Abstract
Introduction
Olfactory reference syndrome (ORS) (halitophobia) is the excessive fear of having bad breath without clinical findings supporting the patient's complaints. In this case report, a low dose of aripiprazole (ARP) successfully improved oral cenesthopathy and then improved ORS.
Case Presentation
A 44-year-old female patient complained of a sensation of astringent film sticking on her tongue. She was also very anxious about her bad breath at work.
We prescribed 0.5 mg of ARP for her symptoms because she worried about potential drowsiness. One week later, the patient reported that the oral sensation had resolved 2 to 3 days after the mediation was administered. No obvious adverse effects were observed except temporary arousal during sleep. Three months after the initial visit, her symptoms worsened, partly because of her job change, so we increased the dose of ARP from 0.5 mg to 1 mg. Later, the patient reported that she was better able to manage the anxiety about her breath. At the 2-year follow-up, her symptoms have continued to improve without medication.
Conclusions
Although the most effective approach to ORS is not established, the current study indicates that a low dose of ARP to treat oral cenesthopathy might improve ORS.
Key Words: olfactory reference syndrome, cenesthopathy, aripiprazole
Most patients with halitosis are managed with dental treatment and oral hygiene instructions; however, some patients persistently complain of halitosis despite a lack of clinical findings, and some are reluctant to treatment. Patients often believe that they have bad breath by misjudging the behavior of people around them and are constantly fixated on it. This so-called halitophobia, its pathogenesis, and treatment have been discussed in previous studies.1–3 Halitophobia is considered an olfactory reference syndrome (ORS). Phillips et al4 reported that about 75% of patients with ORS had halitosis as their chief complaint. The mental disorder classification of ORS has long been debated. The fourth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders describes ORS as a somatic-type delusional disorder and classifies it as a type of cultural jiko-syu-kyofu (a subtype of taijin-kyofu).5 However, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders classifies it as a disorder related to obsessive-compulsive disorder (OCD).6,7 In addition, ORS is classified under the category of “obsessive-compulsive or related disorders” in the 11th edition of the World Health Organization's International Classification of Diseases when deriving morbidity and mortality statistics.8 Olfactory reference syndrome is considered a representative symptom in the borderline area of OCD. Effective treatments include cognitive behavioral therapy, tricyclic antidepressants, and selective serotonin reuptake inhibitors.9,10 However, difficult cases are often seen in patients who drop out of treatment or wander hospitals in search of a suitable treatment.1 Some symptoms of ORS are associated with oral cenesthopathy (OC), such as a “sticky sensation” or “strange taste in the mouth.”11 In this case report, a low dose of aripiprazole (ARP), a reportedly effective treatment for OC,12 successfully improved OC and then ORS.
This study was conducted with the approval of the Ethical Committee of Tokyo Medical and Dental University (number D2013-005), and written informed consent was obtained from the patient.
CASE PRESENTATION
A 44-year-old female patient, working full time in a relatively large company, presented to our clinic with the sensation of an astringent, persimmon-like film on her tongue and complained of a strange taste in her mouth. She was concerned about having bad breath, especially at the office. Aside from Meniere's disease, there was no appreciable illness in her medical history. The only disorder reported in her psychiatric history was dysautonomia, which had been diagnosed 3 months before her first consultation with us. Her family history indicated that her brother had been diagnosed with a psychosomatic disease, but the details were unknown to her.
The patient was employed by her present company after graduating from university. Her family consisted of her husband (living separately from his family for work) and 2 high school children. About 8 years before her first consultation, she reported bad breath with dryness in her mouth. Two years later, she visited a special clinic for breath odor; however, she was not treated at that time because no abnormalities were found during her examination.
During her first consultation with us, her oral hygiene was adequate, but the Saxon test showed a slight decrease in salivary secretion at 1.71 g per 2 minutes. There were no other abnormal organic findings from internal or external oral and panoramic radiographs that were commensurate with her chief complaint. Halitosis was not present based on the interview. At the patient's initial visit, her visual analog scale (VAS) score for the degree of symptoms was 80/100, and her score on the Zung Self-Rating Depression Scale was 30. She was therefore diagnosed with OC and ORS (halitophobia). We explained the symptoms and treatment, and she expressed anxiety that the treatment's adverse effects, including drowsiness and nausea, might affect her work. Therefore, we initially prescribed 0.5 mg of ARP. Two weeks after this first visit, she reported a slight reduction in discomfort and described the sensation as a strange taste of film in her mouth. She reported that she occasionally forgot about her symptoms at work. Her VAS score was reduced to 21. Her Clinical Global Impression—Improvement (CGI-I) score, which represents the clinician's view of patient global functioning before and after initiating a study medication, for OC was 2 (much improved), and the CGI-I for ORS was 3 (minimally improved). The time course of the VAS score and the CGI-I scores for OC and for ORS are shown in Figure 1. She continued the same dose of medication without any report of adverse effects, such as arousal during sleep. Two months after the initial visit, the patient reported that she spent more time worrying about her oral symptoms and that her drowsiness may have been due to her children's examinations and company transfer issues. Three months after the initial visit, she reported that she was going to work at a new location and was still anxious. She thought that she had bad breath when she was nervous; however, the strange taste had become only slightly perceptible. We increased the dose of APR to 1.0 mg. Four months after her initial visit, she reported that the bad breath still bothered her a bit when she got close to people; however, she realized that, upon starting a new job, it was natural to get nervous and experience dry mouth. At this point, she believed that it was acceptable to have a bad breath and that she could handle it. She used to dwell on the idea that her bad breath could be improved, but her perception had changed. For a few months, her condition was relatively stable. However, 9 months after her initial visit, some of her symptoms had returned; she stated that she was very busy at work because of a colleague's retirement, and although the discomfort in her mouth was tolerable, she began to worry that her bad breath was coming back. Ten months after her first visit, she reported that her job was no longer stressful, and her condition improved. Eventually, her job made it difficult for her to visit our clinic; however, when we called her for the 1-year follow-up, she reported she was doing well and was continuing her medication of 1 mg of ARP. Furthermore, when we called her for a 2-year follow-up, she said that her symptoms remained in remission without medication, and we felt that we had obtained a good course of treatment.
FIGURE 1.
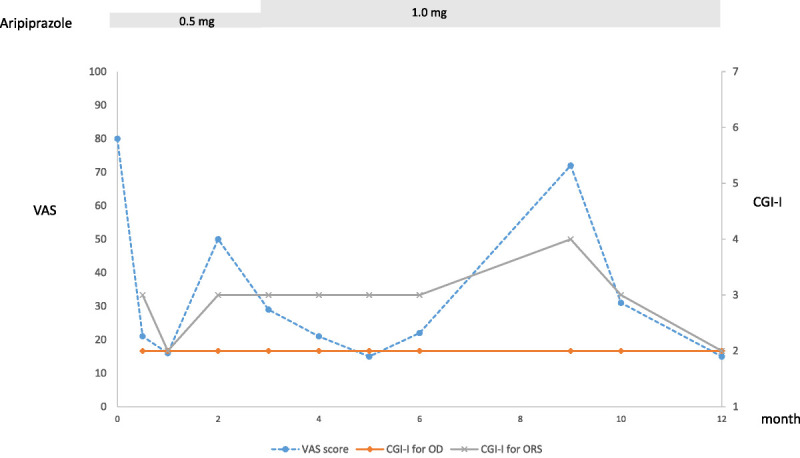
Course of symptoms.
The patient's drug-induced extrapyramidal symptom scale score had remained at 0 throughout her treatment period.
DISCUSSION
Tricyclic antidepressants, selective serotonin reuptake inhibitors,9 and cognitive behavioral therapy10 have been reported to be effective treatments for ORS. In this case, the patient was employed full time and had a strong preference for medication with fewer adverse effects. We therefore selected low-dose ARP for 2 reasons: (1) fewer adverse effects would likely lead to good compliance and continued treatment and (2) existing reports of ARP's effectiveness in treating symptoms of OC.12 We believe that this ultimately led to the successful course of treatment. Furthermore, ORS is an example of an OCD-related disorder, as listed in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, characterized by persistent preoccupation with the belief that one is emitting a perceived foul or offensive body odor. Individuals experience excessive self-consciousness about the perceived odor, often with ideas of reference.6,13 Effectiveness of ARP in treating OCD has been reported.14–17 In addition, the effectiveness of a low dose of ARP has been reported in the context of monotherapy for many psychiatric disorders, including Tourette's syndrome,15 trichotillomania,18 burning mouth syndrome,19,20 chronic pain,21 OC,12 and delusion of ocular or dermatologic parasitosis.22,23 Aripiprazole acts on various receptor systems, especially dopamine and serotonin receptors as a partial agonist or an antagonist.24,25 Aripiprazole used in the present study may have helped to improve symptoms in our patient by acting on the dopamine and serotonin neural circuitry in a fine balance. The prominent effect of low-dose ARP on different symptoms may suggest that ARP acts not on the symptoms directly but on underlying anxiety or a transdiagnostic cognitive process such as rumination.26
We previously reported a case of burning mouth syndrome that improved with a low dose of ARP, which may have been a response to rumination of thoughts about pain and improvement of symptoms.19 In this case, it is possible that ARP may have been a response to the patient's rumination, in which she only thought about halitosis. Indeed, she reported that she did not pay as much attention to her mouth after the treatment. Furthermore, she reported improvement during social activities. Her anxiety and rumination about halitosis improved while taking low-dose ARP as she maintained her life, in which she had many opportunities to interact with various people at work. This suggests that ARP may have been helpful in modifying her cognition and reducing anthropophobic anxiety.
The different time course of improvement for halitophobia and OC is another point of consideration. Miyamoto27 suggested that, in the case of ORS with cenesthopathy, ORS may be the primary symptom, whereas cenesthopathy may be the secondary symptom the patient develops to explain the perceived self-odor. The 2-tiered improvement (cenesthopathy first, halitophobia second) of our case supports this suggestion. In our case, obsessive attention to the mouth may lead to secondary development of uncomfortable oral sensations. Furthermore, once these symptoms are established, rumination may worsen them or help to maintain them. It is also suggested that a low dose of ARP may have been effective for such obsessive thinking in cenesthopathy.
CONCLUSIONS
By treating the symptom expected to improve first (OC), rather than targeting all symptoms at once, we facilitated the patient's acceptance of the treatment. Furthermore, we found that it was important to begin treatment while there were fewer adverse effects from the medication to establish a trusting relationship with the patient. If patients become aware of improvement in their symptoms, they can develop positive expectations regarding future treatment and how it will affect their daily life and work. This may cause patients to modify their thoughts about ORS naturally, without avoiding interpersonal relationships. Further studies are needed to confirm the efficacy of low-dose ARP in ORS.
ACKNOWLEDGMENTS
The authors would like to thank Editage for English language editing.
Footnotes
Conflicts of Interest and Source of Funding: A.T. is currently receiving a grant (19K10328) from JSPS KAKENHI. For the remaining authors, none were declared.
Ethics Approval and Consent to Participate: This study was conducted with the approval of the Ethical Committee of Tokyo Medical and Dental University (number D2013-005), and written informed consent was obtained from the patient.
Competing Interests: The authors declare that they have no competing interests.
Consent for Publication: Written informed consent for publication of the clinical details was obtained from the patient.
Availability of Data and Materials: The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' Contributions: Author M.T. wrote the first draft of the manuscript and managed the literature searches. Authors H.M. and A.T. revised the manuscript critically for important intellectual content. All authors contributed to and have approved the final manuscript.
Contributor Information
Haruhiko Motomura, Email: haru.ompm@tmd.ac.jp.
Akira Toyofuku, Email: toyoompm@tmd.ac.jp.
REFERENCES
- 1.Toyofuku A. Psychosomatic problems in dentistry. Biopsychosoc Med 2016;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aydin M Özen ME Derici Ç, et al. Persistent halitosis can be a part of olfactory reference syndrome: a case report. Br J Pharm Med Res 2019;4:1574–1578. [Google Scholar]
- 3.Greenberg JL Shaw AM Reuman L, et al. Clinical features of olfactory reference syndrome: an internet-based study. J Psychosom Res 2016;80:11–16. [DOI] [PubMed] [Google Scholar]
- 4.Phillips KA, Menard W. Olfactory reference syndrome: demographic and clinical features of imagined body odor. Gen Hosp Psychiatry 2011;33:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K Takei N Iwata Y, et al. Do olfactory reference syndrome and Jiko-shu-kyofu (a subtype of Taijin-kyofu) share a common entity? Acta Psychiatr Scand 2004;109:150–155. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013. [Google Scholar]
- 7.Van Ameringen M, Patterson B, Simpson W. DSM-5 obsessive-compulsive and related disorders: clinical implications of new criteria. Depress Anxiety 2014;31:487–493. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 6B22 Olfactory reference disorder. In: ICD-11 mortality and morbidity statistics [WHO Web site]. September 2020. Available at: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1119008568.
- 9.Toyofuku A Umemoto G Miyagi T, et al. The efficacy of fluvoxamine for “halitophobia”. J Psychosom Dent Japan 2001;16:81–85. [Google Scholar]
- 10.Mrizak J Ouali U Arous A, et al. Successful treatment of halitophobia with cognitive behavioural therapy: a case study. J Contemp Psychother 2019;49:119–125. [Google Scholar]
- 11.Akpata O Omoregie OF Akhigbe K, et al. Evaluation of oral and extra-oral factors predisposing to delusional halitosis. Ghana Med J 2009;43(2):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umezaki Y Miura A Shinohara Y, et al. Clinical characteristics and course of oral somatic delusions: a retrospective chart review of 606 cases in 5 years. Neuropsychiatr Dis Treat 2018;14:2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feusner JD, Phillips KA, Stein DJ. Olfactory reference syndrome: issues for DSM-V. Depress Anxiety 2010;27:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor KM Payne VM Gadde KM, et al. The use of aripiprazole in obsessive-compulsive disorder: preliminary observations in 8 patients. J Clin Psychiatry 2005;66:49–51. [DOI] [PubMed] [Google Scholar]
- 15.Akca OF, Yilmaz S. Effectiveness of low dose aripiprazole as monotherapy on obsessive compulsive disorder and tic disorder comorbidity: a case report. Austin Child Adolesc Psychiatry 2016;1(1):1006. [Google Scholar]
- 16.Coskun M, Karayagmurlu A. Aripiprazole treatment for obsessive compulsive disorder in 2 young subjects who could not tolerate selective serotonin reuptake inhibitors (SSRIs). J Clin Psychopharmacol 2020;40(3):310–312. [DOI] [PubMed] [Google Scholar]
- 17.Coskun M. Aripiprazole monotherapy was effective in treating obsessive-compulsive disorder in a preschool boy. J Clin Psychopharmacol 2017;37(5):636–637. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T, Iyo M. Treatment of puberty trichotillomania with low-dose aripiprazole. Ann Gen Psychiatry 2015;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takenoshita M, Motomura H, Toyofuku A. Low-dose aripiprazole augmentation in amitriptyline-resistant burning mouth syndrome: results from two cases. Pain Med 2017;18:814–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umezaki Y, Takenoshita M, Toyofuku A. Low-dose aripiprazole for refractory burning mouth syndrome. Neuropsychiatr Dis Treat 2016;12:1229–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasahara S Kunii Y Mashiko H, et al. Four cases of chronic pain that improved dramatically following low-dose aripiprazole administration. Prim Care Companion CNS Disord 2011;13(2):PCC.10l01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponson L, Andersson F, El-Hage W. Neural correlates of delusional infestation responding to aripiprazole monotherapy: a case report. Neuropsychiatr Dis Treat 2015;11:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang WL, Chang LR. Aripiprazole in the treatment of delusional parasitosis with ocular and dermatologic presentations. J Clin Psychopharmacol 2013;33(2):272–273. [DOI] [PubMed] [Google Scholar]
- 24.DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther 2004;26(5):649–666. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Narita M. Brain reward circuit and pain. Adv Exp Med Biol 2018;1099:201–210. [DOI] [PubMed] [Google Scholar]
- 26.Kim S Yu BH Lee DS, et al. Ruminative response in clinical patients with major depressive disorder, bipolar disorder, and anxiety disorders. J Affect Disord 2012;136(1–2):e77–e81. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto T. Olfactory reference syndrome: reconsidering symptomatology. J Clin Psychiatry Japan 1976;5(10):29–37. [Google Scholar]


