ABSTRACT
Mycobacterium tuberculosis is a chronic infectious disease pathogen. To date, tuberculosis is a major infectious disease that endangers human health. To better prevent and treat tuberculosis, it is important to study the pathogenesis of M. tuberculosis. Based on early-stage laboratory research results, in this study, we verified the upregulation of sod2 in Bacillus Calmette–Guérin (BCG) and H37Rv infection. By detecting BCG/H37Rv intracellular survival in sod2-silenced and sod2-overexpressing macrophages, sod2 was found to promote the intracellular survival of BCG/H37Rv. miR-495 then was determined to be downregulated by BCG/H37Rv. BCG/H37Rv can upregulate sod2 expression by miR-495 to promote the intracellular survival of BCG/H37Rv through a decline in ROS levels. This study provides a theoretical basis for developing new drug targets and treating tuberculosis.
KEYWORDS: Mycobacterium tuberculosis, SOD2, microRNA, intracellular survival, ROS, miR-495
INTRODUCTION
Mycobacterium tuberculosis is one of the most lethal bacteria. In 2019, approximately 1.4 million people died from tuberculosis (TB) (WHO 2020 global tuberculosis report). Due to the great threat of this disease and the emergence and spread of drug-resistant TB, it is necessary to study innate immunity during M. tuberculosis infection and search for host targets that may be manipulated to downregulate innate immunity.
The infectious process of M. tuberculosis is complicated. In the relatively sterile lower lung, M. tuberculosis masks its pathogen-activated molecular patterns (PAMPs) with the surface lipid phthiocerol dimycocerosate (PDIM) to evade microbicidal macrophages. M. tuberculosis can exploit permissive macrophages and replicate in them. The capability to survive in highly evolved phagocytic host cells is a criterion of pathogenicity (1). The detection of intracellular survival is essential for the measurement of M. tuberculosis pathogenesis.
MicroRNAs (miRNAs) can play an important role in tuberculosis infection by participating in a variety of signal pathways in the innate immune response, regulating the production and response of cytokines and regulating autophagy. For example, miR-337-3p, miR-125b-5p, and miR-365 were found to be involved in the regulation of tuberculosis infection (2, 3).
Based on our previous research (4), superoxide dismutase 2 (sod2) is significantly increased in THP-1 cells infected by H37Rv or Mycobacterium bovis. The superoxide dismutases (SODs) are important enzymes related to intracellular redox reaction. At present, three distinct isoforms of SOD have been identified in mammals: SOD1, SOD2, and SOD3. Among these three isoforms, SOD2 has been localized to the mitochondria of aerobic cells and uses manganese (Mn) as a cofactor (5). This protein can bind to the superoxide by-products of oxidative phosphorylation and transform them to hydrogen peroxide and diatomic oxygen, allowing it to regulate the level of reactive oxygen species (ROS) (6) by participating in the superoxide metabolic process.
ROS are important in many pathogenic infections and exert various therapeutic functions to inhibit bacterial infections. Some ROS are generated as antimicrobial defenses by mammalian hosts (7). Vilchèze et al. reported that vitamin C, which can enhance ROS levels, is lethal to M. tuberculosis (8). Neutrophils can kill microorganisms through ROS production (9). Studies have investigated the interaction of SOD2, ROS, and pathogens, including that of Wang et al., who found that SOD2 regulates antiviral signaling through modulation of ROS levels (10). However, whether SOD2 impacts the intracellular survival of M. tuberculosis through ROS and the regulatory mechanism remain unclear.
In this study, the effect of sod2 on the intracellular survival of BCG/H37Rv was confirmed, and a microRNA, miR-495, was found to be involved in the SOD2 regulatory mechanism during BCG and H37Rv infection.
RESULTS
sod2 expression is increased by H37Rv and BCG infection.
According to a proteomic analysis (4), the expression of sod2 was higher in H37Rv-infected cells than uninfected cells. For further verification, the expression of sod2 in H37Rv- and BCG-infected macrophages, including induced THP-1 and RAW264.7 cells, was detected by quantitative PCR (qPCR) and Western blotting. After 24 h of H37Rv infection, sod2 expression was significantly increased in induced THP-1 cells compared to uninfected cells (Fig. 1A). However, interestingly, H37Rv applied at a multiplicity of infection (MOI) of only 0.5 caused an obvious difference in sod2 expression, while the sod2 increase mediated by BCG was not significant until at least an MOI of 6 (Fig. 1B). SOD2 protein expression also increases after 48 h of BCG/H37Rv infection (Fig. 1C), and the cell viability of THP-1 infected with BCG (MOI, 6, 10, 12, and 20) showed no difference from noninfected cells (Fig. 1D).
FIG 1.
sod2 expression is increased by H37Rv and BCG infection. (A) The mRNA and protein expression of sod2 in macrophages 24 h after H37Rv/BCG infection. (B) The mRNA and protein expression of sod2 in macrophages 24 h after BCG infection. (C) The protein expression of sod2 in macrophages 48 h after H37Rv/BCG infection. (D) Cell viability of THP-1 infected with BCG at different time points. (E) sod2 mRNA and protein expression in RAW264.7 cells infected with BCG (left) and sod2 mRNA expression in lung tissue of mice infected with BCG (right). The numbers below the Western blot lanes are grayscale analyses with cell as the control. *, P ≤ 0.1; **, P ≤ 0.05; ***, P ≤ 0.005 (Student's t test [A, B, D, and E]). Data are from one experiment representative of three (A to E; means ± SEM) independent experiments with similar findings. Data are from 5 mice (E; means ± SEM).
BCG BCG infection mediated the same phenomenon in RAW264.7 cells (Fig. 1E). Regardless of the MOI range, BCG or H37Rv infection can cause an upward trend in sod2 expression. As the increase in sod2 expression is dependent on MOI in induced THP-1 or RAW264.7 cells, this enhancement is largest with the highest-MOI H37Rv or BCG infection.
In addition, in experiments testing the expression of sod2 mRNA in the lung tissue of mice, the sod2 mRNA expression in mice infected with BCG was approximately twice as high as that in the phosphate-buffered saline (PBS) group (Fig. 1E).
sod2 promotes the intracellular survival of BCG/H37Rv in macrophages.
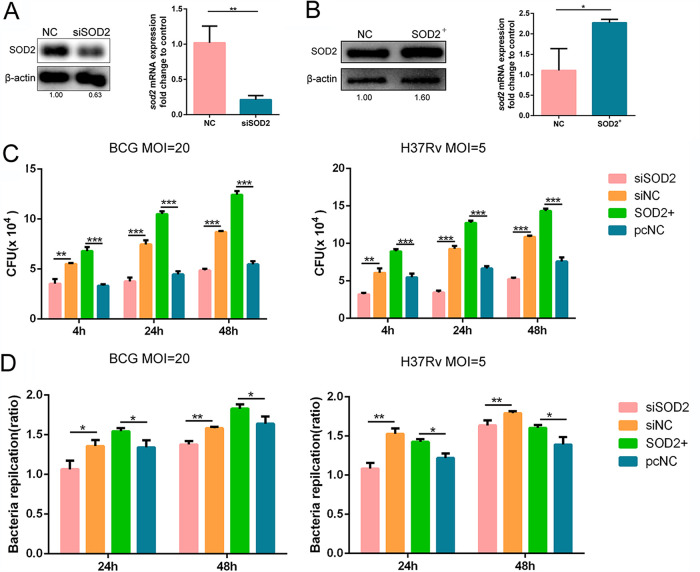
To explore the effect of SOD2 on the intracellular survival of BCG/H37Rv, short interfering RNA (siRNA) to silence sod2 (siSOD2) and a plasmid to overexpress sod2 (SOD2+) were constructed. The levels of sod2 transcription and translation in macrophages were decreased by approximately half after transfection with sod2 siRNA (Fig. 2A). The sod2-overexpressing vector pcDNA3.1-SOD2 was tested by Western blotting and qPCR after transfection into macrophages. The results confirmed that the vector caused nearly twice as much sod2 overexpression as the control (Fig. 2B). The intracellular survival of BCG and H37Rv was detected afterwards. To eliminate the influence of bacterial uptake on the intracellular survival of bacteria at 24 and 48 h postinfection, the results of intracellular bacterial amounts obtained at 24 h and 48 h after infection were divided by the bacterial uptake amount that was obtained at 4 h postinfection. The results showed that the intracellular survival of BCG/H37Rv was inhibited in siSOD2 cells more so than in its control cells, and the intracellular survival of BCG/H37Rv was promoted in SOD2+ cells more so than in its control cells at 24 h or 48 h after infection (Fig. 2C and D). These results verified that sod2 can promote the intracellular survival of BCG and H37Rv in macrophages.
FIG 2.
sod2 promotes the intracellular survival of BCG/H37Rv in macrophages. (A) sod2 translation and transcription levels were tested in THP-1 cells transfected with sod2 siRNA (siSOD2) and negative-control cells. (B) After transfection of the overexpression vector into macrophages (SOD2+), the mRNA and protein expressions levels of sod2 were detected by Western blotting and qPCR. (C) The intracellular survival of BCG/H37Rv in siSOD2/SOD2+ cells were detected at 4 h, 24 h, and 48 h after infection by CFU assay (siNC, macrophages transfected with NC oligonucleotides, the control cells for siSOD2; pcNC, macrophages transfected with empty plasmid, the control cells for SOD2+). (D) The intracellular replication multiple of BCG/H37Rv in siSOD2/SOD2+ cells at 24 h and 48 h after infection. The numbers below the Western blot lanes are grayscale analyses with cell as the control. *, P ≤ 0.1; **, P ≤ 0.05; ***, P ≤ 0.005 (Student's t test [A to D]). Data are from one experiment representative of three (A to D; means ± SEM) independent experiments with similar findings.
SOD2 can regulate the intracellular survival of BCG/H37Rv through ROS.
Considering the close connection between SOD2 and ROS, whether the impact of sod2 is mediated through ROS was explored. The levels of intracellular ROS in sod2-silenced and sod2-overexpressing macrophages without BCG infection were measured by flow cytometry and fluorescence microscopy. The ROS level was increased in sod2-silenced macrophages and decreased in sod2-overexpressing macrophages (see Fig. S1A in the supplemental material), which verified the function of SOD2 as an ROS scavenger.
To explore the connection between intracellular ROS levels and BCG infection and to determine the effect of ROS levels on BCG intracellular survival, intracellular ROS were tested in BCG-infected macrophages by flow cytometry. First, the ROS levels in macrophages infected with BCG at different MOIs were measured by flow cytometry and confocal fluorescence microscopy. The results showed that the mean fluorescence intensity of ROS in macrophages infected with BCG (MOI, 6) was approximately three times that in BCG-infected macrophages (MOI, 20), and with the increase in MOI, the intracellular and mitochondrial ROS level decreased in BCG-infected macrophages, which indicated that the ROS level was negatively related to the MOI of BCG (Fig. 3A and B). Subsequently, ROS levels were measured in siSOD2 macrophages infected with BCG. Compared to NC cells that were also infected with BCG, siSOD2 cells showed approximately 8 times higher ROS levels (Fig. 3C). Finally, BCG intracellular survival was measured in macrophages treated with an ROS inducer (5, 10, and 20 nM), which can promote intracellular ROS levels, in advance. The results showed that ROS can inhibit the intracellular survival of BCG. The ROS inducer caused at least one-third of the BCG intracellular survival loss, and the inhibition was concentration dependent (Fig. 3D). The above-described results suggest that BCG regulates intracellular ROS levels to enhance intracellular survival mainly by SOD2.
FIG 3.
SOD2 can regulate the intracellular survival of BCG through ROS. Intracellular ROS levels (A) and mitochondrial ROS (B) were detected in macrophages infected with BCG of different MOIs 24 h postinfection. (C) ROS levels were detected in macrophages transfected with siRNA (siSOD2) or NC oligonucleotides (siNC) and infected with BCG 24 h postinfection (MOI, 20). (D) The intracellular survival of BCG was tested in macrophages with ROS inducer at incremental concentrations 24 h postinfection (MOI, 20). *, P ≤ 0.1; **, P ≤ 0.05; ***, P ≤ 0.005 (one-way ANOVA [A and D] and Student's t test [C]). Data are from one experiment representative of three (A to D; means ± SEM) independent experiments with similar findings.
miR-495 regulates the sod2 expression.
To explore the mechanism by which BCG or H37Rv regulates sod2 expression in macrophages, microRNA.org was used to study putative miRNAs that can target sod2. Several miRNAs were found to show potential capability on targeting sod2. The expression of these microRNAs was detected in H37Rv-infected macrophages by qPCR, and one stably downregulated microRNA, hsa-miR-495-3P (miR-495), was found (Fig. 4A, Fig. S1C). To validate whether this microRNA can regulate sod2 through direct binding, dual-luciferase reporter vectors containing the wild-type and mutant sod2 3′-untranslated regions (UTRs) were constructed. After cotransfection of the vector and miR-495 mimic into 293T cells, the luciferase intensity was tested and analyzed. The results demonstrated that miR-495 decreased the luciferase intensity 5-fold in cells transfected with miR-495 mimic compared with negative-control cells. However, no difference in luciferase activity was observed in the sod2 3′-UTR-mut group (Fig. 4B), which indicates that miR-495 targets sod2 through direct binding. sod2 expression in macrophages transfected with miR-495 mimic and inhibitor was detected, and the results showed that sod2 expression was downregulated in the miR-495 mimic group and upregulated in the other group (Fig. 4C and D). Thus, it was proven that miR-495 can target sod2 and regulate its expression.
FIG 4.
miR-495 regulates sod2 expression. (A) The wild-type 3′-UTR of sod2 mRNA involves a putative miR-495 binding site, and the red letters represent the mutant sequence. (B) Relative luciferase activity in 293T cells cotransfected with wild-type sod2 3′-UTR-luciferase reporter vector (WT) or mutant-type sod2 3′-UTR-luciferase reporter vector (mut) and miRNA oligonucleotides (mimic NC, miRNA mimic). (C and D) SOD2 expression was detected in macrophages transfected with miR-495 mimic by qPCR and Western blotting. The numbers below the Western blot lanes are grayscale analyses with cell as the control. *, P ≤ 0.1; **, P ≤ 0.05; ***, P ≤ 0.005; ns, no significant difference by Student's t test [B and C]). Data are from one experiment representative of three (A to D; means ± SEM) independent experiments with similar findings.
H37Rv/BCG infection downregulates the expression of miR-495.
The miR-495 sequences in different species were obtained from miRbase. Given the high conservation of miR-495 in different species (Fig. 5A), the level of miR-495 expression in lung tissues of mice infected with BCG was tested, and a decline of approximately half was observed (Fig. 5B). In addition, microRNA expression was measured in serum samples positive for bovine tuberculosis isolated from the clinic (2 healthy bovine serum samples, 3 M. bovis-positive bovine serum samples), and there was about 5 times as much miR-495 expression in 2 healthy samples than in all 3 positive serum samples (Fig. 5C). The miR-495 expression in THP-1 and RAW264.7 cells infected with BCG or H37Rv was also detected. All results showed that H37Rv and BCG at high MOIs can mediate the decrease in miR-495 expression (Fig. 5D, Fig. S1D), and the decrease occurs in an MOI-dependent manner. All the results showed that miR-495 expression is downregulated by BCG or H37Rv infection.
FIG 5.
H37Rv/BCG infection downregulates the expression of miR-495. (A) The conservation of miR-495 in different species. (B) miR-495 expression in mouse lung tissues was tested by qPCR. (C) miR-495 expression in serum from tuberculosis-positive cattle was detected by qPCR (healthy, healthy bovine serum, n = 2; M. bovis, M. bovis-positive bovine serum, n = 3). (D) The expression of miR-495 measured in macrophages infected with H37Rv (MOI, 2, 5) or BCG (MOI, 10, 20) and the expression of miR-495 is decreased in RAW264.7 cells infected with BCG (MOI, 10, 20). *, P ≤ 0.1; **, P ≤ 0.05; ***, P ≤ 0.005 (Student's t test [B and D]). Data are from one experiment representative of three (A, B, and D; means ± SEM) independent experiments with similar findings. (C) Data are from 5 cows.
miR-495 can inhibit the intracellular survival of BCG/H37Rv through ROS.
To determine whether miR-495 can affect the intracellular survival of M. tuberculosis through ROS, the level of intracellular ROS was measured in macrophages transfected with the miR-495 mimic and inhibitor. Macrophages with the miR-495 mimic contain approximately twice as much intracellular ROS as controls, whereas ROS levels in macrophages with the miR-495 inhibitor are decreased by the same factor (Fig. 6A). The intracellular survival of BCG/H37Rv was also detected in these macrophages after 4 h, 24 h, or 48 h of infection, and the intracellular survival of BCG/H37Rv was impaired in miR-495 mimic macrophages and enhanced in miR-495 inhibitor macrophages (Fig. 6B and C). These results indicate that miR-495 can inhibit the intracellular survival of BCG/H37Rv by increasing the intracellular ROS level.
FIG 6.
miR-495 can inhibit the intracellular survival of BCG/H37Rv through ROS. (A) The intracellular ROS level in macrophages transfected with miR-495 mimic, miR-495 inhibitor, and their corresponding negative controls (mimic NC and inhibitor NC). ROS levels were quantified in the right histogram. (B) The intracellular survival of BCG/H37Rv in macrophages with the same treatment at 4 h, 24 h, and 48 h postinfection. (C) The intracellular replication multiple of BCG/H37Rv in miR-495 mimic/inhibitor transfected cells at 24 h and 48 h after infection. *, P ≤ 0.1; **, P ≤ 0.05; ***, P ≤ 0.005 (Student's t test [A to C]). Data are from one experiment representative of three (A and B; means ± SEM) independent experiments with similar findings. (D) The schematic diagram about the regulation process of miR-495/SOD2/ROS in M. tuberculosis infection.
DISCUSSION
SOD2 is an important enzyme that regulates cellular metabolism in various cells. To date, only a few studies have investigated the role of SOD2 in tuberculosis infection. This study aimed to determine the effect of sod2 on H37Rv and BCG infection. The intracellular survival of H37Rv/BCG was explored 4 h, 24 h, and 48 h postinfection, and the data of H37Rv/BCG intracellular survival at 4 h postinfection show that SOD2 can promote the uptake of H37Rv/BCG during infection, which may be caused by the low ROS level, which reduces macrophage defense. The data of H37Rv/BCG intracellular survival at 24 h and 48 h postinfection elucidate that SOD2 can still promote the intracellular survival of H37Rv/BCG after excluding the influence of differential uptake of the bacteria, which may be mediated by the improvement of the bacterial replication level or the decrease of the macrophages’ clearance ability in this process. Although this study has not clarified the role of ROS after the bacteria invade the macrophage, SOD2- and miR-495-related experiments provided valid evidence for the effect of ROS in the process of H37Rv/BCG infection, and it shows that SOD2 can promote the intracellular survival of H37Rv/BCG after the uptake of the bacteria. However, prior to submission of the study, Yabaji et al. reported that sod2 knockdown decreased the M. bovis BCG intracellular load in macrophages by mediating acidification of phagosomes, and the upregulation of SOD2 is dependent on ESAT-6, which does not exist in BCG (11). However, the present study found that BCG wild-type infection with an MOI of at least 6 can also mediate the upregulation of SOD2, which means the SOD2 upregulation is not dependent only on ESAT-6. This study focuses on the relationship between SOD2 and ROS, elucidating the impact of SOD2 from another perspective. In a study investigating the roles of mitochondrial ROS and SOD2 regulation in the zebrafish innate immune response to Pseudomonas aeruginosa infection, sod2 expression was found to increase in response to lipopolysaccharide in mammalian macrophages and neutrophils. Instead of inhibiting bacterial infection, sod2 knockdown results in an increased bacterial burden, as neutrophils that can clear bacteria can be damaged by ROS (12). Moreover, Abuaita et al. found that SOD2 can be delivered by mitochondrion-derived vesicles (MDVs) to phagosomes and enhance methicillin-resistant Staphylococcus aureus (MRSA) killing through phagosome mH2O2 accumulation, since SOD2 is the essential enzyme for H2O2 formation (13). Considering that the antistress bacterial capabilities are different, the regulation of sod2 can affect the viability of bacteria for many reasons, such as influencing the activity of macrophages and neutrophils, promoting mH2O2 accumulation, and scavenging ROS, and it is conceivable that the overexpression of sod2 leads to inverted viability results with M. tuberculosis and MRSA. Generally, BCG cell wall skeleton (BCG-CWS) stimulation can upregulate SOD2 (14). These studies suggest the special roles and different activities of SOD2 during BCG and H37Rv infection, which indicates the differences in tuberculosis therapy relative to other bacterial infections.
ROS regulated by SOD2 are important in various cellular signaling pathways. It has been proven that M. tuberculosis can induce low-density granulocyte generation by promoting neutrophil extracellular trap formation via the ROS pathway, and intracellular ROS and oxidative stress can activate autophagy (15–17), which may explain the decreased viability of M. tuberculosis in macrophages with high levels of ROS, as autophagy is important for antituberculosis responses (18). It is possible that SOD2 regulates the intracellular survival of M. tuberculosis by means other than ROS, since SOD2 has functions besides regulating ROS, e.g., SOD2 overexpression can decrease p53-mediated induction of apoptosis (19). Our results indicate that SOD2 regulates the intracellular survival of M. tuberculosis mainly through ROS.
According to previous studies, SODs are important in pathogen invasion. In most pathogens, extracellular SODs are the first line of defense against host-derived ROS, yet intracellular SODs are also virulence factors for bacterial, fungal, and protozoan pathogens (20). Furthermore, SODA, which combines with Mn and is produced by bacteria, considerably reduces oxygen radicals and the production of NO and impairs cell immunologic function in early infection of M. tuberculosis (21). SODA or SOD2 can have a predominant effect on ROS regulation (22), which indicates the important effects of SODs during pathogenic infection.
Many studies have revealed the mechanisms of sod2 regulation prior to the current study. miR-335, miR-146a, and miR-382-5p can target sod2 and regulate sod2 expression (6, 23, 24). Additionally, SIRT3 can deacetylate and activate SOD2 (25). However, here, we verified a new posttranscriptional regulatory mechanism of sod2.
Before this study, there were no reports on the effect of miR-495 on the M. tuberculosis infection process. According to Wang et al., miR-495 is significantly increased in Caco-2 cells that simulate hypoxic conditions mediating ROS accumulation (26), which indicates a function of miR-495 in regulating ROS levels. A number of reports have confirmed the effects of miR-495 on cell viability, such as inhibiting cell proliferation by targeting different genes and regulating inflammatory reactions involved in a variety of signaling pathways (27–30). Interestingly, miR-495 was found to play a part in many pathological conditions of the lungs (27, 31), and it can be presumed that M. tuberculosis is not necessary to mediate low expression of miR-495. This study first proposes a new function of miR-495 and demonstrates the mechanism by which miR-495 can regulate ROS levels by targeting sod2. It is also found that miR-495 can inhibit the uptake of H37Rv/BCG and impair the intracellular survival of H37Rv/BCG after the uptake of the bacteria.
In summary, we have confirmed that SOD2 can promote the bacterial invasion and intracellular replication of BCG/H37Rv. Importantly, we reveal the mechanism by which miR-495 can regulate the level of ROS by targeting SOD2 (Fig. 6D). This study may offer some insights into M. tuberculosis infection and the immune evasion process. It may also be helpful for tuberculosis drug and vaccine development and therapies for curing tuberculosis.
MATERIALS AND METHODS
Cell culture.
Human THP-1 macrophages were cultured in RPMI 1640 (Gibco, Rockville, MD) with 10% fetal bovine serum (FBS; Gibco, Rockville, MD) and 1% penicillin-streptomycin (pen-strep), and 293T and RAW264.7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 1% pen-strep at 37°C in a humidified atmosphere with 5% CO2 (32).
Preparation of bacteria and cells.
H37Rv and BCG were grown in Middlebrook 7H9 liquid medium supplemented with oleic acid albumin dextrose catalase (OADC) and 0.05% Tween 80 at 37°C as previously described (4). THP-1 and RAW264.7 cells were seeded at 2.0 × 106 cells per well into 6-well plates, and THP-1 were differentiated into macrophages by treatment with 100 nmol/liter phorbol 12-myristate 13-acetate (PMA; Sigma) simultaneously; after that, the cells were cultured for 24 h (33, 34).
Animal experiments.
An appropriate amount of a mid-log-phase culture of BCG in Middlebrook 7H9 liquid medium was resuspended in PBS containing 0.1% Tween 80. After standing still for 30 min, the suspension was processed to obtain supernatant free of visible clumps. This was further diluted with PBS Tween 80 (PBST) for infection.
The experiments conducted on mice had prior approval of the Ethical Review of Experimental Animal Welfare in Hubei Province (approval reference no. 170212). All mice were on the C57BL/6 background and were 6 to 8 weeks of age when infected. Mice were divided into different groups of 4 to 5 mice each. The BCG suspension was prepared as described above. The infected group received a 2.8 × 105 CFU/mouse dose of bacteria, and PBS of the same volume was used for the control group. Mice were anaesthetized with diethyl ether (Sigma, St. Louis, MA) before intranasal delivery, and 20 μl of the infection suspension or PBST was administered into each nostril of the mice of the infected group and control group, respectively. The suspension was released slowly so that no bubbles formed to allow the mouse to inhale naturally.
Mice were sacrificed for lung tissue sampling on day 7 after intranasal infection. The lung lobes from mice of different groups were homogenized in 1 ml TRIzol reagent (Invitrogen, USA). Total RNA was isolated according to the manufacturer's protocol by the TRIzol/chloroform method (35, 36).
Construction of the plasmid for sod2 overexpression.
The sod2 transcript sequence was amplified by using the primers described in Table 1 and cloned into the pcDNA3.1 vector using KpnI and XhoI restriction enzymes (TaKaRa, Japan) to construct the sod2-overexpressing vector pcDNA3.1-SOD2. The effect of pcDNA3.1-SOD2 plasmid was tested by quantitative real-time PCR (qRT-PCR) and Western blotting. SOD2 protein-overexpressing cells (SOD2+) were cultured for further study (37).
TABLE 1.
Primers used for amplification or qRT-PCR
| Gene | Forward primer (5′—3′) | Reverse primer (5′—3′) |
|---|---|---|
| Sod2 (human)b | TGTAATCAACTGGGAGAA | CTTAGAAGACAGGACATTATC |
| Sod2 (human)a (for pcDNA3.1 plasmid) | ACCACGATCGTTATGCTGATCATACCC | GATGGTTGACAGATTCTTTTATTAACA |
| Sod2 (human)a (for pmirGLO plasmid) | GAGCTCACCACGATCGTTATGCTGATCATACCC | GTCGACGATGGTTGACAGATTCTTTTATTAACA |
| β-actin (human)b | TGACGTGGACATCCGCAAAG | CTGGAAGGTGGACAGCGAGG |
| U6b | GTAATACGACTCACTATAGGGAGAAGAG | CGCGCCTGCAGGTCGAC |
| miR-495b | GGAAACAAACATGGTGCACTTCTT | Provided in kit |
| Sod2 (mouse)b | AGAATGTTACTGAAAGATA | ATGCTCTACACTACTATA |
| β-actin (mouse)b | CTTCCTTCTTGGGTATGG | CACTGTGTTGGCATAGAG |
Used for amplification.
Used for qRT-PCR.
Cell infection and transient transfection.
H37Rv/BCG at different MOIs were used to infect macrophages for different durations (4, 24, and 48 h) as previously described (34). Before infection, H37Rv/BCG were centrifuged and resuspended in cell culture medium. After 4 h of interaction of cells and bacteria, cells were gently washed by PBS 3 times to remove nonphagocytosed bacteria. The infected cells would be rested at 37°C for 1 or 2 days or used for CFU assay directly.
For transient transfection, miRNA oligonucleotides including the inhibitor and mimic for miR-495 and their corresponding negative controls (NCs; the corresponding miRNAs with the scrambled sequence) and siRNA oligonucleotides for silencing sod2 were commercially obtained from Gima Inc. (Jiangsu, China). The siRNA oligonucleotides were 5′-CUCAUGCAUGCAAAUCCUU (dTdT)-3′ (sense) and 5′-AAGGAUUUGCAUGCAUGAG (dTdT)-3′ (antisense) (Gima provides). The NC oligonucleotides were 5′-UUCUCCGAACGUGUCACGU (dTdT)-3′ (sense) and 5′-ACGUGACACGUUCGGAGAA (dTdT)-3′ (antisense). Macrophages were transfected with 50 nM the different miRNAs, siRNA, or vector for 36 h using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the supplier’s instructions (34). qRT-PCR analysis and Western blotting were implemented to detect the transcription and translation levels of sod2 to evaluate the effects of transient cell transfection.
qRT-PCR.
According to the manufacturer's protocol, total RNA isolation from cells was performed with TRIzol reagent (Invitrogen), and the RNA was reverse transcribed into cDNA using the 223 kit (Vazyme, China). For the determination of miR-495 levels and the detection of mRNA expression, qRT‐PCR was performed using the miRcute plus miRNA qPCR kit (SYBR green) (Tiangen, Beijing, China) and ChamQ SYBR qPCR master mix as previously described (34). The primers used for amplification are listed in Table 1.
Western blotting.
Western blotting was performed as previously described (34). The transferred membrane was incubated with Tris-HCl buffer salt solution (TBS) in Tween 20 (TBST) containing 5% bovine serum albumin (BSA) at 4°C for 12 h. For immune imprinting, the imprint of SOD2 was first incubated with Homo sapiens anti-SOD2 antibody (1:1,000) (Abcam, UK), while the imprint of β-actin was incubated with mouse anti-β-actin antibody (Beijing Biodragon Immunotechnologies Co., Ltd., China) for 24 h at 4°C. After washing with TBST, membranes were incubated with goat anti-rabbit or goat anti-mouse antibody (1:5,000) (Abclonal, China) for 2 h at room temperature. The membranes were incubated with electrochemiluminescence reagent (Bio-Rad, Inc.) and developed. β-Actin was used as a normalization control.
Dual-luciferase reporter assay.
For the dual-luciferase reporter assay, the sod2 3′-UTR segments containing the putative binding site of miR-495 and the mutated 3′-UTR of sod2 were both amplified and subcloned into a pmirGLO-luciferase promoter vector as previously described (38). The primers used for amplification are listed in Table 1. Human embryonic kidney 293 (HEK293) cells were seeded into 24-well plates and cotransfected with the pmirGLO-luciferase vector (containing the original or mutated 3′-UTR of sod2) and miR-495. Luciferase and Renilla signals were measured 24 h after transfection using a Dual Luciferase reporter assay kit (Promega, Madison, WI, USA).
CFU assay.
BCG/H37Rv survival after infection with macrophages was tested by a CFU assay. As previously described, induced THP-1 cells were infected with BCG or H37Rv for 4, 24, and 48 h at an MOI of 20 or 5, respectively, followed by lysis of the infected cells with 0.025% Triton X-100. Tenfold serial dilutions were used for quantitative culturing with each dilution inoculated on Middlebrook 7H11 agar plates containing 10% OADC (Sigma). BCG/H37Rv were cultured for at least 4 weeks at 37°C, and the numbers of CFU were determined using standard protocols (38).
ROS measurement and induction.
The measurement of ROS was performed by flow cytometry and fluorescence microscopy. For flow cytometry, cells were stained with the fluorescence probe BBoxi O13 according to the instructions of the ROS assay kit (BestBio, China). After removing the culture medium, the cells were gently washed with PBS and then stained with the BBoxi O13 probe for 20 min at 37°C. Cells were collected with PBS after washing with warmed PBS and subjected to flow cytometry analysis. Unstained controls were treated similarly, and dyes were omitted. For microscopy, cells were stained with the same fluorescence probe, BBoxi O13, for 20 min, followed by 4′,6-diamidino-2-phenylindole (DAPI) for 10 min. After three washes, they were observed under a fluorescence microscope (39).
The measurement of mitochondrial ROS was performed by confocal fluorescence microscopy. Cells were incubated with MitoSOX reagent (Thermo Fisher, USA) for 10 min at 37°C, protected from light, and then cells were observed with a confocal fluorescence microscope after three washes with PBS.
The ROS inducer (BestBio, China) was used to increase the intracellular ROS level. According to the instructions, the prepared ROS inducer working solution was added to the cell culture medium at a ratio of 1:1,000 for 20 to 30 min, and subsequent infection experiments were carried out then (40).
Cell viability measurement.
The measurement of cell viability was performed by cell counting kit 8 (CCK-8). Cells incubated in 6-well plates were infected with BCG as indicated, and cell viability was assessed by CCK-8 assay (UE Inc., China) at 24 h and 48 h postinfection by following the manufacturer’s instructions. Optical density was recorded at 450 nm (OD450).
Data analysis.
GraphPad Prism software (GraphPad Software, La Jolla, CA) was used for the statistical analysis. Data are presented as means ± the standard errors of the means (SEM), and the statistical significance of differences was evaluated with the Student's t test. P values of <0.05 were considered statistically significant (indicated by asterisks). For multiple comparisons, analysis of variance (ANOVA) tests were performed. The Tukey test was used as a follow-up test to the ANOVAs.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key R&D Program of China (2017YFD0500303) and the outstanding youth project of Natural Science Foundation in Hubei Province (2019CFA095).
No potential conflict of interest was reported by the authors.
Footnotes
Supplemental material is available online only.
Contributor Information
Chen Tan, Email: tanchen@mail.hzau.edu.cn.
Sabine Ehrt, Weill Cornell Medical College.
REFERENCES
- 1.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. 2011. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 184:957–963. 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen H, Gu J, Xiao H, Liang S, Yang E, Yang R, Huang D, Chen C, Wang F, Shen L, Chen ZW. 2017. Selective destruction of interleukin 23-induced expansion of a major antigen-specific γδ T-cell subset in patients with tuberculosis. J Infect Dis 215:420–430. 10.1093/infdis/jiw511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song Q, Li H, Shao H, Li C, Lu X. 2015. MicroRNA-365 in macrophages regulates Mycobacterium tuberculosis-induced active pulmonary tuberculosis via interleukin-6. Int J Clin Exp Med 8:15458–15465. [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, Wang R, Dong W, Hu L, Zong B, Zhang Y, Wang X, Guo A, Zhang A, Xiang Y, Chen H, Tan C. 2017. Comparative proteomics analysis of human macrophages infected with virulent Mycobacterium bovis. Front Cell Infect Microbiol 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelko IN, Mariani TJ, Folz RJ. 2002. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33:337–349. 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- 6.Rossi C, Zini R, Rontauroli S, Ruberti S, Prudente Z, Barbieri G, Bianchi E, Salati S, Genovese E, Bartalucci N, Guglielmelli P, Tagliafico E, Rosti V, Barosi G, Vannucchi AM, Manfredini R, AGIMM (AIRC-Gruppo Italiano Malattie Mieloproliferative) Investigators. 2018. Role of TGF-β1/miR-382-5p/SOD2 axis in the induction of oxidative stress in CD34+ cells from primary myelofibrosis. Mol Oncol 12:2102–2123. 10.1002/1878-0261.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knaus UG, Hertzberger R, Pircalabioru GG, Yousefi SP, Branco Dos Santos F. 2017. Pathogen control at the intestinal mucosa–H(2)O(2) to the rescue. Gut Microbes 8:67–74. 10.1080/19490976.2017.1279378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilchèze C, Hartman T, Weinrick B, Jacobs WR, Jr.. 2013. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun 4:1881. 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deffert C, Cachat J, Krause KH. 2014. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell Microbiol 16:1168–1178. 10.1111/cmi.12322. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Jin Y, Zeng N, Ruan Q, Qian F. 2017. SOD2 facilitates the antiviral innate immune response by scavenging reactive oxygen species. Viral Immunol 30:582–589. 10.1089/vim.2017.0043. [DOI] [PubMed] [Google Scholar]
- 11.Yabaji SM, Dhamija E, Mishra AK, Srivastava KK. 2020. ESAT-6 regulates autophagous response through SOD-2 and as a result induces intracellular survival of Mycobacterium bovis BCG. Biochim Biophys Acta Proteins Proteom 1868:140470. 10.1016/j.bbapap.2020.140470. [DOI] [PubMed] [Google Scholar]
- 12.Peterman EM, Sullivan C, Goody MF, Rodriguez-Nunez I, Yoder JA, Kim CH. 2015. Neutralization of mitochondrial superoxide by superoxide dismutase 2 promotes bacterial clearance and regulates phagocyte numbers in zebrafish. Infect Immun 83:430–440. 10.1128/IAI.02245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abuaita BH, Schultz TL, O'Riordan MX. 2018. Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control phagosome-localized Staphylococcus aureus. Cell Host Microbe 24:625–636. 10.1016/j.chom.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii K, Kurita-Taniguchi M, Aoki M, Kimura T, Kashiwazaki Y, Matsumoto M, Seya T. 2005. Gene-inducing program of human dendritic cells in response to BCG cell-wall skeleton (CWS), which reflects adjuvancy required for tumor immunotherapy. Immunol Lett 98:280–290. 10.1016/j.imlet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Ezraty B, Gennaris A, Barras F, Collet JF. 2017. Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15:385–396. 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 16.Racanelli AC, Kikkers SA, Choi AMK, Cloonan SM. 2018. Autophagy and inflammation in chronic respiratory disease. Autophagy 14:221–232. 10.1080/15548627.2017.1389823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wible DJ, Bratton SB. 2018. Reciprocity in ROS and autophagic signaling. Curr Opin Toxicol 7:28–36. 10.1016/j.cotox.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paik S, Kim J, Chung C, Jo EJV. 2019. Autophagy: a new strategy for host-directed therapy of tuberculosis. Virulence 10:448–459. 10.1080/21505594.2018.1536598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drane P, Bravard A, Bouvard V, May E. 2001. Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene 20:430–439. 10.1038/sj.onc.1204101. [DOI] [PubMed] [Google Scholar]
- 20.Schatzman SS, Culotta VC. 2018. Chemical warfare at the microorganismal level: a closer look at the superoxide dismutase enzymes of pathogens. ACS Infect Dis 4:893–903. 10.1021/acsinfecdis.8b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao D, Fan Q, Bao L. 2013. The role of superoxide dismutase in the survival of Mycobacterium tuberculosis in macrophages. Jpn J Infect Dis 66:480–488. 10.7883/yoken.66.480. [DOI] [PubMed] [Google Scholar]
- 22.Su R, Peng YP, Deng Z, Deng YT, Ye JQ, Guo Y, Huang ZK, Luo Q, Jiang H, Li JM. 2019. Mycobacterium tuberculosis infection induces low-density granulocyte generation by promoting neutrophil extracellular trap formation via ROS pathway. Front Microbiol 10:1468. 10.3389/fmicb.2019.01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM. 2011. miR-335 and miR-34a promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol 22:1252–1261. 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji G, Lv K, Chen H, Wang T, Wang Y, Zhao D, Qu L, Li Y. 2013. miR-146a regulates SOD2 expression in H2O2 stimulated PC12 cells. PLoS One 8:e69351. 10.1371/journal.pone.0069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Qing W, Sun M, Lv L, Guo D, Jiang Y. 2015. Melatonin protects hepatocytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway. Free Radic Res 49:1275–1284. 10.3109/10715762.2015.1067806. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Yao J, Li Z, Zu G, Feng D, Shan W, Li Y, Hu Y, Zhao Y, Tian X. 2016. miR-34a-5p inhibition alleviates intestinal ischemia/reperfusion-induced reactive oxygen species accumulation and apoptosis via activation of SIRT1 signaling. Antioxid Redox Signal 24:961–973. 10.1089/ars.2015.6492. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Qiao Y, Song T, Wang H. 2019. miR-495 suppresses cell proliferation by directly targeting HMGA2 in lung cancer. Mol Med Rep 19:1463–1470. 10.3892/mmr.2018.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan A, Luo R, Ruan P. 2019. miR-495 promotes apoptosis and inhibits proliferation in endometrial cells via targeting PIK3R1. Pathol Res Pract 215:594–599. 10.1016/j.prp.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Tang KC, Yang ZP, Zeng Q, Wang J, Guo F, Zhao Y. 2018. Effect of miR-495 on lower extremity deep vein thrombosis through the TLR4 signaling pathway by regulation of IL1R1. Biosci Rep 38:BSR20180598. 10.1042/BSR20180598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Feng W, Dong Y, Mao X, Guo F, Luo F. 2018. MicroRNA-495 regulates human gastric cancer cell apoptosis and migration through Akt and mTOR signaling. Oncol Rep 40:3654–3662. 10.3892/or.2018.6722. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi A, Khansarinejad B, Hosseinkhani S, Ghanei M, Mowla SJ. 2017. miR-199a-5p and miR-495 target GRP78 within UPR pathway of lung cancer. Gene 620:15–22. 10.1016/j.gene.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Fu X, Zeng L, Liu Z, Ke X, Lei L, Li G. 2016. MicroRNA-206 regulates the secretion of inflammatory cytokines and MMP9 expression by targeting TIMP3 in Mycobacterium tuberculosis-infected THP-1 human macrophages. Biochem Biophys Res Commun 477:167–173. 10.1016/j.bbrc.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Ghorpade DS, Holla S, Kaveri SV, Bayry J, Patil SA, Balaji KN. 2013. Sonic hedgehog-dependent induction of microRNA 31 and microRNA 150 regulates Mycobacterium bovis BCG-driven toll-like receptor 2 signaling. Mol Cell Biol 33:543–556. 10.1128/MCB.01108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Huang S, Yu T, Liang G, Liu H, Pu D, Peng N. 2019. MiR-140 modulates the inflammatory responses of Mycobacterium tuberculosis-infected macrophages by targeting TRAF6. J Cell Mol Med 23:5642–5653. 10.1111/jcmm.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal P, Pandey P, Sarkar J, Krishnan MY. 2016. Mycobacterium tuberculosis can gain access to adipose depots of mice infected via the intra-nasal route and to lungs of mice with an infected subcutaneous fat implant. Microb Pathog 93:32–37. 10.1016/j.micpath.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Yadav B, Malonia SK, Majumdar SS, Gupta P, Wadhwa N, Badhwar A, Gupta UD, Katoch VM, Chattopadhyay S. 2015. Constitutive expression of SMAR1 confers susceptibility to Mycobacterium tuberculosis infection in a transgenic mouse model. Indian J Med Res 142:732–741. 10.4103/0971-5916.174566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Yao L, Zhang M, Jiang J, Yang M, Wang Y. 2019. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY) 11:7830–7846. 10.18632/aging.102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan Y, Lin D, Feng L, Huang M, Yan H, Li Y, Chen Y, Lin B, Ma Y, Ye Z, Mei Y, Yu X, Zhou K, Zhang Q, Chen T, Zeng J. 2018. Upregulation of miR-196b-5p attenuates BCG uptake via targeting SOCS3 and activating STAT3 in macrophages from patients with long-term cigarette smoking-related active pulmonary tuberculosis. J Transl Med 16:284. 10.1186/s12967-018-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarmiento-Salinas FL, Delgado-Magallón A, Montes-Alvarado JB, Ramírez-Ramírez D, Flores-Alonso JC, Cortés-Hernández P, Reyes-Leyva J, Herrera-Camacho I, Anaya-Ruiz M, Pelayo R, Millán-Pérez-Peña L, Maycotte P. 2019. Breast cancer subtypes present a differential production of reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Front Oncol 9:480. 10.3389/fonc.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu C, Cai Y, Zhu J, Zhang L, Xing A, Pan L, Jia H, Mo S, Feng CG, Shen H, Chen X, Zhang Z. 2019. Histone deacetylase inhibitors impair the host immune response against Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 118:101861. 10.1016/j.tube.2019.101861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download iai.00315-21-s0001.pdf, PDF file, 0.3 MB (338.9KB, pdf)