ABSTRACT
During its progression from the nasopharynx to other sterile and nonsterile niches of its human host, Streptococcus pneumoniae must cope with changes in temperature. We hypothesized that the temperature adaptation is an important facet of pneumococcal survival in the host. Here, we evaluated the effect of temperature on pneumococcus and studied the role of glutamate dehydrogenase (GdhA) in thermal adaptation associated with virulence and survival. Microarray analysis revealed a significant transcriptional response to changes in temperature, affecting the expression of 252 genes in total at 34°C and 40°C relative to at 37°C. One of the differentially regulated genes was gdhA, which is upregulated at 40°C and downregulated at 34°C relative to 37°C. Deletion of gdhA attenuated the growth, cell size, biofilm formation, pH survival, and biosynthesis of proteins associated with virulence in a temperature-dependent manner. Moreover, deletion of gdhA stimulated formate production irrespective of temperature fluctuation. Finally, ΔgdhA grown at 40°C was less virulent than other temperatures or the wild type at the same temperature in a Galleria mellonella infection model, suggesting that GdhA is required for pneumococcal virulence at elevated temperature.
KEYWORDS: Streptococcus pneumoniae, GdhA, CcpA, transcriptional expression, Galleria mellonella, temperature
INTRODUCTION
Streptococcus pneumoniae (the pneumococcus) is responsible for life-threatening diseases of high morbidity and mortality worldwide (1). The bacterium is a resident of the human upper respiratory tract microbiota, and pneumococcal infections generally begin with the asymptomatic colonization of the nasopharynx (2). In favorable conditions, the microbe can disseminate into the middle ear and sinuses, leading to otitis media or sinusitis, and to the lungs, causing pneumonia, for which it is named. The pneumococcus can also disseminate into the bloodstream, brain, or heart, causing sepsis, meningitis, or adverse cardiac events (3). Importantly, it remains enigmatic as to what results in the transition from colonization to invasiveness. One hypothesis is that the ability of the pneumococcus to adapt to changes in local host tissue environments, such as the upper respiratory tract, govern its ability to survive and enable diseased states (4).
Microbes commonly encounter changes in temperature when they move from the external environment to the host and between different host tissues. It has been shown that temperatures range from 30°C to 32°C on the anterior surface of the nasopharynx, and this increases to 34°C in the posterior section of the nasopharynx (5). In the lungs, bloodstream, and central nervous system, the temperature rises to 37°C. The body temperature can increase to 39°C to 40°C as an inflammatory response during infection (6). Therefore, the pneumococcus has to combat distinct temperatures in different tissue sites in health and disease.
Temperature fluctuations have a fundamental impact on microbial physiology, virulence, and survival (7). Therefore, it is important to understand how changes in transcription contribute to pneumococcal adaptation to temperature changes within its host. Temperature increase within a mammalian host may act as a stimulus for a rapid increase in the synthesis of a highly conserved set of proteins referred to as heat shock proteins (HSPs). GroEL, DnaK, and Clp proteins have been identified as major heat shock proteins in the pneumococcus (8) and many other pathogens (9, 10). Further, these are required for S. pneumoniae protein quality, growth, competence, thermal adaptation, and induction of virulence genes, including capsule biosynthesis (cps2A) and cytolytic toxin pneumolysin (ply) (8, 11–13). In a recent study, thermosensing was reported to modulate the synthesis of pneumococcal capsular polysaccharide and factor H binding proteins, which have been linked to complement-mediated evasion of the microbe (5).
In addition to specialized heat shock proteins, the microbes utilize distinct mechanisms to optimize their survival in response to temperature fluctuations. For example, in Streptococcus thermophilus, resistance to heat shock is increased by an insertional mutation of deoD, which encodes a purine nucleoside phosphorylase (PNP), which generates the free purine and deoxyribose-1-phosphate through the phosphorolytic cleavage of nucleosides (14). It was suggested that by regulating the intracellular concentration of ppGpp level, PNP contributes to survival at high temperature.
Mutational analysis of α-amylases has demonstrated that the flexibilities of these enzymes differ as a structural strategy to adapt to environmental temperatures in thermophilic and psychrophilic microorganisms (15). In another example, the polymerization of the cell division protein FtsZ is temperature dependent, and the level of entropy for assembly of the protein changes with temperature in the host (16). Another example is glutamate dehydrogenase, which has been identified as a protein thermosensor in the hyperthermophile Aeropyrum pernix K1 (17).
Glutamate dehydrogenase (GdhA) is a member of a group of oxidoreductase enzymes that catalyze the reversible oxidative deamination of l-glutamate to 2-oxoglutarate (α-ketoglutarate) by connecting amino acid metabolism to tricarboxylic acid (TCA) cycle (18). Pneumococci do not have a functional TCA cycle because the complete set of genes could not be identified in their AT-rich genome (19). Studies on glutamate dehydrogenase demonstrated that it has additional roles in response to various nutritional signals. Girinathan et al. showed that uptake of glutamate is crucial for Clostridium difficile to colonize in the host and that its colonization and pathogenicity of the animal gut were inhibited in the absence of glutamate dehydrogenase (GDH) (20). In Bacillus subtilis, the growth of a rocG (a homologue of gdhA) mutant strain was restricted by a β-lactam antibiotic, cefuroxime (CEF), compared to the wild-type strain, suggesting that glutamate dehydrogenase may be involved in the cell envelope stress response. In further support of this hypothesis, it was also revealed that RocG has an impact on intracellular pH and may protect cells against growth arrest by CEF (21).
To increase our understanding of pneumococcal thermal adaptation, we obtained transcriptional profiles of the pneumococcus at different temperatures. We identified differential expression of a total of 252 genes at 34°C and 40°C at mid-exponential growth phase relative to 37°C. One of the identified genes differentially expressed at 40°C encoded glutamate dehydrogenase (GdhA). In this study, we showed that GdhA is important in pneumococcal adaptation to high temperature as well as for nutrient metabolism and virulence.
RESULTS
Transcriptome analyses of pneumococci grown at different temperatures.
Analysis of the pneumococcal transcriptomes obtained at 34°C and 40°C, relative to 37°C, showed a significant differential expression of a total of 252 genes over the two comparisons (Table S3 in the supplemental material). Compared to 37°C, at 34°C, 97 genes were upregulated, while 35 were downregulated during mid-exponential growth. The expression of 25 operons was affected at 34°C, ranging between 2- to 53-fold upregulation and 2- to 4-fold downregulation (Table 1). On the other hand, at 40°C, 43 genes were upregulated compared to 37°C, and 76 genes were downregulated, representing 21 operons. The expression of these operons ranged between 2- and 32-fold upregulation and 2- and 44-fold downregulation (Table 1). Notably, several operons that were highly upregulated at 34°C were found to be downregulated at 40°C. The differentially expressed genes were functionally diverse, coding for bacteriocin synthesis, competence, transcriptional regulation, and sugar and purine metabolism. In addition, the transcriptional analysis also revealed the differential expression of genes identified previously to be important in temperature homeostasis, including those coding for chaperonin or heat shock proteins coded by SPD_0458-SPD_0461 and SPD_1709-SPD_1710 loci, respectively.
TABLE 1.
Expression levels and putative functions of pneumococcal genes that are sensitive to temperature changes in wild-type D39 at mid-exponential growth phasea
| Locus | Function(s) | Fold change at: |
|
|---|---|---|---|
| 34°C | 40°C | ||
| SPD_0035-SPD_0036 | Hypothetical protein | 2.32 to 3 | −1.93 to −2.18 |
| SPD_0046-SPD_0047 | Bacteriocin synthesis | 9.33 to 9.35 | −7.69 to −7.89 |
| SPD_0049-SPD_0056 | Competence development, purine metabolism | 2.58 to 14.81 | −1.95 to −9.74 |
| SPD_0059-SPD_0060 | Purine metabolism | 1.92 to 2.79 | |
| SPD_0093-SPD_0096 | Hypothetical protein | −2.54 to −3.02 | |
| SPD_0113-SPD_0116 | Hypothetical protein | −2.9 to −4.01 | 19.9 to 32.51 |
| SPD_0132-SPD_0133 | Hypothetical protein | 6.23 to 52.94 | −5.8 to −31.81 |
| SPD_0452-SPD_0453 | Phage integrase protein, restriction/modification subunit | −2.35 to −2.37 | |
| SPD_0447-SPD_0448 | MerR family protein, glutamine synthetase | −3.66 to −4.66 | 2.96 to 4.12 |
| SPD_0466-SPD_0468 | Bacteriocin synthesis | 2.49 to 7.01 | −2.41 to −6.49 |
| SPD_0470-SPD_0474 | Bacteriocin synthesis | 3.38 to 16.54 | −2.02 to −9.59 |
| SPD_0701-SPD_0702 | Histidine kinase, response regulator | 1.96 to 2.06 | −2.24 to −2.59 |
| SPD_0723-SPD_0724 | Sugar metabolism | −1.99 to −1.9 | |
| SPD_0771-SPD_0773 | Sugar metabolism | 1.91 to 2.12 | −2.46 to −3.04 |
| SPD_1098-SPD_1099 | ABC transporter | −2.9 to −3.44 | 2.68 to 2.79 |
| SPD_1175-SPD_1179 | Hypothetical protein, ABC transporter protein | 1.97 to 2.48 | |
| SPD_1380-SPD_1381 | Hypothetical protein, unclassified metabolism | 1.98 to 2.35 | |
| SPD_1514-SPD_1516 | ABC transporter protein, hypothetical protein | 2.74 to 3.99 | |
| SPD_1527-SPD_1528 | Hypothetical protein, ABC transporter protein | 2.06 to 2.9 | −2.02 to −2.29 |
| SPD_1650-SPD_1652 | ABC transporter protein | 2.61 to 4.01 | |
| SPD_1682-SPD_1698 | tRNA synthesis | −2.01 to −2.83 | 2.15 to 3.41 |
| SPD_1740-SPD_1744 | Competence damage protein, hypothetical protein | 3.1 to 5.84 | −3.22 to −5.37 |
| SPD_1830-SPD_1831 | Sugar metabolism, PTS transporter system | 1.97 to 2.21 | |
| SPD_1855-SPD_1856 | Hypothetical protein | 3.71 to 6.18 | −2.42 to −5.38 |
| SPD_1857-SPD_1862 | Competence development | 7.65 to 37.39 | −5.64 to −20.39 |
| SPD_2033-SPD_2035 | Ribosomal surface protein, competence protein, helicase | 2.82 to 3.55 | |
| SPD_2063-SPD_2065 | Competence development | 35.18 to 53.65 | −23.95 to −44.92 |
| SPD_2068-SPD_2069 | Serine protease, transcriptional regulator SpoJ | 4.4 to 6.38 | −3.5 to −3.73 |
Fold changes are approximately ≥2.0 or less than or equal to −2.0 of each operon. All P values are <0.001.
GdhA enzyme activity affects pneumococcal cell size and growth at high temperature.
The transcriptome data in this study showed that gdhA was downregulated at 34°C and upregulated at 40°C (Table S3). Further expression analysis of gdhA by quantitative reverse transcriptase PCR (qRT-PCR) also showed 2.32-fold downregulation at 34°C and an increase of 4.51-fold at 40°C, which was consistent with the microarray data. While the involvement of this enzyme in temperature adaptation in hyperthermophiles was shown (17), in mesophiles, its role has not been studied. Due to the lack of information about its involvement in thermal adaptation in mesophiles, and its temperature-dependent differential expression in the pneumococcus, we hypothesized that GdhA is important in pneumococcal thermal adaptation.
To test this hypothesis, we created an isogenic ΔgdhA strain and a genetically complemented version of it. These strains were then characterized for cell size, growth, and GdhA activity at different temperatures. The results showed that the wild-type D39 strain had a similar cell size at 37°C (0.48 ± 0.014 μm; n = 60) and 34°C (0.44 ± 0.017 μm; n = 60) (P > 0.05) but had a significantly reduced size when incubated at 40°C (0.36 ± 0.003 μm) compared to 34°C and 37°C (P < 0.05) (Table 2). The complemented mutant strain (CompΔgdhA) had a similar cell size to the wild type at all temperatures (for 34°C, 37°C, and 40°C, 0.45 ± 0.03, 0.48 ± 0.06, and 0.34 ± 0.03 μm, respectively; P > 0.05). In contrast, ΔgdhA cells were smaller at 34°C (0.39 ± 0.00 μm; n = 60) and 40°C (0.25 ± 0.01 μm; n = 60) than at 37°C (0.46 ± 0.00 μm; n = 60) (P < 0.05 for both comparisons), and the difference in cell size at 40°C and at 34°C was also significant (P < 0.0001) (Table 2). The results show that incubation temperature has a major impact on cell size at 34°C and at 40°C and that GdhA contributes to regulation of cell size at higher temperature. A representative image of cell measurement at 40°C is shown in Fig. S1.
TABLE 2.
Cell size of wild-type D39, ΔgdhA, and complemented mutant strain, CompΔgdhA, grown in CDM supplemented with 55 mM glucose at 34°C, 37°C, and 40°Ca
| Strain | Cell size (μm) at: |
||
|---|---|---|---|
| 34°C | 37°C | 40°C | |
| D39 (WT) | 0.44 ± 0.01 | 0.48 ± 0.01 | 0.36 ± 0.00 |
| ΔgdhA | 0.39 ± 0.00 | 0.46 ± 0.00 | 0.25 ± 0.01 |
| CompΔgdhA | 0.45 ± 0.03 | 0.48 ± 0.06 | 0.34 ± 0.03 |
Cell size of D39 wild type reduced when grown at 34°C or 40°C compared to 37°C (P < 0.05). A similar size trend was recorded in the absence of gdhA (P < 0.05). While no difference was observed between D39 and ΔgdhA at 37°C, ΔgdhA cells were significantly smaller than the wild type at 34°C or 40°C. No difference between the wild type and complemented mutant strain was seen. The standard error shows the mean of three different experiments for at least 50 individual cell measurements.
Next, at different temperatures, we determined GdhA involvement in pneumococcal growth rate and yield, which refers to the maximal growth difference from the time zero. In brain heart infusion (BHI), Todd-Hewitt broth (THB), and casein-tryptone medium (CAT) media, no significant differences were observed in growth rate and yield of the wild type and ΔgdhA at the different temperatures, showing that the deletion of gdhA does not lead to a growth defect in rich medium (Fig. S2). On the other hand, when tested in chemically defined medium (CDM) supplemented with glucose where the pneumococci were predicted to encounter a more challenging environment, very likely due to limited amount of thermoprotective molecules such as glycine and betaine compared to the growth in rich media, the growth yield (1.19 ± 0.01) and rate (0.30 ± 0.01 h−1) of the wild type decreased significantly (P < 0.0001 for both comparisons) at 40°C compared to 34°C (growth yield, 1.38 ± 0.01, and growth rate, 0.35 ± 0.01 h−1; n = 9) and 37°C (growth yield, 1.48 ± 0.01; growth rate, 0.39 ± 0.01 h−1; n = 9) (Fig. 1) (22). However, there was no significant difference in growth profiles of the wild type at 34°C and 37°C (P > 0.05). As observed for the wild type, ΔgdhA had a significantly lower growth yield and rate at 40°C (growth yield, 0.80 ± 0.02, and growth rate, 0.21 ± 0.02 h−1; n = 9) relative to its growth at 34°C or 37°C (growth yields, 1.19 ± 0.01 or 1.22 ± 0.08, and growth rates, 0.30 ± 0.02 or 0.33 ± 0.01 h−1, respectively; n = 9) (P < 0.05). We did not observe any statistical difference in growth rate of the wild type and mutant at 34°C and 37°C (P > 0.05); however, the wild type had a higher yield than the mutant at 37°C. Moreover, compared to the wild type, ΔgdhA had significantly lower growth rate at 40°C only (P < 0.0001) (Fig. 1). These results show that pneumococcal growth is influenced by increased temperature and that gdhA is required for optimal pneumococcal growth at 40°C.
FIG 1.
Growth profiles of pneumococcal strains in CDM supplemented with 55 mM glucose at different temperatures. Error bars show the standard error of the mean for three individual measurements, each with three replicates (n = 9). Significant differences were seen comparing the growth rate of the mutant strain to the wild-type D39 or among themselves at tested temperatures using ANOVA followed by Tukey’s multiple-comparison test. ****, P < 0.0001.
To determine a link between the observed differences in cell size and growth properties at the high temperature and the GdhA, we determined the enzyme level in the pneumococcal strains grown in CDM supplemented with 55 mM glucose at different temperatures (Table 3). The results showed that the highest GdhA activity in the wild type was at 40°C (74.48 ± 0.83 mU/mg protein) and the lowest at 34°C (16.51 ± 0.65 mU/mg protein) relative to at 37°C (38.19 ± 0.66 mU/mg protein), which was consistent with the transcriptome data. The difference in enzyme activity at 34°C and 40°C relative to at 37°C was significant (P < 0.0001 for comparison of both). There was significantly less GdhA activity in ΔgdhA compared to the wild type at all tested temperatures (for 34°C, 37°C, and 40°C, 1.54 ± 0.35, 2.51 ± 0.19, and 0.4 ± 0.02 mU/mg protein, respectively) (P < 0.001). The genetic complementation in CompΔgdhA reconstituted the activity (for 34°C, 37°C, and 40°C, 13.25 ± 0.79, 28.89 ± 0.51, and 57.59 ± 2.27 mU/mg protein, respectively). These results are consistent with GdhA influencing pneumococcal cell size and growth profiles at high temperature.
TABLE 3.
Glutamate dehydrogenase activity in the wild type (WT), ΔgdhA, and CompΔgdhA at 34°C, 37°C, and 40°C
| Strain | Glutamate dehydrogenase activity (mU/mg protein) ata: |
||
|---|---|---|---|
| 34°C | 37°C | 40°C | |
| WT | 16.51 ± 0.65 | 38.19 ± 0.66 | 74.48 ± 0.83 |
| ΔgdhA | 1.54 ± 0.35 | 2.51 ± 0.19 | 0.04 ± 0.02 |
| CompΔgdhA | 13.25 ± 0.79 | 28.89 ± 0.51 | 57.59 ± 2.27 |
The enzyme activity is expressed as nmol/ml (mU/ml) of NADH released from the substrate per minute at pH 7.6 and normalized against mg protein and expressed for 1 × 108 CFU. The highest induction of GdhA was obtained at 40°C in the wild type and complemented mutant strain CompΔgdhA. The activity in ΔgdhA was negligible at all temperatures.
GdhA activity affects hemolytic activity.
A previous study showed an impact on virulence gene expression that correlated with temperature change (6). Therefore, in addition to the impact of temperature on growth properties of the pneumococcus, we also investigated the effect of temperature and GdhA on the key pneumococcal virulence determinants capsule, and pneumolysin, which is the main determinant of pneumococcal hemolytic activity, directly correlates with its virulence (23). Pneumolysin activity was assessed by measuring the hemolysis of the red blood cells by pneumococcal cell lysates prepared from the cultures grown at different temperatures (23). The results showed that the wild type and the complemented mutant strain (CompΔgdhA) had lower hemolytic activity at 40°C (8.59 ± 0.46 hemolytic units [HU]/mg protein; n = 9) relative to at 37°C (12.56 ± 1.21 HU/mg protein; n = 9) (P < 0.05 and P < 0.01, respectively), while no significant difference was recorded at 34°C (11.44 ± 0.52 HU/mg protein; n = 9) and 37°C (P > 0.05). The lack of gdhA resulted in approximately 1.5-fold lower activity in cultures grown at 40°C (6.22 ± 0.34 HU/mg protein) than 34°C or 37°C (11.19 ± 0.45 and 12.61 ± 1.88 HU/mg protein; n = 9) (P < 0.05) (Fig. 2). Furthermore, at 40°C, ΔgdhA had lower hemolytic activity than the wild type (P < 0.001), whereas when cultured at 34°C or 37°C, the wild type and ΔgdhA had similar activity levels (P > 0.05). We conclude that GdhA is positively associated with pneumolysin activity at higher temperatures.
FIG 2.
Hemolytic activity of pneumococcal strains grown in CDM supplemented with 55 mM glucose at different temperatures. Hemolytic activity assay was performed as a measure of pneumolysin activity and done using 4% (vol/vol) defibrinated sheep blood. Significant differences were seen in pneumococcal strains and at different temperatures using ANOVA and Tukey's multiple-comparison tests. Error bars show the standard error of the mean for three individual measurements, each with three independent biological samples. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Temperature affects capsule production independent of GdhA activity.
The glucuronic acid assay has been used as a direct measurement of capsule polysaccharide content (24). When this assay was applied to our strains in BHI (rich medium), we found that the wild-type pneumococcal strain produced similar amounts of the type 2 capsule subunit, glucuronic acid, at the different temperatures (P > 0.05) (for 34°C, 37°C, and 40°C, 176.1 ± 8.7, 170.2 ± 7.9, and 176.8 ± 6.1 μg protein per 109 CFU, respectively). However, in CDM, the wild-type pneumococcus produced more glucuronic acid at 34°C (190.5 ± 5.4 μg protein per 109 CFU) than at 37°C (171.8 ± 2.7 μg protein per 109 CFU) (P < 0.01) and 40°C (156.0 ± 3.0 μg protein per 109 CFU) (P < 0.0001). The amount of glucuronic acid produced by complemented strain CompΔgdhA was similar to that of the wild type at all temperatures (P > 0.05).
The ΔgdhA strain also produced significantly more glucuronic acid when grown in CDM at 34°C (207.3 ± 3.3 μg protein per 109 CFU) than at 37°C or 40°C (156.4 ± 1.9 and 143.7 ± 4.7 μg protein per 109 CFU, respectively) (P < 0.0001), whereas no difference was observed between 37°C and 40°C (P > 0.05). Compared with the wild type, ΔgdhA produced more glucuronic acid at 34°C (P < 0.05), while there was no significant difference at either 37°C or 40°C (P > 0.05) (Fig. 3). These results did not support a role for GdhA in capsule synthesis at high temperature, whereas it appears to be involved at the lower temperature.
FIG 3.

To access capsule, we measured glucuronic acid concentration of pneumococcal strains grown in CDM supplemented with 55 mM glucose at different temperatures. Significant differences were seen by comparing the amount of capsular polysaccharide produced among pneumococcal strains at different temperatures using ANOVA and Tukey's multiple-comparison tests. Error bars show the standard error of the mean for three individual measurements, each with three independent experiments. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
GdhA contribution to biofilm formation.
Because pneumococci reside in the nasopharynx within biofilm communities, where the temperature is suggested to be around 34°C (25), we evaluated the effect of temperature on pneumococcal biofilm generation using a crystal violet assay on overnight biofilms. The results showed that the wild type formed a similar amount of biofilm at 34°C and 37°C (optical density at 595 nm [OD595], 2.55 ± 0.08 and 2.78 ± 0.05 per 108 CFU, respectively; n = 9), whereas at 40°C (OD595, 2.40 ± 0.04 per 108 CFU; n = 9), the pneumococcus generated significantly less biofilm than at 37°C (P < 0.05). In addition, there was no difference in biofilm formation by the CompΔgdhA strain and the wild type at any of the tested temperatures. Similarly, loss of gdhA did not affect the formation of biofilm at 34°C (OD595, 2.56 ± 0.10 per 108 CFU; n = 9), but the biofilm was reduced at 40°C (OD595, 2.03 ± 0.04 per 108 CFU; n = 9) (P < 0.0001) relative to 37°C (OD595, 2.59 ± 0.07 per 108 CFU; n = 9) (P < 0.0001). A significant difference between the wild type and ΔgdhA strains was also observed at 40°C (P < 0.05), but there was no difference in biofilm formation between the gdhA mutant and the wild type at other temperatures (Fig. 4). Taken together, these data suggest that GdhA could govern biofilm formation in the nasopharynx in a temperature-dependent manner.
FIG 4.
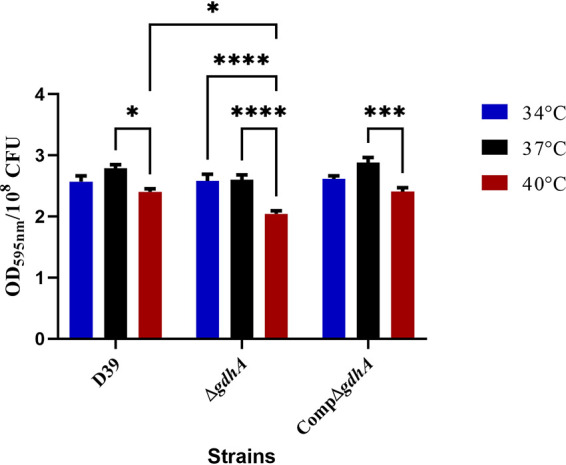
Biofilm formation of pneumococcal strains at different temperatures. Biofilms were measured using crystal violet assay on overnight cultures grown in static conditions. All values are expressed as optical density of stained adherent cells at 595 nm. Each column represents means of three individual measurements each with triplicates with their standard error of means. Mean differences in biofilm formation of the mutant strain were compared to the wild-type strain and among themselves at tested temperatures using ANOVA and Tukey's multiple-comparison tests. *, P < 0.05, ***, P < 0.01; ****, P < 0.001.
Inactivation of GdhA reduces the pH homeostasis at a temperature shift.
Because the external pH has been shown to influence the transcription of gdhA in other systems (26), we wished to test the role of GdhA in pH homeostasis and its synergy with different temperatures in the pneumococcus. This was accomplished by culturing pneumococcal strains at different acidity levels and simultaneously varying the temperature (Fig. 5). The results showed that the survival of the wild-type strain was lowest at pH 5.0 irrespective of temperature, whereby the survival decreased from log10 8.5 to 5.2 CFU/ml within 120 min (P < 0.0001). In addition, in contrast to 34°C and 37°C, incubation at 40°C adversely affected pneumococcal survival at pH 5.4, suggesting that high temperature reduces the tolerance to acidic conditions.
FIG 5.
Acid tolerance of pneumococcal strains at 34°C, 37°C, or 40°C. Data represent the mean of three independent experiments, each with three replicates (n = 9). Mean differences in growth profile of the mutant strain were compared to the wild-type strain at different pH and temperature conditions using two-way ANOVA and Tukey's multiple-comparison tests. *, P < 0.05; ****, P < 0.0001.
Importantly, the acidic conditions had a more severe impact on the survival of the ΔgdhA mutant strain than the wild type. At 34°C and 37°C, ΔgdhA was less tolerable to acidic pH (5.0 to 6.4) than the wild type after 60 min (P < 0.05). On the other hand, at 40°C, the viability of ΔgdhA was significantly reduced at pH range between 5.0 to 6.4 within 30 min (P < 0.0001) compared to the wild type, while no difference was observed in the survival at pH 7.0 to 8.0 (P > 0.05). Hence, the data suggest that environmental pH and temperature have a synergistic adverse impact on the survival ability of pneumococcus and that GdhA plays an influential role in pneumococcal survival at acidic conditions, particularly at high temperature.
Contribution of GdhA to pneumococcal virulence.
The results above demonstrated that GdhA is important for pneumococcal metabolism and adaptation to high temperature. Next, we investigated GdhA’s role in pneumococcal virulence in vivo using a Galleria mellonella infection model. Comparing to other traditional mammalian models, G. mellonella provides technical and logistical advantages (27). The insect is large enough to be injected with defined doses of microbes, and G. mellonella is easy to maintain with a short life span for quick experiments. G. mellonella can be kept at 20°C to 30°C, and infection studies can be performed at below room temperature and over 37°C, which makes it ideal to test the hypotheses of this study.
G. mellonella larvae were infected with pneumococci grown at different temperatures, and the larvae were incubated at 37°C. There was a significant reduction in survival of larvae infected with 5 × 105 CFU wild type or CompΔgdhA that had been cultured at 40°C compared to infection with pneumococci grown at 37°C (P < 0.01) (Fig. 6). No difference was recorded in mortality between the larvae incubated at 34°C and 37°C (P > 0.05). On the other hand, ΔgdhA was significantly less virulent when cultured at 40°C (P < 0.0001) than when grown at 37°C (Fig. 6), and the virulence of ΔgdhA was significantly diminished at growth temperature of 40°C compared to the virulence of wild type, and the reduced virulence was consistent with the low number of recovered ΔgdhA larvae during the course of infection relative to the wild-type counts (Fig. S4) at the same temperature. A representative image of infected larvae is also shown in Fig. S3.
FIG 6.

Survival of G. mellonella infected with 5 × 105 CFU/larvae. Each dot represents the number of dead larvae for individual group (n = 10) at 34°C (blue), 37°C (black), or 40°C (red). Significant differences in mortality numbers are seen comparing with the D39 wild-type strain with the mutant strain and among themselves at tested temperatures using ANOVA and Tukey’s multiple-comparison tests. Error bars show the standard error of the mean. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
GdhA has a temperature-dependent effect on D39 carbon metabolism.
S. pneumoniae is a facultative anaerobe and therefore does not possess an active TCA cycle, preferring to ferment a vast array of sugars. As GdhA has been implicated in the redirection of metabolism in other pathogens (28, 29), we wanted to further investigate the role of GdhA in controlling metabolic flux in response to different temperatures. After feeding the cells a glucose carbon source, metabolic end products of lactate, formate, and acetate were measured, and the results are displayed in Fig. 7.
FIG 7.
Measurement of metabolic end products in pneumococcal strains grown to mid-exponential phase in CDM supplemented with 55 mM glucose at different temperatures. The number of products normalized against 108 CFU. Values (mM) are mean of three independent experiments, each with three replicates and expressed for 108 CFU. ±, SEM; LDH, lactate dehydrogenase; PFL, pyruvate-formate lyase; ACK, acetate kinase.
Results indicated that lactate was the main end product for all the pneumococcal strains, irrespective of temperature. However, the amount of lactate varied among strains and with temperature changes. The difference in lactate concentration at 34°C and 40°C relative to 37°C was significant for all strains (P < 0.0001), but ΔgdhA had significantly less lactate than the wild type and the complemented mutant (CompΔgdhA) at all temperatures (P < 0.01) (Fig. 7), suggesting that GdhA has both a temperature-dependent and -independent impact on pathways affecting lactate production. On the other hand, while the wild type and the complemented mutant generated a negligible level of formate, ΔgdhA generated significantly more formate than the wild type at all tested temperatures (P < 0.0001). In addition, the amount of formate produced by the mutant was significantly higher at 40°C than 34°C and 37°C (P < 0.05 and P < 0.01, respectively), showing that the absence of GdhA leads to what is referred to as mixed acid formation (19). Acetate was also detected only at negligible levels in culture supernatants of all strains at all temperatures. The results above demonstrated that GdhA is important for pneumococcal carbon utilization and metabolism and, importantly, its adaptation to high temperature.
CcpA plays a role in gdhA regulation.
As GdhA clearly impacted carbon utilization (Fig. 7), we reasoned that CcpA, a major regulator of carbon flux in the pneumococcus, and other pathogens (30–32) might be involved. Indeed, CcpA has a major regulatory impact on the expression of ldhA and codes for lactate dehydrogenase, and pflA codes for pyruvate formate lyase, which determine the levels of lactate and formate in the pneumococcus, respectively. We therefore hypothesized that CcpA is also involved in gdhA regulation. Belitsky et al. had identified a putative CcpA binding site (cre) in B. subtilis (33) with the consensus sequence TGWAARCGYTWNCW (where N is any base, W is A or T, R is A or G, and Y is C or T) (34), and in silico analysis showed two cre-like sequences, AGAAAAACGTTCGT and TGAAAAAAAATTCT, in the putative promoter region of gdhA (PgdhA), suggesting that CcpA might control gdhA by binding to a cre site (Fig. S5). EMSA analysis, however, did not show the binding of recombinant CcpA to PgdhA (data not shown), ruling out a direct regulation of gdhA by CcpA.
We then hypothesized that CcpA may control gdhA expression indirectly through its impact on metabolism. To test this, PgdhA::lacZ was introduced into the wild-type, ΔgdhA, and ΔccpA backgrounds. The recombinant strains PgdhA::lacZ-wt, PgdhA::lacZ-ΔgdhA, and PgdhA::lacZ-ΔccpA were grown in CDM supplemented with 55 mM glucose at different temperatures. The results showed that β-galactosidase activity in PgdhA::lacZ-wt was significantly higher at 40°C (190 ± 2.7 MU/108 CFU) than at 34°C and 37°C (68.5 ± 2.3 and 87.8 ± 3.2 MU/108 CFU, respectively; n = 3) (P < 0.0001) (Fig. 8), confirming the microarray data. In PgdhA::lacZ-ΔgdhA, the activity level followed the same pattern as the wild type at 34°C (85.8 ± 2.9 MU/108 CFU) and 37°C (102.4 ± 0.6 MU/108 CFU) (P > 0.05), but at 40°C, PgdhA::lacZ-ΔgdhA had significantly more activity (258.1 ± 4.6 MU/108 CFU) than the wild type (P < 0.0001), suggesting that GdhA represses its own gene expression at high temperature. Furthermore, the amount of β-galactosidase in PgdhA::lacZ-ΔccpA was significantly lower at 37°C (68.4 ± 1.2 MU/108 CFU) and 40°C (91.8 ± 6.9 MU/108 CFU) than the wild type (P < 0.01); however, the reduction was more pronounced at 40°C (P < 0.0001) than at 37°C (P < 0.05). Together, these data suggest that CcpA positively modulates gdhA transcription in a temperature-dependent manner and that this effect is indirect (Fig. 8).
FIG 8.

β-Galactosidase activity of pneumococcal strains grown in CDM supplemented with 55 mM glucose. The activity is expressed in nmol p-nitrophenol/min/ml using mid-exponential-phase cultures. In the ΔccpA, the expression of PgdhA was significantly lower at 37°C than in the WT. Values are average of at least three independent experiments, each with three replicates. Error bars indicate the SEM. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
DISCUSSION
In occupying different anatomical sites within the human host, the pneumococcus encounters fluctuating temperatures and therefore needs to maintain homeostasis at these different temperatures to sustain its survival. Our results show that the pneumococcus is extremely sensitive to changes in temperature fluctuations, as evidenced by differential expression of more than 11% of its gene content. Interestingly, there are dedicated transcriptional profiles for different temperatures. While a block of genes is upregulated, for example, at 34°C, the same set of genes is downregulated at 40°C, suggesting that not only overexpression, but also downregulation of certain genes, is simultaneously required for temperature adaptation and is likely governed by the same set of regulatory factors. Differential expression of genes at different temperatures is consistent with metabolic and genetic reprogramming of the pneumococcal cell, and it is very likely that nutrient availability has a major impact on survival at different temperatures. This is supported by the fact that the pneumococcal growth rate was not affected at high temperature in a nutrient-rich environment, but there was a reduced rate of growth in the nutrient-limited medium, CDM, which better reflects the nutrient-poor environment of the host. We noted that ΔgdhA grew with a shorter lag phase at 37°C than the wild type. This can be linked to the key role played by 2-oxoglutarate (or 2-ketoglutarate), which is converted to glutamate via GdhA activity, in carbon and nitrogen metabolism (35). It is plausible that the lack of GdhA affected the metabolic balance and led to faster ATP generation but reduced ATP yield, which may account for observed growth feature of the mutant.
The metabolite 2-oxoglutarate stands at the crossroads between carbon metabolism and nitrogen metabolism. As one of the substrates of glutamate synthase (GOGAT), 2-oxoglutarate provides the de novo carbon skeleton for the two most important nitrogen-containing compounds in the cell, glutamate and glutamine (36). Moreover, as the product of glutamate metabolism by glutamate dehydrogenase, 2-oxoglutarate can be the entry point into central metabolism for the carbon skeletons of several amino acids (including arginine, ornithine, proline, and histidine), which are catabolized to glutamate (37).
In these studies, temperature-mediated transcriptional alterations revealed important clues about the pneumococcal biology in different anatomical niches and during health and disease. The pneumococcus alters the expression of some of its key pathways, including the expression of competence (com) and bacteriocin (blp) genes in response to temperature shifts. Serving as an excellent internal control, our results are consistent with the modulation of competence with temperature, which is the most optimal between 32 to 34°C and decreases beyond this range (38). Increased expression of bacteriocin coding genes at 34°C is also consistent with their role in microbial competition in the upper respiratory tract (39).
Another noteworthy observation from these studies was the increased expression of purine (pur) metabolism genes at 34°C and their downregulation at 40°C, which could explain the reduced growth rate and cell division at this temperature. A reduced growth rate observed at 40°C was also in line with reduced expression of protein translation genes. In support, it has been reported that approximately half of cellular energy is consumed for translation by ribosomes during cellular proliferation (40), and this points to the reduction in protein synthesis as a possible means to save metabolic energy needed for an unforeseen stress response. Intriguingly, we have detected differential expression of 53 hypothetical genes at different temperatures. Therefore, future studies are required to investigate the involvement of these hypothetical genes in thermal adaptation in detail.
Our findings here indicate the involvement of glutamine metabolism specifically and nitrogen metabolism in general in pneumococcal thermal adaptation. In this study, we focused on gdh’s role in thermal adaptation; its expression went down 2.2-fold at 34°C and increased 2.1-fold at 40°C compared to 37°C. gdhA was shown to be repressed by the regulator of amino acid metabolism CodY and GlnR (also known as MerR), which is shown to repress the expression of genes involved in glutamine synthesis and uptake (35). In this study, we found that the expression of codY increased 2.1-fold at 34°C and did not significantly change at 40°C compared to 37°C in the mutant. On the other hand, the expression of merR went down 3.66-fold at 34°C and increased by 2.96-fold at 40°C in ΔgdhA. These results show that the level of GdhA may contribute to regulation of merR.
The importance of GdhA has been studied in many different microorganisms from the perspective of nutrient metabolism (41, 42); however, its involvement in temperature adaptation has not been studied in mesophiles. In this study, we show that GdhA plays a role in specific phenotypes during temperature adaptation, namely, growth, cell size, pH homeostasis, virulence, and, importantly, metabolic reprogramming. In the case of metabolism, deletion of gdhA resulted in a significant shift in the metabolic end product profile of S. pneumoniae, leading to increased production of formate, particularly at 40°C. We hypothesize that this result could be due to significant upregulation at 40°C of merR, which is known to involve regulation of pyruvate node enzyme pyruvate formate lyase (PflB), whose activity generates formate (43).
Our results shed light on several important facets of pneumococcal survival at different temperatures, which may be relevant for in-host survival during colonization and invasive disease, for example, temperature impact on cell size and biofilm formation. In this regard, we found that temperature shifts have a significant impact on pneumococcal cell size. One hypothesis that could explain this phenomenon would be the linkage between downregulation of certain genes involved in nutrient uptake, such as those required for 6-phospho-beta-glucosidase metabolism (celB) and iron transport (SPD_1651). It can also be speculated that environmental pH impacts cell size by modulating cell division and cell cycle progression controlled by FtsZ protein. It should be noted, however, that microarray data did not indicate significant change in the transcript level of ftsZ (SPD_1479). Moreover, temperature may show its impact on pneumococcal biology through its impact on purine biosynthesis. Our microarray data show downregulation of genes involved in purine biosynthesis at 40°C relative to 37°C. It has been reported that purine biosynthesis is important for bacterial proliferation (44).
Streptococcus pneumoniae forms biofilms in the nasopharynx (45). Chao et al. reported that S. pneumoniae forms denser biofilms at 34°C than at 37°C in vivo (45). Here, we demonstrate that higher temperatures (40°C) attenuate the biofilm formation, which may reflect changes in cell surface architecture (46) and ultimately affect the aggregation of pneumococcal cells (47). It is also possible that the decrease in biofilm formation at high temperature is linked to downregulation of competence genes, which are shown to be upregulated in biofilm-recovered pneumococci (48).
In this study, we also investigated the amount of capsule produced at different temperatures because the pneumococcal polysaccharide capsule is a major virulence factor and microbial capsules can be affected by other environmental factors, such as oxygen concentration and presence of galactose (49). We did not detect any difference in capsule synthesis in BHI irrespective of temperature, but in CDM at 34°C, the pneumococcus produced significantly more capsule than at 37°C or 40°C, despite the fact the expression of cps locus (SPD_0315 to SPD_0327) was not significantly different at 34°C than at 37°C. This inconsistency in capsule locus transcription and synthesis is not clear, but it may be linked to the differences in the rate of posttranscriptional modifications at different temperatures (50).
We investigated the virulence of D39 and ΔgdhA strains at different temperatures in Galleria mellonella. The results showed that the loss of gdhA decreases the virulence at 40°C relative to 37°C, indicating that gdhA is required for virulence of pneumococcus at high temperature. However, it was not clear whether the temperature effect on virulence was due to the incubation temperature of bacteria or larvae. G. mellonella larvae can tolerate up to 42°C (51). A previous study showed that preexposure of larvae to heat induces their immune system and survival. Exposure of larvae to 4°C or 37°C led to increased production of hemocytes and antimicrobial peptides such as gallerimycin and transferrin compared to incubation at 30°C. This mild heat shock increased the resistance to infection by C. albicans (52). It can be concluded that temperature has a strong effect on larval immune system; therefore, variations in experimental temperatures should be minimal to ensure the consistency of results. In this study, we tested 34°C, 37°C, and 40°C to reduce the impact of temperature on larval immune system, which allowed us to investigate the temperature-pathogen interaction only.
In summary, our study shows that temperature fluctuation has a strong impact on transcriptional and phenotypic profiles of S. pneumoniae and that GdhA has a role in maintaining pneumococcal physiology at relatively high temperatures. Detailed knowledge of thermostability of pneumococcal GdhA using protein engineering and biochemical and structural characterization may allow further understanding of the thermal adaptation strategies adapted by S. pneumoniae.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. Routinely, the pneumococcal strains were grown microaerobically in Todd-Hewitt broth (THB) or THB supplemented with 0.5% (wt/vol) yeast extract (THY), brain heart infusion (BHI) broth, casein-tryptone medium (CAT), or on blood agar plates supplemented with 5% (vol/vol) defibrinated horse blood and were incubated at 34°C, 37°C, or 40°C. Chemically defined medium (CDM) supplemented with 55 mM glucose was also used for growth of pneumococcal strains (53). Where appropriate, spectinomycin (100 μg/ml) or kanamycin (250 μg/ml) was added to the culture medium. Escherichia coli strain TOP10 (Invitrogen) was used for cloning and was grown in Luria broth (LB) or on LB agar supplemented with kanamycin (150 μg/ml) or ampicillin (100 μg/ml).
Construction of genetically modified strains and reporter strains.
Oligonucleotides used in this study are listed in Table S2. To construct a gdhA deletion mutant, ΔgdhA, the splicing by overlap extension (SOEing) PCR method was used as previously described (43, 54). Briefly, the genetic locus, including up- and downstream flanking regions surrounding gdhA, was amplified and fused with a spectinomycin resistance gene, which had been amplified with spec/F and spec/R primers from pDL278 (55). The fused fragments were purified and transformed into S. pneumoniae D39. The successful transformation was confirmed by PCR and DNA sequencing. To account for any polar effect of mutagenesis, the ΔgdhA strain was complemented with an intact copy of gdhA as described previously (53, 56). The complemented strain was designated CompΔgdhA. Transcriptional reporter strains were constructed as previously described (53). The putative promoter region of gdhA, which was identified using BPROM software (57), was amplified and cloned into the reporter plasmid pPP2 (58). The recombinant plasmid was then transformed into pneumococcus.
Transcriptome analysis.
Pneumococcal strains were grown to early exponential phase, with an OD600 of approximately 0.3, in CDM supplemented with 55 mM glucose at 34, 37, or 40°C. Three independent biological samples were taken for analysis. The microPREP software package was used to generate the microarray data from the slides. The CyberT implementation of a variant of a t test was performed, and false-discovery rates (FDRs) were calculated (59). For differentially expressed genes, a P value of <0.001 plus an FDR of <0.05 was considered the significance threshold. To identify differentially expressed genes, a Bayesian P value of <0.001 and a fold change cutoff of 2 were used. All other processes for the microarray experiments and data analysis were done as before (60).
Quantitative reverse transcriptase PCR (qRT-PCR) was used to confirm the expression of gdhA as described previously (53). To do this, first-strand cDNA was synthesized using approximately 1 μg of DNase-treated RNA using random hexamers and SuperScript III reverse transcriptase kit (Invitrogen) by following the manufacturer’s instructions.
Hemolytic activity assay.
The hemolytic activity of pneumococcal strains was determined to assay pneumolysin activity (61). Cell lysates were prepared by sonication at an amplitude of 8 μm for 15 s on and 45 s off (Soniprep 150). Twofold dilutions of 50 μl of pneumococcal lysates in phosphate-buffered saline (PBS) (pH 7.0) were made in a 96-well microtiter plate. Red blood cells (RBC), 4% (vol/vol), from defibrinated sheep blood (Oxoid) in PBS were mixed with the lysates and then incubated at 37°C for 30 min. The hemolytic units (HU) were calculated as the highest dilution of lysate causing 50% lysis of RBC in 30 min at 37°C and normalized against milligrams protein.
Glucuronic acid quantification.
The glucuronic acid content was quantified as a measure of capsule production, as described previously (53). Five hundred microliters of mid-exponential phase (approximate OD600 of 0.5 to 0.6 at different temperatures) pneumococcal culture was mixed with 100 μl of 1% (vol/vol) Zwittergent 3-14 detergent (Sigma-Aldrich) in 100 mM citric acid (pH 2.0), and then the mixture was incubated at 50°C for 20 min followed by precipitation with 1 ml of 99% (vol/vol) ethanol. The pellet was dissolved in 200 μl of distilled water and mixed with 1.2 ml of 12.5 mM borax (Sigma-Aldrich) in H2SO4. The suspension was boiled at 100°C for 5 min, cooled to room temperature, and mixed with 20 μl of 0.15% (wt/vol) 3-hydroxydiphenol (Sigma-Aldrich). Absorbance was measured at 520 nm. The glucuronic acid content of the samples was quantified in comparison to a standard curve generated with known concentrations of glucuronic acid and normalized against per μg of protein per 109 CFU.
Biofilm formation assay.
Biofilm formation of pneumococcal strains was analyzed using the crystal violet attachment assay (62). A pellet of overnight pneumococcal cultures grown in THY medium in a 12-well plate was resuspended in fresh 2 ml THY. The culture was then serially diluted to obtain an OD600 of 0.05 to 0.1. After overnight growth, the excess medium was carefully aspirated, and biofilms were washed with 200 μl PBS three times to remove weak or nonadherent bacteria. Attached cells were stained with 50 μl of 0.1% (wt/vol) crystal violet for 15 min, excess stain was discarded, and the biofilms were washed with distilled water three times. Subsequently, biofilm was dissolved in 200 μl of 95% (vol/vol) ethanol, and the absorbance was measured at 595 nm. The amount of biofilm formed was expressed per 108 CFU.
Determination of cell size.
Pneumococcal strains were grown overnight in CDM supplemented with 55 mM glucose. Bacterial suspensions were Gram stained as previously described (62). Pneumococcal cell size was measured lengthwise using a Prior microscope equipped with a digital camera (Infinity) and image analysis software (Infinity). For each assay, at least 50 cells were analyzed.
Enzyme assays.
Glutamate dehydrogenase activity was determined using a commercial kit (Biovision, USA). Briefly, pneumococcal cultures grown at different temperatures in CDM were pelleted and sonicated in ice-cold PBS (pH 7.0). The samples, 50 μl/well, were then mixed with 100 μl of reaction mixture (82 μl assay buffer, 8 μl GDH developer, and 10 μl glutamate [2 M]) and incubated for 3 min at 37°C, and the absorbance was recorded at 450 nm. The plate was incubated for 2 h, and then absorbance was remeasured. The level of Gdh activity in the sample was measured by reference to a NADH standard curve generated with the known concentrations of NADH. One unit of activity is defined as the amount of enzyme to generate 1 μmol of NADH per min at pH 7.6.
β-Galactosidase activity was measured as described before (43), using cells grown anaerobically in CDM supplemented with 55 mM glucose at different temperatures and then harvested in the mid-exponential phase of growth. Miller units of reporter strains were normalized for 108 CFU.
pH survival assay.
Pneumococcal suspensions (20 μl) in PBS (pH 7.0), obtained from cultures incubated at 34°C, 37°C, or 40°C, containing 1 × 108 CFU/ml were incubated in sodium citrate or sodium phosphate buffers covering a pH range of 5.0 to 8.0 for 30, 60, 90, and 120 min in 96-well plates incubated at different temperatures. The CFU/ml was calculated by plating the bacterial suspension.
Fermentation end product analysis.
Pneumococcal cultures (2 ml) grown to mid-exponential phase in CDM supplemented with 55 mM glucose at different temperatures were centrifuged, and the supernatant was collected. The concentrations of l-lactic acid, formic acid, and acetic acid in the supernatant were measured using commercial kits (Megazyme, Ireland), and the amount of end products are given for 108 CFU.
Electrophoretic mobility shift assay.
FAM-labeled DNA amplicon comprising the putative promoter region of gdhA was amplified using gdhA-EMSA-F and gdhA-EMSA-R primers (Table S2). The binding reaction contained 30 ng DNA probe, increasing amounts of purified recombinant CcpA (0 to 1 μM) in binding buffer (20 mM Tris-HCl, pH 7.5, 30 mM KCl, 1 mM dithiothreitol [DTT], 1 mM EDTA, pH 8.0, and 10% [vol/vol] glycerol), and the reaction mixture was incubated for 30 min at room temperature. Then, the mixture was loaded to nondenaturing PAGE (8% [vol/vol]) for 45 min at 200 V. The gel was visualized at 488 nm using a Typhoon Trio+ scanner (GE Healthcare Life Sciences, UK) blue laser. The expression and purification of CcpA were described previously (43).
Galleria mellonella model of pneumococcal infection.
Larvae were acquired from Livefood, UK, and those weighing 25 to 30 mg with white, milky appearance were used for infection. We administered 5 × 105 CFU pneumococci prepared in 10 μl of PBS to the second proleg of the larvae. For each strain at each temperature, 10 larvae were injected. In addition, a control group of larvae was injected with 10 μl of PBS. Infected larvae were incubated at the respective growth temperature, and the survival time was recorded.
Statistical analysis.
GraphPad Prism version 8 (GraphPad, CA, USA) was used for data analysis. The experimental results were described as mean ± standard error of the mean (SEM). One- or two-way analysis of variance (ANOVA) followed by Tukey multiple-comparison test was used to compare the groups. A P value of <0.05 was regarded as statistically significant.
Data availability.
Microarray data have been submitted to the GEO (Gene Expression Omnibus) database under the accession number GSE154888.
ACKNOWLEDGMENT
We are grateful for the support from the NIH (R01 AI139077-01A1 and R01 AI135060-01A1).
Footnotes
Supplemental material is available online only.
Contributor Information
Hasan Yesilkaya, Email: hy3@le.ac.uk.
Nancy E. Freitag, University of Illinois at Chicago
REFERENCES
- 1.Weiser JN, Ferreira DM, Paton JC. 2018. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 16:355–367. 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammerschmidt S. 2006. Adherence molecules of pathogenic pneumococci. Curr Opin Microbiol 9:12–20. 10.1016/j.mib.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell AM, Mitchell TJ. 2010. Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect 16:411–418. 10.1111/j.1469-0691.2010.03183.x. [DOI] [PubMed] [Google Scholar]
- 4.Henriques-Normark B, Tuomanen EI. 2013. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med 3:a010215. 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichner H, Pathak A, Henriques-Normark B, Loh E. 2019. Meningitis pathogens evade immune responses by thermosensing. bioRxiv 10.1101/586131. [DOI]
- 6.Pandya U, Allen CA, Watson DA, Niesel DW. 2005. Global profiling of Streptococcus pneumoniae gene expression at different growth temperatures. Gene 360:45–54. 10.1016/j.gene.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Klinkert B, Narberhaus F. 2009. Microbial thermosensors. Cell Mol Life Sci 66:2661–2676. 10.1007/s00018-009-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon HY, Ogunniyi AD, Choi MH, Pyo SN, Rhee DK, Paton JC. 2004. The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge. Infect Immun 72:5646–5653. 10.1128/IAI.72.10.5646-5653.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourie KR, Wilson HL. 2020. Understanding GroEL and DnaK stress response proteins as antigens for bacterial diseases. Vaccines (Basel) 8:773. 10.3390/vaccines8040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roncarati D, Scarlato V. 2017. Regulation of heat-shock genes in bacteria: from signal sensing to gene expression output. FEMS Microbiol Rev 41:549–574. 10.1093/femsre/fux015. [DOI] [PubMed] [Google Scholar]
- 11.Chastanet A, Prudhomme M, Claverys JP, Msadek T. 2001. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J Bacteriol 183:7295–7307. 10.1128/JB.183.24.7295-7307.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi IH, Shim JH, Kim SW, Kim SN, Pyo SN, Rhee DK. 1999. Limited stress response in Streptococcus pneumoniae. Microbiol Immunol 43:807–812. 10.1111/j.1348-0421.1999.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 13.Park SS, Kwon HY, Tran TD, Choi MH, Jung SH, Lee S, Briles DE, Rhee DK. 2015. ClpL is a chaperone without auxiliary factors. FEBS J 282:1352–1367. 10.1111/febs.13228. [DOI] [PubMed] [Google Scholar]
- 14.Varcamonti M, Graziano MR, Pezzopane R, Naclerio G, Arsenijevic S, De Felice M. 2003. Impaired temperature stress response of a Streptococcus thermophilus deoD mutant. Appl Environ Microbiol 69:1287–1289. 10.1128/AEM.69.2.1287-1289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiteshi K, Gupta R. 2014. Thermal adaptation of alpha-amylases: a review. Extremophiles 18:937–944. 10.1007/s00792-014-0674-5. [DOI] [PubMed] [Google Scholar]
- 16.Concha-Marambio L, Maldonado P, Lagos R, Monasterio O, Montecinos-Franjola F. 2017. Thermal adaptation of mesophilic and thermophilic FtsZ assembly by modulation of the critical concentration. PLoS One 12:e0185707. 10.1371/journal.pone.0185707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhuiya MW, Sakuraba H, Ohshima T. 2002. Temperature dependence of kinetic parameters for hyperthermophilic glutamate dehydrogenase from Aeropyrum pernix K1. Biosci Biotechnol Biochem 66:873–876. 10.1271/bbb.66.873. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira T, Sharkey MA, Engel PC, Khan AR. 2016. Crystal structure of a chimaeric bacterial glutamate dehydrogenase. Acta Crystallogr F Struct Biol Commun 72:462–466. 10.1107/S2053230X16007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yesilkaya H, Spissu F, Carvalho SM, Terra VS, Homer KA, Benisty R, Porat N, Neves AR, Andrew PW. 2009. Pyruvate formate lyase is required for pneumococcal fermentative metabolism and virulence. Infect Immun 77:5418–5427. 10.1128/IAI.00178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girinathan BP, Braun S, Sirigireddy AR, Espinola-Lopez J, Govind R. 2016. Correction: importance of glutamate dehydrogenase (GDH) in Clostridium difficile colonization in vivo. PLoS One 11:e0165579. 10.1371/journal.pone.0165579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YH, Kingston AW, Helmann JD. 2012. Glutamate dehydrogenase affects resistance to cell wall antibiotics in Bacillus subtilis. J Bacteriol 194:993–1001. 10.1128/JB.06547-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldas T, Demont-Caulet N, Ghazi A, Richarme G. 1999. Thermoprotection by glycine betaine and choline. Microbiology (Reading) 145:2543–2548. 10.1099/00221287-145-9-2543. [DOI] [PubMed] [Google Scholar]
- 23.Shak JR, Ludewick HP, Howery KE, Sakai F, Yi H, Harvey RM, Paton JC, Klugman KP, Vidal JE. 2013. Novel role for the Streptococcus pneumoniae toxin pneumolysin in the assembly of biofilms. mBio 4:e00655-13. 10.1128/mBio.00655-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai YC, Peng HL, Chang HY. 2003. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J Bacteriol 185:788–800. 10.1128/JB.185.3.788-800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks LR, Davidson BA, Knight PR, Hakansson AP. 2013. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio 4:e00438-13. 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plaitakis A, Kalef-Ezra E, Kotzamani D, Zaganas I, Spanaki C. 2017. The glutamate dehydrogenase pathway and its roles in cell and tissue biology in health and disease. Biology (Basel) 6:11. 10.3390/biology6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velikova N, Kavanagh K, Wells JM. 2016. Evaluation of Galleria mellonella larvae for studying the virulence of Streptococcus suis. BMC Microbiol 16:291. 10.1186/s12866-016-0905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Commichau FM, Gunka K, Landmann JJ, Stulke J. 2008. Glutamate metabolism in Bacillus subtilis: gene expression and enzyme activities evolved to avoid futile cycles and to allow rapid responses to perturbations of the system. J Bacteriol 190:3557–3564. 10.1128/JB.00099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu K. 2013. Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. ISRN Biochem 2013:645983. 10.1155/2013/645983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho SM, Kloosterman TG, Kuipers OP, Neves AR. 2011. CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PLoS One 6:e26707. 10.1371/journal.pone.0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muscariello L, Marasco R, De Felice M, Sacco M. 2001. The functional ccpA gene is required for carbon catabolite repression in Lactobacillus plantarum. Appl Environ Microbiol 67:2903–2907. 10.1128/AEM.67.7.2903-2907.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobisch S, Zuhlke D, Bernhardt J, Stulke J, Hecker M. 1999. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J Bacteriol 181:6996–7004. 10.1128/JB.181.22.6996-7004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belitsky BR, Kim HJ, Sonenshein AL. 2004. CcpA-dependent regulation of Bacillus subtilis glutamate dehydrogenase gene expression. J Bacteriol 186:3392–3398. 10.1128/JB.186.11.3392-3398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stulke J, Hillen W. 2000. Regulation of carbon catabolism in Bacillus species. Annu Rev Microbiol 54:849–880. 10.1146/annurev.micro.54.1.849. [DOI] [PubMed] [Google Scholar]
- 35.Hendriksen WT, Kloosterman TG, Bootsma HJ, Estevao S, de Groot R, Kuipers OP, Hermans PW. 2008. Site-specific contributions of glutamine-dependent regulator GlnR and GlnR-regulated genes to virulence of Streptococcus pneumoniae. Infect Immun 76:1230–1238. 10.1128/IAI.01004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merrick MJ, Edwards RA. 1995. Nitrogen control in bacteria. Microbiol Rev 59:604–622. 10.1128/mr.59.4.604-622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brosnan JT. 2000. Glutamate, at the interface between amino acid and carbohydrate metabolism. J Nutr 130:988S–990S. 10.1093/jn/130.4.988S. [DOI] [PubMed] [Google Scholar]
- 38.Steinmoen H, Teigen A, Havarstein LS. 2003. Competence-induced cells of Streptococcus pneumoniae lyse competence-deficient cells of the same strain during cocultivation. J Bacteriol 185:7176–7183. 10.1128/JB.185.24.7176-7183.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wholey WY, Abu-Khdeir M, Yu EA, Siddiqui S, Esimai O, Dawid S. 2019. Characterization of the competitive pneumocin peptides of Streptococcus pneumoniae. Front Cell Infect Microbiol 9:55. 10.3389/fcimb.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell JB, Cook GM. 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev 59:48–62. 10.1128/mr.59.1.48-62.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaufay F, Coppine J, Mayard A, Laloux G, De Bolle X, Hallez R. 2015. A NAD-dependent glutamate dehydrogenase coordinates metabolism with cell division in Caulobacter crescentus. EMBO J 34:1786–1800. 10.15252/embj.201490730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Angelis M, Calasso M, Di Cagno R, Siragusa S, Minervini F, Gobbetti M. 2010. NADP-glutamate dehydrogenase activity in nonstarter lactic acid bacteria: effects of temperature, pH and NaCl on enzyme activity and expression. J Appl Microbiol 109:1763–1774. 10.1111/j.1365-2672.2010.04804.x. [DOI] [PubMed] [Google Scholar]
- 43.Al-Bayati FA, Kahya HF, Damianou A, Shafeeq S, Kuipers OP, Andrew PW, Yesilkaya H. 2017. Pneumococcal galactose catabolism is controlled by multiple regulators acting on pyruvate formate lyase. Sci Rep 7:43587. 10.1038/srep43587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaffer CL, Zhang EW, Dudley AG, Dixon B, Guckes KR, Breland EJ, Floyd KA, Casella DP, Algood HMS, Clayton DB, Hadjifrangiskou M. 2017. Purine biosynthesis metabolically constrains intracellular survival of uropathogenic Escherichia coli. Infect Immun 85:e00471-16. 10.1128/IAI.00471-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao Y, Marks LR, Pettigrew MM, Hakansson AP. 2014. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front Cell Infect Microbiol 4:194. 10.3389/fcimb.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kostakioti M, Hadjifrangiskou M, Hultgren SJ. 2013. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3:a010306. 10.1101/cshperspect.a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PW, Orihuela CJ. 2010. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog 6:e1001044. 10.1371/journal.ppat.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trappetti C, Gualdi L, Di Meola L, Jain P, Korir CC, Edmonds P, Iannelli F, Ricci S, Pozzi G, Oggioni MR. 2011. The impact of the competence quorum sensing system on Streptococcus pneumoniae biofilms varies depending on the experimental model. BMC Microbiol 11:75. 10.1186/1471-2180-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rendueles O, Garcia-Garcera M, Neron B, Touchon M, Rocha EPC. 2017. Abundance and co-occurrence of extracellular capsules increase environmental breadth: implications for the emergence of pathogens. PLoS Pathog 13:e1006525. 10.1371/journal.ppat.1006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang SO, Wright JO, Tesorero RA, Lee H, Beall B, Cho KH. 2012. Thermoregulation of capsule production by Streptococcus pyogenes. PLoS One 7:e37367. 10.1371/journal.pone.0037367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnoy S, Gancz H, Zhu Y, Honnold CL, Zurawski DV, Venkatesan MM. 2017. The Galleria mellonella larvae as an in vivo model for evaluation of Shigella virulence. Gut Microbes 8:335–350. 10.1080/19490976.2017.1293225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mowlds P, Kavanagh K. 2008. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathologia 165:5–12. 10.1007/s11046-007-9069-9. [DOI] [PubMed] [Google Scholar]
- 53.Zhi X, Abdullah IT, Gazioglu O, Manzoor I, Shafeeq S, Kuipers OP, Hiller NL, Andrew PW, Yesilkaya H. 2018. Rgg-Shp regulators are important for pneumococcal colonization and invasion through their effect on mannose utilization and capsule synthesis. Sci Rep 8:6369. 10.1038/s41598-018-24910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kahya HF, Andrew PW, Yesilkaya H. 2017. Deacetylation of sialic acid by esterases potentiates pneumococcal neuraminidase activity for mucin utilization, colonization and virulence. PLoS Pathog 13:e1006263. 10.1371/journal.ppat.1006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, Andrew PW. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun 68:2819–2826. 10.1128/IAI.68.5.2819-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motib AS, Al-Bayati FAY, Manzoor I, Shafeeq S, Kadam A, Kuipers OP, Hiller NL, Andrew PW, Yesilkaya H. 2019. TprA/PhrA quorum sensing system has a major effect on pneumococcal survival in respiratory tract and blood, and its activity is controlled by CcpA and GlnR. Front Cell Infect Microbiol 9:326. 10.3389/fcimb.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solovyev VV, Shahmuradov IA, Salamov AA. 2010. Identification of promoter regions and regulatory sites. Methods Mol Biol 674:57–83. 10.1007/978-1-60761-854-6_5. [DOI] [PubMed] [Google Scholar]
- 58.Halfmann A, Hakenbeck R, Bruckner R. 2007. A new integrative reporter plasmid for Streptococcus pneumoniae. FEMS Microbiol Lett 268:217–224. 10.1111/j.1574-6968.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 59.van Hijum SA, de Jong A, Baerends RJ, Karsens HA, Kramer NE, Larsen R, den Hengst CD, Albers CJ, Kok J, Kuipers OP. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 6:77. 10.1186/1471-2164-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hajaj B, Yesilkaya H, Shafeeq S, Zhi X, Benisty R, Tchalah S, Kuipers OP, Porat N. 2017. CodY regulates thiol peroxidase expression as part of the pneumococcal defense mechanism against H2O2 stress. Front Cell Infect Microbiol 7:210. 10.3389/fcimb.2017.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owen RH, Boulnois GJ, Andrew PW, Mitchell TJ. 1994. A role in cell-binding for the C-terminus of pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae. FEMS Microbiol Lett 121:217–221. 10.1111/j.1574-6968.1994.tb07101.x. [DOI] [PubMed] [Google Scholar]
- 62.Feoktistova M, Geserick P, Leverkus M. 2016. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc 2016:pdb.prot087379. 10.1101/pdb.prot087379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download iai.00400-21-s0001.pdf, PDF file, 0.8 MB (777.5KB, pdf)
Data Availability Statement
Microarray data have been submitted to the GEO (Gene Expression Omnibus) database under the accession number GSE154888.






