Abstract
Background
Acute limb ischaemia usually is caused by a blood clot blocking an artery or a bypass graft. Severe acute ischaemia will lead to irreversible damage to muscles and nerves if blood flow is not restored in a few hours. Once irreversible damage occurs, amputation will be necessary and the condition can be life‐threatening. Infusion of clot‐busting drugs (thrombolysis) is a useful tool in the management of acute limb ischaemia. Fibrinolytic drugs are used to disperse blood clots (thrombi) to clear arterial occlusion and restore blood flow. Thrombolysis is less invasive than surgery. A variety of techniques are used to deliver fibrinolytic agents. This is an update of a review first published in 2004.
Objectives
To compare the effects of infusion techniques during peripheral arterial thrombolysis for treatment of patients with acute limb ischaemia.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registries to 20 October 2020. We undertook reference checking to identify additional studies.
Selection criteria
We included all randomised controlled trials (RCTs) comparing infusion techniques for fibrinolytic agents in the treatment of acute limb ischaemia.
Data collection and analysis
We used standard methodological procedures as recommended by Cochrane. We assessed the risk of bias in included trials using the Cochrane 'Risk of bias' tool. We evaluated certainty of evidence using GRADE. For dichotomous outcomes, we calculated the odds ratio (OR) with the corresponding 95% confidence interval (CI). We were not able to carry out meta‐analyses due to clinical heterogeneity, so we have reported the results and performed the comparisons narratively. The main outcomes of interest were amputation‐free survival or limb salvage, amputation, mortality, vessel patency, duration of thrombolysis, and complications such as cerebrovascular accident and major and minor bleeding.
Main results
Nine studies with a total of 671 participants are included in this update. Trials covered a variety of infusion techniques, dosage regimens, and adjunctive agents. We grouped trials according to types of techniques assessed (e.g. intravenous and intra‐arterial delivery of the agent, 'high‐' and 'low‐dose' regimens of the agent, continuous infusion and 'forced infusion' of the agent, use of adjunctive antiplatelet agents). We assessed the certainty of evidence as very low to low due to the limited power of individual studies to deliver clinically relevant results, small and heterogeneous study populations, use of different inclusion criteria by each study in terms of severity and duration of ischaemia, considerably different outcome measures between trials, and use of different fibrinolytic agents. This heterogeneity prevented pooling of data in meta‐analyses.
No regimen has been shown to confer benefit in terms of amputation‐free survival (at 30 days), amputation, or death. For vessel patency, complete success was more likely with intra‐arterial (IA) than with intravenous (IV) infusion (odds ratio (OR) 13.22, 95% confidence interval (CI) 2.79 to 62.67; 1 study, 40 participants; low‐certainty evidence); radiological failure may be more likely with IV infusion (OR 0.02, 95% CI 0.00 to 0.38; 1 study, 40 participants; low‐certainty evidence). Due to the small numbers involved in each arm and design differences between arms, it is not possible to conclude whether any technique offered any advantage over another. None of the treatment strategies clearly affected complications such as cerebrovascular accident or major bleeding requiring surgery or blood transfusion. Minor bleeding complications were more frequent in systemic (intravenous) therapy compared to intra‐arterial infusion (OR 0.03, 95% CI 0.00 to 0.56; 1 study, 40 participants), and in high‐dose compared to low‐dose therapy (OR 0.11, 95% CI 0.01 to 0.96; 1 study, 63 participants).
Limited evidence from individual trials appears to indicate that high‐dose and forced‐infusion regimens reduce the duration of thrombolysis. In one trial, the median duration of infusion was 4 hours (range 0.25 to 46) for the high‐dose group and 20 hours (range 2 to 46) for the low‐dose group. In a second trial, treatment using pulse spray was continued for a median of 120 minutes (range 40 to 310) compared with low‐dose infusion for a median of 25 hours (range 2 to 60). In a third trial, the median duration of therapy was reduced with pulse spray at 195 minutes (range 90 to 1260 minutes) compared to continuous infusion at 1390 minutes (range 300 to 2400 minutes). However, none of the studies individually showed improvement in limb salvage at 30 days nor benefit for the amputation rate related to the technique of drug delivery. Similarly, no studies reported a clear difference in occurrence of cerebrovascular accident or major bleeding. Although 'high‐dose' and 'forced‐infusion' techniques achieved vessel patency in less time than 'low‐dose' infusion, more minor bleeding complications may be associated (OR 0.11, 95% CI 0.01 to 0.96; 1 study, 72 participants; and OR 0.48, 95% CI 0.17 to 1.32; 1 study, 121 participants, respectively). Use of adjunctive platelet glycoprotein IIb/IIIa antagonists did not improve outcomes, and results were limited by inclusion of participants with non‐limb‐threatening ischaemia.
Authors' conclusions
There is insufficient evidence to show that any thrombolytic regimen provides a benefit over any other in terms of amputation‐free survival, amputation, or 30‐day mortality. The rate of CVA or major bleeding requiring surgery or blood transfusion did not clearly differ between regimens but may occur more frequently in high dose and IV regimens. This evidence was limited and of very low certainty. Minor bleeding may be more common with high‐dose and IV regimens.
In this context, thrombolysis may be an acceptable therapy for patients with marginally threatened limbs (Rutherford grade IIa) compared with surgery. Caution is advised for patients who do not have limb‐threatening ischaemia (Rutherford grade I) because of risks of major haemorrhage, cerebrovascular accident, and death from thrombolysis.
Keywords: Humans; Amputation, Surgical; Arteries; Fibrinolytic Agents; Fibrinolytic Agents/therapeutic use; Ischemia; Ischemia/drug therapy; Thrombolytic Therapy
Plain language summary
Infusion techniques for peripheral arterial thrombolysis
Background
Abrupt reduction in blood flow to a limb (acute limb ischaemia) usually is caused by a blood clot (thrombus) blocking an artery or a bypass graft. Severe acute ischaemia will lead to irreversible damage to muscles and nerves if blood flow is not restored in a few hours. Once irreversible damage occurs, amputation will be necessary and the condition can be life‐threatening. Infusion of clot‐busting drugs (thrombolysis) can restore blood flow by dispersing the clot; this approach is less invasive than open surgery.
Is any infusion technique for delivering thrombolysis better than another?
We wanted to know if any method of delivering clot‐busting drugs offered greater benefit compared to another for important outcomes such as preventing amputation and death, restoring blood flow, and reducing length of time needed to deliver drugs; and if any technique caused greater harm than another (such as stroke or bleeding)?
How did we identify and evaluate the evidence?
First, we searched the medical literature for randomised controlled trials (RCTs) ‐ clinical studies where people are randomly put into one of two or more treatment groups. This type of study provides the most robust evidence about effects of treatment. We then compared trial results and summarised the evidence from all studies. Finally, we assessed how certain the evidence was. To do this, we considered factors such as the way studies were conducted, study size, and consistency of findings across studies. Based on our assessments, we categorised the evidence as very low, low, moderate, or high certainty.
What did we find?
We found nine RCTs with a total of 671 participants with varying severity of ischaemia who were randomised to receive thrombolysis by different infusion techniques. These studies used very different trial designs, which prevented pooling of data. Two studies compared intra‐arterial and intravenous drug delivery using different thrombolytic agents. Six studies compared high‐ and low‐dose regimens, or continuous infusion and forced‐infusion (pulse spray) regimens. Studies provided no definition of what high or low dose was, used different agents with or without initial lacing of the clot with a high dose of the agent (bolus), and delivered agents into the artery or the thrombus. One study compared use of additional antiplatelet agents with thrombolysis.
Limited evidence of very low and low certainty from individual studies may indicate that greater benefit is seen when the thrombolytic agent is delivered into the thrombus: systemic intravenous thrombolysis is less effective than intra‐arterial thrombolysis. 'High‐dose' and 'forced‐infusion' techniques, or use of adjunctive agents such as platelet glycoprotein IIb/IIIa inhibitors, may speed up thrombolysis, but these techniques are generally more labour‐intensive and seem to be associated with increased bleeding complications compared to low‐dose regimens, and there is no evidence that they lead to improved outcomes (e.g. lower amputation rates). 'Low‐dose continuous infusion', following initial lacing of the thrombus with a high dose of the thrombolytic agent, is the least labour‐intensive technique. Thrombolysis appears to be an acceptable therapy for patients with marginally threatened limbs (Rutherford grade IIa), but, because of risks of bleeding, stroke, and death, thrombolysis should be used with caution in patients who do not have limb‐threatening ischaemia (Rutherford grade I). Regimens that decrease the time needed to restore blood flow may permit treatment of patients with immediately threatened limbs (Rutherford grade IIb).
More research is needed to confirm these findings.
How up‐to date is this review?
Evidence in this Cochrane Review is current to 20 October 2020.
Summary of findings
Summary of findings 1. Intra‐arterial delivery compared to intravenous delivery for peripheral arterial thrombolysis.
| Is intra‐arterial (IA) delivery or intravenous (IV) delivery of a thrombolytic agent more effective for patients with peripheral arterial thrombosis? | ||||||
| Patient or population: patients with < 30 days' limb ischaemiaa Setting: hospital Intervention: IA delivery of rt‐PA Comparison: IV delivery of rt‐PA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with intravenous | Risk with intra‐arterial | |||||
| 30‐Day amputation‐free survival | Study population | OR 1.71 (0.40 to 7.34) | 40 (1) | ⊕⊝⊝⊝ VERY LOW b,c,d | There was little or no effect on 30‐day amputation‐free survival in the IA group (14/20) compared to the IV group (16/20) | |
| 700 per 1000 | 800 per 1000 (483 to 945) | |||||
|
Amputation (up to 6 months) |
See comment | ‐ | 78 (2) | ⊕⊕⊝⊝ LOW d,e | We were unable to pool the studies due to clinical differences (differences in study population and time point amputation assessed). There was little or no effect on amputation rates in IV and IA groups in either study | |
| 30‐Day mortality | See comment | ‐ | 78 (2) | ⊕⊕⊝⊝ LOW d,e | We were unable to pool the studies due to clinical differences. No deaths occurred in either group in Saroukhani 2015, and there was no clear difference in mortality between IV and IA groups in Berridge 1991 | |
|
Vessel patency ‐ radiological success ‐ complete (to end of treatment) |
Study population | OR 13.22 (2.79 to 62.67) | 40 (1) | ⊕⊕⊝⊝ LOW b,d | We were unable to pool the studies due to clinical differences. Berridge 1991 reported that complete success was more likely with IA than with IV. Saroukhani 2015 reported a higher rate of angiographic improvement in the CDT (IA) group compared to the IV group, but no data were available | |
| 300 per 1000 | 850 per 1000 (545 to 964) | |||||
|
Duration of thrombolysis (to end of treatment) |
See comment | ‐ | 78 (2) | ⊕⊝⊝⊝ VERY LOW d,e,f | We were unable to draw conclusions due to the heterogeneity of methods used. See Table 2 | |
|
CVA (up to 6 months) |
See comment | ‐ | 78 (2) |
⊕⊝⊝⊝ VERY LOW c,d,e | Berridge 1991 reported 1 CVA event in the IV group; no events were reported in the IA group. Saroukhani 2015 reported that no major complications were experienced in IA or IV groups | |
|
Major bleeding (up to 6 months) |
Study population | OR 0.12 (0.01 to 2.53) | 40 (1) | ⊕⊝⊝⊝ VERY LOW b,c,d | Major bleeding may occur more frequently in the IV group (3/20) compared to the IA group (0/20), but the 95% CI indicates there may be no difference | |
| 150 per 1000 | 21 per 1000 (2 to 309) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDT: catheter‐directed thrombolysis; CI: confidence interval; CVA: cerebrovascular accident; IA: intra‐arterial; IV: intravenous; OR: odds ratio; rt‐PA: recombinant tissue plasminogen activator. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStudies differed in the acute thrombotic arterial insufficiency population included.
- Berridge 1991: < 30 days' critical ischaemia of the limb.
- Saroukhani 2015: < 14 days' acute lower limb ischaemia.
bWe downgraded one step for risk of bias concerns (Berridge 1991; no blinding of personnel or outcome assessors). cWe downgraded one step for inconsistency (wide confidence intervals). dWe downgraded one step for imprecision (small numbers of participants with low total event rates). eWe downgraded one step for risk of bias concerns (Berridge 1991; no blinding of personnel or outcome assessors; Saroukhani 2015; other bias concerns). fWe downgraded one step for inconsistency (different methods of thrombolysis).
1. Trials comparing intravenous and intra‐arterial delivery of the agent.
| Berridge 1991 | Saroukhani 2015 | |
| Intervention | IV rt‐PA | IV alteplase (rt‐PA) |
| Comparison | IA rt‐PA | IA (CDT) alteplase |
| 30‐Day amputation‐free survival | IV rt‐PA = 14/20 (70%)a IA rt‐PA = 16/20 (80%)b |
Not reported |
| Amputation | 30 days IV rt‐PA = 0% IA rt‐PA = 1/20 (5%) 3 months IV rt‐PA = 1/20 (5%) IA rt‐PA = 1/20 (5%) |
6 months IV rt‐PA = 6/18 (33%) IA rt‐PA = 4/20 (20%) |
| Mortality | IV rt‐PA = 3/20 (15%) IA rt‐PA = 3/20 (15%) |
No deaths at 6 months |
| Vessel patency | Complete IV rt‐PA = 6/20 (30%) IA rt‐PA = 17/20 (85%) Partial (restoration of flow down to next major distal artery) IV rt‐PA = 3/20 (15%) IA rt‐PA = 3/20 (15%) Failure IV rt‐PA = 11/20 (55%) IA rt‐PA = 0/20 (0%) |
Angiographic improvement reported to be higher in the IA group (P < 0.001) |
| Duration of thrombolysis | IV not reported IA rt‐PA 35 (14 to 64) hours (median and range) |
IV rt‐PA infusion 2 hours IA rt‐PA every 2 hours for 24‐hour period Both regimens repeated day after, if improvement seen |
| CVA | IV rt‐PA = 1/20 (5%) IA rt‐PA = 0/20 (0%) |
Not reported |
| Bleeding (major) | IV rt‐PA = 3/20 (15%) IA rt‐PA = 0/20 (0%) |
IV rt‐PA 0/18 (0%) IA rt‐PA 0/20 (0%) |
| Bleeding (minor) | IV rt‐PA = 9/20 (45%) IA rt‐PA = 0/20 (0%) |
Bleeding not specifically reported IV 0% minor complications IA 4/20 (20%) minor complications |
CDT: catheter‐directed thrombolysis. CVA: cerebrovascular accident. IA: intra‐arterial. IV: intravenous. rt‐PA: recombinant tissue plasminogen activator. a5 of 14 patients had ongoing critical ischaemia. bNo patients had symptomatic ischaemia.
Summary of findings 2. High‐dose compared to low‐dose regimens of thrombolytic agents.
| Is high‐dose or low‐dose delivery of a thrombolytic agent more effective for patients with peripheral arterial thrombosis? | ||||||
| Patient or population: patients with < 42 days' limb ischaemiaa Setting: hospital Intervention: high‐dose thrombolytics Comparison: low‐dose thrombolytics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low‐dose thrombolytics | Risk with high‐dose thrombolytics | |||||
| 30‐Day amputation‐free survival | See comment | ‐ | 302 (4) |
⊕⊝⊝⊝ VERY LOW b,c,d | We were unable to pool data due to heterogeneity between studies. True salvage cannot be calculated given the inclusion of patients with viable limbs and differing severity of disease | |
|
Amputation (follow‐up ranged from 30 days to over 1 year) |
See comment | ‐ | 302 (4) | ⊕⊝⊝⊝ VERY LOW b,c,d | We were unable to pool data due to heterogeneity between studies. Individual studies showed little or no difference in rates of amputation between any of low‐ or high‐dose groups | |
| 30‐Day mortality | See comment | ‐ | 302 (4) | ⊕⊝⊝⊝ VERY LOWb,c,d | We were unable to pool data due to heterogeneity between studies. Individual studies showed little or no difference in mortality between any of low‐ or high‐dose groups | |
|
Vessel patency (at end of treatment) |
See comment | ‐ | 302 (4) | ⊕⊝⊝⊝ VERY LOWb,e | We were unable to pool data due to heterogeneity between studies. Studies used different ways to measure clot lysis. Individual studies showed little or no difference in patency between groups | |
|
Duration of thrombolysis (to end of treatment) |
See comment | ‐ | 302 (4) |
⊕⊝⊝⊝ VERY LOWb,e | We were unable to draw conclusions due to the heterogeneity of methods used. See Table 4 | |
|
CVA (follow‐up ranged from 30 days to over 1 year) |
See comment | ‐ | 302 (4) | ⊕⊝⊝⊝ VERY LOWb,e | We were unable to pool data due to heterogeneity between studies. When reported, little or no effect on CVA events was detected in the individual studies | |
|
Major bleeding (follow‐up ranged from 30 days to over 1 year) |
See comment | ‐ | 302 (4) | ⊕⊝⊝⊝ VERY LOWb,e | We were unable to pool data due to heterogeneity between studies. Individual studies showed little or no difference in major bleeding events between low‐ or high‐dose groups | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVA: cerebrovascular accident. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStudies differed in the acute thrombotic arterial insufficiency population included.
- Braithwaite 1997: < 30 days' acute ischaemia of the lower limb.
- Cragg 1991: acute and chronic ischaemia.
- Plate 2006: < 30 days' acute and sub‐acute lower limb ischaemia.
- Yusuf 1995: < 42 days' lower limb ischaemia.
bWe downgraded one step due to risk of bias concerns (Braithwaite 1997; Cragg 1991; unclear or high risk of selection bias; Braithwaite 1997; Cragg 1991; Plate 2006; Yusuf 1995: no blinding of personnel or outcome assessors). cWe downgraded one step due to inconsistency (clinical heterogeneity between studies prevented meta‐analysis). dWe downgraded one step due to indirectness (studies included participants with varying severity of disease). eWe downgraded two steps due to inconsistency (clinical heterogeneity between studies prevented meta‐analysis; different thrombolysis methods and different ways to measure outcomes).
2. Trials comparing high‐ and low‐dose regimens of the thrombolytic agent.
| Braithwaite 1997 | Cragg 1991 | Plate 2006 | Yusuf 1995 | |
| Intervention | LD infusion (rt‐PA) | LD bolus and LD infusion (urokinase) | CIF (rt‐PA) | CIF (rt‐PA) |
| Comparison | HD bolus and HD infusion (rt‐PA) | HD bolus and HD infusion (urokinase) | HD forced infusion (rt‐PA) | HD forced‐infusion PS (rt‐PA) |
| 30‐Day amputation‐free survival | LD = 37/44 (84%) HD = 39/49 (80%) |
Overall 1 death and 5 amputations, 6/63 (9.5%) | PS = 49/58 (84%) CIF = 54/63 (86%)a |
CIF = 6/9 (67%) PS = 9/9 (100%) |
| Amputation | LD = 2/44 (4.5%) HD = 6/49 (12%) |
5/63 (7.9%) Most were in the HD group |
PS = 4/58 (7%) CIF = 3/63 (5%) |
CIF = 2/9 (22%) PS = 0/9 (0%) |
| Mortality | LD = 5/44 (11%) HD = 4/49 (8%) |
1, but not clear which group | PS = 6/58 (10%) CIF = 7/63 (11%) |
CIF = 1/9 (12%) PS = 0/9 (0%) |
| Vessel patency | Complete lysis LD 21/44 (48%) HD 22/49 (45%) Clinically useful lysis LD 14/44 (32%) HD 10/49 (20%) |
Complete lysis (number and time) LD ‐ NA 12 (71) 26.0 ± 11.2 (24) LD ‐ G 14 (80) 18.2 ± 7.9 (12) HD NA 10 (58) 20.8 ± 13.7 (12) HD ‐ G 15 (83) 16.5 ± 11.9 (12) number (%) mean ± SD (median) hours |
> 75% lysis on completion of thrombolysis (without adjunctive procedure) PS HD 45/58 (78%) CIF LD 41/61 (67%) 30‐Day reocclusion PS HD 8/58 (13%)b CIF LD 9/63 (14%)b 30‐Day incomplete lysis PS 7/58 (12%) CIF 15/63 (24%) 30‐Day reocclusion or "incomplete lysis" requiring either repeat thrombolysis or surgery Reocclusion PS 2/58 (3%) CIF 2/63 (3%) Incomplete lysis PS 2/58 (3%) CIF 8/63 (13%) |
Not reported |
| Duration of thrombolysis | LD 20 (2 to 46) HD 4 (0.25 to 46) Median (range) hours |
LD NA 35.4 ± 14 (24) LD ‐ G 25.3 ± 12.3 (24) HD NA 27.1 ± 15.9 (24) HD ‐ G 22.2 ± 12 (24) mean ± SD (median) hours |
CIF LD 25 (2 to 60) hours PS HD 120 (40 to 310) minutes with additional low dose in 38/58 for 18 (1 to 50) hours median (range) |
CIF 1390 (300 to 2400) minutes PS 195 (90 to 1260) minutes median (range) |
| CVA | LD = 1/44 (2.2%) HD = 0/49 (0%) |
None | PS = 3/58 (5.2%) CIF = 1/63 (1.6%) |
None |
| Bleeding (major) | LD = 3/44 (6.8%) HD = 3/49 (6%) |
HD 2/35 (5.7%) LD 0/37 (0%) |
PS = 4/58 (6.9%) CIF = 8/63 (12.6%) |
CIF = 0/9 (0%) PS = 0/9 (0%) |
| Bleeding (minor) | Not available | HD = 7/35 (20%) LD = 1/37 (2.5%) |
PS = 12/58 (21%) CIF = 7/63 (11%) |
Not available |
CIF: continuous infusion. CVA: cerebrovascular accident. G: graft thrombosis. HD: high dose. LD: low dose. NA: native arterial native occlusion. PS: infusion pulse spray. rt‐PA: recombinant tissue plasminogen activator. SD: standard deviation. a11 (19%) CIF and 7 (11%) PS patients presented with Rutherford grade I ischaemia and hence were not at risk of amputation. bEstimated from Figure 2 in Plate 2006.
Summary of findings 3. Continuous infusion compared to forced (or pulse) infusion of thrombolytic agents.
| Is conventional continuous infusion or forced/pulse infusion delivery of a thrombolytic agent more effective for patients with peripheral arterial thrombosis? | ||||||
| Patient or population: patients with less than 42 days' lower limb ischaemiaa Setting: hospital Intervention: continuous infusion Comparison: forced (or pulse) infusion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with forced infusion | Risk with continuous infusion | |||||
| 30‐Day amputation‐free survival | See comment | ‐ | 304 (4) |
⊕⊝⊝⊝ VERY LOWb,c,d | We were unable to pool data due to heterogeneity between studies. True salvage cannot be calculated given the inclusion of patients with viable limbs and differing severity of disease | |
|
Amputation (follow‐up to 30 days) |
See comment | ‐ | 164 (3) |
⊕⊝⊝⊝ VERY LOWb,c,d | We were unable to pool data due to heterogeneity between studies. Individual studies showed little or no difference in rates of amputation between continuous and forced infusion groups | |
| 30‐Day mortality | See comment | ‐ | 304 (4) |
⊕⊝⊝⊝ VERY LOWb,c,d | We were unable to pool data due to heterogeneity between studies. Individual studies showed little or no difference in mortality between continuous and forced infusion groups | |
|
Vessel patency (up to 30 days) |
See comment | ‐ | 304 (4) |
⊕⊝⊝⊝ VERY LOWb,e | We were unable to pool data due to heterogeneity between studies. Individual studies showed little or no difference in patency between continuous and forced infusion groups | |
|
Duration of thrombolysis (to end of treatment) |
See comment | ‐ | 304 (4) |
⊕⊝⊝⊝ VERY LOWb,e | We were unable to draw conclusions due to the heterogeneity of methods used. See Table 6 and Table 7 | |
|
CVA (follow‐up to 30 days) |
See comment | ‐ | 304 (4) |
⊕⊝⊝⊝ VERY LOWb,e | We were unable to pool data due to heterogeneity between studies. When reported, little or no effect on CVA events was detected in the individual studies | |
|
Major bleeding (follow‐up to 30 days) |
See comment | ‐ | 304 (4) |
⊕⊝⊝⊝ VERY LOWb,e | We were unable to pool data due to heterogeneity between studies. When reported, no clear difference in major bleeding was detected between groups in the individual studies | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVA: cerebrovascular accident. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStudies differed in the acute thrombotic arterial insufficiency population included.
- Braithwaite 1997: < 30 days' acute ischaemia of the lower limb.
- Comerota 2019: < 14 days' limb ischaemia.
- Cragg 1991: acute and chronic ischaemia.
- Kandarpa 1993 < 30 days' limb ischaemia.
- Plate 2006: < 30 days' acute and sub‐acute lower limb ischaemia.
- Yusuf 1995: < 42 days' lower limb ischaemia.
bWe downgraded one step due to risk of bias concerns (Kandarpa 1993; Plate 2006; Yusuf 1995: no blinding of personnel or outcome assessors). cWe downgraded one step due to inconsistency (clinical heterogeneity between studies prevented meta‐analysis). dWe downgraded one step due to indirectness (studies included participants with varying severity of disease). eWe downgraded two steps due to inconsistency (clinical heterogeneity between studies prevented meta‐analysis; different thrombolysis methods and different ways to measure outcomes).
3. Trials comparing continuous infusion with forced infusion of the agent.
| Kandarpa 1993 | Plate 2006 | Yusuf 1995 | |
| Intervention | CIF (urokinase) | CIF (rt‐PA) | CIF (rt‐PA) |
| Comparison | Forced infusion ‐ PS (urokinase) | High‐dose forced infusion ‐ PS (rt‐PA) | High‐dose forced infusion ‐ PS (rt‐PA) |
| 30‐Day amputation‐free survival | CIF = 11/13 (85%) PS = 10/12 (83%) |
PS = 49/58 (84%) CIF = 54/63 (86%)a |
CIF = 6/9 (67%) PS = 9/9 (100%) |
| Amputation | CIF = 2/13 (15%) PS = 0/12 (0%) |
PS = 4/58 (7%) CIF = 3/63 (5%) |
CIF = 2/9 (22%) PS = 0/9 (0%) |
| Mortality | CIF = 0/13 (0%) PS = 2/12 (16.6%) |
PS = 6/58 (10%) CIF = 7/63 (11%) |
CIF = 1/9 (12%) PS = 0/9 (0%) |
| Vessel patency | Patency within 4 hours CIF 9/13 (70%) PS 11/12 (92%) |
> 75% lysis on completion of thrombolysis (without adjunctive procedure) PS HD 45/58 (78%) CIF LD 41/61 (67%) 30‐Day reocclusion or "incomplete lysis" Reocclusion PS 8/58 (13%)b CIF 9/63 (14%)b Incomplete lysis PS 7/58 (12%) CIF 15/63 (24%) 30‐Day reocclusion or "incomplete lysis" requiring either repeat thrombolysis or surgery Reocclusion PS 2/58 (3%) CIF 2/63 (3%) Incomplete lysis PS 2/58 (3%) CIF 8/63 (13%) |
Not reported |
| Duration of thrombolysis | CIF 28 ± 26 hours PS 20 ± 14 hours (mean ± SD) |
CIF 25 (2 to 60) hours PS 120 (40 to 310) minutes with additional LD in 38/58 18 (1 to 50) hours median (range) |
CIF 1390 (300 to 2400) minutes PS 195 (90 to 1260) minutes median (range) |
| CVA | None | PS = 3/58 (5.2%) CIF = 1/63 (1.6%) |
None |
| Bleeding (major) | PS = 3/12 (25%) CIF = 1/13 (7.7%) Required transfusion within 72 hours |
PS = 4/58 (6.9%) CIF = 8/63 (12.6%) |
CIF = 0/9 (0%) PS = 0/9 (0%) |
| Bleeding (minor) | PS = 2/12 (16.6%) CIF = 2/13 (15.3%) |
PS = 12/58 (21%) CIF = 7/63 (11%) |
Not available |
CIF: continuous infusion. CVA: cerebrovascular accident. HD: high dose. LD: low dose. PS: infusion pulse spray. rt‐PA: recombinant tissue plasminogen activator. a11 (19%) CIF and 7 (11%) PS patients presented with Rutherford grade I ischaemia and hence were not at risk of amputation. bEstimated from Figure 2 in Plate 2006.
4. Trials comparing adjunctive antiplatelet agents.
| Duda 2001 | |
| Intervention | urokinase + abciximab |
| Comparison | urokinase alone |
| Amputation‐free survivala | urokinase + abciximab = 48/50 (96%) urokinase alone = 16/20 (80%) |
| Amputation | Not available |
| Mortality | Not available |
| Vessel patency | Initial patency by means of thrombolysis alone urokinase alone: 14/20 (70%) urokinase + abciximab: 33/50 (66%) P = 0.75 Initial patency with thrombolysis and subsequent percutaneous intervention urokinase alone: 20/20 (100%) urokinase + abciximab: 44/50 (88%) P = 0.17 |
| Duration of thrombolysis median (range) |
urokinase + abciximab = 2 (1 to 7.3) hours urokinase alone = 2 (1 to 6) hours |
| CVA | None |
| Bleeding (major) | urokinase + abciximab = 4/50 (8%) urokinase alone = 0/20 (0%) |
| Bleeding (minor) | urokinase + abciximab = 9/50 (18%) urokinase alone = 5/20 (25%) |
aThis is the 90‐day amputation‐free survival rate; 30‐day amputation‐free survival is not given. By 30 days, 4/50 of the abciximab group and 3/20 patients had reached the composite endpoint of surgical revascularisation or limb amputation.
CVA: cerebrovascular accident.
Summary of findings 4. Thrombolysis with or without adjunctive antiplatelet agents.
| Is the use of adjunctive antiplatelet agents with thrombolysis more effective for patients with peripheral arterial thrombosis? | ||||||
| Patient or population: patients with less than 42 days' lower limb ischaemia Setting: hospital Intervention: IA urokinase with adjunctive abciximab Comparison: IA urokinase with placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk without adjunctive antiplatelet agents | Risk with adjunctive antiplatelet agents | |||||
| 30‐Day amputation‐free survival | See comment | ‐ | 70 (1) | ‐ | 30‐Day survival was not reported | |
|
Amputation (follow‐up to over 1 year) |
See comment | ‐ | 70 (1) | ‐ | This could not be calculated from the data provided | |
| 30‐Day mortality | See comment | ‐ | 70 (1) | ‐ | This could not be calculated from the data provided | |
|
Vessel patency (follow‐up over 1 year) |
Study population | OR 1.20 (0.39 to 3.69) | 70 (1) | ⊕⊝⊝⊝ VERY LOWa,b,c | The authors' definition of patency does not clearly distinguish between the presence of thrombus and any underlying stenosis There was little or no difference in patency between treatment groups |
|
| 770 per 1000 | 801 per 1000 (566 to 925) |
|||||
|
Duration of thrombolysis (follow‐up to end of treatment) |
See comment | ‐ | 70 (1) | ⊕⊕⊝⊝ LOWa,b | The median duration of thrombolysis was 120 minutes for each group | |
|
CVA (follow‐up to over 1 year) |
See comment | ‐ | 70 (1) | ⊕⊕⊝⊝ LOWa,b | None was reported | |
|
Major bleeding (follow‐up to over 1 year) |
Study population | OR 0.25 (0.01 to 4.9) | 70 (1) | ⊕⊝⊝⊝ VERY LOWa,b,d | No evidence showed a difference between groups | |
| 80 per 1000 | 21 per 1000 (1 to 299) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVA: cerebrovascular accident; IA: intra‐arterial; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded one step due to risk of bias concerns (Duda 2001; baseline differences between groups). bWe downgraded one step due to imprecision (one small study and low event rate). cWe downgraded one step due to indirectness (definition of outcome does not differentiate between thrombosis and stenosis). dWe downgraded one step due to inconsistency (wide confidence intervals).
Background
Description of the condition
Acute limb ischaemia (ALI) results from an abrupt reduction in blood flow to a limb. It usually is caused by a blood clot (thrombosis) that forms in a diseased artery or a bypass graft (Norgren 2007). A less common cause is embolic occlusion, which is caused by a blood clot that is carried in the circulation until it lodges in a peripheral artery. These thrombi have usually formed in the heart before breaking off into the bloodstream. Clinical manifestations of ALI vary depending on the magnitude of blood flow reduction. For example, occlusion of a small branch vessel may be asymptomatic. A greater degree of ischaemia will result in onset of cramp‐like calf pain during exercise (intermittent claudication). Acute arterial occlusion in a patient with pre‐existing claudication may cause little change in symptoms due to the presence of established collateral circulation. More significant ischaemia leads to pain at rest, and ischaemic damage to nerves and muscles leads to paraesthesia, loss of sensation, and finally paralysis. Severe ischaemia leads to tissue death requiring amputation, if flow is not promptly restored (Norgren 2007).
Description of the intervention
Interventions for ALI are aimed at restoring arterial flow within the limb. A range of treatment options are available, depending on the severity of the ischaemia. Immediate surgery is recommended for immediately threatened limbs (embolectomy, thrombectomy, bypass grafting), and amputation may be required when restoring blood flow to the limbs is not possible. This review focuses on patients whose symptoms require an intervention to relieve severe symptoms or to prevent amputation, when the limb is not immediately threatened. For these patients, revascularisation using peripheral arterial thrombolysis to restore blood flow is the standard treatment (Creager 2012).
How the intervention might work
Peripheral arterial thrombolysis is the process of using fibrinolytic drugs to dissolve occluding thrombi. Thrombolysis has become established as a useful tool in the management of ALI and offers an alternative to open surgery (Darwood 2018). It is particularly useful for those cases of less than two weeks' duration (STILE 1994). Although data from randomised controlled studies are not extensive, much has been learned about indications, risks, and benefits of thrombolysis. Different thrombolytic agents have been used and are the topic of a separate review (Robertson 2013). This review focuses on infusion techniques of drug administration for thrombolysis.
Originally, peripheral arterial thrombolysis was performed via intravenous administration with relatively high doses of the drug used to achieve therapeutic levels at the site of arterial occlusion (blockage). Subsequently, delivery of low doses of the drug directly into the thrombus became more popular (Dotter 1974). This aimed to achieve higher local drug concentrations with a smaller total dose. Success rates appeared much improved and complication rates became more acceptable. In an attempt to achieve faster thrombolysis, high‐dose infusions (McNamara 1984), initial high‐dose bolus (Sullivan 1989), and forced‐injection techniques such as 'pulse spray' were developed (Kandarpa 1988). Forced‐infusion techniques use special catheters with multiple side holes to inject the fibrinolytic agent at high pressure, with the intention that the drug will penetrate deep into the thrombus. Relatively few studies have compared these different techniques, and much debate concerning the optimal method of drug delivery continues (Kessel 2004). Platelets are an important part of the blood clotting cascade, and using adjunctive medication to prevent platelet aggregation and activation may help prevent clot propagation while speeding dissolution (Braithwaite 1995). Percutaneous mechanical devices for aspiration, rheolysis, mechanical fragmentation, and ultrasonography‐assisted fibrinolysis are sometimes used independently or in addition to thrombolysis (Rodgers 2007). It is thought that these shorten the duration of therapy, but data comparing these devices are limited (Araujo 2019).
Why it is important to do this review
Patients with ALI are often elderly and may have significant comorbidities. This review presents available evidence regarding which infusion technique is more effective for initial management of ALI by restoring blood flow rapidly with minimal adverse effects.
Objectives
To compare the effects of infusion techniques during peripheral arterial thrombolysis for treatment of patients with acute limb ischaemia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which participants were randomly allocated to receive peripheral arterial thrombolysis by different techniques. The type of thrombolytic agent used was not of interest because that is the topic of a separate Cochrane Review (Robertson 2013).
Types of participants
We included studies involving patients with limb‐threatening acute limb ischaemia (ALI; Rutherford classification grade IIa or worse) treated with peripheral arterial thrombolysis to restore vessel patency following acute thromboembolic occlusion of a native peripheral artery, or a lower limb arterial bypass graft. We excluded studies including only grade I patients. We included studies involving grade I/IIa/IIb patients, provided most participants were grade II. Patients were included regardless of diabetic status, use of aspirin or anticoagulation post thrombolysis, or use of concurrent heparin.
Only studies that included patients with 'limb‐threatening' ischaemia were considered (i.e. those with Rutherford classification grade IIa or worse). Patients with grade I ischaemia (viable limb) may benefit from therapy to improve the circulation but are unlikely to require amputation if left untreated. In patients with grade I ALI, there is usually no urgency to intervene unless there is a desire to salvage a thrombosed arterial bypass graft. This distinction is important, as many operators consider risks of thrombolysis to outweigh the benefits for patients with intermittent claudication and would defer treatment for a few weeks to allow the thrombus to mature and reduce the risk of embolisation (Braithwaite 1999).
We excluded studies of patients with occluded arteriovenous dialysis fistulae and deep vein thrombosis, as these conditions do not compromise limb blood flow.
Types of interventions
We considered the following regimens with any thrombolytic.
Intravenous infusions.
Intra‐arterial infusions.
Low‐dose infusions.
High‐dose bolus regimens.
Forced‐infusion techniques (when the drug is administered intermittently in high‐pressure pulses intended to force the agent into the thrombus, e.g. pulse spray).
Adjunctive drugs.
Types of outcome measures
Primary outcomes
30‐Day amputation‐free survival or limb salvage (freedom from death or amputation (major or minor) at 30 days)
Amputation
Mortality
Secondary outcomes
Vessel patency
Duration of thrombolysis
Complications (including cerebrovascular accident (CVA), minor and major haemorrhage, distal embolisation)
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Vascular Information Specialist first searched the following databases for relevant trials on 15 October 2015.
Cochrane Vascular Specialised Register.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 10), in the Cochrane Library, via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Information Specialist subsequently conducted further systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year, or publication status restrictions.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS Web, searched on 20 October 2020).
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, Cochrane Register of Studies Online (CRSO 20 October 2020, Issue 9).
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched from 1 January 2017 to 20 October 2020).
Embase Ovid (searched from 1 January 2017 to 20 October 2020).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Ebsco (searched from 1 January 2017 to 20 October 2020).
Allied and Complementary Medicine Database (AMED) Ovid (searched from 1 January 2017 to 20 October 2020).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. When appropriate, these strategies were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6; Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 20 October 2020.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We checked the reference lists of included studies for additional articles.
Data collection and analysis
Selection of studies
For this update, review authors assessed citations retrieved by the search strategies for reports of relevant RCTs. We assessed articles identified by the searches using Covidence software (Covidence). Initial screening was carried out by one review author, or with review author support, and non‐relevant articles were removed (DK, IR, CB, or MS). Potentially relevant, full‐text articles were then assessed independently by two review authors, or with review author support (DK, IR, JP, CB, or MS), according to the Criteria for considering studies for this review. We listed all studies excluded after full‐text assessment and reasons for their exclusion in a Characteristics of excluded studies table. We constructed a PRISMA diagram to illustrate the study selection process. We resolved disagreements by discussion.
Data extraction and management
Data were extracted independently by DK or CB, and were cross‐checked by IR or JP. Any discrepancies were resolved by discussion. The following information was collected.
Participants: age, sex distribution.
Severity of ischaemia: ankle brachial index (ABI), European Consensus definition of critical ischaemia (Consensus Document), Fontaine classification (Fontaine 1954), Ad Hoc Committee Recommendations (Rutherford 1986), Society of Interventional Radiology Standards for Patients With Acute Limb Ischaemia (SIR standards).
Outcome measures: amputation‐free survival or limb salvage, amputation, death, vessel/graft patency, duration of thrombolysis, complications (cerebrovascular accident (CVA), minor and major haemorrhage, distal embolisation).
Assessment of risk of bias in included studies
Two review authors (two of CB, JP, DK) independently assessed methodological quality of included trials using Cochrane's 'Risk of bias' tool and discussed assessment to reach agreement. Assessment of risk of bias in included studies was undertaken in accordance with recommendations described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). These comprise a description and a judgement (low risk, high risk, or unclear risk) for each domain in a risk of bias table. Each entry addresses a specific feature of the study.
Sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective reporting.
Any other bias.
Measures of treatment effect
Measures of effect for dichotomous data were estimated as odds ratios (ORs) and were calculated with 95% confidence intervals (CIs). For continuous data (duration of thrombolysis), we intended to calculate mean differences (MDs) or standardised mean differences (SMDs). Trials used different angiographic endpoints (e.g. estimated clearing of thrombus, restoration of flow, patent to next branch vessel). In addition, frequency of angiographic assessment often differed greatly between control and study groups. This prevented statistical analysis of the outcome 'duration of thrombolysis'.
Unit of analysis issues
In Cragg 1991, 63 patients had 72 episodes of thrombolysis. These represented recurrent thrombosis, and data analysis for this study uses the number of episodes of thrombolysis. In the remaining studies, participants were the unit of analysis. Data from Cragg 1991 were combined only in analyses for complications.
Dealing with missing data
We intended to contact trial authors to request further information when we noted substantial missing data. As no substantial data were missing, we did not contact trial authors to make this request. We did contact these authors to request clarification over unclear and inconsistent reporting (Saroukhani 2015).
Assessment of heterogeneity
We assessed clinical heterogeneity by inspecting individual study characteristics. When possible, we also investigated heterogeneity amongst trials by visually assessing forest plots and CIs and by using the I² statistic (we considered I² ≥ 50% as showing substantial heterogeneity).
Assessment of reporting biases
We intended to use funnel plots for publication bias if more than 10 trials were included in a meta‐analysis (Higgins 2011); however, as insufficient trials were available, we could not do this. We carefully assessed all individual studies to look for selective reporting bias.
Data synthesis
We had planned to carry out meta‐analysis using a fixed‐effect model when heterogeneity was low (determined by I² statistic < 50%), or a random‐effects model when substantial heterogeneity was present (I² statistic ≥ 50%). We reported data using Review Manager 5 (Review Manager 2020). When data could not be pooled due to heterogeneity between trials, we reported and discussed results narratively.
Subgroup analysis and investigation of heterogeneity
For most comparisons, subgroup analysis was not possible because data were insufficient. Such an analysis will be carried out for future versions of this review if data are available. We were able to carry out subgroup analysis on Comerota 2019. We investigated effects on outcomes due to use of a balloon occlusion catheter and duration of the thrombolysis procedure.
Sensitivity analysis
We did not carry out sensitivity analysis, as we identified insufficient trials.
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables to present the findings of this review for the comparisons 'Intra‐arterial delivery compared to intravenous delivery for peripheral arterial thrombolysis' (Table 1); 'High‐dose compared to low‐dose regimens of thrombolytic agents' (Table 3); 'Continuous infusion compared to forced (or pulse) infusion of thrombolytic agents' (Table 5); and 'Thrombolysis with or without adjunctive antiplatelet agents' (Table 8). We selected the most important and most clinically relevant outcomes (both desirable and undesirable) thought to be essential for decision‐making for each 'Summary of findings' table. These include the following.
30‐Day amputation‐free survival or limb salvage.
Amputation.
30‐Day mortality.
Vessel patency.
Duration of thrombolysis.
CVA.
Major bleeding.
We used the system developed by the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) for grading the certainty of evidence as high, moderate, low, and very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of population bias (GRADE 2004).
Results
Description of studies
Results of the search
See Figure 1.
1.

Study flow diagram.
Several searches were carried out since the previous version of this review was published (Kessel 2004). For searches to 2015, results were pre‐screened by the editorial base. We used Cochrane's Screen4Me workflow to help identify potential reports of RCTs in 2019. Results of the Screen4Me assessment process are shown in Figure 2. We then assessed the remaining 2452 'possible' records identified by Screen4Me using Covidence (covidence.org). We also used Covidence to screen search results from additional top‐up searches.
2.
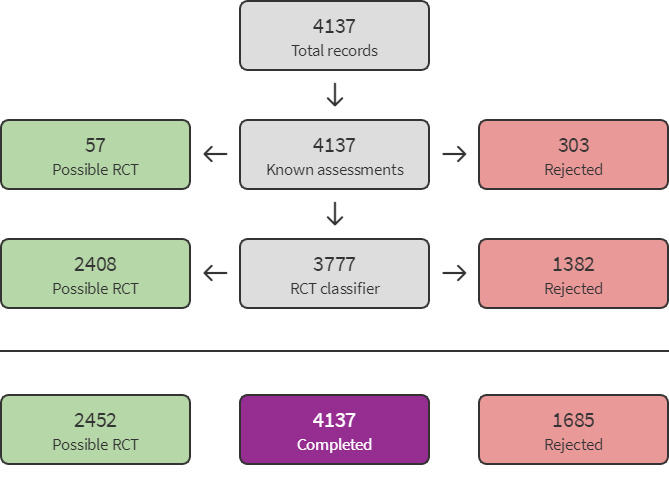
Screen4Me flow diagram.
Included studies
See the Characteristics of included studies table for further details.
We included three additional trials in this update (Comerota 2019; Plate 2006; Saroukhani 2015), making a total of nine RCTs with 671 participants that fulfilled the inclusion criteria for this review (Berridge 1991; Braithwaite 1997; Comerota 2019; Cragg 1991; Duda 2001; Kandarpa 1993; Plate 2006; Saroukhani 2015; Yusuf 1995). These trials covered a variety of thrombolytics, infusion techniques, dosage regimens, and adjunctive agents. Kandarpa 1993 and Yusuf 1995 compared the same techniques but used different thrombolytic agents.
Most of these trials were small and individually had limited power, with the smallest trial involving 25 participants (Kandarpa 1993), and the largest 174 (Comerota 2019). Plate 2006 intended to recruit 590 patients but was stopped prematurely due to low recruitment rates, with only 121 patients, and as a result was underpowered. Trials were carried out internationally, with participants recruited in Belgium, Bulgaria, Czech Republic, Germany, India, Iran, Peru, Poland, Romania, Serbia, Slovakia, Sweden, the UK, Ukraine, and the USA. All studies, except Yusuf 1995, reported numbers of males and females, with a total of 383 men and 164 women included. The mean age of participants ranged from 54 years in Saroukhani 2015 to 72 years in Plate 2006. Comerota 2019 provided mean ages for subgroups, from which we calculated the overall mean age to be 64 years.
There were considerable differences in the clinical status of patient groups and in lesion characteristics in the included studies.
The term 'acute limb ischaemia (ALI)' is ill defined, encompassing both speed of onset and duration of symptoms. Both are likely to have an impact on prognosis. For example, consider a patient with pre‐existing intermittent claudication (IC) on the basis of peripheral arterial disease (PAD) compared with a patient with normal arteries who experiences an embolus. The former may experience a sudden decrease in walking distance if the superficial femoral artery occludes due to in situ thrombosis, but the limb is likely to be viable. Conversely, if the latter experiences an embolus to the superficial femoral artery, the abrupt reduction in blood flow will not be compensated and the patient will require prompt revascularisation to avoid irreversible ischaemia requiring amputation. Thus patients with acute onset of ischaemia due to occlusion of the superficial femoral artery can have vastly different prognoses.
We have noted considerable heterogeneity among participant groups assessed within the reported trials in terms of severity and duration of ischaemia. Most of these studies included some participants with intemittent claudication (grade I ischaemia) (Rutherford 1986); only Saroukhani 2015 clearly included only participants with Rutherford grade IIa and IIb. Patients with grade I ischaemia may require treatment for symptomatic relief, but they will not have limb‐threatening ischaemia (i.e. they are not at risk of amputation). The greater the proportion of participants with intermittent claudication, the greater the risk of bias if amputation rates and amputation‐free survival are used as outcome measures. The same is true when outcomes for participants with chronic limb ischaemia (arbitrarily defined as > 14 days' duration) are considered; these patients have survived the immediate threat of the "acute ischaemic insult". They are effectively "stable", although those with critical ischaemia (rest pain and tissue loss) have high risks of amputation and death within one year. Both Comerota 2019 and Saroukhani 2015 used 14 days of symptoms as an inclusion criterion, with most included studies stating up to 30 days were required for inclusion (Berridge 1991; Braithwaite 1997; Kandarpa 1993; Plate 2006). Cragg 1991 included participants with acute and chronic ischaemia, Plate 2006 included participants with acute and sub‐acute lower limb ischaemia, and Duda 2001 and Yusuf 1995 included participants with ischaemia of duration up to 42 days.
Differences in prognosis are associated with native arterial and bypass graft occlusion, with the latter usually more responsive to thrombolysis.
Kandarpa 1993 included mainly patients with graft occlusion (21/25, 84%), but in Yusuf 1995, patients mainly had native arterial occlusion (14/18, 78%) and the Berridge 1991 cohort comprised only those with native arteries. Berridge 1991 included only patients with critical limb ischaemia, with average occlusion length of 15 cm; in Braithwaite 1997, 20% (19/93) of patients had IC; and the other studies included some patients with non‐critical ischaemia. In the PROMPT study (Duda 2001), 40% of the treatment group were claudicants compared to only 15% in the control group. Saroukhani 2015 reported the cause of ALI to be embolism from another source in four participants and thrombosis in the remaining ones. Plate 2006 included occlusions of embolic origin and accepted in situ thromboses of native vessels and vascular grafts. Thirty‐five patients had occluded grafts (30 with synthetic and five with autogenous graft material) in an aorto‐iliac (8), femoropopliteal (22), or tibial (5) position (Plate 2006). Comerota 2019 accepted thrombosed bypass grafts or native arteries, provided occlusion occurred more than one month after synthetic graft or six months after autologous graft placement.
Different doses of different thrombolytic agents, various outcome measures, and different definitions of success were used in the different studies. Five studies used recombinant tissue plasminogen activator (rt‐PA) (Berridge 1991; Braithwaite 1997; Plate 2006; Saroukhani 2015; Yusuf 1995), and three studies used urokinase (UK) (Cragg 1991; Duda 2001; Kandarpa 1993). One study used plasmin (Comerota 2019).
Inclusion and exclusion criteria were described in all trials, but it was not always possible to discriminate between patients with differing severity of ischaemia.
Included studies used different outcome measures. Berridge 1991; Braithwaite 1997; Comerota 2019; Cragg 1991; Kandarpa 1993; Saroukhani 2015; and Yusuf 1995 had clearly defined outcome measures. Duda 2001 used a composite endpoint of avoidance of surgical revascularisation or amputation and a secondary endpoint of rate of thrombolysis per centimetre of thrombus. Plate 2006 allocated a score of zero to five based on clinical outcome, from no additional treatment (0) to death (5).
Most studies had a follow‐up period of 30 days (Braithwaite 1997; Cragg 1991; Comerota 2019; Kandarpa 1993; Plate 2006; Yusuf 1995); Berridge 1991 reported follow‐up to three months, Saroukhani 2015 six months, and Duda 2001 one year.
Trials comparing intra‐arterial and intravenous delivery of the agent
Berridge 1991 compared intravenous (IV) rt‐PA (variable dose 1 to 10 mg/h) with intra‐arterial (IA) rt‐PA (0.5 mg/h) and IA streptokinase (5000 U/h) in 60 patients with critical ischaemia of the limb of less than 30 days' duration. Only the comparison of IV and IA rt‐PA is considered in this review.
Saroukhani 2015 compared IV alteplase infusion (0.6 mg/kg, 20% as initial bolus) over 2 hours with IA alteplase (5‐mg bolus, then 0.05 mg/kg/h every 2 hours) for 24 hours in 38 patients with grade IIa and IIb ALI, with symptoms less than 14 days and absence of distal runoff. Both regimens were repeated the day after, if improvement was seen in peripheral circulation assessment parameters.
Trials comparing high‐ and low‐dose regimens of the thrombolytic agent
No threshold for low or high dose of a thrombolytic agent has been clearly defined. Different studies used different agents and a variety of treatment regimens.
Braithwaite 1997 compared IA continuous low‐dose infusion (CIF) of rt‐PA (0.5 mg/h or 1 mg/h) with IA high‐dose bolus infusion (3 × 5 mg, then, if needed, infusion at 3.5 mg/h) in 100 patients with ALI of up to 30 days' duration.
Cragg 1991 compared intra‐thrombus high‐dose UK (bolus of 250,000 U, followed by IA infusion of 250,000 U/h for 4 hours, then 125,000 U/h) and low‐dose (bolus of 50,000 U, followed by 50,000 U/h) infusions of UK in 63 patients with a mix of acute and chronic lower limb ischaemia.
Plate 2006 compared high‐dose, intra‐thrombus forced periodic (pulse spray) infusion of rt‐PA (0.33 mg/mL, 2 pulses of 0.13 mg (0.4 mL)/min for up to 2 hours) with low‐dose infusion of rt‐PA (initial intra‐thrombus bolus of 0.25 mg (2.5 mL), then continuous infusion of 0.5 mg (5 mL)/h up to 48 hours plus 600 U/h of heparin) in 121 patients with "sudden onset of unilateral lower limb ischaemia within 30 days". The low dose was delivered by continuous infusion, and the high dose by forced periodic infusion (i.e. dosage and technique differed in the two study groups).
Yusuf 1995 compared IA continuous low‐dose infusion (CIF) of rt‐PA (0.5 mg/h) with high‐dose pulse spray rt‐PA (0.33 mg/mL: 0.2 mL every 15 seconds for the first 15 minutes, then every 30 seconds) in 18 patients with lower limb ischaemia of up to 42 days' duration. The low dose was delivered by continuous infusion, and the high dose by forced periodic infusion (i.e. dosage and technique differed in the two study groups).
Trials comparing continuous infusion with forced infusion or pulse spray of the agent
Kandarpa 1993 compared forced infusion (intra‐thrombus bolus of UK (25,000 IU/10‐cm thrombus), then intra‐thrombus pulse spray of UK (10,000 U/mL)) with continuous infusion (CIF) (intra‐thrombus bolus (25,000 IU/10 cm thrombus), then slow intra‐thrombus UK 3000 U/mL). Overall, UK was used at equivalent dose rates, and the study population included 25 patients with lower limb ischaemia of up to 30 days' duration.
Comerota 2019 compared multiple treatment arms and three separate randomisation cohorts, with eight groups (155 patients) in total receiving IA CIF of 150 mg plasmin. Patients had Society of Vascular Surgery acute ischaemia categories I and IIa of up to 2 weeks' duration. In the first randomised cohort, of the four groups receiving plasmin, three had initial pulse spray infusion and one did not. Three groups had 5‐hour infusions (10, 15, and 30 mL/h), and one group had a 2‐hour infusion (35 mL/h). This cohort also included two control groups ‐ receiving rt‐PA or placebo. Of the other two cohorts, neither had initial pulse spray. Cohort 2 had two groups receiving CIF over 5 and 2 hours, at 60 and 75 mL/h, respectively. Cohort 3 had two groups receiving CIF over 5 and 2 hours, at 30 and 35 mL/h, respectively, with both groups having an additional distal balloon occlusion catheter placed. A fourth cohort reported by the study authors, comprising a single group, was not of interest to this review, as it involved participants who were not randomised.
Plate 2006 compared high‐dose, intra‐thrombus forced infusion of rt‐PA (0.33 mg/mL, 2 pulses of 0.13 mg (0.4 mL)/min for up to 2 hours) with low‐dose continuous infusion of rt‐PA (initial intra‐thrombus bolus of 0.25 mg (2.5 mL), then continuous infusion of 0.5 mg (5 mL)/h until thrombolysis complete or 48 hours plus 600 U/h of heparin) in 121 patients with "sudden onset of unilateral lower limb ischaemia within 30 days". The low dose was delivered by continuous infusion, and the high dose by forced periodic infusion (i.e. dosage and technique differed in the two study groups).
Yusuf 1995 compared IA CIF of rt‐PA (0.5 mg/h) with high‐dose pulse spray rt‐PA (0.33 mg/mL: 0.2 mL every 15 seconds for the first 15 minutes, then every 30 seconds) in 18 patients with lower limb ischaemia of up to 42 days' duration. The low dose was delivered by continuous infusion and the high dose by forced periodic infusion (i.e. dosage and technique differed in the two study groups).
Trials comparing thrombolysis with or without adjunctive antiplatelet agents
Duda 2001 compared an adjunctive antiplatelet agent (abciximab, IV bolus abciximab 0.25 mg/kg, then IV infusion of abciximab) followed by IA pulse spray UK (bolus of 25,000 U/10 cm of thrombus, then infusion of 4000 U/m for 2 hours, then 2000 IU/m for a further 2 hours if needed) with IV 0.9% saline plus initial IA pulse spray bolus of UK 25,000 U/10 cm of thrombus, then infusion of 4000 IU/m for 2 hours, then 2000 IU/m for a further 2 hours if needed.
Excluded studies
We excluded 10 additional trials from this update (Bagan 2013; Han 2009a; Han 2009b; Marder 2012; NCT00073554; NCT00115999; NCT02093468; Verhamme 2012; Yuan 2013; Zhang 2014), bringing the total to 13 excluded studies (Bagan 2013; Han 2009a; Han 2009b; Marder 2012; NCT00073554; NCT00115999; NCT02093468; Poredos 1999; Schweizer 2000; Schweizer 2003; Verhamme 2012; Yuan 2013; Zhang 2014). See the Characteristics of excluded studies table for details.
Risk of bias in included studies
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Six included studies were at unclear or high risk of selection bias (Berridge 1991; Braithwaite 1997; Comerota 2019; Cragg 1991; Kandarpa 1993; Plate 2006). Most studies were unblinded due to use of different techniques to deliver the thrombolytic agent. Trialists were not blind to outcomes, and only Comerota 2019 used independent reference laboratories to assess pre‐intervention and post‐intervention angiograms. All but Cragg 1991 and Comerota 2019 had low risk of bias from incomplete data reporting or selective reporting. Other potential biases included study terminating early due to lack of enrolment (Plate 2006), inconsistent reporting in the article (Kandarpa 1993; Saroukhani 2015), baseline differences (Duda 2001), and deviations from the clinical trial registry protocol (Comerota 2019).
Allocation
Two trials reported adequate methods of randomisation and allocation with appropriate safeguards (e.g. used a random number generator) and so were at low risk of bias (Duda 2001; Yusuf 1995). Four studies described the randomisation process but did not provide sufficient details on allocation so were assessed as being at unclear risk (Berridge 1991; Comerota 2019; Plate 2006; Saroukhani 2015).
Cragg 1991 used alternation to determine treatment groups and so was judged as being at high risk of bias. Braithwaite 1997 used computer‐generated randomisation cards contained in consecutively numbered sealed envelopes, but it was unclear whether the envelopes were opaque, so it is unclear whether this could have introduced selection bias. In Kandarpa 1993, patients were randomised by selection of consecutively numbered sealed envelopes. As it was not clear if the envelopes were opaque, we assessed this study as being at unclear risk of selection bias.
Blinding
Given the interventions being investigated, blinding of both participants and personnel would not always be possible, although blinding of outcome assessors would have been possible. Studies that did not blind personnel or outcome assessors were deemed to have high risk of both performance and detection bias (Berridge 1991; Braithwaite 1997; Cragg 1991; Kandarpa 1993; Plate 2006; Yusuf 1995). Comerota 2019 and Saroukhani 2015 did blind outcome assessors so were judged as having unclear risk of performance bias and low risk of detection bias. Duda 2001 was assessed as having high risk of performance bias and unclear risk of detection bias, as although the angiograms were reviewed by external assessors who were blind to the allocated treatment, external assessors had participated in the treatments.
Incomplete outcome data
No significant loss to follow‐up was reported in any of the studies, and there were no concerns regarding missing data. Outcome reporting within trials was complete to 30 days, except for Cragg 1991, where loss to follow‐up at 30 days was unclear from the data. Comerota 2019 was judged to be at high risk of attrition bias, as a mixture of per‐protocol and intention‐to‐treat analysis was used, and groups were analysed together, without acknowledging the initial cohorts to which they had been randomised.
Selective reporting
All outcomes were reported as planned, so there are no concerns regarding selective reporting within most included trials. Outcome reporting within trials was complete to 30 days, except for Cragg 1991, where loss to follow‐up at 30 days was unclear from the data.
Other potential sources of bias
No evidence of other potential sources of bias was identified in Berridge 1991, Braithwaite 1997, Cragg 1991, or Yusuf 1995. Some inconsistency regarding reporting of data was evident in Kandarpa 1993. Unclear risk of other bias due to differences in the baseline level of participant disease severity was reported in Duda 2001: 40% of the treatment group were claudicants compared to only 15% of the control group. In Saroukhani 2015, the mean age of the catheter‐directed thrombolysis (CDT) group was 55.5 years compared to 88.5 years in the IV group (cutoff age was 75 years), which may indicate potential selection bias. We queried this with the study authors, who explained that this was due to an error in the article. Saroukhani 2015 authors also reported, "Post intervention angiography was not performed in this [IV] group", yet comparisons are reported. Study authors responded to our communication to check this and confirmed that angiography was carried out in both groups. We assessed this study as being at high risk of other potential sources of bias due to these inconsistencies. Changes to the initial protocol were reported in the clinical trial registry protocol of Comerota 2019, and it is unclear if this could represent potential risk of bias. Plate 2006 was terminated early due to enrolment issues, and it is not clear how severity was assessed (participants were described as 'acute and sub‐acute lower limb ischaemia'), so we judged this study to be at unclear risk of other bias.
Effects of interventions
See: Table 1; Table 3; Table 5; Table 8
Clinical heterogeneity and disparate outcome measures used in reporting of the included studies limit their power and their likelihood of allowing meaningful meta‐analysis. This is particularly true for vessel patency and duration of thrombolysis, which were interpreted in different ways in different studies. These factors make direct comparison difficult, but effects have been described textually for each study when possible.
In these circumstances, the most meaningful method of assessment is to compare the most clinically relevant outcome measures (i.e. limb salvage, amputation, mortality, and complications (major and minor bleeding complications and cerebrovascular accident)).
It is important to be aware that limb salvage and amputation figures can be misleading due to inclusion of patients without limb‐threatening ischaemia; these patients were not at risk of amputation. Major bleeding defined as a fall in haemoglobin of more than 2 g/dL or requiring transfusion or surgery is perhaps the best defined of the outcomes.
Trials comparing intra‐arterial and intravenous delivery of the agent
Berridge 1991 and Saroukhani 2015 compared these methods of delivery. See Table 1 and Table 2. In Berridge 1991, intravenous rt‐PA was infused at four different rates, and IA rt‐PA was infused via a catheter embedded in the distal thrombus at a rate of 0.5 mg/h. In Saroukhani 2015, participants received either IV rt‐PA (alteplase, 0.6 mg/kg, 20% as initial bolus for 2 hours) or IA catheter delivery (CDT) of rt‐PA (5‐mg bolus, then 0.05 mg/kg/h every 2 hours for 24 hours).
30‐Day amputation‐free survival
Only Berridge 1991 reported 30‐day amputation‐free survival and described little or no effect on 30‐day amputation‐free survival between methods of delivery (14/20 (70%) IV rt‐PA and 16/20 (80%) IA rt‐PA; odds ratio (OR) 1.71, 95% confidence interval (CI) 0.40 to 7.34; 1 study, 40 participants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Intra‐arterial delivery versus intravenous delivery, Outcome 1: 30‐Day amputation‐free survival
The proportion of patients with asymptomatic limbs was greatest for IA rt‐PA (IV rt‐PA 9/20 (45%); IA rt‐PA 16/20 (80%)). Ongoing critical limb ischaemia (rest pain or tissue loss) was greatest in the IV group. In the IA group, none (0%) of the 16 surviving patients without amputation had critical limb ischaemia compared to 5 of 14 (36%) in the IV group. Study authors reported that an increase in ankle brachial index (ABI) was greater in the IA rt‐PA group than in the IV rt‐PA group (P < 0.001).
Amputation
In Berridge 1991, the major amputation rate was not different between groups at 30 days (IV rt‐PA 0/20 (0%); IA rt‐PA 1/20 (5%)) or at 3 months (IV rt‐PA 1/20 (5%); IA rt‐PA 1/20 (5%)). Saroukhani 2015 reported no clear differences between groups in rates of total amputation at 6 months (IV rt‐PA 6/18 (33%); IA rt‐PA 4/20 (20%)). These figures include forefoot, ankle, and below‐knee amputations (IV: 1, 3, 2; IA: 0, 0, 4, respectively).
Due to clinical heterogeneity, we were unable to combine the results. Amputation rates at 3 months and 6 months indicate little or no difference in effect on amputation rates between IV and IA groups (2 studies, 78 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Intra‐arterial delivery versus intravenous delivery, Outcome 2: Amputation
Mortality
In Berridge 1991, no clear difference in death rates was noted between the two groups at 30 days (IV rt‐PA 3/20 (15%) and IA rt‐PA 3/20 (15%)) (OR 1.00, 95% CI 0.18 to 5.67; 1 study, 40 participants; Analysis 1.3). Saroukhani 2015 analysed all participants at 6 months, so no deaths occurred in this follow‐up period (2 studies, 78 participants; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Intra‐arterial delivery versus intravenous delivery, Outcome 3: 30‐Day mortality
Vessel patency
Radiological success was reported in Berridge 1991 as complete, partial (defined as "lysis of the lesion down to the next major artery distally"), or failure. Among those treated with IA rt‐PA, 17 of 20 patients had complete lysis of the thrombus, 3 of 20 patients had partial lysis of the thrombus, and 0 of 20 procedures were reported as failures. In the IV rt‐PA‐treated group, 6 of 20 patients had complete lysis of the thrombus, 3 of 20 patients had partial lysis, and 11 of 20 procedures were reported as failures. Complete success was more likely with IA than with IV treatment (OR 13.22, 95% CI 2.79 to 62.67; 1 study, 40 participants), and radiological failure may be more likely with IV (OR 0.02, 95% CI 0.00 to 0.38; 1 study, 40 participants; low‐certainty evidence; Analysis 1.4). Saroukhani 2015 reported a higher rate of angiographic improvement in the CDT (IA) group compared to the IV group. Study methods state that the "secondary endpoint ...was complete or near complete recanalization of the occluded artery in angiography". However only a P value is reported (P < 0.001); no further details or results are provided (low‐certainty evidence).
1.4. Analysis.

Comparison 1: Intra‐arterial delivery versus intravenous delivery, Outcome 4: Vessel patency
Duration of thrombolysis
In Berridge 1991, the duration of thrombolysis for IA rt‐PA was 35 (14 to 64) hours (median and range). The duration of thrombolysis for IV rt‐PA is not reported, as rate and duration of the infusion were variable, with a maximum dose of 100 mg infused at 1, 2, 5, or 10 mg/h. In Saroukhani 2015, 80% of 0.6 mg/kg of rt‐PA (alteplase) was given IV over 2 hours (after 20% bolus). The IA group received an initial 5‐mg bolus dose, and the remainder was infused at 0.05 mg/kg/h every 2 hours over a 24‐hour period. Both regimens were repeated the day after, if improvements in the peripheral circulation were seen. See Table 2. We are unable to draw any conclusions due to the heterogeneity of the studies (very low‐certainty evidence).
Complications
Berridge 1991 reported the following complications.
Cerebrovascular accident: 1 intracranial bleed reported in the IV rt‐PA group (1/20, 5%); no events reported in the IA rt‐PA group (0/20, 0%) (OR 0.32, 95% CI 0.01 to 8.26; 1 study, 40 participants; very low‐certainty evidence; Analysis 1.5).
Major bleeding complications: may occur more frequently in the IV rt‐PA group (3/20, 15%) compared to the IA group (0/20, 0%) but the 95% CI indicates that there may be no difference (OR 0.12, 95% CI 0.01 to 2.53; 1 study, 40 participants; very low‐certainty evidence; Analysis 1.6).
Minor bleeding complications: occurred more frequently in the IV rt‐PA group (9/20, 55%) than in the IA group (0/20, 0%) (OR 0.03, 95% CI 0.00 to 0.56; Analysis 1.7).
1.5. Analysis.

Comparison 1: Intra‐arterial delivery versus intravenous delivery, Outcome 5: Cerebrovascular accident
1.6. Analysis.

Comparison 1: Intra‐arterial delivery versus intravenous delivery, Outcome 6: Major bleeding
1.7. Analysis.

Comparison 1: Intra‐arterial delivery versus intravenous delivery, Outcome 7: Minor bleeding
Saroukhani 2015 defined major complications as those requiring transfusion or resulting in disability. Minor complications were described as other haemorrhage, haematoma, or hypersensitivity reactions. Saroukhani 2015 reported that no major complications were experienced in the IA or IV groups. No minor complications were detected in the IV group compared to 4 of 20 (20%) in the CDT IA group. It is not clear exactly what minor events occurred, but these may have included other (non‐major) haemorrhage, haematoma, or hypersensitivity reactions.
Trials comparing high‐ and low‐dose regimens of the thrombolytic agent
Four studies compared high‐ and low‐dose regimens (Braithwaite 1997; Cragg 1991; Plate 2006; Yusuf 1995). It was not possible to pool data because of heterogeneity in the methods and inclusion criteria, so we have presented the study results separately below. See Table 3 and Table 4.
Low‐dose bolus and low‐dose infusion versus high‐dose bolus and high‐dose infusion with urokinase
In Cragg 1991, 72 episodes of thrombolysis were performed in 63 patients (nine patients had two separate admissions). Thirty‐four patients (47%) had native arterial occlusion, and 38 (53%) graft thrombosis. Twenty‐four (71%) of the native arterial occlusions and 37 (97%) of the grafts were treated within four weeks of onset of symptoms.
30‐Day amputation‐free survival
Thirty‐day amputation‐free survival was seen in 57 of 63 (90.5%) participants. Overall one death and five amputations (6/63, 9.5%) were reported. However, it is not clear in which groups these occurred. The true rate of limb salvage cannot be calculated from the data provided, given the inclusion of patients with viable limbs. It is not known how many patients had limbs that would have been at risk of amputation if left untreated (very low‐certainty evidence).
Amputation
Overall, 5 of 63 (7.9%) patients had major amputation; most of these appear to have come from the high‐dose group (only one could have occurred with the low‐dose regimen) (very low‐certainty evidence).
Mortality
A single death occurred, but it is not clear in which group the death occurred (very low‐certainty evidence).
Vessel patency
Study authors describe that the extent of thrombolysis was recorded as "none (less than 10% clot lysis), partial (10% to 90% clot lysis), or complete (antegrade flow with less than 10% residual thrombus)" (Cragg 1991). It is not clear how these proportions were estimated, but only presence or absence of "complete lysis" was reported. The proportion of patients with "complete clot lysis" in the high‐dose group did not differ from that in the low‐dose group (very low‐certainty evidence). See Table 4. Thrombolysis was deemed to be clinically successful if (1) among patients with acute ischaemia, the patient returned to the pre‐ischaemic condition; or (2) among patients with chronic ischaemia, "it aided the performance of a subsequent revascularisation procedure". All groups had similar increases in ABI following lysis, and there was no significant difference in "clinical success".
Duration of thrombolysis
Study authors report time to achieve "complete lysis" and duration of the infusion. It must be noted that the duration of the infusion was longer, reflecting that in some cases, the infusion was continued after "complete lysis" was achieved in an attempt to lyse any residual thrombus. Time to restore antegrade flow or to achieve "complete lysis" in the high‐dose group did not differ from that in the low‐dose group. See Table 4.
Complications
Cerebrovascular accident: no CVA reported
Major bleeding complications: in the high‐dose group, 2 patients with a fall in haemoglobin of more than 2 g/dL, 1 haematoma (localised collection of blood, usually due to bleeding at a site where an artery has been punctured to place a catheter for diagnosis and treatment) requiring evacuation, and 1 patient requiring transfusion for puncture site bleeding. In the low‐dose group, a single patient with a drop in haemoglobin of more than 4 g/L (OR 0.46, 95% CI 0.04 to 5.29; very low‐certainty evidence; Analysis 2.6)
Minor bleeding complications: haematoma and puncture site bleeding were more frequent in the high‐dose group (7/35 (20%)) than in the low‐dose group (1/37 (2.7%)) (OR 0.11, 95% CI 0.01 to 0.96; 1 study, 72 participants; Analysis 2.7)
2.6. Analysis.

Comparison 2: High dose versus low dose, Outcome 6: Major bleeding
2.7. Analysis.

Comparison 2: High dose versus low dose, Outcome 7: Minor bleeding
Low‐dose infusion versus high‐dose bolus and high‐dose infusion with rt‐PA
Braithwaite 1997 reported that 100 patients had ALI of less than 30 days' duration.
30‐Day amputation‐free survival
Thirty‐day amputation‐free survival did not differ between the two groups (high dose 80%; low dose 84%). True limb salvage cannot be calculated from the data provided, given the inclusion of patients with viable limbs (very low‐certainty evidence). See Table 4.
Amputation
The 30‐day amputation rate did not differ between the two groups (high dose 6 (12%); low dose 2 (4.5%)) (OR 0.34, 95% CI 0.07 to 1.79; 1 study, 93 participants; very low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: High dose versus low dose, Outcome 2: Amputation
Mortality
The 30‐day mortality rate did not differ between the two groups (high dose 8%; low dose 11%) (OR 1.44, 95% CI 0.36 to 5.75; 1 study, 93 participants; very low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: High dose versus low dose, Outcome 3: 30‐Day mortality
Vessel patency
Study authors defined "complete lysis" as clearance of the occluded vessel with restoration of flow to runoff and re‐establishment of peripheral pulses.
"Clinically useful" lysis was defined as partial clearance of thrombus, relieving rest pain or improving ABI by 0.2; or as "partial clearance" of thrombus, enabling a procedure to be performed, when none was possible before therapy.
Complete lysis was achieved in 22 (45%) and 21 (48%) patients (OR 1.12, 95% CI 0.50 to 2.54; 1 study, 93 participants), and clinically useful lysis in 10 (20%) and 14 (32%) patients (OR 1.82, 95% CI 0.71 to 4.66; 1 study, 93 participants) in high‐dose and low‐dose groups, respectively. There was no difference between groups (very low‐certainty evidence). See Table 4 and Analysis 2.4.
2.4. Analysis.

Comparison 2: High dose versus low dose, Outcome 4: Vessel patency
Duration of thrombolysis
The median duration of infusion was 4 (range 0.25 to 46) hours for the high‐dose group and 20 (range 2 to 46) hours for the low‐dose group (P < 0.0001; very low‐certainty evidence). See Table 4.
Complications
Cerebrovascular accident: no events reported in the high‐dose group 0/49 (0%). One incident reported in the low‐dose group (1/44, 2.2%) (OR 3.41, 95% CI 0.14 to 85.99; 1 study, 93 participants; very low‐certainty evidence). See Analysis 2.5
Major bleeding complications: occurred with equal frequency in each group (high dose 6%; low dose 6.8%) (OR 1.12, 95% CI 0.21 to 5.87; 1 study, 93 participants; very low‐certainty evidence). See Analysis 2.6
Minor bleeding complications: not specified in Braithwaite 1997
2.5. Analysis.

Comparison 2: High dose versus low dose, Outcome 5: Cerebrovascular accident
Continuous infusion versus high‐dose forced infusion (pulse spray) with rt‐PA
Two studies compared low‐dose continuous infusion (CIF) versus high‐dose forced infusion (pulse spray) with rt‐PA (Plate 2006: Yusuf 1995). It was not possible to pool data because of heterogeneity in the methods and inclusion criteria. See Table 4.
In Plate 2006, 121 patients had acute onset of Rutherford grade I or II ischaemia of less than 30 days' duration.
30‐Day amputation‐free survival
Thirty‐day amputation‐free survival was reported as 49 of 58 (84%) pulse spray and 54 of 63 (86%) low‐dose infusion, but true limb salvage cannot be calculated from the data provided, given inclusion of patients with viable limbs (very low‐certainty evidence). See Analysis 2.1.
2.1. Analysis.

Comparison 2: High dose versus low dose, Outcome 1: 30‐Day amputation‐free survival
Amputation
No difference in amputation rate was detected, with 4 of 58 (7%) in the pulse spray group and 3 of 63 (5%) in the low‐dose continuous infusion group (OR 0.68, 95% CI 0.14 to 3.15; 1 study, 121 participants; very low‐certainty evidence). See Analysis 2.2.
Mortality
No difference in mortality was detected at 30 days, with 6 of 58 (10%) in the pulse spray group and 7 of 63 (11%) in the low‐dose continuous infusion group (OR 1.08, 95% CI 0.34 to 3.43; 1 study, 121 participants; very low‐certainty evidence). See Analysis 2.3.
Vessel patency
The criterion for success was "adequate peripheral blood flow"; minor residual thrombus was considered acceptable. An estimate of the "degree of lysis" was assessed from angiograms obtained immediately after thrombolysis was completed. More than "75% lysis" was accomplished in 45 of 58 patients (78%) in the pulse spray high‐dose group and in 41 of 61 patients (67%) in the low‐dose continuous infusion group on completion of thrombolysis (OR 0.59, 95% CI 0.26 to 1.34; 1 study, 119 participants; very low‐certainty evidence; Analysis 2.4). There was no difference in "incomplete lysis" immediately following completion of thrombolysis. At 30 days, there was little or no effect on "incomplete lysis" or reocclusion overall, or among patients requiring repeat thrombolysis or surgical intervention. See Analysis 2.4 and Table 4.
Duration of thrombolysis
Pulse spray was continued for a median of 120 (range 40 to 310) minutes. Complementary low‐dose infusion was required for a median of 18 (range 1 to 50) hours in 38 of 58 (66%) patients. Low‐dose continuous infusion was administered for a median of 25 (range 2 to 60) hours (very low‐certainty evidence). See Table 4.
Complications
Cerebrovascular accident: 3 of 58 (5.2 %) events detected in the pulse spray group compared to 1 of 63 (1.6 %) in the low‐dose continuous infusion group (OR 0.30, 95% CI 0.03 to 2.93; 1 study, 121 participants; very low‐certainty evidence). See Analysis 2.5
Major bleeding complications: 4 of 58 (6.9%) complications reported in the pulse spray group compared to 8 of 63 (12.6%) in the low‐dose continuous infusion group (OR 1.96, 95% CI 0.56 to 6.91; 1 study, 121 participants; very low‐certainty evidence). See Analysis 2.6
Minor bleeding complications: 12 of 58 (21%) minor bleeding complications reported in the pulse spray group compared to 7 of 63 (11%) in the low‐dose continuous infusion group (OR 0.48, 95% CI 0.17 to 1.32; 1 study, 121 participants). See Analysis 2.7
In Yusuf 1995, 18 patients had less than 42 days' ischaemia.
30‐Day amputation‐free survival
The true rate of limb salvage cannot be calculated from the data provided, given the inclusion of 2 of 9 patients in the pulse spray group and 3 of 9 patients in the continuous infusion group with viable limbs (very low‐certainty evidence). See Analysis 2.1.
Amputation
No clear differences in amputation rates were seen, with 2 of 9 (22%) of patients in the continuous infusion group undergoing amputation compared with 0 of 9 (0%) in the pulse spray group (OR 6.33, 95% CI 0.26 to 152.86; 1 study, 18 participants; very low‐certainty evidence). See Analysis 2.2.
Mortality
One death was reported in the continuous infusion group (1/9, 12%) and none in the pulse spray group (0/9, 0%) (OR 3.35, 95% CI 0.12 to 93.83; 1 study, 18 participants; very low‐certainty evidence). See Analysis 2.3.
Vessel patency
Radiological patency was not reported. "Success" rates were reported by study authors as not significantly different, at 88.8% in the pulse spray group and 55.5% in the continuous infusion group (P < 0.9), probably due to the small numbers involved (n = 18). Criteria for clinical success included radiological evidence of lysis with arterial recanalisation at least as far as the next major arterial collateral branch; increase in ABI greater than 0.2, indicating haemodynamic improvement; limb salvage at 30 days without surgery at the level at which lysis was performed; and no clinical evidence of rethrombosis at 30 days (very low‐certainty evidence).
Duration of thrombolysis
Median duration of therapy was less with pulse spray, at 195 minutes (range 90 to 1260 minutes), compared to continuous infusion, at 1390 minutes (range 300 to 2400 minutes) (P < 0.002; very low‐certainty evidence). See Table 4.
Complications
Cerebrovascular accident: none reported (very low‐certainty evidence)
Major bleeding complications: none in either group (very low‐certainty evidence)
Minor bleeding complications: occurred in each group but not discussed in detail
Trials comparing continuous infusion with forced or pulse spray infusion of the agent
Four studies compared continuous infusion with forced infusion (Comerota 2019; Kandarpa 1993; Plate 2006; Yusuf 1995). It was not possible to combine data because of heterogeneity in methods and inclusion criteria. See Table 5 and Table 6. Results for Plate 2006 and Yusuf 1995 have already been described above, and so are not duplicated below.
Forced‐infusion (pulse spray) bolus followed by continuous infusion versus forced infusion with urokinase
In Kandarpa 1993, 25 participants had less than 30 days' ischaemia. Kandarpa 1993 reported that two patients in the continuous infusion group developed significant muscle swelling (compartment syndrome). "Positive clinical outcome" (i.e. improvement or resolution of ischaemic symptoms) was similar in each group (7 (59%) in the pulse spray (PS) group; 9 (69%) in the continuous infusion group).
30‐Day amputation‐free survival
There was no clear difference between the two groups in amputation‐free survival. Limb salvage occurred in 11 of 13 (85%) patients in the continuous infusion group and in 10 of 12 (83%) in the PS group (OR 1.10, 95% CI 0.13 to 9.34; 1 study, 25 participants; very low‐certainty evidence). See Analysis 3.1.
3.1. Analysis.

Comparison 3: Continuous infusion versus pulse/forced infusion, Outcome 1: 30‐Day amputation‐free survival
Amputation
Two patients in the continuous infusion group underwent amputation (2/13; 15%) compared with no amputations in the PS group (0/12, 0%) (OR 5.43, 95% CI 0.24 to 125.59; 1 study, 25 participants; very low‐certainty evidence). See Analysis 3.2.
3.2. Analysis.

Comparison 3: Continuous infusion versus pulse/forced infusion, Outcome 2: Amputation
Mortality
No deaths were reported in the continuous infusion group (0/13, 0%) compared with two in the PS group (2/12,16.6%) (OR 0.16, 95% CI 0.01 to 3.60; 1 study, 25 participants; very low‐certainty evidence). See Analysis 3.3.
3.3. Analysis.

Comparison 3: Continuous infusion versus pulse/forced infusion, Outcome 3: 30‐Day mortality
Vessel patency
Study authors describe patency as "at least 95% thrombolysis by volume, with brisk antegrade flow" occurring within four hours. There were no clear differences in patency within four hours between the two groups (OR 0.20, 95% CI 0.02 to 2.17; 1 study, 25 participants; very low‐certainty evidence; Analysis 3.4). Ten patients who underwent PS and nine who underwent continuous infusion had residual thrombi prolonging infusion. In the continuous infusion group, infusion was stopped in two participants (one due to bleeding, one due to rethrombosis following vasovagal), and one participant in each group withdrew. There were no clear differences between treatment groups in the proportions of patients achieving complete lysis within 24 hours and beyond 24 hours.
3.4. Analysis.

Comparison 3: Continuous infusion versus pulse/forced infusion, Outcome 4: Vessel patency
Duration of thrombolysis
There were no clear differences between treatment groups in time to complete treatment (very low‐certainty evidence). See Table 6.
Complications
Cerebrovascular accident: none reported (very low‐certainty evidence)
Major bleeding complications: 3 major bleeding complications reported in the PS group (3/12, 25%) compared to 1 in the continuous infusion group (1/13, 7.7%) (OR 0.25, 95% CI 0.02 to 2.82; 1 study, 25 participants; very low‐certainty evidence). See Analysis 3.6
Minor bleeding complications: 2 minor bleeding complications reported in both the PS group (2/12, 16.6%) and the continuous infusion group (2/13,15.3%) (OR 0.91, 95% CI 0.11 to 7.72; 1 study, 25 participants). See Analysis 3.7
Distal embolisation: occurred in 5 patients in each group
3.6. Analysis.

Comparison 3: Continuous infusion versus pulse/forced infusion, Outcome 6: Major bleeding
3.7. Analysis.

Comparison 3: Continuous infusion versus pulse/forced infusion, Outcome 7: Minor bleeding
Continuous infusion with or without additional pulse spray with or without balloon occlusion catheters
Comerota 2019 compared multiple treatment arms; its primary purpose was to optimise plasmin delivery. Eight groups received CIF with different infusion times and regimens. All groups received 150 mg plasmin, four groups had CIF (no pulse), and four had CIF with additional pulse spray. Five had 5‐hour infusions (10 or 15 or 30 or 60 mL/h) and three had 2‐hour infusions (30 or 75 mL/h). Two used additional balloon occlusion catheters (BOCs), and six did not. Results are summarised in Table 9.
5. Comerota 2019 study arms and results.
| Group | Complications ‐ 30 days | |||||||||||||
| ID | Plasmin dose (mg) | Infusion time (h) | Infusion rate (mL/h) | Initial bolus | No. | Amputation‐free survivala | Amputationa |
Mortality, n (N) |
Patency (> 50% thrombolysis, n (N) [%]) |
Major bleeding (%) |
Minor bleeding (%) |
Serious adverse events (%) |
Adverse events (%) |
|
| pulse | A | 150 | 5 | 10 | N | 20 | ‐ | ‐ | 1 (20) | 7 (16) [44] | 15 | 5 | 50 | 70 |
| pulse | B | 150 | 5 | 15 | Y | 20 | ‐ | ‐ | 0 (20) | 9 (19) [47] | 5 | 20 | 20 | 60 |
| no pulse | G | 150 | 5 | 60 | N | 13 | ‐ | ‐ | 0 (13) | 8 (12) [67] | 0 | 46 | 31 | 69 |
| no pulse | H | 150 | 2 | 75 | N | 12 | ‐ | ‐ | 0 (12) | 8 (12) [67] | 0 | 8 | 25 | 58 |
| no pulse | Ib | 150 | 5 | 30 | N | 23 | ‐ | ‐ | 0 (21) | 11 (15) [73] | 10 | 29 | 33 | 67 |
| no pulse | Jb | 150 | 2 | 35 | N | 19 | ‐ | ‐ | 0 (17) | 6 (14) [43] | 0 | 12 | 12 | 59 |
| pulse | C | 150 | 5 | 30 | N | 22 | ‐ | ‐ | 0 (22) | 17 (21) [81] | 5 | 9 | 27 | 81 |
| pulse | D | 150 | 2 | 35 | N | 20 | ‐ | ‐ | 0 (20) | 9 (19) [47] | 0 | 20 | 20 | 70 |
BOC: balloon occlusion catheter. h: hour. ID: group identity letter. No.: number in the group. aAmputation‐free survival and amputation were not mentioned. bThese arms also used BOC.
30‐Day amputation‐free survival and amputations
Amputation survival and amputations were not specifically reported or listed as possible serious adverse events. It is unlikely any occurred during the 30 (± 3)‐day follow‐up (very low‐certainty evidence).
Mortality
One death (1/140) from acute respiratory distress syndrome occurred in the CIF with pulse without BOC group, and was not considered related to treatment (very low‐certainty evidence).
Vessel patency
Data for thrombolysis were reported for each subgroup. We were able to investigate the effects of CIF with or without pulse spray ‐ 5 hours versus 2 hours and with or without additional BOC. The study included participants in analyses only if they received > 90% of the planned thrombolytic agent.
We pooled data from these groups to investigate the effects of 5‐hour infusion versus 2‐hour infusion, and we observed no clear effect among participants with > 50% thrombolysis (OR 1.79, 95% CI 0.83 to 3.84; 1 study, 128 participants; very low‐certainty evidence; Analysis 4.1). We presented results by CIF with or without pulse to investigate whether this impacted the effects; we detected no differences when performing the test for subgroup differences (P = 0.76). This analysis includes data from groups that also received BOC (one in the 5‐hour group and one in the 2‐hour group).
4.1. Analysis.

Comparison 4: Continuous infusion with additional pulse or BOC, Outcome 1: 50% thrombolysis: 5 hours vs 2 hours ± pulse
We pooled data from these groups to investigate the effects of CIF with pulse versus CIF with no pulse, and we observed no clear effect among participants with > 50% thrombolysis (OR 0.67, 95% CI 0.31 to 1.42; 1 study, 128 participants; very low‐certainty evidence; Analysis 4.2). We presented results by infusion time to investigate whether this impacted the effects and detected no differences when performing the test for subgroup differences (P = 0.76). This analysis includes data from groups that also received BOC (both in the no pulse group).
4.2. Analysis.

Comparison 4: Continuous infusion with additional pulse or BOC, Outcome 2: 50% thrombolysis: CIF + pulse vs CIF
We pooled data from these groups to investigate the effects of CIF with BOC versus CIF without BOC, and we observed no clear effect among participants with > 50% thrombolysis (OR 1.09, 95% CI 0.46 to 2.58; 1 study, 128 participants; very low‐certainty evidence; Analysis 4.3). Both subgroups that had BOCs were CIF ‐ one with 5‐hour infusion and one with 2‐hour infusion. Subgroups without BOCs had both CIF and CIF plus pulse.
4.3. Analysis.

Comparison 4: Continuous infusion with additional pulse or BOC, Outcome 3: 50% thrombolysis: BOC vs no BOC
This study also provided data for participants who achieved 90% thrombolysis, revealing there may be a small increase in numbers of participants with 90% thrombolysis following 5‐hour infusion compared with 2‐hour infusion regimens (OR 8.17, 95% CI 1.00 to 66.60; 1 study, 131 participants; Analysis 4.4). The test for subgroup differences does not indicate any effect with or without pulse (P = 0.95).
4.4. Analysis.

Comparison 4: Continuous infusion with additional pulse or BOC, Outcome 4: 90% thrombolysis: 5 hours vs 2 hours ± pulse
There was no clear effect on 90% thrombolysis with or without the use of BOCs (OR 0.56, 95% CI 0.12 to 2.67; 1 study, 131 participants; Analysis 4.5).
4.5. Analysis.

Comparison 4: Continuous infusion with additional pulse or BOC, Outcome 5: 90% thrombolysis: ± BOC
Duration of thrombolysis
Comerota 2019 did not report duration as an outcome but did use groups with different treatment durations. These are reported above.
Complications
Cerebrovascular accident: no cerebral haemorrhage events reported in any of the groups included in this review (very low‐certainty evidence)
Major bleeding: data from groups within Comerota 2019 pooled to investigate any effect of CIF versus additional pulse; no clear effects on numbers of major bleeding events detected (OR 0.64, 95% CI 0.11 to 3.61; 1 study, 131 participants; very low‐certainty evidence; Analysis 3.6). Additional analysis carried out to investigate whether infusion times had an effect on major bleeding. No major bleeding events reported in groups relevant to this review following 2‐hour infusion (0/49) compared to 7 of 96 following 5‐hour infusion (OR 8.30, 95% CI 0.46 to 148.33; 1 study, 131 participants; very low‐certainty evidence; Analysis 4.6)
Minor bleeding: similarly, no clear effects on numbers of minor bleeding events detected (OR 2.02; 95% CI 0.85 to 4.77; 1 study, 131 participants; Analysis 3.7). Data reported are number of participants with minor bleeding events. Some participants had more than one event, presenting by event did not change the overall effect. Additional analysis carried out to investigate whether infusion times had an effect on minor bleeding. No clear differences in minor bleeding events reported in groups relevant to this review following 2‐hour infusion (7/49) and following 5‐hour infusion (19/96) (OR 1.48, 95% CI 0.58 to 3.81; 1 study, 131 participants; Analysis 4.7)
4.6. Analysis.

Comparison 4: Continuous infusion with additional pulse or BOC, Outcome 6: Major bleeding: 5 hours vs 2 hours
4.7. Analysis.

Comparison 4: Continuous infusion with additional pulse or BOC, Outcome 7: Minor bleeding: 5 hours vs 2 hours
Individual study results reported for Plate 2006 and Yusuf 1995 are as reported above and within Table 4 and Table 6.
Trials comparing thrombolysis with or without adjunctive antiplatelet agents
Only Duda 2001 compared adjunctive antiplatelet agents. See Table 8 and Table 7.
Forced‐infusion (pulse spray) bolus followed by continuous forced infusion with urokinase, with or without platelet glycoprotein IIb/IIIa receptor antagonist
Duda 2001 reported on 70 patients with less than 42 days' ischaemia.
30‐Day amputation‐free survival
The 30‐day amputation‐free survival was not reported, but 90‐day amputation‐free survival was reported ‐ abciximab and UK 48 of 50 (96%); UK alone 16 of 20 (80%). Study authors reported that amputation‐free survival was significantly higher at 90 days in the group receiving abciximab than in the group receiving UK alone. However, at the time of treatment, 40% of patients in the abciximab group had non‐limb‐threatening ischaemia compared to only 15% in the UK‐alone group. True limb salvage cannot be calculated from the data provided, given inclusion of patients with viable limbs. By 30 days, 4 of 50 in the abciximab group and 3 of 20 in the UK‐alone group had reached the composite endpoint of surgical revascularisation or limb amputation.
Amputation
This could not be calculated from the data provided.
Mortality
This could not be calculated from the data provided.
Vessel patency
Study authors' definition of patency does not clearly distinguish between the presence of thrombus and any underlying stenosis.
Initial patency by means of thrombolysis alone was reported in 14 of 20 from the UK‐alone group and in 33 of 50 from the abciximab group (OR 1.20, 95% CI 0.39 to 3.69; 1 study, 70 participants; very low‐certainty evidence; Analysis 5.1). Initial patency with thrombolysis and subsequent percutaneous intervention was reported in 20 of 20 from the UK‐alone group and in 44 of 50 in the abciximab group (OR 5.99, 95% CI 0.32 to 111.44; 1 study, 70 participants; Analysis 5.1). There were no clear differences between treatment groups for either of the reported patency outcomes.
5.1. Analysis.

Comparison 5: Urokinase with or without adjunctive antiplatelet agents, Outcome 1: Vessel patency
Duration of thrombolysis
The median duration of thrombolysis was 120 minutes in each group (low‐certainty evidence). See Table 7.
Complications
Cerebrovascular accident: none reported (low‐certainty evidence)
Major bleeding complications: 4 of 50 (8%) in the abciximab group compared with 0 of 20 (0%) in the UK group. Analysis did not show a clear difference between groups (OR 0.25, 95% CI 0.01 to 4.90; 1 study, 70 participants; very low‐certainty evidence; Analysis 5.2)
Minor bleeding complications: 9 of 50 (18%) in the abciximab group compared with 5 of 20 (25%) in the UK group. Analysis did not show a clear difference between groups (OR 1.52, 95% CI 0.44 to 5.26; 1 study, 70 participants; Analysis 5.3)
5.2. Analysis.

Comparison 5: Urokinase with or without adjunctive antiplatelet agents, Outcome 2: Major bleeding
5.3. Analysis.

Comparison 5: Urokinase with or without adjunctive antiplatelet agents, Outcome 3: Minor bleeding
Discussion
Summary of main results
Nine randomised studies detailing different techniques of thrombolysis were included in this review (Berridge 1991; Braithwaite 1997; Comerota 2019; Cragg 1991; Duda 2001; Kandarpa 1993; Plate 2006; Saroukhani 2015; Yusuf 1995). The power of the individual studies to deliver clinically relevant results was limited. Study populations were small and heterogeneous, and each study used different inclusion criteria in terms of severity and duration of ischaemia. Outcome measures also differed considerably between trials. Different agents were used: studies from the UK and Sweden used recombinant tissue plasminogen activator (rt‐PA) (Berridge 1991; Braithwaite 1997; Plate 2006; Yusuf 1995), while those from the USA and Germany used urokinase (Cragg 1991; Duda 2001; Kandarpa 1993). Comerota 2019, an international, multi‐centre trial, used plasmin, and Saroukhani 2015, a single‐centre trial, used alteplase (tissue plasminogen activator).
The combination of these factors made the included studies unsuitable for meaningful statistical comparison. For this reason, the Discussion considers, initially, the most clinically relevant outcome measures of treatment for patients with peripheral arterial ischaemia (limb salvage, amputation, mortality, and complications). Subsequently, other 'surrogate' outcome measures are discussed.
30‐Day amputation free survival ‐ limb salvage
Limb salvage can only truly be identified in Berridge 1991 and Kandarpa 1993. In Braithwaite 1997, the analysis included limb salvage rates, but their validity is questionable, as the study included many patients with intermittent claudication, which is not a limb‐threatening condition. Limb salvage rates were equivalent, at about 80% for each treatment regimen, except for intravenous (IV) 'systemic therapy', for which the limb salvage rate was only 45%. In addition, Berridge 1991 reported an increase in ankle brachial index (ABI) among participants treated with intra‐arterial (IA) rt‐PA compared to IV rt‐PA (0.57 versus 0.18; P < 0.001). This is in keeping with the fact that five (20%) participants in the systemic therapy group had ongoing critical ischaemia at 30 days and indicates that local IA drug delivery may be more effective than systemic IV therapy. The actual method of local delivery (pulse spray, infusion, dose rate) does not appear to affect the rate of limb salvage.
Amputation
Amputation rates varied considerably between studies (0% to 33%). However, no studies showed a clear effect on amputation rate related to the technique of drug delivery. Once again, it is noteworthy that amputation should be required only for patients with critically ischaemic limbs, and inclusion of patients with intermittent claudication makes interpretation of some of the results unclear.
Mortality
Death rates varied considerably between studies (0% to 16%). However, no studies showed a clear effect related to the technique of drug delivery. It would be worrisome if any patients with intermittent claudication were to die as a result of therapy, but unfortunately, these patients could not be extracted from the data set.
Vessel patency
Several studies included the degree of thrombolysis and the volume of thrombus cleared. These features are difficult to quantify objectively and can be calculated only retrospectively. In addition, the concept of thrombus clearance is ambiguous, for example, 50% clearance might refer to reduction in length of the blockage by 50% with no flow within the vessel, or, alternatively, it might refer to the 'volume' of the thrombus, in which case there might be restoration of flow if the lumen was only partially occluded. Both Berridge 1991 and Yusuf 1995 included "radiological evidence of lysis with arterial recanalization at least as far as the next major arterial branch". Although this is an objective measure, it ignores the level of occlusion and does not indicate whether direct in‐line runoff (continuous arterial flow to the foot) was restored. Yusuf 1995 reported that the median ABI increased following thrombolysis, from zero to 0.5 (P < 0.02) in the continuous group, and from zero to 0.9 (P < 0.0001) in the pulse spray group. Patients with an ABI of 0.9 would be expected to be free of ischaemic symptoms, and those with an ABI of 0.5 probably would have intermittent claudication. In Duda 2001, the definition of "patency" did not distinguish between remaining thrombus and underlying stenosis, and no differences were detected between the urokinase group and the abciximab group for either initial patency or patency following subsequent percutaneous interventions. The median ABI the day after intervention was 0.79 (range 0 to 1.18) in the urokinase‐plus‐abciximab group, and 0.79 (range 0.27 to 1.21) in the urokinase‐alone group. Six trials tried to quantify the proportion of thrombus cleared (Berridge 1991; Braithwaite 1997; Comerota 2019; Cragg 1991; Kandarpa 1993; Saroukhani 2015). Three appear to have tried to calculate this from an angiogram (Comerota 2019; Kandarpa 1993; Saroukhani 2015). Broadly speaking, most study authors considered the following categories: no, partial, and complete clearance. Only removal of thrombus with restoration of brisk forward arterial flow is clinically useful. In Berridge 1991, IA infusion was associated with an increase in complete radiological success (100% versus 45%) compared with IV infusion (P < 0.01). Saroukhani 2015 reported a higher rate of angiographic improvement in the catheter‐directed thrombolysis (CDT) (IA) group compared to the IV group (P < 0.001). Braithwaite 1997 reported a non‐significant increase in the median number of arterial segments cleared with low‐dose infusion (three segments) compared to high‐dose infusion (two segments). Cragg 1991 and Kandarpa 1993 failed to show any difference in clearance with different thrombolytic regimens. Levels of thrombolysis varied between groups in Comerota 2019; no differences in numbers of participants with 50% thrombolysis were seen when continuous infusion (CIF) was compared with or without pulsing or balloon occlusion catheters, or when 5‐hour infusions were compared to 2‐hour infusions. Due to the small numbers involved in each arm and design differences between arms, it is not possible to conclude whether any technique offered any advantage over another.
Duration of thrombolysis
The individual trials used different angiographic endpoints (e.g. estimated clearing of thrombus, restoration of flow, patent to next branch vessel). In addition, frequency of angiographic assessment often differed greatly between control and study groups. This prevented further statistical analysis of the duration of thrombolysis. Intuitively, it would seem that the more rapid the restoration of flow, the better, but once again, this has not been shown to affect the key outcome measures of limb salvage, amputation, and death.
Duration of thrombolysis varied considerably between studies. This might well reflect the fact that study endpoints were inconsistent. Kandarpa 1993 continued thrombolysis until all thrombi had been cleared; others stopped when little thrombus remained, or when antegrade flow had been restored. In the PROMPT study, thrombus aspiration and angioplasty were performed if there was no clearing after four hours; the median duration of treatment was 120 minutes for each group (Duda 2001). The trend in these studies was for thrombolysis to be more rapid with high‐dose and pulse spray regimens. Braithwaite 1997 showed a significant reduction in the median duration of infusion with the high‐dose regimen (median 4 hours, range 0.25 to 46 hours) compared to a median of 20 hours in the low‐dose group (range 2 to 46 hours) (P < 0.0001). Similar low‐dose rt‐PA regimens at the same centre yielded different results: median 35 hours, range 14 to 64 (Berridge 1991); median 23 hours, range 5 to 40 (Yusuf 1995). This contrasted with a median duration of lytic therapy of 3.25 hours (range 1.5 to 21) in Yusuf's forced‐infusion group. Kandarpa 1993 failed to show any differences in mean time to recanalisation (40 minutes continuous infusion, 42 minutes forced infusion), mean time to patency (92 versus 95 minutes, respectively), or total duration of thrombolysis (28 ± 26 versus 20 ± 14 hours, respectively) with continuous infusion and pulse spray regimens. Some of the differences between results of Kandarpa 1993 and Yusuf 1995 may be explained by the preliminary use of forced infusion in both control and treatment groups in Kandarpa 1993. Cragg 1991 used another non‐specific endpoint of treatment, namely, "it aided the performance of a subsequent revascularization procedure". This is also subjective and cannot be compared with absolute outcomes such as amputation and mortality. Comerota 2019 had fixed infusion times, depending on the specific group; these are discussed under the outcomes of vessel patency and complications, when appropriate. Likewise, IA and IV groups in Saroukhani 2015 were treated over fixed periods of time and regimens were repeated the day after, if improvements in the peripheral circulation were seen. Duration times from more recent studies seem much shorter than those from earlier studies.
Complications
Major complications
These included cerebrovascular accident and major bleeding (defined as a fall in haemoglobin > 2 g/dL, requiring transfusion or surgery). None of the treatment strategies significantly affected major complications. More incidents of major bleeding may have occurred in the systemic (IV) therapy group in Berridge 1991, and in the "accelerated regimen" groups compared to the continuous treatment groups in Cragg 1991, Duda 2001, and Kandarpa 1993, but these changes may not be meaningful and are not apparent in more recent studies. Saroukhani 2015 reported that no major complications were experienced in either IA or IV groups. Major bleeding in Comerota 2019 ranged from 0% to 15% incidence in different arms. Combining the data from these arms indicates that longer infusion times may result in more bleeding events, but the small numbers involved and differences in design between arms limit our confidence in this.
Minor complications
Minor bleeding complications were more frequent with systemic (IV) therapy ‐ Berridge 1991 ‐ than with high‐dose therapy ‐ Cragg 1991 ‐ and increased with high‐dose forced therapy ‐ Plate 2006. Saroukhani 2015 reported no minor complications in the IV group, compared to 4 of 20 (20%) in the CDT IA group, although some inconsistency in reporting was detected. In Comerota 2019, it is not possible to conclude whether any technique affected minor complications over another.
Summary
None of the different techniques that involve direct delivery of the active agent into the thrombus have been shown to be more effective in terms of restoring patency, preventing limb loss, or preventing death.
This review found evidence indicating that techniques that involve placement of catheters within the occluding thrombus are more effective for reducing ischaemia than those by which treatment is delivered IV (Berridge 1991).
The overall incidence of haemorrhagic and other major complications suggests that thrombolysis should be used with caution in patients presenting with intermittent claudication, which is in keeping with findings reported by Braithwaite 1999. The findings of this review are consistent with those of the most recent European Society of Vascular Surgery (ESVS) guidelines on the management of acute limb ischaemia (ALI) (Björck 2020).
Overall completeness and applicability of evidence
This review set out to determine which is the most effective infusion technique for peripheral arterial thrombolysis and to test the hypothesis that no overall additional benefit is derived from any of the different techniques available to most patients who are suitable for peripheral arterial thrombolysis. The nine included studies cover a variety of techniques of infusion and include patients with variable duration and severity of symptoms, reflecting the spectrum of the disease. However, the power of the individual studies to deliver clinically relevant results was limited. The clinical differences between the studies that prevented pooling have resulted in our inability to clearly report the balance between benefits and complications for any of the comparisons, including high versus low dose.
It is possible that recent advances in techniques, novel thrombolytic agents (second‐ and third‐generation thrombolytic agents), or adjunctive agents will lead to improved outcomes, but only carefully constructed and adequately powered studies will be able to demonstrate this. The development and introduction of newer endovascular technologies, such as mechanical thrombectomy or thrombo‐aspiration devices, may provide alternative strategies or additional adjuncts in the armamentarium of treatment options for ALI. Again, robust comparative studies will be needed to determine whether these technologies provide equivalent or better clinical outcomes when compared to CDT. Recent ESVS guidelines suggest that the potential for more rapid revascularisation with percutaneous mechanical devices can enable non‐surgical techniques to be employed in managing grade IIb ALI in selected patients, previously the preserve of open surgical treatment (Björck 2020). This potential for less invasive treatment also provides further avenues by which to treat patients with more severe degrees of ischaemia without the need for general anaesthesia ‐ something that has become an important consideration during the COVID‐19 pandemic (Jongkind 2021).
Quality of the evidence
This review update includes nine studies with a total of 671 participants. The included studies cover a range of therapeutic strategies for delivery of the thrombolytic agent and use of adjunctive therapies that might influence the outcome. This contrasts with a 2003 systematic review of coronary artery thrombolysis, which identified 142,097 patients in 14 trials and showed clear benefit of systemic thrombolysis in terms of acute mortality and cardiac morbidity (Dundar 2003). Compared to the evidence base for coronary artery thrombolysis, the evidence base for thrombolysis in peripheral arterial ischaemia is very small and trials are underpowered to demonstrate meaningful outcomes.
Study populations were small and heterogeneous; each study used different inclusion criteria in terms of severity and duration of ischaemia. Outcome measures also differed considerably between trials. In addition, different agents were used. We have downgraded our certainty in the evidence presented here for all outcomes to low or very low.
For the comparison 'Intra‐arterial delivery compared to intravenous delivery for peripheral arterial thrombolysis', we downgraded evidence for risk of bias concerns, inconsistency, and imprecision (see Table 1). For the comparison 'High‐dose compared to low‐dose regimens of thrombolytic agents', we downgraded evidence for risk of bias concerns, indirectness, and inconsistency (see Table 3). For the comparison 'Continuous infusion compared to forced (or pulse) infusion of thrombolytic agents', we downgraded evidence for risk of bias concerns, indirectness, and inconsistency (see Table 5). For the comparison 'Thrombolysis with or without adjunctive antiplatelet agents', we downgraded evidence for risk of bias concerns, indirectness, inconsistency, and imprecision (see Table 8).
Potential biases in the review process
It is unlikely that any relevant studies have been missed by the searches. However, there may be publication bias because negative studies might not have been reported. Limb salvage and amputation figures can also be misleading due to inclusion of patients without limb‐threatening ischaemia; these patients were not at risk of amputation to begin with, and results should be interpreted with caution.
Agreements and disagreements with other studies or reviews
The conclusions of this review are in keeping with the findings in other reports. However, it should be noted that data outside randomised trials have not been systematically reviewed.
Braithwaite 1999 reported outcomes of thrombolysis in 108 patients with acute‐onset claudication (Rutherford grade I) due to native artery or bypass graft occlusion. Findings were derived from the NATALI database maintained by the British Thrombolysis Study Group. Outcomes were similar to those in seen in patients with limb‐threatening ischaemia (Rutherford grade II). The incidence of major haemorrhage requiring transfusion or surgery was 6 of 108 (5.5%). The 30‐day mortality was 15 of 108 (14%) and 8 of 108 (10%), and the major amputation rate was 7.8%. It is likely that patients presenting with acute‐onset claudication have a different prognosis from patients with stable claudication and are at greater overall risk. However, as patients with Rutherford grade I ischaemia do not have threatened limbs, Braithwaite concluded that thrombolysis should be reserved for patients who have progressed to critical ischaemia.
Earnshaw 2004 reported outcomes in 1011 patients who had 1031 episodes of thrombolysis documented in the National Audit of Thrombolysis for Acute Leg Ischaemia (NATALI) database. Reports show 140 (12.4%) deaths and 141 (12.4%) amputations. Thirty‐day amputation‐free survival was seen in 852 (75.2%) patients, with patient age > 80 years and diabetes associated with worse outcomes. Major haemorrhage requiring transfusion or surgery occurred during 87 episodes (7.8%) of thrombolysis; 5 of these were fatal. Minor bleeding occurred in a further 70 episodes (6.3%). Cerebrovascular accident occurred in 26 patients (2.3%).
Plate 2009 presented a multi‐variate reanalysis of data from Plate 2006. Life‐threatening complications (cardiac events, cerebrovascular accident (CVA), haemorrhagic events) were seen in 15 (12%) patients within 30 days of treatment; all occurred in patients aged > 70 years.
Byrne 2014 reported outcomes in 147 patients (154 limbs) with ALI treated with thrombolysis. Eighty‐three limbs were treated with continuous infusion, and 71 with pharmacomechanical thrombolysis (forced infusion) ± continuous infusion. As expected, most patients (70.1%) had grade IIa ischaemia, and roughly 20% and 10% had grade IIb and grade I ischaemia, respectively. Major amputation was required in 15.8%, and 30‐day mortality was 5.2%. The most common cause of death was systemic bleeding, which occurred in 5.2%. Fifty‐six of the 71 (79%) limbs treated with pharmacomechanical thrombolysis required additional continuous infusion, and duration of treatment in this group was 23.6 hours, which was almost identical to the 25.5 hours reported in the continuous infusion group. Byrne 2014 concluded that equal benefit was evident in both treatment groups.
Earnshaw 2004 and Plate 2009 attempted to identify factors associated with adverse outcomes of thrombolysis. Both identified increasing age and more severe ischaemia as predictors of amputation, death, and major complications.
Several of the studies in this review include patients with intermittent claudication, as well as those with threatened limbs. No studies separated the outcomes of these patient groups. Findings in this review demonstrate a similar incidence of major haemorrhage and amputation, and, taken in conjunction with Braithwaite 1999 and Byrne 2014, suggest that thrombolysis should be used with caution in patients with intermittent claudication.
Two excluded studies highlight the limitations of small studies to give clear conclusions, in spite of the fact that the techniques used were identical (Schweizer 2000; Schweizer 2003). In the first study (Schweizer 2000), use of abciximab was reported to significantly reduce the duration of thrombolysis to a median of 75 minutes compared to 110 minutes in the control group. In the later study, Schweizer 2003, when abciximab was compared with an alternative platelet glycoprotein IIb/IIIa receptor antagonist tirofiban, the median duration of thrombolysis was 149 and 139 minutes, respectively. It would appear that thrombolysis was not as rapid in Schweizer 2003, regardless of the regimen chosen.
Two studies, both reported within a single paper, compared intra‐thrombus forced infusion of alfimeprase with intra‐thrombus forced infusion of placebo (Han 2009a; Han 2009b). In both studies, patients with acute‐onset lower limb ischaemia Rutherford grade I and IIa within 21 days of randomisation were judged to require vascular surgical intervention if thrombolysis was unsuccessful. No clear differences in amputation, death, or complications were seen. More interesting is the fact that arterial flow restoration was improved in both groups. Almost one in five patients having only guidewire traversal of the thrombus and infusion of placebo proximal to the thrombus improved sufficiently to be judged to no longer require surgical intervention. Almost two in five patients treated with forced infusion of placebo avoided surgery; this was equivalent to findings for alfimeprase and suggests that benefit is conferred by forced infusion into the thrombus, regardless of whether this involves a biologically active agent.
Authors' conclusions
Implications for practice.
There is insufficient evidence to show that any thrombolytic regimen provides a benefit over any other in terms of amputation‐free survival, amputation, or 30‐day mortality. The rate of CVA or major bleeding requiring surgery or blood transfusion did not clearly differ between regimens, but may occur more frequently in high‐dose and IV regimens. This evidence was limited and of very low certainty. Minor bleeding may be more common with high‐dose and IV regimens.
Limited very low‐ and low‐certainty evidence from individual trials appears to indicate that greater benefit is seen when the thrombolytic agent is delivered into the thrombus: systemic intravenous thrombolysis is less effective than intra‐arterial thrombolysis. 'High‐dose' and 'forced‐infusion' techniques, or use of adjunctive agents such as platelet glycoprotein IIb/IIIa inhibitors, may speed up thrombolysis, but these approaches are generally more labour‐intensive and seem to be associated with increased bleeding complications compared to low‐dose regimens; also, no evidence suggests that this leads to improved outcomes (e.g. lower amputation rates, decreased need for adjunctive endovascular or surgical procedures). 'Low‐dose continuous infusion', following initial lacing of the thrombus with a high dose of the thrombolytic agent, is the least labour‐intensive technique. Thrombolysis appears to be an acceptable therapy for patients with marginally threatened limbs (Rutherford grade IIa), but, because of risks of major haemorrhage, CVA, and death, thrombolysis should be used with caution in patients who do not have limb‐threatening ischaemia (Rutherford grade I). Regimens that decrease the time needed to restore blood flow may permit treatment of patients with immediately threatened limbs (Rutherford grade IIb).
Implications for research.
High‐certainty evidence is needed to help clinicians determine the most effective technique for peripheral arterial thrombolysis. Future researchers should be aware that only very large multi‐centre trials comparing different techniques of thrombolysis are likely to be sufficiently powerful to yield clinically significant results. Although the data on IV versus IA thrombolysis is limited, the publication of Berridge 1991 resulted in an almost exclusive move to IA thrombolysis in the setting of acute lower limb ischaemia. Therefore, a large‐scale RCT of IV versus IA thrombolysis is unlikely. Given the increasing use of endovascular technologies such as mechanical thrombectomy or thrombo‐aspiration devices, robust studies are needed to determine whether these provide equivalent or better clinical outcomes when compared to CDT in the treatment of ALI.
Future trials must be carefully constructed to ensure that study populations are truly comparable. Inclusion criteria must be clearly stated, and accepted reporting standards adhered to.
Studies focusing on speed of thrombolysis should include patients with Rutherford grade IIb ischaemia or should provide clear cost/benefit analysis demonstrating a financial advantage of the accelerated treatment regimen.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2023 | Amended | Contributions of authors updated. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 20 October 2020 | New citation required but conclusions have not changed | The most recent search for this review update was performed on 20 October 2020. Three new studies were included and 10 new studies were excluded. Text was updated to reflect current Cochrane recommendations. Risk of bias for all included studies was assessed, and Summary of findings tables were added. No change was made to the conclusions |
| 20 October 2020 | New search has been performed | The most recent search for this review update was performed on 20 October 2020. Three new studies were included and 10 new studies were excluded |
| 20 October 2008 | Amended | Converted to new review format |
| 14 November 2006 | Amended | Edited; CDSR citations updated |
| 20 February 2006 | New search has been performed | No new trials found at last search. Review updated with minor style guide changes only |
| 17 November 2004 | Amended | Synopsis added |
Notes
This is the second of three reviews concerning different aspects of thrombolysis, all of which were originally covered in the generic protocol "Surgery versus thrombolysis for acute limb ischaemia", unique ID 031499080512564323.
The first review is "Surgery versus thrombolysis for initial management of acute limb ischaemia" (Berridge 2013). The third review is "Fibrinolytic agents for peripheral arterial occlusion" (Robertson 2013).
Acknowledgements
The review authors would like to thank Dr David Kessel (DK), Mr David Berridge, and Dr Iain Robertson (IR) for their contributions to this review and to previous versions. Their input is much appreciated. We would like to thank Dr Marlene Stewart (MS) for her help in screening the results of literature searches.
The review authors and the editorial base would like to thank the following peer reviewers for their time and comments: Jos C van den Berg, MD, PhD, Service of Interventional Radiology, Centro Vascolare Ticino, Lugano, Switzerland; Daniel J García‐Martínez, Spain; and Kristin Osika, USA.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | #1 Thrombolytic or Fibrinolytic or Plasminogen AND INREGISTER | 10.10.18 – 4 11.09.19 ‐ 47 20.10.20 ‐ 27 |
| CENTRAL | #1 MESH DESCRIPTOR Arteriosclerosis 947 #2 MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 79 #4 MESH DESCRIPTOR Atherosclerosis 1069 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 821 #6 MESH DESCRIPTOR Intermittent Claudication 828 #7 MESH DESCRIPTOR Ischemia 1553 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2793 #9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY 12157 #10 ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 10513 #11 (peripheral near3 dis*):TI,AB,KY 4844 #12 (claudic* or IC):TI,AB,KY 4116 #13 (isch* or CLI):TI,AB,KY 32142 #14 arteriopathic:TI,AB,KY 7 #15 dysvascular*:TI,AB,KY 23 #16 (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 127 #17 (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 229 #18 ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 98 #19 ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 98 #20 MESH DESCRIPTOR Iliac Artery 159 #21 MESH DESCRIPTOR Popliteal Artery 305 #22 MESH DESCRIPTOR Femoral Artery 907 #23 MESH DESCRIPTOR Tibial Arteries 38 #24 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)):TI,AB,KY 1711 #25 ((bypass or graft) near3 (occlus* or steno* or restenos* or obstuct* or lesio* or block* or obliter*)):TI,AB,KY 1080 #26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 58496 #27 MESH DESCRIPTOR Thrombolytic Therapy EXPLODE ALL TREES 1608 #28 MESH DESCRIPTOR Fibrinolytic Agents 2019 #29 MESH DESCRIPTOR Plasminogen Activators 239 #30 (urokinase or streptokinase or streptase or tenecteplase):TI,AB,KY 2331 #31 (reteplase or alteplase):TI,AB,KY 1037 #32 (anistreplase or prourokinase or retavase or rapilysin):TI,AB,KY 222 #33 (t‐PA or tPA):TI,AB,KY 1661 #34 (r‐PA or rPA):TI,AB,KY 139 #35 (lysis or thrombolysis):TI,AB,KY 4661 #36 (plasminogen near2 activator):TI,AB,KY 4123 #37 (clot near3 (bust* or break* or remov*)):TI,AB,KY 56 #38 #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 10661 #39 #26 AND #38 3519 #40 01/01/2015 TO 10/10/2018:CD 460807 #41 #39 AND #40 1334 |
10.10.18 – 1334 11.09.19 ‐ 1070 20.10.20 ‐ 381 |
| Clinicaltrials.gov | peripheral arterial thrombolysis OR Peripheral Vascular Diseases OR Arteriosclerosis OR Arterial Occlusive Diseases OR Ischemia | Thrombolytic Therapy OR Fibrinolytic Agents OR Plasminogen Activators | 10.10.18 – 253 11.09.19 ‐ 97 20.10.20 ‐ 92 |
| WHO ICTRP Search Portal | peripheral arterial thrombolysis OR Peripheral Vascular Diseases OR Arteriosclerosis OR Arterial Occlusive Diseases OR Ischemia | Thrombolytic Therapy OR Fibrinolytic Agents OR Plasminogen Activators | 10.10.18 ‐ N/A 11.09.19 ‐ 3 20.10.20 ‐ 0 |
| MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) | 1 ARTERIOSCLEROSIS/ 56464 2 exp ARTERIOLOSCLEROSIS/ 151 3 Arteriosclerosis Obliterans/ 3978 4 ATHEROSCLEROSIS/ 31632 5 Arterial Occlusive Diseases/ 26609 6 Intermittent Claudication/ 7640 7 ISCHEMIA/ 47849 8 exp Peripheral Vascular Diseases/ 50459 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 173195 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 144561 11 (peripheral adj3 dis*).ti,ab. 38272 12 (claudic* or IC).ti,ab. 62586 13 (isch* or CLI).ti,ab. 350253 14 arteriopathic.ti,ab. 162 15 dysvascular*.ti,ab. 223 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 714 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1836 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1492 19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 8505 20 exp LEG/bs [Blood Supply] 25070 21 Iliac Artery/ 13451 22 Popliteal Artery/ 9048 23 Femoral Artery/ 27270 24 Tibial Arteries/ 1512 25 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 9813 26 ((bypass or graft) adj3 (occlus* or steno* or restenos* or obstuct* or lesio* or block* or obliter*)).ti,ab. 6857 27 or/1‐26 803523 28 exp Thrombolytic Therapy/ 22642 29 Fibrinolytic Agents/ 28232 30 exp Plasminogen Activators/ 38431 31 (urokinase or streptokinase or streptase or tenecteplase).ti,ab. 20958 32 (reteplase or alteplase).ti,ab. 1914 33 (anistreplase or prourokinase or retavase or rapilysin).ti,ab. 370 34 (t‐PA or tPA).ti,ab. 26233 35 (r‐PA or rPA).ti,ab. 4114 36 (lysis or thrombolysis).ti,ab. 64715 37 (plasminogen adj2 activator).ti,ab. 34519 38 (clot adj3 (bust* or break* or remov*)).ti,ab. 677 39 or/28‐38 154230 40 27 and 39 26346 41 randomized controlled trial.pt. 469353 42 controlled clinical trial.pt. 92682 43 randomized.ab. 423387 44 placebo.ab. 192319 45 drug therapy.fs. 2052184 46 randomly.ab. 298375 47 trial.ab. 441082 48 groups.ab. 1839754 49 or/41‐48 4293737 50 40 and 49 14832 51 (2017* or 2018*).ed. 1716518 52 50 and 51 1163 |
10.10.18 ‐ 1163 10.09.19 ‐ 1058 20.10.20 ‐ 1141 |
| Embase | 1 arteriosclerosis/ 23580 2 exp arteriolosclerosis/ 589 3 peripheral occlusive artery disease/ 31426 4 atherosclerosis/ 135258 5 intermittent claudication/ 8883 6 ischemia/ 72705 7 exp peripheral vascular disease/ 1609458 8 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 232204 9 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 193781 10 (peripheral adj3 dis*).ti,ab. 54066 11 (claudic* or IC).ti,ab. 63363 12 (isch* or CLI).ti,ab. 501942 13 arteriopathic.ti,ab. 181 14 dysvascular*.ti,ab. 245 15 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 970 16 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2672 17 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2030 18 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 11978 19 iliac artery/ 13047 20 popliteal artery/ 7416 21 femoral artery/ 26899 22 tibial artery/ 2654 23 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 13840 24 ((bypass or graft) adj3 (occlus* or steno* or restenos* or obstuct* or lesio* or block* or obliter*)).ti,ab. 8696 25 or/1‐24 1976209 26 exp fibrinolytic therapy/ 22290 27 fibrinolytic agent/ 23713 28 exp plasminogen activator/ 76323 29 (urokinase or streptokinase or streptase or tenecteplase).ti,ab. 24654 30 (reteplase or alteplase).ti,ab. 3344 31 (anistreplase or prourokinase or retavase or rapilysin).ti,ab. 457 32 (t‐PA or tPA).ti,ab. 33937 33 (r‐PA or rPA).ti,ab. 5407 34 (lysis or thrombolysis).ti,ab. 85466 35 (plasminogen adj2 activator).ti,ab. 42669 36 (clot adj3 (bust* or break* or remov*)).ti,ab. 1096 37 or/26‐36 200879 38 25 and 37 79880 39 randomized controlled trial/ 515292 40 controlled clinical trial/ 458015 41 random$.ti,ab. 1333198 42 randomization/ 79498 43 intermethod comparison/ 238459 44 placebo.ti,ab. 276287 45 (compare or compared or comparison).ti. 459799 46 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1796873 47 (open adj label).ti,ab. 66110 48 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 210162 49 double blind procedure/ 153442 50 parallel group$1.ti,ab. 22211 51 (crossover or cross over).ti,ab. 94040 52 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 288139 53 (assigned or allocated).ti,ab. 338625 54 (controlled adj7 (study or design or trial)).ti,ab. 300087 55 (volunteer or volunteers).ti,ab. 226085 56 trial.ti. 252059 57 or/39‐56 4086169 58 38 and 57 19546 59 (2017* or 2018*).dc. 3017993 60 58 and 59 2649 |
10.10.18 ‐ 2649 10.09.19 ‐ 2412 20.10.20 ‐ 2300 |
| CINAHL | S53 S51 AND S52 207 S52 EM 2017 OR EM 2018 442,007 S51 S37 AND S50 1,570 S50 S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 347,742 S49 MH "Random Assignment" 39,518 S48 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" 33,080 S47 MH "Crossover Design" 11,330 S46 MH "Factorial Design" 924 S45 MH "Placebos" 8,403 S44 MH "Clinical Trials" 93,373 S43 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,602 S42 TX crossover OR "cross‐over" 14,749 S41 AB placebo* 28,803 S40 TX random* 222,742 S39 TX trial* 254,903 S38 TX "latin square" 144 S37 S25 AND S36 4,624 S36 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 13,171 S35 TX clot n3 (bust* or break* or remov*) 180 S34 TX plasminogen n2 activator 4,708 S33 TX lysis or thrombolysis 4,673 S32 TX r‐PA or rPA 307 S31 TX t‐PA or tPA 1,316 S30 TX anistreplase or prourokinase or retavase or rapilysin 35 S29 TX urokinase or streptokinase or streptase or tenecteplase 1,042 S28 (MH "Plasminogen Activators+") 3,656 S27 (MH "Fibrinolytic Agents") 4,367 S26 (MH "Thrombolytic Therapy") 4,542 S25 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 92,329 S24 TX (bypass or graft) n3 (occlus* or steno* or restenos* or obstuct* or lesio* or block* or obliter*) 878 S23 TX (femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 1,128 S22 (MH "Tibial Arteries") 147 S21 (MH "Femoral Artery") 1,201 S20 (MH "Popliteal Artery") 362 S19 (MH "Iliac Artery") 458 S18 (MH "Leg/BS") 450 S17 TX (iliac or femoral or popliteal or femoro* or fempop* or crural) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 964 S16 TX (lower n3 extrem*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 122 S15 TX limb n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 283 S14 TX (leg n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ) 130 S13 TX dysvascular* 173 S12 TX arteriopathic 10 S11 TX isch* or CLI 40,062 S10 TX claudic* or IC 5,948 S9 TX peripheral n3 dis* 9,366 S8 TX ((arter* or vascular or vein* or veno* or peripher*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)) 12,817 S7 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD 26,677 S6 (MH "Peripheral Vascular Diseases+") 10,544 S5 (MH "Ischemia") 3,447 S4 (MH "Intermittent Claudication") 846 S3 (MH "Arterial Occlusive Diseases") 1,622 S2 (MH "Atherosclerosis") 3,413 S1 (MH "Arteriosclerosis") 4,818 |
10.10.18 – 207 11.09.19 ‐ 212 20.10.20 ‐ 338 |
| AMED (Allied and Complementary Medicine) | 1 Arteriosclerosis/ 78 2 exp Arteriosclerosis/ 374 3 Atherosclerosis/ 227 4 Arterial occlusive disease/ 89 5 Intermittent claudication/ 75 6 Ischemia/ 268 7 exp Peripheral vascular disease/ 119 8 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 824 9 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 466 10 (peripheral adj3 dis*).ti,ab. 444 11 (claudic* or IC).ti,ab. 1034 12 (isch* or CLI).ti,ab. 1716 13 arteriopathic.ti,ab. 1 14 dysvascular*.ti,ab. 58 15 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 21 16 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 33 17 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 25 18 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 55 19 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 110 20 ((bypass or graft) adj3 (occlus* or steno* or restenos* or obstuct* or lesio* or block* or obliter*)).ti,ab. 20 21 or/1‐20 4451 22 Fibrinolytic Agents/ 7 23 (urokinase or streptokinase or streptase or tenecteplase).ti,ab. 13 24 (reteplase or alteplase).ti,ab. 2 25 (anistreplase or prourokinase or retavase or rapilysin).ti,ab. 1 26 (t‐PA or tPA).ti,ab. 152 27 (r‐PA or rPA).ti,ab. 13 28 (lysis or thrombolysis).ti,ab. 95 29 (plasminogen adj2 activator).ti,ab. 45 30 (clot adj3 (bust* or break* or remov*)).ti,ab. 0 31 or/22‐30 286 32 21 and 31 47 33 exp CLINICAL TRIALS/ 3847 34 RANDOM ALLOCATION/ 318 35 DOUBLE BLIND METHOD/ 676 36 Clinical trial.pt. 1214 37 (clinic* adj trial*).tw. 5511 38 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2906 39 PLACEBOS/ 595 40 placebo*.tw. 3181 41 random*.tw. 18096 42 PROSPECTIVE STUDIES/ 1146 43 or/33‐42 23190 44 32 and 43 11 45 ("2017" or "2018").yr. 5526 46 44 and 45 0 |
10.10.18 – 0 10.09.19 ‐ 1 20.10.20 ‐ 0 |
Appendix 2. CRS search strategy 2015
| Search run on Fri, 9 Oct 2015 | ||
| #1 | MESH DESCRIPTOR Arteriosclerosis | 863 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 69 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 493 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 695 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 669 |
| #7 | MESH DESCRIPTOR Ischemia | 720 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2082 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 7987 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 6756 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 2896 |
| #12 | (claudic* or IC):TI,AB,KY | 2632 |
| #13 | (isch* or CLI):TI,AB,KY | 20299 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 9 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 78 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 119 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 71 |
| #19 | ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 805 |
| #20 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1062 |
| #21 | MESH DESCRIPTOR Iliac Artery | 135 |
| #22 | MESH DESCRIPTOR Popliteal Artery | 248 |
| #23 | MESH DESCRIPTOR Femoral Artery | 726 |
| #24 | MESH DESCRIPTOR Tibial Arteries | 30 |
| #25 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 936 |
| #26 | ((bypass or graft) near3 (occlus* or steno* or restenos* or obstuct* or lesio* or block* or obliter*)):TI,AB,KY | 811 |
| #27 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 | 38306 |
| #28 | MESH DESCRIPTOR Thrombolytic Therapy EXPLODE ALL TREES | 1449 |
| #29 | MESH DESCRIPTOR Fibrinolytic Agents | 1683 |
| #30 | MESH DESCRIPTOR Plasminogen Activators EXPLODE ALL TREES | 2128 |
| #31 | (urokinase or streptokinase or streptase or tenecteplase):TI,AB,KY | 2031 |
| #32 | (reteplase or alteplase):TI,AB,KY | 603 |
| #33 | (anistreplase or prourokinase or retavase or rapilysin):TI,AB,KY | 217 |
| #34 | (t‐PA or tPA):TI,AB,KY | 1172 |
| #35 | (r‐PA or rPA):TI,AB,KY | 82 |
| #36 | (lysis or thrombolysis):TI,AB,KY | 3278 |
| #37 | (plasminogen near2 activator):TI,AB,KY | 3259 |
| #38 | (clot near3 (bust* or break* or remov*)):TI,AB,KY | 29 |
| #39 | #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 | 8027 |
| #40 | #27 AND #39 | 2338 |
Data and analyses
Comparison 1. Intra‐arterial delivery versus intravenous delivery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 30‐Day amputation‐free survival | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.40, 7.34] |
| 1.2 Amputation | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 30 days | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 Up to 6 months | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 30‐Day mortality | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Vessel patency | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Radiological success ‐ complete | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.22 [2.79, 62.67] |
| 1.4.2 Radiological success ‐ partial | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.18, 5.67] |
| 1.4.3 Radiological failure | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.38] |
| 1.5 Cerebrovascular accident | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.26] |
| 1.6 Major bleeding | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.53] |
| 1.7 Minor bleeding | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.56] |
Comparison 2. High dose versus low dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 30‐Day amputation‐free survival | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Amputation | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 30‐Day mortality | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 Vessel patency | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4.1 Complete lysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4.2 Clinically useful lysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4.3 75% lysis ‐ post thrombolysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4.4 Incomplete lysis ‐ 30 days | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5 Cerebrovascular accident | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6 Major bleeding | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7 Minor bleeding | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Continuous infusion versus pulse/forced infusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 30‐Day amputation‐free survival | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Amputation | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 30‐Day mortality | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 Vessel patency | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 90% thrombolysis (4 hours) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 90% thrombolysis (2 or 5 hours) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5 Cerebrovascular accident | 1 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.93] |
| 3.6 Major bleeding | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7 Minor bleeding | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.5. Analysis.

Comparison 3: Continuous infusion versus pulse/forced infusion, Outcome 5: Cerebrovascular accident
Comparison 4. Continuous infusion with additional pulse or BOC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 50% thrombolysis: 5 hours vs 2 hours ± pulse | 1 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.83, 3.84] |
| 4.1.1 CI + pulse | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.56, 4.54] |
| 4.1.2 CI | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.66, 6.30] |
| 4.2 50% thrombolysis: CIF + pulse vs CIF | 1 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.42] |
| 4.2.1 5‐hour infusion | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.23, 1.61] |
| 4.2.2 2‐hour infusion | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.24, 2.52] |
| 4.3 50% thrombolysis: BOC vs no BOC | 1 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.46, 2.58] |
| 4.3.1 5‐hour infusion | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.52, 6.28] |
| 4.3.2 2‐hour infusion | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.17, 2.21] |
| 4.4 90% thrombolysis: 5 hours vs 2 hours ± pulse | 1 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.17 [1.00, 66.60] |
| 4.4.1 CIF + pulse | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.62 [0.48, 154.44] |
| 4.4.2 CIF | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.55 [0.37, 153.42] |
| 4.5 90% thrombolysis: ± BOC | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.6 Major bleeding: 5 hours vs 2 hours | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.7 Minor bleeding: 5 hours vs 2 hours | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Urokinase with or without adjunctive antiplatelet agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Vessel patency | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1.1 Initial patency by means of thrombolysis alone | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1.2 Initial patency with thrombolysis and subsequent percutaneous intervention | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Major bleeding | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.01, 4.90] |
| 5.3 Minor bleeding | 1 | 70 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.44, 5.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berridge 1991.
| Study characteristics | ||
| Methods | Study design: prospective randomised, unblinded Exclusion post randomisation: 6 exclusions post randomisation not included in the analysis Losses to follow‐up: none |
|
| Participants | Country: UK No. of patients: 60 (20 in each group) Age, years: mean 71 Gender: 39 M; 21 F Inclusion criteria: critical lower limb ischaemia of < 30 days' duration Exclusion criteria: recent stroke, surgery, or major trauma; childbearing potential; bleeding diathesis; unable to consent; emboli < 2 days' duration; severe ischaemia with good runoff |
|
| Interventions | Treatment: IA infusion 20 participants rt‐PA: 0.5 mg/h (Boehringer Ingelheim, Bracknell, UK) + 250 units heparin (Unihep Leo 1000, Leo Laboratories, Bucks, UK) 20 participants streptokinase: 5000 units/h (Streptase, Hoescht, Hounslow, UK) + 250 units heparin. The catheter was repositioned periodically during treatment Control: IV infusion 20 participants IV (systemic) rt‐PA: variable dose 1, 2, 5, or 10 mg/h rt‐PA (max dose 100 mg) Therapy was continued until complete lysis was achieved, or the patient deteriorated, or no further discernible lysis occurred either clinically or radiographically after a 12‐hour period |
|
| Outcomes | Primary: 30‐day and 3‐month limb salvage and death Secondary: ABI, duration of thrombolytic therapy, major haemorrhage, CVA 3‐month follow‐up |
|
| Funding | Study funded and rt‐PA supplied by Boehringer Ingelheim Ltd., Bracknell. Berkshire | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed via a random number programme on a Casio FX‐361 calculator with pre‐assigned 'last digits' used for each group |
| Allocation concealment (selection bias) | Unclear risk | No clear details on allocation were provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned |
| Other bias | Low risk | No evidence of other bias |
Braithwaite 1997.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, unblinded Exclusion post randomisation: 7 exclusions post randomisation not included in the analysis (due to duration of ischaemia (1), patent graft hence no lysis (1), moribund patient (1), breach of infusion protocol (4)) Losses to follow‐up: none |
|
| Participants | Country: UK No. of patients: 100 Age, years: mean 71 Gender: 61 M; 32 F Inclusion criteria: acute limb ischaemia of < 30 days' duration Exclusion criteria: recent stroke, surgery, or major trauma; childbearing potential; bleeding diathesis |
|
| Interventions | Treatment: IA high‐dose bolus rt‐PA 3 × 5‐mg doses of tPA (1 mg/mL) were hand‐injected into the thrombus at intervals of 5 to 10 minutes, with check angiography through the same catheter after the third injection. If successful lysis was not achieved after the 3 bolus doses, an infusion of 3.5 mg/h (0.1 mg/mL), through a calibrated infusion pump, was commenced for up to 4 hours. If further thrombolysis was required after another angiogram, the infusion rate was reduced to 0.5 to 1 mg/h Control: IA low‐dose infusion rt‐PA 0.5 mg/h or 1 mg/h rt‐PA (0.1 mg in 1 mL of 0.9% saline) was given via a calibrated infusion pump |
|
| Outcomes | Primary: duration of thrombolytic therapy Secondary: degree of thrombolysis, limb salvage, amputation, major haemorrhage, death, adjunctive procedures |
|
| Funding | ''The authors also thank members of the Thrombolysis Study Group who helped develop the protocol and supported the trial....The Thrombolysis Study Group and the National Audit of Thrombolysis for Acute Leg Ischaemia (NATALI) database were financed by Boehringer Ingelheim Ltd and a grant from the Department of Health'' | |
| Declarations of interest | Not reported | |
| Notes | 30‐day follow‐up. 19 patients were claudicants; the remainder (81%) had ischaemic rest pain. 39 patients had occlusion of a bypass graft | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: computer‐generated randomisation cards in consecutively numbered sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Computer‐generated randomisation cards in consecutively numbered sealed envelopes, but unclear whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned |
| Other bias | Low risk | No evidence of other bias |
Comerota 2019.
| Study characteristics | ||
| Methods | Study design: phase 2, randomised, open‐label study Exclusion post randomisation: none described Losses to follow‐up: 12 (described and accounted for) |
|
| Participants | Country: international (Poland, Romania, Slovakia, Belgium, Bulgaria, Czech Republic, Germany, USA, Peru, India, Serbia, Ukraine) No. of patients: 174 Age, years: mean 64 Gender: 135 M; 39 F Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment (all intra‐arterial)
Control
|
|
| Outcomes | Primary: number of participants with > 50% thrombolysis, safety of plasmin Secondary: major and minor bleeding, death, adverse events, abnormal laboratory values (as a measure of safety and tolerability) |
|
| Funding | This study was funded by Grifols, the sponsor of this clinical trial. The sponsor (Grifols) participated in study design, data collection, analysis, interpretation, and manuscript preparation | |
| Declarations of interest | "Anthony J. Comerota has been paid a consulting fee for responsibility as the principal investigator and has received funding as a study site (Jobst Vascular Institute). Lazar Davidovic has received funding as a study site (University of Belgrade, Clinic for Vascular and Endovascular Surgery, Serbian Clinical Center). Richard Shlansky‐Goldberg has been paid a consulting fee for responsibility as a steering committee member. Kim Hanna and Kecia L. Courtney are employees of Grifols, which is a manufacturer of plasmin. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose" | |
| Notes | 30‐day follow‐up. No reply to email enquiring about patient/condition classification. One study arm not randomised (M, an exploratory group to evaluate higher dose). We have not included data from this arm. The evaluable population included all subjects receiving 90% of their assigned dose of study drug and having both baseline and EOT arteriograms. Subjects receiving plasmin treatment with BOC must have had confirmed cannulation of the target vessel by post‐baseline arteriography and successful BOC inflation. The safety population included all subjects receiving any dose of study drug | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | States "computer‐generated randomization schedule" but no details on allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in published article. Protocol indicated that unblinded pharmacist would prepare and dispense PA or placebo for infusion. Information about dose and volume of the control group would be provided to the investigator after randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States "the central reading facility comprised three radiologists, who performed blinded, retrospective evaluations of arteriogram imaging..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All withdrawals, or losses to follow‐up, reported clearly. Mixture of ITT and per‐protocol analysis used. Results from multiple groups combined and analysed together |
| Selective reporting (reporting bias) | Low risk | All pre‐planned outcomes reported in the clinicaltrials.gov record |
| Other bias | Unclear risk | Changes made post protocol submission (extra treatment arms added) |
Cragg 1991.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, unblinded Exclusion post randomisation: 1 exclusion post randomisation not included in analysis Losses to follow‐up: not described |
|
| Participants | Country: USA No. of patients: 63 (72 infusions) Age, years: mean 61 Gender: 47 M; 16 F Inclusion criteria: acute and chronic peripheral ischaemia in native arteries and bypass grafts Exclusion criteria: unable to traverse occlusion with a guidewire, contraindication to thrombolysis including recent stroke or major surgery, bleeding diathesis |
|
| Interventions | Treatment: high‐dose urokinase; intra‐thrombus UK bolus 250,000 U, then IA 250,000 U/h for 4 hours, then 125,000 U/h up to 24 hours Control: low‐dose urokinase; intra‐thrombus UK bolus 50,000 U, then IA 50,000 U/h up to 24 hours |
|
| Outcomes | Primary: duration of thrombolytic therapy, degree of thrombolysis Secondary: angiographic appearance, clinical grade of ischaemia, clinical success |
|
| Funding | Not reported | |
| Declarations of interest | Not reported | |
| Notes | Upper limb treated in 2 patients; 30‐day follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of randomisation: alternation |
| Allocation concealment (selection bias) | High risk | Allocation based on alternation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. Participants, but not medical staff, were blinded to dose |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Immediate outcomes reported for all participants; unclear whether all participants had 30‐day follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Immediate outcomes reported for all participants; unclear whether all participants had 30‐day follow‐up |
| Other bias | Low risk | No evidence of other bias |
Duda 2001.
| Study characteristics | ||
| Methods | Study design: blinded (patients and outcome assessors), prospective, randomised Exclusion post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Germany No. of patients: 70 Age, years: median 68 Gender: 39 M; 31 F Inclusion criteria: Rutherford grade I‐IIb ischaemia, native arterial and bypass grafts, occlusion for 6 weeks or less (median duration of symptoms 14 days (range 1 to 42)) Exclusion criteria: acute ischaemia requiring immediate revascularisation; recent trauma, stroke, or surgery; childbearing potential; severe hepatic or renal dysfunction; bleeding diathesis; recent thrombolysis |
|
| Interventions | Treatment: urokinase plus abciximab IV bolus injection of abciximab 0.25 mg/kg, then IV infusion of abciximab 0.125 μg/kg/m for 12 hours (max 10 μg/m) Initial IA pulse spray bolus of 25,000 IU per 10 cm of thrombus urokinase, then infusion of 4000 IU/m for 2 hours, then 2000 IU/m for a further 2 hours if needed Control: urokinase plus placebo IV 0.9% saline. Initial IA pulse spray bolus of 25,000 IU per 10 cm of thrombus, then infusion of 4000 IU/m for 2 hours, then 2000 IU/m for a further 2 hours if needed Thrombus aspiration and angioplasty were used if there was no recanalisation at 4 hours |
|
| Outcomes | Primary: rate of major complications (death, major bleeding), amputation‐free survival Secondary: angiographic patency, time to restore flow |
|
| Funding | Centocor and Lilly Deutschland | |
| Declarations of interest | Not reported | |
| Notes | Heterogeneous population; control group had more severe ischaemia at presentation. 40% of treatment group were claudicants compared to only 15% of control group. Follow‐up available to maximum of 427 days. Approximately 80% of patients had native arterial occlusion, with two‐thirds due to thrombosis and one‐third due to embolism | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: independent computer‐generated randomisation; 5:2 ratio study treatment:control |
| Allocation concealment (selection bias) | Low risk | Study medication allocation schedule was computer‐generated by the local Institute for Medical Informatics |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients were blinded, but personnel were not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Post‐treatment angiographic analysis was performed by readers blinded to treatment received, but readers had participated in treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up in initial 30‐day period. Longer‐term follow‐up not relevant to this review |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned |
| Other bias | Unclear risk | Differences in baseline levels of participant disease severity (40% of treatment group were claudicants compared to only 15% of control group) |
Kandarpa 1993.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, not blinded Exclusions post randomisation: none Losses to follow‐up: not described |
|
| Participants | Country: USA No. of patients: 25 (25 acutely ischaemic lower limbs) Age, years: mean 63.9 Gender: 11 M; 14 F Inclusion criteria: lower extremity limb ischaemia due to native arterial or bypass graft occlusion of < 30 days' duration Exclusion criteria: unable to cross lesion with guidewire, irreversible ischaemia, internal bleeding within 3 weeks, major surgery biopsy within 10 days, liver dysfunction, uncontrolled hypertension, trauma/CPR, cardiac emboli, bacterial endocarditis, coagulopathy, recent stroke or CNS tumour or surgery, pregnancy, age < 18 years |
|
| Interventions | All participants had IV heparin (3000 to 5000 U), then intra‐thrombus bolus pulse spray urokinase (25,000 IU/10 cm thrombus delivered as 5 × 0.2‐mL aliquots of 25,000 units/mL solution), then either forced infusion or continuous infusion Forced infusion: intra‐thrombus urokinase 10,000 IU/mL (4000 IU/min × 2 hours to 0.2 mL/30 seconds, then 2000 IU/min × 2 hours to 0.2 mL/60 seconds (Abbokinase; Abbott Laboratories, USA); for this group, both initial bolus and treatment by prototype pulse spray pump (AngioDynamics) Slow continuous infusion: intra‐thrombus urokinase 3000 IU/mL (4000 IU/min × 2 hours to 80 mL/h; 2000 IU/min × 2 hours to 40 mL/h); hand‐injected If further therapy was required, urokinase was infused at 1000 units/min (20 mL/h), as required |
|
| Outcomes | Primary: patency at 4 hours (defined as 95% thrombolysis by volume), duration of thrombolytic therapy Secondary: limb salvage, amputation, major bleeding, death, adjunctive procedures, duration of hospital stay, 30‐day follow‐up |
|
| Funding | Supported by grant from AngioDynamics, New York, USA | |
| Declarations of interest | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation by 'selection of consecutively numbered sealed envelopes' |
| Allocation concealment (selection bias) | Unclear risk | Study authors used consecutively numbered sealed envelopes, but unclear whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned |
| Other bias | Unclear risk | Inconsistent reporting of data in study paper |
Plate 2006.
| Study characteristics | ||
| Methods | Study design: multi‐centre, unblinded, prospective, randomised, following angiography Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: Sweden No. of patients: 121 Age, years: mean 73 (52 to 89) forced infusion; 72 (47 to 97) continuous low‐dose rt‐PA infusion; 72 (47 to 97) all Gender: M:F 28:24 pulse spray high‐dose forced‐infusion rt‐PA; 29:34 continuous infusion; 63:58 all Inclusion criteria: sudden‐onset unilateral lower limb ischaemia of < 30 days' duration with angiographic evidence of thromboembolic occlusion distal to aortic bifurcation; possible to pass guidewire at least 5 cm into occlusion Exclusion criteria: major surgery < 10 days, haematuria < 10 days, gastrointestinal bleeding < 10 days, stroke < 3 months, coagulopathy, pregnancy, brain tumour, malignant hypertension, Dacron prosthesis implanted 3 months, graft infection, irreversible or profound ischaemia, contrast allergy, life expectancy < 30 days, age < 18 years, non‐cooperative |
|
| Interventions | Treatment: intra‐thrombus, forced periodic pulse spray, high ‐dose infusion rt‐PA 0.33 mg/mL, 2 pulses of 0.13 mg (0.4 mL)/min for up to 2 hours, equivalent to 15 mg/h for up to 2 hours or until lysis was complete. Angiography was performed at intervals of 30 minutes, with catheter repositioning as required. If lysis was considered insufficient after 2 hours, complementary low‐dose infusion was initiated as below Control: low‐dose infusion rt‐PA. Initial intra‐thrombus bolus of 0.25 mg (2.5 mL), then continuous infusion of 0.5 mg (5 mL)/h (0.1 mg/mL at a rate of 5 mL/h) until thrombolysis complete or 48 hours. During low‐dose thrombolysis, 600 U/h of heparin continuously infused (adjusting APTT to 60 to 120 seconds). Angiography was performed at intervals of 12 hours to check on progress. After each angiogram, the catheter was repositioned to ensure it remained within the thrombus After thrombolysis was completed, anticoagulation with low‐molecular‐weight heparin was administered subcutaneously twice daily for 3 days and patients were given oral anticoagulants or antiplatelet drugs at the discretion of the treating physician |
|
| Outcomes | Primary endpoints within 30 days: ordinal scale: death = 5, life‐threatening complication = 4, amputation = 3, surgical intervention = 2, reocclusion = 1, none = 0. If more than 1 endpoint reached, single highest endpoint score assigned Secondary endpoints: degree of thrombolysis, patency of occluded vessel, amputation‐free survival |
|
| Funding | ''The study was supported by a generous grant from Stig and Ragna Gorthon’s Foundation. The pulse‐spray infusion pumps were bought at a greatly reduced price from Gothia Medical ABw'' | |
| Declarations of interest | ''There are no conflicts of interest reported'' | |
| Notes | Study terminated prematurely due to poor recruitment; only 121 patients after 3 years. Initial target was 590 patients to be adequately powered to be able to validate study assumption that 30% of control group patients would reach primary endpoint within 30 days at 20% in high‐dose group. Pulse spray infusion pumps were bought at a greatly reduced price from Gothia Medical AB, but no conflicts of interest were declared (rt‐PA, Actilysew, Boehringer‐Ingelheim GmbH, Ingelheim, Germany). Eighteen participants (15%) had viable limbs, and 103 (85%) rest pain or tissue loss | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Randomisation concealed from all participants, but no details on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All surviving patients followed up; no loss to follow‐up at 30 days |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as planned |
| Other bias | Unclear risk | Study terminated prematurely (see notes above). It is not clear how severity of limb ischaemia was measured, and participants are described as 'acute and sub‐acute'. Rutherford classification not reported |
Saroukhani 2015.
| Study characteristics | ||
| Methods | Study design: randomised, single‐blind, clinical trial Exclusions post randomisation: 2 Losses to follow‐up: none |
|
| Participants | Country: Iran No. of patients: 40 (2 were excluded); 38 were assigned to undergo intravenous (n = 18) or CDT (n = 20) Age, years: 54.13 ± 13.5 (ranging from 20 to 75) Gender: 23 (60.5%) men; 15 (39.5%) women Inclusion criteria: < 75 years of age, symptoms of < 14 days' duration, ALI grade IIa and IIb according to Rutherford classification, absence of distal runoff in angiography before the intervention Exclusion criteria: severe anaemia (Hb < 8 g/dL), thrombocytopenia (platelet < 80,000/la), low serum fibrinogen (fibrinogen < 100 mg/dL), severe hypertension (systolic > 160 mmHg, diastolic > 100 mmHg), trauma or surgery within previous 14 days before intervention, history of subarachnoid haemorrhage, life expectancy < 14 months, major internal bleeding < 6 months before intervention, pregnancy, lumbar puncture 2 weeks before intervention |
|
| Interventions | Treatment: systemic IV alteplase (Activase, Genentech, Inc., San Francisco, USA) 0.6 mg/kg (upper limit of 50 mg) of alteplase; 20% was administrated as a bolus, and the remainder was given through IV infusion over a period of 2 hours. Repeat administration a day after initial dose if improvement in peripheral circulation assessment parameters Control: CDT and with IA alteplase (Activase, Genentech, Inc., San Francisco, USA), with infusion rate of 0.05 mg/kg/h. First, 5 mg of alteplase was administrated as a bolus; the remainder was given in divided doses every 2 hours over a period of 24 hours. A similar dosage of alteplase was given a day after initial dose if improvement was seen in peripheral circulation assessment parameters |
|
| Outcomes | Primary: improvement in clinical status, defined as upward shift of at least 1 grade in Rutherford classification, improvement in ABI (≥ 0.1), upward shift of at least 2 scores on VAS (pain severity) Secondary: complete or near‐complete recanalisation of occluded artery in angiography Patency and clinical outcomes were measured according to the Rutherford classification, ABI, and VAS. Adverse events related to the intervention including haemorrhage, haematoma, and hypersensitivity reaction were recorded |
|
| Funding | Supported by Deputy Research of Mashhad University of Medical Sciences | |
| Declarations of interest | ''Conflict of interest: none declared'' | |
| Notes | We contacted study authors to request clarification over inconsistencies in the published article. A response was received clarifying that angiography was carried out in both groups; mean age in the IV group was 58.55 ± 9.98, and "4 patients (10.5%) developed minor side effects (bleeding from puncture site) in the CDT group, no major or minor effect in IV group" 6‐month follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to two study groups using a computer‐based random digit generator" |
| Allocation concealment (selection bias) | Unclear risk | Computer‐based digit randomisation used but no details on allocation provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The interventionists and the patients were not blinded to the route of therapy". Blinding would not be possible given the interventions carried out. Given unclear risk judgement, as outcome assessors were blinded but potential for bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...those recording the outcome and measuring the indices were blinded to the study groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants completed the study and were analysed |
| Selective reporting (reporting bias) | Low risk | All pre‐planned outcomes as described in the methods section are reported |
| Other bias | High risk | Patient baselines: mean age CDT 55.5 compared to 88.5 in IV group, and inclusion criteria listed as 75 years old or younger. Wide SDs and judged to be not significant by study authors. Study authors report that "Post intervention angiography was not performed in [IV] this group", yet comparisons are reported. Inconsistences between text reported and data (i.e. "...no significant difference between two groups .... (P = 0.017)") |
Yusuf 1995.
| Study characteristics | ||
| Methods | Study design: prospective, unblinded, randomised Exclusions post randomisation: none Losses to follow‐up: none |
|
| Participants | Country: UK No. of patients: 18 Age, years: median 71 (pulse spray), 74 (continuous infusion) Gender: M:F not described Inclusion criteria: lower extremity ischaemia for 1 to 42 days Exclusion criteria: active internal bleeding, recent stroke or surgery, infected bypass graft, pregnancy, inability to provide consent, inability to traverse lesion with a guidewire, inability to withstand further ischaemia up to 24 hours, irreversible ischaemia |
|
| Interventions | Treatment: intra‐arterial pulse spray rt‐PA at 0.33 mg/mL: bolus of 0.2 mL (0.066 mg) every 15 seconds for the first 15 minutes and every 30 seconds thereafter, equivalent to 10 mg/h. Clinical observations were taken every 15 minutes, and angiography was performed at intervals of 30 to 120 minutes. Percutaneous transluminal balloon dilatation of any underlying stenosis was performed if required. If any residual thrombus was detected at this stage, the catheter was left for slow infusion of rt‐PA via the end hole at a rate of 1 mg/h Control: intra‐arterial infusion rt‐PA at 0.5 mg/h (0.05 mg/mL at a rate of 10 mL/h). Angiography was performed at intervals of 3 to 8 hours to check on progress. After each angiogram, the catheter was repositioned to ensure it remained within the thrombus rt‐PA protocols: pulse spray forced infusion or low‐dose infusion |
|
| Outcomes | Primary: duration of thrombolytic therapy Secondary: radiological evidence of thrombolysis, increase in ABI, limb salvage at 30 days, amputation, death |
|
| Funding | Not reported | |
| Declarations of interest | Not reported | |
| Notes | 30‐day follow‐up. Five (28%) had viable limbs, and 13 (72%) had threatened limb viability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation using sequentially numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as planned |
| Other bias | Low risk | No evidence of other bias |
ABI: ankle‐brachial index ALI: acute limb ischaemia APTT: activated partial thromboplastin time BOC: balloon occlusion catheter CDT: catheter‐directed thrombolysis CNS: central nervous system CPR: cardiopulmonary resuscitation CVA: cerebrovascular accident IA: intra‐arterial ITT: intention‐to‐treat IV: intra‐venous PA: plasminogen activator rt‐PA: recombinant tissue plasminogen activator VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bagan 2013 | Not a randomised trial. Participants mainly Rutherford grade I intermittent claudication |
| Han 2009a | Compares intra‐thrombus forced infusion of kalimeters with intra‐thrombus forced infusion of placebo and peri‐thrombus placebo. Does not compare infusion techniques of thrombolytics |
| Han 2009b | Compares intra‐thrombus forced infusion of alfimeprase with intra‐thrombus forced infusion of placebo. Does not compare infusion techniques of thrombolytics |
| Marder 2012 | Pilot phase 1 safety study, not a randomised trial |
| NCT00073554 | (HA002/NAPA1) Dose‐escalation study to evaluate the safety and activity of alfimeprase in patients with acute peripheral arterial occlusion |
| NCT00115999 | (HA004/NAPA2) Compares safety and efficacy of intra‐thrombus forced infusion of alfimeprase 0.3 mg/kg with placebo. Does not compare infusion techniques of thrombolytics |
| NCT02093468 | Study aimed to evaluate the safety and efficacy of MST‐188 (CDT rt‐PA) for acute lower limb ischaemia, but study was terminated early (4 participants) Quote: "program discontinued to pursue alternate indications" |
| Poredos 1999 | Mean duration of ischaemia > 30 days. Used Fontaine classification for chronic limb ischaemia rather than Rutherford classification for acute limb ischaemia. Severity of ischaemia not adequately classified. Method of randomisation unclear. Impossible to assess limb salvage. Only immediate post‐procedure outcome; no subsequent follow‐up |
| Schweizer 2000 | Duration of ischaemia unclear. Majority of patients had intermittent claudication, not critical ischaemia. Used Fontaine classification for chronic limb ischaemia rather than Rutherford classification for acute limb ischaemia. Majority of patients classed as grade IIb/III (i.e. short distance claudication and rest pain, when it should be one or the other). Thrombosuction performed before thrombolysis. Details of administration of thrombolysis not specified. Surrogate endpoints: re‐admission to hospital, re‐intervention |
| Schweizer 2003 | Duration of ischaemia unclear. Majority of patients had intermittent claudication, not critical ischaemia. Used Fontaine classification for chronic limb ischaemia rather than Rutherford classification for acute limb ischaemia. Majority of patients classed as grade IIb/III (i.e. short distance claudication and rest pain when it should be one or the other). Thrombosuction performed before thrombolysis. Details of administration of thrombolysis not specified. Surrogate endpoints: re‐admission to hospital, re‐intervention |
| Verhamme 2012 | Not a randomised trial; phase 2a safety and efficacy study |
| Yuan 2013 | Not a randomised trial; review of patients treated before and after a change in dose of thrombolytic agent. Patients mainly chronic stable disease: > 30 days' symptoms (83%), Rutherford grade I intermittent claudication (64%) |
| Zhang 2014 | Participants randomised to receive either 10 or 20 mg of rt‐PA by CDT, but then also received endovascular intervention before outcomes measured. Cannot determine effects of infusion technique |
CDT: catheter‐directed thrombolysis. rt‐PA: recombinant tissue plasminogen activator.
Differences between protocol and review
Types of participants have been clarified by more details on intended types of participants. Some outcomes were rephrased for clarification.
Contributions of authors
CB: identified possible trials and considered them for inclusion; assessed trial quality; extracted data; and wrote the review. JP: identified possible trials and considered them for inclusion; assessed trial quality; double‐checked data; and wrote the review.
Three authors involved in previous published versions of this review between 1998 and 2008 are no longer included on the author byline. Some of the content retained in this review reflects their contributions as follows:
David Berridge: identified possible trials, considered them for inclusion; assessed trial quality (for reviews published between 1998 and 2008).
David Kessel: identified possible trials, considered them for inclusion; assessed trial quality; extracted data and wrote the review (for reviews published between 1998 and 2008).
Iain Robertson: arbitrated in cases where disagreement existed between the authors; provided critique of the text (for reviews published between 2004 and 2008).
The current version of the review includes content and text prepared by David Kessel from earlier unpublished drafts of the 2021 update. David Kessel stepped down as an author prior to completion and publication of the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
Declarations of interest
CB: none known. As CB is based within Cochrane Vascular, editorial tasks for this review update were carried out by other members of the Cochrane Vascular editorial team. JP: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Berridge 1991 {published data only}
- Berridge DC, Gregson RHS, Hopkinson BR, Makin GS. Randomized trial of intra-arterial recombinant tissue plasminogen activator, intravenous recombinant tissue plasminogen activator and intra-arterial streptokinase in peripheral arterial thrombolysis. British Journal of Surgery 1991;78(8):988-95. [DOI] [PubMed] [Google Scholar]
Braithwaite 1997 {published data only}
- Braithwaite BD, Buckenham TM, Galland RB, Heather BP, Earnshaw JJ. Prospective randomized trial of high-dose bolus versus low-dose tissue plasminogen activator infusion in the management of acute limb ischaemia. British Journal of Surgery 1997;84(5):646-50. [PubMed] [Google Scholar]
Comerota 2019 {published data only}
- Comerota AJ, Davidovic L, Hannac K, Courtney KL, Shlansky-Goldberg RD. Phase 2, randomized, open-label study on catheter-directed thrombolysis with plasmin versus rtPA and placebo in acute peripheral arterial occlusion. Journal of Drug Assessment 2019;8(1):43-54. [DOI: 10.1080/21556660.2019.1586402] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EudraCT 2010-019760-36. A phase 2, randomized, open-label (with blinded plasminogen activator and placebo control groups) study to evaluate the effects of different intra-thrombus infusion regimens of plasmin (human) compared to plasminogen activator and placebo in patients with acute lower extremity native artery or bypass graft occlusion. clinicaltrialsregister.eu/ctr-search/trial/2010-019760-36/results (accessed 15 March 2019).
- NCT01222117. A study of intra-thrombus plasmin (human) in acute peripheral arterial occlusion. clinicaltrials.gov/ct2/show/NCT01222117 (first received 18 October 2010).
Cragg 1991 {published data only}
- Cragg AH, Smith TP, Corson JD, Nakagawa N, Castaneda F, Kresowik TF, et al. Two urokinase dose regimens in native arterial and graft occlusions: initial results of a prospective, randomized clinical trial. Radiology 1991;178(3):681-6. [DOI] [PubMed] [Google Scholar]
Duda 2001 {published data only}
- Duda SH, Tepe G, Luz O, Ouriel K, Dietz K, Hahn U, et al. Peripheral artery occlusion: treatment with abciximab plus urokinase versus with urokinase alone - a randomized pilot trial (the PROMPT study). Platelet receptor antibodies in order to manage peripheral artery thrombosis. Radiology 2001;221(3):689-96. [DOI] [PubMed] [Google Scholar]
Kandarpa 1993 {published data only}
- Kandarpa K, Chopra PS, Aruny JE, Polak JF, Donaldson MC, Whittemore AD, et al. Intra-arterial thrombolysis of lower extremity occlusions: prospective, randomized comparison of forced periodic infusion and conventional slow continuous infusion. Radiology 1993;188(3):861-7. [DOI] [PubMed] [Google Scholar]
Plate 2006 {published data only}
- Plate G, Jansson I, Forssell C, Weber P, Oredsson S. Thrombolysis for acute lower limb ischaemia - a prospective, randomised, multicentre study comparing two strategies. European Journal Vascular and Endovascular Surgery 2006;31:651-60. [DOI] [PubMed] [Google Scholar]
Saroukhani 2015 {published data only}
- Saroukhani A, Ravari H, Rad MP. Effects of intravenous and catheter directed thrombolytic therapy with recombinant tissue plasminogen activator (Alteplase) in non-traumatic acute limb ischemia: a randomized double-blind clinical trial. Bulletin of Emergency and Trauma 2015;3(3):86-92. [PMC free article] [PubMed] [Google Scholar]
Yusuf 1995 {published data only}
- Yusuf SW, Whitaker SC, Gregson RHS, Wenham PW, Hopkinson BR, Makin GS. Prospective randomised comparative study of pulse spray and conventional local thrombolysis. European Journal of Vascular and Endovascular Surgery 1995;10(2):136-41. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bagan 2013 {published data only}
- Bagan P, Dakhil B, Lacal P, Couffinhal JC. Acute peripheral arterial occlusion: prospective study evaluating intra-arterial thrombolysis with a micro-porus balloon catheter. Journal of Endovascular Therapy 2013;20:422-6. [DOI] [PubMed] [Google Scholar]
Han 2009a {published data only}
- Han SM, Weaver FA, Comerota AJ, Perler BA, Joing M. Efficacy and safety of alfimeprase in patients with acute peripheral arterial occlusion (PAO). Journal of Vascular Surgery 2010;51(3):600-9. [DOI] [PubMed] [Google Scholar]
Han 2009b {published data only}
- Han SM, Weaver FA, Comerota AJ, Perler BA, Joing M. Efficacy and safety of alfimeprase in patients with acute peripheral arterial occlusion (PAO). Journal of Vascular Surgery 2010;51(3):600-9. [DOI] [PubMed] [Google Scholar]
Marder 2012 {published data only}
- Marder VJ, Comerota AJ, Shlansky-Goldberg RD, Davis JP, Eng CD, Hanna K, et al. Safety of catheter-delivered plasmin in patients with acute lower extremity arterial or bypass graft occlusion: phase Iresults. Journal of Thrombosis and Haemostasis 2012;10(6):985-91. [DOI] [PubMed] [Google Scholar]
NCT00073554 {published data only}
- NCT00073554. Alfimeprase for thrombolysis in acute peripheral arterial occlusion. clinicaltrials.gov/ct2/show/NCT00073554 (first received 26 November 2003).
NCT00115999 {published data only}
- NCT00115999. Study of alfimeprase to rapidly dissolve blood clots in the leg and help prevent the need for surgery on leg arteries. clinicaltrials.gov/ct2/show/NCT00115999 (first received 27 June 2005).
NCT02093468 {published data only}
- NCT02093468. Evaluation of MST-188 in acute lower limb ischemia. clinicaltrials.gov/ct2/show/NCT02093468 (first received 21 March 2014).
Poredos 1999 {published data only}
- Poredos P, Videcnik V. LYS-plasminogen shortens the duration of a local thrombolytic treatment of peripheral arterial occlusions - a randomized controlled trial. Wiener Klinische Wochenschrift 1999;111(1):21-5. [PubMed] [Google Scholar]
Schweizer 2000 {published data only}
- Schweizer J, Kirch W, Koch R, Muller A, Hellner G, Forkmann L. Short- and long-term results of abciximab versus aspirin in conjunction with thrombolysis for patients with peripheral occlusive arterial disease and arterial thrombosis. Angiology 2000;51(11):913-23. [DOI] [PubMed] [Google Scholar]
Schweizer 2003 {published data only}
- Schweizer J, Kirch W, Koch R, Muller A, Hellner G, Forkmann L. Use of abciximab and tirofiban in patients with peripheral arterial occlusive disease and arterial thrombosis. Angiology 2003;54(2):155-61. [DOI] [PubMed] [Google Scholar]
Verhamme 2012 {published data only}
- Verhamme P, Heye S, Peerlinck K, Cahillane G, Tangelder M, Fourneau I, et al. Catheter-directed thrombolysis with microplasmin for acute peripheral arterial occlusion (PAO): an exploratory study. International Angiology 2012;31:289-96. [PubMed] [Google Scholar]
Yuan 2013 {published data only}
- Yuan B, Tan L, Zhang Y, Zhang W, Ren T, Yang B. Effects of delivering recombinant tissue plasminogen activator on a new infusion system during endovascular intervention in patients with lower limb ischemia. Thoracic and Cardiovascular Surgeon 2013;61:445-52. [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y, Zhang W, Li T, Yuan B, Song S. Efficacy of short-term catheter-directed thrombolysis used with rt-PA combined with endovascular interventional therapy in patients with lower limb ischemia. Zhonghua Yi Xue Za Zhi 2014;94(13):1017-20. [PMID: ] [PubMed] [Google Scholar]
Additional references
Araujo 2019
- Araujo ST, Moreno DH, Cacione DG. Percutaneous thrombectomy for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013486. [DOI: 10.1002/14651858.CD013486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berridge 2013
- Berridge DC, Kessel DO, Robertson I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD002784. [DOI: 10.1002/14651858.CD002784.pub2] [DOI] [PubMed] [Google Scholar]
Björck 2020
- Björck M, Earnshaw JJ, Acosta S, Gonçalves FB, Cochennec F, Debus ES, et al. European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. European Journal of Vascular and Endovascular Surgery 2020;59:173-218. [DOI] [PubMed] [Google Scholar]
Braithwaite 1995
- Braithwaite BD, Jones L, Yusuf SW, Dawson K, Berridge DC, Earnshaw JJ. Aspirin improves the outcome of intra-arterial thrombolysis with tissue plasminogen activator. British Journal of Surgery 1995;82:1357-8. [DOI] [PubMed] [Google Scholar]
Braithwaite 1999
- Braithwaite BD, Tomlinson MA, Walker SR, Davies B, Buckenham TM, Earnshaw JJ. Peripheral thrombolysis for acute-onset claudication. British Journal of Surgery 1999;86(6):800-4. [DOI] [PubMed] [Google Scholar]
Byrne 2014
- Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. Journal of Vascular Surgery 2014;59:988-95. [DOI] [PubMed] [Google Scholar]
Consensus Document
- Anonymous. Second European Consensus Document on Chronic Critical Leg Ischemia. Circulation 1991;84(4 Suppl):1-26. [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 11 November 2019. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Creager 2012
- Creager MA, Kaufman JA, Conte MS. Acute limb ischemia. New England Journal of Medicine 2012;366:2198-206. [DOI: 10.1056/NEJMcp1006054] [DOI] [PubMed] [Google Scholar]
Darwood 2018
- Darwood R, Berridge DC, Kessel DO, Robertson I, Forster R. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD002784. [DOI: 10.1002/14651858.CD002784.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dotter 1974
- Dotter CT, Rosch J, Seaman AJ. Selective clot lysis with low dose streptokinase. Radiology 1974;111(1):31-7. [DOI] [PubMed] [Google Scholar]
Dundar 2003
- Dundar Y, Hill R, Dickson R, Whalley T. Comparative efficacy of thrombolytics in acute myocardial infarction: a systematic review. Quarterly Journal of Medicine 2003;96:103-13. [DOI] [PubMed] [Google Scholar]
Earnshaw 2004
- Earnshaw JJ, Whitman B, Foy C. National Audit of Thrombolysis for Acute Leg Ischemia (NATALI): clinical factors associated with early outcome. Journal of Vascular Surgery 2004;39:1018-25. [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;5/6:499-533. [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from training.cochrane.org/handbook/archive/v5.1/.
Jongkind 2021
- Jongkind V, Earnshaw JJ, Gonçalves FB, Cochennec F, Debus ES, Hinchliffe R, et al. Update of the European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia in light of the COVID-19 pandemic, based on a scoping review of the literature. European Journal of Vascular and Endovascular Surgery 2021;(epublished ahead of print):j.ejvs.2021.08.028. [DOI: 10.1016/j.ejvs.2021.08.028] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandarpa 1988
- Kandarpa K, Drinker PA, Singer SJ, Caramore D. Forced pulsatile local infusion of enzyme accelerates thrombolysis: in vivo evaluation of a new delivery system. Radiology 1988;168:739-44. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6. Searching for studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from training.cochrane.org/handbook/archive/v5.1/.
McNamara 1984
- McNamara TO, Fischer JR. Thrombolysis of peripheral arterial and graft occlusions: improved results using high-dose urokinase. American Journal of Roentgenology 1985;144(4):769-75. [DOI] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery 2007;45(1 Suppl S):S5A-66A. [DOI] [PubMed] [Google Scholar]
Plate 2009
- Plate G, Oredsson A, Janke J. When is thrombolysis for acute lower limb ischemia worthwhile? European Journal of Vascular and Endovascular Surgery 2009;37(2):206-12. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. The Cochrane Collaboration, 2020.
Robertson 2013
- Robertson I, Kessel DO, Berridge DC. Fibrinolytic agents for peripheral arterial occlusion. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No: CD001099. [DOI: 10.1002/14651858.CD001099.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodgers 2007
- Rogers JH, Laird JR. Overview of new technologies for lower extremity revascularization. Circulation 2007;116(18):2072-85. [DOI] [PubMed] [Google Scholar]
Rutherford 1986
- Rutherford RB, Flanigan DP, Gupta SK, Johnston KW, Karmody A, Whittemore AD, et al. Suggested standards for reports dealing with lower extremity ischaemia. Journal of Vascular Surgery 1986;4(1):80-94. [PubMed] [Google Scholar]
SIR standards
- Patel N, Sacks D, Patel RI, Moresco KP, Ouriel K, Gray R, et al. SIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. Journal of Vascular and Interventional Radiology 2003;14:S453–S465. [DOI] [PubMed] [Google Scholar]
STILE 1994
- Anonymous. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischaemia of the lower extremity. The STILE trial. Annals of Surgery 1994;220(3):251-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sullivan 1989
- Sullivan KL, Gardiner GA, Shapiro MJ, Bonn J, Levin DC. Acceleration of thrombolysis with a high-dose transthrombus bolus technique. Radiology 1989;173(3):805-8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Berridge 1997
- Berridge DC, Kessel D. Infusion techniques for peripheral arterial thrombolysis. Cochrane Database of Systematic Reviews 1997, Issue 4. Art. No: CD000985. [DOI: 10.1002/14651858.CD000985] [DOI] [PubMed] [Google Scholar]
Kessel 2004
- Kessel DO, Berridge DC, Robertson I. Infusion techniques for peripheral arterial thrombolysis. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD000985. [DOI: 10.1002/14651858.CD000985.pub2] [DOI] [PubMed] [Google Scholar]


