Supplemental digital content is available in the text.
Key Words: HOME-BASED, PHYSICAL ACTIVITY, CANCER SURVIVORS, FATIGUE, DEPRESSION, ANXIETY
ABSTRACT
Purpose
Physical activity (PA) affects fatigue and mental health in cancer survivors favorably, but participation in PA interventions tends to be low. More participants may be reached by home-based PA owing to greater accessibility and self-monitoring. This systematic review therefore evaluated the effects of home-based PA of low to moderate intensity on symptoms of fatigue, depression, and anxiety among cancer survivors.
Methods
PubMed, CINAHL, PsycINFO, and Web of Science were systematically searched for randomized controlled trials. We included investigations of home-based PA interventions in adults treated curatively for cancer and evaluating fatigue, depression, or anxiety as outcomes. We performed a random-effect meta-analysis for the effects of PA interventions on fatigue in the short and long terms. Subgroup analyses were performed for the frequency of counseling. Standardized mean differences (SMD) and 95% confidence intervals are reported.
Results
Eleven articles comprising 1066 participants were included: 77% had a history of breast cancer; 14%, ovarian cancer; 4%, colorectal cancer; 4%, prostate cancer; and 1%, “other” cancer (not specified). Concerning the outcomes, nine articles reported on fatigue and two reported on depression or anxiety. Meta-analyses showed a significant effect of home-based PA on fatigue immediately after the intervention (SMD = 0.22 [0.06–0.37]), at 3 months’ follow-up (SMD = 0.27 [0.04–0.51]), and at 6–9 months’ follow-up (SMD = 0.31 [0.08–0.55]). PA interventions that used frequent counseling were associated with larger improvements in fatigue than those using no or infrequent counseling.
Conclusions
Home-based PA interventions can reduce fatigue among adult cancer survivors for up to 9 months, and frequent counseling may improve the benefits of these interventions.
Exercise oncology has been an upcoming field since around the turn of the century (1). It has led to valuable insights into the favorable role of physical activity (PA) on physical health (2–4) and quality of life (5,6) in cancer survivors. Enhancing PA in this group is warranted because only 20%–30% meet guideline recommendations for activity levels (7,8).
PA may play an essential role in alleviating long-term adverse effects related to cancer or its treatment. Patients frequently encounter symptoms caused by physical deconditioning and problems with psychosocial and mental health (9). Fatigue, depression, and anxiety are three frequently reported symptoms after treatment, with 30%–40% of cancer survivors experiencing fatigue (10–12) and 21% experiencing depression or anxiety (12–14). Results of studies implementing PA interventions show that these can reduce symptoms of fatigue (5,15,16), depression (17,18), and anxiety (5,19). Observational studies have also associated higher levels of PA with lower symptoms of fatigue, depression, and anxiety (20–22).
Despite the beneficial effects, participation in PA interventions is generally low, with fewer than half of eligible participants engaging (23–25). Frequently reported barriers include a lack of time, a lack of motivation or confidence to exercise, feeling unwell or tired, or the PA not meeting a patient’s preferences (26,27). Consequently, study outcomes only apply to participants who have completed the program and not to those who drop out or refuse to participate. PA interventions are also frequently performed at a specific location, and the required travel time can result in them not participating (26). Home-based PA interventions have therefore been introduced to address the various barriers to PA and thereby help to reach more cancer survivors. In these interventions, participants exercise at a self-chosen time and location at or around their own home and without direct supervision. Because of the high accessibility, low costs, and self-monitoring, home-based PA interventions may lead to higher participation and adherence. Furthermore, interventions of low to moderate intensity may be used to target a larger group of cancer survivors.
To date, no reviews have synthesized the effects of home-based PA interventions on fatigue, depression, or anxiety among cancer survivors. Existing reviews of home-based PA interventions either included survivors of breast cancer only (28) or did not evaluate the effects on fatigue, depression, or anxiety (4). The aim of this systematic review was to assess the effect of home-based PA interventions that are of low to moderate intensity on symptoms of fatigue, depression, and anxiety in adult cancer survivors.
METHODS
Protocol and registration
The protocol of this systematic review is available on PROSPERO with registration number CRD42020195618. We adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (29).
Eligibility criteria
Randomized controlled trials meeting the following criteria were included: (i) study sample of cancer survivors (except for nonmelanoma skin cancer); (ii) age 18 yr or older; (iii) completed cancer treatment (chemotherapy, surgery, and or radiotherapy; being on adjuvant hormonal therapy was allowed); (iv) the intervention was designed to increase PA; (v) the intervention did not target other health behaviors (e.g., smoking or nutrition); (vi) the intervention was home-based; (vii) PA was of low to moderate intensity; (viii) PA was not personally supervised and did not include a structured training regime, such as anaerobic training or yoga; (ix) the control group received usual care or a nonphysical intervention; (x) symptoms of fatigue, depression, or anxiety were assessed with a validated questionnaire, a subscale from a validated questionnaire, or a visual analog scale (VAS); and (xi) scores for the questionnaires were reported.
Home-based interventions were defined as those performed by participants in their home environment or in the local community. The level of intensity of PA was determined by metabolic equivalent of task scores (METs) according to the Compendium of Physical Activities (30) or the percentage of maximum heart rate (HRmax) (31). Low intensity was defined as METs <3 and HRmax <63%, moderate intensity as METs 3–6 and HRmax 64%–76%, and high or vigorous intensity as METs >6 and HRmax >77% (30,31).
Search strategy
On May 19, 2020, we searched PubMed, CINAHL, PsycINFO, and Web of Science for relevant studies. A combination of MeSH terms and free-text words was used, including “randomized controlled trial,” “cancer,” “physical activity,” “home-based” or “community-based,” and “fatigue,” “depression,” or “anxiety.” See for the full search strategy Document S1 (see Document, Supplemental Digital Content 1, search strategy, http://links.lww.com/MSS/C359). We applied no restrictions on language or publication date.
Data collection
Articles were first screened by the title and abstract before a full-text review. After a pilot of screening titles and abstracts on 10% of the articles, all articles were screened independently by two authors (F.H. and J.K.O.N). Interrater agreement in study selection was calculated by Cronbach’s α. Discrepancies between the authors were discussed to reach consensus and, if needed, resolved by a third reviewer (D.B.). We checked the references of included articles and of reviews that evaluated PA interventions among cancer survivors to identify additional records.
Detailed information about demographics, disease characteristics, PA interventions, and study outcomes was extracted (Tables 1, 2). Educational level was represented as the percentage of participants with higher education, defined as postsecondary education or higher (e.g., associate degree, college degree, graduate school). The participation rate was defined as the percentage of participants who engaged in the PA intervention in relation to those eligible to participate. Adherence was expressed as the percentages of participants who complied with guidance on the prescribed amount of PA, attended counseling sessions, and used exercise materials. If needed, we contacted the investigators for unreported data.
TABLE 1.
Characteristics of randomized controlled trials evaluating the effects of home-based PA on fatigue.
| Study | Sample Size | Age (yr), % Female, % Caucasian, % Higher Education | Diagnosis (%) | Treatment (%) | Time Since Diagnosisa or Treatmentb | Inclusion and Exclusion Criteria | Intervention and Control | Primary Outcomes | Fatigue Instrument |
|---|---|---|---|---|---|---|---|---|---|
| Baruth et al. (32) | n = 32 (I:20, C: 12) | 56.46 (6.25), 100% female, 75% Caucasian, 91% higher education | Breast cancer | Surgery 91% Chemo 72% Radiation 78% Hormone 66% |
5. 1 (4.1) monthsb | Inclusion: 1) diagnosed with stage I–III cancer, 2) completed adjuvant treatment within the last 12 months, 3) postmenopausal, 4) free of cardiovascular disease and major orthopedic limitations, 5) not regularly active (<5 d·wk−1) Exclusion: — |
I: Home-based walking + counseling Duration: 12 wk Intensity: moderate intensity of RPE 10–11 (at weeks 1–8) and moderate to vigorous intensity of RPE 12–15 (at weeks 8–12) Frequency: from 3 d·wk−1 for 20 min (weeks 1–8) to 5 d·wk−1 for 30 to 40 min (weeks 8–12) Counseling: One in-person counseling session of 30 min (baseline) and five telephone counseling calls of 10–15 min (weeks 1, 2, 4, 7, and 10) provided by public health doctoral students Counseling was based on the social cognitive theory and focused on goal setting and exercise safety Materials: 1) pedometer and 2) activity logs. C: Wait-list control. Asked to remain usual PA levels and received the intervention upon completion of the study |
1) Quality of life (SF-36 and IBCSG-QLC) and 2) fatigue (FACT-F) | FACT-F |
| Pinto et al. (33) | n = 46 (I:20, C: 26) | 57.3 (9.7); 56% female, 98% Caucasian, 76% higher education |
Colon cancer (57%) and rectal cancer (43%) | Surgery 100% Chemo 83% Radiation 43% |
3.0 (1.6) yra | Inclusion: 1) age ≥18 yr, 2) completed primary and adjuvant treatments for colon or rectal cancer, 3) ≤5 yr since treatment completion, 4) able to read and speak English, 5) consent for medical chart review, 6) able to walk unassisted, 7) sedentary (exercising <60 min·wk−1 at moderate intensity or <20 min·wk−1 of vigorous intensity over the past 6 months), 8) access to a telephone Exclusion: 1) history of cancer or 2) a medical or current psychiatric illness |
I: Home-based PA + counseling Duration: 12 wk Intensity: moderate intensity at 64%–76% of HRM Frequency: first few weeks at least 10 min on at least 2 d·wk−1 Gradually increase to 30 min on 5 d·wk−1 Counseling: weekly telephone calls provided by research staff to monitor PA participation, identify relevant health problems, problem solve any barriers to PA, and reinforce participants for their efforts. Counseling was based on the transtheoretical model, the social cognitive theory, and motivational interviewing. Materials: 1) pedometer, 2) activity log, and 3) cancer survivorship tip sheets. C: Attentional control. Received weekly calls to monitor problems and cancer survivorship tip sheets |
1) Self-reported PA (7-d PAR), 2) submaximal fitness (treadwalk test) | FACT-F |
| Pinto et al. (34) | n = 192 (I:106, C: 86) | 60.0 (9.9); 100% female, 94% Caucasian, 76% higher education |
Breast cancer | Surgery 74%–100% Chemo 60% Radiation 72% Hormone 77% |
2.9 (2.1) yra | Inclusion: 1) female age > = 18 yr, 2) completed primary and adjuvant treatment for breast cancer (patients on hormone treatment such as tamoxifen were eligible), 3) = < 5 yr since treatment completion, 4) able to read and speak English, 5) provided consent for medical chart review, 6) able to walk unassisted, 7) relatively inactive (<30 min·wk−1 of vigorous intensity or <90 min·wk−1 of moderate intensity exercise), and 8) had access to a telephone. Exclusion: 1) a history of cancer or 2) a medical or current psychiatric illness |
I: Home-based PA + counseling Duration: 12 wk Intensity: low to moderate intensity at 55%–65% of HRM Frequency: First few weeks: at least 10 min on at least 2 d·wk−1. Gradually increase of goals to 30 min on at least 5 d·wk−1 Counseling: at baseline brief PA advice (<5 min) about PA benefits and recommendation of 30 min of moderate-intensity PA on most days of the week. Counseling was provided by research staff through weekly telephone calls at weeks 1–4, and biweekly calls at weeks 5–12. Counseling was based on the transtheoretical model, the social cognitive theory, and motivational interviewing, and focused on strengthening self-efficacy for PA, self-monitoring of PA, setting PA goals, and planning for exercise. Materials: 1) pedometer, 2) activity log, 3) PA and cancer survivorship tip sheet (weekly), and 4) feedback letter with participant’s progress (weeks 2, 4, 8, 12) C: Attentional control. Received brief PA advice (<5 min) about PA benefits and recommendation of 30 min of moderate-intensity PA on most days of the week, weekly calls during which the Symptom Questionnaire was administered to monitor problems and cancer survivorship tip sheets |
Self-reported PA (7-d PAR) | FACT-F |
| Vallance et al. (35) | n = 377 (PM: 94, PED: 94, COM: 93, C: 96) | 58 (range: 30–90); 100% female, 65% Caucasian, 30% higher education |
Breast cancer | Surgery 100% Chemo 54% Radiation 69% Hormone 67% Hormone current 59% |
3.25 (0.94) yra | Inclusion: 1) histologically confirmed stage I to IIIa breast cancer, 2) physician approval, 3) free of chronic medical and orthopedic conditions that would preclude PA, 4) English language, 5) completion of adjuvant therapy except hormone therapy, and 6) absence of current breast cancer Exclusion: — |
I: Home-based use of exercise materials Duration: 12 wk Intensity/frequency: standard recommendation to perform 30 min of moderate/vigorous PA on 5 d of the week Materials: PM group: exercise guide PED group: 1) pedometer and 2) 12-wk step calendar COM group: 1) pedometer and 2) 12-wk step calendar, and 3) exercise guide C: Usual care. Standard recommendation to perform 30 min of moderate/vigorous PA on 5 d of the week |
Self-reported PA (GLTEQ) | FACT-F |
| Zhou et al. (36) | n = 144 (I:74, C: 70) | 57.3 (8.6); 100% female, 95% Caucasian, 56% higher education |
Ovarian cancer | Chemo 93.1% | 1.7 (1.0) yra | Inclusion: 1) English speaking, 2) age between 18 and 75 yr, 3) diagnosed with ovarian cancer within the past 4 yr, 4) completion of chemotherapy at least 1 month before random assignment, 5) exercising fewer than 90 min· wk−1, and 6) physician consent to start an exercise program Exclusion: — |
I: Home-based PA + counseling Duration: 6 months Intensity: moderate Frequency: 150 min· wk−1 Counseling: weekly telephone counseling provided by a trainer to motivate participants to exercise, and to discuss topics related to exercise and to ovarian cancer survivorship Materials: 1) heart rate monitor and 2) daily activity log C: Attentional control. Received weekly phone calls and a 26-chapter book that contained ovarian cancer survivorship–related information |
1) Quality of life (SF-36) and 2) fatigue (FACT-F) | FACT-F |
| Bennett et al. (37) | n = 56 (I:28, C: 28) | 57.8 (10.0), 89% female, 98% Caucasian, 73% higher education | Breast cancer (77%) and other (25%) | NA | 5.9 (4.68) yra 3.5 (3.64) yrb |
Inclusion: 1) 18 yr or older, 2) completed treatment at least 6 months before enrollment, 3) fatigued or underactive (engaged in planned exercise fewer than 3 d·wk−1 for 20 min), 4) willing to try to increase PA Exclusion: 1) prior transplant treatment for cancer, 2) current immunosuppressive therapy, 3) medical conditions that contraindicated moderate exercise, 4) cognitive difficulties, and 5) psychiatric disorders |
I: Home-based PA + counseling Duration: 6 months Intensity: moderate Frequency: 30 min on most days of the week Counseling: Counseling was provided by a PA counselor, with the first counseling session in person of 30 min, and three telephone calls of 20 min at 2 wk, 2 months, and 4.5 months. Counseling was based on motivational interviewing, with motivational strategies directed at problem solving, offering encouragement, and reformulating goals. Materials: pedometer C: Attentional control. Asked to maintain their current levels of PA and received two telephone calls without motivational interviewing content (at 2 and 4.5 months) |
Self-reported PA (CHAMPS) | SCFS |
| Nyrop et al. (38) | n = 62 (I:31, C: 31) | 63.8 (8.3); 100% female, 74% Caucasian, 77% higher education |
Breast cancer | Surgery: 84%–100% Radiation 74% Chemo 65% Hormone 29% Hormone currently 53%–100% |
2.8 (2.5) yra | Inclusion: 1) adherent to AI prescription for at least 4 wk; 2) age 21 yr or older; 3) not undergoing chemotherapy or radiation treatment during the study period; 4) score of 3 out of 5 on a scale about joint pain, stiffness, or achiness intensity; 5) exercising less than 150 min· wk−1 Exclusion: — |
I: Home-based walking Duration: 6 wk Intensity: NA Frequency: 150 min· wk−1 Materials: 1) daily activity log, 2) workbook with strategies for starting and sustaining a daily walking program, and 3) brochure with topics related to walking, joint pain and treatment, and experiences of women who had completed the walking program before C: Wait-list control. Asked to await further contact from the research team and received intervention after 6 wk |
1) Self-reported walking (minutes per week), 2) joint pain/symptoms (WOMAC, VAS pain, VAS fatigue, VAS stiffness, pain points total), and 3) adherence to AI therapy | VAS fatigue |
| Pinto et al. (39) | n = 86 (I:43, C: 43) | 53.14 (9.76), 100% female, 95% Caucasian, 81% higher education |
Breast cancer | Surgery 49%–93% Chemo 56% Radiation 69% Hormone 62% |
1.84 (1.43) yra | Inclusion: 1) 18 yr or older; 2) sedentary (exercised < one time per week for 20 min at vigorous intensity or < two times per week for 30 min at moderate intensity for the past 6 months); 3) diagnosed with stage 0 to II breast cancer over the last 5 yr; 4) completed surgery, chemotherapy and/or radiation; 5) ambulatory; and 6) willing to be randomized Exclusion: 1) history of cancer (exception: nonmelanoma skin cancer), 2) medical or current psychiatric illness that could make compliance with the study protocol difficult or dangerous |
I: Home-based PA + counseling Duration: 12 wk Intensity: low to moderate intensity at 55%–65% of HRM Frequency: first few weeks at least 10 min on at least 2 d·wk−1. Gradually increase to 30 min on at least 5 d·wk−1 Counseling: During intervention weekly, telephone calls from research staff to monitor PA participation, identify relevant health problems, problem solve any barriers to PA, and reinforce participants for their efforts. Counseling was based on the transtheoretical model. After intervention, monthly calls for 3 months to prompt and reinforce regular PA. Materials: 1) pedometer, 2) activity log, 3) PA and cancer survivorship tip sheet (weekly), and 4) feedback letter with participant’s progress (weeks 2, 4, 8, 12) C: Attentional control. Asked not to change current level of PA and received weekly phone calls and cancer survivorship tip sheet |
1) BMI, 2) self-reported PA (7-d PAR), 3) Rockport 1-mile walk test, 4) PA monitoring (accelerometer), 5) stage of motivational readiness for PA, 6) POMS, 7) fatigue (VAS), 8) body esteem scale | VAS fatigue |
| Pinto et al. (40) | n = 86 (I:43, C: 43) | See Pinto et al. (40) | See Pinto et al. (40) | See Pinto et al. (40) | See Pinto et al. (40) | See Pinto et al. (40) | See Pinto et al. (38) | Self-reported PA (7-d PAR) | See Pinto et al. (40) |
aTime since diagnosis.
bTime since treatment.
7-d PAR, 7-d Physical Activity Recall; AI, aromatase inhibitors; BMI, body mass index; C, control; CHAMPS, Community Health Activities Model Program for Seniors; COM, combined group; FACT-F, Functional Assessment of Cancer Therapy—Fatigue; GLTEQ, Godin Leisure-Time Exercise Questionnaire; HRM, maximum heart rate; I, intervention; IBCSG-QLC, International Breast Cancer Study Group QOL Core Questionnaire; NA, not available; PA, physical activity; PED, pedometer group; PM, print material group; RPE, rating of perceived exertion; SF-36, Short Form Health Survey 36; SCFS, Schwartz Cancer Fatigue Scale; POMS, Profile Of Mood States; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
TABLE 2.
Outcomes of the randomized controlled trials evaluating the effects of home-based PA on fatigue.
| Study | N | Participation | Dropout | Adherence | Fatigue Instrument | Time of Measurement | Intervention | Control | Study Outcomes | PA Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Baruth et al. (32) | n = 32 (I:20, C: 12) | NA | I: 10% (n = 2) C: 0% |
PA: 86.2% (11.9) |
FACT-F | Baseline | 37.5 (9.2)a | 36.7 (9.2)a | Change I vs C = 3.3, d = 0.36, P < 0.05 | I: higher increase in walking energy expenditure (CHAMPS) from baseline to 12 wk than C (d = 2.89). |
| Post: 12 wk | 42.0 (9.2)a | 37.9 (9.2)a | ||||||||
| Pinto et al. (33) | n = 46 (I:20, C: 26) | 27.4% | I: 5% (n = 1) C: 11.5% (n = 3) |
PA: 64.7% |
FACT-F | Baseline | 40.7 (8.7) Adj = 39.1 |
37.9 (10.6) Adj = 39.1 |
Change 3 months I vs C = 0.3, P = ns Change 6 months I vs C = 3.2, P = ns Change 12 months I vs C = 0.5, P = ns |
I: more minutes of moderate PA (7-d PAR) and caloric expenditure (CHAMPS) than C at 3 months (d = 1.93, P = 0.021; d = 0.84, P = 0.009). No differences between I and C on 7-d PAR and CHAMPS at FU of 3 (d = 1.18, P = 0.149; d = 0.49, P = 0.117)) and FU of 9 months (d = 0.98, P = 0.223; d = 0.15, P = 0.626). |
| Post: 3 months | 42.2 (5.81)b | 41.9 (5.57)b | ||||||||
| FU: 3 months | 43.3 (5.08)b | 40.1 (5.55)b | ||||||||
| FU: 9 months | 42.3 (5.0)b | 41.8 (5.55)b | ||||||||
| Pinto et al. (34) | n = 192 (I:106, C: 86) | 71.1% | I: 21.0% (n = 22) C: 9.3% (n = 8) |
NA | FACT-F | Baseline | 39.3 (9.9) Adj = 38.76c |
38.1 (11.6) Adj = 38.76c | Change 3 months I vs C = 1.12, P = ns Change 6 months I vs C = 1.89, P = ns Change 12 months I vs C = 2.44, P = ns |
I: more minutes of moderate PA (7-d PAR) than C at 3 months (P = 0.048) and at FU of 3 months (P = 0.032). No differences between I and C on 7-d PAR at FU of 9 months (P = 0.574). |
| Post: 3 months | 43.29 (7.94)c | 42.17 (8.02)c | ||||||||
| FU: 3 months | 42.60 (7.90)c | 40.71 (8.08)c | ||||||||
| FU: 9 months | 42.45 (7.97)c | 40.20 (7.91)c | ||||||||
| Vallance et al. (35) | n = 377 (PM: 94, PED: 94, COM: 93, C: 96) | 29.5% | PM: 14% (n = 13) PED: 6.3% (n = 6) COM: 9.7% (n = 9) C: 11.5% (n = 11) |
Materials: records pedometer steps on 83.3% of study days | FACT-F | Baseline | PM: 39.7 (9.7) PED: 40.3 (9.9) COM: 39.8 (10.3) |
41.1 (9.3) |
Change COM vs C = 2.3, d = 0.25, P = 0.052 Change PED vs C = 1.2, P = 0.310 Change PM vs C = 0.5, P = 0.673 |
PED and COM: higher increase in moderate to vigorous PA (GLTEQ) from baseline to 12 wk compared with C (d = 0.38, P = 0.017; d = 0.37, P = 0.022), no significant increase in PM compared with C (d = 0.25, P = 0.117). PM, PED and COM: significantly increased in minutes of brisk walking from baseline to 12 wk compared with C (d = 0.48, P = 0.006; d = 0.62, P = 0.000; d = 0.39, P = 0.028). PM, PED, and COM: no increase in steps per day (pedometer) at 12 wk compared with C (P = 0.727; P = 0.885; P = 0.710). Changes in moderate to vigorous PA (r = 0.17, P = 0.002) and brisk walking (r = 0.14, P = 0.013) were associated with changes in fatigue. |
| Post: 12 wk | PM: 42.2 (8.8) PED: 42.8 (7.6) COM: 43.1 (8.9) |
42.6 (8.7) | ||||||||
| Zhou et al. (36) | n = 144 (I:74, C: 70) | 24.2% | I: 17.6% (n = 13) C: 25.7% (n = 18) |
PA: 64.9% Counseling: Adherence to at least 20 out of 28 calls 79.7% I and 73.5% C |
FACT-F | Baseline | 36.7 (11.1) | 35.8 (10.8) | Change I vs C = 2.8, d = 0.26, P = 0.06 | NA |
| Post: 6 months | 40.7 (8.77) | 37.0 (8.26) | ||||||||
| Bennett et al. (37) | n = 56 (I:28, C: 28) | 66.7% | I: 21.4% (n = 6) C: 3.6% (n = 1) |
Counseling: 98% | SCFSd | Baseline | 15.56 (4.64) | 15.52 (3.65) | Change 3 months I vs C = 2.5, P unknown Change 6 months I vs C = −0.50, d = 0.14, P unknown |
I: higher increase in caloric expenditure (CHAMPS) from baseline to 3 and 6 months (d = 0.55, P < 0.05) than C |
| 3 months | 13.43 (4.23) | 11.29 (3.71) | ||||||||
| Post: 6 months | 11.00 (2.90) | 11.46 (3.64) | ||||||||
| Nyrop et al. (38) | n = 62 (I:31, C: 31) | 64.6% | I: 22.6% (n = 7) C: 6.5% (n = 2) |
NA | VASd | Baseline | 4.2 (2.81) | 4.32 (2.82) | Change I vs C = 0.18, d = 0.06, P = ns | I: higher increase in walking time (minutes per week) from baseline to 6 wk than C (d = 1.17, P < 0.01) |
| Post: 6 wk | 4.83 (2.87) | 4.77 (2.82) | ||||||||
| Pinto et al. (39) | n = 86 (I:43, C: 43) | 69.9% | I: 9.3% (n = 4) C: 0% |
NA | VASd | Baseline | 42.47 (23.54) | 41.66 (25.04) | Change I vs C = −16.01, F(1,81) = 12.00, P = 0.001 | I: higher increase in total minutes of PA and total energy expenditure (P = 0.001; P = 0.001), minutes of moderate-intensity PA and moderate energy expenditure (P = 0.001; P = 0.001) and minutes of high-intensity PA and high energy expenditure (P = 0.036; P = 0.033) than C from baseline to 12 wk (7-d PAR). No increase in I compared with C in caloric expenditure (accelerometer) from baseline to 12 wk (P = 0.36) |
| Post: 12 wk | 27.08 (21.41) | 42.28 (26.20) | ||||||||
| Pinto et al. (40) | See Pinto et al. (40) | See Pinto et al. (40) | I: 9.3% (n = 4) C: 9.3% (n = 4) |
See Pinto et al. (40) | See Pinto et al. (40) | FU: 3 months | 34.86 (28.16) | 40.36 (27.47) | Change 6 months I vs C = −5.5, P = 0.310 Change 9 months I vs C = −12.57, P = 0.010 |
No increase in I compared with C in total minutes of moderate-intensity PA (P = 0.765), total energy expenditure (P = 0.097), and moderate energy expenditure (P = 0.748) from baseline to FU of 3 months (7-d PAR) I: higher increase of total minutes of moderate-intensity PA (P = 0.024), total energy expenditure (P = 0.019) and moderate energy expenditure (P = 0.018) than C from baseline to FU of 6 months. |
| FU: 6 months | 32.75 (25.48) | 45.32 (24.17) |
Scores represent mean (SD), study outcomes in bold face represent significant outcomes.
aMean scores are recalculated, SD represents the pooled SD of both groups at baseline, and scores were adjusted for race and radiation treatment.
bScores were adjusted for baseline value’s.
cScores were adjusted for baseline values and full-time employment status.
dHigher score, more symptoms.
7-d PAR, 7-d Physical Activity Recall; Adj, adjusted; C, control; CHAMPS, Community Health Activities Model Program for Seniors; COM, combined group; FACT-F, Functional Assessment of Cancer Therapy—Fatigue; FU, follow-up; GLTEQ, Godin Leisure-Time Exercise Questionnaire; I, intervention; NA, not available; ns, nonsignificant; PA, physical activity; PED, pedometer group; PM, print material group; Post, postintervention; SCFS, Schwartz Cancer Fatigue Scale; POMS, Profile Of Mood States; VAS, visual analog scale.
Data synthesis
We performed meta-analyses for fatigue immediately after the intervention and at 3 and 6–9 months of follow-up. Subgroup analyses were only performed if at least three studies were available. For counseling, subgroup analyses were performed for frequent counseling (<3-wk intervals) and no or infrequent counseling (>8-wk intervals). We calculated standardized mean differences (SMDs) with the means and SD of the intervention and control groups to present the effects at completion and at 3 and 6–9 months of follow-up. We transformed scores to fit into the same direction, if necessary. When single and combined interventions were compared, we used the scores of the combined intervention group for the meta-analysis. Random-effects meta-analyses were conducted for pooled data, using SMDs and 95% confidence intervals for continuous outcomes. Heterogeneity in effect measures between studies was assessed using the I2 statistic.
Risk of bias assessment
The methodological quality of the included studies was evaluated independently by two reviewers (F.H. and D.B.) using the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (41). In case of disagreement, the reviewers aimed to reach consensus, and if needed, discrepancies were resolved by a third reviewer or discussed within the team. We judged single-item rating scales to be at high risk of bias because, compared with multiple-item questionnaires, they can more easily be influenced by knowledge of the intervention received. We judged there as being a high risk of deviation from the intervention if a study lacked an attentional or a wait-list control group, because this could increase the risk of control group contamination. In case of a limited number of studies being included in the meta-analysis (n < 10), we decided not to evaluate the risk of publication bias by funnel plot because the power of the test would be too low to distinguish chance from real asymmetry (42).
RESULTS
Study Selection
In total, 1738 records were found. After removing duplicates, 1302 titles and abstracts were screened. This resulted in 72 full-text records for screening, of which 11 were included in the final review (32,33–40,43,44) (Fig. 1). Interrater agreement was good for the title abstract screening (96.77%; Cohen’s κ = 0.633) and very good for the full-text screening (94.20%; Cohen’s κ = 0.810), respectively (45). Nine studies evaluated fatigue, of which eight did so immediately after the intervention (32,33–37,39), three did so at 3 months of follow-up (33,34,40), and three did so at 6–9 months of follow-up (33,34,40). We found two studies of depression symptoms (43,44) and one of anxiety symptoms (44), the results of which are shown in Tables S2 and S3 (see Tables, Supplemental Digital Content 2, characteristics of the studies for depression and anxiety, http://links.lww.com/MSS/C360; Supplemental Digital Content 3, outcomes of the studies for depression and anxiety, http://links.lww.com/MSS/C361). However, we did not perform a meta-analysis for these outcomes (46).
FIGURE 1.
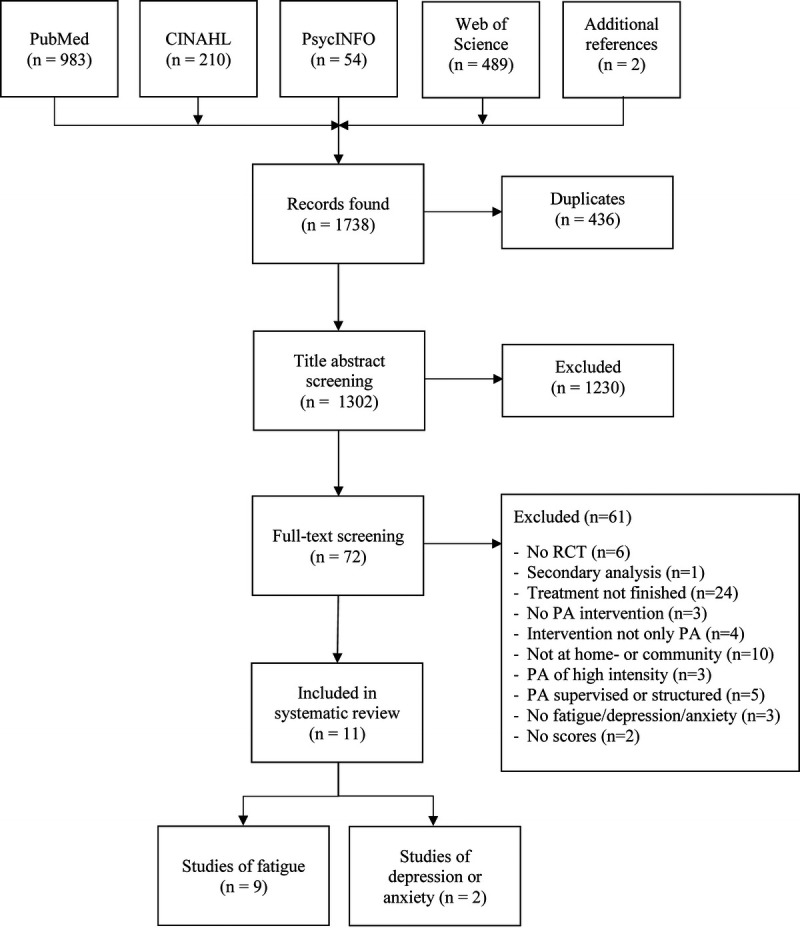
Flowchart of study selection. PA, physical activity; RCT, randomized controlled trial.
Characteristics of the Included Studies
Participants
In total, 1066 participants were included in sample sizes ranging from 30 to 377 (Table 1, Supplement 2, Supplemental Digital Content 2, characteristics of the studies for depression and anxiety, http://links.lww.com/MSS/C360). Seven studies included survivors of breast cancer only (32,34,35,38–40,43), one included survivors of breast and “other” cancers (not specified) (37), and one each included survivors of prostate (44), colorectal (33), and ovarian (36) cancer. The mean age ranged from 53 to 69 yr, and most participants were female (94%) and of Caucasian ethnicity (82%). Fifty-six percent of the participants attended higher education (postsecondary education or higher). Seventy-seven percent of the participants had a history of breast cancer; 14%, ovarian cancer; 4%, colorectal cancer; 4%, prostate cancer; and 1%, “other” cancer (not specified). On average, participants either were diagnosed or had completed their treatment 1.7–5.9 yr before study inclusion, except for one study reporting a mean time of 5.1 months since the end of treatment (32).
Intervention and control
Eight studies allowed participants to choose their preferred home-based PA (33–36,37,39,40,43), and three studies sought to increase walking (32,38,44). All interventions were performed individually in or around the home, except for one study that included community-based group walking sessions (44). The interventions ranged in duration from 6 wk (38) to 6 months (36,37), with the majority lasting 11–12 wk (32,33–35,39,40,43,44). In most studies (32,33–40), the goal of the intervention was to achieve 150 min of PA per week. The remaining studies targeted achieving 30 min of PA on most days of the week (37), 300 min of PA per week (43), or 10,000 steps per day (44). The intensity of the intervention was moderate in six studies (32,33,35,36,37,43) and low to moderate in three studies (34,39,40), but two studies that aimed to increase walking did not specify the required intensity of PA (38,44). Various materials were used to support the interventions, including activity trackers or pedometers (32,33–36,37,39,40,43,44), activity logs (32,33–40,43,44), and information brochures about PA (34,35,38–40).
Counseling was provided in eight studies and was primarily based on the transtheoretical model (33,34,39,40), social cognitive theory (32,33,34), and motivational interviewing (33,34,37). The first session was often supervised in person, with subsequent sessions conducted by telephone. Counseling sessions were offered weekly (33,36,39,40), every 1–2 wk (34), every 2–3 wk (32), every 3 wk (43), or every 2 months (37). Counseling primarily targeted exercise motivation, goal setting, self-efficacy, and problem solving.
In six studies (33,34,36,37,39,40), the control group received an attentional control condition by telephone calls and/or information brochures that were unrelated to PA. Three studies included wait-list control groups (32,38,43), and two included usual care control groups (35,44).
Outcomes
Table 2 shows the intervention outcomes and the scores for fatigue. The Functional Assessment of Cancer Therapy—Fatigue questionnaire (FACT-F) was the most frequently used measure (32,33–36), but a VAS of some form was used in three studies (38–40), and the Schwarz Cancer Fatigue Scale was used in one study (37).
Meta-analysis showed a significant effect in favor of the intervention group immediately after the intervention (SMD = 0.22 [0.06–0.37], Z = 2.76, P = 0.006), at 3 months of follow-up (SMD = 0.27 [0.04–0.51], Z = 2.31, P = 0.02), and at 6–9 months of follow-up (SMD = 0.31 [0.08–0.55], Z = 2.61, P = 0.009; Fig. 2). Heterogeneity between studies in the meta-analyses was small and nonsignificant (I2 = 0%–9%, P = 0.35–0.93). Subgroup analyses showed a difference between the studies that used frequent counseling versus no or infrequent counseling (χ2(1) = 2.70, P = 0.10), with a significant effect for the studies with frequent counseling (SMD = 0.32 [0.12–0.52], Z = 3.08, P = 0.002) and a nonsignificant effect for studies with no or infrequent counseling (SMD = 0.05 [−0.19–0.29], Z = 0.45, P = 0.66; Fig. 3). Subgroup analyses on the type of intervention (walking vs other types of PA), the intensity of PA (low vs moderate), and cancer diagnosis (breast cancer vs other cancers) were not analyzed because too few studies were included per subgroup. Visual inspection revealed no obvious differences between these subgroups.
FIGURE 2.
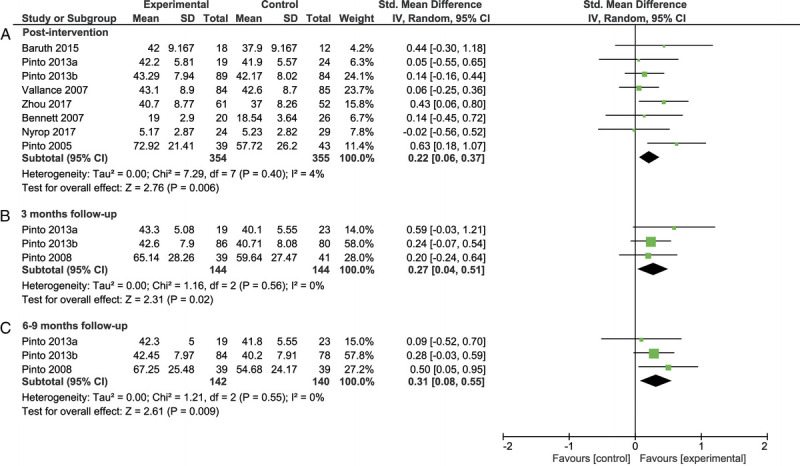
Forest plot of the meta-analysis on fatigue after the intervention (A), at follow-up of 3 months (B), and at follow-up of 6 to 9 months (C). CI, confidence interval.
FIGURE 3.
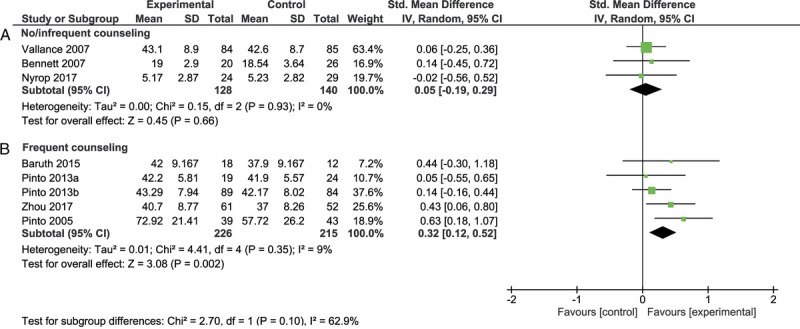
Forest plot of the meta-analysis on fatigue for no or infrequent counseling (A) and frequent counseling (B). CI, confidence interval.
Participation, dropout, and adherence
The participation rate was reported in 10 of the 11 studies and averaged 55.3% (range, 24.2%–84.9%). The average dropout rates in the intervention and control groups were 11.2% (range, 0%–22.6%) and 7.4% (range, 0%–25.7%), respectively.
Adherence was reported in seven studies. Five reported on adherence to the prescribed PA guidelines, for which the average adherence was 77.9% (range, 64.7%–96%). In two studies that reported on adherence to the counseling sessions, one reported 80% adherence to at least 20 of 26 telephone calls, whereas the other reported 98% adherence to 4 telephone calls. Two studies reported on adherence to the use of exercise materials, with 81%–83.3% adherence to recording pedometer steps on study days.
Change in PA
All studies that evaluated change in PA by self-reported questionnaires from baseline to the end of the intervention showed a significant increase in minutes engaged in PA (33–35,39) and in energy expenditure (32,33,37) in the intervention group compared with the control group. However, results varied at 3 and 6–9 months of follow-up (33,34,40), with some studies reporting a higher increase in self-reported PA in the intervention group and others reporting no differences between the intervention and control groups. Varying results were also found in the objective measurement of PA at the end of the intervention and at 3 months of follow-up.
Risk of Bias
The studies by Zhou et al. (36), Bennett et al. (37), and McNeil et al. (43) showed the lowest risk of bias (Fig. 4). The highest risk of bias was observed in the studies by Nyrop et al. (38), Pinto et al. (40), and Pernar et al. (44). Risk of bias was introduced in several ways. First, a single-item VAS scale was used in three studies (38–40), resulting in an increased risk of bias for the outcome measurement. Second, one study was at risk of deviation from the intended interventions because of the lack of an attentional control or wait-list control group (44). Third, two studies had inconsistencies between the outcomes reported in the trial protocol and those reported in the article (34,44). Finally, unequal group sizes led to an increased risk of bias for the randomization process in another study (33).
FIGURE 4.
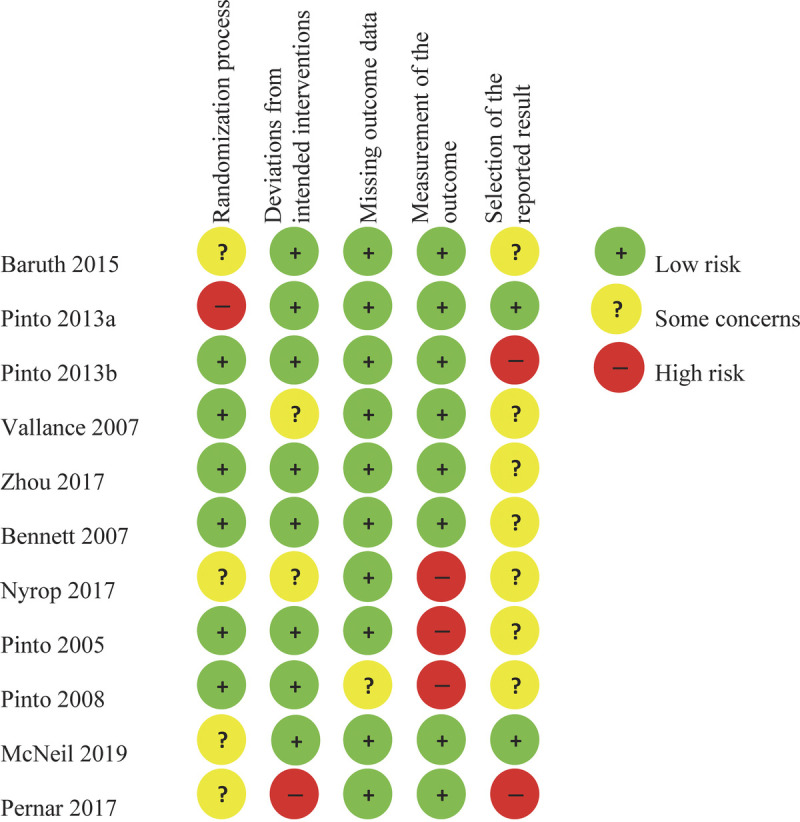
Risk of bias assessment of the included studies.
DISCUSSION
Our results indicate that home-based PA interventions reduce fatigue in adult cancer survivors immediately after the intervention (SMD = 0.22) and at 3 months (SMD = 0.27) and 6–9 months (SMD = 0.31) of follow-up. Those PA interventions that used frequent counseling (SMD = 0.32) also showed improved effects on fatigue compared with interventions in which counseling was infrequent or not provided (SMD = 0.05). Because of the small number of studies included, however, there is insufficient evidence to conclude on how home-based PA affects symptoms of depression and anxiety.
The effects of home-based PA on fatigue at the three assessment points in this study are consistent with those previously reported in research for primarily clinic-based interventions, where the effect sizes ranged from 0.10 to 0.54 (47–49). The improvement in the FACT-F score observed in the intervention group (range, 3.1–4.5) immediately after then intervention is at the lower end of the minimal clinically important difference (50,51). As such, the intervention may clinically alleviate fatigue in cancer survivors, but this cannot be assured. It should also be noted that the studies in this review were not performed in participants with clinical symptoms of fatigue. Implementing a home-based PA intervention in cancer survivors suffering from fatigue may result in larger effect sizes.
Interestingly, we found behavioral counseling in PA interventions to be a potential facilitator in efforts to reduce fatigue. PA interventions with frequent counseling showed improved effects on fatigue (SMD = 0.32), where studies with no or infrequent counseling did not show such effects (SMD = 0.05). This is supported by earlier research showing the added value of counseling and behavioral change strategies in PA interventions on fatigue, depression, and physical function (4,52). PA counseling in the included studies made use of theoretical models to induce behavioral change, with the most common being the transtheoretical model (53), the social cognitive theory (54), and the self-determination theory (55). These models seek to promote increased motivation, self-monitoring, problem solving, and autonomy. PA interventions that include counseling may result in larger health effects by promoting self-efficacy and autonomy. Furthermore, by increasing motivation, self-monitoring, and problem solving in this way, counseling could induce sustainable behavioral change. This may have contributed to the observed long-term effects after 3 and 6–9 months of follow-up.
When seeking to understand how PA can be implemented to alleviate fatigue symptoms, it is important to have knowledge of how fatigue manifests and how we can influence symptom severity. The manifestation of fatigue involves a variety of demographic, medical, biological, psychological, and behavioral factors (56). PA may act primarily on biological, psychological, and behavioral characteristics, with low physical condition and a maladaptive coping style representing significant risk factors for the manifestation of fatigue (56,57). Such coping patterns can include negative feelings of helplessness and worthlessness, low disease acceptance, and low enjoyment (57). PA can contribute in alleviating fatigue not only by improving cardiorespiratory fitness and physical health but also by helping cancer survivors how to cope with their diagnosis and posttreatment recovery by increasing self-efficacy, improving disease acceptance, and reducing negative feelings of helplessness and worthlessness (57). Furthermore, PA can increase enjoyment when it is personalized and adapted to patients’ goals and preferences. The latter, especially, can be of added value in home-based PA interventions.
Unfortunately, too few studies of depression and anxiety were included to allow us to conclude about the effect of home-based PA on symptoms of depression and anxiety. Earlier research showed that one in five cancer survivors experienced symptoms of depression and anxiety (12–14), with clinic-based PA alleviating these symptoms (17–19). It should also be noted that symptoms of fatigue, depression, and anxiety often occur together (58). Some authors even consider these to present in a cluster set as symptoms that are strongly interrelated (57,59,60). The co-occurrence of fatigue, depression, and anxiety is not surprising because all three share underlying components of loss of energy or loss of initiative. Perhaps of even greater interest, however, is that the improvement of one symptom is likely to improve another (60). From that viewpoint, home-based PA may indirectly reduce symptoms of depression and anxiety by alleviating fatigue, and vice versa. There is too little evidence in this review to draw firm conclusions about these effects.
Participation, adherence, and dropout
The participation rate in home-based PA interventions included in this review (mean, 55.3%) was higher than that reported in studies of primarily clinic-based PA interventions both after (37%) (23) and during (41%–43%) treatment (24,25). Also, adherence to PA guidelines was higher than previously reported, with a rate of 77.9% in this review compared with 65% in the earlier review by Bullard et al. (60), who included clinic-based interventions. The mean dropout rates in the intervention (11.2%) and control (7.4%) groups in this review were comparable with those reported by Bullard et al. (61). Participation and adherence may be greater for interventions where participants choose the location (e.g., home or community) and self-monitor engagement compared with clinic-based PA interventions where they have no control over the location or supervision. This may allow for a larger group of cancer survivors to be reached and improving outcomes on fatigue as a result (36,62). Nevertheless, PA interventions must be designed specifically for survivors of cancer because this group typically shows lower adherence, higher dropout rates, and larger variability in adherence and dropout compared with patients who have other chronic diseases (61).
Strengths, limitations, and future research
This is the first review to have evaluated the effects of home-based PA interventions on symptoms of fatigue, depression, and anxiety in cancer survivors. This is also the first review to have differentiated the effects of frequent versus infrequent or no counseling. Nonetheless, several limitations must be considered.
First, a selective group of participants were included in this systematic review, which limits the generalizability of results. Most of the participants were female (94%), of Caucasian ethnicity (82%), and of higher educational level (56%), with breast cancer the most frequent diagnosis (77%). This finding is comparable with other reviews, representing predominantly female breast cancer survivors (5,15,17,18). However, more importantly, with more than half of the participants of high educational level, this group may represent participants of high socioeconomic status with little barriers related to PA. Socioeconomic status is well known to influence PA participation, with people of higher socioeconomic status being more physically active, especially in leisure time PA (63,64). We should strive for PA interventions that reach cancer survivors with low socioeconomic status (63); providing social support to this group may be of particular importance (65).
Second, the studies in this review inevitably have limitations in their design. It was not possible, for example, to blind participants to the intervention or outcome assessment. This could lead to the control group increasing their PA behavior or to the control and PA groups overestimating and underestimating self-reported symptoms, respectively. Such risk can be minimized by offering the control group an attentional control condition or wait-list control. Using multiple-item validated questionnaires, such as the FACT-F, may also help to minimize the influence of patient knowledge of the intervention on the outcome assessment.
Third, although the positive effects at follow-up were promising, it should be noted that these arose from three studies conducted by the same author and that only included breast cancer survivors. Consequently, there may be risks of bias in the study design, study process, and study population.
In the future, research must evaluate the effects of home-based PA interventions on symptoms of depression and anxiety in cancer survivors because there is a lack of research with these outcomes. Furthermore, evaluating symptoms of fatigue, depression, and anxiety over longer-term follow-up (e.g., 12 months) is of great importance because symptoms can persist up to 10 yr after cancer diagnosis (13,66).
CONCLUSIONS
Home-based PA interventions that are of low to moderate intensity reduce fatigue in adult cancer survivors immediately after the intervention, and those favorable effects may even persist for prolonged periods thereafter. Frequent counseling also seems to improve these favorable health outcomes. Therefore, we conclude that home-based PA interventions with frequent counseling offer a suitable and effective intervention for alleviating fatigue in survivors of cancer.
Supplementary Material
Acknowledgments
We would like to thank all authors who responded to our data request for their efforts to provide additional data. We thank Truus van Ittersum for help with developing the search strategy and performing the search. We also thank Dr. Robert Sykes (www.doctored.org.uk) for providing technical editing and writing services for the final draft of this manuscript. This work was supported by the Dutch Cancer Society (KWF Kankerbestrijding; grant number 12375, 2019-1). The funding source had no role in the study design, data collection, analysis, or interpretation.
The authors declare no conflict of interest and declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
NICO-DERK LODEWIJK WESTERINK, Email: n.westerink@umcg.nl.
ANNETTE J. BERENDSEN, Email: a.j.berendsen@umcg.nl.
ANNEMIEK M. E. WALENKAMP, Email: a.walenkamp@umcg.nl.
MATHIEU H. G. DE GREEF, Email: m.h.g.de.greef@rug.nl.
JULIËT K. OUDE NIJEWEEME, Email: j.k.oude.nijeweeme@student.rug.nl.
GEERTRUIDA H. DE BOCK, Email: g.h.de.bock@umcg.nl.
MARJOLEIN Y. BERGER, Email: m.y.berger@umcg.nl.
DAAN BRANDENBARG, Email: d.brandenbarg@umcg.nl.
REFERENCES
- 1.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. 2013;52(2):195–215. [DOI] [PubMed] [Google Scholar]
- 2.Jones LW Liang Y Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013;45(11):2080–90. [DOI] [PubMed] [Google Scholar]
- 4.Swartz MC Lewis ZH Lyons EJ, et al. Effect of home- and community-based physical activity interventions on physical function among cancer survivors: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98(8):1652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra SI Scherer RW Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;2012(8):CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41(1):32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin ML McTiernan A Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Coletta AM Marquez G Thomas P, et al. Clinical factors associated with adherence to aerobic and resistance physical activity guidelines among cancer prevention patients and survivors. PLoS One. 2019;14(8):e0220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson JM, MacPherson CF, Ameringer S, Baggott C, Linder L, Stegenga K. Symptoms and symptom clusters in adolescents receiving cancer treatment: a review of the literature. Int J Nurs Stud. 2013;50(6):847–69. [DOI] [PubMed] [Google Scholar]
- 10.Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CAHHVM, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27(6):965–74. [DOI] [PubMed] [Google Scholar]
- 11.Al Maqbali M, Al Sinani M, Al Naamani Z, Al Badi K, Tanash MI. Prevalence of fatigue in patients with cancer: a systematic review and meta-analysis. J Pain Symptom Manage. 2021;61(1):167–89.e14. [DOI] [PubMed] [Google Scholar]
- 12.Kankerzorg in beeld: over leven met en na kanker [EN: Cancer Care: About Life With and After Cancer]. Amsterdam (the Netherlands): Netherlands Comprehensive Cancer Organisation (IKNL); 2019. [cited 2021 March 25]. Web site [Internet]. Available from: https://www.iknl.nl/kanker-en-leven/gevolgen-van-kanker. [Google Scholar]
- 13.Brandenbarg D Maass SWMC Geerse OP, et al. A systematic review on the prevalence of symptoms of depression, anxiety and distress in long-term cancer survivors: Implications for primary care. Eur J Cancer Care (Engl). 2019;28(3):e13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebber AM Buffart LM Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008;(2):CD006145. [DOI] [PubMed] [Google Scholar]
- 16.McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a meta-analysis. Appl Physiol Nutr Metab. 2011;36(6):892–903. [DOI] [PubMed] [Google Scholar]
- 17.Craft LL, VanIterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JC Huedo-Medina TB Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7(1):e30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170(4):321–31. [DOI] [PubMed] [Google Scholar]
- 20.Ariza-García A, Galiano-Castillo N, Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodríguez L, Arroyo-Morales M. Influence of physical inactivity in psychophysiolocigal state of breast cancer survivors. Eur J Cancer Care. 2013;22(6):738–45. [DOI] [PubMed] [Google Scholar]
- 21.Chipperfield K Fletcher J Millar J, et al. Factors associated with adherence to physical activity guidelines in patients with prostate cancer. Psychooncology. 2013;22(11):2478–86. [DOI] [PubMed] [Google Scholar]
- 22.Grimmett C, Bridgewater J, Steptoe A, Wardle J. Lifestyle and quality of life in colorectal cancer survivors. Qual Life Res. 2011;20(8):1237–45. [DOI] [PubMed] [Google Scholar]
- 23.Kampshoff CS van Mechelen W Schep G, et al. Participation in and adherence to physical exercise after completion of primary cancer treatment. Int J Behav Nutr Phys Act. 2016;13(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Waart H Stuiver MM Van Harten WH, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918–27. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS McKenzie DC Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105(23):1821–32. [DOI] [PubMed] [Google Scholar]
- 26.Hardcastle SJ, Maxwell-Smith C, Kamarova S, Lamb S, Millar L, Cohen PA. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Support Care Cancer. 2018;26(4):1289–95. [DOI] [PubMed] [Google Scholar]
- 27.Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology. 2018;27(3):713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coughlin SS, Caplan L, Stone R, Stewart J. A review of home-based physical activity interventions for breast cancer survivors. Curr Cancer Rep. 2019;1(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D Liberati A Tetzlaff J Altman DG, PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ainsworth BE Haskell WL Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 31.Garber CE Blissmer B Deschenes MR, et al. American College of Sports Medicine Position Stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. [DOI] [PubMed] [Google Scholar]
- 32.Baruth M, Wilcox S, Der Ananian C, Heiney S. Effects of home-based walking on quality of life and fatigue outcomes in early stage breast cancer survivors: a 12-week pilot study. J Phys Act Health. 2015;12(1 Suppl):S110–8. [DOI] [PubMed] [Google Scholar]
- 33.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22(1):54–64. [DOI] [PubMed] [Google Scholar]
- 34.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychol. 2013;32(6):616–26. [DOI] [PubMed] [Google Scholar]
- 35.Vallance JKH, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25(17):2352–9. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y Gottlieb L Cartmel B, et al. Randomized trial of exercise on quality of life and fatigue in women diagnosed with ovarian cancer: The Women’s Activity and Lifestyle Study in Connecticut (WALC). J Clin Oncol. 2017;109(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56(1):18–27. [DOI] [PubMed] [Google Scholar]
- 38.Nyrop KA, Callahan LF, Cleveland RJ, Arbeeva LL, Hackney BS, Muss HB. Randomized controlled trial of a home-based walking program to reduce moderate to severe aromatase inhibitor-associated arthralgia in breast cancer survivors. Oncologist. 2017;22(10):1238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–87. [DOI] [PubMed] [Google Scholar]
- 40.Pinto BM, Rabin C, Papandonatos GD, Frierson GM, Trunzo JJ, Marcus BH. Maintenance of effects of a home-based physical activity program among breast cancer survivors. Support Care Cancer. 2008;16(11):1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterne JAC Savović J Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 42.Higgins JPT Thomas J Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2020. [Google Scholar]
- 43.McNeil J Brenner DR Stone CR, et al. Activity tracker to prescribe various exercise intensities in breast cancer survivors. Med Sci Sports Exerc. 2019;51(5):930–40. [DOI] [PubMed] [Google Scholar]
- 44.Pernar CH Fall K Rider JR, et al. A walking intervention among men with prostate cancer: a pilot study. Clin Genitourin Cancer. 2017;15(6):e1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altman DG. Practical Statistics for Medical Research. 1st ed. London: Chapman and Hall; 1991. pp. 403–9. [Google Scholar]
- 46.Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res Synth Methods. 2017;8(3):290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. [DOI] [PubMed] [Google Scholar]
- 48.Buffart LM, Galvão DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40(2):327–40. [DOI] [PubMed] [Google Scholar]
- 49.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol. 2010;22(3):208–21. [DOI] [PubMed] [Google Scholar]
- 50.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–61. [DOI] [PubMed] [Google Scholar]
- 51.Reddy S, Bruera E, Pace E, Zhang K, Reyes-Gibby CC. Clinically important improvement in the intensity of fatigue in patients with advanced cancer. J Palliat Med. 2007;10(5):1068–75. [DOI] [PubMed] [Google Scholar]
- 52.Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188–94. [DOI] [PubMed] [Google Scholar]
- 53.Prochaska JO, Di Clemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychotherapy. 1982;19(3):276–88. [Google Scholar]
- 54.Bandura A. Social cognitive theory: an agentic perspective. Asian J Soc Psychol. 1999;2:21–41. [DOI] [PubMed] [Google Scholar]
- 55.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. 2000;11(4):227–68. [Google Scholar]
- 56.Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schellekens MPJ, Wolvers MDJ, Schroevers MJ, Bootsma TI, Cramer AOJ, van der Lee ML. Exploring the interconnectedness of fatigue, depression, anxiety and potential risk and protective factors in cancer patients: a network approach. J Behav Med. 2020;43(4):553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell AJ Chan M Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–74. [DOI] [PubMed] [Google Scholar]
- 59.Agasi-Idenburg SC, Thong MS, Punt CJ, Stuiver MM, Aaronson NK. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer. 2017;25(2):625–32. [DOI] [PubMed] [Google Scholar]
- 60.Zhu L Ranchor AV van der Lee M, et al. Co-morbidity of depression, anxiety and fatigue in cancer patients receiving psychological care. Psychooncology. 2017;26(4):444–51. [DOI] [PubMed] [Google Scholar]
- 61.Bullard T, Ji M, An R, Trinh L, MacKenzie M, Mullen SP. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: cancer, cardiovascular disease, and diabetes. BMC Public Health. 2019;19(1):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kessels E, Husson O, van der Feltz-Cornelis CM. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;14:479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Donoghue G Kennedy A Puggina A, et al. Socio-economic determinants of physical activity across the life course: a “DEterminants of DIet and Physical ACtivity” (DEDIPAC) umbrella literature review. PLoS One. 2018;13(1):e0190737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stalsberg R, Pedersen AV. Are differences in physical activity across socioeconomic groups associated with choice of physical activity variables to report? Int J Environ Res Public Health. 2018;15(5):922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craike M, Wiesner G, Hilland TA, Bengoechea EG. Interventions to improve physical activity among socioeconomically disadvantaged groups: an umbrella review. Int J Behav Nutr Phys Act. 2018;15(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bower JE Ganz PA Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


