ABSTRACT
Helicobacter pylori VacA is a secreted toxin that assembles into water-soluble oligomeric structures and forms anion-selective membrane channels. Acidification of purified VacA enhances its activity in cell culture assays. Sites of protomer-protomer contact within VacA oligomers have been identified by cryoelectron microscopy, and in the current study, we validated several of these interactions by chemical cross-linking and mass spectrometry. We then mutated amino acids at these contact sites and analyzed the effects of the alterations on VacA oligomerization and activity. VacA proteins with amino acid charge reversals at interprotomer contact sites retained the capacity to assemble into water-soluble oligomers and retained cell-vacuolating activity. Introduction of paired cysteine substitutions at these sites resulted in formation of disulfide bonds between adjacent protomers. Negative-stain electron microscopy and single-particle two-dimensional class analysis revealed that wild-type VacA oligomers disassemble when exposed to acidic pH, whereas the mutant proteins with paired cysteine substitutions retain an oligomeric state at acidic pH. Acid-activated wild-type VacA caused vacuolation of cultured cells, whereas acid-activated mutant proteins with paired cysteine substitutions lacked cell-vacuolating activity. Treatment of these mutant proteins with both low pH and a reducing agent resulted in VacA binding to cells, VacA internalization, and cell vacuolation. Internalization of a nonoligomerizing mutant form of VacA by host cells was detected without a requirement for acid activation. Collectively, these results enhance our understanding of the molecular interactions required for VacA oligomerization and support a model in which toxin activity depends on interactions of monomeric VacA with host cells.
KEYWORDS: bacterial toxins, pore-forming proteins, oligomerization, membrane channels, gastric cancer, bacterial protein toxin, membrane channel proteins, pore-forming toxins
INTRODUCTION
Helicobacter pylori is a Gram-negative bacterium that colonizes the stomach in about 50% of the human population (1). Persistent H. pylori colonization of the stomach results in gastric inflammation (termed chronic superficial gastritis) and increases the risk for development of gastric adenocarcinoma, peptic ulcer disease, and gastric lymphoma (2, 3). Gastric cancer is currently the third most common cause of cancer-related deaths worldwide (4).
Several strain-specific H. pylori genes or allelic variants have been associated with an increased risk of severe gastric disease (5, 6). For example, colonization of the stomach with H. pylori strains harboring the cag pathogenicity island is associated with an increased risk of peptic ulcer disease or gastric adenocarcinoma compared to the risk in individuals colonized with strains lacking the cag pathogenicity island (7–9). Similarly, H. pylori strains harboring type s1 vacA alleles (encoding forms of VacA that cause cellular alterations in vitro) are associated with increased risk of these diseases compared to strains harboring s2 vacA alleles (which lack activity in most in vitro cell-based assays) (8, 10–12).
VacA was initially identified based on its ability to induce the development of large membrane-bound vacuoles in the cytoplasm of cultured mammalian cells (13). Subsequent studies revealed many additional effects of VacA, including mitochondrial alterations in gastric epithelial cells, apoptotic or necrotic cell death in response to high doses of VacA, and a capacity to cause alterations in many types of immune cells (12, 14–17). VacA binds receptors on the surface of host cells and is subsequently internalized by the cells through a clathrin-independent pathway (18, 19). Most VacA-induced cellular alterations are dependent on the capacity of VacA to form anion-selective channels in cell membranes (20–24) and are attributed to intracellular VacA actions (25–27). In animal models, VacA enhances the capacity of H. pylori to colonize the stomach (28) and exhibits several types of immunomodulatory activity (29–33).
The vacA gene encodes a 140-kDa protein, which undergoes proteolytic processing to yield an 88-kDa protein secreted through a type V (autotransporter) pathway (34–36). The 88-kDa protein consists of two domains (p33 and p55) (36) and contains an N-terminal hydrophobic region (23, 24). Both the p33 domain and the N-terminal portion of the p55 domain are required for intracellular activity of the toxin (37, 38).
The secreted 88-kDa VacA protein has a predominantly β-helical structure (39–42). VacA monomers assemble in solution to form several types of water-soluble oligomeric complexes. Single-layered complexes typically contain 6 or 7 protomers, and double-layered complexes contain 12 or 14 protomers (organized as hexamers or heptamers) (41–46). Mutant forms of VacA (VacA Δ49-57 or Δ346-347) that are unable to form oligomeric structures lack detectable vacuolating activity when added to mammalian cells (40, 47, 48), supporting the view that oligomerization is necessary for VacA activity. Cryoelectron microscopy (cryo-EM) has recently been used to identify intermolecular interactions predicted to be important for VacA oligomerization (41, 42).
When added to mammalian cells, water-soluble VacA oligomers exhibit little or no activity. Acidifying or alkalinizing VacA oligomers (i.e., acid or alkaline activation) markedly increases the activity of the toxin in cell culture-based assays (49–51). Acid activation results in multiple detectable alterations in the physical properties of VacA, including changes in circular dichroism (CD) and fluorescence spectra and changes in limited proteolysis fragmentation patterns (49). Acid or alkaline activation also results in disassembly of VacA oligomers into component 88-kDa monomers (43, 51, 52). VacA monomers reassemble into oligomers in solution at neutral pH or when in contact with membranes (21, 43, 53). Membrane-associated VacA hexamers or heptamers resemble single layers of the water-soluble dodecamers or tetradecamers, except for some structural variation within the central region of the oligomers (21, 44, 53).
Despite recent progress in defining the three-dimensional (3D) structure of soluble VacA oligomers (41, 42), there are important gaps in our understanding of VacA structure-function relationships. For example, there has been relatively little experimental study of the molecular interactions required for VacA oligomerization. Similarly, few studies have compared in detail the capacity of VacA oligomers and VacA monomers to bind membranes, form membrane channels, enter mammalian cells, and cause cellular alterations. Moreover, the mechanisms by which low pH enhances VacA activity are not yet completely understood. Therefore, the goals of the current study were to mutate putative sites of protomer-protomer contact in VacA oligomers and analyze the effects of these alterations on VacA structure and activity.
RESULTS
Protein-protein interactions contributing to VacA oligomerization.
Recent cryo-EM analyses of water-soluble VacA oligomers identified putative sites of intermolecular interaction between adjacent protomers (41). These include two salt bridges (K47/E338 and K55/D346), three side-chain hydrogen bonds (R50/T342, K75/Q343, and K55/D346), and numerous main-chain hydrogen bonds (41). As a complementary approach for defining VacA intermolecular interactions, we subjected purified VacA oligomers to chemical cross-linking with 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDC) or bis(sulfosuccinimidyl)suberate (BS3). EDC generates an amide bond with no spacer between primary amines (e.g., lysines) and carboxylic acid functional groups (e.g., aspartic or glutamic acid), and BS3 cross-links amines to amines with an 11.4-Å spacer. The chemically treated proteins were separated by SDS-PAGE (see Fig. S1 in the supplemental material). Protein bands of various gel mobilities (monomers and oligomers of various sizes) were cut from the gel, digested with trypsin, and analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS) to identify cross-linked amino acids. Cross-linked amino acids identified only in analysis of oligomeric VacA but not in bands corresponding to 88-kDa VacA monomers were likely to represent interprotomer (intermolecular) interactions, whereas cross-linked amino acids detected in both monomer and oligomer bands were likely to represent intramonomer (intramolecular) interactions (Table S1). For example, the K44/E338 cross-link, identified in 10 experimental analyses of oligomeric bands but never identified in monomer bands (Table S1), is predicted to represent an intermolecular interaction.
We next evaluated the results of the experiments described above in the context of the cryo-EM structure of a water-soluble VacA dodecamer, 6nyg (41). This approach allowed us to estimate the distance between the cross-linked amino acids within this VacA oligomeric structure (Table S1). We evaluated distances between each amino acid of interest in chain A with cognate cross-linked amino acids located within the same hexamer (chains A to F) or within the second hexamer (chains G to L). The shortest distances (or, in a few cases, similar distances) are highlighted in Table S1. In many instances, the shortest distances are predicted to be intramonomer cross-links (column A-A in Table S1). All the cross-links predicted to be interprotomer interactions were between protomers of the same hexamer (columns A-B to A-F of Table S1) instead of between protomers of adjacent hexamers. The shortest interprotomer distance (5.8 Å) was between K47 and E338, the site of a putative salt bridge between adjacent protomers (41). The next shortest distance (10.9 Å) was between K44 and E338. Tandem mass spectra supporting the assignment of cross-linked K44 and K47 to E338 are shown in Fig. S2. Other putative interprotomer cross-links included K69/K376, K55/E317, and K75/K146 (Table S1). VacA Δ49-57 and VacA Δ346-347 mutant proteins are each known to be defective in oligomerization (40, 47, 48). Therefore, the results of cryo-EM analysis, biochemical studies, and mutant protein analysis all support a model in which amino acids 44 to 75 (near the amino terminus of the VacA p33 domain) interact with amino acids 338 to 350 (near the amino-terminal end of the p55 domain).
Analysis of mutant VacA proteins with amino acid substitutions corresponding to charge reversals.
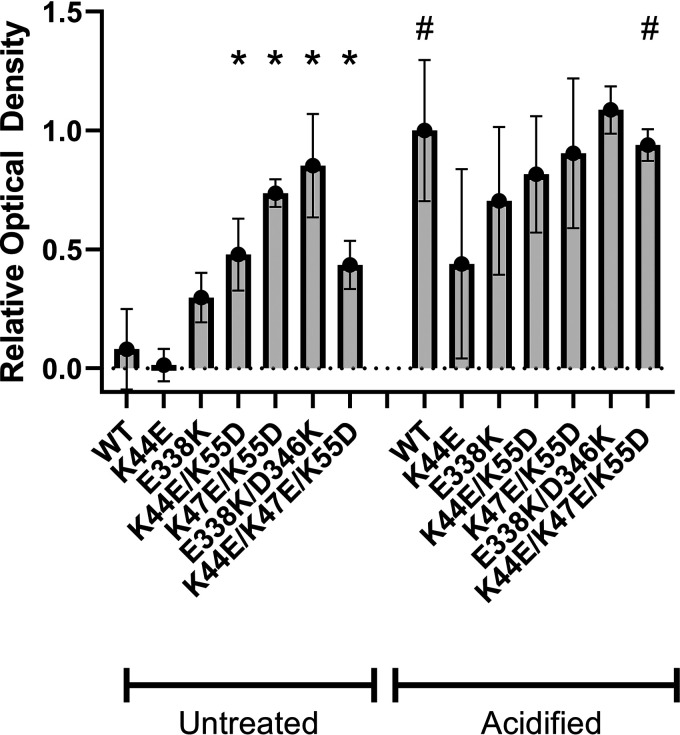
To further investigate the sites of interprotomer contact identified in the experiments described above, we generated H. pylori mutant strains engineered to produce VacA proteins harboring one or more amino acid substitutions at the sites shown in Fig. 1 (K44, K47, K55, E338, and D346). In initial experiments, we manipulated the charges at these sites by changing lysine to aspartic acid or glutamic acid or vice versa. Mutants included K44E, K44E/K55D, K47E/K55D, K44E/K47E/K55D, E338K, and E338K/D346K. We hypothesized that these mutations would disrupt VacA oligomerization and that the mutant proteins would consequently lack toxin activity. VacA proteins were purified from these H. pylori mutant strains and compared to wild-type (WT) VacA. Analysis by negative-stain EM showed that each of the mutant proteins retained the capacity to assemble into water-soluble oligomers at neutral pH (Fig. 2). Standardized concentrations of the purified VacA proteins were then tested for vacuolating activity in a cell culture assay. As expected, acid-activated wild-type VacA caused cell vacuolation, whereas nonactivated wild-type VacA had minimal activity (Fig. 3), consistent with the results of previous studies (49, 50). In contrast to wild-type VacA, the mutant proteins containing multiple substitutions (e.g., K44E/K55D, K47E/K55D, E338K/D346K, and K44E/K47E/K55D) exhibited vacuolating activity in the absence of acid activation (Fig. 3).
FIG 1.
VacA amino acids targeted for mutagenesis. Three pairs of amino acids predicted to be in close proximity in adjacent protomers within a VacA hexamer (41) were selected based on the results of chemical cross-linking experiments and/or cryo-EM analysis of oligomeric VacA. Sites marked in red correspond to the protomer highlighted in pink, while the sites marked in blue correspond to the protomers highlighted in light blue. The indicated amino acid numbers correspond to K44, K47, K55, E338, and D346. Amino acid substitutions were introduced so that the charges of individual amino acids were reversed, or, alternatively, cysteines were introduced to allow disulfide bond formation between adjacent protomers.
FIG 2.
VacA charge-reversal mutants retain an oligomeric structure. Wild-type (WT) VacA and mutant VacA proteins with the indicated amino acid substitutions (corresponding to reversals in amino acid charge) were purified and analyzed by negative-stain EM. The structure of acid-activated WT VacA was compared to that of untreated WT VacA, and the mutant proteins were maintained at neutral pH. The charge change mutants retain an oligomeric structure at neutral pH. Scale bar, 100 nm.
FIG 3.
Charge change mutants exhibit increased vacuolating activity in the absence of acid activation. Aliquots of WT VacA and the indicated mutant VacA proteins were acid activated or left untreated. Standardized concentrations of the VacA proteins (5 μg/ml) were then added to HeLa cells in the presence of 5 mM ammonium chloride for 24 h at 37°C. Neutral red uptake assays were performed to assess cell vacuolation (quantified by measuring optical density at 540 nm). WT VacA exhibited minimal vacuolating activity in the absence of acid activation, whereas most of the charge change mutants exhibited detectable activity in the absence of acid activation. Results were analyzed by analysis of variance with a Dunnett’s post hoc test. Asterisks indicate a P value of <0.005 compared to WT (untreated). #, significant differences when comparing untreated VacA proteins with the corresponding acid-activated proteins (P < 0.0001 for WT VacA and P = 0.002 for K44E/K47E/K55D).
Properties of mutant VacA proteins with paired cysteine substitutions.
We also generated H. pylori mutant strains engineered to produce VacA proteins in which paired cysteine substitutions were predicted to form interprotomer disulfide bonds within VacA oligomers (K44C/E338C, K47C/E338C, and K55C/D346C). Wild-type VacA and the mutant proteins were purified from H. pylori, and we then evaluated the mobility of each protein by SDS-PAGE in the presence or absence of reducing agent. In the presence of a reducing agent, each of the mutant proteins migrated at 88 kDa, similar to wild-type VacA (Fig. 4A). In the absence of a reducing agent, wild-type VacA migrated at 88 kDa, but monomeric VacA bands were nearly absent for the mutant proteins. Instead, a distinct ladder-like series of high-molecular-mass bands was observed (Fig. 4A and B), consistent with the formation of interprotomer disulfide bonds. Disassembly of the mutant proteins into 88-kDa monomers was dependent on the presence of a reducing agent; acidification alone was not sufficient to cause disassembly (Fig. 4C).
FIG 4.
VacA mutant proteins harboring paired cysteine substitutions form high-molecular-mass oligomers in solution. WT VacA and the indicated VacA mutant proteins (harboring paired cysteine substitutions) were purified from H. pylori culture supernatants and analyzed by SDS-PAGE and Coomassie blue staining using loading buffer containing BME (A) or loading buffer lacking BME (B). (C) Aliquots of the 47C/338C mutant protein were left untreated, treated with DTT, acid activated, or treated with both DTT and acid. These VacA preparations were diluted in PBS to a concentration of 5 μg/ml, loaded onto SDS-PAGE gels in the absence or presence of BME, and then analyzed by Western blotting. The 47C/338C mutant retains an oligomeric form under either neutral or acidic conditions in the absence of reducing agent but disassembles into monomers under reducing conditions.
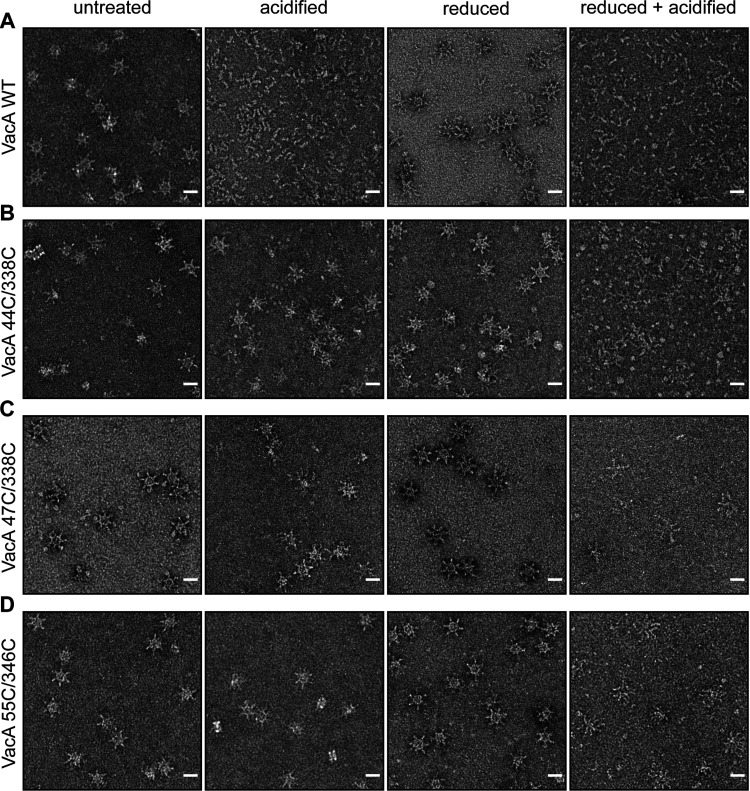
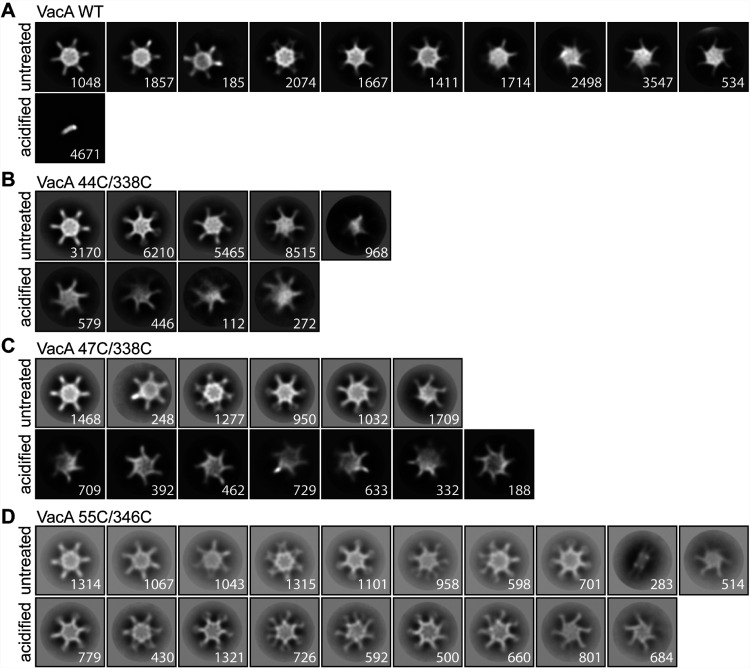
We next imaged wild-type VacA and the mutant VacA proteins by negative-stain EM (Fig. 5 and 6). At neutral pH, the wild-type and mutant VacA proteins formed the expected oligomeric complexes. As expected, acidification of wild-type VacA resulted in disassembly of the oligomeric complexes. In contrast, the K44C/E338C, K47C/E338C, and K55C/D346C mutant proteins retained an oligomeric structure at acidic pH. Analysis of the 2D class averages of the acid-treated mutant proteins did not reveal any uniform well-defined populations of oligomeric structures. Oligomeric forms of the latter proteins disassembled at acidic pH only in the presence of reducing agent.
FIG 5.
Disassembly of VacA oligomers detected by negative-stain EM. WT VacA and the indicated mutants with paired cysteine substitutions were exposed to the indicated conditions (neutral pH with or without 100 mM DTT; acidic pH with or without 100 mM DTT) and then analyzed by negative-stain EM. WT VacA disassembled into monomers at pH 3 in the absence of DTT, whereas the mutant proteins retained an oligomeric structure under these conditions. Both low pH and the reducing agent were required for disassembly of the mutant proteins. Scale bars, 200 Å.
FIG 6.
2D class averages of VacA proteins visualized by negative-stain EM. WT VacA and the indicated VacA mutant proteins containing paired cysteine substitutions were left untreated or were acidified and then were analyzed by negative-stain EM. 2D class averages were generated as described in Materials and Methods. Representative class averages of each protein are shown. WT VacA disassembled into monomers at low pH, whereas the mutants retained an oligomeric structure under these conditions. Numbers of particles are shown in the bottom right corner of each class. Box size, 468 Å.
Vacuolating toxin activity of mutant VacA proteins containing paired cysteine substitutions.
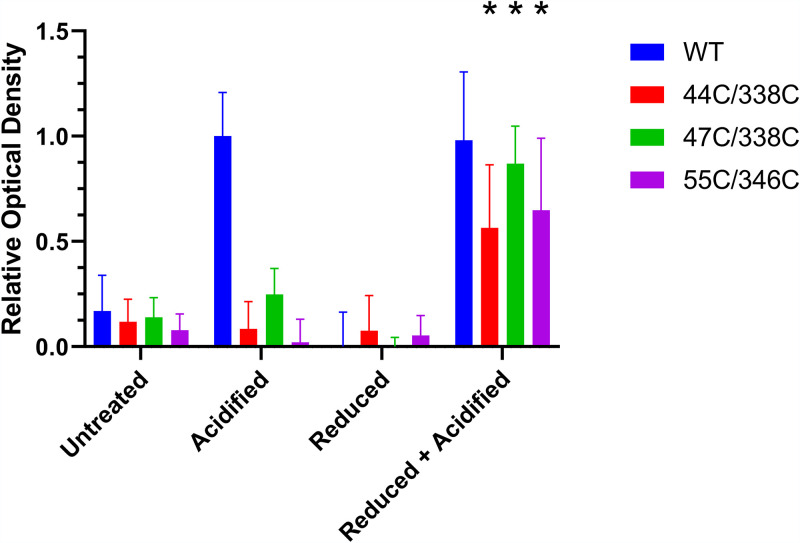
We next assessed the vacuolating toxin activity of the mutant proteins containing paired cysteine substitutions compared to wild-type VacA. Aliquots of the purified VacA protein samples were first subjected to four treatments: acidification, addition of a reducing agent, acid plus a reducing agent, or no treatment (control). The VacA preparations were then tested for cell vacuolating activity. Consistent with previous studies (49, 50), the untreated wild-type VacA protein exhibited very little cell-vacuolating activity, whereas acid-treated wild-type VacA caused cell vacuolation (Fig. 7). Unlike wild-type VacA, the mutant proteins K44C/E338C, K47C/E338C, and K55C/D346C failed to induce cell vacuolation after acid activation. However, treatment of the mutant proteins with both acid and a reducing agent resulted in cell-vacuolating activity (Fig. 7).
FIG 7.
Cell-vacuolating activity of VacA proteins containing paired cysteine substitutions. Aliquots of WT VacA and the indicated mutant proteins containing paired cysteine substitutions were left untreated, acid activated, treated with DTT, or treated with both acid and DTT. The VacA proteins (10 μg/ml final concentration) were then added to HeLa cells in the presence of 5 mM ammonium chloride and incubated for 24 h at 37°C. Neutral red uptake assays were performed to assess cell vacuolation (quantified by measuring optical density at 540 nm). WT VacA exhibited cell-vacuolating activity in response to acid activation, whereas the mutant proteins were only active if treated with both low pH and DTT. Results were analyzed by analysis of variance with a Dunnett’s post hoc test. Asterisks indicate a P value of <0.0001 compared to corresponding preparations treated with acid alone.
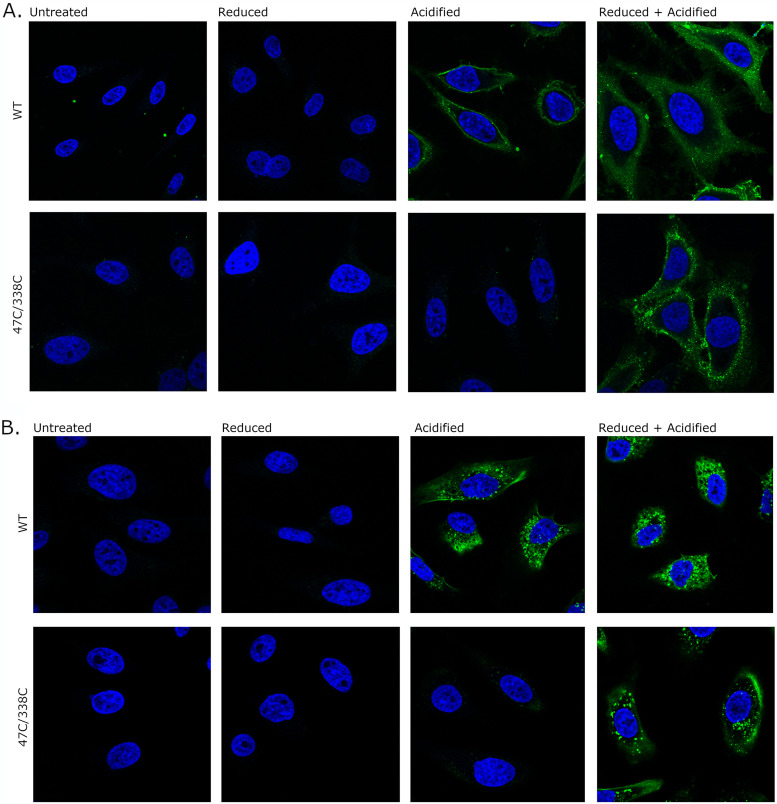
To analyze interactions of the mutant VacA proteins with human cells, we fluorescently labeled wild-type and mutant VacA proteins, added the labeled proteins to HeLa cells, and then assessed VacA localization using confocal microscopy. For studies of VacA binding to cells, the fluorescently labeled VacA proteins were subjected to the treatments described above and then were added to HeLa cells for 1 h at 4°C (Fig. 8A). The untreated wild-type and mutant VacA proteins exhibited very little detectable binding to cells. Binding of wild-type VacA to cells was detected if the protein was acid activated in the presence or absence of reducing agent. Binding of the mutant proteins to cells was detected if the proteins were treated with both acid and a reducing agent but not if the proteins were acid activated in the absence of reducing agent. To assess VacA internalization by the cells, the fluorescently labeled VacA preparations were subjected to the same treatments and incubated with HeLa cells at 37°C as described in Materials and Methods to allow the uptake of bound VacA. In concordance with the binding results, untreated VacA proteins exhibited very little detectable intracellular accumulation within cells. Wild-type VacA was internalized by the cells when treated with acid in the presence or absence of reducing agent. In contrast, internalization of the mutant VacA proteins was not observed unless they were treated with both acid and reducing agent (Fig. 8B).
FIG 8.
Binding and internalization of VacA proteins. WT VacA and the 47C/338C mutant protein were labeled with Alexa Fluor 488 (green) and then were left untreated or were treated with DTT, acid, or acid and DTT. The fluorescently labeled VacA proteins (5 μg/ml final concentration) were incubated with HeLa cells at either 4°C or 37°C, and nuclei were stained with Hoechst 33342 (blue). All images were taken on a Zeiss LSM 880 confocal microscope. (A) Fluorescently labeled VacA proteins were incubated with HeLa cells for 1 h at 4°C. (B) VacA proteins were added to HeLa cells for 5 min at 37°C. The medium containing VacA was replaced with fresh medium supplemented with 5 mM NH4Cl. The cells were then incubated at 37°C for 4 h. Acid-activated WT VacA bound to cells and was internalized, whereas acid-activated mutants did not. Internalization and binding of the mutant VacA proteins required treatment of the proteins with both acid and a reducing agent.
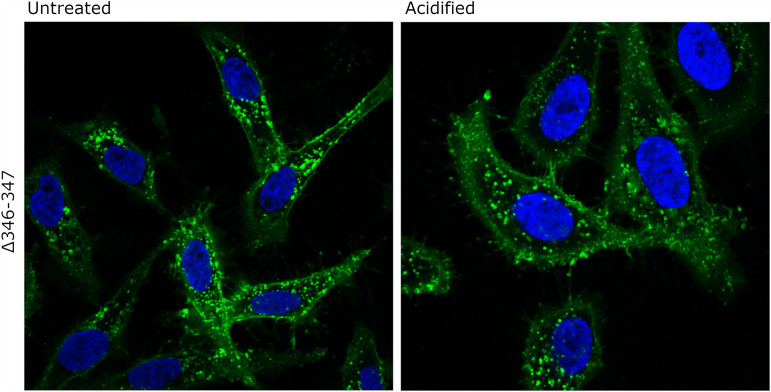
The experiments described above suggested that monomeric forms of VacA (resulting from disassembly of oligomers) can bind to cells and subsequently undergo internalization, whereas water-soluble oligomeric forms of VacA exhibited relatively little detectable binding to cells or intracellular accumulation (Fig. 7 and 8). Similarly, monomeric forms of VacA caused cell vacuolation, whereas the water-soluble oligomeric forms of VacA exhibited relatively little vacuolating activity (Fig. 4). To further evaluate the interactions of monomeric forms of VacA with host cells, we analyzed binding and internalization of the nonoligomerizing Δ346-347 mutant VacA protein, which does not cause cell vacuolation (40). The fluorescently labeled mutant protein was added to HeLa cells, the cells were incubated at 37°C, and the cells were then visualized by confocal microscopy. The Δ346-347 mutant protein interacted with cells and was internalized regardless of whether it was acid activated or not (Fig. 9). This result provides further evidence that monomeric forms of VacA can bind and be internalized by host cells and indicates that monomeric forms of VacA can enter host cells without a requirement for acid activation.
FIG 9.
Internalization of the nonoligomerizing Δ346-347 VacA mutant. The Δ346-347 mutant VacA protein was labeled with Alexa Fluor 488 (green) and was either acidified or left untreated. The labeled protein samples (5 μg/ml final concentration) were then added to HeLa cells for 5 min at 37°C. The medium containing VacA was replaced with fresh medium supplemented with 5 mM NH4Cl. The cells were then incubated at 37°C for 4 h and subsequently stained to visualize cell nuclei (blue). All images were taken on a Zeiss LSM 880 confocal microscope. Both acid-activated and untreated Δ346-347 mutant VacA proteins were internalized by the cells.
DISCUSSION
Recent cryo-EM studies analyzed the structure of water-soluble VacA oligomers and detected multiple interactions between protomers (41, 42). In the current study, we used cross-linking mass spectrometry to validate several interprotomeric interactions detected in the cryo-EM structural analyses, and we analyzed the functional significance of these interactions.
As an initial approach, we generated H. pylori strains that produced VacA proteins with amino acid substitutions corresponding to charge reversals at sites of interprotomeric contact. We hypothesized that such substitutions would inhibit VacA oligomerization and might result in loss of toxin activity. Contrary to our expectations, we found that these mutant proteins retained the capacity to assemble into water-soluble oligomeric structures and retained toxin activity in cell culture assays. In contrast to the purified wild-type VacA protein, which exhibits a marked increase in vacuolating activity following acid activation, most of the mutant proteins with charge reversals exhibited vacuolating toxin activity in the absence of acid activation. One possible explanation is that the charge reversals promote disassembly of water-soluble VacA oligomers into monomers at neutral pH and thereby shift the equilibrium of oligomers and monomers in solution. We did not directly observe an increased proportion of monomers when samples of these mutant VacA proteins were analyzed by negative-stain EM, but we speculate that increased disassembly of these mutant proteins occurs in the cell culture environment. Alternatively, the charge reversal mutations might promote enhanced interactions of the mutant proteins with relevant cell surface receptors.
We also generated H. pylori strains producing VacA proteins with cysteine substitutions at sites of interprotomeric contact. We hypothesized that the substituted cysteine residues would form disulfide bonds and thereby lock VacA in an oligomeric state. Consistent with expectations, these mutant proteins migrated as high-molecular-mass bands when analyzed by SDS-PAGE in the absence of reducing agents. Similarly, EM analysis showed that these mutant proteins retained an oligomeric state when exposed to acidic pH, unlike wild-type VacA, which disassembled into monomers when exposed to acidic pH. VacA assembles into an assortment of double-layer and single-layer water-soluble structures (41–46). The two layers of bilayered oligomers are predicted to interact through hydrogen bonds (41). Therefore, we anticipated that the acid treatment of the mutant oligomers would yield a population of predominantly single-layered structures. Contrary to expectations, analysis of 2D class averages suggested that the acid-induced disassembly of the mutant bilayered oligomers was incomplete. We speculate that the disulfide bonds in the mutant proteins restrict the mobility of protomers within single layers and that this diminishes the separation of bilayered oligomers into single-layered structures.
Acid treatment of wild-type VacA results in an assortment of biochemical changes in the protein, including changes in circular dichroism (CD) and fluorescence spectra, changes in limited proteolysis fragmentation pattern, and disassembly of oligomers into monomers (43, 49, 50, 52). These changes are associated with an increase in toxin activity in cell culture assays (49, 50). Thus far, it has not been possible to dissect which acid-induced changes result in increased toxin activity. In the current study, our analysis of mutant proteins with cysteine substitutions (disulfide bonds) showed that acid activation of VacA oligomers did not enhance toxin activity if the oligomers remain intact. Consistent with this result, we observed that the acid-treated mutant VacA oligomers exhibited minimal binding to cells and were not observed in intracellular sites within cells. Disassembly of the mutant VacA oligomers (resulting from combined treatment with low pH and a reducing agent) was required for VacA binding to cells, intracellular accumulation of the toxin, and toxin activity. These results suggest that acid-induced increases in toxin activity do not result solely from conformational changes in surface-exposed regions of VacA oligomers and suggest that acid-induced disassembly of oligomers is important. Consistent with this view, we observed that a nonoligomerizing mutant form of VacA was internalized by host cells, and this internalization did not require acid treatment of the protein. Previous studies also showed that the nonoligomerized VacA mutant protein was capable of binding liposomes and giant plasma membrane vesicles (53, 54).
The current study shows that VacA locked into an oligomeric state lacks cytotoxic activity. Previous studies have shown that nonoligomerizing mutant forms of VacA also lack cytotoxic activity (40, 47, 48). Collectively, these observations suggest that VacA cytotoxic activity is dependent on the ability of the protein to undergo reversible oligomerization and disassembly.
Multiple models have been proposed to explain the interactions of VacA with host cells. One model proposes that VacA hexamers can interact with cells as a prepore and subsequently insert into the membrane (41). Other models propose that VacA monomers bind to cells and subsequently insert into the plasma membrane and oligomerize or oligomerize on the cell surface and then insert into the membrane (53). The current results support the view that interactions of VacA monomers with host cells are important for toxin binding, intracellular accumulation of the toxin, and toxin activity. However, there are limitations to what can be concluded from the current experiments regarding the relationship between VacA oligomeric state and toxin activity. For example, the lack of activity observed with mutant toxins treated with acid alone (without reducing agent) might not be solely attributable to the blockade in VacA oligomer disassembly and could also result from restricted flexibility of the proteins due to the introduced disulfide bonds. Specifically, we are unable to exclude the possibility that the disulfide bonds interfere with flexibility and mobility of a VacA segment near the N terminus (amino acids 1 to 44) upon contact with cells. This segment includes a hydrophobic region (residues 6 to 27) required for channel formation and toxin activity and an α-helical segment (residues 30 to 37) buried in a protomer-protomer interface (23, 24, 41, 55). In addition, acid treatment of the disulfide mutant VacA proteins failed to completely disassemble bilayered oligomers into single-layered structures. Therefore, we were not able to evaluate functional properties of a uniform population of single-layered VacA oligomers, which might exhibit cell-binding properties different from those of bilayered oligomers. In future studies, it will be important to develop an improved understanding of the sequence of events (binding, oligomerization, membrane insertion, channel formation, and intracellular trafficking) required for VacA toxin activity. It will also be important to further understand how the low pH of intracellular compartments influences the oligomeric state of VacA, VacA membrane interactions, and VacA activity.
The studies reported here provide insight into how pH influences the oligomeric state and activity of VacA in vitro. It will also be important to develop an improved understanding of VacA oligomerization and activity in vivo. Within the normal gastric mucus layer, there is a pH gradient (acidic at the luminal surface and closer to neutral pH at the epithelial surface) (56). Therefore, we speculate that VacA adopts a range of different oligomeric states in vivo. Previous studies have suggested that VacA can inhibit acid production by parietal cells and alter gastric mucus production, thereby altering the normal gastric physiology (57, 58). In addition, a loss of gastric acidity in the setting of atrophic gastritis (which can develop after longstanding H. pylori infection) might alter the VacA oligomeric state in vivo compared to its oligomeric state in the normal acidic stomach. Consequently, we speculate that the effects of VacA on gastric tissue might vary during different stages of H. pylori infection.
MATERIALS AND METHODS
H. pylori culture methods.
H. pylori strains (Table 1) were cultured on Trypticase soy agar plates containing 5% sheep blood at 37°C in room air supplemented with 5% CO2. Liquid cultures were grown in Brucella broth (without sodium bisulfate) supplemented with cholesterol lipid concentrate (Gibco).
TABLE 1.
H. pylori strains analyzed in this study
| Strain description | Reference or source |
|---|---|
| 60190 ΔrdxA VacA-Str808 | 40 |
| 60190 ΔrdxA VacA-Str808 K44E | This study |
| 60190 ΔrdxA VacA-Str808 E338K | This study |
| 60190 ΔrdxA VacA-Str808 K44E/K55D | This study |
| 60190 ΔrdxA VacA-Str808 K47E/K55D | This study |
| 60190 ΔrdxA VacA-Str808 E338K/D346K | This study |
| 60190 ΔrdxA VacA-Str808 K44E/K47E/K55D | This study |
| 60190 ΔrdxA VacA-Str808 44C/338C | This study |
| 60190 ΔrdxA VacA-Str808 47C/338C | This study |
| 60190 ΔrdxA VacA-Str808 55C/346C | This study |
| 60190 ΔrdxA VacA-Str312 Δ346-347 | 40 |
Construction of H. pylori mutant strains.
The amino acid sequence of VacA from strain 60190 (ATCC 49503) is available at accession number Q48245 (34). The VacA numbering system used in the current work designates the amino-terminal alanine of the secreted toxin amino acid 1. H. pylori strain 60190 containing a ΔrdxA mutation and a modified vacA gene (encoding VacA with a strep tag at amino acid 808) (40) was used as the parent strain for generation of mutant strains (Table 1). Mutations were introduced into the chromosomal vacA gene using a negative selection approach (based on use of a cat-rdxA cassette), as described previously (40). VacA Δ346-347 (with a strep tag at amino acid 312) has been described previously (40).
VacA purification.
H. pylori strains were grown in Brucella broth supplemented with cholesterol at 37°C for 2 to 3 days. Bacteria were removed by centrifugation, and proteins in the supernatant were precipitated by addition of a 50% saturated solution of ammonium sulfate and centrifugation. Resuspended proteins were then centrifuged at 4,500 × g for 10 min to remove insoluble components. The supernatant containing strep-tagged VacA was applied to streptactin beads (IBA) in a gravity column. After washing the beads, VacA was eluted with a buffer containing 50 mM Tris, 150 mM NaCl, and 5 mM d-desthiobiotin (pH 7.5) (40). There were no substantial differences in the yields of purified WT VacA compared to yields of purified mutant forms of VacA.
VacA cross-linking and identification of cross-linked amino acids.
Purified VacA preparations (6 μg per sample) were subjected to chemical cross-linking with 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDC) (4 mM) or bis(sulfosuccinimidyl)suberate (BS3) (1 to 4 mM). Cross-linking reactions were carried out in phosphate-buffered saline (PBS) for 2 h at 0°C. Cross-linked products were separated by SDS-PAGE, and bands with a molecular mass higher than that of VacA monomers (>88 kDa) were excised and subjected to in-gel digestion with trypsin. Extracted tryptic peptides were analyzed by nano-LC-MS/MS analysis on a Q Exactive Orbitrap instrument (Thermo Scientific, San Jose, CA) in the Vanderbilt Proteomics Core facility. A data-dependent analysis method consisted of MS1 acquisition (R = 70,000), using an MS automatic gain control target value of 1e6, followed by up to 15 MS/MS scans (R = 17,500) of the most abundant ions detected in the preceding MS scan. The MS2 AGC target value was set to 2e5, with a maximum ion time of 200 ms, a 5% underfill ratio, and intensity threshold of 5e4. High-energy collisional dissociation energy was set to 27, dynamic exclusion was set to 5 s, and peptide match and isotope exclusion were enabled. Data analysis of tandem mass spectrometry data used either StavroX software specifically designed to identify cross-linked peptides or TagRecon in blind PTM search mode (59, 60). Cross-linked peptides identified by StavroX or TagRecon were validated by manual inspection of the tandem mass spectra. Validation criteria included the presence of sequence-specific ions for each peptide as well as the presence of an ion that included the cross-link.
SDS-PAGE and Coomassie staining.
Standardized concentrations of WT VacA and VacA mutant proteins (4 μg of each) were boiled in Laemmli sample buffer with or without β-mercaptoethanol (BME). The proteins were loaded onto a 7.5% SDS-PAGE gel and electrophoresed for 45 min at 180 V. The gels then were stained with SimplyBlue SafeStain (Invitrogen).
Western blotting.
WT and mutant VacA proteins were treated four ways: (i) untreated, (ii) addition of dithiothreitol (DTT) (final concentration, 25 mM), (iii) acidification, or (iv) both acidification and addition of DTT. VacA acidification was accomplished by addition of 200 mM HCl to the samples, resulting in a final pH of 3.0 (50). The samples were diluted to 5 μg/ml in PBS and boiled in Laemmli sample buffer with or without BME, and the protein samples (0.1 μg) were then loaded onto a 10% SDS-PAGE gel. The gel was electrophoresed for 40 min at 180 V and transferred to nitrocellulose paper for 1 h at 100 V. The paper was blocked overnight with 2% milk TBST (Tris-buffered saline [TBS] with 1% Tween 20) and probed with a 1:5,000 dilution of anti-VacA rabbit antiserum 958 (61). The blot was then probed with a 1:5,000 dilution of anti-rabbit IgG horseradish peroxidase conjugate (Promega) and developed using SuperSignal West Pico plus chemiluminescent substrate (Thermo Scientific). As described previously, the anti-VacA antiserum 958 was generated by immunizing rabbits with wild-type oligomeric VacA (61). It reacts specifically with VacA and reacts most strongly with epitopes in the p55 domain (61, 62).
Negative-stain EM methodology.
Wild-type and mutant VacA proteins (3- to 5-μl samples, standardized at 30 μg/ml protein concentration) were adsorbed for 1 min to a glow-discharged (30 s at 5 mA) 400-mesh copper grid (Structure Probe, Inc.) that was coated with collodion film followed by carbon in a carbon evaporator. The grids were washed twice and then negatively stained in 0.75% uranyl formate solution. Transmission electron microscopy (TEM) images were obtained on an FEI Morgagni electron microscope run at 100 keV at a magnification of ×22,000 (2.1 Å/pixel) (Fig. 5 and 6) or ×28,000 (Fig. 2).
For experiments analyzing VacA wild-type and mutant proteins in the presence of a reducing agent, VacA (30 μg/ml concentration) was incubated for 1 h at 37°C with a reducing agent (100 mM DTT final concentration). For analysis of DTT-treated samples at low pH, HCl was added after the 1-h incubation at 37°C. Samples were kept at room temperature and negatively stained within 30 min.
For single-particle 2D classification of VacA wild-type controls and VacA disulfide mutant samples (VacA 44C/338C, VacA 47C/338C, and VacA 55C/346C), VacA samples were negatively stained and imaged with an FEI Tecnai T12 microscope operated at 120 keV. Images were acquired with Leginon (63). Images were recorded at a pixel size of 2.342 Å/px. Contrast transfer function estimation was performed on the images using CTFFIND4 (64). Particles were picked using the crYOLO general negative-stain model and analyzed with the crYOLO box manager using SBGrid (65, 66). Particle extraction (618-Å box size for all samples except VacA wild type, pH 3, 244 Å) and 2D classification (400-Å mask size for all samples except VacA wild type, pH 3, 200 Å) were performed using RELION 3.0.8 (67). Data collection parameters are provided in Table S2 in the supplemental material.
Quantification of VacA-induced cell vacuolation.
HeLa cells were grown in minimal essential medium (MEM) supplemented with 10% fetal bovine serum at 37°C in room air supplemented with 5% CO2. The cells were seeded in 96-well flat-bottom tissue culture-treated plates at a concentration of 2.5 × 105 cells/well and were grown for 24 h prior to addition of VacA preparations, which were standardized based on protein concentration. For analysis of charge change mutants, WT and mutant VacA proteins were either acid activated by addition of HCl to reach a final pH of 3 or left untreated. For analysis of VacA proteins containing paired cysteine mutations, WT and mutant VacA proteins were either (i) untreated; (ii) incubated for 1 h at 37°C in DTT (final concentration, 12.5 mM); (iii) acidified with HCl to reach a final pH of 3; or (iv) incubated with DTT and then acidified. The VacA preparations were then serially diluted and added to cells in medium supplemented with 5 mM NH4Cl. All samples were tested in triplicate for each condition. The next day, neutral red dye was added to cells for 30 min. The cells were then washed three times with 0.9% saline. Acid alcohol (97% ethanol, 3% HCl) was added to extract the dye, and the optical density at 540 nm was measured by a microplate reader (68).
Confocal microscopy.
HeLa cells were plated on glass coverslips in 24-well flat-bottom tissue culture-treated plates at a concentration of 7 × 104 cells per well and incubated overnight until confluent. Mutant and wild-type VacA proteins were labeled with Alexa Fluor 488 using an Alexa Fluor 488 microscale protein labeling kit (Invitrogen) (27). The fluorescently labeled VacA contained 4 to 6 mol dye per mol protein and retained cell-vacuolating activity. The labeled WT and mutant VacA proteins were treated four ways: (i) left untreated; (ii) incubated for 1 h at 37°C in DTT (final concentration, 25 mM); (iii) acidified with HCl; or (iv) incubated with DTT and then acidified. Cells were then incubated with VacA samples (final concentration of 5 μg/ml) in MEM. To assess binding, VacA was incubated with HeLa cells for 1 h at 4°C. To assess internalization, VacA was incubated with cells for 5 min at 37°C, after which cells were washed and the medium replaced with fresh MEM containing 5 mM NH4Cl. The cells were then incubated for 4 h at 37°C. After incubation, the cells for both binding and internalization experiments were washed with PBS and fixed with 4% paraformaldehyde (PFA). Cell nuclei were stained with Hoechst 33342 (NucBlue; Invitrogen) according to the manufacturer’s instructions. The coverslips were mounted on glass slides using Prolong Gold antifade reagent (Invitrogen) and imaged by confocal microscopy (Zeiss LSM 880).
ACKNOWLEDGMENTS
The work described in this paper was supported by NIH CA116087, AI039657, AI118932, T32 AI138932, T32 AI112541, and the Department of Veterans Affairs (I01 BX004447). Microscopy experiments in the Vanderbilt Cell Imaging Shared Resource and proteomic experiments were supported by the Vanderbilt Digestive Diseases Research Center (P30 DK058404) and the Vanderbilt-Ingram Cancer Center (P30 CA068485). Microscope experiments at the University of Michigan were supported by the U-M Biosciences Initiation and NIH S10OD02001.
Footnotes
Supplemental material is available online only.
Contributor Information
Timothy L. Cover, Email: timothy.l.cover@vumc.org.
Victor J. Torres, New York University School of Medicine
REFERENCES
- 1.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. 2017. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153:420–429. 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Cover TL, Blaser MJ. 2009. Helicobacter pylori in health and disease. Gastroenterology 136:1863–1873. 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson K, Atherton JC. 2021. The spectrum of Helicobacter-mediated diseases. Annu Rev Pathol 16:123–144. 10.1146/annurev-pathol-032520-024949. [DOI] [PubMed] [Google Scholar]
- 4.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. 2015. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 136:487–490. 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 5.Cover TL. 2016. Helicobacter pylori diversity and gastric cancer risk. mBio 7:e01869-15. 10.1128/mBio.01869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones KR, Whitmire JM, Merrell DS. 2010. A tale of two toxins: Helicobacter pylori CagA and VacA modulate host pathways that impact disease. Front Microbiol 1:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 55:2111–2115. [PubMed] [Google Scholar]
- 8.Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn L-J, Carneiro F, Sobrinho-Simões M. 2002. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst 94:1680–1687. 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 9.Plummer M, van Doorn L-J, Franceschi S, Kleter B, Canzian F, Vivas J, Lopez G, Colin D, Muñoz N, Kato I. 2007. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J Natl Cancer Inst 99:1328–1334. 10.1093/jnci/djm120. [DOI] [PubMed] [Google Scholar]
- 10.Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 270:17771–17777. 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 11.Van Doorn L-J, Figueiredo C, Mégraud F, Pena S, Midolo P, De Magalhães Queiroz DM, Carneiro F, Vanderborght B, Pegado MDGF, Sanna R, De Boer W, Schneeberger PM, Correa P, Ng EKW, Atherton J, Blaser MJ, Quint WGV. 1999. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology 116:823–830. 10.1016/S0016-5085(99)70065-X. [DOI] [PubMed] [Google Scholar]
- 12.McClain MS, Beckett AC, Cover TL. 2017. Helicobacter pylori vacuolating toxin and gastric cancer. Toxins 9:316. 10.3390/toxins9100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cover TL, Blaser MJ. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem 267:10570–10575. 10.1016/S0021-9258(19)50054-0. [DOI] [PubMed] [Google Scholar]
- 14.Cover TL, Blanke SR. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol 3:320–332. 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 15.Kim IJ, Blanke SR. 2012. Remodeling the host environment: modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA). Front Cell Infect Microbiol 2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foegeding NJ, Caston RR, McClain MS, Ohi MD, Cover TL. 2016. An overview of Helicobacter pylori VacA toxin biology. Toxins 8:173. 10.3390/toxins8060173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I-J, Lee J, Oh SJ, Yoon M-S, Jang S-S, Holland RL, Reno ML, Hamad MN, Maeda T, Chung HJ, Chen J, Blanke SR. 2018. Helicobacter pylori infection modulates host cell metabolism through VacA-dependent inhibition of mTORC1. Cell Host Microbe 23:583–593. 10.1016/j.chom.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta VR, Patel HK, Kostolansky SS, Ballivian RA, Eichberg J, Blanke SR. 2008. Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog 4:e1000073. 10.1371/journal.ppat.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier NC, Monzo P, Kaddai V, Doye A, Ricci V, Boquet P. 2005. Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes. Mol Biol Cell 16:4852–4866. 10.1091/mbc.e05-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabò I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. 1999. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J 18:5517–5527. 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czajkowsky DM, Iwamoto H, Cover TL, Shao Z. 1999. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci USA 96:2001–2006. 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwamoto H, Czajkowsky DM, Cover TL, Szabo G, Shao Z. 1999. VacA from Helicobacter pylori: a hexameric chloride channel. FEBS Lett 450:101–104. 10.1016/s0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- 23.Vinion-Dubiel AD, McClain MS, Czajkowsky DM, Iwamoto H, Ye D, Cao P, Schraw W, Szabo G, Blanke SR, Shao Z, Cover TL. 1999. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J Biol Chem 274:37736–37742. 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- 24.McClain MS, Iwamoto H, Cao P, Vinion-Dubiel AD, Li Y, Szabo G, Shao Z, Cover TL. 2003. Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. J Biol Chem 278:12101–12108. 10.1074/jbc.M212595200. [DOI] [PubMed] [Google Scholar]
- 25.Ye D, Willhite DC, Blanke SR. 1999. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J Biol Chem 274:9277–9282. 10.1074/jbc.274.14.9277. [DOI] [PubMed] [Google Scholar]
- 26.Capurro MI, Greenfield LK, Prashar A, Xia S, Abdullah M, Wong H, Zhong XZ, Bertaux-Skeirik N, Chakrabarti J, Siddiqui I, O'Brien C, Dong X, Robinson L, Peek RM, Philpott DJ, Zavros Y, Helmrath M, Jones NL. 2019. VacA generates a protective intracellular reservoir for Helicobacter pylori that is eliminated by activation of the lysosomal calcium channel TRPML1. Nat Microbiol 4:1411–1423. 10.1038/s41564-019-0441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foegeding NJ, Raghunathan K, Campbell AM, Kim SW, Lau KS, Kenworthy AK, Cover TL, Ohi MD. 2019. Intracellular degradation of Helicobacter pylori VacA toxin as a determinant of gastric epithelial cell viability. Infect Immun 87:e00783-18. 10.1128/IAI.00783-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salama NR, Otto G, Tompkins L, Falkow S. 2001. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun 69:730–736. 10.1128/IAI.69.2.730-736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. 2013. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA 110:3047–3052. 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djekic A, Muller A. 2016. The immunomodulator VacA promotes immune tolerance and persistent Helicobacter pylori infection through its activities on T-cells and antigen-presenting cells. Toxins 8:187. 10.3390/toxins8060187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utsch C, Haas R. 2016. VacA's induction of VacA-containing vacuoles (VCVs) and their immunomodulatory activities on human T cells. Toxins 8:190. 10.3390/toxins8060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altobelli A, Bauer M, Velez K, Cover TL, Muller A. 2019. Helicobacter pylori VacA targets myeloid cells in the gastric lamina propria to promote peripherally induced regulatory T-cell differentiation and persistent infection. mBio 10:e00261-19. 10.1128/mBio.00261-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyburz A, Fallegger A, Zhang X, Altobelli A, Artola-Boran M, Borbet T, Urban S, Paul P, Münz C, Floess S, Huehn J, Cover TL, Blaser MJ, Taube C, Müller A. 2019. Transmaternal Helicobacter pylori exposure reduces allergic airway inflammation in offspring through regulatory T cells. J Allergy Clin Immunol 143:1496–1512. 10.1016/j.jaci.2018.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cover TL, Tummuru MKR, Cao P, Thompson SA, Blaser MJ. 1994. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem 269:10566–10573. 10.1016/S0021-9258(17)34097-8. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt W, Haas R. 1994. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol 12:307–319. 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 36.Telford JL, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce MF, Censini S, Covacci A, Xiang Z. 1994. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med 179:1653–1658. 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres VJ, Ivie SE, McClain MS, Cover TL. 2005. Functional properties of the p33 and p55 domains of the Helicobacter pylori vacuolating cytotoxin. J Biol Chem 280:21107–21114. 10.1074/jbc.M501042200. [DOI] [PubMed] [Google Scholar]
- 38.González-Rivera C, Gangwer KA, McClain MS, Eli IM, Chambers MG, Ohi MD, Lacy DB, Cover TL. 2010. Reconstitution of Helicobacter pylori VacA toxin from purified components. Biochemistry 49:5743–5752. 10.1021/bi100618g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangwer KA, Mushrush DJ, Stauff DL, Spiller B, McClain MS, Cover TL, Lacy DB. 2007. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc Natl Acad Sci USA 104:16293–16298. 10.1073/pnas.0707447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González-Rivera C, Campbell AM, Rutherford SA, Pyburn TM, Foegeding NJ, Barke TL, Spiller BW, McClain MS, Ohi MD, Lacy DB, Cover TL. 2016. A nonoligomerizing mutant form of Helicobacter pylori VacA allows structural analysis of the p33 domain. Infect Immun 84:2662–2670. 10.1128/IAI.00254-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Zhang H, Li S, Pintilie GD, Mou T-C, Gao Y, Zhang Q, van den Bedem H, Schmid MF, Au SWN, Chiu W. 2019. Cryo-EM structures of Helicobacter pylori vacuolating cytotoxin A oligomeric assemblies at near-atomic resolution. Proc Natl Acad Sci USA 116:6800–6805. 10.1073/pnas.1821959116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su M, Erwin AL, Campbell AM, Pyburn TM, Salay LE, Hanks JL, Lacy DB, Akey DL, Cover TL, Ohi MD. 2019. Cryo-EM analysis reveals structural basis of Helicobacter pylori VacA toxin oligomerization. J Mol Biol 431:1956–1965. 10.1016/j.jmb.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cover TL, Hanson PI, Heuser JE. 1997. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J Cell Biol 138:759–769. 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adrian M, Cover TL, Dubochet J, Heuser JE. 2002. Multiple oligomeric states of the Helicobacter pylori vacuolating toxin demonstrated by cryo-electron microscopy. J Mol Biol 318:121–133. 10.1016/S0022-2836(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 45.El-Bez C, Adrian M, Dubochet J, Cover TL. 2005. High resolution structural analysis of Helicobacter pylori VacA toxin oligomers by cryo-negative staining electron microscopy. J Struct Biol 151:215–228. 10.1016/j.jsb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Chambers MG, Pyburn TM, González-Rivera C, Collier SE, Eli I, Yip CK, Takizawa Y, Lacy DB, Cover TL, Ohi MD. 2013. Structural analysis of the oligomeric states of Helicobacter pylori VacA toxin. J Mol Biol 425:524–535. 10.1016/j.jmb.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genisset C, Galeotti CL, Lupetti P, Mercati D, Skibinski DAG, Barone S, Battistutta R, de Bernard M, Telford JL. 2006. A Helicobacter pylori vacuolating toxin mutant that fails to oligomerize has a dominant negative phenotype. Infect Immun 74:1786–1794. 10.1128/IAI.74.3.1786-1794.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivie SE, McClain MS, Torres VJ, Algood HMS, Lacy DB, Yang R, Blanke SR, Cover TL. 2008. Helicobacter pylori VacA subdomain required for intracellular toxin activity and assembly of functional oligomeric complexes. Infect Immun 76:2843–2851. 10.1128/IAI.01664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J, Manetti R, Fontana A, Rappuoli R, Montecucco C. 1995. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem 270:23937–23940. 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 50.McClain MS, Schraw W, Ricci V, Boquet P, Cover TL. 2000. Acid-activation of Helicobacter pylori vacuolating cytotoxin (VacA) results in toxin internalization by eukaryotic cells. Mol Microbiol 37:433–442. 10.1046/j.1365-2958.2000.02013.x. [DOI] [PubMed] [Google Scholar]
- 51.Yahiro K, Niidome T, Kimura M, Hatakeyama T, Aoyagi H, Kurazono H, Imagawa K, Wada A, Moss J, Hirayama T. 1999. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J Biol Chem 274:36693–36699. 10.1074/jbc.274.51.36693. [DOI] [PubMed] [Google Scholar]
- 52.Molinari M, Galli C, de Bernard M, Norais N, Ruysschaert JM, Rappuoli R, Montecucco C. 1998. The acid activation of Helicobacter pylori toxin VacA: structural and membrane binding studies. Biochem Biophys Res Commun 248:334–340. 10.1006/bbrc.1998.8808. [DOI] [PubMed] [Google Scholar]
- 53.Pyburn TM, Foegeding NJ, González-Rivera C, McDonald NA, Gould KL, Cover TL, Ohi MD. 2016. Structural organization of membrane-inserted hexamers formed by Helicobacter pylori VacA toxin. Mol Microbiol 102:22–36. 10.1111/mmi.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raghunathan K, Foegeding NJ, Campbell AM, Cover TL, Ohi MD, Kenworthy AK. 2018. Determinants of raft partitioning of the Helicobacter pylori pore-forming toxin VacA. Infect Immun 86:e00872-17. 10.1128/IAI.00872-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClain MS, Cao P, Iwamoto H, Vinion-Dubiel AD, Szabo G, Shao Z, Cover TL. 2001. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J Bacteriol 183:6499–6508. 10.1128/JB.183.22.6499-6508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schreiber S, Konradt M, Groll C, Scheid P, Hanauer G, Werling H-O, Josenhans C, Suerbaum S. 2004. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci USA 101:5024–5029. 10.1073/pnas.0308386101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F, Xia P, Wu F, Wang D, Wang W, Ward T, Liu Y, Aikhionbare F, Guo Z, Powell M, Liu B, Bi F, Shaw A, Zhu Z, Elmoselhi A, Fan D, Cover TL, Ding X, Yao X. 2008. Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J Biol Chem 283:26714–26725. 10.1074/jbc.M800527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holland RL, Bosi KD, Harpring GH, Luo J, Wallig M, Phillips H, Blanke SR. 2020. Chronic in vivo exposure to Helicobacter pylori VacA: assessing the efficacy of automated and long-term intragastric toxin infusion. Sci Rep 10:9307. 10.1038/s41598-020-65787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Götze M, Pettelkau J, Schaks S, Bosse K, Ihling CH, Krauth F, Fritzsche R, Kühn U, Sinz A. 2012. StavroX–a software for analyzing crosslinked products in protein interaction studies. J Am Soc Mass Spectrom 23:76–87. 10.1007/s13361-011-0261-2. [DOI] [PubMed] [Google Scholar]
- 60.Dasari S, Chambers MC, Slebos RJ, Zimmerman LJ, Ham AJ, Tabb DL. 2010. TagRecon: high-throughput mutation identification through sequence tagging. J Proteome Res 9:1716–1726. 10.1021/pr900850m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schraw W, Li Y, McClain MS, van der Goot FG, Cover TL. 2002. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J Biol Chem 277:34642–34650. 10.1074/jbc.M203466200. [DOI] [PubMed] [Google Scholar]
- 62.Ivie SE, McClain MS, Algood HM, Lacy DB, Cover TL. 2010. Analysis of a beta-helical region in the p55 domain of Helicobacter pylori vacuolating toxin. BMC Microbiol 10:60. 10.1186/1471-2180-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. 2005. Automated molecular microscopy: the new Leginon system. J Struct Biol 151:41–60. 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Rohou A, Grigorieff N. 2015. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J Struct Biol 192:216–221. 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morin A, Eisenbraun B, Key J, Sanschagrin PC, Timony MA, Ottaviano M, Sliz P. 2013. Collaboration gets the most out of software. Elife 2:e01456. 10.7554/eLife.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner T, Merino F, Stabrin M, Moriya T, Antoni C, Apelbaum A, Hagel P, Sitsel O, Raisch T, Prumbaum D, Quentin D, Roderer D, Tacke S, Siebolds B, Schubert E, Shaikh TR, Lill P, Gatsogiannis C, Raunser S. 2019. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun Biol 2:218. 10.1038/s42003-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scheres SH. 2012. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol 180:519–530. 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cover TL, Puryear W, Pérez-Pérez GI, Blaser MJ. 1991. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun 59:1264–1270. 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download iai.00348-21-s0001.pdf, PDF file, 0.4 MB (421.9KB, pdf)