Significance
In animal societies, social life often generates male mating harassment. How do communal animals manage such conflicts without escalating antagonistic relationships? In the Sahara Desert, we studied the mating system of gregarious-behaving desert locusts, the world’s most destructive locust. Despite being widespread and abundant during plagues, its populations are otherwise difficult to access, and its reproductive behaviors in the field are understudied. We show that female locusts behaviorally overcome the costs of male mating harassment: females occupy separate sites before and after mating. Only females with ripe ovaries arrive at male-biased lekking groups. Hence, substantial social conflicts can be simply managed by behaviors. These findings invite to explore the evolutionary hypotheses behind lekking with a density-dependent plastic species.
Keywords: lek, mate choice, mating behavior, operational sex ratio, sexual selection
Abstract
Male mating harassment may occur when females and males do not have the same mating objectives. Communal animals need to manage the costs of male mating harassment. Here, we demonstrate how desert locusts in dense populations reduce such conflicts through behaviors. In transient populations (of solitarious morphology but gregarious behavior), we found that nongravid females occupied separate sites far from males and were not mating, whereas males aggregated on open ground (leks), waiting for gravid females to enter the lekking sites. Once a male mounted a gravid female, no other males attacked the pair; mating pairs were thereby protected during the vulnerable time of oviposition. In comparison, solitarious locusts displayed a balanced sex ratio in low-density populations, and females mated irrespective of their ovarian state. Our results indicate that the mating behaviors of desert locusts are density dependent and that sex-biased behavioral group separation may minimize the costs of male mating harassment and competition.
The maintenance of animal societies depends on conflict management (1–3). Sexual interaction among group members frequently causes competition and male mating harassment (4). Males typically attempt to maximize fitness by fertilizing as many females as possible (5). Consequently, females are frequently harassed by males, with severe male–male competition (6). Communal animals need to manage the costs of male mating harassment as well as male–male competition.
The operational sex ratio (OSR) is usually biased toward males because males tend to recover their reproductive state faster than females (7). Male-biased OSRs cause male–male competition, leading to intense sexual coercion of females by males (i.e., male mating harassment). This phenomenon reduces female fitness by increasing the risk of predation (8) and injury (9). In such cases, a sexually antagonistic coevolution, or “arms race,” of male persistence versus female resistance may occur. This is termed “sexual conflict” (10, 11). Sexual conflicts occur when females and males do not have the same mating objectives (12, 13). However, this phenomenon generates substantial costs, resulting in the loss of energy, time, and mating chance (14). It may also increase predation risk (12), which may negatively influence population dynamics (15). Exaggerated arms races may be counterproductive for communal animals. OSR theory proposes the following simple solution for how animals can resolve these opposing forces: females should live separately from males to prevent male mating harassment (16). According to this theory, nongravid females should occupy sites without males (“time out”), whereas females that are gravid and ready for oviposition should enter male-biased groups (the mating pool) for mating and oviposition (“time in”). In some species, males may form leks (i.e., aggregations), where they engage in competitive displays to entice visiting females that seek partners for copulation (2, 4, 17). The evolutionary mechanism explaining these paradoxical lekking behaviors remains controversial (2, 17). Several competing and nonexclusive models have been proposed by considering various factors including predation risk, habitat fragmentation, resource distribution, and male mating harassment (18). In some cases, males are directly or indirectly evaluated based on their quality by females investing in mate choice (17, 18). However, information about whether this system has evolved in communal animals is limited, especially for species with density-dependent behaviors. Here, we explored how the desert locust, Schistocerca gregaria, resolves male mating harassment and male–male competition in the field.
Desert locusts change from solitary to group life plastically; that is, they change from an initial “solitarious phase,” during which they do not aggregate, to a “gregarious phase,” during which they form swarms that migrate long distances (19, 20). This extreme phenotypic plasticity (termed phase polyphenism) depends on local population density (21, 22), and the associated behavioral and physiological traits may change quickly during the life cycle (23, 24). A behavioral difference is measurable within 1 h and almost completed within 4 h (23). In contrast, other traits such as adult morphometric ratios do not change after adult eclosion. Thus, solitarious-phase looking adult locusts may behave as typical gregarious locusts [referred to as transient (25) or gregarious-behaving locusts; for an explanation of terms, see Study Animal and Terms Used to Describe Populations]. For gregarious-behaving locusts, little is known about how nongravid females prevent mating harassment by males. Although the desert locust is a major pest in over 60 countries in Africa and Asia (26), its mating strategies and management of male mating harassment at the group level remain unclear (19, 27).
During field observations over 9 y in the Sahara Desert of Mauritania, we noticed biased sex ratios of sexually mature groups of desert locusts during the transition from the solitarious to the gregarious phase. In female-biased groups, most females were single (i.e., not guarded by a male), whereas most females were mated in male-biased groups. Researchers previously reported that sexually mature swarms tend to split after oviposition but frequently rejoin when they resume migratory flight between successive oviposition cycles (27). These fragmentary observations were consistent with the “group separation” mating system predicted by the OSR theory (16). Based on these observations and theory, we hypothesized that sexually mature, gregarious-behaving desert locust females and males occupied separate areas (depending on the state of ovarian development) to prevent male mating harassment and to offset costs of male–male competition in mating with gravid females.
We tested this hypothesis by surveying gregarious-behaving populations of desert locusts in the field and by conducting parallel laboratory experiments. Because mating systems vary depending on population density (28), we also examined the mating system of solitarious-phase locusts in low-density populations. Here, we show how desert locusts use a previously unrecognized density-dependent “group separation” mating system to manage male mating harassment and competition without losing sociability.
Results
During field surveys performed in September through December 2012 to 2019, characteristics and behavioral measurements of 11 transient and 3 solitarious reproducing populations were collected. Five swarming, gregarious, nonreproducing populations were measured to compare adult morphometrics. These morphometric analyses indicated that the transient gregarious-behaving populations were formed by originally solitarious adults that grew at a low population density during the nymphal stage (SI Appendix, Fig. S1).
Sex Ratios, Mating States, and Ovarian Development.
To verify the existence of mature sex-biased groups, we measured sex ratios in different life stages: the sex ratio was balanced in hatchlings (percentage of female: mean ± SE; 48.1 ± 1.4, from n = 17 egg pods; total: n = 1,043 hatchlings) and the last instar stage (49.9 ± 1.1% females, from n = 8 night-roosting plants; total: n = 958 hoppers) of transient populations. For solitarious population, sex ratio was balanced for hatchlings (48.3 ± 1.9, from n = 27 egg pods; total: n = 1,413 hatchlings), sexually immature adults (40.0 ± 8.2% females, from n = 28 night-roosting plants; total: n = 45 locusts), and mature populations (SI Appendix, Table S1). However, it substantially varied among the sexually mature transient groups (Fig. 1 A and B and SI Appendix, Table S1). Of the 11 groups, seven formed “leks” with significantly male-biased sex ratios (Table 1 and SI Appendix, Table S1). These seven male-biased lekking groups contained a higher proportion of mating males and females than the nonlek outside groups (Table 1). The other four sites had a female-biased sex ratio, with almost no mating activity, which was consistent with our separation hypothesis.
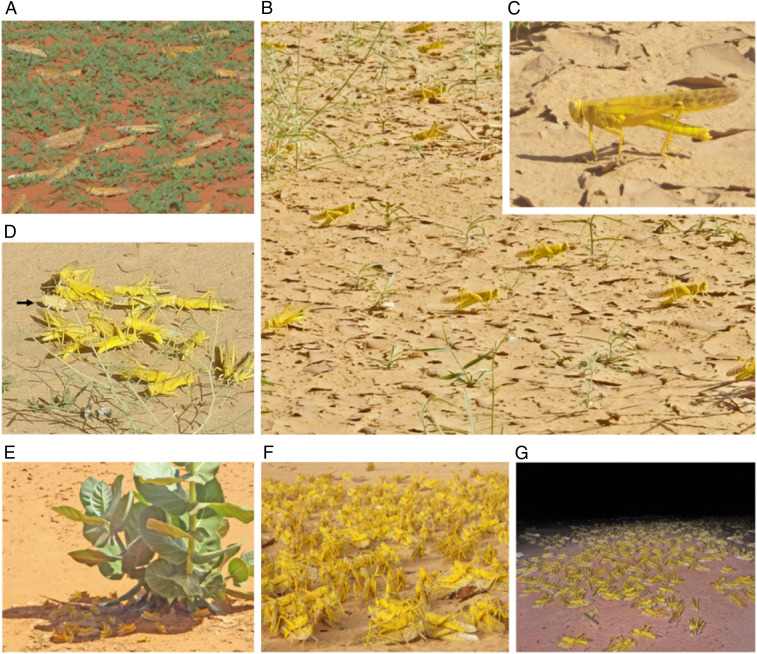
Fig. 1.
Mating systems of sexually mature, transient (gregarious-behaving) desert locust adults. (A) Nongravid females outside a lek. (B) Males aggregating on the hot ground (lek) and waiting for incoming gravid females during the day, (C) by raising their bodies off the ground and orienting the body axis parallel to the sunrays. (D) Males approaching an incoming female (black arrow) and fighting for possession of the female at the onset of pairing. (E) Mating pairs tended to move to the shade at midday and (F) to aggregate near leks near dusk. (G) Group oviposition after dusk.
Table 1.
Locust density, sex ratio, and mating activity in lek sites versus other sites of transient (gregarious-behaving) and solitarious locust populations
| Presence of | Total | Sex ratio | |||||||
| Year | Population type | leks | female and male density | Female density | Male density | (% of females) | Mating female (%) | Mating male (%) | n |
| 2016 | Transient | Lek sites | 0.688 ± 0.032 c | 0.049 ± 0.022 a | 0.639 ± 0.021 b | 7.2 ± 4.2 a | 96.6 ± 5.0 c | 8.2 ± 4.0 a | 65 |
| 2016 | Transient | Outsides | 0.438 ± 0.041 b | 0.41 ± 0.028 b | 0.028 ± 0.028 a | 86.3 ± 5.3 c | 0 a | 0 a | 40 |
| 2013 | Solitarious | None | 0.021 ± 0.037 a | 0.012 ± 0.025 a | 0.010 ± 0.025 a | 54.7 ± 4.8 bc | 3.6 ± 6.4 a | 2.2 ± 6.8 a | 50 |
| 2018 | Solitarious | None | 0.039 ± 0.019 a | 0.020 ± 0.013 a | 0.019 ± 0.013 a | 53.8 ± 2.5 b | 26.9 ± 2.7 b | 29.6 ± 2.8 b | 193 |
| 2019 | Solitarious | None | 0.021 ± 0.070 a | 0.013 ± 0.048 a | 0.009 ± 0.047 a | 60.7 ± 9.1 bc | 11.1 ± 11.3 ab | 16.7 ± 13.3 ab | 14 |
Densities are numbers/m2; sex ratio is the number of females/number of females + males.
Mating is the percentage of locusts mating. Values are means ± SE, and means in a column followed by different letters are significantly different according to the Tukey–Kramer HSD test. n = 7 in lek sites; n = 4 in other sites. The percentage data were arc-sine transformed before analysis.
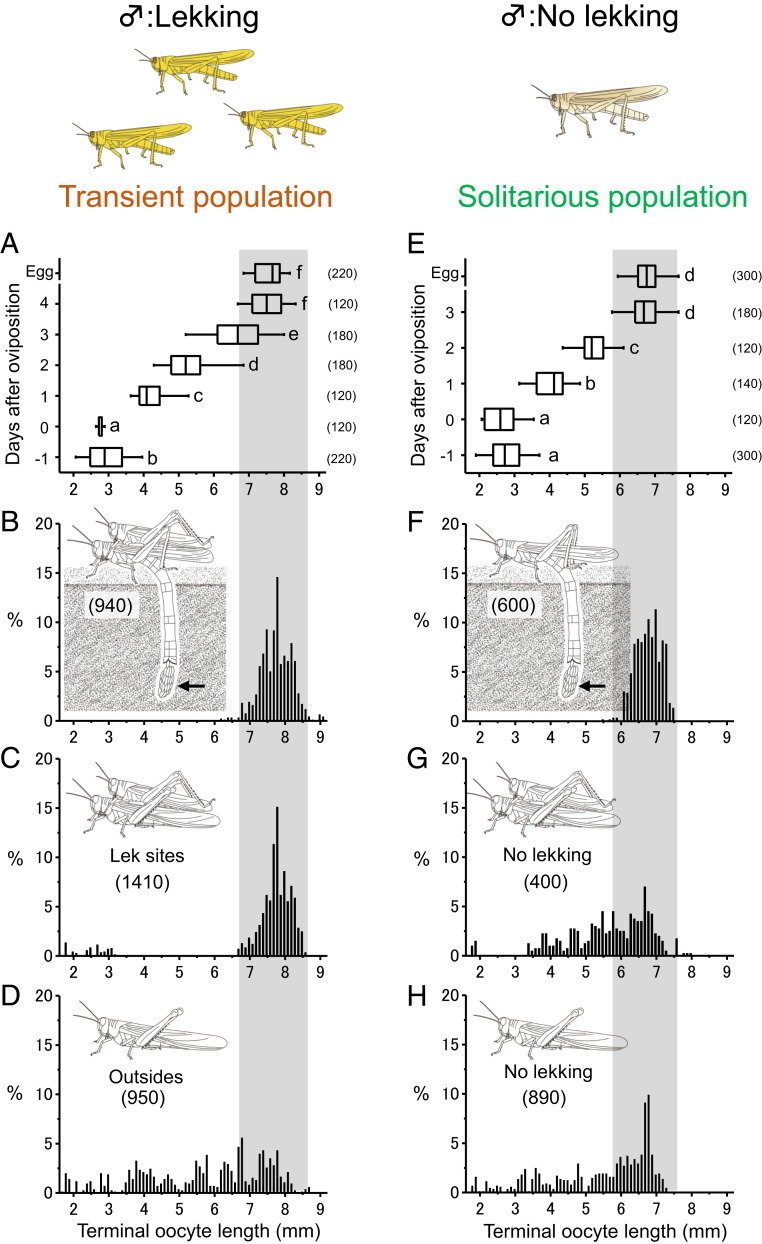
According to the OSR theory, nongravid females should remain outside leks whereas gravid females should enter leks. To determine whether this behavior was exhibited by gregarious-behaving females, we first examined the daily ovarian developmental rate (i.e., developing oocytes within the ovaries during oogenesis) under laboratory conditions. By dissection, we then determined the ovarian states of females from lek and nonlek sites. Fig. 2A shows oocyte development in group-reared (gregarious phase) females during the reproductive cycle under laboratory conditions. The oocytes grew daily. In the females collected from leks and immediately examined, some mating females had small oocytes (<3.2 mm, 7.1%, n = 141, Fig. 2C) that corresponded to the size of oocytes just after oviposition; however, most females had mature eggs (chorionated) in the terminal oocytes or oviducts that were equivalent to the size of laid eggs (Fig. 2 B and C; t test, t = −1.55, f = 2.41, P > 0.05). In comparison, nonmating females outside leks had oocytes of various sizes, which corresponded to oocytes that had been developing for 0 to 4 d (Fig. 2D). Oosorption bodies were found in all females in the lekking groups (100%, n = 141) and in most females in the female-biased group (96.8%, n = 95), indicating that they had laid eggs at least once before. Egg size was significantly smaller in solitarious females (mean ± SE = 6.79 ± 0.02; n = 600) than in gregarious-behaving females in the field (7.77 ± 0.01; n = 940) (Fig. 2 B and F; t test, t = −45.44, f = 2,064.7, P < 0.001) as we previously observed with laboratory-incubated females (24). In contrast, neither mating nor single solitarious locusts showed a clear pattern in oocyte size (Fig. 2 G and H), and most females had already laid eggs at least once (oosorption bodies found in 85.4% of single females, n = 89; and in 92.5% of females in mating pairs, n = 40). This result indicates that mating by solitarious-phase females was unrelated to the maturity of their oocysts.
Fig. 2.
Relationships between mating states and ovarian development of sexually mature transient (gregarious-behaving) (A–D) and solitarious desert locust females (E–H). (A and E) Daily terminal oocyte development of group-reared gregarious-behaving (A) and individually reared solitarious locusts (E). Each box plot displays the median value with the ends of the boxes representing the 25th and 75th percentiles and the ends of the lines representing the 10th and 90th percentiles. Different letters above each box indicate significant differences at P < 0.05 (Tukey–Kramer HSD test). Frequency of different sized eggs (B and F) and length of terminal oocyte or mature eggs in the oviduct of mating pairs (C and G) or single females (i.e., not guarded by a male) (D and H). Numbers in parentheses indicate sample sizes. Gray zones indicate the size of mature eggs. Note that only transient males display lekking behavior, and most mating occurred in leks.
Diel Changes in Mating States in Lekking Sites.
Males in lekking groups aggregated on the ground and remained there during the day (Fig. 1B). When the ground temperature was near 50 °C, the males minimized their exposure to radiation by orienting themselves parallel to the sunrays and straightening their legs and thereby lifting their bodies from the ground (Fig. 1C). With high midday temperatures, some males sheltered in the shade or perched on vegetation to avoid overheating. When a flying gravid female landed on the ground in the middle of a lek, many males immediately approached (Fig. 1D) and attempted to mount the female; this sometimes resulted in a tumbling ball of fighting males, with the female in the middle. One male eventually mounted and paired with the female. Once a male mounted a female, no other male attacked them, and they began to copulate. The winning male remained on the female’s back after copulation and guarded her. Such pairs tended to move to the shade to avoid overheating (Fig. 1E). Around dusk, when the temperature dropped, many pairs aggregated, and the females oviposited in a group (Fig. 1 F and G).
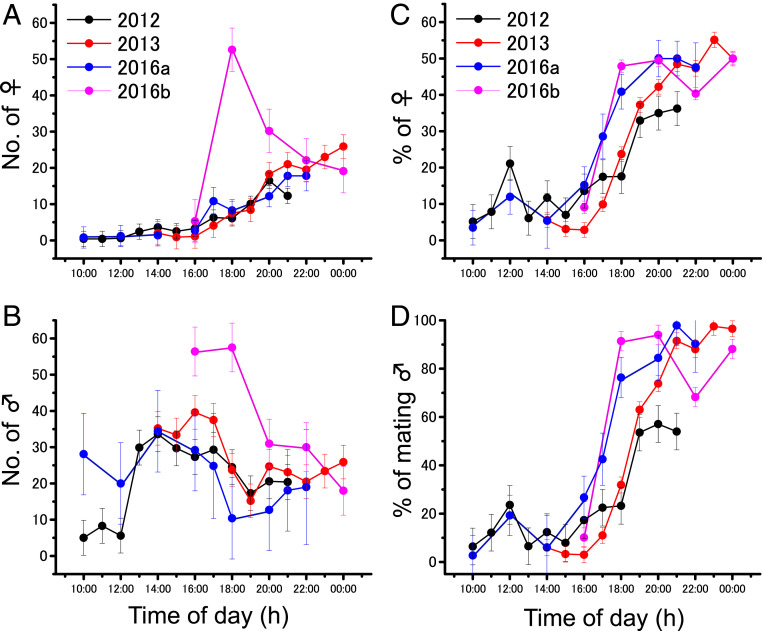
To assess mating dynamics in lekking sites, we observed diel changes in the sex ratio, number of females and males, and mating activity at four sites (Fig. 3). The number of females entering leks increased, and the sex ratios were almost equal after dusk (Fig. 3 A and C). This resulted from a decrease in the number of males because the males that could not mate moved to night-roosting plants after dusk (Fig. 3 B and D). The number of mating pairs increased, with pairs aggregating with each other, and the females then began to oviposit near the lekking sites (Fig. 1G and SI Appendix, Fig. S2). Most females were mate guarded by males during oviposition (2012: 100%, n = 74; 2013: 98.9%, n = 437; 2016a: 100%, n = 24; 2016b: 96.9%, n = 1,056). Males that guarded ovipositing females repelled any approaching ground-wandering males by kicking with their hind legs and successfully protected their mating partners from rivals (100%, n = 100). Oviposition was mostly completed by early morning, but some females continued ovipositing until the following afternoon. After oviposition, the pairs separated, and both sexes flew elsewhere. Thus, lekking and oviposition sites were temporary.
Fig. 3.
Diel changes in the mean numbers of (A) females and (B) males, as well as the percentages of (C) females and (D) mating males of sexually mature, transient (gregarious-behaving) desert locust adults in lek sites within a transect (50 m2). Values are means ± SE.
Mating Avoidance Experiment.
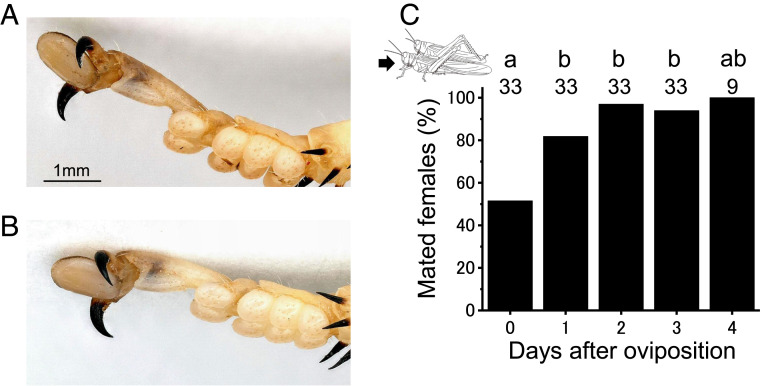
To test whether females could physically prevent unnecessary mating attempts by males, females with oocytes of various sizes were housed with males in a small cage where their mating state (either single or pair) was recorded after 24 h under laboratory conditions. Some females rejected approaching males by kicking them with their hind legs or jumping, but males frequently chased the female and continuously attempted to mate. Mate-guarding males managed to stay on the mated females by hooking onto the female thorax using the fore- and midleg claws (Fig. 4 A and B). Most females were ultimately mounted by males. Although the percentage of mating was significantly lower in females immediately after oviposition (day 0) (Fig. 4C; post hoc Fisher’s exact test after Bonferroni correction, P < 0.05), most females were mounted by males irrespective of the state of ovary development under laboratory conditions.
Fig. 4.
Tip of the male foreleg (A) and midleg (B) that hook onto the mounted female’s thorax, and mate receptivity of group-reared desert locust females as related to ovarian state: that is, days after oviposition (C). Under natural conditions, almost all transient females with mature eggs were mate guarded, whereas most nongravid females were not guarded. In C, values are means, and means with different letters are significantly different at P < 0.05 (post hoc Fisher's exact test after Bonferroni correction). Numbers above bars represent sample sizes.
Does Mate Guarding by Males Impair Fleeing Performance of Females?
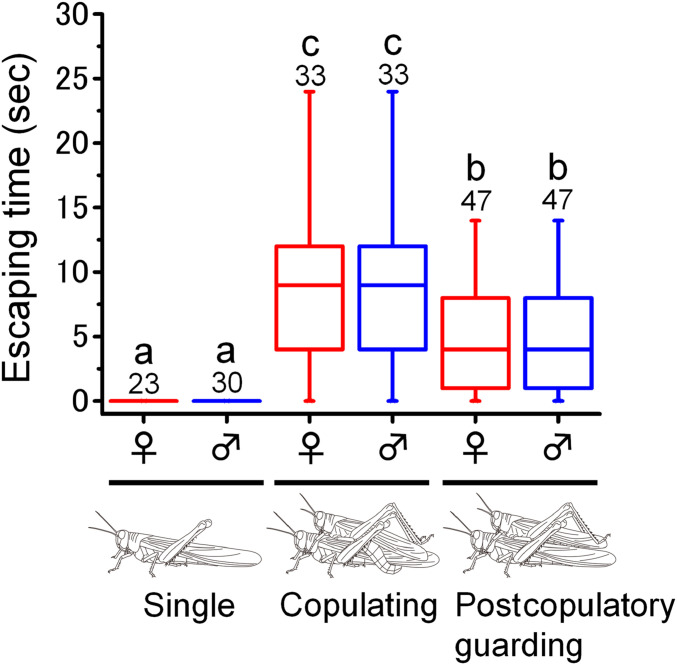
To examine how mate guarding by males interferes with female attempts to flee from predators, fleeing behavior in response to predatory stimuli was recorded in a lek site (Fig. 5). Most single males and females flew in response to approaching simulated predatory stimuli, whereas most mate-guarding males remained on their mates, exhibiting no defensive responses. The copulating females were physically constrained by guarding males. Therefore, the females continuously jumped but could not fly from the chasing predatory stimuli until the males disengaged and then they immediately flew away. The mate-guarding males managed to stay on the mating females during the escaping jumps by hooking onto the female thorax using fore- and midleg claws. As a result, the escape time after the first predatory response was significantly slower for copulating pairs than for single individuals (Fig. 5, Tukey–Kramer Honestly Significant Difference [HSD] test, P < 0.05). A similar trend was observed for postcopulatory guarding pairs (Fig. 5); however, the escape time was significantly faster than that for copulating pairs (Tukey–Kramer HSD test, P < 0.05).
Fig. 5.
Mate guarding by males impairs females fleeing performance. Box plots depict the time required for escape by flying after simulated predatory attacks in single, copulating, and postcopulatory guarding desert locust females and males. Each box plot displays the median value with the ends of the boxes representing the 25th and 75th percentiles and the ends of the lines representing the 10th and 90th percentiles. Different letters above each box indicate significant differences at P < 0.05 (Tukey–Kramer HSD test). Numbers above the bars indicate sample sizes.
Discussion
The results of this study supported our hypothesis that sexually matured, ovary-developing, nongravid gregarious-behaving females occupied areas outside lek sites and were singles (not mating), whereas gravid females entered leks to mate. This “group separation” mating system, which was predicted by the OSR theory (16), allows gregarious-behaving locusts to reduce male mating harassment (i.e., the costs of male mating attempts) and male–male competition. Our study therefore provides insight into how social conflicts can be behaviorally managed by communal animals. Mating systems are shaped by various components, including sex-specific benefits, sexual conflicts, OSR, sperm competition, mate guarding, mate choice, habitat, population density, mobility, and antipredatory strategies (4). In the following sections, we will discuss how the desert locust mating system mitigates conflicts during mating events.
When Females Go Their Own Way.
The most important finding of the present study was that gregarious-behaving locusts formed female- or male-biased groups. These biased sex ratios were behaviorally generated by sexually mature locusts because the sex ratio was otherwise equal throughout the developmental process. Males aggregated and formed lek-like groups on the open ground and almost all mating events occurred there. Similar male-biased groups have been previously documented in other sexually mature, gregarious-phase populations of desert locusts (29, 30). However, leks and female-biased groups were not previously documented. Our dissections revealed that most females in leks were gravid (or had just oviposited), whereas most females outside the leks had developing ovaries. Our morphological studies of ovaries revealed that most females found in both sites had already laid eggs at least once. Our behavioral observations also showed that the sex ratios in lekking sites ranged from being extremely male-biased to almost equal during the course of a day. Roffey and Magor (27) reported that swarms of gregarious locusts often break up as they mature but frequently rejoin when they resume migratory flight between successive cycles of oviposition. However, the latter authors did not clarify the ecological significance of these phenomena. These phenomena can be explained by considering our findings on the “group separation” mating system.
We also found that the mating strategy is different for solitarious populations of the desert locust: the sex ratio was balanced in low-density populations of solitarious locusts, and solitarious females mated irrespective of their ovary development. Additionally, we observed that gregarious-behaving females produced larger eggs than solitarious ones, which agreed with a recent laboratory study showing that individually reared solitarious females increased egg size in response to crowding even in the late-adult stages (24). These results demonstrate that the desert locust flexibly alters both the reproductive and mating strategies in response to population density.
Advantages of Group-Separation Mating Systems.
Some animals use the “group separation” mating system through aggregations of males, that is, leks (17, 18). Males aggregate at species-specific sites and wait for gravid females to arrive and mate. The peculiarity of the desert locust within the “group separation” mating system is that females, when in the gregarious phase, also create groups. This “group separation” helps to reduce costs and has two advantages for them: 1) nongravid females can prevent unnecessary male mating harassment, and 2) males only approach gravid females, which reduces the time required for mate searching and guarding.
Using small cages in our laboratory experiments, we confirmed that most females were physically unable to prevent repeated male sexual coercion. Some females tried to retreat from approaching males by kicking them with their hind legs, as previously observed (28, 31); however, most females were ultimately mounted by males. Therefore, if sexually mature females and males live together as a group, females would incur substantial costs in terms of mating harassment from males. In water striders, grasping and antigrasping morphological traits have coevolved as an arms race (14). Our results tend to show that mating behaviors coevolved due to sexual conflicts (12) in desert locust. Further evolutionary studies are needed to confirm this.
Although male–male competition may be high in leks, males are more likely to encounter gravid females that will soon lay eggs in leks rather than outside leks. Desert locust females are polyandrous (30), and the sperm of the last copulated male is used for fertilization (32). It is therefore adaptive for a male to exhibit postcopulatory guarding to ensure paternity. Gregarious desert locust males have also been reported to release a courtship-inhibition pheromone (phenylacetonitrile) to prevent other males from attempting to court mated females (33). This chemical-dependent mate guarding apparently reduces unnecessary male–male competition (4).
Phase Polyphenism and Lekking System Evolution.
Because the understanding of phase polyphenism evolution is still incomplete (22) and because sexual conflict may be a major evolutional driver shaping any mating system (11, 12), theories about the evolution of lekking behavior could increase our understanding of the reasons for the composite plastic changes in locusts. This is particularly true because mating behavior can be involved in speciation (34).
Among the competing and nonexclusive models that have been proposed to explain the evolution of lekking behavior (18), four are relevant for the desert locust: 1) hotspots, 2) female mate choice, 3) predation risk, and 4) black hole. The “hotspot” model predicts that females might be attracted to leks because lekking sites contain important resources (e.g., food and nesting sites), leading males to encounter the highest number of females in leks (35). In lekking insects, leks are commonly found near oviposition sites used by females (17). We also observed this trend for transient desert locusts: males formed leks on the open ground, and fertilized females laid eggs in lekking sites within 1 d. For oviposition, desert locust females oviposit in moist sandy soil, which is necessary for embryonic development (25). Lekking males that prefer areas with humid soils may have been selected for. Further field work could elucidate whether the capacity of males to detect the best egg-laying area is selected by females.
The “female mate choice” model assumes that receptive females may gain direct or indirect benefits from the opportunities that leks provide in terms of mate choice (4, 36). Female mating rejection via kicking may serve as a direct mate choice in the desert locust (31). Males display certain competitive behaviors that are selected by females, such as endurance (4). Thus, leks are frequently used for indirect mate choice (37). We observed male locusts on the hot ground in leks, with ground temperatures greater than lethal body temperatures (>50 °C), whereas other males sheltered in the shade or vegetation to avoid overheating. Males that are able to remain on such hot ground are likely to be healthy and of high quality. The arrival of females during the afternoon, after several hours of very hot ground conditions, potentially represents an indirect selection of males; that is, females may indirectly use heat endurance as a form of mate choice. During winter when temperatures are cooler, copulation and oviposition show no clear patterns (30). Diel changes in the mating activity of grasshoppers might be associated with individual thermoregulation (38). If seasonal patterns of lekking behavior exist, it would support the inference that this “female mate choice” is an important aspect of desert locust mating behavior.
The “predation risk” model may also explain why receptive females may be attracted to leks. The assumption here is that leks are probably the safest places for females (4, 39). Because desert locust adults can quickly flee in response to a predation threat (40), predation risk is unlikely to be important before mating. However, mating and ovipositing pairs likely experience a high predation risk. We showed that male mate guarding impairs escape by females. Individuals living in groups of conspecifics could reduce the risk of predation via dilution effects (41). Therefore, lekking may have evolved in grasshoppers displaying density-dependent phase polyphenism to facilitate synchronous mating and oviposition in a group, which dilutes predation risk (42).
Finally, the “black hole” model assumes that females randomly enter leks mainly to reduce male mating harassment that could occur in the absence of lekking (18, 42, 43). This model is supported by our observations of harassment under laboratory conditions, that is, females may favor males displaying lekking behaviors to reduce the harassment that can be observed if there is no physical separation. However, male mating harassment could be critical for ovipositing females in leks. If an ovipositing female becomes entangled in a male–male struggle, she could be injured and fail to oviposit (44). Postcopulatory mounting is common in polyandrous animals (4). Desert locust females are not required to mate repeatedly to ensure fertilization, because females can store sperm (45). This raises the question of why gregarious-behaving females mate just before oviposition. As previously described, gregarious males produce courtship-inhibition pheromones, which reduce male–male competition (33) but which may also function as bodyguards for ovipositing females. Thus, by accepting mate guarding by males, gravid females gain various benefits, including avoiding further male mating harassment and achieving safe oviposition. The mate-guarding male also benefits because his sperm is used for fertilization (32). Therefore, the gregarious forms of locusts may have found an evolutionary positive feedback loop by selecting mate-guarding and lekking behaviors that reduce male mating harassment and male competition. In many other lekking species, neighboring lekking males and even nonlekking intruders enter lekking male territories and disrupt courtships (17). The chemical defense system of desert locusts could play, on the contrary, an important role in the lekking system (33).
Importance and Efficacy of Day Spraying of Sexually Mature Locusts.
Our findings could be used to develop strategies to reduce desert locust outbreaks. Preventive controls of the desert locust rely on spraying pesticides (46). Gravid females are the most important target of such sprays. While the mechanism underlying group oviposition remains unclear, leks of yellow males could be used to predict the location of group formation sites. Although ground and aerial control operations have been mainly carried out during the day when males aggregate on the ground, control should probably be implemented at night when large numbers of gravid females will aggregate at the lek site (please see also SI Appendix, Fig. S3 and explanations). Furthermore, if gravid females can be artificially attracted to predefined sites by using pheromone(s), novel environment-friendly control techniques could be developed (47, 48). Future studies should explore 1) how males select lekking sites and 2) how gravid females detect lekking sites. In the present study, we mainly investigated transient and solitarious populations during fall in Mauritania, because typical gregarious populations have not appeared in West Africa since the last upsurge in 2003 to 2005 partly due to success of preventive controls by member countries of the Commission for controlling the Desert Locust in the Western Region (CLCPRO). As behaviors have a tendency to show a gradient of changes from solitarious to gregarious phases in locusts (49), we expect that the mating system described here for transients would be even more pronounced for gregarious populations. Additional research on the mating strategies of typical gregarious populations is needed in other areas, in different seasons, and of different population sizes.
Methods
Study Areas.
This study was conducted in Mauritania (northwest Africa), which is a notorious incubation zone for the formation of gregarious locust swarms (50, 51). Our study sites were located near Akjoujt and Nouakchott in northwestern Mauritania (SI Appendix, Tables S1 and S2). The area is a vast, flat and arid plain supporting various habitats, including dunes, playa, and low, sparse shrublands. Sparse, low grasses and other annuals grow between taller plants. Most low grasses were green. We conducted field surveys in September through December 2012 to 2019.
Study Animal and Terms Used to Describe Populations.
Transient and solitarious-phase populations of the desert locusts (S. gregaria) were studied after the rainy season. Typical populations contained 100 to >100,000 individuals; however, there was a large variation in population size and shape. Phase characteristics are complex, because several characteristics are continuous, reversible, and overlap between the solitarious and gregarious phases with an intermediate transient phase (22). Sexually mature, gregarious male locusts have a characteristic yellow body (45), and all adult males found in our survey site at relatively high densities were yellow (Fig. 1B). However, as described above, our transient population had morphometric characteristics typical of the solitarious phase, that is, a high hind femur length/head width (F/C) ratio and seven eye stripes (extra molting) (SI Appendix, Fig. S1 and Table S2). This suggests that behavioral and morphological gregarization occurred after adult eclosion of solitarious-phase locusts that grew under low population densities during the nymphal stage. Herein, this population is referred to as “transient locusts” or “gregarious-behaving locusts” for convenience. In contrast, solitarious locusts are brown or cream colored, and they occur at low densities (25). Following the Food and Agriculture Organization of the United Nations (FAO) Desert Locust Guidelines (52) and gregarization threshold, which considers the number of adults and vegetation conditions (53), these individuals were defined as typical “solitarious locusts.” In summary, we used the following terms to describe locust populations: solitarious populations contain individuals that have not aggregated or formed swarms; gregarious populations contain individuals that have aggregated and formed swarms; and transient populations are in transition from the solitarious phase to the gregarious phase and contain individuals that became adults in the solitarious phase before advancing to the gregarious phase.
Some of the older literature referred to the swarming crowded phase and the more sedentary isolated ones with the latinized names “gregaria” and “solitaria,” respectively (25). The intermediates were named as phase “transiens.” Currently, the extreme phases are usually named by the nonlatinized terms “gregarious” and “solitary” (or “solitarious”) (49). Although some recent literatures have still used “transiens,” the present study uses “transient.”
Morphometric Measurements.
Adults were collected using sweep nets or by hand to determine the phase states. Their hind femur length (F) and maximum head width (C) were measured using digital calipers (SC-15S; Mitsutoyo Co.) to determine the classical morphometric F/C ratio (25). Desert locusts go through five or six nymphal stadia before reaching the adult stage (25). Adults with five nymphal stadia have six eye strips, and adults with six nymphal stadia (six stadia are considered to result from an extra molt) have seven eye strips. Extra molting is observed only during the solitarious phase, and the number of nymphal stadia influences the F/C ratio in the adult stage (25). Therefore, adults with six and seven eye stripes were analyzed separately. Sexually immature, migrating swarms were found in Nouadhibou in January and February 2013 and in the Banc d’Arguin National Park in December 2013. Based on the F/C ratio of these adults with six stripes, we considered them as gregarious locusts (SI Appendix, Fig. S1). The F/C ratio was analyzed using Steel–Dwass test (the nonparametric form of Tukey’s honest significant difference test).
Determination of Sex Ratio and Mating States.
Systematic field observations were difficult to implement because gregarious desert locusts frequently migrate, with temporary group oviposition. A real-time monitoring system was used to access the locust population in the field. Specifically, highly mobile ground survey teams equipped with long-range radios developed by the Mauritanian National Anti-Locust Center (CNLA) were used. Surveys began immediately after observers encountered locust groups. To determine the sex ratio of each local group, 10 belt transects (2 × 25 m each) were implemented. All males and females in each transect were recorded during the day (10:00 to 17:00 h). Sexing was always conducted by the same observer (K.O.M.) using a binocular or unaided visual observation of external characteristics including body size and coloration (sexually mature, transient males were yellow and smaller than females). Mating state was also recorded as single or mating (copulating or postcopulatory mounting). This procedure was applied for all locust groups that were encountered from October 2012 to 2016. In 2014, 2017, 2018, and 2019, no transient populations were found. From the surveys, the mean sex ratio (total number of females/females + males), density (individuals/m2), and mating activity (percentage of mating individuals) were calculated for each group. A total of 11 transient groups were used in the analysis (SI Appendix, Table S1). In 2013, 2018, and 2019, belt transect (2 × 50 m) surveys were implemented for solitarious locust populations, according to Maeno et al. (54) (SI Appendix, Table S2).
Leks.
According to Höglund and Alatalo (17), a lek can be defined as a mating system where males are more aggregated at the suitable display habitat than could be expected from random male settlement and where females visit these aggregations for the purpose of mating, not because of food or breeding sites within the aggregation. Our field surveys showed that 1) sexually mature, gregarious-behaving males aggregated on open ground, 2) remained on hot ground (nearly 50 °C), and 3) attempted to mate when females landed there (Fig. 1D). Each male maintained a certain distance from its neighbors, and the open ground did not contain specific food resources (Fig. 1B). Thus, these male aggregations were considered to represent “leks.” For the analysis, locust groups were categorized as either “lek site” or “outside.”
States of the Ovaries in the Field.
To determine whether gregarious-behaving, nongravid females occupy locations separate from those of males depending on their ovarian states and whether gravid females entered leks, the relationship between mating state (single versus mating [including copulating, postcopulatory mounting, and digging]) and ovarian state was examined in eight transient groups in 2016. At least 20 females from each group were sampled and dissected at the site or were immediately placed in a cooler box with ice and transported to the CNLA laboratory to determine the states of the ovaries. The ovaries were dissected and maintained in phosphate-buffered saline (T900, Takara co.) before the following were determined: the length of the terminal oocyte and eggs, and the presence or absence of mature eggs in the oviduct. Most females developed oocytes or eggs in the oviduct, which was removed from the ovaries of each female. The length of 10 oocytes per ovary was measured. If locusts laid eggs, small red dots, i.e. corpora lutea or resorption bodies were observed at the base of terminal oocytes (55, 56). To distinguish whether females were either immature or mature, the presence of resorption bodies was also recorded at the same time. The same procedure was performed for four solitarious populations in 2018 and 2019.
Determination of Egg Size.
In 2016, egg pods were collected from the group oviposition sites on the following day and were transported to the CNLA laboratory (Group identity (IDs): 4, 6, 9, and 10). At least 14 egg pods were sampled, and the length of 10 eggs was measured using a stereomicroscope with an ocular micrometer. For the solitarious population, collecting freshly oviposited egg pods in the field was difficult; therefore, solitarious females collected from the field were transported to the CNLA, and the egg pods were obtained from females that were individually reared in a cage (32 × 32 × 30 cm) under semifield conditions (air temperature 50 cm above the ground: 25.4 ± 0.2 °C [18.5 to 38.8 °C] between November 15 and December 1, 2018; 25.9 ± 0.1 °C [13.8 to 40 °C] between October 28 and December 10, 2019). These females were fed lettuce leaves. The first egg pod produced by each female was used in the analysis. This procedure was repeated in 2018 and 2019, and the data were pooled. Eggs collected from four digging females in the field were also included.
Sex Ratio of Other Developmental Stages.
Hatchlings from individual egg pods that were collected from the field in 2016 (Group IDs 6 and 9) were immobilized on ice and sexed with an ocular micrometer. Eggs from a single pod were placed on moist sand in a plastic container (9 cm diameter, 5 cm height) and were maintained in the shade under semifield conditions until hatching (air temperature 50 cm above the ground: 29.3 ± 4.7 °C [22 to 43.7 °C]). More than 50% of the egg pods that hatched were used. The sex ratio of the last instar nymphs in the field was also determined. Female nymphs are larger and slower than male nymphs; thus, sampling tended to be female biased, because male nymphs are more likely to escape. To avoid biased sampling, night-roosting locusts were sampled in November 2015 (19°13′ N, 013°42′ W). All locusts that roosted on the same Calotropis procera bush were regarded as a single group, because stationary nymphs remain on the plants after dusk until early morning (57). The sex ratio of the sexually immature solitarious adults, which recently molted, were also determined by observing night-roosting locusts in April 2011 (20°36′ N, 015°36′ W). Eggs from field collected solitarious locusts were also used for sex ratio determination in 2018.
Diel Changes in the Sex Ratio in Male-Biased Sites (Leks).
Mating pairs were frequently observed to aggregate around dusk and to lay eggs in a group after sunset near lekking sites. If gravid females enter male-biased groups for oviposition, the sex ratios might range from male-biased to equal over the course of 1 d. Diel changes in sex ratios in lekking groups were recorded in the field in 2012 (ID 1), 2013 (ID 3), and 2016 (IDs 4 and 6). The number of individuals of each sex and mating states (single, copulating, postcopulatory mounting, or ovipositing) within each of 10 belt transects (2 × 25 m each) were recorded every 1 or 2 h after a male group was encountered until 24:00 or 01:00 h. The belt transects were sometimes conducted only five times owing to the difficulty in the sampling schedule.
Mate Guarding During Oviposition.
Gregarious males produce the courtship-inhibition pheromone phenylacetonitrile, which reduces male–male competition under laboratory conditions (33). To determine whether phenylacetonitrile also reduces male–male competition in the field, the night-vision mode of a digital video camera (HC-VX870M, Panasonic) was used to record group oviposition and to determine whether rivals attacked ovipositing pairs. Around dusk (18:50 h), an observer carefully approached aggregating pairs and placed the tripod-mounted video camera on open sandy ground nearby. Some pairs responded to the approaching observer, thus record started after 1 h. The central area near the maximum density of group oviposition (70 × 50 cm) was recorded on October 9, 2016 (ID 6), and the number of ovipositing pairs was roughly estimated as 390/100 m2. Some males wandered near ovipositing pairs. We observed whether the first 10 males that visited a group oviposition site displaced the males guarding ovipositing females when the visiting male was within a body length of the copulating pair from 20:00 h.
Effects of Mate Guarding by Males on the Escape Performance of Females.
To assess how mate guarding by males affected the escape performance of females, the modified methods of Maeno et al. (40) were followed. An observer acting as a simulated predator walked slowly toward locusts on the ground. The observer filmed the locusts until they flew away. The adults responded to simulated predator attacks by walking, jumping, or flying as the first response (40). The observer followed the fleeing insects from behind to prompt an escape behavior, until they flew away. The time taken for locusts to fly away from the observer after initially responding to their presence was recorded separately for females and males (escape time was zero when locusts flew away at the first response). Before locusts were approached, the mating states were categorized as 1) single (females or males were on the ground alone), 2) copulating (females were copulated by a mounted male, i.e., their genitalia were physically connected and the female was grasped by male’s fore- and midlegs), and 3) postcopulatory mounting (the genitalia were not connected, but males remained mounted to guard the female from rivals). Communal animals typically detect approaching predators sooner than solitary animals (1). To exclude such a group effect in this study, the targets were directly approached to avoid triggering the fleeing behavior of other locusts (i.e., no locust was located between each target and the observer). All experiments were conducted from 16:00 to 18:20 h at a sufficiently high ambient temperature (50 cm above the ground and in the shade; mean temperature: 40.7 °C [38.1 to 42.8 °C]) on October 18, 2016 (near ID 9).
Determination of Oogenesis.
To determine daily oocyte development during the reproductive cycle, the length of the terminal oocyte was measured for six to eleven group-reared females (gregarious phase). These females were from a strain maintained in the Moroccan National Anti-Locust Center (24). To follow their reproductive history, the group-reared female locusts were marked with white paint (Pentel, EZL31-W) at adult emergence. They were housed with males in a group of ∼40 individuals in a cage (40 × 30 × 30 cm) at 30 ± 2 °C, with a 12:12 h light:dark photoperiod and 40 to 60% relative humidity. They were fed fresh lettuce and cabbage leaves, as well as wheat bran. Plastic cups (diameter, 5 cm; height, 10 cm) were filled with clean moist sand and were placed in the cages to collect the egg pods. Oviposition was recorded every 30 min from 09:00 to 20:00 h, and the oviposition substances were placed during the day to minimize differences in the timing of sampling. Each female laid at least one egg pod.
The length of 20 terminal oocytes from the ovaries was measured for each female using an ocular micrometer on a stereomicroscope, on day 0 (oviposition), 1, 2, 3, 4 (before oviposition), and −1 (mature eggs ovulated in the oviduct). This procedure was performed in the same room for individually reared females (solitarious phase) in separate cages (25 × 15 × 15 cm).
Mating Avoidance Experiment.
To determine whether desert locust females can physically prevent male mating attempts, mating avoidance experiments were conducted using a strain maintained in the Centre de Biologie pour la Gestion des Populations (CBGP) laboratory in Montpellier. The nymphs were housed in groups of ∼100 individuals in large cages (40 × 40 × 42 cm) at 32 ± 1 °C, with a 12:12 h photoperiod. They were fed fresh wheat grass leaves and wheat bran. Females were distinguished by marking them with white paint (Pentel, EZL31-W) at adult emergence. The females were weighed every day to follow their developmental history. Six small cages (20 × 20 × 35 cm) housing 10 females and 10 males (to simulate group-living conditions) were used until the first oviposition. The day of oviposition of each female was recorded by either direct observation during the day or by recording a drastic reduction in body weight. If the body weight of females declined by more than 400 mg when compared with the previous day, they were regarded as having laid eggs. After body weight was measured, the females were returned to a small cage as single. We then presented each of them with males for 24 ± 2 h and recorded whether the females were mated or remained single: that is, whether each female accepted or rejected the male. The same procedure was repeated daily until the next oviposition, which typically happened 4 or 5 d later. By making daily observations, the trials were performed with females with different ovary sizes. Finally, only 33 females that laid eggs twice were used in the analysis.
Statistical Analysis.
The F/C ratios of gregarious, solitarious, and transient populations (populations in transition from the solitarious to the gregarious phase) were compared using the nonparametric, Steel–Dwass test. The sex ratios of the 11 surveyed groups were compared with the expected values derived from the χ2 test. Sex ratios and percentages of mating females or males in the “lek sites,” “outside the lek sites,” and solitarious populations were arc-sine transformed and analyzed using the Tukey–Kramer HSD test. The Tukey–Kramer HSD test was used to evaluate daily ovarian development and terminal oocyte length. To evaluate the effects of ovarian development on the mating activity of females, the percentages of locusts that were mating or single in the mating avoidance experiment were subjected to a post hoc Fisher's exact test after Bonferroni correction using the software package R, version 4.0.1 (58) and JMP (SAS Institute).
Acknowledgments
For their assistance with the field surveys, we thank T. Tidjani, E. Mohamed, A.S. Benahi, and other members of Mauritanian Centre National de Lutte Antiacridienne and S. Nakamura (JIRCAS). We thank C. Mohamed and F. Moumou for assistance and members of the Moroccan Centre National de Lutte Antiacridienne for providing support and encouragement. We also thank N. Lemenager, C. Estienne, and members of Locust Ecology and Management Research at CBGP for helping in various aspects of the research and L. Soldati for photographing the claws of legs at CBGP. K.O.M. sincerely thanks K. Matsuura and members of the Laboratory of Insect Ecology, Graduate School of Agriculture Kyoto University, for their encouragement. We are grateful to Editage (http://www.editage.com) and B. Jaffee for English language editing. T. Maeno kindly provided locust illustrations. D.W. Whitman (Illinois State University) inspired K.O.M. to study locust mating behavior in the field. We greatly appreciate the constructive and valuable comments on the manuscript from D. Cullen and two anonymous reviewers. This study was funded by the Ministry of Agriculture, Forestry, and Fisheries of Japan through the Japan-CGIAR Fellowship Program implemented by JIRCAS and the Sumitomo Foundation (Grant No. 140381). The study was also supported by a research abroad fellowship (No. 128 · 2011) and Grants-in-Aid for Scientific Research (JSPS KAKENHI Grant Numbers 15K18808 and 21K05627) from the Japan Society for the Promotion of Science to K.O.M.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2104673118/-/DCSupplemental.
Data Availability
Excel data have been deposited in Figshare (DOI: 10.6084/m9.figshare.16640113) (59) . All other study data are included in the article and/or SI Appendix.
References
- 1.Krause J., Ruxton G. D., Living in Groups (Oxford University Press, 2002). [Google Scholar]
- 2.Ward A., Webster M., Sociality: The Behaviour of Group-Living Animals (Springer International Publishing, 2016). [Google Scholar]
- 3.Queller D. C., Strassmann J. E., Evolutionary conflict. Annu. Rev. Ecol. Evol. Syst. 49, 73–93 (2018). [Google Scholar]
- 4.Andersson M., Sexual Selection (Princeton University Press, Princeton, NJ, 1994). [Google Scholar]
- 5.Bateman A. J., Intra-sexual selection in Drosophila. Heredity 2, 349–368 (1948). [DOI] [PubMed] [Google Scholar]
- 6.Kokko H., Brooks R., Jennions M. D., Morley J., The evolution of mate choice and mating biases. Proc. Biol. Sci. 270, 653–664 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emlen S. T., Oring L. W., Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Magurran A. E., Nowak M. A., Another battle of the sexes: The consequences of sexual asymmetry in mating costs and predation risk in the guppy, Poecilia reticulata. Proc. Biol. Sci. 246, 31–38 (1991). [DOI] [PubMed] [Google Scholar]
- 9.Mühlhäuser C., Blanckenhorn W. U., The costs of avoiding matings in the dung fly Sepsis cynipsea. Behav. Ecol. 13, 359–365 (2002). [Google Scholar]
- 10.Holland B., Rice W. R., Chase-away sexual selection: Antagonistic seduction versus resistance. Evolution 52, 1–7 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Gavrilets S., Arnqvist G., Friberg U., The evolution of female mate choice by sexual conflict. Proc. Biol. Sci. 268, 531–539 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnquist G., Rowe L., Sexual Conflict (Princeton University Press, Princeton, NJ, 2005). [Google Scholar]
- 13.McLeod D. V., Day T., Female plasticity tends to reduce sexual conflict. Nat. Ecol. Evol. 1, 54 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Arnqvist G., Rowe L., Antagonistic coevolution between the sexes in a group of insects. Nature 415, 787–789 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Le Galliard J.-F., Fitze P. S., Ferrière R., Clobert J., Sex ratio bias, male aggression, and population collapse in lizards. Proc. Natl. Acad. Sci. U.S.A. 102, 18231–18236 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokko H., Klug H., Jennions M. D., Mating Systems. The Evolution of Insect Mating System (Oxford University Press, 2014). [Google Scholar]
- 17.Höglund J., Alatalo R., Leks (Princeton University Press, Princeton, NJ, 1995). [Google Scholar]
- 18.Isvaran K., Mary C. M. St., When should males lek? Insights from a dynamic state variable model. Behav. Ecol. 14, 876–886 (2003). [Google Scholar]
- 19.Uvarov B. P., Grasshoppers and Locusts (Centre for Overseas Pest Research, 1977), vol. 2. [Google Scholar]
- 20.Sword G. A., Lecoq M., Simpson S. J., Phase polyphenism and preventative locust management. J. Insect Physiol. 56, 949–957 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Cullen D. A., Sword G. A., Dodgson T., Simpson S. J., Behavioural phase change in the Australian plague locust, Chortoicetes terminifera, is triggered by tactile stimulation of the antennae. J. Insect Physiol. 56, 937–942 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Ayali A., The puzzle of locust density-dependent phase polyphenism. Curr. Opin. Insect Sci. 35, 41–47 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Rogers S. M., et al., Rapid behavioural gregarization in the desert locust, Schistocerca gregaria entails synchronous changes in both activity and attraction to conspecifics. J. Insect Physiol. 65, 9–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeno K. O., Piou C., Ghaout S., The desert locust, Schistocerca gregaria, plastically manipulates egg size by regulating both egg numbers and production rate according to population density. J. Insect Physiol. 122, 104020 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Uvarov B. P., Grasshoppers and Locusts (Cambridge University. Press, Cambridge, UK, 1966), vol. 1. [Google Scholar]
- 26.Le Gall M., Overson R., Cease A., A global review on locusts (Orthoptera: Acrididae) and their interactions with livestock grazing practices. Front. Ecol. Evol., 10.3389/fevo.2019.00263 (2019). [DOI] [Google Scholar]
- 27.Roffey J., Magor J. I., Desert Locust population parameters. Desert Locust F. Res. Station. Tech. Ser. 30 (FAO, Rome, Italy, 2003). [Google Scholar]
- 28.Golov Y., Rillich J., Douek M., Harari A. R., Ayali A., Sexual behavior of the desert locust during intra- and inter-phase interactions. J. Insect Behav. 31, 629–641 (2018). [Google Scholar]
- 29.Popov G. B., Notes on the behaviour of swarms of the desert locust (Schistocerca gregaria Forskal) during oviposition in Iran. Trans. R. Ent. Soc. Lond. Trans. R. Entomol. Soc. London 105, 65–77 (1954). [Google Scholar]
- 30.Popov G. B., Ecological studies on oviposition by swarms of the desert locust (Schistocerca gregaria Forskal) in eastern Africa. Anti-Locust Bull. 31, 1–70 (1958). [Google Scholar]
- 31.Golov Y., Rillich J., Harari A., Ayali A., Precopulatory behavior and sexual conflict in the desert locust. PeerJ 6, e4356 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter-Jones P., Fertilization of eggs of the desert locust by spermatozoa from successive copulations. Nature 185, 336 (1960). [Google Scholar]
- 33.Seidelmann K., Ferenz H. J., Courtship inhibition pheromone in desert locusts, Schistocerca gregaria. J. Insect Physiol. 48, 991–996 (2002). [DOI] [PubMed] [Google Scholar]
- 34.West-Eberhard M. J., Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (1983). [Google Scholar]
- 35.Beehler B. M., Foster M. S., Hotshots, hotspots, and female preference in the organization of lek mating systems. Am. Nat. 131, 203–219 (1988). [Google Scholar]
- 36.Gibson R. M., Bachman G. C., The costs of female choice in a lekking bird. Behav. Ecol. 3, 300–309 (1992). [Google Scholar]
- 37.Wiley R. H., Poston J., Indirect mate choice, competition for mates, and coevolution of the sexes. Evolution 50, 1371–1381 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Chappell M. A., Whitman D. W., “Grasshopper thermoregulation” in Biology of Grasshoppers, Chapman R. F., Joern A., Eds. (Wiley, New York, 1990), pp. 143–172. [Google Scholar]
- 39.Boyko A. R., Gibson R. M., Lucas J. R., How predation risk affects the temporal dynamics of avian leks: Greater sage grouse versus golden eagles. Am. Nat. 163, 154–165 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Maeno K. O., et al., Defence tactics cycle with diel microhabitat choice and body temperature in the desert locust, Schistocerca gregaria. Ethology 125, 250–261 (2019). [Google Scholar]
- 41.Sword G. A., Lorch P. D., Gwynne D. T., Insect behaviour: Migratory bands give crickets protection. Nature 433, 703 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Stauffer T. W., Whitman D. W., “Grasshopper oviposition” in The Bionomics of Grasshoppers, Katydids and Their Kin (CAB International, Wallingford, 1997), pp. 231–280. [Google Scholar]
- 43.Stillman R. A., Cutton-brock T. H., Sutherland W. J., Black holes, mate retention, and the evolution of ungulate leks. Behav. Ecol. 4, 1–6 (1993). [Google Scholar]
- 44.Clutton-Brock T. H., Parker G. A., Sexual coercion in animal societies. Anim. Behav. 49, 1345–1365 (1995). [Google Scholar]
- 45.Norris M. J., Sexual maturation in the desert locust with special reference to the effects of grouping. Anti-Locust Bull 18, 1–43 (1954). [Google Scholar]
- 46.Zhang L., Lecoq M., Latchininsky A., Hunter D., Locust and grasshopper management. Annu. Rev. Entomol. 64, 15–34 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Guo X., et al., 4-Vinylanisole is an aggregation pheromone in locusts. Nature 584, 584–588 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Hassanali A, et al., Chemical ecology of locusts and related acridids. Annu. Rev. Entomol. 50, 223–245 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Cullen D. A., et al., From molecules to management: Mechanisms and consequences of locust phase polyphenism. Adv. In Insect Phys. 53, 167–285 (2017). [Google Scholar]
- 50.Babah Ebbe M. A., Biogéographie du Criquet pèlerin en Mauritanie (Hermann, 2010). [Google Scholar]
- 51.Piou C., et al., Mapping the spatiotemporal distributions of the Desert Locust in Mauritania and Morocco to improve preventive management. Basic Appl. Ecol. 25, 37–47 (2017). [Google Scholar]
- 52.Symmons P. M., Cressman K., Desert Locust Guidelines 1: Biology and Behavior (FAO, Rome, 2001). [Google Scholar]
- 53.Cisse S., et al., Effect of vegetation on density thresholds of adult desert locust gregarization from survey data in Mauritania. Entomol. Exp. Appl. 149, 159–165 (2013). [Google Scholar]
- 54.Maeno K. O., et al., Daily microhabitat shifting of solitarious-phase desert locust adults: Implications for meaningful population monitoring. Springerplus 5, 107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Launois-Luong M. H., Lecoq M., Sexual maturation and ovarian activity in Rhammatocerus schistocercoides (Orthoptera: Acrididae), a pest grasshopper in the state of Mato Grosso in Brazil. Environ. Entomol. 25, 1045–1051 (1996). [Google Scholar]
- 56.Sundberg S. V., Luong-skovmand M. H., Whitman D. W., Morphology and development of oocyte and follicle resorption bodies in the Lubber grasshopper, Romalea microptera (Beauvois). J. Orthoptera Res. 10, 39–51 (2001). [Google Scholar]
- 57.Maeno K. O., Piou C, Kearney M.R.et al., A general model of the thermal constraints on the world’s most destructive locust, Schistocerca gregaria. Ecol. Appl. 31, e02310 (2021). [DOI] [PubMed] [Google Scholar]
- 58.R Development Core T. , R: A language and environment for statistical computing, version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria, 2020). [Google Scholar]
- 59.Maeno K. O., et al., Density-dependent mating behaviors reduce male mating harassment in locusts. Figshare. 10.6084/m9.figshare.16640113. Deposited 18 September 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Excel data have been deposited in Figshare (DOI: 10.6084/m9.figshare.16640113) (59) . All other study data are included in the article and/or SI Appendix.