Abstract
Background
About 70% to 80% of adults with cancer experience chemotherapy‐induced nausea and vomiting (CINV). CINV remains one of the most distressing symptoms associated with cancer therapy and is associated with decreased adherence to chemotherapy. Combining 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists with corticosteroids or additionally with neurokinin‐1 (NK₁) receptor antagonists is effective in preventing CINV among adults receiving highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC). Various treatment options are available, but direct head‐to‐head comparisons do not allow comparison of all treatments versus another.
Objectives
• In adults with solid cancer or haematological malignancy receiving HEC
‐ To compare the effects of antiemetic treatment combinations including NK₁ receptor antagonists, 5‐HT₃ receptor antagonists, and corticosteroids on prevention of acute phase (Day 1), delayed phase (Days 2 to 5), and overall (Days 1 to 5) chemotherapy‐induced nausea and vomiting in network meta‐analysis (NMA)
‐ To generate a clinically meaningful treatment ranking according to treatment safety and efficacy
• In adults with solid cancer or haematological malignancy receiving MEC
‐ To compare whether antiemetic treatment combinations including NK₁ receptor antagonists, 5‐HT₃ receptor antagonists, and corticosteroids are superior for prevention of acute phase (Day 1), delayed phase (Days 2 to 5), and overall (Days 1 to 5) chemotherapy‐induced nausea and vomiting to treatment combinations including 5‐HT₃ receptor antagonists and corticosteroids solely, in network meta‐analysis
‐ To generate a clinically meaningful treatment ranking according to treatment safety and efficacy
Search methods
We searched CENTRAL, MEDLINE, Embase, conference proceedings, and study registries from 1988 to February 2021 for randomised controlled trials (RCTs).
Selection criteria
We included RCTs including adults with any cancer receiving HEC or MEC (according to the latest definition) and comparing combination therapies of NK₁ and 5‐HT₃ inhibitors and corticosteroids for prevention of CINV.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
We expressed treatment effects as risk ratios (RRs). Prioritised outcomes were complete control of vomiting during delayed and overall phases, complete control of nausea during the overall phase, quality of life, serious adverse events (SAEs), and on‐study mortality. We assessed GRADE and developed 12 'Summary of findings' tables. We report results of most crucial outcomes in the abstract, that is, complete control of vomiting during the overall phase and SAEs. For a comprehensive illustration of results, we randomly chose aprepitant plus granisetron as exemplary reference treatment for HEC, and granisetron as exemplary reference treatment for MEC.
Main results
Highly emetogenic chemotherapy (HEC)
We included 73 studies reporting on 25,275 participants and comparing 14 treatment combinations with NK₁ and 5‐HT₃ inhibitors. All treatment combinations included corticosteroids.
Complete control of vomiting during the overall phase
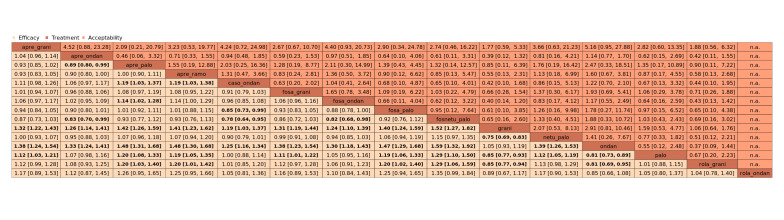
We estimated that 704 of 1000 participants achieve complete control of vomiting in the overall treatment phase (one to five days) when treated with aprepitant + granisetron. Evidence from NMA (39 RCTs, 21,642 participants; 12 treatment combinations with NK₁ and 5‐HT₃ inhibitors) suggests that the following drug combinations are more efficacious than aprepitant + granisetron for completely controlling vomiting during the overall treatment phase (one to five days): fosnetupitant + palonosetron (810 of 1000; RR 1.15, 95% confidence interval (CI) 0.97 to 1.37; moderate certainty), aprepitant + palonosetron (753 of 1000; RR 1.07, 95% CI 1.98 to 1.18; low‐certainty), aprepitant + ramosetron (753 of 1000; RR 1.07, 95% CI 0.95 to 1.21; low certainty), and fosaprepitant + palonosetron (746 of 1000; RR 1.06, 95% CI 0.96 to 1.19; low certainty).
Netupitant + palonosetron (704 of 1000; RR 1.00, 95% CI 0.93 to 1.08; high‐certainty) and fosaprepitant + granisetron (697 of 1000; RR 0.99, 95% CI 0.93 to 1.06; high‐certainty) have little to no impact on complete control of vomiting during the overall treatment phase (one to five days) when compared to aprepitant + granisetron, respectively.
Evidence further suggests that the following drug combinations are less efficacious than aprepitant + granisetron in completely controlling vomiting during the overall treatment phase (one to five days) (ordered by decreasing efficacy): aprepitant + ondansetron (676 of 1000; RR 0.96, 95% CI 0.88 to 1.05; low certainty), fosaprepitant + ondansetron (662 of 1000; RR 0.94, 95% CI 0.85 to 1.04; low certainty), casopitant + ondansetron (634 of 1000; RR 0.90, 95% CI 0.79 to 1.03; low certainty), rolapitant + granisetron (627 of 1000; RR 0.89, 95% CI 0.78 to 1.01; moderate certainty), and rolapitant + ondansetron (598 of 1000; RR 0.85, 95% CI 0.65 to 1.12; low certainty).
We could not include two treatment combinations (ezlopitant + granisetron, aprepitant + tropisetron) in NMA for this outcome because of missing direct comparisons.
Serious adverse events
We estimated that 35 of 1000 participants experience any SAEs when treated with aprepitant + granisetron. Evidence from NMA (23 RCTs, 16,065 participants; 11 treatment combinations) suggests that fewer participants may experience SAEs when treated with the following drug combinations than with aprepitant + granisetron: fosaprepitant + ondansetron (8 of 1000; RR 0.23, 95% CI 0.05 to 1.07; low certainty), casopitant + ondansetron (8 of 1000; RR 0.24, 95% CI 0.04 to 1.39; low certainty), netupitant + palonosetron (9 of 1000; RR 0.27, 95% CI 0.05 to 1.58; low certainty), fosaprepitant + granisetron (13 of 1000; RR 0.37, 95% CI 0.09 to 1.50; low certainty), and rolapitant + granisetron (20 of 1000; RR 0.57, 95% CI 0.19 to 1.70; low certainty).
Evidence is very uncertain about the effects of aprepitant + ondansetron (8 of 1000; RR 0.22, 95% CI 0.04 to 1.14; very low certainty), aprepitant + ramosetron (11 of 1000; RR 0.31, 95% CI 0.05 to 1.90; very low certainty), fosaprepitant + palonosetron (12 of 1000; RR 0.35, 95% CI 0.04 to 2.95; very low certainty), fosnetupitant + palonosetron (13 of 1000; RR 0.36, 95% CI 0.06 to 2.16; very low certainty), and aprepitant + palonosetron (17 of 1000; RR 0.48, 95% CI 0.05 to 4.78; very low certainty) on the risk of SAEs when compared to aprepitant + granisetron, respectively.
We could not include three treatment combinations (ezlopitant + granisetron, aprepitant + tropisetron, rolapitant + ondansetron) in NMA for this outcome because of missing direct comparisons.
Moderately emetogenic chemotherapy (MEC)
We included 38 studies reporting on 12,038 participants and comparing 15 treatment combinations with NK₁ and 5‐HT₃ inhibitors, or 5‐HT₃ inhibitors solely. All treatment combinations included corticosteroids.
Complete control of vomiting during the overall phase
We estimated that 555 of 1000 participants achieve complete control of vomiting in the overall treatment phase (one to five days) when treated with granisetron. Evidence from NMA (22 RCTs, 7800 participants; 11 treatment combinations) suggests that the following drug combinations are more efficacious than granisetron in completely controlling vomiting during the overall treatment phase (one to five days): aprepitant + palonosetron (716 of 1000; RR 1.29, 95% CI 1.00 to 1.66; low certainty), netupitant + palonosetron (694 of 1000; RR 1.25, 95% CI 0.92 to 1.70; low certainty), and rolapitant + granisetron (660 of 1000; RR 1.19, 95% CI 1.06 to 1.33; high certainty).
Palonosetron (588 of 1000; RR 1.06, 95% CI 0.85 to 1.32; low certainty) and aprepitant + granisetron (577 of 1000; RR 1.06, 95% CI 0.85 to 1.32; low certainty) may or may not increase complete response in the overall treatment phase (one to five days) when compared to granisetron, respectively. Azasetron (560 of 1000; RR 1.01, 95% CI 0.76 to 1.34; low certainty) may result in little to no difference in complete response in the overall treatment phase (one to five days) when compared to granisetron.
Evidence further suggests that the following drug combinations are less efficacious than granisetron in completely controlling vomiting during the overall treatment phase (one to five days) (ordered by decreasing efficacy): fosaprepitant + ondansetron (500 of 1000; RR 0.90, 95% CI 0.66 to 1.22; low certainty), aprepitant + ondansetron (477 of 1000; RR 0.86, 95% CI 0.64 to 1.17; low certainty), casopitant + ondansetron (461 of 1000; RR 0.83, 95% CI 0.62 to 1.12; low certainty), and ondansetron (433 of 1000; RR 0.78, 95% CI 0.59 to 1.04; low certainty).
We could not include five treatment combinations (fosaprepitant + granisetron, azasetron, dolasetron, ramosetron, tropisetron) in NMA for this outcome because of missing direct comparisons.
Serious adverse events
We estimated that 153 of 1000 participants experience any SAEs when treated with granisetron. Evidence from pair‐wise comparison (1 RCT, 1344 participants) suggests that more participants may experience SAEs when treated with rolapitant + granisetron (176 of 1000; RR 1.15, 95% CI 0.88 to 1.50; low certainty). NMA was not feasible for this outcome because of missing direct comparisons.
Certainty of evidence
Our main reason for downgrading was serious or very serious imprecision (e.g. due to wide 95% CIs crossing or including unity, few events leading to wide 95% CIs, or small information size). Additional reasons for downgrading some comparisons or whole networks were serious study limitations due to high risk of bias or moderate inconsistency within networks.
Authors' conclusions
This field of supportive cancer care is very well researched. However, new drugs or drug combinations are continuously emerging and need to be systematically researched and assessed.
For people receiving HEC, synthesised evidence does not suggest one superior treatment for prevention and control of chemotherapy‐induced nausea and vomiting.
For people receiving MEC, synthesised evidence does not suggest superiority for treatments including both NK₁ and 5‐HT₃ inhibitors when compared to treatments including 5‐HT₃ inhibitors only. Rather, the results of our NMA suggest that the choice of 5‐HT₃ inhibitor may have an impact on treatment efficacy in preventing CINV.
When interpreting the results of this systematic review, it is important for the reader to understand that NMAs are no substitute for direct head‐to‐head comparisons, and that results of our NMA do not necessarily rule out differences that could be clinically relevant for some individuals.
Keywords: Adult, Humans, Antiemetics, Antiemetics/therapeutic use, Antineoplastic Agents, Antineoplastic Agents/adverse effects, Nausea, Nausea/chemically induced, Nausea/drug therapy, Nausea/prevention & control, Network Meta-Analysis, Palonosetron, Palonosetron/therapeutic use, Randomized Controlled Trials as Topic, Vomiting, Vomiting/chemically induced, Vomiting/drug therapy, Vomiting/prevention & control
Plain language summary
Which drug combinations are best for prevention of nausea and vomiting caused by chemotherapy in adults with cancer?
The burden of nausea and vomiting caused by chemotherapy and what helps to prevent it?
In about 70% to 80% of adults with cancer, chemotherapy induces nausea and vomiting (CINV). Depending on the type of chemotherapy, treatment can cause strong or moderate sickness (hereafter referred to as HEC (highly emetogenic chemotherapy) and MEC (moderately emetogenic chemotherapy)). Multiple drug combinations have showed high benefit for CINV among adults receiving HEC or MEC.
What was the aim of our review?
Using a network meta‐analysis, we aimed to compare the benefits and harms of different drug combinations for prevention of CINV among people receiving HEC or MEC, and to identify treatment ranking. A network meta‐analysis is a technique used to compare different treatments described in already published trials, even when the original individual trial does not describe such comparisons.
What studies did we look at?
We searched selected medical databases and trial registries until February 2021. We included studies comparing multiple drug combinations for prevention of CINV among adults with any type of cancer receiving HEC or MEC that is commonly used in clinical practice. In particular, we looked at drugs inhibiting two specific biochemical receptors (neurokinin receptor and serotonin receptor) that trigger nausea and vomiting after chemotherapy. We looked at the preventative effects of these treatments over five days. This is the period during which the maximum intensity of CINV and further peaks of intensity are expected, after the start of chemotherapy.
Our key results...
...for people receiving HEC
We found 73 studies that reported on the experience of 25,275 participants and compared 14 treatment combinations of our interest.
Benefits. Over five days, investigated treatments helped to prevent any vomiting in 60% to 81% of people on average. Those individuals also had no need for rescue medicines, which are used in case nausea and vomiting occur even though prophylactic treatment has been given. The results of our analysis suggest some differences in effectiveness of different treatments, but overall we had little confidence that those differences would be reflected in real‐world observations.
Harms. We estimated that 1% to 4% of people experience serious side effects. The differences between treatments were small.
...for people receiving MEC
We found 38 studies that reported on the experience of 12,038 participants and compared 15 treatment combinations of our interest.
Benefits. Over five days, investigated treatments helped prevent any vomiting in 43% to 72% of people on average. Those individuals also had no need for rescue medicines. The results of our analysis suggest some differences in the effectiveness of different treatments, but overall, we had little confidence that those differences would be reflected in real‐world observations.
Harms. Few studies reported serious side effects. The ones that did suggest that on average 15% to 18% of people experience such events. Differences between treatments were small. However, we think that future research is needed to rule out potential differences between treatments.
Our confidence in the findings
We assessed how confident we were that there are differences between compared treatments. We had low or very low confidence that one treatment is better or worse than another in preventing CINV. Our confidence in differences between statistical results was mainly limited because measures of variation were wide apart and included both potential advantages and disadvantages, although measures of precision showed no or little effect. We also identified limitations in some of the included studies, which further limited our confidence in the effects. This was mainly the case when study personnel and participants knew which treatments were given and therefore may not adhere to the planned intervention, or may perceive or report effects differently.
Our conclusions
The results of our analysis suggest that there is no superior drug combination for prevention of CINV for people receiving HEC or MEC. However, results suggest that the choice of drugs targeting the serotonin receptor may impact effectiveness, irrespective of whether given with or without a drug targeting the neurokinin receptor. However, when interpreting these results, it is important for the reader to understand that these kinds of multiple‐comparison analyses are no substitute for head‐to‐head comparisons, and that the results do not necessarily rule out differences that could be clinically relevant for some individuals.
How up‐to‐date is this evidence?
Evidence is up‐to‐date to 2 February 2021.
Summary of findings
Summary of findings 1. Summary of findings: complete control of vomiting during the overall phase (HEC) when compared to treatment with aprepitant + granisetron.
| Efficacy | ||||||
| Antiemetics for adults for prevention of nausea and vomiting caused by highly emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by highly emetogenic chemotherapy Settings: inpatient and outpatient care Intervention: neurokinin‐1 (NK₁) receptor antagonist and 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroid Comparison: aprepitant (NK₁) combined with granisetron (5‐HT₃) + corticosteroid Outcome: complete control of vomiting during the overall phase (0 to 120 h of treatment with chemotherapy) RR < 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with aprepitant + granisetron | Corresponding risk with the intervention | |||||
| fosnetupitant + palonosetron | 704 of 1000 | 810 of 1000 (683 to 944) | RR 1.15 (0.97 to 1.37) | 21,642 (39) | ⊕⊕⊕⊝ moderateb |
Fosnetupitant + palonosetron probably increases complete response in the overall phase when compared with aprepitant + granisetron |
| aprepitant + palonosetron | 704 of 1000 | 753 of 1000 (690 to 831) | RR 1.07 (0.98 to 1.18) | 21,642 (39) | ⊕⊕⊝⊝ lowb,c |
Aprepitant + palonosetron may result in a slight increase in complete response in the overall phase when compared with aprepitant + granisetron |
| aprepitant + ramosetron | 704 of 1000 | 753 of 1000 (669 to 852) | RR 1.07 (0.95 to 1.21) | 21,642 (39) | ⊕⊕⊝⊝ lowb,c |
Aprepitant + ramosetron may result in a slight increase in complete response in the overall phase when compared with aprepitant + granisetron |
| fosaprepitant + palonosetron | 704 of 1000 | 746 of 1000 (676 to 838) | RR 1.06 (0.96 to 1.19) | 21,642 (39) | ⊕⊕⊝⊝ lowb,c |
Fosaprepitant + palonosetron may result in a slight increase in complete response in the overall phase when compared with aprepitant + granisetron |
| netupitant + palonosetron | 704 of 1000 | 704 of 1000 (655 to 760) | RR 1.00 (0.93 to 1.08) | 21,642 (39) | ⊕⊕⊕⊕ high |
Netupitant + palonosetron has little to no impact on complete response in the overall phase when compared with aprepitant + granisetron |
| fosaprepitant + granisetron | 704 of 1000 | 697 of 1000 (655 to 746) | RR 0.99 (0.93 to 1.06) | 21,642 (39) | ⊕⊕⊕⊕ high |
Fosaprepitant + granisetron has little to no impact on complete response in the overall phase when compared with aprepitant + granisetron |
| aprepitant + ondansetron | 704 of 1000 | 676 of 1000 (620 to 739) | RR 0.96 (0.88 to 1.05) | 21,642 (39) | ⊕⊕⊝⊝ lowb,c |
Aprepitant + ondansetron may result in a slight decrease in complete response in the overall phase when compared with aprepitant + granisetron |
| fosaprepitant + ondansetron | 704 of 1000 | 662 of 1000 (598 to 732) | RR 0.94 (0.85 to 1.04) | 21,642 (39) | ⊕⊕⊝⊝ lowb,c |
Fosaprepitant + ondansetron may result in a slight decrease in complete response in the overall phase when compared with aprepitant + granisetron |
| casopitant + ondansetron | 704 of 1000 | 634 of 1000 (556 to 725) | RR 0.90 (0.79 to 1.03) | 21,642 (39) | ⊕⊕⊝⊝ lowb,c |
Aprepitant + ondansetron may decrease complete response in the overall phase when compared with aprepitant + granisetron |
| rolapitant + granisetron | 704 of 1000 | 627 of 1000 (549 to 711) | RR 0.89 (0.78 to 1.01) | 21,642 (39) | ⊕⊕⊕⊝ moderateb |
Rolapitant + granisetron probably decreases complete response in the overall phase when compared with aprepitant + granisetron |
| rolapitant + ondansetron | 704 of 1000 | 598 of 1000 (458 to 788) | RR 0.85 (0.65 to 1.12) | 21,642 (39) | ⊕⊕⊝⊝ lowc,d |
Rolapitant + ondansetron may decrease complete response in the overall phase when compared with aprepitant + granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 1312 of 1863 (70.4%) participants treated with aprepitant + granisetron achieved complete response during the overall phase (aprepitant + granisetron was used in 7 studies reporting the outcome). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded once for serious imprecision because 95% CIs cross unity. cDowngraded once for serious study limitations due to high risk of bias. dDowngraded once for serious imprecision due to wide confidence intervals. | ||||||
Summary of findings 2. Summary of findings: serious adverse events (HEC) when compared to treatment with aprepitant + granisetron.
| Safety | ||||||
| Antiemetics for adults for prevention of nausea and vomiting caused by highly emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by highly emetogenic chemotherapy Settings: inpatient and outpatient care Intervention: neurokinin‐1 (NK₁) receptor antagonist and 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroid Comparison: aprepitant (NK₁) combined with granisetron (5‐HT₃) + corticosteroid Outcome: serious adverse events RR < 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with aprepitant + granisetron | Corresponding risk with the intervention | |||||
| aprepitant + ondansetron | 35 of 1000 | 8 of 1000 (1 to 40) | RR 0.22 (0.04 to 1.14) | 16,065 (23) | ⊕⊝⊝⊝ very lowb,c,d |
Evidence is very uncertain about the effect of aprepitant + ondansetron on risk of serious adverse events when compared to aprepitant + granisetron |
| fosaprepitant + ondansetron | 35 of 1000 | 8 of 1000 (2 to 37) | RR 0.23 (0.05 to 1.07) | 16,065 (23) | ⊕⊕⊝⊝ lowb,c |
Fosaprepitant + ondansetron may decrease the risk of serious adverse events slightly when compared to aprepitant + granisetron |
| casopitant + ondansetron | 35 of 1000 | 8 of 1000 (1 to 49) | RR 0.24 (0.04 to 1.39) | 16,065 (23) | ⊕⊕⊝⊝ lowb,c |
Casopitant + ondansetron may decrease the risk of serious adverse events slightly when compared to aprepitant + granisetron |
| netupitant + palonosetron | 35 of 1000 | 9 of 1000 (2 to 55) | RR 0.27 (0.05 to 1.58) | 16,065 (23) | ⊕⊕⊝⊝ lowb,c |
Netupitant + palonosetron may decrease the risk of serious adverse events slightly when compared to aprepitant + granisetron |
| aprepitant + ramosetron | 35 of 1000 | 11 of 1000 (2 to 67) | RR 0.31 (0.05 to 1.90) | 16,065 (23) | ⊕⊝⊝⊝ very lowb,c,d |
Evidence is very uncertain about the effect of aprepitant plus ramosetron on risk of serious adverse events when compared to aprepitant + granisetron |
| fosaprepitant + palonosetron | 35 of 1000 | 12 of 1000 (1 to 103) | RR 0.35 (0.04 to 2.95) | 16,065 (23) | ⊕⊝⊝⊝ very lowb,e |
Evidence is very uncertain about the effect of fosaprepitant + palonosetron on risk of serious adverse events when compared to aprepitant + granisetron |
| fosnetupitant + palonosetron | 35 of 1000 | 13 of 1000 (2 to 76) | RR 0.36 (0.06 to 2.16) | 16,065 (23) | ⊕⊝⊝⊝ very lowb,e |
Evidence is very uncertain about the effect of fosnetupitant + palonosetron on risk of serious adverse events when compared to aprepitant + granisetron |
| fosaprepitant + granisetron | 35 of 1000 | 13 of 1000 (3 to 53) | RR 0.37 (0.09 to 1.50) | 16,065 (23) | ⊕⊕⊝⊝ lowb,c |
Fosaprepitant + granisetron may decrease the risk of serious adverse events slightly when compared to aprepitant + granisetron |
| aprepitant + palonosetron | 35 of 1000 | 17 of 1000 (2 to 167) | RR 0.48 (0.05 to 4.78) | 16,065 (23) | ⊕⊝⊝⊝ very lowb,d,e |
Evidence is very uncertain about the effect of aprepitant + palonosetron on risk of serious adverse events when compared to aprepitant + granisetron |
| rolapitant + granisetron | 35 of 1000 | 20 of 1000 (7 to 60) | RR 0.57 (0.19 to 1.70) | 16,065 (23) | ⊕⊕⊝⊝ lowb,c |
Rolapitant + granisetron may decrease the risk of serious adverse events slightly when compared to aprepitant + granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 20 of 573 (3.5%) participants treated with aprepitant + granisetron experienced at least 1 SAE (aprepitant + granisetron was used in 2 studies reporting the outcome, with follow‐up of up to 29 days). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded once for moderate inconsistency. cDowngraded once for serious imprecision because 95% CIs cross unity and confidence intervals are wide. dDowngraded once for serious study limitations due to high risk of bias. eDowngraded twice for very serious imprecision because 95% CIs cross unity and confidence intervals are very wide, suggesting high possibility of harm. | ||||||
Summary of findings 3. Summary of findings: complete control of vomiting during the overall phase (MEC) when compared to treatment with granisetron.
| Efficacy | ||||||
| Antiemetics for adults for prevention of nausea and vomiting caused by moderately emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by moderately emetogenic chemotherapy Settings: inpatient and outpatient care Intervention
Comparison: granisetron (5‐HT₃) + corticosteroid Outcome: complete control of vomiting during the overall phase (0 to 120 h of treatment with chemotherapy) RR < 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with granisetron | Corresponding risk with the intervention | |||||
| aprepitant + palonosetron | 555 of 1000 | 716 of 1000 (555 to 921) | RR 1.29 (1.00 to 1.66) | 7800 (22) | ⊕⊕⊝⊝ lowb,c |
Aprepitant + palonosetron may increase complete response in the overall phase when compared to granisetron |
| netupitant + palonosetron | 555 of 1000 | 694 of 1000 (510 to 944) | RR 1.25 (0.92 to 1.70) | 7800 (22) | ⊕⊕⊝⊝ lowb,d |
Netupitant + palonosetron may increase complete response in the overall phase when compared to granisetron |
| rolapitant + granisetron | 555 of 1000 | 660 of 1000 (588 to 738) | RR 1.19 (1.06 to 1.33) | 7800 (22) | ⊕⊕⊕⊕ high |
Rolapitant + granisetron results in an increase in complete response in the overall phase when compared to granisetron |
| palonosetron | 555 of 1000 | 588 of 1000 (472 to 733) | RR 1.06 (0.85 to 1.32) | 7800 (22) | ⊕⊕⊝⊝ lowb,d |
Palonosetron may or may not increase complete response in the overall phase when compared to granisetron |
| aprepitant + granisetron | 555 of 1000 | 577 of 1000 (483 to 694) | RR 1.06 (0.85 to 1.32) | 7800 (22) | ⊕⊕⊝⊝ lowb,d |
Aprepitant + palonosetron may or may not increase complete response in the overall phase when compared to granisetron |
| azasetron | 555 of 1000 | 561 of 1000 (422 to 738) | RR 1.01 (0.76 to 1.33) | 7800 (22) | ⊕⊕⊝⊝ lowb,e |
Azasetron may result in little to no difference in complete response in the overall phase when compared to granisetron |
| fosaprepitant + ondansetron | 555 of 1000 | 500 of 1000 (366 to 677) | RR 0.90 (0.66 to 1.22) | 7800 (22) | ⊕⊕⊝⊝ lowb,d |
Fosaprepitant + ondansetron may decrease complete response in the overall phase when compared to granisetron |
| aprepitant + ondansetron | 555 of 1000 | 477 of 1000 (355 to 649) | RR 0.86 (0.64 to 1.17) | 7800 (22) | ⊕⊕⊝⊝ lowb,d |
Aprepitant + ondansetron may decrease complete response in the overall phase when compared to granisetron |
| casopitant + ondansetron | 555 of 1000 | 461 of 1000 (344 to 622) | RR 0.83 (0.62 to 1.12) |
7800 (22) | ⊕⊕⊝⊝ lowb,d |
Casopitant + ondansetron may decrease complete response in the overall phase when compared to granisetron |
| ondansetron | 555 of 1000 | 433 of 1000 (327 to 577) | RR 0.78 (0.59 to 1.04) | 7800 (22) | ⊕⊕⊝⊝ lowb,d |
Ondansetron may decrease complete response in the overall phase when compared to granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 623 of 1123 (55.5%) participants treated with granisetron achieved complete response during the overall phase (granisetron was used in 5 studies reporting the outcome). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded once for serious study limitations due to high risk of bias. cDowngraded once for serious imprecision because 95% CIs included zero effect line. dDowngraded once for serious imprecision because 95% CIs cross unity. eDowngraded once for serious imprecision due to wide confidence intervals. | ||||||
Summary of findings 4. Summary of findings: serious adverse events (MEC) when compared to treatment with granisetron.
| Safety | ||||||
| Antiemetics for adults for prevention of nausea and vomiting caused by moderately emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by moderately emetogenic chemotherapy Settings: inpatient and outpatient care Intervention
Comparison: granisetron (5‐HT₃) + corticosteroid Outcome: serious adverse events RR < 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with granisetron | Corresponding risk with the intervention | |||||
| rolapitant + granisetron | 153 of 1000 | 176 of 1000 (135 to 230) | RR 1.15 (0.88 to 1.50) | 1344 (1) | ⊕⊕⊝⊝ lowb |
Rolapitant + granisetron may increase the risk of serious adverse events slightly when compared to granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 103 of 674 (10.3%) participants treated with granisetron experienced at least 1 SAE (granisetron was used in 1 study reporting the outcome; time frame for reporting safety data was not described). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded twice for very serious imprecision because 95% CIs cross unity, confidence intervals are wide, and information size is small. | ||||||
Background
Description of the condition
Many cancer patients, both with solid tumours and with haematological malignancies, suffer from chemotherapy‐induced nausea and vomiting (CINV), which is an important contributor to morbidity, poor performance status, and decreased quality of life (Feyer 2011; Jordan 2015). The reported age‐adjusted incidence rate of cancer in the USA in 2010 was 464.6 per 100,000, and the mortality rate was 199.8 per 100,000 persons per year (Howlader 2013). Without appropriate antiemetic therapy, 70% to 80% of cancer patients receiving chemotherapy develop CINV (Feyer 2011). This condition is classified into five categories, depending on the start of CINV in relation to the start of chemotherapy and patients’ negative previous experiences (Navari 2016; Tageja 2016).
Acute: nausea and vomiting occurring within the first 24 hours of treatment with chemotherapy, with maximal intensity after five to six hours; activated through a peripheral pathway in which 5‐hydroxytryptamine‐3 (5‐HT₃) receptor activation plays a role.
Delayed: nausea and vomiting occurring from 24 hours to 120 hours of treatment with chemotherapy, with peaks of intensity between 48 and 72 hours; activated through a central pathway in which neurokinin‐1 (NK₁) receptor activation is involved.
Breakthrough: nausea and vomiting occurring although appropriate prophylaxis has been administered.
Anticipatory: conditioned response to the occurrence of CINV in previous chemotherapy cycles resulting in nausea and vomiting.
Refractory: nausea and vomiting recurring in subsequent cycles of chemotherapy, excluding anticipatory CINV.
In this review, we will focus on prevention of acute and delayed CINV.
Several prognostic factors such as younger age, female sex, previous hyperemesis gravidarum or history of vomiting in pregnancy, and motion sickness have been found to increase the likelihood of CINV (Di Mattei 2016; Dranitsaris 2017; Furukawa 2014; Hu 2016; Warr 2014); regular alcohol consumption has been found to reduce the risk of CINV (Hesketh 2010; Hu 2016).
CINV remains one of the most distressing symptoms associated with cancer therapy and can lead to dehydration, electrolyte imbalances, malnutrition, and metabolic disturbances (Viale 2012). Moreover, CINV is associated with decreased adherence to chemotherapy, which could lead to a decreased response resulting in increased risk of death among cancer patients (Wozniak 1998). Therefore, preventing CINV is an important goal for cancer patients.
According to the Multinational Association of Supportive Care in Cancer (MASCC)/European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO), practice focuses on the emetogenicity of chemotherapeutic agents (minimal, low, moderate, high) and the relative doses of antineoplastic agents used (Basch 2012; Jordan 2017; Roila 2016).
Highly emetogenic chemotherapy includes the following agents or combinations of agents (Basch 2012).
Anthracycline/cyclophosphamide combination.
Carmustine.
Cisplatin.
Cyclophosphamide ≥ 1500 mg/m².
Dacarbazine.
Hexamethylmelamine.
Mechlorethamine.
Procarbazine.
Streptozocin.
Moderately emetogenic chemotherapy includes the following agents (Basch 2012).
Alemtuzumab.
Azacitidine.
Bendamustine.
Bosutinib.
Carboplatin.
Ceritinib.
Clofarabine.
Crizotinib.
Cyclophosphamide 1000 mg/m².
Daunorubicin.
Doxorubicin.
Epirubicin.
Idarubicin.
Ifosfamide.
Imatinib.
Irinotecan.
Oxaliplatin.
Romidepsin.
Temozolomide.
Thiotepa.
Trabectedin.
Vinorelbine.
According to the latest MASCC/ESMO guidelines, carboplatin has higher emetogenic potential compared to the other moderately emetogenic agents; patients receiving this drug should receive the same prophylaxis as described for patients with highly emetogenic potential (Jordan 2017; Roila 2016).
With appropriate antiemetic prophylaxis, acute CINV and delayed CINV are clinically significantly reduced. In a recent systematic review, Yuan and colleagues found that the complete response rate of patients receiving an NK₁ receptor antagonist was significantly higher compared to that seen in patients given various control regimens (like 5‐HT₃ receptor antagonists + dexamethasone), with complete response in the acute phase of 85.1% versus 79.6% and complete response in the delayed phase of 71.4% versus 58.2%. According to review authors, the safety profile of NK₁ receptor antagonists was comparable to that of other regimens, with less insomnia but more diarrhoea and hiccups (Yuan 2016).
Description of the intervention
Options for prevention of CINV are 5‐HT₃ receptor antagonists (e.g. ondansetron, granisetron, palonosetron) in combination with corticosteroids (e.g. dexamethasone), or additionally combined with NK₁ receptor antagonists (e.g. aprepitant, fosaprepitant, netupitant, rolapitant). Although antiemetic therapy is common among cancer patients at risk for CINV, recommendations provided in current guidelines are inconsistent. According to ASCO, practice focuses on the emetogenicity of chemotherapeutic agents (minimal, low, moderate, high) and the relative dose of antineoplastic agents used. This guideline recommends 5‐HT₃ receptor antagonists plus dexamethasone for patients administered moderately emetogenic chemotherapy (Basch 2012). The latest update of this guideline for patients receiving highly emetogenic chemotherapy (including anthracycline + cyclophosphamide) recommends a combination of an NK₁ receptor antagonist, a 5‐HT₃ receptor antagonist, and dexamethasone. The oral combination of palonosetron, netupitant, and dexamethasone is one of the specific treatments recommended for these patients (Hesketh 2016). The recommendation in the moderately emetogenic setting is less clear. In the latest MASCC/ESMO guideline report, it was acknowledged that carboplatin‐based chemotherapy might have higher risk of nausea and vomiting compared to other drugs in the category moderately emetogenic chemotherapy (Roila 2016). The MASCC/ESMO guideline recommends the same three‐drug combination as the ASCO guideline for patients receiving highly emetogenic chemotherapy (including anthracycline plus cyclophosphamide) but points out that no published comparative studies have identified differences in efficacy and toxicity between available NK₁ receptor antagonists to recommend one specific drug over another (Roila 2016). In the so called other moderate emetogenic risk group, a 5‐HT₃ receptor antagonist + dexamethasone is still standard of care, although National Comprehensive Cancer Network (NCCN) guidelines broaden the indication for an NK₁ receptor antagonist in this risk category (Ettinger 2017).
Another option for prevention and treatment of CINV‐ or radiotherapy‐induced nausea and vomiting is olanzapine. However, evidence for efficacy and safety of this drug is not yet clear and is being evaluated in a Cochrane Review (Cochrane protocol already published: Sutherland 2017).
How the intervention might work
For 5‐HT₃ receptor antagonists and dexamethasone, solely or in combination with NK₁ receptor antagonists, systematic reviews and meta‐analyses have shown that they improve CINV in cancer patients administered especially highly emetogenic chemotherapy including anthracycline‐cyclophosphamide‐based chemotherapy, with inconclusive evidence on effectiveness and rates of adverse events for one drug compared to another (Celio 2013; dos Santos 2013; Hocking 2014; Jin 2012; Jordan 2016b; Lee 2013; Popovic 2014).
As the central nervous system, the neurotransmitter, and their receptors play a critical role in CINV, both 5‐HT₃ receptor antagonists and NK₁ receptor antagonists inhibit processing of antiemetic signals from the gut to the central nervous system (Janelsins 2013). Both drugs are usually combined with dexamethasone to improve efficacy. A pilot randomised controlled trial (RCT) including 31 patients receiving cisplatin chemotherapy has shown first improved efficacy of ondansetron when combined with dexamethasone and a good safety profile (Smith 1991). Another RCT conducted between 1992 and 1994 for patients receiving moderately emetogenic chemotherapy has shown highest efficacy in terms of complete protection of vomiting and nausea and less delayed vomiting and nausea with the combination of granisetron and dexamethasone compared to granisetron or dexamethasone. No severe adverse events were reported, but constipation and hot flushes were more often found in the granisetron + dexamethasone arm compared to single‐drug arms (Italian Group for Antiemetic Research 1995). Granisetron + dexamethasone has also shown improved efficacy compared to both single drugs only among patients receiving cisplatin chemotherapy (Heron 1994). Thereafter, a 5‐HT₃ receptor antagonist combined with dexamethasone became standard prophylaxis for preventing CINV (Gralla 1999).
As NK₁ receptor antagonists inhibit another receptor in the emetic signal activation, they are combined with 5‐HT₃ receptor antagonists and dexamethasone for patients receiving cisplatin or other highly emetogenic chemotherapeutic agents (Hesketh 2003; Poli‐Bigelli 2003).
Why it is important to do this review
As mentioned above, the decision‐making process for prevention of CINV is usually confusing for patients and physicians, as there are no clear recommendations in international guidelines for a consistent approach to the use of antiemetic agents (Hesketh 2016; Roila 2016). Economic arguments are introduced in discussions on the best strategy, as direct and indirect costs differ enormously for various treatment options, and this could lead to increased healthcare costs (Avritscher 2010; Humphreys 2013). In addition, direct head‐to‐head comparisons of prophylactic options are too sparse to favour one drug or a combined drug regimen over another.
The aim of our systematic review and network analysis is to provide a comprehensive overview on the benefits and harms of antiemetic agents for CINV. By systematically identifying all relevant RCTs conducted to date and critically reviewing their reliability and validity while considering similar trials in the network analysis, we will overcome statistical limitations of individual studies. The network meta‐analysis will allow a hierarchy of therapeutic options, in particular, if the benefits of one option compared to another will translate into a clinically important difference. This comprehensive overview is necessary for clinical decision‐making, and it has the potential to have a great impact on international guidelines and clinical pathways. Moreover, it may contribute to high‐grade decision support for effective therapeutic strategies for the individual person.
The results of this network meta‐analysis will be published in the Cochrane Library and presented at national and international expert meetings and conferences (e.g. American Society of Clinical Oncology, Multinational Association of Supportive Care in Cancer). Results of the network analysis have the potential to influence the design of new RCTs for antiemetic agents. As we have evaluated patient‐related outcomes, a direct impact on patient care and treatment might be expected.
Objectives
-
In adults with solid cancer or haematological malignancy receiving highly emetogenic chemotherapy
To compare the effects of antiemetic treatment combinations including NK₁ receptor antagonists, 5‐HT₃ receptor antagonists, and corticosteroids on prevention of acute phase (Day 1), delayed phase (Days 2 to 5), and overall (Days 1 to 5) chemotherapy‐induced nausea and vomiting in network meta‐analysis
To generate a clinically meaningful treatment ranking according to treatment safety and efficacy
-
In adults with solid cancer or haematological malignancy receiving moderately emetogenic chemotherapy
To compare whether antiemetic treatment combinations including NK₁ receptor antagonists, 5‐HT₃ receptor antagonists, and corticosteroids are superior for prevention of acute phase (Day 1), delayed phase (Days 2 to 5), and overall (Days 1 to 5) chemotherapy‐induced nausea and vomiting to treatment combinations including 5‐HT₃ receptor antagonists and corticosteroids solely, in network meta‐analysis
To generate a clinically meaningful treatment ranking according to treatment safety and efficacy
Methods
Criteria for considering studies for this review
Types of studies
The protocol for this review was previously published in the Cochrane Library (Skoetz 2017). Any differences to the protocol are described in Differences between protocol and review.
We included studies if they were randomised controlled trials (RCTs), which are best designed to minimise bias when evaluating the effectiveness of an intervention. We required full journal publication, with the exception of online clinical trial results and summaries of otherwise unpublished clinical trials and abstracts with sufficient data for analysis. We considered only results from the first cycle, regardless of potential cross‐over. We included blinded and non‐blinded studies, and we addressed the potential impact of blinding in our bias assessment and sensitivity analyses. We applied no limitations with respect to length of follow‐up. However, we considered only results from the first cycle.
We excluded studies that were cluster‐randomised or non‐randomised, as well as case reports and clinical observations.
Types of participants
Studies included trials involving adult patients according to the definition provided in the studies (usually ≧ 18 years of age), with a confirmed diagnosis of cancer, irrespective of type and stage of cancer and gender. We included both patients with solid cancer and patients with haematological malignancies. We included trials that included patients receiving highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC) according to the latest Antineoplastic Agents Emetic Risk Classification (Jordan 2017; Roila 2016). As this classification has changed over the years (e.g. anthracycline and cyclophosphamide combination is nowadays classified as HEC instead of MEC), we used this classification to assess the emetogenic risk of one specific chemotherapeutic agent, irrespective of the emetogenic risk applied by study authors (see section Description of the condition). We performed separate analyses for populations receiving HEC and MEC, according to the definition provided by Multinational Association of Supportive Care in Cancer (MASCC)/European Society of Medical Oncology (ESMO) (Jordan 2017; Roila 2016). We assumed that patients who fulfil the inclusion criteria were equally eligible to be randomised to any of the interventions that we had planned to compare.
We excluded trials including participants not receiving emetogenic chemotherapies at the same level of risk, which did not provide subgroup data for each emetogenic risk group. We excluded trials evaluating participants at risk for radiotherapy‐induced nausea and vomiting. We excluded trials evaluating participants at risk of vomiting and nausea due to underlying disease.
Types of interventions
At the time this review was produced, recommended antiemetics for prophylaxis of chemotherapy‐induced nausea and vomiting (CINV) caused by highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC) included the following drug combinations.
5‐Hydroxytryptamine‐3 (5‐HT₃) receptor antagonists and corticosteroids.
Neurokinin‐1 (NK₁) receptor antagonists, 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists, and corticosteroids.
We compared combinations of these interventions at any dose and by any route versus each other in a full network. We included all RCTs comparing in at least two study arms the intervention of interest ‐ either 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroids, or neurokinin‐1 (NK₁) receptor antagonists in combination with 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroids.
We included only trials that included patients on corticosteroids in both arms.
We analysed prophylaxis for cancer patients administered HEC or MEC separately. We assumed that any participant who met the inclusion criteria was, in principle, equally likely to be randomised to any of the eligible interventions. We grouped interventions by evaluating different drug doses together as one drug of interest, according to the product characteristics.
We excluded trials evaluating solely treatment of nausea and vomiting, meaning that the drug is not given before chemotherapeutic agents are administered to prevent CINV but rather once nausea or vomiting appears. Antiemetic agents to treat CINV might be the same agents used for prevention of CINV, but to include clinically homogenous trials to answer the research question, we focused on prophylaxis only.
Comparisons of direct interest
As mentioned above, current guidelines are highly uncertain about whether to recommend a doublea or tripleb drug combination for cancer patients receiving MEC, and which triple regimen should be administered to cancer patients receiving HEC (Basch 2012; Hesketh 2016; Roila 2016). Therefore the following comparisons are of direct interest.
aDouble drug combination: treatments including a 5‐HT₃ receptor antagonist and corticosteroids.
bTriple drug combination: treatments including an NK₁ receptor antagonist, a 5‐HT₃ receptor antagonist, and corticosteroids.
Comparisons in cancer patients receiving highly emetogenic chemotherapy (HEC)
NK₁ receptor antagonist + 5‐HT₃ receptor antagonist + corticosteroid versus other specific combinations of these drug classes + corticosteroid
We additionally included the following comparisons to strengthen the network.
NK₁ receptor antagonist + 5‐HT₃ receptor antagonist + corticosteroid versus 5‐HT₃ receptor antagonist + corticosteroid.
5‐HT₃ receptor antagonist + corticosteroid versus other specific combinations of this drug class + corticosteroid.
An overview of all included treatment regimens is provided in Table 5.
1. Overview of treatment regimens and treatment abbreviations.
| Drug combinations | Treatment regimena | Abbreviation | Used in HECb setting | Used in MECc setting |
| NK₁ receptor antagonists and 5‐HT₃ receptor antagonists + corticosteroid | aprepitant with granisetron | apre_grani | X | X |
| aprepitant with ondansetron | apre_ondan | X | X | |
| aprepitant with palonosetron | apre_palo | X | X | |
| aprepitant with ramosetron | apre_ramo | X | ||
| aprepitant with tropisetron | apre_tropi | X | ||
| casopitant with ondansetron | caso_ondan | X | X | |
| fosaprepitant with granisetron | fosa_grani | X | X | |
| ezlopitant with granisetron | ezlo_grani | X | ||
| fosaprepitant with ondansetron | fosa_ondan | X | X | |
| fosaprepitant with palonosetron | fosa_palo | X | ||
| fosnetupitant with palonosetron | fosnetu_palo | X | ||
| netupitant with palonosetron | netu_palo | X | X | |
| rolapitant with granisetron | rola_grani | X | X | |
| rolapitant with ondansetron | rola_ondan | X | ||
| 5‐HT₃ receptor antagonists+ corticosteroid | azasetron | aza | X | X |
| dolasetron | dola | X | ||
| granisetron | grani | X | X | |
| ondansetron | ondan | X | X | |
| palonosetron | palo | X | X | |
| ramosetron | ramo | X | X | |
| tropisetron | tropi | X | X |
aAll treatment regimens also include a corticosteroid.
bHighly emetogenic chemotherapy.
cModerately emetogenic chemotherapy.
Comparisons in cancer patients receiving moderately emetogenic chemotherapy (MEC)
NK₁ receptor antagonist + 5‐HT₃ receptor antagonist + corticosteroid versus other specific drug combinations of this drug class + corticosteroid
NK₁ receptor antagonist + 5‐HT₃ receptor antagonist + corticosteroid versus 5‐HT₃ receptor antagonist + corticosteroid
5‐HT₃ receptor antagonist + corticosteroid versus other specific combinations of this drug class + corticosteroid
An overview of all included treatment regimens is provided in Table 5.
We evaluated different intervention doses and different routes of administration together, and we had planned to assess differences in subgroup analyses. However, we were not able to perform these subgroup analyses because networks were not connected when doses and routes were considered separately.
Additional interventions to supplement the analysis
In the HEC setting, we also included trials analysing the following comparisons in addition to the direct comparisons of interest, to increase the amount of available (indirect) information included in the analysis (Ades 2013; Chaimani 2017).
NK₁ receptor antagonist + 5‐HT₃ receptor antagonist + corticosteroid versus 5‐HT₃ receptor antagonist + corticosteroid.
5‐HT₃ receptor antagonist + corticosteroid versus other specific combinations of this drug class + corticosteroid.
Included trials should have been comparable in terms of clinical and methodological criteria to hold for transitivity (Chaimani 2017). Therefore, we excluded trials evaluating in only one arm an intervention of interest but in the control arm different drug classes (e.g. metoclopramide). We excluded these trials, as they evaluated drugs that are no longer recommended for primary prophylaxis of CINV in moderately and highly emetogenic chemotherapy. As these trials might be outdated, the assumption that any participant who met the inclusion criteria was, in principle, equally likely to be randomised to any of the eligible interventions has not been sustained. The efficacy and safety of cannabinoids were evaluated in the Cochrane Review by Smith and colleagues (Smith 2015); cannabinoids are not evaluated in this review.
Types of outcome measures
We included all trials fitting the inclusion criteria mentioned above, irrespective of reported outcomes. We estimated the relative ranking of competing interventions according to each of the following outcomes.
Efficacy
-
Complete control of nausea (no nausea and no significant nausea, as defined on a study levela), determined from reports in participant diaries; in the Results section, we refer to this outcome as "no nausea"
in the acute phase (first 24 hours of treatment with chemotherapy)
in the delayed phase (after 24 to 120 hours of treatment with chemotherapy)
overall (after 0 to 120 hours of treatment with chemotherapy)
-
Complete control of vomiting (no vomiting and no use of rescue medications), determined from reports in participant diaries; this outcome was usually referred to as "complete response" in the studies; we also refer to it in the Results section as "complete response"
in the acute phase (first 24 hours of treatment with chemotherapy)
in the delayed phase (after 24 to 120 hours of treatment with chemotherapy)
overall (after 0 to 120 hours of treatment with chemotherapy)
Quality of life
-
No impairment of quality of life, up to longest follow‐up available, if measured by validated instruments
Functional Living Index–Emesis (FLIE) (Lindley 1992)
Modified Functional Living Index–Emesis (M‐FLIE) (Martin 2003; Martin 2003a)
Safety
On‐study mortality (deaths occurring from randomisation up to 30 days)
Adverse events
Serious adverse events
Neutropenia
Febrile neutropenia
Infections
Local reactions at infusion site
Hiccups
For outcome measurement of any adverse events, we used the longest follow‐up available.
As there are different underlying mechanisms for anticipatory CINV, which do not respond to prophylactic antiemetics, we did not evaluate this outcome.
An overview of all outcomes and prioritisation of outcomes are provided in Table 6.
2. Overview of outcomes.
| Outcome | Definition | Unit of outcome measurement | Referred to as/abbreviation | Prioritisation |
| Complete control of nausea | No nausea and no significant nausea, as defined on a study levela Assessed for:
|
Binary; participants with complete control of nausea | No nausea | Overall phase prioritised for GRADE assessment |
| Complete control of vomiting | No vomiting and no use of rescue medications Assessed for:
|
Binary; participants with complete control of vomiting | Complete response (CR) | Delayed and overall phases prioritised for GRADE assessment Overall phase chosen as most important efficacy outcome |
| Quality of life | No impairment in quality of life during active study period | Binary; participants with no impairment in quality of life | QoL | Prioritised for GRADE assessment |
| On‐study mortality | Deaths occurring from randomisation up to 30 days after the active study period | Binary; participants who died | OSM | Prioritised for GRADE assessment |
| Adverse events | As defined on a study level; during active study period | Binary; participants with at least 1 event | AEs | ‐ |
| Serious adverse events | As defined on a study level; during active study period | Binary; participants with at least 1 event | SAEs | Prioritised for GRADE assessment Chosen as most crucial safety outcome |
| Neutropenia | As defined on a study level; during active study period | Binary; participants with at least 1 event | ‐ | ‐ |
| Febrile neutropenia | As defined on a study level; during active study period | Binary; participants with at least 1 event | ‐ | ‐ |
| Infection | As defined on a study level; during active study period | Binary; participants with at least 1 event | ‐ | ‐ |
| Local reaction at infusion site | As defined on a study level; during active study period | Binary; participants with at least 1 event | ‐ | Prioritised for GRADE assessment |
| Hiccup | As defined on a study level; during active study period | Binary; participants with at least 1 event | ‐ | ‐ |
aStandardised tools are typically used to assess degree of nausea and vomiting (Wood 2011). No nausea and no significant nausea were defined on a study level and typically refer to pre‐defined cutoffs, e.g. in Rapoport 2015 (a) or Schwartzberg 2015, nausea was assessed on a visual analogue scale (VAS; 0 to 100 mm; 0 = no nausea, 100 = severe nausea; < 5 mm = no nausea, < 25 mm = no significant nausea). No significant nausea is typically more subjective because of the wider range on the scale and is therefore less objective, especially in an open‐label study design. To increase comparability of studies and minimise biased results, we were therefore interested in patients with no nausea.
aStandardised tools are typically used to assess degree of nausea and vomiting (Wood 2011). No nausea and no significant nausea were defined on a study level and typically referred to pre‐defined cutoffs (e.g. in Rapoport 2015 (a) or Schwartzberg 2015, nausea was assessed on a visual analogue scale (VAS; 0 to 100 mm; 0 = no nausea, 100 = severe nausea) as < 5 mm for no nausea and < 25 mm for no significant nausea). No significant nausea is typically more subjective because of the wider range on the scale and therefore is less objective, especially with an open‐label study design. To increase comparability of studies and to minimise biased results, we were interested in patients with no nausea.
Search methods for identification of studies
Electronic searches
We searched the following databases without language restrictions. As all intervention arms had to include at least one 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonist, which has been mentioned first in 1988 for treatment of chemotherapy‐induced emesis (Carmichael 1988), we restricted our search from 1988 to present. Only trials that compared at least two of the drug combinations mentioned above are eligible. We searched for all possible comparisons formed by interventions of interest.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 2 of 12), in the Cochrane Library.
Embase (Ovid, 1988 to 2 February 2021).
MEDLINE (Ovid, 1988 to 2 February 2021).
We used medical subject headings (MeSH) or equivalent and text word terms. We did not apply any language restrictions, and we tailored searches to individual databases. The search strategies used can be found in Appendix 1.
Adverse effects
We did not perform a separate search for adverse effects of target interventions. We considered adverse effects only as described in included studies.
Searching other resources
In addition, we searched the following databases/sources.
-
Conference proceedings of annual meetings of the following societies if they were not included in CENTRAL (1988 to 2 February 2021).
American Society of Clinical Oncology (ASCO).
European Society of Medical Oncology (ESMO).
Multinational Association of Supportive Care in Cancer (MASCC).
Databases of ongoing trials.
clinicaltrials.gov (www.clinicaltrials.gov).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/).
Reference lists of reviews and retrieved articles for additional studies (we also performed citation searches on key articles).
We contacted experts in the field for unpublished and ongoing trials. We contacted study authors for additional information when necessary.
Data collection and analysis
Selection of studies
Two review authors independently screened results of the search strategies for eligibility for this review by reading the abstracts using Covidence software (Covidence systematic review software). We coded the abstracts as either 'retrieve' or 'do not retrieve'. In the case of disagreement, or if it was unclear whether we should have retrieved the abstract or not, we obtained the full‐text publication for further discussion. Independent review authors excluded records that clearly did not meet the inclusion criteria and obtained full‐text copies of the remaining records. Two review authors assessed these records independently against our pre‐defined eligibility criteria to identify relevant studies. In the event of disagreement, we adjudicated a third review author. We did not anonymise the studies before assessment. We included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart in the full review, which shows the status of identified studies (Figure 1; Moher 2009), as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Schünemann 2011). We included studies in the review irrespective of whether measured outcome data were reported in a ‘usable’ way.
1.
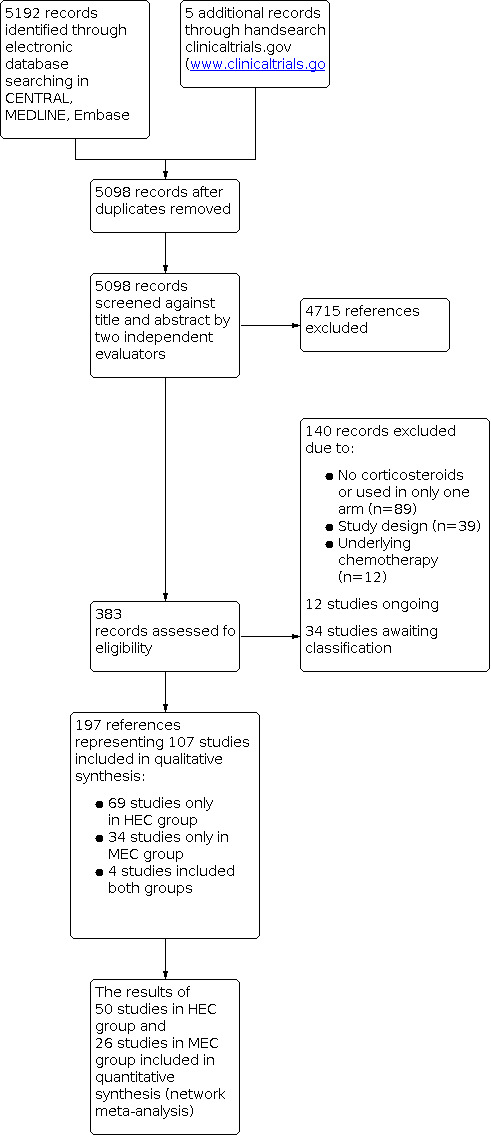
Study flow diagram.
Data extraction and management
Two review authors extracted data using a standardised data extraction form developed in Covidence (Covidence systematic review software). If these authors were unable to reach a consensus, we consulted a third review author for final decision. If required, we contacted the authors of specific studies for supplementary information (Higgins 2011a). After agreement had been reached, we entered data into Review Manager (RevMan 2014). We extracted the following information.
General information: author, title, source, publication date, country, language, duplicate publications.
Risk of bias assessment: sequence generation, allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting, other sources (not pre‐specified) of bias.
Moreover, we extracted the following information, which may have acted as effect modifiers.
Study characteristics: trial design, aims, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, subgroup analysis, statistical methods, power calculations, treatment cross‐overs, compliance with assigned treatment, length of follow‐up, time point of randomisation.
Participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, participants lost to follow‐up, cancer type and stage, additional diagnoses, type and intensity of antineoplastic therapy, emetogenic risk, other patient‐specific prognostic factors, e.g. pregnancy, motion sickness, alcohol intake.
Interventions and comparators: type and dosage of antiemetic agents, duration of prophylaxis, duration of follow‐up.
Outcomes: complete control of nausea (acute, delayed, and overall phases), complete control of vomiting (acute, delayed, and overall phases), on‐study mortality, quality of life, adverse events, and serious adverse events. When possible, we extracted data at arm level, not summary effects.
Notes: sponsorship/funding for trial and notable conflicts of interest of review authors.
We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a table of Characteristics of included studies.
Assessment of risk of bias in included studies
This section was taken from the PaPaS template for protocols and was amended to fit our analysis criteria.
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions ‐ Higgins 2011c ‐ and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with disagreements resolved by discussion. We completed a 'Risk of bias' table for each included study using the 'Risk of bias' tool in RevMan (RevMan 2014).
We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as low risk of bias (any truly random process, e.g. random number table; computer random number generator) or unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or could have been changed after assignment. We assessed these methods as low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes) or unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as low risk of bias (study stated that it was blinded and described the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique) or unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how this was achieved). We considered studies that were not double‐blinded to have high risk of bias. We assessed blinding separately for:
participants; and
personnel.
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as low risk of bias (study had a clear statement that outcome assessors were unaware of treatment allocation, and ideally described how this was achieved) or unclear risk of bias (study stated that outcome assessors were blind to treatment allocation but lacked a clear statement on how this was achieved). We considered studies for which outcome assessment was not blinded as having high risk of bias. We assessed the blinding of outcome assessment for two outcome categories.
Subjective outcomes (patient‐reported outcomes).
Objective outcomes (including mortality and safety).
-
Incomplete outcome data (checking for possible attrition bias due to the quantity, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as low risk (< 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis), unclear risk of bias (used 'last observation carried forward' analysis), or high risk of bias (used 'completer' analysis). We assessed attrition bias for two outcome categories.
Subjective outcomes (patient‐reported outcomes).
Objective outcomes (including mortality and safety).
Selective reporting (checking for reporting bias). We assessed whether primary and secondary outcome measures were pre‐specified, and whether these were consistent with those reported: low risk of bias (study protocol was available and all of the study’s pre‐specified (primary and secondary) outcomes that were of interest in the review have been reported in the pre‐specified way, or the study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were pre‐specified); unclear risk of bias (insufficient information to permit judgement of ‘low risk’ or ‘high risk'); or high risk of bias (not all of the study’s pre‐specified primary outcomes have been reported, or one or more primary outcomes are reported using measurements, analysis methods, or subsets of data that were not pre‐specified, or one or more reported primary outcomes were not pre‐specified, or one or more outcomes of interest in the review are reported incompletely so that they could not be entered into a meta‐analysis, or the study report failed to include results for a key outcome that would have been expected to have been reported for such a study).
-
Other sources of bias: we did not pre‐specify 'other sources of bias' that we were looking for in studies. This item provided us with freedom for potential causes of bias not listed otherwise, such as (but not limited to):
temporary halting of study;
midway protocol amendments, addition or removal of arms, treatment changes; and
additional medications provided based on subjective criteria.
We applied the following rule when making an overall risk of bias judgement per study.
| Overall risk of bias judgement | Criteria |
| Low risk of bias | The study is judged to be at low risk of bias for all domains for this result Or The study is judged to be at low risk of bias for most domains and at unclear risk of bias for selection, performance, and/or detection bias |
| Unclear risk of bias | The study is judged to be at unclear risk of bias in at least 1 of the domains of incomplete outcome data, selective reporting, or other bias for this outcome, but not to be at high risk of bias for any domain |
| High risk of bias | The study is judged to be at high risk of bias in at least 1 domain for this result |
Measures of treatment effect
Relative treatment effect
We used intention‐to‐treat data. For binary outcomes, we used risk ratios (RRs) with 95% confidence intervals (CIs) as the measure of treatment effect. We had planned to calculate continuous outcomes as mean differences (MDs) with 95% CIs, but we did not measure the effect of any outcome using continuous data. In case outcomes would have been reported as continuous data, and different instruments were used, we had planned to use standardised mean differences (SMDs) with 95% CIs to assess the extent of effects.
Relative treatment ranking
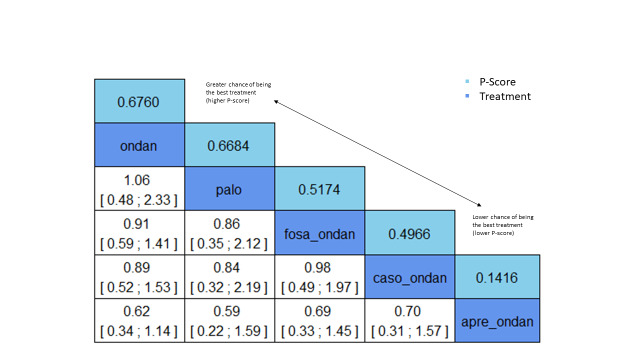
We obtained a treatment hierarchy using P scores (Rücker 2015). P scores allow ranking of treatments on a continuous zero to 1 scale in a frequentist network meta‐analysis.
Unit of analysis issues
We considered only results from the first treatment cycle. RCTs with a cross‐over design were eligible, as long as results had been reported after the first cycle, and therefore before cross‐over.
Studies with multiple treatment groups
As recommended in Chapter 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), for studies with multiple treatment groups, we combined arms as long as they could be regarded as subtypes of the same intervention.
When arms could not be pooled this way, we compared each arm with the common comparator separately. For pair‐wise meta‐analysis, we split the ‘shared’ group into two or more groups with smaller sample sizes, and we included two or more (reasonably independent) comparisons. For this purpose, for dichotomous outcomes, we divided up both the number of events and the total number of participants, and for continuous outcomes, we divided up the total number of participants with unchanged means and standard deviations. For network meta‐analysis, instead of subdividing the common comparator, we used an approach that accounted for the within‐study correlation between effect sizes by re‐weighting all comparisons in each multi‐arm study (Rücker 2012; Rücker 2014).
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), we took the following steps to deal with missing data.
Whenever possible, we contacted the original investigators to request relevant missing data. If the number of participants evaluated for a given outcome was not reported, we used the number of participants randomised per treatment arm as the denominator. If only percentages but no absolute numbers of events were reported for binary outcomes, we calculated numerators using percentages. If estimates for means and standard deviations were missing, we planned to calculate these statistics from reported data whenever possible, using approaches described in Chapter 7.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If standard deviations were missing and we had not been able to calculate them from reported data, we planned to calculate values according to a validated imputation method (Furukawa 2006). If data were not reported numerically but were presented graphically, we estimated missing data from figures. We planned to perform sensitivity analyses to assess how sensitive results were to imputing data in some way. However, as we did not have continuous data, it was not necessary to impute potential missing data, and sensitivity analyses as described were unnecessary. We will apply this approach in future updates when necessary. We addressed in the Discussion the potential impact of missing data on review findings.
Assessment of heterogeneity
Pair‐wise meta‐analyses
For each direct comparison, we used visual inspection of forest plots as well as Cochran’s Q based on the Chi² statistic and the I² statistic to detect the presence of heterogeneity. We interpreted I² values according to Chapter 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We used the following thresholds for interpretation of I².
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
However, we also considered the magnitude and direction of effects and the strength of evidence for heterogeneity. We used the P value of the Chi² test only in describing the extent of heterogeneity ‐ not in determining statistical significance. In addition, we reported Ʈ² ‐ the between‐study variance in random‐effects meta‐analysis.
Network meta‐analysis
A very important pre‐supposition for using network meta‐analysis is to make sure that the network is consistent, meaning that direct evidence and indirect evidence on the same comparisons agree. Inconsistency could be caused by incomparable inclusion and exclusion criteria of trials in the network.
We evaluated the assumption of transitivity epidemiologically by comparing the distribution of potential effect modifiers across the different pair‐wise comparisons. We created a table of important clinical and methodological characteristics, and we assessed whether there were systematic differences between identified comparisons. In particular, we looked at the following potential effect modifiers.
Clinical characteristics: gender, age, chemotherapy, tumour/cancer type.
Methodological characteristics: cross‐over design, blinding, placebo‐controlled, study period, sample size, country, multi‐centre, number of treatment arms, country.
We visually inspected the similarity of these factors, including the inclusion and exclusion criteria of every trial in the network, and we discussed whether the transitivity assumption was met.
To evaluate the presence of inconsistency locally, we used the Bucher method for single loops of evidence (Bucher 1997), as described, for example, in Dias 2013. For each closed loop, we calculated the difference between direct and indirect evidence, together with its 95% CI. We used loop‐specific z‐tests to infer about the presence of inconsistency in each loop. To assess inconsistency, we used graphical representations of direct and indirect estimates together with 95% CIs; we reported the percentage of inconsistent loops in the network and visually examined the forest plots and league tables to assess inconsistency. It should be noted that in a network of evidence, there may be many loops, and with multiple testing, the likelihood that we might find an inconsistent loop by chance was increased. Therefore, we have been cautious when deriving conclusions from the statistical approach and from preferred visual examination to assess inconsistency.
To evaluate the presence of inconsistency in the entire network, we gave the generalised heterogeneity statistic Qtotal and the generalised I² statistic, as described in Rücker 2019. We used the decomp.design command in the R package netmeta (R 2019; netmeta 2016) for decomposition of the heterogeneity statistic into a Q statistic for assessing heterogeneity between studies with the same design, and a Q statistic for assessing design inconsistency to identify the amount of heterogeneity/inconsistency within as well as between designs. Furthermore, we created a netheat plot (Krahn 2013) ‐ a graphical tool for locating inconsistency in network meta‐analysis, using the command netheat in the R package netmeta. We gave Qtotal and its components as well as net heat plots based on fixed‐effect and random‐effects models to identify differences between these approaches (netheat plots and forest plots of fixed‐effect models not shown in the review). For random‐effects models, we reported Ʈ².
In case we identified substantive heterogeneity and/or inconsistency, we explored possible sources by performing pre‐specified sensitivity and subgroup analyses (see below). In addition, we reviewed the evidence base, reconsidered inclusion criteria, and discussed the potential role of unmeasured effect modifiers to identify further sources.
Assessment of reporting biases
In pair‐wise comparisons with at least 10 trials, we planned to examine the presence of small‐study effects graphically by generating funnel plots. We planned to use linear regression tests to test for funnel plot asymmetry (Egger 1997). A P value less than 0.1 would have been considered significant for this test (Sterne 2011). We planned to examine the presence of small‐study effects for the primary outcome only. As described above, we searched study registries to identify completed but not published trials.
Data synthesis
Methods for direct treatment comparisons
We performed analyses according to recommendations provided in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), and we used the statistical software of Cochrane ‐ Review Manager (RevMan 2014) ‐ for analysis. We performed separate analyses for cancer patients receiving HEC or MEC. If applicable, we used R for additional analyses that could not be done with RevMan (R 2019).
Pair‐wise comparisons were part of the network meta‐analysis. However, to outline available direct evidence, we provided forest plots for pair‐wise comparisons with at least 10 trials if trials were clinically homogenous. We performed these standard pair‐wise meta‐analyses using a random‐effects model. We calculated corresponding 95% CIs for all analyses, and we graphically presented the results using forest plots. When trials would have been clinically too heterogenous to be combined, we would have performed only subgroup analyses without calculating an overall estimate.
Methods for indirect and mixed comparisons
If we considered the data sufficiently similar to be combined, we performed a network meta‐analysis using the frequentist weighted least squares approach described by Rücker 2012. We used a random‐effects model, taking into account the correlated treatment effects in multi‐arm studies. We assumed a common estimate for the heterogeneity variance across the different comparisons. To evaluate the extent to which treatments were connected, we gave a network plot for our primary and secondary outcomes. For each comparison, we gave the estimated treatment effect along with its 95% CI. We graphically presented the results using forest plots, with placebo as the reference. We used the R package netmeta for statistical analyses (R 2019; netmeta 2016).
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analysis, if appropriate.
Type of chemotherapy (carboplatin versus other moderately emetogenic chemotherapy, cisplatin versus other highly emetogenic chemotherapy, and anthracycline versus other highly emetogenic chemotherapy).
Cancer type (solid tumours versus haematological malignancies, and breast cancer versus others).
We had planned to use the test for interactions to test for subgroup differences. However, this test is not yet available for network‐meta analysis in netmeta 2016. We will apply this in future updates when possible.
We had planned to conduct the following subgroup analysis but could not do so because of missing information or split networks.
Drug dosage.
Route of administration.
Patient‐specific prognostic factors.
Sensitivity analysis
To test the robustness of the results, we conducted fixed‐effect pair‐wise and network meta‐analyses. We reported estimates of the fixed‐effect model only if they showed a difference from estimates of the random‐effects model. We explored the influence of risk of bias components by considering studies at low risk of bias when compared to all studies. Our rule for an overall risk of bias judgement is described under Assessment of risk of bias in included studies.
Summary of findings and assessment of the certainty of the evidence
'Summary of findings' table
According to Chapter 14 of the updated Cochrane Handbook for Systematic Reviews of Interventions, the “most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes” should be included in the summary of findings table(s) (Schünemann 2019). Together with a clinical expert (KJ), we prioritised the most important and crucial (underlined) outcomes as follows.
Complete control of nausea in the overall phase (Days 1 to 5).
-
Complete control of vomiting.
Delayed phase (Days 2 to 5).
Overall phase (Days 1 to 5).
No impairment of quality of life.
On‐study mortality.
Serious adverse events.
Local reactions at infusion site.
We planned to include a 'Summary of findings' table for each outcome per emetogenic group (HEC and MEC) to present the main findings in a transparent and simple tabular format. Each table includes key information concerning the certainty of evidence and the magnitude of effect of interventions examined. For a comprehensive illustration of our results, we randomly chose aprepitant plus granisetron as exemplary reference treatment for HEC, and granisetron as exemplary reference treatment for MEC. However, theoretically, we could have used every treatment combination as a reference. The estimated absolute effects provided in the 'Summary of findings' table were based on actual event rates reported for the main comparators and summed across studies, and are provided for illustrative purposes only.
We could not include a 'Summary of findings' table for the outcome local reactions at infusion site because in the HEC group, no study reported the outcome for our reference treatment (aprepitant plus granisetron), and in the MEC group, no study reported the outcome at all.
Assessment of the certainty of the evidence
Two review authors independently rated the certainty of evidence of each prioritised outcome for both HEC and MEC groups. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to rank the certainty of evidence using the GRADEprofiler Guideline Development Tool software (GRADEpro GDT 2015), along with the guidelines provided in Chapter 12.2 of the CochraneHandbook for Systematic Reviews of Interventions (Schünemann 2011a), specifically for network meta‐analyses (Puhan 2014). The GRADE Working Group suggests assessment of the certainty of evidence for no more than seven outcomes, and for each outcome included in 'Summary of findings' tables ‐ therefore only for outcomes that are most critical or important for decision‐making (Guyatt 2013).
The GRADE approach used five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess certainty of the body of evidence for each outcome. The GRADE system used the following criteria for assigning grade of evidence.
High = we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate = we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low = our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low = we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The GRADE system used the following criteria for assigning a certainty level to a body of evidence (Chapter 12, Schünemann 2011a).
High: randomised trials; or double‐upgraded observational studies.
Moderate: downgraded randomised trials; or upgraded observational studies.
Low: double‐downgraded randomised trials; or observational studies.
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
We decreased grade if we noted:
serious (‐1) or very serious (‐2) risk of bias;
important inconsistency (‐1);
some (‐1) or major (‐2) uncertainty about directness;
imprecise or sparse data (‐1) or very imprecise or very sparse data (‐2); or
high probability of reporting bias (‐1).
Results
Description of studies
For this systematic review, we identified 383 records for full‐text screening, of which 107 RCTs comprising 197 references, with approximately 37,313 participants, fulfilled the inclusion criteria.
Results of the search
We identified 5192 potentially relevant references through database searches and five additional references through handsearching. After full‐text screening, we concluded that 197 references representing 73 studies in the HEC group (Appendix 2), along with 38 studies in the MEC group (Appendix 3), met the pre‐defined eligibility criteria; thus we selected them for assessment. As some trials were reported in multiple full‐text reports, abstracts, and posters, we indexed these repeating references under the main publications. Four studies included people treated with both highly and moderately emetogenic chemotherapeutic regimens and reported results for both regimens separately; thus we included them in both HEC and MEC groups (Raftopoulos 2015; Ghosh 2010; Ho 2010; Tsubata 2019), resulting in a total of 107 included studies. Two studies in the HEC group were reported in the Korean language (Cho 1998; Lee 1997), and the remaining 105 studies were reported in the English language.
We documented the overall numbers of records screened, identified, selected, excluded, and included in a PRISMA flow diagram (Figure 1, Supplementary Figure 1). Supplementary figures are available from https://osf.io/dr2u7/ (Piechotta 2021).
Included studies
In the HEC group, we included 73 studies reporting on 25,275 participants. Studies were conducted between December 1992 ‐ Roila 1995 ‐ and February 2018 ‐ Li 2019. Stewart 1996 did not report the information that we sought. Sample sizes varied from 15 in Abdel‐Malek 2017 to 2322 participants in Grunberg 2011. In the MEC group, we included 38 studies reporting on 12,038 participants. Studies were conducted between July 1997 for Herrington 2000 and March 2017 for Xiong 2019. Sample sizes varied from 12 in Brohee 1995 to 1369 participants in Schwartzberg 2015.
In the HEC group, 45 studies (62%) included cisplatin > 50 mg/m² alone or in combination for treatment of solid malignancies. Eleven studies (15%) included only breast cancer patients and used the combination of anthracycline and cyclophosphamide in the chemotherapy regimen (Arce‐Salinas 2019; Herrstedt 2009; Kalaycio 1998; Li 2019; Matsumoto 2020; Nakamura 2012; Ohzawa 2015; Rugo 2017; Saito 2017; Warr 2005; Wenzell 2013). Schmitt 2014, Svanberg 2015, Mohammed 2019, and Egerer 2010 used melphalan or ABVD (Adriamycin, Bleomycin, Vinblastine, Dacarbazine) in the chemotherapy regimen for treatment of haematological malignancies. In the MEC group, 13 studies (35%) included patients treated with carboplatin for solid and haematological malignancies (Eisenberg 2003; Endo 2012; Herrington 2000; Ito 2014; Jordan 2016a; Kaushal 2010; Kaushal 2015; Kim 2017; Kusagaya 2015; Maehara 2015; Sugimori 2017; Tanioka 2013; Yahata 2016), eight studies (22%) included patients treated with cyclophosphamide ≤ 1500 mg/m² (Arpornwirat 2009; Brohee 1995; Ghosh 2010; Raftopoulos 2015; Rapoport 2010; Schwartzberg 2015; Song 2017; Yeo 2009), and four studies (11%) included patients treated with oxaliplatin for solid and haematological malignancies (Aridome 2016; Hesketh 2012; Ho 2010; Nishimura 2015). Yeo 2009 and Webb 2010 included patients with breast cancer.
A majority of studies (70%) in the HEC group were double‐blinded (typically reported in journal publication and referring to participants and investigators) or quadruple‐blinded (typically reported in trials registries and referring to participants, care providers, investigators, and outcome assessors), and 27 studies among them were placebo‐controlled. Eleven studies (15%) were open‐label and were not placebo‐controlled (Ando 2016; Arce‐Salinas 2019; Cho 1998; Chua 2000; Ishido 2016; Lee 1997; Mahrous 2020; NCT01640340; Ohzawa 2015; Tsubata 2019; Wenzell 2013). Abdel‐Malek 2017 Kang 2020, and Mohammed 2019 were single‐blinded studies. In the MEC group, 16 studies (43%) were double‐blind with 11 studies (30%) among them placebo‐controlled, and 14 studies (38%) were open‐label. Fifteen studies in the HEC group (Abdel‐Malek 2017; Aksu 2013; Albany 2012; Cho 1998; Chua 2000; Gao 2013; Innocent 2018; Ishido 2016; Kimura 2015; Koizumi 2003; Lee 1997; Nakamura 2012; Ohzawa 2015; Stewart 2000; Wit 2001), as well as four studies in the MEC group (Fujiwara 2015; Jantunen 1992; Kaushal 2015; Seol 2016), were cross‐over studies.
Forty‐nine studies in the HEC group and 26 studies in the MEC group included a triple‐drug combination, including a neurokinin‐1 (NK₁) receptor antagonist, a 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonist, and a corticosteroid, as experimental intervention. The most frequently used NK₁ receptor antagonist was aprepitant, followed by fosaprepitant, rolapitant, netupitant, casopitant, and ezlopitant. As 5‐HT₃ receptor antagonists, palonosetron, granisetron, ondansetron, ramosetron, tropisetron, and azasetron were used. As a corticosteroid, either dexamethasone or methylprednisolone was used. An overview of all included treatment regimens is provided in Table 5.
Nearly all of the studies included individuals ≥ 18 years of age with confirmed malignancy and naïve to emetogenic chemotherapy. Albany 2012, Kimura 2015, and Ghosh 2010 included participants ≥ 15 years of age. Data were not reported separately for participants ≥ 18 years and participants ≥ 15 years in any of these studies. As study authors considered the age of 15 as an appropriate cut‐off and provided no reason to separate the analysis, we assumed that the effects for 15‐ to 17‐year‐olds and ≥ 18‐year‐olds would not differ. Therefore, although the proportions of participants < 18 years old were not reported, we included them in the analysis.
The foremost causes of exclusion were symptomatic malignancy of the central nervous system (CNS) and participant had vomited within 24 hours before treatment Day 1, had an active infection, had an active systemic fungal infection, had any severe concurrent illness except for malignancy, or had abnormal laboratory values.
Seventeen studies from both HEC and MEC groups included participants from Asia, Europe, Africa, North America and South America, Australia, and the Pacific region (Aapro 2006; Arpornwirat 2009; Chawla 2003; Grunberg 2009; Grunberg 2011; Herrstedt 2009 Hesketh 2003; Hesketh 2012; Jordan 2016a; Rapoport 2010; Rapoport 2015 (a); Rugo 2017; Ruhlmann 2017; Warr 2005; Weinstein 2016; Schmoll 2006; Schwartzberg 2015); all were multi‐national and multi‐centred. Most studies were conducted in Asia, including Japan (23%). Other Asian sites were South Korea (Cho 1998; Kim 2015; Kim 2017; Lee 1997; Seol 2016), China (Chua 2000; Ho 2010; Hu 2014; Song 2017; Xiong 2019; Yang 2017; Yeo 2009), and India (Ghosh 2010; Kaushal 2010; Kaushal 2015). Precisely 12 studies included participants only from USA with different ethnicities (Albany 2012; Bubalo 2005; Bubalo 2018; Fox‐Geiman 2001; Herrington 2008; NCT01640340; Raftopoulos 2015; Rapoport 2015 (b); Rapoport 2015 (c); Schnadig 2016; Stiff 2013; Wenzell 2013), and eight studies included participants exclusively from Europe (Brohee 1995; Egerer 2010; Flenghi 2015; Hesketh 2014; Roila 1995; Schmitt 2014; Stewart 2000; Svanberg 2015).
We visually inspected the similarity of clinical and methodological characteristics that could act as potential effect modifiers and assessed whether there were systematic differences between identified comparisons (available from study authors upon request). We decided that for most studies (50 in the HEC group, 26 in the MEC group), it was hypothetically equally likely that any participant could have been randomised to any treatment.
We therefore included 50 studies from the HEC group in the network meta‐analysis (NMA). We could not include 23 HEC studies in the NMA for the following reasons.
Different definitions of efficacy outcomes: Kimura 2015, Svanberg 2015, Fox‐Geiman 2001, Wit 2001, Koizumi 2003, Ghosh 2010, Lee 1997, Chua 2000, Mohammed 2019
Different definitions of acute, delayed, and overall phases: Albany 2012, Ando 2016
Efficacy outcomes reported as a score: Albany 2012, Ando 2016
Results reported over multiple cycles including cross‐over: Abdel‐Malek 2017
Only number of adverse events reported: Albany 2012, Kalaycio 1998
Adverse events divided by severity grade: Kimura 2015, Svanberg 2015
Only abstract with insufficient information available: Arce‐Salinas 2019 Cho 1998, Flenghi 2015, Forni 2000, Gao 2013, Mahrous 2020 Stewart 1996
Full text with insufficient information or without any outcome of interest: Stewart 2000, Egerer 2010
Part of the study population received additional irradiation and results for the subgroup without irradiation have not been separately reported: Bubalo 2018
Adverse events for HEC and MEC reported together: Ghosh 2010.
We included 26 studies from the MEC group in the NMA. We could not include 12 MEC studies in the NMA for the following reasons.
Missing specification of 5‐HT₃ inhibitor: Ito 2014, Maehara 2015, Yahata 2016
Missing specification of 5‐HT₃ and NK₁ inhibitors: Nishimura 2015
Results reported over multiple cycles including cross‐over: Fujiwara 2015, Kitayama 2015
Results reported over multiple cycles: Brohee 1995
Only number of treatment‐related adverse events reported: Seol 2016
Different definitions of efficacy outcomes: Ghosh 2010, Jantunen 1992
Adverse events for HEC and MEC reported together: Ghosh 2010
Only abstract with insufficient information available: Webb 2010, Miyabayashi 2015, Schnadig 2014.
For detailed information regarding the characteristics of included studies, please refer to the Characteristics of included studies table.
Excluded studies
We excluded 140 records of assessed full texts for the following reasons.
We excluded 89 records (63%) because of the antiemetic therapy.
69 used no corticosteroid or corticosteroids in only one arm (Audhuy 1996; Ballatori 1995; Bianchi 1996; Bonneterre 1995; Bubalo 2001; Campora 1994; Dong 2011; Fauser 1995; Fauser 1996; Fauser 1999; Feng 2000; Feng 2002; Fengyi 2002; Gebbia 1994; Goldschmidt 1997; Gralla 1998; Gralla 2003; Hesketh 1996; Huang 2013; Huc 1998; Iihara 2012; Jantunen 1993; Kang 2002; Kim 1998; Kim 2004; Leonardi 1996; Lofters 1995; Martoni 1996; Marty 1995; Matsuoka 2003; Micha 2016; Nasu 2013; Noble 1994; Noda 2002; Ogihara 1999; Park 1997; Pectasides 2007; Perez 1998 (a); Peterson 1996; Poon 1998; Qiu 2011; Ruff 1994; Sheng 2010; Shi 2007; Slabý 2000; Spector 1998; Stewart 1995; Sun 2014; Tang 2013; Tian 2011; Tominaga 1996; Tong 2014; Tremont‐Lukats 2017; Tsavaris 1996; Tsubata 2015; Tsukuda 1995; Weant 2017; Xie 2003; Yalçin 1999; Yang 2005; Yano 2005; Yu 2009; Zeng 2001; Zhang 1996; Zhang 1999; Zhang 2003; Zhang 2003 (a); Zhang 2007; Öge 2000).
9 used other antiemetics (metoclopramide (Italian Group for Antiemetic Research 1993; Italian Group for Antiemetic Research 1995 (a); Italian Group for Antiemetic Research 1995 (b); Van der Vorst 2021), lorazepam (Lacerda 2000; Mandanas 2005), prochlorperazine (Lindley 2005; Matsui 1996), dronabinol (Meiri 2007)).
5 used no 5‐HT₃ inhibitors or 5‐HT₃ inhibitors in only one arm (Belle 2002; Cocquyt 2001; Lavoie 2012; Ohta 1992; Van Belle 2002).
3 did not define which 5‐HT₃ inhibitors or NK₁ inhibitor was used (Nishimura 2015 (a); Nishimura 2015 (b); Yahata 2016 (a)).
1 used corticosteroids as pre‐therapy before chemotherapy (Perez 1998 (b)).
2 compared different preparations of the same drug (Huang 1998; NCT04636632).
We excluded 39 records (28%) because of the study design.
15 were non‐randomised studies of the intervention (Abali 2007; Albany 2014; Choi 2014; Craver 2011; Creed 1999; Huang 2001; Long 2002; Monda 1994; Pater 1997; Perez 1996; Plasencia‐Mota 1993; Rzepecki 2009; Takenaka 2007; Takeshima 2014; Zeidman 1998).
12 were pharmacokinetic studies (Bubalo 2012; Loos 2007; Ottoboni 2014; Suzuki 2015; Tsuji 2016; Vadhan‐Raj 2011; Vadhan‐Raj 2012; Vadhan‐Raj 2014; Vadhan‐Raj 2015; Walko 2012; Zhang 2002; Zhang 2012).
3 were cost‐effectiveness analyses (Barrajon 2000; Humphreys 2013; Moore 2007).
3 were letters or correspondences (Bonneterre 1994; Fedele 1995; Silvestris 2013).
2 were retrospective subset analyses of included studies (Hudis 2003; Uchino 2012).
2 were dose‐finding studies (Roila 2009; Tanimura 1998).
1 was a pooled analysis of different trials (Molassiotis 2013).
1 was a review (Navari 2016).
We excluded 12 records (9%) because of the underlying chemotherapy.
5 did not clearly define the chemotherapy, so it was unclear whether it was HEC or MEC (Chiou 2000; Kilickap 2013; Kim 2012; Lee 2014; Saito 2015).
3 used a concurrent chemoradiotherapy (Kawaguchi 2015; Ruhlmann 2016; Tong 2012).
2 did not provide individual data for HEC and MEC groups (Roscoe 2012; Tan 2004).
1 used a low emetogenic chemotherapy (Dandamudi 2011).
1 used an antiblastic therapy (Adamo 1994).
For further information, please check Characteristics of excluded studies.
Ongoing studies
Of the 12 ongoing studies, four studies are comparing different combinations of NK₁ and 5‐HT₃ inhibitors for prevention and control of nausea and vomiting for people receiving HEC (KTC0001495; UMIN000004021; UMIN000006773; UMIN000007882); three studies are comparing different combinations of NK₁ and 5‐HT₃ inhibitors for prevention and control of nausea and vomiting for people receiving MEC (UMIN000005317; UMIN000005494; UMIN000032860); four studies are comparing a 5‐HT₃ inhibitor versus a combination of NK₁ and 5‐HT₃ inhibitors for prevention and control of nausea and vomiting for people receiving MEC (ChiCTR1900025227; IRCT20191103045317N1; UMIN000012500; UMIN000041004); and one study is comparing different combinations of NK₁ and 5‐HT₃ inhibitors for prevention and control of nausea and vomiting for people receiving both HEC and MEC (NCT03606369).
Please refer to Characteristics of ongoing studies for more detailed information.
Studies awaiting classification
Of the 34 studies awaiting assessment, 10 studies are not recruiting and no results are available for these (four studies using HEC (EUCTR2004‐004956‐38; EUCTR2004‐000371‐34; EUCTR2007‐004043‐30; EUCTR2015‐001800‐74), five studies using MEC (EUCTR2006‐000781‐37; EUCTR2006‐003512‐22; EUCTR2007‐005169‐36; EUCTR2009‐016775‐30; EUCTR2009‐017603‐28), and one study using both HEC and MEC (EUCTR2010‐023297‐39)).
We classified nine further studies with HEC setting and without available results as awaiting assessment because the status is given as "pending" (ChiCTR‐INR‐17010779), "authorised recruitment may be ongoing or finished" (EUCTR2008‐001339‐37), "terminated per PI’s request" (NCT02732015), or "completed" (NCT01101529; NCT03403712; UMIN000004826; UMIN000004863; UMIN000008897), or because status is unknown (PER‐055‐12).
We classified ten further studies with MEC setting and without available results as awaiting assessment because the status is given as "authorised recruitment may be ongoing or finished" (EUCTR2004‐001020‐20), "completed" (CTRI/2017/10/010163; UMIN000004998; UMIN000008041; UMIN000008552; UMIN000010056; UMIN000010186; UMIN000019122), or "terminated" (insufficient accrual)“ (NCT02550119), or because status is unknown (NCT02407600).
Two records were abstracts with insufficient information (Mylonakis 1996; Spina 1995), and one study was completed with no available results and with no information with regard to chemotherapy (NCT00169572).
One study provided insufficient information on the antiemetic regimen in the trial registry (JapicCTI‐194691).
Please refer to Characteristics of studies awaiting classification for more detailed information.
Risk of bias in included studies
Summaries of the risk of bias of included studies for all assessed domains across included studies and per included study are presented in Figure 2 and Figure 3 and in Supplementary Figure 2. Supplementary figures are available from https://osf.io/dr2u7/ (Piechotta 2021).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Among the 107 included studies, 55 (51%) reported a randomised allocation process using simple randomisation, a block randomisation method, computer‐generated randomisation, an interactive voice response system, or a telephone call; therefore we judged them as having low risk of bias for 'Random sequence generation'. The remaining 52 studies (49%) mentioned the random allocation process but did not provided information regarding the sequence generation; therefore, we judged these studies as having unclear risk of bias for 'Random sequence generation'.
Allocation is concealed to ensure that the group assignment of participants is not revealed before they are definitively allocated to their respective groups. We judged the risk of bias for allocation concealment to be low for 17 studies (16%), as these studies used methods such as sealed envelopes, central randomisation, or engaging unrelated personnel/organisations to determine group assignments (Albany 2012; Cheirsilpa 2005; Fox‐Geiman 2001; Grunberg 2011; Hu 2014; Koizumi 2003; Maehara 2015; Matsumoto 2020; Mohammed 2019; Rapoport 2010; Rapoport 2015 (b); Rapoport 2015 (c); Schwartzberg 2015; Sugawara 2019; Svanberg 2015; Weinstein 2016; Zhang 2020). We judged the remaining 90 studies (84%) as having unclear risk of bias, as they provided no sufficient information regarding concealment of allocation.
Blinding
Performance bias
Participants
Regarding blinding of participants towards treatment arms, 71 studies (66%) were reported to be single‐blind, double‐blind, or quadruple‐blind; therefore we judged them as having low risk of bias. We judged nine studies (8%) as having unclear risk of bias, as they provided no information concerning blinding of participants (Aksu 2013; Endo 2012; Forni 2000; Gao 2013; Li 2019; Matsuda 2014; Miyabayashi 2015; Ozaki 2013; Zhang 2018 (b)); we judged the remaining 27 studies (25%) as having high risk of bias because participants were not blinded (Ando 2016; Arce‐Salinas 2019; Aridome 2016; Badar 2015; Cho 1998; Chua 2000; Fujiwara 2015; Herrington 2000; Ishido 2016; Ito 2014; Jantunen 1992; Kaushal 2010; Kaushal 2015; Kusagaya 2015; Lee 1997; Maehara 2015; Mahrous 2020; Mattiuzzi 2007; NCT01640340; Nishimura 2015; Ohzawa 2015; Seol 2016; Song 2017; Sugimori 2017; Tsubata 2019; Wenzell 2013; Xiong 2019).
Personnel
Sixty‐three studies (59%) reported blinding of personnel towards treatment arms; therefore we judged them as having low risk of bias for 'Blinding of personnel'. We judged 11 studies (10%) as having unclear risk of bias, as we found no suggestive information concerning blinding of personnel (Aksu 2013; Brohee 1995; Endo 2012; Forni 2000; Gao 2013; Li 2019; Matsuda 2014; Miyabayashi 2015; Ozaki 2013; Xiong 2019; Zhang 2018 (b)); we judged the remaining 33 studies (31%) as having high risk of bias because personnel were not blinded (Abdel‐Malek 2017; Ando 2016; Arce‐Salinas 2019; Aridome 2016; Badar 2015; Cho 1998; Chua 2000; Fujiwara 2015; Herrington 2000; Ishido 2016; Ito 2014; Jantunen 1992; Kang 2020; Kaushal 2010; Kaushal 2015; Kim 2015; Kimura 2015; Kitayama 2015; Kusagaya 2015; Lee 1997; Maehara 2015; Mahrous 2020; Mattiuzzi 2007; Mohammed 2019; Nakamura 2012; NCT01640340; Nishimura 2015; Ohzawa 2015; Seol 2016; Song 2017; Sugimori 2017; Tsubata 2019; Wenzell 2013).
Detection bias
We assessed blinding of outcome assessment for two outcome categories ‐ subjective and objective outcomes ‐ as previously described.
Subjective outcomes
Assessment of subjective outcomes, such as complete control of nausea, complete control of vomiting, and quality of life, can be influenced by awareness of the intervention. We judged 65 studies (61%) as having low risk of bias because outcome assessors (i.e. participants) were blinded to the intervention. We judged ten studies (9%) as having unclear risk of bias for subjective outcomes, as they provided no information regarding blinding of participants (Aksu 2013; Brohee 1995; Endo 2012; Forni 2000; Gao 2013; Li 2019; Matsuda 2014; Miyabayashi 2015; Ozaki 2013; Zhang 2018 (b)). We judged 32 studies (30%) as having high risk of bias for subjective outcomes, as the unblinded study design might have influenced the outcome assessment (Abdel‐Malek 2017; Ando 2016; Arce‐Salinas 2019; Aridome 2016; Badar 2015; Cho 1998; Chua 2000; Fujiwara 2015; Herrington 2000; Ishido 2016; Ito 2014; Jantunen 1992; Kaushal 2010; Kaushal 2015; Kim 2015; Kimura 2015; Kitayama 2015; Kusagaya 2015; Lee 1997; Maehara 2015; Mahrous 2020; Mattiuzzi 2007; Nakamura 2012; NCT01640340; Nishimura 2015; Ohzawa 2015; Seol 2016; Song 2017; Sugimori 2017; Tsubata 2019; Wenzell 2013; Xiong 2019).
Objective outcomes
Assessment of objective outcomes, such as mortality and adverse events, should not be influenced by awareness of the intervention. We, therefore, judged the risk of detection bias to be low for all studies that assessed or reported objective outcomes. Assessment of detection bias for objective outcomes was not applicable for 41 studies (38%) because objective outcomes were not assessed or reported in those studies (Abdel‐Malek 2017; Aksu 2013; Albany 2012; Ando 2016; Brohee 1995; Bubalo 2005; Cho 1998; Chua 2000; Egerer 2010; Flenghi 2015; Forni 2000; Fox‐Geiman 2001; Fujiwara 2015; Gao 2013; Herrington 2000; Innocent 2018; Jantunen 1992; Jordan 2016a; Kaushal 2010; Kaushal 2015; Li 2019; Maehara 2015; Matsumoto 2020; Mattiuzzi 2007; Miyabayashi 2015; Mohammed 2019; NCT01640340; Ohzawa 2015; Ozaki 2013; Poli‐Bigelli 2003; Rugo 2017; Saito 2017; Schnadig 2014; Stewart 1996; Stewart 2000; Svanberg 2015; Tsubata 2019; Webb 2010; Wenzell 2013; Wit 2001; Zhang 2018 (b)).
Incomplete outcome data
We assessed risk of attrition bias for both outcome categories ‐ subjective and objective outcomes ‐ as previously described. We judged 93 studies (87%) as having low risk of bias for subjective objectives and 60 studies (56%) as having low risk of bias for objective outcomes, as most of the randomised participants (92% to 100%) were included in the antiemetic efficacy and safety analyses in primary studies, and reported study discontinuations were balanced between arms.
Subjective outcomes
We judged 11 studies (10%) as having unclear risk of bias for subjective outcomes, as insufficient information was provided to make an explicit decision whether or not the intention‐to‐treat population was analysed (Aksu 2013; Arce‐Salinas 2019; Cho 1998; Forni 2000; Gao 2013; Lee 1997; Li 2019; Mahrous 2020; Mohammed 2019; Stewart 1996; Zhang 2018 (b)). We judged three studies (3%) as having high risk of bias for subjective outcomes because Kang 2020 used a modified ITT analysis and included only participants who received at least one treatment, because Ozaki 2013 excluded 15 participants (25% of the initial included population) from the efficacy analysis due to insufficient diary entries and protocol violations, and because Seol 2016 analysed the per‐protocol population.
Objective outcomes
We judged five studies (5%) as having unclear risk of bias for objective outcomes because we were not able to evaluate completeness of safety data for Arce‐Salinas 2019 and Mahrous 2020, as only an abstract was available, because Herrington 2008 did not report whether or not serious adverse events occurred, because it was unclear for Matsumoto 2020 who was included in the safety population, and because we were not able to evaluate the completeness of safety data from Lee 1997 due to a language barrier (and we could not identify someone via Cochrane TaskExchange to translate those for us). We judged two studies (2%) as having high risk of bias for objective outcomes because Kang 2020 included the ITT population in safety analysis even though not all participants received at least one study drug, and because Yahata 2016 did only report antiemetic treatment‐related adverse events. We did not assess detection bias for objective outcomes for 40 studies (37%) because objective outcomes were not assessed or reported in those studies (Abdel‐Malek 2017; Aksu 2013; Albany 2012; Ando 2016; Brohee 1995; Bubalo 2005; Cho 1998; Chua 2000; Egerer 2010; Flenghi 2015; Forni 2000; Fox‐Geiman 2001; Fujiwara 2015; Gao 2013; Herrington 2000; Innocent 2018; Jantunen 1992; Jordan 2016a; Kaushal 2010; Kaushal 2015; Li 2019; Maehara 2015; Mattiuzzi 2007; Miyabayashi 2015; Mohammed 2019; NCT01640340; Ohzawa 2015; Ozaki 2013; Poli‐Bigelli 2003; Rugo 2017; Saito 2017; Schnadig 2014; Stewart 1996; Stewart 2000; Svanberg 2015; Tsubata 2019; Webb 2010; Wenzell 2013; Wit 2001; Zhang 2018 (b)).
Selective reporting
We judged 85 studies (79%) as having low risk of bias for selective reporting because outcome reporting seemed to be complete in the study reports for all primary, secondary, and additional outcomes, and we identified no reasons for concern.
We judged 18 studies (17%) as having unclear risk of bias for selective reporting for the following reasons. Ando 2016 reported the pre‐specified outcomes but not according to treatment arms. Herrstedt 2009 was completed and published in 2009. In 2017, the principal investigators (PIs) or Sponsors submitted the results to be published in the trials register. After quality control, results were resubmitted twice, then submission was cancelled at both times (please see https://clinicaltrials.gov/ct2/show/results/NCT00366834). We are not sure whether this means that the quality check revealed some issues with the data and the study authors did not want to resolve those, whether they were not able to resolve those, or whether after this long period of time, the data simply did not meet the quality standard of clinicaltrials.gov, and PIs and Sponsors did not have data needed for the revision because they had never been obtained. We contacted the study authors for clarification and did not receive a response. Svanberg 2015 mentioned that adverse events were not differing between groups but did not provide any supporting data. We could not evaluate selective reporting for Tsubata 2019, as the wrong study registration number was provided in the paper and the correct number was not retrievable. Further, we could not evaluate two studies because of language barriers and we could not identify someone via Cochrane TaskExchange to translate those for us (Cho 1998; Lee 1997). Twelve studies were conference abstracts with insufficient reporting (Arce‐Salinas 2019; Brohee 1995; Flenghi 2015; Forni 2000; Gao 2013; Mahrous 2020; Miyabayashi 2015; Ozaki 2013; Schnadig 2014; Stewart 1996; Webb 2010; Zhang 2018 (b))
We judged five studies (4%) as having high risk of bias for selective reporting. Bubalo 2005 did not provide any results for their pre‐specified secondary outcomes (effects on nausea, appetite and taste changes, and pharmacokinetic interactions). Egerer 2010 mentioned safety assessment in the introduction but did not provide any safety data. Kaushal 2015 and Maehara 2015 did not report any results for pre‐determined safety outcomes. Saito 2017 reported only complete response and quality of life.
Other potential sources of bias
We judged 88 studies (82%) as having low risk for other potential sources of bias because we did not identify any information that would suggest other bias.
We judged 19 studies (18%) as having unclear risk for other potential sources of bias because one study was temporarily halted and the protocol was amended with removal of one arm (Arm C) (Herrington 2008); descriptive statistics of participants in Arm C were not included in the report. Ito 2014 allowed additional antiemetic agents and other supportive treatments at the discretion of the treating physician. Kitayama 2015 did not record the incidence and severity of CINV daily but only on Days 2 and 5. We could not evaluate two studies because of language barriers and we could not identify someone via Cochrane TaskExchange to translate those for us (Cho 1998; Lee 1997). We could not find any full‐text publication for 14 studies and the abstracts did not contain sufficient information to exclude other potential sources of bias (Arce‐Salinas 2019; Brohee 1995; Flenghi 2015; Forni 2000; Gao 2013; Mahrous 2020; Mattiuzzi 2007; Miyabayashi 2015; Ozaki 2013; Saito 2017; Schnadig 2014; Stewart 1996; Webb 2010; Zhang 2018 (b)).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
An overview of all outcomes including prioritisation of outcomes is provided in Table 6. Supplementary figures are available from https://osf.io/dr2u7/ (Piechotta 2021).
Highly emetogenic chemotherapy (HEC)
Our objectives were to compare the effects of antiemetic treatment combinations including NK₁ receptor antagonists, 5‐HT₃ receptor antagonists, and corticosteroids on prevention of acute, delayed, and overall chemotherapy‐induced nausea and vomiting, and to generate a clinically meaningful treatment ranking according to their safety and efficacy. To strengthen the network, we also included treatment arms including 5‐HT₃ receptor antagonists and corticosteroids solely. However, we report outcomes only for comparisons including also NK₁ receptor antagonists.
We describe all comparisons that show evidence of a difference between treatment comparisons including NK₁ and 5‐HT₃ inhibitors and corticosteroids. All other comparisons are provided per outcome in a league table. We do not describe the ranking of treatments because P score rankings also include treatments that include 5‐HT₃ inhibitors and corticosteroids solely. For a comprehensive illustration of our results in forest plots and presentation in 'Summary of findings' tables, we randomly chose aprepitant plus granisetron and a corticosteroid as exemplary reference treatment for HEC. However, theoretically, we could have used every treatment combination as a reference.
Efficacy
Complete control of nausea
We defined complete control of nausea as no nausea and no significant nausea, and we refer to it hereafter as "no nausea".
No nausea and no significant nausea were defined on a study level and typically refer to pre‐defined cutoffs (e.g. in Rapoport 2015 (a) or Schwartzberg 2015, nausea was assessed on a visual analogue scale (VAS; 0 to 100 mm; 0 = no nausea, 100 = severe nausea): < 5 mm for no nausea and < 25 mm for no significant nausea. No significant nausea is typically more subjective because of the wider range on the scale and therefore is less objective, especially in an open‐label study design. To increase comparability of studies and minimise biased results, we were therefore interested in patients with no nausea.
Acute phase (0 to 24 hours)
No nausea in the acute phase was reported in 22 studies including 11,225 participants and comparing a total of 17 treatment regimens. We could include all studies in network meta‐analysis (NMA), and the network was fully connected (see Supplementary Figure 3). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 4 and 5. We observed moderate heterogeneity (I² = 49.9%) between studies in the network. More participants treated with ezlopitant plus granisetron experienced no nausea in the acute phase than participants treated with netupitant plus palonosetron (risk ratio (RR) 1.58, 95% confidence interval (CI) 1.02 to 2.46), and than participants treated with aprepitant plus granisetron (RR 1.64, 95% CI 1.05 to 2.56), respectively. Evidence suggests no other differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We identified no evidence of a difference between direct and indirect estimates in closed loops in the network (see Supplementary Figure 6).
Delayed phase (24 to 120 hours)
No nausea in the delayed phase was reported in 19 studies including 10,545 participants and comparing a total of 15 treatment regimens. We could include all studies in NMA, and the network was fully connected (see Supplementary Figure 7). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 8 and 9. We observed serious heterogeneity (I² = 66.7%) between studies in the network. Evidence suggests no differences between treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We identified no evidence of a difference between direct and indirect estimates in the only closed loop for aprepitant plus granisetron versus granisetron versus palonosetron (P = 0.7668 ) (see Supplementary Figure 10).
Overall phase (0 to 120 hours)
No nausea in the overall phase was reported in 22 studies including 14,588 participants and comparing a total of 14 treatment regimens. We could include all studies in NMA, and the network was fully connected (see Supplementary Figure 11). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 12 and 13. We observed no heterogeneity (I² = 0%) between studies in the network. More participants treated with fosaprepitant plus palonosetron experienced no nausea in the overall phase than participants treated with fosaprepitant plus granisetron (RR 1.43, 95% CI 1.10 to 1.85), aprepitant plus granisetron (RR 1.46, 95% CI 1.12 to 1.90), netupitant plus palonosetron (RR 1.52, 95% CI 1.13 to 2.05), fosaprepitant plus ondansetron (RR 1.62, 95% CI 1.20 to 2.19), aprepitant plus ondansetron (RR 1.68, 95% CI 1.23 to 2.30), and casopitant plus ondansetron (RR 1.83, 95% CI 1.29 to 2.60). Furthermore, evidence suggests that more participants treated with fosnetupitant plus granisetron experience no nausea in the overall phase than participants treated with casopitant plus ondansetron (RR 1.52, 95% CI 1.05 to 2.20); and that more participants treated with rolapitant plus granisetron (RR 1.40, 95% CI 1.02 to 1.93) and fosaprepitant plus granisetron (RR 1.28, 95% CI 1.01 to 1.62) achieve no nausea in the overall phase than participants treated with casopitant plus ondansetron, respectively. Evidence suggests no differences between treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We rated the certainty of evidence for all treatments when compared to aprepitant plus granisetron according to the GRADE system. Using actual reported event rates, we estimated that 896 of 1000 participants achieve complete control of nausea in the overall phase when treated with aprepitant plus granisetron. When compared to treatment with aprepitant plus granisetron, treatment with fosaprepitant plus palonosetron (moderate certainty) probably results in a large increase in complete control of nausea in the overall phase (moderate certainty); treatment with fosnetupitant plus palonosetron (moderate certainty) or rolapitant plus granisetron (moderate certainty) probably increases complete control of nausea in the overall phase; and treatment with ezlopitant plus granisetron may increase complete control of nausea in the overall phase (low certainty). When compared to treatment with aprepitant plus granisetron, treatment with fosaprepitant plus granisetron (high certainty) or netupitant plus palonosetron (high certainty) has little to no effect on complete control of nausea in the overall phase, and treatment with rolapitant plus ondansetron (moderate certainty) likely results in little to no difference. When compared to treatment with aprepitant plus granisetron, treatment with fosaprepitant plus ondansetron (moderate certainty), aprepitant plus ondansetron (moderate certainty), or casopitant plus ondansetron (moderate certainty) probably decreases control of nausea in the overall phase. Our main reasons for downgrading were serious study limitations due to risk of bias and serious or very imprecision. We provide reasons for downgrading per assessment in Table 7.
3. Summary of findings: complete control of nausea during the overall phase (HEC) when compared to treatment with aprepitant + granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by highly emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by highly emetogenic chemotherapy Settings: inpatient and outpatient care Intervention: neurokinin‐1 (NK₁) receptor antagonist and 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroid Comparison: aprepitant (NK₁) combined with granisetron (5‐HT₃) + corticosteroid Outcome: complete control of nausea during the overall phase (0 to 120 h of treatment with chemotherapy) RR < 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with aprepitant + granisetron | Corresponding risk with the intervention | |||||
| fosaprepitant + palonosetron | 896 of 1000 | NE of 1000 (NE to NE) | RR 1.46 (1.12 to 1.90) | 14,588 (22) | ⊕⊕⊕⊝ moderateb |
Fosaprepitant + palonosetron probably results in a large increase in complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| fosnetupitant + palonosetron | 896 of 1000 | NE of 1000 (851 to NE) | RR 1.21 (0.95 to 1.56) | 14,588 (22) | ⊕⊕⊕⊝ moderatec |
Fosnetupitant + palonosetron probably increases complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| ezlopitant + granisetron | 896 of 1000 | NE of 1000 (554 to NE) | RR 1.31 (0.62 to 2.80) | 14,588 (22) | ⊕⊕⊝⊝ lowd |
Ezlopitant + granisetron may increase complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| rolapitant + granisetron | 896 of 1000 | NE of 1000 (860 to NE) | RR 1.12 (0.96 to 1.31) | 14,588 (22) | ⊕⊕⊕⊝ moderatec |
Rolapitant + granisetron probably increases complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| fosaprepitant + granisetron | 896 of 1000 | 914 of 1000 (780 to NE) | RR 1.02 (0.87 to 1.20) | 14,588 (22) | ⊕⊕⊕⊕ high |
Fosaprepitant + granisetron has little to no effect on complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| rolapitant + ondansetron | 896 of 1000 | 860 of 1000 (591 to NE) | RR 0.96 (0.66 to 1.39) | 14,588 (22) | ⊕⊕⊕⊝ moderatec |
Rolapitant + ondansetron probably decreases complete control of nausea slightly in the overall phase when compared with aprepitant + granisetron |
| netupitant + palonosetron | 896 of 1000 | 860 of 1000 (753 to 986) | RR 0.96 (0.84 to 1.10) | 14,588 (22) | ⊕⊕⊕⊕ high |
Netupitant + palonosetron has little to no effect on complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| fosaprepitant + ondansetron | 896 of 1000 | 806 of 1000 (645 to NE) | RR 0.90 (0.72 to 1.13) | 14,588 (22) | ⊕⊕⊕⊝ moderatec |
Fosaprepitant + ondansetron probably decreases complete control of nausea slightly in the overall phase when compared with aprepitant + granisetron |
| aprepitant + ondansetron | 896 of 1000 | 780 of 1000 (609 to NE) | RR 0.87 (0.68 to 1.10) | 14,588 (22) | ⊕⊕⊕⊝ moderatec |
Aprepitant + ondansetron probably decreases complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| casopitant + ondansetron | 896 of 1000 | 717 of 1000 (538 to 950) | RR 0.80 (0.60 to 1.06) | 14,588 (22) | ⊕⊕⊕⊝ moderatec |
Casopitant + ondansetron probably decreases complete control of nausea in the overall phase when compared with aprepitant + granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 412 of 460 (89.6%) participants treated with aprepitant + granisetron experienced no nausea during the overall phase (aprepitant + granisetron was used in 5 studies reporting the outcome). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; NE: not estimable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded once for serious study limitations due to high risk of bias. cDowngraded once for serious imprecision because 95% CIs cross unity and confidence intervals are wide. dDowngraded twice for very serious imprecision because wide confidence intervals suggest both a potentially substantial harm and benefit for the intervention. | ||||||
We identified no evidence for a difference between direct and indirect estimates for both closed loops within this network (see Supplementary Figure 14).
Subgroup analyses
We were able to conduct the following two subgroup analyses.
Type of chemotherapy (cisplatin versus other HEC)
Cisplatin was used for chemotherapy in 16 trials including 9421 participants and comparing a total of 14 treatment regimens. Because of limited direct evidence, not all treatments in the network were connected through direct comparisons but treatments were split into two sub‐networks (figures available from study authors upon request). Sub‐network 1 included ten studies and compared nine treatments versus another. We observed no heterogeneity between studies in this sub‐network. Sub‐network 2 included six studies and compared five treatments versus another. We observed moderate heterogeneity (I² = 48%) between studies in this sub‐network. In subgroup analysis, including cisplatin chemotherapy only, evidence suggests no robust advantage for fosaprepitant plus palonosetron when compared to fosaprepitant plus granisetron (RR 1.49, 95% CI 0.89 to 2.50), aprepitant plus granisetron (RR 1.44, 95% CI 0.92 to 2.27), or netupitant plus palonosetron (RR 1.51, 95% CI 0.94 to 2.41), respectively. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference. Despite the split network, we did not identify any further differences in direction and extent of effect in subgroup analysis between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
Type of cancer (solid versus other)
Seventeen trials included only participants with solid tumours (n = 10,555) and compared 13 treatment regimens. We could include all studies in NMA, and the network was fully connected (figures available from study authors upon request). We observed no heterogeneity between studies in the network. In subgroup analysis, including participants with solid tumours only, evidence suggests no robust advantage for fosaprepitant plus palonosetron when compared with fosaprepitant plus granisetron (RR 1.40, 95% CI 0.85 to 2.31), aprepitant plus granisetron (RR 1.38, 95% CI 0.89 to 2.15), netupitant plus palonosetron (RR 1.44, 95% CI 0.91 to 2.29), fosaprepitant plus ondansetron (RR 1.59, 95% CI 0.95 to 2.68), casopitant plus ondansetron (RR 1.66, 95% CI 0.95 to 2.68), or aprepitant plus ondansetron (RR 1.66, 95% CI 0.98 to 2.81), respectively. Further, evidence from subgroup analysis suggests no robust advantage of fosnetupitant plus palonosetron (RR 1.30, 95% CI 0.79 to 2.15), rolapitant plus granisetron (RR 1.19, 95% CI 0.75 to 1.88), or fosaprepitant plus granisetron (RR 1.18, 95% CI 0.91 to 1.53), when compared with casopitant plus ondansetron, respectively. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference. We did not identify further differences in direction and extent of effect in subgroup analysis between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
Sensitivity analyses
We included in risk of bias (RoB) sensitivity analysis 21 studies with low risk of bias including 13,942 participants and comparing a total of 14 treatment regimens. We could include all studies in NMA, and the network was fully connected (not shown). We observed low heterogeneity (I² = 4.3%) between studies in the network. We did not identify differences in direction and extent of effect in sensitivity analysis compared to the full analysis set.
Complete control of vomiting
We defined complete control of vomiting as no vomiting and no use of rescue medicine. This outcome was usually referred to in the studies as complete response (CR); hereafter we refer to it as CR.
Acute phase (0 to 24 hours)
CR in the acute phase was reported in 41 studies (40 two‐arm studies, one three‐arm study) including 22,400 participants and comparing a total of 18 treatment regimens. The network was not fully connected and consists of two sub‐networks (see Supplementary Figure 15). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Li 2019). Briefly, Li 2019 included 100 participants and compared aprepitant plus tropisetron versus tropisetron. Evidence suggest higher CR in the acute phase for aprepitant plus tropisetron compared to tropisetron (RR 1.24, 95% CI 1.03 to 1.49).
We could include in NMA 40 studies reporting on 22,300 participants and comparing 16 treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 16 and 17. We observed low heterogeneity (I² = 5.6%) between studies in the sub‐network. Fosnetupitant plus palonosetron (RR 1.15, 95% CI 1.05 to 1.27), fosaprepitant plus palonosetron (RR 1.14, 95% CI 1.06 to 1.24), aprepitant plus ramosetron (RR 1.13, 95% CI 1.05 to 1.20), fosaprepitant plus granisetron (RR 1.12, 95% CI 1.04 to 1.20), rolapitant plus granisetron (RR 1.12, 95% CI 1.02 to 1.23), aprepitant plus granisetron (RR 1.11, 95% CI 1.04 to 1.18), aprepitant plus palonosetron (RR 1.11, 95% CI 1.03 to 1.18), fosaprepitant plus ondansetron (RR 1.09, 95% CI 1.03 to 1.16), netupitant plus palonosetron (RR 1.09, 95% CI 1.02 to 1.16), and aprepitant plus ondansetron (RR 1.08, 95% CI 1.03 to 1.14) show higher CR in the acute phase than casopitant plus ondansetron, respectively. Evidence suggests no other differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We identified no evidence of a difference between direct and indirect estimates (see Supplementary Figure 18).
Delayed phase (24 to 120 hours)
CR in the delayed phase was reported in 38 studies (37 two‐arm studies, one three‐arm study) including 21,663 participants and comparing a total of 17 treatment regimens. However, the network was not fully connected and consists of two sub‐networks (see Supplementary Figure 19). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Li 2019). Briefly, Li 2019 included 100 participants and compared aprepitant plus tropisetron versus tropisetron. Evidence suggests higher CR in the delayed phase for aprepitant plus tropisetron compared to tropisetron (RR 1.67, 95% CI 1.21 to 2.30).
We could include in NMA 37 studies reporting on 21,563 participants and comparing 15 treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 20 and 21. We observed moderate heterogeneity (I² = 42.8%) between studies in the sub‐network. Evidence suggests no differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We rated the certainty of evidence for all treatments when compared to aprepitant plus granisetron according to the GRADE system. Using actual reported event rates, we estimated that 694 of 1000 participants achieve complete control of vomiting (CR) in the delayed phase when treated with aprepitant plus granisetron. When compared to treatment with aprepitant plus granisetron, treatment with fosnetupitant plus palonosetron may increase CR in the delayed phase (low certainty). Fosaprepitant plus granisetron (moderate certainty) or netupitant plus palonosetron (moderate certainty) probably results in little to no difference in CR in the delayed phase when compared to aprepitant plus granisetron. Treatment with rolapitant plus granisetron (low certainty), fosaprepitant plus ondansetron (low certainty), or casopitant plus ondansetron (low certainty) may reduce CR in the delayed phase, when compared to aprepitant plus granisetron. Evidence is very uncertain about the effects of fosaprepitant plus palonosetron (very low certainty), aprepitant plus palonosetron (very low certainty), aprepitant plus ramosetron (very low certainty), aprepitant plus ondansetron (very low certainty), and rolapitant plus ondansetron (very low certainty) on CR in the delayed phase when compared to aprepitant plus granisetron. Our main reasons for downgrading were serious study limitations due to risk of bias, moderate inconsistency, and serious imprecision. We provide reasons for downgrading per assessment in Table 8.
4. Summary of findings: complete control of vomiting during the delayed phase (HEC) when compared to treatment with aprepitant + granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by highly emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by highly emetogenic chemotherapy Settings: inpatient and outpatient care Intervention: neurokinin‐1 (NK₁) receptor antagonist and 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroid Comparison: aprepitant (NK₁) combined with granisetron (5‐HT₃) + corticosteroid Outcome: complete control of vomiting during the delayed phase (24 to 120 h of treatment with chemotherapy) RR > 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk aprepitant + granisetron | Corresponding risk with the intervention | |||||
| fosnetupitant + palonosetron | 694 of 1000 | 784 of 1000 (632 to 972) | RR 1.13 (0.91 to 1.40) | 21,563 (37) | ⊕⊕⊝⊝ lowb,c |
Fosnetupitant + palonosetron may increase complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| fosaprepitant + palonosetron | 694 of 1000 | 736 of 1000 (632 to 854) | RR 1.06 (0.91 to 1.23) | 21,563 (37) | ⊕⊝⊝⊝ very lowb,c,d |
Evidence is very uncertain about the effect of fosaprepitant + palonosetron on complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| aprepitant + palonosetron | 694 of 1000 | 722 of 1000 (652 to 798) | RR 1.04 (0.94 to 1.15) | 21,563 (37) | ⊕⊝⊝⊝ very lowb,c,d |
Evidence is very uncertain about the effect of aprepitant + palonosetron on complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| aprepitant + ramosetron | 694 of 1000 | 722 of 1000 (625 to 1.21) | RR 1.04 (0.90 to 1.21) | 21,563 (37) | ⊕⊝⊝⊝ very lowb,c,d |
Evidence is very uncertain about the effect of aprepitant + ramosetron on complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| fosaprepitant + granisetron | 694 of 1000 | 701 of 1000 (632 to 770) | RR 1.01 (0.91 to 1.11) | 21,563 (37) | ⊕⊕⊕⊝ moderateb |
Fosaprepitant + granisetron probably has little to no effect on complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| netupitant + palonosetron | 694 of 1000 | 687 of 1000 (618 to 763) | RR 0.99 (0.89 to 1.10) | 21,563 (37) | ⊕⊕⊕⊝ moderateb |
Netupitant + palonosetron probably has little to no effect on complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| aprepitant + ondansetron | 694 of 1000 | 645 of 1000 (576 to 722) | RR 0.93 (0.83 to 1.04) | 21,563 (37) | ⊕⊝⊝⊝ very lowb,c,d |
Evidence is very uncertain about the effect of aprepitant + ondansetron on complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| rolapitant + granisetron | 694 of 1000 | 632 of 1000 (541 to 736) | RR 0.91 (0.78 to 1.06) | 21,563 (37) | ⊕⊕⊝⊝ lowb,c |
Rolapitant + granisetron may decrease complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| fosaprepitant + ondansetron | 694 of 1000 | 632 of 1000 (548 to 722) | RR 0.91 (0.79 to 1.04) | 21,563 (37) | ⊕⊕⊝⊝ lowb,c |
Fosaprepitant + ondansetron may decrease complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| casopitant + ondansetron | 694 of 1000 | 618 of 1000 (507 to 756) | RR 0.89 (0.73 to 1.09) | 21,563 (37) | ⊕⊕⊝⊝ lowb,c |
Casopitant + ondansetron may decrease complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| rolapitant + ondansetron | 694 of 1000 | 583 of 1000 (437 to 784) | RR 0.84 (0.63 to 1.13) | 21,563 (37) | ⊕⊝⊝⊝ very lowb,c,d |
Evidence is very uncertain about the effect of rolapitant + ondansetron on complete control of vomiting in the delayed phase when compared to aprepitant + granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 1537 of 2215 (69.4%) participants treated with aprepitant + granisetron achieved complete response during the delayed phase (aprepitant + granisetron was used in 10 studies reporting the outcome). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded once for moderate inconsistency. cDowngraded once for serious imprecision because 95% CIs cross unity and confidence intervals are wide. dDowngraded once for serious study limitations due to high risk of bias. | ||||||
We identified no evidence of a difference between direct and indirect estimates (see Supplementary Figure 22).
Overall phase (0 to 120 hours)
CR in the overall phase was reported in 39 studies (38 two‐arm studies, one three‐arm study) including 21,642 participants and comparing a total of 17 treatment regimens. However, the network was not fully connected and consists of two sub‐networks (Figure 4 and Supplementary Figure 23). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Li 2019). Briefly, Li 2019 included 100 participants and compared aprepitant plus tropisetron versus tropisetron. Evidence suggests higher CR in the overall phase for aprepitant plus tropisetron compared to tropisetron (RR 1.67, 95% CI 1.21 to 2.30).
4.
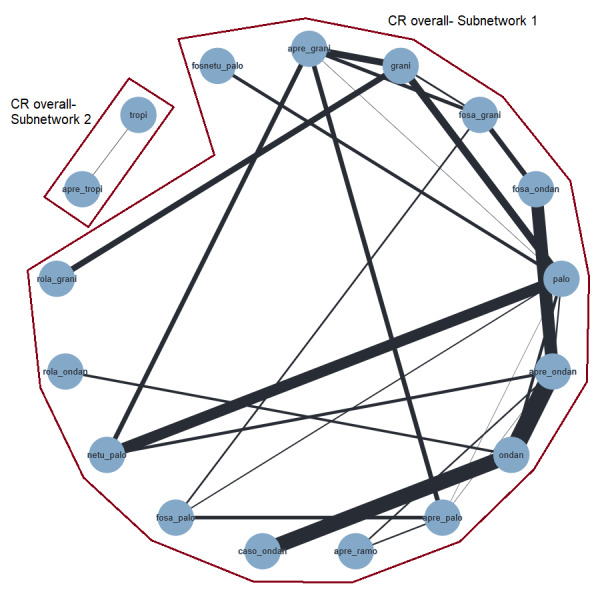
Network graph for the outcome complete control of vomiting in the overall phase (HEC).
A line connects any two treatments when there is at least one study comparing the two treatments. Line width: number of patients.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
We included in NMA 38 studies comprising 21,542 participants and a total of 15 treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Figure 5 and Figure 6 and in Supplementary Figures 24 and 25. We observed low heterogeneity (I² = 11.7%) between studies in the sub‐network. Evidence suggests that more people treated with fosnetupitant plus palonosetron achieve CR in the overall phase than people treated with aprepitant plus ondansetron (RR 1.20, 95% CI 1.01 to 1.43), fosaprepitant plus ondansetron (RR 1.23, 95% CI 1.02 to 1.47), casopitant plus ondansetron (RR 1.28, 95% CI 1.05 to 1.56), and rolapitant plus granisetron (RR 1.29, 95% CI 1.06 to 1.59), respectively. Aprepitant plus palonosetron shows higher CR in the overall phase than aprepitant plus ondansetron (RR 1.12, 95% CI 1.01 to 1.24), fosaprepitant plus ondansetron (RR 1.14, 95% CI 1.02 to 1.28), casopitant plus ondansetron (RR 1.19, 95% CI 1.03 to 1.37), and rolapitant plus granisetron (RR 1.20, 95% CI 1.03 to 1.40), respectively. Treatment with aprepitant plus ramosetron shows higher CR in the overall phase than treatment with casopitant plus ondansetron (RR 1.19, 95% CI 1.03 to 1.38) and rolapitant plus granisetron (RR 1.20, 95% CI 1.01 to 1.42). Treatment with fosaprepitant plus palonosetron shows higher CR in the overall phase than treatment with casopitant plus ondansetron (RR 1.18, 95% CI 1.01 to 1.38) and rolapitant plus granisetron (RR 1.20, 95% CI 1.02 to 1.40). Evidence suggests no other differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
5.
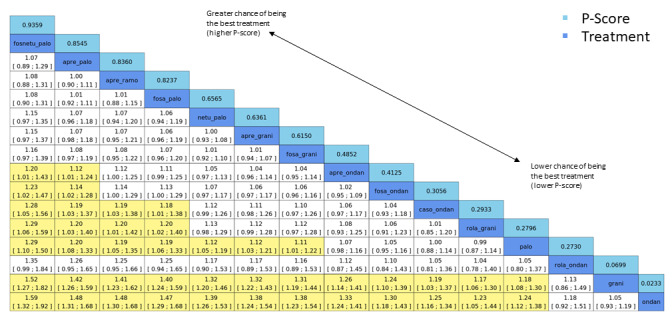
League table for the outcome complete control of vomiting in the overall phase (HEC). Network estimates with 95% CIs are given. Descending P score shows ranking of treatment options. Statistically significant results are marked in yellow. Global approach to check inconsistency/heterogeneity: Q‐statistics, I².
No. of studies: 34. No. of treatments: 14. No. of pair‐wise comparisons: 36. No. of designs: 21.
Qtotal = 23.95, df = 22, P = 0.35/Qwithin = 17.60, df = 13, P = 0.17/Qbetween = 6.34, df = 9, P = 0.71; I² = 8.1%, Tau² = 0.0005. Treatment effects + 95% CIs (risk ratios, random‐effects model).
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
6.
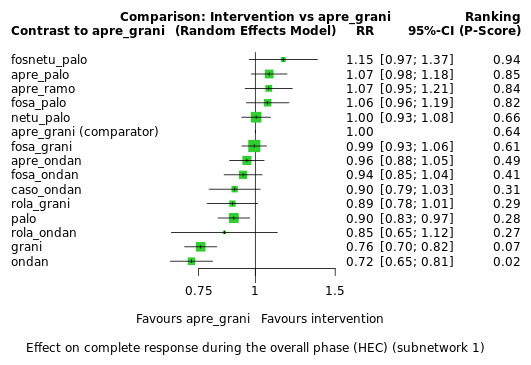
Exemplary network meta‐analysis forest plot for the outcome complete control of vomiting during the overall phase (HEC) (random‐effects model).
Aprepitant + granisetron was used as exemplary reference treatment. Ranking of treatments is ordered by P score (descending).
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
We rated the certainty of evidence for all treatments when compared to aprepitant plus granisetron according to the GRADE system. Using actual reported event rates, we estimated that 704 of 1000 participants achieve complete control of vomiting (CR) in the overall phase when treated with aprepitant plus granisetron. When compared to treatment with aprepitant plus granisetron, treatment with fosnetupitant plus palonosetron probably increases CR in the overall phase. When compared to treatment with aprepitant plus granisetron, treatment with aprepitant plus palonosetron (low certainty), aprepitant plus ramosetron (low certainty), and fosaprepitant plus palonosetron (low certainty) may result in a slight increase in CR in the overall phase. When compared to treatment with aprepitant plus granisetron, treatment with netupitant plus palonosetron (high certainty) or fosaprepitant plus granisetron (high certainty) results in little to no difference in CR in the overall phase. Treatment with aprepitant plus ondansetron (low certainty) or treatment with fosaprepitant plus ondansetron (low certainty) may result in a slight decrease in CR in the overall phase, when compared to treatment with aprepitant plus granisetron. Treatment with casopitant plus ondansetron (low certainty) or rolapitant plus ondansetron (low certainty) may decrease, and treatment with rolapitant plus granisetron (moderate certainty) probably decreases, CR in the overall phase when compared to aprepitant plus granisetron. Our main reasons for downgrading were serious study limitations due to risk of bias and serious imprecision. We provide reasons for downgrading per assessment in Table 1.
We identified no evidence of a difference between direct and indirect estimates (Figure 7 and Supplementary Figure 26).
7.
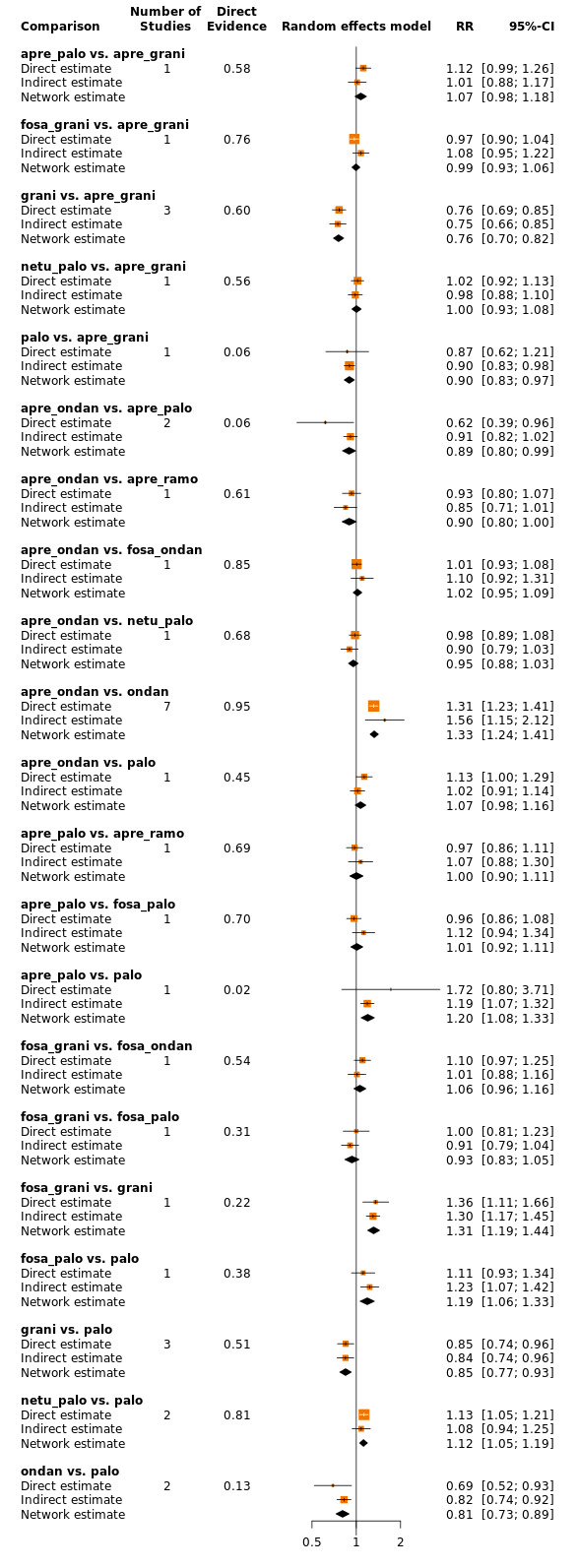
Comparison of direct and indirect evidence (in closed loops) for the outcome complete control of vomiting in the overall phase (HEC). CI: confidence interval; RR: risk ratio.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
Subgroup analyses
We were able to conduct the following three subgroup analyses.
Type of chemotherapy (cisplatin versus other HEC)
Cisplatin was used for chemotherapy in 19 trials including 11,637 participants and comparing a total of 12 treatment regimens. All trials could be included in NMA, and the network was fully connected (not shown). We observed low heterogeneity (I² = 10%) between studies in the network. In subgroup analysis including cisplatin‐based chemotherapy only, evidence suggests no robust advantage for fosnetupitant plus palonosetron when compared with aprepitant plus ondansetron (RR 1.29, 95% CI 0.99 to 1.44) or fosaprepitant plus ondansetron (RR 1.20, 95% CI 0.96 to 1.47), respectively. Evidence further suggests no robust advantage for fosaprepitant plus palonosetron when compared with casopitant plus ondansetron (RR 1.18, 95% CI 0.96 to 1.45); nor for aprepitant plus palonosetron when compared with aprepitant plus ondansetron (RR 1.07, 95% CI 0.92 to 1.25), fosaprepitant plus ondansetron (RR 1.08, 95% CI 0.91 to 1.28), or casopitant plus ondansetron (RR 1.16, 95% CI 0.95 to 1.42), respectively. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference. We did not identify further differences in direction and extent of effect in subgroup analysis between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
Type of cancer (breast cancer versus other)
Eight trials included only participants with breast cancer (n = 5162) and compared ten treatment regimens. The network was not fully connected and consisted of three sub‐networks (figures available from study authors upon request). Sub‐network 1 included six studies and compared six treatments versus another. We observed no heterogeneity between studies in this sub‐network. Sub‐network 2 and Sub‐network 3 each included one study and, respectively, compared two treatments versus another. In comparison to the full analysis set, none of the comparisons of treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor demonstrated any evidence of a difference between comparisons. Confidence intervals of the effect estimates for both analyses were widely overlapping.
Type of cancer (solid (excluding breast cancer) versus other)
Twenty‐five trials included only participants with solid tumours (excluding breast cancer) (n = 13,716) and compared 14 treatment regimens. We could include all studies in NMA, and the network was fully connected (figures available from study authors upon request). We observed low heterogeneity (I² = 29.7%) between studies in the network. In comparison to the full analysis set, none of the comparisons of treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor demonstrated any evidence of a difference between comparisons. Confidence intervals of the effect estimates for both analyses were widely overlapping.
Sensitivity analyses
We included 30 studies with low risk of bias including 20,137 participants and comparing a total of 14 treatment regimens in RoB sensitivity analysis. We could include all studies in NMA, and the network was fully connected (figures available from study authors upon request). We observed low heterogeneity (I² = 27.1%) between studies in the network. When only studies at low risk of bias are considered in NMA, evidence suggests no robust advantage of fosnetupitant plus palonosetron when compared with aprepitant plus ondansetron (RR 1.21, 95% CI 0.99 to 1.46) or fosaprepitant plus ondansetron (RR 1.23, 95% CI 1.00 to 1.51), respectively. Further, evidence suggests no robust advantage for aprepitant plus palonosetron when compared with aprepitant plus ondansetron (RR 1.12, 95% CI 0.97 to 1.30), fosaprepitant plus ondansetron (RR 1.14, 95% CI 0.98 to 1.33), or casopitant plus ondansetron (RR 1.19, 95% CI 1.00 to 1.43), respectively. Sensitivity analysis also suggests no robust advantage for fosaprepitant plus palonosetron when compared with casopitant plus ondansetron (RR 1.18, 95% CI 0.99 to 1.42). However, we identified no evidence of a difference in direction or estimates of effect between the full analysis set and studies with low risk of bias for treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
Quality of life
No impairment in quality of life
We reported and extracted this endpoint as number of participants with no impairment in quality of life (QoL).
QoL was reported in 16 studies (all two‐arm studies) including 8264 participants and comparing a total of 13 treatment regimens. All studies used the Functional Life Index‐Emesis (FLIE) to assess impairment in quality of life. The network was not fully connected and consisted of three sub‐networks (see Supplementary Figure 27). We performed NMA only for Sub‐network 1, as Sub‐networks 2 and 3 consisted of only one pair‐wise comparison (Kang 2020; Li 2019). Briefly, Li 2019 included 100 participants and compared aprepitant plus tropisetron versus tropisetron. Evidence suggests higher QoL for aprepitant plus tropisetron compared to tropisetron (RR 3.00, 95% CI 1.04 to 8.67). Kang 2020 included 270 participants and compared aprepitant plus palonosetron versus aprepitant plus ramosetron. Evidence suggests no differences between treatments (RR 1.01, 95% CI 0.88 to 1.17).
We could include in NMA 14 studies reporting on 7894 participants and comparing nine treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 28 and 29. We observed substantial heterogeneity (I² = 78.1%) between studies in the sub‐network. Evidence suggests no differences in QoL between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We rated the certainty of evidence for all treatments when compared to aprepitant plus granisetron according to the GRADE system. Using actual reported event rates, we estimated that 714 of 1000 participants have no impairment in quality of life when treated with aprepitant plus granisetron. When compared to aprepitant plus granisetron, the evidence is very uncertain about the effects of rolapitant plus ondansetron (very low certainty), netupitant plus palonosetron (very low certainty), casopitant plus ondansetron (very low certainty), rolapitant plus granisetron (very low certainty), and aprepitant plus ondansetron (very low certainty) on QoL. Our main reasons for downgrading were serious study limitations due to risk of bias, inconsistency, and imprecision. We provide reasons for downgrading per assessment in Table 9.
5. Summary of findings: quality of life (HEC) when compared to treatment with aprepitant + granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by highly emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by highly emetogenic chemotherapy Settings: inpatient and outpatient care Intervention: neurokinin‐1 (NK₁) receptor antagonist and 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroid Comparison: aprepitant (NK₁) combined with granisetron (5‐HT₃) + corticosteroid Outcome: no impairment in quality of life RR <1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
|
Interventions (corticosteroids included in all regimens)a |
Illustrative comparative risks* (95% CI) |
Risk ratio (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Assumed risk with aprepitant + granisetron | Corresponding risk with the intervention | |||||
| rolapitant + ondansetron | 714 of 1000 | 893 of 1000 (486 to 1649) | RR 1.25 (0.68 to 2.31) | 7894 (14) | ⊕⊝⊝⊝ very lowb,c |
Evidence is uncertain about the effect of rolapitant + ondansetron on quality of life when compared to aprepitant + granisetron |
| netupitant + palonosetron | 714 of 1000 | 764 of 1000 (585 to 1007) | RR 1.07 (0.82 to 1.41) | 7894 (14) | ⊕⊝⊝⊝ very lowb,d |
Evidence is uncertain about the effect of netupitant + palonosetron on quality of life when compared to aprepitant + granisetron |
| casopitant + ondansetron | 714 of 1000 | 743 of 1000 (421 to 1307) | RR 1.04 (0.59 to 1.83) | 7894 (14) | ⊕⊝⊝⊝ very lowb,c |
Evidence is uncertain about the effect of casopitant + ondansetron on quality of life when compared to aprepitant + granisetron |
| rolapitant + granisetron | 714 of 1000 | 693 of 1000 (521 to 921) | RR 0.97 (0.73 to 1.29) | 7894 (14) | ⊕⊝⊝⊝ very lowb,d |
Evidence is uncertain about the effect of rolapitant + granisetron on quality of life when compared to aprepitant + granisetron |
| aprepitant + ondansetron | 714 of 1000 | 657 of 1000 (393 to 1100) | RR 0.92 (0.55 to 1.54) | 7894 (14) | ⊕⊝⊝⊝ very lowb,c,e |
Evidence is uncertain about the effect of aprepitant + ondansetron on quality of life when compared to aprepitant + granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 569 of 797 participants treated with aprepitant + granisetron experienced no impact on QoL (aprepitant + granisetron was used in 3 studies reporting the outcome, follow‐up on Day 6). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded twice for high inconsistency within the network. cDowngraded twice for very serious imprecision because 95% CIs cross unity and confidence intervals are very wide, suggesting high benefit for the comparator. dDowngraded once for serious imprecision because 95% CIs cross unity and confidence intervals are wide. eDowngraded once for serious study limitations due to high risk of bias. | ||||||
As presented in Figure 27 (supplementary material), there are no closed loops in the network. Therefore, we could not statistically or visually analyse inconsistencies between direct and indirect evidence.
Safety
Safety outcomes were not consistently reported across studies. To be able to meta‐analyse results, we could consider only those that reported the number of participants with at least one event. We could not consider cumulated events or breakdowns in degree of severity, nor further subgroups.
On‐study mortality
On‐study mortality was reported in 18 studies (17 two‐arm studies, one three‐arm study) including 10,392 participants and comparing a total of 11 treatment regimens (see Supplementary Figure 30). We had to exclude from the analysis two studies including a total of 2362 participants due to zero events because no indication is provided of the direction nor the magnitude of the relative treatment effect (Grunberg 2011; NCT01640340). Furthermore, for NMA, we broke down one study ‐ Hesketh 2014 ‐ into two independent studies, as this is a three‐arm study in which two arms did not report any events and therefore one of the three comparisons was not possible. We could include in NMA 16 studies including 8030 participants and nine treatment regimens, and the network was fully connected. Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 31 and 32. We observed no heterogeneity (I² = 0.0%) between studies in the network. Evidence suggests no differences between treatments in on‐study mortality.
We rated the certainty of evidence for all treatments when compared to aprepitant plus granisetron according to the GRADE system. Using actual reported event rates, we estimated that 8 of 1000 participants died during the study when treated with aprepitant plus granisetron. When compared to treatment with aprepitant plus granisetron, treatment with netupitant plus palonosetron (low certainty), aprepitant plus ondansetron (low certainty), casopitant plus ondansetron (low certainty), or rolapitant plus granisetron (low certainty) may reduce on‐study mortality. When compared to treatment with aprepitant plus granisetron, treatment with rolapitant plus ondansetron may increase on‐study mortality (low certainty). Our reason for downgrading was very serious imprecision (please also see Table 10).
6. Summary of findings: on‐study mortality (HEC) when compared to treatment with aprepitant + granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by highly emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by highly emetogenic chemotherapy Settings: inpatient and outpatient care Intervention: neurokinin‐1 (NK₁) receptor antagonist and 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists + corticosteroid Comparison: aprepitant (NK₁) combined with granisetron (5‐HT₃) + corticosteroid Outcome: on‐study mortality RR < 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
|
Interventions (corticosteroids included in all regimens)a |
Illustrative comparative risks* (95% CI) |
Risk ratio (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Assumed risk with aprepitant + granisetron | Corresponding risk with the intervention | |||||
| netupitant + palonosetron | 8 of 1000 | 2 of 1000 (0 to 19) | RR 0.29 (0.04 to 2.34) | 8030 (16) | ⊕⊕⊝⊝ lowb |
Netupitant + palonosetron may have little to no effect on on‐study mortality when compared with aprepitant + granisetron |
| aprepitant + ondansetron | 8 of 1000 | 5 of 1000 (1 to 35) | RR 0.57 (0.07 to 4.39) | 8030 (16) | ⊕⊕⊝⊝ lowb |
Aprepitant + ondansetron may have little to no effect on on‐study mortality when compared with aprepitant + granisetron |
| casopitant + ondansetron | 8 of 1000 | 5 of 1000 (1 to 44) | RR 0.65 (0.08 to 5.53) | 8030 (16) | ⊕⊕⊝⊝ lowb |
Casopitant + ondansetron may have little to no effect on on‐study mortality when compared with aprepitant + granisetron |
| rolapitant + granisetron | 8 of 1000 | 5 of 1000 (1 to 33) | RR 0.66 (0.11 to 4.09) | 8030 (16) | ⊕⊕⊝⊝ lowb |
Rolapitant + granisetron may have little to no effect on on‐study mortality when compared with aprepitant + granisetron |
| rolapitant + ondansetron | 8 of 1000 | 12 of 1000 (1 to 216) | RR 1.56 (0.09 to 26.97) | 8030 (16) | ⊕⊕⊝⊝ lowb |
Rolapitant + ondansetron may have little to no effect on on‐study mortality when compared with aprepitant + granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 7 of 844 (0.08%) participants treated with aprepitant + granisetron died during the study (aprepitant + granisetron was used in 4 studies reporting the outcome, with follow‐up of up to 29 days). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded twice for very serious imprecision due to few events and very wide confidence intervals, suggesting potential benefit and harm for the comparator. | ||||||
We identified no evidence of a difference between direct and indirect estimates within this network (see Supplementary Figure 33).
Adverse events
Participants with at least one adverse event (AE) were reported in 20 studies (19 two‐arm studies, one three‐arm study) including 13,036 participants and comparing a total of 13 treatment regimens. We could include all studies in NMA, and the network was fully connected (see Supplementary Figure 34). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 35 and 36. We observed no heterogeneity (I² = 0.0%) between studies in the network. Evidence suggests similar relative risks of an AE for all treatment comparisons. However, evidence also suggests that participants treated with aprepitant plus granisetron experienced fewer AEs than participants treated with fosaprepitant plus ondansetron (RR 0.93, 95% CI 0.88 to 0.99), fosnetupitant plus palonosetron (RR 0.92, 95% CI 0.88 to 0.97), and fosaprepitant plus granisetron (RR 0.92, 95% CI 0.88 to 0.96), respectively. Evidence suggests no other differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We identified no evidence of a difference between direct and indirect estimates (see Supplementary Figure 37).
Serious adverse events
Participants with at least one serious AE (SAE) were reported in 23 studies (22 two‐arm studies, one three‐arm study) including 16,065 participants and comparing a total of 14 treatment regimens. We could not include in the analysis Cheirsilpa 2005, which included 73 participants, due to zero events. We could include 22 studies in NMA, and the network was fully connected (Figure 8 and Supplementary Figure 38). Results for all network comparisons, including ranking of treatments, are shown in Figure 9 and Figure 10 and in Supplementary Figures 39 and 40. We observed moderate heterogeneity (I² = 50.2%) between studies in the network. Evidence suggests no differences between treatment combinations.
8.
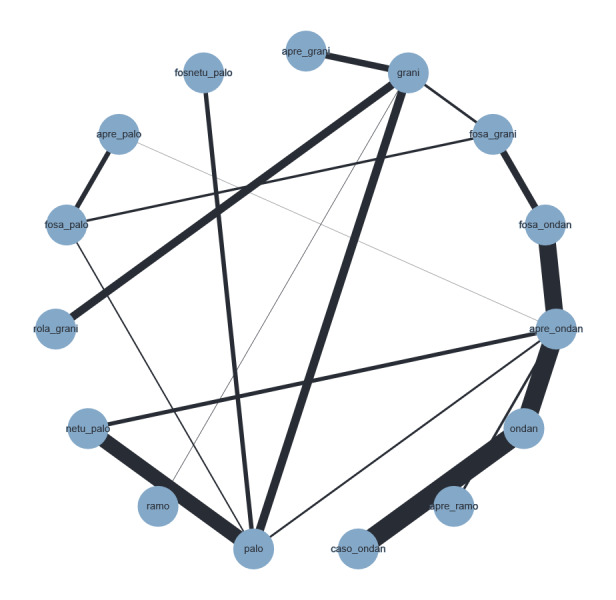
Network graph for the outcome serious adverse events (HEC).
A line connects any 2 treatments when there is at least 1 study comparing the 2 treatments. Line width: number of patients.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
9.
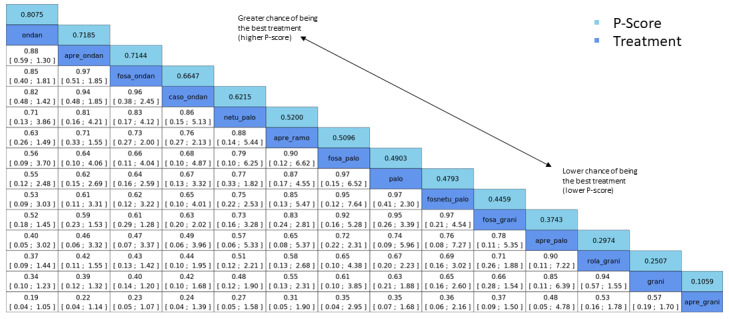
League table for the outcome serious adverse events (HEC). Network estimates with 95% CIs are given. Descending P score shows ranking of treatment options. Statistically significant results are marked in yellow. Global approach to check inconsistency/heterogeneity: Q‐statistics, I².
No. of studies: 20. No. of treatments: 12. No. of pair‐wise comparisons: 22. No. of designs: 13.
Qtotal = 20.26, df = 9, P = 0.016/Qwithin = 16.39, df = 7, P = 0.022/Qbetween = 3.87, df = 2, P = 0.14; I² = 55.6%, Tau² = 0.1057. Treatment effects + 95% CIs (risk ratios, random‐effects model).
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
10.
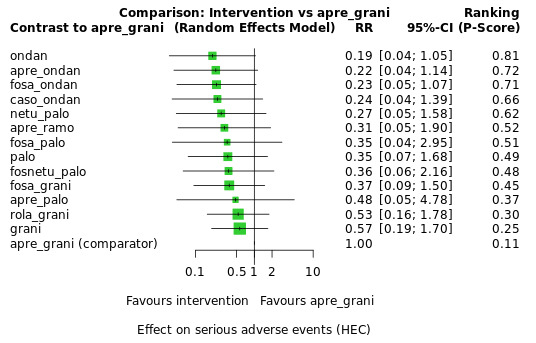
Exemplary network meta‐analysis forest plot for the outcome serious adverse events (HEC) (random‐effects model).
Aprepitant + granisetron was used as exemplary reference treatment. Ranking of treatments is ordered by P score (descending).
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
We rated the certainty of evidence for all treatments when compared to aprepitant plus granisetron according to the GRADE system. Using actual reported event rates, we estimated that 35 of 1000 participants experience SAEs when treated with aprepitant plus granisetron. When compared to treatment with aprepitant plus granisetron, treatment with fosaprepitant plus ondansetron (low certainty), casopitant plus ondansetron (low certainty), netupitant plus palonosetron (low certainty), fosaprepitant plus granisetron (low certainty), or rolapitant plus granisetron (low certainty) may slightly reduce the risk of SAEs. When compared to treatment with aprepitant plus granisetron, evidence is very uncertain about the effects of aprepitant plus ondansetron (very low certainty), aprepitant plus ramosetron (very low certainty), fosaprepitant plus palonosetron (very low certainty), fosnetupitant plus palonosetron (very low certainty), or aprepitant plus palonosetron (very low certainty) on SAEs. Our main reasons for downgrading were serious study limitations due to risk of bias, moderate inconsistency, and serious or very serious imprecision. We provide reasons for downgrading per assessment in Table 2.
We identified no evidence of a difference between direct and indirect estimates (Figure 11 and Supplementary Figure 41).
11.
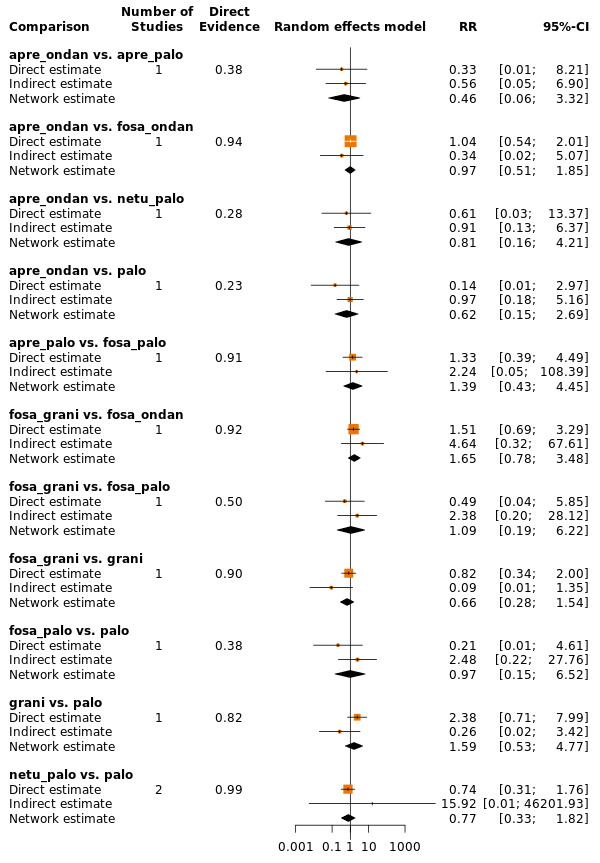
Comparison of direct and indirect evidence (in closed loops) for the outcome serious adverse events (HEC). CI: confidence interval; RR: risk ratio.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
Neutropenia
Neutropenia was reported in 13 studies (all two‐arm studies) including 10,585 participants and comparing a total of 13 treatment regimens. However, the network was not fully connected and consists of two sub‐networks (see Supplementary Figure 42). We performed NMA for Sub‐networks 1 and 2. Results of all network comparisons within Sub‐networks 1 and 2, including ranking of treatments, are shown in Supplementary Figures 43 and 44.
We observed no heterogeneity (I² = 0.0%) between studies in Sub‐network 1. Evidence suggests that fewer participants treated with aprepitant plus ondansetron experienced neutropenia than participants treated with casopitant plus ondansetron (RR 0.35, 95% CI 0.15 to 0.84). Evidence suggests no other differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, within Sub‐network 1.
As presented in Supplementary Figure 42, there are no closed loops in the network. Therefore, we could not statistically or visually analyse inconsistencies between direct and indirect evidence.
We could not analyse generalised heterogeneity statistic Qtotal and generalized I² statistic for Sub‐network 2. Evidence suggests no differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 2.
As presented in Supplementary Figure 42, there are no closed loops in Sub‐network 2. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
Febrile neutropenia
Febrile neutropenia was reported in 19 studies (all two‐arm studies) including 13,873 participants and comparing a total of 14 treatment regimens. We could include all studies in NMA, and the network was fully connected (see Supplementary Figure 45). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 46 and 47. We observed no heterogeneity (I² = 0.0%) between studies in the network. Evidence suggests no differences between treatment combinations.
As presented in Supplementary Figure 45, there are no closed loops in the network. Therefore, we could not statistically or visually analyse inconsistencies between direct and indirect evidence.
Infection
The numbers of participants experiencing any infection were reported in two pair‐wise studies including 999 participants and comparing four treatment combinations (Ishido 2016; Schnadig 2016). Treatment combinations were not connected, and we could not perform NMA (see Supplementary Figure 48) for this outcome. Ishido 2016 included 84 participants and compared aprepitant plus granisetron versus palonosetron. Evidence suggests no differences between aprepitant plus granisetron versus palonosetron (RR 6.68, 95% CI 0.36 to 125.38). Schnadig 2016 included 915 participants and compared fosaprepitant plus granisetron versus fosaprepitant plus ondansetron. Evidence also suggests no differences between fosaprepitant plus granisetron and fosaprepitant plus ondansetron (RR 1.01, 95% CI 0.36 to 2.85).
Local reaction at infusion site
The numbers of participants experiencing any reaction at the infusion site were reported in seven pair‐wise studies including 6522 participants and comparing nine treatment combinations. The network was not fully connected and consisted of three sub‐networks (available from study authors upon request). We could perform NMA for Sub‐network 1 only, as Sub‐networks 2 and 3 consisted of only pair‐wise comparisons. Results for all treatment comparisons are available from study authors upon request, including ranking of treatments for comparisons included in Sub‐network 1.
We could include four studies comparing five treatment combinations and data from 1491 participants in NMA for Sub‐network 1. We could not analyse generalised heterogeneity statistic Qtotal and generalized I² statistic for Sub‐network 1. Evidence suggests no differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 1.
We included data from two studies in Sub‐network 2. Grunberg 2009 and Herrstedt 2009 compared casopitant plus ondansetron versus ondansetron and included a total of 2719 participants. Results of pair‐wise meta‐analysis suggest no differences between casopitant plus ondansetron and ondansetron (RR 5.33, 95% CI 0.70 to 40.83). Sub‐network 3 consisted of only one study. Grunberg 2011 included 2312 participants and compared fosaprepitant plus ondansetron versus aprepitant plus ondansetron. Evidence suggests that participants treated with aprepitant plus ondansetron experienced fewer local reactions at the infusion site than participants treated with fosaprepitant plus ondansetron (RR 0.20, 95% CI 0.08 to 0.51).
We had planned to rate the certainty of evidence for local reactions at the infusion site according to the GRADE system for all treatment regimens compared to aprepitant plus granisetron, respectively. However, as no study reported the outcome for this treatment regimen, we could not rate the certainty of evidence for local reactions at the infusion site.
Hiccups
Hiccups were reported in 18 studies (17 two‐arm studies, one three‐arm study) including 9913 participants and comparing a total of 13 treatment regimens. The network was not fully connected and consisted of two sub‐networks (Supplementary Figure 49). We could perform NMA for Sub‐network 1 only, as Sub‐network 2 consisted of only one pair‐wise comparison. Briefly, Zhang 2020 included 646 participants and compared fosaprepitant plus palonosetron versus aprepitant plus palonosetron. Evidence suggests no differences between treatments (RR 1.17, 95% CI 0.69 to 2.0).
Results for all network comparisons included in Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 50 and 51. We observed no heterogeneity (I² = 0.0%) between studies in the network. Evidence suggests that participants treated with fosaprepitant plus granisetron (RR 0.44, 95% CI 0.24 to 0.81) and aprepitant plus granisetron (RR 0.52, 95% CI 0.29 to 0.92) experienced fewer hiccups than participants treated with rolapitant plus granisetron, respectively. Evidence suggests no other differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
We identified no evidence of a difference between direct and indirect estimates (Supplementary Figure 52).
Efficacy versus acceptability
Optimal treatment should be characterised by both high efficacy and acceptability. Figure 12 and Supplementary Figure 53 illustrate concurrently an exemplary ranking of treatment combinations for the outcome CR during the overall phase, for which we chose to represent efficacy, and the outcome SAEs, for which we chose to represent acceptability. We ordered treatments by P score. Treatment combinations with both high efficacy and high acceptability are located in the upper right corner of this graph. The related league table with all network estimates (RRs and 95% CIs) is given in Figure 13 and in Supplementary Figure 54. According to this ranking, we could not identify superior treatment within this comparison. We could include in this exemplary ranking plot only treatment combinations for which data were available for both outcomes (CR during the overall phase and SAEs).
12.
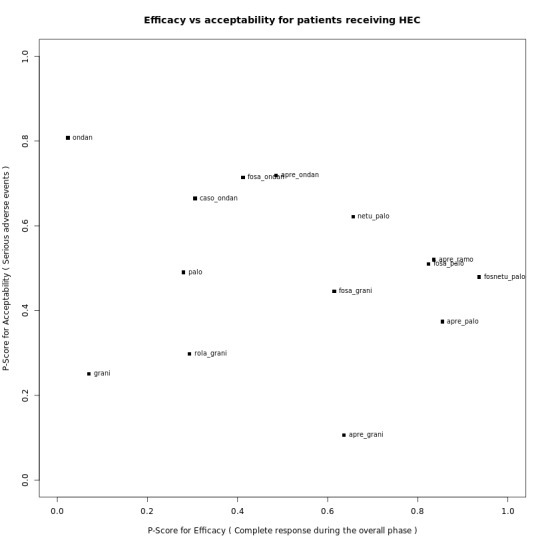
Exemplary ranking plot representing simultaneously the efficacy (x‐axis, CR during the overall phase) and the acceptability (y‐axis, SAEs) of all antiemetic regimens for patients receiving highly emetogenic chemotherapy.
Only antiemetic regimens for which data for both endpoints (CR during the overall phase and SAEs) were available are represented in the ranking plot.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
13.
League table with network estimates (RR with 95% CIs) of all treatment combinations for efficacy (CR during the overall phase) and acceptability (SAEs) (HEC).
Treatments are presented in alphabetical order. For efficacy, RRs > 1 favour the first treatment in alphabetical order. For safety, RRs < 1 favour the first treatment in alphabetical order.
n.a.: no data were available for this comparison
Statistically significant results are marked bold.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
Moderately emetogenic chemotherapy (MEC)
Our objectives were to compare whether antiemetic treatment combinations including NK₁ receptor antagonists, 5‐HT₃ receptor antagonists, and corticosteroids are superior for prevention of acute, delayed, and overall CINV to treatment combinations including 5‐HT₃ receptor antagonists and corticosteroids, and to generate a clinically meaningful treatment ranking according to their safety and efficacy.
We describe all comparisons that show evidence of a difference between treatment combinations including 5‐HT₃ inhibitors and corticosteroids and treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and corticosteroids. All other comparisons are provided per outcome in league tables. We describe the ranking of treatments for those that appear to be most and least effective/safe. Additionally to the P score, we considered the distribution of P scores across rankings, estimated treatment effects, and confidence intervals. For a comprehensive illustration of our results in forest plots and presentation in 'Summary of findings' tables, we randomly chose granisetron as an exemplary reference treatment for MEC. However, theoretically, we could have used every treatment combination as a reference.
Efficacy
Complete control of nausea
We defined complete control of nausea as no nausea and no significant nausea, and we refer to it hereafter as "no nausea".
Acute phase (0 to 24 hours)
No nausea in the acute phase was reported in 13 studies including 4335 participants and comparing eight treatment regimens. We could include all studies in NMA, and the network was fully connected (see Supplementary Figure 55). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 56 and 57. We observed no heterogeneity (I² = 0.0%) between studies in the network. Evidence suggests no differences between treatment combinations including 5‐HT₃ inhibitors, and a corticosteroid versus treatment combination including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid. Ranking of treatments suggests aprepitant plus palonosetron to be most effective in preventing nausea during the acute phase (P score 0.90), and aprepitant plus granisetron (P score 0.11) to be least effective.
The test for incoherence and visual examination showed no evidence of a difference between direct and indirect estimates in the only closed loop in the network (see Supplementary Figure 58).
Delayed phase (24 to 120 hours)
No nausea in the delayed phase was reported in ten studies including 4136 participants and comparing eight treatment regimens. However, the network was not fully connected and consists of two sub‐networks (see Supplementary Figure 59). We performed NMA for Sub‐networks 1 and 2. Results for all network comparisons, including ranking of treatment combinations for both sub‐networks, are shown in Supplementary Figures 60 and 61. We observed substantial heterogeneity (I² = 64.2%) between studies in Sub‐network 1. Aprepitant plus palonosetron shows higher complete control of nausea in the delayed phase than ondansetron (RR 1.63, 95% CI 1.07 to 2.47). Evidence suggests no other differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 1.
We identified no evidence of a difference between direct and indirect estimates in the only closed loop in Sub‐network 1 (see Supplementary Figure 62).
Generalised heterogeneity statistic Qtotal and generalized I² statistic could not be analysed for Sub‐network 2. Evidence suggests no differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 2.
As presented in Supplementary Figure 59, there are no closed loops in Sub‐network 2. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
Overall phase (0 to 120 hours)
No nausea in the overall phase was reported in ten studies including 5036 participants and comparing nine treatment regimens. However, the network was not fully connected and consists of two sub‐networks (see Supplementary Figure 63). We performed NMA for Sub‐networks 1 and 2. Results for all network comparisons, including ranking of treatment combinations for both sub‐networks, are shown in Supplementary Figures 64 and 65. We observed moderate heterogeneity (I² = 41.6%) between studies in Sub‐network 1. Aprepitant plus palonosetron shows higher complete control of nausea in the overall phase than palonosetron (RR 1.68, 95% CI 1.09 to 2.58) and ondansetron (RR 1.91, 95% CI 1.24 to 2.95), respectively. Evidence suggests no other differences between treatment combinations including 5‐HT₃ inhibitors, and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 1.
We identified no evidence of a difference between direct and indirect estimates in the only closed loops in Sub‐network 1 (see Supplementary Figure 66).
We could not analyse generalised heterogeneity statistic Qtotal and generalized I² statistic for Sub‐network 2. Evidence suggests no differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 2.
As presented in Supplementary Figure 63, there are no closed loops in Sub‐network 2. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
We rated the certainty of evidence for all treatments when compared to granisetron according to the GRADE system. Using actual reported event rates, we estimated that 419 of 1000 participants achieve complete control of nausea in the overall phase when treated with granisetron. When compared to treatment with granisetron, treatment with aprepitant plus granisetron likely increases complete control of nausea in the delayed phase (low certainty); treatment with rolapitant plus granisetron likely slightly increases complete control of nausea in the delayed phase (low certainty). Our main reason for downgrading was very serious imprecision (please also see Table 11).
7. Summary of findings: complete control of nausea during the overall phase (MEC) when compared to treatment with granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by moderately emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by moderately emetogenic chemotherapy Settings: inpatient and outpatient care Intervention:
Comparison: granisetron (5‐HT₃) + corticosteroid Outcome: complete control of nausea during the overall phase (0 to 120 h of treatment with chemotherapy) RR < 1 indicates an advantage for the intervention. Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
|
Interventions (corticosteroids included in all regimens)a |
Illustrative comparative risks* (95% CI) |
Risk ratio (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Assumed risk with granisetron | Corresponding risk with the intervention | |||||
| aprepitant + granisetron | 419 of 1000 | 570 of 1000 (365 to 897) | RR 1.36 (0.87 to 2.14) | 1423 (2) | ⊕⊕⊝⊝ lowb |
Aprepitant + granisetron may increase complete control of nausea in the overall phase when compared with granisetron |
| rolapitant + granisetron | 419 of 1000 | 453 of 1000 (402 to 511) | RR 1.08 (0.96 to 1.22) | 1423 (2) | ⊕⊕⊝⊝ lowc |
Rolapitant + granisetron may increase complete control of nausea in the overall phase slightly when compared with granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 298 of 712 (41.9%) participants treated with granisetron experienced no nausea during the overall phase (granisetron was used in 2 studies reporting the outcome). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded twice for very serious imprecision because 95% CIs cross unity, information size is small, and confidence intervals are very wide, suggesting benefit and harm for the comparator. cDowngraded twice for very serious imprecision because 95% CIs cross unity, confidence intervals are wide, and information size is small. | ||||||
Subgroup analyses
We have been able to conduct the following subgroup analyses.
Type of chemotherapy (carboplatin versus other MEC)
Carboplatin was used for chemotherapy in three trials including 211 participants and comparing five treatment regimens. Just as for the full analysis set, the network was not fully connected in subgroup analyses and likewise was divided into two sub‐networks (figure available from study authors upon request). Sub‐network 1 included two studies and compared three treatments versus another. We observed no heterogeneity between studies in this sub‐network. Sub‐network 2 included one study and compared two treatments versus another. In comparison to the overall analysis of Sub‐network 1, evidence from the subgroup analysis does not suggest a robust advantage for aprepitant plus palonosetron compared to palonosetron (RR 1.11, 95% CI 0.53 to 2.34). Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference. The direction and extent of effect of the subgroup analysis for Sub‐network 2 do not deviate from the full analysis set of Sub‐network 2.
Type of cancer (solid versus other)
Nine trials included only participants with solid tumours (n = 4935) and compared nine treatment regimens. Just as for the full analysis set, the network was not fully connected in subgroup analyses and likewise was divided into two sub‐networks (figures available from study authors upon request). Sub‐network 1 included seven studies and compared six treatment regimens. We observed low heterogeneity (I² = 29%) between studies in this sub‐network. Sub‐network 2 included two trials and compared three treatment regimens. We observed no heterogeneity between studies in this sub‐network. In the overall analysis, evidence may suggest an advantage of aprepitant plus ondansetron compared to palonosetron (RR 1.03, 95% CI 0.58 to 1.81), fosaprepitant plus ondansetron (RR 1.11, 95% CI 0.75 to 1.564), ondansetron (RR 1.17, 95% CI 0.85 to 1.62), and casopitant plus ondansetron (RR 1.28, 95% CI 0.88 to 1.85). In subgroup analysis including solid tumours only, the direction of effect changes and evidence may suggest an advantage for palonosetron (RR 1.32, 95% CI 0.66 to 2.62), fosaprepitant plus ondansetron (RR 1.23, 95% CI 0.71 to 2.12), ondansetron (RR 1.16, 95% CI 0.69 to 1.95), and casopitant plus ondansetron (RR 1.05, 95% CI 0.62 to 1.82) compared to aprepitant plus ondansetron. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference. We identified no further differences in direction and extent of effects in subgroup analysis between treatments.
Sensitivity analyses
We included six studies with low risk of bias including 3977 participants and comparing a total of seven treatment regimens in RoB sensitivity analysis. Just as for the full analysis set, the network was not fully connected in subgroup analyses and likewise was divided into two sub‐networks (figures available from study authors upon request). Sub‐network 1 included four studies and compared four treatment regimens. We observed low heterogeneity (I² = 13.1%) between studies in this sub‐network. Sub‐network 2 included two trials and compared three treatment regimens. In the overall analysis, evidence may suggest an advantage of aprepitant plus ondansetron compared to fosaprepitant plus ondansetron (RR 1.11, 95% CI 0.75 to 1.56), ondansetron (RR 1.17, 95% CI 0.85 to 1.62), and casopitant plus ondansetron (RR 1.28, 95% CI 0.88 to 1.85). In subgroup analysis including solid tumours only, the direction of effect changes and evidence may suggest an advantage for fosaprepitant plus ondansetron (RR 1.23, 95% CI 0.73 to 2.06), ondansetron (RR 1.16, 95% CI 0.70 to 1.92), and casopitant plus ondansetron (RR 1.05, 95% CI 0.63 to 1.75) compared to aprepitant plus ondansetron. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference.
Complete control of vomiting
We defined complete control of vomiting as no vomiting and no use of rescue medicine. This outcome was usually referred to in the studies as complete response (CR); hereafter we also refer to this as CR.
Acute phase (0 to 24 hours)
CR in the acute phase was reported in 21 studies (20 two‐arm studies, one three‐arm study) including 7783 participants and comparing 11 treatment regimens. We could include all studies in NMA, and the network was fully connected (see Supplementary Figure 67). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 68 and 69. We observed moderate heterogeneity (I² = 43.5%) between studies in the network. Aprepitant plus palonosetron showed higher CR in the acute phase than ondansetron (RR 1.19, 95% CI 1.08 to 1.31); and rolapitant plus granisetron showed higher CR in the acute phase than ondansetron (RR 1.14, 95% CI 1.01 to 1.29). However, palonosetron showed higher CR in the acute phase than fosaprepitant plus ondansetron (RR 1.14, 95% CI 1.01 to 1.27), aprepitant plus ondansetron (RR 1.14, 95% CI 1.03 to 1.25), and casopitant plus ondansetron (RR 1.15, 95% CI 1.04 to 1.27). Evidence suggests no other differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
Ranking of treatments suggests that aprepitant plus palonosetron (P score 0.91) and palonosetron (P score 0.84) may be most effective in completely controlling vomiting during the acute phase, and ondansetron (P score 0.12) may be least effective.
We identified no evidence of a difference between direct and indirect estimates in closed loops in the network (see Supplementary Figure 70).
Delayed phase (24 to 120 hours)
CR in the delayed phase was reported in 21 studies (20 two‐arm studies, one three‐arm study) including 8421 participants and comparing ten treatment regimens. We could include all studies in NMA, and the network was fully connected (see Supplementary Figure 71). Results for all network comparisons, including ranking of treatments, are shown in Supplementary Figures 72 and 73. We observed low heterogeneity (I² = 6.5%) between studies in the network.
In the delayed phase, aprepitant plus palonosetron shows higher CR than palonosetron (RR 1.22, 95% CI1.08 to 1.37), granisetron (RR 1.29, 95% CI 1.11 to 1.50), and ondansetron (RR 1.51, 95% CI 1.30 to 1.76); rolapitant plus granisetron shows higher CR than granisetron (RR 1.16, 95% CI 1.06 to 1.27) and ondansetron (RR 1.35, 95% CI 1.17 to 1.56); fosaprepitant plus ondansetron shows higher CR than ondansetron (RR 1.15, 95% CI 1.05 to 1.26); and aprepitant plus ondansetron shows higher CR than ondansetron (RR 1.09, 95% CI 1.01 to 1.18). However, palonosetron shows higher CR than casopitant plus ondansetron (RR 1.18, 95% CI 1.04 to 1.34). Evidence suggests no other differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
Ranking of treatments suggests aprepitant plus palonosetron (P score 0.98) and rolapitant plus granisetron (P score 0.85) may be most effective in completely controlling vomiting during the delayed phase, and ondansetron (P score 0.03) may be least effective.
We identified no evidence of a difference between direct and indirect estimates in closed loops in the network (see Supplementary Figure 74).
We rated the certainty of evidence for all treatments when compared to granisetron according to the GRADE system. Using actual reported event rates, we estimated that 641 of 1000 participants achieve complete control of vomiting (CR) in the delayed phase when treated with granisetron. When compared to granisetron, treatment with rolapitant plus granisetron increases CR in the delayed phase (high certainty); and treatment with aprepitant plus palonosetron likely results in a large increase in CR in the delayed phase (moderate certainty). When compared to granisetron, treatment with palonosetron may slightly increase CR in the delayed phase (low certainty); treatment with aprepitant plus granisetron may or may not slightly increase CR in the delayed phase (low certainty); treatment with azasetron may result in little to no difference in CR in the delayed phase (low certainty); treatment with fosaprepitant plus ondansetron probably results in little to no difference in CR in the delayed phase (moderate certainty); treatment with aprepitant plus ondansetron may slightly decrease CR in the delayed phase (low certainty); treatment with casopitant plus ondansetron may decrease CR in the delayed phase (low certainty); and treatment with ondansetron probably decreases CR in the delayed phase (moderate certainty). Our main reasons for downgrading were serious study limitations due to risk of bias and serious imprecision. We provide reasons for downgrading per assessment in Table 12.
8. Summary of findings: complete control of vomiting during the delayed phase (MEC) when compared to treatment with granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by moderately emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by moderately emetogenic chemotherapy Settings: inpatient and outpatient care Intervention:
Comparison: granisetron (5‐HT₃) + corticosteroid Outcome: complete control of vomiting during the delayed phase (24 to 120 h of treatment with chemotherapy) RR > 1 indicates an advantage for the intervention Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) | Risk ratio (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with granisetron | Corresponding risk with the intervention | |||||
| aprepitant + palonosetron | 641 of 1000 | 823 of 1000 (712 to 962) | RR 1.29 (1.11 to 1.50) | 8421 (21) | ⊕⊕⊕⊝ moderateb |
Aprepitant + palonosetron likely results in a large increase of complete control of vomiting during the delayed phase when compared to granisetron |
| rolapitant + granisetron | 641 of 1000 | 744 of 1000 (679 to 814) | RR 1.16 (1.06 to 1.27) | 8421 (21) | ⊕⊕⊕⊕ high |
Rolapitant + granisetron increases complete control of vomiting during the delayed phase when compared to granisetron |
| palonosetron | 641 of 1000 | 679 of 1000 (622 to 750) | RR 1.06 (0.97 to 1.17) | 8421 (21) | ⊕⊕⊝⊝ lowb,c |
Palonosetron may increase complete control of vomiting during the delayed phase slightly when compared to granisetron, but the evidence is uncertain |
| aprepitant + granisetron | 641 of 1000 | 667 of 1000 (564 to 788) | RR 1.04 (0.88 to 1.23) | 8421 (21) | ⊕⊕⊝⊝ lowb,c |
Palonosetron may or may not increase complete control of vomiting during the delayed phase slightly when compared to granisetron, but the evidence is uncertain |
| azasetron | 641 of 1000 | 647 of 1000 (494 to 846) | RR 1.01 (0.77 to 1.32) | 8421 (21) | ⊕⊕⊝⊝ lowb,d |
Azasetron may result in little to no difference in complete control of vomiting during the delayed phase slightly when compared to granisetron, but the evidence is uncertain |
| fosaprepitant + ondansetron | 641 of 1000 | 596 of 1000 (551 to 718) | RR 0.98 (0.86 to 1.12) | 8421 (21) | ⊕⊕⊕⊝ moderateb |
Fosaprepitant + ondansetron probably results in little to no difference in complete control of vomiting during the delayed phase slightly when compared to granisetron |
| aprepitant + ondansetron | 641 of 1000 | 596 of 1000 (526 to 679) | RR 0.93 (0.82 to 1.06) | 8421 (21) | ⊕⊕⊝⊝ lowb,c |
Aprepitant + ondansetron may decrease complete control of vomiting during the delayed phase slightly when compared to granisetron, but the evidence is uncertain |
| casopitant + ondansetron | 641 of 1000 | 570 of 1000 (506 to 647) | RR 0.89 (0.79 to 1.01) | 8421 (21) | ⊕⊕⊝⊝ lowb,d |
Casopitant + ondansetron may decrease complete control of vomiting during the delayed phase when compared to granisetron, but the evidence is uncertain |
| ondansetron | 641 of 1000 | 551 of 1000 (493 to 609) | RR 0.86 (0.77 to 0.95) | 8421 (21) | ⊕⊕⊕⊝ moderateb |
Ondansetron probably decreases complete control of vomiting during the delayed phase when compared to granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 953 of 1486 (64.1%) participants treated with granisetron achieved complete response during the delayed phase (granisetron was used in 7 studies reporting the outcome). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded once for serious study limitations due to high risk of bias. cDowngraded once for serious imprecision because 95% CIs cross unity and include potential advantages and disadvantages. dDowngraded once for serious imprecision due to wide confidence intervals. | ||||||
Overall phase (0 to 120 hours)
CR in the overall phase was reported in 22 studies including 7800 participants and comparing a total of 11 treatment regimens. We could include all studies in NMA, and the network was fully connected (Figure 14 and Supplementary Figure 75). Results for all network comparisons, including ranking of treatments, are shown in Figure 15 and Figure 16 and in Supplementary Figures 76 and 77. We observed low heterogeneity (I² = 13.7%) between studies in the network.
14.
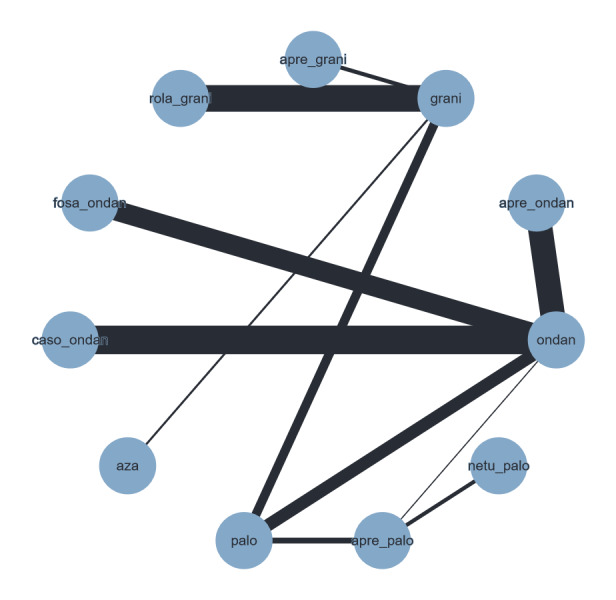
Network graph for the outcome complete control of vomiting during the overall phase (MEC).
A line connects any 2 treatments when there is at least 1 study comparing the 2 treatments. Line width: number of patients.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
15.
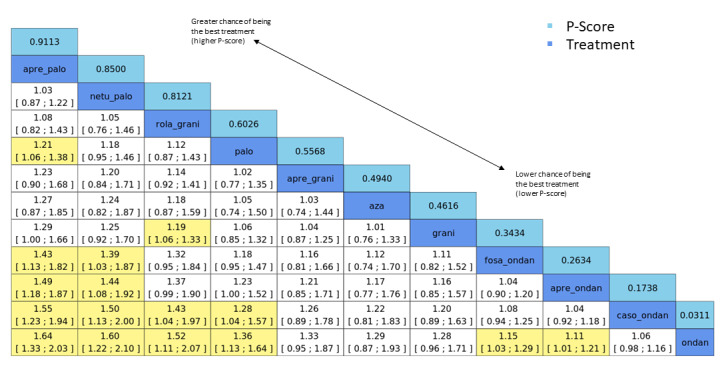
League table for the outcome complete control of vomiting during the overall phase (MEC). Network estimates with 95% CIs are given. Descending P score shows ranking of treatment options. Statistically significant results are marked in yellow. Global approach to check inconsistency/heterogeneity: Q‐statistics, I².
No. of studies: 22. No. of treatments: 11. No. of pair‐wise comparisons: 22. No. of designs: 11.
Qtotal = 13.90, df = 12, P = 0.31/Qwithin = 13.80, df = 11, P = 0.24/Qbetween = 0.09, df = 1, P = 0.76; I² = 13.7%, Tau² = 0.0018.
Treatment effects + 95% CIs (risk ratios, random‐effects model); RR > 1 favours the upper treatment/treatment on the left.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
16.
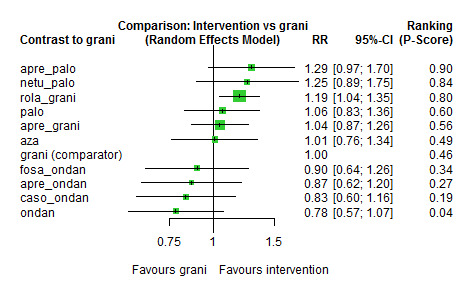
Exemplary network meta‐analysis forest plot for the outcome complete control of vomiting during the overall phase (MEC) (random‐effects model).
Granisetron was used as exemplary reference treatment. Ranking of treatments is ordered by P score (descending).
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
In the overall phase, aprepitant plus palonosetron shows higher CR than palonosetron (RR 1.21, 95% CI 1.06 to 1.38) and ondansetron (RR 1.64, 95% CI 1.33 to 2.03); netupitant plus palonosetron shows higher CR than ondansetron (RR 1.60, 95% CI 1.22 to 2.10); rolapitant plus granisetron shows higher CR than granisetron (RR 1.19, 95% CI 1.06 to 1.33) and ondansetron (RR 1.52, 95% CI 1.11 to 2.07); fosaprepitant plus ondansetron shows higher CR than ondansetron (RR 1.15, 95% CI 1.03 to 1.29); and aprepitant plus ondansetron shows higher CR than ondansetron (RR 1.11, 95% CI 1.01 to 1.21). However, palonosetron shows higher CR than casopitant plus ondansetron (RR 1.28, 95% CI 1.04 to 1.57). Evidence suggests no other differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid.
Ranking of treatments suggests aprepitant plus palonosetron (P score 0.91), netupitant plus palonosetron (P score 0.85), and rolapitant plus granisetron (P score 0.81) may be most effective in completely controlling vomiting during the overall phase, and ondansetron (P score 0.03) may be least effective.
We identified no evidence of a difference between direct and indirect estimates in the only closed loop in the network (Figure 17 and Supplementary Figure 78).
17.
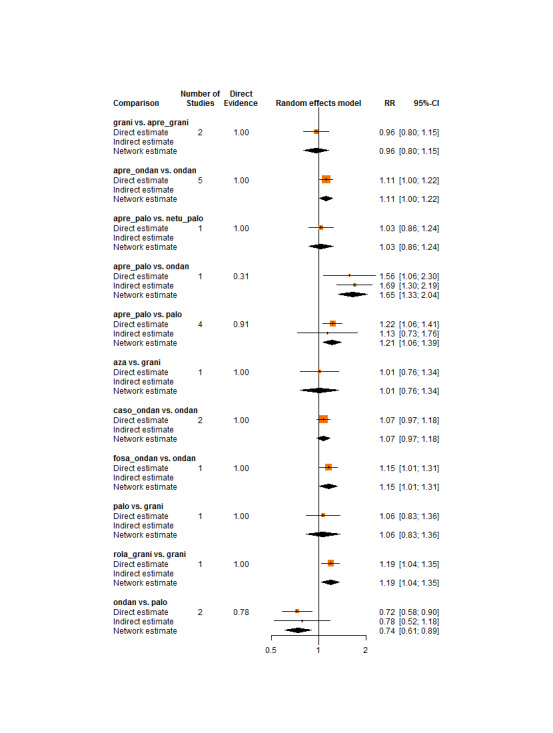
Comparison of direct and indirect evidence (in closed loops) for the outcome complete control of vomiting during the overall phase (MEC). CI: confidence interval; RR: risk ratio.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
We rated the certainty of evidence for all treatments when compared to granisetron according to the GRADE system. Using actual reported event rates, we estimated that 555 of 1000 participants achieve complete control of vomiting (CR) in the overall phase when treated with granisetron. When compared to granisetron, treatment with rolapitant plus granisetron increases CR in the overall phase (high certainty); treatment with aprepitant plus palonosetron (low certainty) and netupitant plus palonosetron may increase CR in the overall phase (low certainty), and treatment with palonosetron (low certainty) or aprepitant plus granisetron (low certainty) may or may not increase CR in the overall phase. When compared to granisetron, treatment with azasetron may result in little to no difference in CR in the overall phase (low certainty). When compared to granisetron, fosaprepitant plus ondansetron (low certainty), aprepitant plus ondansetron (low certainty), casopitant plus ondansetron (low certainty), or ondansetron (low certainty) may reduce CR in the overall phase. Our main reasons for downgrading were serious study limitations due to risk of bias and serious imprecision. We provide reasons for downgrading per assessment in Table 3.
Subgroup analyses
We have been able to conduct the following subgroup analyses.
Type of chemotherapy (carboplatin versus other MEC)
Carboplatin was used for chemotherapy in eight trials including 1628 participants and comparing eight treatment regimens. The network was not fully connected and consisted of two sub‐networks (figures available from study authors upon request). Sub‐network 1 included six studies and compared five treatments to another. No heterogeneity was observed between studies in this sub‐network. Sub‐network 2 included two studies and compared three treatments to another. No heterogeneity was observed between studies in this sub‐network. In comparison to the overall analysis, evidence from the subgroup analysis does not suggest a robust advantage for aprepitant plus palonosetron compared to palonosetron (RR 1.06, 95% CI 0.84 to 1.34) nor for aprepitant plus ondansetron compared to ondansetron (RR 1.04, 95% CI 0.93 to 1.17). However, evidence from subgroup analysis does suggest a robust advantage for palonosetron compared to aprepitant plus ondansetron (RR 1.34, 95% CI 1.08 to 1.67). Despite the split network, we identified no further differences in direction and extent of effects in subgroup analysis. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference.
Type of cancer (solid versus other)
Sixteen trials included only participants with solid tumours (n = 6082) and compared 11 treatment regimens. The network was not fully connected and consisted of two sub‐networks (figures available from study authors upon request). Sub‐network 1 included 12 studies and compared seven treatments to another. We observed moderate heterogeneity (I² = 44.1%) between studies in this sub‐network. Sub‐network 2 included four studies and compared four treatments to another. We observed no heterogeneity between studies in this sub‐network. In comparison to the overall analysis, evidence from subgroup analysis does not suggest a robust advantage for netupitant plus palonosetron compared to fosaprepitant plus ondansetron (RR 1.41, 95% CI 0.97 to 2.05) nor for palonosetron compared to casopitant plus ondansetron (RR 1.26, 95% CI 0.98 to 1.60), fosaprepitant plus ondansetron compared to ondansetron (RR 1.15, 95% CI 0.97 to 1.36), or aprepitant plus ondansetron compared to ondansetron (RR 1.08, 95% CI 0.94 to 1.24). Despite the split network, we identified no further differences in direction and extent of effects in subgroup analysis. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference.
Sensitivity analyses
We included 11 studies with low risk of bias including 6074 participants and comparing a total of ten treatment regimens in RoB sensitivity analysis. The network was not fully connected and consisted of two sub‐networks (figures available from study authors upon request). Sub‐network 1 included ten studies and compared eight treatments to another. We observed moderate heterogeneity (I² = 38.7%) between studies in this sub‐network. Sub‐network 2 included one study only and compared two treatments to another. In comparison to the overall analysis, evidence from the sensitivity analysis does not suggest a robust advantage for rolapitant plus granisetron compared to casopitant plus ondansetron (RR 1.43, 95% CI 0.98 to 2.22), nor for palonosetron compared to casopitant plus ondansetron (RR 1.28, 95% CI 0.98 to 1.67) or aprepitant plus ondansetron compared to ondansetron (RR 1.08, 95% CI 0.97 to 1.21). Despite the split network, we identified no differences in direction and extent of effects in sensitivity analysis between treatments. Confidence intervals of the effect estimates were widely overlapping, and we identified no evidence of a difference.
Quality of life
No impairment in quality of life
We reported and extracted this outcome as number of participants without any impairment in quality of life (QoL).
QoL was reported in four studies including 2783 participants and comparing five treatment regimens. All studies used the Functional Life Index‐Emesis (FLIE) to assess impairment in quality of life. The network was not fully connected and consists of two sub‐networks (Supplementary Figure 79). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Schwartzberg 2015). Briefly, Schwartzberg 2015 included 1212 participants and compared rolapitant plus granisetron versus granisetron. Evidence suggests higher QoL for participants treated with rolapitant plus granisetron compared to participants treated with granisetron (RR 0.92, 95% CI 0.86 to 0.99).
We could include three studies comprising 1671 participants and three treatment regimens in NMA. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 80 and 81. We observed substantial heterogeneity (I² = 78.5%) between studies in the sub‐network. Evidence suggests no differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 1.
We rated the certainty of evidence for all treatments when compared to granisetron according to the GRADE system. Using actual reported event rates, we estimated that 674 of 1000 participants have no impairment in quality of life when treated with granisetron. When compared to granisetron, treatment with rolapitant plus granisetron likely results in a decrease in QoL (moderate certainty). Our main reason for downgrading was serious imprecision (please also see Table 13).
9. Summary of findings: quality of life (MEC) when compared to treatment with granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by moderately emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by moderately emetogenic chemotherapy Settings: inpatient and outpatient care Intervention
Comparison: granisetron (5‐HT₃) + corticosteroid Outcome: no impairment in quality of life RR < 1 indicates an advantage for the intervention. Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network. | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) |
Risk ratio (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Assumed risk with granisetron |
Corresponding risk with the intervention |
|||||
| rolapitant + granisetron | 674 of 1000 | 620 of 1000 (580 to 667) | RR 0.92 (0.86 to 0.99) | 1212 (1) | ⊕⊕⊕⊝ moderateb |
Rolapitant + granisetron probably decreases quality of life slightly when compared to granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 409 of 607 (67.4%) participants treated with granisetron experienced no impact on QoL (granisetron was used in 1 study reporting the outcome, follow‐up on Day 6). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded once for serious imprecision for the small sample size. | ||||||
As presented in Supplementary Figure 79, there are no closed loops in the network. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
Safety
Safety outcomes were not consistently reported across studies. To be able to meta‐analyse results, we could consider safety outcomes only when the number of participants with at least one event was reported. We could not consider cumulated events or breakdown in degree of severity, nor further subgroups.
On‐study mortality
On‐study mortality was reported in five studies including 4149 participants and comparing a total of seven treatment regimens. The network was not fully connected and consists of two sub‐networks (see Supplementary Figure 82). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Schwartzberg 2015). Briefly, Schwartzberg 2015 included 1369 participants and compared rolapitant plus granisetron to granisetron. In the rolapitant plus granisetron group, 12 out of 684 participants died; in the granisetron group, four out of 685 participants died (RR 3.00, 95% CI 0.97 to 9.27).
We could include in NMA four studies reporting on 2780 participants and comparing five treatment regimens. Results for all network comparisons, including ranking of treatments within Sub‐network 1, are shown in Supplementary Figures 83 and 84. We could not analyse generalised heterogeneity statistic Qtotal and generalized I² statistic. Evidence suggests no differences between treatment comparison combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 1.
As presented in Supplementary Figure 82, there are no closed loops in the network. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
We rated the certainty of evidence for all treatments when compared to granisetron according to the GRADE system. Using actual reported event rates, we estimated that 6 of 1000 participants died during the study when treated with granisetron. When compared to granisetron, treatment with rolapitant plus granisetron may result in a large increase in on‐study mortality (low certainty). Our main reason for downgrading was very serious imprecision (please also see Table 14).
10. Summary of findings: on‐study mortality (MEC) when compared to treatment with granisetron.
| Antiemetics for adults for prevention of nausea and vomiting caused by moderately emetogenic chemotherapy | ||||||
|
Patient or population: adult cancer patients at risk for CINV caused by moderately emetogenic chemotherapy Settings: inpatient and outpatient care Intervention
Comparison: granisetron (5‐HT₃) + corticosteroid Outcome: on‐study mortality RR < 1 indicates an advantage for the intervention. Combinations of these interventions at any dose and by any route as mentioned above have been compared to one another in a full network. | ||||||
| Interventions (corticosteroids included in all regimens)a | Illustrative comparative risks* (95% CI) |
Risk ratio (95% CI) |
No. of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
|
Assumed risk with granisetron |
Corresponding risk with the intervention | |||||
| rolapitant + granisetron | 6 of 1000 | 18 of 1000 (6 to 56) | RR 3.00 (0.97 to 9.27) | 1369 (1) | ⊕⊕⊝⊝ lowb |
Rolapitant + granisetron may make little to no difference in on‐study mortality when compared to granisetron |
| *Basis for the assumed risk is actual event rates reported for the main comparator summed across studies: 4 of 685 (0.6%) participants treated with granisetron died during the study (granisetron was used in 1 study reporting the outcome, time frame for reporting safety data was not described). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the risk ratio of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
|
aEither dexamethasone or methylprednisolone was used in all treatment regimens. bDowngraded twice for very serious imprecision because 95% CIs cross unity and because of the small information size. | ||||||
Adverse events
Participants with at least one adverse event (AE) were reported in seven studies including 4394 participants and comparing eight treatment regimens. The network was not fully connected and consists of two sub‐networks (see Supplementary Figure 85). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Kusagaya 2015). Briefly, Kusagaya 2015 included 80 participants and compared aprepitant plus palonosetron versus palonosetron. Of 41 participants who were treated with aprepitant plus palonosetron, 39 experienced at least one AE; of 39 participants who were treated with palonosetron, 37 experienced at least one AE (RR 1.00, 95% CI 0.91 to 1.11).
We could include in NMA six studies comprising 4314 participants and six treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 86 and 87. We observed no heterogeneity (I² = 0.0%) between studies in the sub‐network. Evidence suggests lower risk of AEs for ondansetron compared to rolapitant plus granisetron (RR 0.61, 95% CI 0.41 to 0.91). However, evidence also suggests lower risk of AEs for fosaprepitant plus ondansetron (RR 0.60, 95% CI 0.40 to 0.90), casopitant plus ondansetron (RR 0.60, 95% CI 0.40 to 0.89), and aprepitant plus ondansetron (RR 0.62, 95% CI 0.41 to 0.95), when compared to granisetron. Evidence suggests no other differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within Sub‐network 1.
As presented in Supplementary Figure 85, there are no closed loops in the network. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
Serious adverse events
Participants with at least one serious AE (SAE) were reported in five studies including 4124 participants and comparing seven treatment regimens. The network was not fully connected and consists of two sub‐networks (Figure 18 and Supplementary Figure 88). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Schwartzberg 2015). Briefly, Schwartzberg 2015 included 1344 participants and compared rolapitant plus granisetron versus granisetron. Of 674 participants who were treated with granisetron, 103 experienced at least one SAE; of 670 participants who were treated with rolapitant plus granisetron, 89 experienced at least one SAE (RR 1.15, 95% CI 0.88 to 1.50).
18.
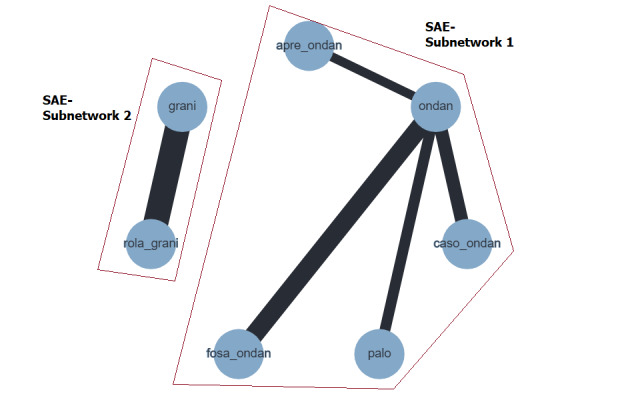
Network graph for the outcome serious adverse events (MEC).
A line connects any 2 treatments when there is at least 1 study comparing the 2 treatments. Line width: number of patients.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
We could include in NMA four studies comprising 2780 participants and five treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Figure 19 and Figure 20 and in Supplementary Figures 89 and 90. We could not analyse generalised heterogeneity statistic Qtotal and generalized I² statistic. Evidence suggests no other differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within this sub‐network.
19.
League table for the outcome serious adverse events (MEC). Network estimates with 95% CIs are given. Descending P score shows ranking of treatment options. Statistically significant results are marked in yellow. Global approach to check inconsistency/heterogeneity: Q‐statistics, I².
No. of studies: 4. No. of treatments: 5. No. of pair‐wise comparisons: 4. No. of designs: 4.
Heterogeneity/inconsistency: Q = 0, df = 0, P = not available; I² = not available, Tau² = not available.
Treatment effects + 95% CIs (risk ratios, random‐effects model).
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
20.
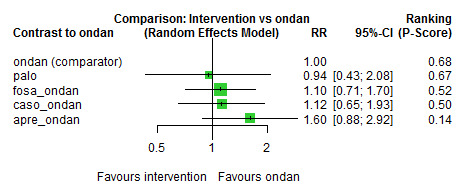
Exemplary network meta‐analysis forest plot for the outcome serious adverse events (MEC) (random‐effects model).
Ondansetron was used as exemplary reference treatment. Ranking of treatments is ordered by P score (descending).
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
We rated the certainty of evidence for all treatments when compared to granisetron according to the GRADE system. Using actual reported event rates, we estimated that 153 of 1000 participants experienced serious adverse events (SAE) when treated with granisetron. When compared to granisetron, treatment with rolapitant plus granisetron may increase SAEs (low certainty). Our main reason for downgrading was very serious imprecision (please also see Table 4).
As presented in Figure 18 and in Supplementary Figure 88, there are no closed loops in the network. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
Neutropenia
Neutropenia was reported in seven studies including 4214 participants and comparing a total of 99 treatment regimens. However, the network was not fully connected and consists of three sub‐networks (Supplementary Figure 91). We performed NMA only for Sub‐networks 1 and 2, as Sub‐network 3 consisted of only one pair‐wise comparison (Kusagaya 2015). Briefly, Kusagaya 2015 included 80 participants and compared aprepitant plus palonosetron versus palonosetron. Of 41 participants who were treated with aprepitant plus palonosetron, 26 experienced neutropenia; of 39 participants who were treated with palonosetron, 19 experienced neutropenia (RR 1.30, 95% CI 0.88 to 1.94).
We could include in NMA of Sub‐network 1 four studies involving 2543 participants and four treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 92 and 93. We observed no heterogeneity (I² = 0.0%) between studies in the sub‐network. Evidence suggests no differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within this sub‐network.
We could include in NMA of Sub‐network 2 two studies involving 1591 participants and three treatment regimens. Results for all network comparisons within Sub‐network 2, including ranking of treatments, are shown in Supplementary Figures 92 and 93. We could not analyse generalised heterogeneity statistic Qtotal and generalised I² statistic. Evidence suggests no differences between treatment comparison combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within this sub‐network.
As presented in Supplementary Figure 91, there are no closed loops in the network. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
Febrile neutropenia
Participants with febrile neutropenia were reported in three studies including 2469 participants and comparing a total of five treatment regimens. The network was not fully connected and consists of two sub‐networks (see Supplementary Figure 94). We performed NMA only for Sub‐network 1, as Sub‐network 2 consisted of only one pair‐wise comparison (Schwartzberg 2015). Briefly, Schwartzberg 2015 included 1344 participants and compared rolapitant plus granisetron versus granisetron. Of 674 participants who were treated with granisetron, 25 experienced febrile neutropenia; of 670 participants who were treated with rolapitant plus granisetron, 14 experienced febrile neutropenia (RR 0.56, 95% CI 0.30 to 1.07).
We could include in NMA two studies comprising 1125 participants and a total of three treatment regimens. Results for all network comparisons within Sub‐network 1, including ranking of treatments, are shown in Supplementary Figures 95 and 96. We could not analyse generalised heterogeneity statistic Qtotal and generalised I² statistic. Evidence suggests no differences between treatment combinations including 5‐HT₃ inhibitors and a corticosteroid versus treatment combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid, within this sub‐network.
As presented in Supplementary Figure 94, there are no closed loops in the network. Therefore, we could not visually nor statistically analyse inconsistencies between direct and indirect evidence.
Infection
None of the included studies reported the number of participants with infection.
Local reaction at the infusion site
None of the included studies reported the number of participants with local reaction at the infusion site.
We had planned to rate the certainty of evidence for local reaction at the infusion site according to the GRADE system for all treatment regimens compared to granisetron, respectively. However, as no study reported this outcome, we could not rate the certainty of evidence for this outcome.
Hiccups
Hiccups were reported in five pair‐wise studies including 1061 participants and comparing six treatment combinations. The network was not fully connected and consisted of three sub‐networks (figures available from study authors upon request). We could not perform NMA, as all sub‐networks consisted of only one pair‐wise comparison. Briefly, Kim 2017 and Song 2017 compared aprepitant plus ondansetron to ondansetron and included a total of 591 participants. Results of pair‐wise meta‐analysis suggest no differences between aprepitant plus ondansetron versus ondansetron (RR 2.08, 95% CI 1.00 to 4.43). Kusagaya 2015 and Xiong 2019 compared aprepitant plus palonosetron versus palonosetron and included a total of 185 participants. Results of pair‐wise meta‐analysis suggest no differences between aprepitant plus palonosetron versus palonosetron (RR 0.77, 95% CI 0.30 to 1.97). Ho 2010 included 285 participants and compared ramosetron to granisetron. Of 144 participants treated with ramosetron, nine experienced hiccups; of 141 participants treated with granisetron, five experienced hiccups (RR 1.76, 95% CI 0.61 to 5.13).
Efficacy versus acceptability
Optimal treatment should be characterised by both high efficacy and acceptability. Figure 21 and Supplementary Figure 97 illustrate concurrently an exemplary ranking of treatment combinations for the outcome CR during the overall phase, which we chose to represent efficacy, and for the outcome SAEs, which we chose to represent acceptability. We ordered treatments by P score. Treatment combinations with both high efficacy and high acceptability are located in the upper right corner of this graph. The related league table with all network estimates (RRs and 95% CIs) is given in Figure 22 and in Supplementary Figure 98. According to this ranking, we considered palonosetron as the most efficacious and acceptable treatment within this comparison. We could include only treatment combinations for which data were available for both outcomes (CR during the overall phase and SAEs) in this exemplary ranking plot.
21.
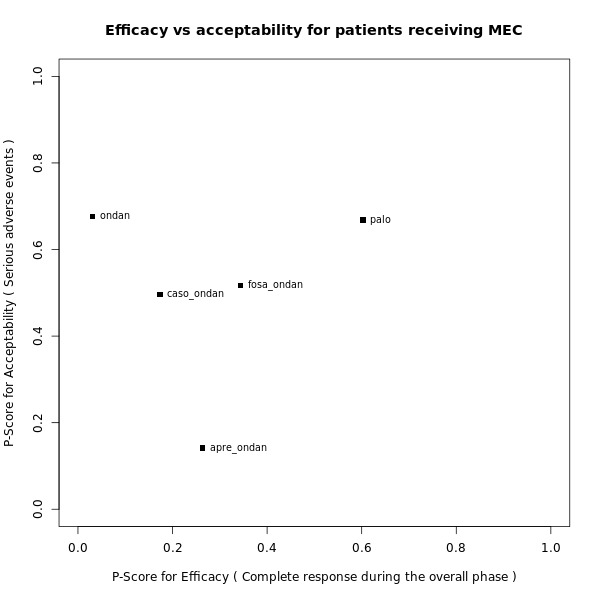
Exemplary ranking plot representing simultaneously the efficacy (x‐axis, CR during overall phase) and the acceptability (y‐axis, SAEs) of all antiemetic regimens for patients receiving moderately emetogenic chemotherapy.
Only antiemetic regimens for which data for both endpoints (CR during the overall phase and SAEs) were available are represented in the ranking plot.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
22.
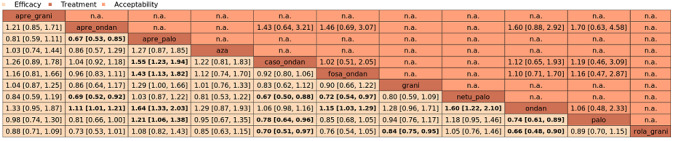
League table with network estimates (RR with 95% CIs) of all treatment combinations for efficacy (CR during the overall phase) and acceptability (SAEs) (MEC).
Treatments are presented in alphabetical order. For efficacy, RRs > 1 favour the first treatment in alphabetical order. For safety, RRs < 1 favour the first treatment in alphabetical order.
n.a.: no data were available for this comparison.
Statistically significant results are marked bold.
A list of treatment abbreviations is provided in Table 5; all treatments included a corticosteroid.
Discussion
Summary of main results
The aim of this systematic review and network meta‐analysis was to synthesise all available evidence on different treatment options for prevention and control of chemotherapy‐induced nausea and vomiting (CINV) in adults with cancer receiving highly or moderately emetogenic chemotherapy (HEC or MEC, respectively). We identified 107 randomised controlled trials (RCTs) that included 37,313 participants from high‐, middle‐, and low‐income countries. Four studies included both HEC and MEC; in total, 73 trials analysed treatment options for prevention of CINV caused by HEC, and 38 analysed treatment options for prevention of CINV caused by MEC.
We investigated 22 different treatment regimens in these studies. Treatment regimens included a 5‐hydroxytryptamine‐3 (5‐HT₃) inhibitor (azasetron, granisetron, ondansetron, palonosetron, ramosetron, or tropisetron) and dexamethasone, and could additionally include a neurokinin‐1 (NK₁) inhibitor (aprepitant, casopitant, ezlopitant, fosaprepitant, fosnetupitant, netupitant (the latter two so far are available only in combination with palonosetron), or rolapitant). All treatment combinations included corticosteroids.
We could include in network meta‐analyses 50 studies in the HEC group and 26 studies in the MEC group. Overall risk of bias was generally low across studies. Results and certainty of evidence for the main outcomes and comparisons are summarised in 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14), and the most crucial outcomes are summarised below. These include complete control of vomiting during the overall treatment phase (Days 1 to 5) and serious adverse events.
Highly emetogenic chemotherapy (HEC)
We included 73 studies reporting on 25,275 participants and comparing 14 treatment combinations with NK₁ and 5‐HT₃ inhibitors. All treatment combinations included corticosteroids.
Complete control of vomiting during the overall phase
We estimated that 704 of 1000 participants achieve complete control of vomiting in the overall treatment phase (one to five days) when treated with aprepitant + granisetron. Evidence from network meta‐analysis (NMA) (39 RCTs, 21,642 participants, 12 treatment combinations with NK₁ and 5‐HT₃ inhibitors) suggests that the following drug combinations are more efficacious than aprepitant + granisetron in completely controlling vomiting during the overall treatment phase (one to five days): fosnetupitant + palonosetron (810 of 1000, risk ratio (RR) 1.15, 95% confidence interval (CI) 0.97 to 1.37; moderate certainty), aprepitant + palonosetron (753 of 1000, RR 1.07, 95% CI 1.98 to 1.18; low certainty), aprepitant + ramosetron (753 of 1000, RR 1.07, 95% CI 0.95 to 1.21; low certainty), and fosaprepitant + palonosetron (746 of 1000, RR 1.06, 95% CI 0.96 to 1.19; low certainty).
Netupitant + palonosetron (704 of 1000, RR 1.00, 95% CI 0.93 to 1.08; high certainty) and fosaprepitant + granisetron (697 of 1000, RR 0.99, 95% CI 0.93 to 1.06; high certainty) have little to no impact on complete control of vomiting during the overall treatment phase (one to five days) when compared to aprepitant + granisetron, respectively.
Evidence further suggests that the following drug combinations are less efficacious than aprepitant + granisetron in completely controlling vomiting during the overall treatment phase (one to five days) (ordered by decreasing efficacy): aprepitant + ondansetron (676 of 1000, RR 0.96, 95% CI 0.88 to 1.05; low certainty), fosaprepitant + ondansetron (662 of 1000, RR 0.94, 95% CI 0.85 to 1.04; low certainty), casopitant + ondansetron (634 of 1000, RR 0.90, 95% CI 0.79 to 1.03; low certainty), rolapitant + granisetron (627 of 1000, RR 0.89, 95% CI 0.78 to 1.01; moderate certainty), and rolapitant + ondansetron (598 of 1000, RR 0.85, 95% CI 0.65 to 1.12; low certainty).
We could not include two treatment combinations (ezlopitant + granisetron, aprepitant + tropisetron) in NMA for this outcome because of missing direct comparisons.
Serious adverse events
We estimated that 35 of 1000 participants experience any serious adverse events (SAEs) when treated with aprepitant + granisetron. Evidence from NMA (23 RCTs, 16,065 participants, 11 treatment combinations) suggests that fewer participants may experience SAEs when treated with the following drug combinations than with aprepitant + granisetron: fosaprepitant + ondansetron (8 of 1000, RR 0.23, 95% CI 0.05 to 1.07; low certainty), casopitant + ondansetron (8 of 1000, RR 0.24, 95% CI 0.04 to 1.39; low certainty), netupitant + palonosetron (9 of 1000, RR 0.27, 95% CI 0.05 to 1.58; low certainty), fosaprepitant + granisetron (13 of 1000, RR 0.37, 95% CI 0.09 to 1.50; low certainty), and rolapitant + granisetron (20 of 1000, RR 0.57, 95% CI 0.19 to 1.70; low certainty).
Evidence is very uncertain about the effects of aprepitant + ondansetron (8 of 1000, RR 0.22, 95% CI 0.04 to 1.14; very low certainty), aprepitant + ramosetron (11 of 1000, RR 0.31, 95% CI 0.05 to 1.90; very low certainty), fosaprepitant + palonosetron (12 of 1000, RR 0.35, 95% CI 0.04 to 2.95; very low certainty), fosnetupitant + palonosetron (13 of 1000, RR 0.36, 95% CI 0.06 to 2.16; very low certainty), and aprepitant + palonosetron (17 of 1000, RR 0.48, 95% CI 0.05 to 4.78; very low certainty) on risk of SAEs when compared to aprepitant + granisetron, respectively.
We could not include three treatment combinations (ezlopitant + granisetron, aprepitant + tropisetron, rolapitant + ondansetron) in NMA for this outcome because of missing direct comparisons.
Moderately emetogenic chemotherapy (MEC)
We included 38 studies reporting on 12,038 participants and comparing 15 treatment combinations with NK₁ and 5‐HT₃ inhibitors, or 5‐HT₃ inhibitors solely. All treatment combinations included corticosteroids.
Complete control of vomiting during the overall phase
We estimated that 555 of 1000 participants achieve complete control of vomiting in the overall treatment phase (one to five days) when treated with granisetron. Evidence from NMA (22 RCTs, 7800 participants, 11 treatment combinations) suggests that the following drug combinations are more efficacious than granisetron in completely controlling vomiting during the overall treatment phase (one to five days): aprepitant + palonosetron (716 of 1000, RR 1.29, 95% CI 1.00 to 1.66; low certainty), netupitant + palonosetron (694 of 1000, RR 1.25, 95% CI 0.92 to 1.70; low certainty), and rolapitant + granisetron (660 of 1000, RR 1.19, 95% CI 1.06 to 1.33; high certainty).
Palonosetron (588 of 1000, RR 1.06, 95% CI 0.85 to 1.32; low certainty) and aprepitant + granisetron (577 of 1000, RR 1.06, 95% CI 0.85 to 1.32; low certainty) may or may not increase complete response in the overall treatment phase (one to five days) when compared to granisetron, respectively. Azasetron (560 of 1000, RR 1.01, 95% CI 0.76 to 1.34; low certainty) may result in little to no difference in complete response in the overall treatment phase (one to five days) when compared to granisetron.
Evidence further suggests that the following drug combinations are less efficacious than granisetron in completely controlling vomiting during the overall treatment phase (one to five days) (ordered by decreasing efficacy): fosaprepitant + ondansetron (500 of 100, RR 0.90, 95% CI 0.66 to 1.22; low certainty), aprepitant + ondansetron (477 of 1000, RR 0.86, 95% CI 0.64 to 1.17; low certainty), casopitant + ondansetron (461 of 1000, RR 0.83, 95% CI 0.62 to 1.12; low certainty), and ondansetron (433 of 1000, RR 0.78, 95% CI 0.59 to 1.04; low certainty).
We could not include five treatment combinations (fosaprepitant + granisetron, azasetron, dolasetron, ramosetron, tropisetron) in NMA for this outcome because of missing direct comparisons.
Serious adverse events
We estimated that 153 of 1000 participants experience any SAEs when treated with granisetron. Evidence from pair‐wise comparison (1 RCT, 1344 participants) suggests that more participants may experience SAEs when treated with rolapitant + granisetron (176 of 1000, RR 1.15, 95% CI 0.88 to 1.50; low certainty). NMA was not feasible for this outcome because of missing direct comparisons.
Overall completeness and applicability of evidence
Robustness of results in subgroup and sensitivity analyses
We were able to compare a total of 21 different treatments combining 5‐HT₃ inhibitors with corticosteroids or additionally an NK₁ inhibitor for prevention and control of CINV in adults with cancer receiving HEC; of these, 14 included both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid. We were able to compare 15 different treatment combinations for adults with cancer receiving MEC; of these, 8 included both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and a corticosteroid. We summarised different types of chemotherapies, drug dosages, routes of administration, cancer types, and patient‐specific prognostic factors, and we had planned to analyse subgroups, if possible. Because of missing information or disconnected networks, we have not been able to analyse subgroups of drug dosages, routes of administration, or patient‐specific prognostic factors. We have been able to conduct subgroup analyses for type of chemotherapy and type of cancer, as well as sensitivity analyses for risk of bias, for both HEC and MEC. We manually compared the results of our subgroup and sensitivity analyses to the results of the full analysis set and investigated whether there were deviations in direction of effects, extent of effects, or both. Most of the effect estimates have been robust in subgroup and sensitivity analyses.
In the HEC group, we noticed that confidence intervals of the effect estimates were widely overlapping, and we found no evidence of a difference between subgroup and sensitivity analyses when compared to the full analysis set, for the outcomes complete control of nausea in the overall phase and complete control of vomiting in the overall phase. The same applies for the outcomes complete control of nausea in the overall phase and complete control of vomiting in the overall phase, which we had investigated in subgroup and sensitivity analyses for the MEC group. However, we have not been able to test for subgroup differences because the test for interactions is not yet available in the software package we used.
Usability of reported outcomes
Not all of the included studies reported the same outcomes. At protocol stage, we defined our efficacy outcomes with the assistance of a clinical expert. However, the definitions of efficacy outcomes used within the included trials did not always correspond with our definitions. For example, we defined the outcome complete control of vomiting as no vomiting and no use of rescue medicine. However, some trials reported the outcome total control of vomiting and defined it as no vomiting, no use of rescue medicine, and no or mild nausea. As a result, we were not able to include these outcomes in our analysis.
Reporting of safety outcomes also differed between studies and led to limited comparability. We decided at protocol stage to include the reported safety data in NMA, if the number of participants experiencing at least one event was reported. We were not able to consider cumulated events or breakdowns in degree of severity or further subgroups (e.g. types of local reactions) in NMA. Not all trials reported the number of participants with at least one event. Therefore, we were not able to compare all identified treatment combinations versus one other for all outcomes. Because of these differences in reporting, we split most safety networks into sub‐networks. Therefore, we could compare only treatments included in the same sub‐network versus each other, reducing the informative value of our results. For the MEC group, the outcomes infection and local reaction at the infusion site have not been reported in any trials; therefore, we could not compare any treatments for these outcomes. We attempted to generate a ranking of treatments comparing efficacy against acceptability; however we could include only a limited number of treatments in this ranking because of the absence of all necessary information.
Consistency of results across phases
We checked whether the ranking of treatments was consistent across acute, delayed, and overall phases. We noticed that for the outcome complete control of nausea in the HEC group, ranking of treatments widely varied across phases. These differences probably originate from the fact that we could include different studies in the analysis of different time periods. However, this indicates discrepancies across the results of different trials and should be further investigated. Until this is done, these findings should be considered with caution.
Consistency within networks per outcome
We detected moderate inconsistency within networks for the outcomes complete control of nausea in the acute phase, complete control of vomiting in the delayed phase, and serious adverse events in the HEC group, and complete control of nausea in delayed and overall phases in the MEC group. We detected substantial inconsistency for the outcome quality of life (QoL) in both HEC and MEC groups. This inconsistency could not be statistically explained nor solved by sensitivity and subgroup analyses and probably originates from the interplay of some effect modifiers, in which our included trials differ slightly (e.g. cancer types, individuals' perceptions of nausea and quality of life, overall morbidity, study start date, study region). These are only minor differences. From a clinical point of view, our included studies therefore remain largely comparable. All trials reporting QoL used the Functional Life Index‐Emesis (FLIE) score to assess any impairments on QoL. We defined at protocol stage that we will compare the numbers of participants without any impairment in QoL. Therefore we did not consider or compare median scores. This may be a reason for inconsistency within networks and may explain why we could not identify differences between treatments.
Entirety of conducted research
In addition to the studies included in this review, we are aware of 46 additional trials that may be eligible for inclusion in our review. Of these, 34 trials are still awaiting assessment, as no results are yet available, and 12 trials are still ongoing. These studies may alter our results, if included in our analysis.
However, despite all these limitations, we were able to identify an extensive number of trials comparing many treatment combinations for multiple outcomes versus one another. We were able to consider the experience of more than 25,000 individuals in the HEC analyses and more than 12,000 individuals in the MEC analyses, emphasising the overall completeness and applicability of our findings.
Quality of the evidence
Rating the certainty of evidence in network meta‐analysis
The advantage and likewise a major challenge of NMA is that it allows us to compare multiple different treatments while considering direct and indirect evidence. We recognise that a comprehensive illustration of results is difficult to present, and that there is no standard approach for assessing the certainty of effect estimates generated by NMA. We followed methods suggested by the GRADE Working Group and discussed our approach with the Cochrane methods support unit.
For a comprehensive presentation and assessment of results, we rated the certainty of network effect estimates for every treatment within networks against one exemplary reference treatment. Because there is not a single standard antiemetic treatment, we had to randomly choose an exemplary reference treatment for both HEC and MEC groups. We chose aprepitant + granisetron as an exemplary reference treatment for all outcomes in the HEC group, and granisetron as an exemplary reference treatment for all outcomes in the MEC group. However, theoretically, we could have used every treatment combination as a reference. We rated the certainty of evidence for prioritised outcomes and all comparisons within networks against the chosen exemplary reference treatment.
For both HEC and MEC, we rated the certainty of evidence for the outcomes complete control of nausea in the overall phase (Days 1 to 5), complete control of vomiting in delayed (Days 2 to 5) and overall phases (Days 1 to 5), no impairment in quality of life, on‐study mortality, and SAEs. We had also planned to rate the certainty of evidence for the outcome local reaction at infusion side but could not do so because in the HEC group, no study reported the outcome for our reference treatment (aprepitant + granisetron), and in the MEC group, no study reported the outcome for any comparison. Overall, our confidence in effect estimates for the most important health outcomes ranged from very low to high certainty.
Highly emetogenic chemotherapy (HEC)
When we compared aprepitant + granisetron with all treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, most of the prioritised outcomes included a range in certainty of evidence across different comparisons. Our reasons for downgrading the evidence varied across comparisons and outcomes.
For the outcome complete control of nausea in the overall phase (Days 1 to 5), our confidence in the evidence was of low to high certainty. Our reasons for downgrading were serious study limitations due to high risk of bias and serious imprecision because the 95% confidence interval (CI) crosses unity and wide confidence intervals.
For the outcome complete control of vomiting in the delayed phase (Days 2 to 5), our confidence in the evidence was of very low to moderate certainty. Our reasons for downgrading were serious study limitations due to high risk of bias, moderate inconsistency within the network, and serious imprecision because the 95% CI crosses unity and wide confidence intervals.
For the outcome complete control of vomiting in the overall phase (Days 1 to 5), our confidence in the evidence was of low to high certainty. Our reasons for downgrading were serious study limitations due to high risk of bias, serious imprecision due to wide confidence intervals, and serious imprecision because the 95% CI crosses unity.
For the outcome no impairment in quality of life, our confidence in the evidence was of very low certainty. Our reasons for downgrading were high inconsistency within the network, very serious imprecision because the 95% CI crosses unity and wide confidence intervals suggesting high benefit for the comparator, serious imprecision because the 95% CI crosses unity, and serious study limitations due to high risk of bias.
For the outcome on‐study mortality, our confidence in the evidence was of low certainty. Our reason for downgrading was very serious imprecision because the 95% CI crosses unity and wide confidence intervals suggesting high benefit for the comparator.
For the outcome serious adverse events, our confidence in the evidence was of very low to low certainty. Our reasons for downgrading were serious study limitations due to high risk of bias, moderate inconsistency within the network, very serious imprecision because the 95% CI crosses unity and wide confidence intervals suggesting high possibility of harm, and serious imprecision because the 95% CI crosses unity and wide confidence intervals.
Moderately emetogenic chemotherapy (MEC)
When we compared granisetron with all treatments, most of the prioritised outcomes included a range of certainty of evidence across the different comparisons. Our reasons for downgrading the evidence also varied across comparisons and outcomes.
For the outcome complete control of nausea in the overall phase (Days 1 to 5), our confidence in the evidence was of low certainty. Our reason for downgrading was very serious imprecision because 95% CIs cross unity, small information size, and wide confidence intervals suggesting high benefit for the comparator, or all.
For the outcome complete control of vomiting in the delayed phase (Days 2 to 5), our confidence in the evidence was of low to high certainty. Our reasons for downgrading were serious study limitations due to high risk of bias, serious imprecision due to wide confidence intervals, and serious imprecision because 95% CIs cross unity and confidence intervals suggesting benefit for the comparator.
For the outcome complete control of vomiting in the overall phase (Days 1 to 5), our confidence in the evidence was of low to high certainty. Our reasons for downgrading were serious study limitations due to high risk of bias, serious imprecision because 95% CIs cross unity, and wide confidence intervals.
For the outcome no impairment in quality of life, our confidence in the evidence was of moderate certainty. Our reason for downgrading was serious imprecision for the small sample size.
For the outcome on‐study mortality, our confidence in the evidence was of low certainty. Our reasons for downgrading were very serious imprecision because 95% CIs cross unity and small information size.
For the outcome serious adverse events. our confidence in the evidence was of low certainty. Our reasons for downgrading were very serious imprecision because 95% CIs cross unity, wide confidence intervals, and small information size.
Potential biases in the review process
Review author IM is an information specialist experienced in medical terminology, who developed the sensitive search strategy with the support of the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group's information specialist. We searched all relevant databases, trial registries, conference proceedings, and reference lists and therefore are confident that we identified all relevant trials.
Although we were able to include 107 studies in this systematic review with network meta‐analysis, we identified insufficient studies to produce funnel plots for pair‐wise comparisons to further investigate potential publication bias. We could have created comparison‐adjusted funnel plots, which requires an assumption regarding differences between small studies and large studies (e.g. newer treatments favoured in small trials, active treatment versus placebo, sponsored versus non‐sponsored) (Chaimani 2013). However, the challenge of NMA is that we would need to take into account several comparisons, which means that we do not have one single line of reference. We therefore decided not to create comparison‐adjusted funnel plots.
To minimise potential biases in the review process, we conducted selection of studies, data extraction, risk of bias assessment, and GRADE assessment in duplicate by two independent review authors and consulted a third review author for cases in which no consensus could be reached. We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. However, grouping of identified records was sometimes difficult, as not all records reported trial registration numbers. In cases for which we were uncertain whether two reports belonged to the same trial, we considered them as representing individual trials.
For a more comprehensive presentation of results, we estimated absolute treatment effects by using actual reported event rates for our randomly chosen main comparators (aprepitant + granisetron for HEC, granisetron for MEC). However, if we would choose another comparator to estimate absolute event rates, all of these effects could change. Thus, when interpreting the results of our NMA, it must be considered that reported absolute event rates are provided for illustrative purposes (using the following comparators: aprepitant + granisetron for HEC, granisetron for MEC) and do not reflect anticipated real‐life event rates.
We complied with Cochrane guidelines for every step of our review and consulted the PaPaS Review Group in cases of methodological uncertainty. With respect to available guidance for NMA, we are not aware of any methodological deficiencies in our review. However, in our opinion, 'Summary of findings' tables are not ideal for summing up such extensive analysis. Also, we surmise that the overall judgement of the quality of included trials and the certainty of evidence could diverge between different review author teams. Both the risk of bias tool and the GRADE approach are sensitive to subjective assessments and can be done more or less stringently.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first comprehensive review with NMA comparing all possible treatment combinations of NK₁ and 5‐HT₃ inhibitors with corticosteroids, or 5‐HT₃ inhibitors with corticosteroids, for prevention and control of CINV caused by HEC and MEC in adults with cancer.
Highly emetogenic chemotherapy (HEC)
We identified one further NMA comparing treatment combinations for prevention and control of CINV caused by HEC (Yokoe 2019). Additionally to our inclusion criteria, Yokoe 2019 included treatment combinations with olanzapine and treatment combinations without corticosteroids. Instead of comparing treatments per used substances versus each other, review authors grouped first‐generation 5‐HT₃ inhibitors into one group (granisetron, ondansetron, azasetron, ramosetron). Palonosetron was considered separately and was further divided into the dosages used. For therapies including an NK₁ inhibitor with a 5‐HT₃ inhibitor and dexamethasone, review authors summarised the NK₁ inhibitors aprepitant, fosaprepitant, and rolapitant into one group, and considered the combination including netupitant and palonosetron separately. In contrast to our inclusion criteria, review authors included only trials investigating antiemetic regimens in adults with solid cancer and excluded haematological malignancies.
Yokoe 2019 included 27 studies, 18 of which have been included in our review as well. We did not include the remaining nine studies in our review for the following reasons. Five studies included olanzapine in the investigational arm. Two studies used a 5‐HT₃ inhibitor without a corticosteroid. One study presented a combined analysis of two trials over multiple cycles and considered two publications of the same study as different studies; we considered this study only once. However, we identified 55 additional studies (Appendix 2), which we have included in our review. Comparability of our NMA and the NMA of Yokoe 2019 is further limited, as Yokoe and colleagues reported only one of our outcomes of interest; i.e. complete response (in acute, delayed, and overall phases).
Yokoe 2019 identified treatment combinations that included olanzapine as most efficient for achieving a complete response across all phases. Among treatment combinations that have also been compared in our analysis, Yokoe 2019 identified netupitant + palonosetron and dexamethasone as most efficient in the overall phase, followed by combinations including any NK₁ inhibitor + palonosetron and dexamethasone, and combinations including any NK₁ inhibitor and any 5‐HT₃ inhibitor with dexamethasone. Treatment combinations including only a 5‐HT₃ inhibitor with or without a corticosteroid performed least effectively.
In contrast to Yokoe 2019, we used only treatments including a 5‐HT₃ inhibitor with a corticosteroid to stabilise the network and focused on differences between treatments combining NK₁ and 5‐HT₃ inhibitors with corticosteroids. However, because immunotherapy agents such as immune checkpoint inhibitors (ICIs) are added to HEC, it is investigated whether the immunosuppressive effects of corticosteroids undermine the action and thus the efficacy of immunotherapy agents (Janowitz 2021). Concerns have been raised that corticosteroid‐including antiemetic prophylaxis reduces the effectiveness of ICI‐HEC. Until the interaction of corticosteroid immunosuppressive effects on the efficacy of immunotherapy agents is better understood, effects of corticosteroid‐sparing or corticosteroid‐free antiemetic treatments might be of particular interest for people receiving ICI‐HEC (Janowitz 2021). Neither our review nor the Yokoe 2019 review has yet been able to adequately answer this open question.
Moderately emetogenic chemotherapy (MEC)
We did not identify any other NMA comparing antiemetic regimens for prevention of CINV caused by MEC. We identified one meta‐analysis investigating the additional benefit of adding an NK₁ inhibitor to a 5‐HT₃ inhibitor for prevention of CINV caused by MEC (Jordan 2018). All 12 included trials of Jordan 2018 have been included in our systematic review as well. We could not include in this NMA the results of five trials, as only the substance class but not the active substance of the NK₁ inhibitor or the 5‐HT₃ inhibitor, or both, was defined in these trials (Aridome 2016; Ito 2014; Maehara 2015; Nishimura 2015; Yahata 2016). We included 25 additional trials in our analysis. Ten trials have not been included by Jordan 2018, as both treatment arms included a combination of 5‐HT₃ inhibitors and corticosteroids solely (Brohee 1995; Eisenberg 2003; Endo 2012; Ghosh 2010; Herrington 2000; Ho 2010; Jantunen 1992; Kaushal 2010; Raftopoulos 2015; Seol 2016); we have not included two further trials, as both treatment arms included a combination including both an NK₁ inhibitor and a 5‐HT₃ inhibitor (Fujiwara 2015; Jordan 2016a), and we could not include four trials as their publication date falls past the search date of Jordan 2018 (Kim 2017; Song 2017; Sugimori 2017; Xiong 2019). We identified ten additional trials in this systematic review (Arpornwirat 2009; Badar 2015; Kitayama 2015; Matsuda 2014; Miyabayashi 2015; Ozaki 2013; Schnadig 2014; Tsubata 2019; Webb 2010; Yeo 2009), three of which reported sufficient results to be included in NMA (Arpornwirat 2009; Badar 2015; Yeo 2009). Badar 2015 included participants with haematological malignancies, which have not been included by Jordan 2018. We analysed potential differences between types of cancer in subgroup analysis and did not identify any substantial disparities in treatment efficacy. Chemotherapy regimens used by Arpornwirat 2009 and Yeo 2009 have been classified as HEC and/or MEC by Jordan 2018 and therefore were excluded from that analysis. Arpornwirat 2009 described that investigators used cyclophosphamide from 500 mg/m² to 1500 mg/m² in combination with another MEC (not further specified) or from 750 mg/m² to 1500 mg/m² alone or in combination with agents that had been claimed by study authors to have low emetogenic potential: oxaliplatin ≥ 85 mg/m², doxorubicin ≥ 60 mg/m², epirubicin ≥ 90 mg/m², FOLFIRI, or carboplatin at an area under the curve (AUC) ≥ 5. According to the latest definition (Basch 2012), chemotherapy is classified as HEC if cyclophosphamide is used at ≥ 1500 mg/m². This was not the case for Arpornwirat 2009. However, chemotherapy is also classified as HEC if cyclophosphamide is used in combination with an anthracycline (Basch 2012). Arpornwirat 2009 described use of the low emetogenic anthracycline doxorubicin in combination with cyclophosphamide from 750 mg/m² to 1500 mg/m². The proportion of participants receiving this HEC combination has not been described in the trial, and results have not been reported separately. Therefore, this study has been mismatched to the MEC group in our review and will be excluded from analysis in an update of this review. Yeo 2009 also used doxorubicin 60 mg/m² + cyclophosphamide 600 mg/m² for chemotherapy. According to the latest definition of HEC, this study has been mismatched to the MEC group in our review. In an update of this review, we will correct this misclassification and will include Yeo 2009 in the HEC group.
The meta‐analysis of Jordan 2018 focused on efficacy outcomes (complete response, no emesis, no nausea) and separately analysed the results for carboplatin regimens, oxaliplatin regimens, and other MEC regimens including neither carboplatin nor oxaliplatin. We analysed all types of MEC together. Jordan 2018 identified benefit for antiemetic regimens including an additional NK₁ inhibitor for the outcome complete response during the overall phase for pure MEC regimens; and for the outcomes complete response during acute, delayed, and overall phases, no emesis during delayed and overall phases, and no nausea during delayed and overall phases for carboplatin‐based chemotherapy. Results of the meta‐analysis suggest no differences in efficacy between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, and treatments including a 5‐HT₃ inhibitor solely, for oxaliplatin‐based chemotherapy. In comparison, results of our NMA do not show a clear trend towards combinations including both an NK₁ inhibitor and a 5‐HT₃ inhibitor, neither for the outcome complete response nor for the outcome no nausea*. On the contrary, results of our NMA suggest that the choice of 5‐HT₃ inhibitor may have an impact on response rates. We analysed potential differences between carboplatin‐based chemotherapy and all MEC regimens in subgroup analysis and did not identify any substantial disparities in treatment efficacy.
Disagreements between the meta‐analysis of Jordan 2018 and our NMA could reflect several reasons. First of all, only 7 of 37 studies have been considered in both analyses. Disagreements between analyses may indicate that the information size of study populations was insufficient to reveal the true effect. However, in the case that there is true benefit for regimens including an NK₁ inhibitor for other MEC and carboplatin‐based chemotherapy, this effect could be covered in our NMA through analyses of all types of MEC together. Unfortunately, we have not been able to conduct a test for subgroup differences as described above (Overall completeness and applicability of evidence). Also, pooling of all NK₁ inhibitors and all 5‐HT₃ inhibitors could reveal differences between individual substances, suggesting overall benefit for treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor.
*We did not investigate the outcome of no emesis in our network meta‐analysis.
Authors' conclusions
Implications for practice.
General
The findings of our systematic review and network‐meta analysis will support clinicians and patients in decision‐making regarding use of NK₁ and 5‐HT₃ inhibitors for prevention of chemotherapy‐induced nausea and vomiting caused by highly or moderately emetogenic chemotherapy. Our results provide a comprehensive overview of all possible treatment options, including a ranking of treatments for each outcome. However, these rankings should be interpreted with caution and the results of all outcomes should be taken into consideration before a decision is made. Because of missing data in the included trials, not all treatment combinations could be compared to each other for every outcome.
When interpreting the results of this systematic review, it is important to understand that network meta‐analyses are no substitute for direct head‐to‐head comparisons. It is also important to consider that the results of our network meta‐analysis do not necessarily rule out differences that could be clinically relevant for some individuals.
For adults receiving highly emetogenic chemotherapy
For people receiving highly emetogenic chemotherapy, synthesised evidence does not suggest one superior treatment for prevention and control of chemotherapy‐induced nausea and vomiting. Results of our network meta‐analysis do not necessarily rule out differences that could be clinically relevant for some individuals.
For adults receiving moderately emetogenic chemotherapy
For people receiving moderately emetogenic chemotherapy, synthesised evidence does not suggest superiority of treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor when compared to treatments including a 5‐HT₃ inhibitor alone. Results of our network meta‐analysis rather suggest that the choice of 5‐HT₃ inhibitor may have an impact on the efficacy of treatment in preventing chemotherapy‐induced nausea and vomiting. Results of our network meta‐analysis do not necessarily rule out differences that could be clinically relevant for some individuals.
For clinicians
For people receiving highly or moderately emetogenic chemotherapy, synthesised evidence does not suggest one superior treatment for prevention and control of chemotherapy‐induced nausea and vomiting. For people receiving moderately emetogenic chemotherapy, the results of our network meta‐analysis rather suggest that the choice of 5‐HT₃ inhibitor may have an impact on the efficacy of treatment in preventing chemotherapy‐induced nausea and vomiting. Results of our network meta‐analysis do not necessarily rule out differences that could be clinically relevant for some individuals.
For policy makers
Highly emetogenic chemotherapy
For prevention of chemotherapy‐induced nausea, evidence suggests with moderate certainty that fosaprepitant + palonosetron is likely the most effective treatment in the overall phase (Days 1 to 5). For prevention of chemotherapy‐induced vomiting, evidence suggests with very low to low certainty that there are small differences between treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor in the delayed phase; and with moderate certainty that aprepitant + palonosetron is likely the most effective treatment in the overall phase. Evidence is very uncertain about differences in effects of NK₁ and 5‐HT₃ inhibitors on quality of life. Primary studies have reported few serious adverse events and deaths during the study period, and very uncertain evidence suggests there may be differences between treatments.
Moderately emetogenic chemotherapy
For people receiving moderately emetogenic chemotherapy, synthesised evidence does not suggest superiority for treatments including both an NK₁ inhibitor and a 5‐HT₃ inhibitor when compared to treatments including a 5‐HT₃ inhibitor alone. For prevention of chemotherapy‐induced nausea, because of limited direct evidence, not all treatments could be compared and ranked in delayed and overall phases; therefore we cannot say whether there are differences between treatments, or to what extent treatments may differ. For prevention of chemotherapy‐induced vomiting, evidence suggests with moderate certainty that aprepitant + palonosetron is likely the most effective treatment in the delayed phase; and with low certainty that aprepitant + palonosetron may be the most effective treatment in the overall phase. We could not compare all treatments versus another for the outcome quality of life. A general interpretation of findings is therefore not possible. Primary studies have reported few serious adverse events and deaths during the study period. However, because of disconnected networks and low certainty of the evidence, we could not compare and rank all treatments for the outcomes serious adverse events and on‐study mortality.
For funders of the intervention
This field of supportive cancer care is very well researched. However, new drugs and new drug combinations are continuously emerging and must be systematically researched and assessed.
Implications for research.
General implications
This is the first network meta‐analysis comparing combination therapies of 5‐HT₃ inhibitors with corticosteroids, or additionally with NK₁ inhibitors, for prevention of chemotherapy‐induced nausea and vomiting caused by highly or moderately emetogenic chemotherapy. This field of supportive cancer care is very well researched, and we could consider the experience of more than 25,000 people receiving highly emetogenic chemotherapy, and more than 12,000 people receiving moderately emetogenic chemotherapy, in our systematic review. Overall, our confidence in the evidence for the most important health outcomes ranged from very low to high certainty. Our confidence in the evidence was mainly affected by missing data.
New antiemetic agents, as well as new antineoplastic treatment concepts, are constantly introduced and this will require an update of our network meta‐analysis. In particular, the addition of immune checkpoint inhibitors to chemotherapy poses the question of negative interaction between corticosteroids and immunotherapy and prompts the need for additional research on the effects of corticosteroid‐sparing or corticosteroid‐free antiemetic treatments.
Design
Most of the included studies were of high methodological quality, and results show low risk of bias. We would appreciate if studies would report allocation and masking more transparently. The main limitation of some of the included studies was an open‐label study design leading to high risk of performance bias and detection bias.
Measurement (endpoints)
Safety outcomes were not consistently reported across studies. To be able to meta‐analyse results, we could consider only those reporting the number of participants with at least one adverse event. We could not consider cumulated events or breakdown in degree of severity, nor further subgroups.
Because of these differences in reporting, not all treatments in the network were connected through direct comparisons, but some were split into sub‐networks. Therefore, we could not compare all identified treatment combinations versus one other for each outcome. This led to limited comparability of treatments, reducing the informative value of our results.
Other
Consistent reporting of core outcomes and outcome data in trials registries would be most helpful for future trials, but also for already completed trials, to ensure comparability of all possible treatment combinations.
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2022 | Amended | Search date amended in PLS. |
History
Protocol first published: Issue 9, 2017 Review first published: Issue 11, 2021
| Date | Event | Description |
|---|---|---|
| 1 December 2021 | Amended | Minor typographical error corrected in Abstract. |
Acknowledgements
This review was published in collaboration with the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). We particularly thank Anna Erskine (Managing Editor) for her ongoing support, along with all members of PaPaS, for their valuable comments, which greatly helped to improve the review. We thank the Cochrane Community Support Team, especially Ursula Gonthier, which helped us overcome methodological and technical obstacles. We thank the Copy Edit Support team, especially Dolores Matthews for their valuable suggestions and edits.
We would like to thank peer reviewers Professor Nicola Stoner, Dr. Ollie Minton, Dr. Alfredo V. Chua Jr., and Sabeeh Kamil (Consumer Reviewer) for their valuable comments, which greatly helped to improve this review. Moreover, we would like to thank Professor Nicola Stoner and Professor Raymond Chan for their valuable comments on the protocol of this review. The review was based in part on suggested wording from the Pain, Palliative and Supportive Care Review Group (PaPaS CRG), the Cochrane Haematology Review Group, and the method templates for a Cochrane intervention review that compares multiple interventions (Chaimani 2014; Chaimani 2017).
We would like to thank Marike Andreas and Selin Altindis, members of our working group, for their support with data extraction and risk of bias assessment.
This project was funded by the German Ministry for Education and Research, grant no 01KG1510.
Cochrane Review Group funding acknowledgment: this project was funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
ID Search
#1 MeSH descriptor: [Antiemetics] explode all trees
#2 (antiemetic* or anti emetic*)
#3 #1 or #2
#4 MeSH descriptor: [Serotonin Antagonists] explode all trees
#5 (5 ht antagonist* or 5ht antagonist*)
#6 (serotonin* near/3 (antagonist* or blockader* or blocker*))
#7 (antiserotonergic* near/2 agent*)
#8 #4 or #5 or #6 or #7
#9 MeSH descriptor: [Serotonin 5‐HT3 Receptor Antagonists] explode all trees
#10 ((5 ht3 or 5ht3) near/2 antagonist*)
#11 #9 or #10
#12 MeSH descriptor: [Granisetron] explode all trees
#13 Granisetron*
#14 (brl‐43694a or brl43694a or brl‐43694 or brl43694)
#15 Kytril*
#16 Sancuso*
#17 #12 or #13 or #14 or #15 or #16
#18 MeSH descriptor: [Ondansetron] explode all trees
#19 Ondansetron*
#20 (gr38032f or gr 38032f)
#21 (sn 307 or sn307)
#22 Zofran*
#23 #18 or #19 or #20 or #21 or #22
#24 Tropisetron*
#25 Navoban*
#26 (ICS 205‐930 or ICS 205930)
#27 #24 or #25 or #26
#28 Dolasetron*
#29 Anzemet*
#30 #28 or #29
#31 Palonosetron*
#32 Aloxi*
#33 #31 or #32
#34 Zatosetron*
#35 (LY277359 or ly 277359)
#36 #34 or #35
#37 Ricasetron*
#38 (BRL‐46470 or BRL46470)
#39 #37 or #38
#40 Bemesetron*
#41 (MDL‐72222 or MDL72222)
#42 #40 or #41
#43 Ramosetron*
#44 Nasea*
#45 (Irribow* or Iribo*)
#46 (Nozia* or IBSet*)
#47 (Ai Ke An* or Lei Mai Xin* or Shan Chen*)
#48 ("Ramea" or Setoral*)
#49 #43 or #44 or #45 or #46 or #47 or #48
#50 "metastron"
#51 (strontium chloride‐89 or Sr‐89)
#52 #50 or #51
#53 itastron*
#54 setron*
#55 #53 or #54
#56 Aprepitant*
#57 Emend*
#58 #56 or #57
#59 Fosaprepitant*
#60 (L‐758,298 or L‐758298)
#61 Fosa
#62 #59 or #60 or #61
#63 Netupitant*
#64 Akynzeo*
#65 "nepa"
#66 #63 or #64 or #65
#67 MeSH descriptor: [Dexamethasone] explode all trees
#68 decameth*
#69 Dexamethason*
#70 Dexamethasone
#71 desamethason*
#72 (dexason* or dexadrol* or dexair* or dexacidin* or dxm*)
#73 (oradexon* or maxidex* or maxitrol* or tobradex* or ak‐dex*)
#74 (hexadecadrol* or oradexon* or decadron* or neodecadron*)
#75 dexametason*
#76 desametason*
#77 (millicorten* or maxidex* or decaspray* or dexpak*)
#78 (hexadrol* or decameth* or methylfluorprednisolon*)
#79 decaject*
#80 #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79
#81 MeSH descriptor: [Nausea] explode all trees
#82 MeSH descriptor: [Vomiting] explode all trees
#83 nause*
#84 vomit*
#85 emesis
#86 emet*
#87 emetogenic*
#88 (antiemetic* or anti‐eme*)
#89 sickness*
#90 #81 or #82 or #83 or #84 or #85 or #86 or #87 or #88 or #89
#91 MeSH descriptor: [Neoplasms by Histologic Type] explode all trees
#92 MeSH descriptor: [Neoplasms by Site] explode all trees
#93 neoplas*
#94 (tumor* or tumour*)
#95 (Krebs* or cancer*)
#96 malignan*
#97 (carcino* or karzino*)
#98 karzinom*
#99 sarcom*
#100 (leukem* or leukaem* or leucem*)
#101 lymphom*
#102 melano*
#103 metastas*
#104 (mesothelio* or mesotelio*)
#105 carcinomatos*
#106 (gliom* or glioblastom*)
#107 osteo*sarcom*
#108 (blastom* or neuroblastom*)
#109 #91 or #92 or #93 or #94 or #95 or #96 or #97 or #98 or #99 or #100 or #101 or #102 or #103 or #104 or #105 or #106 or #107 or #108
#110 #3 or #8 or #11 or #17 or #23 or #27 or #30 or #33 or #36 or #39 or #42 or #49 or #52 or #58 or #62 or #66
#111 #110 or #80
#112 #111 and #90 and #109 in Trials
Embase
(via Ovid)
1. exp antiemetic agent/
2. (antiemetic* or anti emetic*).tw.
3. 1 or 2
4. exp serotonin antagonist/
5. (5 ht antagonist* or 5ht antagonist*).tw.
6. (serotonin* adj3 (antagonist* or blockader* or blocker*)).tw.
7. (antiserotonergic* adj2 agent*).tw.
8. or/4‐7
9. exp serotonin 3 antagonist/
10. ((5 ht3 or 5ht3) adj2 antagonist*).tw.
11. 9 or 10
12. granisetron/
13. (Granisetron* or (brl‐43694a or brl43694a or brl‐43694 or brl43694) or Kytril* or Sancuso*).tw.
14. 12 or 13
15. ondansetron/
16. (Ondansetron* or (gr38032f or gr 38032f) or (sn 307 or sn307) or Zofran*).tw.
17. 15 or 16
18. Tropisetron/ or (Tropisetron* or Navoban* or (ICS 205‐930 or ICS 205930)).tw.
19. Dolasetron/ or (Dolasetron* or Anzemet*).tw.
20. Palonosetron/ or (Palonosetron* or Aloxi*).tw.
21. (Zatosetron* or (LY277359 or ly 277359)).tw.
22. (Ricasetron* or (BRL‐46470 or BRL46470)).tw.
23. (Bemesetron* or (MDL‐72222 or MDL72222)).tw.
24. Ramosetron/ or (Ramosetron* or Nasea* or (Irribow* or Iribo*) or (Nozia* or IBSet*) or (Ai Ke An* or Lei Mai Xin* or Shan Chen*) or "Ramea" or Setoral*).tw.
25. ("metastron" or (strontium chloride‐89 or Sr‐89)).tw.
26. (itastron* or setron*).tw.
27. Aprepitant/ or (Aprepitant* or Emend*).tw.
28. Fosaprepitant/ or (Fosaprepitant* or (L‐758,298 or L‐758298) or Fosa).tw.
29. Netupitant/ or (Netupitant* or Akynzeo* or "nepa").tw.
30. exp dexamethasone/
31. decameth*.tw.
32. (Dexamethason* or Dexamethasone or desamethason*).tw.
33. (dexason* or dexadrol* or dexair* or dexacidin* or dxm*).tw.
34. (oradexon* or maxidex* or maxitrol* or tobradex* or ak‐dex*).tw.
35. (hexadecadrol* or oradexon* or decadron* or neodecadron*).tw.
36. (dexametason* or desametason* or decaject*).tw.
37. (millicorten* or maxidex* or decaspray* or dexpak*).tw.
38. (hexadrol* or decameth* or methylfluorprednisolon*).tw.
39. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40. exp nausea/
41. exp vomiting/
42. (nause* or vomit* or emesis or emet* or emetogenic* or (antiemetic* or anti‐eme*) or sickness*).tw.
43. 40 or 41 or 42
44. exp neoplasm/
45. (neoplas* or tumor* or tumour or Krebs* or cancer* or malignan*).tw.
46. (carcino* or karzino* or karzinom* or sarcom* or leukem* or leukaem* or leucem*).tw.
47. (lymphom* or melano* or metastas* or mesothelio* or mesotelio* or carcinomatos*).tw.
48. (gliom* or glioblastom* or osteo?sarcom* or blastom* or neuroblastom*).tw.
49. 44 or 45 or 46 or 47 or 48
50. 3 or 8 or 11 or 14 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
51. 39 or 50
52. 43 and 49 and 51
53. random$.tw.
54. factorial$.tw.
55. crossover$.tw.
56. cross over$.tw.
57. cross‐over$.tw.
58. placebo$.tw.
59. (doubl$ adj blind$).tw.
60. (singl$ adj blind$).tw.
61. assign$.tw.
62. allocat$.tw.
63. volunteer$.tw.
64. Crossover Procedure/
65. double‐blind procedure.tw.
66. Randomized Controlled Trial/
67. Single Blind Procedure/
68. or/53‐67
69. (animal/ or nonhuman/) not human/
70. 68 not 69
MEDLINE
(via OvidSP) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions
| # | Searches |
| 1 | exp ANTIEMETICS/ |
| 2 | (antiemetic$ or anti emetic$).tw,kf,ot,nm. |
| 3 | or/1‐2 |
| 4 | SEROTONIN ANTAGONISTS/ |
| 5 | (5 ht antagonist$ or 5ht antagonist$).tw,kf,ot,nm. |
| 6 | ((serotonin$ adj3 antagonist$) or (serotonin$ adj3 blocker$) or (serotonin$ adj3 blockader$)).tw,kf,ot,nm. |
| 7 | (antiserotonergic$ adj2 agent$).tw,kf,ot,nm. |
| 8 | or/4‐7 |
| 9 | SEROTONIN 5‐HT3 RECEPTOR ANTAGONISTS/ |
| 10 | ((5 ht3 or 5ht3) adj2 antagonist$).tw,kf,ot,nm. |
| 11 | or/9‐10 |
| 12 | GRANISETRON/ |
| 13 | granisetron$.tw,kf,ot,nm. |
| 14 | (brl‐43694a or brl43694a or brl‐43694 or brl43694).tw,kf,ot,nm. |
| 15 | kytril$.tw,kf,ot. |
| 16 | sancuso$.tw,kf,ot. |
| 17 | or/12‐16 |
| 18 | ONDANSETRON/ |
| 19 | ondansetron$.tw,kf,ot,nm. |
| 20 | (gr38032f or gr 38032f).tw,kf,ot,nm. |
| 21 | (sn 307 or sn307).tw,kf,ot,nm. |
| 22 | zofran$.tw,kf,ot,nm. |
| 23 | or/18‐22 |
| 24 | TROPISETRON/ |
| 25 | (tropisetron$ or navoban$).tw,kf,ot,nm. |
| 26 | (ICS 205‐930 or ICS 205930).tw,kf,ot,nm. |
| 27 | or/24‐26 |
| 28 | DOLASETRON/ |
| 29 | (anzemet$ or dolasetron$).tw,kf,ot. |
| 30 | or/28‐29 |
| 31 | APREPITANT/ |
| 32 | (emend$ or aprepitant$).tw,kf,ot,nm. |
| 33 | or/31‐32 |
| 34 | PALONOSETRON/ |
| 35 | (palonosetron$ or aloxi$).tw,kf,ot,nm. |
| 36 | or/34‐35 |
| 37 | FOSAPREPITANT/ |
| 38 | (L‐758,298 or L‐758298).tw,kf,ot,nm. |
| 39 | (fosaprepitant$ or fosa$).tw,kf,ot,nm. |
| 40 | or/37‐39 |
| 41 | NETUPITANT/ |
| 42 | (netupitant$ or akynzeo$).tw,kf,ot,nm. |
| 43 | "nepa".tw,kf,ot,nm. |
| 44 | or/41‐43 |
| 45 | zatosetron$.tw,kf,ot,nm. |
| 46 | (LY277359 or ly 277359).tw,kf,ot,nm. |
| 47 | or/45‐46 |
| 48 | ricasetron$.tw,kf,ot,nm. |
| 49 | (BRL‐46470 or BRL46470).tw,kf,ot,nm. |
| 50 | or/48‐49 |
| 51 | bemesetron$.tw,kf,ot,nm. |
| 52 | (MDL‐72222 or MDL72222).tw,kf,ot,nm. |
| 53 | or/51‐52 |
| 54 | RAMOSETRON/ |
| 55 | (ramosetron$ or nasea$).tw,kf,ot,nm. |
| 56 | (irribow$ or iribo$ or nozia$ or IBSet$).tw,kf,ot,nm. |
| 57 | ("YM 060" or YM060).tw,kf,ot,nm. |
| 58 | (Ai Ke An$ or Lei Mai Xin$ or Shan Chen$).tw,kf,ot,nm. |
| 59 | ("ramea" or setoral$).tw,kf,ot,nm. |
| 60 | or/54‐59 |
| 61 | mirtazapin$.tw,kf,ot,nm. |
| 62 | (6‐Azamianserin or 6Azamianserin).tw,kf,ot,nm. |
| 63 | mepirzapin$.tw,kf,ot,nm. |
| 64 | or/61‐63 |
| 65 | "metastron".tw,kf,ot,nm. |
| 66 | (strontium chloride‐89 or Sr‐89).tw,kf,ot,nm. |
| 67 | or/65‐66 |
| 68 | itastron$.tw,kf,ot,nm. |
| 69 | setron$.tw,kf,ot,nm. |
| 70 | or/68‐69 |
| 71 | 3 or 8 or 11 or 17 or 23 or 27 or 30 or 33 or 36 or 40 or 44 or 47 or 50 or 53 or 60 or 64 or 67 or 70 |
| 72 | exp DEXAMETHASONE/ |
| 73 | decameth$.tw,kf,ot. |
| 74 | dexamethason$.tw,kf,ot. |
| 75 | dexamethasone.nm. |
| 76 | desamethason$.tw,kf,ot,nm. |
| 77 | (dexason$ or dexadrol$ or dexair$ or dexacidin$ or dxm$).tw,kf,ot,nm. |
| 78 | (oradexon$ or maxidex$ or maxitrol$ or tobradex$ or ak‐dex$).tw,kf,ot,nm. |
| 79 | (hexadecadrol$ or oradexon$ or decadron$ or neodecadron$).tw,kf,ot,nm. |
| 80 | dexametason$.tw,kf,ot,nm. |
| 81 | desametason$.tw,kf,ot,nm. |
| 82 | (millicorten$ or maxidex$ or decaspray$ or dexpak$).tw,kf,ot,nm. |
| 83 | (hexadrol$ or decameth$ or methylfluorprednisolon$).tw,kf,ot. |
| 84 | decaject$.tw,kf,ot,nm. |
| 85 | or/72‐84 |
| 86 | NAUSEA/ |
| 87 | VOMITING/ |
| 88 | nause$.tw,kf,ot,nm. |
| 89 | vomit$.tw,kf,ot,nm. |
| 90 | emesis.tw,kf,ot,nm. |
| 91 | emet$.tw,kf,ot,nm. |
| 92 | emetogenic$.tw,kf,ot,nm. |
| 93 | (antiemetic* or anti‐eme*).tw,kf,ot,nm. |
| 94 | sickness*.tw,kf,ot. |
| 95 | or/86‐94 |
| 96 | exp NEOPLASMS BY HISTOLOGIC TYPE/ |
| 97 | exp NEOPLASMS BY SITE/ |
| 98 | neoplas$.tw,kf,ot. |
| 99 | tumo?r$.tw,kf,ot. |
| 100 | (krebs$ or cancer$).tw,kf,ot. |
| 101 | malignan$.tw,kf,ot. |
| 102 | (carcino$ or karzino$).tw,kf,ot. |
| 103 | karzinom$.tw,kf,ot. |
| 104 | sarcom$.tw,kf,ot. |
| 105 | leuk#?m$.tw,kf,ot. |
| 106 | lymphom$.tw,kf,ot. |
| 107 | melano$.tw,kf,ot. |
| 108 | metastas$.tw,kf,ot. |
| 109 | (mesothelio$ or mesotelio$).tw,kf,ot. |
| 110 | carcinomatos$.tw,kf,ot. |
| 111 | (gliom$ or glioblastom$).tw,kf,ot. |
| 112 | osteo?sarcom$.tw,kf,ot. |
| 113 | (blastom$ or neuroblastom$).tw,kf,ot. |
| 114 | adenocarcinoma$.tw,kf,ot. |
| 115 | myeloma$.tw,kf,ot. |
| 116 | or/96‐115 |
| 117 | randomized controlled trial.pt. |
| 118 | controlled clinical trial.pt. |
| 119 | randomi?ed.ab. |
| 120 | placebo.ab. |
| 121 | drug therapy.fs. |
| 122 | randomly.ab. |
| 123 | trial.ab. |
| 124 | groups.ab. |
| 125 | or/117‐124 |
| 126 | exp ANIMALS/ not HUMANS/ |
| 127 | 125 not 126 |
| 128 | (71 or 85) and 95 and 116 and 127 |
| 129 | limit 128 to ed=19950101‐20000101 |
| 130 | limit 128 to ed=20000101‐20100101 |
| 131 | limit 128 to ed=20100101‐20170216 |
| 132 | limit 128 to ed=20170216‐20171004 |
| 133 | limit 128 to ed=20171004‐20180409 |
| 134 | limit 128 to ed=20180409‐20190403 |
key: exp # /: explode # MeSH subject heading, tw: text word, kf: keyword heading word, ot: original title, ti: title, nm: substance name, pt: publication type, ab: abstract, fs: floating subheading, $: truncation, ?: wildcard, adj#: adjacent within # number of words
Appendix 2. Studies included in HEC group
Abdel‐Malek 2017 (not included in NMA)
Albany 2012 (not included in NMA)
Ando 2016 (not included in NMA)
Arce‐Salinas 2019(not included in NMA)
Bubalo 2018 (not included in NMA)
Cho 1998 (not included in NMA)
Chua 2000 (not included in NMA)
Egerer 2010 (not included in NMA)
Flenghi 2015 (not included in NMA)
Forni 2000 (not included in NMA)
Fox‐Geiman 2001 (not included in NMA)
Gao 2013 (not included in NMA)
Ghosh 2010 (also included in MEC group; not included in NMA)
Ho 2010 (also included in MEC group)
Kalaycio 1998 (not included in NMA)
Kimura 2015 (not included in NMA)
Koizumi 2003 (not included in NMA)
Lee 1997 (not included in NMA)
Mahrous 2020(not included in NMA)
Mohammed 2019(not included in NMA)
Raftopoulos 2015 (also included in MEC group)
Stewart 1996 (not included in NMA)
Stewart 2000 (not included in NMA)
Svanberg 2015 (not included in NMA)
Tsubata 2019(also included in MEC group)
Wit 2001 (not included in NMA)
Appendix 3. Studies included in MEC group
Brohee 1995 (not included in NMA)
Fujiwara 2015 (not included in NMA)
Ghosh 2010 (also included in HEC group; not included in NMA)
Ho 2010 (also included in HEC group)
Ito 2014 (not included in NMA)
Jantunen 1992 (not included in NMA)
Maehara 2015 (not included in NMA)
Miyabayashi 2015 (not included in NMA)
Nishimura 2015 (not included in NMA)
Raftopoulos 2015 (also included in HEC group)
Schnadig 2014 (not included in NMA)
Seol 2016 (not included in NMA)
Tsubata 2019 (also included in HEC group)
Webb 2010 (not included in NMA)
Yahata 2016 (not included in NMA)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aapro 2006.
| Study characteristics | ||
| Methods |
Randomised, stratified, parallel‐group, active‐comparator, phase 3 trial with 3 arms
Enrolment period: July 2000 to December 2001
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: subjects were followed 5 days for efficacy endpoints and 15 days for safety endpoints |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD (range), years: 53.4 ± 13.7 (palonosetron 0.25 mg), 50.6 ± 14.1 (palonosetron 0.75 mg), 50.9 ± 14.2 (ondansetron 32 mg) Gender: male + female Tumour/cancer type: malignant tumour (ovarian cancer, lung cancer, Hodgkin lymphoma, gastric cancer, breast cancer) Chemotherapy regimen: cisplatin, cyclophosphamide, dacarbazine Country/continent: North America, Europe (76 centres) |
|
| Interventions |
Experimental: arm A: palonosetron 0.25 mg palonosetron 0.25 mg i.v. + dexamethasone 20 mg i.v. Experimental: arm B: palonosetron 0.75 mg palonosetron 0.75 mg i.v. + dexamethasone 20 mg i.v. Experimental: arm C: ondansetron 32 mg ondansetron 32 mg i.v. + dexamethasone 20 mg i.v. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind, double‐dummy ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind, double‐dummy ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both participants and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both participants and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the intent‐to‐treat (ITT) cohort included all randomized patients who received chemotherapy and study drug (n = 667)" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety cohort (safety analysis) included all patients who received study drug and had at least one safety assessment after treatment (n = 673)" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Abdel‐Malek 2017.
| Study characteristics | ||
| Methods |
Randomised, cross‐over, placebo‐controlled trial with 2 arms
Study period: January 2015 to June 2015
Masking: single‐blind Baseline patient characteristics: n.r. Follow‐up: yes, time period not mentioned |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Median age (range), years: 45 (38 to 56) Gender: 8 males + 7 females Tumour/cancer type: non‐Hodgkin lymphoma (NHL) Chemotherapy regimen: ESHAP chemotherapy regimen (etoposide 40 mg/m²/d as a 1‐h i.v. infusion from Day 1 to Day 4; cisplatin 25 mg/m²/d as a continuous infusion from Day 1 to Day 4; solumedrol 500 mg/d as a 15‐min i.v. infusion from Day 1 to Day 5; cytarabine 2 g/m² given as a 2‐h i.v. infusion on Day 5) Country: Egypt (single centre) |
|
| Interventions |
Cross‐over trial Experimental: arm A: aprepitant 125/80 mg, then placebo Day 1: aprepitant 125 mg + ondansetron 8 mg + dexamethasone 8 mg Days 2 to 3: aprepitant 80 mg + ondansetron 8 mg + dexamethasone 8 mg Days 4 to 5: ondansetron 8 mg + dexamethasone 8 mg with cross‐over to the opposite treatment in the third and fourth cycles Experimental: arm B: placebo. then aprepitant 125/80 mg Days 1 to 3: placebo + ondansetron 8 mg + dexamethasone 8 mg Days 4 to 5: ondansetron 8 mg + dexamethasone 8 mg with cross‐over to the opposite treatment in the third and fourth cycles |
|
| Outcomes |
Primary endpoint
Secondary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment group assignments were made by block randomization" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... single‐blinded ..." and "... prospective placebo‐controlled cross‐over study ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: personnel were not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: personnel were not blinded towards the intervention, which might influence subjective outcome assessments |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "twelve of the 15 patients who entered the study were fully evaluable and completed 4 cycles of ESHAP chemotherapy regimen" |
| Selective reporting (reporting bias) | Low risk | Comment: outcome measures were described in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Aksu 2013.
| Study characteristics | ||
| Methods |
Randomised, cross‐sectional trial with 2 arms
Recruitment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Median age (range), years: 58 (38 to 72) Gender: male (n = 56, 93%) + female (n = 4, 7%) Tumour/cancer type: non‐small cell lung cancer Chemotherapy regimen: cisplatin (75 mg/m², Day 1) and docetaxel (75 mg/m², Day 1) Country: Turkey |
|
| Interventions |
Experimental: arm A: aprepitant 125/80 aprepitant (125 mg on Day 1, 80 mg on Days 2 and 3) + p.o. ondansetron 4 mg (4 days, beginning on Day 1 of chemotherapy) + dexamethasone (8 mg on Day 1, 4 mg on Days 2 and 3) Control: arm B p.o. ondansetron 4 mg (4 days, beginning on Day 1 of chemotherapy) + dexamethasone (8 mg on Day 1, 4 mg on Days 2 and 3) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... patients were prospectively randomized ..." Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: blinding not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: blinding not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: not reported |
| Selective reporting (reporting bias) | Low risk | Comment: outcome measure was reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Albany 2012.
| Study characteristics | ||
| Methods |
Randomised, placebo‐ controlled, cross‐over, phase 3 trial with 2 arms
Study period: December 2007 to February 2011
Masking: double‐blind (participants, care provider) Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants | Inclusion criteria
Exclusion criteria
Mean age (range), years: 33 years (16 to 62 years) Gender: male Tumour/cancer type: germ cell tumour Chemotherapy regimen: 2 identical courses of standard 5‐day cisplatin‐based chemotherapy regimen Country: United States (multi‐centre, 6) |
|
| Interventions |
Cross‐over study Experimental: arm A: aprepitant 125/80, then placebo Days 1 to 2: dexamethasone 20 mg + 5‐HT₃ RA Day 3: aprepitant 125 mg + 5‐HT₃ RA Days 4 to 5: aprepitant 80 mg + 5‐HT₃ RA Days 6 to 7: aprepitant 80 mg + dexamethasone 4 mg twice per day, then received matched placebo PO daily on Days 3 through 7 during study Cycle 2 Experimental: arm B: placebo, then aprepitant 125/80 Days 1 to 2: dexamethasone 20 mg + 5‐HT₃ RA Day 3: placebo + 5‐HT₃ RA Days 4 to 5: placebo + 5‐HT₃ RA Days 6 to 7: placebo + dexamethasone 8 mg twice per day |
|
| Outcomes |
Primary outcome
Secondary outcome(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment group assignments were made by personnel not otherwise involved with the study to ensure in‐house blinding" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: NCT00572572 reported that both participants and care providers were blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: NCT00572572 reported that both participants and care providers were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "sixty of the 69 patients who entered the study were fully evaluable and completed at least 5 days of both cycles" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ando 2016.
| Study characteristics | ||
| Methods |
Randomised, controlled study with 2 arms
Recruitment period: January 2013 to March 2014
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 61.7 ± 11.7 in aprepitant group, 65.4 ± 10.0 in fosaprepitant group Gender: male (74) + female (19) Tumour/cancer type: malignant tumour (lung cancer, gastric cancer, oesophageal cancer, head and neck cancer) Chemotherapy regimen: CDDP (60 mg/m² or higher) Country: Japan (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg + palonosetron 0.75 mg or granisetron 3 mg or azasetron 10 mg + dexamethasone 6.6 to 9.9 mg Days 2 to 4: aprepitant 80 mg + dexamethasone 3.3 to 6.6 mg Day 5: aprepitant 80 mg Experimental: arm B: fosaprepitant Day 1: fosaprepitant meglumine 150 mg + palonosetron 0.75 mg or granisetron 3 mg or azasetron 10 mg + dexamethasone 6.6 to 9.9 mg Days 2 to 4: dexamethasone 3.3 to 6.6 mg |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no allocation concealment reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all randomised patients were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: in the results section, palonosetron, granisetron, and azasetron were not separately reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Arce‐Salinas 2019.
| Study characteristics | ||
| Methods |
Randomised, open‐label trial
Study period: n.r.
Masking: open‐label Median follow‐up: n.r. ITT analysis: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: n.r. Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: AC, TC, TCH regimens Country: United States (Texas) |
|
| Interventions |
Experimental: arm A: palonosetron, dexamethasone, and fosaprepitant Experimental: arm B: ondansetron, dexamethasone, fosaprepitant |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: outcome assessors (participants) not blinded to intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: outcome robust to blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: conference abstract, not fully evaluable |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Unclear risk | Comment: conference abstract, not fully evaluable |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract, not fully evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not fully evaluable |
Aridome 2016.
| Study characteristics | ||
| Methods |
Randomised, parallel, comparative, phase 2 study with 2 arms
Study period: September 2011 to August 2013
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean years ± SD: 66.46 ± 9.81 in aprepitant group, 63.48 ± 10.23 in control group Gender: male (64) + female (49) Tumour/cancer type: colorectal cancer Chemotherapy regimen: oxaliplatin‐based or irinotecan‐based MEC (FOLFOX, XELOX, or FOLFIRI) Country: Japan (18 institutions, multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. + 5‐HT₃ RAs + dexamethasone 6.6 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 4 mg p.o. Control: arm B Day 1: 5‐HT₃ RAs + dexamethasone 9.9 mg i.v. Days 2 to 3: dexamethasone 8 mg p.o. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomly assigned to the aprepitant (5‑HT3 RA + reduced‑dose dexamethasone + aprepitant) or standard (5‑HT3 + dexamethasone) regimen group according to a computer‑generated, blinded allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis. |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although this was an open‐label study, we assume that knowledge of both patient and personnel had no influence on objective outcomes (e.g. neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "... in total, 113 patients were included in the full analysis set" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all of the included patients were evaluated for objective outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes have been reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Arpornwirat 2009.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, dose‐ranging, phase 2 trial with 6 arms
Enrolment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 57 (22 to 83) in arm A, 58.5 (33 to 88) in arm B, 57.4 (20 to 85) in arm C, 59.2 (26 to 82) in arm D, 57.9 (22 to 84) in arm E, 57.9 (28 to 88) in arm F Gender: male (287) + female (436) Tumour/cancer type: solid malignancy (breast, NSCLC, colon or rectum, ovary, other) Chemotherapy regimen: cyclophosphamide from 500 mg/m² to 1500 mg/m² if given with other MEC, or from 750 mg/m² to 1500 mg/m² if given alone or with agents that had minimal or low emetogenic potential; oxaliplatin ≥ 85 mg/m², doxorubicin ≥ 60 mg/m², epirubicin ≥ 90 mg/m², irinotecan (dosed as part of an irinotecan, leucovorin, 5‐fluorouracil (FOLFIRI) regimen), or carboplatin at an area under the curve (AUC) ≥ 5 Country: 99 centres in 24 countries |
|
| Interventions |
Control: arm A Day 1: 30 min before MEC (casopitant placebo + ondansetron 8 mg p.o. + dexamethasone 8 mg i.v.), 8 h later (ondansetron 8 mg p.o.) Days 2 to 3: casopitant placebo + ondansetron 8 mg p.o. b.i.d. Primary treatment: arm B Day 1: 30 min before MEC (casopitant 50 mg + ondansetron 8 mg p.o. + dexamethasone 8 mg i.v.), 8 h later (ondansetron 8 mg p.o.) Days 2 to 3: casopitant 50 mg + ondansetron 8 mg p.o. b.i.d. Primary treatment: arm C Day 1: 30 min before MEC (casopitant 100 mg + ondansetron 8 mg p.o. + dexamethasone 8 mg i.v.), 8 h later (ondansetron 8 mg p.o.) Days 2 to 3: casopitant 100 mg + ondansetron 8 mg p.o. b.i.d. Primary treatment: arm D Day 1: 30 min before MEC (casopitant 150 mg + ondansetron 8 mg p.o. + dexamethasone 8 mg i.v.), 8 h later (ondansetron 8 mg p.o.) Days 2 to 3: casopitant 150 mg + ondansetron 8 mg p.o. b.i.d. Exploratory: arm E Day 1: 30 min before MEC (casopitant 150 mg + ondansetron 8 mg p.o. + dexamethasone 8 mg i.v.), 8 h later (ondansetron 8 mg p.o.) Days 2 to 3: casopitant placebo + ondansetron 8 mg p.o. b.i.d. Exploratory: arm F Day 1: 30 min before MEC (casopitant 150 mg + ondansetron 16 mg p.o. + dexamethasone 8 mg i.v.), 8 h later (ondansetron placebo) Days 2 to 3: casopitant 150 mg + ondansetron 16 mg q.a.m., ondansetron placebo q.p.m. |
|
| Outcomes |
Primary endpoint(s)
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to 1 of 6 treatment groups (Table 5) by a centralized, automated randomization system and were stratified by sex and chemotherapy treatment (taxane‐based or non taxane‐based)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: 723 patients have been included in the efficacy analysis and primary outcomes have been assessed in the intent‐to‐treat (ITT) population |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety population consisted of the 711 patients who received at least 1 dose of study medication in any treatment cycle" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes have been reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Badar 2015.
| Study characteristics | ||
| Methods |
Randomised, comparative, phase 2 study with 2 arms
Enrolment period: n.r.
Masking: open‐label Baseline patient characteristics: reported Follow‐up: patients were followed for a total of 6 days, starting from first day of chemotherapy |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 49 (21 to 70) in ondansetron + aprepitant arm, 53 (30 to 68) in ondansetron arm Gender: male (55) + female (43) Tumour/cancer type: AML, MDS, CML Chemotherapy regimen: cytarabine‐based chemotherapy at a dose ≥1g/m²/d for at least 3 days Country: n.r. |
|
| Interventions |
Experimental: arm A: aprepitant + ondansetron aprepitant (APREP) 125 mg p.o. plus ondansetron 8 mg i.v. 30 min before cytarabine followed by ondansetron 24 mg i.v. continuous infusion daily until 6 to 12 hours and aprepitant 80 mg p.o. daily until 1 day after the end of last chemotherapy Experimental: arm B: ondansetron Ondansetron (OND) 8 mg i.v. 30 min before cytarabine followed by ondansetron 24 mg i.v. continuous infusion daily until 6 to 12 h after the end of last chemotherapy infusion |
|
| Outcomes |
Primary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although patients and personnel were not blinded, we assume no risk of bias for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "ninety‐eight patients were registered in the study, 49 to each arm. Among them 83 were evaluable for efficacy, 42 in the OND arm and 41 in the OND + APREP arm" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients who had received a single dose of study drug were included for safety analysis (N = 87) |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Brohee 1995.
| Study characteristics | ||
| Methods |
Randomised trial with 2 arms
Enrolment period: n.r.
Masking: single‐blind (patients) Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: male (3) + female (9) Tumour/cancer type: n.r. Chemotherapy regimen: combination therapy with Cy > 600 mg or IFO > 1 g/m² Country: Belgium |
|
| Interventions |
Experimental: arm A: ondansetron 8 mg ondansetron + 10 mg dexamethasone Experimental: arm B: granisetron 3 mg granisetron + 10 mg dexamethasone |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "treatment allocation was randomized in blocks of four" Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "the patients were kept blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding of personnel not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: even though patients were blinded, it is unclear whether personnel were blinded, which could have an impact on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: 12 patients (9 F) enrolled for 53 treatments were included in this study |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract, not evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Bubalo 2005.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, controlled trial with 2 arms
Study period: May 2004 to January 2009
Masking: quadruple‐blind (participant, care provider, investigator, outcomes assessor) Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 46 ± 12.9 in aprepitant group, 46 ± 13 in placebo group Gender: male (28) + female (12) Tumour/cancer type: n.r. Chemotherapy regimen: cyclophosphamide‐containing regimen before transplant Country: United States (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant aprepitant: loading dose of 125‐mg capsule once a day for 1 day, then maintenance dose of 80‐mg capsule daily through Day +4 of bone marrow transplant dexamethasone: for cyclophosphamide total body irradiation (CyTBI) patients: dexamethasone study drug 1 capsule p.o. daily, 1 h before chemotherapy, with aprepitant on total body irradiation (TBI) and cyclophosphamide chemotherapy days; for busulfan cyclophosphamide (BuCy) patients: dexamethasone 1 capsule orally once daily, discontinued after last dose of chemotherapy ondansetron: for CyTBI patients: ondansetron 8 mg orally every 12 h, beginning 1 h before first TBI dose and discontinued after last dose; then ondansetron 8 mg i.v. every 12 h × 4 doses, beginning 30 min before first cyclophosphamide chemotherapy; for BuCy patients: ondansetron 8 mg p.o. every 6 h, beginning 1 h before first busulfan dose and discontinued after last busulfan dose is given; then ondansetron 8 mg i.v. every 12 h × 4 doses, beginning 30 min before first cyclophosphamide chemotherapy Experimental: arm B: placebo placebo comparator: sugar pill: loading dose of 125‐mg capsule once a day for 1 day, then maintenance dose of 80‐mg capsule daily through Day +4 of bone marrow transplant dexamethasone and ondansetron: same doses and routine as arm A |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: NCT00248547 reported that masking was quadruple (participant, care provider, investigator, outcomes assessor) |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: NCT00248547 reported that masking was quadruple (participant, care provider, investigator, outcomes assessor) |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all randomised patients were assessed for the primary outcome |
| Selective reporting (reporting bias) | High risk | Comment: primary outcome reported; "effects on nausea, appetite and taste changes" and "pharmacokinetic interaction" are missing |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Bubalo 2018.
| Study characteristics | ||
| Methods |
Randomised, prospective, placebo‐controlled, parallel, pilot study with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 46 (19 to 60) in aprepitant group, 46 (19 to 63) in placebo group Gender: male (28) + female (12) Tumour/cancer type: acute lymphoblastic leukemia, acute myelogenous leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, non‐Hodgkin lymphoma, myelodysplastic syndrome, myelofibrosis, Waldenstrom’s macroglobulinemia Chemotherapy regimen: very high doses of cyclophosphamide Country: United States (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant aprepitant 125 mg on Day 1 and 80 mg through Day +4 + ondansetron given per institutional guidelines on each day of chemotherapy or radiation as described in appendix A + dexamethasone 12 mg p.o. for 1 dose given on Day 1, and 8 mg p.o. once daily given on subsequent days Experimental: arm B: placebo placebo + ondansetron + dexamethasone |
|
| Outcomes |
Primay objective
Secondary objectives
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a randomization list was generated using a permuted block randomization with a random block size of either 2 or 4" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "the medications were administered on the same schedule to effectively blind the patients and healthcare staff" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "the medications were administered on the same schedule to effectively blind the patients and healthcare staff" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included in the safety analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Campos 2001.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group trial with 4 treatment arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: Days 6 to 8 for adverse events |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 55 ± 16 (group I), 53 ± 14 (group II), 54 ± 14 (group III), 54 ± 13 (group IV) Gender (male:female): 58:42 (group I), 50:50 (group II), 61:39 (group III), 60:40 (group IV) Tumour/cancer type: solid malignancy (lung cancer, gastrointestinal cancer, head and neck cancer, genitourinary cancer, other) Chemotherapy regimen: cisplatin‐based chemotherapy at a dose ≥ 70 mg/m² Country: n.r. (multi‐centre) |
|
| Interventions |
Experimental: treatment group I Day 1: placebo (evening pre‐cisplatin), granisetron (10 µg/kg) + dexamethasone (20 mg placebo) + placebo (treatment pre‐cisplatin) Days 2 to 5: placebo Experimental: treatment group II Day 1: placebo (evening pre‐cisplatin), granisetron (10 µg/kg) + dexamethasone (20 mg placebo) + MK‐869 (400 mg placebo) (treatment pre‐cisplatin) Days 2 to 5: MK‐869 (300 mg placebo) Experimental: treatment group III Day 1: MK‐869 (400 mg placebo) (evening pre‐cisplatin), placebo + dexamethasone (20 mg placebo) + MK‐869 (400 mg placebo) (treatment pre‐cisplatin) Days 2 to 5: MK‐869 (300 mg placebo) Experimental: treatment group IV Day 1: placebo (evening pre‐cisplatin), placebo + dexamethasone (20 mg placebo) + MK‐869 (400 mg placebo) (treatment pre‐cisplatin) Days 2 to 5: MK‐869 (300 mg placebo) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... computer‐generated, randomized allocation schedule ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups, laboratory parameters) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the statistical analysis approach for efficacy was intent‐to‐treat (all patients that had data after cisplatin administration were included). The incidence of emesis in the acute and delayed periods as well as the use of rescue medication in both periods was evaluated" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "all 350 patients who received study medication were included in the analysis of safety" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes measures were described in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Chawla 2003.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled trial with 3 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: Days 19 to 29 post cisplatin for follow‐up examination and laboratory tests |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 56.0 ± 13.0 (aprepitant 125/80), 58.4 ± 13.4 (aprepitant 40/25 mg), 53.7 ± 13.2 (placebo) Gender: male + female Tumour/cancer type: solid tumour of respiratory, urogenital, and other origin Chemotherapy regimen: cisplatin at a dose ≥ 70 mg/m² Country: 21 sites in the United States and 29 sites outside the United States (multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant 125/80 mg + standard therapy Day 1: aprepitant 125 mg + ondansetron 32 mg i.v. + dexamethasone 20 mg p.o. Days 2 to 5: aprepitant 80 mg + dexamethasone 8 mg p.o. Experimental: arm B:aprepitant 40/25 mg + standard therapy Day 1: aprepitant 40 mg + ondansetron 32 mg i.v.+ dexamethasone 20 mg p.o. Days 2 to 5: aprepitant 25 mg + dexamethasone 8 mg p.o. Standard: arm C: placebo + standard therapy Day 1: placebo + ondansetron 32 mg i.v. + dexamethasone 20 mg p.o. Days 2 to 5: placebo + dexamethasone 8 mg p.o. ***Aprepitant 375/250 mg + standard therapy (this arm was replaced by arm B) Day 1: aprepitant 375 mg + ondansetron 32 mg i.v. + dexamethasone 20 mg p.o. Days 2 to 5: aprepitant 250 mg + dexamethasone 8 mg p.o. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
Exploratory endpoints
|
|
| Notes |
***Aprepitant 375/250‐mg dose regimen in the current study was discontinued and was replaced with an aprepitant 40/25‐mg dose regimen, and a new randomisation schedule was generated after the dose group adjustment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... were assigned to 1 of 3 treatment groups according to a computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccup, study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "... all analyses were performed using an intent‐to treat approach ..." |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety analyses are based on data from all patients both before and after the dose‐group adjustment" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Cheirsilpa 2005.
| Study characteristics | ||
| Methods |
Randomised controlled trial with 2 arms
Enrolment period: February 2003 to August 2003
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: ramosetron group 54.53 ± 10.16, granisetron group 53.97 ± 10.50 Gender: male + female Tumour/cancer type: solid malignancy (head and neck, cervix, lung, ovary, stomach‐oesophagus, urinary bladder, testis, other) Chemotherapy regimen: cisplatin at dose ≥ 70 mg/m² Country: Thailand (single centre) |
|
| Interventions |
Experimental: arm A: ramosetron
Active control: arm B: granisetron
|
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "... one nurse prepared for the study drug using identical syringes. The study drug was then sent to another nurse confirming that the study drug was stable and identical in appearance. Then a syringe containing the study drug was handed directly to the investigator. The study drug code was sealed and not opened until all evaluations had been finalized after the completion of treatment" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: adverse events were recorded for all participants |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Cho 1998.
| Study characteristics | ||
| Methods |
Randomised, cross‐over trial with 2 arms
Recruitment period: March 1997 to December 1997
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria
Median age (range), years: 52 (42 to 67) Gender: male + female Tumour/cancer type: solid tumours (stomach, lung, head and neck, ovary, metastasis of unknown origin) Chemotherapy regimen: cisplatin (≥ 50 mg/m²) Country: Korea |
|
| Interventions |
Cross‐over study Experimental: arm A tropisetron + dexamethasone Experimental: arm B ondansetron + dexamethasone |
|
| Outcomes |
Primary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: cannot be determined due to language barrier |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label trial |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label trial |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: cannot be determined due to language barrier |
| Selective reporting (reporting bias) | Unclear risk | Comment: cannot be judged due to language barrier |
| Other bias | Unclear risk | Comment: cannot be judged due to language barrier |
Chua 2000.
| Study characteristics | ||
| Methods |
Randomised, cross‐over trial with 3 arms
Recruitment period: March 1996 to May 1998
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 48 (22 to 74) Gender: male (86.5%) + female (13.5 %) Tumour/cancer type: solid malignancy (nasopharynx, oral cavity, hypopharynx, larynx, ear) Chemotherapy regimen: cisplatin at a dose of 100 mg/m² on Day 1 and 5‐fluorouracil (5‐FU) 1000 mg/m² on Days 1 to 3, repeated every 21 days Country: Hong Kong, China |
|
| Interventions |
Cross‐over study: patients were randomised to receive 1 of the 3 5‐HT₃ antagonists in the first cycle; treatment was crossed over to the other 2 5‐HT₃ antagonists in the second and third cycles Experimental: arm A: granisetron granisetron 3 mg i.v. + dexamethasone 20 mg i.v. Experimental: arm B: tropisetron tropisetron 5 mg i.v. + dexamethasone 20 mg i.v. Experimental: arm C: ondansetron ondansetron 8 mg i.v. + dexamethasone 20 mg i.v. before cisplatin, followed by 2 p.o. doses of 8 mg at 4 and 8 hours after the start of chemotherapy on Day 1 |
|
| Outcomes |
Primary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using a computer‐generated code" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the antiemetic efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Egerer 2010.
| Study characteristics | ||
| Methods |
Randomised, controlled, parallel‐group, placebo‐controlled trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 62.1 (39 to 71) in placebo group, 57.4 (40 to 69) in aprepitant group Gender: male (19) + female (11) Tumour/cancer type: multiple myeloma Chemotherapy regimen: melphalan Country: Germany (single centre) |
|
| Interventions |
Experimental: arm a: aprepitant aprepitant (125 mg) (1 h infusion of 100 mg m‐2) + granisetron (Kevatril) 2 mg once daily on Days 1 to 4 + dexamethasone 8 mg once daily on Day 1 and 4 mg once daily on Days 2 and 3 Experimental: arm B: placebo matched placebo + granisetron (Kevatril) 2 mg once daily on Days 1 to 4 + dexamethasone 8 mg once daily on Day 1 and 4 mg once daily on Days 2 and 3 |
|
| Outcomes |
Primary endpoint
Secondary objectives
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patients were randomized 1:1 to placebo and aprepitant" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: double‐blind |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: double‐blind |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Comment: safety is mentioned in the introduction but no results are provided |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Eisenberg 2003.
| Study characteristics | ||
| Methods |
Randomised, parallel, stratified, comparative phase 3 trial with 3 arms
Study period: May 2000 to December 2001
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: subjects were followed for 15 days |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 53.3 ± 13.1 in palonosetron 0.25 mg group, 55.2 ± 13.1 in palonosetron 0.75 mg group, 53.6 ± 12.7 in dolasetron group Gender: male (102) + female (467) Tumour/cancer type: malignant disease Chemotherapy regimen: any dose of carboplatin, epirubicin, idarubicin, ifosfamide, irinotecan, or mitoxantrone; or methotrexate > 250 mg/m², cyclophosphamide < 1500 mg/m², cyclophosphamide < 1500 mg/m², doxorubicin > 25 mg/m², or cisplatin ≤ 50 mg/m² Country: 61 centres in North America (United States and Mexico) |
|
| Interventions |
Experimental: arm A: palonosetron 0.25 mg palonosetron 0.25 mg + dexamethasone 20 mg (or, if unavailable, a single dose of p.o. dexamethasone 20 mg or i.v. methylprednisolone 125 mg) Experimental: arm B: palonosetron 0.75 mg palonosetron 0.75 mg + dexamethasone 20 mg (or, if unavailable, a single dose of p.o. dexamethasone 20 mg or i.v. methylprednisolone 125 mg) Experimental: arm C: dolasetron dolasetron 100 mg + dexamethasone 20 mg (or, if unavailable, a single dose of p.o. dexamethasone 20 mg or i.v. methylprednisolone 125 mg) |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized using an interactive voice response system across all study sites according to specific procedures" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. on study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "... 569 patients were included in the ITT cohort analysis" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety cohort included all treated patients who had at least one safety assessment after treatment with study drugs" and "the safety cohort comprised 582 patients" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes have been reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Endo 2012.
| Study characteristics | ||
| Methods |
Randomised study with 2 arms
Enrolment period: November 2010 to March 2012
Masking: n.r. Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 67.8 (41 to 85) in azasetron group, 68.0 (44 to 83) in granisetron group Gender: male (80) + female (25) Tumour/cancer type: lung cancer Chemotherapy regimen: carboplatin (area under the concentration vs time curve of 5 to 6) in combination with paclitaxel (200 mg/m²), etoposide (100 mg/m²), gemcitabine (1000 mg/m²), or pemetrexed (500 mg/m²) Country: Japan (single centre) |
|
| Interventions |
Experimental: arm A: azasetron Day 1: p.o. azasetron 10 mg + i.v. dexamethasone 12 mg Days 2 to 3: p.o. dexamethasone 8 mg/d Experimental: arm B: granisetron Day 1: i.v. granisetron 3 mg + i.v. dexamethasone 12 mg Days 2 to 3: p.o. dexamethasone 8 mg/d |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was carried out by using the random number table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: patients were possibly not blinded; therefore we do not know if there is potential for risk of bias |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although blinding was not reported, we assume that for objective outcomes, there would be no potential for risk of bias |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all participants have been included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: safety outcomes were reported for all participants |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Flenghi 2015.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, phase 3 trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean/median age (range), years: n.r. Gender: n.r. Tumor/cancer type: n.r. Chemotherapy regimen: cyclophosphamide i.v. chemotherapy (3 g/m²) Country: Italy (single centre) |
|
| Interventions |
Experimental: arm A aprepitant + palonosetron + dexamethasone Experimental: arm B placebo + palonosetron + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blinded ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blinded ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the analysis was performed according to the intention‐to‐treat principle" |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract, not evaluable |
| Other bias | Unclear risk | Comment: not evaluable |
Forni 2000.
| Study characteristics | ||
| Methods |
Randomised, controlled trial with 3 arms
Recruitment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age (range), years: n.r. Gender: n.r. Tumour/cancer type: osteosarcoma Chemotherapy regimen: cisplatin (120 mg/m², 48‐h CI) followed by doxorubicin (75 mg/m², 24‐h CI); then, in the second cycle, delivered 3 weeks later, ifosfamide 15 g/m² (120‐h CI) Country: n.r. |
|
| Interventions |
Experimental: arm A: granisetron granisetron 2 mg/m² + dexamethasone 8 mg/m² Experimental: arm B: tropisetron tropisetron 5.3 mg/m² + dexamethasone 8 mg/m² Experimental: arm C: ondansetron ondansetron 3.3 mg/m² + dexamethasone 8 mg/m² |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: blinding not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract, not evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Fox‐Geiman 2001.
| Study characteristics | ||
| Methods |
Randomised, comparative trial with 3 arms
Recruitment period: September 1997 to September 1998
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean age, years: 45 (oral ondansetron), 46 (oral granisetron), 49 (intravenous ondansetron) Gender: male + female Tumour/cancer type: n.r. Chemotherapy regimen: STAMP V, TBI/VP/CY, TANC, Bu/Cy, BEAM, BCNU/VP/CY, ICE, Carboplatin/VP, Carboplatin/MTZ/CY, MMT, Thiotepa/CY, TBI/CY Country: United States (single centre) |
|
| Interventions |
Experimental: arm A: oral ondansetron ondansetron 8 mg p.o. (every 8 hours) + dexamethasone 10 mg i.v. (once daily) Experimental: arm B: oral granisetron granisetron 1 mg p.o. (every 12 hours each day) + dexamethasone 10 mg i.v. (once daily) Experimental: arm C: intravenous ondansetron ondansetron 32 mg i.v. (single daily dose) + dexamethasone 10 mg i.v. (once daily) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomization scheme was determined by the study’s biostatistician based on a permuted block design (K = 6)" |
| Allocation concealment (selection bias) | Low risk | Quote: "the treatment allocation scheme was maintained by the study pharmacist, who assumed responsibility for blinded drug distribution" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "... the trial was analysed according to intention to‐treat" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Fujiwara 2015.
| Study characteristics | ||
| Methods |
Randomised, prospective, cross‐over, comparative study with 2 arms
Enrolment period: January 2011 to January 2012
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 57.5 (36 to 76) Gender: female (38) Tumour/cancer type: gynaecological cancer (endometrial cancer, cervical cancer, ovarian or tubal cancer, double endometrial and ovarian cancer) Chemotherapy regimen: TC regimen (paclitaxel and carboplatin) Country: Japan (single centre) |
|
| Interventions |
Cross‐over study Experimental: arm A: palonosetron Day 1: aprepitant 125 mg p.o. + palonosetron 0.75 mg i.v. + dexamethasone 20 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 4 mg p.o. Experimental: arm B: granisetron Day 1: aprepitant 125 mg p.o. + granisetron 3 mg i.v. + dexamethasone 20 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 4 mg p.o. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the group assignment was performed by simple randomization using a table of random numbers and patients were informed of which group (arm A or B) they were assigned" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... non‐blinded ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... non‐blinded ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the intent‐to‐treat population included 19 patients who received palonosetron on Day 1 (Arm A) and 19 patients who received granisetron on Day 1 (Arm B)" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Gao 2013.
| Study characteristics | ||
| Methods |
Randomised, cross‐over, self‐control study with 2 arms
Recruitment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age (range), years: n.r. Gender: n.r. Tumour/cancer type: n.r. Chemotherapy regimen: cisplatin‐based chemotherapy Country: n.r. |
|
| Interventions |
Cross‐over study Experimental: arm A: palonosetron Day 1: palonosetron 0. 25 mg i.v. + dexamethasone 10 mg i.v. Day 2: dexamethasone 10 mg i.v. Day 3: palonosetron 0. 25 mg i.v. + dexamethasone 10 mg i.v. Experimental: arm B: granisetron Days 1 to 3: granisetron 3 mg i.v. + dexamethasone 10 mg i.v. |
|
| Outcomes | not reported | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: blinding not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: blinding not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: no outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract, not evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Ghosh 2010.
| Study characteristics | ||
| Methods |
Randomised, controlled trial with 3 arms
Recruitment period: 5 November 2007 to 30 September 2009
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: patients were followed for 5 days for efficacy endpoints and for 8 days for safety endpoints |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 48 (ondansetron); 49 (granisetron); 47 (palonosetron) Gender: male + female Tumour/cancer type: malignant disease Chemotherapy regimen
Country: India (multi‐centre) |
|
| Interventions |
Experimental: arm A: ondansetron Day 1: ondansetron 8 mg i.v. + dexamethasone 16 mg i.v. Day 2: ondansetron 8 mg i.v. + dexamethasone 4 mg i.v. Day 3: dexamethasone 4 mg i.v. Experimental: arm B: granisetron Day 1: granisetron 3 mg i.v. + dexamethasone 16 mg i.v. Day 2: granisetron 3 mg i.v. + dexamethasone 4 mg i.v. Day 3: dexamethasone 4 mg i.v. Experimental: arm C: palonosetron Day 1: palonosetron 0.75 mg i.v. + dexamethasone 16 mg i.v. Days 2 to 3: dexamethasone 4 mg i.v. Day 3: dexamethasone 4 mg i.v. |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "all study personnel and patients were blinded to the treatment assignment for the duration of the study and the nursing staffs injecting the drugs were prohibited from divulging any information on drug assignment even to the doctors giving the chemotherapeutic drugs" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "all study personnel and patients were blinded to the treatment assignment for the duration of the study and the nursing staffs injecting the drugs were prohibited from divulging any information on drug assignment even to the doctors giving the chemotherapeutic drugs" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all participants were included for the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all participants were included in reporting on adverse events and deaths |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Grunberg 2009.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, phase 3 trial with 3 arms
Recruitment period: 6 November 2006 to 9 October 2007
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Age (range), years: 57 (20 to 84) in 3‐day i.v. + p.o. casopitant group, 59 (18 to 80) in single‐dose p.o. casopitant group, 59 (20 to 82) in control group Gender: male + female Tumour/cancer type: malignant solid tumour (non‐small cell lung, head and neck, small cell lung, ovary, cervix, bladder, other) Chemotherapy regimen: cisplatin, gemcitabine, vinorelbine, fluorouracil, etoposide, paclitaxel, cyclophosphamide, doxorubicin, docetaxel Country: 22 countries (77 centres) |
|
| Interventions |
Experimental: arm A: 3‐day i.v. + p.o. casopitant Day 1: casopitant 90 mg i.v. + casopitant placebo p.o. + ondansetron 32 mg i.v. + dexamethasone 12 mg p.o. + dexamethasone placebo p.o. Days 2 to 3: AM (dexamethasone 8 mg p.o. + casopitant 50 mg p.o.), PM (dexamethasone placebo p.o.) Day 4: AM (dexamethasone 8 mg p.o.), PM (dexamethasone placebo p.o.) Experimental: arm B: single‐dose oral casopitant Day 1: casopitant 150 mg p.o. + casopitant placebo i.v. + ondansetron 32 mg i.v. + dexamethasone 12 mg p.o. + dexamethasone placebo p.o. Days 2 to 3: AM (dexamethasone 8 mg p.o. + casopitant placebo p.o.), PM (dexamethasone 8 mg p.o.) Day 4: AM (dexamethasone 8 mg p.o.), PM (dexamethasone 8 mg p.o.) Control: arm C Day 1: casopitant placebo p.o. + casopitant placebo i.v. + ondansetron 32 mg i.v. + dexamethasone 20 mg p.o. Days 2 to 3: AM (dexamethasone 8 mg p.o. + casopitant placebo p.o.), PM (dexamethasone 8 mg p.o.) Day 4: AM (dexamethasone 8 mg p.o.), PM (dexamethasone 8 mg p.o.) |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were then randomly assigned, through a second telephone call ..." and "randomisation was performed centrally at the study level ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "these supplies were blinded to the pharmacist, the investigator, and the patient" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "these supplies were blinded to the pharmacist, the investigator, and the patient" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia, on‐study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "efficacy analysis were done in the modified intention‐to‐treat population" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "safety analyses were done with all patients who received placebo or study drug" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Grunberg 2011.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, phase 3 trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 56 (19 to 86) in i.v. fosaprepitant group, 57 (19 to 82) in oral aprepitant group Gender: male + female Tumour/cancer type: solid tumour (lung cancer, gastrointestinal cancer, reproductive or genitourinary cancer, miscellaneous or site unspecified, renal and urinary tract cancer, breast cancer, lymphoma, hepatic and biliary cancer, endocrine cancer) Chemotherapy regimen: cisplatin at a dose ≥ 70 mg/m² Country/continent: North America, South America, Europe, Asia‑Pacific, Africa |
|
| Interventions |
Experimental: arm A: i.v. fosaprepitant Day 1: 150 mg fosaprepitant dimeglumine + 32 mg i.v. ondansetron + 12 mg p.o. dexamethasone Day 2: 8 mg p.o. dexamethasone Days 3 to 4: 8 mg p.o. dexamethasone twice a day Experimental: arm B: p.o. aprepitant 125/80 Day 1: 125 mg p.o. aprepitant + 32 mg i.v. ondansetron + 12 mg p.o. dexamethasone Days 2 to 3: 80 mg p.o. aprepitant + 8 mg p.o. dexamethasone Day 4: 8 mg p.o. dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: patients were stratified by sex and were randomly assigned to treatment groups according to a computer‐generated, blinded allocation schedule |
| Allocation concealment (selection bias) | Low risk | Comment: to ensure in‐house blinding, treatment group assignments were made by personnel not otherwise involved with the study |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‑blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‑blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. infusion site reaction) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included from the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all included patients were checked for adverse events |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hashimoto 2013.
| Study characteristics | ||
| Methods |
Randomised, phase 3 trial with 2 arms
Enrolment period: July 2011 to June 2012
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: n.r. Gender: n.r. Tumour/cancer type: malignant solid tumour Chemotherapy regimen: HEC containing ≥ 50 mg/m² cisplatin (CDDP) Country: Japan |
|
| Interventions |
Experimental: arm A: palonosetron Day 1: aprepitant 125 mg p.o. + palonosetron 0.75 mg i.v. + dexamethasone 9.9 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 6.6 mg i.v. Day 4: dexamethasone 6.6 mg i.v. Experimental: arm B: granisetron Day 1: aprepitant 125 mg p.o. + granisetron 1 mg i.v. + dexamethasone 9.9 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 6.6 mg i.v. Day 4: dexamethasone 6.6 mg i.v. |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done centrally using the minimization method with stratification with respect to center, age (< 60 versus ≥ 60 years), gender, and cisplatin dose (< 70 versus ≥ 70 mg/m2)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "at each center, one pharmacist was pre‐designated who was notified of treatment label ... All other staffs (physicians, study pharmacists, and nurses involved in the study) as well as patients were kept blinded to the assigned treatments" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "at each center, one pharmacist was pre‐designated who was notified of treatment label ... All other staffs (physicians, study pharmacists, and nurses involved in the study) as well as patients were kept blinded to the assigned treatments" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Quote: "...our study designated study pharmacists at each center who were blinded (masked) to treatment allocation and who evaluated efficacy end points for each patient every day based on diary data and interview" |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included for safety analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐specified outcomes were reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Herrington 2000.
| Study characteristics | ||
| Methods |
Randomised study with 2 arms
Enrolment period: July 1997 to February 1999
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 59 (20 to 91) in ondansetron group, 62.5 (25 to 84) in granisetron group Gender: male (15) + female (46) Tumour/cancer type: breast, lymphoma, multiple myeloma, other Chemotherapy regimen: moderately emetogenic chemotherapy (carboplatin ≥ 300 mg/m², cisplatin ≥ 20 to < 50 mg/m², cyclophosphamide, p.o. ≥ 100 mg/m², cyclophosphamide, i.v. ≥ 500 to < 1000 mg/m², dacarbazine ≥ 350 to < 500 mg/m², daunorubicin ≥ 45 mg/m², doxorubicin ≥ 40 mg/m² as a single agent or ≥ 25 mg/m² combined with other chemotherapeutic agents, idarubicin ≥ 12 mg/m², ifosfamide ≥ 1000 mg/m², methotrexate ≥ 250 mg/m², mitoxantrone ≥ 12 mg/m²) Country: n.r. (multi‐centre) |
|
| Interventions |
Experimental: arm A: ondansetron p.o. single‐dose ondansetron 16 mg + dexamethasone 12 mg Experimental: arm B: granisetron p.o. granisetron 1 mg + dexamethasone 12 mg |
|
| Outcomes |
Primary objective
Secondary objective(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "sixty‐five patients (75% women) took part in the study, but only 61 were included in the analysis (Table 6)" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Herrington 2008.
| Study characteristics | ||
| Methods |
Randomised, pilot, placebo‐controlled, comparative trial with 3 arms
Patient evaluation period: June 2005 to May 2007
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Age ± SD, years: 59.6 ± 10.7 (palonosetron + 3‐day aprepitant), 58.3 ± 10.5 (palonosetron + 1‐day aprepitant), 56.1 ± 12.6 (palonosetron + placebo) Gender: male + female Tumour/cancer type: solid malignancy (breast cancer, lung cancer, head and neck cancer, other) Chemotherapy regimen
Country: United States (single institute) |
|
| Interventions |
Experimental: arm A: palonosetron + 3‐day aprepitant Day 1: aprepitant 125 mg p.o. + palonosetron 0.25 mg i.v. + dexamethasone 12 mg p.o. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 8 mg p.o. Day 4: dexamethasone 8 mg p.o. Experimental: arm B: palonosetron + 1‐day aprepitant Day 1: aprepitant 125 mg p.o. + palonosetron 0.25 mg i.v. + dexamethasone 12 mg p.o. Days 2 to 3: matching placebo + dexamethasone 8 mg p.o. Day 4: dexamethasone 8 mg p.o. Experimental: arm C: palonosetron + placebo Day 1: placebo + palonosetron 0.25 mg i.v. + dexamethasone 18 mg p.o. Days 2 to 4: dexamethasone 8 mg p.o. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included for efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Unclear risk | Quote: "there were no reports of serious adverse events that were related to study medication" Probably for all patients |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Unclear risk | Comment: the study was temporarily halted, and the protocol was amended with removal of arm C. Descriptive statistics for patients in arm C were not included in the report |
Herrstedt 2009.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, parallel‐group, phase 3 controlled trial with 4 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 51 in single p.o. dose group, 51 in 3‐day p.o. group, 53 in 3‐day i.v./p.o. group, 52 in control group Gender: male (40) + female (1893) Tumour/cancer type: breast cancer and other Chemotherapy regimen: anthracycline and cyclophosphamide (AC)‐based regimen Country: 196 centres in 32 countries |
|
| Interventions |
Experimental: arm A: casopitant single oral dosage Day 1: casopitant 150 mg p.o. + casopitant placebo i.v. + ondansetron 8 mg p.o. twice daily + dexamethasone 8 mg i.v. Days 2 to 3: casopitant placebo p.o. + ondansetron 8 mg p.o. twice daily Experimental: arm B: casopitant 3‐day oral dosage Day 1: casopitant 150 mg p.o. + casopitant placebo i.v. + ondansetron 8 mg p.o. twice daily + dexamethasone 8 mg i.v. Days 2 to 3: casopitant 50 mg p.o. + ondansetron 8 mg p.o. twice daily Experimental: arm C: casopitant 3‐day intravenous/oral dosage Day 1: casopitant placebo p.o. + casopitant 90 mg i.v. + ondansetron 8 mg p.o. twice daily + dexamethasone 8 mg i.v. Days 2 to 3: casopitant 50 mg p.o. + ondansetron 8 mg p.o. twice daily Control: arm D Day 1: casopitant placebo p.o. + casopitant placebo i.v. + ondansetron 8 mg p.o. twice daily + dexamethasone 8 mg i.v. Days 2 to 3: casopitant placebo p.o. + ondansetron 8 mg p.o. twice daily |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "after the initial screening visit, investigators used a Randomisation and Medication system to register patients for the trial ..." Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. study mortality, infusion/injection site reaction) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "of the 1,933 patients randomly assigned to the study, a modified intent to treat (mITT) population (n=1,917) was used for the efficacy analysis" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety population (n=1,920) included all patients randomly assigned to a study arm who received any investigational drug (casopitant or casopitant placebo)"; and "there was a single death in each study arm, none of which were attributed to the investigational drug by the investigator" |
| Selective reporting (reporting bias) | Unclear risk | Comment: most outcome measures were reported in the results section. Results were submitted to study registry, then were cancelled. Satisfaction and impact on daily life were not reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hesketh 1999.
| Study characteristics | ||
| Methods |
Randomised, phase 2 trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 66 (36 to 81) in ezlopitant group, 62 (40 to 76) in placebo group Gender: male + female Tumour/cancer type: solid tumour (lung cancer, ovary cancer, oesophagus/oropharynx cancer, other/unspecified) Chemotherapy regimen: cisplatin at a dose ≥ 100 mg/m² Country: n.r. (10 institutions, multi‐centre) |
|
| Interventions |
Experimental: arm A: Gran/Dex + CJ‐11,974 granisetron 10 μg/kg i.v. 30 min before cisplatin + dexamethasone 20 mg i.v. 30 min before cisplatin + CJ‐11,974 100 mg orally 30 min before and 12 h after cisplatin, and twice daily on Days 2 through 5 after cisplatin Experimental: arm B: Gran/Dex + placebo granisetron 10 μg/kg i.v. 30 min before cisplatin + dexamethasone 20 mg i.v. 30 min before cisplatin + identical‐appearing placebo capsules orally 30 min before and 12 h after cisplatin, and twice daily on Days 2 through 5 after cisplatin |
|
| Outcomes |
Primary efficacy endpoint
Secondary efficacy endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "three (two from group 1 and one from group 2) of the 61 total patients who received the study drug were not included in efficacy analyses because assessments were not recorded..." |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "all 61 patients treated on the study were considered assessable for adverse events. Safety results are listed in Table 8" |
| Selective reporting (reporting bias) | Low risk | Comment: all efficacy measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hesketh 2003.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range) ± SD, years: 59 (18 to 84) ± 12, aprepitant 125/80 regimen; 58 (19 to 83) ± 12, placebo group Gender: male + female Tumour/cancer type: solid malignancy (respiratory cancer, urogenital cancer, others) Chemotherapy regimen: cisplatin ≥ 70 mg/m² Country: 15 centres in United States, 14 centres in other countries |
|
| Interventions |
Experimental: arm A: aprepitant 125/80 Day 1: p.o. aprepitant 125 mg + i.v. ondansetron 32 mg + p.o. dexamethasone 12 mg Days 2 to 3: p.o. aprepitant 80 mg + p.o. dexamethasone 8 mg Day 4: p.o. dexamethasone 8 mg Standard therapy: arm B Day 1: i.v. ondansetron 32 mg + p.o. dexamethasone 20 mg Days 2 to 4: p.o. dexamethasone 8 mg twice per day |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... computer‐generated random assignment schedule ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: a modified intent‐to‐treat approach, which included all patients who received cisplatin, took study drug, and had at least 1 post‐treatment assessment, was used to analyse the data |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "all patients who received cisplatin and at least one dose of study drug were included in the statistical analyses for safety" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hesketh 2012.
| Study characteristics | ||
| Methods |
Randomised, active‐controlled, parallel‐group, phase 3 study with 2 arms
Study period: 2008 March 10 to 13 April 2009
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (SD), years: 61.3 (11.03) in casopitant group, 61.3 (10.77) in placebo group Gender: male (392) + female (318) Tumour/cancer type: colorectal cancer Chemotherapy regimen: oxaliplatin doses between 85 and 130 mg/m² Country: 89 centres (hospitals or outpatient clinics) in 11 countries (multi‐centre) |
|
| Interventions |
Experimental: arm A: casopitant casopitant + ondansetron + dexamethasone Control: arm B: placebo placebo + ondansetron + dexamethasone |
|
| Outcomes |
Primary outcome
Secondary outcome(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the efficacy endpoints were analyzed for the modified intention to treat (MITT) population which comprised the subset of the intention to treat (ITT) population who received any investigational product and had oxaliplatin administered" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety population included all subjects who received any investigational product" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section and in the study registry |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hesketh 2014.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, phase 2 trial with 5 arms
Recruitment period: 2008
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 55 (PALO), 55 (NEPA100), 55 (NEPA200), 53 (NEPA300), 55.5 (APR + OND) Gender: male (386) + female (291) Tumour/cancer type: solid tumour (lung/respiratory, head and neck, ovarian, other urogenital, gastric, other GI, breast, other) Chemotherapy regimen: cisplatin‐based chemotherapy at a dose ≥ 50 mg/m² alone or in combination with other chemotherapy agents Country: 29 sites in Russia, 15 sites in Ukraine |
|
| Interventions |
Experimental: arm A: PALO Day 1: p.o. palonosetron 0.50 mg + p.o. dexamethasone 20 mg + placebo Days 2 to 4: p.o. dexamethasone 8 mg b.i.d. Experimental: arm B: NEPA100 Day 1: p.o. netupitant 100 mg + p.o. palonosetron 0.50 mg + p.o. dexamethasone 12 mg Days 2 to 4: p.o. dexamethasone 4 mg b.i.d. Experimental: arm C: NEPA200 Day 1: p.o. netupitant 200 mg + p.o. palonosetron 0.50 mg + p.o. dexamethasone 12 mg Days 2 to 4: p.o. dexamethasone 4 mg b.i.d. Experimental: arm D: NEPA300 Day 1: p.o. netupitant 300 mg + p.o. palonosetron 0.50 mg + p.o. dexamethasone 12 mg Days 2 to 4: p.o. dexamethasone 4 mg b.i.d. Experimental: arm E: APR + OND Day 1: p.o. aprepitant 125 mg + i.v. ondansetron 32 mg + p.o. dexamethasone 12 mg Days 2 to 3: p.o. aprepitant 80 mg in morning + p.o. dexamethasone 4 mg b.i.d. Day 4: p.o. dexamethasone 4 mg b.i.d. |
|
| Outcomes |
Primary efficacy endpoint
Secondary efficacy endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups, study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "15 patients did not receive study treatment and were not included in the safety population and 677 (98%) patients were included in the full analysis set" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "15 patients did not receive study treatment and were not included in the safety population and 677 (98%) patients were included in the full analysis set"; and "one patient (NEPA100) died during the study due to multiple organ failure. His death was not considered related to study medication" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ho 2010.
| Study characteristics | ||
| Methods |
Randomised, parallel, comparative, active‐control trial with 2 arms
Recruitment period: January 2006 to December 2007
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 51 (29 to 73) in ramosetron + dexamethasone group, 51 (22 to 74) in granisetron + dexamethasone group Gender: male (110) + female (175) Tumour/cancer type: solid malignancy (breast, lung, nasopharynx, mouth, rectum, liver, bladder, stomach, oesophagus, testis, brain, other) Chemotherapy regimen: cisplatin, doxorubicin, epirubicin, or oxaliplatin Country: Taiwan (4 centres) |
|
| Interventions |
Experimental: arm A: ramosetron + dexamethasone ramosetron 0.3 mg + dexamethasone 20 mg Experimental: arm B: granisetron + dexamethasone granisetron 3 mg + dexamethasone 20 mg |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s) To be evaluated during first, second, third, and fourth 6‐h durations and total 24‐h period after the start of chemotherapy
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: Both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included for the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: safety data were reported for all randomised patients who received the study drug |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Hu 2014.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, phase 3 trial with 2 arms
Study period: August 2009 to April 2010
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: follow‐up on Days 19 to 29 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years : 53.1 ± 10.1 (aprepitant regimen), 53.6 ± 10.6 (placebo group) Median age, years: 56 (aprepitant regimen), 54 (placebo group) Gender: male + female Tumour/cancer type: solid tumour (lung cancer, nasopharyngeal cancer, gastrointestinal cancer, reproductive cancer, breast cancer, lymphoma, other) Chemotherapy regimen: cisplatin (≥ 70 mg/m²) Country: China (16 independent centres) |
|
| Interventions |
Experimental: arm A: aprepitant regimen Day 1: aprepitant 125 mg p.o. + granisetron 3 mg i.v. + dexamethasone 6 mg p.o. Day 2: aprepitant 80 mg p.o. + dexamethasone 3.75 mg p.o. Day 3: aprepitant 80 mg p.o. + dexamethasone 3.75 mg p.o. Day 4: dexamethasone 3.75 mg p.o. Standard: arm A Day 1: placebo + granisetron 3 mg i.v. + dexamethasone 10.5 mg p.o. Day 2: placebo + dexamethasone 7.5 mg p.o. Day 3: placebo + dexamethasone 7.5 mg p.o. Day 4: dexamethasone 7.5 mg p.o. |
|
| Outcomes |
Primary endpoint
Secondary endpoints ·
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "... computer‐generated blinded allocation ..." |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "all patients treated with cisplatin or aprepitant who underwent one or more posttreatment assessments were included in the modified intent‐to‐treat analysis" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: safety data were reported for all randomised patients who received the study drug |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Innocent 2018.
| Study characteristics | ||
| Methods |
Randomised, cross‐over study with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean age, years: 53.5 Gender: male (10) + female (24) Tumour/cancer type: cervical cancer and head and neck cancers were predominant Chemotherapy regimen: cisplatin‐based Country: n.r. |
|
| Interventions |
Cross‐over study Experimental: arm A: ondansetron Day 1: ondansetron 12 mg i.v. + dexamethasone 8 mg i.v. Days 2 to 5: ondansetron 8 mg p.o. b.d. + dexamethasone 4 mg p.o. b.d. Experimental: arm B: granisetron Day 1: granisetron 12 mg i.v. + dexamethasone 8 mg i.v. Days 2 to 5: granisetron 1 mg p.o. b.d. + dexamethasone 4 mg p.o. b.d. |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised study but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote. "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote. "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the patient‐reported outcome analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ishido 2016.
| Study characteristics | ||
| Methods |
Randomised, cross‐over, phase 2 trial with 2 arms
Recruitment period: November 2010 to August 2013
Masking: open‐label Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 65 (30 to 75) in aprepitant + granisetron + dexamethasone group, 64 (33 to 77) in palonosetron + dexamethasone group Gender: male + female Tumour/cancer type: advanced or recurrent oesophageal or gastric cancer Chemotherapy regimen
Country: Japan (single centre) |
|
| Interventions |
Cross‐over trial Experimental: arm A: aprepitant + granisetron + dexamethasone, then palonosetron + dexamethasone 1 h before start of treatment with cisplatin: 125 mg aprepitant (administered p.o.) + 3 mg granisetron (administered i.v.) + 6.6 mg dexamethasone (administered i.v.) after 24 h and 48 h: 80 mg aprepitant (administered p.o.) + 4 mg dexamethasone (administered p.o.) during second cycle, study treatments were crossed over, that is, aprepitant + granisetron + dexamethasone group received palonosetron + dexamethasone Experimental: arm B: palonosetron + dexamethasone, then aprepitant + granisetron + dexamethasone before treatment with cisplatin: 0.75 mg palonosetron (administered i.v.) + 13.2 mg dexamethasone (administered i.v.) after 24 h and 48 h: 8 mg dexamethasone (administered p.o.) during second cycle, study treatments were crossed over, that is, palonosetron + dexamethasone group received aprepitant + granisetron + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients scheduled to receive chemotherapy who provided informed consent were assigned randomly to receive AGD or PD in a 1: 1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open ‐no one is blinded ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open ‐no one is blinded ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although this was an open‐label study, both patients and personnel had no influence on objective outcomes (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "effectiveness and safety were evaluated in the remaining 84 patients ..." |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "effectiveness and safety were evaluated in the remaining 84 patients ..." |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were described in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ito 2014.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, phase 2 study with 2 arms
Enrolment period: n.r.
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 67 (34 to 84) in aprepitant group, 66 (44 to 81) in control group Gender: male (110) + female (24) Tumour/cancer type: adenocarcinoma, squamous cell carcinoma, other Chemotherapy regimen: carboplatin + paclitaxel, carboplatin + paclitaxel + bevacizumab, carboplatin + pemetrexed, carboplatin + pemetrexed + bevacizumab Country: Japan (multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg + first‐generation 5‐HT₃ antagonist + dexamethasone 8 mg Days 2 to 3: aprepitant 80 mg + dexamethasone 8 mg Control: arm B Day 1: first‐generation 5‐HT₃ antagonist + dexamethasone 8 mg Days 2 to 3: dexamethasone 8 mg |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done centrally using a computer program and stratified by sex and non‐platinum agent" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although this was an open‐label study, both patients and personnel had no influence on objective outcomes (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: 1 patient discontinued due to anaphylactic shock, and remaining 133 patients were included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "all 134 patients (including one patient who could not complete chemotherapy because of anaphylactic shock due to paclitaxel) were included in the safety analysis" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Unclear risk | Quote: "additional antiemetic agents and other supportive treatments were administered at the discretion of the treating physicians" |
Jantunen 1992.
| Study characteristics | ||
| Methods |
Randomised, prospective, cross‐over study with 2 arms
Enrolment period: n.r.
Masking: open‐label Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 58.3 in males, 49.5 in females Gender: male (14) + female (33) Tumour/cancer type: solid tumour (breast, lung, melanoma, other) Chemotherapy regimen: non‐cisplatin‐containing chemotherapy (CNF, CMF, CEF, VAC, carboplatin‐containing, DTIC‐containing, epirubicin‐containing, MTX‐5‐FU, MMM) Country: n.r. |
|
| Interventions |
Cross‐over study Experimental: arm A: ondansetron 8 mg ondansetron + 10 mg dexamethasone (loading dose of ondansetron was followed by 8 mg ondansetron given orally twice at 8‐h intervals) Experimental: arm B: tropisetron 5 mg tropisetron + 10 mg dexamethasone |
|
| Outcomes | control of vomiting during first 24 h was scored as
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "thirty‐nine patients were evaluable for cross‐over analysis" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Jordan 2016a.
| Study characteristics | ||
| Methods |
Randomised, parallel, phase 3 study with 2 arms comparison of netupitant + palonosetron + dexamethasone vs aprepitant + palonosetron + dexamethasone Enrolment period: July 2011 to September 2012
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 57 ± 10 in NEPA group, 58 ± 11 in APR + PALO group Gender: male (106) + female (90) Tumour/cancer type: solid malignancy (lung/respiratory, gynaecological, head and neck, breast, other) Chemotherapy regimen: carboplatin Country: multi‐national, multi‐centre |
|
| Interventions |
Experimental: arm A: netupitant + palonosetron + dexamethasone oral netupitant/palonosetron (300 mg/0.50 mg) hard capsule (on Day 1) with oral dexamethasone before each scheduled chemotherapy cycle Active comparator: arm B: aprepitant + palonosetron + dexamethasone oral aprepitant hard capsule 125 mg (on Day 1) + 80 mg daily (for the following 2 days) and oral palonosetron soft capsule 0.50 mg (on Day 1) given with oral dexamethasone at each scheduled chemotherapy cycle |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated in a 3:1 ratio to receive one of the following treatments ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all included patients have been analysed in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kalaycio 1998.
| Study characteristics | ||
| Methods |
Randomised trial with 2 arms
Enrolment period: September 1994 to April 1996
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 43.5 in granisetron group, 43 in ondansetron group Gender: n.r. Tumour/cancer type: breast cancer Chemotherapy regimen: 1500 mg cyclophosphamide/m²/d, 125 mg thiotepa/m²/d, 200 mg carboplatin/m²/d Country: n.r. |
|
| Interventions |
Experimental: arm A: granisetron granisetron as a 0.5‐mg i.v. bolus 30 min before chemotherapy followed by continuous infusion of 0.04 mg/h (1 mg/d) for 7 days + 10 mg dexamethasone/d i.v. for 7 days Experimental: arm B: ondansetron ondansetron as 8‐mg i.v. bolus 30 min before chemotherapy followed by continuous infusion of 1 mg/h (24 mg/d) for 7 days + 10 mg dexamethasone/d i.v. for 7 days |
|
| Outcomes |
Primary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... computer generated a list whereby each patient was assigned to either arm 1 or arm 2 as they were enrolled on the trial" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "both patient and treatment team remained blinded to the identity of the study drug throughout the patient's hospitalization" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "both patient and treatment team remained blinded to the identity of the study drug throughout the patient's hospitalization" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote. "of the 48 patients enrolled, 45 were evaluable" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included for assessed adverse events Comment: not reported |
| Selective reporting (reporting bias) | Low risk | Comment: outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kang 2020.
| Study characteristics | ||
| Methods |
Randomised trial with 2 arms
Enrolment period: August 2015 to September 2017
Masking: single‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (SD), years: 59.4 (12.0) in ramosetron group, 60.3 (11.8) in palonosetron group Gender: 37.2% (62.8% male) female in ramosetron group, 38.7% female (61.3% male) in palonosetron group Tumour/cancer type: solid tumours (lung and thymus, breast, head and neck, gynaecological and genitourinary, gastrointestinal, others) Chemotherapy regimen: individual highly emetogenic chemotherapies: 71.5% in ramosetron group, 72.5% in palonosetron group received cisplatin Country: Korea, multi‐centre |
|
| Interventions |
Experimental: arm A: ramosetron aprepitant (Day 1, 125 mg p.o. 1 h before chemotherapy; Days 2 to 3, 80 mg p.o.), ramosetron (Day 1, 0.3 mg i.v. 30 min before chemotherapy), and dexamethasone (Day 1, 12 mg p.o. or i.v. 30 min before chemotherapy; Days 2 to 4, 8 mg p.o.) Experimental: arm B: palonosetron aprepitant (Day 1, 125 mg p.o. 1 h before chemotherapy; Days 2 to 3, 80 mg p.o.), palonosetron (Day 1, 0.25 mg i.v. 30 min before chemotherapy), and dexamethasone (Day 1, 12 mg p.o. or i.v. 30 min before chemotherapy; Days 2 to 4, 8 mg p.o.) |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "stratified block randomization was conducted with a 1:1 ratio between groups, randomly mixing block sizes of 2 and 4, considering (1) chemotherapeutic regimen (cisplatin vs.non‐cisplatin), (2) treatment schedule (single‐day vs. multiple‐ day), and (3) sex (male vs. female), as stratification factors"; "patients were assigned according to a pre‐defined randomization sequence created by an independent investigator with no clinical involvement in the trial" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Single‐blinded; participants were not aware of treatment |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Physicians were aware of treatment |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Outcome assessors (participants) were blinded to intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Outcome was robust to blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | High risk | Modified ITT was analysed (participants who received at least 1 treatment) |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | High risk | Safety population (ITT) analysed, but not all participants received intervention |
| Selective reporting (reporting bias) | Low risk | No reasons for any concerns detected |
| Other bias | Low risk | No other sources of bias detected |
Kaushal 2010.
| Study characteristics | ||
| Methods |
Randomised, cross‐over study with 2 arms
Enrolment period: n.r.
Masking: open‐label Baseline patient characteristics: not reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: head and neck cancer Chemotherapy regimen: moderately emetogenic cancer chemotherapeutic drugs (i.v. docetaxel 60 mg/m², i.v. carboplatin 300 mg/m², and i.v. 5‐fluorouracil 600 mg/m²) Country: India (single centre) |
|
| Interventions |
Cross‐over study Experimental: arm A: palonosetron palonosetron 0.25 mg i.v. + dexamethasone 16 mg i.v. (half hour before chemotherapy) Experimental: arm B: ondansetron ondansetron 16 mg i.v. + dexamethasone 16 mg i.v. (half hour before chemotherapy) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all 30 patients have been included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kaushal 2015.
| Study characteristics | ||
| Methods |
Randomised, prospective study with 2 arms
Enrolment period: n.r.
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range): 52 (36 to 70) in PDA group, 51 (34 to 69) in OD group Gender: male (52) + female (8) Tumour/cancer type: head and neck cancer (squamous cell carcinoma of head and neck) Chemotherapy regimen: docetaxel 60 mg/m² intravenously (i.v.), carboplatin 300 mg/m² i.v., and 5‐FU (5‐fluorouracil) 600 mg/m² i.v. Country: India (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant + palonosetron + dexamethasone (PDA) Day 1: p.o. aprepitant 125 mg + palonosetron 0.25 mg i.v. + dexamethasone 12 mg i.v. Days 2 to 3: capsule aprepitant 80 mg o.d. + tablet dexamethasone 8 mg b.d. Control: arm B: ondansetron + dexamethasone (OD) Day 1: ondansetron 16 mg i.v. + dexamethasone 12 mg i.v. + ondansetron 8 mg b.d. (after chemotherapy) Days 2 to 3: ondansetron 8 mg b.d. + dexamethasone 8 mg b.d. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | Comment: the result of safety analysis was not reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kim 2015.
| Study characteristics | ||
| Methods |
Randomised, prospective, phase 3 trial with 2 arms
Enrolment period: June 2011 to September 2012
Masking: single‐blind (participant) Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 58.9 ± 10.4 in ramosetron group, 59.0 ± 11.6 in ondansetron group Gender: male + female Tumour/cancer type: solid tumour (lung cancer, lymphoma, stomach cancer, head and neck cancer, breast cancer, oesophagus cancer, hepatobiliary and pancreas cancer, other) Chemotherapy regimen: highly emetogenic chemotherapeutic agents (NCCN Guideline v1.0 2011 antiemesis) Country: Korea (17 institutions, multi‐centre) |
|
| Interventions |
Experimental: arm A: ramosetron Day 1: ramosetron 0.3 mg i.v. + aprepitant 125 mg p.o. + dexamethasone 12 mg p.o. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 8 mg p.o. Day 4: dexamethasone 8 mg p.o. Experimental: arm B: ondansetron Day 1: ondansetron 16 mg i.v. + aprepitant 125 mg p.o. + dexamethasone 12 mg p.o. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 8 mg p.o. Day 4: dexamethasone 8 mg p.o. |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to the RAD or OAD groups (1:1 ratio) according to a stratified block randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... single‐blind ..." Comment: patients were blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... single‐blind ..." Comment: only patients were blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: although patients were blinded, unblinded personnel might have an influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although this was a single‐blind study, both patients and personnel had no influence on objective outcomes (e.g. study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "... an m‐ITTpopulation of 299 was subjected to the efficacy analysis ..." |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "three deaths of OAD patients during the study were considered unrelated to the medication" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kim 2017.
| Study characteristics | ||
| Methods |
Randomised, active‐comparator, phase 4 trial (MK‐0869‐225) with 2 arms
Study period: 2012 December 28 to 4 August 2014
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD (range), years: 59.7 ±11.4 (23 to 84) in aprepitant group, 60.9 ± 11.5 (28 to 85) in control group Gender: male (263) + female (217) Tumour/cancer type: solid malignancy (gastrointestinal, lung, gynaecological, other) Chemotherapy regimen: carboplatin, oxaliplatin, irinotecan‐based Country: South Korea (multi‐centre, 20 sites) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg capsule p.o. + ondansetron 16 mg i.v. + dexamethasone 12 mg p.o. Days 2 to 3: aprepitant 80 mg capsule p.o. + placebo for ondansetron 8 mg p.o. twice daily (b.i.d.) Control: arm B Day 1: aprepitant placebo capsule p.o. + ondansetron 16 mg i.v. + dexamethasone 20 mg p.o. Days 2 to 3: aprepitant placebo capsule p.o. + ondansetron 8 mg p.o. b.i.d. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
Exploratory endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible patients were randomised (1:1) to receive either a 3‐day aprepitant or control regimen" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia, hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "of the 494 randomized subjects, 480 were included in the modified intent‐to‐treat population" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the all‐patients‐as treated (APaT) population was used for safety analyses" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kimura 2015.
| Study characteristics | ||
| Methods |
Randomised, cross‐over trial with 2 arms
Enrolment period: 1 April 2011 to 31 March 2013
Masking: single‐blind Baseline patient characteristics: reported Follow‐up: patients were followed up for 10 days during each course for efficacy and safety endpoints |
|
| Participants |
Inclusion criteria
Exclusion criteria
Age (range), years: 36.1 (15 to 65) in palonosetron arm, 50.6 (18 to 70) in granisetron arm Gender: male + female Tumour/cancer type: osteosarcoma, malignant fibrous histiocytoma, synovial sarcoma, leiomyosarcoma, rhabdomyosarcoma, dedifferentiated liposarcoma, myxoid liposarcoma, clear cell sarcoma Chemotherapy regimen: cisplatin + doxorubicin, ifosfamide + doxorubicin/ifosfamide + etoposide Country: Japan |
|
| Interventions |
Cross‐over study Experimental: arm A: palonosetron Day 1: p.o. 125 mg aprepitant + i.v. 0.75 mg palonosetron + i.v. 6.6 mg dexamethasone Days 2 to 5: 80 mg aprepitant + 6.6 mg dexamethasone Experimental: arm B: granisetron Day 1: p.o. 125 mg aprepitant + i.v. 3 mg × 2 granisetron + i.v. 6.6 mg dexamethasone Days 2 to 5: 80 mg aprepitant + 3 mg × 2 granisetron + 6.6 mg dexamethasone |
|
| Outcomes |
Primary endpoints
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a single randomization method was used to assign eligible patients to the palonosetron or granisetron arm" |
| Allocation concealment (selection bias) | Unclear risk | Commen: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "all participants were blinded to the antiemetic treatment assignments for the duration of the study" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: only patients were blinded to the study intervention |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: although patients were blinded, unblinded personnel might have an influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although this was a single‐blind study, we assume that both patients and personnel had no influence on objective outcomes (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "all patients were eligible for efficacy analysis ..." |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "safety was assessed for all patients who received treatment" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kitayama 2015.
| Study characteristics | ||
| Methods |
Randomised, cross‐over study with 2 arms
Study period: April 2013 to November 2014
Masking: single‐blind Baseline patient characteristics: yes Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
All patients provided written informed consent before entering the study Mean/median age, years: 80% ≥ 50 years Gender: 37% male Tumour/cancer type: colorectal, breast, other Chemotherapy regimen: MEC (oxaliplatin base, irinotecan base, other) Country: n.r. |
|
| Interventions |
Experimental: arm A: fosaprepitant Day 1: fosaprepitant 150 mg + granisetron 3 mg + dexamethasone 4.95 mg Experimental: arm B: palonosetron Day 1: i.v. palonosetron 0.75 mg + dexamethasone 9.9 mg |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: "... minimization method ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: single‐blind |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: single‐blind only |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: single‐blind study; knowledge of treatment may have affected outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: single‐blind study; we assume that knowledge of treatment would not influence objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the total of 35 patients and 70 therapies was available for analysis" and "we analyzed the per‐protocol cohort including all patients who received the study medication and completed the follow‐up period" Comment: 2 participants did not start study treatment; 2 withdrew because they could not complete chemotherapy |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: adverse events reported for all patients who completed chemotherapy and study treatment |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes only for the overall phase have been reported, as no significant difference was found in any other evaluation points |
| Other bias | Unclear risk | Comment: participants did not record the incidence and severity of CINV daily, but only on Days 2 and 5 |
Koizumi 2003.
| Study characteristics | ||
| Methods |
Randomised, cross‐over trial with 2 arms
Patient hospitalisation period: March 1998 to June 1999
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: recorded daily for 7 days after the start of chemotherapy |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 61.2 (26 to 81) in granisetron‐ramosetron group, 58.7 (33 to 77) in ramosetron‐granisetron group Gender: male + female Tumour/cancer type: solid tumour (gastric cancer, oesophageal cancer) Chemotherapy regimen: 2 courses of combined chemotherapy (including ≥ 60 mg/m² cisplatin) Country: Japan |
|
| Interventions |
Cross‐over study Experimental: arm A: granisetron‐ramosetron granisetron 3 mg i.v. during treatment phase 1, followed by ramosetron 0.3 mg i.v. during treatment phase 2 + methylprednisolone sodium (Solumedrol) 250 mg i.v. immediately before and 6 h after chemotherapy Experimental: arm B: ramosetron‐granisetron ramosetron 0.3 mg i.v. during treatment phase 1, followed by granisetron 3 mg i.v. during treatment phase 2 + methylprednisolone sodium (Solumedrol) 250 mg i.v. immediately before and 6 h after chemotherapy |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "the study drug code was sealed, preserved at the registration center and not opened until all evaluations had been finalized, after the completion of treatment phase 2" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: "clinical response was evaluated in the remaining 30 patients" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all included patients recorded adverse events |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Kusagaya 2015.
| Study characteristics | ||
| Methods |
Randomised, controlled, prospective, parallel‐group trial with 2 arms
Enrolment period: April 2013 to February 2015
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 70 (57 to 90) in aprepitant group, 73 (43 to 84) in control group Gender: male (57) + female (23) Tumour/cancer type: non‐small cell lung cancer Chemotherapy regimen: carboplatin + paclitaxel, carboplatin + paclitaxel + bevacizumab, carboplatin + pemetrexed, carboplatin + pemetrexed + bevacizumab, carboplatin + S‐1 Country: Japan (multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg + palonosetron 0.75 mg + dexamethasone 8 mg Days 2 to 3: aprepitant 80 mg + dexamethasone 8 mg Control: arm B Day 1: palonosetron 0.75 mg + dexamethasone 8 mg Days 2 to 3: dexamethasone 8 mg |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed centrally by computer software and stratified by sex, age, and non‐platinum chemotherapy agent" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although this was an open‐label study, both patients and personnel had no influence on objective outcomes (e.g. neutropenia, hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "one patient withdrew consent before chemotherapy, and 80 patients (41 in the aprepitant group and 39 in the control group) were assessed for efficacy and safety" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "one patient withdrew consent before chemotherapy, and 80 patients (41 in the aprepitant group and 39 in the control group) were assessed for efficacy and safety" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Lee 1997.
| Study characteristics | ||
| Methods |
Randomised, cross‐over trial with 2 arms
Recruitment period: n.r.
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 61 (32 to 77) in arm A, 58.5 (30 to 71) in arm B Gender: male (29) + female (10) Tumour/cancer type: solid tumour (lung cancer, head and neck tumour, unknown tumour, oesophageal cancer, gastric cancer, malignant lymphoma) Chemotherapy regimen: cisplatin (≥ 50 mg/m²), ifosfamide (≥ 3000 mg/m²), mitomycin (6 mg/m²), fluorouracil (1000 mg/m²), etoposide (80 mg/m²) Country: Korea (multi‐centre) |
|
| Interventions |
Cross‐over study Experimental: arm A first ondansetron + dexamethasone, then tropisetron + dexamethasone Experimental: arm B first tropisetron + dexamethasone, then ondansetron + dexamethasone |
|
| Outcomes | not readable | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not evaluable due to language barrier |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: for objective outcomes, we assume that not blinding participants and personnel would not influence risk of bias |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: not evaluable because of language barrier |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Unclear risk | Comment: not evaluable because of language barrier |
| Selective reporting (reporting bias) | Unclear risk | Comment: not evaluable because of language barrier |
| Other bias | Unclear risk | Comment: not evaluable because of language barrier |
Li 2019.
| Study characteristics | ||
| Methods |
Randomised, prospective study with 2 arms
Recruitment period: June 2015 to February 2018
Masking: n.r. Baseline patient characteristics: reported Follow‐up: patients were followed on Days 6 to 8 and on Days 19 to 21 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 51.74 ± 7.082 in aprepitant group, 47.46 ± 8.180 in standard group Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: anthracycline (30 mg/m²/d for pirarubicin or 45 mg/m²/d for epirubicin) and cyclophosphamide Country: Mongolia, China (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. + tropisetron 5 mg i.v. + dexamethasone 6 mg p.o. Day 2: aprepitant 80 mg p.o. + tropisetron 5 mg i.v. + dexamethasone 3.75 mg p.o. Day 3: aprepitant 80 mg p.o. + dexamethasone 3.75 mg p.o. Day 4: dexamethasone 3.75 mg p.o. Standard: arm B Day 1: tropisetron 5 mg i.v. + dexamethasone 10.5 mg p.o. Day 2: tropisetron 5 mg i.v. + dexamethasone 7.5 mg p.o. Days 3 to 4: dexamethasone 7.5 mg p.o. |
|
| Outcomes |
Primary endpoints
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised study but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: blinding not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: blinding was not reported; therefore it is possible that knowledge of allocated treatment could have posed a risk of bias |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: not reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Maehara 2015.
| Study characteristics | ||
| Methods |
Randomised, parallel‐design study with 2 arms
Enrolment period: November 2010 to October 2012
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 54.5 ± 11.9 in control group, 62.6 ± 12.7 in aprepitant group Gender: n.r. Tumour/cancer type: ovarian cancer, endometrial cancer Chemotherapy regimen: paclitaxel and carboplatin (TC) Country: Japan (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. + first‐generation 5‐HT₃ antagonist 3 mg + dexamethasone 8 or 16 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 8 or 4 mg p.o. Control: arm B Day 1: first‐generation 5‐HT₃ antagonist 3 mg + dexamethasone 8 or 4 mg i.v. Days 2 to 3: dexamethasone 8 or 16 mg p.o. |
|
| Outcomes |
Primary outcome
Secondary outcome(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "twenty‐three eligible patients were divided randomly into two groups (A and B) using sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all 23 patients were included in patient‐reported efficacy analysis |
| Selective reporting (reporting bias) | High risk | Quote: "safety was evaluated using general laboratory tests" Comment: no results regarding the safety analysis were reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Mahrous 2020.
| Study characteristics | ||
| Methods |
Randomised, open‐label study
Study period: n.r.
Masking: open‐label Baseline patient characteristics: n.r. Median follow‐up: n.r. ITT analysis: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: n.r. Gender: n.r. Tumour/cancer type: n.r. Chemotherapy regimen: cisplatin‐based, or combination of cyclophosphamide and anthracyclines Country: Egypt |
|
| Interventions |
Experimental: arm A: granisetron with dexamethasone Experimental: arm B: palonosetron with dexamethasone |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures: adverse events |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: outcome assessors (participants) not blinded to intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: outcome robust to blinding |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: conference abstract, not fully evaluable |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Unclear risk | Comment: conference abstract, not fully evaluable |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract, not fully evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not fully evaluable |
Matsuda 2014.
| Study characteristics | ||
| Methods |
Randomised study with 2 arms
Enrolment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: n.r. Chemotherapy regimen: n.r. Country: Japan |
|
| Interventions |
Experimental: arm A: aprepitant aprepitant + palonosetron + dexamethasone Control: arm B palonosetron + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: blinding not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: blinding not reported; however, this should not affect objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | All patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: not reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Matsumoto 2020.
| Study characteristics | ||
| Methods |
Randomised trial with 2 arms
Enrolment period: December 2012 to October 2014
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 54 (27 to 82) in granisetron group, 54 (30 to 74) in palonosetron group Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: anthracycline plus cyclophosphamide (AC) regimen Country: Japan |
|
| Interventions |
Experimental: arm A: palonosetron fosaprepitant + palonosetron 0.75 mg + dexamethasone Experimental: arm B: granisetron fosaprepitant + granisetron 1 mg + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised study but method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "concealment: central registration" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: 326 patients were evaluable |
| Selective reporting (reporting bias) | High risk | Comment: safety was planned but not reported; complete response for nausea in acute and overall phases not reported |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Mattiuzzi 2007.
| Study characteristics | ||
| Methods |
Randomised, phase 2 trial with 3 arms
Recruitment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age (range), years: n.r. Gender: n.r. Tumour/cancer type: acute myeloid leukemia, myelodysplastic syndrome Chemotherapy regimen: MD‐HD‐CHEMO with HDAC‐containing regimens Country: n.r. |
|
| Interventions |
Experimental: arm A: ONDA 1 to 5 ONDA 8 mg i.v. bolus, then 24 mg i.v., continuous infusion on Day 1 through Day 5 and for 12 h after Ara C infusion ends Experimental: arm B: PALO 1 to 5 PALO 0.25 mg i.v. bolus over 30 seconds on Day 1 through Day 5 of Ara C infusion Experimental: arm C: PALO 1, 3, 5 PALO 0.25 mg i.v. bolus over 30 seconds on Days 1, 3, and 5 of Ara C infusion All patients received Solumedrol 40 mg i.v. before Ara C |
|
| Outcomes |
Primary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "open randomized comparative trial" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "open randomized comparative trial" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: 95 patients were evaluable |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Miyabayashi 2015.
| Study characteristics | ||
| Methods |
Randomised, prospective, phase 2 study with 2 arms
Study period: January 2011 to April 2014
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: lung cancer Chemotherapy regimen: MEC Country: n.r. |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. + palonosetron 0.75 mg i.v. + dexamethasone 4.95 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 4 mg p.o. Experimental: arm B Day 1: granisetron 3 mg i.v. + dexamethasone 9.9 mg i.v. Days 2 to 3: dexamethasone 8 mg p.o. |
|
| Outcomes |
Primary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract only |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Mohammed 2019.
| Study characteristics | ||
| Methods |
Randomised, parallel‐design study with 2 arms
Enrolment period: January to December 2015
Masking: single‐blind Baseline patient characteristics: sex and age reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (SD), years: 47.8 (14.4) Gender: 63.5% female, 36.5% male Tumour/cancer type: Hodgkin lymphoma Chemotherapy regimen: Adriamycin, Bleomycin, Vinblastine, Dacarbazine Country: Iraq (single centre) |
|
| Interventions |
Experimental: arm A oral aprepitant on Days 1 to 3 (Day 1, 125 mg 1 h before chemotherapy; Days 2 to 3, 80 mg), ondansetron on Day 1 (Day 1, 32 mg i.v. infusion over 15 min at 30 to 60 min before chemotherapy), oral dexamethasone on Days 1 to 4 (Day 1, 12 mg 30 min before chemotherapy; Days 2 to 4, 8 mg in the morning) Control: arm B ondansetron on Days 1 to 4 (Day 1, 32 mg i.v. infusion over 15 min at 30 to 60 min before chemotherapy; Days 2 to 4, 8 mg p.o. twice daily), p.o. dexamethasone on Days 1 to 4 (Day 1, 20 mg 20 min before chemotherapy; Days 2 to 4, 8 mg twice daily) |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated, random allocation schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "computer‐generated, random allocation schedule" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: single‐blinded; participants not aware of assigned treatment |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "the selection of both groups was known by the researcher after taking the agreement of physician responsible for patients' treatment" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Outcome assessors (participants) blinded to intervention |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: mean scores reported, but unclear whether data on all participants were included in analysis |
| Selective reporting (reporting bias) | Low risk | Comment: no reasons for any concerns detected |
| Other bias | Low risk | Comment: no reasons for any concerns detected |
Nakamura 2012.
| Study characteristics | ||
| Methods |
Randomised, cross‐over trial with 2 arms
Recruitment period: March 2009 to April 2010
Masking: single‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria:
Exclusion criteria
Median age (range), years: 51 (36 to 73) in azasetron first group, 50 (42 to 68) in granisetron NK first group Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: FEC100 (5‐FU (500 mg/m²), epirubicin hydrochloride (100 mg/m²), and cyclophosphamide hydrate (500 mg/m²) on Day 1, every 3 weeks) Country: Japan (single centre) |
|
| Interventions |
Cross‐over study Experimental: arm A: azasetron first azasetron 10 mg mixed with dexamethasone and intravenously infused over 30 min before administration of FEC100 regimen granisetron hydrochloride 2 mg and dexamethasone 8 mg p.o. administered for 3 days after FEC100 regimen Experimental: arm B: granisetron NK first granisetron NK 3 mg mixed with dexamethasone and intravenously infused over 30 min before administration of FEC100 regimen granisetron hydrochloride 2 mg and dexamethasone 8 mg p.o. administered for 3 days after FEC100 regimen |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... single‐blind ..." Comment: patients were blinded |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... single‐blind ..." Comment: personnel were not blinded |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: although patients were blinded, unblinded personnel might have had an influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although personnel were not blinded, we assume that this had no effect on objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included in analysis of adverse events |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
NCT01640340.
| Study characteristics | ||
| Methods |
Randomised study with 2 arms
Study period: January 2011 to August 2011
Masking: open‐label Baseline patient characteristics: yes Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: female Tumour/cancer type: malignant neoplasm Chemotherapy regimen: HEC Country: United States (single centre) |
|
| Interventions |
Experimental: arm A: palonosetron palonosetron 0.25 mg Day 1; aprepitant 125 mg Day 1, 80 mg Days 2 to 3; dexamethasone 12 mg Day 1, 8 mg Days 2 to 4 Experimental: arm B: ondansetron ondansetron 24 mg Day 1; aprepitant 125 mg Day 1, 80 mg Days 2 to 3; dexamethasone 12 mg Day 1, 8 mg Days 2 to 4 |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the study results tab |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Nishimura 2015.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group study with 2 arms
Study period: April 2011 to March 2014
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age, years: 64.1 in aprepitant group, 64.2 in control group Gender: male (252) + female (161) Tumour/cancer type: colorectal cancer Chemotherapy regimen: oxaliplatin‐based chemotherapy (FOLFOX, XELOX, or SOX regimen including oxaliplatin at ≥ 85 mg/m²) Country: 25 hospitals in Japan (multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant or fosaprepitant Day 1: p.o. aprepitant 125 mg + i.v. 5‐HT₃ receptor antagonist + dexamethasone 6.6 mg Days 2 to 3: aprepitant 80 mg + p.o. dexamethasone 2 mg twice daily Day 1: i.v. fosaprepitant 150 mg + 5‐HT₃ receptor antagonist + dexamethasone 6.6 mg Day 2: p.o. dexamethasone 2 mg twice daily Day 3: p.o. dexamethasone 4 mg twice daily Control: arm B Day 1: i.v. 5‐HT₃ receptor antagonist + dexamethasone 9.9 mg Days 2 to 3: p.o. dexamethasone (4 mg) twice daily |
|
| Outcomes |
Primary outcome
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the allocation of patients was performed using the minimisation method with age, sex, chemotherapy‐naive/non‐naive status, regimen (FOLFOX, XELOX or SOX) and study centre as the adjustment factors" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although this was an open‐label study, both patients and personnel had no influence on objective outcomes (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all 413 patients have been included in the full analysis set (Fig. 1) |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "tolerability analyses included 398 patients who received oxaliplatin‐based chemotherapy in the first cycle" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ohzawa 2015.
| Study characteristics | ||
| Methods |
Prospective, stratified, randomised, cross‐over, comparative trial with 2 arms
Recruitment period: n.r.
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Median age (range), years: 53 (35 to 75) in palonosetron‐first group, 53 (40 to 71) in granisetron‐first group Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen AC treatment, adriamycin (60 mg/m²) and cyclophosphamide (600 mg/m²) EC treatment, epirubicin (90 mg/m²) and cyclophosphamide (600 mg/m²) FEC treatment, 5‐fluorouracil (500 mg/m²), epirubicin (90 mg/m²), and cyclophosphamide (500 mg/m²) Country: Japan (single centre) |
|
| Interventions |
Cross‐over study Experimental: arm A: palonosetron‐first Day 1: aprepitant p.o. 125 mg + palonosetron i.v. 0.75 mg + dexamethasone i.v. 13.2 mg Days 2 to 3: aprepitant p.o. 80 mg + dexamethasone p.o. 8 mg Day 4: dexamethasone p.o. 8 mg Experimental: arm A: granisetron‐first Day 1: aprepitant p.o. 125 mg + granisetron i.v. 3 mg + dexamethasone i.v. 13.2 mg Days 2 to 3: aprepitant p.o. 80 mg + dexamethasone p.o. 8 mg Day 4: dexamethasone p.o. 8 mg |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... stratified randomization ...", "... using a table of random numbers ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... non‑blinded ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... non‑blinded ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were described in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ozaki 2013.
| Study characteristics | ||
| Methods |
Randomised, phase 2 study with 2 arms
Enrolment period: August 2011 to March 2013
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: colorectal cancer Chemotherapy regimen: standard MEC regimen Country: Japan (multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant aprepitant + palonosetron + dexamethasone Control: arm B palonosetron + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: blinding not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: blinding not reported |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | High risk | Quote: "fifteen patients were excluded because of insufficient diary and protocol violation. Finally, we analyzed 45 patients (26 male, 19 female) for this study" Comment: the percentage of excluded patients was 25% of the initial included population |
| Selective reporting (reporting bias) | Unclear risk | Comment: only complete response was reported in detail; other secondary outcomes were not reported with statistical figures, as no significant differences were observed between the 2 groups. Conference abstract only, not evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Poli‐Bigelli 2003.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, phase 3, placebo‐controlled trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD (range), years: 54 ± 13 (18 to 82) in aprepitant regimen group, 53 ± 14 (18 to 81) in standard therapy regimen group Gender: male + female Tumour/cancer type: solid tumours (respiratory, urogenital, eyes/ears/nose/throat, other) Chemotherapy regimen: chemotherapy regimen that included cisplatin 70 mg/m² Country: Argentina, Brazil, Chile, Colombia, Guatemala, Mexico, Peru, Venezuela (18 centres) |
|
| Interventions |
Experimental: arm A: aprepitant 125/80 mg Day 1: p.o. aprepitant 125 mg + i.v. ondansetron 32 mg + p.o. dexamethasone 12 mg Days 2 to 3: p.o. aprepitant 80 mg + p.o. dexamethasone 8 mg Day 4: p.o. dexamethasone 8 mg Standard: arm B Day 1: i.v. ondansetron 32 mg + p.o. dexamethasone 20 mg Days 2 to 4: p.o. dexamethasone 8 mg twice daily |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... computer‐generated randomization ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: a modified intent‐to‐treat approach was used to analyse efficacy data |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Raftopoulos 2015.
| Study characteristics | ||
| Methods |
Randomised, prospective, parallel‐group, phase 3 trial with 3 arms
Study period: June 2006 to February 2009
Masking: double‐blind (participant, investigator) Baseline patient characteristics: n.r. Follow‐up: after completion of study treatment, patients are followed at approximately 30 days |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean/median age (range), years: MEC: APF530 250 mg: 60.3 (SD 12.5); APF530 500 mg: 59.1 (SD 13.3); palonosetron 0.25 mg: 60.4 (12.8); HEC: APF530 250 mg: 53.0 (SD 12.7); APF530 500 mg: 52.8 (SD 11.9); palonosetron 0.25 mg: 54.5 (SD 12.8) Gender: male + female Tumour/cancer type: solid tumour (lung cancer, breast cancer, ovarian cancer, lymphoma) Chemotherapy regimen: cyclophosphamide + doxorubicin or epirubicin (78% of MEC participants and 75% of HEC participants) Country: United States (52 study locations, multi‐centre) |
|
| Interventions |
Active comparator: arm A Day 1 of chemotherapy course 1: palonosetron hydrochloride i.v. + placebo subcutaneously (SC) + dexamethasone i.v. patients in high‐risk (level 5) stratum also receive oral dexamethasone on Days 2 to 4 of all treatment courses Experimental: arm B Day 1 of chemotherapy course 1: APF530 SC + placebo i.v. + dexamethasone i.v. Day 1 of chemotherapy courses 2 to 4: APF530 SC + dexamethasone i.v. patients in high‐risk (level 5) stratum also receive oral dexamethasone as in arm A Experimental: arm C Day 1 of chemotherapy course 1: APF530 SC at higher dose + placebo i.v. + dexamethasone i.v. Day 1 of chemotherapy courses 2 to 4: APF530 SC (at same higher dose) + dexamethasone i.v. patients in high‐risk (level 5) stratum also receive oral dexamethasone as in arm A |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized 1:1:1 to ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. injection site reaction) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "efficacy analyses were performed separately for ASCO‐derived MEC and HEC strata and were based on a modified intent‐to‐treat (mITT) population" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety population in this reanalysis comprised all 1395 patients who were randomized and received study drug ..." |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Rapoport 2010.
| Study characteristics | ||
| Methods |
Randomised, prospective, parallel‐group, controlled trial with 2 arms
Study period: January 2007 to December 2008
Masking: double‐blind (participant, investigator) Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 57.1 ± 11.8 in aprepitant group, 55.9 ± 12.6 in control group Gender: male (196) + female (652) Tumour/cancer type: solid malignancy (breast cancer, colorectal cancer, lung cancer, ovarian cancer) Chemotherapy regimen: non‐AC (anthracycline (doxorubicin and epirubicin) and cyclophosphamide), AC (anthracycline (doxorubicin and epirubicin) and cyclophosphamide) Countries: USA, Mexico, Canada, Chile, Brazil, Peru, Colombia, Panama, Hong Kong, Australia, South Africa, France, Germany, Israel, Russia |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. 1 h before chemotherapy + ondansetron 8 mg p.o. 30 to 60 min before chemotherapy; 8 mg p.o. 8 h after first dose + dexamethasone 12 mg p.o. 30 min before chemotherapy Days 2 to 3: aprepitant 80 mg p.o. + ondansetron placebo p.o. b.i.d. Control: arm B: placebo Day 1: aprepitant placebo p.o. 1 h before chemotherapy + ondansetron 8 mg p.o. 30 to 60 min before chemotherapy; 8 mg p.o. 8 h after first dose + dexamethasone 20 mg p.o. 30 min before chemotherapy Days 2 to 3: aprepitant placebo p.o. + ondansetron 8 mg p.o. b.i.d. |
|
| Outcomes |
Primary outcome measure
Secondary outcome measure
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... computer‐generated, random, blinded allocation schedule ..." |
| Allocation concealment (selection bias) | Low risk | Comment: allocation was blinded |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: double‐blind (participant, investigator) |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: double‐blind (participant, investigator) |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the analysis of efficacy was based on the full analysis set population, which included those patients who received MEC, took a dose of study drug, and completed at least one posttreatment efficacy assessment" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "all patients who received at least one dose of study drug were included in the safety analyses" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Rapoport 2015 (a).
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, placebo‐controlled, dose‐ranging,phase 2 trial with 5 arms
Study period: September 2006 to March 2008
Masking: double‐blind (participant, investigator) Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 55 (22 to 86) in rolapitant 9‐mg group, 53 (26 to 76) in rolapitant 22.5‐mg group, 57 (19 to 79) in rolapitant 90‐mg group, 56 (20 to 75) in rolapitant 180‐mg group, 54 (18 to 77) in control group Gender: male (244) + female (210) Tumor/cancer type: n.r. Chemotherapy regimen: HEC (≥ 70 mg/m² cisplatin‐based chemotherapy) Country: 75 sites in 21 countries |
|
| Interventions |
Experimental: arm A: rolapitant 9 mg Day 1: rolapitant 9 mg administered approximately 2 h before first dose of chemotherapeutic agent on Day 1 of Cycle 1 + i.v. ondansetron 32 mg 0.5 h before initiation of chemotherapy + oral dexamethasone 20 mg 0.5 h before initiation of chemotherapy Days 2 to 4: dexamethasone 8 mg twice daily Experimental: arm B: rolapitant 22.5 mg Day 1: rolapitant 22.5 mg administered approximately 2 h before first dose of chemotherapeutic agent on Day 1 of Cycle 1 + i.v. ondansetron 32 mg 0.5 h before initiation of chemotherapy + oral dexamethasone 20 mg 0.5 h before initiation of chemotherapy Days 2 to 4: dexamethasone 8 mg twice daily Experimental: arm C: rolapitant 90 mg Day 1: rolapitant 90 mg administered approximately 2 h before first dose of chemotherapeutic agent on Day 1 of Cycle 1 + i.v. ondansetron 32 mg 0.5 h before initiation of chemotherapy + oral dexamethasone 20 mg 0.5 h before initiation of chemotherapy Days 2 to 4: dexamethasone 8 mg twice daily Experimental: arm D: rolapitant 180 mg Day 1: rolapitant 180 mg administered approximately 2 h before first dose of chemotherapeutic agent on Day 1 of Cycle 1 + i.v. ondansetron 32 mg 0.5 h before initiation of chemotherapy + oral dexamethasone 20 mg 0.5 h before initiation of chemotherapy Days 2 to 4: dexamethasone 8 mg twice daily Control: arm E Day 1: placebo + i.v. ondansetron 32 mg 0.5 h before initiation of chemotherapy + oral dexamethasone 20 mg 0.5 h before initiation of chemotherapy Days 2 to 4: dexamethasone 8 mg twice daily |
|
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: double‐blind (participant, investigator) |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: double‐blind (participant, investigator) |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. febrile neutropenia, neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included for the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included for the safety analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Rapoport 2015 (b).
| Study characteristics | ||
| Methods |
Randomised, active‐controlled, parallel‐group, phase 3 trial with 2 arms
Study period: February 2012 to May 2014
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 57.0 ± 10.08 in rolapitant group, 57.7 ± 11.15 in placebo group Gender: male (304) + female (222) Tumour/cancer type: solid tumour (breast, colon or rectum, head and neck, lung, ovary, stomach, and other tumours) Chemotherapy regimen: cisplatin‐based chemotherapy (≥ 60 mg/m²) Country: United States (multi‐centre) |
|
| Interventions |
Experimental: arm A: rolapitant Day 1: oral dose of rolapitant 180 mg (equivalent to 200 mg rolapitant hydrochloride monohydrate) 1 to 2 h before administration of chemotherapy + granisetron (10 µg/kg i.v.) + dexamethasone (20 mg p.o.) Days 2 to 4: dexamethasone (8 mg p.o.) to be administered orally b.i.d. Control: arm B Day 1: placebo + granisetron (10 µg/kg i.v.) + dexamethasone (20 mg p.o.) Days 2 to 4: dexamethasone (8 mg p.o.) to be administered orally b.i.d. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "for randomisation of patients, we used an interactive web‐based randomisation system (IWRS) at cycle 1" |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent group not involved with study implementation created a randomisation schedule for study drug labelling" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: double‐blind (participant, investigator) |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: double‐blind (participant, investigator) |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. febrile neutropenia, neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: analysis included modified ITT population |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety population included all patients who were randomly allocated to a treatment group and who received at least one dose of study drug" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Rapoport 2015 (c).
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, active‐controlled, phase 3 trial with 2 arms
Study period: February 2012 to March 2014
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 58.5 ± 10.05 in rolapitant group, 58.5 ± 9.25 in placebo group Gender: male (369) + female (175) Tumour/cancer type: solid tumour (breast, colon or rectum, head and neck, lung, ovary, stomach, and other tumours) Chemotherapy regimen: cisplatin‐based chemotherapy (≥ 60 mg/m²) Country: United States (multi‐centre) |
|
| Interventions |
Experimental: arm A: rolapitant Day 1: oral dose of rolapitant 180 mg (equivalent to 200 mg rolapitant hydrochloride monohydrate) 1 to 2 h before administration of chemotherapy + granisetron (10 µg/kg i.v.) + dexamethasone (20 mg p.o.) Days 2 to 4: dexamethasone (8 mg p.o.) to be administered orally b.i.d. Control: arm B Day 1: placebo + granisetron (10 µg/kg i.v.) + dexamethasone (20 mg p.o.) Days 2 to 4: dexamethasone (8 mg p.o.) to be administered orally b.i.d. |
|
| Outcomes |
Primary endpoints
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "for randomisation of patients, we used an interactive web‐based randomisation system (IWRS) at cycle 1" |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent group not involved with study implementation created a randomisation schedule for study drug labelling" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "double‐blind (participant, investigator)" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "double‐blind (participant, investigator)" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention; therefore they probably had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. febrile neutropenia, neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: analysis included modified ITT population |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety population included all patients who were randomly allocated to a treatment group and who received at least one dose of study drug" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Roila 1995.
| Study characteristics | ||
| Methods |
Double‐blind, multi‐centre, randomised trial with 2 arms
Recruitment period: December 1992 to July 1994
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age (range), years: 61 Gender: male + female Tumour/cancer type: solid tumours Chemotherapy regimen: cisplatin at doses ≥ 50 mg/m², used alone or in combination with other antineoplastic agents Country: Italy (multi‐centre) |
|
| Interventions |
Experimental: arm A: ondansetron ondansetron 8 mg i.v. diluted in 50 mL normal saline and administered 15 min, 30 min before chemotherapy + dexamethasone 20 mg i.v. added to 5‐HT₃ antagonist and administered 15 min, 45 min before chemotherapy Experimental: arm B: granisetron granisetron 3 mg i.v. diluted in 50 mL normal saline and administered 15 min, 30 min before chemotherapy + dexamethasone 20 mg i.v. added to 5‐HT₃ antagonist and administered 15 min, 45 min before chemotherapy |
|
| Outcomes |
Primary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a centralized list of computer‐generated random permuted blocks of 10 patients for each centre ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: double‐blind study |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: double‐blind study |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "a total of 973 patients took part in the study and 966 of them were evaluated for efficacy according to the intention‐to‐treat principle" Comment: some patients missing without reasons provided; however, we judged this number to be small |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: 967 participants were included for adverse events assessment; this number represents nearly the entire enrolled cohort |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Rugo 2017.
| Study characteristics | ||
| Methods |
Randomised, active‐controlled, parallel‐group, phase 3 trial with 2 arms
Study period: April 2011 to November 2012
Masking: quadruple‐blind (participant, care provider, investigator, outcomes assessor) Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
The following inclusion criteria must be checked before inclusion at each cycle of the multiple‐cycle extension
Exclusion criteria
The following exclusion criteria must be checked before inclusion at each cycle of the multiple‐cycle extension
Mean age (SD), years: 53.7 (10.66) in netupitant + palonosetron group, 54.1 (10.65) in palonosetron group Gender: male + female Tumour/cancer type: breast cancer Chemotherapy regimen: anthracycline and cyclophosphamide‐containing chemotherapy Country: United States (28), Argentina (9), Belarus (6), Brazil (12), Bulgaria (12), Croatia (9), Germany (11), Hungary (8), India (13), Italy (5), Mexico (5), Poland (10), Romania (10), Russian Federation (22), Ukraine (12) (172 centres) |
|
| Interventions |
Experimental: arm A: NEPA + dexamethasone Day 1: oral netupitant/palonosetron (300 mg/0.50 mg) hard capsule with oral dexamethasone before each scheduled chemotherapy cycle Experimental: arm B: palonosetron + dexamethasone Day 1: oral palonosetron 0.50 mg (Aloxi) with oral dexamethasone before each scheduled chemotherapy cycle |
|
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "quadruple (participant, care provider, investigator, outcomes assessor)" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "quadruple (participant, care provider, investigator, outcomes assessor)" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: patients, personnel, investigator, and outcome assessor were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all randomised patients were included in the ITT population |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Ruhlmann 2017.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, phase 3 study with 2 arms
Recruitment period: June 2010 to March 2015
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years (median, range): fosaprepitant group: 48 (25 to 74); placebo group: 47 (26 to 77) Gender: female Tumour/cancer type: cervical cancer Chemotherapy regimen: cisplatin 40 mg/m² Country: 8 centres in 4 countries (Germany, Australia, Norway, Denmark) |
|
| Interventions |
Experimental: arm A: fosaprepitant fosaprepitant + palonosetron + dexamethasone Experimental: arm B: placebo placebo + palonosetron + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation numbers were generated by an independent research staff member using a web randomisation sequence generator" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the blocks, in sealed envelopes, were provided to the pharmacists who were not masked to treatment at the study centres, and sealed blinding envelopes unique to each randomisation number were provided to the centres."; "To avoid treatments being visually distinguishable, study drug and placebo were provided in identically wrapped and labelled infusion bags of the same volume" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutrophil count decreased) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "... the 234 patients receiving study medication represented the modified intention‐to‐treat population (figure 1) and were all included in the analysis of the primary and secondary outcomes and in the safety analysis" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "... the 234 patients receiving study medication represented the modified intention‐to‐treat population (figure 1) and were all included in the analysis of the primary and secondary outcomes and in the safety analysis" |
| Selective reporting (reporting bias) | Low risk | Comment: study authors mentioned explicitly that FLIE results are not reported in this article |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Saito 2009.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, comparative, phase 3, active‐comparator trial with 2 arms
Study period: July 2006 to May 2007
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 58.4 ± 10.4 in palonosetron arm, 58 ± 10.5 in granisetron arm Gender: male + female Tumour/cancer type: solid tumour (non‐small cell lung cancer, small cell lung cancer, breast cancer, others) Chemotherapy regimen: cisplatin, doxorubicin + cyclophosphamide/epirubicin + cyclophosphamide Country: Japan (75 centres) |
|
| Interventions |
Experimental: arm A: palonosetron i.v. injection of 0.75 mg palonosetron (5 mL), then granisetron placebo before administration of highly emetogenic chemotherapy, concomitantly administered with corticosteroids Experimental: arm B granisetron i.v. injection of palonosetron placebo, then 40 μg/kg granisetron hydrochloride before administration of highly emetogenic chemotherapy, concomitantly administered with corticosteroids |
|
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was done centrally by computer ..."; "a non‐deterministic minimisation method with a stochastic‐biased coin was applied to the randomisation of patients" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the modified ITT cohort was used for the primary efficacy analysis" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "... including one death caused by bleeding from pulmonary carcinoma in the granisetron group..." |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Saito 2013.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, parallel, phase 3 trial with 2 arms
Recruitment period: August 2009 to December 2009
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 62 (26 to 86) in fosaprepitant group, 63 (25 to 85) in placebo group Gender: male (257) + female (90) Tumour/cancer type: solid tumour (respiratory, genitourinary, digestive, head and neck, other) Chemotherapy regimen: chemotherapy including cisplatin (≥ 70 mg/m²) Country: Japan (68 institutions) |
|
| Interventions |
Experimental: arm A: fosaprepitant Day 1: i.v. fosaprepitant (150 mg) + granisetron (40 μg/kg) + dexamethasone phosphate (10 mg) Day 2: dexamethasone phosphate (4 mg) Day 3: dexamethasone phosphate (8 mg) Experimental: arm B: placebo Day 1: placebo + granisetron (40 μg/kg body weight) + dexamethasone phosphate (20 mg) Days 2 to 3: dexamethasone phosphate (8 mg) |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. study mortality, neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the efficacy analysis was carried out on the modified intention‐to‐treat (ITT) population" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "therefore, 340 patients (167 in the standard arm and 173 in the fosaprepitant arm) were assessable in the modified ITT analysis, and 344 patients were included in the safety analysis" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Saito 2017.
| Study characteristics | ||
| Methods |
Randomised, comparative, interventional, parallel, phase 3 trial with 2 arms
Recruitment period: 2012 to 2015
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age (range), years: n.r. Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: anthracycline and cyclophosphamide‐containing regimens (AC) Country: Japan (11 institutions) |
|
| Interventions |
Experimental: arm A: palonosetron Day 1: aprepitant (125 mg) + palonosetron (0.75 mg) + dexamethasone (9.9 mg) Days 2 to 3: aprepitant (80 mg) Experimental: arm B: granisetron Day 1: aprepitant (125 mg) + granisetron (40 μg/kg) + dexamethasone (9.9 mg) Days 2 to 3: aprepitant (80 mg) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included for patient‐reported outcomes analysis |
| Selective reporting (reporting bias) | High risk | Comment: only complete response and quality of life were reported in the results section |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Schmitt 2014.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, phase 3 trial with 2 arms
Recruitment period: July 2005 to January 2012
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: Days 5 to 7 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 58.3 (27 to 72) in aprepitant arm, 57.9 (35 to 72) in placebo arm Gender: male + female Tumour/cancer type: multiple myeloma Chemotherapy regimen: high‐dose melphalan therapy Country: Heidelberg University Hospital, Heidelberg, Germany (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg + granisetron 2 mg + dexamethasone 4 mg Days 2 to 3: aprepitant 80 mg + granisetron 2 mg + dexamethasone 2 mg Day 4: aprepitant 80 mg + granisetron 2 mg Experimental: arm B: placebo Day 1: placebo 125 mg + granisetron 2 mg + dexamethasone 8 mg Days 2 to 3: placebo 80 mg + granisetron 2 mg + dexamethasone 4 mg Day 4: placebo 80 mg + granisetron 2 mg |
|
| Outcomes |
Primary endpoint
Secondary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... computer‐generated randomization list ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "362 patients underwent ITT analysis out of 363 randomly assigned patients" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: ITT data set used for safety analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Schmoll 2006.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group trial with 2 arms
Enrolment period: patients were enrolled from 2 January 2004 and were followed up until 30 September 2004
Masking: double‐blind, with sponsor blinding Baseline patient characteristics: reported Follow‐up: follow‐up visit on Days 19 to 29 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD (range), years: 59 ± 11 (20 to 79) in aprepitant regimen, 58 ±11 (23 to 82) in control regimen Gender: male + female Tumour/cancer type: solid tumour (respiratory, urogenital, gastrointestinal, eyes/ears/nose/throat, other) Chemotherapy regimen: chemotherapy including cisplatin ≥ 70 mg/m²in Cycle 1 Country: 56 investigator sites in Europe, North America, South America, and Korea |
|
| Interventions |
Experimental: arm A: aprepitant 125/80 mg Day 1: p.o. aprepitant 125 mg + i.v. ondansetron 32 mg + p.o. dexamethasone 12 mg Days 2 to 3: p.o. aprepitant 80 mg + p.o. ondansetron placebo twice daily + p.o. dexamethasone 8 mg in the morning and placebo in the evening Day 4: p.o. ondansetron placebo twice daily + p.o. dexamethasone 8 mg in the morning and placebo in the evening Control: arm B Day 1: aprepitant placebo + i.v. ondansetron 32 mg + p.o. dexamethasone 20 mg Days 2 to 3: aprepitant placebo + p.o. ondansetron 8 mg twice daily + p.o. dexamethasone 8 mg twice daily Day 4: p.o. ondansetron 8 mg twice daily + p.o. dexamethasone 8 mg twice daily |
|
| Outcomes |
Primary efficacy endpoint
Secondary endpoints
Exploratory endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients received either the aprepitant or the control regimen in a 1:1 ratio according to a sponsor‐supplied, computer‐generated, random allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind, parallel‐group trial with sponsor blinding ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind, parallel‐group trial with sponsor blinding ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. febrile neutropenia, hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the efficacy analyses used a modified intention‐to‐treat (mITT) population ..." |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included for the tolerability analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were described in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Schnadig 2014.
| Study characteristics | ||
| Methods |
Randomised, phase 3 study with 2 arms
Enrolment period: n.r.
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: n.r. Chemotherapy regimen: MEC (cyclophosphamide, doxorubicin, epirubicin, carboplatin, irinotecan, daunorubicin, or cytarabine) Country: n.r. |
|
| Interventions |
Experimental: arm A: rolapitant rolapitant + granisetron + dexamethasone Experimental: arm B: placebo placebo + granisetron + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...were randomized 1:1 to either ..." Comment: sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: one thousand three hundred forty‐four evaluable patients were included for efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract only, not evaluable |
| Other bias | Unclear risk | Comment: conference abstract only, not evaluable |
Schnadig 2016.
| Study characteristics | ||
| Methods |
Randomised, prospective, double‐dummy, parallel‐group, phase 3 trial with 2 arms
Study period: March 2014 to May 2015
Masking: quadruple‐blind (participant, care provider, investigator, outcomes assessor) Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 55.7 ± 11.75 in granisetron group, 55.6 ± 11.94 in ondansetron group Gender: male + female Tumour/cancer type: malignant disease Chemotherapy regimen: anthracycline + cyclophosphamide Country: United States (77 centres) |
|
| Interventions |
Experimental: arm A: fosaprepitant + granisetron + dexamethasone Day 1: single SC dose of APF530 500 mg (10 mg granisetron) + placebo for ondansetron i.v. + fosaprepitant 150 mg i.v. + dexamethasone 12 mg i.v. Day 2: dexamethasone 8 mg p.o. QD Days 3 to 4: dexamethasone 8 mg p.o. b.i.d. Experimental: arm B: fosaprepitant + ondansetron + dexamethasone Day 1: single i.v. dose of ondansetron 0.15 mg/kg (to maximum of 16 mg) + placebo for APF530 SC + fosaprepitant 150 mg i.v. + dexamethasone 12 mg i.v. Day 2: dexamethasone 8 mg p.o. QD Days 3 to 4: dexamethasone 8 mg p.o. b.i.d. |
|
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "masking: quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "masking: quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. injection‐site reactions, neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "efficacy analyses were performed on the modified intent‐to‐treat (mITT) population, comprising all randomized patients who received study drug and an HEC regimen and had post baseline efficacy data" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "safety analyses were based on the safety population, comprising all randomized patients who received study drug" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Schwartzberg 2015.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, active‐controlled, phase 3 study with 2 arms
Study period: 5 March to 6 September 2013
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 58 (22 to 86) in rolapitant group, 56 (22 to 88) in active control group Gender: male (265) + female (1067) Tumour/cancer type: malignant solid tumour (breast, colon or rectum, head and neck, lung, ovary, stomach, other tumours) Chemotherapy regimen: MEC including ≥ 1 of the following agents: cyclophosphamide i.v. (< 1500 mg/m²), doxorubicin, epirubicin, carboplatin, idarubicin, ifosfamide, irinotecan, daunorubicin, cytarabine i.v. (> 1 g/m²) Country: 170 cancer centres in 23 countries (multi‐centre) |
|
| Interventions |
Experimental: arm A: rolapitant Day 1: rolapitant (200 mg p.o.) + granisetron (2 mg p.o.) + dexamethasone (20 mg p.o.) Days 2 to 3: granisetron (2 mg p.o.) administered orally Control: arm B Day 1: placebo + granisetron (2 mg p.o.) + dexamethasone (20 mg p.o.) Days 2 to 3: granisetron (2 mg p.o.) administered orally |
|
| Outcomes |
Primary outcome
Secondary outcome(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "for randomisation of patients, we used an interactive web‐based randomisation system (IWRS) at cycle 1" |
| Allocation concealment (selection bias) | Low risk | Quote: "for masking, a double‐blind technique was used. Placebo capsules were identical in appearance to rolapitant capsules. Through out the study, neither the patient nor the investigators and assessors knew which treatment the patient was receiving."; "... an independent group with no further role in study implementation ..." |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia, febrile neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "therefore, in cycle 1, the modified intention‐to‐treat population comprised 666 patients in each treatment group" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety population consisted of all patients who received at least one dose of study drug" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Seol 2016.
| Study characteristics | ||
| Methods |
Randomised, cross‐over, active‐controlled, phase 4 study with 2 arms
Enrolment period: 17 August 2012 to 14 February 2014
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age± SD, years: 58.88 ± 10.53 in granisetron and palonosetron group, 60.41 ± 10.06 in palonosetron and granisetron group Gender: male (117) + female (71) Tumour/cancer type: n.r. Chemotherapy regimen: MEC, FOLFIRI, or FOLFOX Country: 6 tertiary referral hospitals in Korea (multi‐centre) |
|
| Interventions |
Cross‐over study Experimental: arm A: granisetron and palonosetron transdermal granisetron (1 GTDS patch, 7 days) in the first cycle, palonosetron (i.v. 0.25 mg/d, 1 day) in the second cycle before receiving MEC in 2 consecutive cycles prophylactic dexamethasone (i.v. 10 mg) within 30 min before chemotherapy on Day 1 Experimental: arm B: palonosetron and granisetron palonosetron in the first cycle and GTDS in the second cycle prophylactic dexamethasone (i.v. 10 mg) within 30 min before chemotherapy on Day 1 |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: blinding should not affect risk of bias of objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | High risk | Quote: "three hundred thirty‐three cycles were included in the per protocol analysis ‐ 165 cycles for the GTDS and 168 cycles for the palonosetron were analyzed (Fig. 2)" Comment: per‐protocol analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: safety assessed for all completed cycles |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Song 2017.
| Study characteristics | ||
| Methods |
Randomised, prospective, comparative clinical trial with 2 arms
Enrolment period: October 2013 to March 2015
Masking: open‐label Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 41.2 ± 4.57 in aprepitant group, 39.6 ± 6.85 in control group Gender: male (74) + female (34) Tumour/cancer type: B‐ or T‐cell non‐Hodgkin lymphoma Chemotherapy regimen: cyclophosphamide (750 mg/m²), epirubicin (60 mg/m²), vincristine (1.4 mg/m²) Country: China (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: p.o. aprepitant 125 mg + i.v. ondansetron 24 mg + p.o. prednisone 100 mg Days 2 to 3: p.o. aprepitant 80 mg + prednisone 100 mg once daily Days 4 to 5: p.o. prednisone 100 mg once daily Control: arm B Day 1: i.v. ondansetron 24 mg + p.o. prednisone 100 mg Days 2 to 5: p.o. prednisone 100 mg once daily |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to one of the two treatment groups, according to a computer‐generated random assignment schedule" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although it was an open‐label study, both patients and personnel had no influence on objective outcomes (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: 101 patients have been included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: safety data reported for all 101 patients |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Stewart 1996.
| Study characteristics | ||
| Methods |
Randomised trial with 2 arms
Recruitment period: n.r. Enrolled/randomised patient number: n.r. Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age (range), years: n.r. Gender: n.r. Tumour/cancer type: n.r. Chemotherapy regimen: cisplatin Country: n.r. |
|
| Interventions |
Experimental: arm A: granisetron granisetron + dexamethasone (8 mg i.v., then treatment 4 mg t.d.s. for 3 days afterwards) Experimental: arm B: ondansetron ondansetron + dexamethasone (8 mg i.v., then treatment 4 mg t.d.s. for 3 days afterwards) |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised study but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: double‐blind |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: double‐blind |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: not evaluable, conference abstract only |
| Other bias | Unclear risk | Conference abstract, not evaluable |
Stewart 2000.
| Study characteristics | ||
| Methods |
Randomised, cross‐over, controlled trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 56 (37 to 74) for all patients Gender: male (9) + female (12) Tumour/cancer type: n.r. Chemotherapy regimen: highly emetogenic chemotherapy, including cisplatin, mean dose 74 mg/m² Country: UK |
|
| Interventions |
Cross‐over study Experimental: arm A: ondansetron ondansetron 8 mg + i.v. bolus dexamethasone 8 mg immediately before chemotherapy and p.o. dexamethasone 4 mg 3 times a day on Days 2 to 4 Experimental: arm B: granisetron granisetron 3 mg by infusion + i.v. bolus dexamethasone 8 mg immediately before chemotherapy and p.o. dexamethasone 4 mg 3 times a day on Days 2 to 4 |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... random allocation using a Latin square design in sets of four" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Comment: double‐blind |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Comment: double‐blind |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all patients were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Comment: outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Stiff 2013.
| Study characteristics | ||
| Methods |
Randomized, comparative, phase 3 trial with 2 arms
Registration period: September 2004 to July 2008
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 50 (20 to 75) in aprepitant 125/80 mg group, 51 (19 to 79) in placebo group Gender: male + female Tumour/cancer type: malignant disease (non‐Hodgkin lymphoma, AML, multiple myeloma, ALL, Hodgkin lymphoma, CML, myelodysplastic syndrome, myeloproliferative disorder, chronic lymphocytic leukaemia, myelofibrosis) Chemotherapy regimen: high‐dose cyclophosphamide preparative regimens Country: United States (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant 125/80 mg aprepitant 125 mg p.o. Day 1, then 80 mg daily during preparative regimen + 3 days dexamethasone 7.5 mg i.v. daily during preparative regimen + 1 day ondansetron 8 mg p.o. q8h daily during preparative regimen + 1 day Experimental: arm B: placebo placebo p.o. daily during preparative regimen + 3 days dexamethasone 10 mg i.v. daily during preparative regimen + 1 day ondansetron 8 mg p.o. q8h daily during preparative regimen + 1 day |
|
| Outcomes |
Primary endpoints
Secondary endpoints
Additional analyses
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: patients who received the study drug were included in the intent‐to‐treat analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "five patients died in the aprepitant arm due to sepsis (3 patients), toxic epidermal necrolysis and sepsis (1 patient), and veno‐occlusive disease of the liver (1 patient), whereas 2 patients died in the control arm due to viral pneumonia/encephalitis (1 patient) and fungal pneumonia (1 patient) within 30 days" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Sugawara 2019.
| Study characteristics | ||
| Methods |
Multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group, phase 2 study with 3 arms
Registration period: September 2016 to November 2017
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 67 (36 to 79) in placebo group, 66 (41 to 76) in fosnetupitant 81‐mg group, 67 (37 to 78) in fosnetupitant 235‐mg group Gender: 24.2% female (75.8% male) in placebo group, 25.1% female (74.9% male) in fosnetupitant 81‐mg group, 24.1% female (75.9% male) in fosnetupitant 235‐mg group Tumour/cancer type: confirmed malignant solid tumour Chemotherapy regimen: cisplatin‐based, cisplatin at a dose ≥ 70 mg/m² Country: Japan (multi‐centre) |
|
| Interventions |
Experimental: arm A: fosnetupitant 81 mg Fosnetupitant (81 mg intravenously, approximately 60 min before administration of cisplatin and infused for 30 min on Day 1), palonosetron (0.75 mg intravenously, approximately 60 min before administration of cisplatin and infused for 30 min on Day 1), dexamethasone (Day 1, 60 min before administration of cisplatin, 9.9 mg; Days 2 to 4, 6.6 mg administered intravenously in the morning) Experimental: arm B: fosnetupitant 235 mg Fosnetupitant (235 mg intravenously, approximately 60 min before administration of cisplatin and infused for 30 min on Day 1), palonosetron (0.75 mg intravenously, approximately 60 min before administration of cisplatin and infused for 30 min on Day 1), dexamethasone (Day 1, 60 min before administration of cisplatin, 9.9 mg; Days 2 to 4, 6.6 mg administered intravenously in the morning) Control: arm C: placebo Placebo, palonosetron (0.75 mg intravenously, approximately 60 min before administration of cisplatin and infused for 30 min on Day 1), dexamethasone (Day 1, 60 min before administration of cisplatin,13.2 mg; Days 2 to 4, 6.6 mg administered intravenously in the morning) |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation method used for random allocation, stratified by sex and age class (age < 55 years vs age ≥ 55 years) |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment assignment was masked from all patients, investigators, and study personnel except for the pharmacists who were preparing the study drugs at the institutions, who were prohibited from divulging any information regarding drug assignment" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "treatment assignment was masked from all patients, investigators, and study personnel except for the pharmacists who were preparing the study drugs at the institutions, who were prohibited from divulging any information regarding drug assignment" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "treatment assignment was masked from all patients, investigators, and study personnel except for the pharmacists who were preparing the study drugs at the institutions, who were prohibited from divulging any information regarding drug assignment" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "treatment assignment was masked from all patients, investigators, and study personnel except for the pharmacists who were preparing the study drugs at the institutions, who were prohibited from divulging any information regarding drug assignment" |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Quote: "treatment assignment was masked from all patients, investigators, and study personnel except for the pharmacists who were preparing the study drugs at the institutions, who were prohibited from divulging any information regarding drug assignment" |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Data available for nearly all participants (194/197 in placebo group, 195/199 in fosnetu 81‐mg group, 195/198 in fosnetu 235‐mg group) |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | All participants who received ≥ 1 dose included in safety analysis |
| Selective reporting (reporting bias) | Low risk | No reason for any concern detected |
| Other bias | Low risk | No other bias detected |
Sugimori 2017.
| Study characteristics | ||
| Methods |
Randomised, prospective, phase 2 study with 2 arms
Enrolment period: November 2010 to March 2014
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 58.5 in aprepitant group, 57.7 in control group Gender: female Tumour/cancer type: gynaecological cancer Chemotherapy regimen: carboplatin target area under the curve of 5 and 175 mg/m² paclitaxel (TC) therapy Country: Japan (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg + palonosetron 0.75 mg + dexamethasone 6.6 mg Days 2 to 3: aprepitant 80 mg + dexamethasone 8 mg Control: arm B Day 1: palonosetron 0.75 mg + dexamethasone 13.2 mg Days 2 to 3: dexamethasone 8 mg |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "... open‐label ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Quote: "... open‐label ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although participants were not blinded, we assume that this does not affect objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: all 78 patients were included in the efficacy analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: side effects reported as percentage of all patients |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Svanberg 2015.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled trial with 2 arms
Recruitment period: June 2010 to June 2012
Masking: double‐blind (attending nurse and patient) Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years : 58.11 ± 8.84 (experimental group), 56.52 ± 8.25 (control group) Gender: 64 male + 32 female Tumour/cancer type: lymphoma (38) and myeloma (58) Chemotherapy regimen: BEAM, BEAC for lymphoma patients and high‐dose melphalan, BBM for myeloma patients Country: Sweden (single centre) |
|
| Interventions |
Experimental: arm A 6 mg of betamethasone (T Betapred 0.5 mg, 12 tablets daily) + T Navobane (tropisetron) (5 mg) + aprepitant (Emend), started 1 h before first HDCT dose for SCT and administered daily until 7 days after the end of chemotherapy Control: arm B 6 mg of betamethasone (T Betapred 0.5 mg, 12 tablets daily) + T Navobane (tropisetron) (5 mg) + placebo, started 1 h before first HDCT dose for SCT and administered daily until 7 days after the end of chemotherapy |
|
| Outcomes |
Primary efficacy endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "a random assignment to the experimental (EXP) or control (CTR) group was performed by research nurses not participating in any other way in the study" |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "study drug or placebo was unknown to the attending nurse and the patient in the study" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "study drug or placebo was unknown to the attending nurse and the patient in the study" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the analysis is made on an intention‐to‐treat basis" |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse events mentioned as not differing, no proportions provided. All other outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Takahashi 2010.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, parallel, comparative, phase 2 trial with 3 arms
Study start date: August 2005
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years : 63.3 ± 9.4 (aprepitant 40 ⁄ 25 + standard therapy), 60.5 ± 9.7 (aprepitant 125 ⁄ 80 mg + standard therapy), 62.2 ± 9.8 (standard therapy) Gender: male + female Tumour/cancer type: solid malignant tumour Chemotherapy regimen: cisplatin at a dose ≥ 70 mg/m² Country: Japan (9 facilities) |
|
| Interventions |
Experimental: arm A: aprepitant 40 ⁄ 25 + standard therapy Day 1: aprepitant 40 mg + dexamethasone 8 mg + granisetron 40 µg/kg Days 2 to 3: aprepitant 25 mg + dexamethasone 6 mg Days 4 to 5: aprepitant 25 mg Experimental: arm B: aprepitant 125 ⁄ 80 mg + standard therapy Day 1: aprepitant 125 mg + dexamethasone 6 mg + granisetron 40 µg/kg Days 2 to 3: aprepitant 80 mg + dexamethasone 4 mg Days 4 to 5: aprepitant 80 mg Standard: arm C Day 1: placebo + dexamethasone 12 mg + granisetron 40 µg/kg Days 2 to 3: placebo + dexamethasone 8 mg Days 4 to 5: placebo |
|
| Outcomes |
Primary endpoint
Secondary endpoints
Both primary and secondary endpoints were assessed in the overall phase (Days 1 to 5), the acute phase (Day 1), and the delayed phase (Days 2 to 5) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment assignment (dynamic allocation) was performed using a minimization method for balancing four factors (sex, presence or absence of at least one emetogenic antitumour agent used in combination with cisplatin, presence or absence of previous treatment with cisplatin, and institution) between the treatment and control groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "of these, 449 patients were included in the safety analysis set, 439 subjects were included in the FAS" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "all 453 enrolled subjects were included in the safety analysis" Quote: "serious adverse events led to the death of one patient in the standard therapy group and one in the 125 ⁄80 mg group. The former died of febrile neutropenia, acute respiratory distress syndrome (ARDS) and septic shock, and the latter died of cardiac failure" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Tanioka 2013.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, phase 2 study with 2 arms
Study period: January 2011 to September 2012
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 53 (36 to 67) in aprepitant group, 59 (33 to 69) in placebo group Gender: female Tumour/cancer type: ovarian cancer (early/advanced), endometrial cancer, other, ascites or peritoneal dissemination Chemotherapy regimen: carboplatin + intravenous cytotoxic anti‐tumour drugs such as paclitaxel and pemetrexed; carboplatin + paclitaxel; carboplatin + liposomal doxorubicin; irinotecan + fluorouracil, bevacizumab, or cetuximab Country: Japan (multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. + granisetron 1 mg i.v. + dexamethasone 12 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 4 mg i.v. Experimental: arm B: placebo Day 1: placebo 0 mg p.o. + granisetron 1 mg i.v. + dexamethasone 20 mg i.v. Days 2 to 3: placebo 0 mg p.o. + dexamethasone 8 mg i.v. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomly assigned to the aprepitant group or placebo group according to a computer‐generated, blinded allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Comment: precise allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "of these, 91 patients were included in the full analysis set" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "safety was evaluated in all the 92 subjects who were assigned to treatment, including the patient who discontinued chemotherapy due to a hypersensitivity reaction" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Tsubata 2019.
| Study characteristics | ||
| Methods |
Randomised, prospective, single‐centre, comparative, phase 3 trial
Study period: October 2010 to January 2013
Masking: open‐label Baseline patient characteristics: reported Median follow‐up: n.r. ITT analysis: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age, years: 68 Gender: 22 men and 13 women Tumour/cancer type: NSCLC, SCLC, BC Chemotherapy regimen: HEC or MEC; not further specified Country: Japan (single centre) |
|
| Interventions |
|
|
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified and 1:1 randomised; method not further described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Outcome assessors (participants) not blinded to intervention |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Data available for all randomised participants |
| Selective reporting (reporting bias) | Unclear risk | Wrong UMIN ID provided in paper; UMIN ID 000005268 is a clinical trial to evaluate effect of spectacle lens that reduces myopia progression |
| Other bias | Low risk | No other sources of bias detected |
Warr 2005.
| Study characteristics | ||
| Methods |
Randomised, parallel‐group, placebo‐controlled, phase 3 trial with 2 arms
Recruitment period: October 2002 to December 2003
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 53.1 (aprepitant regimen), 52.1 (control regimen) Gender: male (2) + female (864) Tumour/cancer type: breast cancer Chemotherapy regimen: anthracycline plus cyclophosphamide‐based chemotherapy regimen (the following agents were administered alone or in combination: i.v. cyclophosphamide 750 to 1500 mg/m² (± 5%); i.v. cyclophosphamide 500 to 1500 mg/m² (± 5%) and i.v. doxorubicin ≤ 60 mg/m² (± 5%); i.v. cyclophosphamide 500 to 1500 mg/m² (± 5%) and i.v. epirubicin ≤ 100 mg/m² (± 5%). Other chemotherapeutic agents, Hesketh level 2 or lower, could be added to the above chemotherapeutic regimens) Country: 95 centres in the United States, Germany, Austria, Canada, Hong Kong, Hungary, Spain, United Kingdom, Italy, Australia, and Greece |
|
| Interventions |
Experimental: arm A: aprepitant 125/80 mg Day 1: p.o. aprepitant 125 mg + p.o. ondansetron 8 mg (30 to 60 min before chemotherapy and again 8 h after chemotherapy) + p.o. dexamethasone 12 mg Days 2 to 3: p.o. aprepitant 80 mg Control: arm B: placebo Day 1: placebo + p.o. ondansetron 8 mg (30 to 60 min before chemotherapy and again 8 h after chemotherapy) + p.o. dexamethasone 20 mg Days 2 to 3: placebo + p.o. ondansetron 8 mg |
|
| Outcomes |
Primary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... using a computer‐generated allocation schedule with a block size of four" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "... modified intent‐to‐treat analysis was conducted, including all patients who received chemotherapy, took the study drug, and had at least one post‐treatment assessment" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included in the safety analysis |
| Selective reporting (reporting bias) | Low risk | Comment: outcome measure was reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Webb 2010.
| Study characteristics | ||
| Methods |
Randomised study with 2 arms
Enrolment period: n.r.
Masking: double‐blind Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: MEC Country: n.r. |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. + ondansetron 8 mg p.o. b.i.d. + dexamethasone 12 mg p.o. Days 2 to 3: aprepitant 80 mg p.o. Control: arm B: ondansetron Day 1: ondansetron 8 mg p.o. b.i.d. + dexamethasone 20 mg p.o. Days 2 to 3: ondansetron 8 mg p.o. b.i.d. |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: breast cancer patients were also included in the modified intent‐to‐treat population |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract only, not evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Weinstein 2016.
| Study characteristics | ||
| Methods |
Randomised, active‐comparator, parallel‐group, phase 3 superiority trial (PN031) with 2 arms
Enrolment period: 30 October 2012 to 03 November 2014
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 60.0 ± 11.8 in fosaprepitant group, 59.1 ± 12.3 in control group Gender: male (409) + female (591) Tumour/cancer type: malignant disease (lung, breast, colorectal, gynaecological, gastrointestinal, head and neck, other) Chemotherapy regimen: MEC agents except for the combination of anthracycline and cyclophosphamide Country: 125 sites across 30 countries |
|
| Interventions |
Experimental: arm A: fosaprepitant Day 1: fosaprepitant 150 mg intravenous (i.v.) infusion, ~ 30 minutes before chemotherapy + dexamethasone 12 mg orally (p.o.) ~ 30 minutes before chemotherapy + ondansetron 16 mg total dose: 8 mg p.o. ~ 30 to 60 minutes before chemotherapy, followed by 8 mg p.o. 8 hours after first dose + dexamethasone placebo, p.o. ~ 30 minutes before chemotherapy Days 2 to 3: ondansetron placebo, p.o. every 12 hours Control: arm B: fosaprepitant Day 1: fosaprepitant placebo, 150 mL i.v. infusion, ~ 30 minutes before chemotherapy + dexamethasone 20 mg, p.o. ~ 30 minutes before chemotherapy + ondansetron 16 mg total dose: 8 mg p.o. ~ 30 to 60 minutes before chemotherapy; followed by 8 mg p.o. 8 hours after first dose Days 2 to 3: ondansetron 8 mg p.o. every 12 hours |
|
| Outcomes |
Primary endpoint(s)
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized (1:1) to the single‐dose fosaprepitant or control regimen via an interactive voice response system/interactive web response system, and stratified based on sex" |
| Allocation concealment (selection bias) | Low risk | Quote: "study medications were supplied in a blinded manner as fosaprepitant/placebo i.v. bags, ..." |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the ITT and ASaT populations comprised 1000 and 1001 subjects, respectively (Figure 1)" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the ITT and ASaT populations comprised 1000 and 1001 subjects, respectively (Figure 1)" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Wenzell 2013.
| Study characteristics | ||
| Methods |
Randomised, prospective, pilot study with 2 arms
Study period: January 2011 to July 2011
Masking: open‐label Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 52.9 ± 12.7 in ondansetron group, 50.9 ± 9.2 in palonosetron group Gender: female Tumour/cancer type: breast cancer (N = 39), lymphoma (N = 1) Chemotherapy regimen: AC (doxorubicin/cyclophosphamide), AC plus bevacizumab, ABVD Country: USA (single centre) |
|
| Interventions |
Experimental: arm A: palonosetron Day 1: aprepitant 125 mg p.o. + palonosetron 0.25 mg i.v. + dexamethasone 12 mg p.o. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 8 mg p.o. Day 4: dexamethasone 8 mg p.o. Experimental: arm B: ondansetron Day 1: aprepitant 125 mg p.o. + ondansetron 24 mg p.o. Days 2 to 3: aprepitant 80 mg p.o. + dexamethasone 8 mg p.o. Day 4: dexamethasone 8 mg p.o. |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomized to one of two treatments according to a permuted block‐design with block sizes of 2, 4, or 6" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Comment: open‐label |
| Blinding of participants and personnel (performance bias) Blinding of personnel | High risk | Comment: open‐label |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: patients and personnel were not blinded towards the intervention and therefore might influence subjective outcomes analysis |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "an intention to‐treat approach was used to evaluate patients" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Wit 2001.
| Study characteristics | ||
| Methods |
Randomised, cross‐over trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Median age (range), years: 46 (29 to 71) in granisetron, 46 (30 to 73) in ondansetron Gender: male (4) + female (36) Tumour/cancer type: solid tumour (breast, ovarian, lung, other) Chemotherapy regimen: cisplatin ≥ 50 mg m–2 or cyclophosphamide ≥ 500 mg m–2 Country: n.r. |
|
| Interventions |
Cross‐over study Experimental: arm A: granisetron granisetron 3 mg i.v. + dexamethasone 10 mg i.v. Experimental: arm B: ondansetron patients with previous treatment failure continued treatment with ondansetron 8 mg i.v. + dexamethasone 10 mg i.v. |
|
| Outcomes |
Primary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but the method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: 40 patients were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Xiong 2019.
| Study characteristics | ||
| Methods |
Randomised trial with 2 arms
Recruitment period: March 2014 to March 2017
Masking: open‐label Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean ± SD, years: 39.8 ± 8.6 in aprepitant group, 41.5 ± 9.4 in control group Gender: male (54) + female (51) Tumour/cancer type: bone or soft tissue sarcoma Chemotherapy regimen: 30 mg/m² on Days 1 and 2 for doxorubicin and 3 g/m² on Days 1 to 3 for ifosfamide Country: China |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: 125 mg p.o. aprepitant + 0.25 mg i.v. palonosetron + 5 mg i.v. dexamethasone Days 2 to 3: 80 mg p.o. aprepitant + 5 mg i.v. dexamethasone Control: arm B Day 1: 0.25 mg i.v. palonosetron + 10 mg i.v. dexamethasone Days 2 to 3: 10 mg i.v. dexamethasone |
|
| Outcomes |
Primary endpoints
Secondary endpoint
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... using a computer‐generated allocation schedule with a block size of four" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | High risk | Quote: "we have no placebo, and the administration of dexamethasone is different in the two groups, so it is difficult for us to make this trial double blinded" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Quote: "we have no placebo, and the administration of dexamethasone is different in the two groups, so it is difficult for us to make this trial double blinded" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | High risk | Comment: study was not blinded; knowledge of treatment may affect outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: although the study was not blinded, we assume that knowledge of treatment had no effect on outcome assessment for objective outcomes |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "105 patients (51 patients in the aprepitant group and 54 patients in the control group) were included in the efficacy analyses"; "three patients were excluded from both the efficacy and safety analyses because they did not receive at least 1 day’s dose of study drug"; modified ITT analysis |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: "three patients were excluded from both the efficacy and safety analyses because they did not receive at least 1 day’s dose of study drug"; modified ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Yahata 2016.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled, parallel‐group trial with 2 arms
Study period: April 2011 to December 2013
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (range), years: 59 (26 to 77) in aprepitant group, 59 (24 to 79) in placebo group Gender: female Tumour/cancer type: gynaecological cancer (ovarian cancer, endometrial cancer, cervical cancer, peritoneal cancer, tubal cancer) Chemotherapy regimen: TC combination chemotherapy (paclitaxel and carboplatin) Country: Japan (9 institutes, multi‐centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg p.o. + granisetron/ondansetron ¼ mg + dexamethasone 20 mg i.v. Days 2 to 3: aprepitant 80 mg p.o. Experimental: arm B: placebo Day 1: placebo 0 mg p.o. + granisetron/ondansetron ¼ mg + dexamethasone 20 mg i.v. |
|
| Outcomes |
Primary endpoint
Secondary endpoint(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised trial but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "efficacy analyses were conducted with the full analysis set, which was defined as all randomized patients who received at least one dose of the study drugs" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | High risk | Quote: "safety was evaluated in all patients taking the study drugs", but "adverse events obviously caused by paclitaxel and/or carboplatin (e.g. alopecia, neutropenia) were excluded, and only toxicities not related to the study drugs were recorded" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Yang 2017.
| Study characteristics | ||
| Methods |
Randomised, active‐control, parallel‐group, phase 3 trial with 2 arms
Recruitment period: November 2014 to July 2015
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: yes |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 55 (20 to 79) in fosaprepitant group, 53 (18 to 74) in aprepitant group Gender: male (326) + female (319) Tumour/cancer type: n.r. Chemotherapy regimen: HEC (according to NCCN Clinical Practice Guidelines in Oncology: Antiemesis version 1.2014) Country: China (21 centres) |
|
| Interventions |
Experimental: arm A: fosaprepitant Day 1: fosaprepitant 150 mg i.v. + granisetron 3 mg i.v. + dexamethasone 6 mg p.o. or i.v. Day 2: dexamethasone 3.75 mg p.o. Day 3: dexamethasone 3.75 mg p.o. every 12 h Day 4: dexamethasone 3.75 mg p.o. every 12 h Experimental: arm B: aprepitant Day 1: aprepitant 125 mg p.o. + granisetron 3 mg i.v. + dexamethasone 6 mg p.o. or i.v. Day 2: aprepitant 80 mg p.o. + dexamethasone 3.75 mg p.o. Day 3: aprepitant 80 mg p.o. + dexamethasone 3.75 mg p.o. Day 4: dexamethasone 3.75 mg p.o. |
|
| Outcomes |
Primary efficacy endpoint
Secondary efficacy endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized in a 1:1 ratio using a central randomization system ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. hiccups) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "assessments of efficacy, tolerability and safety variables were performed for 5 days after the start of chemotherapy (0–120 hr), including the acute and delayed phases" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "a total of 645 patients were included in the safety study" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Yeo 2009.
| Study characteristics | ||
| Methods |
Randomised, placebo‐controlled study with 2 arms
Enrolment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Median age (range), years: 46.5 (32 to 66) in aprepitant group, 48.5 (26 to 68) in standard group Gender: female (124) Tumour/cancer type: invasive ductal carcinoma, invasive lobular carcinoma, other Chemotherapy regimen: doxorubicin 60 mg/m² + cyclophosphamide 600 mg/m² Country: Hong Kong, China (single centre) |
|
| Interventions |
Experimental: arm A: aprepitant Day 1: aprepitant 125 mg, ondansetron 8 mg, dexamethasone 12 mg, before chemotherapy and ondansetron 8 mg 8 h later Days 2 to 3: aprepitant 80 QD Standard: arm B: ondansetron Day 1: ondansetron 8 mg and dexamethasone 20 mg before chemotherapy and ondansetron 8 mg 8 h later Days 2 to 3: ondansetron 8 mg b.i.d. |
|
| Outcomes |
Primary objective
Secondary objective(s)
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to 1 of 2 anti‐emetic regimens according to an in‐house blinding and allocation schedule of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. neutropenia, febrile neutropenia) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "thus, data from 124 evaluable patients (62 in each arm) was available for analysis. The modified intention‐to‐treat (mITT) approach was used for all efficacy analyses" |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Comment: all patients were included for safety analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Zhang 2018 (a).
| Study characteristics | ||
| Methods |
Randomised, single initial cycle, parallel‐group, international, phase 3 trial with 2 arms
Recruitment period: n.r.
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age ± SD, years: 54.6 ± 9.63 in NEPA group, 54.5 ± 10.24 in APR/GRAN group Gender: male (589) + female (240) Tumour/cancer type: solid tumour (lung, head and neck, and other) Chemotherapy regimen: cisplatin‐based chemotherapy at a dose ≥ 50 mg/m² alone or in combination with other chemotherapy agents Country: 30 sites in China, 5 sites in Taiwan, 3 sites in Thailand, 8 sites in Korea (46 centres) |
|
| Interventions |
Experimental: arm A: NEPA Day 1: NEPA (300 mg netupitant and 0.5 mg palonosetron) + dexamethasone 12 mg Days 2 to 4: dexamethasone 8 mg daily Experimental: arm B: APR/GRAN Day 1: aprepitant 125 mg + 3 mg i.v. granisetron + dexamethasone 12 mg Days 2 to 3: aprepitant 80 mg daily + dexamethasone 8 mg daily Day 4: dexamethasone 8 mg daily |
|
| Outcomes |
Primary efficacy endpoint
Secondary efficacy endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were stratified by gender and randomly assigned (1:1) to receive either NEPA or APR/GRAN treatment ..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "... double‐blind ..." |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "... double‐blind ..." |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Comment: both patients and personnel were blinded towards the intervention and thus had no influence on outcome assessment (e.g. study mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "the full analysis set (FAS) population (efficacy analyses) was defined as all patients who were randomized and received protocol‐required cisplatin and study treatment ...": of 334 randomised, 328 were included in FAS |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | Quote: "the safety analysis population consisted of all patients who received study treatment" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported in the results section |
| Other bias | Low risk | Comment: no information to suggest other sources of bias |
Zhang 2018 (b).
| Study characteristics | ||
| Methods |
Randomised study with 2 arms
Recruitment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: locally advanced or metastatic lung cancer Chemotherapy regimen: cisplatin‐based combination chemotherapy Country: China |
|
| Interventions |
Experimental: arm A: aprepitant aprepitant + palonosetron + dexamethasone Experimental: arm B: placebo placebo + palonosetron + dexamethasone |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random sequence generation not reported" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) Blinding of participants | Unclear risk | Comment: blinding not reported |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Unclear risk | Comment: blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: blinding not reported; therefore we do not know if this was a risk of bias |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Unclear risk | Comment: unclear whether all randomised patients were included for efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: conference abstract, not evaluable |
| Other bias | Unclear risk | Comment: conference abstract, not evaluable |
Zhang 2020.
| Study characteristics | ||
| Methods |
Randomised study with 2 arms
Recruitment period: October 2014 to November 2015
Masking: double‐blind Baseline patient characteristics: reported Follow‐up: n.r. |
|
| Participants |
Inclusion criteria
Exclusion criteria
Mean age (SD), years: 55.88 (10.37) in fosaprepitant group, 55.88 (10.19) in aprepitant group Gender: 40.50% female (59.50% male) in fosaprepitant group, 40.87% female (59.13% male) in aprepitant group Tumour/cancer type: solid malignant tumour Chemotherapy regimen: single day of cisplatin (dosage ≥ 50 mg/m² and infusion time ≤ 3 hours) Country: China |
|
| Interventions |
Experimental: arm A: fosaprepitant fosaprepitant (Day 1, 150 mg i.v.) + palonosetron (0.25 mg i.v.) + dexamethasone (6 mg on Day 1 followed by 3.75 mg on Day 2 and 3.75 mg p.o. every 12 hours on Days 3 to 4) + aprepitant simulating agents (placebo, scheduled as received in aprepitant group) Experimental: arm B: aprepitant aprepitant (Day 1, 125 mg, p.o.; Days 2 to 3, 80 mg p.o.) + palonosetron + dexamethasone (6 mg on Day 1 followed 3.75 mg on Days 2 to 4, with dexamethasone simulation agent 3.75 mg p.o. on Days 3 to 4), fosaprepitant simulating agent (placebo, scheduled as received in fosaprepitant group) |
|
| Outcomes |
Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated, blinded allocation schedule. Patients firstly stratified by gender and then based on whether the first administration of chemotherapy or not and the emetogenic potential of anticancer agents (excluding cisplatin) were randomized to different treatment groups" |
| Allocation concealment (selection bias) | Low risk | Computer‐generated, blinded allocation schedule |
| Blinding of participants and personnel (performance bias) Blinding of participants | Low risk | Quote: "both patients and researchers were blinded to the therapeutic grouping" |
| Blinding of participants and personnel (performance bias) Blinding of personnel | Low risk | Quote: "both patients and researchers were blinded to the therapeutic grouping" |
| Blinding of outcome assessment (detection bias) Subjective outcomes (Patient reported outcomes) | Low risk | Quote: "both patients and researchers were blinded to the therapeutic grouping" |
| Blinding of outcome assessment (detection bias) Objective outcomes (including mortality and safety) | Low risk | Quote: "both patients and researchers were blinded to the therapeutic grouping" |
| Incomplete outcome data (attrition bias) Subjective outcomes (Patient reported outcomes) | Low risk | Data available for nearly all participants (1/322 participants in fosa group and 3/326 participants from apre group excluded because no study drug received) |
| Incomplete outcome data (attrition bias) Objective outcomes (including mortality and safety data) | Low risk | All participants who received at least 1 dose included in safety analysis |
| Selective reporting (reporting bias) | Low risk | No reasons for any concern detected |
| Other bias | Low risk | No other sources of bias detected |
5‐HT₃: serotonin.
5‐HT₃ RA: 5‐HT₃ receptor antagonist.
ABVD: doxorubicin (Adriamycin), bleomycin, vinblastine (Velbe), dacarbazine (DTIC).
AC: doxorubicin, cyclophosphamide.
ALT: alanine aminotransferase.
AML: acute myeloid leukemia.
APF: granisetron.
AST: aspartate aminotransferase.
AUC: area under the curve.
BBM: berbamine.
BCNU: carmustine.
BEAM: carmustine, etoposide, cytarabine, and melphalan.
BEAC: carmustine, etoposide, cytarabine, cyclophosphamide.
β‐hCG: β‐subunit of human chorionic gonadotropin.
b.i.d.: twice daily.
Bu: busulfan.
CDDP: cisplatin.
CEF: cyclophosphamide, epirubicin, 5‐fluorouracil.
CINV: chemotherapy‐induced nausea and vomiting.
CNF: cyclophosphamide, novantrone, and 5‐fluorouracil.
CNS: central nervous system.
CMF: cyclophosphamide, mitoxantrone, and 5‐fluorouracil.
CML: chronic myeloid leukemia.
CR: complete response.
CrCl: creatinine clearance.
CTCAE: Common Terminology Criteria for Adverse Events.
Cy: cyclophosphamide.
CyTBI: cyclophosphamide total body irradiation.
EC: epirubicin, cyclophosphamide.
ECG: electrocardiogram.
ECOG: Eastern Cooperative Oncology Group.
ESHAP: multi‐day cisplatin along with etoposide, methylprednisolone, high‐dose cytarabine.
FAC: 5‐fluorouracil (5‐FU) + AC.
FEC: fluorouracil, epirubicin, cyclophosphamide.
FLIE: Functional Living Index‐Emesis.
FOLFOX: 5‐fluorouracil + leucovorin + oxaliplatin.
g/m²: gram per square meter.
GTDS: granisetron transdermal delivery system.
h: hour.
hCG: human chorionic gonadotropin.
HDCT: high‐dose chemotherapy.
HEC: highly emetogenic chemotherapy.
HSCT: haematopoietic stem cell transplantation.
IFO: ifosfamide.
ITT: intention‐to‐treat.
IU/L: international units per litre.
i.v.: intravenous.
L: litre.
MEC: moderately emetogenic chemotherapy.
μg: microgram.
mg: milligram.
mg/dL: milligrams per decilitre.
mg/m²: milligram per square meter.
min: minutes.
mITT: modified intention‐to‐treat.
MMT: paclitaxel.
msec: millisecond.
MTZ: mitoxantrone.
NCCN: National Comprehensive Cancer Network.
NSCLC: non‐small cell lung carcinoma.
NK₁: neurokinin‐1.
n.r.: not reported.
PBSC: peripheral blood stem cell.
p.o.: per os (orally).
PRN: medicine as required.
q8h: every 8 hours.
QAM: in the morning.
QD: once a day.
QoL: quality of life.
QPM: in the evening.
SC: subcutaneously.
SCT: stem cell transplantation.
SOX: S‐1, oxaliplatin.
SPAMP V: cyclophosphamide, thiotepa, carboplatin.
TAC: docetaxel, doxorubicin, cyclophosphamide.
TANC: paclitaxel, mitoxantrone, carboplatin.
TBI: total body irradiation.
TC: paclitaxel, carboplatin.
ULN: upper limit of normal.
VAS: visual analogue scale.
VP: etoposide.
vs: versus.
WBC: white blood cell count.
XELOX: capecitabine, oxaliplatin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abali 2007 | non‐randomised study |
| Adamo 1994 | antiblastic therapy used as treatment regimen |
| Albany 2014 | non‐randomised study |
| Audhuy 1996 | application of dexamethasone has not been reported |
| Ballatori 1995 | dexamethasone has been used in only 1 arm |
| Barrajon 2000 | cost‐benefit analysis |
| Belle 2002 | no use of 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonists in first 2 arms, along with neurokinin‐1 (NK₁) receptor antagonist |
| Bianchi 1996 | application of dexamethasone has not been reported |
| Bonneterre 1994 | a letter |
| Bonneterre 1995 | application of dexamethasone has not been reported |
| Bubalo 2001 | application of dexamethasone has not been reported |
| Bubalo 2012 | objectives of this article are (1) to assess the pharmacokinetics of aprepitant in cancer patients undergoing HSCT, and (2) to examine the potential drug–drug interaction between aprepitant and cyclophosphamide |
| Campora 1994 | application of dexamethasone has not been reported |
| Chiou 2000 | chemotherapy regimen is not clearly reported |
| Choi 2014 | non‐randomised study |
| Cocquyt 2001 | use of 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonist has been reported in only 1 arm |
| Craver 2011 | non‐randomised study |
| Creed 1999 | no information given regarding randomisation, no comparator against ondansetron |
| Dandamudi 2011 | docetaxel belongs to low emetogenic chemotherapy regimen |
| Dong 2011 | dexamethasone was permitted as rescue medication |
| Fauser 1995 | application of dexamethasone has not been reported |
| Fauser 1996 | application of dexamethasone has not been reported |
| Fauser 1999 | dexamethasone has been used in only 1 arm |
| Fedele 1995 | correspondence |
| Feng 2000 | application of dexamethasone has not been reported |
| Feng 2002 | application of dexamethasone has not been reported |
| Fengyi 2002 | application of dexamethasone has not been reported |
| Gebbia 1994 | application of dexamethasone has not been reported |
| Goldschmidt 1997 | no use of dexamethasone reported |
| Gralla 1998 | application of dexamethasone has not been reported |
| Gralla 2003 | application of dexamethasone has not been reported |
| Hesketh 1996 | application of dexamethasone has not been reported |
| Huang 1998 | comparison of identical dose of Zudan and Zofran; both are ondansetron |
| Huang 2001 | no information available regarding randomisation |
| Huang 2013 | application of dexamethasone has not been reported |
| Huc 1998 | application of dexamethasone has not been reported |
| Hudis 2003 | retrospective subset analysis |
| Humphreys 2013 | cost‐effectiveness study |
| Iihara 2012 | application of dexamethasone has not been reported |
| Italian Group for Antiemetic Research 1993 | use of metoclopramide in 1 arm |
| Italian Group for Antiemetic Research 1995 (a) | on Days 2 to 4 after chemotherapy, all patients received oral metoclopramide + intramuscular dexamethasone as antiemetic prophylaxis for delayed emesis |
| Italian Group for Antiemetic Research 1995 (b) | use of metoclopramide |
| Jantunen 1993 | application of dexamethasone has not been reported |
| Kang 2002 | application of dexamethasone has not been reported |
| Kawaguchi 2015 | concurrent chemoradiotherapy |
| Kilickap 2013 | chemotherapy regimen is not clearly reported |
| Kim 1998 | application of dexamethasone has not been reported |
| Kim 2004 | application of dexamethasone has not been reported |
| Kim 2012 | detailed chemotherapy regimens within the HEC group have not been reported |
| Lacerda 2000 | addition of lorazepam in all treatment arms |
| Lavoie 2012 | use of 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonist has not been reported |
| Lee 2014 | chemotherapy regimen is not clearly mentioned |
| Leonardi 1996 | application of dexamethasone has not been reported |
| Lindley 2005 | comparison of ondansetron, prochlorperazine, and dexamethasone in 3 individual arms |
| Lofters 1995 | dolasetron (dol) vs ondansetron (ond) with and without dexamethasone (dex) to evaluate additive effects of i.v. DEX with each drug |
| Long 2002 | no information available regarding randomisation |
| Loos 2007 | pharmacokinetic study |
| Mandanas 2005 | addition of lorazepam in all treatment arms |
| Martoni 1996 | application of dexamethasone has not been reported |
| Marty 1995 | application of dexamethasone has not been reported |
| Matsui 1996 | patients were randomly assigned to receive granisetron alone (arm 1) or granisetron, dexamethasone, and prochlorperazine (arm 2) |
| Matsuoka 2003 | treatment arms include granisetron + dexamethasone vs granisetron alone |
| Meiri 2007 | efficacy determination of dronabinol alone and in combination with ondansetron vs ondansetron alone |
| Micha 2016 | application of dexamethasone has not been reported in Cycle 1 |
| Molassiotis 2013 | study type: pooled analysis of different trials |
| Monda 1994 | no information given regarding randomisation |
| Moore 2007 | cost‐effectiveness study |
| Nasu 2013 | application of dexamethasone has not been reported |
| Navari 2016 | review |
| NCT04636632 | comparison of different preparations of the same drug |
| Nishimura 2015 (a) | 5‐HT₃ not defined and use of both fosaprepitant and aprepitant in 1 group reported |
| Nishimura 2015 (b) | 5‐HT₃ not defined and use of both fosaprepitant and aprepitant in 1 group reported |
| Noble 1994 | application of dexamethasone has not been reported |
| Noda 2002 | application of dexamethasone has not been reported |
| Öge 2000 | application of dexamethasone has not been reported |
| Ogihara 1999 | dexamethasone has been reported in only 1 group |
| Ohta 1992 | ondansetron or saline injection has been given to patients |
| Ottoboni 2014 | pharmacokinetic and dose‐finding study |
| Park 1997 | application of dexamethasone has not been reported |
| Pater 1997 | non‐randomised study |
| Pectasides 2007 | application of dexamethasone has not been reported |
| Perez 1996 | no information given regarding randomisation |
| Perez 1998 (a) | application of dexamethasone has not been reported |
| Perez 1998 (b) | dexamethasone or methylprednisolone was permitted as a prophylactic component of pre‐therapy |
| Peterson 1996 | dexamethasone has been reported in only 1 group |
| Plasencia‐Mota 1993 | no information given regarding randomisation |
| Poon 1998 | application of dexamethasone has not been reported |
| Qiu 2011 | application of dexamethasone has not been reported |
| Roila 2009 | dose‐finding study |
| Roscoe 2012 | no individual data have been provided for HEC and MEC regimens |
| Ruff 1994 | application of dexamethasone has not been reported |
| Ruhlmann 2016 | 5 weeks of fractionated radiotherapy and concomitant weekly cisplatin have been used |
| Rzepecki 2009 | non‐randomised study; historical control group |
| Saito 2015 | chemotherapy regimen is not clearly reported |
| Sheng 2010 | application of dexamethasone and chemotherapy regimen have not been reported |
| Shi 2007 | application of dexamethasone has not been reported |
| Silvestris 2013 | a letter |
| Slabý 2000 | application of dexamethasone has not been reported |
| Spector 1998 | application of dexamethasone has not been reported |
| Stewart 1995 | application of dexamethasone has not been reported |
| Sun 2014 | application of dexamethasone has not been reported |
| Suzuki 2015 | pharmacogenomics study |
| Takenaka 2007 | no information given regarding randomisation |
| Takeshima 2014 | non‐randomised study and no comparator has been used |
| Tan 2004 | no individual data have been provided for HEC and MEC regimens |
| Tang 2013 | application of dexamethasone has not been reported |
| Tanimura 1998 | dose‐finding study |
| Tian 2011 | application of dexamethasone has not been reported |
| Tominaga 1996 | application of dexamethasone has not been reported |
| Tong 2012 | concurrent radiochemotherapy |
| Tong 2014 | application of dexamethasone and chemotherapy regimen have not been reported |
| Tremont‐Lukats 2017 | application of dexamethasone has not been reported |
| Tsavaris 1996 | application of dexamethasone has not been reported |
| Tsubata 2015 | no use of dexamethasone has been reported |
| Tsuji 2016 | pharmacogenomics study |
| Tsukuda 1995 | application of dexamethasone has not been reported |
| Uchino 2012 | retrospective study |
| Vadhan‐Raj 2011 | pharmacological study |
| Vadhan‐Raj 2012 | pharmacological study |
| Vadhan‐Raj 2014 | pharmacological study |
| Vadhan‐Raj 2015 | pharmacological study |
| Van Belle 2002 | no use of 5‐hydroxytryptamine‐3 (5‐HT₃) receptor antagonist has been reported in 2 of 3 arms |
| Van der Vorst 2021 | metoclopramide used as part of the antiemetic regimen |
| Walko 2012 | pharmacological study |
| Weant 2017 | application of dexamethasone has not been reported |
| Xie 2003 | use of dexamethasone has not been reported |
| Yahata 2016 (a) | no clear distinction in presenting results for granisetron and ondansetron |
| Yalçin 1999 | application of dexamethasone has not been reported |
| Yang 2005 | application of dexamethasone has not been reported |
| Yano 2005 | application of dexamethasone has not been reported |
| Yu 2009 | application of dexamethasone has not been reported |
| Zeidman 1998 | non‐randomised study |
| Zeng 2001 | application of dexamethasone and chemotherapy regimen have not been reported |
| Zhang 1996 | application of dexamethasone has not been reported |
| Zhang 1999 | application of dexamethasone has not been reported |
| Zhang 2002 | phase 1, pharmacokinetic study |
| Zhang 2003 | use of dexamethasone has not been reported |
| Zhang 2003 (a) | application of dexamethasone has not been reported |
| Zhang 2007 | application of dexamethasone has not been reported |
| Zhang 2012 | phase 1, pharmacokinetic study |
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR‐INR‐17010779.
| Methods |
Randomised, cross‐over study with 2 arms
Study period: April 2017 to March 2020
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: histologically or cytologically confirmed malignant tumour Chemotherapy regimen: cisplatin, doxorubicin ≥ 60 mg/m², epirubicin > 90 mg/m², ifosfamide ≥ 2 g/m² Country: China |
| Interventions |
Cross‐over study Experimental: arm A Cycle 1: palonosetron 0.25 mg on Day 1, Day 3 + dexamethasone 10 mg on Day 1, 5 mg on Days 2 to 5 Cycle 2: tropisetron 5 mg on Days 1 to 3/5 + dexamethasone 10 mg on Day 1, 5 mg on Days 2 to 5 Experimental: arm B Cycle 1: tropisetron 5 mg on Days 1 to 3/5 + dexamethasone 10 mg on Day 1, 5 mg on Days 2 to 5 Cycle 2: palonosetron 0.25 mg on Day 1, Day 3 + dexamethasone 10 mg on Day 1, 5 mg on Days 2 to 5 |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
CTRI/2017/10/010163.
| Methods |
Randomised, interventional, parallel study with 2 arms
Recruitment period: November 2017 to April 2018
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: malignancy Chemotherapy regimen: moderate or low emetogenic potential chemotherapeutic agents except for carboplatin Country: India |
| Interventions |
Experimental: arm A: granisetron Day 1: i.v. injections of granisetron 1 mg + i.v. dexamethasone 8 mg given 30 min before administration of moderate emetogenic and mild emetogenic chemotherapy agents Days 2 to 5: p.o. granisetron 1 mg once daily before food Control: arm B: ondansetron Day 1: i.v. injections of ondansetron 8 mg + i.v. dexamethasone 8 mg given 30 min before administration of moderate emetogenic chemotherapy agents and low chemotherapeutic agents Days 2 to 5: p.o. ondansetron 8 mg once daily before food |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
|
EUCTR2004‐000371‐34.
| Methods |
Randomised, phase 2, placebo‐ and active‐controlled study with 3 arms
Masking: double‐blind Baseline patient characteristics: reported |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid tumours Chemotherapy regimen: cisplatin‐based chemotherapy Country: 47 centres in 18 countries (multi‐centre) |
| Interventions |
Experimental: arm A casopitant 50 mg + ondansetron + dexamethasone Experimental: arm B casopitant 100 mg + ondansetron + dexamethasone Experimental: arm C casopitant 150 mg + ondansetron + dexamethasone |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
EUCTR2004‐001020‐20.
| Methods |
Randomised, placebo‐controlled, dose‐ranging study Recruitment period: February 2005 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid malignancy Chemotherapy regimen: MEC Country: Czech Republic, Germany, Hungary, Ireland, Spain, United Kingdom |
| Interventions | oral neurokinin₁ receptor antagonist, GW679769, administered as 50 mg, 100 mg, and 150 mg oral tablets in combination with ondansetron hydrochloride and dexamethasone |
| Outcomes |
Primary endpoints
Secondary objectives
|
| Notes |
|
EUCTR2004‐004956‐38.
| Methods |
Randomised, placebo‐controlled study with 2 arms
Recruitment period: June 2005 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: multiple myeloma Chemotherapy regimen: high‐dose melphalan Country: Germany (single centre) |
| Interventions |
Experimental: arm A aprepitant + granisetron + dexamethasone Experimental: arm B placebo + granisetron + dexamethasone |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
EUCTR 2005‐000137‐37‐cz 2005.
| Methods |
Randomised, parallel‐group, cross‐over study with 3 arms
Recruitment period: September 2009 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: histologically or cytologically confirmed malignant disease Chemotherapy regimen
Country: n.r. |
| Interventions |
Cross‐over study Experimental: arm A palonosetron 0.25 mg + dexamethasone 8 mg Experimental: arm B palonosetron 0.50 mg + dexamethasone 8 mg Experimental: arm C palonosetron 0.75 mg + dexamethasone 8 mg |
| Outcomes |
Primary endpoint
Secondary endpoints
Additional secondary outcomes
|
| Notes |
|
EUCTR2006‐000781‐37.
| Methods |
Randomised, phase 3, active‐controlled study Masking: double‐blind Baseline patient characteristics: reported |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: n.r. Chemotherapy regimen: MEC Country: 196 centres in 32 countries (multi‐centre) |
| Interventions | casopitant + ondansetron + dexamethasone |
| Outcomes |
Primary endpoints
Secondary endpoints for Cycle 1
|
| Notes |
|
EUCTR2006‐003512‐22.
| Methods |
Randomised, parallel‐group study Recruitment period: November 2006 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid malignancy Chemotherapy regimen
Country: France, Germamy |
| Interventions | aprepitant, fosaprepitant, ondansetron, dexamethasone |
| Outcomes |
Primary endpoints
Secondary objectives
|
| Notes |
|
EUCTR2007‐004043‐30.
| Methods |
Randomised, active‐controlled, parallel‐group study Recruitment period: March 2008 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid malignancy Chemotherapy regimen: cisplatin Country: Denmark, Germany, Hungary, Italy, Lithuania, Netherlands, Poland, Portugal, Spain, Sweden |
| Interventions | single dose of intravenous MK‐0517 |
| Outcomes |
Primary endpoints
Secondary objectives
|
| Notes |
|
EUCTR2007‐005169‐36.
| Methods |
Randomised, parallel‐group study Recruitment period: February 2008 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: colorectal cancer Chemotherapy regimen: oxaliplatin at a dose between 85 mg/m² and 130 mg/m² Country: Bulgaria, Czech Republic, Germany, Hungary. Italy, Slovakia |
| Interventions | casopitant + ondansetron + dexamethasone |
| Outcomes |
Primary endpoint
Secondary objectives
|
| Notes |
|
EUCTR2008‐001339‐37.
| Methods |
Randomised, parallel‐group study Recruitment period: April 2008 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: malignancy Chemotherapy regimen: cisplatin Country: Italy |
| Interventions | aprepitant |
| Outcomes |
Primary endpoint
Secondary objectives
|
| Notes |
|
EUCTR2009‐016775‐30.
| Methods |
Randomised, phase 3, active‐controlled, parallel‐group study with 2 arms
Recruitment period: April 2011 to November 2012 Sample size: 726 Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid tumours Chemotherapy regimen: MEC Countries: Argentina, Brazil, Bulgaria, Croatia, Germany, Hungary, India, Italy, Mexico, Poland, Romania, Russian Federation, Ukraine, United States |
| Interventions |
Experimental: arm A netupitant + palonosetron + dexamethasone Experimental: arm B palonosetron + dexamethasone |
| Outcomes |
Primary endpoint
Secondary endpoints
|
| Notes |
|
EUCTR2009‐017603‐28.
| Methods |
Randomised, phase 3, placebo‐controlled study Recruitment period: February 2010 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: multiple myeloma, Hodgkin lymphoma, non‐Hodgkin lymphoma Chemotherapy regimen: cyclophosphamide i.v. chemotherapy (3 g/m²) Country: Italy |
| Interventions | aprepitant |
| Outcomes |
Primary endpoint
Secondary objective
|
| Notes |
|
EUCTR2010‐023297‐39.
| Methods |
Randomised, phase 3, double‐blind, active‐control study Recruitment period: July 2011 to September 2012 Sample size: 309 Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: malignant tumours Chemotherapy regimen HEC: cisplatin, mechlorethamine, streptozocin, cyclophosphamide ≥ 1500 mg/m², carmustine, dacarbazine MEC: any i.v. dose of oxaliplatin, carboplatin, epirubicin, idarubicin, ifosfamide, irinotecan, daunorubicin, or doxorubicin; i.v. cyclophosphamide (< 1500 mg/m²), i.v. cytarabine (> 1 g/m²); azacitidine, alemtuzumab, bendamustine, or clofarabine Countries: Bulgaria, Czech Republic, Germany, Hungary, India, Poland, Russian Federation, Serbia, Ukraine, United States |
| Interventions |
Experimental: arm A netupitant + palonosetron + dexamethasone Experimental: arm B aprepitant + palonosetron + dexamethasone |
| Outcomes |
Primary objective
Secondary objectives
|
| Notes |
|
EUCTR2015‐001800‐74.
| Methods |
Randomised, phase 3, active‐controlled study Recruitment period: December 2015 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid tumour malignancy Chemotherapy regimen
Country: Austria, Croatia, Czech Republic, Germany, Israel, Italy, Poland, Serbia, South Africa, Spain, Ukraine, United States |
| Interventions | pro‐netupitant (260 mg) + palonosetron (0.25 mg) + dexamethasone |
| Outcomes |
Primary endpoints
Secondary endpoints
|
| Notes |
|
JapicCTI‐194691.
| Methods |
Randomised, interventional, phase 3 study with 2 arms
Target sample size: 100 Masking: n.r. Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: minimum 20 years Gender: male and female Tumour/cancer type: not specified Chemotherapy regimen: AC/EC Country: Japan |
| Interventions |
Experimental: arm A Fosnetupitant 235 mg i.v. before start of chemotherapy Active control: arm B Fosaprepitant 150 mg i.v. before start of chemotherapy |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
Mylonakis 1996.
| Methods |
Randomised, comparative study with 2 arms
Recruitment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: n.r. Chemotherapy regimen: moderately emetogenic chemotherapy regimen Country: n.r. |
| Interventions |
Experimental: arm A ondansetron Experimental: arm B tropisetron |
| Outcomes | n.r. |
| Notes |
|
NCT00169572.
| Methods |
Randomised, phase 2, parallel‐assignment study Study start date: February 2005 Study completion date: not provided
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid malignancy Chemotherapy regimen: n.r. Country: Argentina, Austria, Belgium, Chile, Croatia, Czech Republic, Hong Kong, Hungary, Italy, Mexico, Netherlands, Pakistan, Peru, Philippines, Poland, Romania, Singapore, Slovakia, Taiwan |
| Interventions |
Drug: aprepitant, ondansetron, GW679769, dexamethasone Study arms: not provided |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
NCT01101529.
| Methods |
Randomised, phase 2 study with 2 arms
Study period: May 2010 to December 2012
Masking: triple (participant, care provider, investigator) Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: lymphoproliferative disease Chemotherapy regimen: myeloablative chemotherapy + autologous stem cell transplantation Country: Sweden |
| Interventions |
Experimental: arm A aprepitant given orally 125 mg the first day, then 80 mg daily during the chemotherapy course + 1/dexamethasone 6 mg daily during chemotherapy days + 2/tropisetron (Navoban) 5 mg daily during chemotherapy and 2 days after Control: arm B placebo + 1/dexamethasone 6 mg daily during chemotherapy days + 2/tropisetron (Navoban) 5 mg daily during chemotherapy and 2 days after |
| Outcomes |
Primary outcome
Secondary outcome
|
| Notes |
|
NCT02407600.
| Methods |
Randomised, placebo‐controlled, cross‐over study with 2 arms Recruitment period: April 2015 to February 2018
Masking: quadruple (participant, care provider, investigator, outcomes assessor) Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: non‐small cell lung cancer Chemotherapy regimen: carboplatin‐based combination chemotherapy Country: United States |
| Interventions |
Cross‐over study Experimental: arm A fosaprepitant (Emend) for injection; 150 mg was administered, 1 time, i.v. on Day 1 only, as an infusion with a duration of 30 minutes Experimental: arm B saline placebo intravenously on Day 1 of first chemotherapy cycle |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
NCT02550119.
| Methods |
Randomised study with 2 arms
Recruitment period: 19 April 2006 to 1 April 2010
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: gastrointestinal malignancy Chemotherapy regimen: oxaliplatin‐containing regimen in combination with 5‐fluorouracil (combinations such as fluorouracil, oxaliplatin, and leucovorin calcium (FOLFOX), FOLFOX + bevacizumab, FOLFOX + cetuximab) Country: United States |
| Interventions |
Experimental: arm A dolasetron mesylate p.o. or i.v., dexamethasone p.o. or i.v., and aprepitant p.o. 1 day before chemotherapy; dexamethasone p.o. and aprepitant p.o. on Days 2 and 3 after chemotherapy begins during Course 2 to 3 Experimental: arm B dolasetron mesylate and dexamethasone as in arm A and placebo p.o. 1 day before chemotherapy; dexamethasone p.o. and placebo p.o. on Days 2 and 3 after chemotherapy begins during Courses 2 to 3 |
| Outcomes |
Primary outcome
Secondary outcome
|
| Notes |
|
NCT02732015.
| Methods |
Randomised, interventional, phase 2, parallel study with 2 arms
Estimated enrolled patients: 91, terminated per principal investigator's request after enrolment of 37 participants Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: male + female Tumour/cancer type: sarcoma Chemotherapy regimen: doxorubicin + ifosfamide (AI) or ifosfamide (AI) and vincristine (VAI) is indicated Country: United States |
| Interventions |
Experimental: arm A: rolapitant i.v. dexamethasone daily and ondansetron i.v. on Days 1 to 5, and p.o. rolapitant hydrochloride on Day 1 treatment repeats every 21 days for 6 cycles in the absence of disease progression or unacceptable toxicity Experimental: arm B: fosaprepitant i.v. dexamethasone and i.v. ondansetron on Days 1 to 5, and i.v. fosaprepitant dimeglumine over 30 min on Day 1 Cycle 2. Treatment repeats every 21 days for 6 cycles in the absence of disease progression or unacceptable toxicity |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
|
NCT03403712.
| Methods |
Randomised, phase 3b study with 2 arms
Study period: 16 March 2018 to 19 September 2018
Masking: quadruple (participant, care provider, investigator, outcomes assessor) Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: anthracycline + cyclophosphamide Country: United States, Georgia (multi‐centre) |
| Interventions |
Experimental: arm A i.v. fosnetupitant/ palonosetron (260 mg/0.25 mg) fixed‐dose combination, administered as a 30‐min infusion of a 50‐mL solution on Day 1 of each cycle p.o. dexamethasone will be administered on Day 1 of each cycle (12 mg) Control: arm B p.o. netupitant/palonosetron (300 mg/0.50 mg) fixed‐dose combination on Day 1 of each cycle p.o. dexamethasone will be administered on Day 1 of each cycle (12 mg) |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
|
PER‐055‐12.
| Methods |
Randomised, multi‐centre, parallel‐group, active‐controlled, phase 3 study Recruitment period
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: 18 to 99 Gender: male + female Tumour/cancer type: n.r. Chemotherapy regimen: HEC Country: n.r. (multi‐centre) |
| Interventions |
|
| Outcomes |
|
| Notes |
|
Spina 1995.
| Methods |
Randomised study with 2 arms
Recruitment period: n.r.
Masking: n.r. Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria: n.r. Exclusion criteria: n.r. Mean/median age, years: n.r. Gender: n.r. Tumour/cancer type: HIV‐related non‐Hodgkin lymphoma (HIV‐NHL) Chemotherapy regimen: moderately emetogenic chemotherapy regimen Country: n.r. |
| Interventions |
Experimental: arm A granisetron Experimental: arm B ondansetron |
| Outcomes | n.r. |
| Notes |
|
UMIN000004826.
| Methods |
Randomised, interventional, phase 2 study with 2 arms
Recruitment period: October 2010 to n.r.
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: head and neck cancer Chemotherapy regimen: cisplatin ≥ 60 mg/m² Country: Japan |
| Interventions |
Cross‐over study Experimental: arm A: granisetron aprepitant was administered p.o. at 125 mg/body 1 h or 1 h and a half before cisplatin administration. On Days 2 and 3, aprepitant was administrated p.o. at 80 mg/body granisetron 40 μg/body and dexamethasone 12.3 mg/body were administered i.v. 30 min before cisplatin administration. On Days 2 and 3, granisetron 40 μg/body and dexamethasone 6.6 mg/body were administered Experimental: arm B: palonosetron aprepitant was administered p.o. at 125 mg/body 1 h or 1 h and a half before cisplatin administration. On Days 2 and 3, aprepitant was administrated p.o. at 80 mg/body palonosetron 0.75 mg/body and dexamethasone 12.3 mg/body were administered i.v. 30 min before cisplatin administration. On Days 2 and 3, dexamethasone 6.6 mg/body was administered |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
|
UMIN000004863.
| Methods |
Randomised, phase 3, active‐controlled study with 2 arms
Study period: May 2011 to June 2012
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: solid malignancy (lung cancer, gastric cancer, oesophagus cancer, cervical cancer, endometrial cancer, head and neck cancer, etc.) Chemotherapy regimen: cisplatin ≥ 50 mg/m² Country: Japan |
| Interventions |
Experimental: arm A: granisetron granisetron 1 mg + dexamethasone (Days 1 to 4) + aprepitant (Days 1 to 3) Experimental: arm B: palonosetron palonosetron 0.75 mg + dexamethasone (Days 1 to 4) + aprepitant (Days 1 to 3) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
UMIN000004998.
| Methods |
Randomised, phase 2 study with 2 arms
Recruitment period: January 2011 to January 2013
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: female Tumour/cancer type: haematology and clinical oncology Chemotherapy regimen: carboplatin‐ or irinotecan‐ (> 150 mg/m²) based regimens Country: Japan |
| Interventions |
Experimental: arm A Day 1, aprepitant 125 mg, granisetron 1 mg, and dexamethasone 12 mg before chemotherapy; Days 2 through 3, aprepitant 80 mg QD and dexamethasone 4 mg QD Control: arm B Day 1, granisetron 1 mg and dexamethasone 20 mg before chemotherapy; Days 2 through 3, dexamethasone 8 mg QD |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
UMIN000008041.
| Methods |
Randomised, cross‐over, active‐controlled study with 2 arms Recruitment period: February 2012 to n.r.
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: advanced/recurrent gastric cancer or colorectal cancer Chemotherapy regimen: FOLFILI, CPT‐11 monotherapy, FOLFOX, or XELOX regimen Country: Japan |
| Interventions |
Cross‐over study Experimental: arm A
Experimental: arm B
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
|
UMIN000008552.
| Methods |
Randomised, active‐controlled, cross‐over study with 2 arms
Recruitment period: August 2012 to n.r.
Masking: single‐blind (participants were blinded) Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: female Tumour/cancer type: gynaecological cancer Chemotherapy regimen: carboplatin (> AUC 5) Country: Japan |
| Interventions |
Cross‐over study Experimental: arm A NK₁ receptor antagonist (fosaprepitant) + 5‐HT₃ receptor antagonist + dexamethasone Experimental: arm B palonosetron + dexamethasone |
| Outcomes |
Primary outcome
|
| Notes |
|
UMIN000008897.
| Methods |
Randomised, interventional, phase 3 study with 2 arms
Recruitment period: November 2012 to n.r.
Masking: double‐blind Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: breast cancer Chemotherapy regimen: AC/EC/FAC/FEC chemotherapy Country: Japan |
| Interventions |
Experimental: arm A: granisetron granisetron + dexamethasone Experimental: arm B: palonosetron palonosetron + dexamethasone |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
UMIN000010056.
| Methods |
Randomised, active‐controlled, phase 2 study with 2 arms
Recruitment period: April 2014 to n.r.
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: lung cancer Chemotherapy regimen: carboplatin Country: Japan |
| Interventions |
Experimental: arm A aprepitant + palonosetron + dexamethasone Experimental: arm B palonosetron + dexamethasone |
| Outcomes | n.r. |
| Notes |
|
UMIN000010186.
| Methods |
Randomised study with 2 arms
Recruitment period: May 2013 to October 2015
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: male + female Tumour/cancer type: n.r. Chemotherapy regimen: carboplatin (CBDCA)‐based moderately emetogenic chemotherapy (MEC) Country: Japan |
| Interventions |
Experimental: arm A aprepitant (Day 1: 125 mg, Days 2 to 3: 80 mg) + granisetron 3 mg + dexamethasone (Day 1: 6.6 mg i.v., Days 2 to 3: 2 mg POX2) Experimental: arm B palonosetron 0.75 mg + dexamethasone (Day 1: 9.9 mg i.v., Days 2 to 3: 4 mg POX2) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
|
UMIN000019122.
| Methods |
Randomised, phase 2 study with 2 arms
Recruitment period: November 2011 to n.r.
Masking: open‐label Baseline patient characteristics: n.r. |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: n.r. Gender: female Tumour/cancer type: gynaecological cancer Chemotherapy regimen: carboplatin (area under the concentration curve of 5) and paclitaxel at 175 mg/m² Country: Japan |
| Interventions |
Experimental: arm A p.o. administration of 125 mg aprepitant 90 min before administration of chemotherapy drug on Day 1 and of 80 mg on Days 2 and 3, and 0.75 mg + palonosetron administered i.v. on Day 1 + 6 mg dexamethasone administered i.v. on Day 1 and 4 mg dexamethasone administered p.o. on Days 2 and 3 Experimental: arm B 0.75 mg palonosetron administered i.v. on Day 1 + 6 mg dexamethasone administered i.v. on Day 1 and 4 mg dexamethasone administered p.o. on Days 2 and 3 |
| Outcomes |
Primary outcome
|
| Notes |
|
5‐FU: 5‐fluorouracil.
5‐HT₃: serotonin (5‐hydroxytryptamine).
AC: doxorubicin, cyclophosphamide.
ALT: alanine aminotransferase.
AST: aspartate aminotransferase.
CNS: central nervous system.
Cr: creatinine.
CPT‐11: camptothecin‐11.
CTCAE: Common Terminology Criteria for Adverse Events.
d: day (e.g., d1, d3).
EC: epirubicin.
ECG: electrocardiogram.
ECOG: Eastern Cooperative Oncology Group.
ESMO: European Society for Medical Oncology.
FAC: 5‐fluorouracil (5FU) + AC.
FEC: fluorouracil, epirubicin, cyclophosphamide.
FLIE: Functional Living Index‐Emesis.
FOLFILI: folinic acid, fluorouracil, and irinotecan.
FOLFOX: folinic acid, fluorouracil, and oxaliplatin.
g/L: gram per litre.
g/m²: gram per square meter.
h: hour.
Hb: haemoglobin.
HBV: hepatitis B virus.
HCV: hepatitis C virus.
HEC: highly emetogenic chemotherapy.
HIV: human immunodeficiency virus.
IU: international unit.
i.v.: intravenous.
L: litre.
MASCC: Annual Meeting on Supportive Cancer Care.
MEC: moderately emetogenic chemotherapy.
mg: milligram.
mL/min: millilitre per minute.
msec: millisecond.
NCI: National Cancer Institute.
NK₁: neurokinin‐1.
n.r.: not reported.
NSCLC: non‐small cell lung carcinoma.
PLT: platelets.
p.o.: oral.
QD: once a day.
QTc: QT interval corrected for heart rate.
SOX: S‐1 plus oxaliplatin.
TBIL: total bilirubin.
ULN: upper limit of normal.
VAS: visual analogue scale.
VP‐16: etoposide.
VM‐26: teniposide.
vs: versus.
WBC: white blood cell count.
XELOX: capecitabine, oxaliplatin.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900025227.
| Study name | A randomized, open, parallel controlled phase II clinical study comparing the efficacy and safety of dexamethasone, palonosetron, or aprepitant in the control of acute and delayed vomiting in non‐small cell lung cancer patients receiving multiple moderately emetogenic chemotherapy regimens |
| Methods |
Randomised, interventional, active‐controlled study with 2 arms
Target sample size: 100 Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: both Tumour/cancer type: non‐small cell lung cancer Chemotherapy regimen: not reported, moderately emetogenic chemotherapy Country: China |
| Interventions |
Experimental: arm A: aprepitant/palonosetron/dexamethasone Intervention details not reported Experimental: arm B: palonosetron/dexamethasone Intervention details not reported |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 15 August 2019 |
| Contact information | Research and public contact: Zhang Lemeng (Hunan Cancer Hospital, 283 Tongzipo Road, Yuelu District, Changsha, Hu'nan, China, email: zhanglemeng@hnca.org.cn) |
| Notes |
|
IRCT20191103045317N1.
| Study name | Comparison between the effect of Triplet Aprepitant/Dexamethasone/Ondansetron vs doublet Dexamethasone/Ondansetron for prevention of moderately emetogenic chemotherapy: placebo‐controlled double blind, randomised clinical trial of efficacy |
| Methods |
Randomised, interventional, active‐controlled study with 2 arms
Target sample size: 90 (actual sample size reached: 160) Masking: double‐blind Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: not available Tumour/cancer type: not available Chemotherapy regimen: not available, moderately emetogenic chemotherapy Country: Iran |
| Interventions |
Experimental: arm A: aprepitant/dexamethasone/ondansetron p.o. administration of 125 mg aprepitant, i.v. injection of 12 mg dexamethasone and 8 mg ondansetron on Day 1, followed by 80 mg Abitant on Days 2 and 3 Experimental: arm B: dexamethasone/ondansetron i.v. injection of 12 mg dexamethasone and 8 mg ondansetron on Day 1 |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 21 December 2018 |
| Contact information | Research and public contact: Mania Rajabzadeh Kheradmardi (Shahid Beheshti University of Medical Sciences, Jorjani Center of Radiation Oncology, Imam Husein Hospital, Shahid Madani Ave, Nezam Abad Town, Tehran, Iran, email: mania_008@yahoo.com) |
| Notes |
|
KTC0001495.
| Study name | A randomized, double‐blind, double‐dummy, parallel group, international multi center study assessing the efficacy and safety of a netupitant‐palonosetron Fixed Dose Combination (FDC) compared to an extemporary combination of granisetron and aprepitant on the prevention of highly emetogenic chemotherapy‐induced nausea and vomiting in patients with cancer |
| Methods |
Randomised, interventional, phase 3 study with 2 arms
Target sample size: 832 Masking: double‐blind Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: male + female Tumour/cancer type: solid tumour malignancy Chemotherapy regimen: cisplatin‐based chemotherapy regimen Country: multi‐national (7 centres) |
| Interventions |
Experimental: arm A p.o. administration of NETU‐PALO FDC (containing 300 mg netupitant and 0.5 mg palonosetron) on Day 1 (with adjusted dexamethasone regimen: 12 mg on Day 1 + 8 mg daily from Day 2 to Day 4) Active control: arm B p.o. aprepitant 125 mg (on Day 1) + 80 mg daily (on Days 2 and day 3) and 3 mg i.v. granisetron on Day 1 (with adjusted dexamethasone regimen: 12 mg on Day 1 + 8 mg daily from Day 2 to Day 4) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 15 January 2015 |
| Contact information | Younyoung Cho, Konkuk University Medical Center |
| Notes |
|
NCT03606369.
| Study name | Effectiveness and quality of life analysis of palonosetron against ondansetron combined with dexamethasone and fosaprepitant in prevention of acute and delayed emesis associated to chemotherapy moderately and highly emetogenic in breast cancer |
| Methods |
Randomised, interventional, parallel study with 2 arms
Estimated enrolled patients: 560 Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: anthracyclines combined with cyclophosphamide or carboplatin combined with docetaxel or docetaxel combined with cyclophosphamide Country: Mexico |
| Interventions |
Experimental: arm A: palonosetron early emesis: palonosetron 0.25 mg i.v. + dexamethasone 12 mg i.v. + fosaprepitant 150 mg i.v. delayed emesis: dexamethasone 8 mg p.o. on Days 2, 3, and 4 Experimental: arm B: ondansetron early emesis: ondansetron 16 mg i.v. + dexamethasone 12 mg i.v. + fosaprepitant 150 mg i.v. delayed emesis: metoclopramide 10 mg p.o. every 6 hours + dexamethasone 8 mg p.o. every 24 hours |
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | 5 November 2015 |
| Contact information | Contact: Claudia H. Arce Salinas, MD; phone: 56280400 ext 12065; email: c.arce.salinas@gmail.com Contact: Juan P González Serrano, BD; phone: 5519480352; email: jpablogs_9@hotmail.com |
| Notes |
|
UMIN000004021.
| Study name | Study of oral neurokinin‐1 antagonist, aprepitant for the prevention of nausea and vomiting in patients receiving chemotherapy with irinotecan alone or combination of irinotecan plus cisplatin for unresectable gastric cancer |
| Methods |
Randomised, cross‐over, interventional, placebo‐controlled study with 2 arms
Target sample size: 80 Masking: double‐blind Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: male + female Tumour/cancer type: gastric cancer Chemotherapy regimen: irinotecan alone or combination of irinotecan plus cisplatin Country: Japan |
| Interventions |
Cross‐over study Experimental: arm A: aprepitant aprepitant: 125 mg p.o./d on Day 1 followed by 80 mg p.o./d on Days 2 and 3 dexamethasone: 3.3 mg i.v./body on Days 1 to 3 granisetron: 40 μg i.v./kg on Day 1 Experimental: arm B: placebo placebo: placebo capsules p.o. on Days 1 to 3 dexamethasone: 6.6 mg i.v./body on Days 1 to 3 granisetron: 40 μg i.v./kg on Day 1 |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 1 August 2010 |
| Contact information |
Research contact: Hiroto Miwa (Division of Upper Gastroenterology, Department of Internal Medicine; Hyogo College of Medicine; 1‐1 Mukogawa‐cho, Nishinomiya, Hyogo, Japan) Public contact: Junji Tanaka (Division of Upper Gastroenterology, Department of Internal Medicine; Hyogo College of Medicine; 1‐1 Mukogawa‐cho, Nishinomiya, Hyogo, Japan) |
| Notes |
|
UMIN000005317.
| Study name | Effect of oral neurokinin‐1 antagonist, aprepitant for chemotherapy‐induced nausea and vomiting in patients with gynecologic cancer receiving carboplatin/paclitaxel chemotherapy |
| Methods |
Randomised, interventional, parallel, active‐controlled study with 2 arms
Target sample size: 60 Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: female Tumour/cancer type: gynaecological cancer Chemotherapy regimen: carboplatin/paclitaxel Country: Japan |
| Interventions |
Experimental: arm A: aprepitant aprepitant 125 mg p.o. on Day 1 aprepitant 80 mg p.o. on Days 2 to 3 granisetron 3 mg i.v. on Day 1 dexamethasone 16 mg or 8 mg i.v. on Day 1 dexamethasone 4 mg p.o. on Days 2 to 3 Experimental: arm B granisetron 3 mg i.v. on Day 1 dexamethasone 16 mg or 8 mg i.v. on Day 1 dexamethasone 8 mg p.o. on Days 2 to 3 |
| Outcomes |
Primary outcome
|
| Starting date | 29 November 2010 |
| Contact information | Research contact: Hiroshi Tsujioka (Department of Gynecology; Fukuoka University Hospital; 7‐45‐1 Nanakuma, Jonan‐ku, Fukuoka, Japan) |
| Notes |
|
UMIN000005494.
| Study name | Aprepitant for nausea, vomiting with the TC therapy of the gynecology cancer patient or the DC therapy, fosaprepitant, granisetron, protective efficacy of the dexamethasone combination therapy |
| Methods |
Randomised, interventional, active‐controlled study with 2 arms
Target sample size: 140 Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: female Tumour/cancer type: uterine cancer, ovarian cancer Chemotherapy regimen: paclitaxel/carboplatin or docetaxel/carboplatin Country: Japan |
| Interventions |
Experimental: arm A: aprepitant/fosaprepitant aprepitant on Days 1 to 3 (or fosaprepitant on Day 1) granisetron on Day 1 dexamethasone on Days 1 to 4 Experimental: arm B granisetron on Day 1 dexamethasone on Days 1 to 4 |
| Outcomes |
Primary outcome
|
| Starting date | 1 May 2011 |
| Contact information |
Research contact: Hideaki Masuzaki (Department of Obstetrics and Gynecology; Nagasaki University (graduate school); 1‐7‐1 Sakamoto, Nagasaki, Japan) Public contact: Shuhei Abe (Department of Obstetrics and Gynecology; Nagasaki University (graduate school); 1‐7‐1 Sakamoto, Nagasaki, Japan; email: koutabe@yahoo.co.jp) |
| Notes |
|
UMIN000006773.
| Study name | Randomized phase II study of aprepitant in patients with colorectal cancer receiving FOLFOX, FOLFIRI, or XELOX chemotherapy regimen |
| Methods |
Randomised, phase 2, parallel, interventional controlled study with 2 arms
Target sample size: 100 Masking: n.r. Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: male + female Tumour/cancer type: colon and rectal cancer Chemotherapy regimen: FOLFOX, FOLFIRI, or XELOX chemotherapy regimen Country: Japan |
| Interventions |
Experimental: arm A: aprepitant aprepitant 125 mg p.o. on Day 1 aprepitant 80 mg p.o. on Days 2 and 3 5‐HT₃ receptor antagonist i.v. on Day 1 dexamethasone 6.6 mg i.v. on Day 1 dexamethasone 4 mg p.o. on Days 2 and 3 Experimental: arm B 5‐HT₃ receptor antagonist i.v. on Day 1 dexamethasone 9.9 mg i.v. on Day 1 dexamethasone 8 mg p.o. on Days 2 and 3 |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 1 November 2011 |
| Contact information |
Contact 1: Shoji Natsugoe (Department of Digestive Surgery, Breast and Thyroid Surgery; Kagoshima University Graduate School of Medical and Dental Sciences; 8‐35‐1 Sakuragaoka, Kagoshima, Japan; telephone: 099‐275‐5358) Contact 2: Sumiya Ishigami (Department of Digestive Surgery, Breast and Thyroid Surgery; Kagoshima University Graduate School of Medical and Dental Sciences; 8‐35‐1 Sakuragaoka, Kagoshima, Japan; telephone: 099‐275‐5360) |
| Notes |
|
UMIN000007882.
| Study name | Multicenter double‐blind randomized comparative parallel study with concomitant therapy of 3 drugs, aprepitant + dexamethasone+palonosetron or aprepitant + dexamethasone+ granisetron, for prevention of nausea/vomiting in breast cancer patients receiving AC therapy |
| Methods |
Randomised, interventional, controlled study with 2 arms
Target sample size: 660 Masking: double blind Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: doxorubicin hydrochloride (adriamycin) and cyclophosphamide Country: Japan |
| Interventions |
Experimental: arm A: granisetron aprepitant (Day 1: 125 mg, Days 2 to 3: 80 mg) + dexamethasone (Day 1: 9.9 mg) + granisetron (Day 1: 40 (microgram)/kg) Experimental: arm B: palonosetron aprepitant (Day 1: 125 mg, Days 2 to 3: 80 mg) + dexamethasone (Day 1: 9.9 mg) + palonosetron (Day 1: 0.75 mg) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 1 June 2012 |
| Contact information | Research and public contact: Mitsue Saito (Department of Breast Oncology; Juntendo University Hospital; 3‐1‐3, Hongo, Bunkyo‐ku, Tokyo 113‐8421; email: mitsue@juntendo.ac.jp) |
| Notes |
|
UMIN000012500.
| Study name | Effect of aprepitant for nausea and vomiting during paclitaxel + carboplatin (TC) therapy |
| Methods |
Randomised, cross‐over, interventional study with 2 arms
Target sample size: 50 Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: female Tumour/cancer type: gynaecological malignancy Chemotherapy regimen: paclitaxel + carboplatin (TC) regimen Country: Japan |
| Interventions |
Cross‐over study Experimental: arm A first course 5‐HT₃ receptor antagonist + dexamethasone i.v. second course 5‐HT₃ receptor antagonist + dexamethasone i.v. + aprepitant p.o. Experimental: arm B first course 5‐HT₃ receptor antagonist + dexamethasone i.v. + aprepitant p.o. second course 5‐HT₃ receptor antagonist + dexamethasone i.v. |
| Outcomes |
Objective
|
| Starting date | 18 January 2011 |
| Contact information |
Research contact: Masahide Ohmichi; Department of Obstetrics and Gynecology; Osaka Medical College; 2‐7, Daigaku‐machi, Takatsuki, Osaka; email: m‐ohmichi@poh.osaka‐med.ac.jp Public contact: Masanori Kanemura; Department of Obstetrics and Gynecology; Osaka Medical College; 2‐7, Daigaku‐machi, Takatsuki, Osaka; email: gyn044@poh.osaka‐med.ac.jp |
| Notes |
|
UMIN000032860.
| Study name | Palonosetron versus granisetron in combination with fosaprepitant and dexamethasone for TC therapy in patients with gynecologic cancer |
| Methods |
Randomised, factorial, active‐controlled study with 2 arms
Target sample size: 240 Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: female Tumour/cancer type: gynaecological cancer Chemotherapy regimen: paclitaxel + carboplatin (TC) regimen Country: Japan |
| Interventions |
Experimental: arm A: palonosetron fosaprepitant + palonosetron + dexamethasone Experimental: arm B: granisetron fosaprepitant + granisetron + dexamethasone |
| Outcomes |
Primary outcome
|
| Starting date | 10 June 2018 |
| Contact information | Research and contact person: Soshi Kusunoki; Department of Obstetrics and Gynecology; Faculty of Medicine, Juntendo University; Bunkyoku, Tokyo, Japan; email: skusuno@juntendo.ac.jp |
| Notes |
|
UMIN000041004.
| Study name | To establish of optimal antiemetic therapy for trastuzumab deruxtecan therapy‐induced nausea and vomiting in patients with breast cancer: an open‐label, randomized pilot study |
| Methods |
Randomised, interventional, controlled study with 2 arms
Target sample size: 40 Masking: open‐label Baseline patient characteristics: not available |
| Participants |
Inclusion criteria
Exclusion criteria
Mean/median age, years: not available Gender: female Tumour/cancer type: breast cancer Chemotherapy regimen: trastuzumab deruxtecan Country: Japan |
| Interventions |
Experimental: arm A granisetron + dexamethasone + aprepitant (fosaprepitant) Experimental: arm B granisetron + dexamethasone |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 1 July 2020 |
| Contact information | Research and public contact: Hirotoshi Iihara (Gifu University Hospital, Department of Pharmacy, 500‐1194 1‐1 Yanagido, Gifu, email: dai0920@gifu‐u.ac.jp) |
| Notes |
|
AC: doxorubicin, cyclophosphamide.
ALT: alanine transaminase.
AST: aspartate aminotransferase.
CINV: chemotherapy‐induced nausea and vomiting.
ECG: electrocardiogram.
ECOG: Eastern Cooperative Oncology Group.
dL: decilitre.
FOLFILI: folinic acid, fluorouracil, and irinotecan.
FOLFOX: folinic acid, fluorouracil, and oxaliplatin.
g: gram.
i.v.: intravenous.
kg: kilogram.
min: minute.
mL: millilitre.
NK₁: neurokinin‐1.
p.o.: oral.
QoL: quality of life.
ULN: upper limit of normal.
VAS: visual analogue scale.
WBC: white blood cell count.
XELOX: capecitabine, oxaliplatin.
Differences between protocol and review
Criteria for considering studies for this review
Types of interventions
At protocol stage, we defined that the corticosteroid dexamethasone had to be included in the antiemetic treatment regimen. We named dexamethasone because this is the corticosteroid that is most widely used. However, we had missed that methylprednisolone is sometimes used as the corticosteroid in these antiemetic regimens. We discussed this with a clinical expert (KJ) and decided that because the mechanism of action and the antiemetic role are comparable for these corticosteroids, trials using other corticosteroids (e.g. methylprednisolone) were eligible for inclusion.
Types of outcome measures
We re‐ordered outcome measures and grouped them into efficacy, quality of life, and safety because we think differentiating between primary and secondary outcomes is more appropriate for clinical trials. We added an overview table of all outcomes including their prioritisation according to consumer relevance.
We retrospectively added the outcome "serious adverse events", as we realised during preparation of our data extraction sheet that we missed listing it during preparation of the protocol.
Search methods for identification of studies
Searching other resources
We had planned to search the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct) for ongoing or completed, but not yet published, trials. We could not do so because the study register is no longer available.
Data collection and analysis
Assessment of risk of bias in included studies
At protocol stage, we had planned to consider sample size as one domain in our bias assessment. Because this is no longer recommended by the PaPaS Group, we removed this domain and considered other sources of bias instead. We did not pre‐specify this item, to provide us with some freedom to consider potential causes of bias that are not addressed in the other domains.
Summary of findings and assessment of the certainty of the evidence
At protocol stage, we had planned to include two 'Summary of findings' tables (one for HEC and one for MEC). Because it was not possible to include the information for all prioritised outcomes and all comparisons within a network in one comprehensive table, we discussed with the PaPaS Group and the Method Support Unit to include instead one 'Summary of findings' table per outcome. For a comprehensive illustration of our results, we randomly chose an exemplary reference treatment.
At protocol stage, we had planned to rate the certainty of evidence for each outcome using the GRADE approach. Because the latest guidance of the GRADE Working Group suggests that the certainty of evidence of no more than seven outcomes should be assessed (Guyatt 2013), we graded only the outcomes that are most critical or important for decision‐making. These are the outcomes that we have included in the 'Summary of findings' tables.
Based on recommendations by the PaPaS Group, we decided to downgrade twice for imprecision in case of very imprecise or very sparse data.
Contributions of authors
| Drafting the protocol | NS |
| Developing and running the search strategy | IM PaPaS Information Specialist provided support |
| Providing clinical advice | KJ |
| Obtaining copies of studies | NS, MH |
| Selecting which studies to include (2 people) | NS, MH, BS |
| Extracting data from studies (2 people) | VP, MH, BS, NK |
| Assessing risk of bias | MH, NK |
| Entering data into RevMan | VP, MH |
| Carrying out the analysis | AA, KK |
| Interpreting the analysis | VP, NS |
| Performing GRADE assessment | VP, BS |
| Drafting the final review | VP, MH |
| Taking responsibility for updates | NS, VP |
Sources of support
Internal sources
-
University Hospital Cologne, Germany
Cochrane Cancer, Department I of Internal Medicine
External sources
-
German Ministry for Education and Research (BMBF), Germany
Grant no: 01KG1510
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS)
Declarations of interest
VP: none known.
AA: none known.
MH: none known.
BS: none known.
NK: none known.
IM: none known.
KJ is a specialist oncology physician who manages patients with CINV. She received consultancy fees or honoraria from Hexal (2016‐2020), Riemser (2016‐2021), Pfizer (2016‐2017), and Voluntis (2016‐2021). Moreover, she was an investigator on Jordan 2016a; Rapoport 2010; and Weinstein 2016. She was not involved in study selection, bias assessment, or data extraction or interpretation, but served as a content expert.
KK: none known.
NS: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Aapro 2006 {published data only}
- Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Annals of Oncology 2006;17(9):1441-9. [DOI] [PubMed] [Google Scholar]
Abdel‐Malek 2017 {published data only}
- Abdel-Malek R, Abbas N, Shohdy KS, Ismail M, Fawzy R, Salem DS, et al. Addition of 3-day aprepitant to ondansetron and dexamethasone for prophylaxis of chemotherapy-induced nausea and vomiting among patients with diffuse large B cell lymphoma receiving 5-day cisplatin-based chemotherapy. Journal of Egyptian National Cancer Institute 2017;29:155-8. [DOI] [PubMed] [Google Scholar]
Aksu 2013 {published data only}
- Aksu G, Dolasik I, Ensaroglu F, Sener SY, Aydin FH, Temiz S, et al. Evaluation of the efficacy of aprepitant on the prevention of chemotherapy-induced nausea and vomiting and quality of life with functional living index emesis. Balkan Medical Journal 2013;30:64-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albany 2012 {published data only}
- Albany C, Brames MJ, Fausel C, Johnson CS, Picus J, Einhorn LH. Randomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a Hoosier Oncology Group study. Journal of Clinical Oncology 2012;30:3998-4003. [CLINICALTRIALS.GOV IDENTIFIER: NCT00572572] [DOI] [PubMed] [Google Scholar]
- Brames M, Picus J, Yu M, Johnston E, Bottema B, Williams C, et al. Phase III double-blind placebo-controlled study evaluating 5HT3 antagonist + dexamethasone +/- aprepitant in germ cell tumors receiving 5-day cisplatin chemotherapies. In: Supportive Care in Cancer. 2011:S140.
Ando 2016 {published data only}
- Ando Y, Hayashi T, Ito K, Suzuki E, Mine N, Miyamoto A, et al. Comparison between 5-day aprepitant and single-dose fosaprepitant meglumine for preventing nausea and vomiting induced by cisplatin-based chemotherapy. Support Care Cancer 2016;24(2):871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arce‐Salinas 2019 {published data only}
- Arce-Salinas C, Deneken-Hernandez Z, Flores-Diaz D, Gonzalez-Serrano JP, Matus-Santos JA, Ruiz-Garcia E, et al. Efficacy and quality of life analysis of palonosetron vs ondansetron for high and moderate emetogenic chemotherapy for breast cancer. Cancer Research 2019;79(4):Supplement.
Aridome 2016 {published data only}
- Aridome K, Mori SI, Baba K, Yanagi M, Hamanoue M, Miyazono F, et al. A phase II, randomized study of aprepitant in the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapies in colorectal cancer patients. Molecular and Clinical Oncology 2016;4(3):393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arpornwirat 2009 {published data only}
- Arpornwirat W, Albert I, Hansen VL, Levin J, Bandekar RR, Grunberg SM. Phase 2 trial results with the novel neurokinin-1 receptor antagonist casopitant in combination with ondansetron and dexamethasone for the prevention of chemotherapy-induced nausea and vomiting in cancer patients receiving moderately emetogenic chemotherapy. Cancer 2009;115(24):5807-16. [DOI] [PubMed] [Google Scholar]
Badar 2015 {published data only}
- Badar T, Cortes J, Borthakur G, O'Brien S, Wierda W, Garcia-Manero G, et al. Phase II, open label, randomized comparative trial of ondansetron alone versus the combination of ondansetron and aprepitant for the prevention of nausea and vomiting in patients with hematologic malignancies receiving regimens containing high-dose cytarabine. Biomed Research International 2015:497-597. [DOI] [PMC free article] [PubMed]
Brohee 1995 {published data only}
- Brohee D. Comparison of dexamethasone (DXM) + granisetron (G) or + ondansetron (O) in cancer patients treated with moderately emetic cytotoxics [abstract no: 1231]. In: European Journal of Cancer. Vol. 31. 1995:S257.
Bubalo 2005 {published data only}
- Bubalo J. Randomized pilot study of aprepitant in combination with standard antiemetic therapy comprising ondansetron and dexamethasone for the control of nausea and vomiting in patients undergoing hematopoietic stem cell transplantation, 2005. https://clinicaltrials.gov/ct2/show/NCT00248547. [CLINICALTRIALS.GOV IDENTIFIER: NCT00248547]
Bubalo 2018 {published data only}
- Bubalo J, Mulverhill K, Meyers G, Hayes-Lattin B, Maziarz R. A randomized, placebo-controlled pilot trial of aprepitant combined with standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting in patients undergoing cyclophosphamide-based conditioning regimens prior to hematopoietic stem cell transplant (HSCT). Bone Marrow Transplant 2018;53:1010-8. [DOI] [PubMed] [Google Scholar]
- Bubalo JS, Cherala G, McCune JS, Munar MY, Tse S, Maziarz R. Aprepitant pharmacokinetics and assessing the impact of aprepitant on cyclophosphamide metabolism in cancer patients undergoing hematopoietic stem cell transplantation. Journal of Clinical Pharmacology 2012;52(4):586-94. [DOI] [PubMed] [Google Scholar]
Campos 2001 {published data only}
- Campos D, Pereira JR, Reinhardt RR, Carracedo C, Poli S, Vogel C, Martinez-Cedillo J, et al. Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. Journal of Clinical Oncology 2001;19(6):1759-67. [DOI] [PubMed] [Google Scholar]
Chawla 2003 {published data only}
- Chawla SP, Grunberg SM, Gralla RJ, Hesketh PJ, Rittenberg C, Elmer ME, et al. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer 2003;97:2290-300. [DOI] [PubMed] [Google Scholar]
- Martin A, Cai B, Horgan K, Elmer M, Pearson J. Patients treated with an NK1 receptor antagonist report less hardship due to chemotherapy-induced nausea and vomiting compared to those on standard antiemetic therapy [abstract]. In: European Journal of Cancer. Vol. 37. 2001:S280.
- Martin AR, Carides AD, Pearson JD, Horgan K, Elmer M, Schmidt C, et al. Functional relevance of antiemetic control. Experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. European Journal of Cancer 2003;97:1395-401. [DOI] [PubMed] [Google Scholar]
- Wit R, Herrstedt J, Rapoport B, Carides AD, Carides G, Elmer M, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. Journal of Clinical Oncology 2003;21(22):4105-11. [DOI] [PubMed] [Google Scholar]
Cheirsilpa 2005 {published data only}
- Cheirsilpa A, Sinthusake T, Songsakkaesorn A, Visawaprasit S, Chulaka K, Changkuingdee N. Comparison of ramosetron and granisetron for the prevention of acute and delayed emesis in cisplatin-based chemotherapy: a randomized controlled trial. Japanese Journal of Clinical Oncology 2005;35(12):695-9. [DOI] [PubMed] [Google Scholar]
Cho 1998 {published data only}
- Cho ST, Hwang BK, Kim CK, Ko BM, Bae SH, Bong JD. A comparison of the acute antiemetic effect of tropisetron with ondansetron in patients receiving cisplatin. Journal of the Korean Cancer Association 1998;(6):1240-8.
Chua 2000 {published data only}
- Chua DT, Sham JS, Kwong DL, Kwok CC, Yue A, Foo YC, et al. Comparative efficacy of three 5-HT3 antagonists (granisetron, ondansetron, and tropisetron) plus dexamethasone for the prevention of cisplatin-induced acute emesis: a randomized crossover study. American Journal of Clinical Oncology 2000;(2):185-91. [DOI] [PubMed]
Egerer 2010 {published data only}
- Egerer G, Eisenlohr K, Gronkowski M, Burhenne J, Riedel KD, Mikus G. The NK1 receptor antagonist aprepitant does not alter the pharmacokinetics of high-dose melphalan chemotherapy in patients with multiple myeloma. British Journal of Clinical Pharmacology 2010;70(6):903-7. [EUDRACTI-NO: EudraCT 2004-004956-38] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenberg 2003 {published data only}
- Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 2003;98(11):2473-82. [DOI] [PubMed] [Google Scholar]
Endo 2012 {published data only}
- Endo J, Iihara H, Yamada M, Yanase K, Kamiya F, Ito F, et al. A randomized controlled non-inferiority study comparing the antiemetic effect between intravenous granisetron and oral azasetron based on estimated 5-HT3 receptor occupancy. Anticancer Research 2012;32(9):3939-47. [PubMed] [Google Scholar]
Flenghi 2015 {published data only}
- Flenghi L, Bonifacio E, Reboldi G, Muzi C, Ballanti S, Falcinelli F, et al. Aprepitant prevents nausea and vomiting after HD-CTX for PBSC harvesting: a phase III, double-blinded, randomized, placebo-controlled trial. In: Haematological Oncology. 2015:308.
Forni 2000 {published data only}
- Forni C, Ferrari S, Loro L, Mazzei T, Beghelli C, Biolchini A, et al. Granisetron, tropisetron, and ondansetron in the prevention of acute emesis induced by a combination of cisplatin-Adriamycin and by high-dose ifosfamide delivered in multiple-day continuous infusions. Supportive Care in Cancer 2000;(2):131-3. [DOI] [PubMed]
Fox‐Geiman 2001 {published data only}
- Fox-Geiman MP, Fisher SG, Kiley K, Fletcher-Gonzalez D, Porter N, Stiff P. Double-blind comparative trial of oral ondansetron versus oral granisetron versus IV ondansetron in the prevention of nausea and vomiting associated with highly emetogenic preparative regimens prior to stem cell transplantation. Biology of Blood and Marrow Transplantation 2001;7(11):596-603. [DOI] [PubMed] [Google Scholar]
Fujiwara 2015 {published data only}
- Fujiwara S, Terai Y, Tsunetoh S, Sasaki H, Kanemura M, Ohmichi M. Palonosetron versus granisetron in combination with aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with gynecologic cancer. Journal of Gynecologic Oncology 2015;26(4):311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2013 {published data only}
- Gao Y-Q, Chen Y-D, Feng L-H. Effect of palonosetron in prevention of nausea and vomiting induced by cisplatin-based chemotherapy. [Chinese]. Journal of Practical Oncology 2013;28(6):654-6.
- NCT02445872. Safety and efficacy of aprepitant for CINV in patients with lung cancer receiving multiple-day cisplatin chemotherapy, 2015. https://clinicaltrials.gov/show/nct02445872.
Ghosh 2010 {published data only}
- Ghosh S, Dey S. Comparing different antiemetic regimens for chemotherapy induced nausea and vomiting. International Journal of Collaborative Research on Internal Medicine & Public Health 2010;2(5):142-56.
Grunberg 2009 {published data only}
- Gridelli C, Haiderali AM, Russo M W, Blackburn LM, Lykopoulos K. Casopitant improves the quality of life in patients receiving highly emetogenic chemotherapy. Supportive Care in Cancer 2010;18(11):1437-44. [DOI] [PubMed] [Google Scholar]
- Grunberg SM, Rolski J, Strausz J, Aziz Z, Lane S, Russo MW, et al. Efficacy and safety of casopitant mesylate, a neurokinin 1 (NK1)-receptor antagonist, in prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy: a randomised, double-blind, placebo-controlled trial. Lancet Oncology 2009;10(6):549-58. [CLINICALTRIALS.GOV IDENTIFIER: NCT00431236] [DOI] [PubMed] [Google Scholar]
Grunberg 2011 {published data only}
- Grunberg S, Chua D, Maru A, Dinis J, Vandry SD, Boice JA, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol - EASE. Journal of Clinical Oncology 2011;29:1495-501. [CLINICALTRIALS.GOV IDENTIFIER: NCT00619359] [DOI] [PubMed] [Google Scholar]
- Maru A, Gangadharan VP, Desai CJ, Mohapatra RK, Carides AD. A phase 3, randomized, double-blind study of single-dose fosaprepitant for prevention of cisplatin-induced nausea and vomiting: results of an Indian population subanalysis. Indian Journal of cancer 2013;50(4):285-91. [DOI] [PubMed] [Google Scholar]
Hashimoto 2013 {published data only}
- Hashimoto H, Yamanaka T, Shimada Y, Arata K, Matsui R, Goto K, et al. Palonosetron (PALO) versus granisetron (GRA) in the triplet regimen with dexamethasone (DEX) and aprepitant (APR) for preventing chemotherapy-induced nausea and vomiting (CINV) in patients (pts) receiving highly emetogenic chemotherapy (HEC) with cisplatin (CDDP): a randomized, double-blind, phase III trial [conference proceeding]. Journal of Clinical Oncology 2013;(15 Suppl 1). [UNIQUE ID ISSUED BY UMIN: UMIN000004863]
- Suzuki K, Yamanaka T, Hashimoto H, Shimada Y, Arata K, Matsui R, et al. Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Annals of Oncology 2016;27:1601–6. [DOI] [PubMed] [Google Scholar]
- Tsuji D, Yokoi M, Suzuki K, Daimon T, Nakao M, Ayuhara H, et al. Influence of ABCB1 and ABCG2 polymorphisms on the antiemetic efficacy in patients with cancer receiving cisplatin-based chemotherapy: a TRIPLE pharmacogenomics study. Pharmacogenomics Journal 2016;31:31. [DOI] [PubMed] [Google Scholar]
Herrington 2000 {published data only}
- Herrington JD, Kwan P, Young RR, Lagow E, Lagrone L, Riggs MW. Randomized, multicenter comparison of oral granisetron and oral ondansetron for emetogenic chemotherapy. Pharmacotherapy 2000;20(11):1318-23. [DOI] [PubMed] [Google Scholar]
Herrington 2008 {published data only}
- Herrington JD, Jaskiewicz AD, Song J. Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer 2008;112(9):2080-7. [DOI] [PubMed] [Google Scholar]
Herrstedt 2009 {published data only}
- Aziz Z, Arpornwirat W, Herrstedt J, Camlett I, Piontek T, Ranganathan S, et al. Phase III results for the novel neurokinin-1 (NK-1) receptor antagonist, casopitant: 3-day IV/oral dosing regimen for chemotherapy-induced nausea and vomiting (CINV) in patients (Pts) receiving moderately emetogenic chemotherapy (MEC). Journal of Clinical Oncology 2008;26(15 Suppl):20512. [Google Scholar]
- Ewer M, Grunberg S, Ranganathan S, Lane S, Russo M. Cardiac safety data for casopitant, a neurokinin-1 receptor antagonist, given with anthracycline. In: Cancer Research. Vol. 69 (24 Suppl). 2010:1118.
- Grunberg SM, Aziz Z, Shaharyar A, Herrstedt J, Roila F, VanBelle S, et al. Phase III results of a novel oral neurokinin-1 (NK-1) receptor antagonist, casopitant: single oral and 3-day oral dosing regimens for chemotherapy-induced nausea and vomiting (CINV) in patients (pts) receiving moderately emetogenic chemotherapy (MEC). Journal of Clinical Oncology 2008;26(15 Suppl):9540. [Google Scholar]
- Herrstedt J, Apornwirat W, Shaharyar A, Aziz Z, Roila F, Van Belle S, et al. Phase III trial of casopitant, a novel neurokinin-1 receptor antagonist, for the prevention of nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Journal of Clinical Oncology 2009;27(32):5363-9. [DOI] [PubMed] [Google Scholar]
Hesketh 1999 {published data only}
- Hesketh PJ, Gralla RJ, Webb RT, Ueno W, DelPrete S, Bachinsky ME, et al. Randomized phase II study of the neurokinin 1 receptor antagonist CJ-11,974 in the control of cisplatin-induced emesis. Journal of Clinical Oncology 1999;17:338-43. [DOI] [PubMed] [Google Scholar]
Hesketh 2003 {published data only}
- Aapro M, Street JC, Carides AD. Efficacy of aprepitant in management of chemotherapy-induced nausea and vomiting (CINV) in cancers of the digestive and urogenital systems [Abstract No. 892P]. Annals of Oncology 2009;(Suppl 8):274.
- Gralla RJ, Wit R, Herrstedt J, Carides AD, Ianus J, Guoguang-Ma J, et al. Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two Phase III randomized clinical trials. Cancer 2005;104(4):864-8. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin - the Aprepitant Protocol 052 Study Group. Journal of Clinical Oncology 2003;21(22):4112-9. [DOI] [PubMed] [Google Scholar]
- Warr DG, Grunberg SM, Gralla RJ, Hesketh PJ, Roila F, Wit R, et al. The oral NK1 antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from 2 randomised, double-blind trials. European Journal of Cancer 2005a;41(2005):1278–85. [DOI] [PubMed] [Google Scholar]
Hesketh 2012 {published data only}
- Hesketh PJ, Moiseyenko V, Rosati G, Makhson A, Levin J, Russo MW. Phase III study of single-dose casopitant in combination with ondansetron and dexamethasone for the prevention of oxaliplatin-induced nausea and vomiting. Journal of Clinical Oncology 2011;29(15 Suppl 1):9019-19. [DOI] [PubMed]
- Hesketh PJ, Wright O, Rosati G, Russo M, Levin J, Lane S, et al. Single-dose intravenous casopitant in combination with ondansetron and dexamethasone for the prevention of oxaliplatin-induced nausea and vomiting: a multicenter, randomized, double-blind, active-controlled, two arm, parallel group study. Supportive Care in Cancer 2012;20(7):1471-8. [CLINICALTRIALS.GOV IDENTIFIER: NCT00601172] [DOI] [PubMed] [Google Scholar]
Hesketh 2014 {published data only}
- Hesketh PJ, Rossi G, Rizzi G, Palmas M, Alyasova A, Bondarenko I, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Annals of Oncology 2014;25(7):1340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2010 {published data only}
- Ho C-L, Su W-C, Hsieh RK, Lin Z-Z, Chao T-Y. A randomized, double-blind, parallel, comparative study to evaluate the efficacy and safety of ramosetron plus dexamethasone injection for the prevention of acute chemotherapy-induced nausea and vomiting. Japanese Journal of Clinical Oncology 2010;40(4):294-301. [DOI] [PubMed] [Google Scholar]
Hu 2014 {published data only}
- Cheng Y, Zhang L, Zhang H, Zhou C, Han B, Zhang Y, et al. The effect of a 3-day oral aprepitant regimen on the prevention of CINV over standard therapy in Chinese patients receiving high-dose cisplatin. In: Journal of Clinical Oncology. Vol. 29. 2011:9105.
- Hu Z, Cheng Y, Zhang H, Zhou C, Han B, Zhang Y, et al. Aprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trial. In: Supportive Care in Cancer. 2014:979-87. [CLINICALTRIALS.GOV IDENTIFIER: NCT00952341] [DOI] [PubMed]
- Zhang L, Cheng Y, Zhang HY, Zhou C, Han B, Zhang Y, et al. A 3-day oral aprepitant regimen provides superior prevention of CINV over standard therapy in Chinese patients receiving high-dose cisplatin. In: Journal of Thoracic Oncology. 2011:S458-9.
Innocent 2018 {published data only}
- Innocent M, Karimi PN, Nyamu DG, Maranga ISO. Efficacy and cost of granisetron versus ondansetron in the prevention of chemotherapy induced nausea and vomiting among cancer patients at Kenyatta National Hospital. International Journal of Pharmaceutical Sciences and Research 2018;9(4):1644-9. [Google Scholar]
Ishido 2016 {published data only}
- Ishido K, Higuchi K, Azuma M, Sasaki T, Tanabe S, Katada C, et al. Aprepitant, granisetron, and dexamethasone versus palonosetron and dexamethasone for prophylaxis of cisplatin-induced nausea and vomiting in patients with upper gastrointestinal cancer: a randomized crossover phase II trial (KDOG 1002). Anticancer Drugs 2016;27:884-90. [UNIQUE ID ISSUED BY UMIN: UMIN 000005623] [DOI] [PubMed] [Google Scholar]
Ito 2014 {published data only}
- Ito Y, Karayama M, Inui N, Kuroishi S, Nakano H, Nakamura Y, et al. Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer 2014;84(3):259-64. [UNIQUE ID ISSUED BY UMIN: UMIN000010018] [DOI] [PubMed] [Google Scholar]
Jantunen 1992 {published data only}
- Jantunen IT, Kataja VV, Johansson RT. Ondansetron and tropisetron with dexamethasone in the prophylaxis of acute vomiting induced by non-cisplatin-containing chemotherapy. Acta Oncologica 1992;31(5):573-5. [DOI] [PubMed] [Google Scholar]
Jordan 2016a {published data only}
- Gralla RJ, Bosnjak SM, Balser C, Rizzi G, Borroni ME, Rossi G, et al. Prevention of chemotherapy-induced nausea and vomiting (CINV) over repeated chemotherapy cycles: results of a phase 3 trial of NEPA, a fixed oral dose combination of netupitant and palonosetron. In: European Journal of Cancer. Vol. 49. 2013:S267. [CLINICALTRIALS.GOV IDENTIFIER: NCT01376297]
- Gralla RJ, Bosnjak SM, Hontsa A, Balser C, Rizzi G, Rossi G, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Annals of Oncology 2014;25(7):1333-9. [CLINICALTRIALS.GOV IDENTIFIER: NCT01376297] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K, Gralla R, Rizzi G, Kashef K. Efficacy benefit of an NK1 receptor antagonist (NK1RA) in patients receiving carboplatin: supportive evidence with NEPA (a fixed combination of the NK1RA, netupitant, and palonosetron) and aprepitant regimens. In: Supportive Care in Cancer. Vol. 24. 2016:4617-25. [CLINICALTRIALS.GOV IDENTIFIER: NCT01376297] [DOI] [PubMed]
- Jordan K, Gralla R, Rossi G, Borroni ME, Rizzi G. Is the addition of an NK1 receptor antagonist beneficial in patients receiving carboplatin? Supplementary data with nepa, a fixed-dose combination of netupitant and palonosetron. In: Supportive Care in Cancer. Vol. 22. 2014:S107.
- Jordan K, Gralla RJ, Rizzi G. Should all antiemetic guidelines recommend adding a NK1 receptor antagonist (NK1RA) in patients (pts) receiving carboplatin (carbo)? Efficacy evaluation of NEPA, a fixed combination of the NK1RA, netupitant, and palonosetron. Journal of Clinical Oncology 2015;33(15 Suppl 1):9597. [CLINICALTRIALS.GOV IDENTIFIER: NCT01376297 ] [Google Scholar]
- Jordan K, Rizzi G, Borroni ME, Palmas M, Gralla R. Phase 3 study of NEPA (fixed-dose combination of netupitant and palonosetron) for prevention of CINV following repeated moderately (MEC) and highly (HEC) emetogenic chemotherapy cycles. In: Supportive Care in Cancer. Vol. 21. 2013:S152.
- Rugo H S, Rossi G, Rizzi G, Aapro M. Efficacy of NEPA (netupitant/palonosetron) across multiple cycles of chemotherapy in breast cancer patients: a subanalysis from two phase III trials. Breast 2017;33:76-82. [CLINICALTRIALS.GOV IDENTIFIER: NCT01376297] [DOI] [PubMed] [Google Scholar]
Kalaycio 1998 {published data only}
- Kalaycio M, Mendez Z, Pohlman B, Overmoyer B, Boparai N, Jones E, et al. Continuous-infusion granisetron compared to ondansetron for the prevention of nausea and vomiting after high-dose chemotherapy. Journal of Cancer Research and Clinical Oncology 1998;124(5):265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2020 {published data only}
- Kang JH, Kwon JH, Lee YG, Park KU, An HJ, Sohn J, et al. Ramosetron versus palonosetron in combination with aprepitant and dexamethasone for the control of highly-emetogenic chemotherapy-induced nausea and vomiting. Cancer Research and Treatment 2020;52(3):907-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JH, Kang JH, Lee YG, Park KU, An HJ, Sohn J, et al. Ramosetron versus palonosetron in combination with aprepitant and dexamethasone for the control of highly emetogenic chemotherapy-induced nausea and vomiting. Annals of Oncology 2018;29:viii610. [DOI] [PMC free article] [PubMed]
Kaushal 2010 {published data only}
- Kaushal J, Gupta MC, Kaushal V, Bhutani G, Dhankar R, Atri R, et al. Clinical evaluation of two antiemetic combinations palonosetron dexamethasone versus ondansetron dexamethasone in chemotherapy of head and neck cancer. Singapore Medical Journal 2010;51(11):871-5. [PubMed] [Google Scholar]
Kaushal 2015 {published data only}
- Kaushal P, Atri R, Soni A, Kaushal V. Comparative evaluation of triplet antiemetic schedule versus doublet antiemetic schedule in chemotherapy-induced emesis in head and neck cancer patients. ecancermedicalscience 2015;9:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim HJ, Shin SW, Song EK, Lee NR, Kim JS, Ahn JS, et al. Ramosetron versus ondansetron in combination with aprepitant and dexamethasone for the prevention of highly emetogenic chemotherapy-induced nausea and vomiting: a multicenter, randomized phase III trial, KCSG PC10-21. Oncologist 2015;20:1440-7. [CLINICALTRIALS.GOV IDENTIFIER: NCT01536691] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song EK, Kim JS, Ahn JS, Yun HJ, Cho YH, Park KU, et al. A prospective multicenter, single blind, randomized phase III trial to compare ramosetron, aprepitant and dexamethasone with ondansetron, aprepitant and dexamethasone for preventing CINV: KCSG PC10-21. In: Supportive Care in Cancer. 2013:S228-9.
Kim 2017 {published data only}
- Kim JE, Jang JS, Kim JW, Sung YL, Cho CH, Lee MA, et al. Efficacy and safety of aprepitant for the prevention of chemotherapy-induced nausea and vomiting during the first cycle of moderately emetogenic chemotherapy in Korean patients with a broad range of tumor types. Supportive Care in Cancer 2017;25(3):801-9. [CLINICALTRIALS.GOV IDENTIFIER: NCT01636947] [DOI] [PubMed] [Google Scholar]
Kimura 2015 {published data only}
- Kimura H, Yamamoto N, Shirai T, Nishida H, Hayashi K, Tanzawa Y, et al. Efficacy of triplet regimen antiemetic therapy for chemotherapy-induced nausea and vomiting (CINV) in bone and soft tissue sarcoma patients receiving highly emetogenic chemotherapy, and an efficacy comparison of single-shot palonosetron and consecutive-day granisetron for CINV in a randomized, single-blinded crossover study. Cancer Medicine 2015;4:333-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitayama 2015 {published data only}
- Kitayama H, Tsuji Y, Sugiyama J, Doi A, Kondo T, Hirayama M. Efficacy of palonosetron and 1-day dexamethasone in moderately emetogenic chemotherapy compared with fosaprepitant, granisetron, and dexamethasone: a randomized crossover study. In: Supportive Care in Cancer. 2015:S135. [DOI] [PubMed]
- Kitayama H, Tsuji Y, Sugiyama Y, Doi A, Kondo T, Hirayama M. Efficacy of palonosetron and 1‑day dexamethasone in moderately emetogenic chemotherapy compared with fosaprepitant, granisetron, and dexamethasone: a prospective randomized crossover study. International Journal of Clinical Oncology 2015;20(6):1051-6. [DOI] [PubMed] [Google Scholar]
Koizumi 2003 {published data only}
- Koizumi W, Tanabe S, Nagaba S, Higuchi K, Nakayama N, Saigenji K, et al. A double-blind, crossover, randomized comparison of granisetron and ramosetron for the prevention of acute and delayed cisplatin-induced emesis in patients with gastrointestinal cancer: is patient preference a better primary endpoint? Chemotherapy 2003;49(6):316-23. [DOI] [PubMed] [Google Scholar]
Kusagaya 2015 {published data only}
- Kusagaya H, Inui N, Karayama M, Fujisawa T, Enomoto N, Kuroishi S, et al. Evaluation of palonosetron and dexamethasone with or without aprepitant to prevent carboplatin-induced nausea and vomiting in patients with advanced non-small-cell lung cancer. Lung Cancer 2015;90(3):410-6. [UNIQUE ID ISSUED BY UMIN: UMIN ID 10056] [DOI] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee KS, Han JY, Moon H, Lee BK, Cho SG, Jin JY, et al. Comparison of tropisetron with ondansetron in the prevention of cisplatin-induced nausea and vomiting. Journal of the Korean Cancer Association 1997;29(2):332-9. [Google Scholar]
Li 2019 {published data only}
- Li Q, Wu Y, Wang W, Deng S, Jiang C, Chen F, et al. Effectiveness and safety of combined neurokinin-1 antagonist aprepitant treatment for multiple-day anthracycline-induced nausea and vomiting. Current Problems in Cancer. 2019;43(6):100462. [DOI] [PubMed] [Google Scholar]
Maehara 2015 {published data only}
- Maehara M, Ueda T, Miyahara D, Takahashi Y, Miyata K, Nam SO, et al. Clinical efficacy of aprepitant in patients with gynecological cancer after chemotherapy using paclitaxel and carboplatin. Anticancer Research 2015;35(8):4527-34. [PubMed] [Google Scholar]
Mahrous 2020 {published data only}
- Mahrous MA. Comparative study between the clinical effect of palonosetron and granisetron as antiemetic therapy for patients receiving highly emetogenic chemotherapy regimens. Annals of Oncology 2020;31(Suppl 2):S86-7.
Matsuda 2014 {published data only}
- Matsuda Y, Sugimori Y, Murata K, Sato A, Ichiyama T, Sakamoto S, et al. A phase II study to evaluate efficacy of aprepitant in preventing chemotherapy-induced nausea and vomiting. In: International Journal of Gynecological Cancer. 2014:1179-80.
Matsumoto 2020 {published data only}
- Matsumoto K, Takahashi M, Sato K, Osaki A, Takano T, Naito Y, et al. A double-blind, randomized, multicenter phase 3 study of palonosetron vs granisetron combined with dexamethasone and fosaprepitant to prevent chemotherapy-induced nausea and vomiting in patients with breast cancer receiving anthracycline and cyclophosphamide. Cancer Medicine 2020;9:3319-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Takahashi M, Sato K, Takano T, Ryushima Y, Doi M, et al. Palonosetron or granisetron for prevention of CINV in patients with breast cancer receiving dexamethasone and fosaprepitant following anthracycline plus cyclophosphamide (AC) regimen. In: Journal of Clinical Oncology. 2015. [UNIQUE ID ISSUED BY UMIN: UMIN000008897]
Mattiuzzi 2007 {published data only}
- Mattiuzzi G, Cortes J, Cassat J, Blamble D, Bekele B N, Beran M, et al. An interim analysis of phase II, open randomized comparative trial: alternate or multiple-day dosing of palonosetron (PALO) is effective and safe in the prevention of chemotherapy induced nausea and vomiting (CINV) in patients (pts) with leukemia receiving high dose Ara-C (HDAC). In: Blood. Vol. 110. 2007.
Miyabayashi 2015 {published data only}
- Miyabayashi T, Miura S, Tanaka H, Ishida T, Watanabe S, Makino M, et al. A randomized phase II trial of triplet or doublet antiemetic therapy in lung cancer patients receiving MEC (NLCTG1002). In: Annals of Oncology. Vol. 26. 2015:vii93.
- Tanaka H, Miura S, Abe T, Tsukada H, Ishida T, Watanabe S, et al. A randomized phase 2 study of triplet or doublet antiemetic therapy for chemotherapy-induced nausea and vomiting in lung cancer patients receiving moderately emetogenic chemotherapy (NLCTG1002). In: Supportive Care in Cancer. 2015:S139-40.
Mohammed 2019 {published data only}
- Mohammed DA, Jabar EG, Al-Sabbagh MS, Fawzi HA. Comparison of acute and delayed antiemetic effect of adding aprepitant to ondansetron and dexamethasone regimen in patients with Hodgkin lymphoma receiving highly emetogenic chemotherapy. International Journal of Research in Pharmaceutical Sciences 2019;10(2):1397-404. [Google Scholar]
Nakamura 2012 {published data only}
- Nakamura K, Onikubo T, Nakamura M, Tauchi K. Antiemetic effect of granisetron NK, a generic 5-HT3 receptor antagonist in comparison with azasetron for prevention of chemotherapy-induced nausea and vomiting in cancer patient - a crossover randomized controlled clinical trial. Annals of Cancer Research and Therapy 2012;20(2):63-7. [Google Scholar]
NCT01640340 {published data only}
- NCT01640340. Ondansetron versus palonosetron antiemetic regimen prior to highly emetogenic chemotherapy (HEC). https://clinicaltrials.gov/show/nct01640340 (accessed 15 October 2019).
Nishimura 2015 {published data only}
- Fukunaga M, Nishimura J, Satoh T, Takemoto H, Nakata K, Ide Y, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (Senri Trial): a multicenter, randomized, controlled phase 3 trial. In: Supportive Care in Cancer. Vol. 23. 2015:S38-9. [CLINICALTRIALS.GOV IDENTIFIER: NCT01344304] [DOI] [PubMed]
- Nishimura J, Satoh T, Fukunaga M, Takemoto H, Nakata K, Ide Y, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled phase 3 trial. European Journal of Cancer 2015;51(10):1274-82. [CLINICALTRIALS.GOV IDENTIFIER: NCT01344304] [DOI] [PubMed] [Google Scholar]
- Takemoto H, Nishimura J, Komori T, Kim HM, Ota H, Suzuki R, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea. International Journal of Clinical Oncology 2017;22(1):88-95. [CLINICALTRIALS.GOV IDENTIFIER: NCT01344304] [DOI] [PubMed] [Google Scholar]
- UMIN000005431. Multicenter randomized controlled trial of combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000005431 2011.
Ohzawa 2015 {published data only}
- Ohzawa H, Miki A, Hozumi Y, Miyazaki C, Sagara Y, Tanaka Y, et al. Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline-based regimens. Oncology Letters 2015;9(1):119-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozaki 2013 {published data only}
- Ozaki Y, Horimatsu T, Nozaki A, Hasegawa S, Matsumoto S, Sakai Y, et al. The efficacy of palonosetron/dexamethasone plus NK1 receptor antagonist (aprepitant) therapy for prevention of chemotherapy induced nausea and vomiting in colorectal cancer patients. In: European Journal of Cancer. Vol. 49. 2013:S524.
Poli‐Bigelli 2003 {published data only}
- Aapro M, Street JC, Carides AD. Efficacy of aprepitant in management of chemotherapy-induced nausea and vomiting (CINV) in cancers of the digestive and urogenital systems [Abstract No. 892P]. In: Annals of Oncology. Vol. 19. 2009:274.
- Gralla RJ, Wit R, Herrstedt J, Carides AD, Ianus J, Guoguang-Ma J, et al. Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two Phase III randomized clinical trials. Cancer 2005;104(4):864-8. [DOI] [PubMed] [Google Scholar]
- Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 2003;97:3090-8. [DOI] [PubMed] [Google Scholar]
- Warr DG, Grunberg SM, Gralla RJ, Hesketh PJ, Roila F, Wit R, et al. The oral NK1 antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from 2 randomised, double-blind, placebo controlled trials. European Journal of Cancer 2005;41(2005):1278–85. [DOI] [PubMed] [Google Scholar]
Raftopoulos 2015 {published data only}
- Boccia R, Cooper W, O'Boyle E. Sustained antiemetic responses with APF530 (sustained-release granisetron) during multiple cycles of emetogenic chemotherapy. Journal of Community and Supportive Oncology 2015;13(2):38-46. [DOI] [PubMed] [Google Scholar]
- Boccia R, O'Boyle E, Cooper W. Randomized phase III trial of APF530 versus palonosetron in the prevention of chemotherapy-induced nausea and vomiting in a subset of patients with breast cancer receiving moderately or highly emetogenic chemotherapy. BioMed Central Cancer 2016;16:166. [CLINICALTRIALS.GOV IDENTIFIER: NCT00343460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia RV, Cooper W, O'Boyle E. Phase 3 trial of APF530 versus palonosetron (PALO) in preventing chemotherapy-induced nausea and vomiting (CINV): efficacy in breast cancer patients (pts) receiving moderately (MEC) or highly (HEC) emetogenic chemotherapy. In: Journal of Clinical Oncology. Vol. 32. 2014:Suppl 1. [CLINICALTRIALS.GOV IDENTIFIER: NCT00343460]
- Raftopoulos H, Boccia R, Cooper W, O'Boyle E, Gralla R J. Slow-release granisetron (APF530) versus palonosetron for chemotherapy-induced nausea/vomiting: analysis by American Society of Clinical Oncology emetogenicity criteria. Future Oncology 2015;11(18):2541-51. [CLINICALTRIALS.GOV IDENTIFIER: NCT00343460] [DOI] [PubMed] [Google Scholar]
- Raftopoulos H, Cooper W, O'Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Supportive Care in Cancer 2015;23(3):723-32. [CLINICALTRIALS.GOV IDENTIFIER: NCT00343460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapoport 2010 {published data only}
- Rapoport BL, Jordan K, Boice JA, Taylor A, Brown C, Hardwick JS, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Supportive Care in Cancer 2010;18(4):423-31. [CLINICALTRIALS.GOV IDENTIFIER: NCT00337727] [DOI] [PubMed] [Google Scholar]
- Rapoport BL. Efficacy of a triple antiemetic regimen with aprepitant for the prevention of chemotherapy-induced nausea and vomiting: effects of gender, age, and region. Current Medical Research and Opinion 2014;30(9):1875-81. [CLINICALTRIALS.GOV IDENTIFIER: NCT00337727] [DOI] [PubMed] [Google Scholar]
Rapoport 2015 (a) {published data only}
- Chua D, Poma A, Hedley ML. Efficacy and safety of rolapitant for the prevention of chemotherapy-induced nausea and vomiting in subjects receiving highly emetogenic chemotherapy. In: Supportive Care in Cancer. Vol. 20. 2012:S102.
- Chua D, Poma A, Hedley ML. Rolapitant, a novel NK-1 receptor antagonist, for the prevention of nausea in subjects receiving highly emetogenic chemotherapy (HEC). In: Supportive Care in Cancer. Vol. 20. 2012:S110. [DOI: 10.1007/s00520-012-1479-7] [DOI] [PubMed]
- Fein LE, Poma A, Hedley ML. Efficacy and safety of rolapitant, a novel NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting in subjects receiving highly emetogenic chemotherapy. In: Journal of Clinical Oncology. Vol. 30. 2012.
- Rapoport B, Chua D, Poma A, Arora S, Wang Y, Fein LE. Study of rolapitant, a novel, long-acting, NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Supportive Care in Cancer 2015;23(11):3281-8. [CLINICALTRIALS.GOV IDENTIFIER: NCT00394966] [DOI: ] [DOI] [PubMed] [Google Scholar]
- Rapoport B, Schwartzberg L, Chasen M, Powers D, Arora S, Navari R, et al. Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. European Journal of Cancer 2016;57:23-30. [CLINICALTRIALS.GOV IDENTIFIER: NCT00394966] [DOI] [PubMed] [Google Scholar]
Rapoport 2015 (b) {published data only}
- Chasen M, Urban L, Schnadig I, Rapoport B, Powers D, Arora S, et al. Rolapitant improves quality of life of patients receiving highly or moderately emetogenic chemotherapy. Supportive Care in Cancer 2016;25:85-92. [CLINICALTRIALS.GOV IDENTIFIER: NCT01499849] [DOI: DOI 10.1007/s00520-016-3388-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport B, Schwartzberg L, Chasen M, Powers D, Arora S, Navari R, et al. Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. European Journal of Cancer 2016;57:23-30. [CLINICALTRIALS.GOV IDENTIFIER: NCT01499849] [DOI] [PubMed] [Google Scholar]
- Rapoport BL, Chasen MR, Gridelli C, Urban L, Modiano MR, Schnadig ID, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncology 2015a;16(9):1079-89. [CLINICALTRIALS.GOV IDENTIFIER: NCT01499849] [DOI] [PubMed] [Google Scholar]
Rapoport 2015 (c) {published data only}
- Chasen M, Urban L, Schnadig I, Rapoport B, Powers D, Arora S, et al. Rolapitant improves quality of life of patients receiving highly or moderately emetogenic chemotherapy. Supportive Care in Cancer 2016;25(1):85-92. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500213 ] [DOI] [PMC free article] [PubMed]
- Rapoport B, Poma A, Hedley ML, Martell R, Navari R. Phase 3 trial results for rolapitant, a novel NK-1 receptor antagonist, in the prevention of chemotherapy-induced nausea and vomiting (CINV) in subjects receiving highly emetogenic chemotherapy (HEC). In: Supportive Care in Cancer. Vol. 22. 2014:S100. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500213 ]
- Rapoport B, Schwartzberg L, Chasen M, Powers D, Arora S, Navari R, et al. Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. European Journal of Cancer 2016;57:23-30. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500213 ] [DOI] [PubMed] [Google Scholar]
- Rapoport BL, Chasen MR, Gridelli C, Urban L, Modiano MR, Schnadig ID, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncology 2015;16(9):1079-89. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500213 ] [DOI] [PubMed] [Google Scholar]
Roila 1995 {published data only}
- Roila F, Angelis V, Marinis F, Scarfone G, Scagliotti G, Donati D, et al, Italian Group for Antiemetic Research. Ondansetron vs granisetron, both combined with dexamethasone in the prevention of cisplatin-induced emesis. Annals of Oncology 1995;6:805-10. [PubMed] [Google Scholar]
Rugo 2017 {published data only}
- Aapro M, Karthaus M, Schwartzberg L, Bondarenko I, Sarosiek T, Oprean C, et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy-induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: results of a randomized, double-blind, phase 3 trial versus oral palonosetron. Supportive Care in Cancer 2017;25:1127-35. [CLINICALTRIALS.GOV IDENTIFIER: NCT01339260] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aapro M, Karthaus M, Schwartzberg L, Rossi G, Rizzi G, Borroni ME, et al. Multiple cycle CINV control and safety of NEPA, a capsule containing netupitant and palonosetron administered once per cycle of moderately emetogenic chemotherapy (MEC). In: Supportive Care in Cancer. 2014:S108.
- Aapro M, Rossi G, Rizzi G, Borroni M E, Lorusso V, Karthaus M, et al. Efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately emetogenic chemotherapy (MEC). In: European Journal of Cancer. 2013:S266. [CLINICALTRIALS.GOV IDENTIFIER: NCT01339260]
- Aapro M, Rugo H, Rizzi G. Evaluation of sustained antiemetic efficacy over repeated cycles of anthracycline-cyclophosphamide (AC)-based chemotherapy: a phase 3 study of NEPA, a fixed-dose combination of netupitant and palonosetron for prevention of chemotherapy-induced nausea and. In: Cancer Research. Vol. 75. 2015:9 Suppl 1.
- Aapro M, Rugo H, Rizzi G. NEPA, a new combination antiemetic, exhibits sustained efficacy over repeated chemotherapy cycles. In: Breast. 2015:S88.
- Aapro M, Rugo H, Rossi G, Rizzi G, Borroni ME, Bondarenko I, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Annals of Oncology 2014;25:1328-33. [CLINICALTRIALS.GOV IDENTIFIER: NCT01339260] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aapro MS, Rossi G, Rizzi G, Borroni ME, Nagl NG. Impact on patients' daily life activities of NEPA, the oral fixed-dose combination of netupitant, a new NK1 receptor antagonist (RA), and palonosetron (PALO) plus dexamethasone (DEX) versus PALO plus DEX for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately emetogenic chemotherapy (MEC). In: Journal of Clinical Oncology. 2013.
- Rugo HS, Rossi G, Rizzi G, Aapro M. Efficacy of NEPA (netupitant/palonosetron) across multiple cycles of chemotherapy in breast cancer patients: a subanalysis from two phase III trials. Breast 2017;33:76-82. [CLINICALTRIALS.GOV IDENTIFIER: NCT01339260] [DOI] [PubMed] [Google Scholar]
- Rugo HS, Rossi G, Rizzi G, Borroni ME, Lorusso V, Karthaus M, et al. NEPA, a fixed-dose combination of netupitant and palonosetron, prevents chemotherapy-induced nausea and vomiting (CINV) more effectively and reduces the impact on daily living for breast cancer patients compared with palonosetron. In: Cancer Research. Vol. 73. 2013:24 Suppl. 1.
Ruhlmann 2017 {published data only}
- Ruhlmann CH, Christensen TB, Hammer Dohn L, Paludan M, Roennengart E, Hilpert F, et al. Fosaprepitant reduces the impact of nausea on daily function during five weeks of chemo-radiotherapy: a sub-study of the GAND-emesis trial. Annals of Oncology 2017;28 (Suppl 5):v543. [CTG: NCT 01074697] [Google Scholar]
Saito 2009 {published data only}
- Kubota K, Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, et al. Control of nausea with palonosetron versus granisetron, both combined with dexamethasone, in patients receiving cisplatin or anthracycline plus cyclophosphamide-based regimens. Supportive Care in Cancer 2016;14:4025-33. [CLINICALTRIALS.GOV IDENTIFIER: NCT00359567] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncology 2009;10(2):115-24. [CLINICALTRIALS.GOV IDENTIFIER: NCT00359567] [DOI] [PubMed] [Google Scholar]
Saito 2013 {published data only}
- Saito H, Yoshizawa H, Yoshimori K, Katakami N, Katsumata N, Kawahara M, et al. Efficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Annals of Oncology 2013;24(4):1067-73. [JAPICCTI-NO: JapicCTI090829] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saito 2017 {published data only}
- Ogata H, Saito M, Tsuneizumi M, Kutomi G, Hosoya K, Kawai Y, et al. Difference between 1st and 2nd generation serotonin receptor antagonists in triplet antiemetic therapy for highly emetogenic chemotherapy in breast cancer patients-according to recent multi-institutional double-blind randomized clinical research on the AC regimen. In: Cancer Research. Vol. 77(4). 2017:Suppl, Abstract nr P5-11-03. [DOI: 10.1158/1538-7445.SABCS16-P5-11-03] [UMIN: UMIN000007882] [DOI]
- Saito M, Tsuneizumi M, Kutomi G, Ogata H, Sugizaki K, Katsumata N, et al. Interim analysis of a randomized double blind comparative trial of triplet antiemetic therapy (TTT) for AC - historical comparison of CR with doublet antiemetic study. In: Supportive Care in Cancer. Vol. 23. 2015:S137.
- Saito M, Uomori T, Miura K, Taguchi R, Kurata M, Tsuneizumi M, et al. Comparison between 1st and 2nd generation serotonin receptor antagonists in triplet antiemetic therapy in breast cancer patients-according to recent multi-institutional double-blind randomized study - is this part of the title? In: Supportive Care in Cancer. Vol. 25, Suppl 2. 2017:S57-8. [UNIQUE ID ISSUED BY UMIN: UMIN000007882]
- Tsuneizumi M, Saito M, Ogata H, Kutomi G, Hosoya K, Kawai Y, et al. Possible mechanisms of serotonin and aprepitant actions in chemotherapy induced nausea and vomiting (CINV): insights into the mechanisms of serotonin and aprepitant actions in CINV-According to recent multi-institutional double-blind randomized clinical research on the AC regimen- is this part of the title? In: Journal of Clinical Oncology. Vol. 35. 2017:Suppl 15, 6587. [DOI: DOI: 10.1200/JCO.2017.35.15_suppl.6587]
Schmitt 2014 {published data only}
- Schmitt T, Goldschmidt H, Neben K, Freiberger A, Hüsing J, Gronkowski M, et al. Aprepitant, granisetron, and dexamethasone for prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: results of a randomized, placebo-controlled phase III trial. Journal of Clinical Oncology 2014;32(30):3413-20. [CLINICALTRIALS.GOV IDENTIFIER: NCT00571168] [DOI] [PubMed] [Google Scholar]
Schmoll 2006 {published data only}
- Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Annals of Oncology 2006;17(6):1000-6. [CLINICALTRIALS.GOV IDENTIFIER: NCT00090207] [DOI] [PubMed] [Google Scholar]
Schnadig 2014 {published data only}
- Hesketh P, Schwartzberg L, Modiano M, Arora S, Poma A, Schnadig I. Rolapitant for prevention of chemotherapy-induced nausea and vomiting (CINV) in non-anthracycline/cyclophosphamide (A/C) moderately emetogenic therapy (MEC). In: Supportive Care in Cancer. 2015:S141.
- Schnadig I, Modiano M, Poma A, Hedley ML, Martell R, Schwartzberg L. Phase 3 trial results for rolapitant, a novel NK-1 receptor antagonist, in the prevention of chemotherapy-induced nausea and vomiting (CINV) in subjects receiving moderately emetogenic chemotherapy (MEC). In: Supportive Care in Cancer. Vol. 22. 2014:S100-1. [DOI: 10.1007/s00520-014-2222-3] [DOI]
Schnadig 2016 {published data only}
- Schnadig ID, Agajanian R, Dakhil C, Gabrail N, Vacirca J, Taylor C, et al. APF530 versus ondansetron, each in a guideline-recommended three-drug regimen, for the prevention of chemotherapy-induced nausea and vomiting due to anthracycline plus cyclophosphamide-based highly emetogenic chemotherapy regimens: a post hoc subgroup analysis of the phase III randomized MAGIC trial. Cancer Management Research 2017;9:179-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnadig ID, Agajanian R, Dakhil C, Gabrail NY, Smith RE, Taylor C, et al. APF530 (granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncology 2016;12(12):1469-81. [CLINICALTRIALS.GOV IDENTIFIER: NCT02106494] [DOI] [PubMed] [Google Scholar]
- Schwartzberg L, Mosier M, Geller RB, Klepper MJ, Schnadig I, Vogelzang NJ. APF530 for nausea and vomiting prevention following cisplatin: phase 3 MAGIC trial analysis. Journal of Community and Supportive Oncology 2017;15(2):82-8. [Google Scholar]
Schwartzberg 2015 {published data only}
- Chasen M, Urban L, Schnadig I, Rapoport B, Powers D, Arora S, et al. Rolapitant improves quality of life of patients receiving highly or moderately emetogenic chemotherapy. Supportive Care in Cancer 2016;25:85-92. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500226] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh PJ, Schnadig ID, Schwartzberg LS, Modiano MR, Jordan K, Arora S, et al. Efficacy of the neurokinin-1 receptor antagonist rolapitant in preventing nausea and vomiting in patients receiving carboplatin-based chemotherapy. Cancer 2016;122(15):2418-25. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500226] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D, Schnadig ID, Modiano MR, Poma A, Schwartzberg LS. Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients (pts) receiving anthracycline-cyclophosphamide (AC)-based chemotherapy. In: Journal of Clinical Oncology. Vol. 33. 2015:208. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500226]
- Rapoport B, Schwartzberg L, Chasen M, Powers D, Arora S, Navari R, et al. Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. European Journal of Cancer 2016;57:23-30. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500226] [DOI] [PubMed] [Google Scholar]
- Schwartzberg LS, Modiano MR, Rapoport BL, Chasen MR, Gridelli C, Urban L, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncology 2015;16(9):1071-8. [CLINICALTRIALS.GOV IDENTIFIER: NCT01500226] [DOI] [PubMed] [Google Scholar]
- Urban L, Poma A, Dardeno M, Martell R. Safety of rolapitant, a novel NK-1 receptor-antagonist, for prevention of chemotherapy-induced nausea and vomiting (CINV) in subjects receiving moderately or highly emetogenic chemotherapy (MEC or HEC). In: Supportive Care in Cancer. Vol. 22. 2014:S100.
Seol 2016 {published data only}
- Seol YM, Kim HJ, Choi YJ, Lee EM, Kim YS, Oh SY, et al. Transdermal granisetron versus palonosetron for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: a multicenter, randomized, open-label, cross-over, active-controlled, and phase IV study. Supportive Care in Cancer 2016;24(2):945-52. [DOI] [PubMed] [Google Scholar]
Song 2017 {published data only}
- Song Z, Wang H, Zhang H, Zhao K, Zhang M, Yang F. Efficacy and safety of triple therapy with aprepitant, ondansetron, and prednisone for preventing nausea and vomiting induced by R-CEOP or CEOP chemotherapy regimen for non-Hodgkin lymphoma: a phase 2 open-label, randomized comparative trial. Leukemia & Lymphoma 2017;58(4):816-21. [DOI] [PubMed] [Google Scholar]
Stewart 1996 {published data only}
- Stewart L. A double-blind randomised trial of granisetron + dexamethasone (Dex) v ondansetron-dex in the treatment of cisplatin associated nausea and vomiting [abstract]. In: British Journal of Cancer. Vol. 73. 1996:51.
Stewart 2000 {published data only}
- Stewart L, Crawford SM, Taylor PA. The comparative effectiveness of ondansetron and granisetron in a once daily dosage in the prevention of nausea and vomiting caused by cisplatin: a double-blind clinical trial. The Pharmaceutical Journal 2000;165(7104):59-62. [Google Scholar]
Stiff 2013 {published data only}
- Stiff PJ, Fox-Geiman MP, Kiley K, Rychlik K, Parthasarathy M, Fletcher-Gonzalez D, et al. Prevention of nausea and vomiting associated with stem cell transplant: results of a prospective, randomized trial of aprepitant used with highly emetogenic preparative regimens. Biology of Blood and Marrow Transplantation 2013;19:49-55 e1. [CLINICALTRIALS.GOV IDENTIFIER: NCT00781768] [DOI] [PubMed] [Google Scholar]
Sugawara 2019 {published data only}
- Sugawara S, Inui N, Kanehara M, Morise M, Yoshimori K Kumagai T, et al. Multicenter, placebo-controlled, double-blind, randomized study of fosnetupitant in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Cancer 2019;125(22):4076-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugimori 2017 {published data only}
- Sugimori Y, Ota T, Ujihira T, Ishiguro T, Ogishima D. A phase II randomised study to evaluate the efficacy of aprepitant plus palonosetron for preventing delayed-phase CINV associated with TC therapy in gynaecological cancer. Journal of Obstetrics & Gynaecology Research 2017;43(9):1454-9. [UNIQUE ID ISSUED BY UMIN: UMIN000019122] [DOI] [PubMed] [Google Scholar]
Svanberg 2015 {published data only}
- Svanberg A, Birgegard G. Addition of aprepitant (Emend) to standard antiemetic regimen continued for 7 days after chemotherapy for stem cell transplantation provides significant reduction of vomiting. Oncology 2015;89:31-6. [DOI] [PubMed] [Google Scholar]
Takahashi 2010 {published data only}
- Takahashi T, Hoshi E, Takagi M, Katsumata N, Kawahara M, Eguchi K. Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Science 2010;101:2455-61. [CLINICALTRIALS.GOV IDENTIFIER: NCT00212602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanioka 2013 {published data only}
- Tanioka M, Kitao A, Matsumoto K, Shibata N, Yamaguchi S, Fujiwara K, et al. A randomised, placebo-controlled, double-blind study of aprepitant in nondrinking women younger than 70 years receiving moderately emetogenic chemotherapy. British Journal of Cancer 2013;109(4):859-65. [UMIN: UMIN000004998] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsubata 2019 {published data only}
- Tsubata Y, Nakao M, Amano Y, Hotta T, Hamaguchi M, Okimoto T, et al. Translational and randomized study of 5-HT3 receptor antagonists for evaluation of chemotherapy-induced nausea and vomiting related biomarkers. Journal of Medical Investigation 2019;66(3.4):269-74. [DOI] [PubMed] [Google Scholar]
Warr 2005 {published data only}
- Herrstedt J, Muss HB, Warr DG, Hesketh PJ, Eisenberg PD, Raftopoulos H, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. [Erratum appears in Cancer. 2006 Apr 1;106(7):1641]. Cancer 2005;104(7):1548-55. [DOI] [PubMed] [Google Scholar]
- Muss HB, Herrstedt J, Hesketh P, Warr D, Gabriel M, Rodgers A, et al. Randomized, double-blind trial comparing the effect of an aprepitant regimen versus a standard antiemetic regimen during four cycles of moderately emetogenic chemotherapy. Value in Health 2004;10(6):23-31. [Google Scholar]
- Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. [Erratum appears in J Clin Oncol. 2005 Aug 20;23(24):5851. Note: Dosage error in published abstract; MEDLINE/PubMed abstract corrected]. Journal of Clinical Oncology 2005;23(12):2822-30. [DOI] [PubMed] [Google Scholar]
- Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Supportive Care in Cancer 2011;19(6):807-13. [DOI] [PubMed] [Google Scholar]
Webb 2010 {published data only}
- Webb T, Hardwick J, Carides A, Street J. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting (CINV) associated with moderately emetogenic (MEC) chemotherapy in patients with breast cancer. In: Cancer Research. Vol. 69. 2010.
Weinstein 2016 {published data only}
- Rapoport B, Weinstein C, Camacho ES, Khanani SA, Beckford-Brathwaite E, Kevill L, et al. A randomized, phase 3, double-blind study of intravenous fosaprepitant as a single dose for preventing chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy. In: Supportive Care in Cancer. Vol. 23. 2015:S385. [CLINICALTRIALS.GOV IDENTIFIERCLINICALTRIALS.GOV IDENTIFIER: NCT01594749]
- Weinstein C, Jordan K, Green SA, Camacho E, Khanani S, Beckford-Brathwaite E, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: results of a randomized, double-blind phase III trial. Annals of Oncology 2016;27(1):172-8. [CLINICALTRIALS.GOV IDENTIFIER: NCT01594749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenzell 2013 {published data only}
- Wenzell C, Berger M, Blazer M, Crawford B, Griffith N, Lustberg M B, et al. Pilot study on the efficacy of ondansetron and palonosetron-containing antiemetic regimens prior to highly emetogenic chemotherapy. In: Journal of Oncology Pharmacy Practice. 2012:16-7. [DOI] [PMC free article] [PubMed]
- Wenzell CM, Berger MJ, Blazer MA, Crawford BS, Griffith NL, Wesolowski R, et al. Pilot study on the efficacy of an ondansetron- versus palonosetron-containing antiemetic regimen prior to highly emetogenic chemotherapy. Supportive Care in Cancer 2013;21:2845-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wit 2001 {published data only}
- Wit R, Boer AC, Linden GHM, Stoter G, Sparreboom A, Verweij J. Effective cross-over to granisetron after failure to ondansetron, a randomized double blind study in patients failing ondansetron plus dexamethasone during the first 24 hours following highly emetogenic chemotherapy. British Journal of Cancer 2001;85(8):1099-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiong 2019 {published data only}
- Xiong J, Zhao G, Yang S, Chen J. Efficacy, tolerability and pharmacokinetic impact of aprepitant in sarcoma patients receiving ifosfamide and doxorubicin chemotherapy: a randomized controlled trial. Advances in Therapy 2019;36(2):355-64. [DOI] [PubMed] [Google Scholar]
Yahata 2016 {published data only}
- Yahata H, Kobayashi H, Sonoda K, Shimokawa M, Ohgami T, Saito T, et al. Efficacy of aprepitant for the prevention of chemotherapy-induced nausea and vomiting with a moderately emetogenic chemotherapy regimen: a multicenter, placebo-controlled, double-blind, randomized study in patients with gynecologic cancer receiving paclitaxel and carboplatin. International Journal of Clinical Oncology 2016;21(3):491-7. [UNIQUE ID ISSUED BY UMIN: UMIN 000005215] [DOI] [PubMed] [Google Scholar]
Yang 2017 {published data only}
- Yang LQ, Sun XC, Qin SK, Cheng Y, Shi JH, Chen ZD, et al. Efficacy and safety of fosaprepitant in the prevention of nausea and vomiting following highly emetogenic chemotherapy in Chinese people: a randomized, double-blind, phase III study. European Journal of Cancer Care 2017;26(6):e12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeo 2009 {published data only}
- Yeo W, Mo FK, Suen JJ, Ho WM, Chan SL, Lau W, et al. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Research and Treatment 2009;113(3):529-35. [DOI] [PubMed] [Google Scholar]
- Zee BC, Yeo W, Mo FK, Lau W, Poon A, Chan L, et al. Quality of life (QOL) for breast cancer patients receiving aprepitant versus placebo for prevention of chemotherapy induced nausea and vomiting. Journal of Clinical Oncology 2008;26(15 Suppl):20549. [Google Scholar]
Zhang 2018 (a) {published data only}
- Zhang L, Lu S, Feng J, Dechaphunkul A, Chang J, Wang D, et al. A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Annals of Oncology 2018;29(2):452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lu S, Feng J F, Dechaphunkul A, Chessari S, Lanzarotti C, et al. Phase III study of NEPA, a fixed combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV). In: Journal of Clinical Oncology. Vol. 35. 2017:15 Suppl. 10090. [DOI: 10.1200/JCO.2017.35.15_suppl.10090] [DOI]
Zhang 2018 (b) {published data only}
Zhang 2020 {published data only}
- Zhang Z, Yang Y, Lu P, Li X, Chang J, Zheng R, et al. Fosaprepitant versus aprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based chemotherapy: a multicenter, randomized, double-blind, double-simulated, positive-controlled phase III trial. Annals of Translational Medicine 2020;8(5):234-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abali 2007 {published data only}
- Abali H, Celik I. Tropisetron, ondansetron, and granisetron for control of chemotherapy-induced emesis in Turkish cancer patients: a comparison of efficacy, side-effect profile, and cost. Cancer investigation 2007;25(3):135-9. [DOI] [PubMed] [Google Scholar]
Adamo 1994 {published data only}
- Adamo V, Aiello R, Altavilla G, Cammarata N, Carreca I, Carroccio E, et al. [Granisetron vs ondansetron for the control of acute vomiting during antiblastic therapy: a randomized trial]. Tumori 1994;80(Suppl):131. [Google Scholar]
Albany 2014 {published data only}
- Albany C, Hanna NH, Picus J, Hauke RJ, Fausel CA, Liu Z, et al. Phase II study of fosaprepitant plus 5HT3 receptor antagonists plus dexamethasone in patients with germ cell tumors undergoing 5-day cisplatin-based chemotherapy: Hoosier Oncology Group QL12-153. In: Journal of Clinical Oncology. Vol. 32. 2014.
Audhuy 1996 {published data only}
- Audhuy B, Cappelaere P, Claverie N. Double-blind comparison of the antiemetic efficacy of two single IV doses of dolasetron and one IV dose of granisetron after cisplatin (80 mg/m2) chemotherapy. European Journal of Cancer 2000;32(5):807-13. [PubMed] [Google Scholar]
- Audhuy B, Cappelaere P, Martin M, Cervantes A, Fabbro M, Rivière A, et al. A double-blind, randomised comparison of the anti-emetic efficacy of two intravenous doses of dolasetron mesilate and granisetron in patients receiving high dose cisplatin chemotherapy. Eurpoean Journal of Cancer 1996;32(5):807-13. [PubMed] [Google Scholar]
- Audhuy B. Double-blind, comparative trial of the antiemetic efficacy of two IV doses of dolasetron mesilate (DM) and granisetron (G) after infusion of high-dose cisplatin chemotherapy (CT). In: European Journal of Cancer. Vol. 31A. 1995:S253-4.
Ballatori 1995 {published data only}
- Ballatori E, Roila F, Angelis V, Campora E, Cognetti F, Sabbatini R, et al, Italian Group for Antiemetic Research. Persistence of efficacy of three antiemetic regimens and prognostic factors in patients submitted to moderately emetogenic chemotherapy. Supportive Care in Cancer 1995;3(Suppl):338. [Google Scholar]
Barrajon 2000 {published data only}
Belle 2002 {published data only}
- Belle S, Lichinitser MR, Navari RM, Garin AM, Decramer MLA, Riviere A, et al. Prevention of cisplatin-induced acute and delayed emesis by the selective neurokinin-1 antagonists, L-758,298 and MK-869: a randomized controlled trial. Cancer 2002;94(11):3032-41. [DOI] [PubMed] [Google Scholar]
Bianchi 1996 {published data only}
- Bianchi A, Maccio A, Curreli L, Ghiani M, Santona MC, Astara G. Comparison of granisetron vs ondansetron vs tropisetron in the prophylaxis of acute nausea and vomiting induced by high-dose cisplatin for treatment of primary head and neck cancer: an open randomized controlled trial. In: Annals of Oncology. Vol. 7. 1996:135. [PubMed]
- Mantovani G, Macciò A, Bianchi A, Curreli L, Ghiani M, Proto E, et al. Comparison of granisetron, ondansetron, and tropisetron in the prophylaxis of acute nausea and vomiting induced by cisplatin for the treatment of head and neck cancer: a randomized controlled trial. Cancer 1996;77(5):941-8. [PubMed] [Google Scholar]
- Mantovani G, Maccio A, Bianchi A, Curreli L, Ghiani M, Santoma MC, et al. Comparison of granisetron vs ondansetron vs tropisetron in the prophylaxis of acute nausea and vomiting induced by highly emetogenic chemotherapy (high-dose cisplatin) for treatment of primary head and neck cancer: an open cross-over randomized controlled trial. In: Annals of Oncology. Vol. 31 A. 1995:S252. Abs. 1206.
Bonneterre 1994 {published data only}
Bonneterre 1995 {published data only}
- Bonneterre J, Hecquet B. Granisetron (IV) compared with ondansetron (IV plus oral) in the prevention of nausea and vomiting induced by moderately-emetogenic chemotherapy. A cross-over study. Bulletin du Cancer 1995;82(12):1038-43. [PubMed] [Google Scholar]
Bubalo 2001 {published data only}
- Bubalo J, Seelig F, Karbowicz S, Maziarz RT. Randomized open-label trial of dolasetron for the control of nausea and vomiting associated with high-dose chemotherapy with hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 2001;7(8):439-45. [DOI] [PubMed] [Google Scholar]
Bubalo 2012 {published data only}
- Bubalo JS, Cherala G, McCune JS, Munar MY, Tse S, Maziarz R. Aprepitant pharmacokinetics and assessing the impact of aprepitant on cyclophosphamide metabolism in cancer patients undergoing hematopoietic stem cell transplantation. Journal of Clinical Pharmacology 2012;52(4):586-94. [DOI] [PubMed] [Google Scholar]
Campora 1994 {published data only}
- Campora E, Simoni C, Rosso R. [Tropisetron versus ondansetron in the prevention and control of emesis in patients undergoing chemotherapy with FAC/FEC for metastatic or surgically treated breast carcinoma]. Minerva Medica 1994;85(1-2):25-31. [PubMed] [Google Scholar]
Chiou 2000 {published data only}
- Chiou TJ, Tzeng WF, Wang WS, Yen CC, Fan FS, Liu JH, et al. Comparison of the efficacy and safety of oral granisetron plus dexamethasone with intravenous ondansetron plus dexamethasone to control nausea and vomiting induced by moderate/severe emetogenic chemotherapy. Chinese Medical Journal 2000;63(10):729-36. [PubMed] [Google Scholar]
Choi 2014 {published data only}
- Choi CH, Kim MK, Park JY, Yoon A, Kim HJ, Lee YY, et al. Safety and efficacy of aprepitant, ramosetron, and dexamethasone for chemotherapy-induced nausea and vomiting in patients with ovarian cancer treated with paclitaxel/carboplatin. Supportive Care in Cancer 2014;22(5):1181-7. [DOI] [PubMed] [Google Scholar]
Cocquyt 2001 {published data only}
- Cocquyt V, Belle S, Reinhardt RR, Decramer ML, O'Brien M, Schellens JH, et al. Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. European Journal of Cancer 2001;37(7):835-42. [DOI] [PubMed] [Google Scholar]
Craver 2011 {published data only}
- Craver C, Gayle J, Balu S, Buchner D. Palonosetron versus other 5-HT3 receptor antagonists for prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies treated with emetogenic chemotherapy in a hospital outpatient setting in the United States. Journal of Medical Economics 2011;14(3):341-9. [DOI] [PubMed] [Google Scholar]
Creed 1999 {published data only}
- Creed M, Brogden J, Ames M, Bryson J. Oral ondansetron (OND) for the prevention of acute nausea and vomiting (N/V) in highly emetogenic cisplatin (CDDP)-based chemotherapy regimens. In: Supportive Care in Cancer. Vol. 7. 1999:176.
Dandamudi 2011 {published data only}
- Dandamudi UB, Adams LM, Johnson B, Bauman J, Morris S, Murray S, et al. Lack of effect of casopitant on the pharmacokinetics of docetaxel in patients with cancer. Cancer Chemotherapy and Pharmacology 2011;67(4):783-90. [DOI] [PubMed] [Google Scholar]
Dong 2011 {published data only}
- Dong X, Huang J, Cao R, Liu L. Palonosetron for prevention of acute and delayed nausea and vomiting in non-small-cell lung carcinoma patients. Medical Oncology 2011;28:1425-9. [DOI] [PubMed] [Google Scholar]
Fauser 1995 {published data only}
- Fauser AA, Bergerat JP, Cocquyt V, Chemaissani A, Favero A, Dressler H. Double-blind, comparative trial of four single oral doses of dolasetron mesilate (DM) and multiple doses of ondansetron (OND) for emesis prevention after moderately emetogenic chemotherapy (CT). In: European Journal of Cancer. Vol. 31. 1995:254.
- Favero A, Bergerat J, Chemaissani A, Dressler H. Single oral doses of dolasetron versus multiple doses of ondansetron in preventing emesis after moderately emetogenic chemotherapy. In: Supportive Care in Cancer. Vol. 3. 1995:337.
Fauser 1996 {published data only}
- Fauser AA, Duclos B, Chemaissani A, Del Favero A, Cognetti F, Diaz-Rubio E, et al, European Dolasetron Comparative Study Group. Therapeutic equivalence of single oral doses of dolasetron mesilate and multiple doses of ondansetron for the prevention of emesis after moderately emetogenic chemotherapy. European Journal of Cancer 1996;32A(9):1523-9. [DOI] [PubMed] [Google Scholar]
Fauser 1999 {published data only}
Fedele 1995 {published data only}
- Fedele P, Spina M, Valentini M, Collini D, Lucia C, Manicone M, et al. Ondansetron versus granisetron in the prevention of chemotherapy-induced nausea and vomiting. Cancer 1995;76(3):535-6. [DOI] [PubMed] [Google Scholar]
Feng 2000 {published data only}
- Feng FY, Zhang P, He YJ, Li YH, Zhou MZ, Cheng G, et al. Comparison of the selective serotonin3 antagonists ramosetron and granisetron in treating acute chemotherapy-induced emesis, nausea, and anorexia: a single-blind, randomized, crossover study. Current Therapeutic Research 2000;61(12):901-9. [Google Scholar]
Feng 2002 {published data only}
- Feng F, Zhang P, He Y, Li Y, Zhou M, Chen G, et al. Clinical comparison of the selective serotonin3 antagonists ramosetron and granisetron in treating acute chemotherapy-induced emesis, nausea and anorexia. Chinese Medical Sciences Journal 2002;17:168-72. [PubMed] [Google Scholar]
Fengyi 2002 {published data only}
- Fengyi F, Pin Z, Youjian H, Yuhong L, Meizhen Z, Gang C, et al. Clinical comparison of the selective serotonin3 antagonists ramosetron and granisetron in treating acute chemotherapy-induced emesis, nausea and anorexia. Chinese Medical Sciences Journal 2002;17(3):168-72. [PubMed] [Google Scholar]
Gebbia 1994 {published data only}
- Gebbia V, Cannata G, Testa A, Curto G, Valenza R, Cipolla C, et al. Ondansetron versus granisetron in the prevention of chemotherapy-induced nausea and vomiting. Results of a prospective randomized trial. Cancer 1994;74(7):1945-52. [DOI] [PubMed] [Google Scholar]
Goldschmidt 1997 {published data only}
- Goldschmidt H, Salwender H, Egerer G, Kempe R, Voigt T. Comparison of oral itasetron with oral ondansetron: results of a double-blind, active-controlled phase II study in chemotherapy-naive patients receiving moderately emetogenic chemotherapy. Anti-Cancer Drugs 1997;8(5):436-44. [PubMed] [Google Scholar]
Gralla 1998 {published data only}
- Gralla RJ, Navari RM, Hesketh PJ, Popovic W, Strupp J, Noy J, et al. Single-dose oral granisetron has equivalent antiemetic efficacy to intravenous ondansetron for highly emetogenic cisplatin-based chemotherapy. Journal of Clinical Oncology 1998;16(4):1568-73. [DOI] [PubMed] [Google Scholar]
Gralla 2003 {published data only}
- Gralla R, Lichinitser M, Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Annals of Oncology 2003;14(10):1570-7. [DOI] [PubMed] [Google Scholar]
Hesketh 1996 {published data only}
- Hesketh P, Navari R, Grote T, Gralla R, Hainsworth J, Kris M, et al, Dolasetron Comparative Chemotherapy-Induced Emesis Prevention Group. Double-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. Journal of Clinical Oncology 1996;14(8):2242-9. [DOI] [PubMed] [Google Scholar]
Huang 1998 {published data only}
- Huang J, Wang Z, Zhou L. [A randomized trial of Zudan in the prophylaxis of nausea and vomiting induced by cisplatin]. Chinese Journal of Oncology 1998;20(2):153-4. [PubMed] [Google Scholar]
Huang 2001 {published data only}
- Huang F, Zhang ML. Effect of ondansetron in prevention of nausea and vomiting induced by cancer chemotherapy. Shanxi Medical Journal 2001;30(9):546-8. [Google Scholar]
Huang 2013 {published data only}
- Huang J, Wang XJ, Yu D, Jin YN, Zhen LZ, Xu N, et al. The effect of palonosetron hydrochloride in the prevention of chemotherapy-induced moderate and severe nausea and vomiting. Experimental and Therapeutic Medicine 2013;5(5):1418-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huc 1998 {published data only}
- Huc P, Block S, Carlier D, Darloy F, Bonneterre ME, Bleuse JP, et al. Granisetron (per os) compared with ondansetron (per os) in the prevention of nausea and vomiting induced by mildly emetogenic chemotherapies. Groupe de Recherches en Cancerologie du Nord [Granisétron (per os) comparé à ondansétron (per os) dans la prévention des nausées et vomissements induits par des chimiothérapies moyennement émétisantes]. Bulletin du Cancer 1998;85(6):562-8. [PubMed] [Google Scholar]
Hudis 2003 {published data only}
- Hudis CA, Hainsworth JD, Perez EA, Macciocchi A. Palonosetron is more effective than ondansetron/dolasetron in preventing chemotherapy-induced nausea and vomiting in patients with breast cancer: combined results of 2 phase 3 trials. In: CENTRAL. 2003. [CENTRAL: CN-00990041]
Humphreys 2013 {published data only}
- Humphreys S, Pellissier J, Jones A. Cost-effectiveness of an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer in the UK. Cancer Management and Research 2013;5:215-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iihara 2012 {published data only}
- Iihara H, Endo J, Yamada M, Kitaichi K, Yanase K, Kamiya F, et al. The antiemetic effects of oral azasetron in lung cancer patients treated with moderately emetogenic chemotherapy: comparison with intravenous granisetron. In: European Respiratory Journal. Vol. 40. 2012:Suppl 56, 228s.
Italian Group for Antiemetic Research 1993 {published data only}
- Italian Group for Antiemetic Research. Difference in persistence of efficacy of two antiemetic regimens on acute emesis during cisplatin chemotherapy. Journal of Clinical Oncology 1993;11(12):2396-404. [DOI] [PubMed] [Google Scholar]
Italian Group for Antiemetic Research 1995 (a) {published data only}
- Italian Group for Antiemetic Research. Ondansetron versus granisetron, both combined with dexamethasone, in the prevention of cisplatin-induced emesis. Annals of Oncology 1995;6:805-10. [PubMed] [Google Scholar]
Italian Group for Antiemetic Research 1995 (b) {published data only}
- Italian Group for Antiemetic Research. Persistence of efficacy of three antiemetic regimens and prognostic factors in patients undergoing moderately emetogenic chemotherapy. Journal of Clinical Oncology 1995;13(9):2417-26. [DOI] [PubMed] [Google Scholar]
Jantunen 1993 {published data only}
- Jantunen IT, Muhonen TT, Kataja VV, Flander MK, Teerenhovi L. 5-HT3 receptor antagonists in the prophylaxis of acute vomiting induced by moderately emetogenic chemotherapy - a randomised study. European Journal of Cancer 1993;29A(12):1669-72. [DOI] [PubMed] [Google Scholar]
Kang 2002 {published data only}
- Kang YK, Park YH, Ryoo BY, Bang YJ, Cho KS, Shin DB, et al. Ramosetron for the prevention of cisplatin-induced acute emesis: a prospective randomized comparison with granisetron. Journal of International Medical Research 2002;30(3):220-9. [DOI] [PubMed] [Google Scholar]
Kawaguchi 2015 {published data only}
- Kawaguchi R, Tanase Y, Haruta S, Yoshida S, Furukawa N, Kobayashi H. Addition of aprepitant to standard therapy for prevention of nausea and vomiting among patients with cervical cancer undergoing concurrent chemoradiotherapy. International Journal of Gynaecology & Obstetrics 2015;131(3):312-3. [DOI] [PubMed] [Google Scholar]
Kilickap 2013 {published data only}
- Kilickap S, Kacan T, Akgul Babacan N. The efficacy of palonosetron compared with granisetron in preventing chemotherapy-induced nausea and vomiting: a randomized study. In: European Journal of Cancer. Vol. 49, Suppl 2. 2013:S272.
Kim 1998 {published data only}
- Kim YB, Kim JW, Seo DG, Lee CM, Park NH, Song YS. A randomized comparison of tropisetron versus ondansetron in the control of nausea and vomiting in gynecologic cancer patients undergoing cisplatin-based chemotherapy. Journal of Obstetrics and Gynecology 1998;41(10):2544-50. [Google Scholar]
Kim 2004 {published data only}
- Kim JS, Baek JY, Park SR, Choi IS, Kim SI, Kim DW, et al. Open-label, randomized comparison of the efficacy of intravenous dolasetron mesylate and ondansetron in the prevention of acute and delayed cisplatin-induced emesis in cancer patients. Cancer Research 2004;36(6):372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim KI, Lee DE, Cho I, Yoon JH, Yoon SS, Lee HS, et al. Effectiveness of palonosetron versus other serotonin 5-HT3 receptor antagonists in triple antiemetic regimens during multiday highly emetogenic chemotherapy. Annals of Pharmacotherapy 2012;46(12):1637-44. [DOI] [PubMed] [Google Scholar]
Lacerda 2000 {published data only}
- Lacerda JF, Martins C, Carmo JA, Lourenço MF, Araújo Pereira ME, Rodrigues A, et al. Randomized trial of ondansetron, granisetron, and tropisetron in the prevention of acute nausea and vomiting. Transplantation Proceedings 2000;32(8):2680-1. [DOI] [PubMed] [Google Scholar]
Lavoie 2012 {published data only}
- Lavoie A, Grenier ME, Longpre-Girard J, Messier C, Letarte N, Ayoub JP, et al. Assessing the impact of aprepitant with dexamethasone in preventing nausea and vomiting induced by moderately emetogenic chemotherapies - PADENAUVO pilot study. In: Supportive Care in Cancer. Vol. 20. 2012:S147-8.
Lee 2014 {published data only}
- Lee HY, Kim HK, Lee KH, Kim BS, Song HS, Yang SH, et al. A randomized double-blind, double-dummy, multicenter trial of azasetron versus ondansetron to evaluate efficacy and safety in the prevention of delayed nausea and vomiting induced by chemotherapy. Cancer Research and Treatment 2014;46(1):19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonardi 1996 {published data only}
Lindley 2005 {published data only}
- Lindley C, Goodin S, McCune J, Kane M, Amamoo MA, Shord S, et al. Prevention of delayed chemotherapy-induced nausea and vomiting after moderately high to highly emetogenic chemotherapy: comparison of ondansetron, prochlorperazine, and dexamethasone. American Journal of Clinical Oncology 2005;28(3):270-6. [DOI] [PubMed] [Google Scholar]
Lofters 1995 {published data only}
- Lofters WS, Zee B. Dolasetron (DOL) vs ondansetron (OND) with and without dexamethasone (DEX) in the prevention of nausea (N) and vomiting (V) in patients (PTS) receiving moderately emetogenic chemotherapy (MEC). European Journal of Cancer 1995;31(Suppl 6):S252.
Long 2002 {published data only}
- Long WH. Effect of tropisetron on preventing lung cancer patients from nausea and vomiting caused by chemotherapy. Chinese Journal of New Drugs and Clinical Remedies 2002;21(7):425-7.
Loos 2007 {published data only}
- Loos WJ, Wit R, Freedman SJ, Dyck K, Gambale JJ, Li S, et al. Aprepitant when added to a standard antiemetic regimen consisting of ondansetron and dexamethasone does not affect vinorelbine pharmacokinetics in cancer patients. Cancer Chemotherapy and Pharmacology 2007;59:407-12. [DOI] [PubMed] [Google Scholar]
Mandanas 2005 {published data only}
- Mandanas RA, Beveridge R, Rifkin RM, Wallace H, Greenspan A, Asmar L. A randomized, multicenter, open-label comparison of the antiemetic efficacy of dolasetron versus ondansetron for the prevention of nausea and vomiting during high-dose myeloablative chemotherapy. Supportive Cancer Therapy 2005;2(2):114-21. [DOI] [PubMed] [Google Scholar]
Martoni 1996 {published data only}
- Martoni A, Angelelli B, Guaraldi M, Strocchi E, Pannuti F. An open randomised cross-over study on granisetron versus ondansetron in the prevention of acute emesis induced by moderate dose cisplatin-containing regimens. European Journal of Cancer 1996;32A(1):82-5. [DOI] [PubMed] [Google Scholar]
Marty 1995 {published data only}
- Marty M, Kleisbauer JP, Fournel P, Vergnenegre A, Carles P, Loria-Kanza Y, et al, The French Navoban Study Group. Is Navoban (tropisetron) as effective as Zofran (ondansetron) in cisplatin-induced emesis? In: Anti-cancer Drugs. Vol. 6. 1995:15-21. [PubMed]
Matsui 1996 {published data only}
- Matsui K, Fukuoka M, Takada M, Kusunoki Y, Yana T, Tamura K, et al. Randomised trial for the prevention of delayed emesis in patients receiving high-dose cisplatin. British Journal of Cancer 1996;73(2):217-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuoka 2003 {published data only}
- Matsuoka S, Okamoto S, Watanabe R, Mori T, Nagayama H, Hamano Y, et al. Granisetron plus dexamethasone versus granisetron alone in the prevention of vomiting induced by conditioning for stem cell transplantation: a prospective randomized study. International Journal of Hematology 2003;77:86-90. [DOI] [PubMed] [Google Scholar]
Meiri 2007 {published data only}
- Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Current Medical Research and Opinion 2007;23(3):533-43. [DOI] [PubMed] [Google Scholar]
Micha 2016 {published data only}
- Micha JP, Rettenmaier MA, Brown JV 3rd, Mendivil A, Abaid LN, Lopez KL, et al. A randomized controlled pilot study comparing the impact of aprepitant and fosaprepitant on chemotherapy induced nausea and vomiting in patients treated for gynecologic cancer. International Journal of Gynecological Cancer 2016;26(2):389-93. [DOI] [PubMed] [Google Scholar]
Molassiotis 2013 {published data only}
- Molassiotis A, Nguyen AM, Rittenberg CN, Makalinao A, Carides A. Analysis of aprepitant for prevention of chemotherapy-induced nausea and vomiting with moderately and highly emetogenic chemotherapy. Future Medicine 2013;9(10):1443-50. [DOI] [PubMed] [Google Scholar]
Monda 1994 {published data only}
- Monda MdGM, Vita F, Della Vittoria M, Frattolillo A, Catalano G. [Tropisetron vs granisetron in acute delayed emesis induced by cisplatin: preliminary data]. Tumori 1994;(80):150. [Google Scholar]
Moore 2007 {published data only}
- Moore S, Tumeh J, Wojtanowski S, Flowers C. Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health 2007;10(1):23-31. [DOI] [PubMed] [Google Scholar]
Nasu 2013 {published data only}
- Nasu R, Nannya Y, Ichikawa M, Kurokawa M. Randomized controlled trial to evaluate the efficacy of aprepitant in hematological malignancy. In: Annals of Oncology. Vol. 24. 2013:ix51.
- Nasu R, Nannya Y, Yoshimi A, Hosoi M, Ueda K, Yoshiki Y, et al. A randomized controlled study evaluating the efficacy of aprepitant for highly emetogenic chemotherapies in hematological malignancies. In: Blood. Vol. 122. 2013:1790.
Navari 2016 {published data only}
- Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. New England Journal of Medicine 2016;374(14):1356-67. [DOI] [PubMed] [Google Scholar]
NCT04636632 {published data only}
- NCT04636632. Fosaprepitant for the prevention of nausea and emesis during concurrent chemoradiotherapy for nasopharyngeal carcinoma [2020]. https://clinicaltrials.gov/ct2/show/NCT04636632 (last accessed 21 July 2021).
Nishimura 2015 (a) {published data only}
- Nishimura J, Satoh T, Fukunaga M, Takemoto H, Nakata K, Ide Y, et al. A phase III trial of aprepitant in colorectal cancer patients receiving oxaliplatin-based chemotherapy (SENRI Trial). In: Annals of Oncology. Vol. 26. 2015:iv108.
Nishimura 2015 (b) {published data only}
- Nishimura J, Satoh T, Fukunaga M, Takemoto H, Nakata K, Ide Y, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled phase 3 trial. European Journal of Cancer 2015;51(10):1274-82. [DOI] [PubMed] [Google Scholar]
Noble 1994 {published data only}
- Noble A, Bremer K, Goedhals L, Cupissol D, Dilly SG, The Granisetron Study Group. A double-blind, randomised, crossover comparison of granisetron and ondansetron in 5-day fractionated chemotherapy: assessment of efficacy, safety and patient preference. European Journal of Cancer 1994;30A(8):1083-8. [DOI] [PubMed] [Google Scholar]
Noda 2002 {published data only}
- Noda K, Ikeda M, Taguchi T, Kanamaru R, Ichijyo M, Okada K, et al. Clinical assessment of ramosetron HCl oral preparation in the treatment of nausea and vomiting induced by cisplatin: a multicenter, randomized, parallel-design, double-blind comparative study with ondansetron HCl. Current Therapeutic Research 2002;63(10):636-48.
Öge 2000 {published data only}
- Oge A, Alki N, Oge O, Kartum A. Comparison of granisetron, ondansetron and tropisetron for control of vomiting and nausea induced by cisplatin. Journal of Chemotherapy 2000;12(1):105-8. [DOI] [PubMed] [Google Scholar]
Ogihara 1999 {published data only}
- Ogihara M, Suzuki T, Yanagida T, Tsuruya Y, Ishibashi K, Yamaguchi O. Clinical assessment of granisetron and methyl-prednisolone as a prophylactic antiemetic in cisplatin-induced delayed emesis. [Japanese]. Japanese Journal of Clinical Urology 1999;53(2):141-5. [Google Scholar]
Ohta 1992 {published data only}
- Ohta J, Taguchi T, Furue H, Niitani H, Ota K, Tsukagoshi S, et al. [Anti-emetic effect and safety of single dose of ondansetron injection in double-blind comparison study with placebo]. Gan To Kagaku Ryoho 1992;19(12):2041-55. [PubMed] [Google Scholar]
Ottoboni 2014 {published data only}
- Ottoboni T, Gelder MS, O'Boyle E. Biochronomer[TM] technology and the development of APF530, a sustained release formulation of granisetron. Journal of Experimental Pharmacology 2014;6:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 1997 {published data only}
- Park JO, Rha SY, Yoo NC, Kim JH, Roh JK, Min JS, et al. A comparative study of intravenous granisetron versus intravenous and oral ondansetron in the prevention of nausea and vomiting associated with moderately emetogenic chemotherapy. American Journal of Clinical Oncology 1997;20(6):569-72. [DOI] [PubMed] [Google Scholar]
Pater 1997 {published data only}
- Lofters WS, Pater JL, Zee B, Dempsey E, Walde D, Moquin JP, et al. Phase III double-blind comparison of dolasetron mesylate and ondansetron and an evaluation of the additive role of dexamethasone in the prevention of acute and delayed nausea and vomiting due to moderately emetogenic chemotherapy. Journal of Clinical Oncology 1997;15(8):2966-73. [DOI] [PubMed] [Google Scholar]
- Pater JL, Lofters WS, Zee B, Dempsey E, Walde D, Moquin JP, et al. The role of the 5-HT3 antagonists ondansetron and dolasetron in the control of delayed onset nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Annals of Oncology 1997;8(2):181-5. [DOI] [PubMed] [Google Scholar]
Pectasides 2007 {published data only}
- Pectasides D, Dafni U, Aravantinos G, Timotheadou E, Skarlos DV, Pavlidis N, et al. A randomized trial to compare the efficacy and safety of antiemetic treatment with ondansetron and ondansetron zydis in patients with breast cancer treated with high-dose epirubicin. Anticancer Research 2007;27(6c):4411-7. [PubMed] [Google Scholar]
Perez 1996 {published data only}
- Perez EA, Lembersky B, Kaywin P, Kalman L, Friedman C. Intravenous granisetron vs ondansetron in the prevention of cyclophosphamide-doxorubicin-induced emesis in breast cancer patients: a double-blind crossover study [abstract]. In: Proceedings of the American Society of Clinical Oncology. Vol. 15. 1996:543, Abstract 1764.
Perez 1998 (a) {published data only}
- Perez EA, Lembersky B, Kaywin P, Kalman L, Yocom K, Friedman C. Comparable safety and antiemetic efficacy of a brief (30-second bolus) intravenous granisetron infusion and a standard (15-minute) intravenous ondansetron infusion in breast cancer patients receiving moderately emetogenic chemotherapy. Cancer Journal from Scientific American 1998;4(1):52-8. [PubMed] [Google Scholar]
Perez 1998 (b) {published data only}
- Perez EA, Hesketh P, Sandbach J, Reeves J, Chawla S, Markman M, et al. Comparison of single-dose oral granisetron versus intravenous ondansetron in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy: a multicenter, double-blind, randomized parallel study. Journal of Clinical Oncology 1998;16(2):754-60. [DOI] [PubMed] [Google Scholar]
Peterson 1996 {published data only}
- Peterson C, Hursti TJ, Börjeson S, Avall-Lundqvist E, Fredrikson M, Fürst CJ, et al. Single high-dose dexamethasone improves the effect of ondansetron on acute chemotherapy-induced nausea and vomiting but impairs the control of delayed symptoms. Supportive Care in Cancer 1996;4(6):440-6. [DOI] [PubMed] [Google Scholar]
Plasencia‐Mota 1993 {published data only}
- Plasencia-Mota A, García-Vidrios V, Rivas-Vera S, Velez-Rodríguez S, Silveyra-Gómez C, Hernández-Hernández A. An evaluation of the effectiveness of ondansetron vs. triple antiemetic drug in patients with hematologic neoplasias [Evaluación de la efectividad de ondansetron vs triple droga antiemética en pacientes con neoplasias hematológicas]. In: Sangre. Vol. 38. 1993:85. [CENTRAL: CN-00402257]
Poon 1998 {published data only}
- Poon RT, Chow LW. Comparison of antiemetic efficacy of granisetron and ondansetron in Oriental patients: a randomized crossover study. British Journal of Cancer 1998;77(10):1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiu 2011 {published data only}
- Qiu LH, Wang HQ, Yu Z, Li L, Wang XH, Wang SJ, et al. Efficacy and safety of palonosetron versus tropisetron in the prevention of highly emetogenic chemotherapy-induced acute and delayed vomiting in Chinese cancer patients. [Chinese]. Zhonghua Yi Xue Za Zhi 2011;91(36):2555-7. [PubMed] [Google Scholar]
Roila 2009 {published data only}
- Roila F, Rolski J, Ramlau R, Dediu M, Russo MW, Bandekar RR, et al. Randomized, double-blind, dose-ranging trial of the oral neurokinin-1 receptor antagonist casopitant mesylate for the prevention of cisplatin-induced nausea and vomiting. Annals of Oncology 2009;20(11):1867-73. [DOI] [PubMed] [Google Scholar]
Roscoe 2012 {published data only}
- Roscoe JA, Heckler CE, Morrow GR, Mohile SG, Dakhil SR, Wade JL, et al. Prevention of delayed nausea: a University of Rochester Cancer Center Community Clinical Oncology Program study of patients receiving chemotherapy. Journal of Clinical Oncology 2012;30(27):3389-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruff 1994 {published data only}
- Ruff P, Paska W, Goedhals L, Pouillart P, Riviere A, Vorobiof D, et al, The Ondansetron and Granisetron Emesis Study Group. Ondansetron compared with granisetron in the prophylaxis of cisplatin-induced acute emesis: a multicentre double-blind, randomised, parallel-group study [Erratum appears in Oncology 1994 May-Jun;51(3):243]. Oncology 1994;51(1):113-8. [DOI] [PubMed] [Google Scholar]
Ruhlmann 2016 {published data only}
- Ruhlmann CH, Christensen TB, Dohn LH, Paludan M, Ronnengart E, Halekoh U, et al. Efficacy and safety of fosaprepitant for the prevention of nausea and emesis during 5 weeks of chemoradiotherapy for cervical cancer (the GAND-emesis study): a multinational, randomised, placebo-controlled, double-blind, phase 3 trial. Lancet Oncology 2016;17(4):509-18. [DOI] [PubMed] [Google Scholar]
Rzepecki 2009 {published data only}
- Rzepecki P, Pielichowski W, Oborska S, Barzal J, Mlot B. Palonosetron in prevention of nausea and vomiting after highly emetogenic chemotherapy before haematopoietic stem cell transplantation-single center experience. Transplantation Proceedings 2009;41(8):3247-9. [DOI] [PubMed] [Google Scholar]
Saito 2015 {published data only}
- Saito M, Tsuneizumi M, Kutomi G, Ogata H, Sugizaki K, Katsumata N, et al. Interim analysis of a randomized double blind comparative trial of triplet antiemetic therapy (TTT) for. In: Supportive Care in Cancer. Vol. 23. 2015:S137. [DOI: 10.1007/s00520-015-2712-y] [DOI]
Sheng 2010 {published data only}
- Sheng GF, Bi YZ, Zeng DX, Xu Z, Dong YZ, Song HL, et al. Clinical trial of treating nausea and vomiting caused by chemotherapy with palonosetron. [Chinese]. Chinese Journal of Cancer Prevention and Treatment 2010;17(23):1976-7. [Google Scholar]
Shi 2007 {published data only}
- Shi Y, He X, Yang S, Ai B, Zhang C, Huang D, et al. Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: a prospective randomized controlled study. Chemotherapy 2007;53(1):44-50. [DOI] [PubMed] [Google Scholar]
Silvestris 2013 {published data only}
- Silvestris N, Brunetti AE, Russano M, Nardulli P. Optimal control of nausea and vomiting with a three-drug antiemetic regimen with aprepitant in metastatic pancreatic cancer patients treated with first-line modified FOLFIRINOX. Supportive Care in Cancer 2013;21(11):2955-6. [DOI] [PubMed] [Google Scholar]
Slabý 2000 {published data only}
- Lacerda JMF, Matrins C, Carmo JA, Lourenco MF, Araujo-Pereira ME, Rodrigues A, et al. Randomized trial of ondansetron (OND), granisetron (GRA) and tropisetron (TRO) in the prevention of acute nausea and vomiting in stem cell transplantation (SCT) [abstract]. In: Blood. Vol. 94. 1999:150a.
- Slabý J, Trnený M, Procházka B, Klener P. Antiemetic efficacy of three serotonin antagonists during high-dose chemotherapy and autologous stem cell transplantation in malignant lymphoma. Neoplasma 2000;47(5):319-22. [PubMed] [Google Scholar]
Spector 1998 {published data only}
- Spector JI, Lester EP, Chevlen EM, Sciortino D, Harvey JH, Whaley W, et al. A comparison of oral ondansetron and intravenous granisetron for the prevention of nausea and emesis associated with cisplatin-based chemotherapy. Oncologist 1998;3(6):432-8. [PubMed] [Google Scholar]
Stewart 1995 {published data only}
- Stewart A, McQuade B, Cronje JD, Goedhals L, Gudgeon A, Corette L, et al, Emesis Study Group for Ondansetron and Granisetron in Breast Cancer Patients. Ondansetron compared with granisetron in the prophylaxis of cyclophosphamide-induced emesis in out-patients: a multicentre, double-blind, double-dummy, randomised, parallel-group study. Oncology 1995;52(3):202-10. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun DS, Ko YH, Jin JY, Woo IS, Park YS, Kang JH, et al. Clinical impacts of granisetron transdermal system:sustained anti-emetic efficacy and quality of life in control of nausea and vomiting induced by highly emetogenic chemotherapy. In: Supportive Care in Cancer. Vol. 22. 2014:S106-s107.
Suzuki 2015 {published data only}
- Suzuki K, Tsuji D, Yokoi M, Daimon T, Nakao M, Ayuhara H, et al. Influence of ABCB1 and ABCG2 polymorphisms on the antiemetic efficacy of a triple antiemetic combination in cancer patients receiving cisplatin-based chemotherapy: TRIPLE Pharmacogenomics Study. In: European Journal of Cancer. Vol. 51. 2015:S231. [DOI] [PubMed]
Takenaka 2007 {published data only}
- Takenaka M, Okamoto Y, Ikeda K, Hashimoto R, Ueda T, Kurokawa N, et al. [Comparison of antiemetic efficacy of 5-HT3 receptor antagonists in orthopedics cancer patients receiving high-dose chemotherapy]. Gan To Kagaku Ryoho 2007;34(3):403-7. [PubMed] [Google Scholar]
Takeshima 2014 {published data only}
- Takeshima N, Matoda M, Abe M, Hirashima Y, Kai K, Nasu K, et al. Efficacy and safety of triple therapy with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy for gynecological cancer: KCOG-G1003 phase II trial. Supportive Care in Cancer 2014;22(11):2891-8. [DOI] [PubMed] [Google Scholar]
Tan 2004 {published data only}
- Tan M, Xu R, Seth R. Granisetron vs dolasetron for acute chemotherapy-induced nausea and vomiting (CINV) in high and moderately high emetogenic chemotherapy: an open-label pilot study. Current Medical Research & Opinion 2004;20(6):879-82. [DOI] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang L, Lin F, Yao Y. Efficacy of palonosetron hydrochloride injection in preventing gastrointestinal reactions caused by high-dose chemotherapy in osteosarcoma patients. [Chinese]. Chinese Journal of Clinical Oncology 2013;40(3):168-170, 177. [Google Scholar]
Tanimura 1998 {published data only}
- Tanimura S, Banba J, Tomoyasu H, Masaki M. [Effect of concurrent use of ondansetron hydrochloride and dexamethasone against nausea and vomiting in lung cancer patients receiving cisplatin]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1998;25(14):2275-81. [PubMed] [Google Scholar]
Tian 2011 {published data only}
- Tian W, Wang Z, Zhou J, Zhang S, Wang J, Chen Q, et al. Randomized, double-blind, crossover study of palonosetron compared with granisetron for the prevention of chemotherapy-induced nausea and vomiting in a Chinese population. Medical Oncology 2011;28(1):71-8. [DOI] [PubMed] [Google Scholar]
Tominaga 1996 {published data only}
- Tominaga T, Furuse K, Fukuda T, Yamaguchi T, Horai T, Oizumi K, et al. [Clinical evaluation of azasetron tablets against nausea and vomiting induced by anticancer drugs: multicenter double blind test with ondansetron tablets as control]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1996;12(14):3089-112. [Google Scholar]
Tong 2012 {published data only}
- Tong Q, He Z-Y, Sun J-Y. The efficacy of palonosetron for prevention of concurrent radiochemotherapy induced nausea and vomit in patients with locally advanced nasopharyngeal carcinoma. In: Journal of Chinese Oncology. Vol. 4. 2012:011.
Tong 2014 {published data only}
- Tong Z, Li S, Zheng R, He Z, Zhang L, Ouyang X, et al. Effect of palonosetron in preventing chemotherapy-induced vomiting. [Chinese]. Chinese Journal of Clinical Oncology 2014;41(20):1323-7. [Google Scholar]
Tremont‐Lukats 2017 {published data only}
- Tremont-Lukats IW, Teixeira GM. Comparison of granisetron, ondansetron, and tropisetron in the prophylaxis of acute nausea and vomiting induced by cisplatin for the treatment of head and neck cancer. A randomized controlled trial. Cancer 2017;78(11):2450-3. [DOI] [PubMed] [Google Scholar]
Tsavaris 1996 {published data only}
- Tsavaris N, Kosmas C, Samarkos M, Stratigaki-Alexiou E, Kondos A, Arvaniti-Bofili E, et al. Randomized comparative study of antiemetic activity of metoclopramide (M) vs ondansetron (Od) vs tropisetron vs granisetron (G) in patients receiving moderately emetogenic chemotherapy. In: Supportive Care in Cancer. Vol. 4. 1996:252.
Tsubata 2015 {published data only}
- Tsubata Y, Mori Y, Nakao M, Amano Y, Hotta T, Koba N, et al. Randomized translational study of 5HT3 receptor antagonists for evaluation of chemotherapy-induced nausea and vomiting (CINV) related biomarker and prevention of CINV using questionnaires. In: Journal of Clinical Oncology. Vol. 33. 2015.
Tsuji 2016 {published data only}
- Tsuji D, Yokoi M, Suzuki K, Daimon T, Nakao M, Ayuhara H, et al. Influence of ABCB1 and ABCG2 polymorphisms on the antiemetic efficacy in patients with cancer receiving cisplatin-based chemotherapy: a TRIPLE pharmacogenomics study. Pharmacogenomics Journal 2016;17:435-40. [DOI] [PubMed] [Google Scholar]
Tsukuda 1995 {published data only}
- Tsukuda M, Mochimatsu I, Furukawa M, Kohno H, Kawai S, Enomoto H, et al. [A randomized crossover comparison of azasetron and granisetron in the prophylaxis of emesis induced by chemotherapy including cisplatin]. Japanese Journal of Cancer & Chemotherapy 1995;22(13):1959-67. [PubMed] [Google Scholar]
Uchino 2012 {published data only}
- Uchino J, Hirano R, Tashiro N, Yoshida Y, Ushijima S, Matsumoto T, et al. Efficacy of aprepitant in patients with advanced or recurrent lung cancer receiving moderately emetogenic chemotherapy. Asian Pacific Journal of Cancer Prevention 2012;13(8):4187-90. [DOI] [PubMed] [Google Scholar]
Vadhan‐Raj 2011 {published data only}
- Vadhan-Raj S, Spasojevic I, Zhou X, Romaguera JE, Fanale MA, Fayad LE, et al. Effects of aprepitant on drug metabolism in lymphoma patients receiving multi-day chemotherapy regimen of cyclophosphamide, doxorubicin, vincristine, prednisone, + rituxan (R/CHOP): randomized, cross-over study. In: Blood. Vol. 118. 2011:Abstract 1613.
Vadhan‐Raj 2012 {published data only}
- Vadhan-Raj S, Spasojevic I, Zhou X, Romaguera J, Fanale M, Fayad L, et al. Effects of aprepitant on drug metabolism in patients receiving chemotherapy of cyclophosphamide, doxorubicin, vincristine, prednisone, rituxan (R/CHOP): randomized, crossover study. In: Supportive Care in Cancer. Vol. 20. 2012:S262-3.
Vadhan‐Raj 2014 {published data only}
- Vadhan-Raj S, Zhou X, Araujo D M, Somaiah N, Conley A P, Ravi V, et al. Effects of fosaprepitant (Fosa) administered as single dose versus two doses, on nausea/vomiting (N/V) in patients receiving multiday chemotherapy (CT) with a highly emetogenic regimen of doxorubicin and ifosfamide (AI): randomized cross-over study. In: Journal of Clinical Oncology. Vol. 35. 2014.
Vadhan‐Raj 2015 {published data only}
- Vadhan-Raj S, Zhou X, Spasojevic I, Ravi V, Araujo D, Somaiah N, et al. Randomised, cross over study of fosaprepitant (single dose vs two doses) for nausea and vomiting in Sarcoma patients receiving multi-day chemotherapy. In: Supportive Care in Cancer. Vol. 23. 2015:S131-2.
Van Belle 2002 {published data only}
- Van Belle S, Lichinitser MR, Navari RM, Garin AM, Decramer ML, Riviere A, et al. Prevention of cisplatin-induced acute and delayed emesis by the selective neurokinin-1 antagonists, L-758,298 and MK-869. Cancer 2002;94(11):3032-41. [DOI] [PubMed] [Google Scholar]
Van der Vorst 2021 {published data only}
- Van der Vorst MJDL, Toffoli EC, Beusink M, Van Linde ME, Van Voorthuizen T, Brouwer S, et al. Metoclopramide, dexamethasone, or palonosetron for prevention of delayed chemotherapy-induced nausea and vomiting after moderately emetogenic chemotherapy (MEDEA): a randomized, phase III, noninferiority trial. Oncologist 2021;26(1):e173-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walko 2012 {published data only}
- Walko CM, Combest AJ, Spasojevic I, Yu AY, Bhushan S, Hull JH, et al. The effect of aprepitant and race on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemotherapy and Pharmacology 2012;69(5):1189-96. [DOI] [PubMed] [Google Scholar]
Weant 2017 {published data only}
- Weant M, Woodring S, Randazzo DM, Friedman HS, Desjardins A, Vlahovic G, et al. Randomized open-label-phase-ii trial of aprepitant plus ondansetron compared to ondansetron alone in prevention of chemotherapy-induced-nausea-vomiting (CINV) in glioma patients receiving adjuvant temozolomide. In: Supportive Care in Cancer. Vol. 25. 2017:S128. [DOI] [PubMed]
Xie 2003 {published data only}
- Xie XD, Zheng ZD, Liu DW, Liu YY, Shan X. [Effects of nausea on prevention of gastrointestinal side effects caused by chemotherapeutic drugs]. Chinese Medical Journal 2003;83(13):1180-2. [PubMed] [Google Scholar]
Yahata 2016 (a) {published data only}
- Yahata H, Kobayashi H, Sonoda K, Shimokawa M, Ohgami T, Saito T, et al. Efficacy of aprepitant for the prevention of chemotherapy-induced nausea and vomiting with a moderately emetogenic chemotherapy regimen: a multicenter, placebo-controlled, double-blind, randomized study in patients with gynecologic cancer receiving paclitaxel and carboplatin. International Journal of Clinical Oncology, 2016;21(3):491-7. [DOI] [PubMed] [Google Scholar]
Yalçin 1999 {published data only}
- Yalçin S, Tekuzman G, Baltali E, Ozişik Y, Barişta I. Serotonin receptor antagonists in prophylaxis of acute and delayed emesis induced by moderately emetogenic, single-day chemotherapy: a randomized study. American Journal of Clinical Oncology 1999;22(1):94-6. [DOI] [PubMed] [Google Scholar]
- Yalþin S, Tekuzman G, Baltali E, Ízisik Y, Barista I, Zengin N. 5-HT3 receptor antagonists in prophylaxis of acute and delayed emesis induced by moderately emetogenic single day chemotherapy. In: ESMO. 1998:Abstract #718. [DOI] [PubMed]
Yang 2005 {published data only}
- Yang X, Zhang S. [A randomized control study on the clinical effects of ramosetron in prophylaxis of nausea and vomiting induced by cisplatin chemotherapy in patients with lung cancer]. Chinese Journal of Lung Cancer 2005;8(4):322-5. [DOI] [PubMed] [Google Scholar]
Yano 2005 {published data only}
- Yano S, Makino K, Nakamura H, Kai Y, Morioka M, Hamada J, et al. Comparative clinical study of the anti-emetic effects of oral ramosetron and injected granisetron in patients with malignant glioma undergoing ACNU chemotherapy. Neurologia Medico-Chirurgica 2005;45(6):294-8; discussion 298. [DOI] [PubMed] [Google Scholar]
Yu 2009 {published data only}
- Yu Z, Liu W, Wang L, Liang H, Huang Y, Si X, et al. The efficacy and safety of palonosetron compared with granisetron in preventing highly emetogenic chemotherapy-induced vomiting in the Chinese cancer patients: a phase II, multicenter, randomized, double-blind, parallel, comparative clinical trial. Supportive Care in Cancer 2009;17(1):99-102. [DOI] [PubMed] [Google Scholar]
Zeidman 1998 {published data only}
- Zeidman A, Ben Dayan D, Ben Zion T, Kaufman O, Cohen AM, Mittelman M. Granisetron and ondansetron for chemotherapy-related nausea and vomiting. Haematologia 1998;29(1):25-31. [PubMed] [Google Scholar]
Zeng 2001 {published data only}
Zhang 1996 {published data only}
- Zhang P, Sun Y, Zhang H. [A randomized trial of tropisetron in the prophylaxis of nausea and vomiting induced by chemotherapy]. Chinese Journal of Oncology 1996;18(2):154-6. [PubMed] [Google Scholar]
Zhang 1999 {published data only}
- Zhang S, Hu F, Yang X. A randomized trial of Zudan in the prophylaxis of chemotherapy induced nausea and vomiting. [Chinese]. Chinese Journal of Clinical Oncology 1999;26(3):214-5. [Google Scholar]
Zhang 2002 {published data only}
- Zhang P, Feng F, He Y, Li Y, Zhou M, Cheng G, et al. [Nausea disintegrating buccal tablet in the prevention of gastrointestinal reaction induced by anticancer drugs]. Chinese Journal of Oncology 2002;24(5):504-7. [PubMed] [Google Scholar]
Zhang 2003 {published data only}
- Zhang JD, Liu YP, Teng YE, Shi J. [Preventive effects of ramosetron and granisetron in prevention of gastrointestinal reaction associated with chemotherapeutic agents: a comparative study]. Zhonghua Yi Xue Za Zhi 2003;83(23):2058-60. [PubMed] [Google Scholar]
Zhang 2003 (a) {published data only}
- Zhang JD, Liu YP, Teng YE, Shi J. [Preventive effects of ramosetron and granisetron in prevention of gastrointestinal reaction associated with chemotherapeutic agents: a comparative study]. Chinese Medical Journal 2003;83(23):2058-60. [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang WD, Wang XW, Chen ZD, Qin FZ, Shu YQ, Pan LX, et al. [Tropisetron hydrochloride in preventing and treating chemotherapy-induced nausea and vomiting: a phase II, randomized, multicenter, double-blinded, comparative clinical trial]. Chinese Journal of Cancer 2007;26(8):870-3. [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang D, Mita M, Shapiro G I, Poon J, Small K, Tzontcheva A, et al. Effect of aprepitant on the pharmacokinetics of the cyclin-dependent kinase inhibitor dinaciclib in patients with advanced malignancies. Cancer Chemotherapy and Pharmacology 2012;70(6):891-8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR‐INR‐17010779 {published data only}
- ChiCTR-INR-17010779. Efficacy and safety of the first generation of 5-HT3 receptor antagonist compare with the second generation of 5-HT3 receptor antagonists in preventing multiday-based highly emetogenic chemotherapy-induced nausea and vomiting: a randomized, open, cross-over control, multi-center study, 2017. http://www.who.int/trialsearch/trial2.aspx?Trialid=chictr-inr-17010779 (accessed 15 October 2019).
CTRI/2017/10/010163 {published data only}
- CTRI/2017/10/010163. Effect of granisetron and ondansetron in prevention of nausea and vomiting in cancer patients, 2017. http://www.who.int/trialsearch/trial2.aspx?Trialid=ctri/2017/10/010163 (accessed 15 October 2019).
EUCTR2004‐000371‐34 {published data only}
- EUCTR 2004-000371-34. A phase II multicentre, randomised, double-blind, placebo and active-controlled, dose-ranging, parallel group study of the safety and efficacy of the oral neurokinin-1 receptor antagonist, GW679769 when administered at daily doses of 50 mg, 100 mg, and 150 mg oral tablets in combination with ondansetron hydrochloride and dexamethasone for the prevention of chemotherapy-induced nausea and vomiting in cancer subjects receiving highly emetogenic cisplatin-based chemotherapy, 2004. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2004-000371-34-sk (accessed 15 October 2019).
EUCTR2004‐001020‐20 {published data only}
- EUCTR2004-001020-20-ES. A phase II multicenter, randomized, double-blind, placebo-controlled, dose ranging, parallel group study of the safety and efficacy of the oral neurokinin-1 receptor antagonist, GW679769, when administered as 50 mg, 100 mg and 150 mg oral tablets in combination with ondansetron hydrochloride and dexamethasone for the prevention of chemotherapy-induced nausea and vomiting in cancer subjects receiving moderately emetogenic chemotherapy, 2005. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2004-001020-20-es (accessed 15 October 2019).
EUCTR2004‐004956‐38 {published data only}
- EUCTR2004-004956-38-de. Randomised, placebo controlled, single-center, double-blind clinical trial to investigate efficacy and safety of Aprepitant combined with Kevatril and Dexamethasone versus Placebo combined with Kevatril and Dexamethasone in prevention of acute and delayed high-dose chemotherapy-induced nausea and vomiting in subjects with multiple myeloma receiving an autologous peripheral blood stem cell transplantation - EmNa, 2005. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2004-004956-38-de (accessed 15 October 2019).
EUCTR 2005‐000137‐37‐cz 2005 {published data only}
- EUCTR 2005-000137-37-cz 2005. Single dose, randomized, double-blind, parallel group, multicenter study of palonosetron 0.25 mg, 0.50 mg and 0.75 mg administered by the oral route versus palonosetron 0.25 mg IV for the prevention of moderately emetogenic chemotherapy-induced nausea and vomiting in patients with cancer, 2005. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2005-000137-37-cz (accessed 15 October 2019).
EUCTR2006‐000781‐37 {published data only}
- EUCTR2006-000781-37-sk. A phase III, multicenter, randomized, double-blind, active controlled, parallel group study of the safety and efficacy of the intravenous and oral formulations of the neurokinin-1 receptor antagonist, casopitant (GW679769) in combination with ondansetron and dexamethasone for the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy, 2006. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2006-000781-37-sk (accessed 15 October 2019).
EUCTR2006‐003512‐22 {published data only}
- EUCTR2006-003512-22-FR. A randomized, double-blind, parallel-group study conducted under in-house blinding conditions to determine the efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy (study #2), 2006. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2006-003512-22-fr (accessed 15 October 2019).
EUCTR2007‐004043‐30 {published data only}
- EUCTR2007-004043-30. A phase III, randomized, double-blind, active-controlled, parallel-group study, conducted under in-house blinding conditions, to examine the safety, tolerability, and efficacy of a single dose of intravenous MK-0517 for the prevention of chemotherapy-induced nausea and vomiting (CINV) associated with cisplatin chemotherapy - CINV single dose study, 2007 [Estudio en fase III aleatorizado, doble ciego, con control activo y de grupos paralelos, realizado en condiciones de enmascaramiento interno, para examinar la seguridad, la tolerabilidad y la eficacia de una dosis única de MK 0517 por vía intravenosa para la prevención de las náuseas y los vómitos inducidos por la quimioterapia (NVIQ) que se asocian a la quimioterapia con cisplatino]. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2007-004043-30-es (accessed 15 October 2019).
EUCTR2007‐005169‐36 {published data only}
- EUCTR2007-005169-36-sk. NKV110721, a study of single dose intravenous casopitant in combination with ondansetron and dexamethasone for the prevention of oxaliplatin-induced nausea and vomiting, 2008. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2007-005169-36-sk (accessed 15 October 2019). [DOI] [PubMed]
EUCTR2008‐001339‐37 {published data only}
- EUCTR2008-001339-37. Aprepitant in the prevention of cisplatin-induced delayed emesis: a double-blind randomized study - aprepitant for cisplatin-induced delayed emesis, 2009. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2008-001339-37-it (accessed 15 October 2019).
EUCTR2009‐016775‐30 {published data only}
- EUCTR2009-016775-30. A phase III multicenter, randomized, double-blind, double-dummy, active-controlled, parallel group study of the efficacy and safety of oral netupitant administered in combination with palonosetron and dexamethasone compared to oral palonosetron and dexamethasone for the prevention of nausea and vomiting in cancer patients receiving moderately emetogenic chemotherapy, 2011. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2009-016775-30-pl (accessed 15 October 2019).
EUCTR2009‐017603‐28 {published data only}
- EUCTR2009-017603-28. Aprepitant for prevention of acute and delayed nausea and vomiting: a phase III, double-blind, randomized, placebo-controlled trial in patients receiving a high-emetogenic dose of cyclophosphamide for peripheral blood stem cells harvesting - ND, 2010. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2009-017603-28-it (accessed 15 October 2019).
EUCTR2010‐023297‐39 {published data only}
- EUCTR2010-023297-39. A phase III, multicenter, randomized, double-blind, unbalanced (3:1) active control study to assess the safety and describe the efficacy of netupitant and palonosetron for the prevention of chemotherapy-induced nausea and vomiting in repeated chemotherapy cycles, 2011. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2010-023297-39-pl (accessed 15 October 2019).
EUCTR2015‐001800‐74 {published data only}
- EUCTR2015-001800-74. A study to evaluate the safety and efficacy of a combination of pro-netupitant/palonosetron intravenously administered for the prevention of chemotherapy-induced nausea and vomiting, 2015. http://www.who.int/trialsearch/trial2.aspx?Trialid=euctr2015-001800-74-de (accessed 15 October 2019).
JapicCTI‐194691 {published data only}
- JapicCTI-194691. A randomized, double-blind, multicenter, phase III study of Pro-NETU for the prevention of chemotherapy induced nausea and vomiting (CINV) in patients receiving AC/EC based highly emetogenic chemotherapy. https://rctportal.niph.go.jp/en/detail?trial_id=JapicCTI-194691 (last accessed: 21 July 2021).
Mylonakis 1996 {published data only}
- Mylonakis N, Tsavaris N, Karabelis A, Stefis J, Kosmidis P. A randomized comparative study of antiemetic activity of Ondansetron (Ond) vs Tropisetron (Tr) in patients receiving moderately emetogenic chemotherapy. In: Supportive Care in Cancer. Vol. 4 Suppl.. 1996:252.
NCT00169572 {published data only}
- NCT00169572. Study for the prevention of nausea in cancer patients receiving highly emetogenic cisplatin based chemotherapy, 2005. https://clinicaltrials.gov/show/nct00169572 (accessed 15 October 2019).
NCT01101529 {published data only}
- NCT01101529. Treatment of chemotherapy-induced nausea and vomiting, 2010. https://clinicaltrials.gov/show/nct01101529 (accessed 15 October 2019).
NCT02407600 {published data only}
- NCT02407600. Study assessing fosaprepitant in advanced NSCLC patients treated with carboplatin based chemotherapy, 2015. https://clinicaltrials.gov/show/nct02407600 (accessed 15 October 2019).
NCT02550119 {published data only}
- NCT02550119. Dolasetron mesylate and dexamethasone with or without aprepitant in preventing nausea and vomiting in patients undergoing oxaliplatin-containing chemotherapy for gastrointestinal malignancy, 2015. https://clinicaltrials.gov/show/nct02550119 (accessed 15 October 2019).
NCT02732015 {published data only}
- NCT02732015. Effects of rolapitant on nausea/vomiting in patients with sarcoma receiving multi-day Highly Emetogenic Chemotherapy (HEC) dith Doxorubicin and ifosfamide regimen (AI), 2016. https://clinicaltrials.gov/show/nct02732015 (accessed 15 October 2019).
NCT03403712 {published data only}
- NCT03403712. A study to assess the safety and the efficacy of IV fosnetupitant/palonosetron (260 mg/0.25 mg) combination compared to oral netupitant/palonosetron (300 mg/0.5 mg) combination for the prevention of CINV in ac chemotherapy in women with breast cancer, 2018. https://clinicaltrials.gov/show/nct03403712 (accessed 15 October 2019).
PER‐055‐12 {published data only}
- PER-055-12. A phase 3, multicenter, randomized, double-blind, active-controlled study of the safety and efficacy of rolapitant for the prevention of chemotherapy- induced nausea and vomiting (CINV) in subjects receiving highly emetogenic chemotherapy (HEC), 2012. http://www.who.int/trialsearch/trial2.aspx?Trialid=per-055-12 (accessed 15 October 2019).
Spina 1995 {published data only}
- Spina M, Valentini M, Fedele P, Bernardi D, Nasti G, Zuccarino L, et al. Randomized comparison of granisetron vs ondansetron in patients (pts) with HIV-related non-Hodgkin's lymphoma (HIV-NHL) receiving moderately emetogenic chemotherapy (CT) regimens [abstract]. In: Proceedings of the American Society of Clinical Oncology. 1995:532.
UMIN000004826 {published data only}
- UMIN000004826. Randomized crossover trial of Granisetron/Dexamethasone/Aprepitant versus Palonosetron/Dexamethasone/Aprepitant for the prevention of nausea and vomiting in patients receiving Cisplatin containing chemotherapy for head and neck cancer, 2011. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000004826 (accessed 15 October 2019).
UMIN000004863 {published data only}
- UMIN000004863. A double-blind randomized controlled trial comparing 0.75mg of Palonosetron with 1mg of Granisetron for the control of highly emetogenic chemotherapy-induced emesis, 2011. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000004863 (accessed 15 October 2019).
UMIN000004998 {published data only}
- UMIN000004998. A multicenter, double-blind, placebo-controlled phase II study of aprepitant for prevention of chemotherapy-induced nausea and vomiting (CINV) following moderately emetogenic chemotherapy (MEC) in women younger than 70 years without alcohol drinking habit, 2011. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000004998 (accessed 15 October 2019).
UMIN000008041 {published data only}
- UMIN000008041. Evaluation of combinational effect of Aprepitant on nausea and vomiting induced by chemotherapy (moderate risk) in patients with gastric cancer or colorectal cancer, 2012. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000008041 (accessed 15 October 2019).
UMIN000008552 {published data only}
- UMIN000008552. A single-blind randomized controlled trial comparing Aprepitant plus (Granisetron) 1st 5-HT3 receptor antagonist and Palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapies including CBDCA in the gynecology cancer patients, 2012. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000008552 (accessed 15 October 2019).
UMIN000008897 {published data only}
- UMIN000008897. Randomized double-blind phase 3 study of granisetron vs palonosetron combined with dexamethasone plus fosaprepitant for patient with breast cancer treated with peri-operative AC/EC/FAC/FEC chemotherapy, 2012. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000008897 (accessed 15 October 2019).
UMIN000010056 {published data only}
- UMIN000010056. Comparison of antiemetic effectiveness and safety of palonosetron and dexamethasone with palonosetron, dexamethasone and aprepitant in patients with lung cancer receiving combination therapy with carboplatin: a phase II randomized study, 2013. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000010056 (accessed 15 October 2019).
UMIN000010186 {published data only}
- UMIN000010186. Prospective, open-label, comparative study on the efficacy of triple (aprepitant + granisetron 3 mg + dexamethasone) versus double (palonosetron 0.75 mg + dexamethasone) combination therapy for nausea and vomiting during moderately emetogenic chemotherapy containing carboplatin: CAP Study, 2013. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000010186 (accessed 15 October 2019).
UMIN000019122 {published data only}
- UMIN000019122. A phase II randomised study to evaluate the efficacy of aprepitant plus palonosetron for preventing delayed-phase CINV associated with TC therapy in gynaecological cancer, 2015. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000019122 (accessed 15 October 2019). [DOI] [PubMed]
References to ongoing studies
ChiCTR1900025227 {published data only}
- ChiCTR1900025227. A randomized, open, parallel controlled phase II clinical study comparing the efficacy and safety of dexamethasone, palonosetron or aprepitant in the control of acute and delayed vomiting in non-small cell lung cancer patients receiving multiple moderately emetogenic chemotherapy regimens, 2019. http://www.chictr.org.cn/showprojen.aspx?proj=42128 (accessed 12 May 2021).
IRCT20191103045317N1 {published data only}
- IRCT20191103045317N1. Comparison between the effect of Triplet Aprepitant/Dexamethasone/Ondansetron vs. doublet Dexamethasone/Ondansetron for prevention of moderately emetogenic chemotherapy: placebo-controlled double blind, randomised clinical trial of efficacy, 2021. https://www.irct.ir/trial/43388 (accessed 12 May 2021).
KTC0001495 {published data only}
- KTC0001495. A randomized, double-blind, double-dummy, parallel group, international multi center study assessing the efficacy and safety of a netupitant-palonosetron Fixed Dose Combination (FDC) compared to an extemporary combination of granisetron and aprepitant on the prevention of highly emetogenic chemotherapy-induced nausea and vomiting in patients with cancer, 2015. http://www.who.int/trialsearch/trial2.aspx?Trialid=kct0001495 (accessed 15 October 2019).
NCT03606369 {published data only}
- NCT03606369. Effectiveness and quality of life analysis of palonosetron against ondansetron combined with dexamethasone and fosaprepitant in prevention of acute and delayed emesis associated to chemotherapy moderate and highly emetogenic in breast cancer, 2018. https://clinicaltrials.gov/show/nct03606369 (accessed 15 October 2019).
UMIN000004021 {published data only}
- UMIN000004021. Study of oral neurokinin-1 antagonist, aprepitant for the prevention of nausea and vomiting in patients receiving chemotherapy with irinotecan alone or combination of irinotecan plus cisplatin for unresectable gastric cancer, 2010. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000004021 (accessed 15 October 2019).
UMIN000005317 {published data only}
- UMIN000005317. Effect of oral neurokinin-1 antagonist, aprepitant for chemotherapy-induced nausea and vomiting in patients with gynecologic cancer receiving carboplatin/paclitaxel chemotherapy, 2011. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000005317 (accessed 15 October 2019).
UMIN000005494 {published data only}
- UMIN000005494. Aprepitant for nausea, vomiting with the TC therapy of the gynecology cancer patient or the DC therapy, fosaprepitant, granisetron, protective efficacy of the dexamethasone combination therapy, 2011. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000005494 (accessed 15 October 2019).
- UMIN000005494. Aprepitant for nausea, vomiting with the TC therapy of the gynecology cancer patient or the DC therapy, fosaprepitant, granisetron, protective efficacy of the dexamethasone combination therapy, 2011. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000006477 (accessed 15 October 2019).
UMIN000006773 {published data only}
- UMIN000006773. Randomized phase II study of aprepitant in patients with colorectal cancer receiving FOLFOX, FOLFIRI, or XELOX chemotherapy regimen, 2011. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000006773 (accessed 15 October 2019).
UMIN000007882 {published data only}
- UMIN000007882. Multicenter double-blind randomized comparative parallel study with concomitant therapy of 3 drugs, aprepitant + dexamethasone + palonosetron or aprepitant + dexamethasone + granisetron, for prevention of nausea/vomiting in breast cancer patients receiving AC therapy, 2012. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000007882 (accessed 15 October 2019).
UMIN000012500 {published data only}
- UMIN000012500. Effect of aprepitant for nausea and vomiting during Paclitaxel + Carboplatin (TC) therapy, 2013. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000012500 (accessed 15 October 2019).
UMIN000032860 {published data only}
- UMIN000032860. Palonosetron versus granisetron in combination with fosaprepitant and dexamethasone for TC therapy in patients with gynecologic cancer, 2018. http://www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000032860 (accessed 15 October 2019).
UMIN000041004 {published data only}
- UMIN000041004. To establish of optimal antiemetic therapy for trastuzumab deruxtecan therapy-induced nausea and vomiting in patients with breast cancer: an open-label, randomized pilot study, 2020. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000041004 (accessed 12 May 2021).
Additional references
Ades 2013
- Ades AE, Caldwell DM, Reken S, Welton NJ, Sutton AJ, Dias S. Evidence synthesis for decision making 7: a reviewer's checklist. Medical Decision Making 2013;33(5):679-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Avritscher 2010
- Avritscher EB, Shih YC, Sun CC, Gralla RJ, Grunberg SM, Xu Y, et al. Cost-utility analysis of palonosetron-based therapy in preventing emesis among breast cancer patients. Journal of Supportive Oncology 2010;8:242-51. [DOI] [PubMed] [Google Scholar]
Basch 2012
- Basch E, Prestrud AA, Hesketh PJ, Kris MG, Somerfield MR, Lyman GH. Antiemetic use in Oncology: Updated guideline recommendations from ASCO. American Society of Clinical Oncology Educational Book 2012;32:532-40. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carmichael 1988
- Carmichael J, Cantwell BM, Edwards CM, Rapeport WG, Harris AL. The serotonin type 3 receptor antagonist BRL 43694 and nausea and vomiting induced by cisplatin. BMJ 1988;297(6641):110-1. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Celio 2013
- Celio L, Bonizzoni E, Bajetta E, Sebastiani S, Perrone T, Aapro MS. Palonosetron plus single-dose dexamethasone for the prevention of nausea and vomiting in women receiving anthracycline/cyclophosphamide-containing chemotherapy: meta-analysis of individual patient data examining the effect of age on outcome in two phase III trials. Supportive Care in Cancer 2013;21:565-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLOS ONE 2013;8(10):e76654. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2014
- Chaimani A, Salanti G, Becker L, Caldwell D, Higgins J, Li T. Protocol template for a Cochrane intervention review that compares multiple interventions, 2014. methods.cochrane.org/sites/methods.cochrane.org.cmi/files/public/uploads/Protocol%20for%20Cochrane%20Reviews%20with%20Multiple%20Interventions.pdf.
Chaimani 2017
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence systematic review software [Computer program]
- Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, Available at www.covidence.org.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Dias 2013
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Medical Decision Making 2013;33(5):641-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Mattei 2016
- Di Mattei VE, Carnelli L, Carrara L, Bernardi M, Crespi G, Rancoita PM, et al. Chemotherapy-induced nausea and vomiting in women with gynecological cancer: a preliminary single-center study investigating medical and psychosocial risk factors. Cancer Nursing 2016;39(6):E52-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
dos Santos 2013
- dos Santos LV, Brunetto AT, Sasse AD, Souza FH, Lima JP. NK1 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting: an updated meta-analysis. In: Journal of Clinical Oncology. Vol. 31. 2013:e20506.
Dranitsaris 2017
- Dranitsaris G, Molassiotis A, Clemons M, Roeland E, Schwartzberg L, Dielenseger P, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Annals of Oncology 2017;28(6):1260-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ettinger 2017
Feyer 2011
- Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Annals of Oncology 2011;22:30-8. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Furukawa 2014
- Furukawa N, Akasaka J, Shigemitsu A, Sasaki Y, Nagai A, Kawaguchi R, et al. Evaluation of the relation between patient characteristics and the state of chemotherapy-induced nausea and vomiting in patients with gynecologic cancer receiving paclitaxel and carboplatin. Archives of Gynecology and Obstetrics 2014;289(4):859-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc), 2015. Available from www.gradepro.org, 2015.
Gralla 1999
- Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. Journal of Clinical Oncology 1999;17(9):2971-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt GH, Oxmanc AD, Satesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables - binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158-72. [DOI: ] [DOI] [PubMed] [Google Scholar]
Heron 1994
- Heron JF, Goedhals L, Jordaan JP, Cunningham J, Cedar E, The Granisetron Study Group. Oral granisetron alone and in combination with dexamethasone: a double-blind randomized comparison against high-dose metoclopramide plus dexamethasone in prevention of cisplatin-induced emesis. Annals of Oncology 1994;5(7):579-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hesketh 2010
- Hesketh PJ, Aapro M, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Supportive Care in Cancer 2010;18:1171–7. [DOI] [PubMed] [Google Scholar]
Hesketh 2016
- Hesketh PJ, Bohlke K, Lyman GH, Basch E, Chesney M, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology Focused Guideline Update. Journal of Clinical Oncology 2016;34(4):381-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available from www.cochrane-handbook.org edition. The Cochrane Collaboration, 2009 [Updated 2011]. [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
Higgins 2011c
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
Hocking 2014
- Hocking CM, Kichenadasse G. Olanzapine for chemotherapy-induced nausea and vomiting: a systematic review. Supportive Care in Cancer 2014;22:1143-51. [DOI] [PubMed] [Google Scholar]
Howlader 2013
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute 2013;http://seer.cancer.gov/csr/1975_2010/.
Hu 2016
- Hu Z, Liang W, Yang Y, Keefe D, Ma Y, Zhao Y, et al. Personalized estimate of chemotherapy-induced nausea and vomiting: development and external validation of a nomogram in cancer patients receiving highly/moderately emetogenic chemotherapy. Medicine 2016;95(2):e2476. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Italian Group for Antiemetic Research 1995
- Italian Group for Antiemetic Research. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. New England Journal of Medicine 1995;332(1):1-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Janelsins 2013
- Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opinion on Pharmacotherapy 2013;14(6):757-66. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janowitz 2021
- Janowitz T, Kleeman S, Vonderheide RH. Reconsidering dexamethasone for antiemesis when combining chemotherapy and immunotherapy. The Oncologist 2021;26(4):269-73. [DOI: 10.1002/onco.13680] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 2012
- Jin Y, Wu X, Guan Y, Gu D, Shen Y, Xu Z, et al. Efficacy and safety of aprepitant in the prevention of chemotherapy-induced nausea and vomiting: a pooled analysis. Supportive Care in Cancer 2012;20:1815-22. [DOI] [PubMed] [Google Scholar]
Jordan 2015
- Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Annals of Oncology 2015;26(6):1081-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jordan 2016b
- Jordan K, Warr DG, Hinke A, Sun L, Hesketh PJ. Defining the efficacy of neurokinin-1 receptor antagonists in controlling chemotherapy-induced nausea and vomiting in different emetogenic settings - a meta-analysis. Supportive Care in Cancer 2016;24(5):1941-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jordan 2017
- Jordan K, Chan A, Gralla RJ, Jahn F, Rapoport B, Warr D, et al. 2016 Updated MASCC/ESMO Consensus Recommendations: emetic risk classification and evaluation of the emetogenicity of antineoplastic agents. Supportive Care in Cancer 2017;25(1):271-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jordan 2018
- Jordan K, Blattermann L, Hinke A, Muller-Tidow C, Jahn F. Is the addition of a neurokinin-1 receptor antagonist beneficial in moderately emetogenic chemotherapy? A systematic review and meta-analysis. Supportive Care in Cancer 2018;26(1):21-32. [DOI] [PubMed] [Google Scholar]
Krahn 2013
- Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Medical Research Methodology 2013;13:35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013
- Lee J, Oh H. Ginger as an antiemetic modality for chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis. Oncology Nursing Forum 2013;40:163-70. [DOI] [PubMed] [Google Scholar]
Lindley 1992
- Lindley CM, Hirsch JD, O'Neill CV, Transau MC, Gilbert CS, Osterhaus JT. Quality of life consequences of chemotherapy-induced emesis. Quality of Life Research 1992;1(5):331-40. [DOI] [PubMed] [Google Scholar]
Martin 2003
- Martin AR, Carides AD, Pearson JD, Horgan K, Elmer M, Schmidt C, et al. Functional relevance of antiemetic control. Experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. European Journal of Cancer 2003;39(10):1395-401. [DOI] [PubMed] [Google Scholar]
Martin 2003a
- Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients' daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Supportive Care in Cancer 2003;11(8):522-7. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
netmeta 2016 [Computer program]
- netmeta. Network Meta-Analysis using Frequentist Methods. R package version 0.9-2. Rücker G, Schwarzer G, Krahn U, König J. http://CRAN.R-project.org/package=netmeta, 2016.
Popovic 2014
- Popovic M, Warr DG, Deangelis C, Tsao M, Chan KK, Poon M, et al. Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Supportive Care in Cancer 2014;22(6):1685-97. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (Clinical research ed.) 2014;349:g5630. [PMID: ] [DOI] [PubMed] [Google Scholar]
R 2019 [Computer program]
- R: A language and environment for statistical computing. R Core Team, Version 3.3.2. Vienna, Austria: R Foundation for Statistical Computing, 2019. http://www.R-project.org/.
RevMan 2014 [Computer program]
- Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roila 2016
- Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Annals of Oncology 2016;27(Suppl 5):v119-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rücker 2012
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3(4):312-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rücker 2014
- Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Statistics in Medicine 2014;33(25):4353-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rücker 2015
- Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Medical Research Methodology 2015;15:58. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2019 [Computer program]
- netmeta. network meta-analysis using frequentist methods. R package version 1.1-0. Rücker G, Krahn U, König J, Efthimiou o, Schwarzer G. The Comprehensive R Archive Network, 2019.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JT, Vist GE, Glasziou P, Guyatt GH. Chapter 11. Presenting results and 'Summary of findings tables'. In: Higgins JT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration, 2011. [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration, 2011. [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.. [Google Scholar]
Smith 1991
- Smith DB, Newlands ES, Rustin GJ, Begent RH, Howells N, McQuade B, et al. Comparison of ondansetron and ondansetron plus dexamethasone as antiemetic prophylaxis during cisplatin-containing chemotherapy. Lancet 1991;338(8765):487-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2015
- Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD009464. [DOI: 10.1002/14651858.CD009464.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10. Addressing reporting biases. In: Higgins JT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Intervention Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sutherland 2017
- Sutherland A, Naessens K, Plugge E, Head K, Burton Martin J, Wee B. Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults. Cochrane Database of Systematic Reviews 2017;(2). [DOI: 10.1002/14651858.CD012555] [CD012555] [DOI] [PMC free article] [PubMed]
Tageja 2016
- Tageja N, Groninger H. Chemotherapy-induced nausea and vomiting: an overview and comparison of three consensus guidelines. Postgraduate Medical Journal 2016;92(1083):34-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Viale 2012
- Viale PH, Grande C, Moore S. Efficacy and cost: avoiding undertreatment of chemotherapy-induced nausea and vomiting. Clinical Journal of Oncology Nursing 2012;16:E133-41. [DOI] [PubMed] [Google Scholar]
Warr 2014
- Warr D. Prognostic factors for chemotherapy induced nausea and vomiting. European Journal of Pharmacology 2014;722:192-6. [DOI] [PubMed] [Google Scholar]
Wood 2011
- Wood JM, Chapman K, Eilers J. BC tools for assessing nausea, vomiting, and retching. Cancer Nursing 2011;34(1):E14-E24. [DOI] [PubMed] [Google Scholar]
Wozniak 1998
- Wozniak AJ, Crowley JJ, Balcerzak SP, Weiss GR, Spiridonidis CH, Baker LH, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. Journal of Clinical Onoclogy 1998;16:2459-65. [DOI] [PubMed] [Google Scholar]
Yokoe 2019
- Yokoe T, Hayashida T, Nagayama A, Nakashoji A, Maeda H, Seki T, et al. Effectiveness of antiemetic regimens for highly emetogenic chemotherapy-induced nausea and vomiting: a systematic review and network meta-analysis. The Oncologist 2019;24(6):e347-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuan 2016
- Yuan DM, Li Q, Zhang Q, Xiao XW, Yao YW, Zhang Y, et al. Efficacy and safety of Neurokinin-1 receptor antagonists for prevention of chemotherapy-induced nausea and vomiting: systematic review and meta-analysis of randomized controlled trials. Asian Pacific Journal of Cancer Prevention 2016;17(4):1661-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Piechotta 2021
- Piechotta V, Adams A, Haque M, Scheckel B, Kreuzberger N, Monsef I, et al. Supplementary material to 'Piechotta et al. Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis'. available from: https://osf.io/dr2u7/ 2021. [DOI: DOI 10.17605/OSF.IO/DR2U7] [DOI] [PMC free article] [PubMed]
Skoetz 2017
- Skoetz N, Haque M, Weigl A, Kuhr K, Monsef I, Becker I, et al. Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta‐analysis. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012775. [DOI: 10.1002/14651858.CD012775] [DOI] [PMC free article] [PubMed] [Google Scholar]