Abstract
Background:
Mutations in the TMPRSS3 gene, although rare, can cause high frequency hearing loss with residual hearing at low frequencies. Several previous studies have reported cochlear implant (CI) outcomes for adults with TMPRSS3 mutation with mixed results. Although some studies have suggested that TMPRSS3 is expressed in spiral ganglion cells, it remains unclear if previously reported poor CI outcomes in this population were secondary to long durations of deafness or to the effects of the TMPRSS3 mutation. To date, no studies in the literature have reported CI outcomes for children with TMPRSS3 mutation treated with CI.
Objective:
The current case series aimed to describe outcomes for three children with sloping hearing loss caused by TMPRSS3 mutation who underwent bilateral CI.
Study Design:
Case series.
Setting:
Academic medical center.
Patients:
Three children (3–4 yr) with TMPRSS3 mutation and normal sloping to profound high frequency hearing loss.
Interventions:
CI and electric acoustic stimulation (EAS).
Main Outcome Measures:
Outcome measures were residual hearing thresholds, speech recognition scores, and electrode placement determined via intraoperative CT imaging.
Results:
All three children maintained residual acoustic hearing and received benefit from EAS. Mean change in low-frequency pure-tone average was 17 dB. Mean postoperative word and sentence recognition scores in the bilateral EAS condition were 80 and 75%, respectively.
Conclusions:
Results indicate that CI with EAS is an appropriate treatment for children with TMPRSS3 genetic mutation. Pediatric results from this case series show more favorable CI outcomes than are currently reported for adults with TMPRSS3 mutation suggesting that the intervention may be time sensitive.
Keywords: Audiology, Candidacy, Cochlear implant, Electric acoustic stimulation, Genetic hearing loss, Pediatric, Residual hearing, TMPRSS3
The most common form of hereditary hearing loss is autosomal recessive nonsyndromic hearing loss (ARNSHL), which accounts for about 80% of cases of genetic hearing loss (1). Children with ARNSHL are highly variable in terms of clinical presentation. ARNSHL most commonly presents as prelingual severe to profound, non-progressive hearing loss such as in the phenotype for the frequently mutated gene GJB2. In cases of typical ARNSHL, cochlear implant (CI) is the standard of care to treat severe-to-profound hearing loss. However, in less typical cases of ARNSHL, children may present with progressive, sloping losses, for which best audiologic practice is more ambiguous.
The most frequent cause of ARNSHL involves mutation of GJB2 (Connexin 26), followed by mutations of the SLC26A4, MYO7A, OTOF, CDH23, and TMC1 genes (2). Mutations in the TMPRSS3 gene are much rarer, accounting for less than 1% of ARNSHL in Caucasians (3). The TMPRSS3 gene, first identified by Scott et al. (4), is named after the protein that it encodes: transmembrane protease, serine 3, the function of which is unknown in humans. Previous studies have shown that this gene is likely expressed in inner and outer hair cells and possibly spiral ganglion cells (5-7). Mutations in TMPRSS3 have been identified in ARNSHL at the DFNB8 and DFNB10 loci (4,8,9), and all variants associated with deafness appear to disrupt the proteolytic activity of TMPRSS3. These mutations typically result in congenital severe-to-profound deafness (DFNB10) or post-lingual onset, high frequency hearing loss with residual hearing at low frequencies (DFNB8) (7).
Several previous studies have reported CI outcomes for adults with TMPRSS3 gene mutations with mixed results (10-15). To date, no studies in the literature have reported CI outcomes for children with TMPRSS3 mutation treated with CI. This population is at a unique disadvantage because hearing aids do not provide adequate auditory access to mid-to-high frequencies necessary for speech and language development; however, many of these children do not meet the Food and Drug Administration (FDA) labeled candidacy criteria for a CI. Therefore, there is ambiguity surrounding best audiologic practice for these patients. The current case series describes three children with sloping hearing loss caused by TMPRSS3 mutation who underwent bilateral CI before age 5 and derived significant benefit from bilateral electric acoustic stimulation (EAS).
METHODS
The methods used in this study were in accordance with the ethical standards of the institutional review board at our university (IRB approval: 090155), and informed consent and assent were obtained before study enrollment. Children with hearing loss caused by a mutation in the TMPRSS3 gene who went on to receive a CI were eligible for inclusion in this case series. Three children were identified before implantation. Audiometric data were collected prospectively before and after implantation. Specifically, residual hearing, CI-aided detection thresholds, and speech recognition data were collected to assess outcomes. Each child also received genetic testing specific to mutations associated with hearing loss and an intraoperative computerized tomography (CT) scan to confirm CI device placement.
Despite significant speech and language delays, none of the three children met FDA labeled indications for pediatric CI. The term ‘‘FDA labeled indications’’ refers to the indications of a particular CI, which are proposed by the implant manufacturer and approved by the FDA. Importantly, FDA labeling governs manufacturers, not well-informed clinicians. Off-label use of a CI is allowable if the treating audiologist and surgeon are well-informed, use is based on firm scientific rationale/evidence, and the process is well-documented. A recent study by Carlson et al. (16) showed that 78% of CI surgeons are routinely implanting off-label. They posit that this high percentage is driven by compelling evidence showing that current labeled indications for CI use are overly restrictive, and several different patient groups who do not meet labeled indications show significant benefit from implantation (i.e., greater residual hearing in the implanted ear, implantation before 9 months of age, single-sided deafness in pediatric population). In light of our center’s extensive experience with hearing preservation CI surgery and fitting of electric acoustic systems, we elected to proceed with implantation for the three children in this case series.
RESULTS
Table 1 shows demographic information for the three children with TMPRSS3 gene mutation in this series. Mean age at first implantation was 4 years. All three children passed their newborn hearing screening and subsequently experienced a rapidly progressive hearing loss starting in the high frequencies. Preoperatively, all three children had audiometric thresholds in the normal-hearing range for 125 through at least 500 Hz with precipitously sloping high-frequency loss reaching a profound degree. As a result of this profound high frequency hearing loss, all three girls demonstrated significant delays in articulation, receptive language, and expressive language preoperatively. Before implantation, all patients were appropriately fitted with hearing aids, which they used consistently as verified via data logging.
TABLE 1.
Patient demographics
| Patient | Sex | Age at Implantation | CI Left | CI Right | Left Δ LFPTA (dB) |
Right Δ LFPTA (dB) |
|---|---|---|---|---|---|---|
| 1 | F | 54 months | Cochlear 532 | Cochlear 522 | 26.7 | 11.7 |
| 2 | F | 47 months | Cochlear 522 | Cochlear 522 | 51.7 | 8.3 |
| 3 | F | 43 months | Cochlear 532 | Cochlear 532 | 0 | 3.4 |
CI indicates cochlear implant; LFPTA, low frequency pure-tone average (125, 250, 500 Hz).
Patient 1
Patient 1 was a 4-year-old woman who traveled 7 hours to our center to seek a second opinion regarding CI. After passing a newborn hearing screening, she was identified with hearing loss and fit with hearing aids at the age of 3. During her candidacy evaluation at our center, she scored 32% with her left hearing aid and 44% with her right hearing aid on a four-choice, closed-set picture pointing task (Northwestern University-Children’s Perception of Speech, NU-CHIPS (17)) where 25% represents chance. Her preoperative audiogram is shown in Figure 1A in black. She demonstrated delayed receptive and expressive language and severely delayed articulation. Her preoperative standard scores for the Preschool Language Scale-5 (PLS-5) (18) were 89, 86, and 86 for Auditory Comprehension, Expressive Communication, and Total Language, respectively. Her preoperative standard score for the Goldman-Fristoe-3 (19) test of articulation was 69. Her nonverbal IQ standard score for the Kaufman Brief Intelligence Test 2nd Edition (KBIT-2) (20) was 121, representing above-average nonverbal IQ. Overall, her preoperative test results were consistent with poor speech recognition and severely delayed receptive and expressive language in the presence of above-average cognition.
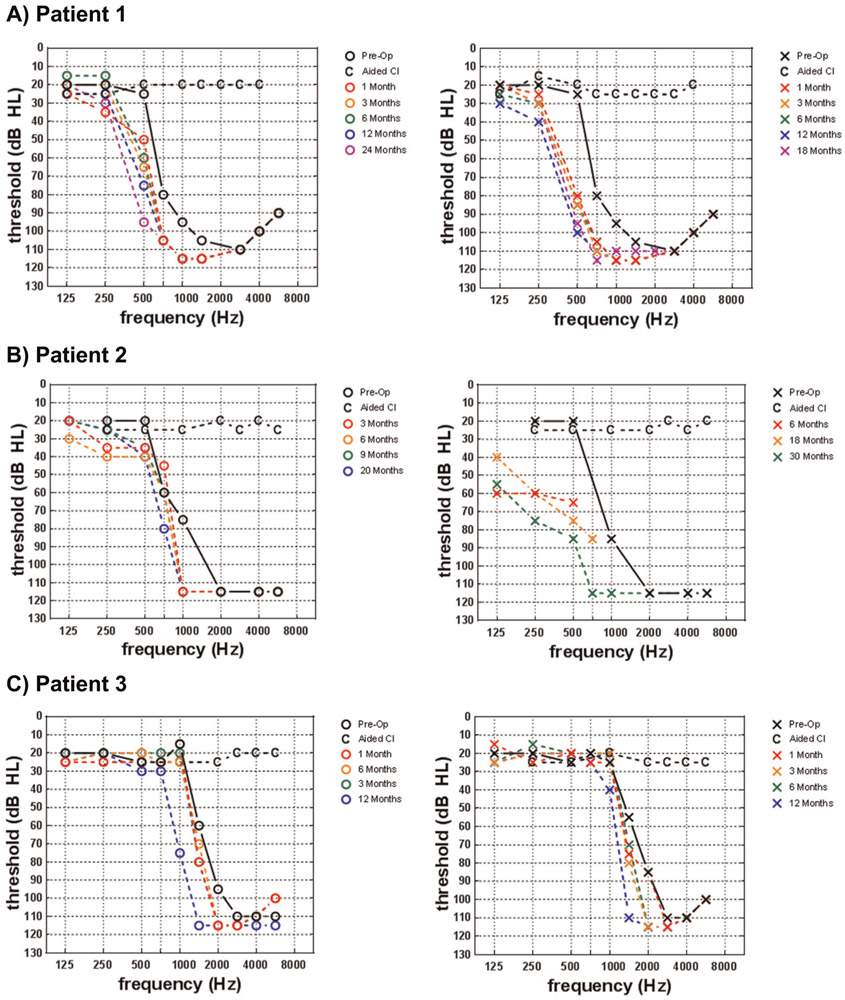
FIG. 1.
Preoperative and postoperative residual hearing thresholds as well as aided detection thresholds are shown for the A) Patient 1, B) Patient 2, and C) Patient 3.
Based on poor access to high frequency speech stimuli with appropriately fitted hearing aids and delayed speech and language, we elected to first implant her right ear with a Cochlear Nucleus 522 at 54 months of age. Four months later we implanted her left ear with a Cochlear Nucleus 532 at 58 months of age. Both surgeries were straightforward, and intraoperative CT scan confirmed appropriate device placement within the scala tympani except for electrode 1 in the right ear, which was extracochlear and deactivated. Shortly after activation, her N7 sound processors were equipped with commercially available acoustic components (Cochlear Hybrid Receiver, Cochlear Ltd., Sydney, Australia) affording low-frequency acoustic amplification, bilaterally. With the acoustic components active, the electric-only bandwidth was set to 438 to 7938 Hz, bilaterally. The acoustic components were removed after 16 and 12 months of CI use in the right and left ears, respectively. The reason is that at this time point, her 500-Hz threshold exceeded 80 dB HL, yet she exhibited normal hearing sensitivity at 125 and 250 Hz. At this time, her map was changed to full-electric simulation (188–7938 Hz), but she was still demonstrating benefit from the acoustic input via her residual, unaided hearing. Her most recent speech recognition scores at 18 months post-activation for her right ear and 24 months post-activation for her left ear are shown in Table 2. Progression of her residual hearing is shown in Figure 1A. She has shown significant improvement in speech recognition abilities, as she is now approaching ceiling level performance in quiet and in background noise (+5 dB SNR). She has also demonstrated significant improvement in her speech and language abilities as evidenced by scores ranging from 92 to 100% on an articulation assessment (Identifying Early Phonological Needs in Children with Hearing Impairment, IEPNCHI) (21), and she is now outperforming her peers with typical hearing as evidenced by standard scores well over 100 on the PLS-5 (Auditory Comprehension=112, Expressive Communication=115, Total Language Score=115).
TABLE 2.
Postoperative speech recognition scores are shown for Patient 1 at 18 months post-activation for the left ear and 24 months post-activation for the right ear
| CNC Words | BabyBio Quiet | BabyBio +5 dB SNR |
BabyBio +2 dB SNR | BabyBio 0 dB SNR |
|
|---|---|---|---|---|---|
| Right CI (EAS) | 68% | 92% | 87% | – | – |
| Left CI (EAS) | 75% | 94% | 82% | – | – |
| Bilateral CI (EAS) | 84% | – | – | 79% | 52% |
| Bilateral electric only | – | – | – | 72% | – |
| Bilateral electric + R acoustic | – | – | – | 79% | – |
| Bilateral electric + L acoustic | – | – | – | 77% | – |
CI indicates cochlear implant; CNC, consonant-nucleus-consonant; EAS, electric acoustic stimulation; L, left; R, right; SNR, signal-to-noise ratio.
Patient 2
Patient 2 was a 3-year-old woman who was referred to our center to seek a second opinion regarding a CI evaluation. After passing a newborn hearing screening, she was identified with hearing loss and fit with hearing aids at the age of 3. Her preoperative audiogram is shown in Figure 1B in black. At her preoperative evaluation, she was unable to detect /s/ and /sh/ even with frequency lowering technology activated in her hearing aids. She demonstrated delayed receptive and expressive language and severely delayed articulation. The PLS-5 (18) was administered when she was 3.5 years of age, and her scores indicated that she was functioning 2 years behind her chronological age.
Based on poor access to high frequency speech stimuli with appropriately fitted hearing aids and delayed speech and language, we elected to first implant her left ear with a Cochlear Nucleus 522 at 47 months of age. Eleven months later we implanted her right ear with a Cochlear Nucleus 522 at 58 months of age. Though the Nucleus 532 electrode was preferred for both surgeries, the angle of the round window prevented insertion of the 532. Both surgeries were straightforward with the 522, and intraoperative CT scan confirmed appropriate device placement within the scala tympani except for electrodes 1 and 2 in the right ear, which were extracochlear and thus deactivated at activation. Shortly after activation, her N7 sound processors were equipped with acoustic components affording low-frequency acoustic amplification, bilaterally. With the acoustic components active, the electric-only bandwidth was set to 813 to 7938 Hz for the right ear and 438 to 7938 for the left ear. Her most recent speech recognition scores at 20 months post-activation for her right ear and 30 months post-activation for her left ear are shown in Table 3. Progression of her residual hearing is shown in Figure 1B. Overall, she is performing well with single-word repetition (80%) but is struggling with auditory memory and language skills necessary for sentence repetition (64%). Note that further speech recognition data could not be obtained due to the patient’s age, proximity to implant center, and amount of testing required for pediatric EAS programming and evaluations.
TABLE 3.
Postoperative speech recognition scores are shown for Patient 2 at 30 months, post-activation for the left ear, and 20 months post-activation for the right ear
| LNT Words Hard |
CNC Words |
BabyBio Quiet |
|
|---|---|---|---|
| Right CI (EAS) | 80% | – | 64% |
| Left CI (EAS) | 80% | – | 64% |
| Bilateral CI (EAS) | – | 72% | 55% |
CI indicates cochlear implant; CNC, consonant-nucleus-consonant; EAS, electric acoustic stimulation; LNT, Lexical Neighborhood Test.
Patient 3
Patient 3 is the younger sister of Patient 2. Patient 3 also passed her newborn hearing screening, and she was identified with hearing loss and fit with hearing aids at the age of 2. Patient 3’s hearing loss was monitored at our center following her sister’s implantation. Patient 3 was successfully using hearing aids for some time until her hearing loss progressed to the audiogram shown in Figure 1C at which point she could no longer detect / s/ and /sh/ with frequency lowering technology activated in her hearing aids.
Based on poor access to high frequency speech stimuli with appropriately fitted hearing aids, rapid progression of hearing loss, and observed progression of hearing loss in her older sister, we elected to proceed with bilateral implantation of the Cochlear Nucleus 532 device at 43 months of age. Surgery was straightforward, and intraoperative CT scan confirmed appropriate device placement within the scala tympani except for electrode 1 in the left ear, which was extracochlear and deactivated at activation. Patient 3 was not fitted with acoustic components because her residual hearing was preserved in the normal-hearing range. Instead, the low-frequency electric cut-off was set to 700 Hz in both ears, and her natural acoustic hearing was used for the lower frequencies. This type of configuration has also been termed ‘‘electro-natural hearing’’ in the literature (22). Progression of her residual hearing through the 12-month postoperative timepoint is shown in Figure 1C. If her hearing sensitivity declines, acoustic components will be added to her processors in the future. Her most recent speech recognition scores at 12 months post-activation are shown in Table 4. Much like her sister, she is performing well on tasks of single-word repetition (82–92%) but is struggling with the auditory memory and language skills required for sentence repetition (62%). Note that further speech recognition data could not be obtained due to the patient’s age, proximity to implant center, and amount of testing required for pediatric EAS programming and evaluations.
TABLE 4.
Postoperative speech recognition scores are shown for Patient 3 at 12 months post-activation
| LNT Easy Words |
LNT Hard Words |
HINT-C Sentences |
|
|---|---|---|---|
| Right CI (EAS) | 82% | – | – |
| Left CI (EAS) | 92% | – | – |
| Bilateral CI (EAS) | – | 76% | 62% |
CI indicates cochlear implant; EAS, electric acoustic stimulation; HINT-C, Hearing in Noise Test for Children; LNT, Lexical Neighborhood Test.
DISCUSSION
The results of the three cases presented in this series provide support for early implantation for children with hearing loss due to TMPRSS3 mutation and for expanded indications for CI in the pediatric population. All three children were evaluated at other centers and determined to be ineligible for a CI based on their normal low-frequency hearing, yet they were all struggling with their hearing aids and exhibiting significant speech and language delay. If we were to apply the criterion proposed by Leigh et al. (23) (pure-tone average >65 dB HL), Patients 1 and 2 would have met this criterion with a greater than 75% chance of performing better with a CI than a hearing aid. Patient 3 would not have met this criterion; however, it is important to note that because Patient 3 was the sibling of Patient 2 with genetic confirmation of TMPRSS3 mutation, we elected to proactively implant Patient 3 based upon progression and results observed with her sister in addition to her speech and language delay. Overall, all three patients benefitted from CI in the presence of significant residual hearing; however, there are some factors that should be considered regarding young children with residual hearing and patients with TMPRSS3 gene mutation.
Within this relatively homogeneous case series, we observed considerable variability in speech recognition, speech production, and language skills. Patient 1 showed the most favorable outcomes. While a number of factors could contribute to her success, most notably, Patient 1 wore her processors most consistently (12.6 h per day), and she received weekly speech therapy, whereas the other two patients did not. The importance of speech and language therapy is also evidenced by Patient 2 and 3’s sentence recognition scores. While their word recognition scores were excellent, they struggled with auditory memory and sentence structure skills required to repeat a sentence, a skill that could be targeted in speech/language therapy. Patient 3 had the advantage of being implanted at the youngest age with the most residual hearing; however, her daily processor usage is poorer than the other two patients at 8.0 hours per day. Based on previous studies of daily CI use in the pediatric population (24,25), it is likely that this amount of processor usage is hindering her potential auditory, speech, and language development. It is possible that she has the lowest daily CI use because of her ability to get by with her residual hearing especially in the presence of visual cues. This is a point that may require additional counseling and effort on the family’s part to guard against this potential habit.
CI outcomes for patients with hearing loss caused by TMPRSS3 mutation are sparsely reported and inconsistent. Until this case series, outcomes have only been reported in adults. Tropitzsch et al. (10), Eppsteiner et al. (11), and Shearer et al. (12) report poor CI outcomes for 10 total adults, while others report favorable outcomes for 13 adults (13-15). There are two possible reasons for poorer CI outcomes in the adult population. The first is that many of the patients included in studies to date have a long duration of deafness, which has been shown to be associated with poorer outcomes (26,27). For example, the two patients reported by Eppsteiner et al. (11) had a duration of deafness greater than 50 years, a significant confounding factor. The second possible reason for poor outcomes is loss of spiral ganglion cells, which have been shown in animal models to express TMPRSS3 (7,11). Spiral ganglion cell degeneration would reduce available targets for neural stimulation and therefore diminish CI efficacy. However, insufficient human models exist to assess the role of TMPRSS3 in spiral ganglion cells. Furthermore, despite some progression of hearing loss in this cohort, all three children have shown steady improvement in speech recognition scores through the 2.5-year post-implantation mark for patients 1 and 2 and the 1-year post-implantation mark for patient 3. In other words, we have not seen any evidence of theoretical spiral ganglion cell degeneration leading to a decline in performance in our case series. Regardless of potential reason for poor performance, both possibilities support aggressive treatment via CI for children with TMPRSS3 mutation. If duration of deafness is the cause for poor performance, children can obviate this via earlier, off-label implantation, and if spiral ganglion cell degeneration is the cause of poor performance, CI may be a therapeutic option for slowing or reversing degeneration.
CONCLUSION
Results indicate that CI with EAS is an appropriate treatment for children with sloping hearing loss secondary to TMPRSS3 genetic mutation. Pediatric results from this case series show more favorable CI outcomes than are currently reported for adults with TMPRSS3 mutation suggesting that the intervention may be time-sensitive. Children with TMPRSS3 should be proactively evaluated, monitored, and considered for off-label implantation to avoid delays in acquisition of speech and language skills.
Financial Material & Support:
NIH R01 DC13117; PI: R.H.G., PhD. Institutional Review Board: IRB# 130229.
Footnotes
Conflict(S) of Interest to Declare: None directly related to this case series.
REFERENCES
- 1.Camp GV, Willems PJ, Smith RJ. Nonsyndromic hearing impairment: unparalleled heterogeneity. Am J Hum Genet 1997;60:758–64. [PMC free article] [PubMed] [Google Scholar]
- 2.Battelino S, Klancar G, Kovac J, Battelino T, Trebusak Podkrajsek K. TMPRSS3 mutations in autosomal recessive nonsyndromic hearing loss. Eur Arch Otorhinolaryngol 2016;273:1151–4. [DOI] [PubMed] [Google Scholar]
- 3.Wattenhofer M, Di Iorio M, Rabionet R, et al. Mutations in the TMPRSS3 gene are a rare cause of childhood nonsyndromic deafness in Caucasian patients. J Mol Med 2002;80:124–31. [DOI] [PubMed] [Google Scholar]
- 4.Scott HS, Kudoh J, Wattenhofer M, et al. Insertion of β-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nat Genet 2001;27:59–63. [DOI] [PubMed] [Google Scholar]
- 5.Guipponi M, Toh MY, Tan J, et al. An integrated genetic and functional analysis of the role of type II transmembrane serine proteases (TMPRSSs) in hearing loss. Hum Mutat 2008;29:130–41. [DOI] [PubMed] [Google Scholar]
- 6.Guipponi M, Vuagniaux G, Wattenhofer M, et al. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet 2002;11:2829–36. [DOI] [PubMed] [Google Scholar]
- 7.Fasquelle L, Scott HS, Lenoir M, et al. Tmprss3, a transmembrane serine protease deficient in human DFNB8/10 deafness, is critical for cochlear hair cell survival at the onset of hearing. J Biol Chem 2011;286:17383–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Yosef T, Wattenhofer M, Riazuddin S, et al. Novel mutations of TMPRSS3 in four DFNB8/B10 families segregating congenital autosomal recessive deafness. J Med Genet 2001;38:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wattenhofer M, Sahin-Calapoglu N, Andreasen D, et al. A novel TMPRSS3 missense mutation in a DFNB8/10 family prevents proteolytic activation of the protein. Hum Genet 2005;117:528–35. [DOI] [PubMed] [Google Scholar]
- 10.Tropitzsch A, Knoblich N, Müller M, et al. Cochlear implant performance in patients with TMPRSS3 mutations. Laryngorhinootologie 2018;97:S272. Forschung Heute – Zukunft Morgen. [Google Scholar]
- 11.Eppsteiner RW, Shearer AE, Hildebrand MS, et al. Prediction of cochlear implant performance by genetic mutation: the spiral ganglion hypothesis. Hear Res 2012;292:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer AE, Tejani VD, Brown CJ, et al. In vivo electrocochleography in hybrid cochlear implant users implicates TMPRSS3 in spiral ganglion function. Sci Rep 2018;8:14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyagawa M, Nishio SY, Sakurai Y, et al. The patients associated with TMPRSS3 mutations are good candidates for electric acoustic stimulation. Ann Otol Rhinol Laryngol 2015;124:193S–204S. [DOI] [PubMed] [Google Scholar]
- 14.Weegerink NJD, Schraders M, Oostrik J, et al. Genotype-phenotype correlation in DFNB8/10 families with TMPRSS3 mutations. J Assoc Res Otolaryngol 2011;12:753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbracht M, Senderek J, Eggermann T, et al. Autosomal recessive postlingual hearing loss (DFNB8): compound heterozygosity for two novel TMPRSS3 mutations in German siblings. J Med Genet 2007;44:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson ML, O’Connell BP, Lohse CM, Driscoll CL, Sweeney AD. Survey of the American Neurotology Society on cochlear implantation. Otol Neurotol 2018;39:e20–7. [DOI] [PubMed] [Google Scholar]
- 17.Elliott L, Katz D. Northwestern University Children’sPerception of Speech: (NU-CHIPS). Auditec of St. Louis; 1980. [Google Scholar]
- 18.Zimmerman I, Steiner V, Pond R. Preschool Langauge Scales-Fifth Edition (PLS-5). San Antonio: Pearson; 2011. [Google Scholar]
- 19.Goldman R, Fristoe M. Goldman Fristoe 3: Test of Articulation. Pearson; 2015. Available at: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Developmental-Early-Childhood/Goldman-Fristoe-Test-of-Articulation-3/p/100001202.html. Accessed July 9, 2019. [Google Scholar]
- 20.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. second edition American Guidance Services; Circle Pines, MN: 2004 [Google Scholar]
- 21.Paden EP, Brown CJ. Identifying Early Phonological Needs in Children with Hearing-Impairment. Innsbruck, Austria: Med-El; 1992. [Google Scholar]
- 22.Skarzynski H, Lorens A, Dziendziel B, Skarzynski PH. Expanding pediatric cochlear implant candidacy: a case study of electro-natural stimulation (ENS) in partial deafness treatment. Int J Pediatr Otorhinolaryngol 2015;79:1896–900. [DOI] [PubMed] [Google Scholar]
- 23.Leigh JR, Dettman SJ, Dowell RC. Evidence-based guidelines for recommending cochlear implantation for young children: audiological criteria and optimizing age at implantation. Int J Audiol 2016;55 (suppl):S9–18. [DOI] [PubMed] [Google Scholar]
- 24.Easwar V, Sanfilippo J, Papsin B, Gordon K. Impact of consistency in daily device use on speech perception abilities in children with cochlear implants: datalogging evidence. J Am Acad Audiol 2018;29:835–46. [DOI] [PubMed] [Google Scholar]
- 25.Guerzoni L, Cuda D. Speech processor data logging helps in predicting early linguistic outcomes in implanted children. Int J Pediatr Otorhinolaryngol 2017;101:81–6. [DOI] [PubMed] [Google Scholar]
- 26.Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol 1999;20:445–52. [PubMed] [Google Scholar]
- 27.Green KMJ, Julyan PJ, Hastings DL, Ramsden RT. Auditory cortical activation and speech perception in cochlear implant users: effects of implant experience and duration of deafness. Hear Res 2005;205:184–92. [DOI] [PubMed] [Google Scholar]