Abstract
Purpose:
To determine classification criteria for sarcoidosis-associated uveitis
Design:
Machine learning of cases with sarcoid uveitis and 15 other uveitides.
Methods:
Cases of anterior, intermediate, and panuveitides were collected in an informatics-designed preliminary database, and a final database was constructed of cases achieving supermajority agreement on the diagnosis, using formal consensus techniques. Cases were analyzed by anatomic class, and each class was split into a training set and a validation set. Machine learning using multinomial logistic regression was used on the training sets to determine a parsimonious set of criteria that minimized the misclassification rate among the intermediate uveitides. The resulting criteria were evaluated on the validation sets.
Results:
One thousand eighty-three anterior uveitides, 589 intermediate uveitides, and 1012 panuveitides, including 278 cases of sarcoidosis-associated uveitis, were evaluated by machine learning. Key criteria for sarcoidosis-associated uveitis included a compatible uveitic syndrome of any anatomic class and evidence of sarcoidosis, either 1) a tissue biopsy demonstrating non-caseating granulomata or 2) bilateral hilar adenopathy on chest imaging. The overall accuracy of the diagnosis of sarcoidosis-associated uveitis in the validation set was 99.7% (95% confidence interval 98.8, 99.9).The misclassification rates for sarcoidosis-associated uveitis in the training sets were: anterior uveitis 3.2%, intermediate uveitis 2.6%, and panuveitis 1.2%; in the validation sets the misclassification rates were: anterior uveitis 0%, intermediate uveitis 0%, and panuveitis 0%, respectively.
Conclusions:
The criteria for sarcoidosis-associated uveitis had a low misclassification rate and appeared to perform sufficiently well for use in clinical and translational research.
PRECIS
Using a formalized approach to developing classification criteria, including informatics-based case collection, consensus-technique-based case selection, and machine learning, classification criteria for sarcoidosis-associated uveitis were developed. Key criteria included a compatible uveitic syndrome and evidence of sarcoidosis with either a tissue biopsy demonstrating non-caseating granulomata or chest imaging demonstrating bilateral hilar adenopathy. The resulting classification criteria had a low misclassification rate.
The American Thoracic Society, the European Respiratory Society, and the World Association of Sarcoidosis and Other Granulomatous Diseases have defined sarcoidosis as a multi-system disease of unknown etiology, characterized by granuloma formation, and with a predilection for pulmonary involvement. They further note that “the presence of non-caseating granulomata in a single organ … does not establish the diagnosis of sarcoidosis,” and that the diagnosis of sarcoidosis requires a compatible clinical syndrome.1 Sarcoidosis is present worldwide. In the United States, the incidence has been estimated at 5.9/100,000/year for men and 6.3/100,000/year for women. In the United States, sarcoidosis is more common among African Americans than Caucasians. The cumulative lifetime risk has been estimated at 0.85% for whites and 2.4% for blacks, and the prevalence as 10.9/100,000 for whites and 35.5/100,000 for blacks.1 Pulmonary disease is the most common abnormality with bilateral hilar adenopathy the most characteristic feature on chest imaging (either chest radiograph or computerized tomography [CT]) and parenchymal lung disease having the most negative effect on pulmonary function. In multidisciplinary clinical settings, pulmonary involvement is seen in ~85% to 95% of patients. Involvement of the liver, spleen, or lymph nodes is reported in 25% to 35%, and of the skin in 12% to 25%. Erythema nodosum is reported as present in 4% to 30%, but is not specific for a diagnosis of sarcoidosis, as it occurs with other diseases. Neurologic involvement is present in only ~5%. It is likely that some of this variation represents regional and racial/ethnic variation and that some of the variation represents referral bias. Ocular disease typically is reported as present in ~12% to 25% of patients with documented sarcoidosis with variable frequencies reported depending on the extent of examination (e.g. whether aqueous tear deficiency is evaluated).2,3 Uveitis typically is the most common ocular manifestation of ocular sarcoidosis. In a population-based study in Olmstead County, Minnesota, USA 7% of patients with sarcoidosis had ocular involvement, uveitis was the most common form of ocular sarcoid (61%), and anterior uveitis (71% of uveitis) was the most common anatomic class of uveitis.4 Conversely, sarcoidosis-associated uveitis accounts for ~5% to 10% of uveitis presenting to tertiary care eye centers in the United States.2,5,6
Although anterior uveitis is the most common anatomic class of uveitis seen with sarcoidosis-associated uveitis in the United States, any anatomic class of uveitis may be seen with sarcoidosis, including intermediate, a mixed anterior/intermediate type, posterior, and panuveitis,2,6–11 and in some parts of the world, intermediate uveitis and panuveitis may be more common.9,11 Vitreous inflammatory manifestations include snowballs and “string of pearls” inflammatory debris. Posterior segment clinical findings include choroidal nodules, optic nerve nodules, multifocal choroiditis, and perivascular sheathing (e.g. “candle wax drippings”), occasionally with vascular occlusion.2,6–11 Among patients with sarcoidosis-associated uveitis, the reported frequencies of ocular manifestations typically are: anterior uveitis, 65% to 70%; iris nodules, 11% to 16%; vitritis, 3% to 25%; periphlebitis, 10% to 17%; paucifocal, typically elevated, choroidal nodules (sometimes inappropriately termed “sarcoid granulomas”), 4% to 5%, and multifocal choroiditis ~11%.2 Among patients with sarcoidosis-associated anterior uveitis, both acute anterior uveitis and chronic anterior uveitis have been reported.2
The Standardization of Uveitis Nomenclature (SUN) Working Group is an international collaboration, which has developed classification criteria for the leading 25 uveitides using a formal approach to development and classification.12–17 Among the uveitides studied was sarcoidosis-associated uveitis.
Methods
The SUN Developing Classification Criteria for the Uveitides project proceeded in four phases as previously described: 1) informatics, 2) case collection, 3) case selection, and 4) machine learning.13–16
Informatics.
As previously described, the consensus-based informatics phase permitted the development of a standardized vocabulary and the development of a standardized, menu-driven hierarchical case collection instrument.13
Case collection and case selection.
De-identified information was entered into the SUN preliminary database by the 76 contributing investigators for each disease as previously described.13–16 Cases in the preliminary database were reviewed by committees of 9 investigators for selection into the final database, using formal consensus techniques described in the accompanying article.15,16 Because the goal was to develop classification criteria,17 only cases with a supermajority agreement (>75%) that the case was the disease in question were retained in the final database (i.e. were “selected”).15,16
Machine learning.
The final database was analyzed by anatomic class; cases for each class were randomly separated into a training set (~85% of the cases) and a validation set (~15% of the cases) for each disease as described in the accompanying article.16 Relevant cases of sarcoidosis-associated uveitis were analyzed in the anterior uveitides, intermediate uveitides, and panuveitides. Machine learning was used on the training sets to determine criteria that minimized misclassification. The criteria then were tested on the validation sets; for both the training sets and the validation sets, the misclassification rate was calculated for each disease. The misclassification rate was the proportion of cases classified incorrectly by the machine learning algorithm when compared to the consensus diagnosis.
Cases of sarcoidosis-associated anterior, intermediate, and panuveitis were evaluated in the machine learning for anterior uveitides (cytomegalovirus anterior uveitis, herpes simplex virus anterior uveitis, juvenile idiopathic arthritis-associated anterior uveitis, syphilitic anterior uveitis, spondyloarthritis/HLA-B7-associated anterior uveitis, tubulointerstitial nephritis with uveitis, varicella zoster virus anterior uveitis), intermediate uveitides (multiple-sclerosis-associated intermediate uveitis, pars planitis, intermediate uveitis, non-pars planitis type, syphilitic intermediate uveitis), and panuveitides, (Behçet disease, syphilitic panuveitis, sympathetic ophthalmia, Vogt-Koyanagi-Harada disease, tuberculous panuveitis) respectively. Although “isolated” posterior sarcoidosis-associated uveitis cases were included in the machine learning of posterior uveitides, there were too few cases (N=12) for reliable statistical inferences.
The study adhered to the principles of the Declaration of Helsinki. Institutional Review Boards (IRBs) at each participating center reviewed and approved the study; the study typically was considered either minimal risk or exempt by the individual IRBs.
Results
Three hundred eighty-three cases of sarcoidosis-associated uveitis were collected, and 278 (73%) achieved supermajority agreement on the diagnosis during the “selection” phase and were used in the machine learning phase. They were compared to 971 other anterior uveitides, 537 other intermediate uveitides, and 910 other panuveitides. The details of the machine learning results for these diseases are outlined in the accompanying article.16 The characteristics of cases with sarcoid-associated uveitis listed in Table 1. Biopsy confirmation of the diagnosis of sarcoidosis was obtained in 58%, and 79% had bilateral hilar adenopathy on chest imaging. Bilateral hilar adenopathy was detected in 72% of 242 cases with reported chest radiography results and 82% of 164 cases with reported chest CT scan results. Of 156 cases with both chest radiography and chest CT results reported, 116 had bilateral hilar adenopathy on both imaging modalities, 24 cases had no evidence of bilateral hilar adenopathy of both imaging modalities, and 16 cases had bilateral hilar adenopathy identified on chest CT imaging but not chest radiography. The characteristics of cases of sarcoid-associated uveitis by anatomic class are listed in Table 2. The criteria developed after machine learning are listed in Table 3. The key features of the criteria are a compatible uveitic syndrome and evidence of sarcoidosis. Compatible uveitic syndromes included anterior uveitis (Figure 1), intermediate uveitis (Figure 2), posterior uveitis with either focal choroidal nodule (Figure 3) or multifocal choroiditis (Figure 4), and panuveitis with either choroiditis or retinal vascular sheathing (Figure 5) and/or occlusion. Evidence of sarcoidosis was either tissue biopsy demonstrating non-caseating granulomata or chest imaging (either chest radiography or chest CT) demonstrating bilateral hilar adenopathy. The overall accuracies by anatomic class were: anterior uveitides, training set 97.5% and validation set 96.7% (95% confidence interval [CI] 92.4, 98.6); intermediate uveitides, training set 99.8% and validation set 99.3% (95% CI 96.1, 99.9); and panuveitides, training set 96.3% and validation set 94.0% (95% CI 89.0, 96.8).16 The overall accuracy of the diagnosis of sarcoidosis-associated uveitis in the validation set was 99.6% (95% CI 98.8, 99.9). The misclassification rates for sarcoid-associated uveitis in the training set were as follows: against anterior uveitides 3.2%, intermediate uveitides 2.6%, and non-infectious panuveitides 1.2%. There were too few cases of isolated posterior sarcoidosis-associated uveitis for formal testing, although they were included in the testing against the other diseases. In the validation set the misclassification rates were as follows: against anterior uveitides 0%, intermediate uveitides 0%, and non-infectious panuveitides 0%.
Table 1.
Characteristics of Patients with Sarcoid Uveitis
| Characteristic | Result |
|---|---|
| Number cases | 278 |
| Demographics | |
| Age, median, years (25th 75th percentile) | 49 (39, 61) |
| Gender (%) | |
| Men | 29 |
| Women | 71 |
| Race/ethnicity (%) | |
| White, non-Hispanic | 37 |
| Black, non-Hispanic | 26 |
| Hispanic | 1 |
| Asian, Pacific Islander | 24 |
| Other | 9 |
| Missing | 3 |
| Uveitis History | |
| Uveitis course (%) | |
| Acute, monophasic | 5 |
| Acute, recurrent | 7 |
| Chronic | 80 |
| Indeterminate | 8 |
| Laterality (%) | |
| Unilateral | 18 |
| Unilateral, alternating | 1 |
| Bilateral | 82 |
| Ophthalmic examination | |
| Keratic precipitates (%) | |
| None | 52 |
| Fine | 18 |
| Round | 6 |
| Stellate | 0 |
| Mutton Fat | 23 |
| Anterior chamber cells (%) | |
| Grade 0 | 15 |
| ½+ | 24 |
| 1+ | 28 |
| 2+ | 25 |
| 3+ | 7 |
| 4+ | 1 |
| Hypopyon (%) | 1 |
| Anterior chamber flare (%) | |
| Grade 0 | 60 |
| 1+ | 30 |
| 2+ | 9 |
| 3+ | 1 |
| 4+ | 0 |
| Iris (%) | |
| Normal | 64 |
| Posterior synechiae | 27 |
| Iris nodules | 12 |
| Sectoral iris atrophy | 0 |
| Patchy iris atrophy | 1 |
| Diffuse iris atrophy | 0 |
| Heterochromia | 0 |
| Intraocular pressure (IOP), involved eyes | |
| Median, mm Hg (25th, 75th percentile) | 16 (13, 19) |
| Proportion patients with IOP>24 mm Hg either eye (%) | 10 |
| Vitreous cells (%) | |
| Grade 0 | 31 |
| ½+ | 21 |
| 1+ | 31 |
| 2+ | 14 |
| 3+ | 3 |
| 4+ | 0 |
| Vitreous haze (%) | |
| Grade 0 | 61 |
| ½+ | 11 |
| 1+ | 20 |
| 2+ | 5 |
| 3+ | 2 |
| 4+ | 0 |
| Vitreous snowballs (%) | 17 |
| Pars plana snowbanks (%) | 1 |
| Choroidal nodule (%) | 2 |
| Multifocal choroiditis (%) | 30 |
| Retinal vascular sheathing (%) | 18 |
| Anatomic class (%) | |
| Anterior uveitis | 40 |
| Intermediate uveitis | 19 |
| Posterior uveitis | 4 |
| Panuveitis | 37 |
| Evidence of sarcoidosis (%) | |
| Non-caseating granuloma on tissue biopsy* | 58 |
| Bilateral hilar adenopathy of chest imaging† | 79 |
| Non-specific tests for sarcoidosis (%) | |
| Angiotensin converting enzyme (ACE) | 52 |
| Lysozyme | 12 |
161 of 161 patients biopsied had a “positive” biopsy demonstrating non-caseating granulomata.
174 of 242 patients (72%) had a chest radiograph with bilateral hilar adenopathy, and 134 of 164 patients (82%) undergoing computerized tomography had bilateral hilar adenopathy.
Table 2.
Characteristics of Sarcoid Uveitis by Anatomic Class of the Uveitis
| Characteristic/Anatomic Class | Anterior uveitis | Intermediate uveitis | Posterior Uveitis | Panuveitis |
|---|---|---|---|---|
| Number cases | 112 | 52 | 12 | 102 |
| Demographics | ||||
| Age, median, years (25th 75th percentile) | 46 (37, 55) | 52 (43, 67) | 53 (50, 64) | 51 (35, 63) |
| Gender (%) | ||||
| Men | 24 | 29 | 33 | 33 |
| Women | 76 | 71 | 67 | 67 |
| Race/ethnicity (%) | ||||
| White, non-Hispanic | 30 | 63 | 42 | 31 |
| Black, non-Hispanic | 49 | 6 | 0 | 15 |
| Hispanic | 0 | 2 | 0 | 2 |
| Asian, Pacific Islander | 7 | 13 | 33 | 45 |
| Other | 7 | 12 | 25 | 4 |
| Missing | 7 | 4 | 0 | 3 |
| Uveitis History | ||||
| Uveitis course (%) | ||||
| Acute, monophasic | 10 | 0 | 0 | 3 |
| Acute, recurrent | 14 | 2 | 0 | 3 |
| Chronic | 63 | 96 | 92 | 78 |
| Indeterminate | 13 | 2 | 8 | 16 |
| Laterality (%) | ||||
| Unilateral | 24 | 19 | 42 | 7 |
| Unilateral, alternating | 2 | 0 | 0 | 0 |
| Bilateral | 74 | 81 | 58 | 93 |
| Ophthalmic examination | ||||
| Keratic precipitates (%) | ||||
| None | 46 | 75 | 92 | 43 |
| Fine | 19 | 15 | 8 | 20 |
| Round | 8 | 0 | 0 | 8 |
| Stellate | 1 | 0 | 0 | 0 |
| Mutton Fat | 27 | 10 | 0 | 29 |
| Anterior chamber cells (%) | ||||
| Grade 0 | 4 | 35 | 75 | 12 |
| ½+ | 25 | 27 | 17 | 24 |
| 1+ | 32 | 13 | 8 | 32 |
| 2+ | 30 | 19 | 0 | 24 |
| 3+ | 7 | 6 | 0 | 8 |
| 4+ | 2 | 0 | 0 | 1 |
| Hypopyon (%) | 1 | 0 | 0 | 0 |
| Anterior chamber flare (%) | ||||
| Grade 0 | 63 | 81 | 100 | 42 |
| 1+ | 29 | 15 | 0 | 39 |
| 2+ | 6 | 2 | 0 | 18 |
| 3+ | 2 | 0 | 0 | 1 |
| 4+ | 0 | 2 | 0 | 0 |
| Iris (%) | ||||
| Normal | 61 | 60 | 100 | 66 |
| Posterior synechiae | 33 | 29 | 0 | 23 |
| Iris nodules | 13 | 10 | 0 | 16 |
| Sectoral iris atrophy | 0 | 0 | 0 | 0 |
| Patchy iris atrophy | 1 | 2 | 0 | 2 |
| Intraocular pressure (IOP), involved eyes | ||||
| Median, mm Hg (25th, 75th percentile) | 16 (13, 19) | 16 (14, 18) | 15 (14, 17) | 16 (13, 18) |
| Percent patients with IOP>24 mm Hg either eye | 8 | 8 | 0 | 16 |
| Vitreous cells (%) | ||||
| Grade 0 | 55 | 10 | 25 | 17 |
| ½+ | 27 | 21 | 17 | 14 |
| 1+ | 14 | 52 | 33 | 39 |
| 2+ | 3 | 15 | 25 | 24 |
| 3+ | 1 | 2 | 0 | 6 |
| Vitreous haze (%) | ||||
| Grade 0 | 86 | 46 | 42 | 44 |
| ½+ | 6 | 17 | 17 | 14 |
| 1+ | 5 | 29 | 33 | 28 |
| 2+ | 1 | 4 | 8 | 11 |
| 3+ | 1 | 4 | 0 | 3 |
| Vitreous snowballs (%) | 0 | 58 | 8 | 26 |
| Pars plana snowbanks (%) | 0 | 4 | 0 | 0 |
| Choroidal nodule (%) | 0 | 0 | 17 | 5 |
| Multifocal choroiditis (%) | 0 | 0 | 92 | 73 |
| Retinal vascular sheathing (%) | 0 | 27 | 49 | 28 |
| Evidence of sarcoidosis (%) | ||||
| Non-caseating granuloma on tissue biopsy | 60 | 54 | 58 | 59 |
| Bilateral hilar adenopathy of chest imaging | 82 | 85 | 75 | 74 |
| Non-specific tests for sarcoidosis (%) | ||||
| Angiotensin converting enzyme (ACE) | 45 | 51 | 58 | 59 |
| Lysozyme | 14 | 0 | 0 | 17 |
Table 3.
Classification Criteria for Sarcoid Uveitis
| Criteria |
| 1. Compatible uveitic picture, either |
| a. Anterior uveitis OR |
| b. Intermediate or anterior/intermediate uveitis OR |
| c. Posterior uveitis with either choroiditis (paucifocal choroidal nodule(s) or multifocal choroiditis) OR |
| d. Panuveitis with choroiditis or retinal vascular sheathing or retinal vascular occlusion |
| AND |
| 2. Evidence of sarcoidosis, either |
| a. Tissue biopsy demonstrating non-caseating granulomata OR |
| b. Bilateral hilar adenopathy on chest imaging |
| Exclusions |
| 1. Positive serology for syphilis using a treponemal test |
| 2. Evidence of infection with Mycobacterium tuberculosis,* either |
| a. Histologically- or microbiologically-confirmed infection with M. tuberculosis† OR |
| b. Positive interferon-Ɣ release assay (IGRA)‡ OR |
| c. Positive tuberculin skin test§ |
Routine exclusion of tuberculosis is not required in areas where tuberculosis is non-endemic but should be performed in areas where tuberculosis is endemic or in tuberculosis-exposed patients. With evidence of latent tuberculosis in a patient with a uveitic syndrome compatible with either sarcoidosis or tubercular uveitis and bilateral hilar adenopathy, the classification as sarcoid uveitis can be made only with biopsy confirmation of sarcoidosis (and therefore exclusion of tuberculosis).
E.g. biopsy, fluorochrome stain, culture, or polymerase chain reaction based assay.
E.g. Quantiferon-gold or T-spot.
E.g. Purified protein derivative (PPD) skin test; a positive result should be >10 mm induration.
Figure 1.
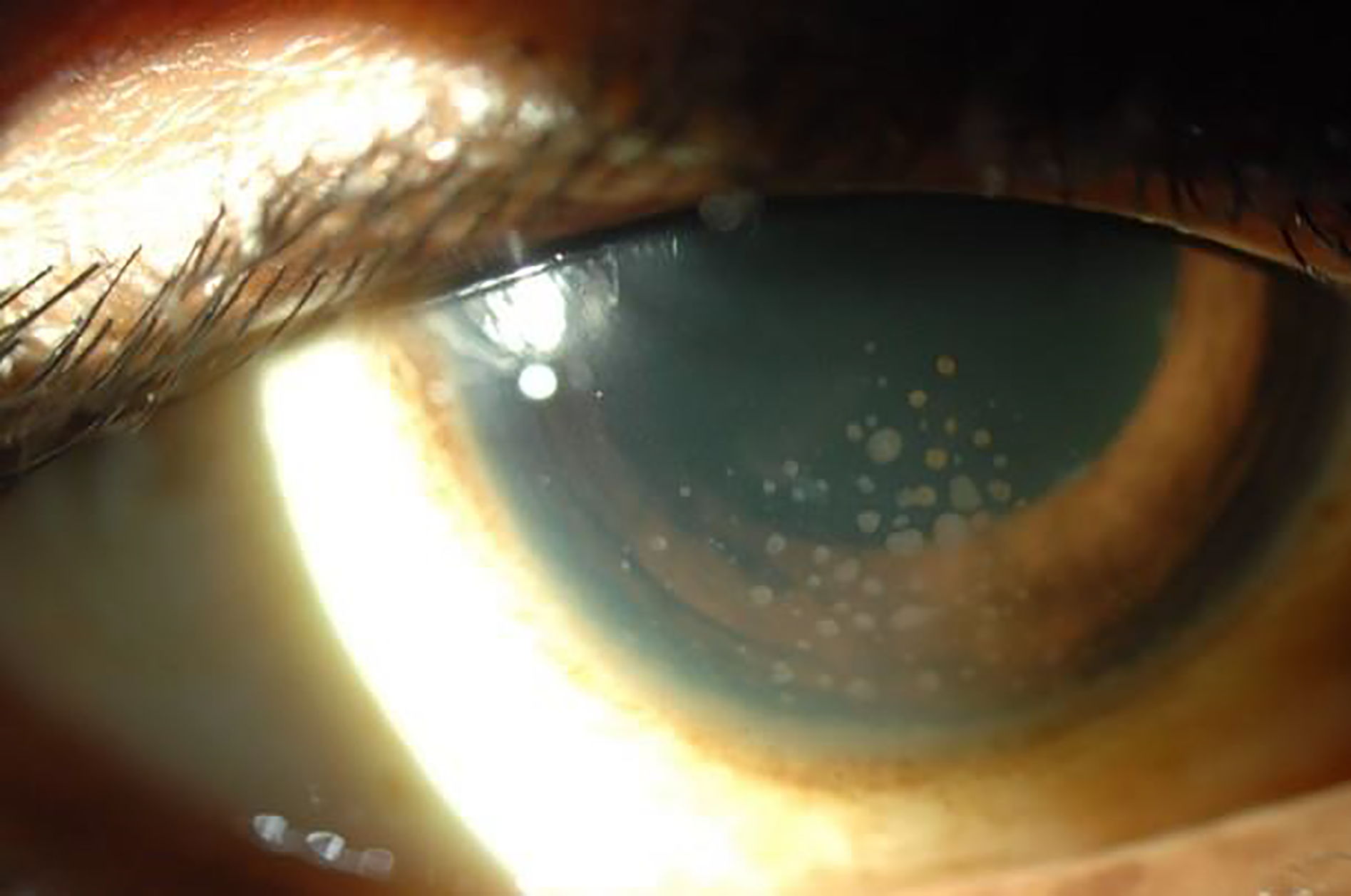
Sarcoidosis-associated anterior uveitis with mutton-fat keratic precipitates.
Figure 2.
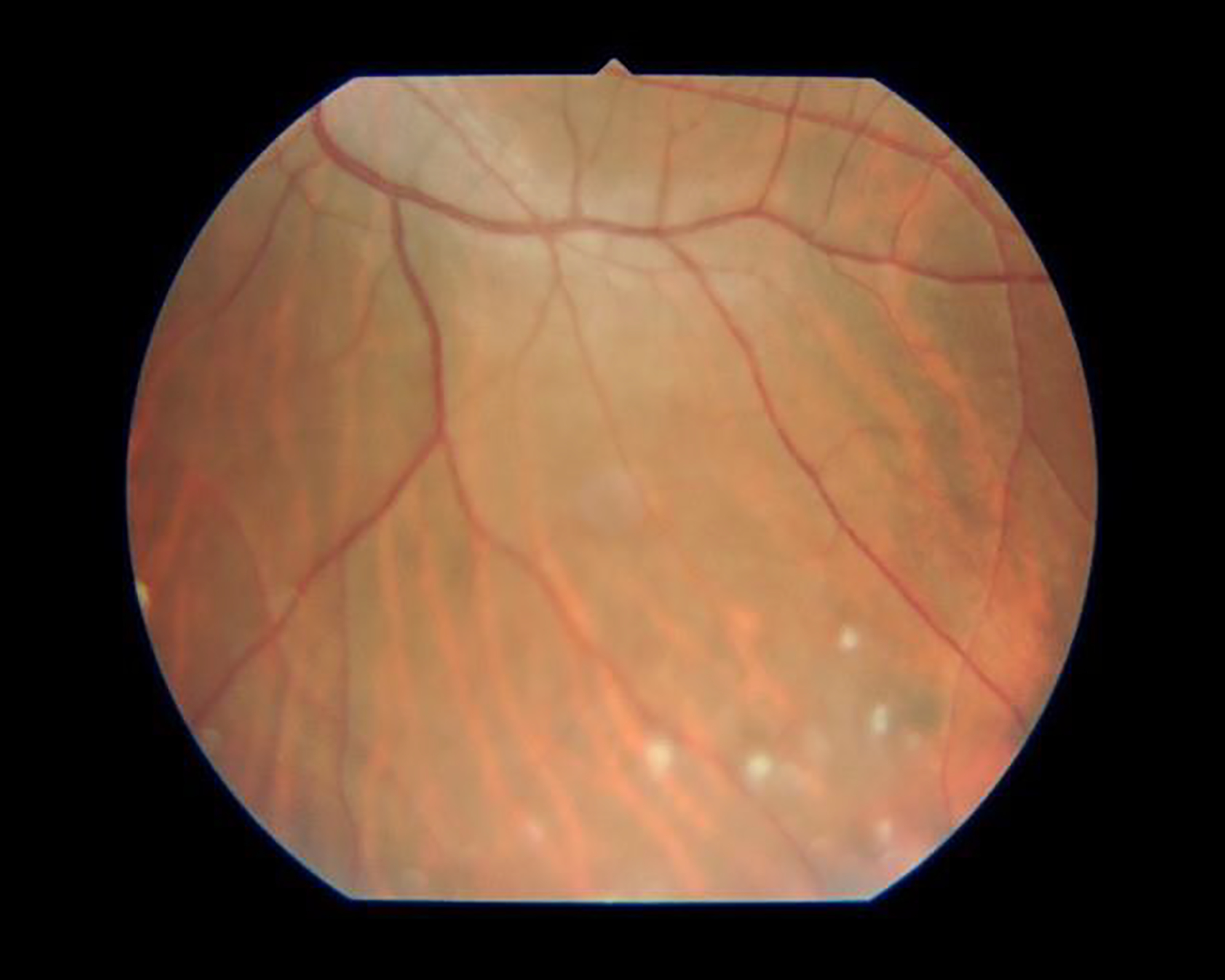
Sarcoidosis-associated uveitis with vitritis.
Figure 3.
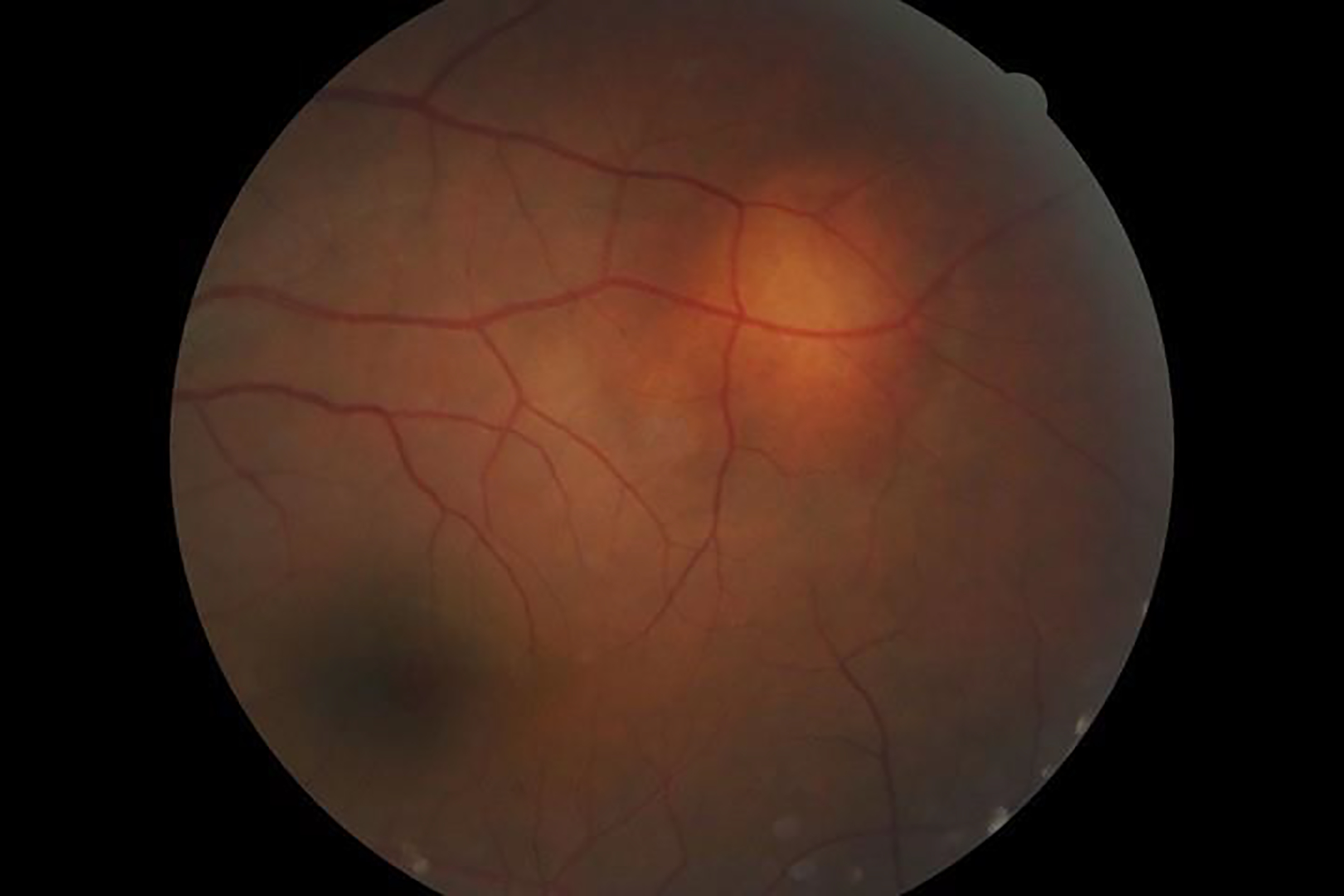
Sarcoidosis-associated uveitis with a focal choroidal nodule.
Figure 4.
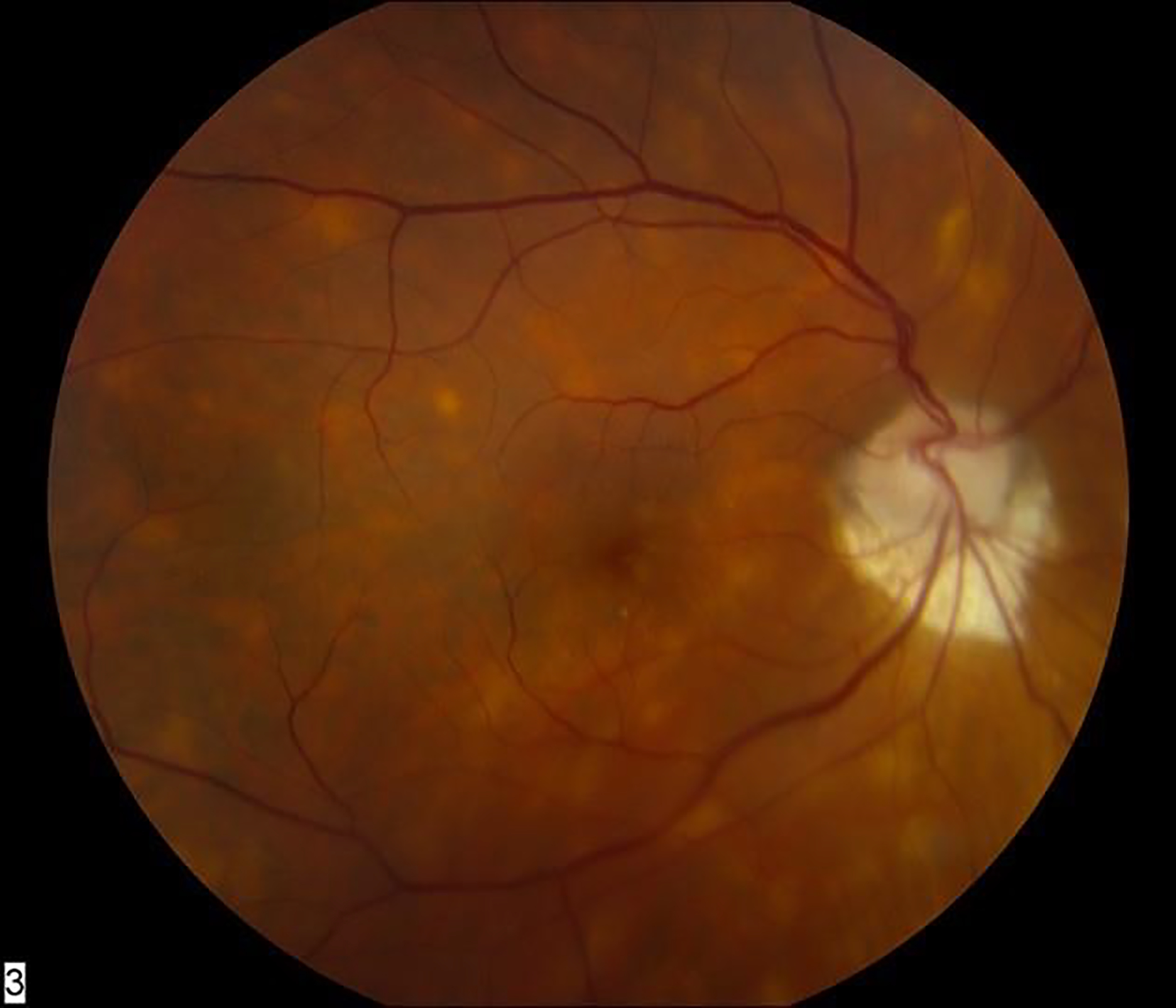
Sarcoidosis-associated uveitis with multifocal choroiditis.
Figure 5.
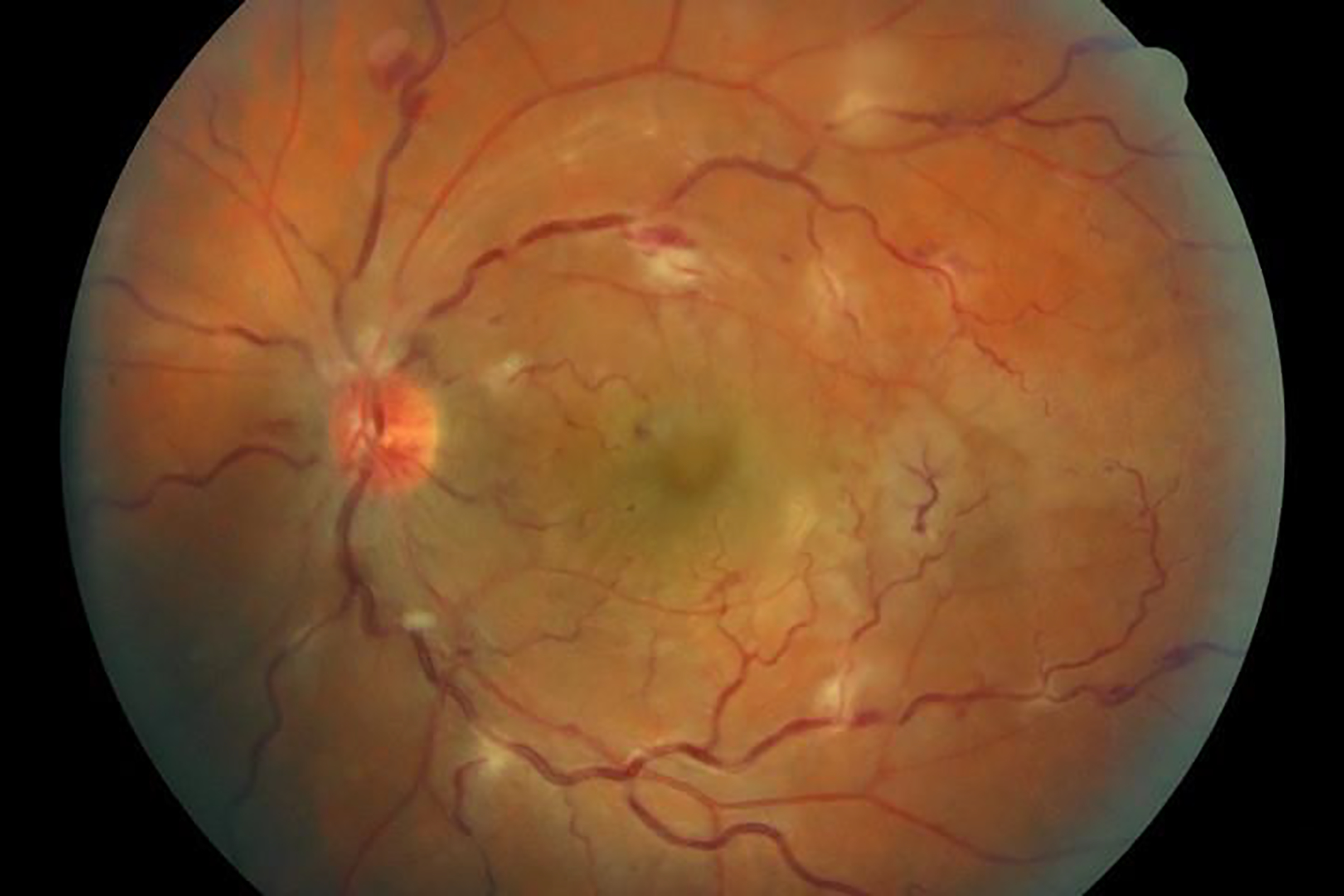
Sarcoidosis-associated uveitis with retinal vascular sheathing.
Discussion
The classification criteria developed by the SUN Working Group for sarcoidosis-associated uveitis have a low misclassification rate, indicating good discriminatory performance against other uveitides.
The diagnosis of sarcoidosis is most straight forward when there is compatible pulmonary disease and a “confirmatory” biopsy demonstrating non-caseating granulomata. In regions where tuberculosis in not endemic, patients with asymptomatic bilateral hilar adenopathy or bilateral hilar adenopathy and uveitis nearly always have sarcoidosis when a pulmonary biopsy is performed.18 However, in regions where tuberculosis is endemic or in patients from endemic regions (with > 6 months residence there), tuberculosis needs to be excluded, as both diseases may produce a similar picture on chest imaging.19 In these situations, if the patient has evidence of latent tuberculosis (e.g. the tuberculin skin test is positive or an interferon-Ɣ release assay [IGRA] is positive), the only way to confirm the diagnosis is biopsy. In the SUN database 6.1% of cases of TB uveitis had bilateral hilar adenopathy on chest imaging, of whom 76% were from Asian countries (and therefore presumably from a TB-endemic country).20 A study of patients with uveitis and a positive IGRA in a non-endemic country suggested that when a biopsy (or bronchoalveolar lavage) is performed ~75% of these patients will have sarcoidosis and not TB.21 Nevertheless, 36% of the patients with uveitis and bilateral hilar adenopathy in this study did not undergo additional testing and were presumed to have ocular TB. As such, patients with a uveitis compatible either with sarcoidosis or with TB uveitis (e.g. chronic anterior uveitis with iris nodules), bilateral hilar adenopathy, and a positive tuberculin skin test or IGRA cannot be reliably diagnosed without biopsy or microbiologic confirmation of the diagnosis.
Although a patient with uveitis reasonably may be presumed to have sarcoidosis when there is a compatible clinical picture and chest imaging, not all patients with ocular sarcoidosis will have an abnormal chest radiograph or CT scan.22 Hence, there have been attempts to create diagnostic criteria and to evaluate serological tests for sarcoidosis, including the serum angiotensin-1 converting enzyme level and the serum lysozyme level.23 The International Workshop on Ocular Sarcoidosis (IWOS) published criteria in 2009.24 The IWOS Criteria included four levels of certainty: definite (biopsy-confirmed), presumed (bilateral hilar adenopathy and uveitis), probable (neither biopsy-confirmed, nor bilateral hilar adenopathy, but fulfilling several ocular and systemic criteria, the latter relating to anergy and serological tests), and possible ocular sarcoidosis. Evaluation of the IWOS Criteria by an international group demonstrated problems with the performance of the IWOS Criteria,11 which subsequently were revised (“Revised IWOS Criteria”) but kept the different levels of certainty.25 The SUN Criteria for sarcoidosis-associated uveitis are similar to the definite and presumed ocular sarcoidosis classes of the IWOS Criteria. The SUN Criteria for sarcoidosis-associated uveitis did not include probable and possible cases of IWOS Criteria-diagnosed ocular sarcoidosis, because only ~62% of those with probable ocular sarcoidosis using the IWOS criteria will have sarcoidosis when a biopsy is performed22,24 (and presumably a lower percentage of possible cases), which reflects the difference between classification criteria developed by the SUN Working Group and diagnostic criteria developed by the IWOS Group.
The presence of any of the exclusions in Table 3 suggests an alternate diagnosis, and the diagnosis of sarcoidosis-associated uveitis should not be made in their presence. In prospective studies many of these tests will be performed routinely, and the alternative diagnoses excluded. However, in retrospective studies based on clinical care, not all of these tests may have been performed. Hence the presence of an exclusionary criterion excludes pars planitis, but the absence of such testing does not always exclude the diagnosis of sarcoidosis-associated uveitis if the criteria for the diagnosis are met. The exception is that in areas where TB is endemic or in patients emmigrating from areas in which TB is endemic, TB should be excluded.
Neither elevated serum ACE nor elevated serum lysozyme level was selected by the machine learning for the SUN criteria set of sarcoidosis-associated uveitis. Sensitivity of an elevated serum ACE for detecting sarcoidosis has been reported variably from 22% to 84%, and of an elevated serum lysozyme as 42% to 60%.10,23,24,26,27 Reported positive predictive values for an elevated serum ACE have ranged from 18% to 90% and for an elevated serum lysozyme as 12%.10,25,28,29 The highest value for the positive predictive value of an ACE was derived from a case series enriched for sarcoidosis and over half of the cases had probable or possible IWOS Criteria-diagnosed ocular sarcoidosis.27 Had the percentage of sarcoidosis cases been at the more typical 5%, the positive predictive value would have dropped to 52%. As such neither the SUN process nor the literature supports the inclusion of these serological tests in classification criteria.
More recently, serum soluble interleukin (IL)-2 receptor (sIL-2R) has been evaluated as a possible diagnostic test for sarcoidosis.27 Case series data suggest high sensitivity and specificity (98% and 94%, respectively). In a sarcoidosis enriched population of patients with uveitis, the positive predictive value was 77%,27 but in a population of patients with uveitis where sarcoidosis accounted for 5% of cases, the positive predictive value would be 46%. Although the SUN database did not have sIL-2R data for evaluation, the positive predictive values suggest that it may have a limited role in classification criteria. However, in both situations, the negative predictive value would be 99%, suggesting that it may be a reasonable test for excluding sarcoidosis in those clinical settings where the test is available.
Because sarcoidosis is in the differential diagnosis of most classes of uveitis, its exclusion is an important part of the criteria for many other uveitic diseases.8 Although serologic tests to date have performed too poorly to be used for diagnosing sarcoidosis, as noted above, they may potentially have value for excluding sarcoidosis, and some clinical centers use a two-step approach by screening with an ACE and obtaining chest imaging only in those with an elevated ACE or high suspicion. Reported negative predictive values have ranged from 87% to 97%.10,23,26,28 Because the agreement among uveitis experts on uveitic diagnoses is moderate at best,15 prospective series using standardized classification criteria should be used to evaluate this strategy.
The identification of bilateral hilar adenopathy on chest imaging is important in establishing the diagnosis of sarcoidosis, but other findings on chest imaging (e.g. nodular disease, interstitial pneumonitis without bilateral hilar adenopathy) are not specific and should not be used to diagnose sarcoidosis absent biopsy confirmation.18,19 Traditionally screening has been performed with chest radiographs and chest CT scanning used in cases of equivocal chest radiographs or cases with high suspicion on other grounds. Nevertheless, there are data to suggest that chest CT scanning may be superior for the detection of bilateral hilar adenopathy.29,30 Whether chest CT scanning should replace chest radiography as a screening tool is an open question, and the SUN data do not provide a definitive answer. However, among cases with both chest imaging results, chest radiography detected 88% of the cases of bilateral hilar adenopathy seen on chest CT imaging, suggesting that for “screening” purposes, the more traditional approach may be adequate. In a retrospective case series of 709 patients with uveitis, among whom 10.7% had sarcoidosis, chest CT had superior sensitivity to chest radiography, but the positive predictive value for both was 100% and the negative predictive values for chest radiograph were 94.4% and for chest CT 98.2%, again suggesting that the chest radiograph may be adequate as a screening tool.28 Nevertheless, there may be selected clinical situations in which a chest CT is preferred.30 Prospective studies involving both chest imaging techniques and using standardized classification criteria might resolve this issue.
Classification criteria are employed to diagnose individual diseases for research purposes.17 Classification criteria differ from clinical diagnostic criteria, in that although both seek to minimize misclassification, when a trade-off is needed, diagnostic criteria typically emphasize sensitivity, whereas classification criteria emphasize specificity,17 in order to define a homogeneous group of patients for inclusion in research studies and limit the inclusion of patients without the disease in question that might confound the data. The machine learning process employed did not explicitly use sensitivity and specificity; instead it minimized the misclassification rate. Because we were developing classification criteria and because the typical agreement between two uveitis experts on diagnosis is moderate at best,15 the selection of cases for the final database (“case selection”) included only cases which achieved supermajority agreement on the diagnosis. As such, some cases which clinicians would diagnose with sarcoidosis-associated uveitis will not be so classified by classification criteria.
In conclusion, the criteria for sarcoidosis-associated uveitis outlined in Table 3 appear to perform sufficiently well for use as classification criteria in clinical research.
Acknowledgments
Grant support: Supported by grant R01 EY026593 from the National Eye Institute, the National Institutes of Health, Bethesda, MD, USA; the David Brown Fund, New York, NY, USA; the Jillian M. And Lawrence A. Neubauer Foundation, New York, NY, USA; and the New York Eye and Ear Foundation, New York, NY, USA.
Footnotes
Writing committee: Douglas A. Jabs, MD, MBA2,3; Nisha R. Acharya, MD4; Alastair K. Denniston, PhD, MRCP, FRCOphth5; Susan L. Lightman, PhD, FRCP, FRCOphth6,7; Peter McCluskey, MD8; Neal Oden, PhD9; Annabelle A. Okada, MD, DMSc10; Alan G. Palestine, MD11; Jennifer E. Thorne, MD, PhD2,3; Brett E. Trusko, PhD, MBA12; Albert Vitale, MD13
Affiliations: 1Members of the SUN Working Group are listed online at ajo.com. From 2the Department of Epidemiology, the Johns Hopkins University Bloomberg School of Public Health, and 3the Wilmer Eye Institute, the Department of Ophthalmology, the Johns Hopkins University School of Medicine, Baltimore, MD, USA; 4the Francis I. Proctor Foundation, the University of California, San Francisco, San Francisco, CA, USA; 5the Academic Unit of Ophthalmology, the University of Birmingham, Birmingham, UK; 6Moorfields Eye Hospital, London, UK; 7Institute of Ophthalmology, University College London, London, UK; 8the Save Sight Institute, Department of Ophthalmology, University of Sydney School of Medicine, Sydney, NSW, Australia; 9the Emmes Company, LLC, Rockville, MD, USA; 10the Department of Ophthalmology, Kyorin University School of Medicine, Tokyo, Japan 11the Department of Ophthalmology, University of Colorado School of Medicine, Aurora, Co, USA; 12the Department of Medicine, Texas A&M University, College Station, TX, USA; 13the Department of Ophthalmology, the University of Utah School of Medicine, Salt Lake City, UT, USA.
Conflict of Interest: Douglas A. Jabs: none; Nisha A. Acharya: none; Alastair K. Denniston; none; Susan L. Lightman: none; Peter McCluskey: none; Neal Oden: none; Annabelle A. Okada: consultant: AbbVie Japan, Astellas Pharma Japan, Bayer AG, Daiichi Sankyo; lecture fees: Alcon Pharma Japan, Mitsubishi Tanabe Pharma, Novartis Pharma Japan, Santen Pharmaceutical Corporation, Senju Pharmaceutical Corporation; grant support from Alcon Pharma Japan, Bayer Yakuhin, Mitsubishi Tanabe Pharma; Alan G. Palestine: none; Jennifer E. Thorne: Dr. Thorne engaged in a part of this research as a consultant and was compensated for the consulting service; Brett E. Trusko: none; Albert Vitale: none.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Statement on sarcoidosis. Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adoped by the ATS Board of Dirrectors and they ERS Executive Committee, February 1999. Am J Resp Crit Care Med 1999;160:736–55. [DOI] [PubMed] [Google Scholar]
- 2.Sepah YJ, Agarwal A, Jabs DA, Nguyen QD. Sarcoidosis. In Schachat AP, Wilkinson CP, Hinton DR, Sada SVR, Wiedemann P, eds. Ryan’s Retina, 6th ed. Elsevier, New York, 2018, pp.1572–85. [Google Scholar]
- 3.Baughman RP, Teirsten AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care Med 2001;164:1885–9. [DOI] [PubMed] [Google Scholar]
- 4.Ungprasert P, Tooley AA, Crowson CS, Matteson EL, Smith WM. Clinical characteristics of ocular sarcoidosis: a population-based study 1976–2013. Ocular Immunol Inflamm 2019;27:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderly DE, Genstler AJ, Smith RE, Rao NA. Changing patterns of uveitis. Am J Ophthalmol 1987;103:131–6. [DOI] [PubMed] [Google Scholar]
- 6.Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol 2013;156:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obenauf CD, Shaw HE, Syndor CF, Klintworth GK. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol 1978;86:648–55. [DOI] [PubMed] [Google Scholar]
- 8.Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol 1986;102:297–301. [DOI] [PubMed] [Google Scholar]
- 9.Rothova A Ocular involvement in sarcoidosis. Br J Ophthalmol 2000;84:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum AD, Oh FS, Chakrabarti A, Tessler HH, Goldstein D. Clinical features and diagnostic evaluation of biopsy-proven ocular sarcoidosis. Arch Ophthalmol 2011;129:409–13. [DOI] [PubMed] [Google Scholar]
- 11.Acharya NR, Browne EN, Rao N, Mochizuki M, for the International Ocular Sarcoidosis Working Group. Distinguishing features of ocular sarcoidosis in an international cohort of uveitis patients. Ophthalmology 2018;125:119–26. [DOI] [PubMed] [Google Scholar]
- 12.Jabs DA, Rosenbaum JT, Nussenblatt RB, the Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Report of the first international workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trusko B, Thorne J, Jabs D, et al. Standardization of Uveitis Nomenclature Working Group. The SUN Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inform Med 2013;52:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada AA, Jabs DA. The SUN Project. The future is here. Arch Ophthalmol 2013;131:787–9. [DOI] [PubMed] [Google Scholar]
- 15.Jabs DA, Dick A, Doucette JT, Gupta A, Lightman S, McCluskey P, Okada AA, Palestine AG, Rosenbaum JT, Saleem SM, Thorne J, Trusko B for the Standardization of Uveitis Nomenclature Working Group. Interobserver agreement among uveitis experts on uveitic diagnoses: the Standard of Uveitis Nomenclature Experience. Am J Ophthalmol 2018; 186:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Standardization of Uveitis Nomenclature (SUN) Working Group. Development of classification criteria for the uveitides. Am J Ophthalmo 2020;volume:pp. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria. Arthritis Care Res 2015;67:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winterbauer RH, Belic N, Moores KD. Clinical interpretation of bilateral hilar adenopathy. Ann Intern Med 1973. 78:65–71. [DOI] [PubMed] [Google Scholar]
- 19.Babu K, Shukla SB, Philips M. High resolution chest computerized tomography in the diagnosis of ocular sarcoidosis in a high TB endemic population. Ocular Immunol Inflamm 2017;25:253–8. [DOI] [PubMed] [Google Scholar]
- 20.The Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for tubercular uveitis. Am J Ophthalmol 2020;volume:pp. [DOI] [PubMed] [Google Scholar]
- 21.LaDista NR, van Velthoven ME, Ten Dam-vanLoon NH, et al. Clinical manifestations of patients with intraocular inflammation and positive Quanteriferon-TB gold in-tube test in a country non-endemic for tuberculosis. Am J Ophthalmol 2014;137:754. [DOI] [PubMed] [Google Scholar]
- 22.Ohara K, Okubo A, Kamata K, Sasaki H, Kobayashi J, Kitamura S. Transbronchial lung biopsy in the diagnosis of suspected ocular sarcoidosis. Arch Ophthalmol 1993;111:642–4. [DOI] [PubMed] [Google Scholar]
- 23.Baarsma GS, La Hey E, Glasius E, de Vries J, Kilstra A. The predictive value of serum angiotensin converting enzyme and lysozyme levels in the diagnosis of ocular sarcoidosis. Am J Ophthalmol 1987;104:211–7. [DOI] [PubMed] [Google Scholar]
- 24.Herbort CP, Mochizuki M, Rao NA, the members of the Scientific Committee of the First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: results of the First International Workshop on Ocular Sarcoidosis (IWOS). Ocular Immunol Inflamm 2009;17:160–9. [DOI] [PubMed] [Google Scholar]
- 25.Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA, for the International Workshop on Ocular Sarcoidosis Study Group. Br J Ophthalmol 2019; ePub ahead of print [Google Scholar]
- 26.Niederer RL, Al-Janabi A, Lightman SL, Tomkins-Netzer O. Serum angiotensin-converting enzyme has a high negative predictive value in the investigation for systemic sarcoidosis. Am J Ophthalmol 2018;194:82–7. [DOI] [PubMed] [Google Scholar]
- 27.Gundlach E, Hoffman MM, Prasse A, Heinzelman S. Interleukin-2 receptor and angiotensin converting enzyme as markers for ocular sarcoidosis. PLoS ONE; 2016:11:e0147258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niederer RL, Sims JL. Utility of screening investigations for systemic sarcoidosis in undifferentiated uveitis. Am J Ophthalmol 2019;206:149–53. [DOI] [PubMed] [Google Scholar]
- 29.Kosmorsky GS, Meisler DM, Rice TW, Meziane MA, Lowder CY. Chest computed tomography and mediastinoscopy in the diagnosis of sarcoidosis-associated uveitis. Am J Ophthalmol 1998;126:132–4. [DOI] [PubMed] [Google Scholar]
- 30.Han YS, Rivera-Grana E, Salek S, Rosenbaum JT. Distinguishing uveitis secondary to sarcoidosis from idiopathic disease: cardiac implications. JAMA Ophthalmol 2018;136:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


