Abstract
Several vaccines have been approved worldwide for the prevention of morbidity and mortality against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, the development of these vaccines has raised concerns regarding their adverse effects. Herein, we report the first case of intracerebral hemorrhage (ICH) due to vasculitis after the first dose of mRNA vaccine (BNT162b2, Pfizer/BioN-Tech). Although this case cannot demonstrate a direct relationship between COVID-19 vaccination and vasculitis, the clinical and histological features of this patient are highly consistent with the adverse effects of COVID-19 vaccine.
Keywords: Intracerebral hemorrhage, COVID-19, Vaccination, Vasculitis
Introduction
The coronavirus disease (COVID-19) pandemic has negatively affected people with acute cerebrovascular diseases including stroke [9]. Several vaccines have been approved worldwide for the prevention of morbidity and mortality against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3, 11, 15]. However, the development of these vaccines has raised concerns regarding their adverse effects. For example, studies have reported cases of stroke due to thromboembolism in the central nervous system (CNS) [7, 17]. Herein, we report the first case of intracerebral hemorrhage (ICH) due to vasculitis after the first dose of mRNA vaccine (BNT162b2, Pfizer/BioN-Tech).
Case report
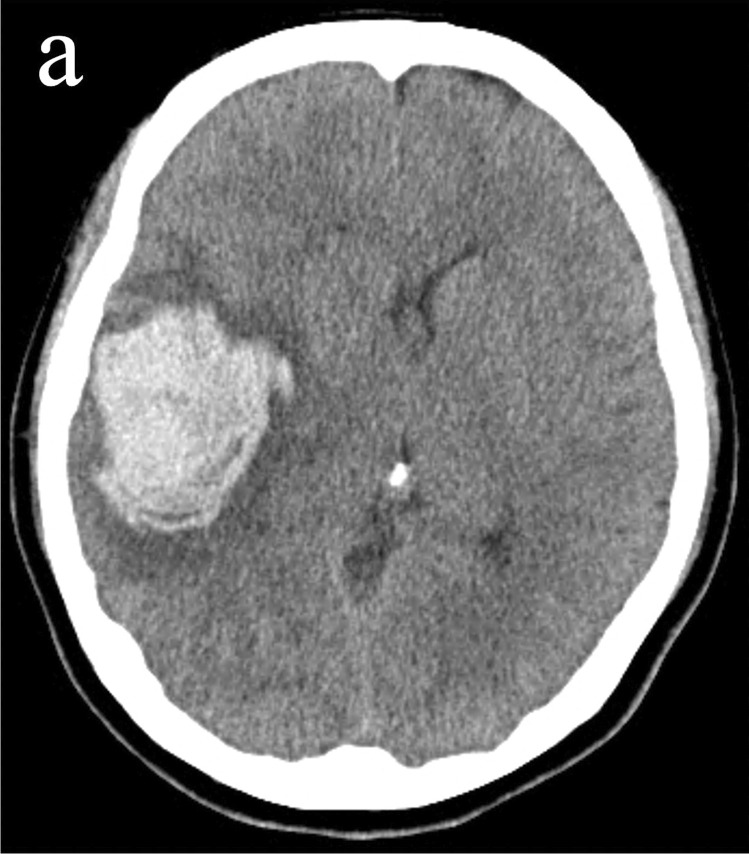
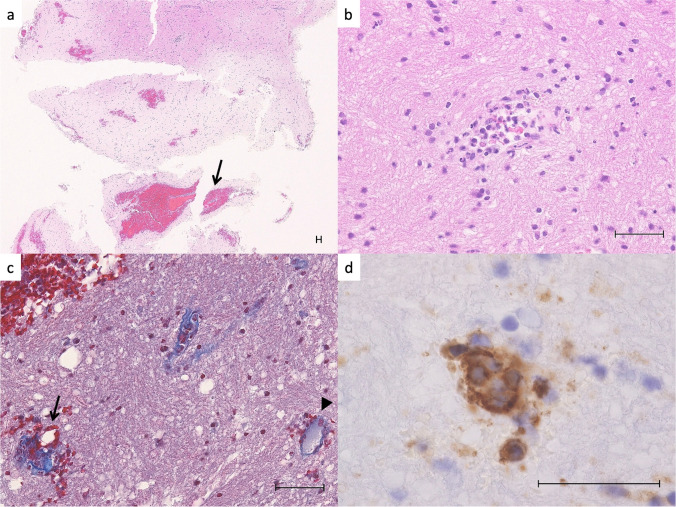
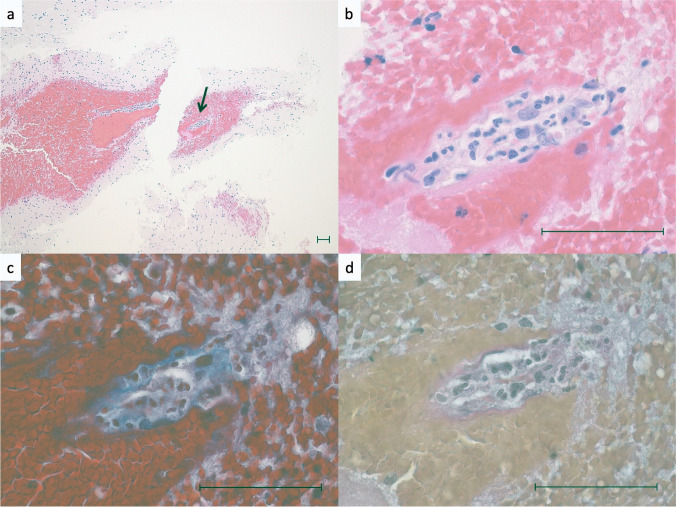
A 48-year-old woman with no significant previous medical history, including allergy, vasculitis, or autoimmune disorders, received the first dose of the mRNA COVID-19 vaccine (BNT162b2, Pfizer/BioNTech). She noticed a gradually progressing left hemiparesis and was transferred to our hospital because of worsening consciousness and anisocoria from another hospital 2 days after vaccination. Her systolic blood pressure was 119. Head computed tomography (CT) showed ICH of up to 5.6 cm and edema surrounding it in the right temporal lobe (Fig. 1). CT angiography showed no abnormal vascular lesions, including vascular malformations or cerebral venous thrombosis (CVT). No rash was observed on her body and a PCR test for SARS-CoV-2 was negative. Laboratory tests, including complete blood count, were normal except for a hemoglobin concentration of 9.6 g/dL. Moreover, coagulation tests of PT and APTT and serum levels of ANCA and CRP were normal. We performed a right frontotemporal craniotomy to evacuate the ICH. The clot was removed successfully. No abnormal bleeding was found, except for mild oozing from the hematoma wall. Pathological findings from the hematoma wall and cerebral tissue revealed neutrophilic infiltrates in small vessels with disruption of vascular architecture, few cell debris, erythrocyte leakage, and endothelial nuclear enlargement (Figs. 2 and 3). Congo red staining revealed no amyloid deposition in the vessel walls. No granuloma or fibrinoid necrosis was observed in the vessels. The background cerebral tissue showed no necrosis and no suppurative inflammatory findings suggestive of infection. Elastica van Gieson staining, Masson trichrome staining, desmin immunostaining, and CD31 immunostaining showed that the affected blood vessel walls contained only thin collagen fibers and endothelial cells, with no elastic fibers or smooth muscle, suggesting that it was neutrophilic vasculitis at the level of capillary to postcapillary venules. Postoperative additional blood examination revealed unremarkable prothrombotic and autoimmune findings. Enhanced MRI revealed no abnormal lesions except for the hematoma. Rest of the clinical course was favorable, and the patient was discharged 4 weeks after onset.
Fig. 1.
Computed tomography revealed intracerebral hemorrhage and edema surrounding it in the right temporal lobe
Fig. 2.
Histology of the hematoma wall and cerebral tissue. a H&E stain shows scattered petechial hemorrhages and perivascular hematoma (arrows) in the cerebral cortex. b Typical angiocentric neutrophilic infiltration with few cell fragments and mild extravasation of red blood cells. There is no necrosis in the surrounding brain tissue. c Masson Trichrome stain shows that the small blood vessel in the hemorrhage area (arrow) is disrupted and has collagen fiber tears and extravasation of red blood cells compared to the open, uninvolved small blood vessel (arrowhead). d High power view of the arrow area in Fig. 2c. CD31 immunostaining shows that the nuclei of vascular endothelial cells are enlarged and disorganized. Scale bars are 50 μm
Fig. 3.
Histological features of vasculitis. a Middle power view of the perivascular hematoma. b High power view of the arrow area in Fig. 3a. The small blood vessel is compressed and occluded by the perivascular hematoma. Neutrophilic infiltration is observed in the vessel wall, and endothelial cell enlargement and fibrin deposition are shown. c Same area as in Fig. 3b. Masson Trichrome stain shows tearing and obscuration of collagen fibers in the wall of the small vessel. d Same area as in Fig. 3b. Elastica van Gieson staining shows elastic fibers and smooth muscle are absent in the vessel wall. Scale bars are 50 μm
Discussion
During the COVID-19 pandemic, several vaccines against SARS-CoV-2 have been approved after the demonstration of safety and efficacy [3, 11, 15]. However, several adverse effects have also been reported. Typical reactogenicity included mild-to-moderate pain at the injection site, fatigue, headache, and fever. In addition, some rare cases of vaccine-induced CNS diseases have been reported. Particularly, stroke due to thromboembolism after COVID-19 vaccination is a major concern [7, 17]. The mechanism of vaccine-induced thromboembolism is usually the same as that of SARS-CoV-2, which is a hypercoagulable state. However, some rare cases of thrombotic thrombocytopenia as the cause of stroke have also been demonstrated [4]. In these cases, venous thrombosis may appear more frequently than arterial thrombosis [2]. Our study is the first to report a case of ICH due to vasculitis following COVID-19 vaccination.
Vasculitis after COVID-19 vaccination has been reported in different dermatological areas [5, 6]. A recent study demonstrated that the frequencies of vasculitis within approximately two weeks after the first dose of Pfizer (New York, NY, USA)-BioNTech (Mainz, Germany) (BNT162b2) and Moderna (mRNA-1273) vaccine were 2.9% and 0.7%, respectively [8]. Pathological findings include small vasculitis, characterized by fibrin deposits, fibrinoid necrosis associated with neutrophil infiltration of the vessel walls, and leukocyte destruction. The pathogenesis of vasculitis following vaccination remains unclear, although an autoimmune mechanism mediated by vaccine proteins has been proposed [1]. Therefore, similar to influenza vaccination, the COVID-19 vaccination may result in vasculitis in several organs [16].
Clinicopathological differential diagnoses include hypertensive cerebral hemorrhage, amyloid angiopathy, angiitis of the central nervous system (ACNS), secondary vasculitis, and an adverse effect of the COVID-19 vaccine. The patient’s blood pressure was within normal limits, and there was no history of hypertension. Amyloid angiopathy is associated with aging, and amyloid deposits are found mainly in the small arteries and arterioles. Although amyloid deposition may be accompanied by granulomatous vasculitis, there was no amyloid deposition or granuloma in the vessel wall in the present case [12]. In typical ACNS, small- to medium-sized arteries are involved that present with histological findings of granulomatous vasculitis, necrotizing polyarteritis, and lymphoplasmacytic vasculitis [13]. However, the histological findings observed in this case were different. Lastly, secondary vasculitis is an incidental histological finding in any organ and is usually associated with necrosis or viral/bacterial infection, including in COVID-19. Indeed, SARS-CoV-2 itself induces a hyperinflammatory response in endothelial cells, which causes vasculitis in the kidneys, heart, lungs, and small bowel [14]. However, no necrosis or pyogenic inflammation was observed in the cerebral tissue of this patient [10]. Although there is no direct proof that vasculitis was caused by the vaccination, the clinical and histologic features of this patient are highly consistent with an adverse effect of the COVID-19 vaccine. Thus, further studies are necessary to ensure the safety of COVID-19 vaccination.
Conclusions
This is the first report of ICH possibly due to COVID-19 vaccine-induced vasculitis. Histological assessment is required for the definitive diagnosis of ICH associated with COVID-19 vaccine-induced vasculitis.
Author contribution
Kenji Fukuda: Conception and design of this study; Data curation; Writing, original draft preparation; Visualization; Project administration. Ryuhei Takeyama: Writing, original draft preparation; Data collection. Yuki Kouzaki: Data collection. Takahisa Koga: Data collection. Shuji Hayashi: Supervision. Hiroshi Ohtani: Writing, original draft preparation; Reviewing and editing, Supervision. Tooru Inoue: Supervision.
Data availability
The data that support the findings of this study are openly available.
Declarations
Informed consent
Informed consent of participation and publication was obtained from the patient.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Vascular Neurosurgery—Other
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agmon-Levin N, Paz Z, Israeli E, Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. 2009;5:648–652. doi: 10.1038/nrrheum.2009.196. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ, Gull D, Jager HR, Scully MA, Werring DJ. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;92:1247–1248. doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Group CS Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(403):416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19:1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostan E, Gulseren D, Gokoz O. New-onset leukocytoclastic vasculitis after COVID-19 vaccine. Int J Dermatol. 2021;60:1305–1306. doi: 10.1111/ijd.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen SR, Prussick L, Kahn JS, Gao DX, Radfar A, Rosmarin D. Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int J Dermatol. 2021;60:1032–1033. doi: 10.1111/ijd.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias L, Soares-Dos-Reis R, Meira J, Ferrao D, Soares PR, Pastor A, Gama G, Fonseca L, Fagundes V, Carvalho M. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021;30:105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, Desai SR, French LE, Lim HW, Thiers BH, Hruza GJ, Blumenthal KG, Fox LP, Freeman EE. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiris S, Mesa H, Aysola A, Manivel J, Toledo J, Borges-Sa M, Aldighieri S, Reveiz L. Pathological findings in organs and tissues of patients with COVID-19: a systematic review. PLoS One. 2021;16:e0250708. doi: 10.1371/journal.pone.0250708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(2603):2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvarani C, Brown RD, Jr, Calamia KT, Christianson TJ, Huston J, 3rd, Meschia JF, Giannini C, Miller DV, Hunder GG. Primary central nervous system vasculitis: comparison of patients with and without cerebral amyloid angiopathy. Rheumatology (Oxford) 2008;47:1671–1677. doi: 10.1093/rheumatology/ken328. [DOI] [PubMed] [Google Scholar]
- 13.Salvarani C, Brown RD, Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet. 2012;380:767–777. doi: 10.1016/S0140-6736(12)60069-5. [DOI] [PubMed] [Google Scholar]
- 14.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Clutterbuck EA, Collins AM, Cutland CL, Darton TC, Dheda K, Dold C, Duncan CJA, Emary KRW, Ewer KJ, Flaxman A, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Galiza E, Goodman AL, Green CM, Green CA, Greenland M, Hill C, Hill HC, Hirsch I, Izu A, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Libri V, Lillie PJ, Marchevsky NG, Marshall RP, Mendes AVA, Milan EP, Minassian AM, McGregor A, Mujadidi YF, Nana A, Padayachee SD, Phillips DJ, Pittella A, Plested E, Pollock KM, Ramasamy MN, Ritchie AJ, Robinson H, Schwarzbold AV, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Thomson EC, Torok ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, White T, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Oxford CVTG. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T. Vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 2017;13:188–196. doi: 10.2174/1573397113666170517155443. [DOI] [PubMed] [Google Scholar]
- 17.Zakaria Z, Sapiai NA, Ghani ARI. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir (Wien) 2021;163:2359–2362. doi: 10.1007/s00701-021-04860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available.