Abstract
The prevalence of ear disorders has spurred efforts to develop drug delivery systems to treat these conditions. Here, recent advances in drug delivery systems that access the ear through the tympanic membrane (TM) are reviewed. Such methods are either non-invasive (placed on the surface of the TM), or invasive (placed in the middle ear, ideally on the round window [RW]). The major hurdles to otic drug delivery are identified and highlighted the representative examples of drug delivery systems used for drug delivery across the TM to the middle and (crossing the RW also) inner ear.
Keywords: drug delivery, invasive, noninvasive, trans-tympanic
1. Introduction
Diseases of the middle ear, such as middle ear infections (otitis media [OM]) are generally treated by systemic drug administration, that is, by pills, syrups, and intramuscular injections, since direct instillation of drugs into the middle ear is usually inhibited by the presence of the tympanic membrane (TM), which is impermeable to most compounds.[1] These systemic drugs are distributed throughout the body even though the intended targets are two relatively tiny portions of the body. Systemic distribution limits the drug levels that can be achieved within the middle ear, particularly since systemic distribution will entail side effects.[2] In the case of OM, side effects can include rashes and diarrhea, and can limit treatment. The frequency of prescribing of antibiotics in such a prevalent condition is believed to be potentially responsible for the development of antibiotic resistant bacteria.[3] Moreover, the administration of antibiotics three times a day for ten days to uncooperative toddlers is challenging, as can be attested to by any parent. The potential utility of local treatment of middle ear conditions is seen in the efficacy of simple formulations such as Ciprodex solution, a combination of an antibiotic and an anti-inflammatory agent, that is instilled into the outer ear of patients with tympanostomy tubes (tubes through the TM) to treat middle ear conditions, or analogous formulations deposited directly into the middle ear.[4] However, the majority of patients with middle ear disease do not have tympanostomy tubes or perforated TMs. Consequently, drug delivery systems have been developed for noninvasive trans-tympanic drug delivery, that is, delivery across an intact TM to the middle ear. (In this review, all delivery across the TM will be termed trans-tympanic, the distinction being made between those that are non-invasive and those that are not. We have preferred not to use intratympanic which, by analogy with the terminology for skin, would mean delivery into the TM.)
Delivery to the inner ear is in some ways even more difficult. Systemic drug delivery to the inner ear is limited by barriers between the blood and the fluid-filled spaces in the inner ear.[5] Direct delivery would entail either going through the TM to get into the middle ear and then through the round window (RW), or drilling directly through the skull. Invasive trans-tympanic drug delivery systems have been developed that would be implanted or injected into the middle ear (ideally on the RW) for delivery to the inner ear. This review will not address systemic drug delivery to the ear, nor systems that deliver drugs directly into the inner ear.
Invasive and noninvasive trans-tympanic drug delivery to the inner ear are analogous, in that both involve transit of drugs from an air-filled space across a barrier into a fluid-filled space (assuming that flux across the TM into the middle ear requires the presence of fluid). Consequently, the governing principles (such as determinants of flux) and design principles (such as the use of specific types of drug delivery systems) for the one have some bearing on the other. Another common feature is that variability in the anatomy of the human external ear canal, in the thickness of the various barriers to delivery, and in the accumulation of normal debris (e.g., ear wax) can affect delivery.[6]
This progress report focuses on relatively recent developments in the field. Consequently, significant older contributions may have been omitted; this is not intended to diminish their importance. Moreover, inclusion of specific technologies in this report is intended to provide an appreciation of the range of approaches that have been adopted, and is not an implicit endorsement of any given system (including our own).
2. Structure of the Ear and Factors Influencing Drug Delivery
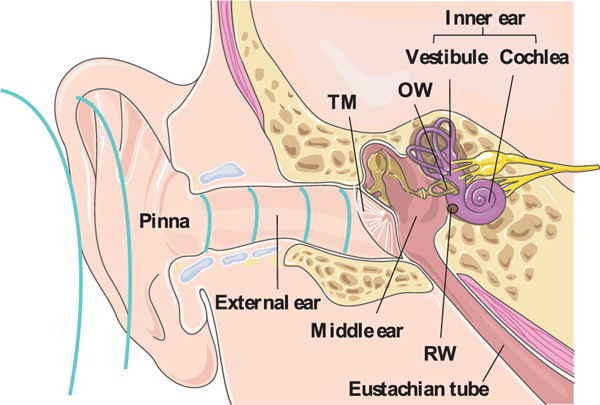
The human ear is separated into the external ear, the middle ear, and the inner ear (Figure 1). The external ear extends from the pinna along the external ear canal, and ends at the TM, which creates a barrier with the middle ear.[7] The TM comprises three layers: (1) the outer layer that is a stratified squamous keratinized epithelium;[1a] (2) a middle fibro-elastic connective tissue rich in collagen; and (3) the innermost layer, a simple cuboidal mucosal epithelium.[8] The thickness of the human TM is not uniform in any given individual, and there is variability in its mean thickness (typically 80–100 μm with surface are a around 64.3 mm2).[9] It is impenetrable to all except some small and moderately lipophilic molecules because of its keratin- and lipid-rich stratum corneum (SC).[10] Perforation of the TM may induce middle ear infection and partial hearing loss.[11]
Figure 1.
Anatomy of the human ear. TM: tympanic membrane; OW: oval window; RW: round window. The ear anatomy image was adapted under the terms and conditions of the CC BY 3.0 Unported License. Copyright Servier Medical Art.
The middle ear is (usually) an air-filled cavity surrounded by the temporal bone of the skull,[12] separated from the outer ear by the TM and the inner ear by the oval and RWs (Figure 1).[13] It is connected to the middle ear by the Eustachian tube,[14] through which drugs and other materials administered in the middle ear cavity can be cleared. While drug delivery through both oval and RWs have been described,[15] the majority of reports use the RW, perhaps because the oval window is partly occluded by the third ossicle.[16] The RW is a three-layer semi-permeable membrane composed of (1) a single cell layer outer epithelium (on the side of the middle ear, and continuous with its lining); (2) a connective tissue layer with fibroblasts, blood vessels, collagen, and elastic fibers; and (3) the innermost layer (bounding the inner ear) of squamous cells.[17] The human RW has an average thickness of 70 μm with a surface area of ≈2.2 mm2.[18] In trans-tympanic drug delivery to the inner ear, the drug is generally injected through the TM and deposited in the middle ear,[19] from which drugs diffuse through the RW into the fluid spaces of the inner ear.[20] The RW is located behind mucosal folds which, with other structures, form the mucosal niche.[21] Placing materials precisely in the niche so that they appose the RW can be challenging.[16b]
The inner ear contains the cochlea (auditory organ) and the vestibular system (organ of balance), which are embedded deep in the petrous bone.[22] Once drugs cross the RW and get into the inner ear, further distribution depends on their diffusion within the fluids of the inner ear,[23] crossing other barriers such as the vestibular membrane, and other factors; this subject will not be covered here.
3. Trans-Tympanic Drug Delivery Systems
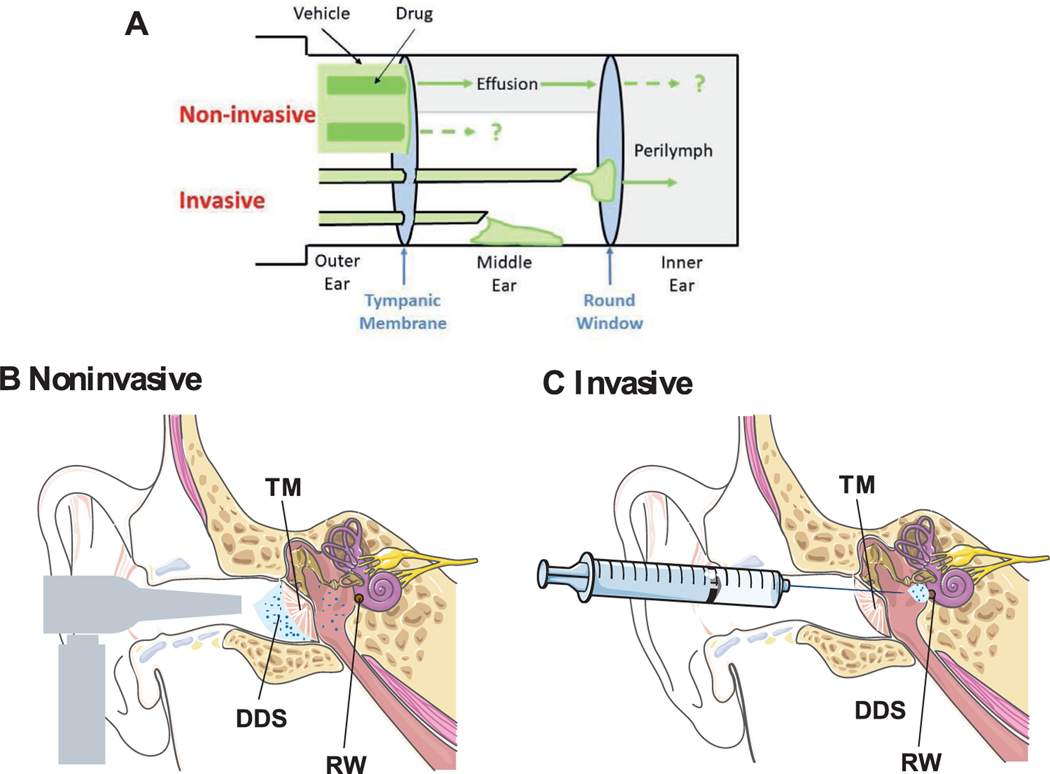
In noninvasive trans-tympanic delivery, drugs or drug delivery systems are applied to the TM so that they diffuse into the middle ear (Figure 2A,B).[24] Invasive trans-tympanic delivery has two principal goals: (1) injection of a drug or drug delivery system into the middle ear cavity through the TM or a device that crosses the TM, so that drugs are delivered to the middle ear (2) placement of drug delivery systems on the RW so that drugs diffuse into the inner ear (Figure 2A,C).
Figure 2.
Trans-tympanic drug delivery. A) Schematic of trans-tympanic drug delivery systems. In non-invasive delivery, drug fluxes into middle ear fluid, with substantial dilution. It is unknown whether substantial drug flux can occur into the inner ear, or into the middle ear in the absence of fluid. Invasive delivery is via injection or incision into the middle ear, or in the round window niche, for drug flux into the middle or (more commonly) inner ear. Anatomy of B) noninvasive trans-tympanic drug delivery systems (TM: tympanic membrane, DDS: drug delivery system), C) invasive trans-tympanic drug delivery systems. RW: Round window. The ear anatomy and syringe images were adapted under the terms and conditions of the CC BY 3.0 Unported License. Copyright Servier Medical Art.
Drug transfer across the TM and RW both relate to the more general problem of getting drugs across barriers. The RW does not seem as impermeable to drugs as the TM,[21] perhaps because it does not have a SC. Moreover, drug transport across the RW may entail processes other than those detailed in this section, such as pinocytosis. Drug flux across barriers has been best studied in the context of transdermal drug delivery, where the principal physical barrier to drug permeation is the outer layer, the 10–20 μm thick SC. The SC is composed of dead corneocytes and lipids organized in lamellar crystalline bilayers. Permeation of drug through skin can occur through the lipid bilayers (intercellular path), across the corneocytes (intracellular), or through hair follicles or sebaceous and/or sweat ducts (transcellular). Most molecules penetrate through skin via the intercellular path and many permeation enhancement techniques therefore aim to affect the molecular architecture of that path.[25] In general, a low molecular weight and specific log P value (neither too hydrophilic nor-hydrophobic, perhaps 0–5) is favorable for skin permeation. There are similar considerations with regard to the TM and RW.[10a] The TM is impermeable to virtually all molecules except some small and moderately hydrophobic molecules. It is suggested that smaller, cationic, and lipophilic particles preferentially pass through the RW.[26]
Transport across barriers of this type can in general be considered to be governed by Fick’s first law of diffusion (at steady state):
| (1) |
where J is the transfer (flux) rate of a diffusing substance; A is the unit area of the membrane; D is the diffusion coefficient and (dc/dx) is concentration gradient across the membrane. (The negative sign in this equation is because diffusion occurs away from the area of high drug concentration.) Thus, the goal of many drug delivery systems is to maintain the highest possible concentration outside the barrier for the longest possible period, to use drugs for which the permeability of the membrane is the greatest to that the diffusion coefficient is maximized, and to apply the system over the greatest possible area. The diffusion coefficient can also be increased by agents that can enhance diffusion of drugs through barriers.[27]
Many of the drug delivery systems used to cross the TM and RW bear some resemblance to those used for transdermal drug delivery. These have included hydrogels, from which drugs diffuse passively across the barrier; nanoparticles (NPs), which can themselves cross the barriers; and chemical permeation enhancers (CPEs), which enhance the flux of a second drug across barriers. CPEs have been widely used in skin, but also to get drugs across the TM and RW.[28] Inflammation itself can enhance flux across barriers in the ear, as has been shown in the TM and RW,[29] and by the use of inflammatory mediators such as histamine on the RW.[30]
The fate of drugs after they cross barriers is not always easy to predict. For example, it is not known whether drugs applied to the TM will spread within the middle ear in the absence of fluid (e.g., an effusion, as in OM). It is also not known whether drugs that accumulate in middle ear fluid by noninvasive trans-tympanic delivery will do so in sufficient concentration to be able to diffuse across the RW at effective concentrations.
All of the systems described below are examples of local drug delivery (i.e., systems administered locally for local effect). An important feature that they therefore share (ideally) is the absence of systemic distribution and therefore systemic side effects. As one example, noninvasive trans-tympanic delivery of antibiotics from a hydrogel containing CPEs resulted in supratherapeutic drug levels in middle ear fluid in a chinchilla model of OM, but with undetectable drug levels (by high performance liquid chromatography) in blood.[29c] This system also had minimal effect on the gut microbiome, whereas systemic (oral) antibiotics affected it profoundly.[31]
3.1. Noninvasive Trans-Tympanic Drug Delivery Systems
Not many such systems have been described. They are deposited on the TM, often with a means—such as a hydrogel—to immobilize them in situ.
3.1.1. Reverse Thermal Gelling Hydrogels
There are numerous design characteristics of noninvasive trans-tympanic delivery apart from the crossing of the TM. The drugs that will be applied to the TM, and the components that may help them get across (see section 3.1.2), need to remain in contact with the TM for an extended period. Many of the patients for such applications are children, who are often uncooperative with application of a material to the ear, and may not be willing to remain still. Consequently, what may be needed is a system that is easy to apply (i.e., a liquid during application) but gels quickly upon contacting the TM. While there are a number of in situ gelling polymer strategies that could be employed, we have selected reverse thermal gelling hydrogels for this purpose, particularly poloxamers (such hydrogels gel when heated). Poloxamer 407 (P407) is an ABA-triblock polymer composed of a hydrophobic central block of polypropylene glycol and two hydrophilic polyethylene glycol (PEG) units.[32] P407 is a solution at room temperature and is therefore easily administered, forming a depot (gelling) upon contacting the warm (37 °C) TM, allowing it to remain there for an extended period and provide prolonged release.[33] Our group has formulated a hydrogel formulation containing CPEs (see section 3.1.2) and P407 for trans-tympanic delivery of antibiotics to the middle ear to treat OM, and showed that it could enhance flux across the TM (Figure 3A).[28c] However, interaction between the CPEs and P407 impaired the mechanical properties of P407 (gelation and the dwell time on the TM), reducing efficacy.[29c] A thermosensitive penta-block copolymer P407-polybutylphosphoester (P407-PBP) with more hydrophobic domains was designed to address this issue (Figure 3B).
Figure 3.
A formulation for noninvasive trans-tympanic delivery to the middle ear. A) The formulation included a polymer with reverse thermal gelling properties, a combination of chemical permeation enhancers (3CPE), and an antibiotic, ciprofloxacin. B) The mechanical properties of P407 and P407-PBP with or without the combination of chemical permeation enhancers in panel A (3CPE) as a function of temperature. Without CPEs, P407 gels (G′ > G″) at 27 °C; addition of 3CPE prevents gelation. In contrast, 3CPE enhanced the gelation of P407-PBP. C) Time course of elimination of bacteria (CFU: colony-forming units, a metric of bacterial presence) from middle ear fluid of chinchillas with OM after trans-tympanic treatment with different formulations. (B and C) Reproduced with permission.[29c] Copyright 2016, AAAS.
Such systems can affect hearing, in proportion to the amount that is placed on the TM. For example, in chinchillas, 50 μL of P407 had no effect on hearing (as assessed by acoustic brainstem responses),[28c] while 200 μL of P407-PBP resulted in a mild hearing deficit analogous to the effect of ear wax.[29c]
3.1.2. Chemical Permeation Enhancers
Hydrogels and other depot-type delivery systems can maintain drugs at the desired location, but apart from that function, they generally do not inherently increase flux across barriers. In fact, the need for the drug to diffuse out of the hydrogel generally reduces flux across the TM compared to the flux from a free solution.[29c] Due to the structural similarity of the TM to skin, the flux of therapeutic agents across the intact TM can be enhanced via methods similar to those developed for skin. CPEs are widely applied in transdermal systems to help drugs cross the skin.[34] CPEs are a variety of molecules—usually small, often surfactants—that can interact with the skin’s SC layer and reversibly decrease its barrier effect. The enhancement mechanisms of CPEs and their pros and cons have been reviewed.[35] The presence of CPEs, for example, a combination of 2% limonene, 1% sodium dodecyl sulfate (SDS) and 0.5% bupivacaine (a local anesthetic with CPE properties), enhanced the flux of the antibiotic ciprofloxacin across the TM (Figure 3A).[36] A single application of a trans-tympanic drug delivery system comprising these CPEs in P407-PBP completely eradicated OM from nontypable Haemophilus influenzae in chinchillas, compared with only 62.5% clearance of infection in animals receiving a single application of ciprofloxacin solution (Figure 3C). (This and other observations confirmed the importance of a) CPEs and b) maintaining close apposition with the TM.) One interesting fact was that although the TM thickened fivefold in OM, antibiotic flux across it also increased fivefold. Ciprofloxacin was undetectable by high performance liquid chromatography in plasma samples from blood, indicating the absence of systemic distribution.[29c] Tissue reaction to the gel and its components was benign. The hydrogel system had degraded completely within three weeks after application.
The system was subsequently shown to be effective against another bacterium commonly found in OM, Streptococcus pneumoniae. This system was able to deliver a ciprofloxacin concentration in middle ear fluid ≈100-fold greater than the minimum inhibitor concentration for S. Pneumoniae, even though that concentration was 100-fold less than that in the hydrogel outside the TM. Further studies found that this hydrogel-CPE formulation could successfully increase delivery of anesthetics, such as bupivacaine and tetrodotoxin, across intact TMs ex vivo. Isobolographic analysis indicated that strong synergistic effects existed among the CPEs (SDS, limonene, and bupivacaine), and that combination of the CPEs could greatly increase the peak drug flux across the TM ex vivo.[37]
3.1.3. Nanocarriers
NPs are drug delivery vehicles < 1 μm in diameter,[38] with a wide range of functions in drug delivery,[39] including acting as local depot systems and crossing biological barriers.[1a] Deformable nanocarriers that have been used in transdermal delivery have also been used in noninvasive trans-tympanic delivery. For example, nanovesicular systems composed of an edge activator (component that softens lipid bilayers) and a surfactant (in spanlastics) or phospholipid (in transfersomes) have been used to cross the TM. Ex vivo permeation studies showed that these formulations caused higher ciprofloxacin permeation through the rabbit TM compared to commercial ciprofloxacin drops (Ciprocin).[40] Their ability to cross skin and the TM has been attributed to their elasticity.[41]
Levofloxacin has been incorporated into nanoliposomes decorated with PEG, and demonstrated a 4.29 fold increase in drug permeation through the rabbit TMs in vivo compared to free levofloxacin. The treated TMs had normal structure without inflammation.[42] Other nanoparticulate approaches to crossing the TM have been described in abstract form,[43] including two types of ionic NPs including Ag2S quantum dots (QD) (≈8 nm, −26 mV surface charge) and Gd2O3 NPs doped with 1% Ne (≈100 nm, +42 mV surface charge). The cationic Gd2O3 NPs showed much higher diffusion across the chinchilla TM ex vivo (5%) than the anionic Ag2S QD (0.05%). The Gd2O3 NPs were then used to deliver dexamethasone and ceftriaxone across the TM ex vivo.
3.1.4. Peptides
Recently, peptides that can transit across the TM in rat OM were identified by phage display.[1b] Those with the greatest ability to cross the TM were found to have shared peptide sequences and structural similarities. The peptides do not appear to have adverse effects on the external ear, TM, middle ear, or inner ear by histology, nor was hearing impaired as assessed by acoustic brain responses. TM-transiting peptides placed on/near the RW showed minimal penetration into the inner ear.[44] Peptides could also cross the uninfected TMs of rats, guinea pigs, rabbits, and humans ex vivo.[45]
3.1.5. Physical Means
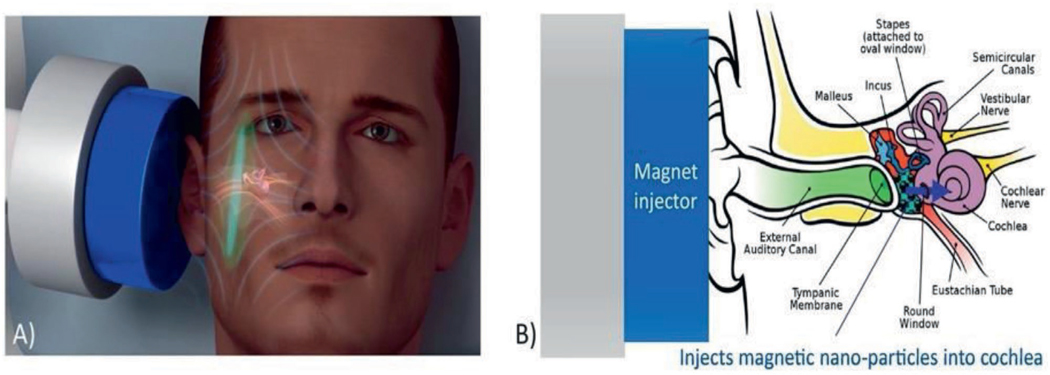
Physical (vs chemical) means have been applied to improve drug flux across the TM.[46] Recently, a hollow ≈15 μm microtube (termed a microshotgun; Figure 4) was prepared, loaded with Fe3O4 NPs and solid dispersion propellant (dry citric acid and sodium bicarbonate). The microshotgun was placed on the surface of the TM (also the RW). Application of water triggered a reaction in the propellant, ejecting the Fe3O4 NPs so that they penetrated the outer epithelial layer of the TM (Figure 4). Subsequently, application of an external magnetic field enhanced the penetration of NPs through the TM. This system improved the flux of Fe3O4 NPs across the TM and RW ex vivo compared to the flux from passive diffusion. Actual drug delivery was not reported.[47]
Figure 4.
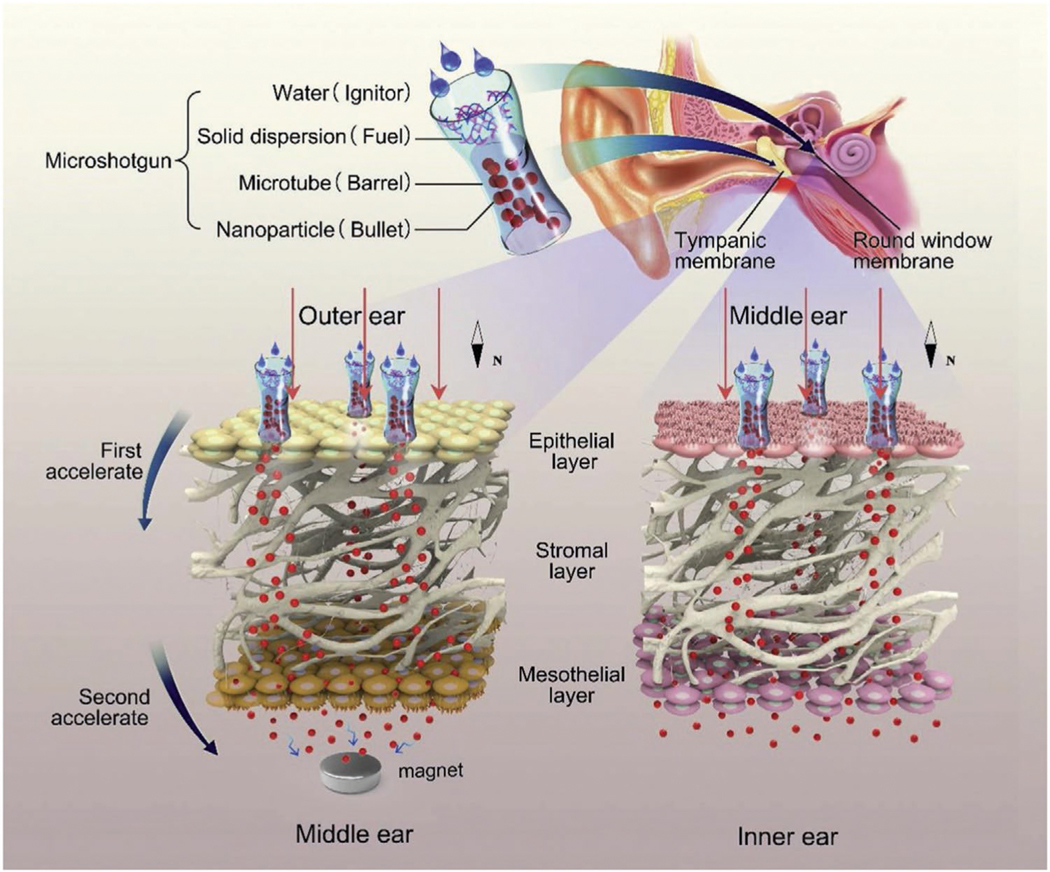
A microshotgun delivery device for the noninvasive trans-tympanic delivery of magnetic NPs. Reproduced with permission.[83] Copyright 2020, Elsevier.
Magnetic fields been also been used to push or pull drugcoated magnetic particles across the TM (the physics of those two distinct modalities has been reviewed elsewhere[16b]). In vivo studies have shown that 100 nm diameter magnetic particles loaded with prednisolone were able to traverse the rat TM by this method, but with highly variable results.[16b]
3.2. Invasive Trans-Tympanic Drug Delivery Systems
Invasive trans-tympanic delivery is comparatively well established, experimentally and clinically. As noted above, Ciprodex drops are applied in the ears of patients with tympanostomy tubes, and there are numerous formulations for administration into the middle ear undergoing clinical trials (Table 1[48,49]). If used for drug delivery to the middle ear, the pharmacokinetics of these systems are straightforward, analogous to simple topical delivery. If placed on the RW to deliver drug into the middle ear, then the pharmacokinetics are analogous to those for non-invasive trans-tympanic delivery, as described above. Excessive volumes placed in the middle ear can affect hearing. Here we describe some systems that have been used for invasive trans-tympanic drug delivery.
Table 1.
Summary of hydrogel-based drug delivery system (applied at RW unless indicated) development pipeline.
| Product/description | Active agent | Polymer | Indication | Status |
|---|---|---|---|---|
| IGF-1 | Insulin-like growth factor | Gelatin | SNHL | Clinical trial |
| OTIPRIOa) | Ciprofloxacin | Poloxamer 407 | Otitis media with effusion | FDA approved |
| OTIVIDEX | Dexamethasone | Poloxamer 407 | Meniere’s Disease | Phase 3 |
| OTO-311 | NMDA receptor | Poloxamer 407 | Tinnitus | Phase 1 |
| OTO-413 | Brain-derived neurotrophic factor | Undisclosed | Hidden hearing loss | Phase 1/2 |
| OTO-510 | Undisclosed otoprotectant | Undisclosed | Cisplatin-induced hearing loss | Pre-clinical |
| Pedmark | Sodium thiosulfate | None | Cisplatin-induced hearing loss | Phase 3 |
| STR-001 | Pioglitazone | Poloxamer 407 | Hearing loss | Phase 3 |
| Sonsuvi | Cell-penetrating peptide | Hyaluronic acid gel | SNHL or otitis media | Phase 3 |
| Keyzilen | NMDA receptor antagonist | Hyaluronic acid gel | Acute inner ear tinnitus | Phase 3 |
SNHL, sensorineural hearing loss; NMDA, N-Methyl-D-Aspartate.
Applied in middle ear.
3.2.1. Hydrogels
The residence time of drugs in the middle ear is normally short because of clearance via the Eustachian tube.[26b,50] Hydrogels have a high viscosity that can reduce the clearance of incorporated drugs via the Eustachian tube, allowing longer residence times in the middle ear,[51] extending duration of effect, and reducing the frequency of injections.[17a]
Otiprio is a P407 hydrogel containing ciprofloxacin to treat OM. A single dose of Otiprio placed in the middle ear of chinchillas resulted in clinical cure of S. pneumoniae OM within 18 h.[48a] P407 has also been used to deliver N-acetylcysteine (NAC), an antioxidant potentially protective against cisplatin-induced hearing loss. Application of the 4% P407-NAC hydrogel to the RW in albino guinea pigs resulted in a fourfold increase of NAC in perilymph over administration of an equally concentrated NAC solution. The perilymph NAC level was 122 times higher than the corresponding plasma level, and NAC was undetectable in the cerebrospinal fluid, suggesting minimal systemic and cerebral exposure.[52] Clinical studies have been carried out using P407 placed on the RW to deliver drugs into the inner ear for a variety of diseases (Table 1).[10a,48b]
Hyaluronic acid is a naturally occurring mucoadhesive and biodegradable polymer that can form viscous gels in aqueous media.[53] Hyaluronic acid gels have shear-thinning properties: their viscosity decreases under shear, for example, during injection, and recovers rapidly after injection.[54] High molecular weight (≈1.5 MDa) hyaluronic acid delivered to the middle ear remains in prolonged contact with the mucosa, prolonging the dwell time of drug after injection.[55] Two hyaluronic acid-based hydrogels are currently in Phase 3 clinical trials.[49] Keyzilen is a small molecule noncompetitive NMDA receptor antagonist in a hyaluronic acid gel, which was developed for the treatment of acute inner ear tinnitus. Sonsuvi contains the drug brimapitide in a hyaluronic acid gel, and was developed to treat sudden hearing loss.
Chitosan is a cationic polymer derived from chitin.[56] The chitosan derivative, chitosan glycerophosphate (CGP) has been used as a hydrogel carrier for drug delivery due to its favorable thermo-responsive gelation properties and its ability to harbor a variety of payloads.[6a,57] In addition, its positive charge promotes electrostatic interactions with negatively charged mucosal surfaces in the middle ear, allowing a prolonged residence time on the RW.[58] The delivery of therapeutic compounds from the CGP hydrogel can be terminated by delivery of exogenous chitosanase, which hydrolyzes the chitosan chains in the gel, resulting in the clearance of drug via the Eustachian tube;[59] such clearance could be helpful in case of impending drugrelated toxicity. The CGP hydrogel has been used to deliver gentamicin and dexamethasone into the inner ear via the RW.[19b,60]
Several other hydrogel-forming polymers have been used for drug delivery to the inner ear via the RW. Application of gelatin containing topical insulin-like growth factor-1 (IGF-1, MW ≈7650 Daltons) in patients with sensorineural hearing loss had therapeutic effects superior to dexamethasone applied the same way, and a favorable safety profile.[61] The thermogelling triblock copolymer PLGA-PEG-PLGA has also been developed for trans-tympanic drug delivery to the inner ear. In vitro, the PLGA-PEG-PLGA copolymer systems showed sustained release of cidofovir (a drug to treat sensorineural hearing loss from cytomegalovirus) for four days. The PLGA-PEG-PLGA copolymer did not show toxicity to the inner ear by ABR testing at the dose tested (0.05 mL).[62] Silk fibroin-PEG hydrogel has been used to deliver micronized dexamethasone to the inner ear of guinea pigs in vivo after deposition on the RW. The dexamethasone concentration in perilymph remained above 100 ng mL−1 (i.e., above the minimal effective concentration, 40 ng mL−1) for at least 10 days after application, compared to less than 12 h after administration of free dexamethasone. No adverse effects were observed in the middle and inner ear histologically, and hearing thresholds returned to pre-operative baseline levels by 14 day after application.[63]
3.2.2. Nanoparticles
After administration into the middle ear, drugs in NPs can reach the inner ear by diffusion of released free drug or by passage of drug-loaded NPs through the RW into the cochlea.[64] The effect of surface modifications of NPs on flux into the inner ear has been explored.[65] Dexamethasone was delivered across the RW in four different phospholipid-based NPs: neutral, anionic, cationic, and cationic-polyethyleneglycol (PEGylated cationic NPs). The PEG was attached to the positively charged NPs to prolong particle circulation in the perilymph by preventing non-specific binding of proteins. Nanoparticle accumulation in HEI-OC1 cells (an organ of Corti cell line) in vitro and in the organ of Corti in C57/BL6 mice was higher after administration of cationic-PEG NPs than with the other formulations. Cationic-PEG NPs loaded with dexamethasone (Cat-PEG-Dex) improved hearing loss at all tested frequencies to a greater degree than did dexamethasone phosphate solution. Quantitative PCR indicated that Cat-PEG-Dex reduced the levels of pro-inflammatory cytokines in the mouse cochlea to a greater degree than did free dexamethasone.[66]
Nanoparticle flux across the RW has been enhanced by conjugating cell penetrating peptides to the nanoparticle surface.[67] In one example, the cell penetrating peptide oligoarginine (Arg8) was conjugated to the surface of a nanoparticle composed of poly(amino acid) (PHEA). Oligoarginine-conjugated NPs entered into HEI-OC1 cells in vitro at higher levels than did unmodified NPs, and oligoarginine-modified NPs showed comparable or better transfection capabilities than the commercially available transfection reagent Lipofectamine. The penetration of oligoarginine-modified NPs across the RWM and transgenic expression of green fluorescent protein (loaded into NPs) was observed in vivo in C57/BL6 mice.[68]
pH-sensitive polymeric NPs have been widely used to transport and release drugs within inflamed tissues.[69] Inflammation contributes to hair cell apoptosis in cisplatin-induced hearing loss, and the pH of inflamed tissues is 0.2–0.6 units lower than that of normal tissues due to the high rate of glycolysis and resulting increase in lactic acid concentration in affected cells. Recently, NPs have been designed that would enhance release of payloads within the inflamed middle ear. The NPs were created by self-assembly of a) a copolymer of a methacrylate derivative of the anti-inflammatory drug ibuprofen and 1-vinylimidazol (which, with a pKa of 6, imparted pH sensitivity), and b) a copolymer of N-vinylpyrrolidone with methacrylate derivatives of either α-tocopheryl succinate or α-tocopherol (ROS scavengers). The NPs were loaded with dexamethasone, and used to treat cisplatin (CDDP)-induced hearing loss. In vitro tests in cell culture indicated that NP formulation reduced CDDP-induced toxicity and intracellular ROS accumulation. In vivo experiments in mice (where NP were deposited into the middle ear by bullostomy) demonstrated reduced hearing loss from CDDP in rats treated with dexamethasone loaded NPs.[70]
NPs injected in the middle ear are readily cleared through the Eustachian tube. Clearance can be slowed by incorporation into hydrogels.[71] Recently, a CGP hydrogel was developed and loaded with NPs containing the c-Jun N-terminal kinase inhibitor, D-JNKi-1, for targeted therapy of noise-induced hearing loss (NIHL). D-JNKi-1 inhibitors are anti-apoptosis agents and can limit hair cell death following an acute insult. The NPs’ surfaces were decorated with a peptide that recognized prestin, a protein exclusively expressed on the membranes of inner ear hair cells. Application of this system to the RW successfully delivered D-JNKi-1 to the hair cells, and improved protection from NIHL in CBA/J mice in vivo compared to untargeted NPs.[72] Poly (lactic-co-glycolic acid) (PLGA) NPs (≈290 nm) containing interferon α−2b (IFN) have been loaded into a CGP hydrogel and deposited on the RW. In guinea pigs, CGP-NPs prolonged the duration of drug exposure in the cochlea 1.5- to 3-fold compared to the durations achieved by an IFN solution, IFN NPs, and an IFN-loaded CGP hydrogel. Histological evaluation showed no adverse effects on inner ear histology.[73] Hyaluronic acid gels have also been used to deliver PEGylated liposomes loaded with dexamethasone phosphate. Application of this gel to the RW achieved release of the drug into the perilymph of guinea pigs for 30 days in vivo, at therapeutic levels.[74]
3.2.3. Magnetically Driven Nanoparticles
Magnetic iron oxide NPs have been used to deliver drugs through the RW. For example, in animals with cisplatin-induced hearing loss, 300 nm drug-loaded magnetic NPs were injected into the middle ear and crossed the RW in a magnetic field (Figure 5).[75] Once in the inner ear, the steroid payload was released, resulting in a greater mitigation of hearing loss than was achieved by deposition of an equivalent dose of free steroid in the middle ear. There was only a mild and transient effect on hearing (assessed by acoustic brain response) from the therapy, and inflammation was mild and limited to the middle ear.[76]
Figure 5.
Magnetically driven nanoparticles. A) The placement of a proposed magnet device for inner drug delivery. B) A schematic of injection of magnetic nanoparticles through the RW for inner ear drug delivery. (A and B) Reproduced with permission.[76] Copyright 2019, Elsevier.
3.2.4. Ultrasound-Driven Devices
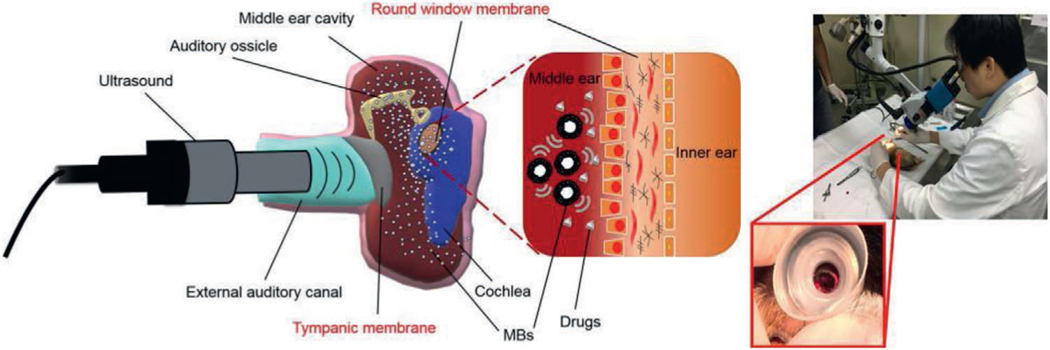
Ultrasound-induced microbubble (USMB) cavitation has been used to facilitate drug delivery across the RW (Figure 6). A solution of drug and microbubbles was injected into the middle ear with a needle, then ultrasound was applied into the saline-filled external auditory canal, driving active agents through the RW and into the inner ear. In guinea pigs, the application of ultrasound induced a 2.8-fold increase in delivery of fluorescently labeled biotin to the inner ear.[77] Application of ultrasound transcranially (vs through the auditory canal) also increased flux across the RW, although to a lesser degree.
Figure 6.
Ultrasound-driven devices. Schematic of ultrasound-induced microbubble (USMB) cavitation to facilitate drug delivery to the inner ear. Reproduced with permission.[77] Copyright 2020, ASCI.
3.2.5. Pump- and Catheter-Based Systems
A number of pump- and catheter-based systems have been developed, primarily for use in animal models where precise control of low infusion rates is desired. In one recent example, a fully implantable peristaltic micropump was fabricated to provide programmable and precise drug delivery via microtubing to the middle ear side of the RW (Figure 7). The micropump was built by 3D-printing on a printed circuit board; electronic components enabled wireless control. The micropump was implanted in the scalp of animals and the microtubing was inserted into the middle ear through a hole drilled through the skull, so that the catheter tip was positioned at the RW. The micropump could achieve precise flow rates of 10–100 nL min−1 in the presence of backpressures up to 10 times larger than what might occur physiologically. This system was able to infuse a known ototoxic compound (sodium salicylate) to the RW in mice at 50 nL min−1 for 20 min. The micropump prototype did not induce inflammation or infection after implantation for one month.[78]
Figure 7.
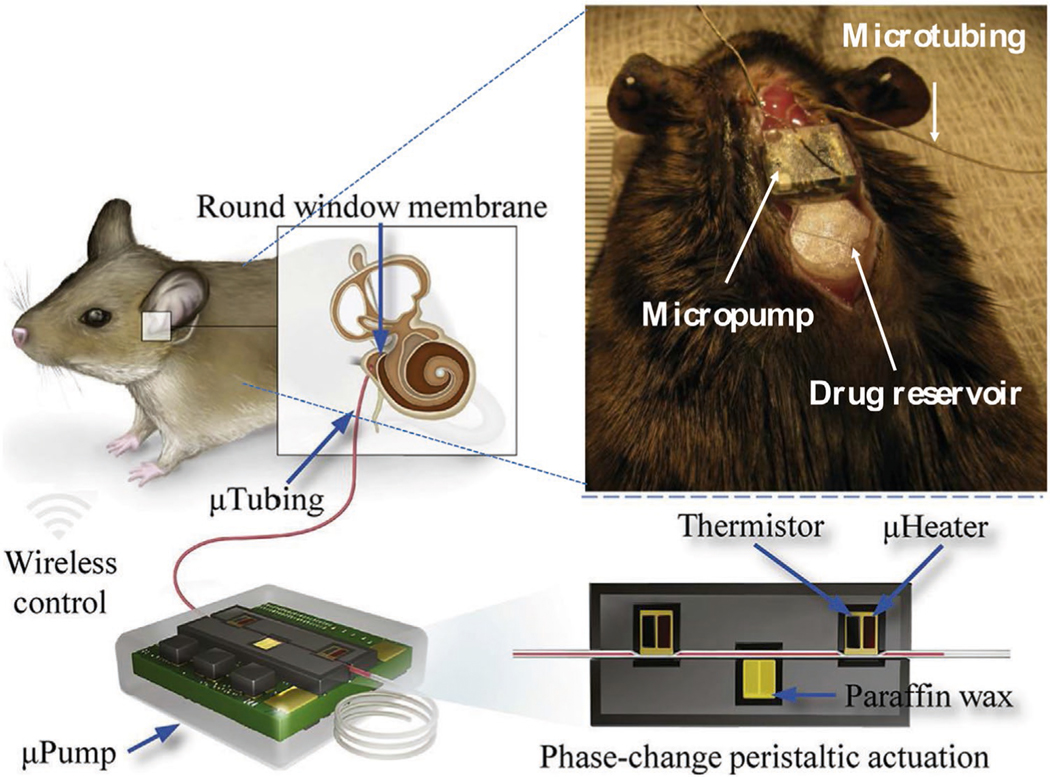
Pump- and catheter-based systems. Schematic illustration of the peristaltic micropump designed for drug delivery across the round window. Reproduced with permission.[78] Copyright 2019, Elsevier.
3.2.6. Other Devices
A variety of relatively macroscopic devices have been developed, and even deployed in humans. Many of these have suffered from technical difficulties with administration, obstruction, and dislocation, or medical problems such as hearing loss, granuloma formation in the middle ear, and permanent TM perforation. The Silverstein MicroWick is an example of such a device, developed to treat Meniere’s related vertigo by perfusion of gentamicin. This device is a polyvinyl acetate wick 1 mm in diameter by 9 mm in length, that is inserted across the TM (e.g., by myringotomy) and placed in contact with the RW. The medication (e.g., gentamicin) is administered in the outer ear; this can be done by patients.
Drug delivery into the middle ear has also been achieved from implantable devices whose primary purpose was not drug delivery itself. Chronic OM may lead to the destruction of the middle ear bones such that prostheses have to be implanted to restore sound transmission; these may become infected. To treat and prevent infections, ciprofloxacin was loaded into a surface coating of nanoporous silica on middle ear prostheses. In a rabbit model, Pseudomonas aeruginosa was inoculated into the middle ear of rabbits to induce OM, and the prostheses loaded with ciprofloxacin were then implanted into the infected middle ears. P. aeruginosa was almost completely eliminated after application of ciprofloxacin-loaded prostheses, but not in untreated animals.[79]
4. Perspective
Although rapid progress has been made in the study of trans-tympanic drug delivery systems in the last decade, there are still many challenges to overcome.
The use of noninvasive trans-tympanic delivery across the TM is still in its infancy. The range of drug delivery systems that have been applied to the RWM have not yet been applied there. The use of such systems could have beneficial effects, including increasing drug flux, or perhaps extending the duration of effect from a single dose. One important consideration for all such systems, however, is that the competition in the context of the most common middle ear disease—OM—is a simple oral formulation. Therefore, even though a local treatment for OM has long been sought for, acceptance of such a treatment is likely to be impacted by ease of application, need for specialized equipment and training, overhead cost, pain, and other factors. Those constraints may be less significant in the treatment of chronic middle ear conditions which might, for example, warrant performing myringotomy to place formulations directly in the middle ear. (These constraints also generally may not apply to inner ear diseases, which are often more severe and intractable.) Validation of the therapeutic efficacy and potential reduction of systemic toxicity of noninvasive systems in humans will be important.
Noninvasive trans-tympanic delivery might only be usable for diseases of the middle ear, due to the steep (100-fold) drop in drug concentration across the TM.[80] Moreover, it is likely that this approach will be restricted to clinical situations where the middle ear is fluid-filled. Using non-invasive methods to cross both the TM and RWM would likely involve two decrements in concentration in series (and require a fluid-filled middle ear), making attainment of therapeutic drug concentrations in the inner ear hard to achieve.
As we have seen, a broad range of methods for invasive trans-tympanic drug delivery to the inner ear have been attempted, with therapeutic effect shown in animals. Here, as in noninvasive approaches, technical refinement will be important, including combination of drug delivery systems. One common issue for such approaches is going to be the fate of drugs and NPs once they enter the perilymph, for example. whether they can successfully diffuse throughout the inner ear and into tissues. Here also, assessment in humans will be important.
Although it is beyond the scope of this update, it bears mentioning that a variety of approaches have been used to target intravenously-administered drugs and NPs to specific anatomical locations such as tumors, the brain, the eye, and the heart.[81] In many of those, there is a blood-tissue barrier as there is in the ear, which can be disrupted by disease or by an external stimulus such as heat or ultrasound. However, such approaches require a relatively tissue-specific ligand and/or for the stimulus to be able to reach the target tissue. The latter would be limited by the fact that the inner ear is encased in bone.
In all cases, the biocompatibility of the system will be of great importance.[82] All drug delivery systems have the potential to cause inflammation, wherever they are placed. The drugs within could also have adverse tissue effects, particularly if they are present at high concentration for extended periods. It will be important to study the fate of drug delivery systems, especially if composed of non-degradable materials. Studying the impact of formulations on hearing (whether by blocking sound conduction or cochlear ototoxicity) will also be important.
Acknowledgements
The authors would like to thank Prof. Rong Yang for contributing schematic art. This work was funded by NIH grants DC015050 and DC015557 to D.S.K.
Biography

Zipei Zhang obtained his Ph.D. in Food Science from the University of Massachusetts, Amherst in 2019. He is currently a postdoctoral researcher in the Laboratory for Biomaterials and Drug Delivery at Boston Children’s Hospital at Harvard Medical School. His current research focuses on the design and fabrication of novel drug delivery systems to treat otitis media. His research interests also include colloid and interfacial science and gastrointestinal digestion.

Daniel S. Kohane obtained his M.D. and Ph.D. in Physiology from Boston University. He is a Professor of Anaesthesia at Harvard Medical School and a Senior Associate in Pediatric Critical Care in the Department of Anesthesiology at Boston Children’s Hospital, where he directs the Laboratory for Biomaterials and Drug Delivery and is the Vice Chair for Research. His research focuses on drug delivery, biomaterials, and nanomedicine.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].a) Yang R, Wei T, Goldberg H, Wang W, Cullion K, Kohane DS, Adv. Mater. 2017, 29, 1606596; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kurabi A, Pak KK, Bernhardt M, Baird A, Ryan AF, Sci. Rep. 2016, 6, 22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Hoberman A, Paradise JL, Rockette HE, Shaikh N, Wald ER, Kearney DH, Colborn DK, Kurs-Lasky M, Bhatnagar S, Haralam MA, Cunha BA, Med. Clin. North Am. 2001, 85, 149;11190350 [Google Scholar]; c) Cox LM, Blaser MJ, Nat. Rev. Endocrinol. 2015, 11, 182; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) McLane J, J. Am. Acad. Dermatol. 2001, 45, S188; [DOI] [PubMed] [Google Scholar]; e) Çetinkaya A, Akova YA, Am. J. Ophthalmol. 2006, 142, 816. [DOI] [PubMed] [Google Scholar]
- [3].a) Zielnik-Jurkiewicz B, Bielicka A, Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2129; [DOI] [PubMed] [Google Scholar]; b) Anderson RM, Nat. Med. 1999, 5, 147; [DOI] [PubMed] [Google Scholar]; c) Barkai G, Greenberg D, Givon-Lavi N, Dreifuss E, Vardy D, Dagan R, Emerging Infect. Dis. 2005, 11, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Wang X, Fernandez R, Tsivkovskaia N, Harrop-Jones A, Hou HJ, Dellamary L, Dolan DF, Altschuler RA, LeBel C, Piu F, Otol. Neurotol. 2014, 35, 459; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Spektor Z, Jasek MC, Jasheway D, Dahlin DC, Kay DJ, Mitchell R, Faulkner R, Wall GM, Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 97. [DOI] [PubMed] [Google Scholar]
- [5].a) Juhn SK, Acta Oto-Laryngol. 1988, 105, 79; [Google Scholar]; b) Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A, Sci. Transl. Med. 2019, 11, eaao0935; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Juhn SK, Hunter BA, Odland RM, Int. Tinnitus J. 2001, 7, 72. [PubMed] [Google Scholar]
- [6].a) Rathnam C, Chueng S-TD, Ying Y-LM, Lee K-B, Kwan KY, Front. Cell. Neurosci. 2019, 13, 493; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Volandri G, Di Puccio F, Forte P, Carmignani C, J. Biomech. 2011, 44, 1219. [DOI] [PubMed] [Google Scholar]
- [7].a) Anthwal N, Thompson H, J. Anat. 2016, 228, 217; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Musiek FE, Baran JA, The Auditory System: Anatomy, Physiology, and Clinical Correlates, Plural Publishing, San Diego, CA: 2018; [Google Scholar]; c) Tarabichi M, Marchioni D, Presutti L, Nogueira JF, Pothier D, Otolaryngol. Clin. North Am. 2013, 46, 131; [DOI] [PubMed] [Google Scholar]; d) Qing Z, Mao-Li D, J. Otol. 2009, 4, 7; [Google Scholar]; e) Tavolga WN, Popper AN, Fay RR, Hearing and Sound Communication in Fishes, Springer Science & Business Media, Berlin: 2012. [Google Scholar]
- [8].Lambert E, Roy S, Pediatr. Clin. North Am. 2013, 60, 809. [DOI] [PubMed] [Google Scholar]
- [9].a) Van der Jeught S, Dirckx JJJ, Aerts JRM, Bradu A, Podoleanu AG, Buytaert JAN, J. Assoc. Res. Otolaryngol. 2013, 14, 483; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Trakimas DR, Ishai R, Ghanad I, Black NL, Kozin ED, Cheng JT, Remenschneider AK, Laryngoscope 2018, 128, E351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Liu X, Li M, Smyth H, Zhang F, Drug Dev. Ind. Pharm. 2018, 44, 1395; [DOI] [PubMed] [Google Scholar]; b) Kuypers LC, Decraemer WF, Dirckx JJJ, Otol. Neurotol. 2006, 27, 256; [DOI] [PubMed] [Google Scholar]; c) Doyle WJ, Alper CM, Seroky JT, Karnavas WJ, Acta Oto-Laryngol. 1998, 118, 567. [DOI] [PubMed] [Google Scholar]
- [11].a) Hao J, Li SK, Eur. J. Pharm. Sci 2019, 126, 82; [DOI] [PubMed] [Google Scholar]; b) Decraemer WF, Funnell WRJ, in Chronic Otitis Media. Pathogenesis-Oriented Therapeutic Management, (Ed: Ars B), Kugler Publications, Amsterdam, Netherlands, 2008, p. 51; [Google Scholar]; c) Aernouts J, Couckuyt I, Crombecq K, Dirckx JJJ, Int. J. Eng. Sci. 2010, 48, 599. [Google Scholar]
- [12].a) Li L, Chao T, Brant J, O’Malley B Jr., Tsourkas A, Li D, Adv. Drug Delivery Rev. 2017, 108, 2; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Voss SE, Rosowski JJ, Merchant SN, Peake WT, Hear. Res. 2000, 150, 43; [DOI] [PubMed] [Google Scholar]; c) Gan RZ, Feng B, Sun Q, Ann. Biomed. Eng. 2004, 32, 847; [DOI] [PubMed] [Google Scholar]; d) Thompson H, Tucker AS, Science 2013, 339, 1453. [DOI] [PubMed] [Google Scholar]
- [13].a) Thomson S, Madani G, Clin. Radiol. 2014, 69, e146; [DOI] [PubMed] [Google Scholar]; b) Goelzer B, Hansen CH, Sehrndt G, Occupational Exposure to Noise: Evaluation, Prevention and Control, World Health Organisation, Geneva, Switzerland: 2001. [Google Scholar]
- [14].Seibert JW, Danner CJ, Otolaryngol. Clin. North Am. 2006, 39, 1221. [DOI] [PubMed] [Google Scholar]
- [15].a) Zou J, Poe D, Ramadan UA, Pyykkö I, Ann. Otol., Rhinol., Laryngol 2012, 121, 119; [DOI] [PubMed] [Google Scholar]; b) Zou J, Sood R, Ranjan S, Poe D, Ramadan UA, Pyykkö I, Kinnunen PKJ, Otol. Neurotol. 2012, 33, 666. [DOI] [PubMed] [Google Scholar]
- [16].a) Salt AN, Hirose K, Hear. Res. 2018, 362, 25; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shapiro B, Kulkarni S, Nacev A, Sarwar A, Preciado D, Depireux DA, Annu. Rev. Biomed. Eng. 2014, 16, 455. [DOI] [PubMed] [Google Scholar]
- [17].a) El Kechai N, Agnely F, Mamelle E, Nguyen Y, Ferrary E, Bochot A, Int. J. Pharm. 2015, 494, 83; [DOI] [PubMed] [Google Scholar]; b) Marchioni D, Molteni G, Presutti L, Indian J. Otolaryngol 2011, 63, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Okuno H, Sando I, Acta Oto-Laryngol. 1988, 106, 55; [DOI] [PubMed] [Google Scholar]; b) Staecker H, Rodgers B, Expert Opin. Drug Delivery 2013, 10, 639. [DOI] [PubMed] [Google Scholar]
- [19].a) Arnold W, Senn P, Hennig M, Michaelis C, Deingruber K, Scheler R, Steinhoff H-J, Riphagen F, Lamm K, Audiol. Neurotol. 2005, 10, 53; [DOI] [PubMed] [Google Scholar]; b) Xu L, Heldrich J, Wang H, Yamashita T, Miyamoto S, Li A, Uboh CE, You Y, Bigelow D, Ruckenstein M, Otol. Neurotol. 2010, 31, 1115. [DOI] [PubMed] [Google Scholar]
- [20].a) Salt AN, Volta Rev. 2005, 105, 277; [PMC free article] [PubMed] [Google Scholar]; b) Saber A, Strand SP, Ulfendahl M, Eur. J. Pharm. Sci 2010, 39, 110; [DOI] [PubMed] [Google Scholar]; c) Borden RC, Saunders JE, Berryhill WE, Krempl GA, Thompson DM, Queimado L, Audiol. Neurotol. 2011, 16, 1; [DOI] [PubMed] [Google Scholar]; d) Wang X, Dellamary L, Fernandez R, Harrop A, Keithley EM, Harris JP, Ye Q, Lichter J, LeBel C, Piu F, Audiol. Neurotol. 2009, 14, 393; [DOI] [PubMed] [Google Scholar]; e) Buckiová D, Ranjan S, Newman TA, Johnston AH, Sood R, Kinnunen PKJ, Popelář J, Chumak T, Syka J, Nanomedicine 2012, 7, 1339. [DOI] [PubMed] [Google Scholar]
- [21].Duan M.-l., Zhi-Qiang C, J. Otol. 2009, 4, 34. [Google Scholar]
- [22].a) Merchant SN, Nadol JB Schuknecht’s Pathology of the Ear, PMPH, USA: 2010; [Google Scholar]; b) Erixon E, Högstorp H, Wadin K, Rask-Andersen H, Otol. Neurotol. 2009, 30, 14; [DOI] [PubMed] [Google Scholar]; c) Dallos P, Fay RR, The Cochlea, Springer Science & Business Media, Berlin: 2012. [Google Scholar]
- [23].a) Patuzzi R, Hear. Res. 2011, 277, 4; [DOI] [PubMed] [Google Scholar]; b) Eckhard A, Gleiser C, Arnold H, Rask-Andersen H, Kumagami H, Müller M, Hirt B, Löwenheim H, Mol. Aspects Med. 2012, 33, 612. [DOI] [PubMed] [Google Scholar]
- [24].a) Borkholder DA, Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 472; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ, Otol. Neurotol. 2007, 28, 1124. [DOI] [PubMed] [Google Scholar]
- [25].Barry BW, Eur. J. Pharm. Sci 2001, 14, 101. [DOI] [PubMed] [Google Scholar]
- [26].a) Goycoolea MV, Acta Oto-Laryngol., Suppl 1992, 493, 43; [PubMed] [Google Scholar]; b) Wang X, Dellamary L, Fernandez R, Ye Q, LeBel C, Piu F,Laryngoscope 2011, 121, 385; [DOI] [PubMed] [Google Scholar]; c) Goycoolea MV, Lundman L, Microsc. Res. Tech 1997, 36, 201; [DOI] [PubMed] [Google Scholar]; d) Zou J, Pyykkö I, Hyttinen J, J. Otol 2016, 11, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lane ME, Int. J. Pharm 2013, 447, 12. [DOI] [PubMed] [Google Scholar]
- [28].a) Hoskison E, Daniel M, Al-Zahid S, Shakesheff KM, Bayston R, Birchall JP, Ther. Delivery 2013, 4, 115; [DOI] [PubMed] [Google Scholar]; b) Mikulec AA, Hartsock JJ, Salt AN, Otol. Neurotol 2008, 29, 1020; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Khoo X, Simons EJ, Chiang HH, Hickey JM, Sabharwal V, Pelton SI, Rosowski JJ, Langer R, Kohane DS, Biomaterials 2013, 34, 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].a) Nordang L, Linder B, Anniko M, Otol. Neurotol 2003, 24, 339; [DOI] [PubMed] [Google Scholar]; b) Banerjee A, Parnes LS, Otolaryngol. Clin. North Am 2004, 37, 1035; [DOI] [PubMed] [Google Scholar]; c) Yang R, Sabharwal V, Okonkwo OS, Shlykova N, Tong R, Lin LY, Wang W, Guo S, Rosowski JJ, Pelton SI, Sci. Transl. Med 2016, 8, 356ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chandrasekhar SS, Rubinstein RY, Kwartler JA, Gatz M, Connelly PE, Huang E, Baredes S, Otolaryngol. Head Neck Surg 2000, 122, 521. [DOI] [PubMed] [Google Scholar]
- [31].Sabharwal YRV, Kohane DS, Pelton SI, presented at Int. Symp. on Recent Advances in Otitis Media, Los Angeles, CA, June 2019. [Google Scholar]
- [32].Dumortier G, Grossiord JL, Agnely F, Chaumeil JC, Pharm. Res 2006, 23, 2709. [DOI] [PubMed] [Google Scholar]
- [33].a) Zarrintaj P, Ramsey JD, Samadi A, Atoufi Z, Yazdi MK, Ganjali MR, Amirabad LM, Zangene E, Farokhi M, Formela K, Acta Biomater. 2020, 110, 37; [DOI] [PubMed] [Google Scholar]; b) Moghimi SM, Hunter AC, Trends Biotechnol. 2000, 18, 412. [DOI] [PubMed] [Google Scholar]
- [34].Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S, Proc. Natl. Acad. Sci. USA 2005, 102, 4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].a) Sloan KB, Wasdo SC, Rautio J, Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S, Subramony JA, Sharma A, Drug Delivery 2005, 102, 4688; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pilgram GSK, Van der Meulen J, Gooris GS, Koerten HK, Bouwstra JA, Biochim. Biophys. Acta Biomembr 2001, 1511, 244; [DOI] [PubMed] [Google Scholar]; c) Cornwell PA, Barry BW, Bouwstra JA, Gooris GS, Int. J. Pharm 1996, 127, 9. [Google Scholar]
- [36].Yang R, Saarinen R, Okonkwo OS, Hao Y, Mehta M, Kohane DS, Mol. Pharmaceutics 2019, 16, 1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang R, Okonkwo OS, Zurakowski D, Kohane DS, J. Controlled Release 2018, 289, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].De Jong WH, Borm PJA, Int. J. Nanomed 2008, 3, 133. [Google Scholar]
- [39].Valente F, Astolfi L, Simoni E, Danti S, Franceschini V, Chicca M, Martini A, J. Drug Delivery Sci. Technol 2017, 39, 28. [Google Scholar]
- [40].Al-Mahallawi AM, Khowessah OM, Shoukri RA, Int. J. Pharm 2017, 522, 157. [DOI] [PubMed] [Google Scholar]
- [41].a) El Zaafarany GM, Awad GAS, Holayel SM, Mortada ND, Int. J. Pharm 2010, 397, 164; [DOI] [PubMed] [Google Scholar]; b) Muzzalupo R, Tavano L, Res. Rep. Transdermal Drug Delivery 2015, 4, 23. [Google Scholar]
- [42].Abdelbary AA, Abd-Elsalam WH, Al-Mahallawi AM, Int. J. Pharm 2019, 559, 201. [DOI] [PubMed] [Google Scholar]
- [43].Sharma PAKG, Khampang P, Hong W, Kerschner J, Joshi A, presented at Int. Symp. on Recent Advances in Otitis Media, Los Angeles, CA, June 2019. [Google Scholar]
- [44].Kurabi A, Beasley KA, Chang L, McCann J, Pak K, Ryan AF, PLoS One 2017, 12, e0172158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kurabi A, Schaerer D, Noack V, Bernhardt M, Pak K, Alexander T, Husseman J, Nguyen Q, Harris JP, Ryan AF, Sci. Rep 2018, 8, 11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nguyen K, Kempfle JS, Jung DH, McKenna CE, Expert Opin. Ther. Pat 2017, 27, 191. [DOI] [PubMed] [Google Scholar]
- [47].Liang Z, Yu H, Lai J, Wen L, Chen G. J. J. o. C. R., J. Controlled Release 2020, 321, 119. [DOI] [PubMed] [Google Scholar]
- [48].a) Edmunds AL, Pharm. Ther 2017, 42, 307; [Google Scholar]; b) Otonomy, https://www.otonomy.com/press/otonomy-initiates-phase-1-clinical-trial-fortinnitus-product-candidate-oto-311/, accessed: May 2020;; c) Otonomy, https://www.otonomy.com/pipeline/, accessed: April 2020;; d) Fennecpharma, https://fennecpharma.com/product-candidates/pedmark/clinical-trials/, accessed: May 2020;; e) Aurismedical, https://aurismedical.com/product-candidates/am-111, accessed: May 2020.
- [49].Staecker H, Jokovic G, Karpishchenko S, Kienle-Gogolok A, Krzyzaniak A, Lin C-D, Navratil P, Tzvetkov V, Wright N, Meyer T, Otol. Neurotol 2019, 40, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].a) Nakagawa T, Ito J, Acta Oto-Laryngol. 2007, 127, 30; [DOI] [PubMed] [Google Scholar]; b) Young S, Wong M, Tabata Y, Mikos AG, J. Controlled Release 2005,109, 256. [DOI] [PubMed] [Google Scholar]
- [51].McCall AA, Swan EEL, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ, Ear Hear. 2010, 31, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gausterer JC, Saidov N, Ahmadi N, Zhu C, Wirth M, Reznicek G, Arnoldner C, Gabor F, Honeder C, Eur. J. Pharm. Biopharm 2020, 150, 143. [DOI] [PubMed] [Google Scholar]
- [53].a) Vasvani S, Kulkarni P, Rawtani D, Int. J. Biol. Macromol 2020, 151, 1012; [DOI] [PubMed] [Google Scholar]; b) Burdick JA, Prestwich GD, Adv. Mater 2011, 23, H41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].El Kechai N, Bochot A, Huang N, Nguyen Y, Ferrary E, Agnely F, Int. J. Pharm 2015, 487, 187. [DOI] [PubMed] [Google Scholar]
- [55].Girish KS, Kemparaju K, Life Sci. 2007, 80, 1921. [DOI] [PubMed] [Google Scholar]
- [56].Rinaudo M, Prog. Polym. Sci 2006, 31, 603. [Google Scholar]
- [57].Jyoti K, Malik G, Chaudhary M, Sharma M, Goswami M, Katare OP, Singh SB, Madan J, Int. J. Biol. Macromol 2020, 161, 325. [DOI] [PubMed] [Google Scholar]
- [58].Sreenivas SA, Pai KV, Trop. J. Pharm. Res 2008, 7, 1077. [Google Scholar]
- [59].Lajud SA, Han Z, Chi F-L, Gu R, Nagda DA, Bezpalko O, Sanyal S, Bur A, Han Z, O’Malley BW Jr., J. Controlled Release 2013, 166, 268. [DOI] [PubMed] [Google Scholar]
- [60].a) Paulson DP, Abuzeid W, Jiang H, Oe T, O’Malley BW, Li D, Laryngoscope 2008, 118, 706; [DOI] [PubMed] [Google Scholar]; b) Luo J, Xu L, Ann. Otol., Rhinol., Laryngol 2012, 121, 208. [DOI] [PubMed] [Google Scholar]
- [61].Nakagawa T, Kumakawa K, Usami S.-i., Hato N, Tabuchi K, Takahashi M, Fujiwara K, Sasaki A, Komune S, Sakamoto T, BMC Med. 2014, 12, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].a) Liu H, Feng L, Tolia G, Liddell MR, Hao J, Kevin Li S, Drug Dev. Ind. Pharm 2014, 40, 896; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Feng L, A Ward J, Li SK, Tolia G, Hao J, Choo DI, Curr. Drug Delivery 2014, 11, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yu D, Sun C, Zheng Z, Wang X, Chen D, Wu H, Wang X, Shi F, Int. J. Pharm 2016, 503, 229. [DOI] [PubMed] [Google Scholar]
- [64].Mäder K, Lehner E, Liebau A, Plontke SK, Hear. Res 2018, 368, 49. [DOI] [PubMed] [Google Scholar]
- [65].Praetorius M, Brunner C, Lehnert B, Klingmann C, Schmidt H, Staecker H, Schick B, Acta Oto-Laryngol. 2007, 127, 486. [DOI] [PubMed] [Google Scholar]
- [66].Yang K-J, Son J, Jung SY, Yi G, Yoo J, Kim D-K, Koo H, Biomaterials 2018, 171, 133. [DOI] [PubMed] [Google Scholar]
- [67].Roy S, Johnston AH, Newman TA, Glueckert R, Dudas J, Bitsche M, Corbacella E, Rieger G, Martini A, Schrott-Fischer A, Int. J. Pharm 2010, 390, 214. [DOI] [PubMed] [Google Scholar]
- [68].Yoon JY, Yang K-J, Lee K-Y, Park S-N, Kim D-K, Kim J-D, Biomaterials 2015, 73, 243. [DOI] [PubMed] [Google Scholar]
- [69].a) Yu Z, Ma L, Ye S, Li G, Zhang M, Carbohydr. Polym 2020, 236, 115972; [DOI] [PubMed] [Google Scholar]; b) Pu H-L, Chiang W-L, Maiti B, Liao Z-X, Ho Y-C, Shim MS, Chuang E-Y, Xia Y, Sung H-W, ACS Nano 2014, 8, 1213; [DOI] [PubMed] [Google Scholar]; c) Li C, Li H, Wang Q, Zhou M, Li M, Gong T, Zhang Z, Sun X, J. Controlled Release 2017, 246, 133. [DOI] [PubMed] [Google Scholar]
- [70].Martín-Saldaña S, Palao-Suay R, Aguilar MR, García-Fernández L, Arévalo H, Trinidad A, Ramírez-Camacho R, San Román J, J. Controlled Release 2018, 270, 53. [DOI] [PubMed] [Google Scholar]
- [71].Mittal R, Pena SA, Zhu A, Eshraghi N, Fesharaki A, Horesh EJ, Mittal J, Eshraghi AA, Artif. Cells, Nanomed., Biotechnol 2019, 47, 1312. [DOI] [PubMed] [Google Scholar]
- [72].Kayyali MN, Wooltorton JRA, Ramsey AJ, Lin M, Chao TN, Tsourkas A, O’Malley BW Jr., Li D, J. Controlled Release 2018, 279, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dai J, Long W, Liang Z, Wen L, Yang F, Chen G, Drug Dev. Ind. Pharm 2018, 44, 89. [DOI] [PubMed] [Google Scholar]
- [74].El Kechai N, Mamelle E, Nguyen Y, Huang N, Nicolas V, Chaminade P, Yen-Nicolaÿ S, Gueutin C, Granger B, Ferrary E, J. Controlled Release 2016, 226, 248. [DOI] [PubMed] [Google Scholar]
- [75].Ramaswamy B, Roy S, Apolo AB, Shapiro B, Depireux DA, Front. Cell. Neurosci 2017, 11, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shimoji M, Ramaswamy B, Shukoor MI, Benhal P, Broda A, Kulkarni S, Malik P, McCaffrey B, Lafond JF, Nacev A, Eur. J. Pharm. Sci 2019, 126, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liao A-H, Wang C-H, Weng P-Y, Lin Y-C, Wang H, Chen H-K, Liu H-L, Chuang H-C, Shih C-P, JCI Insight 2020, 5, e132880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Forouzandeh F, Zhu X, Alfadhel A, Ding B, Walton JP, Cormier D, Frisina RD, Borkholder DA, J. Controlled Release 2019, 298, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lensing R, Bleich A, Smoczek A, Glage S, Ehlert N, Luessenhop T, Behrens P, Müller PP, Kietzmann M, Stieve M, Acta Biomater. 2013, 9, 4815. [DOI] [PubMed] [Google Scholar]
- [80].Yang R, Sabharwal V, Shlykova N, Okonkwo OS, Pelton SI, Kohane DS, JCI Insight 2018, 3, e123415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].a) Wang Y, Kohane DS, Nat. Rev. Mater 2017, 2, 17020; [Google Scholar]; b) Wang Y, Liu C-H, Ji T, Mehta M, Wang W, Marino E, Chen J,Kohane DS, Nat. Commun 2019, 10, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kohane DS, Langer R, Chem. Sci 2010, 1, 441. [Google Scholar]
- [83].Liang Z, Yu H, Lai J, Wen L, Chen G, J. Controlled Release 2020, 321, 119. [DOI] [PubMed] [Google Scholar]