Abstract
Purpose:
To determine the relationship of tumoral and nontumoral radiation dose to response and toxicity after transarterial radioembolization (TARE) of breast cancer liver metastasis.
Methods:
This retrospective study evaluated all patients with breast cancer liver metastases treated with TARE (2/2011—6/2019). Extent of disease was measured as unilobar or bilobar on baseline PET/CT prior to TARE. Response was assessed for targeted regions with modified PERCIST criteria on first follow-up PET/CT. Tumoral and nontumoral liver dosimetry was evaluated by performing volumetric segmentation on post-TARE Bremsstrahlung SPECT/CT. ≥Grade 3 hepatotoxicity was defined as ≥grade 3 bilirubin/AST/ALT elevation or ascites requiring intervention. Fisher’s exact tests, Wilcoxon rank sum tests, and Kaplan-Meier survival analysis were performed.
Results:
Among 64 women, 60 patients had pre- and post-TARE PET/CT, of whom 46/60 (77%) achieved objective response (OR). Responders received higher tumoral dose with a median (interquartile range) of 167 (96—217) vs. 54 (45—62) Gy (p<0.001). ≥Grade 3 hepatotoxicity occurred in 8/64 (12.5%) and was associated with higher pre-treatment bilirubin levels of 0.9 (0.9—1.1) vs. 0.5 (0.4—0.7) mg/dL (p=0.013). Median overall survival (OS) was 11 (95% CI 10—19) months. Bilobar disease (Hazard Ratio [HR]: 2.77, 95% CI 1.11—6.89, p=0.028) and elevated pre-TARE AST (HR 1.02, 95% CI 1.01—1.03, p<0.001) were independently associated with shorter survival. ≥Grade 3 hepatotoxicity was associated with reduced survival (p<0.001). OR was associated with longer OS of 17 months, compared with 10 months (p=0.027).
Conclusion:
In TARE for breast cancer liver metastasis, higher tumoral radiation dose (>79.5 Gy) was associated with OR, which was associated with longer survival. Pre-existing liver dysfunction was associated with hepatotoxicity, which was associated with decreased survival.
Keywords: Radioembolization, breast cancer, dosimetry, hepatotoxicity
Introduction
Yttrium-90 (Y90) transarterial radioembolization (TARE) is an emerging locoregional therapy for liver-dominant metastatic breast cancer [1]. Breast cancer patients with objective response (OR) after TARE live longer than those without OR [2]. Though most patients achieve OR [3], TARE causes up to 10% rate of ≥grade 3 adverse events [4–6]. Reliable predictors of OR are unknown, limiting patient selection and potentially putting patients at unnecessary risk of toxicities.
The impact of radiation dose to tumor on response and survival after TARE has been studied in hepatocellular carcinoma (HCC) and colorectal cancer. In these diseases, tumoral doses exceeding approximately 100 Gy are associated with OR and doses exceeding approximately 200 Gy are considered tumoricidal. In colorectal cancer, >40—60 Gy tumoral dose was associated with >50% reduction in metabolic activity [7]. In one colorectal cancer patient, dose to different portions of tumor was evaluated, suggesting a 100-Gy threshold to prevent future progression [8]. In infiltrative HCC, tumor dose >100 Gy predicted longer survival [9], and >110 Gy has been associated with 75% OR rate by enhancement-based imaging criteria [10]. A high percentage of pre-transplant patients achieved complete pathologic necrosis when >190 Gy was administered to tumor [11]. This “tumoricidal” target was validated prospectively using mRECIST criteria, identifying a 200-Gy threshold [12], and by a retrospective study showing >205-Gy was associated with significantly longer progression-free survival [13].
Whereas higher doses to tumor are associated with better responses, increased dose to normal liver parenchyma is associated with hepatotoxicity. There is conflicting data in studies of HCC regarding dose thresholds for radiotoxicity: one study reported a 100-Gy threshold in Okuda stage I/II patients [14]; another report suggested a threshold of 75 Gy in Child-Pugh A patients [15]; whereas, another study of Child-Pugh A and B patients suggested a 54-Gy threshold [16]. In another study, a dose to normal liver parenchyma >52 Gy was associated with a 50% probability of ≥grade 2 hepatotoxicity [10]. One TARE guideline statement for both HCC and metastatic tumors suggested an overall dose threshold goal of >120 Gy to tumor, and a limit of <50—70 Gy to normal parenchyma [17].
The relationship of radiation dose to post-TARE outcomes has not been addressed in patients with breast cancer. The hypothesis was that radiation dose to tumor is associated with imaging response, whereas dose to nontumoral liver is associated with hepatotoxicity. Specifically, higher tumoral radiation dose was hypothesized to correlate with objective metabolic imaging response, and higher dose to nontumoral liver was hypothesized to correlate with grade 3 and higher elevation of liver function tests, ascites requiring drainage or aspiration, and potentially radiation-induced liver disease. This study’s purpose was to determine the relationship of radiation dose to imaging response, survival, and toxicity after TARE of breast cancer liver metastasis.
Materials and Methods
Study design and patient population
This retrospective single-center study reviewed all breast cancer patients who underwent TARE of liver metastases (2/2011—6/2019). This research was partly funded through the NIH/NCI Cancer Center Support Grant P30 CA008748, and the Breast Cancer Research Foundation. Informed consent was waived for this Health Insurance Portability and Accountability Act-compliant, institutional review board-approved study. Indications for TARE were intolerance of systemic therapy or liver-predominant disease progression on systemic treatment with ineligibility for resection or ablation. Additionally, extrahepatic metastasis must have been stable or decreasing in size or metabolic activity. The number of systemic therapy lines received and anticoagulant/antiplatelet treatment within 6 months of TARE were abstracted from medical charts. Images were reviewed for hepatic burden (bilobar or unilobar) and number of extrahepatic organs involved by metastasis.
Radioembolization technique
TARE was performed [3] using glass (TheraSpheres, MDS Nordion, Ottawa, Ontario, Canada) or resin microspheres (SirSpheres, Sirtex SIR-Spheres Pty Ltd., Lane Cove, Australia) based on operator preference. Glass dosimetry targeted a 120-Gy dose using the medical internal radiation dosimetry model; resin dosimetry applied calculations based on body surface area and tumor volume. Systemic therapy was held at least ±1 week of TARE.
Absorbed dose estimation
A bremsstrahlung SPECT/CT-based semi-quantitative method estimated absorbed dose in tumoral and nontumoral parenchyma [9] (Figure 1). SPECT/CT performed within 6 hours of TARE was loaded into Hybrid Recon v1.1.2 (Hermes Medical Solutions, Stockholm, Sweden). The liver and tumors were manually segmented on SPECT/CT referencing pre-procedural by a radiologist blind to patient outcomes.
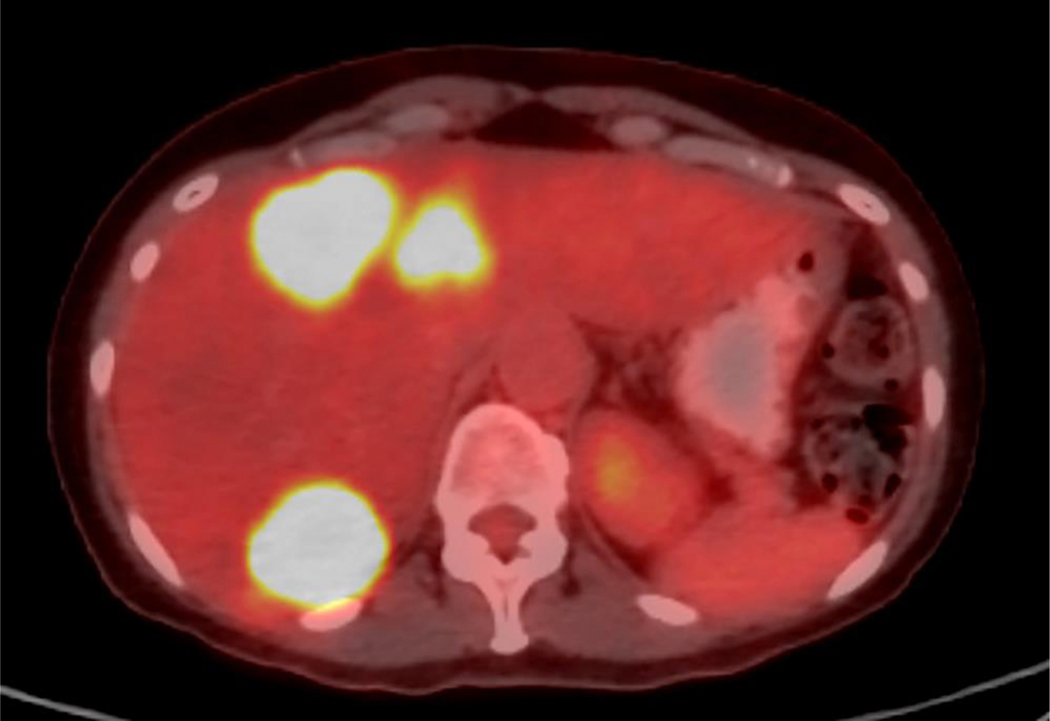
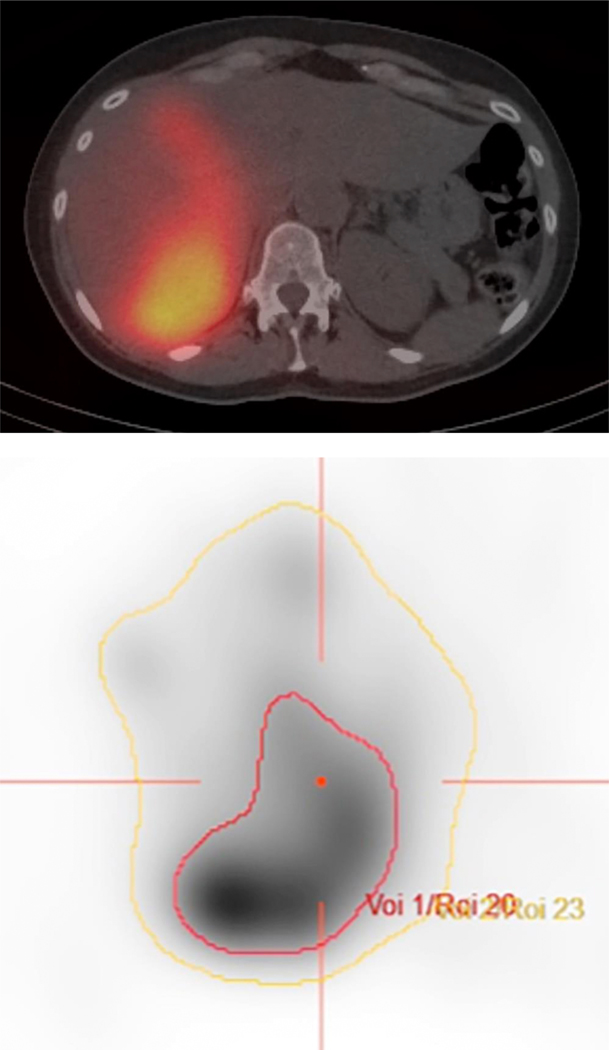
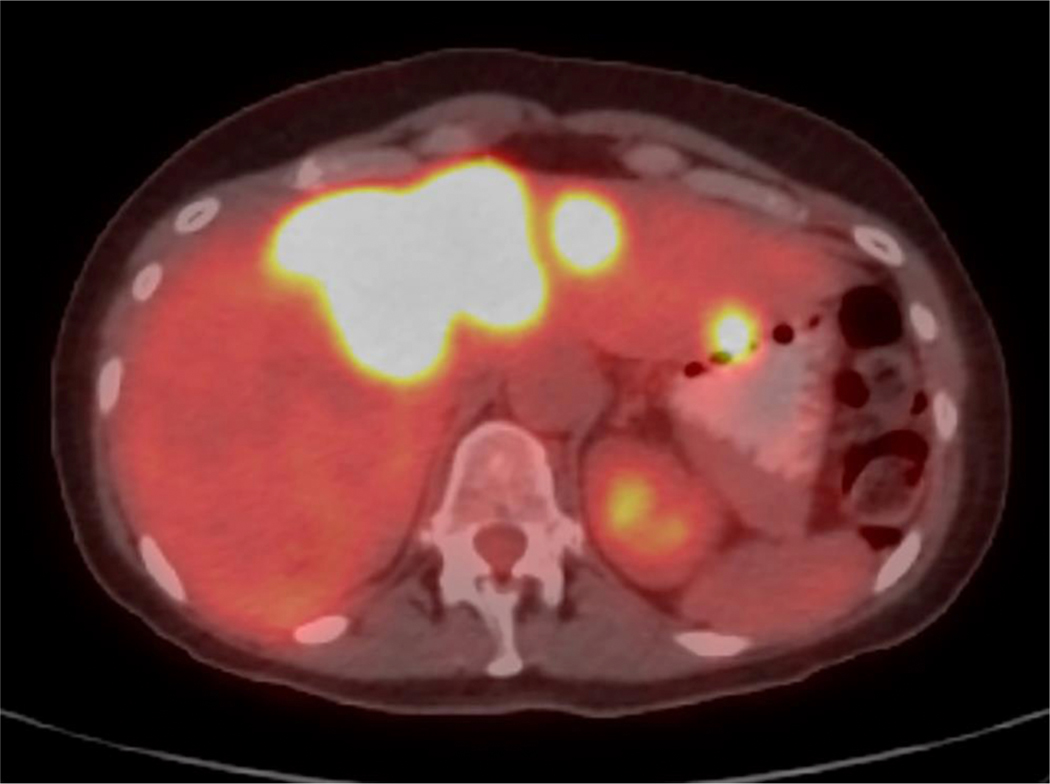
Figure 1. Case example.
A. Axial PET/CT image demonstrates bilobar FDG-avid hepatic metastases in a 57-year-old woman with liver-dominant metastatic breast cancer. The patient was treated with radioembolization, starting with the right lobe. B. Immediate post-procedure SPECT/CT demonstrates preferential tumoral uptake of yttrium-90. C. PET/CT images were loaded into Hermes software, and the liver (yellow line) and tumors (red line) were contoured. In this case, an estimated 212 Gy was delivered to tumor, whereas 72 Gy was delivered to nontumoral parenchyma. D. Follow-up PET/CTdemonstrates complete response in right hepatic tumors, with interval progression of the untreated left lobe tumors.
The total number of counts was computed in the whole liver and segmented tumor volumes. Whole liver total count was equated to total activity delivered to the liver, considering the lung shunt fraction (LSF). Activity for tumor volume was calculated [9]:
The activity for nontumoral liver parenchyma was estimated:
Because of the average range of β-particles (main contribution to absorbed dose) and low SPECT/CT resolution, β-particle energy emitted within a voxel range is considered absorbed within the same voxel range [18]. Absorbed dose in each volume was computed by multiplying the activity concentration by a constant, 50 Gy•kg/GBq [19]. A liver tissue density constant of 1.03 g/cm3 was applied to convert volume to mass:
and
Assessment of response and survival
Patients were followed with laboratory assessment at 2 and 4—6 weeks, and then as needed. Imaging was performed at approximately 3-month intervals. PERCIST criteria [20] were modified to score response in targeted hepatic regions by two radiologists blind to dosimetry characteristics of the treatment. OR was defined as complete or partial response, quantified as at least 30% reduction in metabolic activity, comparing the first post-TARE PET/CT to the most recent pre-TARE PET/CT.
Toxicity assessment
Total bilirubin, AST, ALT, and platelet count were recorded from within 1 week of TARE until death or loss of follow up. Chart review determined whether ascites developed requiring aspiration or drainage after TARE. ≥Grade 3 hepatotoxicity was defined as AST, ALT, or total bilirubin elevation by ≥3x baseline, platelet count <50×109/L, or ascites requiring intervention, within 6 months of TARE [21]. Five patients developed ≥grade 3 hepatotoxicity due to hepatic or peritoneal progression and were not attributed to TARE.
Statistical analysis
Primary endpoints were imaging response and measures of hepatotoxicity. Secondary endpoints included overall survival (OS). Radiation dose and other clinical factors were summarized descriptively and evaluated for association with response and hepatotoxicity using Fisher’s exact or Wilcoxon rank sum tests. Means were presented as mean±standard deviation. Due to small samples, multivariable analysis was not performed for response or hepatotoxicity. OS was calculated from the date of TARE until the date of death or last follow-up visit, and estimated using Kaplan-Meier methods. Radiation dose and other clinical factors were evaluated for association with OS using Cox proportional hazards regression. Multivariable analysis was performed on factors with p<0.05 in univariable analysis.
A landmark analysis (landmark time of 2 months after TARE) estimated the relationship between OR and OS, because OR was not determined until the first follow-up scan. Analysis excluded patients without imaging response assessment (n=4) or who were not followed for at least 2 months (n=2). Hepatotoxicity was evaluated as a baseline variable since median interval to hepatotoxicity was 5 days (IQR 0—10).
Receiver operating characteristic (ROC) curve analysis [22] assessed whether radiation dose differentiated patients with and without OR. The area under the curve (AUC) was computed using the trapezoidal rule and used as a summary measure of discrimination. Optimal radiation dose threshold was identified using Youden’s index, maximizing the sum of sensitivity and specificity [23]. Analysis was by statisticians (MH, CM) using R, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A p value <0.05 was considered significant.
Results
Sixty-four women with metastatic breast cancer, of mean age 51±11 years (range: 29—76), were treated with TARE (Table 1). Of the 64 patients, 73% were estrogen receptor-positive, 56% were progesterone receptor-positive, and 17% were HER2-positive. Forty-eight/64 (75%) had bilobar disease (Table 1). Ten (16%) patients had only liver metastases, 16 (25%) had 1 other site of metastatic organ involvement, 17 (27%) had 2 other sites, and 21 (32%) had more than 2 other sites. Nineteen/64 (30%) patients were on anticoagulation or antiplatelet therapy within 6 months of TARE.
Table 1. Patient and treatment characteristics.
Chart and imaging review revealed patient and treatment characteristics, summarized descriptively. Statistics are presented as median [minimum—maximum]; n (%).
| Characteristic | n=64 |
|---|---|
| Dose to tumor (Gy) | 136 [19—445] |
| Unknown | 6 |
| Dose to normal parenchyma (Gy) | 68 [12—244] |
| Unknown | 6 |
| Number of extrahepatic metastatic sites | |
| 0 | 10 (16%) |
| 1 | 16 ( 25%) |
| 2 | 17 (2 '%) |
| 3 | 15 (23%) |
| 4 | 5(7.8%) |
| 5 | 1 (1.6%) |
| TARE device | |
| Glass microspheres | 42 (66%) |
| Resin microspheres | 22 (34%) |
| Extent of disease | |
| Unilobar | 16 (25%) |
| Bilobar | 48 (75%) |
| Lung shunt fraction (%) | 3 [0–10] |
| Number of systemic therapy lines | 8 [1–19] |
| Hormone receptor st ‘us | |
| ER+ | 47 (73%) |
| PR+ | 36 (56%) |
| HEr 2 + | 11 (17%) |
| Pre-TARE laboratory values | |
| Total bilirubin (mg/dL) | 0.55 [0.20—2.50] |
| AST (units/L) | 35 [12—373] |
| ALT (units/L) | 34 [9—193] |
| Platelets (x10^9/L) | 201 [54—590] |
| Percent of normal parenchyma treated | 50 [11—83] |
| Unknown | 6 |
| Anticoagulation | 19 (30%) |
Glass microspheres were used in 42 patients (66%) and resin microspheres in 22 patients (34%). The average activity administered was 31.58±12.21 mCi for resin and 89.31±46.5 mCi for glass treatments. Of 22 resin microsphere treatments, 6 (27%) did not receive the full prescribed dose due to early stasis. Mean LSF was 4±2% (range 0—10%). Overall median dose to tumor was 136 Gy, and median dose to normal liver parenchyma was 68 Gy.
Sixty/64 (94%) patients had response assessment by modified PERCIST. PET/CT was performed on average 33±28 days before and 57±54 days after TARE. Among the 60 patients with imaging response assessment, 46 (77%, 95% CI 64—86%) achieved OR, demonstrating a 30% or greater reduction in metabolic activity (Table 2). Among the patients with response assessment, 42/46 who had OR and 12/14 without OR had post-TARE SPECT/CT allowing for estimation of tumoral and nontumoral radiation dose. Patients with OR had higher median dose delivered to tumor (167 Gy) compared to patients without OR (54 Gy) (p<0.001). The patients with OR received higher dose delivered to nontumoral liver parenchyma (85 Gy) compared to patients without OR (32 Gy) (p=0.002). Treatment with glass microspheres was associated with significantly higher OR (92%) compared to resin microspheres (45%) (p<0.001). Patients with fewer extrahepatic metastatic organs involved were more likely to have OR than those with more sites (p=0.014). Other factors, such as hormone receptor status, anticoagulant therapy, pre-TARE liver function tests, number of prior systemic therapies, and extent of hepatic metastasis, did not significantly impact imaging response.
Table 2. Association of radiation dose and clinical factors with objective response (OR).
Sixty patients had follow-up PET/CT imaging available to determine response, with 46 patients achieving OR. Wilcoxon rank-sum tests and Fisher’s exact tests were performed, with median (IQR); n (%) presented.
| Characteristic | No objective response (n=14) | Objective response (n=46) | p-value |
|---|---|---|---|
| Dose to tumor (Gy) | 54.0 (45.2—61.5) | 167.0 (97.0—216.8) | <0.001 |
| Unknown | 2 | 4 | |
| Dose to normal parenchyma (Gy) | 31.5 (27.5—52.8) | 85.0 (46.2—107.8) | 0.002 |
| Unknown | 2 | 4 | |
| Number of extrahepatic metastatic sites | 2.5 (2.0—3.0) | 1.5 (1.0—2.8) | 0.014 |
| TARE device | <0.001 | ||
| Glass microspheres | 3 (7.5,) | 37 (92%) | |
| Resin microspheres | 11 (. 5%) | 9 (45%) | |
| Extent of disease | 0.3 | ||
| Unilobar | 2 (12%) | 14 (88%) | |
| Bilobar | 12 (27%) | 32 (73%) | |
| Number of systemic therapy lines | 8.0 (6.2—12.5) | 8.5 (5.0, 12.0) | 0.5 |
| Hormone receptor status | |||
| Estrogen receptor | 0.5 | ||
| ER-negative | 2 (13%) | 13 (87%) | |
| ER-positive | 12 (27%) | 33 (73%) | |
| Progesterone receptor | >0.9 | ||
| PR-negative | 6 (24%) | 19 (76%) | |
| PR-positive | 8 (23%) | 27 (77%) | |
| HER2 receptor | 0.4 | ||
| HER2-negative | 13 (26%) | 37 (74%) | |
| HER2-positive | 1 (10%) | 9 (90%) | |
| Pre-TARE laboratory values | |||
| Total bilirubin (mg/dL) | 0.6 (0.4—1.0) | 0.5 (0.3—0.9) | 0.2 |
| AST (units/L) | 57.5 (28.0—73.8) | 30.5 (21.5—72.0) | 0.2 |
| ALT (units/L) | 39.0 (23.2—54.0) | 33.5 (18.0—53.8) | 0.5 |
| Platelets (x10^9/L) | 197.0 (156.5—287.8) | 202.5 (156.0—268.5) | 0.9 |
| Percent of normal parenchyma treated | 45 (28—64) | 50 (34—69) | 0.6 |
| Unknown | 2 | 4 | |
| Anticoagulation | 0.7 | ||
| Not anticoagulated | 11 (25%) | 33 (75%) | |
| Anticoagulated | 3 (19%) | 13 (81%) |
The radiation dose AUC was 0.916 (95% CI 0.841—0.991). At a threshold of 79.5 Gy, the sensitivity (proportion of responders who received >79.5 Gy) was 83% and the specificity (proportion of non-responders who received ≤79.5 Gy) was 92%; 97% of patients who received >79.5 Gy to tumor achieved OR, whereas 61% of patients who received <79.5 Gy had stable or progressive disease.
Of the 64 patients, 8 (12.5%) developed ≥grade 3 hepatotoxicity attributable to TARE. Of these, 5 received glass microspheres and 3 received resin microspheres. The 8 patients included 4 patients with ≥grade 3 hyperbilirubinemia, noted on average 23 days after TARE; 1 of these patients also had ≥grade 3 transaminitis. Three patients had ≥grade 3 thrombocytopenia noted on average 42 days after TARE. One patient had ascites requiring aspiration 64 days after TARE. Of the 8 patients with ≥grade 3 hepatotoxicity, 6 patients recovered, on average 98 days following TARE, including 3/4 patients with hyperbilirubinemia and 3/3 patients with thrombocytopenia.
Several factors were assessed to determine which were associated with ≥grade 3 hepatotoxicity (Table 3). Patients who developed ≥grade 3 hepatotoxicity had higher radiation dose to nontumoral liver parenchyma (89 Gy) compared with those that did not (64 Gy), though the difference was not significant (p=0.068). Patients who developed ≥grade 3 hepatotoxicity due to TARE had higher pre-TARE total bilirubin (0.9 mg/dL) compared to those that did not (0.5 mg/dL) (p=0.013). Five/19 (26%) patients on anticoagulation developed ≥grade 3 hepatotoxicity, compared with 3/45 (7%) not on anticoagulation (p=0.044). Extent of disease, number of lines of systemic therapy, hormone receptor status, and number of other sites of metastatic disease were not associated with hepatotoxicity after TARE.
Table 3. Association of radiation dose and clinical factors with ≥grade 3 hepatotoxicity.
Eight patients (12.5%) developed ≥grade 3 hepatotoxicity attributable to TARE. Wilcoxon rank-sum tests and Fisher’s exact tests were performed to compare groups, with median (IQR); n (%) presented.
| Characteristic | No hepatotoxicity (n=56) | Hepatotoxicity (n=8) | p-value |
|---|---|---|---|
| Dose to tumor (Gy) | 140.0 (64.0—200.0) | 97.0 (73.0—218.0) | 0.9 |
| Unknown | 5 | 1 | |
| Dose to normal parenchyma (Gy) | 64.0 (34.5—100.0) | 89.0 (68.5—129.5) | 0.068 |
| Unknown | 5 | 1 | |
| Number of extrahepatic metastatic sites | 2.0 (1.0—3.0) | 2.0 (1.0—3.0) | 0.7 |
| TARE device | >0.9 | ||
| Glass microspheres | 37 (88%) | 5 (12%) | |
| Resin micropheres | 19 (86 %) | 3 (14%) | |
| Extent of disease | 0.2 | ||
| Unilobar | 16 (100%) | 0 (0%) | |
| Bilobar | 40 (83%) | 8 (17%) | |
| Number of systemic therapy lines | 8.0 (5.0—11.2) | 7.5 (5.5—14.2) | >0.9 |
| Hormone receptor status | |||
| Estrogen receptor | 0.4 | ||
| ER-negative | 14 (82%) | 3 (18%) | |
| ER-positive | 42 (89%) | 5 (11%) | |
| Progesterone receptor | 0.3 | ||
| PR-negative | 23 (82%) | 5 (18%) | |
| PR-positive | 33 (92%) | 3 (8.3%) | |
| HER2 receptor | >0.9 | ||
| HER2-negative | 46 (87%) | 7 (13%) | |
| HER2-positive | 10 (91%) | 1 (9.1%) | |
| Pre-TARE laboratory values | |||
| Total bilirubin (mg/dL) | 0.5 (0.4—0.7) | 0.9 (0.9—1.1) | 0.013 |
| AST (units/L) | 33.0 (23.0—66.2) | 75.5 (28.5—118.0) | 0.13 |
| ALT (units/L) | 30.0 (18.8—50.2) | 47.0 (35.0—57.5) | 0.3 |
| Platelets (x10^9/L) | 201.5 (159.8—271.0) | 184.5 (134.5—343.2) | 0.7 |
| Percent of normal parenchyma treated | 49.0 (34.0—67.5) | 59.0 (43.5—69.0) | 0.5 |
| Unknown | 5 | 1 | |
| Anticoagulation | 0.044 | ||
| Not anticoagulated | 42 (93%) | 3 (6.7%) | |
| Anticoagulated | 14 (74%) | 5 (26%) |
Median OS was 10.9 (95% CI 9.5—18.7) months, with a median follow-up of 19.1 (95% CI 0.7—38.6) months. Univariable analysis (Table 4) identified bilobar disease, pre-TARE AST, and pre-TARE ALT as factors associated with reduced survival (p<0.05). In multivariable analysis, bilobar disease (HR: 2.7 [95% CI 1.1—6.9; p=0.028]) and higher pre-TARE AST (HR: 1.02 [95% CI 1.01—1.03; p<0.001]) were independently associated with increased risk of death.
Table 4. Association of radiation dose and clinical factors with overall survival.
Univariable analysis was performed using Cox proportional hazards regression. HR: Hazard Ratio, CI: Confidence Interval.
| Covariate | n | HR | 95% CI | p-value |
|---|---|---|---|---|
| Dose to tumor (Gy) | 58 | 1.00 | 0.99—1.00 | 0.13 |
| Dose to normal parenchyma (Gy) | 58 | 1.00 | 0.99—1.01 | >0.9 |
| Number of extrahepatic metastatic sites | 64 | 1.17 | 0.91—1.51 | 0.2 |
| TARE device | 64 | |||
| Glass microspheres | — | — | ||
| Resin microspheres | 1.42 | 0.78—2.58 | 0.3 | |
| Extent of disease | 64 | |||
| Unilobar | — | — | ||
| Bilobar | 3.35 | 1.41—7.96 | 0.006 | |
| Number of systemic therapy lines | 64 | 1.01 | 0.95—1.08 | 0.7 |
| Hormone receptor status | ||||
| ER-positive | 64 | 0.67 | 0.35—1.30 | 0.2 |
| PR-positive | 64 | 0.96 | 0.53—1.75 | >0.9 |
| HER2-positive | 64 | 0.69 | 0.29—1.66 | 0.4 |
| Pre-TARE laboratory values | ||||
| Total bilirubin (mg/dL) | 64 | 1.17 | 0.57—2.40 | 0.7 |
| AST (units/L) | 64 | 1.02 | 1.01—1.02 | <0.001 |
| ALT (units/L) | 64 | 1.01 | 1.00—1.02 | 0.032 |
| Platelets (x10^9/L) | 64 | 1.00 | 1.00—1.01 | 0.11 |
| Percent of normal parenchyma treated | 58 | 1.00 | 0.99—1.02 | >0.9 |
| Anticoagulation | 64 | 1.18 | 0.64—2.21 | 0.6 |
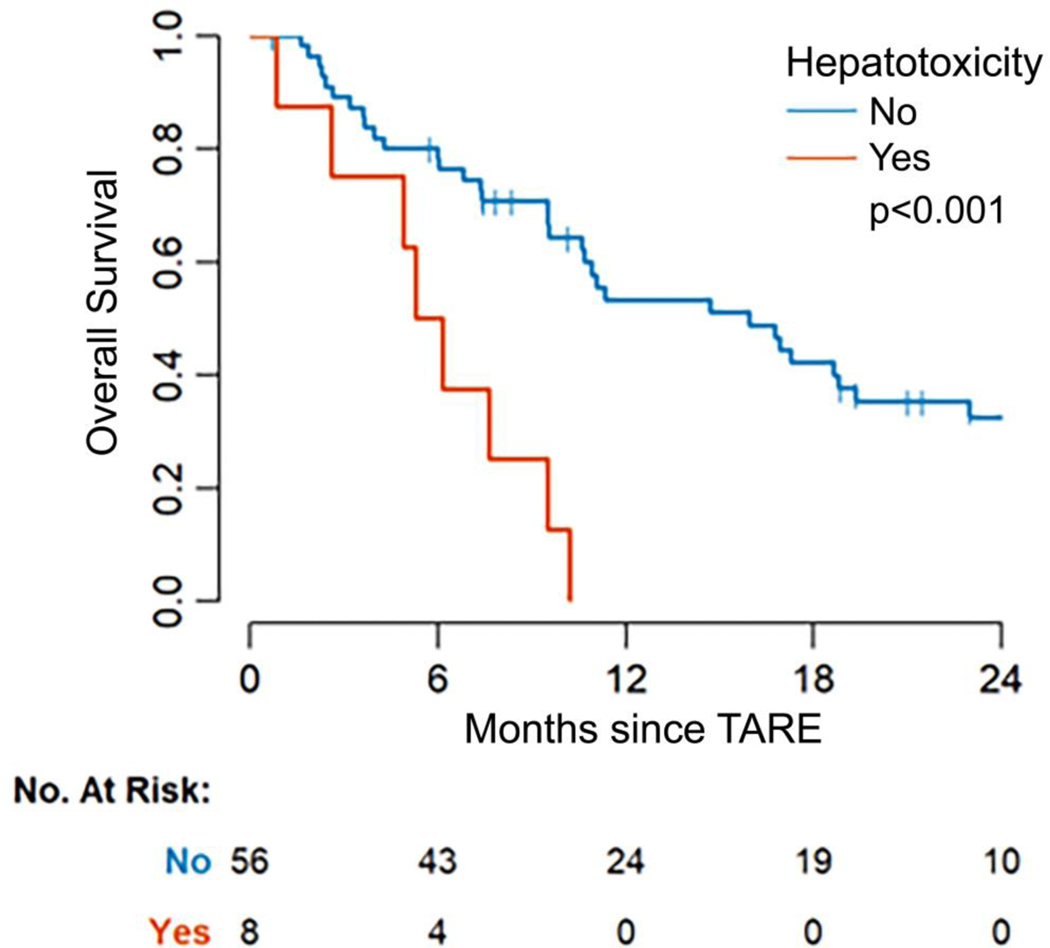
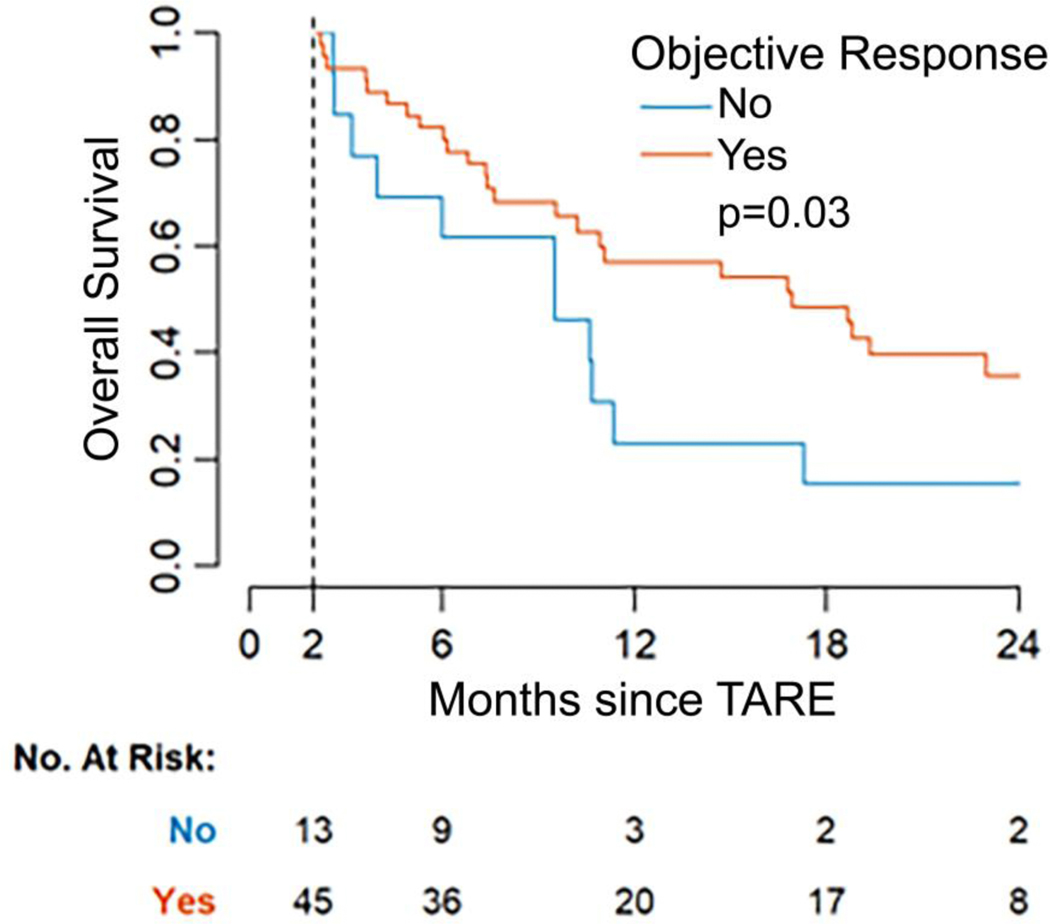
Grade 3 and greater hepatotoxicity was associated with worse OS (HR: 4.26, 95% CI 1.84—9.86; p<0.001). Median OS for patients with ≥grade 3 hepatotoxicity was 6 months (95% CI 5, —), compared to a median of 16 months (95% CI 11—23) for patients without hepatotoxicity (Figure 2). OR was associated with longer OS (HR: 0.48, 95% CI 0.24—0.93, p=0.03). Median OS was 17 months (95% CI 10, —) among responders compared to 10 months (95% CI 4, —) among patients without OR (Figure 3).
Figure 2. Kaplan-Meier curve of overall survival by hepatotoxicity.
Patients with ≥grade 3 hepatotoxicity attributed to transarterial radioembolization (TARE) had a significantly lower median survival of 6 months compared with patients without toxicity who survived a median of 16 months (p<0.001).
Figure 3. Kaplan-Meier curve of overall survival (OS) by objective response (OR).
Patients who achieved OR after transarterial radioembolization (TARE) had a significantly longer median OS of 17 months compared with patients that did not who survived a median of 10 months (p=0.03). Response was landmarked at 2 months after TARE, since response status was not known until the first follow-up PET/CT. The analysis includes patients with imaging response assessment who were followed for at least 2 months (n=58).
Discussion
In this study on TARE performed to treat breast cancer liver metastasis, higher radiation dose to tumor resulted in higher OR rates by metabolic imaging criteria. A correlation with higher tumoral dose and outcomes has been reported in HCC with proposed dose thresholds from 100 to 200 Gy [9–13], and in colorectal cancer with proposed dose thresholds from 40 to 100 Gy [7, 8]. The relationship of dose to tumor and response has not been well studied in breast cancer. The proposed 79.5-Gy threshold here is lower than thresholds suggested for HCC, possibly because of differences in assessing outcomes (anatomical vs. metabolic response vs. survival), estimating dose, tumor biology, or the relatively small sample size in the present study. In this study, dose to tumor and normal parenchyma was estimated semi-quantitatively by analyzing post-TARE bremsstrahlung SPECT/CT [9], which results in poorer spatial resolution than PET/CT [24, 25]. Though most β-particles deposit energy locally, the activity measured from the broad-spectrum bremsstrahlung photons may reduce the accuracy of SPECT.
In this study, OR rates were higher after glass compared with resin microsphere treatments. This might reflect higher radiation doses delivered with glass microspheres, or that 27% of resin treatments in this study reached stasis before delivering the entire prescribed dose, a phenomenon reported previously in breast cancer patients undergoing resin TARE [5]. Other possible factors impacting response were assessed; patients with fewer sites of extrahepatic metastasis were more likely to achieve OR after TARE, possibly reflecting underlying tumor biology [2].
In this study, 12.5% of patients developed ≥grade 3 hepatotoxicity attributable to TARE, manifest as grade 3 or greater elevation in liver function tests or ascites requiring intervention. However, 75% recovered in an average of 3 months. Concordant with prior reports, higher pre-TARE bilirubin levels were associated with post-TARE ≥grade 3 hepatotoxicity [26]. There was no association with the number of lines of systemic therapy. Unlike prior research in colorectal cancer patients undergoing TARE [27], anticoagulant therapy was associated with increased rates of ≥grade 3 hepatotoxicity. This discordance may reflect differences between breast and colorectal cancer or the use of pentoxifylline and ursodeoxycholic acid in addition to low molecular weight heparin [27]; future trials may eliminate the anticoagulant and achieve similar if not better results. In HCC, dose thresholds of 50—100 Gy to normal parenchyma have been proposed as predictors of hepatotoxicity [14–16]. In this study, the association of dose to nontumoral liver parenchyma with ≥grade 3 hepatotoxicity was not significant, so a dose threshold could not be estimated. Patients who developed ≥grade 3 hepatotoxicity received a median dose to normal parenchyma of 89 Gy, whereas those who did not received 64 Gy.
In this study, poorer pre-TARE liver function and increased tumor burden were associated with shorter survival, similar to prior reports [28, 29]. Findings here confirm that breast cancer patients who achieve OR on short interval follow-up PET/CT after TARE have prolonged survival, compared with those that do not [2]. Combined with the observation that anatomic/size-based imaging response criteria do not reflect post-treatment pathological response in hypovascular metastatic tumors [30], these observations highlight that PET/CT response assessments are essential prognostication tools. Metabolic imaging may better represent pathologic response and predict survival. The repeated observation that PET/CT response predicts survival outcomes suggests that strategies aimed at optimizing imaging response after TARE may confer a survival benefit. Maximizing radiation dose to tumor may be one such strategy. Predicting how Y90 microspheres will distribute into tumor vs. normal liver parenchyma is challenging, particularly in hypovascular tumors such as breast cancer. Dose distribution is poorly predicted during mapping, because technetium-99m macroaggregated albumin and Y90 differ significantly in regard to size, with post-mapping SPECT/CT overestimating eventual dose delivered to tumor [31]. Mapping and treating with the same particle, such as holmium, could more accurately predict TARE dosimetry during mapping prior to administration and improve patient selection [32].
Study limitations reflect its retrospective nature and small sample size. Patients’ imaging follow-up was conducted at different times, introducing variability and potential bias into outcome assessment. The use of different types of microspheres is another study limitation, representing a potential confounding variable that should be addressed in larger prospective studies. The SPECT semi-quantitative method is limited, but more accurate quantitative methods (e.g., direct Monte Carlo reconstruction or dose kernel convolution) are complex, requiring more computing power [33, 34]. These preliminary results are hypothesis-generating; prospective studies involving PET/CT may provide clarity on dose thresholds for response and toxicity. Proposed radiation dose thresholds must be validated in future independent data from other institutions.
Conclusions
In summary, higher radiation doses to tumor were associated with increased rates of OR assessed by metabolic imaging response, which in turn were associated with longer OS. Most patients achieve OR when at least 79.5 Gy is administered to tumor. These findings suggest the need for accurate techniques to calculate expected dose to tumor vs. nontumoral liver prior to treatment, to better select patients and potentially avoid toxicities including abnormalities in liver function tests and development of ascites requiring intervention.
Highlights.
Higher radiation dose to breast cancer liver metastases during radioembolization is associated with objective response
A dose threshold to tumor of 79.5 Gy is proposed to achieve objective response
Longer survival is associated with objective response
Shorter survival is associated with worse pre-radioembolization liver function, bilobar disease, and radioembolization-associated liver impairment
Accurate pre-treatment dosimetry may help optimize response to radioembolization of breast cancer liver metastasis
Acknowledgments:
The authors thank Joseph O’Donoghue of Medical Physics for his input regarding the methodology of dosimetry and access to post-processing software.
Funding Sources: This research was funded through the NIH/NCI Cancer Center Support Grant P30 CA008748, and the Breast Cancer Research Foundation.
Footnotes
Credit Author Statement
Fourat Ridouani, MD: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Visualization; Roles/Writing – original draft; Writing – review & editing
Mohamed M. Soliman, MD: Data curation; Formal analysis; Investigation
Ryan England, MD: Conceptualization; Writing – review & editing
Meier Hsu, MS: Data curation; Formal analysis; Visualization; Roles/Writing – original draft; Writing – review & editing
Chaya S. Moskowitz, PhD: Data curation; Formal analysis; Visualization; Roles/Writing – original draft; Writing – review & editing; Supervision
Raphael Doustaly, MS: Conceptualization; Formal analysis; Methodology; Software; Visualization; Writing – review & editing
Constantinos Sofocleous, MD, PhD: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing
F. Edward Boas, MD, PhD: Investigation; Writing – review & editing
Hooman Yarmohammadi, MD: Investigation; Writing – review & editing
Amy Deipolyi, MD, PhD: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing – original draft; Writing – review & editing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mouli SK, Gupta R, Sheth N, Gordon AC, Lewandowski RJ, Locoregional therapies for the treatment of hepatic metastases from breast and gynecologic cancers, Seminars in interventional radiology, Thieme Medical Publishers, 2018, pp. 029–034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Deipolyi AR, Riedl CC, Bromberg J, Chandarlapaty S, Klebanoff CA, Sofocleous CT, Yarmohammadi H, Brody LA, Boas FE, Ziv E, Association of PI3K Pathway Mutations with Early Positron-Emission Tomography/CT Imaging Response after Radioembolization for Breast Cancer Liver Metastases: Results of a Single-Center Retrospective Pilot Study, J Vasc Interv Radiol 29 (9) (2018) 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Deipolyi AR, England RW, Ridouani F, Riedl CC, Kunin HS, Boas FE, Yarmohammadi H, Sofocleous CT, PET/CT imaging characteristics after radioembolization of hepatic metastasis from breast cancer, Cardiovascular and interventional radiology 43 (3) (2020) 488–494. [DOI] [PubMed] [Google Scholar]
- [4].Saxena A, Kapoor J, Meteling B, Morris DL, Bester L, Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients, Ann Surg Oncol 21 (4) (2014) 1296–303. [DOI] [PubMed] [Google Scholar]
- [5].Pieper CC, Meyer C, Wilhelm KE, Block W, Nadal J, Ahmadzadehfar H, Willinek WA, Schild HH, Yttrium-90 Radioembolization of Advanced, Unresectable Breast Cancer Liver Metastases-A Single-Center Experience, J Vasc Interv Radiol 27 (9) (2016) 1305–15. [DOI] [PubMed] [Google Scholar]
- [6].Fendler WP, Lechner H, Todica A, Paprottka KJ, Paprottka PM, Jakobs TF, Michl M, Bartenstein P, Lehner S, Haug AR, Safety, Efficacy, and Prognostic Factors After Radioembolization of Hepatic Metastases from Breast Cancer: A Large Single-Center Experience in 81 Patients, J Nucl Med 57 (4) (2016) 517–23. [DOI] [PubMed] [Google Scholar]
- [7].van den Hoven AF, Rosenbaum CE, Elias SG, de Jong HW, Koopman M, Verkooijen HM, Alavi A, van den Bosch MA, Lam MG, Insights into the dose–response relationship of radioembolization with resin 90Y-microspheres: a prospective cohort study in patients with colorectal cancer liver metastases, Journal of Nuclear Medicine 57 (7) (2016) 1014–1019. [DOI] [PubMed] [Google Scholar]
- [8].D’Arienzo M, Filippi L, Chiaramida P, Chiacchiararelli L, Cianni R, Salvatori R, Scopinaro F, Bagni O, Absorbed dose to lesion and clinical outcome after liver radioembolization with 90 Y microspheres: a case report of PET-based dosimetry, Annals of nuclear medicine 27 (7) (2013) 676–680. [DOI] [PubMed] [Google Scholar]
- [9].Kokabi N, Galt JR, Xing M, Camacho JC, Barron BJ, Schuster DM, Kim HS, A simple method for estimating dose delivered to hepatocellular carcinoma after yttrium-90 glass-based radioembolization therapy: preliminary results of a proof of concept study, Journal of Vascular and Interventional Radiology 25 (2) (2014) 277–287. [DOI] [PubMed] [Google Scholar]
- [10].Strigari L, Sciuto R, Rea S, Carpanese L, Pizzi G, Soriani A, Iaccarino G, Benassi M, Ettorre GM, Maini CL, Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: radiobiologic considerations, Journal of Nuclear Medicine 51 (9) (2010) 1377–1385. [DOI] [PubMed] [Google Scholar]
- [11].Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, Mulcahy M, Baker T, Abecassis M, Sato KT, Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: Multicenter radiology‐pathology correlation and survival of radiation segmentectomy, Hepatology 60 (1) (2014) 192–201. [DOI] [PubMed] [Google Scholar]
- [12].Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, Wilson AE, Lewis DH, Padia SA, Prospective trial using internal pair-production positron emission tomography to establish the yttrium-90 radioembolization dose required for response of hepatocellular carcinoma, International Journal of Radiation Oncology* Biology* Physics 101 (2) (2018) 358–365. [DOI] [PubMed] [Google Scholar]
- [13].Garin E, Lenoir L, Rolland Y, Edeline J, Mesbah H, Laffont S, Porée P, Clément B, Raoul J-L, Boucher E, Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: preliminary results, Journal of Nuclear Medicine 53 (2) (2012) 255–263. [DOI] [PubMed] [Google Scholar]
- [14].Dancey JE, Shepherd FA, Paul K, Sniderman KW, Houle S, Gabrys J, Hendler AL, Goin JE, Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres, Journal of nuclear medicine 41 (10) (2000) 1673–1681. [PubMed] [Google Scholar]
- [15].Chiesa C, Mira M, Maccauro M, Spreafico C, Romito R, Morosi C, Camerini T, Carrara M, Pellizzari S, Negri A, Radioembolization of hepatocarcinoma with 90 Y glass microspheres: development of an individualized treatment planning strategy based on dosimetry and radiobiology, European journal of nuclear medicine and molecular imaging 42 (11) (2015) 1718–1738. [DOI] [PubMed] [Google Scholar]
- [16].Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, Wilson AE, Lewis DH, Padia SA, Hepatotoxic dose thresholds by positron-emission tomography after Yttrium-90 Radioembolization of liver tumors: a prospective single-arm observational study, Cardiovascular and interventional radiology 41 (9) (2018) 1363–1372. [DOI] [PubMed] [Google Scholar]
- [17].Lau W-Y, Kennedy AS, Kim YH, Lai HK, Lee R-C, Leung TW, Liu C-S, Salem R, Sangro B, Shuter B, Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres, International Journal of Radiation Oncology* Biology* Physics 82 (1) (2012) 401–407. [DOI] [PubMed] [Google Scholar]
- [18].O’ Doherty J, A review of 3D image-based dosimetry, technical considerations and emerging perspectives in (90)Y microsphere therapy, J Diagn Imaging Ther 2 (2) (2015) 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pasciak AS, Bourgeois AC, Bradley YC, A Comparison of Techniques for (90)Y PET/CT Image-Based Dosimetry Following Radioembolization with Resin Microspheres, Front Oncol 4 (2014) 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].O JH, Lodge MA, Wahl RL, Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0, Radiology 280 (2) (2016) 576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].U.D.o. Health, H. Services, National Cancer Institute Common Terminology Criteria For Adverse Events (CTCAE), Version 5.0 2017, Online at https://ctep/cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 Google Scholar There is no corresponding record for this reference (2019).
- [22].Schöder H, Noy A, Gönen M, Weng L, Green D, Erdi YE, Larson SM, Yeung HW, Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma, Journal of Clinical Oncology 23 (21) (2005) 4643–4651. [DOI] [PubMed] [Google Scholar]
- [23].Youden WJ, Index for rating diagnostic tests, Cancer 3 (1) (1950) 32–5. [DOI] [PubMed] [Google Scholar]
- [24].Elschot M, Vermolen BJ, Lam MG, de Keizer B, van den Bosch MA, de Jong HW, Quantitative comparison of PET and Bremsstrahlung SPECT for imaging the in vivo yttrium-90 microsphere distribution after liver radioembolization, PloS one 8 (2) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tafti BA, Padia SA, Dosimetry of Y-90 microspheres utilizing Tc-99m SPECT and Y-90 PET, Seminars in nuclear medicine, Elsevier, 2019. [DOI] [PubMed] [Google Scholar]
- [26].Cianni R, Pelle G, Notarianni E, Saltarelli A, Rabuffi P, Bagni O, Filippi L, Cortesi E, Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer, Eur Radiol 23 (1) (2013) 182–9. [DOI] [PubMed] [Google Scholar]
- [27].Seidensticker M, Seidensticker R, Damm R, Mohnike K, Pech M, Sangro B, Hass P, Wust P, Kropf S, Gademann G, Prospective randomized trial of enoxaparin, pentoxifylline and ursodeoxycholic acid for prevention of radiation-induced liver toxicity, PloS one 9 (11) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gordon AC, Gradishar WJ, Kaklamani VG, Thuluvath AJ, Ryu RK, Sato KT, Gates VL, Salem R, Lewandowski RJ, Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy, J Vasc Interv Radiol 25 (10) (2014) 1523–32, 1532 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Paprottka K, Schoeppe F, Ingrisch M, Rübenthaler J, Sommer N, De Toni E, Ilhan H, Zacherl M, Todica A, Paprottka P, Pre-therapeutic factors for predicting survival after radioembolization: a single-center experience in 389 patients, European journal of nuclear medicine and molecular imaging 44 (7) (2017) 1185–1193. [DOI] [PubMed] [Google Scholar]
- [30].Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers, Nature medicine 26 (4) (2020) 566–576. [DOI] [PubMed] [Google Scholar]
- [31].Song YS, Paeng JC, Kim H-C, Chung JW, Cheon GJ, Chung J-K, Lee DS, Kang KW, PET/CT-based dosimetry in 90Y-microsphere selective internal radiation therapy: single cohort comparison with pretreatment planning on 99mTc-MAA imaging and correlation with treatment efficacy, Medicine 94 (23) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Smits ML, Nijsen JF, van den Bosch MA, Lam MG, Vente MA, Mali WP, van het Schip AD, Zonnenberg BA, Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study, The lancet oncology 13 (10) (2012) 1025–1034. [DOI] [PubMed] [Google Scholar]
- [33].Dewaraja YK, Chun SY, Srinivasa RN, Kaza RK, Cuneo KC, Majdalany BS, Novelli PM, Ljungberg M, Fessler JA, Improved quantitative (90) Y bremsstrahlung SPECT/CT reconstruction with Monte Carlo scatter modeling, Med Phys 44 (12) (2017) 6364–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elschot M, Lam MG, van den Bosch MA, Viergever MA, de Jong HW, Quantitative Monte Carlo-based 90Y SPECT reconstruction, J Nucl Med 54 (9) (2013) 1557–63. [DOI] [PubMed] [Google Scholar]