Abstract
Background
The combination of benzoyl peroxide and a new topical therapy, such as topical niacinamide, reduces facial sebum production and also has a skin-lightening effect. This combined treatment might lead to improved efficacy in the treatment of facial acne vulgaris while also promoting the resolution of postacne erythema and postinflammatory hyperpigmentation.
Objective
The primary objective was to evaluate and compare the clinical efficacy of topical 2.5% benzoyl peroxide plus 5% niacinamide and 2.5% benzoyl peroxide with cream base for mild to moderate facial acne vulgaris. Secondary objectives were to evaluate and compare clinical efficacy regarding postinflammatory hyperpigmentation, postacne erythema, reduction of facial sebum production, and side effects. METHODS: Patients with mild to moderate facial acne vulgaris and aged 18 to 40 years were enrolled. Treatment was randomly assigned to the left or right side of the face for 12 weeks. Both inflammatory and noninflammatory acne lesions were counted by a physician, and the postinflammatory hyperpigmentation score and postacne erythema score were calculated using an Antera 3D® camera (Miravex, Dublin, Ireland). Sebum casual level was measured using a Sebumeter® (Courage+Khazaka Electronic, Köln, Germany) every two weeks. Physician improvement score, patient satisfaction index, and side effects were assessed by evaluation forms every two weeks.
Results
At Week 12, the niacinamide group (5% niacinamide+2.5% benzoyl peroxide) showed significant reduction in both the acne lesion count and sebum casual levels from baseline (p=0.000 and p=0.001, respectively). The reduction in noninflammatory lesion count in the niacinamide group was better than that in the cream base group (2.5% benzoyl peroxide+cream base), with a statistically significant difference (p=0.004). However, the reduction in inflammatory lesions was not significantly different between the two groups. The sebum casual level in the niacinamide group was reduced faster than that in the cream base group. The postacne erythema score was reduced from baseline in both groups, with no statistically significant difference within or between the two groups. The postinflammatory hyperpigmentation score showed increases in both groups above the baseline, with a statistically significant difference in the cream base group (p=0.000) but no such difference in the niacinamide group (p=0.58). There was no statistically significant difference between the two groups. Furthermore, no statistically significant differences were found between the two groups at every follow-up visit in terms of physician improvement scale, patient satisfaction index, or side effects.
Conclusion
The combination of 2.5% benzoyl peroxide and 5% niacinamide is more effective than 2.5% benzoyl peroxide alone for mild to moderate facial acne vulgaris.
Keywords: Niacinamide, benzoyl peroxide, acne vulgaris
Acne vulgaris is an inflammatory disorder caused by many factors that affects the pilosebaceous units of the skin. The condition is commonly found in dermatologic outpatient departments. The main pathogenic factors include increased sebum production, follicular hyperkeratinization leading to obstruction of the sebaceous follicles, Propionibacterium acnes colonization in pilosebaceous units, and inflammation around the follicles.1 Clinical presentation of acne vulgaris ranges from mild comedones to severe pustules and cysts. Topical therapy includes the use of many agents as monotherapy or combined. Commonly used topical acne therapies include benzoyl peroxide, retinoids, antibiotics, salicylic acid, azelaic acid, dapsone, combination antibiotics with benzoyl peroxide, retinoids with benzoyl peroxide, and retinoids with antibiotic. Therapeutic decisions can depend on the patient’s age, area of involvement, severity of disease, and patient preference. In the treatment of mild to moderate acne vulgaris, recommendations based on "Guidelines of Care for the Management of Acne Vulgaris" by the American Academy of Dermatology include topical combination therapy.2 Benzoyl peroxide is an antibacterial drug; it releases free oxygen radicals that can kill P. acnes and also has a mild comedolytic effect. There are no reports concerning drug resistance. Combinations of benzoyl peroxide with topical antibiotic treatment give better results and can reduce the development of antibiotic resistance. Benzoyl peroxide has some side effects, including irritation with concentration dependence, staining and bleaching of clothes, and uncommon allergic contact dermatitis.3 Topical niacinamide, or nicotinamide, is an amide of vitamin B3 (niacin) with anti-inflammatory effects that reduces facial sebum production and also induces skin lightening.4 It is widely used in cosmetic preparations and is considered safe up to 5%.5 This study compared a combination of 2.5% benzoyl peroxide plus 5% niacinamide with 2.5% benzoyl peroxide plus cream base for the treatment of acne vulgaris. We aimed to evaluate whether 5% niacinamide was an effective drug for better treatment results in mild to moderate acne vulgaris.
METHODS
This study was a double-blinded, randomized, controlled, split-face trial. Neither the investigator nor the participants knew which drugs were administered. A randomization code list was generated by the research assistant for labeling the study medication and producing randomized code envelopes. Participants received all study medications from the research assistant at the Thai Tobacco Monopoly Hospital. The protocol was approved by Thammasat University Institutional Review Board and conducted at the outpatient department of Tobacco Monopoly Hospital, Thailand, from November 2018 to April 2019. Informed consent was signed by each subject prior to the treatment procedure.
Patients aged 18 years or older with mild to moderate facial acne vulgaris as evaluated by the Leeds Revised Acne Grading System were included in the study. Patients were excluded from the study if they had used topical acne medication two weeks prior to the study, received oral retinoids one year prior to the study, received dermabrasion, underwent laser resurfacing two months prior to the study, had underlying diseases, such as endocrine disease, liver disease, active gastrointestinal ulcers, or a history of gastrointestinal ulcers or gout, had received intramuscular injections of contraceptives, estrogen, or steroids within three months prior to the study, used medical soap or anti-acne cream within seven days of the study, had historical allergies to topical benzoyl peroxide or niacinamide, and/or were pregnant or breastfeeding.
Patients were asked to apply a 2.5% benzoyl peroxide water-based gel 2 FTU (1g) on their entire face for 10 minutes before facial washing. After washing off the 2.5% benzoyl peroxide water-based gel, the patients were randomly assigned to apply 5% niacinamide cream 1 FTU (0.5 g) on one half of the face and the cream base 1 FTU (0.5g) on the other half. This procedure was performed twice daily for 12 weeks.
Evaluation of efficacy using total lesion count, sebum casual level using a Sebumeter® (Courage + Khazaka Electronic, Köln, Germany), postacne erythema score, and postinflammatory hyperpigmentation score by an Antera 3D® camera (Miravex, Dublin, Ireland) were assessed at baseline and two, four, six, eight, 10, and 12 weeks. Evaluation of satisfaction using the physician's improvement scale and patient satisfaction index and evaluation of side effects using a patient evaluation form were conducted at two, four, six, eight, 10, and 12 weeks (Table 1).
TABLE 1.
Statistical analysis and comparison of physician's improvement scale scores at each visit between the niacinamide group and cream base group
| VARIABLE | NIACINAMIDE | CREAM BASE | P-VALUE |
|---|---|---|---|
| Week 2 | |||
| 0 | 4 (21%) | 3 (16%) | 1 |
| 1 | 11 (58%) | 12 (63%) | |
| 2 | 4 (21%) | 4 (21%) | |
| Week 4 | |||
| 1 | 6 (32%) | 10 (53%) | 1 |
| 2 | 9 (47%) | 6 (32%) | |
| 3 | 4 (21%) | 3 (16%) | |
| Week 6 | |||
| 0 | 0 (0%) | 1 (6%) | 0.09 |
| 1 | 4 (22%) | 2 (11%) | |
| 2 | 4 (22%) | 10 (56%) | |
| 3 | 10 (56%) | 5 (28%) | |
| Week 8 | |||
| 1 | 2 (12%) | 2 (12%) | 0.7 |
| 2 | 4 (24%) | 4 (24%) | |
| 3 | 10 (59%) | 10 (59%) | |
| 4 | 1 (3%) | 1 (6%) | |
| Week 10 | |||
| 1 | 1 (6%) | 3 (18%) | 0.09 |
| 2 | 4 (24%) | 3 (18%) | |
| 3 | 10 (59%) | 9 (53%) | |
| 4 | 2 (12%) | 2 (12%) | |
| Week 12 | |||
| 2 | 2 (12%) | 3 (18%) | 0.09 |
| 3 | 7 (41%) | 11 (65%) | |
| 4 | 8 (47%) | 3 (18%) | |
RESULTS
Study population. A total of 21 patients (16 women, 5 men) with facial acne vulgaris, including seven patients with moderate facial acne vulgaris and 14 patients with mild facial acne vulgaris per the Leeds Revised Grading System were included in this study. Patient age ranged from 18 to 36 years.
Efficacy. Inflammatory lesions and noninflammatory lesions were counted. At baseline, the mean lesion count of inflammatory lesions and noninflammatory lesions were not different between the two groups (p=0.24 and p=0.2, respectively). After 12 weeks of treatment, both types of lesion in the niacinamide group (2.5% benzoyl peroxide+5% niacinamide) showed a significant gradual decrease from baseline, similar to the cream base group (2.5% benzoyl peroxide+cream base). At the end of the treatment, percentage of inflammatory lesion change from baseline was not statistically significantly different between the two groups (Figure 1). Interestingly, percentage of noninflammatory lesion change from baseline was statistically different between the two groups (niacinamide group decreased 51% vs. cream base group decreased 34%; p=0.004) (Figure 2).
FIGURE 1.
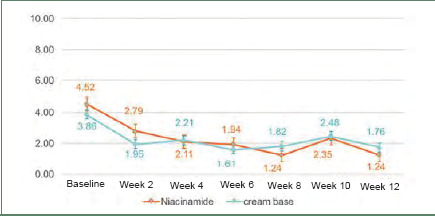
Graph of inflammatory lesion counts
FIGURE 2.
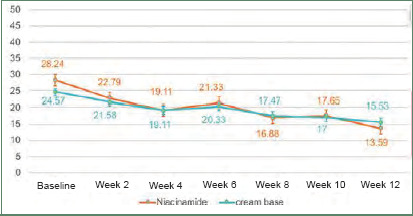
Graph of noninflammatory lesion counts
Sebum casual level was measured using a Sebumeter®. Room temperature was controlled at 23°C to 25°C with humidity at 40% to 60% in the Dermatology Outpatient Department at the Thai Tobacco Monopoly Hospital, measured by a sensor RTH 100. Sebum casual level was reduced significantly from baseline from Week 6 and continued to decrease in later follow-up visits in the niacinamide group, as compared with in the cream base group, where the level was decreased significantly only at Weeks 8 and 12. Decreases in sebum casual level at Week 12 were 26 percent and 20 percent from baseline in the niacinamide group and cream base group, respectively. However, these reductions were not statistically different (p=0.26) (Figure 3).
FIGURE 3.
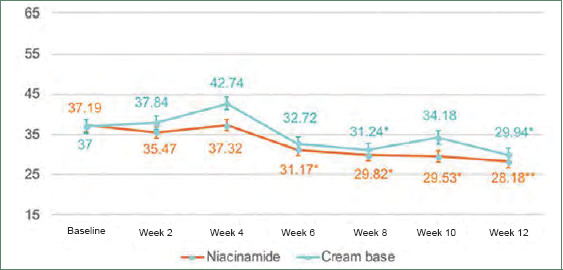
Graph of sebum casual level
Postacne erythema and postinflammatory hyperpigmentation scores were recorded by an Antera 3D® camera (Figures 4–7). Postacne erythema scores were not statistically different from baseline or between the two groups at every follow-up visit. Change in postacne erythema score at Week 12 decreased 0.5 percent (Figure 8) and increased 1.5 percent (FFigure 9) from baseline in the niacinamide group and cream base group, respectively; however, change between the two groups was not statistically different (p=0.98).
FIGURE 4.
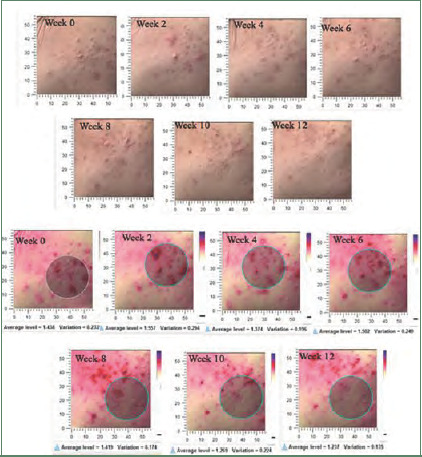
Postacne erythema scores and images taken with the Antera 3D® camera of the niacinamide-treated side of study participant NO. 17
FIGURE 7.
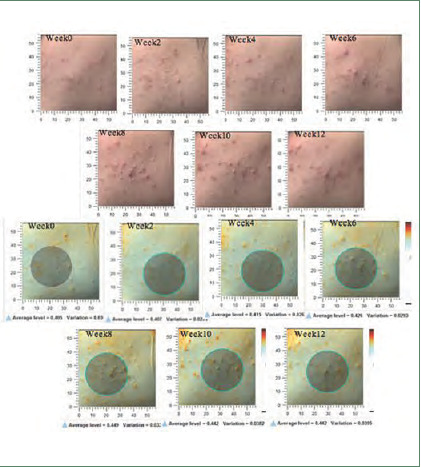
Postinflammatory hyperpigmentation scores and images taken with the Antera 3D® camera of the cream base–treated side of study participant NO. 17
FIGURE 8.
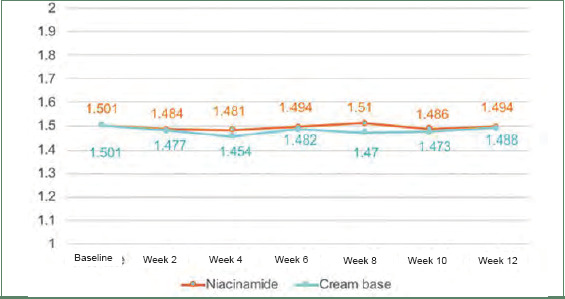
Graph of postacne erythema scores
FIGURE 9.
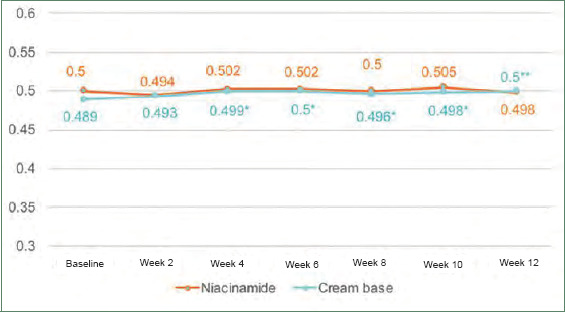
Graph of postinflammatory hyperpigmentation score of acne skin.
FIGURE 5.
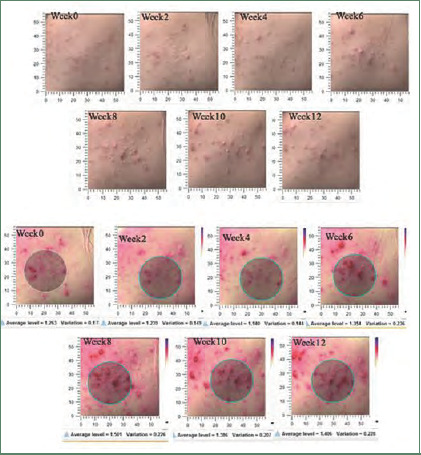
Postacne erythema scores and images taken with the Antera 3D® camera of the cream base-treated side of study participant NO. 17
FIGURE 6.
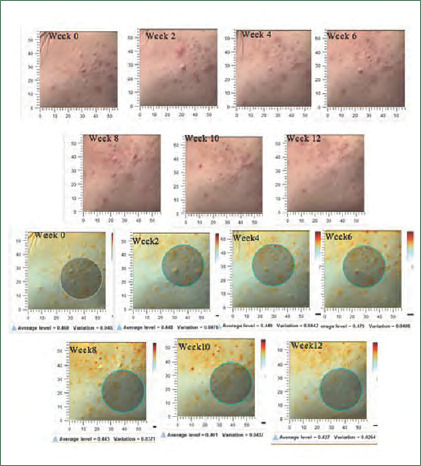
Postinflammatory hyperpigmentation scores and images taken with the Antera 3D® camera of the niacinamide-treated side of study participant NO. 17
Evaluation of satisfaction. A satisfactory evaluation was performed by the doctor and patients at every follow-up visit at Weeks 2, 4, 6, 8, 10, and 12. Scales ranged from 0 to 4, where 0 indicated no change, 1 indicated slight improvement, 2 indicated moderate improvement, 3 indicated significant improvement, and 4 indicated excellent improvement (Table 2).
TABLE 2.
Statistical analysis and comparison of patient satisfaction index scores at each visit between the niacinamide group and cream base group
| VARIABLE | NIACINAMIDE | CREAM BASE | P-VALUE |
|---|---|---|---|
| Week 2 | |||
| 0 | 5 (26%) | 3 (10%) | 0.46 |
| 1 | 5 (26%) | 10 (53%) | |
| 2 | 4 (21%) | 4 (21%) | |
| 3 | 4 (21%) | 2 (11%) | |
| 4 | 1 (5%) | 1 (5%) | |
| Week 4 | |||
| 0 | 1 (5%) | 1 (5%) | 0.98 |
| 1 | 5 (26%) | 5 (26%) | |
| 2 | 5 (26%) | 5 (26%) | |
| 3 | 7 (37%) | 7 (37%) | |
| 4 | 1 (5%) | 1 (5%) | |
| Week 6 | |||
| 0 | 0 (0%) | 1 (5%) | 0.62 |
| 1 | 4 (22%) | 1 (5%) | |
| 2 | 6 (33%) | 8 (44%) | |
| 3 | 4 (22%) | 4 (22%) | |
| 4 | 4 (22%) | 4 (22%) | |
| Week 8 | |||
| 1 | 3 (18%) | 3 (18%) | 1 |
| 2 | 4 (24%) | 3 (18%) | |
| 3 | 6 (35%) | 6 (35%) | |
| 4 | 4 (24%) | 5 (29%) | |
| Week 10 | |||
| 1 | 2 (12%) | 1 (5%) | 1 |
| 2 | 3 (18%) | 3 (18%) | |
| 3 | 6 (35%) | 7 (41%) | |
| 4 | 6 (35%) | 6 (35%) | |
| Week 12 | |||
| 1 | 2 (12%) | 1 (5%) | 0.77 |
| 2 | 1 (6%) | 1 (5%) | |
| 3 | 3 (18%) | 6 (35%) | |
| 4 | 11 (65%) | 9 (53%) | |
Side effects. At every follow-up visit, patients were asked if they had experienced itching, burning, crusting, greasy skin, or dermatitis (Table 3).
TABLE 3.
Statistical analysis and comparison of side effects at each visit between the niacinamide group and cream base group
| VARIABLE | NIACINAMIDE | CREAM BASE | P-VALUE |
|---|---|---|---|
| Week 2 | |||
| Itching | 4 (21%) | 4 (21%) | 1 |
| Burning | 5 (26%) | 5 (26%) | 1 |
| Crusting | 4 (21%) | 3 (16%) | 1 |
| Greasy skin | 7 (37%) | 6 (32%) | 0.73 |
| Dermatitis | 6 (31%) | 6 (31%) | 1 |
| Week 4 | |||
| Itching | 3 (16%) | 3 (16%) | 1 |
| Burning | 1 (5%) | 2 (10%) | 1 |
| Crusting | 5 (26%) | 6 (32%) | 0.721 |
| Greasy skin | 8 (42%) | 8 (42%) | 1 |
| Dermatitis | 0 (0%) | 0 (0%) | - |
| Week 6 | |||
| Itching | 2 (11%) | 1 (5%) | 1 |
| Burning | 2 (11%) | 0 (0%) | 0.49 |
| Crusting | 5 (28%) | 4 (22%) | 1 |
| Greasy skin | 10 (56%) | 10 (56%) | 1 |
| Dermatitis | 2 (11%) | 1 (6%) | 1 |
| Week 8 | |||
| Itching | 1 (6%) | 2 (11%) | 1 |
| Burning | 1 (6%) | 1 (6%) | 1 |
| Crusting | 2 (11%) | 0 (0%) | 0.49 |
| Greasy skin | 7 (41%) | 5 (29%) | 0.47 |
| Dermatitis | 1 (6%) | 0 (0%) | 1 |
| Week 10 | |||
| Itching | 2 (12%) | 2 (12%) | 1 |
| Burning | 0 (0%) | 0 (0%) | 0 |
| Crusting | 1 (6%) | 0 (0%) | 1 |
| Greasy skin | 8 (47%) | 8 (47%) | 1 |
| Dermatitis | 0 (0%) | 0 (0%) | - |
| Week 12 | |||
| Itching | 0 (0%) | 0 (0%) | - |
| Burning | 0 (0%) | 0 (0%) | - |
| Crusting | 0 (0%) | 0 (0%) | - |
| Greasy skin | 5 (29%) | 5 (29%) | 1 |
| Dermatitis | 1 (6%) | 2 (11%) | 1 |
DISCUSSION
Acne is a common chronic inflammatory skin disorder. A number of factors lead to the onset of acne, but the basic process involves four steps.1 First, the epithelium of the infundibulum becomes hyperkeratotic. The keratinocytes show enhanced cohesion and produce excess cells; their adhesive nature causes them to form a plug, which blocks the follicular ostium. As a result, a gradual accumulation of sebum, keratin, and bacteria in the follicle causes a microcomedo to form as the upper hair follicle dilates. Secondly, excess sebum production from lipoperoxides activates the peroxisome proliferator-activated receptor (PPARs) pathway and also the action of androgenic hormones on sebocyte proliferation and differentiation. Thirdly, P. acnes binds to the toll-like receptor 2 (TLR-2) on both monocytes and polymorphonuclear cells located around the sebaceous follicle. This causes the release of a number of proinflammatory cytokines, including interleukin (IL)-1β, IL-8, IL-12, and tumor necrosis factor (TNF)-α. Finally, inflammation is the response to this sequence of events. Triglycerides, which are a component of sebum, can then be broken down to form free fatty acids by P. acnes, which is typically found in the pilosebaceous unit. These free fatty acids promote bacterial clumping, allowing colonization by P. acnes, which then stimulates further inflammation.1
Management of acne vulgaris depends on the infection severity. Treatment for mild acne patients begins with topical therapy, while moderate severity starts with topical combination therapy together with oral antibiotics. Patients with severe acne are usually treated with topical combination therapy and oral antibiotics or oral isotretinoin. Topical therapy often involves benzoyl peroxide, salicylic acid and antibiotics, such as clindamycin and erythromycin. Oral therapy consists of oral antibiotics—for example, tetracycline, erythromycin, and penicillin group hormonal agents and isotretinoin.2
Niacinamide/nicotinamide is an amide of vitamin B3 or niacin. It has many pharmacological properties related to the dermatologic field, including an anti-inflammatory effect.4 Niacinamide reduces inflammation in many pathways. The nuclear factor kappa light-chain-enhancer of activated B-cells (NF-κB) pathway is essential for the expression of adhesion molecules, chemokines, inflammatory cytokines, and pro-inflammatory mediators. This pathway is regulated by the nuclear poly(ADP-ribose) polymerase-1 (PARP-1), which is inhibited by niacinamide. Niacinamide can also inhibit the expression of MHC-II and the production of TNF-α, IL-1, IL-6, IL-8, and nitric oxide in in-vitro and animal models.6 Niacinamide can also inhibit leucocyte chemotaxis, lysosomal enzyme release, lymphocyte transformation, and IL-8 production from P. acnes through NF-κB and mitogen-activated protein kinase pathways.7 Niacinamide has a sebosuppressive effect and can induce the tube that connects the sebaceous gland and follicular ostium at the skin surface to become exfoliated and encourage sebum reflux to the skin surface faster, leading to a lack of reservoir of sebum in the duct and possibly decreasing sebum excretion from the skin. A double-blinded, randomized clinical trial in 130 participants compared a 2% niacinamide moisturizer and placebo moisturizer. Results showed that the 2% niacinamide moisturizer significantly reduced sebum excretion rates.8 Niacinamide also indicated a lightening effect by inhibiting the transfer of melanosomes from melanocytes to keratinocytes in an in-vitro cell cocultured study.9 Niacinamide has a barrier-protective antipruritic effect by acting on the key enzyme for sphingolipid synthesis, called serine palmitoyltransferase, which increases the rate of ceramide synthesis.10 Niacinamide also inhibits cAMP-phosphodiesterase, stabilizes mast cells, and reduces histamine release, which has an antipruritic effect.11 Its antimicrobial properties include a tubercuolostatic effect by inhibition of the class III NAD-dependent deacetylase-protein family (Sir2),12 antiretroviral effects against human immunodeficiency virus by inhibition of the nuclear PARP,13 and fungistatic effects by decreasing the activity of enzymes produced by fungi.14 Moreover, an in-vitro study found that niacinamide inhibits P. acnes biofilm formation and increases biofilm degradation.15 Lastly, niacinamide has a photoprotective effect against both photocarcinogenesis by unblocking glycolysis and replenishing adenosine triphosphate in ultraviolet-irradiated cultured human keratinocytes and ultraviolet-induced immunosuppression. This mechanism can include alterations in complement, energy metabolism, and apoptosis.16 As a result, niacinamide has been used to combat many dermatologic diseases, especially acne vulgaris. Previous studies used topical niacinamide and randomized patients to treatment with niacinamide only or clindamycin only. Results showed that the niacinamide group experienced significant improvement in acne vulgaris from baseline with results comparable to the clindamycin-treated group.17
This double-blinded, randomized study was conducted to assess the clinical efficacy of 2.5% benzoyl peroxide and 5% niacinamide versus that of 2.5% benzoyl peroxide and cream base. Our results indicated that both noninflammatory lesion and inflammatory lesion counts were decreased significantly from baseline at the end of the study in both treatment groups. The reduction of noninflammatory lesion counts was 51 percent in the niacinamide group and 34 percent in the cream base group, respectively. This difference was statistically significant (p=0.004), while the difference in the reduction of inflammatory lesions was not significant (p=0.63). The greater efficacy of 2.5% benzoyl peroxide plus 5% niacinamide as compared with 2.5% benzoyl peroxide plus cream base might be because all four pathogeneses of acne were eliminated. Benzoyl peroxide can kill P. acnes and has a comedolytic effect. Niacinamide has an anti-inflammatory effect by inhibiting interleukin-8 production from P. acnes and can reduce facial sebum production (Figure 10).
FIGURE 10.
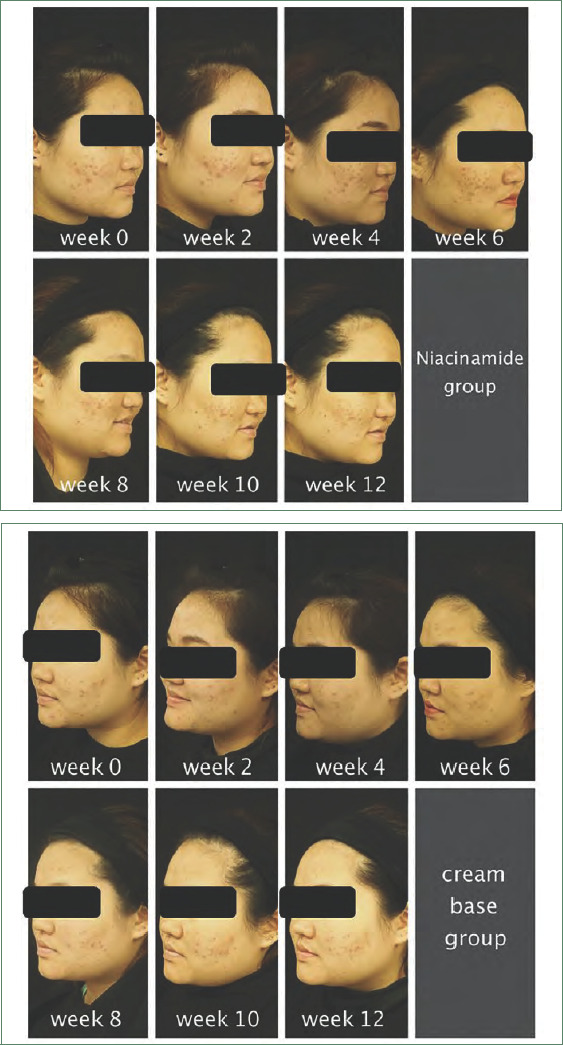
Clinical photos of the niacinamide-treated and cream base-treated sides of study participant NO. 17
Sebum casual level of the niacinamide group started to decrease significantly from baseline in six weeks and continued to reduce further at each follow-up visit at a faster and more sustainable rate than the cream base group, which showed a significant decrease only at Week 8 and Week 12. At the end of 12 weeks, both groups showed a significant reduction in sebum casual level, with no statistically significant difference between them. This could have resulted from the use of benzoyl peroxide. Previous studies showed that benzoyl peroxide might affect sebum excretion rate in some patients. The postacne erythema and postinflammatory hyperpigmentation scores were not statistically different from baseline or between the two groups. This might be because acne vulgaris is a chronic inflammatory disease and can cause new erythema and hyperpigmentation. Therefore, studying the effect on postacne erythema score and postinflammatory hyperpigmentation should only be carried out when the disease is controlled.
In this study, we also evaluated acne improvement using a physician's improvement scale and patient satisfaction scale. Results revealed a good clinical response in both treatment groups but no statistically significant differences between the niacinamide group and cream base group.
Both treatments were well tolerated. A few patients experienced itchiness, burning, crusting, greasy skin, and dermatitis, but these symptoms decreased by the end of the treatment in both groups, with no statistically significant differences between groups.
CONCLUSION
The combination of 2.5% benzoyl peroxide with 5% niacinamide was found to be more effective for the treatment of mild to moderate facial acne vulgaris. This combination reduced noninflammatory lesions and improved noninflammatory lesions and facial sebum reduction more quickly relative to 2.5% benzoyl peroxide with a cream base and gave minimal side effects. However, the efficacy of 2.5% benzoyl peroxide with 5% niacinamide for treating inflammatory lesions, postacne erythema, and postinflammatory hyperpigmentation was comparable to that of 2.5% benzoyl peroxide with a cream base.
Recommendation for clinical application. Niacinamide can be used as an additional topical medication for mild to moderate facial acne vulgaris for better reduction of noninflammatory lesions with minimal side effects.
Recommendations for further studies. Additional research is required to confirm our findings. Further studies with a larger number of participants can provide increased precision and accuracy. Further studies with different concentrations of niacinamide should be carried out to optimize efficacy of the treatment.
REFERENCES
- Goldsmith LA. New York, NY: McGraw-Hill; 2012. Fitzpatrick’s Dermatology in General Medicine 8th ed. [Google Scholar]
- Zaenglein AL, Pah AL, Schlosser BJ. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- Cunliffe WJ. Benzoyl peroxide in acne. J Am Acad Dermatol. 1989;20(1317):518–520. doi: 10.1016/s0190-9622(89)80098-2. [DOI] [PubMed] [Google Scholar]
- Wohlrab J. Niacinamide—mechanism of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27(6):311–315. doi: 10.1159/000359974. [DOI] [PubMed] [Google Scholar]
- Burnett CL, Bergfeld WF, Belsito DV et al. Final report of the safety assessment of Kojic acid as used in cosmetics. Int J Toxicol. 2010;29(6 Suppl):244S–273S. doi: 10.1177/1091581810385956. [DOI] [PubMed] [Google Scholar]
- Ungerstedt JS, Blomback M, Soderstrom T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin Exp Immunol. 2003;131(1):48–52. doi: 10.1046/j.1365-2249.2003.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange PA, Raingeaud J, Calvez V, Dupin N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J Dermatol Sci. 2009;56(2):106–112. doi: 10.1016/j.jdermsci.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Draelos ZD, Matsubara M, Smiles K. The effect of 2% niacinamide on facial sebum production. J Cosmet Laser Ther. 2006;8(2):96–101. doi: 10.1080/14764170600717704. [DOI] [PubMed] [Google Scholar]
- Greatens A, Hakozaki T, Koshoffer A et al. Effective inhibition of melanosome transfer to keratinocytes by lectins and niacinamide is reversible. Exp Dermatol. 2005;14(7):498–508. doi: 10.1111/j.0906-6705.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- Tanno O, Ota Y, Kitamura N et al. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br J Dermatol. 2000;143(3):524–531. doi: 10.1111/j.1365-2133.2000.03705.x. [DOI] [PubMed] [Google Scholar]
- Parrot JL, Wyczolkowska Y, Santais MC et al. The action of nicotinamide on the release of histamine. Agents Actions. 1974;4(3):202. doi: 10.1007/BF01970280. [DOI] [PubMed] [Google Scholar]
- Boshoff HI, Xu X, Tahlan K et al. Biosynthesis and recycling of nicotinamide cofactors in mycobacterium tuberculosis. An essential role for NAD in nonreplicating bacilli. J Biol Chem. 2008;283(28):19329–19341. doi: 10.1074/jbc.M800694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HS, Sang WW, Wang YO, Liu W. Nicotinamide phosphoribosyltransferase/sirtuin 1 pathway is involved in human immunodeficiency virus type 1 Tat-mediated long terminal repeat transactivation. J Cell Biochem, 2010;110(6):1464–1470. doi: 10.1002/jcb.22704. [DOI] [PubMed] [Google Scholar]
- Ciebiada-Adamiec A, Malafiej E, Ciebiada I. Inhibitory effect of nicotinamide on enzymatic activity of selected fungal strains causing skin infection. Mycoses. 2010;53(3):204–207. doi: 10.1111/j.1439-0507.2009.01696.x. [DOI] [PubMed] [Google Scholar]
- Shih YH. Influence of nicotinamide on the development of Propionibacterium acnes biofilm: a preliminary study. J Am Acad Dermatol. 2015;72(S1):AB8. [Google Scholar]
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29(2):157–162. doi: 10.1080/01635589709514618. [DOI] [PubMed] [Google Scholar]
- Shahmoradi Z, Iraji F, Hossein Siadat A, Ghorbaini A. Comparison of topical 5% nicotinamid gel versus 2% clindamycin gel in the treatment of the mild-moderate acne vulgaris: a double-blinded randomized clinical trial. J Res Med Sci. 2013;18(2):115–117. [PMC free article] [PubMed] [Google Scholar]


