Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, corticosteroid response, critical illness, glucocorticoid receptor alpha, glucocorticoid-inducible leucine zipper
Abstract
OBJECTIVES:
Critical illness is characterized by increased serum cortisol concentrations and bioavailability resulting from the activation of the hypothalamic-pituitary-adrenal axis, which constitutes an essential part of the stress response. The actions of glucocorticoids are mediated by a ubiquitous intracellular receptor protein, the glucocorticoid receptor. So far, data on coronavirus disease 2019 and glucocorticoid receptor alpha expression are lacking.
DESIGN:
Prospective observational study.
SETTING:
One academic multidisciplinary ICU.
SUBJECTS:
Twenty-six adult coronavirus disease 2019 patients; 33 adult noncoronavirus disease 2019 patients, matched for age, sex, and disease severity, constituted the control group. All patients were steroid-free.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Glucocorticoid receptor alpha, glucocorticoid-inducible leucine zipper expression, and serum cortisol were measured on ICU admission. In coronavirus disease 2019 patients, glucocorticoid receptor alpha and glucocorticoid-inducible leucine zipper messenger RNA expression were upregulated (4.7-fold, p < 0.01 and 14-fold, p < 0.0001, respectively), and cortisol was higher (20.3 vs 14.3 μg/dL, p < 0.01) compared with the control group.
CONCLUSIONS:
ICU coronavirus disease 2019 patients showed upregulated glucocorticoid receptor alpha and glucocorticoid-inducible leucine zipper expression, along with cortisol levels, compared with ICU noncoronavirus disease 2019 patients. Thus, on ICU admission, critical coronavirus disease 2019 appears to be associated with hypercortisolemia, and increased synthesis of glucocorticoid receptor alpha and induced proteins.
The clinical spectrum of coronavirus disease 2019 (COVID-19) is heterogeneous, ranging from a flu-like syndrome to severe pneumonia, requiring monitoring and support in an ICU (1). Critical illness is an acute stress condition, characterized by increased concentration of cortisol due to activation of the hypothalamic-pituitary-adrenal (HPA) axis (2). Most of the immunologic, metabolic, and hemodynamic actions of glucocorticoids (GCs) are mediated by a ubiquitous intracellular receptor protein, the glucocorticoid receptor (GCR). In humans, glucocorticoid receptor alpha (GCR-α) is the predominant and functionally active receptor (3). The cortisol-GCR complex translocates to the nucleus and exerts transcriptional activation or repression of GC-target genes, such as the glucocorticoid-inducible leucine zipper (GILZ). The end-result is inhibition of the proinflammatory response.
A previous study in a non-ICU COVID-19 cohort showed activation of the HPA axis; patients exhibited an increase in cortisol, which was significantly higher than in those without COVID-19 infection (4). However, so far, data on COVID-19 and GCR-α expression are lacking.
In view of the above, in the present study, we measured whole blood expression of the key receptor, GCR-α, the expression of a GC-target gene, GILZ, and the levels of the signaling molecule, cortisol, in ICU COVID-19 patients.
MATERIALS AND METHODS
This prospective, observational study was approved by the “Evangelismos” Hospital Research Ethics Committee (170/24-4-2020), and all procedures carried out were in compliance with the Helsinki Declaration. Informed written consent was obtained from all patients’ next-of-kin. Enrolment took place between May and October 2020. A Consolidated Standards of Reporting Trials flow diagram shows the enrolment process (Supplementary Fig. S1, http://links.lww.com/CCM/G486). A control group of 33 adult ICU non-COVID-19 patients, matched for age, sex, and disease severity, was also included. None of the patients had received corticosteroids, including dexamethasone, prior to blood sampling.
GCR-α and GILZ expression, and serum cortisol were measured on ICU admission. Detailed methods are given in the Online Supplementary Material (http://links.lww.com/CCM/G486).
RESULTS
Characteristics of the Study Population
In Greece, the first pandemic wave did not hit hard the country, possibly due to the early lockdown on March 10, 2020. Between March and April, there were between 30 and 130 daily new cases, which declined in May. In August, cases started rising again, reaching up to 1,000 in October.
Twenty-six adult ICU COVID-19 patients were enrolled (Supplementary Fig. S1, http://links.lww.com/CCM/G486). The ICU non-COVID-19 patients consisted of trauma (n = 17), medical (n = 7; CNS-related pathologies), and surgical (n = 9) cases. Eleven of the patients (33%) had sepsis or septic shock on ICU admission. Characteristics of the two patient groups are presented in Table 1. In ICU COVID-19 patients, comorbidities were more frequent, oxygenation was worse, whereas fibrinogen and lactate dehydrogenase were higher. ICU mortality in COVID-19 was similar to our overall ICU mortality (~30%). This mortality compares favorably with the 40–50% mortality rate reported worldwide for ICU admissions during this period and could be possibly explained by the fact that not all patients were mechanically ventilated.
TABLE 1.
Clinical Characteristics and Laboratory Data of Critically Ill Patients on ICU Admission
| Characteristics | ICU COVID-19 | ICU Non-COVID-19 | p | Reference Values |
|---|---|---|---|---|
| Number of patients, n | 26 | 33 | ||
| Age (yr), median (IQR) | 62 (55–75) | 57 (43–74) | 0.2 | |
| Sex, n (%) | ||||
| Male | 19 (73.1) | 20 (60.6) | 0.3 | |
| Female | 7 (26.9) | 13 (39.4) | ||
| Comorbidities, n (%) | 18 (69.2) | 12 (36.4) | 0.01 | |
| Hypertension | 13 | 7 | ||
| Hyperlipidemia | 6 | 0 | ||
| Diabetes | 3 | 1 | ||
| Coronary artery disease | 2 | 2 | ||
| Chronic obstructive pulmonary disease | 0 | 1 | ||
| Chronic kidney disease | 0 | 1 | ||
| Acute Physiology and Chronic Health Evaluation II score, mean ± sd | 15 ± 5 | 17 ± 6 | 0.2 | |
| Pao2/Fio2 (mm Hg), median (IQR) | 178 (127–261) | 245 (189–353) | 0.003 | |
| Vitals signs | ||||
| Heart rate (beats/min), median (IQR) | 92 (82–106) | 82 (64–104) | 0.05 | |
| Mean arterial pressure (mm Hg), median (IQR) | 75 (71–86) | 75 (69–97) | 0.9 | |
| Temperature (°C), median (IQR) | 37.9 (37.4–38.2) | 37.0 (35.5–37.9) | 0.005 | |
| Laboratory data | ||||
| Hemoglobin, mean ± sd | 13.1 ± 1.8 | 10.0 ± 2.3 | <0.0001 | 12–17.5 |
| WBC count (per μL), mean ± sd | 10,480 ± 5,000 | 14,170 ± 5,850 | 0.02 | 4–10.5 × 103 |
| Neutrophils (%), mean ± sd | 81.6 ± 6.7 | 81.2 ± 6.7 | 0.8 | 40–70 |
| Lymphocytes (%), mean ± sd | 13.0 ± 6.2 | 10.1 ± 4.7 | 0.08 | 25–45 |
| Platelets (per μL), median (IQR) | 220,000 (143,000–270,000) | 163,000 (126,000–230,000) | 0.04 | 140–450 × 103 |
| Urea (mg/dL), median (IQR) | 31 (25–53) | 31 (21–37) | 0.5 | 10–50 |
| Creatinine (mg/dL), median (IQR) | 0.9 (0.7–1.2) | 0.7 (0.6–0.9) | 0.05 | 0.6–1.4 |
| Glucose (mg/dL), median (IQR) | 137 (119–187) | 122 (100–183) | 0.1 | 70–110 |
| Total bilirubin (mg/dL), median (IQR) | 0.7 (0.4–1.1) | 0.6 (0.4–1.0) | 0.5 | <1 |
| C-reactive protein (mg/dL), median (IQR) | 9.7 (4.4–20.8) | 9.9 (5.8–17.5) | 0.9 | <0.5 |
| Fibrinogen (mg/dL), mean ± sd | 631 ± 161 | 421 ± 167 | <0.0001 | 200–400 |
| Lactate dehydrogenase (U/L), median (IQR) | 465 (345–632) | 290 (206–460) | 0.001 | <225 |
| Inotropes (mean dose, µg/kg/min), median (IQR) | 0.2 (0.0–0.3) | 0.3 (0.1–0.5) | 0.2 | |
| Outcomes | ||||
| Length of stay in the ICU (d), median (IQR) | 23 (15–40) | 21 (10–31) | 0.2 | |
| Mechanical ventilation, n (%) | 21 (80.8) | 33 (100) | 0.01 | |
| Mechanical ventilation duration (d), median (IQR) | 15 (5–37) | 10 (5–23) | 0.2 | |
| ICU mortality, n (%) | 8 (30.8) | 11 (33.3) | 0.8 | |
COVID-19 = coronavirus disease 2019, IQR = interquartile range.
Data are expressed as percentages of total related variable (%) and mean ± sd for normally distributed variables and median (IQR) for skewed data.
Patients’ outcome and demographics are also shown.
GCR-α Expression, Signaling, and Cortisol Levels in ICU COVID-19 Patients
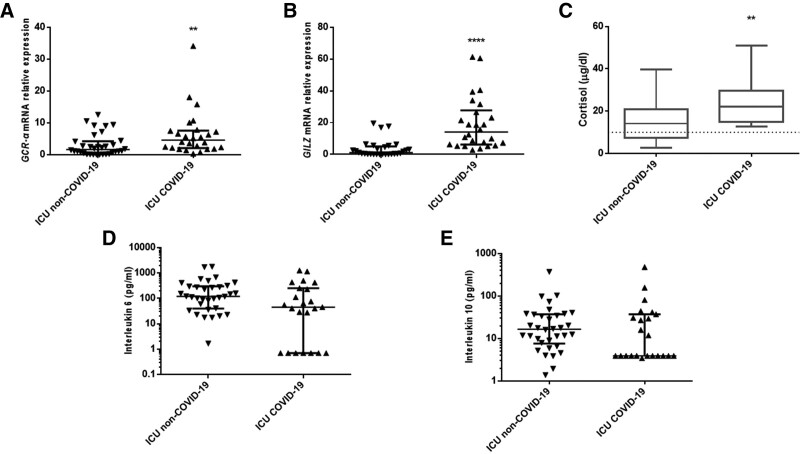
At admission and compared with the non-COVID-19 controls, COVID-19 patients exhibited a 4.7-fold higher GCR-α messenger RNA expression (interquartile range [IQR], 2.1–7.8; p < 0.01) (Fig. 1A). Furthermore, GILZ expression was upregulated by 14.1-fold (IQR, 6.2–27.8; p < 0.0001) (Fig. 1B). Cortisol was also higher (20.3 vs 14.3 µg/dL; p < 0.01) (Fig. 1C), and none of the patients had cortisol less than 10 µg/dL (Fig. 1C). Interleukin (IL)-6 and IL-10 levels were comparable among the groups (Fig. 1, D and E).
Figure 1.
Corticosteroid response in coronavirus disease 2019 (COVID-19). Distribution of glucocorticoid receptor alpha (GCR-α) (A) and glucocorticoid-inducible leucine zipper (GILZ) (B) expression in 26 COVID-19 critically ill patients and 33 non-COVID-19 critically ill patients on ICU admission. Data are presented as scatter plots. Line in the middle: median value; lower and upper lines: 25th–75th percentiles. **p < 0.01, ****p < 0.0001 by Mann-Whitney U test. C, Morning cortisol levels were measured in 26 COVID-19 critically ill patients and in 33 non-COVID-19 critically ill patients on ICU admission. Data are presented as box plots. Line in the box: median value; box edges: 25th to 75th percentiles; whiskers: range of values; horizontal line: cortisol levels 10 µg/dL. **p < 0.01 by Mann-Whitney U test. Distribution of interleukin-6 (D) and interleukin-10 (E) in 26 COVID-19 critically ill patients and 33 non-COVID-19 critically ill patients on ICU admission. Data are presented as scatter plots. Line in the middle: median value; lower and upper lines: 25th–75th percentiles. mRNA = messenger RNA.
GCR-α and GILZ messenger RNA (mRNA) expression did not correlate with cortisol (rs = –0.028, p = 0.9 and rs = 0.159, p = 0.4, respectively). Furthermore, GILZ expression did not correlate with IL-6 or IL-10 (rs = 0.172, p = 0.4 and rs = 0.362, p = 0.1, respectively). However, GILZ expression strongly correlated with GCR-α expression (rs = 0.551, p = 0.004).
DISCUSSION
In this prospective study, we measured GCR-α expression, signaling, and serum cortisol levels, in ICU COVID-19 patients relatively to a general ICU population of similar disease severity. The novel finding is that receptor expression and signaling, and cortisol were higher in ICU COVID-19 patients. Furthermore, according to baseline cortisol, none of the patients with COVID-19 exhibited critical-illness–related corticosteroid insufficiency (CIRCI).
Nearly every tissue in the body expresses GCR, highlighting its critical role in homeostasis and survival. Data on GCR-α expression and critical illness in humans point toward GC resistance in sepsis; specifically, results have shown lower GCR-α expression in septic patients, thereby limiting the anti-inflammatory effects of GCs (5). In our ICU COVID-19 patients, GCR-α expression was elevated, indicating that GC resistance may not constitute a problem. Furthermore, downstream GCR-α signaling occurred, as confirmed by transactivation of the GC-inducible gene GILZ. These might be regarded as beneficial responses to increase the effectiveness of corticosteroid therapy on target cells (6). According to recent data, dexamethasone’s beneficial effect on survival is mainly attributed to its potent immunosuppressive effects (7). Dexamethasone at the recommended dose (6 mg) would probably not have a substantial effect if GC resistance was present; our data showing a functional GCR-a support the results of studies, showing increased survival rates when administered while the disease is dominated by immunopathological elements.
Cortisol secretion is essential for surviving acute illness. One immunoevasive strategy used by other viruses, such as the severe acute respiratory syndrome (SARS) coronavirus or the influenza virus, is to inhibit its host’s corticosteroid stress response (8). These viruses express amino acid sequences homologous to human adrenocorticotropic hormone (ACTH). The host produces antibodies against viral antigens, which, however, may bind to the host’s ACTH, resulting in an inability of ACTH to stimulate cortisol secretion. The overall result is adrenal cortex dysfunction (8). Furthermore, viruses, in particular SARS coronavirus 2, may cause hemorrhage, necrosis, or thrombosis at the adrenal level causing acute hypoadrenalism (9). These assumptions provide compelling evidence that the HPA axis is a potential target of COVID-19. Indeed, in critically ill patients, cortisol was lower in COVID-19 patients compared with non-COVID-19 patients (10), whereas hospitalized patients with more severe disease had lower cortisol and ACTH levels, suggesting a direct link between COVID-19 infection and impaired GC response (11). On the other hand, noncritically ill COVID-19 patients exhibited extremely high cortisol, which was associated with higher mortality rates (4). Similarly, in our ICU COVID-19 patients, cortisol was higher than that in their counterparts without COVID-19, despite similar disease severity. CIRCI is characterized by the inability of the organism to produce sufficient cortisol or resistance of the tissues to its actions, or both (12). We did not assess adrenal function following stimulation with synthetic ACTH; instead, we used the alternative for CIRCI definition (morning baseline cortisol levels <10 μg/dL) (13). We found that none of our patients fulfilled the CIRCI criteria, contrasting the well-established knowledge that a subset of critically ill patients might exhibit adrenal dysfunction (12). Whether cortisol may be useful as a biomarker to monitor COVID-19 severity and ICU outcomes remains to be investigated.
Critical illness is often accompanied by hypercortisolemia, mainly attributed to stress-induced activation of the HPA axis. However, higher cortisol in critically ill patients may also be a consequence of normal production levels but reduced cortisol metabolism (14, 15). Data on the disturbance of the pulsatile and circadian nature of the HPA axis and its potential clinically important impact on gene expression rhythms of core clock genes are emerging (16).
A major limitation of this article is that GILZ measurement in whole blood reflects both neutrophil and monocyte production, perhaps even more the former. Since monocytes are the cells of interest in the immunomodulatory effects of GCs, it would be more appropriate to look at isolated monocytes. Another limitation of our study was the relatively small number of patients but similar to other investigations on the role of GCR mRNA expression levels in critical illness and sepsis.
CONCLUSIONS
Our data provide novel evidence on the upregulated expression and signaling of the functionally active GCR-α isoform in the peripheral blood of ICU COVID-19 patients. Circulating baseline cortisol was high, and consequently, our patients did not meet CIRCI criteria. Overall, it seems that critical infection with COVID-19 appears to be associated with hypercortisolemia, and increased synthesis of GCR-α and induced proteins.
Supplementary Material
Footnotes
*See also p. 2157.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
This work was funded, in part, by the nonprofit institute “THORAX” Research Centre for Intensive and Emergency Thoracic Medicine, Athens, Greece.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Wong CKH, Wong JYH, Tang EHM, et al. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: A systematic review and meta-analysis. Sci Rep. 2020; 10:19765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Téblick A, Peeters B, Langouche L, et al. Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol. 2019; 15:417–427 [DOI] [PubMed] [Google Scholar]
- 3.Hollenberg SM, Weinberger C, Ong ES, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985; 318:635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan T, Khoo B, Mills EG, et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020; 8:659–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Xie M, Yu Y, et al. Leukocyte glucocorticoid receptor expression and related transcriptomic gene signatures during early sepsis. Clin Immunol. 2021; 223:108660. [DOI] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheatland R. Molecular mimicry of ACTH in SARS - implications for corticosteroid treatment and prophylaxis. Med Hypotheses. 2004; 63:855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellastella G, Maiorino MI, Esposito K. Endocrine complications of COVID-19: What happens to the thyroid and adrenal glands? J Endocrinol Invest. 2020; 43:1169–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao Y, Xu B, Guan W, et al. The adrenal cortex, an underestimated site of SARS-CoV-2 infection. Front Endocrinol (Lausanne). 2020; 11:593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alzahrani AS, Mukhtar N, Aljomaiah A, et al. The impact of COVID-19 viral infection on the hypothalamic-pituitary-adrenal axis. Endocr Pract. 2021; 27:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marik PE, Pastores SM, Annane D, et al. ; American College of Critical Care Medicine: Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008; 36:1937–1949 [DOI] [PubMed] [Google Scholar]
- 13.Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med. 2017; 45:2078–2088 [DOI] [PubMed] [Google Scholar]
- 14.Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013; 368:1477–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melby JC, Spink WW. Comparative studies on adrenal cortical function and cortisol metabolism in healthy adults and in patients with shock due to infection. J Clin Invest. 1958; 37:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maas MB, Iwanaszko M, Lizza BD, et al. Circadian gene expression rhythms during critical illness. Crit Care Med. 2020; 48:e1294–e1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.