Abstract
Background
Antibiotic and antiviral agents may be prescribed in patients with suspected or confirmed coronavirus disease 2019 (COVID-19) infections because of in vitro evidence of cessation of viral replication, potential bacterial secondary or coinfection, and inability to distinguish COVID-19 infections from common bacterial infections. The objective of this study was to evaluate antimicrobial prescribing patterns in the outpatient setting during the initial peak of COVID-19 in New York City.
Methods
This single-center, retrospective chart review included patients at least 18 years old who were prescribed oral antimicrobial agents in outpatient primary care clinics between March and May 2020. Data were compared with prescribing patterns from March to May 2019. The primary outcome was the number of antimicrobial prescriptions per 1000 patient visits. Secondary outcomes included documented indication, incidence of confirmed infections, mortality, and/or hospital admission within 90 days. Descriptive statistics were used.
Results
The overall antimicrobial prescribing rate increased from 31.94 prescriptions per 1000 visits in 2019 to 57.48 prescriptions per 1000 visits in 2020. Agents that were more commonly prescribed during the initial peak of COVID-19 include cefpodoxime, hydroxychloroquine, doxycycline, and sulfamethoxazole-trimethoprim. COVID-19 represented 7 (6%) documented antimicrobial indications in 2020, with agents such as azithromycin, hydroxychloroquine, doxycycline, cefpodoxime, and oseltamivir prescribed.
Conclusions
Overall antimicrobial prescribing rates in outpatient primary care clinics increased during the first peak of COVID-19 in an area with high infection burden. This increase may have been influenced by restricted patient evaluation, changes in patient management, and a decrease in overall patient visits.
Key Words: antibiotics, antivirals, prescribing, outpatient, coronavirus disease 2019, COVID-19, New York City
Antibiotics may be prescribed in patients with suspected or confirmed coronavirus disease 2019 (COVID-19) infections because of in vitro evidence of cessation of viral replication, as well as the risk of potential bacterial coinfection or bacterial secondary infection.1 During the initial peak of COVID-19 in New York City, increased telehealth visits limited patient evaluation and high inpatient census created a greater need to prevent hospitalizations. The inability to distinguish COVID-19 infections from common bacterial infections, such as community-acquired pneumonia, may have influenced antimicrobial prescribing patterns in the outpatient setting. This may be due to similar clinical presentation, limited severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen testing capacity during the initial peak, and lack of diagnostic tests in the outpatient and telehealth setting. In patients without confirmed COVID-19, empiric treatment for bacterial pathogens may be appropriate in patients with suspected pneumonia.2 During the initial months of the pandemic, antibiotic and antiviral agents that were of interest for therapeutic treatment of COVID-19 included azithromycin, hydroxychloroquine, tetracyclines, and oseltamivir.3–5 Although the combination was a common course of therapy, the National Institutes of Health recommends against the use of hydroxychloroquine plus azithromycin in nonhospitalized patients, except in clinical trials.4 The Infectious Diseases Society of America also recommends against use of hydroxychloroquine with or without azithromycin.6 Doxycycline has yet to be studied in clinical trials; however, tetracyclines have been proposed as potential therapeutic agents based on their mechanisms of action.5
Literature reporting the incidence of bacterial coinfections and secondary infections for COVID-19 estimates low rates of coinfection at 3.5% and secondary infections at 15.5%.1 Analysis for all coronaviruses (SARS-1, Middle East Respiratory Syndrome, SARS-CoV-2, and others) report antibiotic used in 79% of patients, despite an estimated incidence of only 8% for bacterial or fungal coinfections.7 In New York City, bacterial and fungal coinfections were reported in 3.6% of patients at one academic medical center; 79% of these patients received antibiotics.8 Most of the reported incidences of coinfection, secondary infection, and antibiotic use are focused on inpatient admissions in communities with varying COVID-19 prevalences.1,7,8 A subgroup analysis of patients treated in the ambulatory setting estimated an outpatient antimicrobial prescribing rate of 3%.8
Within the United States, New York City was considered one of the epicenters early in the COVID-19 pandemic. Brooklyn had the second highest number of cases out of the 5 boroughs from February 29 to June 1, 2020.9 COVID-19 infections within the University Hospital of Brooklyn at SUNY Downstate Health Sciences University first peaked between March and May 2020. In addition, the University Hospital of Brooklyn was officially designated as a COVID-19–only facility from March 28 to June 5, 2020. This study evaluated outpatient antimicrobial prescribing during the initial peak of COVID-19 early on in the pandemic, when health care services rapidly changed to face the challenges of a pandemic.
MATERIALS AND METHODS
A single-center, retrospective chart review at an academic medical center that evaluated antimicrobial prescribing patterns in the outpatient setting during COVID-19 was completed. This study was determined to be exempt from the SUNY Downstate Medical Center Institutional Review Board and Privacy Board. Patients were identified using computer-generated reports of outpatient prescriptions sent from 3 primary care clinics. Patients were included in the study if they were at least 18 years old and were prescribed oral antibiotics and/or antiviral agents during an outpatient visit in a primary care clinic. Patients who were excluded include those who received prescriptions written for the same regimen within 5 days of the original prescription, as these are likely duplicate prescriptions sent to the pharmacy for various reasons but most likely for electronic prescribing failures. Patients receiving additional prescriptions for the same regimen with a duration of 30 days or more were excluded to eliminate repetition of chronic antibiotic and/or antiviral agents. Patients prescribed antimicrobials from March 2020 to May 2020 represented prescribing patterns during COVID-19. Data from March 2019 to May 2019 represented antimicrobial prescribing patterns before the pandemic for comparison. These dates were chosen to eliminate the potential of separate identifiable infections because of seasonal fluctuations.
Charts were reviewed for patient demographics including age, sex, race, and comorbidities. The primary outcome was the number of antibiotic and/or antiviral prescriptions per 1000 patient visits. Antimicrobial prescribing was reported for each individual agent and as a total of all antibiotic and/or antiviral agents. Secondary outcomes evaluated include hospital admission at the University Hospital of Brooklyn within 90 days, mortality within 90 days, incidence of confirmed infections, and documented indication. Confirmed infections included a diagnosis of a bacterial and/or viral infection supported by laboratory, culture, or imaging results. Hospital admissions and mortality within 90 days were reported based on the total number of patients. Incidence of confirmed infections was reported based on total number of prescriptions, as some patients were prescribed multiple agents. Descriptive statistics were used because this represented census data.
RESULTS
In the 2019 prepandemic group, 283 patients with 288 prescriptions from a total 9011 clinic visits were included. The 2020 COVID-19 group included 277 patients with 305 prescriptions from a total 5307 clinic visits (Fig. 1). The average age was approximately 56 years, and roughly 70% of patients were female in both groups. In 2019 and 2020, respectively, 90.5% and 92.1% of patients were Black. The most common comorbidities were hypertension (61.1%, 58.8%), diabetes mellitus (33.9%, 34.7%), hyperlipidemia (33.6%, 26.7%), and asthma (22.6%, 17.3%). Ninety-eight percent of visits in 2019 were in-person compared with only 36.8% of visits in 2020. The number of telehealth visits increased from 2.1% in 2019 to 63.2% in 2020 (Table 1).
FIGURE 1.
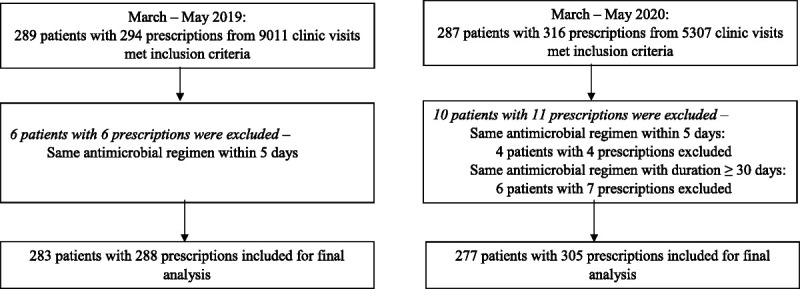
Flowchart of patient inclusion.
TABLE 1.
Demographics
| 2019 (n = 283) | 2020 (n = 277) | |
|---|---|---|
| Age, mean ± SD, y | 56.0 ± 19.0 | 56.5 ± 17.7 |
| Sex, n (%) | ||
| Female | 198 (70.0) | 203 (73.3) |
| Male | 85 (30.0) | 74 (26.7) |
| Race, n (%) | ||
| Black | 256 (90.5) | 255 (92.1) |
| White | 21 (7.4) | 12 (4.3) |
| Asian | 2 (0.7) | 4 (1.4) |
| Hispanic | 0 (0.0) | 1 (0.4) |
| Undisclosed | 4 (1.4) | 5 (1.8) |
| Comorbidities, n (%) | ||
| Hypertension | 173 (61.1) | 163 (58.8) |
| Diabetes mellitus | 96 (33.9) | 96 (34.7) |
| Hyperlipidemia | 95 (33.6) | 74 (26.7) |
| Asthma | 64 (22.6) | 48 (17.3) |
| Heart failure | 29 (10.2) | 19 (6.9) |
| CAD | 26 (9.2) | 19 (6.9) |
| COPD | 21 (7.4) | 15 (5.5) |
| Cancer | 21 (7.4) | 17 (6.1) |
| Renal disease | 18 (6.4) | 24 (8.7) |
| Type of visit, n (%) | ||
| In-person | 277 (97.9) | 102 (36.8) |
| Telehealth* | 6 (2.1) | 175 (63.2) |
*Telehealth visits included televideo and audio-only visits.
CAD indicates coronary artery disease; COPD, chronic obstructive pulmonary disease.
The overall antimicrobial prescribing rate increased from 31.94 prescriptions per 1000 visits in 2019 to 57.48 prescriptions per 1000 visits during the initial peak. Agents that were more commonly prescribed during the initial peak of COVID-19 include cefpodoxime, hydroxychloroquine, doxycycline, and sulfamethoxazole-trimethoprim (Table 2). There was only 1 prescription ordered for cefpodoxime in 2019 (0.11 prescriptions/1000 visits) compared with 10 cefpodoxime prescriptions (1.88 prescriptions/1000 visits) in 2020. Hydroxychloroquine prescribing increased from 8 prescriptions in 2019 (0.89 prescriptions/1000 visits) to 27 prescriptions in 2020 (5.09 prescriptions/1000 visits). An increase in doxycycline was seen with 14 prescriptions in 2019 (1.55 prescriptions/1000 visits) versus 32 prescriptions in 2020 (6.03 prescriptions/1000 visits). There were 47 prescriptions in 2020 (8.86 prescriptions/1000 visits) for sulfamethoxazole/trimethoprim, which was increased from 34 prescriptions in 2019 (3.77 prescriptions/1000 visits). Of note, overall fluoroquinolone prescribing decreased in 2020, from 32 to 25 prescriptions, but there was a slight increase in the use of levofloxacin, an antibiotic commonly used for community acquired pneumonia.
TABLE 2.
Antimicrobial Prescribing Rates
| 2019 | 2020 | |||
|---|---|---|---|---|
| n (%) | Rate Per 1000 Visits | n (%) | Rate Per 1000 Visits | |
| Penicillins | ||||
| Penicillin VK | 2 (0.7) | 0.22 | 2 (0.7) | 0.38 |
| Ampicillin | 4 (1.4) | 0.44 | 1 (0.3) | 0.19 |
| Amoxicillin | 16 (5.6) | 1.78 | 18 (5.9) | 3.39 |
| AMX-C | 33 (11.5) | 3.66 | 26 (8.5) | 4.90 |
| Cephalosporins | ||||
| Cephalexin | 9 (3.1) | 1.00 | 7 (2.3) | 1.32 |
| Cefuroxime | 1 (0.4) | 0.11 | 2 (0.7) | 0.38 |
| Cefdinir | 1 (0.4) | 0.11 | 0 (0.0) | 0.00 |
| Cefixime | 1 (0.4) | 0.11 | 0 (0.0) | 0.00 |
| Cefpodoxime | 1 (0.4) | 0.11 | 10 (3.3) | 1.88 |
| Fluoroquinolones | ||||
| Ciprofloxacin | 30 (10.4) | 3.33 | 18 (5.9) | 3.39 |
| Levofloxacin | 2 (0.7) | 0.22 | 7 (2.3) | 1.32 |
| Macrolides | ||||
| Azithromycin | 53 (18.4) | 5.88 | 38 (12.5) | 7.16 |
| Clarithromycin | 0 (0.0) | 0.00 | 1 (0.3) | 0.19 |
| Other antibiotics | ||||
| SMZ-TMP | 34 (11.8) | 3.77 | 47 (15.4) | 8.86 |
| Clindamycin | 14 (4.9) | 1.55 | 7 (2.3) | 1.32 |
| Doxycycline | 14 (4.9) | 1.55 | 32 (10.5) | 6.03 |
| Metronidazole | 18 (6.3) | 2.00 | 18 (5.9) | 3.39 |
| Nitrofurantoin | 21 (7.3) | 2.33 | 13 (4.3) | 2.45 |
| Vancomycin | 1 (0.4) | 0.11 | 0 (0.0) | 0.00 |
| Antiviral agents | ||||
| Acyclovir | 2 (0.7) | 0.22 | 5 (1.6) | 0.94 |
| Famciclovir | 1 (0.4) | 0.11 | 0 (0.0) | 0.00 |
| Valacyclovir | 17 (5.9) | 1.89 | 24 (7.9) | 4.52 |
| Oseltamivir | 5 (1.7) | 0.55 | 2 (0.7) | 0.38 |
| Hydroxychloroquine | 8 (2.8) | 0.89 | 27 (8.9) | 5.09 |
| Total | 288 | 31.94 | 305 | 57.48 |
AMX-C indicates amoxicillin-clavulanic acid; SMZ-TMP, sulfamethoxazole-trimethoprim.
The most common indication for antimicrobial prescriptions was acute respiratory infections in 2019 (25%) and urinary tract infection in 2020 (16%; Fig. 2). Twenty-nine percent of prescriptions in the 2020 group did not have an indication documented in the visit note, an increase from 20% in 2019. COVID-19 represented 7 (6%) documented antimicrobial indications in 2020, with agents such as azithromycin, hydroxychloroquine, doxycycline, cefpodoxime, and oseltamivir prescribed.
FIGURE 2.
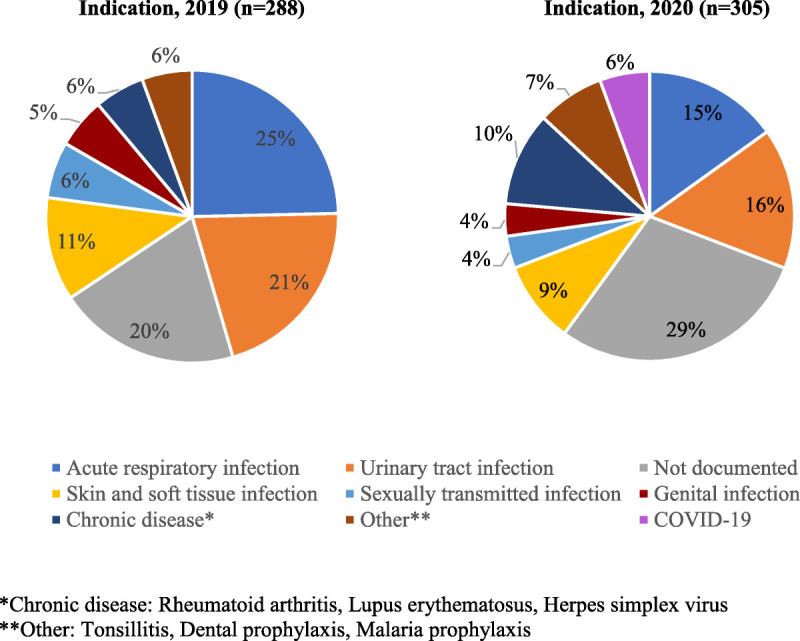
Documented indication for prescribed antimicrobial agents.
Confirmed infections based on a diagnosis supported by laboratory, culture, or imaging results were higher in 2019 (26.0%) versus 2020 (16.4%). A small number of patients were admitted to the University Hospital of Brooklyn within 90 days with 6 patients (2.1%) in 2019 and 8 patients (2.9%) in 2020. No reports of mortality within 90 days were captured in the 2019 group, although 4 patients died within that time frame in 2020.
DISCUSSION
Overall antimicrobial prescribing rates in the outpatient primary care clinics at the University Hospital of Brooklyn increased during the first peak of COVID-19. This increase in overall prescribing rate may have been influenced by a decrease in overall patient visits. With routine primary care visits being suspended to prioritize urgent patient care, a majority of the outpatient primary care visits are presumed to be for acute complaints. The number of antimicrobial prescriptions during the initial peak was similar to the number of prescriptions in 2019, suggesting that the number of patients presenting with potential infections may have been similar to prepandemic times. A primary transition to telehealth visits in 2020 may have influenced prescribing patterns owing to restrictions for patient evaluation. A decrease in the percentage of confirmed infections by laboratory or imaging results is an example of the impact of telehealth restrictions, limited laboratory hours of operation, and restrictions set forth by limited nonessential travel. Empiric and/or unnecessary prescribing of antibiotics and antivirals may have increased as a consequence.
A retrospective evaluation of antimicrobial use during the initial months of the pandemic illustrated high antibiotic prescribing rates with low incidence of bacterial coinfections and secondary infections.1,7,8,10 This finding was relevant within our patient population as well. The inability to distinguish COVID-19 from other respiratory infections may have been a contributing factor to prescribing patterns presented within our study. SARS-CoV-2 testing supply limitations and delays early in the pandemic prohibited definitive diagnosis in the outpatient setting. In East Brooklyn, where many of our patients reside, a high percent positivity paired with low to medium testing rates may indicate underreporting in the number of COVID-19 infections.9 The prescribing of agents for an indication of COVID-19 treatment was reported often based on symptoms alone. In the early months of the pandemic, there was a growing interest in using agents such as azithromycin, hydroxychloroquine, doxycycline, and oseltamivir based on in vitro evidence of cessation of viral replication or clinical use. Providers had few management options for treating these patients, as there was much still unknown about the disease, creating an opportunity for experimental therapy to be used.
A recent study by Buehrle et al11 reported that prescribing for the 10 most common outpatient antibiotics significantly decreased in April 2020, with the cessation of many nonessential health care services. This reduction in prescribing was followed by an increase in prescribing through July 2020 that went beyond prepandemic rates for several of those antibiotics. These data represented nationwide practice, which varied greatly depending on the incidence of COVID-19 infections and its impact on health care services. There have not been any reports that evaluate outpatient antibiotic prescribing specifically during a period in which a geographical area had a high burden of COVID-19 infections. This study provides contrasting data that illustrate increases in prescribing rates in an area and time period with high infection rates. Antimicrobial prescribing rates increased despite decreased patient visits.
Limitations of this study include limited documentation of the identified indication for antimicrobial therapy and underreporting of secondary outcomes because of patient utilization of neighboring hospitals, urgent care facilities, and clinics for medical care during the initial peak of COVID-19. The data from this retrospective chart review provide insight into the impact of COVID-19 on antimicrobial prescribing in the setting of restricted patient evaluation and pressure to prevent hospitalizations. In addition to reported increases in inpatient antibiotic prescribing, the consequences of increased outpatient antimicrobial prescribing during COVID-19 are important to consider for antimicrobial resistance in the community in the future. Future studies should look into determining the appropriateness of antimicrobial prescribing during COVID-19 and the influence on antimicrobial resistance.
Footnotes
The authors have no funding and conflicts of interest to disclose.
Contributor Information
Stanley Moy, Email: stanley.moy@downstate.edu.
Nubriel Hernandez, Email: nubriel.hernandez@downstate.edu.
REFERENCES
- 1.Langford BJ So M Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metlay JP, Waterer GW. Treatment of community-acquired pneumonia during the coronavirus disease 2019 (COVID-19) pandemic. Ann Intern Med. 2020;173(4):304–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiba S. Effect of early oseltamivir on outpatients without hypoxia with suspected COVID-19. Wien Klin Wochenschr. 2021;133(7-8):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Treatment Guidelines Panel . Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed April 19, 2021. [PubMed]
- 5.Sodhi M, Etminan M. Therapeutic potential for tetracyclines in the treatment of COVID-19. Pharmacotherapy. 2020;40:487–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhimraj A Morgan RL Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Infect Dis Soc Am. 2021; Version 4.3.0. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed June 14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawson TM Moore LSP Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nori P Cowman K Chen V, et al. Bacterial and fungal co-infections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42(1):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson CN Baumgartner J Pichardo C, et al. COVID-19 outbreak—New York City, February 29–June 1, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1725–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens RW Jensen K O'Horo JC, et al. Antimicrobial prescribing practices at a tertiary-care center in patients diagnosed with COVID-19 across the continuum of care. Infect Control Hosp Epidemiol. 2021;42(1):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buehrle DJ Nguyen MH Wagener MM, et al. Impact of the coronavirus disease 2019 pandemic on outpatient antibiotic prescriptions in the United States. Open Forum Infect Dis. 2020;7(12):ofaa575. [DOI] [PMC free article] [PubMed] [Google Scholar]


