Abstract
Many aspects of the SARS-CoV-2 virus remain poorly understood, including its rapid mutation and its effects on populations of different ages. The present literature of review is focused on the effectiveness of current available vaccines in view of immerging several SARS-CoV-2 variants. The most dangerous and infectious SARS-CoV-2 strain, B117, was recently discovered in the United Kingdom, and another new variant, 501.V2, was discovered in South Africa. In countries such as the United States, Japan, India, and Brazil, the variant B117 spread far more quickly than the original strain. The new SARS-CoV-2 mutations have made producing a universal and effective vaccine more difficult. SARS-CoV-2’s S protein, which aids in receptor identification and membrane fusion, is a primary target for vaccine development using its mRNA or inactivated virus. Currently, in the interval of few days new more infectious SARS-CoV-2 mutant is detected, started from SARS-CoV-2 Alpha (B.1.1.7), beta (B.1.351), delta (B.1.617.2), delta plus, gamma (P.1) and now variant lamda. The variant detected first in Peru and spread almost 27 countries including UK that accounts for 82% of new infections. These mutant variants are posing new challenge even to the fully vaccinated individuals and a challenge for the public health. Thus, a need to review current treatment vaccination guideline and strategy as early as possible. Reporting all new SARS-CoV-2 variants and their effectiveness in response to several available vaccines, we would like to draw the attention of health care provider, and all developed countries health care agencies including WHO to frame new guidelines for vaccination and immediate intervention to control the development of new SARS-CoV-2 variants from the third world countries by providing vaccines to the poor countries as early as possible.
Keywords: B117, COVID-19, mutations, SARS-CoV-2, therapies, Vaccines
Introduction
In December 2019, SARS-CoV-2 emerged in Wuhan, China 1 and spread to various nations around the world (Figure 1). A new SARS-CoV-2 strain, B117, was first observed in southeastern England in September 2020. It forced another round of tight lockdowns as patients overwhelmed hospitals in these regions and has since spread to the United States, France, Brazil, Canada, and India (Figure 2). Experts are concerned that mutation will hinder ongoing immunization efforts. As a result of the mutant SARS-CoV-2 strain discovered in the United Kingdom, more than 80 countries closed their borders to the island nation. Travelers from that country will be required to show a negative COVID-19 test before boarding flights to most of the countries in the World. Worldwide, nearly 4,031,709 people have died in the pandemic. With the emergence of this new strain, the number of cases among children has risen; however, there is no conclusive evidence that the novel SARS-CoV-2 variant has a unique propensity to infect or cause sickness in younger patients.
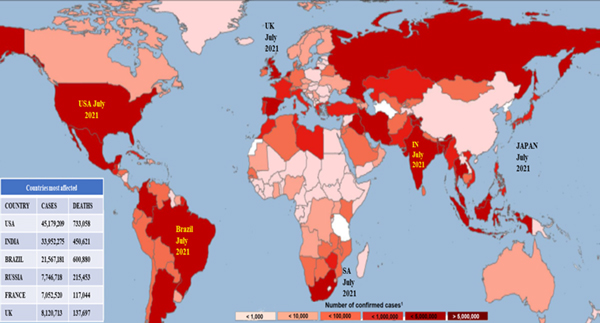
Figure 1.
SARS-Cov2 mediated COVID-19 infection across the globe as of July 31st, 2021
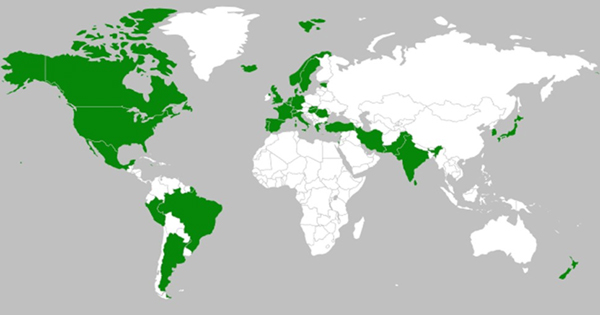
Figure 2.
SARS-Cov2 (B117) variant infections spread in various countries of the globe.
The new SARS-CoV-2 strain includes significant gene alterations in its spike (S) protein that make it easier for the virus to spread from person to person: The B117 mutant strain is approximately 50% more contagious than earlier variants. Scientists are concerned that this new strain could result in a significant increase in the number of cases and deaths. These surges will occur at a time when many hospitals are already overburdened, causing fatality rates to skyrocket as patients who may otherwise have survived succumb to a lack of resources such as staff, equipment, and beds. According to the US CDC’s models, the new B117 strain of SARS-CoV-2 in the UK and 501.V2 in South Africa may resurface in late April any viral infection. Because DCs are prevalent in the respiratory tract and respond to inflammation, they have been identified as a potential candidate in antigen presentation during SARS infection and in understanding the immunopathology of SARS 11.
To cause coronavirus infection, innate immune cells produce an effective antiviral response by viral infection receptors known as pattern recognition receptors (PRRs), which recognize pathogen-associated molecular patterns (PAMPs). C-type lectin-like receptors, Toll-like receptors (TLR), NOD-like receptors (NLR), RIG-I-like receptors (RLR), and cytoplasmic free-molecule receptors such as cGAS-STING, IFI16, and others are examples of PRRs 12. Endosomal RNA receptors TLR3 and TLR7, as well as the cytosolic RNA sensor RIG-I/MDA5, identify RNA viruses like coronavirus, viral genomic RNA, or replication intermediates like double-stranded RNA (dsRNA). This identification activates many signaling pathways, causing inflammation and the development of a cellular immune response. Interferon regulatory factor 3 (IRF3), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and AP-1 are all transcription factors activated by these pathways. As a result, these factors encourage the synthesis of interferons of type I (IFN-I), inflammatory cytokines, and chemokines13. In SARS patients, a cytokine storm is known to occur because of an immune system overreaction. The use of recombinant IFN-I and IFN-II as antiviral cytokines that limit viral replication in targeted cells is one treatment for this condition. Cyclosporine-targeting medicines like cyclosporine A and alisporivir are two further options for controlling host immune response in COVID infection. Through cyclophilin A and cyclophilin B, the CD147 receptor modulates cytokine production and chemotaxis in inflammatory cells 14. B cells, with the help of T cells, develop into plasma cells after being influenced by virus immune responses to mediate antibody production. Plasma cells subsequently create antibodies specific to a viral antigen. Neutralizing antibodies are effective in inhibiting the virus fromentering host cells and limiting infection, and they play antibodies specific to a viral antigen. Neutralizing antibodies are effective in inhibiting the virus from entering host cells and limiting infection, and they playa critical protective role later in infection, preventing relapse. Infected cells, on the other hand, show a cellular immune response, which is mediated by T-lymphocytes. Helper T cells oversee the entire adaptive immune response, while cytotoxic T cells are essential for the clearing and cleansing of virally infected cells. Lung epithelial cells, alveolar macrophages, and neutrophils initiate the innate immune response. Adaptive immune responses involving T and B cells are triggered in the next stage to complete the immune response. When it comes to viral infections, the innate immune system is the first line of defense. When a virus infects a cell, it produces and secretes interferon (IFN) molecules. IFN-α and IFN-β operate as signaling molecules, activating an antiviral response in surrounding cells and rendering them immune to infection 15. SARS coronavirus (SARS-CoV), which is comparable to SARS-CoV-2, has been shown to decrease antiviral type I interferon induction 16.
Beyond ACE 2, integrins as potential receptors for SARS-CoV-2 entrance
Integrins are a group of cell surface receptors that are created by the non-covalent connection of type I transmembrane glycoproteins, the α and the β subunit. There are currently 18-α subunits and 8-β subunits recognized, which are found in slightly over 20 integrins. Integrins are found all over the body, and each nucleated cell has its own integrin signature. Integrins may play an inhibitory function in SARS-CoV-2 and SARS-CoV entrance, according to present studies. Integrins are a heterodimeric family of cell surface receptors that connect extracellular matrix proteins to the cytoskeleton within the cell. They have a key role in gene expression, cell proliferation, differentiation, migration, and apoptosis regulation. Activated myofibroblasts form specialized focal adhesions with high amounts of integrins 5, 1, and 3. The angiotensin-converting enzymes and integrin 1 (ITGB1) cross the plasma membrane, and the angiotensin-converting enzymes are also found in soluble form in the plasma when they are shed from the cell membrane. There are three ways for viruses to connect with integrins on the cell surface. In the first scenario, the virion functions as an antagonist by binding without internalization or by initiating integrin-mediated pathways. The second paradigm involves binding that does not result in internalization but does result in the activation of integrin-mediated pathways. In this case, the virion takes on the role of agonist. Finally, internalization of the virus can lead to infection and viral replication. 17
Cytokines induced inflammation in Covid-19.
In SARS-CoV infection, immune effector cells release significant amounts of pro-inflammatory cytokines such as IFN-I, IFN-II, IL-1, IL-6, IL-12, IL-18, IL-33, TNF-α, TGF, and chemokines such as CCL2, CCL3, CCL5, CXCL8, CXCL9, and CXCL10, culminating in a lethal uncontrolled inflammatory response18. COVID-19 patients have varying levels of cytokines and chemokines as the disease progresses from mild to severe. Initial plasma levels of IL-1, IL-1ra, IL-7, IL-8, IL-10, IFN-, monocyte chemoattractant peptide (MCP)-1, macrophage inflammatory protein (MIP)-1A, MIP-1B, granulocyte colony stimulating factor (G-CSF), and tumor necrosis factor-alpha (TNF-α) are increased in SARS-CoV-2-infected patients, according to a retrospective analysis 19. Further research revealed that ICU patients have greater plasma concentrations of IL-2, IL-7, IL-17, IL-10, MCP1, MIP-1A, and TNF-α than non-ICU patients 15. Furthermore, in severe infection, plasma levels of IL-2, IL-6, IL-8, IL-10, and TNF-α are much higher than in non-severe illness 20,21. Plasma levels of IL-6, a cytokine thought to play a role in macrophage activation syndrome (MAS), rise in both moderate and severe COVID-19 patient groups 22. Furthermore, the vast area of lung injury (50%) is closely connected with elevated levels of IL-6 and the subgroup of lymphocytes in peripheral blood, according to an assessment of pulmonary infiltration in patients with ARDS 23.
Long term effect of Covid-19
COVID-19 patients with and without prior neurologic disorders experience neurologic symptoms 24. After COVID-19 infection, neuropsychiatric disorders are more common than respiratory infections or flu. Within 6 months of infection, 34% of patients are diagnosed with a neurological disease, 2.1% have a stroke, 0.6% percent have brain hemorrhage, and 0.7% experience confusion 25. When compared to other health events such as elevated heart rate, influenza, other respiratory tract infections, skin infection, cholelithiasis, urolithiasis, and fracture of a large bone in patients with no prior psychiatric history, diagnosis of COVID-19 was associated with an increased incidence of a first psychiatric diagnosis in the following 14–90 days. During that period, 18% of COVID-19 patients are diagnosed with a mental health disorder, 58% of whom had no prior diagnosis. In adults over 65 years old, the incidence of a first diagnosis of dementia in the 14–90 days following a COVID-19 diagnosis was 16% 26.
Severity of COVID-19 disease is linked to a mutation in the SARS-CoV-2 virus
In cultured immortalized cells and primary human airway epithelial cells, the new G614 mutant virus replicates more efficiently than the ancestral D614 (Wuhan-HU-1/2019) virus, while in hamsters, the G614 mutant virus (B117 variant) replicates to higher titers in nasal-wash samples than the D614 variant 27. The G614 strain has a reproduction rate 31% higher than the D614 strain 28. In September 2020, B117 infections were discovered in the United Kingdom. The B117 lineage includes a substantial number of genetic mutations in the spike protein 29. Other mutations in the spike protein P681H include a 69/70 deletion with a conformational change near the S1/S2 furin cleavage site with a mutation in ORF8 and a 69/70 deletion with a conformational change in the spike protein P681H near the S1/S2 furin cleavage site with a mutation in ORF8.
The B.1.351 lineage (20H/501Y.V2) variant has various mutations in the spike protein, including K417T, E484K, and N501Y, and lacks the deletion at 69/70. The human angiotensin-converting enzyme 2 (ACE2) receptor may bind more strongly to this variation, which was initially discovered in South Africa. The P.1 lineage (also known as 20J/501Y.V3) variant has 17 unique amino acid alterations and three deletions in the spike protein receptor-binding domain: K417T, E484K, and N501Y. It was initially discovered in Japan (Figure 3). Details of these mutations were recently published on the CDC’s website at https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-emerging-variant.html. The BNT162b2 mRNA-based COVID-19 vaccine showed comparable neutralizing titers to the spike N501Y substitution 30; nevertheless, its effect on patients afflicted with B117 and N501Y variations remains to be validated.
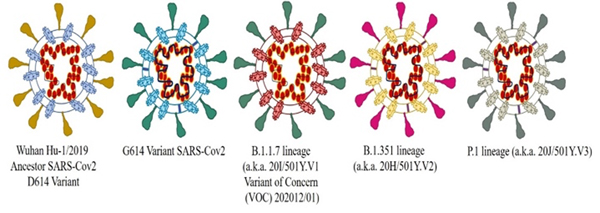
Figure 3.
Diagrammatic representation of reported SARS-Cov2 variants following mutation from Wuhan SARS-Cov2 (D614).
The mutant strain more contagious and spread via air.
In hamsters, the mutant strain was more likely to spread through the air from one cage to another, showing that the G strain virus is more infectious. Once detected in the UK, it spread quickly throughout Europe, Asia, and the United States. The D strain is still present, although it only accounts for roughly 2% of all infections worldwide. The concern now is whether the G strain can evolve resistance to the newly discovered protein used to combat D strain immunizations. However, as immunity develops, it is possible that the new strain will be forced to evolve. Evolution favors viruses that can evade antibodies as time passes and more people develop immunity.
New G strain of virus may change the host.
The chance that the new variant could spread to one of the world’s 1,400 bat species is a source of concern. Bats are good hosts for coronaviruses and are still considered the most likely source of SARS-CoV-2. Currently, investigators are concerned that if the virus’s antigenic structure changes, the produced antibodies Protein subunit vaccines will no longer identify it. However, it is possible that this new virus will evolve into a common cold virus. After penetrating the lower respiratory system and beyond, vaccine- and infection-induced immunity will most likely protect people from this new strain of virus. It would then join the four common corona viruses that are currently circulating among the population and cause colds.
Double/Triple mutant
Cases of the B.1.617 strain have surged dramatically in India since the beginning of 2021. This virus, dubbed the “double mutant virus,” has two important alterations: E484Q and L452R. B.1.617 has a number of mutations: G142D and E154K in the N-terminal antigenic super site, L452R and E484Q in the receptor binding domain within the polybasic furin cleavage site at the S1/S2 boundary (P681R), 1 variant of the spike protein, including G142D and E154K in the N-terminal antigenic supersite, L452R and E484Q in the receptor binding domain within the polybasic furin cleavage site at the S1 31. A “triple mutant variation” is created when three virus mutations combine to form a new variant. E154K, P681R, and Q1071H are some of the mutations found in the triple variation (B.1.618) newly discovered in West Bengal, India. E484K, a significant immune-escape mutation with two deletions in its spike protein, H146del and Y145del, characterizes the B.1.618 strain 32. In addition to the usual symptoms of sore throat, body ache, fever, and loss of smell and taste, symptoms of the novel coronavirus triple-mutant variant include diarrhea, abdominal discomfort, rashes, conjunctivitis, confusion, and bleeding from the nose and throat. Mucormycosis (black fungus), an uncommon, life-threatening fungal infection caused by Rhizopus arrhizus, is associated with a variety of clinical disorders33. Recent reports of black fungus infection in COVID-infected patients in India may be due to unsanitary conditions and contaminated equipment.
Types of SARS-CoV-2 vaccines
The challenge of developing and implementing largescale vaccine production is rapidly increasing around the world. It requires a series of coordinating activities that must be organized in parallel but should be useful for decades with proper preclinical testing, phased clinical trials, planned production, and distribution. The immune system and implications for protective immunity may be good targets for vaccines. Vaccines that have been approved or are currently under development are divided into six categories (Table 1–5).
Table 1.
DNA-based Vaccines and stages of development
| Developer / Researcher | Stage of Development | Product Description |
|---|---|---|
| Zydus Cadila Healthcare Limited | Phase III | DNA; (ZyCoV-D) plasmid vaccine |
| Inovio Pharmaceuticals/ Beijing Advaccine Biotechnology/ VGXI Inc./ Richter-Helm BioLogics/ Ology Bioservices/ International Vaccine Institute/ Seoul National University Hospital/ Thermo Fisher Scientific/ Kaneka Eurogentec | Phase II/III | DNA; (INO-4800) plasmid vaccine with electroporation 50 |
| Osaka University/ AnGes/ Takara Bio/ Cytiva/ Brickell Biotech | Phase II/III | DNA; (AG0301 & AG0302) plasmid vaccine + adjuvant |
| GeneOne and Life Science | Phase I/II | DNA; (GLS-5310) |
| Genexine Consortium (GenNBio, International Vaccine Institute, Korea Advanced Institute of Science and Technology (KAIST), Pohang University of Science and Technology (POSTECH)/ Binex/ PT Kalbe Pharma | Phase I/II | DNA; (formerly GX-19) (GX-19N) |
| OncoSec Medical Incorporated / Providence Cancer Institute | Phase I | (CORVax12), IL-12 expression platform + “S” glycoprotein |
| Symvivo | Phase I | DNA; bacTRL-Spike |
| BioNet Asia/ Technovalia/ Vax4COVID/ The University of Sydney/ The University of Western Australia/ Telethon Kids Institute/ PharmaJet | Pre-clinical | DNA; (COVIGEN) needle-free delivery |
| Chula Vaccine Research Center | Pre-clinical | DNA with electroporation |
| Ege University Drug Development and Pharmacokinetic Research Application Center (ARGEFAR)/ Scientific and Technological Research Council of Turkey (TUBITAK) | Pre-clinical | DNA |
| Entos Pharmaceuticals/ Cytiva | Pre-clinical | DNA; (Covigenix) |
| Globe Biotech Limited, Bangladesh | Pre-clinical | DNA plasmid vaccine |
| Immunomic Therapeutics/ EpiVax/ PharmaJet | Pre-clinical | DNA; plasmid vaccine, needle-free delivery |
| Mediphage Bioceuticals/ University of Waterloo/ Lambton College | Pre-clinical | DNA; msDNA-VLP |
| National Research Centre, Egypt | Pre-clinical | DNA; plasmid vaccine S, S1, S2, RBD & N |
| National Institute of Chemistry, Slovenia | Pre-clinical | Plasmid DNA, nanostructured RBD |
| OPENCORONA Project: Karolinska Institute/ Justus Liebig University Giessen/ Public Health Agency of Sweden (FoHM)/ IGEA/ Cobra Biologics/ Adlego Biomedical/ Region Stockholm | Pre-clinical | DNA with electroporation |
| Scancell/ University of Nottingham/ Nottingham Trent University | Pre-clinical | DNA; plasmid vaccine RBD&N |
| Statens Serum Institute, Denmark | Pre-clinical | DNA; (CoVAXIX) plasmid vaccine |
| Takis/ Applied DNA Sciences/ Evvivax/ Rottapharm Biotech | Pre-clinical | DNA (COVID-eVax) |
| University of Cambridge/ DIOSynVax/ PharmaJet | Pre-clinical | DNA; (DIOS-CoVax2) synthetic gene inserts compatible with multiple delivery systems |
Table 5.
Protein based subunit vaccines and stages of development
| Developer / Researcher | Stage of Development | Product Description |
|---|---|---|
| BioNTech/ Pfizer/ Fosun Pharma/ Rentschler Biopharma | Authorized | 3 LNP-mRNAs; BNT162 59; 60;61;62 |
| Moderna/ National Institute of Allergy and Infectious Diseases (NIAID)/ Biomedical Advanced Research and Development Authority (BARDA)/ Lonza/ Catalent/ Rovi/ Medidata/ BIOQUAL/ Baxter BioPharma Solutions | Authorized | RNA; LNP-encapsulated mRNA (mRNA 1273), (TAK-919)63;64 |
| CureVac/Bayer/Novartis | Phase III | RNA; mRNA (CVnCoV) |
| Arcturus/Duke-NUS/ Catalent | Phase I/II | RNA; mRNA; (LUNAR-COV19) |
| Imperial College London/ VacEquity Global Health | Phase I/II | RNA; LNP-nCoVsaRNA |
| People’s Liberation Army (PLA) Academy of Military Sciences/ Walvax Biotech | Phase I | mRNA (ARCoV) |
| Providence Therapeutics Holdings Inc. | Phase I | PTX-COVID19-B vaccine |
| BIOCAD | Pre-clinical | RNA; liposome-encapsulated mRNA |
| CanSino Biologics/Precision Nanosystems | Pre-clinical | RNA; mRNA lipid nanoparticle (mRNA-LNP) |
| Centro Nacional Biotecnologia (CNB-CSIC), Spain | Pre-clinical | RNA: Replicating defective SARS-CoV-2 derived RNAs |
| Chimeron Bio/ George Mason University’s National Center for Biodefense and Infectious Disease | Pre-clinical | Self-amplifying RNA, self-assembling delivery system |
| China CDC / Tongji University / Stermina | Pre-clinical | RNA; mRNA |
| Chula Vaccine Research Center/University of Pennsylvania | Pre-clinical | LNP-mRNA; (ChulaCov19) |
| Curevac/GSK | Pre-clinical | Next-generation multi-valent mRNA-based vaccines |
| CureVac/UK Government (Vaccines Taskforce) | Pre-clinical | Multiple mRNA vaccine candidates against SARS-CoV-2 variants |
| Daiichi-Sankyo/ University of Tokyo’s Institute of Medical Science | Pre-clinical | RNA; mRNA (DS-5670) |
| Elixirgen Therapeutics/ Fujita Health University | Pre-clinical | srRNA (EXG-5003) |
| Federal Budgetary Research Institution (FBRI) State Research Center of Virology and Biotechnology “VECTOR” | Pre-clinical | RNA; mRNA |
| Fudan University / Shanghai JiaoTong University / RNACure Biopharma | Pre-clinical | RNA; LNP-encapsulated mRNA cocktail encoding VLP |
| Fudan University / Shanghai JiaoTong University / RNACure Biopharma | Pre-clinical | RNA; LNP-encapsulated mRNA cocktail encoding RBD |
| GeneOne Life Science / Houston Methodist | Pre-clinical | mRNA; (GLS-3000) |
| Gennova | Pre-clinical | Self-amplifying RNA |
| Globe Biotech Limited, Bangladesh | Pre-clinical | D614G variant LNP-encapsulated mRNA; (Bangavax) |
| Greenlight Biosciences | Pre-clinical | mRNA |
| IDIBAPS- Hospital Clinic, Spain | Pre-clinical | mRNA |
| Infectious Disease Research Institute/ Amyris, Inc. | Pre-clinical | saRNA formulated in a NLC |
| Max-Planck Institute of Colloids and Interfaces | Pre-clinical | LNP-encapsulated mRNA encoding S |
| RNA immune, Inc. | Pre-clinical | RNA; several mRNA candidates |
| Sanofi Pasteur/ Translate Bio | Pre-clinical | LNP-mRNA |
Inactivated Virus Vaccines
These vaccines are often created by exposing a virulent virus to a physical or chemical substance that preserves the virus’s immunogenicity while also acting as an immunogen (Table 2).
Table 2.
Inactivated/ Live attenuated virus Vaccines and stages of development
| Developer / Researcher | Stage of Development | Product Description |
|---|---|---|
| Beijing Institute of Biological Products/ Sinopharm | Phase III | Inactivated, (BBIBP-CorV)51 |
| Bharat Biotech/ Indian Council of Medical Research/ National Institute of Virology/ Ocugen/ Precisa Medicamentos | Phase III | Inactivated; whole virion (COVAXIN) (BBV152)52; 53; 54 |
| Institute of Medical Biology, Chinese Academy of Medical Sciences | Phase III | Inactivated55 |
| Research Institute for Biological Safety Problems, Republic of Kazakhstan | Phase III | Inactivated, (QazCovid-in®) |
| Sinovac/ Instituto Butantan/ Bio Farma | Phase III | Inactivated (inactivated + alum); CoronaVac (formerly PiCoVacc)52,56; 57 |
| Wuhan Institute of Biological Products/ Sinopharm | Phase III | Inactivated 58 |
| Erciyes University | Phase II | Inactivated; (ERUCOV-VAC) |
| Shenzhen Kangtai Biological Products Co.,Ltd./ Beijing Minhai Biotechnology Co., Ltd. | Phase II | Inactivated SARS-CoV-2 vaccine (Vero cell) |
| Valneva/ Dynavax/ National Institute for Health Research, United Kingdom | Phase I/II | Inactivated (Inactivated + CpG 1018), VLA2001 |
| Shifa Pharmed Industrial Co | Phase I | COVID-19 inactivated vaccine (COVIran Barekat) |
| Government Pharmaceutical Organization (GPO; Thailand) / Dynavax / PATH | Pre-clinical | Egg-based, inactivated, whole chimeric Newcastle Disease Virus (NDV) expressing membrane-anchored pre-fusion-stabilized trimeric SARS-CoV-2 S protein (Hexapro) + CpG 1018 |
| Institute Butantan (Brazil) / Dynavax / PATH | Pre-clinical | |
| Institute of Vaccines and Medical Biologicals (IVAC; Vietnam) / Dynavax / PATH | Pre-clinical | |
| KM Biologics | Pre-clinical | Inactivated (inactivated + alum) |
| Kocak Farma Ilac ve Kimya San. A.S. | Pre-clinical | Inactivated |
| National Research Centre, Egypt | Pre-clinical | Inactivated whole virus |
| Osaka University / BIKEN / NIBIOHN | Pre-clinical | Inactivated |
| Selcuk University | Pre-clinical | Inactivated |
| Sinovac/ Dynavax | Pre-clinical | Inactivated + adjuvant (CpG 1018) |
| Live attenuated virus Vaccines and stages of development | ||
| Codagenix / Serum Institute of India | Phase I | Single-dose, intranasal, live attenuated vaccine, (COVI-VAC) |
| Indian Immunologicals Ltd/ Griffith University | Pre-clinical | Codon deoptimized live attenuated virus |
| Mehmet Ali Aydinlar University/ Acıbadem Labmed Health Services A.S. | Pre-clinical | Codon deoptimized live attenuated vaccines |
| Meissa Vaccines | Pre-clinical | MV-014-210 |
Virus- like particles (VLPs)
Virus-like particles (VLPs) are multiprotein structures that lack a viral genome but are co-expressed as non-infectious particles as a vaccine immunogen, sometimes yielding a safer vaccine option. Vaccines made in vitro from bacteria, yeast, or mammalian cells and containing only important viral proteins or peptides (Table 3).
Table 3.
Viral like particle Vaccines and stages of development
| Developer / Researcher | Stage of Development | Product Description |
|---|---|---|
| Medicago Inc. | Phase II/III | VLP; plant derived VLP adjuvanted with GSK or Dynavax adjuvants; (CoVLP)65 |
| Serum Institute of India/ Accelagen Pty/ SpyBiotech | Phase I/II | RBD SARS-CoV-2 HBsAg VLP vaccine |
| Arizona State University | Pre-clinical | Myxoma virus co-expressing S, M, N and E proteins |
| Arizona State University | Pre-clinical | Plasmid driven production of virus like particles (VLPs) containing S, M, N and E proteins of SARS-CoV-2 |
| ARTES Biotechnology | Pre-clinical | VLP; eVLP |
| Bezmialem Vakif University | Pre-clinical | VLP |
| Doherty Institute | Pre-clinical | VLP; unknown |
| Icosavax | Pre-clinical | VLP displaying the SARS-CoV-2 receptor-binding domain (RBD); (IVX-411) |
| Imophoron Ltd / Bristol University’s Max Planck Centre | Pre-clinical | VLP; ADDomerTM multiepitope display |
| IrsiCaixa AIDS Research/ IRTA-CReSA/ Barcelona Supercomputing Centre/ Grifols | Pre-clinical | S protein integrated in HIV VLPs |
| Mahidol University/ The Government Pharmaceutical Organization (GPO)/ Siriraj Hospital | Pre-clinical | VLP + Adjuvant |
| Max-Planck Institute for Dynamics of Complex Technical Systems | Pre-clinical | VLP |
| Medicago Inc./ GSK | Pre-clinical | VLP (CoVLP)+ Adjuvant |
| Medicago Inc./ Dynavax | Pre-clinical | VLP (CoVLP)+ Adjuvant (CpG 1018) |
| Middle East Technical University | Pre-clinical | VLP |
| Navarrabiomed, Oncoimmunology group | Pre-clinical | Virus-like particles, lentivirus, and baculovirus vehicles |
| OSIVAX | Pre-clinical | VLP (COVID-19 and SARS1) |
| Saiba GmbH | Pre-clinical | VLP; virus-like particle, based on RBD displayed on virus-like particle |
| Tampere University | Pre-clinical | VLPs produced in BEVS |
| University of Manitoba | Pre-clinical | Virus-like particle-based dendritic cell-targeting vaccine |
| University of Sao Paulo | Pre-clinical | VLPs peptides/whole virus |
| VBI Vaccines / National Research Council of Canada/ Therapure Biomanufacturing | Pre-clinical | Enveloped virus-like particle (eVLP): Pan-coronavirus vaccine candidate, targeting COVID-19, SARS, and MERS, spike protein (VBI-2900) |
Protein subunit vaccines
Vaccines made in vitro from bacteria, yeast, or mammalian cells and containing only important viral proteins or peptides (Table 5)
Virus- vectored vaccines
Viral vector-based vaccines are the vaccines without antigens, but they use the body’s cells to produce them. These antigen (s) are produced by transduced host cells after immunization.
DNA and mRNA vaccines
Viral antigens are encoded by a recombinant DNA plasmid and generated in host cells via a sequential transcription-to-translation process in DNA vaccines. mRNA vaccines, on the other hand, are produced in vitro and create viral antigens in the cytoplasm through direct protein translation in vivo (Table 1, 4).
Table 4.
RNA based Vaccines and stages of development
| Developer / Researcher | Stage of Development | Product Description |
|---|---|---|
| Anhui Zhifei Longcom Biopharmaceutical/ Institute of Microbiology, Chinese Academy of Sciences | Phase III | Adjuvanted recombinant protein (RBD-Dimer). (ZF2001) |
| Instituto Finlay de Vacunas | Phase III | rRBD produced in CHO-cell chemically conjugate to tetanus toxoid; (FINLAY-FR-2) (SOBERANA 02) |
| Novavax/Emergent Biosolutions/ Praha Vaccines/ Biofabri/ Fujifilm Diosynth Biotechnologies/ FDB/ Serum Institute of India/ SK bioscience/ Takeda Pharmaceutical Company Limited/ AGC Biologics/ PolyPeptide Group/ Endo | Phase III | Protein subunit; Full length recombinant SARs COV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M; (NVX-CoV2373) (SARS-CoV-2 rS) |
| Medigen Vaccine Biologics Corp/ NIAID/ Dynavax | Phase II | MVC-COV1901 vaccine injection; S-2 P protein + CpG 1018 |
| Sanofi Pasteur/ GSK | Phase II | Protein subunit; S protein, baculovirus production |
| West China Hospital, Sichuan University | Phase II | RBD (baculovirus production expressed in Sf9 cells) |
| Biological E Ltd/ Dynavax/ Baylor College of Medicine | Phase I/II | Protein subunit; (BECOV2) |
| Center for Genetic Engineering and Biotechnology (CIGB), Havana | Phase I/II | CIGB-669 (RBD-AgnHB) |
| Federal Budgetary Research Institution (FBRI) State Research Center of Virology and Biotechnology “VECTOR” | Phase I/II | Peptide vaccine, EpiVacCorona |
| Instituto Finlay de Vacunas | Phase I/II | RBD + Adjuvant (FINLAY-FR-1) (SOBERANA 01) |
| Nanogen Pharmaceutical Biotechnology | Phase I/II | Recombinant SARS-CoV-2 spike protein, aluminum adjuvanted |
| Shionogi & Co., Ltd./ National Institute of Infectious Disease, Japan | Phase I/II | Recombinant protein vaccine S-268019, baculovirus expression |
| VIDO-InterVac, University of Saskatchewan | Phase I/II | Protein subunit, adjuvanted microsphere peptide, (COVAC-1 & COVAC-2) |
| Adimmune Corporation | Phase I | Baculovirus-insect cells expression system, spike (S) protein (tAdimrSC-2f) |
| Center for Genetic Engineering and Biotechnology (CIGB), Havana | Phase I | CIGB-66 (RBD + aluminum hydroxide) |
| Clover Biopharmaceuticals Inc./ Dynavax | Phase I | Protein subunit, native like trimeric subunit spike protein; (SCB-2019) |
| Covaxx/ University of Nebraska Medical Center (UNMC)/ DASA/ United Biomedical Inc. Asia | Phase I | S1-RBD-protein; Multitope Peptide-Based Vaccine (MVP); UB-612 |
| Razi Vaccine and Serum Research Institute | Phase I | SARS-CoV-2 recombinant Spike protein vaccine (Razi Cov Pars) |
| University Hospital Tuebingen | Phase I | SARS-CoV-2 HLA-DR peptides, (CoVAC-1) |
| Vaxine Pty Ltd/ Flinders University/ Oracle/ Medytox/ Sypharma/ Oxford Expression Technologies | Phase I | Protein subunit; recombinant spike protein with Advax adjuvant (COVAX-19) |
Live- attenuated virus vaccines
In this case, the virus is suppressed through in numbers can cause confusion, the World Health Organization (WHO) simplified them: The UK variant B.1.117 is known as Alpha, South African variant B1.351 is Beta, the Brazil variant P.1 is gamma, and the Indian variant B1.617.2 is Delta. According to the WHO, more than 4,500 sequences of variants from more than 40 countries have been uploaded to a coronavirus database and could be less. However, many countries, including India, are sequencing only a fraction of their virus cases, so it is difficult to determine just how large a role the variant is playing in surges. Recent reports indicate that inactivated vaccines, such as those developed in China, seem to induce lower effective, as currently reported in cases in Seychelles. It is too early to know how replication-vitro or in vivo passage and genetic mutagenesis. By simulating live virus infection, the resultant virus becomes non-pathogenic or weakly pathogenic while retaining immunogenicity (Table 2).
Vaccines and treatments for COVID-19
For effective treatment of COVID-19 and related disease, numerous treatment methods are available, and others are being tested in clinical trials. Depending on the severity of the disease and other medical conditions, bamlanivimab, casirivimab plus imdevimab, remdesivir, and dexamethasone with or without remdesivir may be used 34. Antibodies against SARS-CoV-2 may be present in the plasma of recovered COVID-19 patients, which could help fight the virus and its inflammatory response, but the safety and efficacy of COVID-19 convalescent plasma in pregnant and pediatric patients has not yet been determined 35. Mesenchymal stem cells are also being studied extensively as a therapeutic approach for COVID-19 patients with lung infection and inflammation 36. Interleukins play a vital role in the cytokine storm common in severe COVID-19, and interleukin inhibitors such as anakinra (which inhibits IL-1), sarilumab, tocilizumab (which inhibits IL-6), and interferons are being studied 37. Alternatives to dexamethasone include prednisone, methylprednisolone, and hydrocortisone, all of which may help prevent SARS-CoV-2-induced systemic inflammation, lung damage, and multisystem organ dysfunction 38. Vitamins C, D, and zinc are also indicated for COVID-19 patients who are not critically ill, although their exact role has yet to be determined 39. With the goal of developing a safe and effective vaccine for COVID-19, researchers are looking into DNA-based and RNA-based techniques as well as replicating and nonreplicating vector strategies. SARS-CoV-2 vaccines based on mRNA are currently licensed. Antibodies or immune cells bind to molecules on the virus’s surface to provide immunity. If alterations in these components on the virus’s surface occur, antibodies will be unable to bind to them as strongly, allowing the virus to escape, which is why the seasonal flu vaccine needs to be updated every year. If this occurs, a COVID vaccine would need to be updated on a regular basis. There are over 200 COVID vaccine candidates in various phases of research but is too early to know how many have evolution-proof characteristics. The vaccine from AstraZeneca has been linked to significant and occasionally fatal blood clots in rare situations 40. Patients who experienced clots after vaccination had antibodies that activated their platelets, and younger people appear to be more susceptible than older people. Vaccines that only provide temporary relief put patients at risk and may also render other vaccines ineffective if viruses evolve that are resistant to many vaccines at the same time.
Vaccine efficacy by coronavirus variant
The first significant change in SARS-CoV-2 occurred early in the pandemic, in March and April 2020, when the original strain was replaced globally by a new variant known as D614G 41. The important mutation in this variant, which is found in the S protein, has been proven to improve the virus’s replication efficiency and transmissibility 42. Although neutralizing antibodies (NAbs) did not recognize this variant, it served as a warning. Another variant began to spread in August 2020 in the United Kingdom (where such events are closely monitored), and infections in that country climbed substantially from November 2020 to January 2021. This variant has now been identified in several countries, including the USA. The first variant “UK strain,” known as B.1.1.7. N501Y, contains a critical sequence alteration in the S protein that appears to boost the transmissibility of SARS-CoV-2, but in a somewhat different way than D614G. Fortunately, the site of the N501Y alteration renders it unlikely to influence most of the NAb binding sites on the receptor-binding domain (RBD) in terms of vaccine protection. Another new variant, N501Y.V2 (or B.1.351), has been discovered in South Africa, Japan, and Brazil (P.1) with similar infectious properties. The N501Y.V2 strain causes more sequence changes than both the D614G and the B.1.1.7 variants, and these changes are more worrying because they are located inside or close to the RBD 43. Recently, India reported a variant B.1.617.2 which spreads more easily, with transmission rates 30–100% higher than the other reported variants in the UK, South Africa, or Brazil. Most recently, the US identified two variants in California, B1.427 and B1.429, that reduce the effectiveness of antibodies generated by COVID-19 infection or by vaccines (Table 6). Since these variant identification default vaccines (Johnson & Johnson, Janssen, AstraZeneca), or adjuvant purified protein vaccines (Novavax and Sanofi- NAb levels GSK) may be affected by simian or human adenovirus vaccine replication defeat. Researchers across the world are currently working to better understand how N501Y.V2 an related variants affect these vaccines. In addition to avoiding immunity recognition due to vaccine, variants are also less susceptible to. monoclonal antibody (nMAb) neutralization. For instance, changes to N501Y in the B.1.1.7 variant almost eliminate the activity of several nMAbs, and a study by a South African team shows that almost all the nMAbs tested against N501Y.V2 are no longer effective, but recently two new variants like delta plus and Lamda is identified in several countries including UK that accounts for 82% of new infections and still not sure that current vaccinated population is completely protected with this new variants 44. The efficacy of the top 10 vaccines has been shown in the Table 7.
Table 6.
Neutralizing antibodies for Covid-19.
| Antibody Name | Source | Neutralizing activity | Neutralizing mechanism |
|---|---|---|---|
| S230.15 And m396 | Human | Neutralize human and palm civet SARS-CoV | Recognize epitopes (residues408, 442, 443, 460, 475) on SARS-CoV S1 protein, interfering with RBD–ACE2 receptor interaction66 |
| S109.8S 277. 14S230.15 | Human | Neutralize human (Urbani, GZ02, CUHK-W1), palm civet (HC/SZ/61/03), and raccoon dog(A031G) SARS-CoV infectious clones containing S variants | Inhibit the binding of SARS-CoV RBD–ACE2 receptor67 |
| 80RscFv | Human | Neutralize live SARS-CoV (strain Urbani) infection | Epitopes on SARS-CoV S1 (residues 261–672), blocking RBD–ACE2 binding and inhibiting syncytium formation68 |
| CR3022 CR3014 scFv | Human | Neutralize live SARS-CoV (strainHKU-39849) infection; CR3022 could neutralize CR3014 escape variants | Recognize epitopes on SARS-CoV RBD (residues 318–510); CR3022 binds SARS-CoV-2 RBD with high affinity69; 70 |
| Tocilizumab and Sarilumab | Recombinant humanized monoclonal antibody | IL-6 receptor antagonists | Targeting IL-6 and its receptor (IL6R) by tocilizumab and siltuximab monoclonal antibodies could mitigate cytokine storm71 |
| Bevacizumab | Recombinant humanized monoclonal antibody | VEGF inhibitor | Target vascular endothelial growth factor (VEGF), inhibit it and prevents acute lung injury (ALI) and ARDS72 |
Table 7.
Efficacy of vaccines in response to corona virus variant.
| Vaccine | Efficacy at preventing disease: D614G & B.1.1.7 | Efficacy at preventing infection: D614G &B.1.1.7 | preventing disease: B.1.351, P.1, B.1.617 | Efficacy at preventing infection: B.1.351, P.1, B.1.617 |
|---|---|---|---|---|
| Pfizer/BioNTech | 91% | 86% | 86% | 82% |
| Moderna | 94% | 89% | 89% | 85% |
| AstraZeneca | 74% | 52% | 35% | 31% |
| Johnson & Johnson (Janssen) | 72% | 72% | 64% | 57% |
| Sputnik-V | 92% | 81% | 59% | 52% |
| Novavax | 89% | 79% | 49% | 43% |
| CoronaVac | 50% | 44% | 32% | 28% |
| Sinopharm | 73% | 65% | 47% | 41% |
| Tianjin CanSino | 66% | 58% | 42% | 37% |
| Covaxin | 78% | 69% | 50% | 44% |
| Other mRNA vaccines | 91% | 86% | 86% | 82% |
| All other vaccines | 75% | 66% | 57% | 50% |
Do the New SARS-CoV-2 Variants Affect Vaccine Efficacy?
So far, the new variants tend to affect COVID-19’s potential to spread but have no effect on how sick people get from the virus, and currently available vaccines appear to be effective against the novel mutations. Vaccines are designed to produce a wide range of antibodies to multiple portions of the virus, so that even if one portion of the virus mutates, the antibodies will still identify another. It is likely that a variant will emerge that diminishes vaccine efficacy, 52.0%.and manufacturers are working to develop new vaccines that will protect against new SARS-CoV-2 strains.
Efficacy of the vaccines
Numerous vaccines are being developed, but only few are showing good results in clinical trials. The clinical trials of Pfizer/BioNTech and Moderna vaccines showed 95% and ‘94.1% efficacy, respectively, whereas the Johnson & Johnson vaccine, which was tested in the United States, Brazil, and South Africa, had 66% efficacy
Vaccine efficacy by coronavirus variant
The first significant change in SARS-CoV-2 occurred early in the pandemic, in March and April 2020, when overall and 85% efficacy against severe disease 45,46.
Pfizer-BioNTech
This is an mRNA vaccine that codes for the virus’s spike protein and is encapsulated in a lipid nanoparticle. Once injected, the cells churn out the spike protein, triggering the body’s immune system to recognize the virus. It has demonstrated 95% efficacy.
Moderna
Messenger RNA (mRNA) is a highly unstable molecule that is used to create proteins in cells. mRNA can be synthesized in a laboratory and, when injected into cells, can cause pieces of proteins to be made. When these small pieces of protein (peptides) leave the cell, the body can develop an immune reaction to them. The Moderna vaccine is stable at 36–46° F and has an efficacy rate of 94.5%.
Why two doses of Pfizer-BioNTech and Moderna
The first dose helps to prime your immune system. It introduces it to the spike protein and allows it to generate a small immune response prior to the second dose. Time is needed to allow this process to develop properly 47.
Johnson & Johnson’s Single-Dose COVID-19 Vaccine
This option uses a modified adenovirus (Ad26.COV2. S) to spur antibody production in its recipients. Overall efficacy was 66% but in the severe critical Covid-19 it showed an efficacy rate of 76% and in the variant studies (20H/501Y.V2), the vaccine efficacy was 52.0%.
NVX-CoV2373 Covid-19 Vaccine against the B.1.351
For this vaccine researchers used a baculovirus for the first time to insert into the gene for the SARS-CoV-2 spike protein into moth cells, which produced spikes on their cell membranes. They then harvested the spike proteins and mixed them with a synthetic soap-like particle (saponin) in which the spikes embed. The vaccine efficacy was 60.1% in the normal population 48.
Nasal Spray (Ab’s) to Beat COVID-19 as a game changer
IgM has been engineered as a promising drug model with powerful neutralization, wide coverage of variants, with desirable pharmacokinetics and safety profiles. We intend that developed; IgM, administered could provide both for COVID-19 and other respiratory viral conditions as new therapeutic platform 49.
Normal working life is affected due to Covid-19
The remote workers before the Covid-19 pandemic were less than 4% of the US workforce; however, after the SARS-CoV-2 outbreak, it shot up to 56% of the US. Approximately, 14 times increase within a single quarter. A remote work-study found that 86% took advantage of those who had the option to work remotely. A study indicated that even when offices can open back up, around one-third of professionals predict up to 75% of the workforce might go fully remote in the next 15 years. However, if the current vaccines work as well as hoped and the virus becomes the sniffles, still the situation of working style may not change.
Funding.
Dr. Mishra is the Endowed Schlieder Chair. All authors would like to thank Edward G. Schlieder Educational Foundation for the major support. The work is also partially supported by the NIH R01 AI080581 grant (AM).
Footnotes
Conflict of Interest
The author has no conflicts of interest associated with the material presented in this paper.
Ethics Statement
This paper is a perspective, so it did not need ethical approval.
References
- 1.Kandikattu HK, Venkateshaiah SU, Kumar S, Mishra A. IL-15 immunotherapy is a viable strategy for COVID-19. Cytokine & Growth Factor Reviews 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman S Another decade, another coronavirus. Mass Medical Soc; 2020. [Google Scholar]
- 5.Finlay BB, See RH, Brunham RC. Rapid response research to emerging infectious diseases: lessons from SARS. Nature Reviews Microbiology 2004;2:602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Z-D, Wang Z-Y, Zhang S-F, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging infectious diseases 2020;26:1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JF-W, Kok K-H, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging microbes & infections 2020;9:221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah N, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral research 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuyama S, Taguchi F. Two-step conformational changes in a coronavirus envelope glycoprotein mediated by receptor binding and proteolysis. Journal of virology 2009;83:11133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367:1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau Y-L, Peiris J, Law H. Role of dendritic cells in SARS coronavirus infection. Hong Kong Med J 2012;18:28–30. [PubMed] [Google Scholar]
- 12.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. Journal of medical virology 2020;92:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuerth JD, Weber F. Phleboviruses and the type I interferon response. Viruses 2016;8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Zhu D. Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19. Medicine in Drug Discovery 2020:100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cologne G The innate and adaptive immune systems [Updated 2020 Jul 30]. Institute for Quality and Efficiency in Health Care (IQWiG; ): 2006 2020. [Google Scholar]
- 17.Makowski L, Olson-Sidford W, W-Weisel J. Biological and clinical consequences of integrin binding via a rogue RGD motif in the SARS CoV-2 spike protein. Viruses 2021;13:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus research 2008;133:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators of inflammation 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong C, Lam C, Wu A, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical & Experimental Immunology 2004;136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Shen C, Li J, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. MedRxiv 2020. [Google Scholar]
- 22.Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging microbes & infections 2020;9:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, He J, Wu S. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients: disease characteristics and retrospective analysis. Medrxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayanappa A, Chastain WH, Paz M, et al. SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine & growth factor reviews 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet Psychiatry 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet Psychiatry 2021;8:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plante JA, Liu Y, Liu J, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung K, Pei Y, Leung GM, Lam TT, Wu JT. Empirical transmission advantage of the D614G mutant strain of SARS-CoV-2. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rambaut A, Loman N, Pybus O, et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. Genom Epidemiol 2020. [Google Scholar]
- 30.Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nature Medicine 2021;27:620–1. [DOI] [PubMed] [Google Scholar]
- 31.Edara V-V, Lai L, Sahoo M, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B. 1.617. 1 variant. bioRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahoo JP, Mishra AP, Samal KC. Triple Mutant Bengal Strain (B. 1.618) of Coronavirus and the Worst COVID Outbreak in India. Biotica Research Today 2021;3:261–5. [Google Scholar]
- 33.Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Medical mycology case reports 2021;32:64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nhean S, Varela ME, Nguyen Y-N, et al. COVID-19: A Review of Potential Treatments (Corticosteroids, Remdesivir, Tocilizumab, Bamlanivimab/Etesevimab, and Casirivimab/Imdevimab) and Pharmacological Considerations. Journal of Pharmacy Practice 2020;182:812–27. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luchsinger LL, Ransegnola BP, Jin DK, et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. Journal of clinical microbiology 2020;58:e02005–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coelho A, Alvites RD, Branquinho MV, Guerreiro SG, Maurício AC. Mesenchymal stem cells (MSCs) as a potential therapeutic strategy in COVID-19 patients: literature research. Frontiers in cell and developmental biology 2020;8:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open biology 2020;10:200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voto C, Berkner P, Brenner C. Overview of the pathogenesis and treatment of SARS-CoV-2 for clinicians: a comprehensive literature review. Cureus 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAuliffe S, Ray S, Fallon E, Bradfield J, Eden T, Kohlmeier M. Dietary micronutrients in the wake of COVID-19: an appraisal of evidence with a focus on high-risk groups and preventative healthcare. BMJ nutrition, prevention & health 2020;3:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ON MO, PRE N, SOUTH S. COVID vaccines and blood clots: what researchers know so far. Nature 2021;596. [DOI] [PubMed] [Google Scholar]
- 41.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020;182:812–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou YJ, Chiba S, Halfmann P, et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 2020;370:1464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starr TN, Greaney AJ, Hilton SK, et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell 2020;182:1295–310. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y. V2 escapes neutralization by South African COVID-19 donor plasma. Nature medicine 2021;27:622- [DOI] [PubMed] [Google Scholar]
- 45.Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. bmj 2021;373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenforde MW. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged≥ 65 Years—United States, January–March 2021. MMWR Morbidity and mortality weekly report 2021;70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livingston EH. Necessity of 2 doses of the Pfizer and Moderna COVID-19 vaccines. JAMA 2021;325:898-. [DOI] [PubMed] [Google Scholar]
- 48.Shinde V, Bhikha S, Hossain Z, et al. Preliminary efficacy of the NVX-CoV2373 Covid-19 vaccine against the B. 1.351 variant. MedRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ku Z, Xie X, Hinton PR, et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature 2021:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel A, Walters J, Reuschel EL, et al. Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model. bioRxiv 2020. [Google Scholar]
- 51.Isakova-Sivak I, Rudenko L. A promising inactivated whole-virion SARS-CoV-2 vaccine. The Lancet Infectious Diseases 2021;21:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. The Lancet Infectious Diseases 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity trial of an inactivated SARS-CoV-2 vaccine-BBV152: a phase 1, double-blind, randomised control trial. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sapkal GN, Yadav P, Ella R, et al. Neutralization of UK-variant VUI-202012/01 with COVAXIN vaccinated human serum. bioRxiv 2021. [Google Scholar]
- 55.Pu J, Yu Q, Yin Z, et al. An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine. medRxiv 2020. [Google Scholar]
- 56.Zhang Y-J, Zeng G, Pan H-X, et al. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medrxiv 2020. [Google Scholar]
- 57.Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. Jama 2020;324:951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. MedRxiv 2020. [Google Scholar]
- 60.Sahin U, Muik A, Derhovanessian E, et al. Concurrent human antibody and TH1 type T-cell responses elicited by a COVID-19 RNA vaccine. MedRxiv 2020. [Google Scholar]
- 61.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020;586:589–93. [DOI] [PubMed] [Google Scholar]
- 62.Walsh EE, Frenck R, Falsey AR, et al. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. Medrxiv 2020. [Google Scholar]
- 63.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. New England Journal of Medicine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. New England Journal of Medicine 2020;383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward BJ, Gobeil P, Seguin A, et al. Phase 1 trial of a Candidate Recombinant Virus-Like Particle Vaccine for Covid-19 Disease Produced in Plants. medRxiv 2020. 2021:08971900211048139. [Google Scholar]
- 66.Zhu Z, Chakraborti S, He Y, et al. Potent cross reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proceedings of the National Academy of Sciences 2007;104:12123–8. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rockx B, Corti D, Donaldson E, et al. Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge. Journal of virology 2008;82:3220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sui J, Li W, Murakami A, et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proceedings of the National Academy of Sciences 2004;101:2536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian X, Li C, Huang A, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerging microbes & infections 2020;9:382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ter Meulen J, Bakker AB, Van Den Brink EN, et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. The Lancet 2004;363:2139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu T, Zhang J, Yang Y, et al. The potential role of IL-6 in monitoring coronavirus disease 2019. Available at SSRN 3548761 2020. [Google Scholar]
- 72.Pang J, Xu F, Aondio G, et al. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nature communications 2021;12:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]