Abstract
Background
Coenzyme Q10 is one of the most widely sold nutritional supplements in the United States. Coenzyme Q10 is available in both its oxidized form (ubiquinone) and its reduced form (ubiquinol). The predominant marketing of Coenzyme Q10 to physicians and patients asserts that the ubiquinol form of Coenzyme Q10 has superior absorption to the ubiquinone form. This study has been designed to compare and contrast the stability and absorption of ubiquinol supplements, as well as the claims made for ubiquinol compared with ubiquinone.
Ubiquinol, the reduced state of Coenzyme Q10, is commercially available as a nutritional supplement; however, ubiquinol, by its nature as an electron donor, is much less stable than ubiquinone, the oxidized state of Coenzyme Q10. The absorption, bioavailability and efficacy of ubiquinol products has been much less often tested in clinical trials. Consequently, insufficiently documented marketing claims are being made for ubiquinol supplements.
Methods
In Part 1 of this report on the instability of the lipid-soluble antioxidant ubiquinol, SIBR Research presented data from lab studies showing that oral ubiquinol is likely to be oxidized to ubiquinone and absorbed as ubiquinone. In this Part 2, SIBR Research conducted a study of the transfer and absorption of orally ingested ubiquinol in large dogs.
Results
In the dog studies, the percentage of ubiquinol converted to ubiquinone increased as the capsule contents passed through the stomach and small intestines and into the lymph system.
Conclusions
The dog studies demonstrate that oral ubiquinol in commercial nutritional supplements is not stable in the gastrointestinal tract of large dogs. Based on these results, it seems likely that in humans also, most of the ubiquinol from capsules will be oxidized to ubiquinone in the acid profile between the stomach and the small intestines, where there is a wide range of acidity. The ubiquinol from the supplement will be absorbed in the ubiquinone state and will pass into the lymph system as ubiquinone, where it will be reduced back to ubiquinol. It will pass from the lymph system into the blood circulation as ubiquinol.
Introduction
Ubiquinone (fully oxidized state) and ubiquinol (fully reduced state) are the major states of the lipid-soluble vitamin-like bionutrient known as Coenzyme Q10 (CoQ10). There is also a transient intermediate state, ubisemiquinone (also known as semiquinone) that has multiple unexplained biologic functions.1
In American usage, the terms CoQ10 and CoQH2 are commonly used for ubiquinone and ubiquinol, respectively. In European usage, the designations Q10 and QH2 are more commonly used.
Both the ubiquinone state and the ubiquinol state are bioactive redox forms of Coenzyme Q10. Ubiquinone plays an essential role in the production of cellular ATP energy. Ubiquinol plays an important role as a lipid-soluble antioxidant. As such, ubiquinol helps prevent the peroxidation of the LDL lipoproteins in the blood.2
It is misleading to claim that ubiquinol is more important than ubiquinone.2 Both states of Coenzyme Q10 are important. That ubiquinol is the predominant form of Coenzyme Q10 in the blood makes sense in that there is relatively little need for the bioenergetics form (ubiquinone) in the circulation while there is a manifest need for the antioxidant form (ubiquinol).2
When Coenzyme Q10 in the blood passes into the tissues, there are numerous enzyme systems available to to maintain adequate levels of ubiquinol. Mantle and Dybring list 5 enzyme systems that facilitate this conversion: NAD(P)H dehydrogenase quinone 1 (known as NQO1), glutathione reductase, lipoamide dehydrogenase, cytochrome b5 reductase and thioredoxin reductase.3
Ubiquinol: The Inherently Unstable State of Coenzyme Q10
Because ubiquinol has the property of being an electron donor, it is useful as an antioxidant, and a lipid-soluble antioxidant at that. When it donates 2 electrons, it is oxidized to the ubiquinone form. It is this ability to act as an electron donor that makes the ubiquinol form inherently unstable.2
Biological Functions of Coenzyme Q10
As mentioned above, Coenzyme Q10 has vitamin-like effects and functions in the body, but it cannot be considered a vitamin as the cells are able to synthesize Coenzyme Q10 in the same mevalonate pathway that produces cholesterol. Among the vital biologic functions of Coenzyme Q10 are that it:4
acts as a co-factor in the cellular process of mitochondrial oxidative phosphorylation and thus in the process of adenosine triphosphate (ATP) energy production
functions as an antioxidant that protects the cells against damage from harmful free radicals
improves endothelial function and thus protects against atherosclerosis
promotes cell signaling through its effect on the expression of genes that code for the involved proteins
has demonstrated anti-inflammatory effects
Ubiquinone: The Form of Coenzyme Q10 Synthesized in the Cells
The form of Coenzyme Q10 that is synthesized in the cells is ubiquinone. Ubiquinol is, then, the product of redox reactions involving ubiquinone.2,5 Ingested ubiquinone in rodents has been shown to increase the ubiquinol concentration in the lymph.6 It is not necessary to take a ubiquinol supplement in order to get enough ubiquinol in the blood circulation.5,6
Studies have shown that cellular biosynthesis of CoQ10 peaks in early adulthood, typically in the 20s, and then, with considerable inter-individual variability, steadily decreases with increasing age.7 Both the ageing process and the use of various prescription medications, chief among them statins and bisphosphonates, contribute to the decline in the endogenous biosynthesis of Coenzyme Q10.8-10 It has been hypothesized that the use of statin medications can deplete the supply of Coenzyme Q10 and result in the calcification of coronary arteries and the impairment of heart muscle function.11
It is not feasible to eat enough food or to change one’s diet to make up for the loss of endogenously produced Coenzyme Q10. For example, it would be necessary to eat nearly 3 lb of steak or 2 lb of liver to meet the need for a daily intake of 3.5 mg of Coenzyme Q10.12
Consequently, daily Coenzyme Q10 supplementation is necessary. However, commercially available Coenzyme Q10 supplements vary considerably in their absorption and bioavailability and, thus, in their efficacy.
Differences in the manufacturer’s method of solubilization of the active ingredient Coenzyme Q10, in the manufacturer’s choices of the carrier oils for the Coenzyme Q10 and in the manufacturer’s heating and cooling processes during the production of the Coenzyme Q10 capsules are decisive with respect to the absorption of the contents of the capsules. Even when they are produced with the same Coenzyme Q10 raw material, Coenzyme Q10 supplements are not equally well absorbed.13
Coenzyme Q10 Status Needed for Therapeutic Benefits
Reference values for plasma or serum Coenzyme Q10 status range widely from 0.36 to 1.59 mg/L.12 A normal healthy individual who is not elderly and not taking a supplement is expected to have a plasma Coenzyme Q10 status of 0.8 mg/L.14
At the Ninth Conference of the International Coenzyme Q10 Association in New York in 2018, the assembled Coenzyme Q10 researchers generally agreed that a plasma Coenzyme Q10 status of 2.0 to 2.5 mg/L is needed for an individual to realize significant improvement in Coenzyme Q10 as an adjunctive treatment of heart failure.14
Misleading Marketing Claims for Ubiquinol Supplements
American companies marketing ubiquinol supplements make the following claims, for which more documentation from clinical trials is needed:
better absorbed form
more bioactive form
form needed by people over age 40 years
more water-soluble form10
At best, claims of this nature are only partially documented. These claims can be misleading for the consumer of Coenzyme Q10 supplements who is not familiar with the state of Coenzyme Q10 research. SIBR Research will address these marketing claims in Part 3 of this report.
For the moment, let it suffice to say that the formulation of Coenzyme Q10 supplements seems to be much more important for absorption and bio-availability than the form of the supplement, ie, whether the supplement uses ubiquinone or ubiquinol as the active ingredient.13
Ubiquinol Is Easily Oxidized Back to Ubiquinone
The production of Coenzyme Q10 capsules is difficult in and of itself, and the production of ubiquinol capsules is even more difficult. In order to prevent the oxidation of the ubiquinol to ubiquinone already in the process of filling the capsule, conscientious ubiquinol supplement manufacturers need to encapsulate the ubiquinol under nitrogen.
Ubiquinol will be oxidized to ubiquinone if the ubiquinol is exposed to room air during the manufacturing process. The results of the lab studies reported in Part 1—studies of the capsule contents of 13 commercially available ubiquinol products—show that, for the most part, the ubiquinol in the capsule remains in the ubiquinol state.15
It is easy for the consumer to check the contents of a ubiquinol capsule. They should cut open the capsule and examine the fluid contents. If the fluid is orange in color or becomes orange within an hour after being exposed to air, the contents of the ubiquinol capsule have been oxidized to the ubiquinone state. If the capsule contents are white, resembling milk, then the ubiquinol from the capsule is still in its ubiquinol state.16
The lab studies reported in Part 1 of this article indicate that in many cases the ubiquinol from the capsule does not remain in its reduced form as it passes through 2.2 pH and 8.2 pH solutions simulating the conditions in the stomach and the small intestines.15
In 2 different studies, SIBR Research has investigated the question of the stability of ubiquinol sold in capsules:
Part 1. The Instability of the Lipid-Soluble Antioxidant Ubiquinol: Lab Studies
Part 2. The Instability of the Lipid-Soluble Antioxidant Ubiquinol: Dog Studies
The 2 studies represent SIBR Research’s search for an answer to the question: is oral ubiquinol from supplements oxidized to the ubiquinone state prior to Coenzyme Q10 arriving at the intestinal absorption cells?
In other words, the 2 studies were conducted to learn whether the oral ubiquinol from supplement capsules is absorbed as ubiquinol or is converted to ubiquinone and absorbed as ubiquinone prior to arriving in the lymph vessels.
Materials and Methods
In-Vivo Study
In the in vivo studies, 6 dogs were trained to lie quietly on a lab table. The dogs were fed a high fat diet for 3 days to make the lymph vessels appear a milky white color. Catheters (Medical Supply House, 21-gauge) were inserted into the stomach, small intestines, distal lymph vessels from the absorption cells, proximal lymph vessels channeling the lymph to the systemic circulation and the brachial vein. Catheters were exteriorized to the back, flushed with a saline heparin mix and sealed in a protective cover. After 3 days, each dog was fed a vegetable diet and rested overnight.
On the study day, the dogs were given a cup of vegetable dog chow in water (Chewy Pet Foods) and 100 mg of Coenzyme Q10 (Product name available upon request). Control samples were collected from each catheter. A total of 3 ml samples were collected from each site at hourly intervals, rapidly frozen at -82°C, and stored. With completion of all 6 dog studies, the samples were shipped overnight to the analytical lab for analysis of total Coenzyme Q10 content, ubiquinol content and ubiquinone content.
The analytical reports were sent to the statistical lab for analysis. Group means and standard deviations for total Coenzyme Q10 content, ubiquinol content, and ubiquinone content were calculated for each site on the dogs and are presented in this report. A P value of .05 was considered statistically significant.
Results
In Vitro Lab Studies
The results of the lab studies were reported in Part 1 of this article.15
In Vivo Study in Large Dogs
The data from the large dog study show that the administered ubiquinol was oxidized more and more as it passed through the stomach and the small intestines on its way to the enterocytes. This finding is consistent with the findings in the in vitro studies reported above.
The percentage of ingested ubiquinol that was converted to ubiquinone increased as the Coenzyme Q10 passed from the capsule and traversed the stomach and small intestines until it reached the enterocytes. The percentage of ubiquinol in the ubiquinol capsules administered to the dogs was 93%. After 5 minutes in the stomach, the group mean percentage of ubiquinol was 87% of the total Coenzyme Q10, and after 60 minutes, the group mean percentage of ubiquinol was 55% of the total Coenzyme Q10.
In the dogs’ small intestines, the group mean percentage of ubiquinol fell to a low of 8% of the total Coenzyme Q10. Thereafter, in the lymph collecting duct adjacent to the absorption cells, the group mean percentage of ubiquinone decreased to 54% in the distal lymph nodes, 10% in the abdominal lymph nodes, and 4.9% in the thoracic lymph nodes before entering the blood.
After absorption predominantly in the ubiquinone state, the Coenzyme Q10 first appeared in the abdominal lymph nodes as ubiquinone but was rapidly converted back to ubiquinol, as it traversed the lymphatic system before entering the venous blood. In the venous blood, the ubiquinol was measured at 96% while the ubiquinone was measured as 4%.
Discussion
In Vitro Study
The data from the in vitro study show that the ubiquinol in ubiquinol supplements is generally stable while still in the capsule but is considerably less stable in 2.2 pH and 8.2 pH solutions simulating the stomach and the duodenum.
Given these results from an investigation of 13 commercially available ubiquinol supplements, the purchaser of a typical ubiquinol supplement can expect that three-fourths or more of the ubiquinol that they bought will be oxidized to the ubiquinone state of Coenzyme Q10 before the capsule contents reach the intestinal absorption cells.
In Vivo Study
The results of the dog study show that the ingested ubiquinol is mostly (92%) oxidized in the small intestines before it reaches the enterocytes. The results suggest that the fraction of ingested ubiquinol that does get absorbed is mostly absorbed in the ubiquinone form.
Then, entering the lymph nodes from the enterocytes, the ubiquinone (converted from the ingested ubiquinol) is fairly rapidly converted back to ubiquinol. It traverses the lymph system and enters the blood circulation predominantly in the ubiquinol state.12
That Coenzyme Q10 is primarily in the ubiquinol state in the blood can be explained by the need for the antioxidant form of Coenzyme Q10 to protect against the peroxidation of the lipoproteins in the blood. In the blood, there is relatively little need for the oxidized form of Coenzyme Q10, which is the essential form needed for the process of ATP energy generation.12
The lipid solubility and the large molecular size of the Coenzyme Q10 molecules make absorption a difficult process. The relatively long period (5 to 8 hours) after absorption that it takes for ingested Coenzyme Q10 to reach a maximum concentration (Cmax) in the blood plasma is caused primarily by the slow flow of the lymph. There is less pressure in the lymph vessels than in the blood vessels. A good estimate is that a sample of the lymph distal to the enterocytes will show the Cmax of exogenous Coenzyme Q10 as soon as 2 hours after ingestion.12
Implications for Ubiquinol Marketing Claims
At this point, it is relevant to address the various marketing claims which assert that ubiquinol is the superior and/or necessary form for Coenzyme Q10; eg, the claims for ubiquinol on the website ubiquinol.org.
SIBR Research will address what it regards as misleading marketing claims for ubiquinol supplements in Part 3 of this article.
Conclusions
The in vitro studies reported in Part 1 of this article showed that between 76% and 84% of the ubiquinol in 13 commercially-available ubiquinol supplements is oxidized to ubiquinone in pH solutions, simulating the pH in the stomach and in the small intestines.
The low pH in the gastric juice is characterized by the presence of many free hydrogen ions, and the somewhat elevated pH in the small intestines is characterized by the presence of many free hydroxyl ions. Ubiquinol acts as an electron donor in the gastric and small intestine juices; accordingly, the ubiquinol from supplement capsules is converted to ubiquinone, the oxidized form of Coenzyme Q10.
In the dog study, the measurements of the relative percentages of ubiquinol and ubiquinone at various stages show that the ingested ubiquinol is largely oxidized to ubiquinone before it reaches the enterocytes. When it passes from the enterocytes into the lymph system, it is converted back to ubiquinol, and it is predominantly as ubiquinol that it enters the blood circulation.
As far as SIBR Research can ascertain, this is the first published report of the oxidation of orally ingested ubiquinol to ubiquinone prior to arrival at the intestinal absorption cells.
Figure 1.
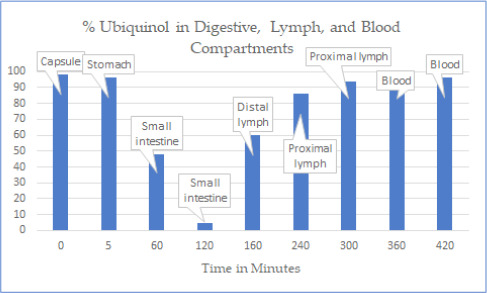
The ingested ubiquinol was oxidized to ubiquinone in the stomach and small intestines. It was reduced back to ubiquinol in the lymph nodes.
Figure 2.
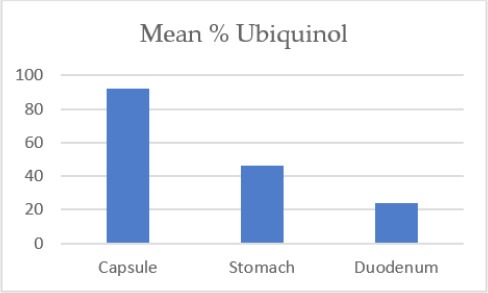
Ubiquinol as a percentage of total CoQ10 declines as the orally ingested ubiquinol passes through the gastrointestinal tract.
Acknowledgements
The author wishes to thank Richard Morrill, editor, Q10facts.com for assistance in the writing of thise paper.
Footnotes
Ethical Approval
SIBR Research received ethical approval for the large dog study from the Institutional Review Board at the University of Texas in Austin.
Conflicts of Interest
The author declares no conflicts of interest.
References
- 1.Linnane AW, Kios M, Vitetta L. Coenzyme Q(10) – its role as a prooxidant in the formation of superoxide anion/hydrogen peroxide and the regulation of the metabolome. Mitochondrion. 2007;7(Suppl):S51-S61. [DOI] [PubMed] [Google Scholar]
- 2.Judy WV, Stogsdill WW, Judy DS, Judy JS. Coenzyme Q10 Facts or Fabrications. NovaQ10. www.medicatrix.be/download/ubiquinone_ubiquinol_biodisponibilite.pdf. Accessed October 21, 2021.
- 3.Mantle D, Dybring A. Bioavailability of Coenzyme Q10: an overview of the absorption process and subsequent metabolism. Antioxidants. 2020;9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of Coenzyme q10: recent developments. Mol Biotechnol. 2007;37:31-37. [DOI] [PubMed] [Google Scholar]
- 5.Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta.1992;1126(3):247-254. [DOI] [PubMed] [Google Scholar]
- 6.Mohr D, Umeda Y, Redgrave TG, Stocker R. Antioxidant defenses in rat intestine and mesenteric lymph. Redox Rep. 1999;4(3):79-87. [DOI] [PubMed] [Google Scholar]
- 7.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579-84. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Camacho JD, Bernier M, López-Lluch G, Navas P. Coenzyme Q10 supplementation in aging and disease. Front Physio. 2018;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayer A, Macdonald P, Stocker R. CoQ10 function and role in heart failure and ischemic heart disease. Ann Rev Nutr. 2015;35:175-213. [DOI] [PubMed] [Google Scholar]
- 10.Judy WV, Hall JH, Dugan W, Toth PD, Folkers K. Coenzyme Q10 reduction of Adriamycin cardiotoxicity. In: Folkers K, Yamamura Y. (eds.) Biomedical and Clinical Aspects of Coenzyme Q. Vol 4. Amsterdam, the Netherlands: Elsevier/North-Holland Biomedical Press. 1984;pp 231-241. [Google Scholar]
- 11.Okuyama H, Langsjoen PH, Hamazaki T, Ogushi Y, Hama R, Uchino H. Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Rev Clin Pharmacol. 2015;8:189-199. [DOI] [PubMed] [Google Scholar]
- 12.Judy WV. Coenzyme Q10: The Substances That Powers Life, An Insider’s Guide. Haderslev, Denmark: Ny Videnskab,. 2018; pp 35-37. ISBN: 978-87-7776-186-7. [Google Scholar]
- 13.López-Lluch G, Del Pozo-Cruz J, Sánchez-Cuesta A, Cortés-Rodríguez AB, Navas P. Bioavailability of Coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition. 2019;57:133-140. [DOI] [PubMed] [Google Scholar]
- 14.Langsjoen PH, Langsjoen AM. Comparison study of plasma Coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2014;3:13-17. [DOI] [PubMed] [Google Scholar]
- 15.Judy WV. The Instability of the Lipid-Soluble Antioxidant Ubiquinol: Part 1-Lab Studies. Integr Med (Encinitas). 2021. Aug;20(4):24-28. PMID: 34602873; PMCID: PMC8483252. [PMC free article] [PubMed] [Google Scholar]
- 16.Freye E, Strobel HP. The whole truth about coenzyme Q10 you may not find elsewhere. Adv Compl Alt Med. 2018;2:107-116. [Google Scholar]


