This work found that after 14 years of the human papillomavirus vaccine implementation, a major obstacle to human papillomavirus vaccination is still lack of knowledge about it among adolescents.
Supplemental digital content is available in the text.
Abstract
Background
Cervical and oropharyngeal cancers are associated with human papillomavirus (HPV) infection, which can be prevented with the vaccines. However, uptake of the HPV vaccine remains low in many countries. There is a need to better understand the barriers to and facilitators of HPV vaccination from young people's perspectives.
Methods
Five electronic databases were searched for original publications (dated January, 2006–December, 2019) reporting barriers to and facilitators of HPV vaccination among young people. All articles were screened against prespecified eligibility criteria, and data were extracted against prespecified form.
Results
A total of 13 studies that were published in international peer-reviewed journals and met the stated eligibility criteria were identified. The barriers reported were centralized around lack of knowledge about HPV and the HPV vaccine, fear about the safety and efficacy of the HPV vaccine, fear about not being able to pay for the HPV vaccine, and discrimination regarding to the HPV vaccine. The facilitators reported were centralized around trust in the efficacy and safety of the HPV vaccine, discounted price of vaccination, positive recommendations from others, perceived risk of HPV infection, and benefits of vaccine.
Conclusions
After their introduction 14 years ago, knowledge deficiency of the HPV vaccine is still a critical barrier to vaccination. Educational initiatives aimed at adolescents and young adults were urgently needed. Understanding factors that arbitrate in early HPV vaccination is critical for improving the HPV vaccination rate.
Persistent infection with high-risk types of human papillomavirus (HPV) is known to cause cervical, oropharyngeal, vaginal, vulvar, penile, anal, and rectal cancers.1 Among them, cervical cancer has the highest incidence (7.4 per 100,000), and the second is oropharyngeal cancer (4.5 per 100,000).2 Approximately 99.7% of cervical cancers and 80% of oropharyngeal cancers are now attributed to high-risk types of HPV.3,4 Two international studies show the decreasing incidence rate of cervical cancer and the rising incidence rate of oropharyngeal cancer.5,6 It is noteworthy that cervical cancer incidence remains high, although a declining trend was observed.6 Fortunately, cervical and oropharyngeal cancers are vaccine-preventable diseases.7
Since 2006, many pivotal clinical trials have shown that both bivalent and quadrivalent HPV vaccines have significantly high effectiveness (>90%) in preventing HPV infections and related diseases caused by vaccine-targeted HPV genotypes.8–11 The Advisory Committee on Immunization Practices recommends HPV vaccine initiation at ages 9 to 26 years.12,13 One study on modeled evaluations of the cost-effectiveness of HPV vaccination indicated that an HPV vaccination coverage of 70% in women has been regarded as the threshold for optimum cost-effectiveness.14 As of October 2019, 100 countries and territories have introduced the HPV vaccine into their national immunization schedules.15
However, global estimates of HPV vaccination coverage indicated that only 33.6% of young females aged 10 to 20 years had received the full course of the HPV vaccine in more developed regions compared with only 2.7% of females in less developed regions.16 There is a huge gap between real-world HPV vaccine coverage and recommended threshold coverage.
Identifying barriers and facilitators to HPV vaccination from key stakeholders' perspectives is paramount. Barriers at the policy level mainly include nonmandatory HPV vaccination and incomplete insurance coverage.17 Health care providers and parents both reported knowledge gaps and financial concerns as barriers.18 The former also mentioned parents’ negative attitudes regarding vaccination. In contrast, parents reported not receiving caregivers’ recommendations and concerns about vaccine safety as their additional barriers to HPV vaccination.18 There are many studies about providers' and parents' perceived barriers to having their patients and children vaccinated, but few studies focus on the vaccine recipient's own perspectives. To increase the uptake of HPV vaccination, a better understanding of the factors that obstruct early HPV vaccination, as experienced by the vaccine recipient, is urgently needed. The purpose of this study is to systematically review self-reported barriers and facilitators to HPV vaccination among young men and women aged 9 to 26 years to inform future efforts to improve HPV vaccination initiation and uptake. The data from this study will enable more precise and accurate assessments of vaccination experience from the vaccine recipient perspective so that future interventions can be developed to improve the HPV vaccination rate and cancer prevention.
METHODS
A protocol for this systematic review was not registered with a database. However, this review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Supplemental File 3, http://links.lww.com/OLQ/A648).19
Search Strategy
We conducted a systematic review of the literature to summarize the barriers to and facilitators of HPV vaccination among adolescents and young adults. Given the HPV vaccine became available in 2006, we included only peer-reviewed journal articles published from January 1, 2006, to December 31, 2019. Five databases, namely, PubMed, Web of Science, Scopus, Medline (EBSCOhost), and PsycINFO (EBSCOhost), were searched for studies published in English. All searches were conducted in a combination of Medical Subject Headings and free terms including “papillomavirus vaccines,” “vaccination,” “cross-sectional studies,” “young adult” and “adolescent.” A medical librarian was consulted to verify the search strategy, and the search was customized for each database separately. See Supplementary File 1 (http://links.lww.com/OLQ/A646) for the complete retrieval strategy.
Eligibility Screening
To be eligible for inclusion, studies had to report perceived barriers and facilitators of HPV vaccination among young people aged 9 to 26 years. Studies that described the HPV vaccine uptake, and attitudes toward or knowledge about the HPV vaccine without reporting the barriers and facilitators to HPV vaccination were excluded. Studies that included solely parents, health care providers, and state leaders, without enrolling adolescents and young adults aged 9 to 26 years were also excluded. In addition, our review also excluded sexual minority populations including transgenders or gay or lesbian individuals because their barriers and facilitators to HPV vaccination should be substantially different from the general adolescents and young adults. Primary studies that used qualitative, quantitative, and mixed methods were included in the review. Review articles, case reports, abstracts, and conference proceedings were excluded.
Data Extraction
The search was performed independently by 2 authors. The authors screened the titles and abstracts of articles retrieved in the initial search based on the inclusion criteria of this study. Full texts of relevant studies were then screened for the final inclusion. Disagreements between the authors were resolved by consensus. To gather all existing evidence, we extracted data into a prespecified data extraction formed by L.Z. and checked by J.W., including country, objectives, study design, respondents, and sample size. We also extracted the self-reported barriers to and facilitators of HPV vaccination mentioned by young respondents.
Quality Assessment
The quality and validity of each article comprising this analysis were assessed using the Agency for Healthcare Research and Quality (AHRQ) criteria for observational studies, which contains 11 items.20 All questions were answered “yes” (scored as 1), “no” (0), or “unclear” (0). Rating criteria for the AHRQ were as follows: low quality, 0–3; moderate quality, 4–7; and high quality, 8–11.
Data Analysis
Data were collected from 13 included studies using Microsoft Excel (Microsoft), with respect to study characteristics, quality assessment, and study results. Given the heterogeneity of included studies, we conducted a narrative synthesis of abstracted data instead of statistical meta-analysis.
RESULTS
Characteristics of Included Studies
Using the predefined search terms, 568 potential articles were identified (Fig. 1). After initial review for relevance and duplication, 440 abstracts remained to be screened for eligibility. A total of 151 articles were retrieved for full-text analysis using the same inclusion criteria. The characteristics of all included studies are shown in Table 1. Of the 13 articles, 2 were conducted by qualitative interviews, and the remainder were questionnaire-based surveys in study design. All studies were published in English and conducted after licensure of the HPV vaccine (after 2006). The mean age of survey respondents in 13 included articles was younger than 26 years. The geographical distribution of these studies was as follows: the United States (N = 2), Malaysia (n = 2), Hong Kong, China (n = 1), United Arab Emirates (n = 1), Germany (n = 1), Uganda (n = 1), India (n = 1), Greece (n = 1), Canada (n = 1), Brasilia (n = 1), and Singapore (n = 1). Five included studies only examined females, 2 studies only examined males, and the other studies included both males and females. All of the studies provided information on barriers, whereas only 4 studies reported facilitators in their decision-making process. As shown in Table 2, the analysis using the AHRQ criteria demonstrated that the studies were of good quality, as the eligible articles were all of moderate or high quality. All 13 articles scored between 4 and 8 points on the quality measure.
Figure 1.
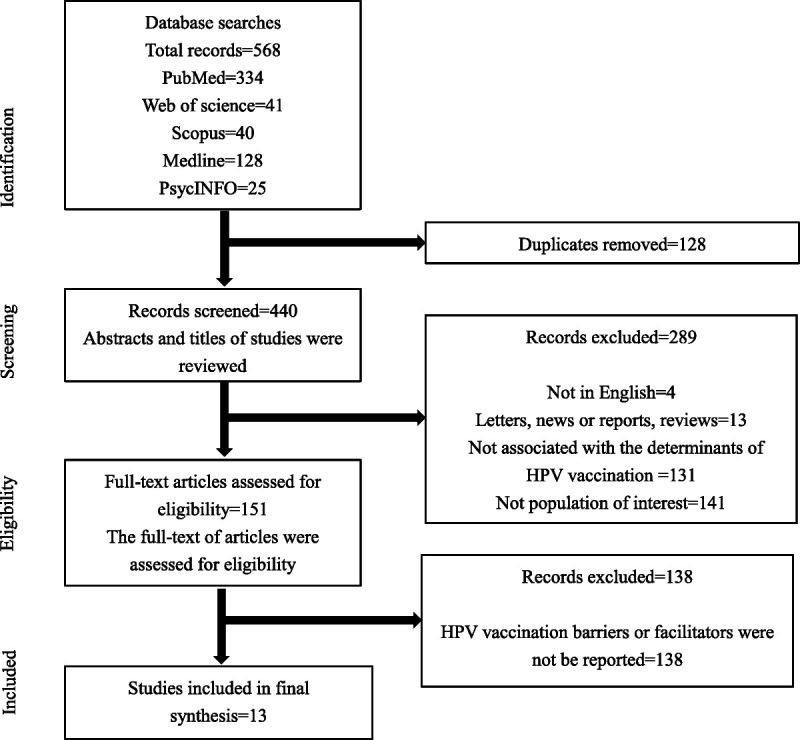
Flowchart for the identification and inclusion of articles in the systematic review.
TABLE 1.
General Characteristics of 13 Included Cross-Sectional Studies
| References | Country | Objectives | Study Design | Respondents (Age, y) | Sample Size | |
|---|---|---|---|---|---|---|
| 1 | Rashwan et al.21 | Malaysia | To assess the knowledge of HPV infection, cervical, cancer and HPV vaccination among medical, dentistry, and pharmacy students and to assess their attitude and practice toward HPV vaccination | Questionnaire survey | Pharmacy students (22–23) | 305 |
| 2 | Ortashi et al.22 | United Arab Emirates | To assess the knowledge about and acceptability of HPV vaccination among male university students in the United Arab Emirates | Questionnaire survey | Male students (21 ± 1.5) | 356 |
| 3 | Lee et al.23 | Hong Kong, China | To update the potential factors influencing the initiation of HPV vaccination among Chinese adolescent girls in HK without universal coverage | Questionnaire survey | Female students (12–19) | 1414 |
| 4 | Remschmidt et al.24 | Germany | To assess the strategy of recruiting young females in Germany via a social media site and to explore the impact of different targeting strategies on study sample characteristics; to assess awareness, knowledge, and attitude related to HPV-infection and HPV vaccine uptake and to identify factors associated with receipt of the HPV vaccine and completion of the HPV vaccination series | Questionnaire survey | Female (18–25) | 1161 |
| 5 | Turiho et al.25 | Uganda | To assess girls' knowledge of cervical cancer and HPV vaccine, and their acceptance of future vaccination of friends and hypothetical daughters | Qualitative interview | Girls (9–19) | 777 |
| 6 | Swarnapriya et al.26 | India | To assess the knowledge, attitude, and practices regarding cervical cancer screening and HPV vaccination among medical and paramedical students. To analyze the factors influencing the knowledge about HPV vaccination among the medical and paramedical students | Questionnaire survey | Medical and paramedical students (19.25 ± 1.64) | 957 |
| 7 | Mammas et al.27 | Greece | To assess HPV vaccination uptake among female adolescents in Greece during the period from 2008 to 2014 To investigate sociodemographic reasons for declining HPV vaccination | Questionnaire survey | Female adolescents (13.2 ± 9) | 632 |
| 8 | Fernandes et al.31s | Canada | To estimate the proportion of women in this age group who have been vaccinated with an HPV vaccine to estimate the proportion of women who would be interested in receiving the vaccine, among those who have not been vaccinated to determine the main barriers that prevent and factors that promote HPV vaccination | Questionnaire survey | Undergraduate women (18–25) | 401 |
| 9 | da Silva Wanderley et al.28 | Brasilia | To explore how medical students differ regarding HPV vaccination status according to their demographics, sexuality, medical school year, and sources of information regarding the vaccine | Questionnaire survey | Medical students (21.8 ± 3.1) | 379 |
| 10 | Widjaja29 | Malaysia | To evaluate the level of awareness, knowledge and attitudes of HPV and its vaccine within university student scope in urban area | Questionnaire survey | Students (21–24) | 425 |
| 11 | Schmidt-Grimminger et al.33s | United States | To determine the knowledge, attitudes, and beliefs related to the HPV vaccine and factors that facilitate or hinder vaccination among important stakeholder subgroups in the community | A mixed-research method to include both qualitative (focus groups) and quantitative (survey) data | Young adults and teens (14–26) | 36 |
| 12 | Zhuang et al.30 | Singapore | To survey the knowledge, attitudes, and practices regarding HPV vaccination among young women in Singapore | Questionnaire survey | Students (15–26; 27–34) | 251 (98.4%) + 4 (1.6%) |
| 13 | Katz et al.32s | United States | To better understand male college students' HPV: (i) knowledge, (ii) perceived risk, (iii) sources of information, and (iv) barriers to and communication about vaccination | Questionnaire survey | Male undergraduate students (16–26) | 165 |
TABLE 2.
Quality Assessment of Observational Studies Using the Agency for Healthcare Research and Quality Criteria
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rashwan et al.21 | Y | N | Y | Y | N | Y | Y | Y | N | Y | N | 7 |
| Ortashi et al.22 | Y | N | Y | Y | N | Y | Y | N | N | Y | N | 6 |
| Lee et al.23 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N | 8 |
| Remschmidt et al.24 | Y | N | Y | Y | N | Y | Y | Y | N | Y | N | 7 |
| Turiho et al.25 | Y | Y | Y | N | N | Y | Y | Y | N | Y | N | 7 |
| Swarnapriya et al.26 | Y | N | Y | Y | N | Y | Y | Y | N | Y | N | 7 |
| Mammas et al.27 | Y | Y | Y | Y | N | Y | Y | N | N | Y | N | 7 |
| Fernandes et al.31s | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | 8 |
| Wanderley et al.28 | Y | N | Y | Y | N | Y | Y | N | N | Y | N | 6 |
| Widjaja29 | Y | N | N | Y | N | Y | Y | N | N | Y | N | 5 |
| Schmidt-Grimminger et al.33s | Y | N | N | N | N | Y | Y | N | N | Y | N | 4 |
| Zhuang et al.30 | Y | N | N | Y | N | Y | Y | N | N | N | N | 4 |
| Katz et al.32s | Y | N | N | Y | N | Y | Y | N | N | N | N | 4 |
Criteria: yes (Y), 1; no (N), 0; unclear (U), 0.
1 = Define the source of information (survey, record review); 2 = list inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications; 3 = indicate time period used for identifying patients; 4 = indicate whether or not subjects were consecutive if not population-based; 5 = indicate if evaluators of subjective components of study were masked to other aspects of the status of the participants; 6 = describe any assessments undertaken for quality assurance purposes (e.g., test/retest of primary outcome measurements); 7 = explain any patient exclusions from analysis; 8 = describe how confounding was assessed and/or controlled; 9 = if applicable, explain how missing data were handled in the analysis; 10 = summarize patient response rates and completeness of data collection; 11 = clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained.
Perceived Barriers From Adolescents and Young Adults
At this level, we organized the study results into the following primary categories: (1) lack of knowledge about HPV and the HPV vaccine, (2) fear about the safety and efficacy of the HPV vaccine, (3) fear about not being able to pay for the HPV vaccine, (4) discrimination regarding to the HPV vaccine, and (5) other barriers, such as lack of time, fear of pain, and a negative experience with vaccinations in general (Table 3).
TABLE 3.
Barriers to and Facilitators of HPV Vaccination
| References | Population Group Concerned (Age, y) | Barriers Encountered | Facilitators Reported |
|---|---|---|---|
| Widjaja29 | Students (21–24) | 1. I feel shy to talk about it to my parent/health care professional 2. I do not have time to bare 3 injections to the hospital 3. My parents do not allow me to take the vaccine 4. Safety and efficacy regarding the vaccine 5. Price 6. I'm not sure where to get the vaccination |
1. Suggested by health care professional 2. Benefits of vaccination regardless of the pricing 3. I can be sexually active to everyone after the injection 4. Forced by parent/partner 5. Self-awareness regarding HPV infection 6. Discounted price of vaccination |
| Turiho et al.25 | Girls (9–19) | Several negative rumors | 1. Appreciation of its preventive role against cervical cancer 2. Realized that side effects were rare |
| Ortashi et al.22 | Male students (21 ± 1.5) | 1. If it has side effects 2. If it has no clear benefits for myself 3. Objections from my family and friends 4. Objections from religious authority 5. I'm not sexually active |
1. It protects my partner from cervical cancer 2. If it is safe 3. Protects me from getting cancer 4. I'm sexually active 5. If it is recommended by family or friends 6. Recommended by doctor 7. If it is recommended by religious authority |
| Lee et al.23 | Female students (12–19) | 1. Cost of vaccination 2. Side effects 3. Being too young for vaccination 4. Low perceived needs 5. Fear of injection |
1. The perceived risk of getting cervical cancer 2. Recommendations from credible sources including doctors and parents 3. The efficacy of the vaccine |
| Remschmidt et al.24 | Female (18–25) | 1. The receipt of recommendations against the vaccination 2. A negative experience with vaccinations in general 3. Fear of injections 4. Not aware of the HPV vaccine |
— |
| Rashwan et al.21 | Pharmacy students (22–23) | 1. Not necessary because you are not prone to be infected 2. HPV vaccine is still new 3. Not cost-effective 4. Afraid of side effect 5. Never get any suggestion 6. Never heard about HPV vaccination 7. Do not have time to complete all 3 |
— |
| Swarnapriya et al.26 | Medical and paramedical students (19.25 ± 1.64) | 1. Doubts regarding efficacy of the vaccine 2. Fear of side effects 3. Cost 4. Minimal risk of carcinoma cervix |
— |
| Mammas et al.27 | Female adolescents (13.2 ± 9) | 1. Fears of side effects 2. Financial issues 3. Lack of knowledge 4. Busy schedule 5. Vaccination not deemed necessary 6. Religious taboos 7. Fear of exposure to needles 8. Medical contraindications 9. Physician advising against vaccination |
— |
| Fernandes et al.31s | Undergraduate women (18–25) | 1. I do not know enough about the vaccine's potential side effects 2. I do not know enough about the vaccine 3. I do not know if the vaccine works 4. The vaccine costs too much 5. The vaccine is not covered by my health insurance |
— |
| Wanderley et al.28 | Medical students (21.8 ± 3.1) | 1. The high cost of the vaccine 2. Not advised about vaccination 3. Doubt about vaccine effects 4. Feeling safe from infection 5. Low efficacy of vaccination |
— |
| Zhuang et al.30 | Students (15–26; 27–34) | 1. Lack of information 2. Cost 3. Side effects 4. Parents 5. Religion 6. Race |
— |
| Schmidt-Grimminger et al.33s | Young adults and teens (14–26) | 1. Negative perceptions about HPV in the community 2. Recommendations from parents |
— |
| Katz et al.32s | Male undergraduate students (16–26) | 1. Long-term effects 2. Only get vaccine if covered by health insurance 3. Cost 4. Hard to find time to talk to health care provider 5. Difficult to get to health care provider's office 6. Difficult to find time to talk to health care provider 7. Discomfort with injection 8. Vaccine is painful 9. Health care provider is too busy to talk to me |
— |
Nearly all included articles (11/13) reported that a lack of knowledge on HPV, cervical cancer, and HPV vaccines affected adolescents’ willingness to initiate HPV vaccination programs.21–30,31s According to our findings, HPV-related knowledge among people aged 9 to 26 years is rather limited. In 2012, 6 years after the first HPV vaccine officially launched by the Food and Drug Administration, there were still people who said they never heard about HPV vaccination.21 Some people said they were not aware of the HPV vaccine and felt shy about discussing it with parents or health care providers.24,29 People may also be misled by some messages, such as that students (aged 9–26 years) are too young for vaccination.28 We also found that many people think vaccination is not deemed necessary because of the lack of a perceived risk of HPV infection.27 They said that they were not sexually active and were not prone to infection.21,22,26,28 Moreover, there was little difference between medical and nonmedical students in accessing HPV-related information.
Many studies (10/13) on factors influencing the intention of adolescents or young adults to get HPV vaccines reported concerns about the safety and efficacy of the HPV vaccine.21–23,26–30,31s,32s Concerns about adverse effects were the most common reason for rejecting HPV vaccinations among people aged 9 to 26 years. More than half of the included articles (8/13) reported concern about adverse effects as a barrier to receiving HPV vaccination.21–23,26,27,30,31s,32s Students from Malaysia indicated that they also had fears about the safety of vaccine and thought it was still new.21,29 One study on undergraduate women indicated that they had not yet been vaccinated because they do not know enough about the vaccine's potential adverse effects and whether the vaccine works.31s Even medical students also reported doubts about the efficacy of the HPV vaccine.28
More than two-thirds of studies (9/13) reported that the cost of vaccines was a concern.21,23,26–30,31s,32s Studies conducted in developed regions (the United States, Greece, Canada, Singapore, and Hong Kong, China) refer to financial issues as a barrier to receiving the HPV vaccine; this was also a concern in some developing countries (Malaysia, India, Brasilia). Among these, 2 questionnaire surveys conducted in America and Canada both noted that the vaccine costs too much and is not covered by personal health insurance.31s,32s Moreover, one study conducted in Malaysia also reported that HPV vaccination was not cost-effective.21
Nearly half of the articles (6/13) reported that discrimination against the HPV vaccine also influenced the decision regarding vaccination.22,24,27,29,30,33s Previous research found that others' recommendations against vaccination played a key role in the decision regarding HPV vaccination. Some students said that their parents would not allow them to take the vaccine; notably, vaccination against HPV is not recommended by some physicians either.22,27,29,30,33s Negative perceptions about HPV will spread throughout the community.33s In addition, discrimination regarding sexually transmitted diseases from religious authority was also reported as a barrier to HPV vaccination.22,27,30 One study conducted in Greece explicitly described HPV vaccination as a religious taboo.27
In addition to the 4 main barriers mentioned previously, there are also some other obstacles that will be described in detail here. First, questionnaire surveys on young women reported that fear of pain was one of the reasons for refusing HPV vaccination.23,24,27,32s Moreover, most people aged 9 to 26 years were still attending school, whose busy schedule would preclude them from talking to health care providers about vaccination or complete all 3 doses of the HPV vaccine.21,27,29,32s Finally, some people also mentioned medical contraindications and not knowing where to get vaccinated as their reasons for not initiating HPV vaccination.27,29
Perceived Facilitators From Adolescents and Young Adults
At this level, we organized the study results into the following primary categories: (1) trust in the safety and efficacy of the HPV vaccine, (2) discounted price of vaccination, (3) positive recommendations from others, and (4) perceived risk of HPV infection and benefits of vaccine (Table 3).
Many students reported that recommendations from others have a positive impact on increasing vaccination uptake and credible sources, including doctors, parents, friends, and religious authority.22–24 Otherwise, awareness of vaccines’ preventive role against cervical cancer, for self and others, was a facilitator of receiving the HPV vaccine.22,25
DISCUSSION
Although there is substantial literature on factors associated with higher and lower HPV vaccination uptake, the body of literature reporting barriers from the vaccine recipient's perspectives that are critical to HPV vaccination is relatively sparse. This is a systematic review that reviews the literature on barriers to and facilitators of HPV vaccination among adolescents and young adults. Our systematic review found that the barriers to vaccination were mainly concerns about the lack of knowledge about HPV and the HPV vaccine, fear about the safety and efficacy of the vaccine, fear about not being able to pay for the HPV vaccine, and discrimination regarding the HPV vaccine. Second, trust in the efficacy and safety of the HPV vaccine, discounted price of vaccination, positive recommendations from others, perceived risk of HPV infection, and benefits of the vaccine are the facilitators of HPV vaccination.
The lack of knowledge or information on cervical cancer and HPV infection was the most commonly reported barriers when considering the uptake of HPV vaccination. This provides an opportunity for the spread of misinformation in social networks. Compared with other populations, adolescents and young adults are more vulnerable to misinformation. For instance, many adolescents refused to receive the HPV vaccine because they indicated that they were not sexually active and were not prone to infection. Unfortunately, approximately 80% of women will acquire HPV infection during their lifetime.34s As a result, educational initiatives aimed at people aged 9 to 26 years may be most successful if designed to increase awareness of susceptibility to HPV infection and HPV transmission. School-based meetings are perhaps an essential sensitization strategy to increase the amount and quality of knowledge and information on cervical cancer and the HPV vaccine.35s Previous studies have shown that integrating school immunization provision with general practice provides a convenient location for parents and their children to discuss the immunization program.36s
Our results also indicated that concerns about the safety and efficacy of the HPV vaccine constitute a barrier to HPV vaccination among the target population. In contrast, trust in the efficacy and safety of vaccines may play a role as vaccination activators. In fact, many randomized controlled trials have confirmed the safety and efficacy of HPV vaccination.37s–39s No serious vaccine-related adverse effects were reported in the clinical study.37s As previously reported, concerns about the safety and effectiveness of the vaccine is related to lack of knowledge.40s This knowledge gap reveals an additional priority for education. Health literacy initiatives in adolescents and young adults should focus on the efficacy of the HPV vaccine and highlight its established safety at the same time.
Another barrier is the cost of vaccination. In contrast, the discounted price of vaccination has a positive effect on initiating HPV vaccination. Previous studies have also shown that the highest HPV vaccination coverage rates are observed in countries where vaccines are funded from the national budget.41s This was the case in Japan, where a high uptake rate for individual HPV vaccination was obtained.42s The main source of HPV vaccination program costs in developed countries is the government budget, whereas in developing countries, international donors such as the Global Alliance for Vaccines and Immunization, can be an important source.43s Actually, a record low price of as little as US $4.50 per dose for low-income countries compared with more than $100 in high-income countries was announced by the Global Alliance for Vaccines and Immunization as early as 2013.44s
Additional obstacles to vaccination include discrimination against the HPV vaccine and practical barriers. Discrimination against the HPV vaccine refers to religious taboo, cultural biases; practical barriers refer to fear of needles, busy schedule, limited access to health services, medical contraindications. Many of these factors are related to all kinds of vaccines. However, cultural and religious sensitivity seems to be specific to the HPV vaccine. Two studies conducted in the United Arab Emirates and Singapore mentioned religious authority as a barrier to HPV vaccination.23,29 A previous study has also revealed that sex-related issues are taboo topics in many countries around the world, especially in Asian communities.45s However, a survey conducted in Scotland indicated that some women would like to receive the information on HPV delivered within the religious community, not within other places for social gathering.46s Therefore, health literacy initiatives addressing HPV vaccination gaps also needs to be culturally tailored. It is perhaps a good approach that develop education programs with the help of local community and religious leaders.46s
Of the 4 facilitators of vaccination mentioned in our review, positive recommendations from others were most commonly reported. Adolescents and young adults usually receive recommendations for HPV vaccination from their physicians, parents, and friends. Furthermore, health care providers' recommendations are more convincing to the vaccine recipient than recommendations from other sources.47s According to a study of 17,264 girls aged 12 to 17 years in the United States, girls who receive a clinician recommendation to vaccinate are 23 times more likely to be vaccinated than those not counseled.48s It is also notable that physician effects could be both positive and negative. Thus, acceptability for vaccination of adolescents and young adults could be improved by increasing providers positive recommendations. Physicians should use this influence to disseminate HPV-related knowledge to youths, including the transmission of virus, the likelihood of infection, and its oncogenicity.
Our findings should be interpreted with the following limitations. First, we only included peer-reviewed studies published in the English language. Therefore, gray literature was excluded from the review, which may have biased the results. Second, all studies were cross-sectional, precluding an understanding of changes in knowledge, attitudes, and practices over time. In addition, most studies recruited convenience samples, thus limiting the generalizability of the findings. Furthermore, another limitation relates to the medical background of the study population. A quarter of the retrieved articles concern studies performed in medical or pharmacy student populations. However, nearly three-quarters of the studies were conducted in populations without a medical background. This knowledge gap may also affect outcome.
This review provides valuable data about adolescents’ and young adults' self-reported barriers and facilitators to initiating HPV vaccination. After their introduction 14 years ago, knowledge deficiency of the HPV vaccine is still a critical barrier to vaccination. Educational initiatives aimed at adolescents and young adults were urgently needed. School-based meetings and health care providers’ recommendations were identified as key cues to action. Efforts to better understand determinants of HPV vaccination among adolescents and young adults could potentially increase vaccination rates and decrease morbidity and mortality due to HPV-related cancers.
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A647.
Footnotes
Conflict of Interest and Sources of Funding: The authors declare that they have no known competing financial interests or personal relationships that could have seemed to influence the work reported in this article. The authors report no funding sources.
Author Contributions: L.Z. and J.W. developed the review protocol with input from all authors. L.Z. developed the search strategy with input from J.W. and M.Z. Database searches were performed by L.Z., and screening of search results according to eligibility criteria was performed by L.Z. and J.W. L.Z. extracted data from included articles, produced data tables, performed data analyses, and conducted a narrative synthesis. J.W. contributed to the planning of data presentation and analyses. Final data tables and analyses were verified by M.Z., and all authors reviewed and agreed upon the narrative synthesis. Quality assessment of included studies was conducted by L.Z. and reviewed and agreed upon by all authors. Writing of the manuscript was led by J.W., and all authors contributed.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Luyan Zheng, Email: luyanzheng@zju.edu.cn.
Jie Wu, Email: zjwujie@zju.edu.cn.
REFERENCES
- 1.Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol 2017; 40:80–85. [PubMed] [Google Scholar]
- 2.Viens LJ Henley SJ Watson M, et al. Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016; 65:661–666. [DOI] [PubMed] [Google Scholar]
- 3.de Martel C Plummer M Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141:664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci 2006; 110:525–541. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK Anderson WF Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol 2013; 31:4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbyn M Weiderpass E Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health 2020; 8:e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017; 123:2219–2229. [DOI] [PubMed] [Google Scholar]
- 8.Lehtinen M Paavonen J Wheeler CM, et al. HPV PATRICIA Study Group . Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-Year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89–99. [DOI] [PubMed] [Google Scholar]
- 9.Munoz N Kjaer SK Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102:325–339. [DOI] [PubMed] [Google Scholar]
- 10.Herrero R Wacholder S Rodríguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: A community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov 2011; 1:408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaer SK Sigurdsson K Iversen O-E, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009; 2:868–878. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) . Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1705–1708. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) . FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629. [PubMed] [Google Scholar]
- 14.Canfell K Chesson H Kulasingam SL, et al. Modeling preventative strategies against human papillomavirus–related disease in developed countries. Vaccine 2012; 30 Suppl 5:F157–F167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . Major milestone reached as 100 countries have introduced HPV vaccine into national schedule. Available at: https://www.who.int/news/item/31-10-2019-major-milestone-reached-as-100-countries-have-introduced-hpv-vaccine-into-national-schedule. Accessed December 16, 2019.
- 16.Bruni L Diaz M Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob Health 2016; 4:e453–e463. [DOI] [PubMed] [Google Scholar]
- 17.Carhart MY Schminkey DL Mitchell EM, et al. Barriers and facilitators to improving Virginia's HPV vaccination rate: A stakeholder analysis with implications for pediatric nurses. J Pediatr Nurs 2018; 42(1532–8449):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holman DM Benard V Roland KB, et al. Barriers to human papillomavirus vaccination among us adolescents a systematic review of the literature. JAMA Pediatr 2014; 168:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D Liberati A Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339:332–336. [PMC free article] [PubMed] [Google Scholar]
- 20.Rostom A, Dube C, Cranney A. Evidence Report/Technology Assessment No. 104. Celiac Dis Evid Reports/Technology Assessments [Online]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK35156/. Accessed April 22, 2020.
- 21.Rashwan HH, Saat NZNM, Manan DNA. Knowledge, attitude and practice of Malaysian medical and pharmacy students towards human papillomavirus vaccination. Asian Pac J Cancer Prev 2012; 13:2279–2283. [DOI] [PubMed] [Google Scholar]
- 22.Ortashi O, Raheel H, Khamis J. Acceptability of human papillomavirus vaccination among male university students in the United Arab Emirates. Vaccine 2013; 31:5141–5144. [DOI] [PubMed] [Google Scholar]
- 23.Lee A Ho M Cheung CKM, et al. Factors influencing adolescent girls’ decision in initiation for human papillomavirus vaccination: A cross-sectional study in Hong Kong. BMC Public Health 2014; 14:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remschmidt C Walter D Schmich P, et al. Knowledge, attitude, and uptake related to human papillomavirus vaccination among young women in Germany recruited via a social media site. Hum Vaccin Immunother 2014; 10:2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turiho AK Okello ES Muhwezi WW, et al. Effect of school-based human papillomavirus (HPV) vaccination on adolescent girls' knowledge and acceptability of the HPV vaccine in Ibanda District in Uganda. Afr J Reprod Health 2014; 18:45–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Swarnapriya K, Kavitha D, Mohan Reddy GM. Knowledge, attitude and practices regarding HPV vaccination among medical and para medical in students, India a cross sectional study. Asian Pac J Cancer Prev 2016; 16:8473–8477. [DOI] [PubMed] [Google Scholar]
- 27.Mammas IN Theodoridou M Koutsaftiki C, et al. Vaccination against human papillomavirus in relation to financial crisis: The “Evaluation and Education of Greek Female Adolescents on Human Papillomaviruses' Prevention Strategies” ELEFTHERIA study. J Pediatr Adolesc Gynecol 2016; 29:362–366. [DOI] [PubMed] [Google Scholar]
- 28.da Silva Wanderley M Sobral DT de Azevedo Levino L, et al. Students' HPV vaccination rates are associated with demographics, sexuality, and source of advice but not level of study in medical school. Rev Inst Med Trop Sao Paulo 2019; 61:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widjaja VN. Awareness, knowledge and attitudes of human papillomavirus (HPV) among private university students—Malaysia perspective. Asian Pac J Cancer Prev 2019; 20:2045–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuang QY Wong RX Chen WMD, et al. Knowledge, attitudes and practices regarding human papillomavirus vaccination among young women attending a tertiary institution in Singapore. Singapore Med J 2016; 57:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]


