiology. 2018;315(4):F1042–F1057. 10.1152/ajprenal.00072.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. March/2005 2005;1(3):191–200. [DOI] [PubMed] [Google Scholar]
- 16.Qiao B, Sugianto P, Fung E, et al. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. June 6 2012;15(6):918–24. 10.1016/j.cmet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science. 2004;306(5704):2090–2093. 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 18.Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. February 22 2018;131(8):899–910. 10.1182/blood-2017-05-786590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billesbølle CB, Azumaya CM, Kretsch RC, et al. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature. October 2020;586(7831):807–811. 10.1038/s41586-020-2668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metabolism. 2005/March/01/ 2005;1(3):191–200. 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Pietrangelo A Ferroportin disease: pathogenesis, diagnosis and treatment. Haematologica. December 2017;102(12):1972–1984. 10.3324/haematol.2017.170720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao JJ, Krzyzanski W, Wang YM, et al. Pharmacokinetics of anti-hepcidin monoclonal antibody Ab 12B9m and hepcidin in cynomolgus monkeys. Aaps j. December 2010;12(4):646–57. 10.1208/s12248-010-9222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. September 15 2005;106(6):2196–9. 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. November 15 2008;112(10):4292–7. 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. April 1 2003;101(7):2461–3. 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 26.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. December 1 2006;108(12):3730–5. 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodnough JB, Ramos E, Nemeth E, Ganz T. Inhibition of hepcidin transcription by growth factors. Hepatology. July 2012;56(1):291–9. 10.1002/hep.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. April 2013;12(2):280–91. 10.1111/acel.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latour C, Kautz L, Besson-Fournier C, et al. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology. February 2014;59(2):683–94. 10.1002/hep.26648. [DOI] [PubMed] [Google Scholar]
- 30.Urist MR. Bone: formation by autoinduction. Science. November 12 1965;150(3698):893–9. 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 31.Senn on the Healing of Aseptic Bone Cavities by Implantation of Antiseptic Decalcified Bone. Ann Surg. November 1889;10(5):352–68. 10.1097/00000658-188907000-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. Nov-Dec 1971;50(6):1392–406. 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- 33.Hinck AP, Mueller TD, Springer TA. Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb Perspect Biol. December 1 2016;8(12). 10.1101/cshperspect.a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelsen SM, Christian JL. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem. October 2 2009;284(40):27157–66. 10.1074/jbc.M109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. The Journal of Biochemistry. 2009;147(1):35–51. 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 36.Rosenzweig BL, Imamura T, Okadome T, et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. August 15 1995;92(17):7632–6. 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babitt JL, Zhang Y, Samad TA, et al. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem. August 19 2005;280(33):29820–7. 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 38.Samad TA, Rebbapragada A, Bell E, et al. DRAGON, a bone morphogenetic protein co-receptor. J Biol Chem. April 8 2005;280(14):14122–9. 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- 39.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. May 2006;38(5):531–9. 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. June 27 1997;89(7):1165–73. 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 41.Nakao A, Afrakhte M, Morn A, et al. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature. 1997/October/01 1997;389(6651):631–635. 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 42.Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12(2):186–197. 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imamura T, Takase M, Nishihara A, et al. Smad6 inhibits signalling by the TGF-β superfamily. Nature. 1997/October/01 1997;389(6651):622–626. 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 44.Lin X, Liang YY, Sun B, et al. Smad6 recruits transcription corepressor CtBP to repress bone morphogenetic protein-induced transcription. Mol Cell Biol. December 2003;23(24):9081–93. 10.1128/mcb.23.24.9081-9093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Fei T, Zhang L, et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. June 2007;27(12):4488–99. 10.1128/mcb.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kavsak P, Rasmussen RK, Causing CG, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. December 2000;6(6):1365–75. 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 47.Ebisawa T, Fukuchi M, Murakami G, et al. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem. April 20 2001;276(16):12477–80. 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 48.Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell. July 2003;14(7):2809–17. 10.1091/mbc.e02-07-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J Cell Biol. December 10 2001;155(6):1017–27. 10.1083/jcb.200106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itóh S, Landström M, Hermansson A, et al. Transforming growth factor beta1 induces nuclear export of inhibitory Smad7. J Biol Chem. October 30 1998;273(44):29195–201. 10.1074/jbc.273.44.29195. [DOI] [PubMed] [Google Scholar]
- 51.Ishisaki A, Yamato K, Hashimoto S, et al. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J Biol Chem. May 7 1999;274(19):13637–42. 10.1074/jbc.274.19.13637. [DOI] [PubMed] [Google Scholar]
- 52.Xiao X, Alfaro-Magallanes VM, Babitt JL. Bone morphogenic proteins in iron homeostasis. Bone. 2020/September/01/ 2020;138:115495. 10.1016/j.bone.2020.115495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. Journal of Molecular Medicine. 2009/May/01 2009;87(5):471–480. 10.1007/s00109-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang C-Y, Xiao X, Bayer A, et al. Ablation of Hepatocyte Smad1, Smad5, and Smad8 Causes Severe Tissue Iron Loading and Liver Fibrosis in Mice. Hepatology. 2019;70(6):1986–2002. 10.1002/hep.30780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang CY, Core AB, Canali S, et al. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood. July 6 2017;130(1):73–83. 10.1182/blood-2016-12-759423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. December 2005;2(6):399–409. 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Canali S, Wang CY, Zumbrennen-Bullough KB, Bayer A, Babitt JL. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am J Hematol. November 2017;92(11):1204–1213. 10.1002/ajh.24888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canali S, Zumbrennen-Bullough KB, Core AB, et al. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood. January 26 2017;129(4):405–414. 10.1182/blood-2016-06-721571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch PS, Olsavszky V, Ulbrich F, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. January 26 2017;129(4):415–419. 10.1182/blood-2016-07-729822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayeur C, Leyton PA, Kolodziej SA, Yu B, Bloch KD. BMP type II receptors have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism. Blood. September 25 2014;124(13):2116–23. 10.1182/blood-2014-04-572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. January 2004;36(1):77–82. 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 62.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. October 13 2011;118(15):4224–30. 10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. April 2009;41(4):478–81. 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 64.Andriopoulos B Jr., Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. April 2009;41(4):482–7. 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daher R, Kannengiesser C, Houamel D, et al. Heterozygous Mutations in BMP6 Pro-peptide Lead to Inappropriate Hepcidin Synthesis and Moderate Iron Overload in Humans. Gastroenterology. March 2016;150(3):672–683.e4. 10.1053/j.gastro.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 66.Bignell P, Atoyebi W, Robson K. Heterozygous BMP6 Variants Coupled With HFE Variants. Gastroenterology. October 2016;151(4):769. 10.1053/j.gastro.2016.02.088. [DOI] [PubMed] [Google Scholar]
- 67.Le Gac G, Gourlaouen I, Ka C, Férec C. The p.Leu96Pro Missense Mutation in the BMP6 Gene Is Repeatedly Associated With Hyperferritinemia in Patients of French Origin. Gastroenterology. October 2016;151(4):769–70. 10.1053/j.gastro.2016.03.054. [DOI] [PubMed] [Google Scholar]
- 68.Piubelli C, Castagna A, Marchi G, et al. Identification of new BMP6 pro-peptide mutations in patients with iron overload. Am J Hematol. June 2017;92(6):562–568. 10.1002/ajh.24730. [DOI] [PubMed] [Google Scholar]
- 69.Mleczko-Sanecka K, Casanovas G, Ragab A, et al. SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood. April 1 2010;115(13):2657–65. 10.1182/blood-2009-09-238105. [DOI] [PubMed] [Google Scholar]
- 70.An P, Wang H, Wu Q, et al. Smad7 deficiency decreases iron and haemoglobin through hepcidin up-regulation by multilayer compensatory mechanisms. J Cell Mol Med. June 2018;22(6):3035–3044. 10.1111/jcmm.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kautz L, Meynard D, Monnier A, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112(4):1503–1509. 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 72.Ramos E, Kautz L, Rodriguez R, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. April 2011;53(4):1333–41. 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corradini E, Meynard D, Wu Q, et al. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54(1):273–284. 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao X, Dev S, Canali S, et al. Endothelial Bone Morphogenetic Protein 2 (Bmp2) Knockout Exacerbates Hemochromatosis in Homeostatic Iron Regulator (Hfe) Knockout Mice but not Bmp6 Knockout Mice. Hepatology. 2020;72(2):642–655. 10.1002/hep.31048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y, Alfaro-Magallanes VM, Babitt JL. Physiological and pathophysiological mechanisms of hepcidin regulation: clinical implications for iron disorders. Br J Haematol. December 14 2020. 10.1111/bjh.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. February/01 2020;105(2):260–272. 10.3324/haematol.2019.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hawula ZJ, Wallace DF, Subramaniam VN, Rishi G. Therapeutic Advances in Regulating the Hepcidin/Ferroportin Axis. Pharmaceuticals. 2019;12(4):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ginzburg YZ. Hepcidin-ferroportin axis in health and disease. Vitam Horm. 2019;110:17–45. 10.1016/bs.vh.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. January 2003;33(1):21–2. 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 80.Kearney SL, Nemeth E, Neufeld EJ, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. January 2007;48(1):57–63. 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 81.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. May 2007;92(5):583–8. 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 82.Taher AT, Saliba AN. Iron overload in thalassemia: different organs at different rates. Hematology Am Soc Hematol Educ Program. December 8 2017;2017(1):265–271. 10.1182/asheducation-2017.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Camaschella C, Roetto A, Calì A, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. May 2000;25(1):14–5. 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 84.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I–like gene is mutated in patients with hereditary haemochromatosis. Nature Genetics. 1996/August/01 1996;13(4):399–408. 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 85.Roetto A, Totaro A, Piperno A, et al. New mutations inactivating transferrin receptor 2 in hemochromatosis type 3. Blood. May 1 2001;97(9):2555–60. 10.1182/blood.v97.9.2555. [DOI] [PubMed] [Google Scholar]
- 86.Corradini E, Garuti C, Montosi G, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. October 2009;137(4):1489–97. 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corradini E, Rozier M, Meynard D, et al. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology. November 2011;141(5):1907–14. 10.1053/j.gastro.2011.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. December 2009;50(6):1992–2000. 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 89.Bolondi G, Garuti C, Corradini E, et al. Altered hepatic BMP signaling pathway in human HFE hemochromatosis. Blood Cells Mol Dis. December 15 2010;45(4):308–12. 10.1016/j.bcmd.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryan JD, Ryan E, Fabre A, Lawless MW, Crowe J. Defective bone morphogenic protein signaling underlies hepcidin deficiency in HFE hereditary hemochromatosis. Hepatology. 2010;52(4):1266–1273. 10.1002/hep.23814. [DOI] [PubMed] [Google Scholar]
- 91.Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, Loréal O. Haemochromatosis. Nat Rev Dis Primers. April 5 2018;4:18016. 10.1038/nrdp.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. July 2014;46(7):678–84. 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kautz L, Jung G, Du X, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. October 22 2015;126(17):2031–7. 10.1182/blood-2015-07-658419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arezes J, Foy N, McHugh K, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. October 4 2018;132(14):1473–1477. 10.1182/blood-2018-06-857995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang CY, Xu Y, Traeger L, et al. Erythroferrone lowers hepcidin by sequestering BMP2/6 heterodimer from binding to the BMP type I receptor ALK3. Blood. February 6 2020;135(6):453–456. 10.1182/blood.2019002620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramos E, Ruchala P, Goodnough JB, et al. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. November 1 2012;120(18):3829–36. 10.1182/blood-2012-07-440743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corradini E, Schmidt PJ, Meynard D, et al. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. November 2010;139(5):1721–9. 10.1053/j.gastro.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Preza GC, Ruchala P, Pinon R, et al. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. The Journal of Clinical Investigation. December/01/ 2011;121(12):4880–4888. 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casu C, Oikonomidou PR, Chen H, et al. Minihepcidin peptides as disease modifiers in mice affected by β-thalassemia and polycythemia vera. Blood. July 14 2016;128(2):265–76. 10.1182/blood-2015-10-676742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casu C, Chessa R, Liu A, et al. Minihepcidins improve ineffective erythropoiesis and splenomegaly in a new mouse model of adult β-thalassemia major. Haematologica. July/01 2020;105(7):1835–1844. 10.3324/haematol.2018.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J, Liu W, Liu Y, et al. New thiazolidinones reduce iron overload in mouse models of hereditary hemochromatosis and β-thalassemia. Haematologica. September/01 2019;104(9):1768–1781. 10.3324/haematol.2018.209874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nicolas G, Viatte L, Lou DQ, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. May 2003;34(1):97–101. 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 103.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. The Journal of clinical investigation. 2010;120(12):4466–4477. 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nai A, Pagani A, Mandelli G, et al. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of β-thalassemia. Blood. May 24 2012;119(21):5021–9. 10.1182/blood-2012-01-401885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. April 28 2011;117(17):4590–9. 10.1182/blood-2010-10-315507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The Serine Protease Matriptase-2 (TMPRSS6) Inhibits Hepcidin Activation by Cleaving Membrane Hemojuvelin. Cell Metabolism. 2008;8(6):502–511. 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. May 1 2007;109(9):4038–44. 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ganz T. Anemia of Inflammation. New England Journal of Medicine. 2019;381(12):1148–1157. 10.1056/NEJMra1804281. [DOI] [PubMed] [Google Scholar]
- 109.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. May 2004;113(9):1271–6. 10.1172/jci20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. November 1 2006;108(9):3204–9. 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mayeur C, Lohmeyer LK, Leyton P, et al. The type I BMP receptor Alk3 is required for the induction of hepatic hepcidin gene expression by interleukin-6. Blood. April 3 2014;123(14):2261–8. 10.1182/blood-2013-02-480095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Latour C, Besson-Fournier C, Gourbeyre O, Meynard D, Roth MP, Coppin H. Deletion of BMP6 worsens the phenotype of HJV-deficient mice and attenuates hepcidin levels reached after LPS challenge. Blood. November 23 2017;130(21):2339–2343. 10.1182/blood-2017-07-795658. [DOI] [PubMed] [Google Scholar]
- 113.Schooley JC, Kullgren B, Allison AC. Inhibition by interleukin-1 of the action of erythropoietin on erythroid precursors and its possible role in the pathogenesis of hypoplastic anaemias. Br J Haematol. September 1987;67(1):11–7. 10.1111/j.1365-2141.1987.tb02289.x. [DOI] [PubMed] [Google Scholar]
- 114.Rusten LS, Jacobsen SE. Tumor necrosis factor (TNF)-alpha directly inhibits human erythropoiesis in vitro: role of p55 and p75 TNF receptors. Blood. February 15 1995;85(4):989–96. [PubMed] [Google Scholar]
- 115.Libregts SF, Gutiérrez L, de Bruin AM, et al. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. September 1 2011;118(9):2578–88. 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 116.Guida C, Altamura S, Klein FA, et al. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood. April 2 2015;125(14):2265–75. 10.1182/blood-2014-08-595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song S-NJ, Iwahashi M, Tomosugi N, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Research & Therapy. 2013/October/02 2013;15(5):R141. 10.1186/ar4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15(6):R204. 10.1186/ar4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nature Genetics. 2008/May/01 2008;40(5):569–571. 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. May 23 2008;320(5879):1088–92. 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Folgueras AR, de Lara FM, Pendás AM, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. September 15 2008;112(6):2539–45. 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 122.Kovac S, Böser P, Cui Y, et al. Anti-hemojuvelin antibody corrects anemia caused by inappropriately high hepcidin levels. Haematologica. 2016;101(5):e173–e176. 10.3324/haematol.2015.140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cooke KS, Hinkle B, Salimi-Moosavi H, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. October 24 2013;122(17):3054–61. 10.1182/blood-2013-06-505792. [DOI] [PubMed] [Google Scholar]
- 124.Schwoebel F, van Eijk LT, Zboralski D, et al. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. March 21 2013;121(12):2311–5. 10.1182/blood-2012-09-456756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Theurl I, Schroll A, Sonnweber T, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. November 3 2011;118(18):4977–84. 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steinbicker AU, Sachidanandan C, Vonner AJ, et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. May 5 2011;117(18):4915–23. 10.1182/blood-2010-10-313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vadhan-Raj S, Abonour R, Goldman JW, et al. A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. Journal of Hematology & Oncology. 2017/March/21 2017;10(1):73. 10.1186/s13045-017-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang CY, Canali S, Bayer A, Dev S, Agarwal A, Babitt JL. Iron, erythropoietin, and inflammation regulate hepcidin in Bmp2-deficient mice, but serum iron fails to induce hepcidin in Bmp6-deficient mice. Am J Hematol. February 2019;94(2):240–248. 10.1002/ajh.25366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rausa M, Pagani A, Nai A, et al. Bmp6 expression in murine liver non parenchymal cells: a mechanism to control their high iron exporter activity and protect hepatocytes from iron overload? PLoS One. 2015;10(4):e0122696. 10.1371/journal.pone.0122696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang AS, Anderson SA, Wang J, et al. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood. February 3 2011;117(5):1687–99. 10.1182/blood-2010-06-287292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Feng Q, Migas MC, Waheed A, Britton RS, Fleming RE. Ferritin upregulates hepatic expression of bone morphogenetic protein 6 and hepcidin in mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;302(12):G1397–G1404. 10.1152/ajpgi.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kautz L, Besson-Fournier C, Meynard D, Latour C, Roth MP, Coppin H. Iron overload induces BMP6 expression in the liver but not in the duodenum. Haematologica. February 2011;96(2):199–203. 10.3324/haematol.2010.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(S1):S54–S62. 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 134.Poisson J, Lemoinne S, Boulanger C, et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. Journal of Hepatology. 2017/January/01/ 2017;66(1):212–227. 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 135.MacParland SA, Liu JC, Ma XZ, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. October 22 2018;9(1):4383. 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lim PJ, Duarte TL, Arezes J, et al. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat Metab. May 2019;1(5):519–531. 10.1038/s42255-019-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jenkitkasemwong S, Wang CY, Coffey R, et al. SLC39A14 Is Required for the Development of Hepatocellular Iron Overload in Murine Models of Hereditary Hemochromatosis. Cell Metab. July 7 2015;22(1):138–50. 10.1016/j.cmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fillebeen C, Charlebois E, Wagner J, et al. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood. January 24 2019;133(4):344–355. 10.1182/blood-2018-05-850404. [DOI] [PubMed] [Google Scholar]
- 139.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. May 2009;23(3):95–104. 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rouault TA, Hentze MW, Caughman SW, Harford JB, Klausner RD. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. September 2 1988;241(4870):1207–10. 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- 141.Truman-Rosentsvit M, Berenbaum D, Spektor L, et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood. January 18 2018;131(3):342–352. 10.1182/blood-2017-02-768580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Payne S, De Val S, Neal A. Endothelial-Specific Cre Mouse Models. Arterioscler Thromb Vasc Biol. November 2018;38(11):2550–2561. 10.1161/atvbaha.118.309669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. April 1999;21(4):396–9. 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- 144.Ned RM, Swat W, Andrews NC. Transferrin receptor 1 is differentially required in lymphocyte development. Blood. November 15 2003;102(10):3711–8. 10.1182/blood-2003-04-1086. [DOI] [PubMed] [Google Scholar]
- 145.Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics. December 2002;80(6):630–45. 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- 146.Wang CY, Jenkitkasemwong S, Duarte S, et al. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem. October 5 2012;287(41):34032–43. 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He L, Girijashanker K, Dalton TP, et al. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. July 2006;70(1):171–80. 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 148.Wang B, He L, Dong H, Dalton TP, Nebert DW. Generation of a Slc39a8 hypomorph mouse: markedly decreased ZIP8 Zn2+/(HCO3−)2 transporter expression. Biochem Biophys Res Commun. July 1 2011;410(2):289–94. 10.1016/j.bbrc.2011.05.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin W, Li D, Cheng L, et al. Zinc transporter Slc39a8 is essential for cardiac ventricular compaction. The Journal of Clinical Investigation. February/01/ 2018;128(2):826–833. 10.1172/JCI96993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dalton TP, He L, Wang B, et al. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(9):3401–3406. 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Steimle BL, Smith FM, Kosman DJ. The solute carriers ZIP8 and ZIP14 regulate manganese accumulation in brain microvascular endothelial cells and control brain manganese levels. J Biol Chem. December 13 2019;294(50):19197–19208. 10.1074/jbc.RA119.009371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997/July/01 1997;388(6641):482–488. 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 153.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. May 2005;115(5):1258–66. 10.1172/jci24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen TT, Li L, Chung DH, et al. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med. October 3 2005;202(7):955–65. 10.1084/jem.20042433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li L, Fang CJ, Ryan JC, et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A. February 23 2010;107(8):3505–10. 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li JY, Paragas N, Ned RM, et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. January 2009;16(1):35–46. 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Silva-Gomes S, Santos AG, Caldas C, et al. Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J Hepatol. February 2014;60(2):354–61. 10.1016/j.jhep.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 158.Walter PB, Knutson MD, Paler-Martinez A, et al. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proceedings of the National Academy of Sciences. 2002;99(4):2264–2269. 10.1073/pnas.261708798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Inoue H, Hanawa N, Katsumata SI, Katsumata-Tsuboi R, Takahashi N, Uehara M. Iron deficiency induces autophagy and activates Nrf2 signal through modulating p62/SQSTM. Biomed Res. 2017;38(6):343–350. 10.2220/biomedres.38.343. [DOI] [PubMed] [Google Scholar]
- 160.Inoue H, Kobayashi K-I, Ndong M, et al. Activation of Nrf2/Keap1 signaling and autophagy induction against oxidative stress in heart in iron deficiency. Bioscience, Biotechnology, and Biochemistry. 2015/August/03 2015;79(8):1366–1368. 10.1080/09168451.2015.1018125. [DOI] [PubMed] [Google Scholar]
- 161.Kautz L, Meynard D, Besson-Fournier C, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009/September/17/ 2009;114(12):2515–2520. 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 162.Pagani A, Mariateresa P, Colucci S, et al. Hemochromatosis proteins are dispensable for the acute hepcidin response to BMP2. Haematologica. 2020;105(10):e493–e493. 10.3324/haematol.2019.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kleven MD, Jue S, Enns CA. Transferrin Receptors TfR1 and TfR2 Bind Transferrin through Differing Mechanisms. Biochemistry. March 6 2018;57(9):1552–1559. 10.1021/acs.biochem.8b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.West AP Jr., Bennett MJ, Sellers VM, Andrews NC, Enns CA, Bjorkman PJ. Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE. J Biol Chem. December 8 2000;275(49):38135–8. 10.1074/jbc.C000664200. [DOI] [PubMed] [Google Scholar]
- 165.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. December 15 2004;104(13):4287–93. 10.1182/blood-2004-06-2477. [DOI] [PubMed] [Google Scholar]
- 166.Johnson MB, Chen J, Murchison N, Green FA, Enns CA. Transferrin receptor 2: evidence for ligand-induced stabilization and redirection to a recycling pathway. Mol Biol Cell. March 2007;18(3):743–54. 10.1091/mbc.e06-09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pagani A, Vieillevoye M, Nai A, et al. Regulation of cell surface transferrin receptor-2 by iron-dependent cleavage and release of a soluble form. Haematologica. April 2015;100(4):458–65. 10.3324/haematol.2014.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.West AP Jr., Giannetti AM, Herr AB, et al. Mutational analysis of the transferrin receptor reveals overlapping HFE and transferrin binding sites. J Mol Biol. October 19 2001;313(2):385–97. 10.1006/jmbi.2001.5048. [DOI] [PubMed] [Google Scholar]
- 169.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. March 2008;7(3):205–14. 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. March 2009;9(3):217–27. 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schmidt PJ, Fleming MD. Transgenic HFE-dependent induction of hepcidin in mice does not require transferrin receptor-2. Am J Hematol. June 2012;87(6):588–95. 10.1002/ajh.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.D'Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. November 2012;57(5):1052–60. 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 173.Wu XG, Wang Y, Wu Q, et al. HFE interacts with the BMP type I receptor ALK3 to regulate hepcidin expression. Blood. August 21 2014;124(8):1335–43. 10.1182/blood-2014-01-552281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao N, Nizzi CP, Anderson SA, et al. Low intracellular iron increases the stability of matriptase-2. J Biol Chem. February 13 2015;290(7):4432–46. 10.1074/jbc.M114.611913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Finberg KE, Whittlesey RL, Fleming MD, Andrews NC. Down-regulation of Bmp/Smad signaling by Tmprss6 is required for maintenance of systemic iron homeostasis. Blood. May 6 2010;115(18):3817–26. 10.1182/blood-2009-05-224808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wahedi M, Wortham AM, Kleven MD, et al. Matriptase-2 suppresses hepcidin expression by cleaving multiple components of the hepcidin induction pathway. J Biol Chem. November 3 2017;292(44):18354–18371. 10.1074/jbc.M117.801795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee P, Hsu M-H, Welser-Alves J, Peng H. Severe microcytic anemia but increased erythropoiesis in mice lacking Hfe or Tfr2 and Tmprss6. Blood cells, molecules & diseases. 2012;48(3):173–178. 10.1016/j.bcmd.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lenoir A, Deschemin J-C, Kautz L, et al. Iron-deficiency anemia from matriptase-2 inactivation is dependent on the presence of functional Bmp6. Blood. 2011;117(2):647–650. 10.1182/blood-2010-07-295147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Enns CA, Jue S, Zhang AS. The ectodomain of matriptase-2 plays an important nonproteolytic role in suppressing hepcidin expression in mice. Blood. August 20 2020;136(8):989–1001. 10.1182/blood.2020005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Constam DB. Regulation of TGFβ and related signals by precursor processing. Semin Cell Dev Biol. August 2014;32:85–97. 10.1016/j.semcdb.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 181.Harrison CA, Al-Musawi SL, Walton KL. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-β superfamily ligands. Growth Factors. October 2011;29(5):174–86. 10.3109/08977194.2011.608666. [DOI] [PubMed] [Google Scholar]
- 182.Valera E, Isaacs MJ, Kawakami Y, Izpisúa Belmonte JC, Choe S. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS One. June 17 2010;5(6):e11167. 10.1371/journal.pone.0011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Israel DI, Nove J, Kerns KM, et al. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13(3-4):291–300. 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 184.Tajer B, Dutko JA, Little SC, Mullins MC. BMP heterodimers signal via distinct type I receptor class functions. Proc Natl Acad Sci U S A. April 13 2021;118(15). 10.1073/pnas.2017952118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim HS, Neugebauer J, McKnite A, Tilak A, Christian JL. BMP7 functions predominantly as a heterodimer with BMP2 or BMP4 during mammalian embryogenesis. Elife. September 30 2019;8. 10.7554/eLife.48872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tillet E, Ouarné M, Desroches-Castan A, et al. A heterodimer formed by bone morphogenetic protein 9 (BMP9) and BMP10 provides most BMP biological activity in plasma. J Biol Chem. July 13 2018;293(28):10963–10974. 10.1074/jbc.RA118.002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. May 2009;11(5):637–43. 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Latour C, Besson-Fournier C, Meynard D, et al. Differing impact of the deletion of hemochromatosis-associated molecules HFE and transferrin receptor-2 on the iron phenotype of mice lacking bone morphogenetic protein 6 or hemojuvelin. Hepatology. January 2016;63(1):126–37. 10.1002/hep.28254. [DOI] [PubMed] [Google Scholar]
- 189.Arezes J, Foy N, McHugh K, et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood. February 20 2020;135(8):547–557. 10.1182/blood.2019003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu Q, Sun CC, Lin HY, Babitt JL. Repulsive guidance molecule (RGM) family proteins exhibit differential binding kinetics for bone morphogenetic proteins (BMPs). PLoS One. 2012;7(9):e46307. 10.1371/journal.pone.0046307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Canali S, Core AB, Zumbrennen-Bullough KB, et al. Activin B Induces Noncanonical SMAD1/5/8 Signaling via BMP Type I Receptors in Hepatocytes: Evidence for a Role in Hepcidin Induction by Inflammation in Male Mice. Endocrinology. March 2016;157(3):1146–62. 10.1210/en.2015-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Healey EG, Bishop B, Elegheert J, Bell CH, Padilla-Parra S, Siebold C. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat Struct Mol Biol. June 2015;22(6):458–65. 10.1038/nsmb.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Traeger L, Gallitz I, Sekhri R, et al. ALK3 undergoes ligand-independent homodimerization and BMP-induced heterodimerization with ALK2. Free Radic Biol Med. December 2018;129:127–137. 10.1016/j.freeradbiomed.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee DH, Zhou LJ, Zhou Z, et al. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood. April 15 2010;115(15):3136–45. 10.1182/blood-2009-11-251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Enns CA, Jue S, Zhang AS. Hepatocyte Neogenin Is Required for Hemojuvelin-Mediated Hepcidin Expression and Iron Homeostasis in Mice. Blood. April 6 2021. 10.1182/blood.2020009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao N, Maxson JE, Zhang RH, Wahedi M, Enns CA, Zhang AS. Neogenin Facilitates the Induction of Hepcidin Expression by Hemojuvelin in the Liver. J Biol Chem. June 3 2016;291(23):12322–35. 10.1074/jbc.M116.721191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. August 29 2005;24(37):5742–50. 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 198.Calzolari A, Raggi C, Deaglio S, et al. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci. November 1 2006;119(Pt 21):4486–98. 10.1242/jcs.03228. [DOI] [PubMed] [Google Scholar]
- 199.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. June 2009;94(6):765–72. 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poli M, Luscieti S, Gandini V, et al. Transferrin receptor 2 and HFE regulate furin expression via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling. Implications for transferrin-dependent hepcidin regulation. Haematologica. November 2010;95(11):1832–40. 10.3324/haematol.2010.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Waheed A, Parkkila S, Zhou XY, et al. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci U S A. November 11 1997;94(23):12384–9. 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Feder JN, Tsuchihashi Z, Irrinki A, et al. The hemochromatosis founder mutation in HLA-H disrupts beta2-microglobulin interaction and cell surface expression. J Biol Chem. May 30 1997;272(22):14025–8. 10.1074/jbc.272.22.14025. [DOI] [PubMed] [Google Scholar]
- 203.Díaz V, Gammella E, Recalcati S, et al. Liver iron modulates hepcidin expression during chronically elevated erythropoiesis in mice. Hepatology. December 2013;58(6):2122–32. 10.1002/hep.26550. [DOI] [PubMed] [Google Scholar]
- 204.Tangudu NK, Buth N, Strnad P, Cirstea IC, Spasić MV. Deregulation of Hepatic Mek1/2−Erk1/2 Signaling Module in Iron Overload Conditions. Pharmaceuticals (Basel). May 7 2019;12(2). 10.3390/ph12020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen H, Choesang T, Li H, et al. Increased hepcidin in transferrin-treated thalassemic mice correlates with increased liver BMP2 expression and decreased hepatocyte ERK activation. Haematologica. 2016;101(3):297–308. 10.3324/haematol.2015.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Meyer J, Gonelle-Gispert C, Morel P, Bühler L. Methods for Isolation and Purification of Murine Liver Sinusoidal Endothelial Cells: A Systematic Review. PLOS ONE. 2016;11(3):e0151945. 10.1371/journal.pone.0151945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang Z, Xu Q, Zhang N, Du X, Xu G, Yan X. CD146, from a melanoma cell adhesion molecule to a signaling receptor. Signal Transduction and Targeted Therapy. 2020/August/11 2020;5(1):148. 10.1038/s41392-020-00259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004/October/01 2004;287(4):G757–G763. 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 209.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110(6):2182–2189. 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kawabata H, Fleming RE, Gui D, et al. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. January 1 2005;105(1):376–81. 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- 211.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. July 2007;117(7):1933–9. 10.1172/jci31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro [ 10.1002/hep.23085]. Hepatology. 2009/September/01 2009;50(3):920–928. 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ramachandran SD, Schirmer K, Münst B, et al. In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS One. 2015;10(10):e0139345. 10.1371/journal.pone.0139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pettinato G, Lehoux S, Ramanathan R, et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Scientific Reports. 2019/June/20 2019;9(1):8920. 10.1038/s41598-019-45514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kang YB, Sodunke TR, Lamontagne J, et al. Liver sinusoid on a chip: Long-term layered co-culture of primary rat hepatocytes and endothelial cells in microfluidic platforms [ 10.1002/bit.25659]. Biotechnology and Bioengineering. 2015/December/01 2015;112(12):2571–2582. 10.1002/bit.25659. [DOI] [PubMed] [Google Scholar]
- 216.Elvevold K, Smedsrød B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008/February/01 2008;294(2):G391–G400. 10.1152/ajpgi.00167.2007. [DOI] [PubMed] [Google Scholar]
- 217.Martinez I, Nedredal GI, Øie CI, et al. The influence of oxygen tension on the structure and function of isolated liver sinusoidal endothelial cells. Comparative Hepatology. 2008/May/05 2008;7(1):4. 10.1186/1476-5926-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Guo R, Xu X, Lu Y, Xie X. Physiological oxygen tension reduces hepatocyte dedifferentiation in in vitro culture. Scientific Reports. 2017/July/19 2017;7(1):5923. 10.1038/s41598-017-06433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]