Abstract
Purpose:
To determine classification criteria for varicella zoster virus (VZV) anterior uveitis
Design:
Machine learning of cases with VZV anterior uveitis and 8 other anterior uveitides.
Methods:
Cases of anterior uveitides were collected in an informatics-designed preliminary database, and a final database was constructed of cases achieving supermajority agreement on the diagnosis, using formal consensus techniques. Cases were split into a training set and a validation set. Machine learning using multinomial logistic regression was used on the training set to determine a parsimonious set of criteria that minimized the misclassification rate among the anterior uveitides. The resulting criteria were evaluated on the validation set.
Results:
One thousand eighty-three cases of anterior uveitides, including 123 cases of VZV anterior uveitis, were evaluated by machine learning. The overall accuracy for anterior uveitides was 97.5% in the training set and 96.7% in the validation set (95% confidence interval 92.4, 98.6). Key criteria for VZV anterior uveitis included unilateral anterior uveitis with either 1) positive aqueous humor polymerase chain reaction assay for VZV; 2) sectoral iris atrophy in a patient ≥60 years of age; or 3) concurrent or recent dermatomal herpes zoster. The misclassification rates for VZV anterior uveitis were 0.9% in the training set and 0% in the validation set, respectively.
Conclusions:
The criteria for VZV anterior uveitis had a low misclassification rate and appeared to perform sufficiently well for use in clinical and translational research.
PRECIS
Using a formalized approach to developing classification criteria, including informatics-based case collection, consensus-technique-based case selection, and machine learning, classification criteria for varicella zoster virus (VZV) anterior uveitis were developed. Key criteria included unilateral anterior uveitis with either 1) positive aqueous humor polymerase chain reaction assay for VZV; 2) sectoral iris atrophy in a patient ≥60 years of age; or 3) concurrent or recent dermatomal herpes zoster. The resulting criteria had an acceptable misclassification rate.
Varicella zoster virus (VZV) is a common herpes family DNA virus, causing varicella (“chicken pox”) in children, herpes zoster in adults, and, in immunocompromised adults, disseminated herpes zoster. Herpes zoster may erupt along the distribution of the first branch of the trigeminal nerve, resulting in herpes zoster ophthalmicus. Herpes zoster is estimated to affect 20% to 30% of the population at some point during their lifetime, and 10% to 20% of these individuals are estimated to have herpes zoster ophthalmicus.1 Ocular disease due to herpes zoster ophthalmicus is common, affecting an estimated 50% of patients with trigeminal nerve dermatomal herpes zoster, and one of the most common manifestations is anterior uveitis.1–3 A population-based study from Taiwan estimated the incidence of anterior uveitis at 0.3%/person-year after any herpes zoster with a 13-fold increased risk of uveitis for herpes zoster ophthalmicus.4 Prospective trials of acyclovir for herpes zoster ophthalmicus estimated the risk of anterior uveitis in patients not treated with antivirals at ~60%, which was substantially reduced by the early use of antiviral agents, such as acyclovir and valacyclovir.5–7 One study in 2014 from North Africa similarly estimated that ~60% of patients with herpes zoster ophthalmicus would develop anterior uveitis.8
Varicella zoster virus anterior uveitis is presumed to be due to active viral replication in the eye, as evidenced by the detection of VZV DNA in the anterior chamber using polymerase chain reaction analysis of aqueous obtained by paracentesis9–13 and the response to antiviral therapy.5–7 Anterior uveitis due to VZV in the absence of dermatomal herpes zoster occurs, albeit less commonly, and can be diagnosed by PCR analysis of an aqueous specimen.11,12 In one case series of patients with VZV anterior uveitis, 6% of cases of VZV anterior uveitis occurred without dermatomal zoster.12 A syndrome of herpetic anterior uveitis with sectoral iris atrophy is due to either herpes simplex virus (HSV) or VZV in over 95% of cases. In younger patients (<50 years of age) it typically is due to HSV, and in older patients (≥60 years of age) overwhelmingly to VZV.13
The Standardization of Uveitis Nomenclature (SUN) Working Group is an international collaboration, which has developed classification criteria for 25 of the most common uveitides using a formal approach to development and classification.14–20 Among the anterior uveitides being studied was VZV anterior uveitis.
Methods
The SUN Developing Classification Criteria for the Uveitides project proceeded in four phases as previously described: 1) informatics, 2) case collection, 3) case selection, and 4) machine learning.16–18.20
Informatics.
As previously described, the consensus-based informatics phase permitted the development of a standardized vocabulary and the development of a standardized, menu-driven hierarchical case collection instrument.16
Case collection and case selection.
De-identified information was entered into the SUN preliminary database by the 76 contributing investigators for each disease as previously described.18,20 Cases in the preliminary database were reviewed by committees of 9 investigators for selection into the final database, using formal consensus techniques described in the accompanying article.18,20 Because the goal was to develop classification criteria, only cases with a supermajority agreement (>75%) that the case was the disease in question were retained in the final database (i.e. were “selected”).
Machine learning.
The final database then was randomly separated into a training set (~85% of cases) and a validation set (~15% of cases) for each disease as described in the accompanying article.20 Machine learning was used on the training set to determine criteria that minimized misclassification. The criteria then were tested on the validation set; for both the training set and the validation set, the misclassification rate was calculated for each disease. The misclassification rate was the proportion of cases classified incorrectly by the machine learning algorithm when compared to the consensus diagnosis. For VZV anterior uveitis, the diseases against which it was evaluated were: cytomegalovirus (CMV) anterior uveitis, HSV anterior uveitis, juvenile idiopathic arthritis (JIA)-associated anterior uveitis, spondylitis/HLA-B27-associated anterior uveitis, tubulointerstitial nephritis with uveitis (TINU), Fuchs uveitis syndrome, sarcoidosis-associated anterior uveitis, and of syphilitic anterior uveitis.
Comparison of cases with and without dermatomal herpes zoster and with and without PCR confirmation of VZV in the anterior chamber.
Comparison of cases with and without dermatomal herpes zoster and with and without PCR confirmation of VZV in the anterior chamber for categorical variables was performed with the chi-square test or the Fisher’s exact test if a cell was less than 5. For continuous variables, the Wilcoxon rank sum test was used. P-values are nominal and two-sided.
Results
One hundred sixty-three cases of VZV anterior uveitis were collected, and 123 (76%) achieved supermajority agreement on the diagnosis during the “selection” phase and were used in the machine learning phase. These cases of VZV anterior uveitis were compared to 960 cases of other anterior uveitides, including 89 cases of CMV anterior uveitis, 101 cases of HSV anterior uveitis, 146 cases of Fuchs Uveitis Syndrome, 202 cases of JIA-associated anterior uveitis, 184 cases of spondylitis/HLA-B27-associated anterior uveitis, 94 cases of TINU, 112 cases of sarcoidosis-associated anterior uveitis, and 32 cases of syphilitic anterior uveitis. The characteristics at presentation to a SUN Working Group Investigator of cases with VZV anterior uveitis are listed in Table 1. A comparison of cases with and without dermatomal zoster is listed in Table 2, and a comparison of cases with and without PCR testing for VZV is listed in Table 3. Differences between cases with and without dermatomal zoster included the following for those without dermatomal zoster: younger age, more often men, more often non-White, less often normal iris, more vitritis, and more likely to have undergone paracentesis for PCR testing. Differences between cases with and without PCR testing included the following for those with PCR testing: more often non-White, suggestion of a normal iris less often (P=0.06), and dermatomal zoster less often. As 98% of cases without PCR testing had dermatomal zoster, it appears that PCR testing was used in those without dermatomal zoster and more atypical cases. Other than the iris appearance, there were no differences in the appearance of the uveitis between those with and without dermatomal zoster and those with and without PCR testing. The criteria developed after machine learning are listed in Table 4. Key features for the diagnosis of VZV anterior uveitis included: 1) positive PCR for VZV in the aqueous obtained on paracentesis, or 2) dermatomal Herpes zoster; or 3) anterior uveitis with sectoral iris atrophy in a patient ≥60 years of age. The overall accuracy for anterior uveitides was 97.5% in the training set and 96.7% in the validation set (95% confidence interval 92.4, 98.6).20 The misclassification rate for VZV anterior uveitis in the training set was 0.9% and in the validation set 0%.
Table 1.
Characteristics of Cases with Varicella Zoster Virus Anterior Uveitis
| Characteristic | Result |
|---|---|
| Number cases | 123 |
| Demographics | |
| Age, median, years (25th 75th percentile) | 63 (54, 73) |
| Age category, years (%) | |
| ≤16 | 2 |
| 17–50 | 15 |
| 51–60 | 23 |
| >60 | 61 |
| Gender (%) | |
| Men | 40 |
| Women | 60 |
| Race/ethnicity (%) | |
| White, non-Hispanic | 72 |
| Black, non-Hispanic | 3 |
| Hispanic | 3 |
| Asian, Pacific Islander | 11 |
| Other | 3 |
| Missing/unknown | 8 |
| Uveitis History | |
| Uveitis course (%) | |
| Acute, monophasic | 38 |
| Acute, recurrent | 6 |
| Chronic | 41 |
| Indeterminate | 15 |
| Laterality (%) | |
| Unilateral | 96 |
| Unilateral, alternating | 0 |
| Bilateral | 4 |
| Ophthalmic examination | |
| Cornea | |
| No keratitis | 71 |
| Keratitis | 29 |
| Keratic precipitates (%) | |
| None | 30 |
| Fine | 42 |
| Round | 11 |
| Stellate | 3 |
| Mutton Fat | 13 |
| Other | 1 |
| Anterior chamber cells (%) | |
| Grade ½+ | 29 |
| 1+ | 41 |
| 2+ | 23 |
| 3+ | 5 |
| 4+ | 0 |
| Hypopyon (%) | 0 |
| Anterior chamber flare (%) | |
| Grade 0 | 55 |
| 1+ | 34 |
| 2+ | 11 |
| 3+ | 0 |
| 4+ | 0 |
| Iris (%) | |
| Normal | 79 |
| Posterior synechiae | 7 |
| Sectoral iris atrophy | 11 |
| Patch iris atrophy | 3 |
| Diffuse iris atrophy | 2 |
| Heterochromia | 2 |
| Intraocular pressure (IOP), involved eyes | |
| Median, mm Hg (25th, 75th percentile) | 14 (12, 20) |
| Proportion patients with IOP>24 mm Hg either eye (%) | 26 |
| Vitreous cells (%) | |
| Grade 0 | 88 |
| ½+ | 10 |
| 1+ | 2 |
| 2+ | 1 |
| 3+ | 0 |
| 4+ | 0 |
| Dermatomal Herpes zoster (%) | 86 |
| Immune compromised host (%) | 7 |
| Laboratory | |
| Aqueous PCR positive for VZV* (%) | 20 |
PCR = polymerase chain reaction; VZV=varicella zoster virus; 25 patients tested and 25 (100%) were positive.
Table 2.
Characteristics of Varicella Zoster Virus Anterior Uveitis Cases with and without Dermatomal Herpes Zoster
| Characteristic | With Dermatomal Herpes zoster | Without Dermatomal Herpes zoster | P-value |
|---|---|---|---|
| Number cases | 106 | 17 | |
| Demographics | |||
| Age, median, years (25th 75th percentile) | 62 (50, 74) | 54 (43, 63) | 0.05 |
| Age category, years (%) | 0.08 | ||
| ≤16 | 2 | 0 | |
| 17–50 | 12 | 29 | |
| 51–60 | 21 | 35 | |
| >60 | 60 | 29 | |
| Gender (%) | 0.03 | ||
| Men | 36 | 64 | |
| Women | 64 | 36 | |
| Race/ethnicity (%) | 0.002 | ||
| White, non-Hispanic | 80 | 41 | |
| Black, non-Hispanic | 3 | 6 | |
| Hispanic | 3 | 0 | |
| Asian, Pacific Islander | 10 | 24 | |
| Other | 1 | 11 | |
| Missing/unknown | 3 | 18 | |
| Uveitis History | |||
| Uveitis course (%) | 0.64 | ||
| Acute, monophasic | 37 | 41 | |
| Acute, recurrent | 5 | 12 | |
| Chronic | 42 | 35 | |
| Indeterminate | 16 | 12 | |
| Laterality (%) | 0.22 | ||
| Unilateral | 96 | 94 | |
| Bilateral | 4 | 6 | |
| Ophthalmic examination | |||
| Cornea | 0.58 | ||
| No keratitis | 70 | 76 | |
| Keratitis | 30 | 24 | |
| Keratic precipitates (%) | 0.27 | ||
| None | 29 | 35 | |
| Fine | 45 | 24 | |
| Round | 8 | 24 | |
| Stellate | 4 | 0 | |
| Mutton Fat | 12 | 17 | |
| Other | 2 | 0 | |
| Anterior chamber cells (%) | 0.11 | ||
| Grade ½+ | 31 | 12 | |
| 1+ | 40 | 35 | |
| 2+ | 22 | 29 | |
| 3+ | 4 | 12 | |
| Anterior chamber flare (%) | 0.69 | ||
| Grade 0 | 57 | 47 | |
| 1+ | 33 | 41 | |
| 2+ | 10 | 12 | |
| Iris (%) | |||
| Normal | 83 | 53 | 0.005 |
| Posterior synechiae | 4 | 24 | 0.01 |
| Sectoral iris atrophy | 8 | 23 | 0.08 |
| Other iris abnormality | 7 | 24 | 0.03 |
| Intraocular pressure (IOP), involved eyes | |||
| Median, mm Hg (25th, 75th percentile) | 15 (12, 20) | 18 (14, 22) | 0.80 |
| Percent patients with IOP>24 mm Hg either eye | 22 | 41 | 0.21 |
| Vitreous cells (%) | 0.01 | ||
| Grade 0 | 91 | 71 | |
| ½+ | 8 | 18 | |
| ≥1+ | 1 | 12 | |
| Immune compromised host (%) | 7 | 12 | 0.42 |
| Laboratory | |||
| Aqueous PCR positive for VZV* (%) | 9† | 88‡ | <0.0001 |
PCR = polymerase chain reaction; VZV=varicella zoster virus.
10 patients tested and 10 (100%) were positive.
15 patients tested and 15 (100%) were positive.
Table 3.
Characteristics of Cases with Varicella Zoster Anterior Uveitis with and without Aqueous Polymerase Chain Reaction Testing for Varicella Zoster Virus
| Characteristic | Without PCR* Testing | With PCR* Testing | P-value |
|---|---|---|---|
| Number cases | 98 | 25 | |
| Demographics | |||
| Age, median, years (25th 75th percentile) | 66 (56, 74) | 55 (50, 70) | 0.07 |
| Age category, years (%) | 0.13 | ||
| ≤16 | 2 | 0 | |
| 17–50 | 12 | 24 | |
| 51–60 | 25 | 32 | |
| >60 | 61 | 44 | |
| Gender (%) | 0.16 | ||
| Men | 37 | 52 | |
| Women | 63 | 48 | |
| Race/ethnicity (%) | 0.01 | ||
| White, non-Hispanic | 81 | 52 | |
| Black, non-Hispanic | 3 | 4 | |
| Hispanic | 3 | 0 | |
| Asian, Pacific Islander | 10 | 20 | |
| Other | 1 | 8 | |
| Missing/unknown | 2 | 16 | |
| Uveitis History | |||
| Uveitis course (%) | 0.62 | ||
| Acute, monophasic | 34 | 46 | |
| Acute, recurrent | 5 | 8 | |
| Chronic | 42 | 38 | |
| Indeterminate | 18 | 8 | |
| Laterality (%) | 0.91 | ||
| Unilateral | 96 | 96 | |
| Bilateral | 4 | 4 | |
| Ophthalmic examination | |||
| Cornea | 0.74 | ||
| No keratitis | 71 | 68 | |
| Keratitis | 29 | 32 | |
| Keratic precipitates (%) | 0.63 | ||
| None | 31 | 28 | |
| Fine | 44 | 36 | |
| Round | 9 | 16 | |
| Stellate | 4 | 0 | |
| Mutton Fat | 11 | 20 | |
| Other | 1 | 0 | |
| Anterior chamber cells (%) | 0.33 | ||
| Grade ½+ | 32 | 8 | |
| 1+ | 37 | 16 | |
| 2+ | 23 | 48 | |
| 3+ | 4 | 20 | |
| Anterior chamber flare (%) | 0.76 | ||
| Grade 0 | 54 | 60 | |
| 1+ | 36 | 28 | |
| 2+ | 10 | 12 | |
| Iris (%) | |||
| Normal | 83 | 64 | 0.06 |
| Posterior synechiae | 6 | 8 | 0.66 |
| Sectoral iris atrophy | 9 | 16 | 0.25 |
| Other iris abnormality | 6 | 24 | 0.001 |
| Intraocular pressure (IOP), involved eyes | |||
| Median, mm Hg (25th, 75th percentile) | 15 (12, 20) | 16 (14, 30) | 0.12 |
| Percent patients with IOP>24 mm Hg either eye | 21 | 36 | 0.29 |
| Vitreous cells (%) | 0.08 | ||
| Grade 0 | 89 | 84 | |
| ½+ | 10 | 8 | |
| ≥1+ | 1 | 8 | |
| Immune compromised host (%) | 8 | 5 | 0.22 |
| Dermatomal Herpes zoster (%) | 98 | 40 | <0.0001 |
PCR = polymerase chain reaction; testing for varicella zoster virus in aqueous.
Table 4.
Classification Criteria for Varicella Zoster Virus Anterior Uveitis
| Criteria |
| 1. Evidence of anterior uveitis |
| a. anterior chamber cells |
| b. if anterior vitreous cells are present, severity is less than anterior chamber inflammation |
| c. no evidence of retinitis |
| AND |
| 2. Unilateral uveitis (unless there is a positive aqueous PCR* for varicella zoster virus) |
| AND |
| 3. Evidence of varicella zoster virus infection in the eye |
| a. aqueous humor PCR positive for varicella zoster virus OR |
| b. sectoral iris atrophy in a patient ≥ 60 years of age OR |
| c. concurrent or recent dermatomal Herpes zoster |
| Exclusions |
| 1. Positive serology for syphilis using a treponemal test |
| 2. Evidence of sarcoidosis (either bilateral hilar adenopathy on chest imaging or tissue biopsy demonstrating non-caseating granulomata) |
| 3. Aqueous specimen PCR positive for cytomegalovirus or herpes simplex virus |
PCR = polymerase chain reaction
Discussion
Varicella zoster anterior uveitis concurrent with or following Herpes zoster ophthalmicus is an anterior uveitis ipsilateral to the cutaneous disease.1–3,5–7 Anterior uveitis in the context of herpes zoster ophthalmicus has been taken as prima facie evidence that the uveitis is due to VZV,1–3,5–7 and studies employing PCR of the aqueous humor support this approach.9,10,12 Rare cases of bilateral disease have been described, but typically require aqueous humor PCR testing for confirmation of VZV. In this series 96% of cases were unilateral. Herpes zoster can occur at any age but tends to occur in older patients with ~50% of reported cases over 60 years of age,2,8,21,22 as was present in the cases in this series. Morphologically, no single feature of the uveitis reliably diagnosed VZV anterior uveitis alone, and it can have an appearance similar to HSV anterior uveitis and occasionally CMV anterior uveitis.22–25 Studies using PCR analysis of aqueous suggested that a combination of age and sectoral iris atrophy can be used to morphologically diagnose VZV anterior uveitis and to distinguish it from HSV anterior uveitis.13,22 However, in the 50 to 59 year age bracket there is sufficient overlap that only ages below 50 years (HSV) and 60 years or above (VZV) can be used for diagnosis. Cytomegalovirus anterior uveitis more often has endothelial cell loss than HSV and VZV anterior uveitis and infrequently has sectoral iris atrophy. Nodular “coin-shaped” endothelial lesions are strongly suggestive of CMV anterior uveitis but are present in a minority of these patients;22,24,25 when present they should lead to paracentesis for PCR testing of aqueous, as currently that is the only way to reliably diagnose CMV anterior uveitis.26 However, because of the low yield on routine use, paracentesis for PCR analysis of aqueous for viruses is not always performed,27 and diagnoses may be made morphologically. The classification criteria for VZV anterior uveitis developed herein performed reasonably well with a low misclassification rate.
Varicella zoster virus and herpes simplex virus are causes of the acute retinal necrosis syndrome, which although primarily a retinitis, typically has anterior chamber and vitreous inflammation.28,29 Furthermore, unlike CMV retinitis, which nearly always occurs in patients with immune compromise, acute retinal necrosis occurs in both immunologically “normal” and immune compromised hosts. Therefore, it is important to exclude retinitis with ophthalmoscopy though a dilated pupil before concluding that the diagnosis is VZV anterior uveitis.
The presence of any of the exclusions in Table 4 suggests an alternate diagnosis, and the diagnosis of VZV anterior uveitis should not be made in their presence. In prospective studies many of these tests will be performed routinely, and the alternative diagnoses excluded. However, in retrospective studies based on clinical care, not all of these tests may have been performed. Hence the presence of an exclusionary criterion excludes VZV anterior uveitis, but the absence of such testing does not exclude the diagnosis of VZV anterior uveitis if the criteria for the diagnosis are met.
Classification criteria are employed to diagnose individual diseases for research purposes.19 Classification criteria differ from clinical diagnostic criteria, in that although both seek to minimize misclassification, when a trade-off is needed, diagnostic criteria typically emphasize sensitivity, whereas classification criteria emphasize specificity,19 in order to define a homogeneous group of patients for inclusion in research studies and limit the inclusion of patients without the disease in question that might confound the data. The machine learning process employed did not explicitly use sensitivity and specificity; instead it minimized the misclassification rate. Because we were developing classification criteria and because the typical agreement between two uveitis experts on diagnosis is moderate at best,18 the selection of cases for the final database (“case selection”) included only cases which achieved supermajority agreement on the diagnosis. Because of this, some patients diagnosed by clinicians as having VZV anterior uveitis may not satisfy these criteria.
In sum, the criteria for VZV anterior uveitis outlined in Table 4 appear to perform adequately well for use as classification criteria.
Figure 1.
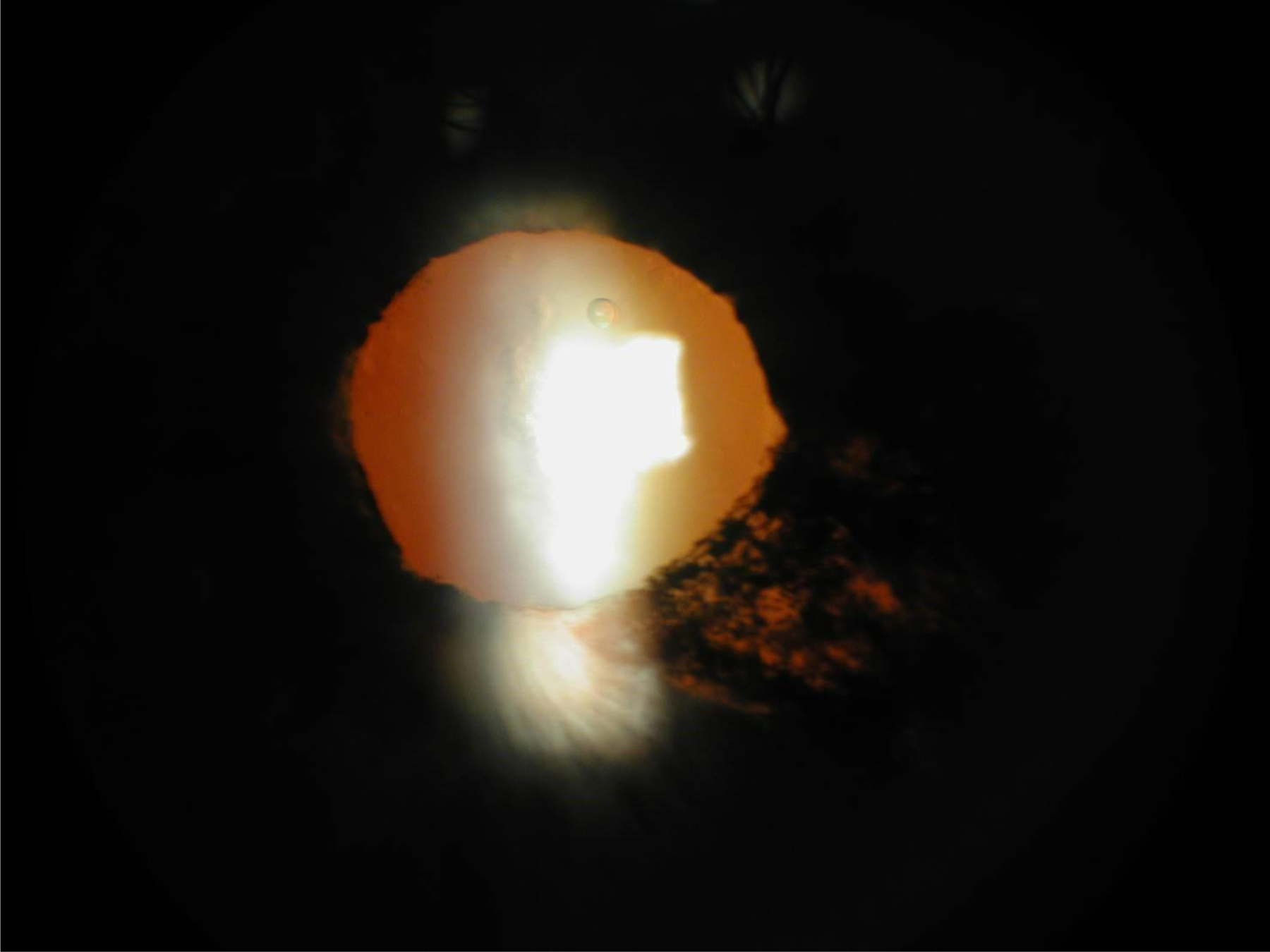
Sectoral iris atrophy in a patient with varicella zoster virus anterior uveitis
Acknowledgments
Grant support: Supported by grant R01 EY026593 from the National Eye Institute, the National Institutes of Health, Bethesda, MD, USA; the David Brown Fund, New York, NY, USA; the Jillian M. and Lawrence A. Neubauer Foundation, New York, NY, USA; and the New York Eye and Ear Foundation, New York, NY, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT roles: Douglas A. Jabs, MD, MBA: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing--Review and editing, Visualization, Supervision, Project administration, Funding acquisition. Laure Caspers, MD: Investigation, Writing--Original draft, Writing--Review and editing. Soon-Phaik Chee, FRCOphth, FRCS (G), FRCS (Ed), MMed (Singapore): Investigation, Writing--Review and editing. Debra Goldstein, MD: Investigation, Writing--Review and editing. Peter McCluskey, MD: Investigation, Data curation, Writing--Review and editing. Philip I. Murray, PhD, FRCP, FRCS, FRCOphth: Investigation, Writing--Review and editing. Neal Oden, PhD: Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing--Review and editing. Alan G. Palestine, MD: Investigation, Writing--Review and editing. James T. Rosenbaum, MD: Investigation, Writing--Review and editing. Jennifer E. Thorne, MD, PhD: Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing--Review and editing. Brett E. Trusko, PhD, MBA: Methodology, Software, Resources, Data curation, Investigation, Writing--Review and editing.
REFERENCES
- 1.Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008;115 (Suppl):S3–12. [DOI] [PubMed] [Google Scholar]
- 2.Thean JH, Hall AJ, Stawell RJ. Uveitis in Herpes zoster ophthalmicus. Clin Exp Ophthalmol 2001;29:406–10. [DOI] [PubMed] [Google Scholar]
- 3.Tugal-Tutkun I, Cimino L, Akova YA. Review for disease of the year: Varicella zoster virus-induced anterior uveitis. Ocul Immunol Inflamm 2018;26:171–7. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Hu CC, Lin HC. Increased risk of anterior uveitis following herpes zoster: a nationwide population-based study. Arch Ophthalmol 2012;130:451–5. [DOI] [PubMed] [Google Scholar]
- 5.Cobo LM, Foulks GN, Lass J, et al. Oral acyclovir in the therapy of acute herpes zoster ophthalmicus. An interim report. Ophthalmology 1985;92:1574–83. [DOI] [PubMed] [Google Scholar]
- 6.Cobo LM, Foulks GN, Liesegang T, et al. Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology 1986;93:763–70. [DOI] [PubMed] [Google Scholar]
- 7.Colin J, Prisant O, Cochener B, Lescale O, Rolland B, Hoang-Xuan T. Comparison of the efficacy and safety of valacicolvir and acyclovir for the treatment of herpes zoster ophthalmicus. Ophthalmology 2000;107:1507–11. [DOI] [PubMed] [Google Scholar]
- 8.Kahloun R, Attia S, Jelliti B, et al. Ocular involvement and visual outcome of herpes zoster ophthalmicus: review of 45 patients from Tunisia, North Africa. J Ophthalmic Inflamm Infect 2014;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S, Tada R, Shimomura Y, et al. Detecting varicella-zoster virus DNA in iridocyclitis using polymerase chain reaction. Arch Ophthalmol 1995;113:1358–9. [DOI] [PubMed] [Google Scholar]
- 10.Sugita S, Shimizu N, Watanabe K, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol 2008;92:928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kido S, Sugita S, Horie S, et al. Association of varicella zoster virus load in the aqueous humor with clinical manifestations of anterior uveitis in herpes zoster ophthalmicus and zoster sine herpete. Br J Ophthalmol 2008; 92:505–8. [DOI] [PubMed] [Google Scholar]
- 12.Neumann R, Barquet D, Rosenblatt A, et al. Herpetci anterior uveitis – analysis of presumed and PCR proven cases. Ocular Immunol Inflamm 2019; 27:211–8. [DOI] [PubMed] [Google Scholar]
- 13.Van der Lelij A, Ooijman FM, Kilstra A, Rogthova A. Anterior uveitis with sectoral iris atrophy in the absence of keratitis. A distinct clinical entity among herpetic eye diseases. Ophthalmology 2000;107:1164–70. [DOI] [PubMed] [Google Scholar]
- 14.Jabs DA, Rosenbaum JT, Nussenblatt RB, the Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Report of the first international workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol 2013;156:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trusko B, Thorne J, Jabs D, et al. Standardization of Uveitis Nomenclature Working Group. The SUN Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med 2013;52:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada AA, Jabs DA. The SUN Project. The future is here. Arch Ophthalmol 2013;131:787–9. [DOI] [PubMed] [Google Scholar]
- 18.Jabs DA, Dick A, Doucette JT, Gupta A, Lightman S, McCluskey P, Okada AA, Palestine AG, Rosenbaum JT, Saleem SM, Thorne J, Trusko B for the Standardization of Uveitis Nomenclature Working Group. Interobserver agreement among uveitis experts on uveitic diagnoses: the Standard of Uveitis Nomenclature experience. Am J Ophthalmol 2018; 186:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria. Arthritis Care Res 2015;67:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Standardization of Uveitis Nomenclature (SUN) Working Group. Development of classification criteria for the uveitides. Am J Ophthalmol 2020;volume:pp. [DOI] [PubMed] [Google Scholar]
- 21.Ghaznawi N, Virdi A, Dayan A, et al. Herpes zoster ophthalmicus: comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology 2011:118:2242–50. [DOI] [PubMed] [Google Scholar]
- 22.Takase H, Kubono R, Terada Y et al. Comparison of ocular characteristics of anterior uveitis caused by herpes simplex, varicella zoster, and cytomegalovirus. Jpn J Ophthalmol 2014;58:473–82. [DOI] [PubMed] [Google Scholar]
- 23.Miserocchi E, Waheed NK, Dios E, et al. Visual outcome in herpes simplex virus and varicella zoster virus uveitis: a clinical evaluation and comparison. Ophthalmology 2002;109:1532–7. [DOI] [PubMed] [Google Scholar]
- 24.Wensig B, Relvas LM, Caspers LE, et al. Comparison or rubella virus- and herpes virus-associated anterior uveitis: clinical manifestations and visual prognosis. Ophthalmology 2011;118:1905–10. [DOI] [PubMed] [Google Scholar]
- 25.Relvas LJ, Caspers L, Chee SP, Zierhut M, Willermain F. Differential diagnosis of viral-induced anterior uveitis. Ocul Immunol Inflamm 2018;26:726–31. [DOI] [PubMed] [Google Scholar]
- 26.The Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for cytomegalovirus anterior uveitis. Am J Ophthalmol 2020;vol:pp. [DOI] [PubMed] [Google Scholar]
- 27.Anwar Z, Galor A, Albini TA, Miller D, Perez V, Davis JL. The diagnostic utility of anterior chamber paradentesis with polymerase chain reaction in anterior uveitis. Am J Ophthalmol 2013;155:781–6. [DOI] [PubMed] [Google Scholar]
- 28.Ganatra JB, chandler D, Santos C, Kupperman B, Margolis TP. Viral causes of acute retinal necrosis syndrome. Am J Ophthalmol 2000;129:166–72. [DOI] [PubMed] [Google Scholar]
- 29.Wong R, Pavesio CE, Laidlaw DA, Williamson TH, Graham EM, Stanford MR. Acute retinal necrosis: the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology 2010;117:556–60. [DOI] [PubMed] [Google Scholar]


