Abstract
Purpose:
To determine classification criteria for toxoplasmic retinitis.
Design:
Machine learning of cases with toxoplasmic retinitis and 4 other infectious posterior/panuveitides.
Methods:
Cases of infectious posterior/panuveitides were collected in an informatics-designed preliminary database, and a final database was constructed of cases achieving supermajority agreement on diagnosis, using formal consensus techniques. Cases were split into a training set and a validation set. Machine learning using multinomial logistic regression was used on the training set to determine a parsimonious set of criteria that minimized the misclassification rate among the infectious posterior/panuveitides. The resulting criteria were evaluated on the validation set.
Results:
Eight hundred three cases of infectious posterior/panuveitides, including 174 cases of toxoplasmic retinitis, were evaluated by machine learning. Key criteria for toxoplasmic retinitis included: 1) focal or paucifocal necrotizing retinitis and either; 2) positive polymerase chain reaction assay for Toxoplasma gondii from an intraocular specimen or 3) the characteristic clinical picture of a round or oval retinitis lesion proximal to a hyperpigmented and/or atrophic chorioretinal scar. Overall accuracy for infectious posterior/panuveitides was 92.1% in the training set and 93.3% (95% confidence interval 88.2, 96.3) in the validation set. The misclassification rates for toxoplasmic retinitis were 8.2% in the training set and 10% in the validation set.
Conclusions:
The criteria for toxoplasmic retinitis had a low misclassification rate and appeared to perform sufficiently well for use in clinical and translational research.
PRECIS
Using a formalized approach to developing classification criteria, including informatics-based case collection, consensus-technique-based case selection, and machine learning, classification criteria for toxoplasmic retinitis were developed. Key criteria included focal or paucifocal necrotizing retinitis and either positive PCR assay for Toxoplasma gondii from an intraocular specimen or characteristic clinical picture of a round or oval retinitis lesion proximal to a hyperpigmented and/or atrophic chorioretinal scar. The resulting classification criteria had a reasonably low misclassification rate.
Toxoplasma gondii is a ubiquitous parasite worldwide and is the most common cause of retinal infection in most populations, resulting in a substantial burden of eye disease and vision loss.1,2 Toxoplasma gondii reproduces sexually only in the gut of felines, but can reproduce asexually in most other mammals and in birds. Infection occurs via one of several routes: through ingestion of materials contaminated with cat feces that contain öocysts; by eating raw or undercooked tissue of infected intermediate hosts; or vertically from mother to an unborn child during pregnancy. With rare exception, vertical transmission occurs only when the mother is first infected during the pregnancy.
In intermediate hosts, including food animals and human beings, öocysts become tachyzoites, the proliferative form of the parasite that can cause clinical disease, but parasites eventually encyst in various tissues, including the retina. These tissue cysts contain the bradyzoite form of parasite that does not induce clinical disease, but remains viable for prolonged periods of time. Tissue cysts reactivate from time-to-time, releasing bradyzoites, which again convert back to tachyzoites, but the factors that cause reactivation are poorly understood. Proliferation of tachyzoites is self-limited in people with normal immune function. People with post-natally acquired infections may develop a transient illness characterized by lymphadenopathy, fever, and sore throat, but the initial infection often is asymptomatic. Ocular involvement may occur at the time of initial systemic infection or months to years later.
In the United States the age-adjusted seroprevalence of anti-T. gondii antibodies during the period 2011–2014 was approximately 10%.3 Seroprevalence increases with age, and is higher among men, socioeconomically disadvantaged groups, and individuals born outside the United States.3 It is estimated that, overall, 2% of T. gondii-infected individuals in the United States have ocular involvement.1 The risk of ocular involvement also is believed to be higher among Hispanic immigrants,1 presumably because they are infected in their countries of origin by endemic parasites of greater virulence (see below). In 2010, it was estimated that nearly 5000 people in the United States would develop symptomatic ocular toxoplasmosis each year.4
The rates of both infection and ocular involvement are higher in many other parts of the world; for example, in the area of Erechim, in southern Brazil, results of a population-based study showed that 21.3% of individuals over the age of 13 years had ocular toxoplasmosis,5 and in a prior study from the same area, 98 of 100 children aged 10–15 were infected with T. gondii.6
The primary ocular site of T. gondii infection is the retina and eventually may result in full-thickness retinal necrosis. The subjacent choroid can also be destroyed, presumably by accompanying inflammation, which ultimately results in an atrophic scar with white center, due to exposure of the sclera, and a variably pigmented border as the lesion becomes inactive. Tissue cysts are believed to persist at scar borders after resolution of an active episode. Recurrences arising from these tissue cysts account for the classic appearance of toxoplasmic retinitis: a focus of intense tissue inflammation adjacent to a pre-existing retinochoroidal scar. Not all lesions arise from scars, however. “Primary lesions” (those arising from normal appearing retina) may occur at the time of an initial infection or may occur later; these late primary lesions are thought to arise from organisms that encyst in the retina at the time of initial infection, but do not immediately cause clinically apparent disease.7,8
The clinical appearance of toxoplasmic retinal lesions may vary, based on the duration of parasite proliferation before encystment, and on the severity of associated inflammation2,9–11 Infections that resolve early, with minimal inflammation, may result in only multiple small outer retinal opacities, a presentation of disease termed “punctate outer retinal toxoplasmosis”.12 Conversely, persistent infection, as may occur in immune-compromised individuals, may result in large areas of retinal necrosis, possibly mimicking other forms of necrotizing retinitis, such as cytomegalovirus (CMV) retinitis.13 Occasionally elderly individuals may develop extensive lesions.2,14,15
Variation in prevalence of infection and risk for ocular involvement among otherwise healthy individuals appears to reflect parasite strains of different virulence.16,17 Genotypes of parasites endemic to different geographic areas vary considerably; the presence of more virulent strains in food animals of southern Brazil is thought to explain the fact that ocular toxoplasmosis is more prevalent and more severe in that region than in the United States.
Because of the relatively high seroprevalence of T. gondii antibodies in the general population and the relatively low risk of ocular involvement, the presence of IgG antibodies to T. gondii typically is not a useful feature for diagnosing toxoplasmic retinitis; however, a negative serologic test may help to exclude toxoplasmic retinochoroiditis in a patient with a non-specific focus of retinal inflammation. Conversely, the presence of IgM antibodies may provide information about recently acquired systemic toxoplasmosis. Polymerase chain reaction techniques can be used to identify T. gondii DNA in ocular fluids, and are particularly helpful in diagnosing ocular toxoplasmosis in patients with unusual presentations of disease.18
Although retinal lesions are self-limited in otherwise healthy individuals, it is believed that treatment with a combination of antimicrobial agents and corticosteroid will reduce tissue damage from associated inflammation. There is no consensus regarding the best antimicrobial agents; most common is use of both a dihydrofolate reductase inhibitor and a sulfonamide, such as pyrimethamine and sulfadiazine or combination trimethoprim-sulfamethoxazole.19 Despite the absence of class I clinical trials demonstrating the efficacy of antimicrobial treatment of ocular toxoplasmosis, one comparative trial in which treatment was assigned by clinical center reported that treatment with pyrimethamine and sulfadiazine resulted in smaller scars than did no treatment, suggesting efficacy in limiting retinal damage. In this trial the recurrence rate was unaffected by the short-term course of treatment.20 Subsequent small clinical trials suggested efficacy similar to pyrimethamine and sulfadiazine for trimethoprim-sulfamethoxazole, for pyrimethamine and azithromycin, and for intravitreal clindamycin and dexamethasone.21–23 Retrospective cohort data suggest that treatment of ocular toxoplasmosis with corticosteroids alone is associated with increased risks of fulminant toxoplasmic retinitis,24 ocular recurrences, and worse visual outcomes;10 therefore, such management generally is discouraged. Severely immunocompromised patients can be treated with an antimicrobial agent alone, and are likely to require continued antimicrobial therapy to maintain lesion inactivity.25,26 Treatment with currently available drugs does not eliminate tissue cysts, but continued treatment with an antimicrobial agent, such as trimethoprim-sulfamethoxazole, reduces the risk of recurrences.27,28
The Standardization of Uveitis Nomenclature (SUN) Working Group is an international collaboration, which has developed classification criteria for 25 of the most common uveitides using a formal approach to development and classification. Among the diseases studied was toxoplasmic retinitis.29–35
Methods
The SUN Developing Classification Criteria for the Uveitides project proceeded in four phases as previously described: 1) informatics, 2) case collection, 3) case selection, and 4) machine learning.31–34
Informatics.
As previously described, the consensus-based informatics phase permitted the development of a standardized vocabulary and the development of a standardized, menu-driven hierarchical case collection instrument.31
Case collection and case selection.
De-identified information was entered into the SUN preliminary database by the 76 contributing investigators for each disease as previously described.32,34 Cases in the preliminary database were reviewed by committees of 9 investigators for selection into the final database, using formal consensus techniques described in the accompanying article..33,34 Because the goal was to develop classification criteria,35 only cases with a supermajority agreement (>75%) that the case was the disease in question were retained in the final database (i.e. were “selected”).33,34
Machine learning.
The final database then was randomly separated into a training set (~85% of the cases) and a validation set (~15% of the cases) for each disease as described in the accompanying article.34 Machine learning was used on the training set to determine criteria that minimized misclassification. The criteria then were tested on the validation set; for both the training set and the validation set, the misclassification rate was calculated for each disease. The misclassification rate was the proportion of cases classified incorrectly by the machine learning algorithm when compared to the consensus diagnosis. For infectious posterior and panuveitides, the diseases against which toxoplasmic retinitis was evaluated were: acute retinal necrosis (ARN), cytomegalovirus (CMV) retinitis, syphilitic uveitis, and tubercular uveitis.
The study adhered to the principles of the Declaration of Helsinki. Institutional Review Boards (IRBs) at each participating center reviewed and approved the study; the study typically was considered either minimal risk or exempt by the individual IRBs.
Results
Two hundred thirteen cases of toxoplasmic retinitis were collected and 174 (82%) achieved supermajority agreement on the diagnosis during the “selection” phase and were used in the machine learning phase. These cases of toxoplasmic retinitis were compared to cases of infectious posterior/panuveitides, including 186 cases of ARN, 211 cases of CMV retinitis, 35 cases of syphilitic posterior uveitis and 197 cases of tubercular uveitis. The details of the machine learning results for these diseases are outlined in the accompanying article.34 The characteristics of cases with ocular toxoplasmosis are listed in Table 1, and the classification criteria developed after machine learning are listed in Table 2. Key features of the criteria include a unifocal or paucifocal (<5 lesions) active retinitis and either: 1) evidence of infection with T. gondii, either from PCR of an intraocular fluid specimen or serum IgM antibodies to T. gondii (evidence of acute infection), or 2) classic clinical picture (Figure 1) with hyperpigmented and/or atrophic scar accompanied by either a) a round or oval area of active retinitis or b) a recurrent area of active retinitis. The overall accuracy for infectious posterior/panuveitides was 92.1% in the training set and 93.3% (95% confidence interval 88.2, 96.3%) in the validation set. The misclassification rate for toxoplasmic retinitis in the training set was 8.2% and in the validation set 10%. In the training set the disease with which it was most often confused was CMV retinitis, whereas in the validation set no one disease predominated.
Table 1.
Characteristics of Cases with Toxoplasmic Retinitis
| Characteristic | Result |
|---|---|
| Number cases | 174 |
| Demographics | |
| Age, median, years (25th 75th percentile) | 28 (21, 43) |
| Gender (%) | |
| Men | 50 |
| Women | 50 |
| Race/ethnicity (%) | |
| White, non-Hispanic | 55 |
| Black, non-Hispanic | 8 |
| Hispanic | 9 |
| Asian, Pacific Islander | 11 |
| Other | 13 |
| Missing | 4 |
| Uveitis History | |
| Uveitis course (%) | |
| Acute, monophasic | 43 |
| Acute, recurrent | 24 |
| Chronic | 26 |
| Indeterminate | 7 |
| Laterality (%) | |
| Unilateral | 88 |
| Unilateral, alternating | 0 |
| Bilateral | 12 |
| Ophthalmic examination | |
| Keratic precipitates (%) | |
| None | 61 |
| Fine | 18 |
| Round | 10 |
| Stellate | 1 |
| Mutton Fat | 9 |
| Other | 1 |
| Anterior chamber cells (%) | |
| Grade 0 | 45 |
| ½+ | 14 |
| 1+ | 16 |
| 2+ | 12 |
| 3+ | 10 |
| 4+ | 3 |
| Anterior chamber flare (%) | |
| Grade 0 | 63 |
| 1+ | 25 |
| 2+ | 9 |
| 3+ | 2 |
| 4+ | 1 |
| Iris (%) | |
| Normal | 97 |
| Posterior synechiae | 3 |
| Iris nodules | 0 |
| Iris atrophy (sectoral, patchy or diffuse) | 0 |
| Heterochromia | 0 |
| Intraocular pressure (IOP), involved eyes | |
| Median, mm Hg (25th, 75th percentile) | 16 (13, 18) |
| Proportion patients with IOP>24 mm Hg either eye (%) | 7 |
| Vitreous cells (%) | |
| Grade 0 | 21 |
| ½+ | 13 |
| 1+ | 30 |
| 2+ | 27 |
| 3+ | 7 |
| 4+ | 2 |
| Vitreous haze (%) | |
| Grade 0 | 30 |
| ½+ | 19 |
| 1+ | 27 |
| 2+ | 14 |
| 3+ | 9 |
| 4+ | 1 |
| Retinitis characteristics | |
| Number lesions per eye, including active lesions & scars (%)* | |
| Unifocal (one) | 5 |
| Paucifocal (2 to 4) | 82 |
| Multifocal (≥5) | 8 |
| Indeterminate (lesion not photographed or dense vitritis) | 5 |
| Number active lesions per eye (%)* | |
| Unifocal (one) | 78 |
| Paucifocal (2 to 4) | 2 |
| Multifocal (≥5) | 0 |
| Indeterminate (lesion not photographed or dense vitritis) | 20 |
| Proximate/adjacent hyperpigmented/atrophic scars (%)* | |
| Present | 82 |
| Absent (active lesion only) | 16 |
| Indeterminate (dense vitritis) | 2 |
| Lesion shape (%) | |
| Round or ovoid | 59 |
| Placoid | 16 |
| Ameboid | 9 |
| Wedge-shaped | 3 |
| Punctate | 2 |
| Missing | 11 |
| Lesion character (%) | |
| Circumferential | 1 |
| Confluent | 7 |
| Granular | 1 |
| Lesion location (%) | |
| Posterior pole involved | 52 |
| Mid-periphery and/or periphery only | 48 |
| Lesion size (%) | |
| <250 μm | 6 |
| 250–500 μm | 10 |
| >500 μm | 84 |
| Other features (%) | |
| Retinal vascular sheathing or leakage or occlusion | 17 |
| Retinal hemorrhage | 6 |
| Systemic disease | |
| Immunocompromised patients (%) | 6 |
| Human immunodeficiency virus infection | 5 |
| Organ transplant | 0 |
| Chemotherapy or other immunosuppression | 1 |
| Laboratory data (%) | |
| Aqueous or vitreous specimen PCR† positive for Toxoplasma gondii | 10 |
| Positive serology for antibodies to Toxoplasma gondii‡ | 75 |
| Positive IgM antibodies to Toxoplasma gondii | 21 |
| Positive IgG antibodies to Toxoplasma gondii | 74 |
Based on evaluation of photographs of 158 cases.
PCR = polymerase chain reaction; 17 of 20 cases tested (85%) were positive.
Either IgG or IgM antibodies to Toxoplasma gondii were present in 131 of 131 cases tested (100%). IgM antibodies were present in 36/131 cases tested (21%) and IgG antibodies were present in 128/131 cases tested (98%).
Table 2.
Classification Criteria for Toxoplasmic Retinitis
| Criteria |
| 1. Focal or paucifocal necrotizing retinitis* |
| AND (#2 or #3) |
| 2. Evidence of infection with Toxoplasma gondii |
| a. Positive PCR† for Toxoplasma gondii from either the aqueous or vitreous specimen OR |
| b. Positive serum IgM antibodies against Toxoplasma gondii |
| OR |
| 3. Characteristic clinical ocular features |
| a. Hyperpigmented and/or atrophic chorioretinal scar (“toxoplasmic scar”) AND (b. or c.) |
| b. Round or oval retinitis lesions OR |
| c. Recurrent acute (episodic) course |
| Exclusions |
| 1. Both negative IgG AND IgM antibodies against Toxoplasma gondii (unless there is a positive PCR for Toxoplasma gondii from an aqueous or vitreous specimen) |
| 2. Positive serology for syphilis using a treponemal test |
| 3. Intraocular specimen PCR-positive for herpes simplex virus, varicella zoster virus or cytomegalovirus (unless there is immune compromise, morphologic evidence for >1 infection, the characteristic picture of toxoplasmic retinitis, and the intraocular fluid specimen also has a positive PCR for T. gondii) |
“Active” retinitis lesions in immunocompetent patients. Immunocompromised patients may have a multifocal retinitis or a diffuse necrotizing retinitis. Number of scars may be ≥5.
PCR = polymerase chain reaction.
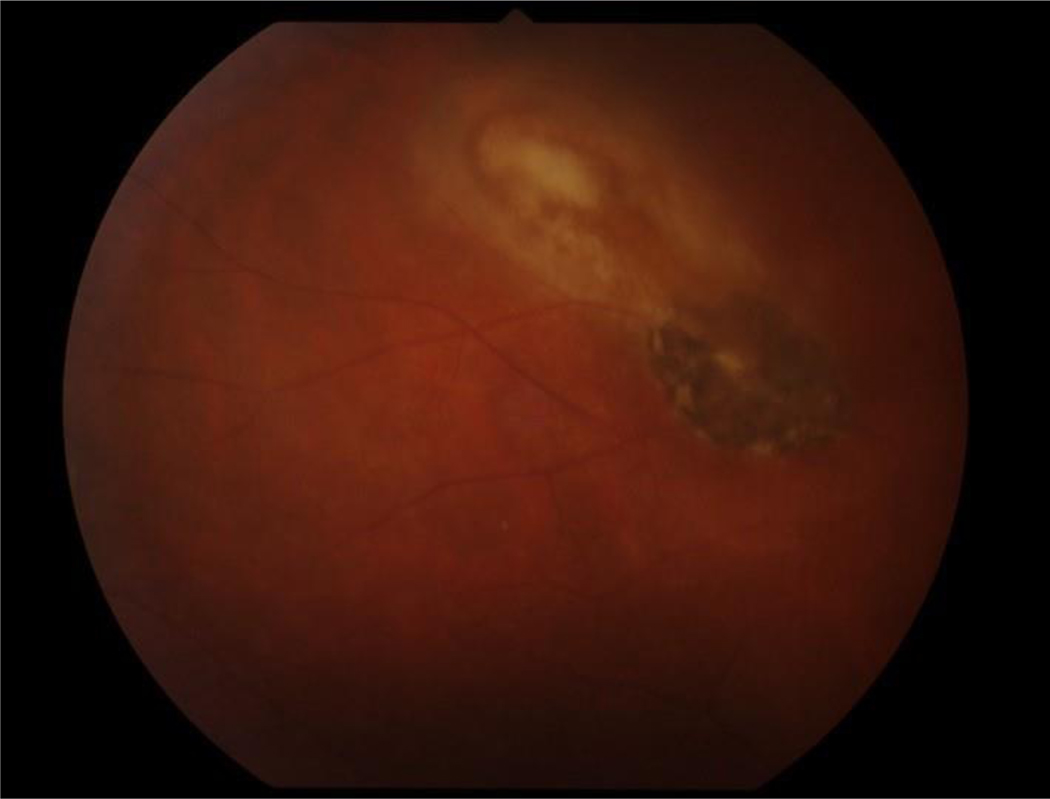
Figure 1.
Fundus photograph of a case of toxoplasmic retinitis with an area of focal retinitis characterized by retinal necrosis and edema, adjacent to a hyperpigmented chorioretinal scar.
Discussion
Necrotizing retinitides are characterized by full thickness retinal necrosis with or without inflammation, which, upon resolution, leave an atrophic and gliotic scar in the involved areas. Clinically, the initial presentation is white to yellow retinal edema and opacity with or without hemorrhage. Necrotizing retinitides may have relatively well demarcated borders, as in the case of ARN, or have satellites extending into adjacent retina, as is seen in CMV retinitis. The classification criteria developed by the SUN Working Group for toxoplasmic retinitis have a relatively low misclassification rate, indicating reasonably good discriminatory performance against other infectious posterior and pan-uveitides.
The criteria were developed to diagnose active toxoplasmic retinitis but do not address the diagnosis of chorioretinal scars in the absence of active retinitis. Although these scars may have an appearance similar to those described in the criteria and a reasonable inference made, the scars also may be non-specific. This limitation is applicable to congenital toxoplasmosis, where the eye exam may demonstrate chorioretinal scars without active retinitis.
In ocular toxoplasmosis, the retinitis appears to be due to the proliferation of tachyzoites, whereas the anterior uveitis, vitritis, and vascular sheathing appear to be due to the immunologic response to T. gondii.2,36 As such the clinical appearance will vary depending on the immunologic status of the host.13,25,26 Nevertheless, in immune-competent adults, a characteristic picture is present permitting diagnosis based on the clinical morphologic appearance: a single round or oval area of active retinitis is adjacent to an atrophic and hyperpigmented scar or scars and is associated with vitritis. The presumed reason for this appearance is that the immune response typically causes T. gondii to encyst again without ongoing cell lysis, resulting in well demarcated scars. In contrast, viral retinitides spread in a brushfire manner due to persistent viral replications with expanding areas of retinal necrosis.
The cases in the SUN database have characteristics similar to those from other case series, suggesting good generalizability, including presence of retinochoroidal scars, anterior segment inflammation, vitritis, disease course, and lesion location.3,9–11,37,38 Although the macula represents only 5% of the retinal area, the posterior pole is disproportionally affected by ocular toxoplasmosis: 52% of cases in the SUN database had posterior pole involvement, a result seen in case series of congenitally-acquired ocular toxoplasmosis37 and to a slightly lesser extent in international case series of ocular toxoplasmosis.38 The reasons for this disproportionate involvement of the posterior pole are unknown, but theoretically could relate to the density of the retinal vasculature and the presence of parasitemia during the initial systemic infection.38–40
There can be variation in the clinical presentation, including lesion size, shape, pigmentation, and presence of scars.2,9–14 Although multiple factors likely contribute to this variability, the host’s immune status and age are two of the more important ones. Persons with immune compromise (e.g. AIDS, organ transplant, etc.) may have large, persistently active lesions, attributable to the failure of the immune response, continued tachyzoite proliferation, and the resultant ongoing tissue destruction.13,24,25 Ocular lesions also may be more severe in newborns, who have immature immune defenses, and have been reported to be more severe in the elderly.14,15 Similarly, patients with newly-acquired ocular toxoplasmosis may have focal necrotizing retinitis without an adjacent scar,2,8 as may some patients with remote infection without active retinal disease resulting in tissue cysts but no scars.10,37,38 In atypical presentations confirmation of intraocular infection with T. gondii, by assaying an intraocular fluid specimen (e.g. by PCR) may be required,18 and in cases without an adjacent toxoplasmosis scar due to recently acquired systemic toxoplasmosis, confirmation of acute systemic infection by detection of IgM antibodies to T. gondii in the serum may be helpful. Because of the high prevalence of IgG antibodies to T. gondii in the general population, the presence of IgG antibodies to T. gondii generally is not helpful in establishing the diagnosis.
Aqueous humor can be sampled to detect presumed intraocular antibody production using the Goldmann-Witmer coefficient (GWC) analysis. An elevated GWC for pathogens has been taken as evidence of intraocular infection, although it actually suggests an immunologic antibody response to the pathogen and does not detect the pathogen itself. Several studies have suggested that analysis of intraocular fluid using GWC analysis may be beneficial in the evaluation of patients with intraocular infections.41–43 However, these retrospective studies suffer from a lack of a “gold standard” and a presumption of superior accuracy of the GWC analysis. Nevertheless, there appears to be value in its use, and GWC analysis may be complementary to PCR analysis, in that PCR detection of pathogen DNA may decline with treatment. Although used extensively in some clinics, unlike PCR analysis of intraocular fluid, GWC coefficient analysis is not widely used throughout the world. In the SUN machine learning, the GWC analysis did not emerge as a useful factor, perhaps because of its limited use by SUN investigators. Prospective studies against standardized diagnosis may demonstrate its utility and lead to its inclusion in the criteria in the future.
The presence of any of the exclusions in Table 2 suggests an alternate diagnosis, and the diagnosis of toxoplasmic retinitis should not be made in their presence. In prospective studies many of these tests will be performed routinely, and the alternative diagnoses excluded. However, in retrospective studies based on clinical care, not all of these tests may have been performed. Hence the presence of an exclusionary criterion excludes toxoplasmic retinitis, but the absence of such testing does not always exclude the diagnosis of toxoplasmic retinitis if the criteria for the diagnosis are met.
Classification criteria are employed to diagnose individual diseases for research purposes.35 Classification criteria differ from clinical diagnostic criteria, in that although both seek to minimize misclassification, when a trade-off is needed, diagnostic criteria typically emphasize sensitivity, whereas classification criteria emphasize specificity,35 in order to define a homogeneous group of patients for inclusion in research studies and limit the inclusion of patients without the disease in question that might confound the data. The machine learning process employed did not explicitly use sensitivity and specificity; instead it minimized the misclassification rate. Because we were developing classification criteria and because the typical agreement between two uveitis experts on diagnosis is moderate at best,32 the selection of cases for the final database (“case selection”) included only cases which achieved supermajority agreement on the diagnosis. As such, some cases which clinicians would diagnose with toxoplasmic retinitis may not be so classified by classification criteria.
In conclusion, the criteria for toxoplasmic retinitis outlined in Table 2 appear to perform sufficiently well for use as classification criteria in clinical research.34
Acknowledgments
Grant support: Supported by grant R01 EY026593 from the National Eye Institute, the National Institutes of Health, Bethesda, MD, USA; the David Brown Fund, New York, NY, USA; the Jillian M. And Lawrence A. Neubauer Foundation, New York, NY, USA; and the New York Eye and Ear Foundation, New York, NY, USA.
Footnotes
Conflict of Interest: Douglas A. Jabs: none; Rubens Belfort, Jr.: none; Bahram Bodaghi: none; Elizabeth Graham: none; Gary Holland: none; Susan L. Lightman: none; Neal Oden: none; Alan G. Palestine: none; Justine R. Smith: none; Jennifer E. Thorne: Dr. Thorne engaged in a portion of this research as a consultant and was compensated for the consulting service; Brett E. Trusko: none.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Holland GN. Ocular toxoplasmosis: a global reassessment. Part I. epidemiology and course of the disease. Am J Ophthalmol 2003;136:973–88. [DOI] [PubMed] [Google Scholar]
- 2.Holland GN. Ocular toxoplasmosis; a global reassessment. Part II. disease manifestations and management. Am J Ophthalmol 2004;137:1–17. [PubMed] [Google Scholar]
- 3.Jones JL, Kruszon-Moran D, Elder S, et al. Toxoplasma gondii infection in the United States. Am J Trop Med Hygiene 2018;98:551–7. Erratum in: Am J Trop Med Hygiene 2018;99:241–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hygiene 2010;82:464–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glassner PD, Silveira C, Kruszon-Moran D, et al. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol 1992;114:136–44. [DOI] [PubMed] [Google Scholar]
- 6.Silveira C, Belfort R Jr, Burnier M Jr, Nussenblatt R. Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. Am J Ophthalmol 1988;106:362–4. [DOI] [PubMed] [Google Scholar]
- 7.Silveira C, Belfort R Jr, Muccioli C, et al. A follow-up study of Toxoplasma gondii infection in southern Brazil. Am J Ophthalmol 2001;131:351–4. [DOI] [PubMed] [Google Scholar]
- 8.Arantes TE, Silveira C, Holland GN, et al. Ocular involvement following post-natally acquired Toxoplasma gondii infection in Southern Brazil: a 28-year experience. Am J Ophthalmol 2015;159:1002–1012. [DOI] [PubMed] [Google Scholar]
- 9.Friedman CT, Knox DL. Variations in recurrent active toxoplasmic retinochoroiditis. Arch Ophthalmol 1969;81:481–93. [DOI] [PubMed] [Google Scholar]
- 10.Bosch-Driessen LH, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 2002;109:869–78. [DOI] [PubMed] [Google Scholar]
- 11.Smith JR, Cunningham ET Jr. Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol 2002;13:387–92. [DOI] [PubMed] [Google Scholar]
- 12.Doft BH, Gass DM. Punctate outer retinal toxoplasmosis. Arch Ophthalmol 1985;103:1332–6. [DOI] [PubMed] [Google Scholar]
- 13.Elkins BS, Holland GN, Opremcak EM, et al. Ocular toxoplasmosis misdiagnosed as cytomegalovirus retinopathy in immunocompromised patients. Ophthalmology 1994;101:499–507. [DOI] [PubMed] [Google Scholar]
- 14.Labalette P, Delhaes L, Margaron F, Fortier B, Rouland JF. Ocular toxoplasmosis after the fifth decade. Am J Ophthalmol 2002;133:506–15. [DOI] [PubMed] [Google Scholar]
- 15.Holland GN. Ocular toxoplasmosis: the influence of patient age. Mem Inst Oswaldo Cruz 2009;104:351–7. [DOI] [PubMed] [Google Scholar]
- 16.Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis 2001;184:633–9. [DOI] [PubMed] [Google Scholar]
- 17.Shobab L, Pleyer U, Johnsen J, et al. Toxoplasma serotype is associated with development of ocular toxoplasmosis. J Infect Dis 2013;208:1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montoya JG, Parmley S, Liesenfeld O, Jaffe JG, Remington JS. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology 1999;106:1554–63. [DOI] [PubMed] [Google Scholar]
- 19.Holland GN, Lewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 2002;134:102–14. [DOI] [PubMed] [Google Scholar]
- 20.Rothova A, Meenken C, Buitenhuis HJ, et al. Therapy for ocular toxoplasmosis. Am J Ophthalmol 1993;115:517–23. [DOI] [PubMed] [Google Scholar]
- 21.Sohelian M, Sadoughi MM, Ghajamia M, et al. Prospective randomized trial of trimethoprim/dulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 2005;112:1876–82. [DOI] [PubMed] [Google Scholar]
- 22.Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisone in treatment of ocular toxoplasmosis. Ophthalmology 2011;118:134–41. [DOI] [PubMed] [Google Scholar]
- 23.Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MS, et al. A prospective, randomized trial of pyimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol 2002;134:34–40. [DOI] [PubMed] [Google Scholar]
- 24.Sabates R, Pruett RC, Brockhurst RJ. Fulminant ocular toxoplasmosis. Am J Ophthalmol 1981;92:497–503. [DOI] [PubMed] [Google Scholar]
- 25.Holland GN, Engstrom RE Jr, Glasgow BJ, et al. Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol 1988;106:653–67. [DOI] [PubMed] [Google Scholar]
- 26.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 1995;623–83. [PMC free article] [PubMed] [Google Scholar]
- 27.Silveira C, Belfort R Jr, Muccioli C, et al. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol 134:41–6. [DOI] [PubMed] [Google Scholar]
- 28.Felix JP, Lira RP, Zacchia RS, Toribio JM, Nascimento MA, Arieta CE. Trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: randomized controlled clinical trial. Am J Ophthalmol 2014;157:762–6. [DOI] [PubMed] [Google Scholar]
- 29.Jabs DA, Rosenbaum JT, Nussenblatt RB, the Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Report of the first international workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol 2013;156:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trusko B, Thorne J, Jabs D, et al. Standardization of Uveitis Nomenclature Working Group. The SUN Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med 2013;52:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada AA, Jabs DA. The SUN Project. The future is here. Arch Ophthalmol 2013;131:787–9. [DOI] [PubMed] [Google Scholar]
- 33.Jabs DA, Dick A, Doucette JT, Gupta A, Lightman S, McCluskey P, Okada AA, Palestine AG, Rosenbaum JT, Saleem SM, Thorne J, Trusko, B for the Standardization of Uveitis Nomenclature Working Group. Interobserver agreement among uveitis experts on uveitic diagnoses: the Standard of Uveitis Nomenclature experience. Am J Ophthalmol 2018; 186:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Standardization of Uveitis Nomenclature (SUN) Working Group. Development of classification criteria for the uveitides. Am J Ophthalmol 2020;volume:pp. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria. Arthritis Care Res 2015;67:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman PE, Ghosheh R, Tabbara KF, O’Connor GR, Stern W. The role of hypersensitivity reactions to toxoplasma antigens in experimental ocular toxoplasmosis in nonhuman primates. Am J Ophthalmol 1982;94:159–64. [DOI] [PubMed] [Google Scholar]
- 37.Mets MB, Holfels E, Boyer KM, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol 1997;123:1–16. [DOI] [PubMed] [Google Scholar]
- 38.Dodds EM, Holland GN, Stanford MR, et al. Intraocular inflammation associated with ocular toxoplasmosis: relationships at initial examination. Am J Ophthalmol 2008;146:856–65. [DOI] [PubMed] [Google Scholar]
- 39.Burney DP, Lappin MR, Spilker M, McReynolds L. Detection of Toxoplasma gondii parasitemia in experimentally inoculated cats. J Parasitol 1999;85:947–51. [PubMed] [Google Scholar]
- 40.Filice GA, Hitt JA, Mitchell CD, Blackstad M, Sorensen SW. Diagnosis of Toxoplasma parasitemia in patients with AIDS by gene detection after amplification with polymerase chain reaction. J Clin Microbiol 1993;31:2327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Groot-Mijnes JD, Rothova A, Van Loon AM, et al. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol 2006;141:313–8. [DOI] [PubMed] [Google Scholar]
- 42.Rothova A, de Boer JH, ten Dam-van Loon NH, et al. Usefulness of aqueous humor analysis for diagnosis of posterior uveitis. Ophthalmology 2008;115:306–11. [DOI] [PubMed] [Google Scholar]
- 43.Fekkar A, Bodaghi B, Touafek F, Le Hoang P, Mazier D, Paris L. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol 2008;46:1965–7. [DOI] [PMC free article] [PubMed] [Google Scholar]