Abstract
Studies of the regenerative capacity of the liver have converged on the Hippo pathway, a serine/threonine kinase cascade discovered in Drosophila and conserved from unicellular organisms to mammals. Genetic studies of mouse and rat livers have revealed that the Hippo pathway is a key regulator of liver size, regeneration, development, metabolism, and homeostasis and that perturbations in the Hippo pathway can lead to the development of common liver diseases, such as fatty liver disease and liver cancer. In turn, pharmacological targeting of the Hippo pathway may be utilized to boost regeneration and to prevent the development and progression of liver diseases. We review current insights provided by the Hippo pathway into liver pathophysiology. Furthermore, we present a path forward for future studies to understand how newly identified components of the Hippo pathway may control liver physiology and how the Hippo pathway is regulated in the liver.
Keywords: Hippo pathway, YAP/TAZ, liver cancer, fatty liver disease, regeneration, metabolism
INTRODUCTION
The liver, which acts as a key checkpoint for circulation from the digestive tract, is a highly complex organ that serves diverse roles such as maintaining plasma glucose and ammonia levels, detoxifying drugs, synthesizing bile, and storing and processing key nutrients. The importance of the liver in whole-body homeostasis can be dramatically recognized in individuals with liver damage, who often exhibit diverse symptoms such as fatigue and lethargy, swelling and ascites, encephalopathy, and jaundice. Threats to the liver come in the form of alcohol, cancer, viral and other infections, drugs and toxins, obesity and metabolic syndrome, genetic diseases, and autoimmune conditions (1). As such, liver cirrhosis, the end stage for chronically injured liver, has been estimated to account for 1 million deaths worldwide per year (2).
Because of its position to defend the body against toxic threats, the liver has an evolutionarily conserved and remarkable capacity to regenerate, an ability immortalized in the ancient Greek myth of the punishment of Prometheus, whose liver is said to be lost and regenerated every day (3). Indeed, classical experiments showed that after surgical resection of three of the five lobes of the liver, rats can recover the original biomass of their liver within two weeks (4). This amazing regenerative property in humans has been utilized for clinical benefit in the process of split-organ transplantation, in which one liver donor grants two liver allografts, and in resections due to oncological indications (5). Further inherent to this regenerative capacity is the tight control of the size of the liver; livers, even those from baboons, transplanted into humans can both grow and shrink to match the body size of the recipient (6, 7).
Over the past two decades, the discovery of a growth regulatory pathway in Drosophila, termed the Hippo pathway (Figure 1), has provided insight into a genetically encoded size-control and regeneration program in the liver. Elucidation of the Hippo pathway in the mammalian liver has unraveled how perturbations in this regenerative pathway drive diseases, from fatty liver disease to liver cancer (8–10). Consequently, many researchers have turned to the Hippo pathway as a potential therapeutic target to enhance liver regeneration and to prevent and cure liver disease. This review explores our common understanding of the Hippo pathway, with a particular emphasis on its role in the physiology of the liver, highlighting potential opportunities for therapy. We begin by discussing key molecules of the Hippo pathway in mammals and then delineate their roles in liver size, regeneration, homeostasis, development, metabolism, and disease.
Figure 1.
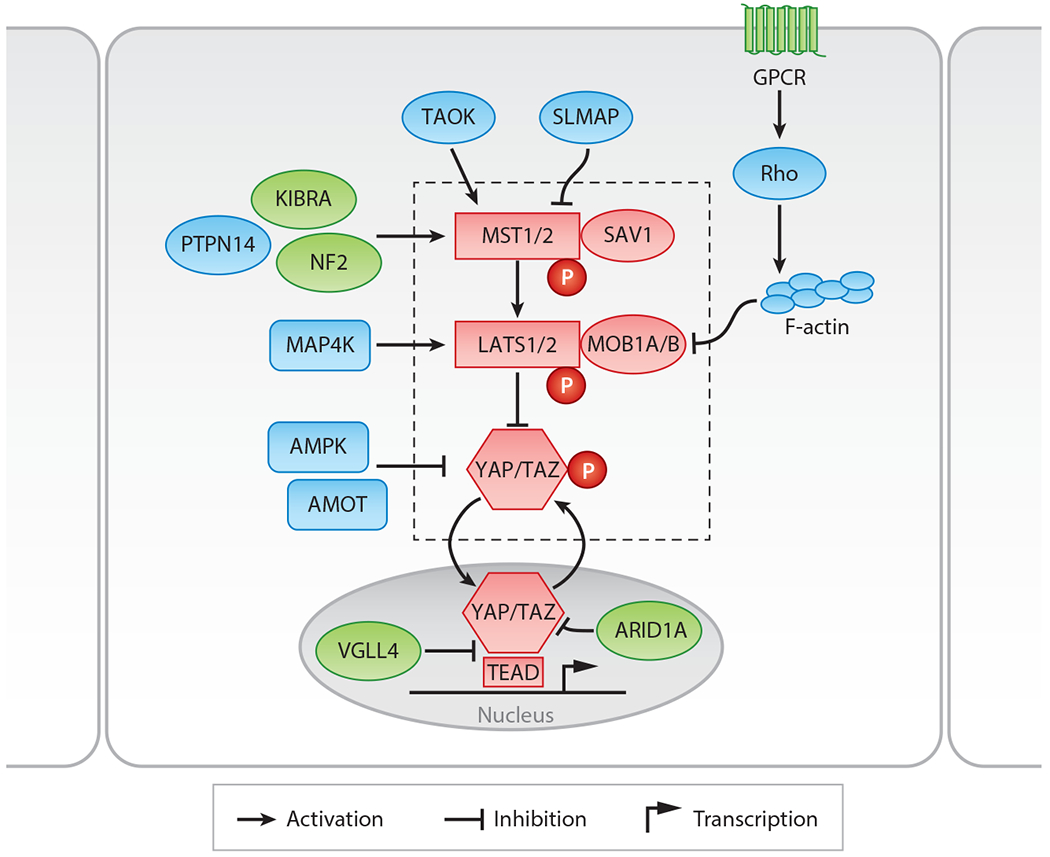
The Hippo pathway in mammals. Components in red denote the core proteins of the Hippo pathway, and components within the dotted rectangle indicate components of the canonical kinase cascade. Components in green are regulators of the Hippo pathway that are important in the liver, and components in blue denote regulators identified in Drosophila or in mammals that have not yet been tested in the liver but are discussed in this review.
THE MAMMALIAN HIPPO PATHWAY
The Hippo pathway was first discovered at the beginning of the twenty-first century as a key signaling pathway that suppresses the growth of tissues in Drosophila. From unbiased mutagenesis screens using genetic mosaics, four tumor suppressor genes—warts (LATS1/2 in mammals) (11, 12), salvador (SAV1 in mammals) (13, 14), mob as tumor suppressor (MOB1A/B in mammals) (15), and hippo (MST1/2 in mammals) (16–20)—were identified on the basis of their ability to promote massive overgrowth as homozygous loss-of-function mutant clones. In the fly, these tumor suppressors constitute a kinase cascade (20) that impinges on a transcriptional coactivator, Yorkie (YAP and TAZ in mammals), which is the substrate of Warts (LATS1/2) (21). Yorkie was then shown to bind to Scalloped (TEAD1/2/3/4 in mammals), which acts as the transcription factor that binds to DNA and controls gene transcription (22, 23). The pathway was so named because of the newly identified upstream kinase and tumor suppressor hippo, and because of the role of the pathway in regulating tissue size. Soon after identification of the genes in Drosophila, the mammalian orthologs of the Hippo pathway and their functional significance were validated in mammalian cells (8, 24, 25). The duplication and evolutionary conservation of many of the core Hippo pathway proteins in mammals emphasize the critical role of the pathway in size control and other physiological functions.
The canonical mammalian Hippo pathway kinase cascade consists of the MST1/2–SAV1 complex phosphorylating and activating the LATS1/2–MOB1A/B complex, which then phosphorylates and inactivates YAP and TAZ (Figure 1; Table 1). MST1/2 and LATS1/2 are the core kinases of the cascade, and SAV1 and MOB1A/B act as adaptor proteins to enhance the activation and phosphorylation of MST1/2 and LATS1/2 (26). The phosphorylation-controlled inactivation of YAP and TAZ is mediated by cytoplasmic sequestration of phosphorylated YAP/TAZ by 14-3-3 proteins as well as by proteasome-mediated degradation of phosphorylated YAP/TAZ (8, 24, 25, 27, 28). Genetic deletion of MST1/2, SAV1, LATS1/2, or MOB1A/B thus leads to increased nuclear enrichment of YAP and TAZ and to their increased activity as transcriptional coactivators. Conversely, overexpression of MST1/2, SAV1, LATS1/2, or MOB1A/B leads to increased cytoplasmic localization and degradation of YAP and TAZ. Therefore, the protein level and subcellular localization of YAP/TAZ are often used as readouts of Hippo pathway activity.
Table 1.
Core components of the Hippo pathway and their phenotypes after genetic manipulation in mice
| Hippo pathway component | Manipulation | Phenotype | Reference(s) |
|---|---|---|---|
| YAP | Overexpression in hepatocytes | Hepatomegaly | 8, 48 |
| Drives the formation of hepatocellular carcinoma, cholangiocarcinoma, or both; drives hepatoblastoma with additional WNT activation | 8, 47, 73, 83, 85 | ||
| Dedifferentiation of hepatocytes to cholangiocytes | 47 | ||
| Peritumoral activation of YAP restrains adjacent tumor growth |
112 | ||
| Loss in hepatocytes | Prevents progression of hepatocellular carcinoma | 82 | |
| Hepatomegaly partially through increased fibrogenesis | 35, 59 | ||
| Prevents proper regenerative response in combination with deletion of TAZ | 59 | ||
| Prevents ductular response to injury | 63 | ||
| Loss in cholangiocytes | Hypoplastic biliary ducts after development-specific deletion | 35, 59 | |
| Prevents ductular response to injury | 63, 64 | ||
| Causes loss of bile ducts after deletion in adulthood | 63 | ||
| TAZ/WWTR1 | Overexpression | Drives the formation of hepatocellular carcinoma | 73 |
| Drives inflammation | 70, 73 | ||
| Promotes progression of NASH | 70 | ||
| LATS1/2 | Loss | Hepatomegaly | 49, 50 |
| Peritumoral loss of LATS1/2 restrains adjacent tumor growth | 112 | ||
| Shift differentiation potential of hepatoblasts to cholangiocytes | 50, 75 | ||
| Spontaneous development of fatty liver disease | 118 | ||
| MST1/2 | Overexpression | Suppresses hepatocellular carcinoma growth | 53 |
| Suppresses lipid accumulation in Akt-driven fatty liver disease |
117 | ||
| Loss | Hepatomegaly | 51, 52 | |
| Drives the formation of hepatocellular carcinoma and/or cholangiocarcinoma | 51–53, 74 | ||
| Promotes macrophage infiltration and inflammation | 74 | ||
| Promotes regeneration | 155 | ||
| Promotes fatty liver disease | 119 | ||
| SAV1 | Loss | Hepatomegaly | 51, 54 |
| Drives the formation of hepatocellular carcinoma and/or cholangiocarcinoma | 51, 54 | ||
| Promotes nonalcoholic fatty liver disease | 71, 117 | ||
| MOB1A/1B | Loss | Hyperplasia of cholangiocytes | 55 |
| Drives the formation of hepatocellular carcinoma and/or cholangiocarcinoma | 55 | ||
| NF2 | Loss | Drives the formation of hepatocellular carcinoma and/or cholangiocarcinoma | 35, 47 |
| Leads to the dedifferentiation of hepatocytes | 47 | ||
| KIBRA | Loss | Hepatomegaly and combined hepatocellular carcinoma and cholangiocarcinoma |
56 |
| Inflammation | 56 |
When Hippo signaling is low, YAP and TAZ enter the nucleus and interact with the TEF/TEAD family of DNA-binding transcription factors, composed of TEAD1/2/3/4, to drive Hippo target genes, such as CTGF (29). The importance of this interaction can be highlighted by the co-occupancy of YAP/TAZ and TEAD genome wide at particular sites on chromatin (30, 31). Furthermore, a disease-causing point mutation in TEAD that affects its binding with YAP and TAZ underlies Sveinsson chorioretinal atrophy (32). The TEAD family transcription factors, when not bound to YAP and TAZ, bind to transcriptional corepressors such as VGLL4 to suppress Hippo target gene expression (33). Transcriptional activity of YAP and TAZ can then be antagonized both by Hippo pathway activity and by VGLL4 expression.
Many other proteins also feed into the Hippo pathway to regulate the phosphorylation or localization of the Hippo kinases or of YAP and TAZ. Those studied most intensely in the liver are the proteins neurofibromin 2 (NF2), a well-known tumor suppressor that, when defective, leads to the autosomal dominant disorder neurofibromatosis type II (34), and kidney and brain expressed protein (KIBRA). NF2 increases Hippo pathway activity to restrict YAP and TAZ activity (35, 36) by targeting LATS1/2 to the plasma membrane and increasing its activation and phosphorylation by MST1/2 (37). KIBRA also activates the Hippo pathway to restrain YAP and TAZ by interacting with NF2 and other tumor suppressors (38). Together, NF2 and KIBRA are thought to regulate Hippo signaling at cell junctions, particularly at the apical surface of epithelial cells (26).
In diverse contexts, the Hippo pathway is tumor suppressive, and YAP/TAZ regulate both cell proliferation and cell survival. Many studies have identified target genes of YAP/TAZ to be those that control cell proliferation, cell migration, epithelial-mesenchymal transition, extracellular matrix organization, and cytoskeletal organization (30, 39, 40). YAP/TAZ expression is both required and sufficient to confer cancer stem cell traits (41), and YAP/TAZ promote tumor immune evasion by regulating PD-L1 (42). Furthermore, YAP/TAZ can drive resistance in many different cancers to targeted therapies (43). Importantly, YAP/TAZ regulate many other oncogenes, most notably c-Myc, in both Drosophila and mammals (30, 44). YAP/TAZ and the Hippo pathway have also been suggested to regulate other oncogenic pathways in particular cell types and contexts, such as Wnt (45, 46) and Notch (47).
THE HIPPO PATHWAY IN LIVER SIZE AND HEPATO-BILIARY REGENERATION
In Drosophila, the Hippo pathway was discovered to be a key regulator of tissue size, and manipulations of the Hippo pathway in the mouse liver resulted in a similarly striking phenotype. Transgenic overexpression of YAP in the liver causes massive hepatomegaly, producing livers up to five times their normal size (8, 48). This overgrowth is mediated by an increase in hepatocyte proliferation coordinated with a decrease in hepatocyte death. Importantly, cessation of YAP overexpression causes a reversal in this phenotype, with the liver rapidly returning to its normal size (8, 48). Extended overexpression of YAP results in the formation of hepatocellular carcinoma (HCC) (8), a topic that is discussed in the section titled The Hippo Pathway in Liver Cancer. Thus, YAP/TAZ act as key signaling molecules to control the overall size of the liver (Figure 2).
Figure 2.
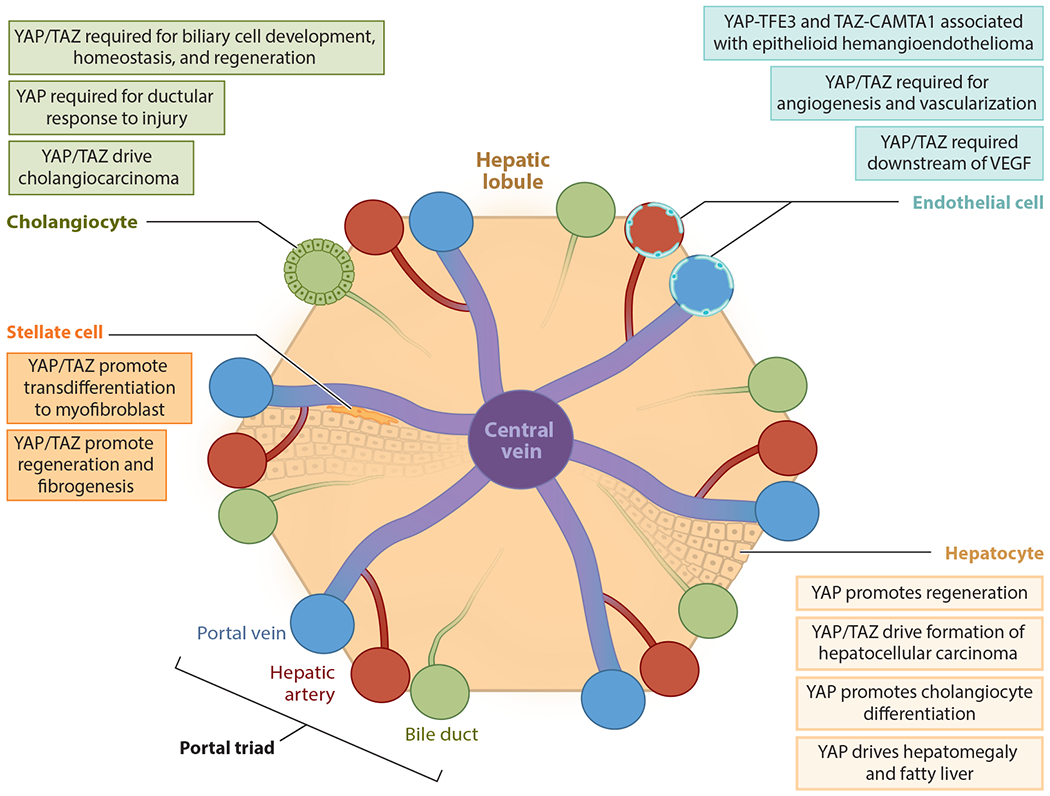
Cell-type-specific functions of the Hippo pathway. The Hippo pathway and YAP/TAZ play key roles in hepatocytes, cholangiocytes, endothelial cells, and stellate cells within the liver, which are depicted here in a representation of a hepatic lobule. Shared features include that YAP/TAZ regulate liver regeneration in the context of the stellate cell, hepatocyte, and cholangiocyte and are involved in cancers that originate from liver bile duct cells, hepatocytes, and endothelial cells.
Further proving the importance of the Hippo pathway in liver pathophysiology, manipulation of the upstream components of the Hippo pathway that control YAP/TAZ activation results in similar phenotypes. Deletion of Lats1/2 (49, 50), Mst1/2 (51–53), Sav1 (51, 54), Mob1a/b (55), Nf2 (35), or Kibra (56) also drives the formation of hepatomegaly and cancers (Table 1). Additional evidence that supports that YAP and TAZ act through the TEAD family of proteins is that expression of a dominant-negative TEAD2 can suppress YAP-induced hepatomegaly and cancer (57). Additionally, overexpression of Vgll4, which antagonizes Yap’s binding to the TEAD family of transcription factors, suppresses YAP-induced liver overgrowth and cancer (33).
Consistent with the functional role of the Hippo pathway in regulating liver size, YAP and TAZ are important mediators of the liver regenerative response (58, 59) (Figure 2). One day after partial hepatectomy in rats, Yap target genes are upregulated, and Yap is enriched in the nucleus, likely due to an observed decrease in Hippo pathway kinase activation. Further showing that the dynamic regulation of the Hippo pathway is critical for liver regeneration, Mst1/2 and Lats1/2 are again activated after a return to the normal liver–to–body weight ratio, and Yap activity is concurrently decreased (58). Liver-specific Albumin-Cre-mediated genetic deletion of Yap and Taz hampers regeneration and prevents return to the normal liver–to–body weight ratio after 2/3 partial hepatectomy (59). Therefore, YAP/TAZ function to regulate both liver size and liver regeneration.
Central to their role in liver regeneration, YAP and TAZ have been investigated for their response to damage and for their roles in other common liver diseases. Prominently, YAP/TAZ have been implicated in the development of biliary ductular reactions. Ductular reactions are hyperplastic biliary ducts that develop in response to liver injury, and are associated with both fibrogenesis and regeneration (60). Evidence for the importance of YAP in the response to injury and in ductular reactions comes from studies demonstrating an increase in YAP activity in the ductular response to injury correlated with the severity of nonalcoholic fatty liver disease (NAFLD) (10), in the presence of biliary atresia in neonates (61), and in bile ductular reactions to primary sclerosing cholangitis and primary biliary cirrhosis (62). In mouse models of biliary cholestasis induced by bile duct ligation, loss of Yap prevented the ductular response to injury, inhibited hepatocyte proliferation, and increased hepatocyte death (62).
Indeed, recent studies have identified the regenerative program of chronically injured liver to be defined by a unique pattern of Hippo pathway activation (63, 64). Single-cell RNA-sequencing analysis of biliary epithelial cells revealed particular cells defined by activation of Yap and Taz target genes. This compartment of Yap/Taz-activated cells increased after chronic liver injury induced by treatment with 3,5-diethoxycarbonyl-1,4-dihydrocollididine (DDC). In hepatocytes, activation of a Yap transcriptional program was also revealed by single-cell RNA sequencing after treatment with DDC, suggesting that Yap is induced in both hepatocytes and cholangiocytes after injury. Ablation of Yap by treatment with a hepatocyte-specific adenovirus abrogated the ductular response to injury. From this result, Pepe-Mooney et al. (63) concluded that Yap may control differentiation from hepatocytes to biliary epithelial cells (discussed in the section titled YAP/TAZ in Liver Development, Homeostasis, and Organoid Formation) and may also have non-cell autonomous effects on proliferating biliary epithelial cells. A concurrent study, using a CRISPR screen in biliary epithelial cell organoids, concluded that the Hippo pathway, but not LGR4/5-dependent WNT signaling, is a key mediator of biliary expansion and is required for the ductular response after chronic liver injury, partially by controlling biliary epithelial cell proliferation (64).
THE HIPPO PATHWAY IN LIVER INFLAMMATION AND FIBROSIS
Although YAP and TAZ promote regeneration in the liver, emerging evidence suggests that these transcriptional coactivators may also drive fibrosis and inflammation, which can be detrimental to the regenerating liver. In particular, YAP/TAZ are thought to regulate liver fibrosis via the stellate cell (65–67). Stellate cells are tissue-resident mesenchymal cells that, upon activation, transdifferentiate to a myofibroblastic state, in which they secrete factors that aid in inflammation and wound repair (68). Yap accumulates in the nucleus and becomes activated in hepatic stellate cells in mice after administration of the liver-damaging agent CCl4. YAP was also activated in hepatic stellate cells in patients with hepatitis C infection. Importantly, pharmacological inhibition of Yap was sufficient to prevent hepatic stellate cell activation and fibrogenesis. Thus, YAP activation in hepatic stellate cells may contribute to the architectural distortion of the liver due to chemical or infectious injury (65). In contrast, others have argued that activation of YAP in hepatic stellate cells is beneficial to liver regeneration. Pharmacological treatment of regenerating liver after ischemia-reperfusion injury with a YAP inhibitor reduced stellate cell proliferation and significantly impaired regeneration (66). Preventing hepatic stellate cell activation and Yap activation by manipulating Hedgehog signaling also inhibited liver regeneration and hepatocyte proliferation (67). Therefore, it seems that YAP activation in hepatic stellate cells drives beneficial non-cell-autonomous effects in the short term but detrimental effects in the long term.
Although Yap activation in a hepatocyte alone is not sufficient to drive proliferation without inflammation (69), other researchers have identified YAP and TAZ as critical components of liver inflammation. TAZ is increased in the hepatocytes of individuals with nonalcoholic steatohepatitis (NASH), and Taz is also increased in several mouse models of NASH (70). Suppression of Taz in mouse models of NASH was sufficient to prevent inflammation, fibrosis, and cell death, and Taz expression promoted progression from steatosis to NASH. One potential mechanism for this phenotype is that Taz controls the expression of Ihh (Indian hedgehog), which leads to the non-cell-autonomous activation of stellate cells (70). Similarly, a transposon screen in mouse liver identified Sav1 as a critical suppressor for the development of NAFLD and hepatitis-B-virus-induced HCC. Kodama et al. (71) proposed that Sav1 may prevent NAFLD through its role in turning off Yap/Taz activation and their fibrogenic program.
An emerging suggestion is that TAZ and YAP may not be completely redundant and may have different transcriptional targets (72, 73). This distinction was observed in the liver, in which the expression of Taz, but not Yap, was associated with the development of inflammation. Taz expression in hepatocytes promotes myeloid infiltration, and mouse and human tumors with increased TAZ show an increase in proinflammatory cytokines (73). Similarly, loss of Mst1/2 leads to liver inflammation, particularly through upregulation of monocyte chemoattractant protein-1 (Mcp-1) (51, 74). However, in this model, suppression of Yap was sufficient to lead to ablation of inflammation (74). Thus, how YAP/TAZ coordinate or diverge to control inflammation in the liver remains unclear, but their dysregulation is sufficient to drive myeloid infiltration and inflammation.
YAP/TAZ IN LIVER DEVELOPMENT, HOMEOSTASIS, AND ORGANOID FORMATION
After discovery of its role in controlling liver size and regeneration, the Hippo pathway has been revealed to be a key mediator in the development of the liver. Albumin-Cre driven Yap deletion results in elevated serum bilirubin and alanine aminotransferase concurrent with hepatomegaly due to macrovesicular steatosis and progressive fibrosis. Yap deletion also leads to hypoplastic biliary ducts and increased hepatocyte turnover (35). Deletion of both Yap and Taz leads to phenotypes similar to those observed in Yap-specific depletion, suggesting that Yap is more important than Taz for liver development (59). Yap is also required during adult biliary cell homeostasis, as deletion of Yap leads to loss of bile duct morphology and integrity and widespread liver damage. However, Pepe-Mooney et al. (63) did not observe any phenotype in any other organ or cell type, including hepatocytes, after adult deletion of Yap, suggesting that in adult biliary epithelial cells, Yap might be specifically required.
Additionally, YAP has been suggested to promote biliary cell identity (47, 50, 75). Biliary epithelial cells express high levels of Yap and have higher expression of YAP/TAZ target genes (47). In vitro or in vivo depletion of Lats1/2 in hepatoblasts is sufficient to shift the differentiation program toward the expansion and development of bile ducts, potentially through suppression of the hepatocyte-specific transcription factor Hnf4α (50, 75). Expression of an activated YAP (YAPS127A) or deletion of Nf2 also induced transdifferentiation of hepatocytes to a bile duct fate, potentially through the activation of Notch signaling (47). Altogether, these results suggest that Yap and Taz are required for liver cell specification and that Hippo signaling controls the differentiation program from hepatoblasts to hepatocytes to biliary epithelial cells. Thus, dysregulation of this pathway, even in adulthood, is sufficient to promote pathology.
Although not exclusively studied, it is also likely that YAP/TAZ and the Hippo pathway play a critical role in the development and maintenance of liver sinusoidal endothelial cells. YAP/TAZ are required for angiogenesis downstream of VEGF signaling (76, 77). Similarly, Yap/Taz deletion in endothelial cells results in embryonic lethality due to poor vascularization (76). Using retinal angiogenesis as a model, many groups have shown that Yap and Taz are required for angiogenic sprouting and branching and that Lats1/2 deletion results in an increase in sprouting and branching (78–80). In total, specific YAP/TAZ depletion in liver sinusoidal endothelial cells may reveal new ways in which YAP/TAZ control liver regeneration, development, and homeostasis.
Also, YAP/TAZ and their regulation by the Hippo pathway seem to be critical components for primary hepatocyte culture and organoid formation. YAP overexpression in mouse liver promotes the formation, after dissection, of liver organoids (47), and Yap is required for the formation of biliary organoids. Similarly, loss of Sav1 and Lats1 promotes biliary organoid formation (64). Furthermore, one of the primary limitations of primary hepatocyte culture is maintaining hepatocyte identity. Recent work has argued that cell spreading, which induces Yap activation through mechanical force, induces hepatocyte dedifferentiation. Confining cell spreading or pharmacologically limiting the mechanical tension is sufficient to maintain Yap inactivation and hepatocyte differentiation (81). Therefore, pharmacological inhibition of YAP activity may be a mechanism to increase the efficiency and maintenance of liver organoids.
THE HIPPO PATHWAY IN LIVER CANCER
YAP has long been implicated in the formation of HCC. Yap is overexpressed and required for progression in c-Myc and Akt1-driven HCC (82). Hepatocyte-specific YAP overexpression is also sufficient to drive the formation of HCC (8). Likewise, hydrodynamic tail injection–mediated overexpression of Yap or Taz and NRas can drive HCC formation (73, 83). Emphasizing the role of the Hippo pathway in restraining the development of liver cancer, depletion of Mst1/2 (51–53), Sav1 (54), Nf2 (35, 47),Mob1a/b (55), or Kibra (Wwc1/2) (56) is sufficient to lead to the development of HCC, cholangiocarcinoma (CC), or mixed HCC and CC. Additionally, activation of Yap was shown to be an early and required event in carcinogen-induced liver cancer in rats (84).
Furthermore, Yap is important in additional liver cancers. YAP is overexpressed in human hepatoblastomas and is required for hepatoblastoma cell line proliferation. Furthermore, YAP and β-catenin overexpression in adult mouse liver is sufficient to lead to the development of hepatoblastoma (85, 86), and withdrawal of activated YAP in established hepatoblastoma induces tumor regression (87). This is particularly interesting in light of evidence that individuals with germline mutations in APC and familial adenomatous polyposis have an increased risk of hepatoblastoma (88). In studies of APC-deficient adenomas in the intestine, APC independently negatively regulates Yap and β-catenin (89). Therefore, genetic loss of APC in the liver may also result in activated YAP and β-catenin, increasing the likelihood of developing hepatoblastoma in individuals with familial adenomatous polyposis. Additionally, an endothelial cell–derived vascular tumor, epithet-lioid hemangioendothelioma, most commonly presents in the liver and is defined by chromosomal translocation-derived fusion genes, TAZ-CAMTA1 and YAP-TFE3, which are thought to evade Hippo-mediated suppression and to drive an activated YAP/TAZ-like gene program (90–93).
Activated YAP/TAZ are thought to drive expression of important TEAD-dependent targets that sustain growth and survival of these various liver cancers. For example, YAP/TAZ promote expression of the antiapoptotic gene BIRC5, which is required for the survival of liver cancer cells (8). Another known target of YAP in the liver is the adenosine monophosphate-activated kinase (AMPK) protein family member NUAK2, which is required for YAP-dependent liver cancer growth and sustains activation of YAP (94). YAP also activates expression of Notch pathway genes, such as Notch2, and activation of Notch is required for YAP-dependent hepatocyte reprogramming and CC development (43). Furthermore, a YAP/TAZ gene signature that can predict poor prognosis in patients has even been developed, though how many of these genes, composed of well-known YAP/TAZ targets such as Ctgf, may promote liver cancer development and progression remains unknown (95).
Despite strong genetic evidence for the role of the Hippo pathway and YAP and TAZ in the development of liver cancer, how the Hippo pathway becomes dysregulated to drive liver cancer has remained elusive. Many studies have shown that increased YAP and TAZ levels correlate with worse prognosis in liver cancer (94–100) and that most (62%) patients with HCC exhibit YAP overexpression. One study found up to 12% of patients with CC had focal deletions in SAV1 (101) and another study saw up to 5% of patients with NF2 loss (102). Others observed frequent decreases in MST1 levels and concomitant decreases in MOB1 and YAP phosphorylation in HCC (53).
One attractive hypothesis is that more common mutations in liver cancer either directly or indirectly lead to Hippo pathway dysregulation and YAP and TAZ activation. ARID1A is mutated in 10–15% of patients with HCC and in 20% of patients with combined HCC and CC (103–105). A recent study has argued that Arid1a binds to and suppresses the transcriptional output of Yap. Additionally, in mice, loss of Arid1a dramatically promotes the formation of combined HCC and CC in the Nf2-deleted background, and Yap and Taz depletion can rescue bile duct proliferation mediated by the loss of Arid1a (106). Loss of p53, which is also very common in liver cancer (104, 105), may also influence YAP and TAZ activity by decreasing the expression of a known negative regulator of YAP and TAZ, PTPN14 (107). KRAS, which is frequently mutated in liver cancer and is sufficient to drive the formation of CC in combination with p53 mutations (108), also drives Yap activity that can sustain tumor progression even after removal of Kras expression (39, 109). Epigenetic changes that lead to changes in the expression of known YAP/TAZ or Hippo pathway regulators may also be important for the activation of YAP and TAZ in liver cancer. The upregulation of SET1A, which is observed in many cancers, can lead to the methylation and increased nuclear localization and activity of YAP (110).
A newer perspective on the role of YAP/TAZ in the development of liver cancer comes from studies of cell competition. Overexpression of yorkie, the Drosophila homolog of YAP, in cell clones (the winner cell) can result in the elimination of wild-type neighboring cells (loser cells) (44, 111). From this perspective, recent work has shown that YAP and TAZ are activated in peritumoral hepatocytes in both mice and humans, and this activation actually works to restrain progression of the tumor, as loss of Yap and Taz in neighboring tissues leads to faster progression of the cancer. Furthermore, overexpression of YAP and TAZ in hepatocytes adjacent to tumors driven by N-AKT could prevent progression of the cancer. These results suggest that it is the relative level of YAP/TAZ in the tumor to the surrounding tissue that defines the winner and loser cells that is responsible for cancer progression (112).
YAP/TAZ AND THE HIPPO PATHWAY IN LIVER METABOLISM
As one of the key organs in regulating host metabolism, the liver is particularly susceptible to metabolic disorders. As YAP and TAZ are required for and promote key liver processes, from development to transformation, there is emerging evidence that the Hippo pathway regulates diverse metabolic processes. Although Hippo pathway–regulated metabolism is still an emerging field, it is clear that abnormalities of these metabolic processes induced by Hippo pathway dysregulation can drive disease. Furthermore, understanding Hippo-regulated metabolism may reveal new therapeutic targets to inhibit tumorigenesis or to boost regeneration.
Glucose
Particularly important to cellular transformation is the reprogramming of glycolysis, which allows cells to survive in nutrient-poor environments. Furthermore, a hallmark of cancer cells is the Warburg effect, in which cancer cells have a high rate of glucose utilization (113). Therefore, it has not been particularly surprising that one function of YAP is to drive glycolysis. YAP drives glycolysis partially through its regulation of a direct target gene, the glucose transporter GLUT3, which promotes glucose uptake (114). Others showed that the long noncoding RNA BCAR4 is required for YAP-induced glycolysis by promoting the expression of hexokinase 2 and 6-phosphofructo-2 kinase. Loss of BCAR4 could suppress YAP-induced glycolysis and tumorigenesis (115).
Together with its critical role in driving glycolysis, YAP regulates blood levels of glucose by suppressing gluconeogenesis, a normal process in which the liver produces glucose and increases blood glucose levels in the fasted state (116). YAP activation can almost completely antagonize the function of glucagon, which normally stimulates gluconeogenesis, or the effects of the administration of dexamethasone, a glucocorticoid that normally stimulates gluconeogenesis in the liver. In turn, YAP suppression can enhance the function of glucagon. Importantly, YAP suppresses the transcription of Pgc1α, a key transcriptional regulator of gluconeogenesis, which lowers the expression of glucose-6-phosphatase catalytic subunit (G6pc) and phosphoenolpyruvate carboxykinase (Pck1), two enzymes directly involved in gluconeogenesis. This suppression of gluconeogenesis appears to be important for the development of HCC, as the expression of Pgc1α in YAP-activated liver cancer cells inhibited growth (116).
In addition to regulating glucose, YAP has been suggested to regulate insulin signaling, and the Hippo pathway may restrain the development of fatty liver and liver cancer. YAP activation through the genetic deletion of Sav1 cooperates with Akt in the Pten knockout background to drive NAFLD, NASH, and then liver cancer. YAP/TAZ activation can upregulate insulin receptor substrate 2 (Irs2), thereby promoting Akt activation, and the downregulation of Irs2 can suppress Akt and Yap-driven HCC. Indeed, YAP expression correlates with IRS2 expression in HCC derived from patients. Altogether, this suggests that YAP can reprogram insulin signaling to regulate AKT through IRS2, which results in the formation of fatty liver and liver cancer (117).
Cholesterol
Further demonstrating the role of the Hippo pathway in the development of fatty liver, deletion of Lats2 or Mst1 is sufficient to induce fatty liver disease in mice, potentially by inducing constitutive sterol regulatory-element binding protein (SREBP) activity and the accumulation of cholesterol (118, 119). Supporting the involvement of Hippo signaling in restraining fatty liver disease, steatosis induced by loss of Pten could be rescued by overexpression of Mst1 (117). Therefore, the Hippo pathway plays a key role in the development of fatty liver, potentially through the regulation of SREBP. It is likely that future studies will reveal new insights into the Hippo pathway’s control of fatty acids.
Glutamine
Particularly important to the liver, YAP plays a critical role in the reprogramming of glutamine metabolism, which in turn promotes growth (120). Yap1 in zebrafish liver directly promotes the expression of glula and glulb, the zebrafish orthologs of glutamine synthetase, which increases glutamine levels and leads to the production of nucleotides. Importantly, genetic loss of glula/glulb could suppress Yap-induced hepatomegaly, and pharmacological impairment of Yap or glutamine synthetase could suppress the growth of liver cancer cells. Therefore, Cox et al. (120) suggest that YAP-induced metabolic reprogramming grants YAP-activated cells a growth advantage by increasing the cellular concentration of key metabolites. Another mechanism of Yap-induced glutamine utilization has been proposed in a study of YAP and β-catenin-driven hepatoblastomas. Liu et al. (121) identified the amino acid transporter SLC38A1 as a direct transcriptional target of YAP that is induced in hepatoblastoma. Upregulation of SLC38A1 in turn led to the activation of mTORC1, which helped drive tumor growth.
In contrast, YAP has also been linked to the breakdown of glutamine during the process of regeneration and fibrosis. Importantly, glutaminolysis is a well-understood marker of malignancy, as it directly feeds into the TCA cycle and produces ATP and other metabolites (122). As discussed above, YAP plays a critical role in the activation of hepatic stellate cells and their transdifferentiation to myofibroblasts. It has been argued that this transdifferentiation process is partially controlled by glutaminolysis, and inhibition of glutaminolysis prevents the formation of myofibroblasts. Correspondingly, patients with liver fibrosis showed activation of glutaminolysis. Yap knockdown suppressed the activation of hepatic stellate cells and also suppressed glutaminolysis by inhibiting the expression of glutaminase (123). Therefore, YAP activation likely drives glutamine breakdown in hepatic stellate cells to enhance their transdifferentiation to myofibroblasts. Indeed, studies of breast cancer discovered that YAP increases transcription of glutamine-converting enzymes and that these enzymes are required for cancer cell survival (124).
Thus, YAP/TAZ and the Hippo pathway are fundamental transcription factors that regulate glycolysis, gluconeogenesis, amino acid metabolism, and fatty acid accumulation in the liver. Dys-regulation of the Hippo pathway is sufficient to drive the formation of fatty liver and the progression to liver cancer. Taken together, regulation of the Hippo pathway may be utilized for clinical benefit in patients to prevent or treat the development of liver diseases.
REGULATION OF HIPPO PATHWAY ACTIVITY
As described above, the Hippo pathway serves as a key molecular pathway that controls liver size, metabolism, development, and regeneration. Furthermore, dysregulation of the Hippo pathway is sufficient to induce fatty liver disease and liver cancer. These findings raise the question of how the Hippo pathway is physiologically regulated in the liver. Emerging evidence suggests that an increasing number of physiological signals and growth pathways feed into the liver to control Hippo signaling (Figure 3). Perturbations in these signals may then lead to disease.
Figure 3.
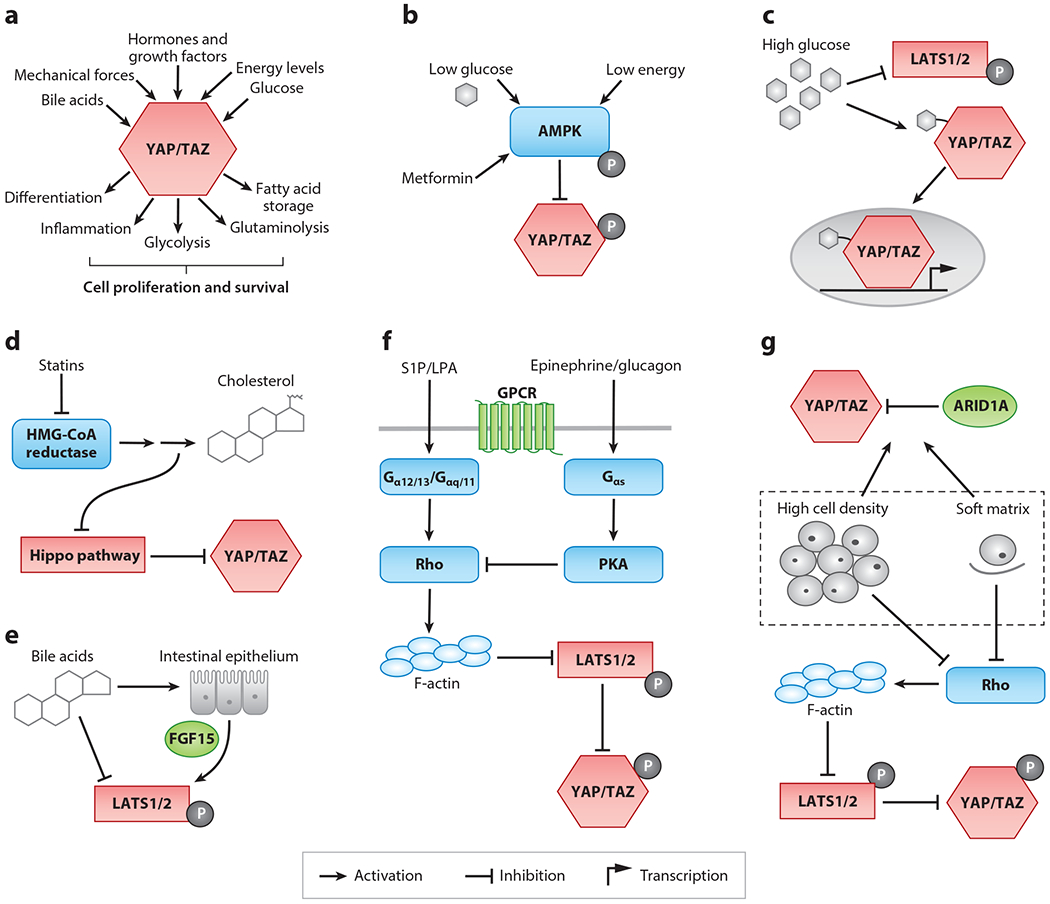
Regulation of the Hippo pathway. (a) Summary of inputs and outputs of the Hippo pathway that converge on YAP/TAZ. Schematics of regulation of the Hippo pathway by (b) energy levels and AMPK, (c) high glucose, (d) the mevalonate pathway, (e) bile acids, (f) GPCRs and their ligands, and (g) mechanical forces. Components in red denote the core proteins of the Hippo pathway, and components in green are regulators of the Hippo pathway that are important in the liver. Blue components denote regulators identified in Drosophila or in mammals that have not yet been tested in the liver but are discussed in this review.
Metabolic Regulation
Although YAP/TAZ and the Hippo pathway itself regulate metabolism in the liver, an increasing number of studies suggest that metabolites also feed back to regulate Hippo pathway activity. Prominently, while YAP itself drives glycolysis, multiple studies have demonstrated that glucose levels regulate Hippo pathway activity. Cells grown in low-glucose medium show increased Hippo pathway phosphorylation and increased YAP/TAZ cytoplasmic localization (114, 125–127). YAP is also required for liver tumorigenesis driven by high glucose (128). One possible explanation for how YAP becomes activated in high-glucose conditions is that YAP may become modified with O-linked β-N-acetylglucosamine (O-GlcNAc), a sugar attachment that gets added to many proteins in the presence of high glucose. Modification of YAP with O-GlcNAc disrupts its interaction with the Hippo pathway and its ability to become degraded (128, 129). Therefore, high glucose may drive YAP activity by posttranslational modification with O-GlcNAc (Figure 3).
Another mechanism that links glucose levels to YAP activity involves the key metabolic enzyme AMPK (Figure 3). Glucose starvation leads to increased levels of AMP, which activates AMPK. When activated, AMPK promotes the uptake of nutrients, turns off metabolic synthesis reactions, and promotes catabolism of cellular fuels (125, 127, 130). Multiple studies have demonstrated that AMPK can directly phosphorylate YAP and upstream Hippo pathway regulators to inhibit YAP activity. Indeed, metformin, a commonly taken drug to help prevent glucose intolerance in type II diabetes and that activates AMPK, can lead to YAP phosphorylation (127, 129). Thus, glucose levels can increase YAP activity by promoting O-GlcNAc modification or by inhibiting AMPK. This finding suggests that dysregulation of blood glucose in metabolic syndromes such as obesity and diabetes may promote excessive YAP activity and consequently YAP-induced proliferation and transformation.
Aside from glucose, YAP/TAZ can also seemingly be regulated by the mevalonate pathway (Figure 3). The discovery of this effect came from a small-molecule screen that identified statins as inhibitors of YAP/TAZ by promoting their cytoplasmic localization. Statins, which lower cholesterol levels in patients with hypercholesterolemia and are one of the most commonly taken drugs, inhibit the enzyme HMG-CoA reductase, which catalyzes the production of cholesterol from mevalonic acid. Additional products of the mevalonate pathway, such as geranylgeranyl, are added as posttranslational modifications to upstream Hippo pathway regulators, and these modifications likely feedback to inhibit the Hippo pathway (131–133). As mutant p53 drives the mevalonate pathway through SREBP, statins may be an effective therapy to inhibit YAP/TAZ activity in cancers with high HMG-CoA reductase activity (131). However, whether statins can reduce YAP/TAZ activity in the liver remains unexplored.
G Protein–Coupled Receptor Regulation
G protein–coupled receptors (GPCRs) are a highly diverse group of cellular surface receptors that respond to a wide variety of physiological signals and are highly susceptible to different therapeutic drugs. As major regulators of intracellular signaling, GPCRs have also been discovered to control Hippo and YAP/TAZ signaling, both positively and negatively (134). The first implication that GPCRs can regulate LATS1/2 and consequently YAP/TAZ came from a study in which serum starvation of cells in culture induced LATS1/2-dependent phosphorylation and cytoplasmic translocation of YAP/TAZ. Further screening showed that phospholipids, such as lysophosphatidic acid, that are present in serum activate GPCRs, inhibit LATS1/2, and drive YAP/TAZ activation (135). Later studies showed that gain-of-function mutations in GNAQ and GNA11, which are activated in 5% of tumors and 83% of uveal melanomas and which cause constitutive activation of GPCR signaling, drive YAP/TAZ activation. Furthermore, YAP/TAZ activation is required for GNAQ-driven uveal melanomas (136, 137), and activated YAP/TAZ, induced by LATS1/2 loss, are themselves sufficient to drive the formation of uveal melanoma (138). All in all, this shows that YAP/TAZ are regulated by GPCR signaling and that this dysregulation can drive disease (Figure 3).
As such, GPCR regulation of YAP/TAZ has emerged as a driver or as a potential therapeutic axis in liver cancer. One such GPCR is angiotensin II type 1 receptor (AT1R), which regulates the renin–angiotensin–aldosterone system that controls systemic blood pressure and blood volume. One study found that losartan, which blocks AT1R activity, could inactivate YAP/TAZ and suppress the growth of CC (139). Another study found that sphingosine-1-phosphate (S1P), a phospholipid-like lysophosphatidic acid that activates a specific GPCR, activated YAP and could drive HCC proliferation (140). Similarly, prostaglandin E2, through a YAP-dependent axis, can promote HCC proliferation (141). Each of these studies raises promising therapeutic options to target YAP/TAZ-activated liver cancer, but these methods have not yet been tested in vivo.
As discussed above, activated YAP can completely antagonize the function of glucagon (116), so it is not surprising that YAP itself can be potently regulated by glucagon. Glucagon, through activation of its GPCR, can increase LATS1/2 activity and YAP phosphorylation (142). Epinephrine, the fight-or-flight hormone activated due to stress, can also inactivate YAP through a LATS1/2-dependent mechanism. As both hormones drive glucose availability and increase blood glucose levels (135, 142), these hormones likely act via their GPCRs to inhibit YAP/TAZ and to ultimately prevent glycolysis and cell proliferation in times of stress and starvation (130).
Particularly relevant to liver, YAP activity also seems to be regulated by the presence of bile acids (143, 144). Mice genetically engineered to express high levels of bile acids develop spontaneous HCCs similar to those seen from Mst1/2 knockout mice. Importantly, high bile acids in vivo or the administration of cholic acids to hepatocytes in vitro seems to drive Yap activation through the downregulation of Mst1/2 and Lats1/2. Anakk et al. (143) further report a correlation between cholestasis, YAP activity, and the development of HCC. However, it remains unknown whether this effect is mediated by the bile acid GPCR TGR5 (130). Recently, it has been argued that bile acids stimulate the production of Fgf15 from intestinal enterocytes, which travel through the enterohepatic circulation to activate Mst1/2 phosphorylation (144). In support of this evidence, mice deficient in their response to Fgf15 show hepatomegaly, similar to mice in which YAP is overexpressed (145).
Mechanical Regulation
That the Hippo pathway is required for proper liver size and liver regeneration suggests that there exists some intrinsic property of the tissue that can regulate Hippo signaling. An emerging paradigm is that the Hippo pathway and YAP/TAZ are regulated by mechanical forces, such as cell stretching. As a tissue expands or contracts, changing mechanical forces could then feed back onto YAP/TAZ activity to either limit or promote cellular proliferation and differentiation. Several lines of evidence support this idea. The localization of YAP/TAZ is acutely sensitive to cell density; YAP/TAZ are localized to the nucleus in sparse cultures and to the cytoplasm in dense cultures (25). Additionally, cells grown on a stiff extracellular matrix show nuclear localization of YAP/TAZ, whereas cells grown on soft substrates show cytoplasmic localization (146). Moreover, others have observed changes in YAP/TAZ localization due to fluid flow and to direct stretching (147–150).
Although the effect of mechanical forces on YAP and TAZ have been observed in many different contexts, the actual mechanism of these forces remains elusive; however, NF2 is believed to play a role in sensing cytoskeletal tension (37). Progress in understanding how mechanical force–induced YAP activation affects liver pathology is hindered by the lack of in vivo mouse models in which cellular tension is manipulated. Still, the interaction between YAP and ARID1A, which is important in liver tumorigenesis, is regulated by mechanical forces, in which sparse conditions increase the interaction between ARID1A and YAP, decreasing YAP’s activation (106). Similarly, mechanical force seems to influence cell identity and hepatocyte differentiation in liver organoids through YAP (81). Some studies seeking to understand how the extracellular matrix affects liver size have found that loss of integrin-linked kinase (ILK) leads to increased liver size and regeneration following partial hepatectomy and that loss of ILK leads to increased YAP (151). However, other studies of ILK came to the opposite conclusion: Loss of ILK actually inhibits YAP (152). Going forward, liver organoid models may be particularly useful in elucidating how mechanical force and its effect on YAP can influence liver development, homeostasis, regeneration, and tumorigenesis. Furthermore, it is likely that changing organ stiffness enacted through chronic liver damage and fibrogenesis may lead to the dysregulation of the Hippo pathway and YAP/TAZ.
TARGETED YAP/TAZ THERAPY
Studies of the effects of YAP and TAZ and Hippo pathway dysregulation on the liver have revealed context-dependent effects on disease. For example, although increased YAP and TAZ activity are sufficient to result in progression of fatty liver disease and the development of liver cancer, they also are required for liver regeneration. This suggests that therapeutics that both inhibit and activate the Hippo pathway will have therapeutic uses. Common strategies to affect Hippo pathway activity may work to enhance or inhibit Hippo kinase phosphorylation, to impact YAP or TAZ localization, or to prevent or enhance the binding of YAP and TAZ to the transcription factors TEAD1/2/3/4 or their transcriptional machinery.
As reviewed above, several commonly used drugs indirectly affect YAP/TAZ activity. Statins (131), metformin (127, 129), and angiotensin inhibitors (139) indirectly affect YAP/TAZ localization. In addition to these drugs, tankyrase inhibitors inhibit YAP by stabilizing a known negative regulator of YAP termed angiomotin. Tankyrase inhibitors, via angiomotin, enrich YAP in the cytoplasm (153). However, in vivo data for each of these treatments are lacking, and these drugs affect other processes outside of YAP.
For the direct positive regulation of YAP/TAZ activity, a small-molecule inhibitor of MST1/2, XMU-MP-1, has been developed (154). This inhibitor increased the rate of mouse liver regeneration after partial hepatectomy and boosted the growth of human hepatocytes transplanted into mouse livers. Furthermore, XMU-MP-1 could prevent liver damage, ameliorate acetaminophen-induced liver injury, and prevent fibrosis and cell death associated with chronic liver injury (154). Another study found that targeting Mst1/2 in older mice by liposomal small interfering RNA could restore regeneration in nonregenerating livers (155). Future studies aimed at targeting the Hippo pathway may reveal new pharmacological treatments that will prevent common causes of liver injury and aid in liver repair.
For the negative regulation of YAP/TAZ activity, several different strategies have led to drug discovery. Screening for molecules that inhibit the interaction of YAP with TEAD led to the discovery of verteporfin, which can inhibit YAP-induced hepatomegaly or bile expansion due to Nf2 loss (57). Development of a polypeptide that mimics VGLL4 and prevents the binding of YAP to TEAD can suppress the growth of gastric cancer xenografts (156). Additionally, the molecule MYF-01-37 covalently attaches to TEAD2 and inhibits its interaction with YAP, suppressing YAP-induced drug resistance (157). These strategies and others will likely be effective for producing new therapeutic drugs for the treatment of liver cancer and other malignancies.
NEW MOLECULAR REGULATORS AND TARGETS OF THE HIPPO PATHWAY IN THE LIVER
As an increasing number of molecular regulators of the Hippo pathway are discovered in Drosophila and in mammalian cells, comparatively few have been tested for their phenotype or physiological relevance in the liver. For each newly discovered regulator, there is the potential for a new therapeutic target to either boost regeneration or inhibit the development of fatty liver disease and cancer. As expression of YAP/TAZ in the liver can cause hepatomegaly, cell proliferation, fatty acid accumulation, and tumorigenesis, each of these molecular components can be tested for their ability to either drive, enhance, or restrict these phenotypes. Revealing which of these molecules are important in the liver will also further reveal how the Hippo pathway is regulated in the liver by mechanical forces, metabolism, hormones, and other growth factors.
Particularly important to the Hippo pathway is the question of how the phosphorylation of MST1/2 and LATS1/2 are controlled under different physiological conditions. TAOK1/2/3 have been identified as upstream kinases that catalyze activation of MST1/2 to drive Hippo pathway activation (158, 159). To the contrary, the striatin-interacting phosphatase and kinase (STRIPAK) complex dephosphorylates and inactivates MST1/2 and Hippo pathway activity (160, 161). How TAOK1/2/3 and the STRIPAK complex are physiologically regulated and how they relate to liver pathology remain unexplored. Additionally, there is evidence of an alternative, MST1/2-independent kinase cascade to LATS1/2 in flies and mammals that is mediated by the MAP4K subfamily of kinases (162–164). Whether MAP4K can directly phosphorylate LATS1/2 in the liver has not been reported.
Downstream of both mechanical forces and GPCRs, Rho, a small GTPase, regulates the Hippo pathway in many different contexts (135, 165, 166). The GPCR-coupled proteins Gα12/13 and Gαq/11 activate actin polymerization through Rho, which results in the downregulation of LATS1/2 activity, suggesting how activating mutations in these G proteins leads to uveal melanoma and other cancers (135–137). Alternatively, Gαs activates protein kinase A (PKA) to inactivate Rho and to activate LATS1/2 (142, 167). Through mechanical tension, the Rho-ROCK (Rho-associated protein kinase)-MLC (nonmuscle myosin II light chain) pathway controls YAP/TAZ activity, and pharmacological inhibition of Rho can induce YAP/TAZ phosphorylation (165, 166). Although Rho GTPases and their activation have been implicated in liver carcinogenesis (168), their role in regulating the Hippo pathway in the liver remains unvalidated.
Another promising avenue of research is determining which targets downstream of YAP/TAZ in the liver are required for particular disease processes, such as GLUL in hepatomegaly and liver cancer (120). Another promising target is the protein kinase NUAK2, an AMPK that is a direct target gene of YAP in the liver (94). Future screens in liver cell lines and organoids and analyses of YAP targets in mouse liver will likely reveal additional interesting targets that will allow us to better understand how YAP/TAZ can drive liver disease.
CONCLUSIONS
Emerging from genetic screens in the simple model organism Drosophila, the Hippo pathway has provided myriad insights into the physiology of the liver. At its core, the Hippo pathway controls cellular proliferation within the context of signals provided by the entire tissue. Thus, responding to cellular forces, metabolic constraints, and signals from neighboring tissues, the Hippo pathway fundamentally regulates the size of the liver. Under normal conditions, the Hippo pathway promotes development into an appropriately sized liver with the correct distribution of hepatocytes and cholangiocytes. However, in the context of Hippo pathway dysregulation induced from genetic mutations or aberrant physiological signals, the size of the liver also becomes dysregulated, promoting poor regeneration, hepatomegaly, or liver cancer.
Although it is well understood that the Hippo pathway and YAP/TAZ regulate normal and pathological liver physiology, the important signaling molecules both upstream of Hippo and downstream of YAP and TAZ are little known. As increasingly more Hippo pathway regulators are discovered and put in the context of other signaling molecules, comparatively few have been tested for their effects on liver pathology. For example, even though GPCRs are well-known regulators of Hippo, can activating mutations in these receptors drive hepatomegaly or cancer? Furthermore, how do liver cells sense biomechanical signals, and does this mechanoregulation contribute to the final organ size of the liver?
We know that many common liver diseases are driven or modulated by YAP and TAZ, but how the physiological inputs responsible for these diseases ultimately synapse onto YAP/TAZ activation remains largely unknown. Furthermore, from a therapeutic standpoint, discovering the required genes downstream of YAP/TAZ that drive hepatomegaly, metabolic dysfunction, cancer, or even regeneration will likely reveal new druggable targets. As the rates of liver diseases throughout the world increase, therapeutically targeting the Hippo pathway takes on a sense of urgency. Can we fine-tune Hippo to boost regeneration and also to treat liver cancer?
ACKNOWLEDGMENTS
We apologize to our colleagues whose work we could not discuss and cite due to space constraints. We would like to thank Yonggang Zheng for a critical review of an earlier draft of the article. Research in the Pan laboratory is supported in part by grants from the National Institutes of Health (EY015708) and the Department of Defense (PR190360). D.P. is an investigator of the Howard Hughes Medical Institute.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Schuppan D, Afdhal NH. 2008. Liver cirrhosis. Lancet 371:838–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AA, Lopez AD, Shahraz S, Lozano R,Mokdad AH, et al. 2014. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalopoulos GK, DeFrances MC. 1997. Liver regeneration. Science 276:60–66 [DOI] [PubMed] [Google Scholar]
- 4.Higgins GM, Anderson RM. 1931. Experimental pathology of liver: restoration of liver in the white rat following partial surgical removal. Arch. Pathol 12:186–202 [Google Scholar]
- 5.Emre S, Umman V. 2011. Split liver transplantation: an overview. Transplant. Proc 43:884–87 [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, et al. 1993. Baboon-to-human liver transplantation. Lancet 341:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawasaki S,Makuuchi M, Ishizone S,Matsunami H, Terada M, Kawarazaki H. 1992. Liver regeneration in recipients and donors after transplantation. Lancet 339:580–81 [DOI] [PubMed] [Google Scholar]
- 8.Dong J, Feldmann G, Huang J, Wu S, Zhang N, et al. 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130:1120–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan D 2010. The Hippo signaling pathway in development and cancer. Dev. Cell 19:491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado MV, Michelotti GA, Pereira TA, Xie G, Premont R, et al. 2015. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J. Hepatol 63:962–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9:534–46 [DOI] [PubMed] [Google Scholar]
- 12.Xu T, Wang W, Zhang S, Stewart RA, Yu W. 1995. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development 121:1053–63 [DOI] [PubMed] [Google Scholar]
- 13.Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, et al. 2002. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129:5719–30 [DOI] [PubMed] [Google Scholar]
- 14.Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, et al. 2002. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110:467–78 [DOI] [PubMed] [Google Scholar]
- 15.Lai Z-C, Wei X, Shimizu T, Ramos E, Rohrbaugh M, et al. 2005. Control of cell proliferation and apoptosis by Mob as tumor suppressor, Mats. Cell 120:675–85 [DOI] [PubMed] [Google Scholar]
- 16.Harvey KF, Pfleger CM, Hariharan IK. 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114:457–67 [DOI] [PubMed] [Google Scholar]
- 17.Jia J, Zhang W, Wang B, Trinko R,Jiang J. 2003. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17:2514–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantalacci S, Tapon N, Líopold P. 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol 5:921–27 [DOI] [PubMed] [Google Scholar]
- 19.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. 2003. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol 5:914–20 [DOI] [PubMed] [Google Scholar]
- 20.Wu S, Huang J, Dong J, Pan D. 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114:445–56 [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Wu S, Barrera J, Matthews K, Pan D. 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122:421–34 [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. 2008. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 14:377–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Liu Y, Zheng Y, Dong J, Pan D. 2008. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 14:388–98 [DOI] [PubMed] [Google Scholar]
- 24.Lei Q-Y, Zhang H, Zhao B, Zha Z-Y, Bai F, et al. 2008. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol 28:2426–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao B, Wei X, Li W, Udan RS, Yang Q, et al. 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21:2747–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Pan D. 2019. The Hippo signaling pathway in development and disease. Dev. Cell 50:264–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C-Y, Zha Z-Y, Zhou X, Zhang H, Huang W, et al. 2010. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFβ-TrCP E3 ligase. J. Biol. Chem 285:37159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao B, Li L, Tumaneng K, Wang C-Y, Guan K-L. 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TrCP. Genes Dev. 24:72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B, Ye X, Yu J, Li L, Li W, et al. 2008. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22:1962–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, et al. 2015. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol 17:1218–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galli GG, Carrara M, Yuan WC, Valdes-Quezada C, Gurung B, et al. 2015. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Mol. Cell 60:328–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa M 2007. A Sveinsson’s chorioretinal atrophy-associated missense mutation in mouse Tead1 affects its interaction with the co-factors YAP and TAZ. Biochem. Biophys. Res. Commun 361:1022–26 [DOI] [PubMed] [Google Scholar]
- 33.Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, et al. 2013. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev. Cell 25:388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalamarides M, Acosta MT, Babovic-Vuksanovic D, Carpen O, Cichowski K, et al. 2012. Neurofibromatosis 2011:a report of the Children’s Tumor Foundation annual meeting. Acta Neuropathol. 123:369–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Bai H, David KK, Dong J, Zheng Y, et al. 2010. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, et al. 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol 8:27–36 [DOI] [PubMed] [Google Scholar]
- 37.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. 2013. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154:1342–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. 2010. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell 18:300–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor A, Yao W, Ying H, Hua S, Liewen A, et al. 2014. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 158:185–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh H, Slattery M, Ma L, Crofts A, White KP, et al. 2013. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep. 3:309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, et al. 2011. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147:759–72 [DOI] [PubMed] [Google Scholar]
- 42.Janse van Rensburg HJ, Azad T, Ling M, Hao Y, Snetsinger B, et al. 2018. The Hippo pathway component TAZ promotes immune evasion in human cancer through PD-L1. Cancer Res. 78:1457–70 [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Yang X. 2015.The Hippo pathway in chemotherapeutic drug resistance. Int. J. Cancer 137:2767–73 [DOI] [PubMed] [Google Scholar]
- 44.Neto-Silva RM, de Beco S, Johnston LA. 2010. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev. Cell 19:507–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park HW, Kim YC, Yu B, Moroishi T, Mo J-S, et al. 2015.Alternative Wnt signaling activates YAP/TAZ. Cell 162:780–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, et al. 2014. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158:157–70 [DOI] [PubMed] [Google Scholar]
- 47.Yimlamai D, Christodoulou C, Galli GG,Yanger K, Pepe-Mooney B, et al. 2014. Hippo pathway activity influences liver cell fate. Cell 157:1324–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, et al. 2007. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol 17:2054–60 [DOI] [PubMed] [Google Scholar]
- 49.Chen Q, Zhang N, Xie R, Wang W, Cai J, et al. 2015. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 29:1285–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi J, Lu L, Yanger K, Wang W, Sohn BH, et al. 2016. Large tumor suppressor homologs 1 and 2 regulate mouse liver progenitor cell proliferation and maturation through antagonism of the coactivators YAP and TAZ. Hepatology 64:1757–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, et al. 2010. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. PNAS 107:1437–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song H, Mak KK, Topol L, Yun K, Hu J, et al. 2010. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. PNAS 107:1431–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou D, Conrad C,Xia F,Park J-S,Payer B,et al. 2009.Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16:425–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee K-P, Lee J-H, Kim T-S, Kim T-H, Park H-D, et al. 2010. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. PNAS 107:8248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishio M, Sugimachi K, Goto H, Wang J, Morikawa T, et al. 2016. Dysregulated YAP1/TAZ and TGF-β signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. PNAS 113:E71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermann A, Wennmann DO, Gromnitza S, Edeling M, VanMarck V, et al. 2018.WW and C2 domain-containing proteins regulate hepatic cell differentiation and tumorigenesis through the hippo signaling pathway. Hepatology 67:1546–59 [DOI] [PubMed] [Google Scholar]
- 57.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee S-J, et al. 2012. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26:1300–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, et al. 2014. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol 307:G196–204 [DOI] [PubMed] [Google Scholar]
- 59.Lu L, Finegold MJ, Johnson RL. 2018. Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Exp. Mol. Med 50:e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato K, Marzioni M, Meng F, Francis H, Glaser S, Alpini G. 2019. Ductular reaction in liver diseases: pathological mechanisms and translational significances. Hepatology 69:420–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurda GT, Zhu Q, Bai H, Pan D, Schwarz KB, Anders RA. 2014. The use of Yes-associated protein expression in the diagnosis of persistent neonatal cholestatic liver disease. Hum. Pathol 45:1057–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai H, Zhang N, Xu Y, Chen Q, Khan M, et al. 2012. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 56:1097–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pepe-Mooney BJ, Dill MT, Alemany A, Ordovas-Montanes J, Matsushita Y,et al. 2019. Single-cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration. Cell Stem Cell 25:23–38.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Planas-Paz L, Sun T, Pikiolek M, Cochran NR, Bergling S, et al. 2019. YAP, but not RSPO-LGR4/5, signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell 25:39–53.e10 [DOI] [PubMed] [Google Scholar]
- 65.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, et al. 2015. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J. Hepatol 63:679–88 [DOI] [PubMed] [Google Scholar]
- 66.Konishi T, Schuster RM, Lentsch AB. 2018. Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol 314:G471–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swiderska-Syn M, Xie G, Michelotti GA, Jewell ML, Premont RT, et al. 2016. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology 64:232–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherman MH. 2018. Stellate cells in tissue repair, inflammation, and cancer. Annu. Rev. Cell Dev. Biol 34:333–55 [DOI] [PubMed] [Google Scholar]
- 69.Su T, Bondar T, Zhou X, Zhang C, He H, Medzhitov R. 2015. Two-signal requirement for growth-promoting function of Yap in hepatocytes. eLife 4:e02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, et al. 2016. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 24:848–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kodama T, Yi J, Newberg JY, Tien JC, Wu H, et al. 2018.Molecular profiling of nonalcoholic fatty liver disease-associated hepatocellular carcinoma using SB transposon mutagenesis. PNAS 115:E10417–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plouffe SW, Lin KC, Moore JL 3rd, Tan FE, Ma S, et al. 2018. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem 293:11230–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagenbeek TJ, Webster JD, Kljavin NM, Chang MT, Pham T, et al. 2018. The Hippo pathway effector TAZ induces TEAD-dependent liver inflammation and tumors. Sci. Signal 11:eaaj1757. [DOI] [PubMed] [Google Scholar]
- 74.Kim W, Khan SK, Liu Y, Xu R, Park O, et al. 2018. Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut 67:1692–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee D-H, Park JO, Kim T-S, Kim S-K, Kim T-h, et al. 2016. LATS-YAP/TAZ controls lineage specification by regulating TGFβ signaling and Hnf4a expression during liver development. Nat. Commun 7:11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, Freire Valls A, Schermann G, Shen Y, Moya IM, et al. 2017. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell 42:462–78.e7 [DOI] [PubMed] [Google Scholar]
- 77.Azad T,Janse van Rensburg HJ, Lightbody ED, Neveu B, Champagne A, et al. 2018.ALATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat. Commun 9:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neto F, Klaus-Bergmann A, Ong YT, Alt S, Vion A-C, et al. 2018. YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. eLife 7:e31037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, Kim YH, Kim J, Park DY, Bae H, et al. 2017. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Investig 127:3441–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakabe M, Fan J, Odaka Y, Liu N, Hassan A, et al. 2017. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. PNAS 114:10918–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun P, Zhang G, Su X,Jin C, Yu B, et al. 2019. Maintenance of primary hepatocyte functions in vitro by inhibiting mechanical tension-induced YAP activation. Cell Rep. 29:3212–22.e4 [DOI] [PubMed] [Google Scholar]
- 82.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, et al. 2006. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125:1253–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen S, Guo X,Yan H, Lu Y,Ji X, et al.2015. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 25:997–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perra A, Kowalik MA, Ghiso E, Ledda-Columbano GM, Di Tommaso L, et al. 2014. YAP activation is an early event and a potential therapeutic target in liver cancer development. J. Hepatol 61:1088–96 [DOI] [PubMed] [Google Scholar]
- 85.Tao J, Calvisi DF, Ranganathan S, Cigliano A, Zhou L, et al. 2014. Activation of β-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology 147:690–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J, Liu P, Tao J, Wang P, Zhang Y, et al. 2019. TEA domain transcription factor 4 is the major mediator of Yes-associated protein oncogenic activity in mouse and human hepatoblastoma. Am. J. Pathol 189:1077–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith JL, Rodríguez TC, Mou H, Kwan S-Y, Pratt H, et al. 2020. YAP1 withdrawal in hepatoblastoma drives therapeutic differentiation of tumor cells to functional hepatocyte-like cells. Hepatology. In press. 10.1002/hep.31389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomlinson GE, Kappler R. 2012. Genetics and epigenetics of hepatoblastoma. Pediatr. Blood Cancer 59:785–92 [DOI] [PubMed] [Google Scholar]
- 89.Cai J, Maitra A, Anders RA, Taketo MM, Pan D. 2015. β-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 29:1493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, et al. 2011. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci. Transl. Med 3:98ra82. [DOI] [PubMed] [Google Scholar]
- 91.Errani C, Zhang L, Sung YS, Hajdu M, Singer S, et al. 2011.Anovel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50:644–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antonescu CR, Le Loarer F,Mosquera JM, Sboner A, Zhang L, et al.2013. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 52:775–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanas MR, Ma S, Jadaan FO, Ng CK, Weigelt B, et al. 2016. Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein. Oncogene 35:929–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan W-C, Pepe-Mooney B, Galli GG, Dill MT, Huang H-T, et al. 2018. NUAK2 is a critical YAP target in liver cancer. Nat. Commun 9:4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sohn BH, Shim JJ, Kim SB, Jang KY, Kim SM, et al. 2016. Inactivation of Hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin. Cancer Res 22:1256–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo Y, Pan Q, Zhang J, Xu X, Liu X, et al.2015. Functional and clinical evidence that TAZ is a candidate oncogene in hepatocellular carcinoma. J. Cell Biochem 116:2465–75 [DOI] [PubMed] [Google Scholar]
- 97.Han S-x, Bai E, Jin G-h, He C-c, Guo X-j, et al. 2014. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J. Immunol. Res 2014:261365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim GJ, Kim H, Park YN. 2013. Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLOS ONE 8:e75449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao H, Jiang N, Zhou B, Liu Q, Du C. 2015. TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 106:151–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, et al. 2009. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 115:4576–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, et al. 2013. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 144:829–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang N, Zhao Z, Long J, Li H, Zhang B, et al. 2017. Molecular alterations of the NF2 gene in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncol. Rep 38:3650–58 [DOI] [PubMed] [Google Scholar]
- 103.Xue R, Chen L, Zhang C, Fujita M, Li R, et al. 2019. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell 35:932–47.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, et al. 2012. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat. Genet 44:760–64 [DOI] [PubMed] [Google Scholar]
- 105.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, et al. 2012. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet 44:694–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang L, Azzolin L, Di Biagio D, Zanconato F, Battilana G, et al. 2018. The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature 563:265–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mello SS, Valente LJ, Raj N, Seoane JA, Flowers BM, et al. 2017.A p53 super-tumor suppressor reveals a tumor suppressive p53-Ptpn14-Yap axis in pancreatic cancer. Cancer Cell 32:460–73.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hill MA, Alexander WB, Guo B, Kato Y, Patra K, et al. 2018. Kras and Tp53 mutations cause cholangiocyte- and hepatocyte-derived cholangiocarcinoma. Cancer Res. 78:4445–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, et al. 2014. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158:171–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fang L, Teng H, Wang Y, Liao G, Weng L, et al. 2018. SET1A-mediated mono-methylation at K342 regulates YAP activation by blocking its nuclear export and promotes tumorigenesis. Cancer Cell 34:103–18.e9 [DOI] [PubMed] [Google Scholar]
- 111.Ziosi M, Baena-López LA, Grifoni D, Froldi F, Pession A, et al. 2010. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLOS Genet. 6:e1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moya IM, Castaldo SA, Van den Mooter L, Soheily S, Sansores-Garcia L,et al. 2019.Peritumoral activation of the Hippo pathway effectors YAP and TAZ suppresses liver cancer in mice. Science 366:1029–34 [DOI] [PubMed] [Google Scholar]
- 113.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. 2008. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7:11–20 [DOI] [PubMed] [Google Scholar]
- 114.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, et al. 2015. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol 17:490–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng X, Han H, Liu G-P, Ma Y-X, Pan R-L, et al. 2017. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 36:3325–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu Y, Shin D-J, Pan H, Lin Z, Dreyfuss JM, et al. 2017. YAP suppresses gluconeogenic gene expression through PGC1α. Hepatology 66:2029–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeong SH, Kim HB, Kim MC, Lee JM, Lee J-H, et al. 2018. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. J. Clin. Investig 128:1010–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aylon Y, Gershoni A, Rotkopf R, Biton IE, Porat Z, et al. 2016. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 30:786–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geng C, Zhang Y, Gao Y, Tao W, Zhang H, et al. 2016. Mst1 regulates hepatic lipid metabolism by inhibiting Sirt1 ubiquitination in mice. Biochem. Biophys. Res. Commun 471:444–49 [DOI] [PubMed] [Google Scholar]
- 120.Cox AG, Hwang KL, Brown KK, Evason K, Beltz S, et al. 2016. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat. Cell Biol 18:886–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu P, Calvisi DF, Kiss A, Cigliano A, Schaff Z, et al. 2017. Central role of mTORC1 downstream of YAP/TAZ in hepatoblastoma development. Oncotarget 8:73433–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hensley CT, Wasti AT, DeBerardinis RJ. 2013. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Investig 123:3678–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du K, Hyun J, Premont RT, Choi SS, Michelotti GA, et al. 2018. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology 154:1465–79.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang C-S, Stampouloglou E, Kingston NM, Zhang L, Monti S, Varelas X. 2018. Glutamine-utilizing transaminases are a metabolic vulnerability of TAZ/YAP-activated cancer cells. EMBO Rep. 19:e43577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, et al. 2014. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 9:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, et al. 2015. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 34:1349–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mo J-S, Meng Z, Kim YC, Park HW, Hansen CG, et al. 2015. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol 17:500–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, et al. 2017. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat. Commun 8:15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, et al. 2017. Regulation of the Hippo-YAP pathway by glucose sensor O-GlcNAcylation. Mol. Cell 68:591–604.e5 [DOI] [PubMed] [Google Scholar]
- 130.Koo JH, Guan K-L. 2018. Interplay between YAP/TAZ and metabolism. Cell Metab. 28:196–206 [DOI] [PubMed] [Google Scholar]
- 131.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, et al. 2014. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol 16:357–66 [DOI] [PubMed] [Google Scholar]
- 132.Wang Z, Wu Y, Wang H, Zhang Y, Mei L, et al. 2014. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. PNAS 111:E89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mi W, Lin Q, Childress C, Sudol M, Robishaw J, et al. 2015. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene 34:3095–106 [DOI] [PubMed] [Google Scholar]
- 134.Luo J, Yu F-X. 2019. GPCR-Hippo signaling in cancer. Cells 8:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu F-X, Zhao B, Panupinthu N, Jewell JL, Lian I, et al. 2012. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150:780–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP,Molinolo AA, et al. 2014. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated Rho GTPase signaling circuitry Cancer Cell 25:831–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu F-X, Luo J, Mo J-S, Liu G, Kim YC, et al. 2014. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 25:822–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li H, Li Q, Dang K, Ma S, Cotton JL, et al. 2019. YAP/TAZ activation drives uveal melanoma initiation and progression. Cell Rep. 29:3200–11.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saikawa S, Kaji K, Nishimura N, Seki K, Sato S, et al. 2018. Angiotensin receptor blockade attenuates cholangiocarcinoma cell growth by inhibiting the oncogenic activity of Yes-associated protein. Cancer Lett. 434:120–29 [DOI] [PubMed] [Google Scholar]
- 140.Cheng JC, Wang EY, Yi Y, Thakur A, Tsai SH, Hoodless PA. 2018. S1P stimulates proliferation by upregulating CTGF expression through S1PR2-mediated YAP activation. Mol. Cancer Res 16:1543–55 [DOI] [PubMed] [Google Scholar]
- 141.Xu G, Wang Y, Li W, Cao Y, Xu J, et al. 2018. COX-2 forms regulatory loop with YAP to promote proliferation and tumorigenesis of hepatocellular carcinoma cells. Neoplasia 20:324–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu F-X, Zhang Y, Park HW, Jewell JL, Chen Q, et al. 2013. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 27:1223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Anakk S, Bhosale M, Schmidt VA, Johnson RL, Finegold MJ, Moore DD. 2013. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 5:1060–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ji S, Liu Q, Zhang S, Chen Q, Wang C, et al. 2019. FGF15 activates Hippo signaling to suppress bile acid metabolism and liver tumorigenesis. Dev. Cell 48:460–74.e9 [DOI] [PubMed] [Google Scholar]
- 145.Naugler WE, Tarlow BD, Fedorov LM, Taylor M, Pelz C,et al. 2015. Fibroblast growth factor signaling controls liver size in mice with humanized livers. Gastroenterology 149:728–40.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. 2011. Role of YAP/TAZ in mechanotrans-duction. Nature 474:179–83 [DOI] [PubMed] [Google Scholar]
- 147.Codelia VA, Sun G, Irvine KD. 2014. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr. Biol 24:2012–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, et al. 2017. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171:1397–410.e14 [DOI] [PubMed] [Google Scholar]
- 149.Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, et al. 2017. Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev. Cell 40:523–36.e6 [DOI] [PubMed] [Google Scholar]
- 150.Wang L, Luo J-Y, Li B, Tian XY, Chen L-J, et al. 2016. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540:579–82 [DOI] [PubMed] [Google Scholar]
- 151.Apte U, Gkretsi V, Bowen WC, Mars WM, Luo J-H, et al. 2009. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 50:844–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. 2013. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat. Commun 4:2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang W, Li N, Li X, Tran MK, Han X, Chen J. 2015. Tankyrase inhibitors target YAP by stabilizing angiomotin family proteins. Cell Rep. 13:524–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fan F, He Z, Kong LL, Chen Q, Yuan Q, et al. 2016. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci. Transl. Med 8:352ra108. [DOI] [PubMed] [Google Scholar]
- 155.Loforese G, Malinka T, Keogh A, Baier F, Simillion C, et al. 2017. Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2. EMBO Mol. Med 9:46–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiao S, Wang H, Shi Z, Dong A, Zhang W, et al. 2014. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25:166–80 [DOI] [PubMed] [Google Scholar]
- 157.Kurppa KJ, Liu Y, To C, Zhang T, Fan M, et al. 2020. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell 37:104–22.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boggiano JC, Vanderzalm PJ, Fehon RG. 2011. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev. Cell 21:888–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Poon CL, Lin JI, Zhang X, Harvey KF. 2011.The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev. Cell 21:896–906 [DOI] [PubMed] [Google Scholar]
- 160.Zheng Y, Liu B, Wang L, Lei H, Pulgar Prieto KD, Pan D. 2017. Homeostatic control of Hpo/MST kinase activity through autophosphorylation-dependent recruitment of the STRIPAK PP2A phosphatase complex. Cell Rep. 21:3612–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bae SJ, Ni L, Osinski A, Tomchick DR, Brautigam CA, Luo X. 2017. SAV1 promotes Hippo kinase activation through antagonizing the PP2A phosphatase STRIPAK. eLife 6:e30278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, et al. 2014. The conserved Misshapen-Warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev. Cell 31:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, et al. 2015.MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun 6:8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. 2015. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev. Cell 34:642–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cai J, Song X, Wang W, Watnick T, Pei Y, et al. 2018. A RhoA-YAP-c-Myc signaling axis promotes the development of polycystic kidney disease. Genes Dev. 32:781–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao B, Li L,Wang L,Wang C-Y, Yu J, Guan K-L. 2012. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26:54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim M, Kim M, Lee S, Kuninaka S, Saya H, et al. 2013.cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 32:1543–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grise F, Bidaud A, Moreau V. 2009. Rho GTPases in hepatocellular carcinoma. Biochim. Biophys. Acta Rev. Cancer 1795:137–51 [DOI] [PubMed] [Google Scholar]


