ABSTRACT
The type IV secretion system of Neisseria gonorrhoeae translocates single-stranded DNA into the extracellular space, facilitating horizontal gene transfer and initiating biofilm formation. Expression of this system has been observed to be low under laboratory conditions, and multiple levels of regulation have been identified. We used a translational fusion of lacZ to traD, the gene for the type IV secretion system coupling protein, to screen for increased type IV secretion system expression. We identified several physiologically relevant conditions, including surface adherence, decreased manganese or iron, and increased zinc or copper, which increase gonococcal type IV secretion system protein levels through transcriptional and/or translational mechanisms. These metal treatments are reminiscent of the conditions in the macrophage phagosome. The ferric uptake regulator, Fur, was found to repress traD transcript levels but to also have a second role, acting to allow TraD protein levels to increase only in the absence of iron. To better understand type IV secretion system regulation during infection, we examined transcriptomic data from active urethral infection samples from five men. The data demonstrated differential expression of 20 of 21 type IV secretion system genes during infection, indicating upregulation of genes necessary for DNA secretion during host infection.
KEYWORDS: Neisseria gonorrhoeae, biofilms, copper, iron regulation, phagosomes, transcriptional regulation, transcriptome, type IV secretion, urethral infection, zinc
INTRODUCTION
Neisseria gonorrhoeae, the bacterial agent of the sexually transmitted infection gonorrhea, establishes thriving infections on human mucosal surfaces (e.g., the urogenital tract, rectum, nasopharynx, and eyes) (1). Symptomatic gonorrhea infections are characterized by robust inflammatory responses, with extensive influx of both activated neutrophils and macrophages (2, 3). N. gonorrhoeae has been shown to invade and survive in epithelial cells (4, 5) and neutrophils (6–8) and to cause programmed cell death in macrophages (3, 9). The wide variety of tissues and cell types that N. gonorrhoeae interacts with throughout the course of any given infection reflects the bacterium’s capacity to respond to diverse environments.
Many N. gonorrhoeae strains possess the 59-kb gonococcal genetic island (GGI) on their ∼2.2-Mb chromosome (10). This island has been found in 60 to 80% of isolates and encodes a unique type IV secretion system (T4SS) (10–13). T4SSs are a diverse subset of protein nanomachines that can be used for protein secretion, conjugation, DNA uptake, and, in the case of N. gonorrhoeae, DNA secretion (12). The gonococcal T4SS exports single-stranded DNA (ssDNA) directly into the extracellular space independent of contact with a host or neighboring cell (10, 11, 14). This unique method of DNA secretion has been shown to serve many purposes in N. gonorrhoeae populations. The T4SS plays a role in biofilm establishment, largely by contributing an abundance of “sticky” DNA to nucleate and build up the biofilm matrix (15). The secreted DNA has also been observed to be taken up by neighboring gonococci and stably incorporated into the chromosome, acting as a mechanism of horizontal gene transfer between gonococci (16–18). This process has been implicated in the spread of antibiotic resistance in gonococcal populations; GGI-containing strains of N. gonorrhoeae are more likely to have increased resistance to antibiotics (19). While detectable genetic exchange does occur under laboratory conditions, detecting T4SS proteins and secreted DNA substrates in the laboratory has proven challenging due to low levels of expression in this environment (10, 11, 18, 20, 21).
Only 21 of the 66 GGI genes (located on only 4 of 12 identified operons) are necessary for the T4SS to be active (Fig. 1A) (11, 22–24). Many of the gonococcal T4SS proteins, especially the structural components, are homologous to those of F-like plasmid-encoded conjugation systems (22). The remainder of the GGI is largely comprised of genes of unknown function (11, 22). While some regulatory components have been characterized within the GGI, no homologues of F-like T4SS regulators have been found, and a holistic picture of regulation is lacking in this system (20, 21, 25). The aforementioned low levels of T4SS expression have compounded the challenge of investigating regulation of the T4SS.
FIG 1.

Gonococcal genetic island (GGI) and traD::lacZ reporter construct. (A) Map of the GGI. Genes necessary for secretion are shown in green. Transcripts measured in qRT-PCR experiments are indicated with stars. Promoters (green arrows) are marked for operons necessary for secretion only. tra and trb genes, as well as ltgX, are homologous to genes found on the E. coli F-plasmid. To date, no putative regulators have been identified. Blue brackets indicate the 4.4-kb region from exp1-ydcA. (B) Gene map of traD::lacZ translational fusion reporter construct. The origin of transfer (oriT) is indicated by a stem-loop.
In this study, we found that the low expression seen in the laboratory is not representative of T4SS expression during host infection. We hypothesize that N. gonorrhoeae may invest in building the T4SS DNA pump only in response to signals specific to host environmental niches. Similar temporospatial determinants of expression have been identified in other T4SSs. For example, intracellular growth conditions signal Legionella to express a ubiquitin ligase that regulates T4SS protein levels via the host cell proteasome, and Bartonella henselae’s VirB/D4 T4SS is induced by interwoven signals from host cell pH and the bacterial stringent response (26, 27). Building on these observations, we aimed to determine how the environment of infection affects T4SS expression in gonococci.
By screening an array of compounds, we identified several conditions that alter expression of the type IV coupling protein, TraD. We followed up this screen with quantitative transcript and protein measurements and determined that iron chelation, zinc, and copper all enhance TraD expression, whereas manganese downregulates T4SS expression. We also found that surface adherence upregulates gene expression across the GGI. Our findings, along with the previous reports from our laboratory that piliation enhances type IV secretion (T4S) (25), are consistent with a model of increased GGI expression in response to specific conditions encountered within the human host. We tested this hypothesis by performing a transcriptomic analysis using publicly available data from active urethral infections (28) and discovered significant changes in GGI expression during infection, including increased expression of multiple T4SS genes.
RESULTS
Screen for conditions affecting TraD expression.
Gonococci isolated from infections are always piliated, and piliated gonococci are more sensitive to antibiotics in the growth medium than nonpiliated variants (29, 30). Moreover, previous studies have demonstrated that piliated gonococcal strains secrete more DNA and exhibit increased expression of traD (encoding the type IV coupling protein ATPase), traI (encoding the type IV secretion relaxase), and the GGI gene encoding the hypothetical protein YdhB (25). Due to its increased expression in the more robustly secreting piliated cells and its necessity for secretion, we selected TraD as a representative T4SS protein and made a traD::lacZ translational fusion expressed at the native traD locus in gonococci (AKK520) (22, 25) (Fig. 1B). Using this LacZ reporter in piliated cells, we tested a panel of infection-relevant compounds on TraD expression using disk diffusion assays on agar plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Table 1).
TABLE 1.
Effects of compounds on TraD expressiona
| Category | Test condition | Description | Observed blue color |
|---|---|---|---|
| Carbon sources | Glucose | 40 mg/ml | No effect |
| Lactate | 60% (wt/wt) sodium dl-lactate solution | No effect | |
| Acetate | 3 M NaOAc | No effect | |
| Antibiotics | Ciprofloxacin | 8 mg/ml | No effect |
| Streptomycin | 100 mg/ml | No effect | |
| Extracellular material | Spent medium | 10 μl of filter-sterilized supernatant, N. gonorrhoeae MS11 in cGCBL | No effect |
| DNA | DNA | 1.5 μg of N. gonorrhoeae gDNA | No effect |
| recA4 b | recA loss-of-function allele (43) | ↑ | |
| Reactive oxygen/nitrogen species | Hydrogen peroxide | 3% solution | ↑ |
| Nitrite | 500 mM NaNO2 | No effect | |
| SNAP | NO donor, S-nitroso-N-acetyl-dl-penicillamine, 90 mM | No effect | |
| GSNO | NO donor, S-nitrosoglutathione, 50 mM | No effect | |
| Infection/transmission | Spermidine | 100 mg/ml | No effect |
| Seminal plasma | ≥3 pooled donors per sample | ↑ | |
| Metals | Iron | 1.2 μM Fe(NO3)3 | No effect |
| Deferoxamine mesylate (Desferal) | Iron chelator, 25 mM | ↑ | |
| fur-1 b | fur point mutation, partially functional Fur | ↑ | |
| Magnesium | 100 mM MgCl2 | No effect | |
| Manganese | 100 mM MnCl2 | ↓ | |
| Copper | 100 mM CuSO4 | ↑ | |
| Cobalt | 100 mM CoCl2 | No effect | |
| Zinc | 100 mM ZnSO4 | ↑ | |
| EDTA | 250 mM | ↑ |
N. gonorrhoeae organisms expressing the traD::lacZ reporter at the native traD locus were spread onto GCB agar plates containing X-Gal, and compounds of interest were screened by disk diffusion assay with blue colony color employed as the metric of protein expression. “No effect” indicates no visible changes in colony color in response to the compound-suffused disk. “↑” indicates increased color with proximity to disk (or increased color in the tested mutant). “↓” indicates decreased color with proximity to disk.
Side-by-side comparison of mutant and wild-type bacteria, as opposed to disk diffusion, was used to determine difference in blue color.
Gonococci infect a variety of environmentally distinct niches, and it is possible that the T4SS is active only at certain infection sites. The host typically sequesters metal at sites of infection; pathogen killing via nutritional immunity strategies has been documented for iron, zinc, and manganese (31–33). N. gonorrhoeae utilizes several TonB-dependent and ABC transport systems to obtain necessary metals, and it modulates expression of these scavenging and import systems in response to metals in its environment (34, 35). Supplemental iron had no visible effect on TraD (Table 1); however, Gonococcal Base Medium (GCB) agar has a high iron content due to the addition of Kellogg’s supplements, so additional iron may have been superfluous for regulation. In contrast, the iron chelator deferoxamine (Desferal) increased TraD reporter signal, suggesting that low iron availability increases TraD expression. EDTA increased TraD similarly (Table 1). We expanded our screen to include other metals, including copper, cobalt, magnesium, and zinc. Solutions of copper or zinc each increased β-galactosidase activity in our screen (Table 1). The only compound tested that visibly decreased signal was manganese (Table 1). No effect on β-galactosidase activity was seen in a control strain constitutively expressing lacZ. Based on these observations, we hypothesize that T4SS expression changes in response to stimuli, including metal availability.
Next, we tested carbon sources metabolized by N. gonorrhoeae. Neither solutions of glucose, its preferred carbon source, nor lactate, an additional carbon source utilized for growth during infection (36, 37), altered TraD levels in the disk diffusion LacZ reporter assay (Table 1). Acetate, a metabolizable by-product of growth (38), also showed no effect on TraD levels (Table 1).
Transcriptional changes in response to subinhibitory levels of antibiotics have been identified in a number of bacterial pathogens, including Salmonella enterica serovar Typhimurium and N. gonorrhoeae (39, 40). Clinical isolates of N. gonorrhoeae have demonstrated resistance to all classes of antibiotics, making antibiotic response an especially salient issue for gene regulation (41). However, we found no effect from ciprofloxacin or streptomycin on TraD expression in the disk diffusion assay (Table 1).
We also tested genomic DNA, the substrate of the T4SS, and spent medium from cultures of wild-type GGI+ gonococci, which contained DNA from both autolysis and secretion as well as other growth and lysis products. Neither affected TraD expression (Table 1). Transformation of DNA, which may be facilitated by the T4SS, is speculated to provide material for DNA repair (42). We probed the role of repair in regulating T4S by testing a DNA recombination-deficient mutant (recA4) [43] in our disk diffusion assay. The recombination mutant did have stronger blue color than wild-type recA+ cells, indicating some potential regulatory overlap between these systems (Table 1).
We tested pH and seminal plasma, compounds characteristic of specific infections sites, to gain insight into T4SS expression in various contexts. pH varies greatly between infection niches: engulfed bacteria encounter variable acidification by host cell phagosomes, whereas the average pH of semen is ∼8.2 (44, 45). Liquid β-galactosidase assays of cultures exposed to decreased or increased medium pH conditions at which N. gonorrhoeae can survive in vitro (6.3 or 8.0, respectively) showed no significant changes in TraD expression (see Fig. S1 in the supplemental material). Spermidine, an abundant polyamine in the male urogenital tract that helps gonococci persist against innate immune factors (46), had no effect on TraD expression. However, whole seminal plasma as would be encountered in semen-mediated transmission of gonorrhea visibly increased TraD reporter expression on plates (Table 1).
During host infection, N. gonorrhoeae must combat reactive oxygen and nitrogen species produced by both the host immune response and bacterial metabolism (8, 47–50). TraD expression increased with proximity to a disk of 3% hydrogen peroxide (Table 1). None of the nitrogen species tested (nitrite, SNAP, and GSNO) elicited a change in TraD expression (Table 1). Based on these initial experiments, we proceeded to further investigate compounds with the most profound effects on TraD, beginning with the metals iron, zinc, copper, and manganese.
Iron availability and T4SS expression.
Like many bacteria, gonococci employ a ferric uptake regulator, Fur, to control gene expression in response to intracellular iron (51). We found that a fur point mutant, the fur-1 mutant, wherein iron response is compromised due to impaired functionality of Fur, had increased levels of TraD in the disk diffusion assay compared to the wild type (Table 1). Canonically, the presence of iron allows Fur monomers to dimerize, and these dimers bind promoters to repress transcription (52, 53).
To confirm and quantify the results from the disk diffusion assays, we performed β-galactosidase assays on N. gonorrhoeae expressing traD::lacZ, grown in liquid cultures. These assays demonstrated that the fur-1 strain (AKK548) exhibited a significant increase in TraD expression, with nearly 2-fold more β-galactosidase made than in the wild type (Fig. 2A). The fur-1 mutant showed no difference in β-galactosidase activity between iron-replete [GCBL (Gonococcal Base Liquid Medium) plus 1.2 μM Fe(NO3)3] and iron-depleted (GCBL plus 100 μM deferoxamine) media, behaving as expected for this iron-unresponsive mutant. A 100 μM concentration of deferoxamine is in excess of the amount necessary to chelate all Fe3+ in complete GCBL (cGCBL) and was sufficient to lead to strong β-galactosidase activity in a control strain carrying a tbpA::lacZ transcriptional fusion (Fig. S2B).
FIG 2.
Iron sensing plays a regulatory role in T4S. (A) β-Galactosidase assay detecting TraD-LacZ expression from strains wild type (WT) for fur versus the fur-1 mutant in both iron-replete and iron-depleted growth media. *, P < 0.05 in Student’s t test comparing sample to wild-type fur in iron-replete media. Data are averages of three experiments; error bars show SEM. (B) Representative Western blot against the FLAG tag in TraD-FLAG3 strains with wild-type fur and fur-1 in iron-replete media. Arrow indicates the TraD-FLAG3 band. (C) qRT-PCR comparing GGI transcripts from the wild type and the fur-1 mutant in both iron-replete [cGCBL + 1.2 μM Fe(NO3)3] and iron-depleted (GCBL plus 100 μM deferoxamine) growth media. *, P < 0.05 in Student’s t test comparing ΔCT values to those of the wild type in iron-replete medium. Data are averages of three experiments; error bars show 95% confidence intervals. Transcripts were normalized to rmp. (D) Quantification of DNA in supernatants from the wild type (MS11), a ΔGGI strain (ND500), and the fur-1 mutant. Values were corrected for extracellular DNA resultant from lysis by subtracting ΔGGI strain (ND500) values in each experimental replicate. Student’s t test determined no significant differences from MS11. Data are averages of three experiments; error bars show SEM.
Western blots detecting an epitope-tagged TraD also showed increased TraD protein in the fur-1 strain (Fig. 2B). Transcript levels of traD (located on GGI operon 1) and traK (located on operon 2) (Fig. 1A) in the fur-1 mutant exhibited modest increases over wild-type levels regardless of iron availability (Fig. 2C), offering some explanation for the observed increase in protein. We were able to quantify secreted DNA for both the wild type and fur-1 strain, although DNA quantification is often highly variable (21). The fur-1 strain exhibited a nonsignificant trend of increased DNA secretion compared to the wild type (Fig. 2D), indicating a potential change in T4SS activity in response to the increased protein expression.
Iron depletion alone only partially recapitulated expression differences observed in the fur-1 mutant. β-Galactosidase assays using the traD::lacZ translational reporter revealed that TraD protein was significantly more abundant under iron chelation conditions than under the supplemental-iron condition (Fig. 2A). Addition of iron back to deferoxamine-treated media at a 1:1 molar ratio was sufficient to rescue β-galactosidase activity in the traD::lacZ-expressing strain (Fig. S2A). The effect of iron depletion was corroborated by Western blotting, in which TraD-FLAG3 was more abundant in cells grown in iron-chelated medium (Fig. S2C). However, for both traD and traK, transcript levels were the same under iron-replete and -deplete conditions (Fig. 2C). These data suggest a model in which Fur plays two roles. First, Fur represses traD transcription regardless of iron availability, perhaps from low-affinity or transient Apo-Fur repression, wherein Fur could bind promoters independent of iron (54, 55). While a BLAST search did not identify consensus Fur boxes in the GGI, the defining characteristics of AT richness and inverted repeats are present in some GGI promoters (56, 57). Second, Fur and iron levels might interact, perhaps through a second factor, to keep TraD protein levels low under iron-replete conditions but allow TraD levels to rise during iron depletion.
Copper, zinc, and cooperative upregulation of TraD expression.
Niches occupied by gonococci vary highly in metal availability, and metal concentrations are often not well known. For example, Goullé et al. found a median zinc concentration in human plasma of ∼11.1 mM, whereas Owen and Katz did an extensive scan of reported literature values for zinc concentration in human semen and found it to be an order of magnitude higher, at 224 ± 53 mM (58, 59). We tested multiple concentrations of both zinc and copper, striving to add enough to elicit an effect without disrupting in vitro growth.
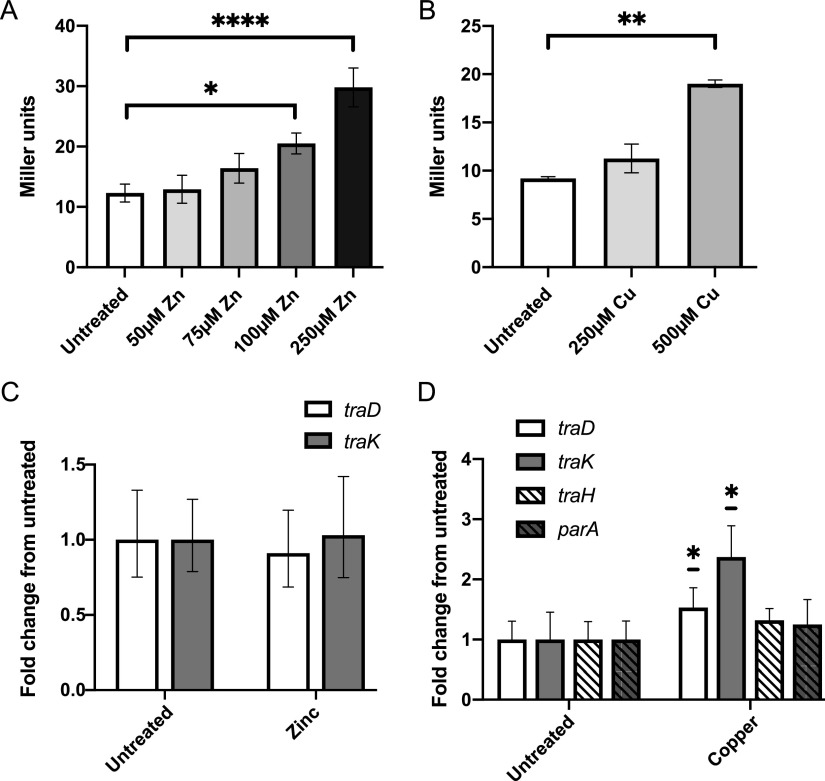
Disk diffusion assays with both ZnSO4 and CuSO4 demonstrated an increase in β-galactosidase activity for the traD::lacZ strain with increasing concentrations of these metals. Quantitative β-galactosidase assays confirmed that supplementary zinc and copper both increased TraD production (Fig. 3A and B). TraD upregulation was concentration dependent, with the highest TraD expression observed at 250 μM ZnSO4 and 500 μM CuSO4 (Fig. 3A and B). Zinc treatment did not significantly alter transcript levels for traD or traK (Fig. 3C).
FIG 3.
Zinc and copper both alter TraD expression. (A and B) β-Galactosidase assays of traD::lacZ gonococci with various concentrations of ZnSO4 (A) or CuSO4 (B). Significance was determined by one-way analysis of variance (ANOVA) and Dunnett’s posttest. Data are averages of three experiments; error bars show SEM. (C and D) qRT-PCR of GGI transcripts in response to 100 μM ZnSO4 (C) or 500 μM CuSO4 (D). Significance was determined by Student’s t test comparing ΔCT values. Data are averages of three experiments; error bars show 95% confidence interval. Transcripts were normalized to rpoB. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
We tested the effect of copper treatment on traD and traK transcript levels, as well as those of traH (operon 3) and parA (operon 4), representing all four GGI operons necessary for secretion (Fig. 1A). All of the tested transcripts exhibited small increases with copper treatment, with traD and traK being statistically significantly higher (∼1.5- and 2.3-fold, respectively) (Fig. 3D). The effects of increased concentrations of zinc and copper look very similar to those of iron chelation: small (if any) transcriptional changes and more pronounced protein increases, suggesting largely posttranscriptional upregulation of TraD protein expression.
Copper and zinc toxicities have both been identified as mechanisms of bacterial killing in the phagosome (60–63). We next asked if zinc and copper could act cooperatively to further increase TraD expression. Since the phagosome also employs iron sequestration to kill bacteria, we included deferoxamine in β-galactosidase assays of traD::lacZ with combination treatments. All binary combinations of deferoxamine, zinc, and copper, as well as treatment with all three, significantly increased TraD expression (Fig. 4A). No significant changes in growth were observed in response to these treatments, as assessed by quantifying protein concentration in the cultures after growth (Fig. S4 and S5). Protein accumulation in cell pellets is a preferred method of growth assessment for N. gonorrhoeae, as it is robust to changes in piliation, opacity protein, and lipooligosaccharide (LOS) variation (16, 25, 64). We used Western blotting against the FLAG epitope tag to further explore the specificity of posttranscriptional metal regulation. Gonococci with 3×FLAG-tagged traD and traK were grown in GCBL and treated with either deferoxamine and zinc or deferoxamine, zinc, and copper. The traK construct was overexpressed from a complementation locus to provide ample transcript and allow visualization by Western blotting. TraD protein was distinctly increased by both metal treatments, whereas TraK had no change in abundance (Fig. 4B). As with the individual metal conditions, the combination deferoxamine, zinc, and copper treatment had no significant effect of GGI transcript levels (Fig. 4C). Experimental efforts were made to directly measure the effects of iron-chelated, high-zinc, high-copper media on secreted DNA output; however, the ssDNA detection methods require the use of defined media and were not sufficient to discern ssDNA from double-stranded DNA (dsDNA) resultant from cell lysis, which was increased in these cultures.
FIG 4.
Semicooperative upregulation of TraD by zinc, copper, and iron chelation using 100 μM deferoxamine, 250 μM ZnSO4, and 500 μM CuSO4. (A) β-Galactosidase assay detecting TraD-LacZ expression. Significance was determined by Student’s t test comparing samples to untreated cultures. *, P < 0.05; **, P < 0.01; ****, P < 0.0001. Data are averages of three experiments; error bars show SEM. (B) Representative Western blot against the FLAG epitope. Native expression of traD::FLAG3 (top) and induced overexpression of traK::FLAG3 (bottom) are shown. The WT has no FLAG tag. (C) qRT-PCR showing no significant differences between all tested GGI transcripts by Student’s t test comparing ΔCT values. Data are averages of four experiments; bars show 95% confidence interval. Transcripts were normalized to rpoB.
The mechanism of toxicity for copper and zinc is often mismetallation of proteins (61, 65). Since Fur is an active regulator in this system, we performed β-galactosidase assays to determine whether the effects of zinc and copper on TraD expression were a result of Fur mismetallation. The traD::lacZ fur-1 mutant strain responded to both zinc and copper treatments by expressing more TraD, indicating that these two metals do not act by disrupting the activity of Fur (Fig. S3).
Manganese represses GGI expression.
The only compound in our disk diffusion screen that downregulated TraD expression was manganese chloride (Table 1). Similar to the case with all tested metals in this study, manganese concentrations vary highly between body sites and have not been characterized in the context of Neisseria infections. One study reports median manganese concentrations in whole blood to be ∼138 μM, while isolated plasma had only ∼20 μM (58). We performed Western blotting with a TraD-FLAG3 strain (AKK556) and detected less TraD in cultures grown with supplemental manganese (cGCBL plus 100 μM MnCl2), confirming that TraD is expressed less in response to this manganese treatment (Fig. 5A). Quantification of extracellular DNA in wild-type cells (strain MS11) revealed that cultures treated with manganese had consistently less DNA in the supernatant than untreated cultures (Fig. 5B), though manganese did not affect bacterial growth (Fig. S5). A GGI deletion strain (ND500) was used as a control for lysis in these experiments. Surprisingly, quantitative reverse transcription-PCR (qRT-PCR) demonstrated that transcript levels for traD and traK were significantly decreased in cultures grown with 100 μM MnCl2 (Fig. 5C). This result indicates that manganese was working in a unique manner from the other metals identified in our screen, acting at the transcriptional level to downregulate GGI expression.
FIG 5.
Manganese represses T4S. (A) Representative Western blot for TraD-FLAG3 demonstrates a visible decrease in TraD in cultures treated with 100 μM MnCl2. Arrow indicates the TraD-FLAG3 band. (B) Extracellular DNA quantification shows less DNA is secreted in manganese-treated cultures. Values were corrected for extracellular DNA resultant from lysis by subtracting ΔGGI strain (ND500) values in each experimental replicate. Data are averages of three experiments; error bars show SEM. (C) qRT-PCR showing decreased traD and traK transcript with 100 μM MnCl2. **, P < 0.01 in Student’s t test comparing ΔCT values. Data are averages of three experiments; error bars show 95% confidence interval. Transcripts were normalized to rpoB.
GGI genes are expressed during urethral infection.
Many of the factors that we have found to alter T4SS protein expression are physiologically relevant to gonorrhea infection. We postulate that the low expression levels observed under standard laboratory conditions are not representative of expression in the human host. Furthermore, most of the effects that were identified in the compound screen appear to act at the posttranscriptional level. This finding was somewhat surprising and made us question whether there are noteworthy changes in GGI transcripts between the lab and the host environment. In a recent study, Nudel et al. collected urethral swabs from infected males and performed transcriptome sequencing (RNA-Seq) on N. gonorrhoeae isolates both during host infection and as a monoculture in laboratory chemically defined medium CDM (GEO accession GSE113290) (28). We identified five GGI+ urethral isolates in the resultant data set (Table S3) and used Rockhopper (66, 67) to compare GGI expression in the urethral swabs and growth of the pure isolates in medium.
The GGI exhibited distinct gene expression profiles between laboratory culture and urethral infection (Fig. 6). Of the 62 GGI genes detected by Rockhopper, 35 (∼56%) had significantly higher expression during urethral infection. Furthermore, 20 of the 21 genes that are essential for T4SS-dependent DNA secretion were more highly expressed in the urethral infections, half of these significantly so. The exception, traH, had significantly higher expression in culture medium though it was still expressed at detectable levels during infection.
FIG 6.
The GGI is differentially expressed during natural urethral infection in humans. Shown is a heat map of GGI gene expression in laboratory-grown gonococcal isolates and urethral swab samples. Brackets on the left indicate genes necessary for DNA secretion in laboratory culture. n = 5. *, q value < 0.05.
The portion of the GGI spanning ych-ydcA, which consists largely of genes of unknown function (Fig. 1A), has been previously demonstrated to be dispensable for DNA secretion (22). This region, spanning ∼4.4 kb, was notably downregulated during urethral infection compared to laboratory growth conditions (Fig. 6). Furthermore, Rockhopper identified several novel antisense transcripts in this region, and many of these antisense transcripts were significantly increased in the urethral swab samples. Hypothesizing that the 4.4-kb region might contain genes that negatively regulate the T4SS, we deleted the exp1-ydcA region from the gonococcal chromosome (Fig. 1A). Contrary to our hypothesis, measurements of traD, traK, and parA expression did not show increased transcript levels in the deletion mutant. Overall, we conclude from the RNA-Seq analysis that while the T4SS is not robustly expressed in the laboratory, its transcription is upregulated during infection of the male urethra in a specific and consistent manner.
Surface adherence enhances GGI expression.
At some infection sites gonococci are capable of biofilm formation, creating a matrix consisting partly of extracellular DNA atop epithelial cells (68–70). The gonococcal T4SS has been shown to contribute to biofilm establishment, although it is not essential for biofilm formation (15). However, the effect of the biofilm environment on T4SS expression has not heretofore been investigated. We grew the bacteria in stationary 12-well tissue culture plates for 6, 12, and 24 h to elucidate potential effects of planktonic versus surface-adhered cellular lifestyles on the secretion system. Planktonic cells were separated from cells adhered to the bottom of the wells by gentle pipetting, and then the two populations of cells were analyzed in parallel. Due to the limited number of cells obtained from growth in this manner, we were unable to detect β-galactosidase activity from the traD::lacZ reporter strain in either planktonic or surface-adhered cells. However, qRT-PCR indicated that adhered cells had significantly increased transcript levels at 6 and 12 h of stationary growth, with at least a 4-fold increase in transcript level over their planktonic counterparts (Fig. 7). Increases were less pronounced, but still over 2-fold, after 24 h of growth. Cellular lifestyle thus appears to exhibit a regulatory effect on GGI transcription, with growth under adherent conditions increasing T4SS expression especially at early time points. This observation is relevant to infection at many host sites, where gonococci adhere to host cells and form biofilms on host tissues.
FIG 7.
GGI transcript levels are elevated in surface-adhered cells. Shown is a qRT-PCR analysis of representative GGI genes. #, P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.001 in Student’s t test comparing ΔCT values. Data are averages of four experiments; error bars show 95% confidence interval. Transcripts were normalized to rpoB.
DISCUSSION
Type IV secretion systems are a diverse family of protein machines that play myriad roles in host-pathogen interactions. The effects of the gonococcal T4SS have been observed both in the laboratory and at the population level; however, observable expression of this system has been low (11, 13, 16, 19, 20). We expect that the gonococcal T4SS might be assembled or otherwise activated by specific environmental signals during infection, especially because it is likely a costly apparatus to build and run. In this study, we investigated gonococcal responses to conditions that are likely encountered during infection and bacterial regulatory machinery that alters expression or activity of the T4SS.
Upregulation of T4SS protein expression by phagosome-like metal conditions.
After testing a variety of compounds, we found effects on GGI protein expression from four metals—manganese, iron, zinc, and copper—some acting at the transcriptional and others at the posttranscriptional level. Manganese depressed TraD expression at the transcriptional level and repressed traK transcript levels (operon 2) as well.
Iron sensing and response in gonococci are controlled by the ferric uptake regulator, Fur. Fur dimerizes when iron is bound and binds Fur boxes at promoters, where it can to directly repress transcription, directly activate transcription, or indirectly activate transcription by repressing a repressor (71). The fur-1 gonococcal strain, which has a point mutation in the fur gene leading to partially functional Fur (72), had significantly increased TraD protein. Transcript levels for traD were modestly increased in the fur-1 mutant compared to wild-type N. gonorrhoeae under iron-replete conditions (Fig. 2C), suggesting that Fur slightly represses traD transcription. Interestingly, iron chelation by deferoxamine treatment significantly increased TraD protein levels but had no effect on levels of traD transcript (Fig. 2C and Fig. S2C), indicating that Fur might contribute both in conjunction with iron and as an independent factor.
Synthesizing these data, we suggest a model (Fig. 8) in which iron-dependent Fur regulation acts at the posttranscriptional or translational level but does not affect traD transcription. This could be achieved through an intermediate regulator such as a protein or small RNA (sRNA). These regulatory elements have been identified as mechanisms of Fur activity previously in the pathogenic Neisseria (71, 73, 74).
FIG 8.
Model of TraD regulation by metals. When iron (orange circles) availability is high, Fur represses expression of the type IV secretion system coupling protein, TraD, through an unknown intermediate (yellow, depicted here as either an sRNA or a protein). In the phagosome, iron sequestration releases Fur-mediated TraD repression, resulting in increased TraD protein levels. Residual apo-Fur binding may happen transiently at the traD promoter, with small effects on traD transcript levels. High levels of manganese (pink rhombuses) repress traD transcription, but the phagosome pumps manganese away from intracellular gonococci, allowing transcription to proceed. Zinc (purple squares) and copper (blue triangles) contribute to the upregulation of TraD in an undefined manner; here we depict several points at which this regulation could occur: repression of a repressor or protease, direct stabilization of TraD, or enhancement of a TraD chaperone.
Apart from regulation by manganese and iron, both zinc and copper supplementation increased TraD protein levels. Zinc elicited no transcriptional changes, whereas copper yielded small but significant increases in traD and traK, suggesting that these metals act differently within the GGI regulon (Fig. 3C and D). Pairwise combinations of deferoxamine, zinc, and copper treatments, as well as treatment with all three, all enhanced TraD expression beyond that of individual treatments (Fig. 4A). Zinc and copper could act at a number of points in the regulatory network: they could enhance TraD protein stability, perhaps by inhibiting the activity of a protease or by activating a chaperone protein for TraD, or they could act upon another regulator within this system, potentially one that is also controlled by iron-dependent Fur regulation (65) (Fig. 8).
Our finding that TraD protein expression is increased specifically and semicooperatively by iron chelation combined with zinc and copper indicates that there may exist a specific and complex infection niche in which gonococci activate the T4SS in response to these combined signals. The host environment during symptomatic gonorrhea is highly inflammatory, and the host response to gonococci usually includes an influx of both macrophages and neutrophils. Gonococci have long been known to survive and replicate in neutrophils (6–8, 75, 76), and more recent work has determined that they remain viable after macrophage engulfment as well (3, 9, 77). In both types of immune cell, the phagosome employs active nutritional immunity strategies to sequester iron and manganese away from bacteria (77–79). A transcriptional response to facilitate iron acquisition has been recorded in response to professional phagocytes and growth during human infection (77, 80). At the same time, the toxic cations zinc and copper (observed only in macrophages to date) are pumped into the phagosome (60–62, 81, 82). Sensing and responding to these types of stressors are essential for maintaining metal homeostasis, and a variety of mechanisms and responses have been observed in bacteria (83). We propose that upon engulfment by a host immune cell, N. gonorrhoeae organisms in the phagosome respond to these specific metal conditions by synthesizing TraD and potentially other T4SS proteins. As TraD is the coupling protein, it could then recruit substrates for secretion by the T4SS. Protein stoichiometry has not been elucidated in this system; it is possible that TraD is a limiting factor for T4S that is turned on upon entrance into the host cell phagosome for immediate secretion of DNA or possibly protein effectors.
Expressing the T4SS at this time could confer several advantages to the bacterial invader. First, every new host has the possibility of harboring new bacterial populations, both existing populations of N. gonorrhoeae (especially in cases of asymptomatic gonorrhea) and commensal microbiota. These present an opportunity for T4SS-mediated horizontal gene transfer, which would enhance survival of gonococcal genes, including GGI genes, beyond the duration of gonococcal infection. Additionally, one could imagine that in the face of toxic cationic metals and host defense molecules, a bacterium surrounded by secreted, negatively charged DNA may be less susceptible to killing.
The gonococcal T4SS was previously noted to be expressed during intracellular infection of epithelial cells in tissue culture. Zola et al. found that gonococcal mutants lacking tonB could survive if they carried the GGI, whereas strains lacking the GGI would not survive intracellularly (84). Furthermore, mutations affecting structural components of the T4SS eliminated intracellular survival in the absence of TonB. Since all gonococci in the wild do express TonB and use it for metal acquisition, the significance of the ability of GGI+ gonococci to bypass TonB was not clear. However, the current findings suggesting that phagosome-like metal conditions upregulate type IV secretion, together with the knowledge that the T4SS is expressed during intracellular growth, suggest that the phagosome may be a previously unappreciated niche for gonococcal type IV secretion. VirB-type T4SSs have been noted to play important roles in intracellular growth for multiple pathogens, such as Legionella pneumophila and Brucella abortus, and a T4SS inner membrane complex in nontypeable Haemophilus influenzae facilitates the release of DNA and proteins needed to form biofilms (85–89).
Increased GGI expression during urethral infection and surface adherence.
The infection niche of the male urethra is complex and multifaceted; bacteria can survive in extracellular biofilms or intracellularly in both immune and epithelial cells (4–9, 68, 69). The significant increases in GGI transcript levels found in newly surface-adhered cells suggest that adherence plays a role in triggering GGI gene expression, ultimately leading to DNA secretion. Secreted DNA has been shown to nucleate biofilm formation, and within biofilms gonococci have higher rates of horizontal gene transfer (15, 90).
The transcriptional response of gonococci during urethral infection provides some information about nutrient availability and stressors in this space. Nudel et al. reported that multiple iron scavenging genes were significantly upregulated in male infection compared to in vitro growth, including fbpA (NGO_0217), tbpA, (NGO_1495), and tbpB (NGO_1496) (28). The authors concluded that gonococci experienced an iron-depleted environment during infection in men. Also, oxidative stress genes were significantly increased in men, including one for a glutaredoxin, trx1 (thioredoxin), and msrAB. The encoded proteins act to repair proteins damaged by oxidation as would occur when the bacteria encounter neutrophils or are present in the host cell phagosome (28). Further examination of the data set of differential transcript levels between in vitro and in vivo gonococci revealed several additional metal-related expression changes. The genes for the three proteins that comprise the TonB complex, TonB, ExbB, and ExbD, were enriched in vivo (4.24-, 3.35-, and 2.60-fold) (GEO accession number GSE113290) (28). Expression of ZnuA, part of the ABC transporter for uptake of zinc, was downregulated in male urethral swabs compared to in vitro conditions (0.34-fold). In addition, NGO_2127, a CadD family cadmium resistance transporter, was enriched 3.06-fold in male urethral swabs. Since cadmium has no known function in higher organisms and is toxic to humans, it seems probable that this protein provides resistance to a different metal, possibly Zn2+, which is chemically similar to Cd2+. Regulators and transporters often recognize both zinc and cadmium, and other bacterial efflux systems provide resistance to both of these metals (91, 92). The decreased expression of a zinc importer coupled with the increased expression of a putative zinc exporter suggests that the male reproductive tract represents a high-zinc environment during gonococcal infection. ArsR (NGO_1562) is upregulated 2.2-fold in vivo. This protein is a member of the ArsR-SmtB family of helix-turn-helix transcriptional regulators that are involved in the stress response to heavy metals and may sense intracellular levels of zinc (93). Overall, these data suggest that gonococci in the male urethra experience some of the same conditions in vivo that we have identified as regulators of T4SS expression in vitro, including low iron and increased zinc.
Consistent with these environmental conditions and our in vitro findings regarding metal regulation, our investigation of transcriptional changes of the GGI during urethral infection found that the transcriptional profile of the GGI was markedly increased in urethral infection isolates of N. gonorrhoeae compared to the same isolates grown in laboratory media (Fig. 6; GEO accession number GSE113290). The upregulation of almost all genes essential for DNA secretion indicates an increase in T4SS expression during urethral infection. The consequences of the T4SS and the extent to which its activity may shape the infection process are subjects for future study in this field.
This study has identified several environmental factors that work in combination to regulate the gonococcal T4SS. Regulation appears to act at multiple levels, potentially differing between intracellular and extracellular bacteria. Metal regulators map onto phagosomal conditions, which involve a combination of nutrient sequestration and metal toxicity to kill invading bacteria. Additionally, surface adherence increases GGI expression and may be a contributing factor for the robust upregulation of the GGI observed during urethral infection. The specific and complex upregulation of the T4SS within multiple infection niches indicates that the T4SS is part of the gonococcal response to the human host and points to a potential role for this system in the infection process.
MATERIALS AND METHODS
Bacteria and growth conditions.
Gonococci were grown on GCB agar plates with Kellogg’s supplements, in liquid GCBL medium with Kellogg’s supplements and 0.042% sodium bicarbonate (called complete GCBL [cGCBL]) (94), or in Graver-Wade (GW) medium (95). Bacterial strains used in this study are listed in Table S1. All mutants are derived from the wild-type strain MS11. Primer sequences are listed in Table S2. Where needed, kanamycin was used at 40 μg ml−1 for Escherichia coli and 80 μg ml−1 for N. gonorrhoeae. Unless otherwise noted, deferoxamine mesylate was used at 100 μM and metals were used at the following concentrations: 100 μM MnCl2, 250 μM ZnSO4, 500 μM CuSO4.
Construction of traD::lacZ reporter strain.
The 3′ region of traD was PCR amplified from MS11 chromosomal DNA with primers PstI-traDlacZ-F and 76-R and then digested with HindIII and PstI, resulting in a 0.75-kb fragment. This fragment was ligated into the HindIII and PstI sites of pPK1008, generating pKL5 which contained a traD-lacZ translational fusion. DNA downstream from traD, containing yaa truncated at the 5′ end, was PCR amplified with primers PspOMI-83F and 86-R, digested with PspOMI and SpeI, and ligated into the NotI and SpeI sites in pKL5, generating pKL10. The aph3 kanamycin resistance gene from pKH99 was cloned into the SpeI and AgeI sites in pKL10, immediately downstream from the traD-lacZ fusion, generating the reporter plasmid pAKK1. N. gonorrhoeae strain MS11 was transformed with linearized pAKK1, and transformants containing the traD-lacZ reporter recombined in the GGI were selected on GCB agar containing Kan, resulting in strain AKK500. The aph3 gene was removed by transforming AKK500 with pKL10 and screening for transformants that were sensitive to Kan, resulting in reporter strain AKK520.
Construction of a TraD-FLAG3 N. gonorrhoeae strain.
To create the TraD-FLAG3 construct, a 0.27-kb fragment containing a linker fused to the triple-epitope FLAG tag was obtained from pMR100 by cutting with PstI, blunting with T4 DNA polymerase, and then digesting with XbaI. This fragment was cloned into the SmaI/SpeI sites of pAKK1, resulting in the final C-terminal FLAG3-tagged TraD construct (pAKK28). N. gonorrhoeae MS11 was transformed with pAKK28, resulting in AKK556 with TraD-FLAG3 at the native traD site.
Construction of fur-1 mutants.
The fur-1 allele from N. gonorrhoeae MCV403 (gift from C. Cornelissen) was PCR amplified with primers fur_up-F and fur_down-R and transformed into N. gonorrhoeae MS11, AKK520, and AKK556. Putative transformants were identified by their smaller colony size and confirmed by sequencing the fur gene. The fur-1 allele in MCV403 contains a tyrosine 82-to-cysteine substitution, which was present in the original fur-1 mutant isolated previously (72). Our sequencing of the fur allele in MCV403 revealed that it had two additional mutations: a silent ATT-to-ATC base change within isoleucine 7 and a GAG-to-GGG mutation changing glutamate 48 to glycine. The fur-1 alleles in AKK550, AKK548 (traD::lacZ), and AKK573 (traD-FLAG3) contain all three mutations.
Disk diffusion and blue/white screening.
AKK520 was grown overnight from frozen stocks on GCB agar plates, 16 to 20 h. Bacteria were swabbed into 2 to 4 ml of cGCBL and serially diluted to 10−5. Volumes (80 μl) of the 10−4 and/or 10−5 dilutions were plated on plates with GCB plus 40 μg ml−1 of X-Gal plates. A 0.25-in. disk (Hardy Diagnostics) was placed near the bottom of the plate and suffused with 10 μl of the test compound at a single concentration, listed in Table 1. Diffusion of compound from this disk creates a concentration gradient across the plate. Plates were incubated at 37°C with 5% CO2. Blue color was assessed after 2 days.
RNA-Seq data analysis.
We obtained the publicly available PacBio whole-genome sequence data from reference 28 (BioProject PRJNA329501 and Gene Expression Omnibus accession number GSE113290). We assembled the raw sequence data using Flye 2.7 with two rounds of polishing (96). We evaluated assembly quality using QUAST (97). Using BLAST 2.9.0+ (56), we identified GGI genes in each de novo assembly; individual GGI gene sequences from reference strain NCCP11945 (GenBank accession number CP001050.1) were used as the query sequences (98).
We proceeded with transcriptomic analysis using the GGI+ isolates from male patients only, as the GGI was identified in only one cervicovaginal lavage sample. Reads were aligned to the genome of NCCP11945 using Rockhopper with default parameters (66, 67). Gene expression is reported as reads per kilobase per million (RPKM). GGI genes with a q value of ≤0.05 were considered significantly different between groups.
β-Galactosidase assays.
The β-galactosidase assays were performed as described previously (21). Cultures were started from 16 to 20 h of N. gonorrhoeae overnight growth on GCB plates suspended in cGCBL at an optical density at 540 nm (OD540) of ∼0.25 and grown for 3 h with aeration with treatments as noted throughout. For assays testing change in medium pH, gonococci were grown as described above in 3 ml of cGCBL at pH 7.3 for 2 h. Bacteria were collected by centrifugation and resuspended in 3 ml of fresh cGCBL at pH 6.3, 7.3, or 8.0 and then grown 1 h. After growth, cultures were placed on ice 20 min, and 0.5 ml was removed for protein quantification via Bradford assay. After chilling, 2 ml of culture was harvested by centrifugation, resuspended in Z buffer, and exposed to o-nitrophenyl-β-d-galactopyranoside (ONPG) in 96-well plates. Absorbance was read with a BioTek Synergy HT plate reader. Protein concentration was used in place of optical density in the Miller equation.
qRT-PCR.
N. gonorrhoeae cultures in cGCBL were grown 3 h at 37°C with rotation from an OD540 of ∼0.25. Where noted, static growth samples were prepared as in the static surface adherence assay. A volume of 0.75 ml of culture was combined with 0.75 ml of −20°C methanol and centrifuged at 17,000 × g for 1 min. This procedure was repeated a total of two times, to harvest cells from 1.5 ml of culture. RNA isolation was performed as described previously (21). DNA was removed with the TURBO DNA-free kit (Invitrogen) and cDNA prepared using the iScript cDNA synthesis kit (Bio-Rad). qRT-PCR was performed using SYBR green reagents (Bio-Rad) and the threshold cycle (ΔΔCT) method (99). Statistical significance was determined with the two-tailed Student’s t test comparing ΔCT statistics according to reference 100. The housekeeping gene rpoB was used for normalization in all cases except tests involving manipulation of iron levels, because rpoB was observed to respond to changes in iron levels. In these specific tests, rmp was used for normalization instead (101).
Static surface adherence assays.
Surface-adhered gonococci were grown in the manner of (102–105). N. gonorrhoeae cells from overnight growth were swabbed then suspended in cGCBL and adjusted to an OD540 of ∼0.1. A volume of 1 ml of culture was transferred to a sterile 12-well tissue culture plate in triplicate. The plate lid was coated in Triton solution and Parafilm was used for sealing. The plate was incubated at 37°C and 5% CO2 for 6, 12, or 24 h without agitation. To separate planktonic from adhered cells, the plate was tilted and liquid was gently removed from wells with a pipette, constituting the liquid (or planktonic) fraction. A volume of 1 ml of GCBL was added to each well, and adhered cells were resuspended using a cell scraper and agitation with a Pipetman. These cells were then collected as the surface-adhered fraction.
DNA secretion assays.
DNA secretion assays were performed similarly to assays described previously (11, 14). Briefly, gonococci were grown on GCB agar plates overnight from frozen stocks of piliated bacteria. A piliated colony was restreaked if reversion to nonpiliation was observed on the plate. Bacteria were swabbed into GW medium prepared without spermidine, and the OD540 was adjusted to 0.15 to 0.2. Cultures (3 ml) were grown for 1.5 h at 37°C in 15-ml Falcon tubes with rotation. After initial growth, 0.5 ml of culture was diluted into 2.5 ml of fresh GW medium and vortexed thoroughly, and then 0.5 ml was removed for analysis. The remaining culture was grown as before for 2 h, at which time another 0.5 ml was removed for analysis. Samples were harvested by centrifugation for 2 min at 17,000 × g immediately after collection. A 0.4-ml supernatant sample was removed to quantify DNA using Quanti-iT Pico green fluorescent dye (Life Technologies). Remaining liquid and pellet were resuspended in water and protein was quantified using the Bio-Rad protein assay.
Western blotting.
(i) Detection of TraD-FLAG3 in wild-type, manganese-treated, and fur-1 strains.
To assay wild-type versus fur-1 TraD expression, overnight growth of N. gonorrhoeae AKK556 and AKK573 on GCB agar plates was swabbed into GCBL. To assay the effects of manganese treatment, overnight growth of AKK556 on GCB agar plates was swabbed into cGCBL, the OD540 was adjusted to ∼0.2, and 3-ml cultures were grown with rotation at 37°C for 3 h. For all assays, cells were collected by centrifugation and resuspended in sterile Milli-Q water and sonicated to obtain whole-cell lysates. Protein was quantified by a Bradford assay (Bio-Rad), and equivalent quantities were electrophoresed on 10% SDS-PAGE gels. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane and blocked overnight with 5% nonfat dry milk in Tris-buffered saline containing 0.5% Tween 20 (TBST). Membranes were incubated for 1 h with primary antibody M2 anti-FLAG (Sigma) at a 1:10,000 dilution and then washed 3 times with TBST. Blots were then incubated with goat anti-mouse peroxidase-conjugated antibody (1:10,000 dilution) (Santa Cruz Biotechnology), washed 4 times with TBST, then developed with the Immobilon Western chemiluminescent horseradish peroxidase (HRP) substrate (Millipore), and exposed to film.
(ii) Detection of TraD-FLAG3 following deferoxamine, copper, and zinc treatment.
Liquid N. gonorrhoeae cultures were inoculated from 16 to 20 h of overnight growth on GCB agar plates into cGCBL at an OD540 of ∼0.25 and grown for 3 h at 37°C with rotation. Cultures of 3 ml were pelleted by centrifugation in a swinging-bucket tabletop centrifuge (Jouan C3i) in their entirety, washed once with cold phosphate-buffered saline (PBS), resuspended in 500 μl of water, and sonicated. Lysate samples were resolved on precast 10% SDS-PAGE gels (Bio-Rad). Proteins were transferred to a PDVF membrane, blocked with 5% milk in TBST for 1 h, and incubated with 1:20,000 M2 antibody overnight at 4°C. Secondary antibody goat anti-mouse HRP was diluted 1:20,000 and administered for 1 h. Blots were developed using the Li-Cor Odyssey Fc imaging system.
Data availability.
The data that support the findings of this study are available in the Gene Expression Omnibus, BioProject PRJNA329501, accession number GSE113290. Additional data are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
This work was funded by NIH grant R01AI047958.
We thank Cynthia Cornelissen for the fur-1 mutant.
We declare no conflict of interest.
J.P.D. conceived the study. M.M.C., A.K.K., and J.K. acquired, analyzed, and interpreted data. A.K.K. built strains and plasmids. A.C.S., aided by C.S.P., determined GGI presence/absence and assisted RNA-Seq analysis. M.M.C. wrote the manuscript. J.P.D. and A.K.K. provided critical reading and revision.
Footnotes
Supplemental material is available online only.
Contributor Information
Joseph P. Dillard, Email: jpdillard@wisc.edu.
Manuela Raffatellu, University of California San Diego School of Medicine.
REFERENCES
- 1.Unemo M, Seifert HS, Hook EW, Hawkes S, Ndowa F, Dillon J-AR. 2019. Gonorrhoea. Nat Rev Dis Primers 5:79. 10.1038/s41572-019-0128-6. [DOI] [PubMed] [Google Scholar]
- 2.Sintsova A, Sarantis H, Islam EA, Sun CX, Amin M, Chan CHF, Stanners CP, Glogauer M, Gray-Owen SD. 2014. Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog 10:e1004341. 10.1371/journal.ppat.1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Château A, Seifert HS. 2016. Neisseria gonorrhoeae survives within and modulates apoptosis and inflammatory cytokine production of human macrophages. Cell Microbiol 18:546–560. 10.1111/cmi.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merz AJ, Rifenbery DB, Arvidson CG, So M. 1996. Traversal of a polarized epithelium by pathogenic Neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol Med 2:745–754. 10.1007/BF03401658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol Microbiol 42:659–672. 10.1046/j.1365-2958.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 6.King G, James JF, Swanson J. 1978. Studies on gonococcus infection. XI. Comparison of in vivo and in vitro association of Neisseria gonorrhoeae with human neutrophils. J Infect Dis 137:38–43. 10.1093/infdis/137.1.38. [DOI] [PubMed] [Google Scholar]
- 7.Apicella MA, Ketterer M, Lee FKN, Zhou D, Rice PA, Blake MS. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J Infect Dis 173:636–646. 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- 8.Criss AK, Katz BZ, Seifert HS. 2009. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol 11:1074–1087. 10.1111/j.1462-5822.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritter JL, Genco CA. 2018. Neisseria gonorrhoeae–induced inflammatory pyroptosis in human macrophages is dependent on intracellular gonococci and lipooligosaccharide. J Cell Death 11:1179066017750902. 10.1177/1179066017750902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillard JP, Seifert HS. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol 41:263–277. 10.1046/j.1365-2958.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton HL, Domínguez NM, Schwartz KJ, Hackett KT, Dillard JP. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol 55:1704–1721. 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan MM, Heilers J, van der Does C, Dillard JP. 2017. Secretion of chromosomal DNA by the Neisseria gonorrhoeae type IV secretion system. Curr Top Microbiol Immunol 413:323–345. 10.1007/978-3-319-75241-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shockey AC. 2019. Genomics of bacterial pathogens across evolutionary scales. PhD thesis. University of Wisconsin—Madison, Madison, WI. [Google Scholar]
- 14.Salgado-Pabón W, Jain S, Turner N, van der Does C, Dillard JP. 2007. A novel relaxase homologue is involved in chromosomal DNA processing for type IV secretion in Neisseria gonorrhoeae. Mol Microbiol 66:930–947. 10.1111/j.1365-2958.2007.05966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zweig M, Schork S, Koerdt A, Siewering K, Sternberg C, Thormann K, Albers SV, Molin S, Van der Does C. 2014. Secreted single-stranded DNA is involved in the initial phase of biofilm formation by Neisseria gonorrhoeae. Environ Microbiol 16:1040–1052. 10.1111/1462-2920.12291. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton HL, Schwartz KJ, Dillard JP. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J Bacteriol 183:4718–4726. 10.1128/JB.183.16.4718-4726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton HL, Dillard JP. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol Microbiol 59:376–385. 10.1111/j.1365-2958.2005.04964.x. [DOI] [PubMed] [Google Scholar]
- 18.Kohler PL, Chan YA, Hackett KT, Turner N, Holly LH, Cloud-Hansen C, Dillard JP. 2013. Mating pair formation homologue TraG is a variable membrane protein essential for contact-independent type IV secretion of chromosomal DNA by Neisseria gonorrhoeae. J Bacteriol 195:1666–1679. 10.1128/JB.02098-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison OB, Clemence M, Dillard JP, Tang CM, Trees D, Grad YH, Maiden MCJ. 2016. Genomic analyses of Neisseria gonorrhoeae reveal an association of the gonococcal genetic island with antimicrobial resistance. J Infect 73:578–587. 10.1016/j.jinf.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsey ME, Hackett KT, Bender T, Kotha C, van der Does C, Dillard JP. 2014. TraK and TraB are conserved outer membrane proteins of the Neisseria gonorrhoeae type IV secretion system and are expressed at low levels in wild-type cells. J Bacteriol 196:2954–2968. 10.1128/JB.01825-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey ME, Bender T, Klimowicz AK, Hackett KT, Yamamoto A, Jolicoeur A, Callaghan MM, Wassarman KM, Van Der Does C, Dillard JP. 2015. Targeted mutagenesis of intergenic regions in the Neisseria gonorrhoeae gonococcal genetic island reveals multiple regulatory mechanisms controlling type IV secretion. Mol Microbiol 97:1168–1185. 10.1111/mmi.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pachulec E, Siewering K, Bender T, Heller E-M, Salgado-Pabon W, Schmoller SK, Woodhams KL, Dillard JP, van der Does C. 2014. Functional analysis of the gonococcal genetic island of Neisseria gonorrhoeae. PLoS One 9:e109613. 10.1371/journal.pone.0109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain S, Zweig M, Peeters E, Siewering K, Hackett KT, Dillard JP, van der Does C. 2012. Characterization of the single stranded DNA binding protein SsbB encoded in the gonoccocal [sic] genetic island. PLoS One 7:e35285. 10.1371/journal.pone.0035285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remmele CW, Xian Y, Albrecht M, Faulstich M, Fraunholz M, Heinrichs E, Dittrich MT, Müller T, Reinhardt R, Rudel T. 2014. Transcriptional landscape and essential genes of Neisseria gonorrhoeae. Nucleic Acids Res 42:10579–10595. 10.1093/nar/gku762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salgado-Pabón W, Du Y, Hackett KT, Lyons KM, Arvidson CG, Dillard JP. 2010. Increased expression of the type IV secretion system in piliated Neisseria gonorrhoeae variants. J Bacteriol 192:1912–1920. 10.1128/JB.01357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubori T, Shinzawa N, Kanuka H, Nagai H. 2010. Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog 6:e1001216. 10.1371/journal.ppat.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Québatte M, Dick MS, Kaever V, Schmidt A, Dehio C. 2013. Dual input control: activation of the Bartonella henselae VirB/D4 type IV secretion system by the stringent sigma factor RpoH1 and the BatR/BatS two-component system. Mol Microbiol 90:756–775. 10.1111/mmi.12396. [DOI] [PubMed] [Google Scholar]
- 28.Nudel K, McClure R, Moreau M, Briars E, Abrams AJ, Tjaden B, Su X-H, Trees D, Rice PA, Massari P, Genco CA. 2018. Transcriptome analysis of Neisseria gonorrhoeae during natural infection reveals differential expression of antibiotic resistance determinants between men and women. mSphere 3:e00312-18. 10.1128/mSphereDirect.00312-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey JM. 1987. Gonococcal pilin variants in experimental gonorrhea. J Exp Med 165:1344–1357. 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbs CP, Reimann B-Y, Schultz E, Kaufmann A, Haas R, Meyer TF. 1989. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature 338:651–652. 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- 31.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. 2013. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci USA 110:3841–3846. 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zygiel EM, Nelson CE, Brewer LK, Oglesby-Sherrouse AG, Nolan EM. 2019. The human innate immune protein calprotectin induces iron starvation responses in Pseudomonas aeruginosa. J Biol Chem 294:3549–3562. 10.1074/jbc.RA118.006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornelissen CN. 2018. Subversion of nutritional immunity by the pathogenic Neisseriae. Pathog Dis 76:ftx112. 10.1093/femspd/ftx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurakis S, Keller K, Maxwell CN, Pereira K, Chazin WJ, Criss AK, Cornelissen CN. 2019. The novel interaction between Neisseria gonorrhoeae TdfJ and human S100A7 allows gonococci to subvert host zinc restriction. PLoS Pathog 15:e1007937. 10.1371/journal.ppat.1007937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morse SA, Williams RP. 1978. The biology of the gonococcus. CRC Crit Rev Microbiol 7:93–189. 10.3109/10408417909083071. [DOI] [PubMed] [Google Scholar]
- 37.Exley RM, Wu H, Shaw J, Schneider MC, Smith H, Jerse AE, Tang CM. 2007. Lactate acquisition promotes successful colonization of the murine genital tract by Neisseria gonorrhoeae. Infect Immun 75:1318–1324. 10.1128/IAI.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hebeler BH, Morse SA. 1976. Physiology and metabolism of pathogenic Neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol 128:192–201. 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arvidson CG, Tumbrink J, Lenz J, Fantacone ML. 2006. Sublethal concentrations of ciprofloxacin induces genes implicated in horizontal gene transfer in Neisseria gonorrhoeae, p 38. 15th International Pathogenic Neisseria Conference, Cairns, Australia.
- 40.Goh EB, Yim G, Tsui W, McClure JA, Surette MG, Davies J. 2002. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Natl Acad Sci USA 99:17025–17030. 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidsen T, Rødland EA, Lagesen K, Seeberg E, Rognes T, Tønjum T. 2004. Biased distribution of DNA uptake sequences towards genome maintenance genes. Nucleic Acids Res 32:1050–1058. 10.1093/nar/gkh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seifert HS. 1997. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene 188:215–220. 10.1016/s0378-1119(96)00810-4. [DOI] [PubMed] [Google Scholar]
- 44.Haugen TB, Grotmol T. 1998. pH of human semen. Int J Androl 21:105–108. 10.1046/j.1365-2605.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee WL, Harrison RE, Grinstein S. 2003. Phagocytosis by neutrophils. Microbes Infect 5:1299–1306. 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Goytia M, Shafer WM. 2010. Polyamines can increase resistance of Neisseria gonorrhoeae to mediators of the innate human host defense. Infect Immun 78:3187–3195. 10.1128/IAI.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Archibald FS, Duong M-N. 1986. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun 51:631–641. 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardinale JA, Clark VL. 2005. Determinants of nitric oxide steady-state levels during anaerobic respiration by Neisseria gonorrhoeae. Mol Microbiol 58:177–188. 10.1111/j.1365-2958.2005.04807.x. [DOI] [PubMed] [Google Scholar]
- 49.Seib KL, Wu H-J, Kidd SP, Apicella MA, Jennings MP, McEwan AG. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev 70:344–361. 10.1128/MMBR.00044-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winterbourn CC, Kettle AJ, Hampton MB. 2016. Reactive oxygen species and neutrophil function. Annu Rev Biochem 85:765–792. 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 51.Berish SA, Subbarao S, Chen CY, Trees DL, Morse SA. 1993. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect Immun 61:4599–4606. 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bagg A, Neilands JB. 1987. Ferric uptake regulation protein acts as a repressor, employing iron(II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26:5471–5477. 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- 53.De Lorenzo V, Wee S, Herrero M, Neilands JB. 1987. Operator sequences of the aerobactin operon of plasmid colV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol 169:2624–2630. 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu C, Genco CA. 2012. Fur-mediated activation of gene transcription in the human pathogen Neisseria gonorrhoeae. J Bacteriol 194:1730–1742. 10.1128/JB.06176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClure R, Sunkavalli A, Balzano PM, Massari P, Cho C, Nauseef WM, Apicella MA, Genco CA. 2020. Global network analysis of Neisseria gonorrhoeae identifies coordination between pathways, processes, and regulators expressed during human infection. mSystems 5:e00729-19. 10.1128/mSystems.00729-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 57.Rice P, Longden L, Bleasby A. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16:276–277. 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 58.Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, Bouige D, Lacroix C. 2005. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair: reference values. Forensic Sci Int 153:39–44. 10.1016/j.forsciint.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Owen DH, Katz DF. 2005. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl 26:459–469. 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 60.Neyrolles O, Wolschendorf F, Mitra A, Niederweis M. 2015. Mycobacteria, metals, and the macrophage. Immunol Rev 264:249–263. 10.1111/imr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheldon JR, Skaar EP. 2019. Metals as phagocyte antimicrobial effectors. Curr Opin Immunol 60:1–9. 10.1016/j.coi.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner D, Maser J, Lai B, Cai Z, Barry CE, Höner zu Bentrup K, Russell DG, Bermudez LE. 2005. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol 174:1491–1500. 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 63.White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 284:33949–33956. 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia DL, Dillard JP. 2006. AmiC functions as an N-acetylmuramyl-L-alanine amidase necessary for cell separation and can promote autolysis in Neisseria gonorrhoeae. J Bacteriol 188:7211–7221. 10.1128/JB.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baksh KA, Zamble DB. 2020. Allosteric control of metal-responsive transcriptional regulators in bacteria. J Biol Chem 295:1673–1684. 10.1074/jbc.REV119.011444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mcclure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B. 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41:e140. 10.1093/nar/gkt444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tjaden B. 2015. De novo assembly of bacterial transcriptomes from RNA-seq data. Genome Biol 16:1. 10.1186/s13059-014-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greiner LL, Edwards JL, Shao J, Rabinak C, Entz D, Apicella MA. 2005. Biofilm formation by Neisseria gonorrhoeae. Infect Immun 73:1964–1970. 10.1128/IAI.73.4.1964-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steichen CT, Shao JQ, Ketterer MR, Apicella MA. 2008. Gonococcal cervicitis: a role for biofilm in pathogenesis. J Infect Dis 198:1856–1861. 10.1086/593336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steichen CT, Cho C, Shao JQ, Apicella MA. 2011. The Neisseria gonorrhoeae biofilm matrix contains DNA, and an endogenous nuclease controls its incorporation. Infect Immun 79:1504–1511. 10.1128/IAI.01162-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu C, McClure R, Nudel K, Daou N, Genco CA. 2016. Characterization of the Neisseria gonorrhoeae iron and Fur regulatory network. J Bacteriol 198:2180–2191. 10.1128/JB.00166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas CE, Sparling FP. 1996. Isolation and analysis of a fur mutant of Neisseria gonorrhoeae. J Bacteriol 178:4224–4232. 10.1128/jb.178.14.4224-4232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson LA, Day M, Allen J, Scott E, Dyer DW. 2017. Iron-regulated small RNA expression as Neisseria gonorrhoeae FA 1090 transitions into stationary phase growth. BMC Genomics 18:317. 10.1186/s12864-017-3684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mellin JR, Goswami S, Grogan S, Tjaden B, Genco CA. 2007. A novel Fur- and iron-regulated small RNA, NrrF, is required for indirect Fur-mediated regulation of the sdhA and sdhC genes in Neisseria meningitidis. J Bacteriol 189:3686–3694. 10.1128/JB.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casey SG, Shafer WM, Spitznagel JK. 1986. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect Immun 52:384–389. 10.1128/iai.52.2.384-389.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmer A, Criss AK. 2018. Gonococcal defenses against antimicrobial activities of neutrophils. Trends Microbiol 26:1022–1034. 10.1016/j.tim.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zughaier SM, Kandler JL, Shafer WM. 2014. Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages. PLoS One 9:e87688. 10.1371/journal.pone.0087688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canonne-Hergaux F, Calafat J, Richer E, Cellier M, Grinstein S, Borregaard N, Gros P. 2002. Expression and subcellular localization of NRAMP1 in human neutrophil granules. Blood 100:268–275. 10.1182/blood.v100.1.268. [DOI] [PubMed] [Google Scholar]
- 79.Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. 2000. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (NRAMP1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med 192:1237–1247. 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McClure R, Nudel K, Massari P, Tjaden B, Su X, Rice PA, Genco CA. 2015. The gonococcal transcriptome during infection of the lower genital tract in women. PLoS One 10:e0133982. 10.1371/journal.pone.0133982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. 2011. Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10:248–259. 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ong CLY, Berking O, Walker MJ, McEwan AG. 2018. New insights into the role of zinc acquisition and zinc tolerance in group A streptococcal infection. Infect Immun 86:e00048-18. 10.1128/IAI.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chandrangsu P, Rensing C, Helmann JD. 2017. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 15:338–350. 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zola TA, Strange HR, Dominguez NM, Dillard JP, Cornelissen CN. 2010. Type IV secretion machinery promotes ton-independent intracellular survival of Neisseria gonorrhoeae within cervical epithelial cells. Infect Immun 78:2429–2437. 10.1128/IAI.00228-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vogel JP, Andrews HL, Wong SK, Isberg RR. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873–876. 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 86.Berger KH, Isberg RR. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol 7:7–19. 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 87.O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol 33:1210–1220. 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 88.Celli J, De Chastellier C, Franchini D-M, Pizarro-Cerda J, Moreno E, Gorvel J-P. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med 198:545–556. 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jurcisek JA, Brockman KL, Novotny LA, Goodman SD, Bakaletz LO. 2017. Nontypeable Haemophilus influenzae releases DNA and DNABII proteins via a T4SS-like complex and ComE of the type IV pilus machinery. Proc Natl Acad Sci USA 114:E6632–E6641. 10.1073/pnas.1705508114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kouzel N, Oldewurtel ER, Maier B. 2015. Gene transfer efficiency in gonococcal biofilms: role of biofilm age, architecture, and pilin antigenic variation. J Bacteriol 197:2422–2431. 10.1128/JB.00171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nies DH. 1992. Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid 27:17–28. 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- 92.Rensing C, Mitra B. 2007. Zinc, cadmium, and lead resistance and homeostasis, p 321–341. In Nies DH, Silver S (ed), Molecular microbiology of heavy metals. Springer, Berlin, Germany. [Google Scholar]
- 93.Saha RP, Samanta S, Patra S, Sarkar D, Saha A, Singh MK. 2017. Metal homeostasis in bacteria: the role of ArsR–SmtB family of transcriptional repressors in combating varying metal concentrations in the environment. BioMetals 30:459–503. 10.1007/s10534-017-0020-3. [DOI] [PubMed] [Google Scholar]
- 94.Kellogg DS, Peacock WL, Deacon WE, Brown L, Pirkle DI. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol 85:1274–1279. 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wade JJ, Graver MA. 2007. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol Lett 273:35–37. 10.1111/j.1574-6968.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 96.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37:540–546. 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 97.Mikheenko A, Prjibelski A, Saveliev V, Antipov D, Gurevich A. 2018. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 34:i142–i150. 10.1093/bioinformatics/bty266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung GT, Yoo JS, Oh HB, Yeong SL, Cha SH, Kim SJ, Yoo CK. 2008. Complete genome sequence of Neisseria gonorrhoeae NCCP11945. J Bacteriol 190:6035–6036. 10.1128/JB.00566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Applied Biosystems. 1997. User bulletin #2. Relative quantitation of gene expression introduction. Applied Biosystems, Foster City, CA. [Google Scholar]
- 100.Yuan JS, Reed A, Chen F, Stewart CN. 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85. 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agarwal S, Sebastian S, Szmigielski B, Rice PA, Genco CA. 2008. Expression of the gonococcal global regulatory protein Fur and genes encompassing the Fur and iron regulon during in vitro and in vivo infection in women. J Bacteriol 190:3129–3139. 10.1128/JB.01830-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304. 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 103.Goytia M, Dhulipala VL, Shafer WM. 2013. Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol Lett 343:64–69. 10.1111/1574-6968.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kwiatek A, Mrozek A, Bacal P, Piekarowicz A, Adamczyk-Popławska M. 2015. Type III methyltransferase M.NgoAX from Neisseria gonorrhoeae FA1090 regulates biofilm formation and interactions with human cells. Front Microbiol 6:1426. 10.3389/fmicb.2015.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taktikos J, Lin YT, Stark H, Biais N, Zaburdaev V. 2015. Pili-induced clustering of N. gonorrhoeae bacteria. PLoS One 10:e0137661. 10.1371/journal.pone.0137661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download iai.00519-21-s0001.pdf, PDF file, 0.6 MB (628.7KB, pdf)
Data Availability Statement
The data that support the findings of this study are available in the Gene Expression Omnibus, BioProject PRJNA329501, accession number GSE113290. Additional data are available from the corresponding author upon reasonable request.