Abstract
The t(2;13) chromosomal translocation in alveolar rhabdomyosarcoma tumors (ARMS) creates an oncogenic transcriptional activator by fusion of PAX3 DNA binding motifs to a COOH-terminal activation domain derived from the FKHR gene. The dominant oncogenic potential of the PAX3-FKHR fusion protein is dependent on the FKHR activation domain. We have fused the KRAB repression module to the PAX3 DNA binding domain as a strategy to suppress the activity of the PAX3-FKHR oncogene. The PAX3-KRAB protein bound PAX3 target DNA sequences and repressed PAX3-dependent reporter plasmids. Stable expression of the PAX3-KRAB protein in ARMS cell lines resulted in loss of the ability of the cells to grow in low-serum or soft agar and to form tumors in SCID mice. Stable expression of a PAX3-KRAB mutant, which lacks repression function, or a KRAB protein alone, lacking a PAX3 DNA binding domain, failed to suppress the ARMS malignant phenotype. These data suggest that the PAX3-KRAB repressor functions as a DNA-binding-dependent suppressor of the transformed phenotype of ARMS cells, probably via competition with the endogenous PAX3-FKHR oncogene and repression of target genes required for ARMS tumorigenesis. The engineered repressor approach that directs a transcriptional repression domain to target genes deregulated by the PAX3-FKHR oncogene may be a useful strategy to identify the target genes critical for ARMS tumorigenesis.
Tumor-specific chromosomal translocations involving transcription factor genes often result in the fusion of DNA binding domains to new transcriptional effector domains (see reviews, references 15 and 65). Since the DNA binding domains are unaltered, it is likely that the natural genes are targeted. However, the new effector domains may constitute either a gain or a loss of function relative to the natural level of gene activity (15, 63). In some of the cases, the aberrant transcriptional regulation is attributed to a dominant-negative mode of action (58, 71). In several other studies, the oncogenicity of the chimeric transcription factors has been correlated with the gain of a transcription activation potential (41, 53). Chimeric transcription factors have been shown to initiate or predispose cells to a variety of oncogenic mechanisms: perturbation of growth factor signaling, deregulation of the cell cycle, protection from apoptosis, or blockade of differentiation (15, 42). We have focused on a pediatric solid tumor, alveolar rhabdomyosarcoma (ARMS), which provides a model system for analysis of a transcription factor that gains a dominant activation function.
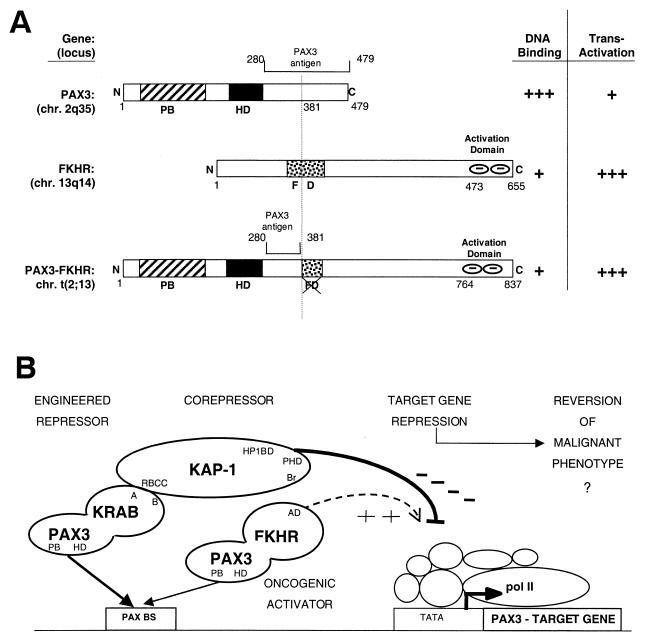
ARMS is cytogenetically characterized by a t(2;13) chromosomal translocation and less frequently by a t(1;13) chromosomal translocation (4). As a result, the t(2;13) the DNA binding motifs of PAX3 (paired box and homeodomain) are fused to a COOH-terminal activation domain of the forkhead gene, FKHR (Fig. 1A) (31, 35). In the tumors carrying the t(1;13) translocation, the PAX7 DNA binding domains are fused to the same activation domain of FKHR (20). We have shown that the PAX3-FKHR fusion protein localizes to the nucleus of cultured ARMS cells, binds to canonical PAX3 binding sites in vitro, and activates transcription of a reporter gene containing these binding sites. The PAX3-FKHR fusion protein is a much more potent transcriptional activator than wild-type PAX3 and may function as a dominant oncogene (31, 68). The increased transactivation capacity maps to the COOH terminus of the FKHR region and is dominant over the cis-acting repression domains retained in the chimeras (7, 8). All t(2;13) translocations result in truncation of the FKHR DNA binding domain, known to bind to insulin response elements (IREs) (4, 35). Thus, PAX3-FKHR would not be predicted to bind to IREs and would lose the function attributed to FKHR as an activator of genes in insulin signaling pathways regulating apoptosis (3, 19, 25). Whether the normal proapoptotic cellular function mediated by the wild-type FKHR allele is affected by the PAX3-FKHR fusion is unknown. However, the PAX3-FKHR protein is clearly not subject to the normal posttranslational regulation that governs the wild-type FKHR (60). PAX3 and PAX7 have nearly identical DNA binding motifs and bind similar PAX recognition elements in vitro (66). A reasonable prediction is that both PAX3-FKHR and PAX7-FKHR proteins function as strong activators of natural PAX3 or PAX7 target genes, and this deregulation results in ARMS tumorigenesis.
FIG. 1.
(A) The PAX3-FKHR chimeric transcription factor generated by the t(2;13) translocation in alveolar rhabdomyosarcoma (ARMS) retains the PAX3 paired box (PB) and homeodomain (HD) DNA binding motifs, has a disrupted forkhead (FD) DNA binding domain, and acquires a COOH-terminal FKHR-derived activation domain. (B) An engineered transcriptional repressor-corepressor recruitment strategy for inhibiting oncogenic activator function. The PAX3-KRAB repressor was designed to directly compete with PAX3-FKHR for binding and silence PAX3 target genes.
PAX3 and PAX7 are members of the PAX (paired box) gene family, consisting of nine developmentally regulated transcription factors with critical roles as master regulators of organogenesis (17, 49). For PAX3, this view is based on analysis of embryonic expression patterns and the developmental defect syndromes associated with specific mutations: the splotch mouse and the human Waardenburg syndrome (12, 72). Pax-3 and Pax-7 exhibit partially overlapping patterns of early expression in developing mouse central nervous system and during formation of dermomyotome and limb bud mesenchyme (50, 67). Current evidence suggests that PAX3 protects cells from apoptosis (9, 10, 61) and regulates cellular proliferation signals during epithelial-mesenchyme transitions (17, 67). Thus, an understanding of the normal role of PAX3 and PAX7 in development will undoubtedly shed light on the oncogenic potential of the PAX-FKHR fusions.
Based on correlation with PAX3 expression during development, a number of target genes have been proposed, including c-MET, MYOD, NCAM, MITF, TRP-1, and others (52, 70). In support of c-MET, MITF, and TRP-1 as direct targets is evidence that PAX3 can bind directly to sites in the promoters and can activate specific promoter reporters (27, 34, 74). However, most of the candidate promoters lack recognition elements for both the paired box and homeodomain motifs of PAX3, and direct binding has not been demonstrated (6, 62). Definition of the nucleotide sequence requirements for binding by PAX3 has been hampered by the fact that both the paired box and the homeodomain DNA binding motifs can accommodate considerable degeneracy (14). An added level of complexity is that PAX3 may function either as a transcription activator or as a repressor (13, 27). PAX3 effects on transcription may also be modulated in a cell-specific manner and can depend on the association with corepressors such as RB, HIRA, or DAXX (40, 48, 75). Clearly, there is a need for systems that can define physiologically relevant target genes for PAX3 that contribute to the normal biological phenotype and tumorigenesis.
We were interested in evaluating whether the transformed phenotype in ARMS was actually dependent on the transcriptional properties of PAX3-FKHR by using a strategy designed to repress PAX3 target genes. If increased activation of downstream target genes by PAX3-FKHR results in transformation, then repression of those target genes might result in reversion of the malignant phenotype (Fig. 1B). We approached this by engineering a PAX3 protein that functions as a strong constitutive repressor. The highly potent repression module we used is the KRAB domain of the KOX1 protein (Fig. 2A) which we have previously characterized (32, 51). Applied use of KRAB-mediated repression depends on recruitment of the corepressor, KAP-1, a KRAB binding protein that is ubiquitously expressed (32, 59, 64). We show that the PAX3-KRAB repressor can repress PAX3 reporter plasmids and inhibit the malignant growth of Rh30 ARMS cells in vitro and in vivo.
FIG. 2.
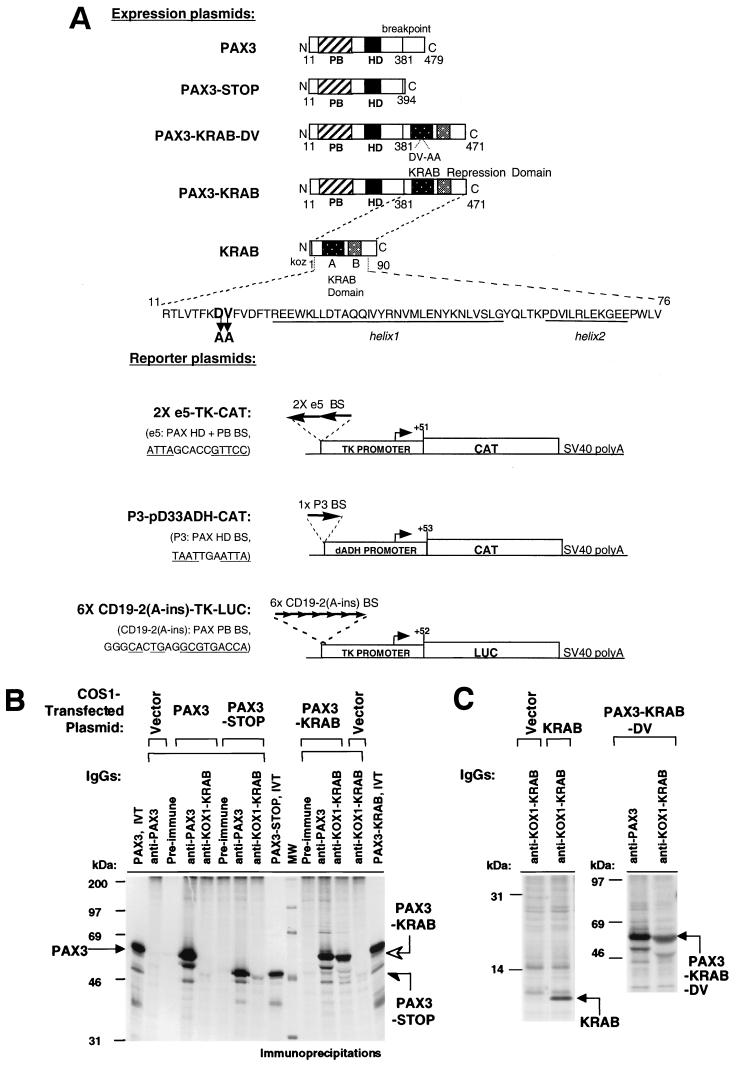
(A) Schematic representation of expression and reporter plasmids. The PAX3-KRAB repressor was created from PAX3-FKHR by removing the FKHR sequences and fusing a KRAB repression domain to the PAX3 DNA binding domains. PAX3-KRAB-DV contains an inactive mutant KRAB domain. The control plasmid, PAX3-STOP, lacks a KRAB domain, and the KRAB plasmid lacks PAX3 DNA binding domains. The 2xe5-TKCAT reporter plasmid has two e5 sites (homeodomain plus paired box binding sites, HD+PB BS), a minimal TK promoter, and a CAT gene. The P3-pD33ADH-CAT plasmid has an HD binding site, a distal ADH promoter, and a CAT gene. The 6x-CD19-2(A-ins)LUC reporter plasmid has six CD19-2(A-ins) PB binding sites, a minimal TK promoter, and a luciferase gene. (B and C) Immunoprecipitation of proteins expressed in COS1 cells after transient transfection and metabolic labeling. PAX3 (53 kDa) and the PAX3-STOP (48 kDa) are detected by anti-PAX3 IgG but not by preimmune or anti-KOX1-KRAB IgGs. Both anti-PAX3 and anti-KOX1-KRAB IgGs detect PAX3-KRAB (open arrow) and PAX3-KRAB-DV proteins (53 kDa). The expression of the KRAB protein (10 kDa) was detectable only with anti-KOX1-KRAB IgG.
MATERIALS AND METHODS
Expression plasmids.
The previously described pcDNA3-PAX3-FKHR plasmid (31, 35) which expresses a mouse-human PAX3-FKHR hybrid protein was used as the base to construct the PAX3-KRAB wild-type and mutant fusion genes. It was digested with EcoRI and XbaI to remove the FKHR sequences. The wild-type KRAB or the mutant KRAB (KOX1 amino acids 18 and 19 changed from DV to AA) domains were then inserted by ligation of an EcoRI-XbaI fragment encoding amino acids 1 to 90 of the KOX1 cDNA (32, 51). The KRAB domain DNAs were generated by PCR amplification from the PM1-KOX1(1–161) template using a 5′ oligonucleotide primer to incorporate an EcoRI site immediately before the KOX1 initiator methionine and a 3′ oligonucleotide primer to incorporate a TGA stop codon after amino acid 90 of KOX1, followed by XhoI and XbaI sites. The resulting expression plasmids will hereafter be referred to as PAX3-KRAB, and PAX3-KRAB-DV (Fig. 2A). For the KRAB plasmid, a 5′ oligonucleotide primer containing a HindIII site and a Kozak consensus sequence and the 3′ primer described above was used for PCR amplification. The HindIII-XbaI-digested PCR product was inserted into the pcDNA3 vector. The murine Pax-3 cDNA has been previously described (31, 38). The PAX3-STOP plasmid was constructed by digesting the PAX3-FKHR plasmid with EcoRI and XbaI, followed by incubation with Klenow enzyme and deoxynucleoside triphosphates and blunt-end ligation. The PAX3-STOP protein expressed from this pcDNA3 plasmid contains PAX3 sequence (encoding amino acids 11 to 381) followed by the additional vector-derived COOH-terminal sequence NSRGPYSIVSPKC before an in-frame TGA stop codon. The PAX3-KAP-1 plasmid was constructed by insertion of an EcoRI fragment encoding amino acids 293 to 835 of KAP-1 derived from the pM2-KAP-1 plasmid (32) into the EcoRI site of the PAX3-STOP plasmid in frame with the PAX3 DNA binding domain. All PCR-derived segments of DNA were sequenced on both strands. The PAX3 reporter plasmids P3-pD33ADH-CAT and p6x-CD19-2(A-ins)-TK-Luc have been described (16, 76). The 2xe5-TKCAT reporter contains two tandem e5 sites (5′-ATTAGCACCGTTCC-3′) with a 3′-to-5′ orientation at the SalI site upstream of the TK promoter of pBL2TKCAT (46) (Fig. 2A).
Cell lines.
NIH 3T3 mouse cells (ATCC CRL 1658), human Rh30 ARMS cells which contain the t(2;13) translocation (a kind gift of Peter Houghton and Peter Dias), and COS1 monkey kidney cells (ATCC CRL1650) were grown and transfected as described previously (31). The human RD embryonal rhabdomyosarcoma cell line which lacks the t(2;13) translocation (36) was maintained in Dulbecco modified Eagle medium supplemented with 2 mM l-glutamine and 13% fetal bovine serum (FBS) and was kindly provided by J. Biegel of the University of Pennsylvania. All media and supplements for cell culture were obtained from GIBCO (Gaithersburg, Md.).
Antisera and immunoprecipitation.
The procedures for metabolic labeling, radioimmunoprecipitation assay cell lysate preparation, immunoprecipitation from cell lysates, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and fluorography have been previously described (31). Purification of polyclonal immunoglobulin G (IgG) fractions from sera raised against PAX3 (amino acids 280 to 479), KOX1 (amino acids 1 to 161), and KAP-1 (amino acids 423 to 584) antigens has been previously described (31, 32, 51).
Electrophoretic mobility shift assays (EMSA).
The Promega TnT T7 transcription and translation system was used to prepare in vitro-translated protein lysates that were [35S]methionine (New England Nuclear) labeled for use in immunoprecipitations or were left unlabeled for use in the EMSA. Quantitation of labeled proteins within in vitro translation lysates was used to normalize amounts of unlabeled lysate used for EMSA, as previously described (31). The procedures for preparation of crude nuclear extracts and for EMSA have been previously described (31, 32).
Transfections and reporter assays.
The transcription assays were performed via transient transfection of NIH 3T3 or Rh30 cells with calcium phosphate-DNA precipitates containing pcDNA3 PAX3-derivative expression plasmids (Fig. 2A), p0N260-CMV β-d-galactosidase plasmid, and PAX-specific chloramphenicol acetyltransferase (CAT) or luciferase reporter plasmids, as previously described (31, 32). Luciferase assays were performed using the Promega Luciferase Assay kit with a Monolight 2010 Luminometer. β-d-Galactosidase activity was used to normalize transfection efficiency in cell extracts for both CAT and luciferase assays.
Stable cell lines, colony formation, and cell proliferation assays.
Rh30 cells were transfected with 10 μg of pcDNA3 CMV-based expression plasmids as depicted in Fig. 2A. At 48 h posttransfection, 3 × 105 cells were plated into triplicate 10-cm dishes containing media for selection of geneticin-resistant colonies (G418, 550 μg/ml [active]; GIBCO). After 2 weeks (with medium changes every 3 days), the G418-resistant colonies were isolated using cell cloning rings. The clones were maintained in selection, expanded, cryopreserved, and evaluated for stable expression of protein by radiolabeling and immunoprecipitation. Stable human RD cell clones were selected and maintained in medium with 750 μg of G418 (active) per ml. For colony formation assays the colonies were fixed and stained with methylene blue in 70% ethanol. The proliferation of Rh30 cell clones was evaluated by cell counting. Briefly, 5 × 104 cells were plated in 60-mm dishes, and cells from triplicate dishes were counted at regular intervals over a 10-day period using a hemocytometer.
Soft-agar and poly-HEMA growth assays.
Soft-agar assays were employed to evaluate anchorage-independent growth (69). Briefly, 5 × 104 viable cells (determined by trypan blue exclusion) were suspended in complete medium plus 10% FBS and 0.4% SeaPlaque GTG agar (FMC). The agar-cell mixtures were plated in triplicate in six-well plates between bottom and top layers of complete growth medium with 0.8% agar. After 2 to 3 weeks, the agar assays were stained with p-iodonitrotetrazolium violet and scored for viable colonies in the 0.4% agar layer. Growth in suspension culture conditions was evaluated using poly-HEMA (2-hydroxyethyl methacrylate; Sigma)-coated plates as previously described (34). Briefly, culture wells of six-well plates were coated with a 5-mg/ml solution of poly-HEMA and allowed to dry. The Rh30 cells (5 × 104 cells) were seeded in 4 ml of complete growth media onto poly-HEMA-coated wells. Cell proliferation was measured at intervals of increasing time in culture by direct cell counting of single-cell suspensions using a hemocytometer or by standard MTT assay of metabolic activity associated with viable cells (57).
Tumor formation.
The tumorigenic potentials of Rh30 cells were evaluated by injection of cell suspensions into SCID mice. For each cell clone tested, five mice received dorsal midline subcutaneous injections of 2 × 106 cells in a volume of 0.2 ml of complete growth media. Tumor formation was evaluated at 25 and 50 days postimplantation at which time the mice were sacrificed by cervical dislocation and the tumors were immediately harvested.
RESULTS
Construction and characterization of a PAX3-KRAB transcriptional repressor.
To test the hypothesis that PAX3 target genes are aberrantly activated by the PAX3-FKHR fusion protein in ARMS, we created a PAX3-KRAB transcriptional repressor that would directly compete with the endogenous PAX3-FKHR chimeric activator in the Rh30 ARMS cell line (Fig. 1B). The 90-amino-acid KRAB repression domain was fused in frame to the COOH terminus of PAX3 at the breakpoint junction (Fig. 2A). We have previously demonstrated that this KRAB(1–90) polypeptide is necessary and sufficient for recruitment of the corepressor KAP-1 (32, 51). As an important negative control, we used the PAX3-KRAB-DV plasmid, which encodes a KRAB domain that cannot bind KAP-1 and is inactive as a transcription repressor (32, 51, 59, 64). PAX3-STOP lacks the FKHR activation domain and lacks the KRAB domain. Additional specificity control was provided by the KOX1-KRAB plasmid lacking PAX3 DNA binding domains.
The chimeric proteins were properly expressed in vivo after transient transfection in COS1 cells and [35S]methionine labeling (Fig. 2B and C). Each protein was detected by immunoprecipitation using either anti-PAX3 and/or anti-KOX1-KRAB sera. As predicted, the PAX3-STOP (half arrow) protein was of lower molecular weight than PAX3 (solid arrow) and was detected by anti-PAX3 but not anti-KOX1-KRAB IgG. The PAX3-KRAB and PAX3-KRAB-DV proteins were detected by both anti-PAX3 and anti-KOX1-KRAB sera, confirming that stable fusion polypeptides were produced. The KRAB(1–90) protein was detected by the anti-KOX1-KRAB IgG as a 10-kDa protein (Fig. 2C). Immunofluorescence microscopy of transfected cells demonstrated that all proteins exhibited nuclear immunoreactivity with the appropriate antibodies (data not shown).
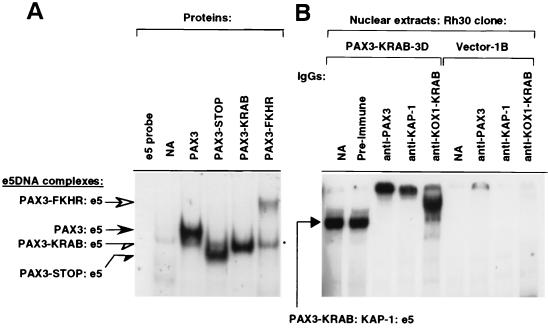
To compare the DNA binding properties of these proteins, each was produced in vitro and tested for binding to the e5 oligonucleotide PAX3 binding site probe in gel shift assays using normalized amounts of lysate (38). The PAX3-KRAB protein binds as well as full-length PAX3 or the truncated PAX3-STOP, but PAX3-FKHR binds less well than wild-type PAX3, as we have previously observed with Rh30 cells (Fig. 3A) (31). Antibody supershift studies verified that the shifted bands in Fig. 3A contained the indicated proteins (data not shown). When the PAX3-KRAB protein was expressed in a stable Rh30 cell clone (clone 3B, see results below) (Fig. 3B) a specific ternary complex was formed with the endogenous KAP-1 corepressor. We could specifically supershift this complex with antisera against PAX3, KOX1-KRAB, or KAP-1 (32). We have previously shown that the PAX3-KRAB-DV protein does not complex with the endogenous KAP-1 corepressor (32, 64). These results confirmed that the Rh30 cell line expresses the endogenous KAP-1 and that the PAX3-KRAB protein is competent both in DNA binding and in corepressor recruitment.
FIG. 3.
(A) EMSA evaluation of binding activities of in vitro-translated PAX3, PAX3-STOP, PAX3-KRAB, and PAX3-FKHR proteins to e5 DNA probe, 5′-ATTAGCACCGTTCC-3′. For the e5 DNA, protein complexes are indicated for PAX3 (solid arrow), PAX3-FKHR (open arrow), PAX3-STOP (solid half arrow), and PAX3-KRAB (open half arrow). The asterisk indicates a nonspecific complex. (B) EMSA analysis of PAX3-KRAB–KAP-1–e5 DNA supershifted complexes (solid arrow) from nuclear extracts of stable PAX3-KRAB Rh30 cell clone 3D but not from vector-transfected clone 1B. The PAX3-KRAB–KAP-1–e5 DNA ternary complex can be supershifted with anti-PAX3, anti-KOX1-KRAB, and anti-KAP-1 IgG antibodies but is unaffected by the addition of preimmune IgGs.
PAX3-KRAB is a repressor in transient-transfection assays.
We next determined the ability of each protein to regulate transcription in transfected cells. Three different reporter plasmids (described in the legend) were used to evaluate whether binding sites for both the paired box and the homeodomain motifs were required together or whether binding sites for either motif alone would be sufficient (Fig. 2A).
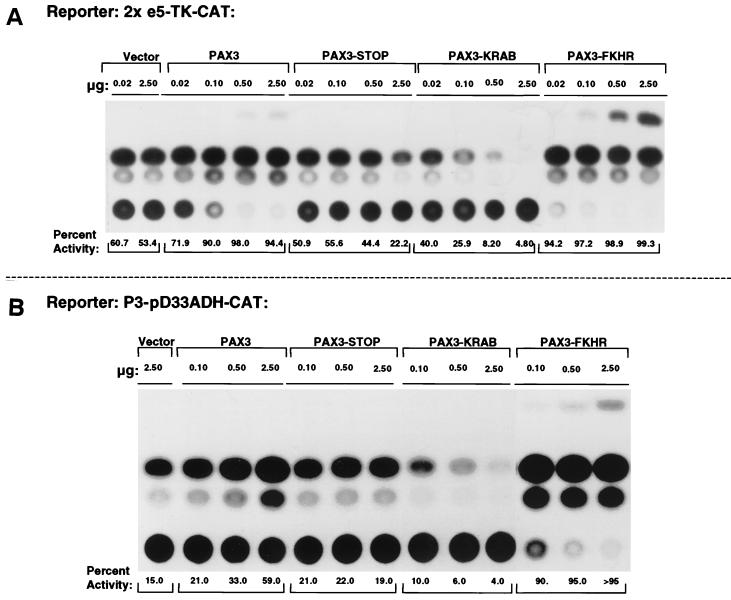
The 2xe5-TK-CAT reporter showed a high basal level activity in NIH 3T3 cells which was unaffected by the empty expression vector over a range of 0.02 to 2.50 μg (Fig. 4A). The same range of cotransfected PAX3 plasmid showed an intermediate level of activation of this reporter. However, PAX3-STOP was essentially neutral (except at 2.5 μg where a slight repression was seen). The PAX3-FKHR plasmid was an extremely potent activator that gave 100% activity with as little as 0.02 μg. Most importantly, PAX3-KRAB functioned as a potent repressor since reporter gene activity was almost abolished at 0.10 μg (Fig. 4A). Identical results were obtained in Rh30 cells (32). We have previously shown that PAX3-based transcriptional regulation shows a dependence on the presence of e5 binding sites in the reporter vectors (data not shown). Furthermore, we have also shown that the repression function is abolished using the PAX3-KRAB-DV mutant protein and that the KRAB domain alone cannot confer repression (32, 51). Hence, it is evident that fusion of the KRAB domain to the neutral PAX3-STOP molecule creates a very potent repressor of transcription. The repressive effect is >25-fold (compare 0.10 μg of PAX3-KRAB to 2.50 μg of PAX3-STOP).
FIG. 4.
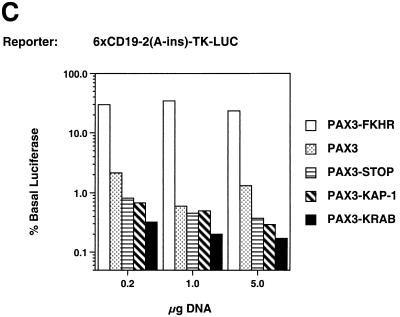
Transcriptional regulatory potentials of the PAX3, PAX3-STOP, PAX3-KRAB, and PAX3-FKHR proteins. Cells were transiently transfected with the indicated amount of expression plasmids along with a constant amount (2.5 μg) of the reporter plasmids (Fig. 2A). (A) 2xe5-TK-CAT reporter activity in NIH 3T3 cells. (B) P3-pD33ADH-CAT reporter activity in Rh30 cells. (C) 6x-CD19-2(A-ins)LUC reporter activity in NIH 3T3 cells. The percentage of basal luciferase refers to the ratio of activity with the indicated expression plasmids to the activity with the vector (4,578.4 relative light units).
Qualitatively, identical results were obtained using the same spectrum of expression plasmids and the P3-pD33ADH-CAT homeodomain reporter in Rh30 cells (Fig. 4B) and the 6xCD19-2(A-ins)LUC reporter in NIH 3T3 cells (Fig. 4C). Thus, the PAX3-KRAB would be expected to repress endogenous target genes with diverse PAX3 binding sites. Furthermore, the results from Rh30 cells imply that PAX3-KRAB-mediated repression is dominant over effects of the endogenous PAX3-FKHR activator.
In efforts to construct more powerful repressors, we also tested a PAX3-KAP-1 plasmid, which was a less-effective repressor than PAX3-KRAB (Fig. 4C). Other repressors with different configurations of the KRAB domain relative to the PAX3 DNA binding domains were also evaluated: KRAB-PAX3, KRAB-PAX3-KRAB, and PAX3-KRAB-KRAB-KRAB. The repression capacities of these other repressors did not exceed that of PAX3-KRAB, as judged by transient transfection (data not shown).
PAX3-KRAB suppresses the transformed phenotype of Rh30 cell clones.
The Rh30 cell exhibits serum-independent and anchorage-independent growth in vitro and is tumorigenic in mice (26, 69). To determine whether stable expression of PAX3-KRAB in Rh30 cells could alter aspects of their malignant growth, we transfected them with the indicated expression vectors (Fig. 2A) and selected for clonal cell lines.
The results of colony formation assays from a pool of transfectants show that the PAX3-KRAB repressor inhibits colony formation in Rh30 cells (Fig. 5). Colony formation was robust and equivalent for populations that received vector, PAX3-STOP, or the PAX3-KRAB-DV mutant. The colony inhibition effects of PAX3-KRAB were dependent on the presence of a functional KRAB repression domain since PAX3-STOP and the PAX3-KRAB-DV mutant had no demonstrable effect. Despite obvious inhibition of colony formation in PAX3-KRAB-transfected populations, a modest number of surviving colonies did arise with kinetics similar to vector or control transfected populations. Further analyses were restricted to clones selected for growth in complete medium (clones that were unable to break through the colony-forming barrier were not studied).
FIG. 5.
PAX3-KRAB inhibits colony formation of Rh30 cells. Equal numbers of Rh30 cells were transfected with equal amounts of the indicated pcDNA3 PAX3 expression plasmids. After 2 weeks, G418-resistant colonies were either stained with methylene blue or isolated as clonal cell lines from a parallel set of unstained dishes.
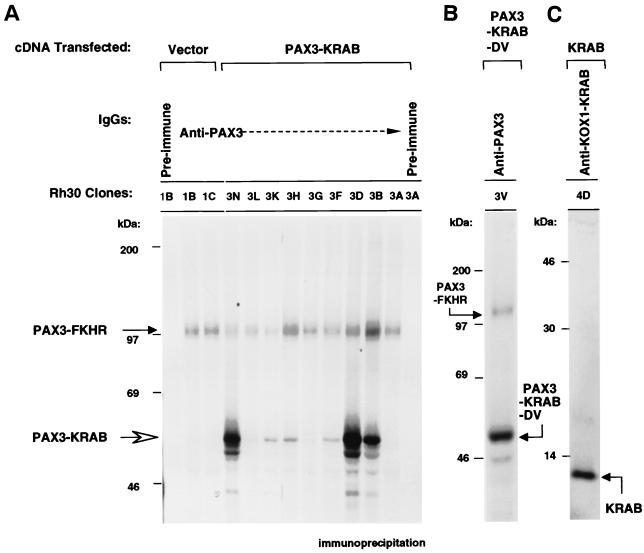
We isolated a spectrum of surviving colonies, expanded them into cell lines, and evaluated each for expression of PAX3-KRAB, as well as of the endogenous PAX3-FKHR protein. Equal numbers of cells were subjected to metabolic labeling and immunoprecipitation with anti-PAX3 sera (Fig. 6A). All clones contain the endogenous 97-kDa PAX3-FKHR protein at roughly equivalent levels. No correlation was observed between expression levels of PAX3-FKHR and PAX3-KRAB, which indicates that PAX3-KRAB does not affect the endogenous PAX3-FKHR level. It is clear that different clones show different levels of PAX3-KRAB expression: clones 3A and 3G do not express detectable PAX3-KRAB, clones 3H and 3K express low levels, and clones 3B, 3N, and 3D express the highest levels (Fig. 6A). PAX3-KRAB expression was also verified using anti-KOX1-KRAB sera (data not shown). Immunoprecipitation analysis was conducted for cell clones that displayed stable high-level expression of PAX3-STOP (data not shown) or for PAX3-KRAB-DV (clone 3V) (Fig. 6B) and for the KRAB domain alone (clone 4D) (Fig. 6C).
FIG. 6.
Expression of PAX3-KRAB, PAX3-KRAB-DV, and the KRAB domain protein in stable Rh30 cell clones. Equal numbers of Rh30 clones were metabolically labeled, and cell lysates were immunoprecipitated with anti-PAX3 IgG (A and B) or anti-KOX1-KRAB (C). (A) PAX3-KRAB protein expression in Rh30 clones. All clones express the endogenous 97-kDa PAX3-FKHR fusion protein (solid arrow). High-level PAX3-KRAB expression was observed from Rh30 clones 3N, 3D, and 3B (open arrow). (B) Expression of the PAX3-KRAB-DV protein in the Rh30 clone 3V. (C) Expression of the KRAB domain protein in the Rh30 cell clone 4D.
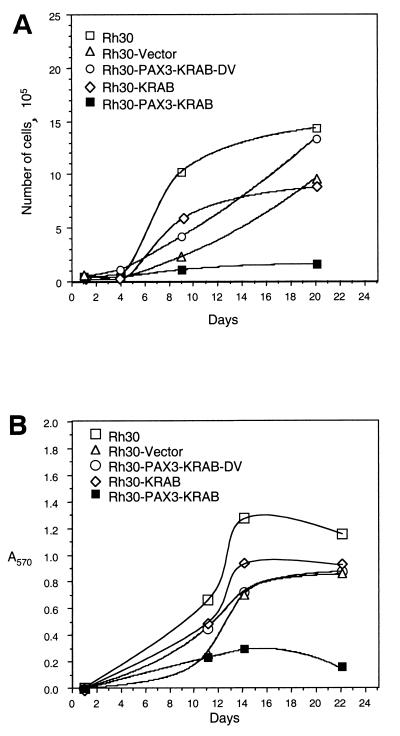
PAX3-KRAB reduces proliferation of Rh30 ARMS cells in low serum.
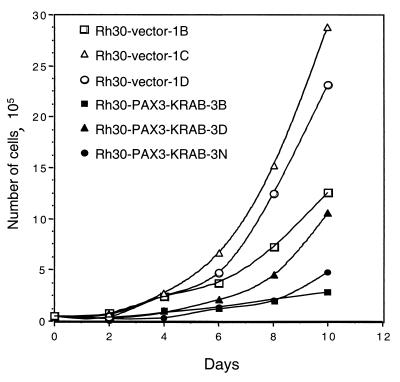
Clones were examined for a correlation between expression of the transfected protein and attributes of the transformed phenotype. All of the clones shown in Fig. 6A, regardless of PAX3-KRAB expression levels, displayed roughly similar growth rates (data not shown) when assayed in complete growth medium and 10% FBS, consistent with the conditions of original colony selection. However, when growth rates were assayed in low-serum conditions (1% FBS), the clones that expressed high levels of PAX3-KRAB had significantly reduced growth potential compared to the vector clones (Fig. 7). This suggests that ARMS cell proliferation becomes critically sensitive to serum growth factors in the presence of PAX3-KRAB. Thus, PAX3 or PAX3-FKHR might couple growth factor signaling pathways to proliferation. Preliminary experiments have shown that control Rh30 clones grow well in defined medium (lacking serum) but that growth of PAX3-KRAB clones was impaired (data not shown).
FIG. 7.
Rh30 cell clones expressing PAX3-KRAB (3B, 3D, and 3N, solid symbols) show reduced proliferation in low-serum media compared with vector clones (1B, 1C, and 1D, open symbols). Equal numbers of each Rh30 cell clone were seeded into triplicate dishes supplemented with 1% FBS and were then counted at 48-h intervals.
PAX3-KRAB converts Rh30 ARMS cells to an anchorage-dependent phenotype.
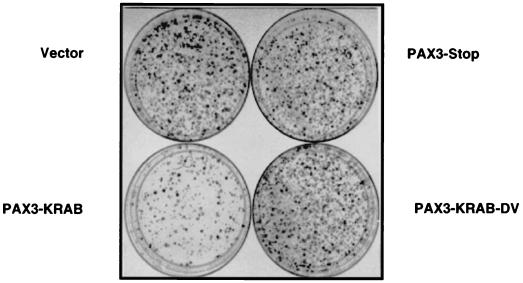
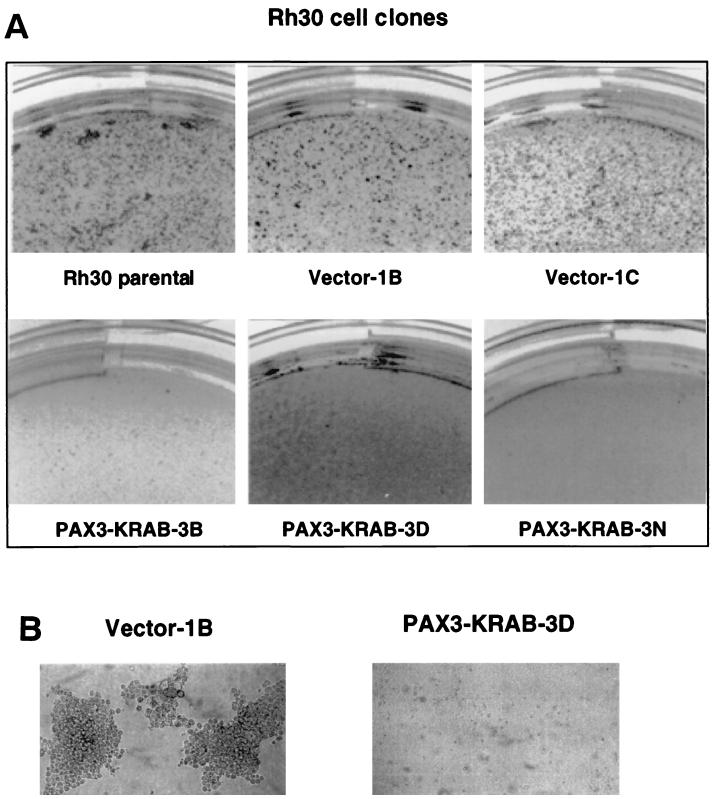
To determine if anchorage-independent growth was affected in the PAX3-KRAB transfectants. Rh30 clones were plated into soft-agar assays with complete growth medium and 10% FBS. Growth was scored and compared to parental Rh30 cells or vector-transfected clones. Representative photographs of the soft-agar assays are demonstrated in Fig. 8. Data for all soft-agar experiments are summarized in Table 1. In Fig. 8A, clones 3B, 3D, and 3N which show moderate to high-level expression of PAX3-KRAB (Fig. 6A) fail to form colonies in soft agar. Overall, 90% of the clones (9 of 10) expressing PAX3-KRAB showed inhibition of soft-agar growth, whereas 85% of PAX3-KRAB expression-negative clones (11 of 13) showed positive colony growth. Vector-transfected clones 1B and 1C formed robust colonies in soft agar similar to the parental Rh30 cells, as did all vector-transfected clones (15 of 15) (Table 1). For Rh30 cell clones expressing a low level of PAX3-KRAB (e.g., 3H and 3K in Fig. 6A), partial inhibitory effects were observed on soft-agar growth (data not shown). The Rh30 clone expressing the PAX3-KRAB-DV mutant repressor, e.g., 3V, and those clones expressing the KRAB domain alone, e.g., clone 4D in Fig. 6A, revealed no impairment of colony-forming potential. One Rh30 clone that expressed a very high level of PAX3-STOP failed to grow in soft agar; however, two clones which showed no PAX3-STOP expression also failed to grow in soft agar.
FIG. 8.
(A) Rh30 cell clones expressing PAX3-KRAB (3B, 3D, and 3N) show reduced colony formation in soft agar compared with vector clones (1B and 1C) or parental Rh30 cells. An equal number of the indicated Rh30 clones (5 × 104 cells) was suspended in 0.4% agarose, and colonies were photographed at 14 to 21 days. (B) Soft-agar colonies of Rh30 cell clones (vector 1B versus PAX3-KRAB-3D). Phase-contrast magnification, ×20.
TABLE 1.
Summary of Rh30 cell clone growth in soft agar
| Cell clone | No. of clones | Expression status: no. of clonesa | No. of clones showing growth in soft agar/total no. |
|---|---|---|---|
| Vector | 15 | NA | 15/15 |
| PAX3-KRAB | 23 | Positive: 10 | 1/10 |
| Negative: 13 | 11/13 | ||
| PAX3-KRAB-DV | 6 | Positive: 1 | 1/1 |
| Negative: 5 | 5/5 | ||
| PAX3-STOP | 6 | Positive: 1 | 0/1 |
| Negative: 5 | 3/5 | ||
| KRAB | 6 | Positive: 3 | 3/3 |
| Negative: 3 | 3/3 |
NA, not applicable.
Cell proliferation assays in poly-HEMA-coated dishes were conducted as an independent evaluation of the anchorage dependency of the Rh30 cell clones. The results for cell number and for viable cells by MTT assay are plotted over time in culture in Fig. 9A and B, respectively. These data further show specificity of inhibition in that growth in poly-HEMA for the PAX3-KRAB-DV clone and the KRAB-domain-only clone was equivalent to parental Rh30 or vector controls. These additional findings confirm that PAX3-KRAB expression converts Rh30 cells from an anchorage-independent to an anchorage-dependent phenotype and are consistent with the notion that PAX3-KRAB may have repressed target gene(s) with a critical role in ARMS tumorigenicity.
FIG. 9.
Inhibition of anchorage-independent growth of PAX3-KRAB Rh30 clone 3D (■) in poly-HEMA-coated plates compared to control Rh30 clones (□, parental cells; ▵, vector clone 1B; ○, PAX3-KRAB-DV clone 3V; ◊, KRAB clone 4D). Equal numbers of the indicated Rh30 clones (5 × 104 cells) were seeded into poly-HEMA-coated wells in RPMI 1640 plus 10% FBS. Cell proliferation was measured over time in culture by direct cell counting (A) or by MTT assay (B).
We tested whether PAX3-KRAB would inhibit the growth of the embryonal rhabdomyosarcoma RD cell line. Although of similar myogenic lineage, RD cells do not contain the t(2;13) translocation (or any other known alteration of PAX genes) and hence do not express PAX3-FKHR, although they do express a low level of the PAX3 protein (data not shown). Transfection of PAX3-KRAB into the RD cells had no effect on colony survival (data not shown). We isolated stable RD clones that expressed PAX3-KRAB and found that PAX3-KRAB-transfected RD cells grew as well in soft agar as vector-transfected control clones (data not shown). The absence of effect on RD cell growth suggests that PAX3-KRAB is not a generalized inhibitor of cell growth and that its inhibitory activity is specific to the ARMS malignant phenotype.
PAX3-KRAB suppresses the tumorigenicity of Rh30 cells.
These results suggest a strong correlation between expression of the PAX3-KRAB gene and suppression of the in vitro malignant growth characteristics of Rh30 ARMS cells. To determine if this suppression extended to tumor formation in vivo, we evaluated the potential of stable Rh30 PAX3-KRAB cell clones to form tumors in SCID mice. The tumor incidence data are summarized in Table 2. The Rh30 cell clones transfected with vector alone (clone 1B) or clones expressing either no detectable PAX3-KRAB (clone 3A) or high levels (clone 3D) were implanted into SCID mice. Four of five mice that received vector clone 1B cells and all five mice that received clone 3A cells displayed grossly palpable tumors within the 25- to 50-day period of evaluation. In contrast, only one of the five -mice that received the PAX3-KRAB-expressing cells (clone 3D) showed evidence of tumor formation. Tumors from clones 1B and 3A arose much earlier and were much larger (data not shown) than the tumor which arose with long latency in the mouse that received PAX3-KRAB clone 3D cells. It was also observed that the control Rh30 tumors derived from clones 1B and 3A (PAX3-KRAB negative clones) were loosely structured upon excision and hemorrhagic. The tumor from the PAX3-KRAB-positive cells was nonhemorrhagic, and the boundaries were more circumscribed. These results suggest a compelling correlation between expression of PAX3-KRAB repressor and suppression of the tumorigenic phenotype in Rh30 ARMS cells.
TABLE 2.
Incidence of Rh30 cell clone tumors in SCID mice
| Rh30 cell clone | PAX3-KRAB expression | Tumor incidencea at:
|
Totalb | |
|---|---|---|---|---|
| Day 25 | Day 50 | |||
| Vector 1B | NAc | 3/5 | 1/5 | 4/5 |
| PAX3-KRAB-3A | Negative | 3/5 | 2/5 | 5/5 |
| PAX3-KRAB-3D | Positive | 0/5 | 1/5 | 1/5 |
Number of animals with tumors/total number of animals.
Total number of animals with tumors/total number of animals.
NA, not applicable.
DISCUSSION
In order to evaluate the dependence of the ARMS Rh30 malignant phenotype on the transcriptional activation potential of the endogenous PAX3-FKHR generated by the t(2;13) translocation, we employed a strategy designed to directly compete with the oncogenic transcriptional activator and repress PAX3 target genes with an engineered repressor, PAX3-KRAB. Dominantly interfering with endogenous cellular activators using heterologous repressors is an approach which has also been successfully used in other studies (5, 47). Our biochemical comparisons showed that PAX3-KRAB protein bound to a canonical PAX3 target DNA sequence with similar affinity to wild-type PAX3 protein and greater affinity than the PAX3-FKHR protein. In transient-transfection assays, PAX3-KRAB could efficiently repress reporter plasmids containing different types of PAX binding sites: either paired domain sites alone, homeodomain sites alone, or both paired and homeodomain sites together. Thus, PAX3-KRAB-mediated repression did not show an obvious bias toward a particular type of recognition site. Furthermore, PAX3-KRAB was also found to dominantly repress PAX3 reporters in Rh30 cells containing the endogenous PAX3-FKHR transcriptional activator. These findings lend confidence to our interpretation that the PAX3-KRAB repressor would be well suited to compete with PAX3-FKHR and cause repression of endogenous PAX3 target genes.
Our data clearly show that expression of the PAX3-KRAB repressor alters the malignant growth properties of ARMS cells that express the endogenous PAX3-FKHR protein. The Rh30 cell line was transfected with the PAX3-KRAB plasmid, and stable cell lines able to grow as colonies were expanded and analyzed. The colony-forming ability of PAX3-KRAB-transfected Rh30 cells was diminished compared with cells transfected with control plasmids (vector, PAX3-STOP, or the PAX3-KRAB-DV, with a mutant KRAB domain). Clonal cell lines were tested for (i) expression of the transfected genes (PAX3-KRAB, PAX3-KRAB-DV, PAX3-STOP, or KRAB), (ii) growth in low-serum media, (iii) anchorage-independent growth in soft-agar or poly-HEMA-coated dishes, and (iv) tumorigenicity in SCID mice. Rh30 vector clones grew well in low serum, readily formed colonies in soft agar, and grew as tumors in SCID mice. The stable PAX3-KRAB Rh30 clones exhibited reduced growth in low-serum media, an inhibited anchorage-independent growth potential, and a suppressed tumorigenicity in SCID mice. However, anchorage-independent growth was not affected for the PAX3-KRAB-DV Rh30 clones lacking functional KRAB domains or the clones with a KRAB domain lacking PAX3 DNA binding domains. Furthermore, PAX3-KRAB did not inhibit colony formation or anchorage-independent growth of RD cells that lack a t(2:13) translocation. Our data show that the PAX3-KRAB repressor has inhibited the malignant growth of Rh30 cells, which underscores the hypothesis that the malignant phenotype depends on cellular growth pathways that are aberrantly regulated by the translocation-generated oncogenic activator PAX3-FKHR.
In spite of findings that PAX3-FKHR can transform heterologous cells in vitro, current evidence supports the idea that PAX3-FKHR may require other factors for full tumorigenesis. CEF cells infected with a PAX3-FKHR virus can form transformed foci and display anchorage-independent growth in vitro but do not form chick wing web tumors (68). Furthermore, the presence of several defects in ARMS tumors and cell lines involving other pathways associated with transformation has made interpretation of the specific contributions of PAX3-FKHR to the overall malignant phenotype difficult (2). The Rh30 cell line is known to contain mutated p53 and elevated expression of the N-MYC, GLI, and CDK4 genes (24, 44, 45). These other alterations may have aided in obtaining stable Rh30 clones that expressed high levels of PAX3-KRAB yet were able to grow as colonies in vitro. However, PAX3-KRAB expression would not be expected to reverse the effect of these other genetic alterations. Although other factors in addition to PAX3-FKHR may contribute to the malignant phenotype of ARMS, PAX3-KRAB exerts a dominant inhibitory effect on growth.
One of the additional factors believed to cooperate with PAX3-FKHR in malignant transformation in ARMS is insulin-like growth factor II (IGF-II) (36, 55, 73). Cell biological studies have elucidated that ARMS cell lines are highly dependent on IGF-II–IGF-IR signaling pathways for autocrine growth regulation (26, 56, 69). A relationship between IGF-II and PAX3-FKHR is supported by recent evidence that PAX3-FKHR-transfected NIH 3T3 cells have elevated expression of IGF-II and IGF Binding Protein 5 (IGFBP5) (43). IGFBP5 is involved in the regulation of IGF-I- and IGF-II-mediated cellular proliferation responses (30). Our preliminary studies have found altered levels of IGF-II and IGF-I receptor transcripts in PAX3-KRAB Rh30 clones (W. J. Fredericks and F. J. Rauscher III, unpublished data). It will be important to clarify whether IGF-II and IGFBP5 are direct targets for PAX3-FKHR in ARMS.
We have shown that constitutive expression of PAX3-KRAB in Rh30 cells has altered both serum-independent and anchorage-independent growth phenotypes. Rh30 cell colony-forming ability (under full serum conditions) was inhibited by PAX3-KRAB; yet, once selected for colony growth, the PAX3-KRAB clones were able to grow under full serum conditions at a rate similar to the vector clones. However, the PAX3-KRAB cell clones were inhibited under low-serum (and serum-free) conditions. PAX3-KRAB may be affecting a component of a signaling pathway that is compensated for by growth in high serum. Full serum conditions, however, did not suffice to prevent anchorage-independent growth inhibition; hence, these two phenotypes are separable. It will be interesting to determine whether the previously described Rh30 cell autocrine growth mechanisms involving IGF-II and the hepatocyte scatter factor ligand for the c-MET receptor are affected in PAX3-KRAB-expressing cells (26, 29). PAX3-KRAB has probably inhibited transcriptional pathways important for both serum-independent and for anchorage-independent growth. Validation of our hypothesis will require future identification of target genes that are aberrantly activated by PAX3-FKHR and repressed by PAX3-KRAB.
Studies of gene expression defects in mutant Pax-3 splotch mice have brought attention to several candidate target genes with possible functional association with proliferation or tumorigenesis. These include the DNA replication licensing factor, cdc-46 (39), and the receptor for platelet-derived growth factor α (pdgfrα) (21, 28). Recognition that ARMS may be derived from myogenic precursor cells suggests that studies of PAX3-dependent pathways in muscle development might clarify how deregulation by PAX3-FKHR could play a role in ARMS (1, 10, 22).
Early in murine muscle development, Pax-3 expression correlates with expression of the c-Met and Lbx1 genes involved in cellular migration to the limb bud (18, 23, 67). Later, expression of Pax-3 commits limb myoblasts to a program of cell cycle exit and a cascade of induction of myogenic terminal differentiation factors: Myf-5, MyoD, and myogenin (11). In contrast, ARMS tumor cells are simultaneously proliferative and express differentiation markers (MYOD, myogenin, and myofibrillar proteins) (2, 43). Understanding the defects in ARMS that allow coexistence of these typically opposite states will undoubtedly require development of focused biological systems for definition of the target genes aberrantly regulated by PAX3-FKHR.
The c-MET proto-oncogene has an established role in tumor invasiveness and angiogenesis (29). Elevated c-MET expression in ARMS tumor specimens and in PAX3-FKHR-transfected ERMS RD cells supports the idea that c-MET might be deregulated by PAX3-FKHR in ARMS (37). Our preliminary studies have found that c-MET transcript levels were unchanged in PAX3-KRAB Rh30 clones, suggesting that PAX3-KRAB-mediated inhibition of the malignant phenotype was not due to repression of c-MET (Fredericks and Rauscher, unpublished). Amplification of the c-MET gene in the Rh30 cell line may have altered its potential for repression by PAX3-KRAB (29). The fact that splotch mice still contain c-Met-positive migratory cells suggests that other factors in addition to PAX3 can promote c-MET expression (54).
In summary, we have described a new system for targeting oncogenic transcriptional activators with engineered repressors. This, in combination with a new conditional PAX3-repressor system (currently under development), should be useful for definition of differentially repressed target genes involved in reversion of the malignant phenotype in ARMS.
ACKNOWLEDGMENTS
We thank Giovanni Rovera, Naomi Galili, and Sunil Mukopadhyay for the anti-FKHR antiserum and the PAX3-FKHR mouse-human hybrid plasmid. We thank J. Biegel for the RD cell line. We also thank Beat Schafer for the 6x-CD19-2(A-ins)LUC reporter plasmid and Claude Desplan for the P3-pD33ADH-CAT reporter plasmid. Peter Sobaille of the Dr. Meenhard Herlyn laboratory at the Wistar Institute provided assistance with tumorigenicity studies in SCID mice. George Prendergast, Jennifer Morris, and Laura Benjamin provided helpful suggestions. We are grateful to Thanos Halazonetis, Mark Lechner, Hongzhuang Peng, and David Schultz for their editorial comments.
W.J.F. was supported by the Wistar Basic Cancer Research Training Grant CA 09171. J.R.F. was supported by the Medical Science Training Program, University of Pennsylvania School of Medicine. F.J.R. is supported in part by National Institutes of Health grants CA 52009, Core grant CA 10815, DK 49210, GM 54220, DAMD 17-96-1-6141, and ACS NP-954; the Irving A. Hansen Memorial Foundation; the Mary A. Rumsey Memorial Foundation; and the Pew Scholars Program in the Biomedical Sciences.
REFERENCES
- 1.Amthor H, Christ B, Patel K. A molecular mechanism enabling continuous embryonic muscle growth—a balance between proliferation and differentiation. Development. 1999;126:1041–1053. doi: 10.1242/dev.126.5.1041. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J, Gordon A, Pritchard-Jones K, Shipley J. Genes, chromosomes, and rhabdomyosarcoma. Genes Chromosomes Cancer. 1999;26:275–285. [PubMed] [Google Scholar]
- 3.Ayala J E, Streeper R S, Desgrosellier J S, Durham S K, Suwanichkul A, Svitek C A, Goldman J K, Barr F G, Powell D R, O'Brien R M. Conservation of an insulin response unit between mouse and human glucose-6-phosphatase catalytic subunit gene promoters: transcription factor FKHR binds the insulin response sequence. Diabetes. 1999;48:1885–1889. doi: 10.2337/diabetes.48.9.1885. [DOI] [PubMed] [Google Scholar]
- 4.Barr F G. The role of chimeric paired box transcription factors in the pathogenesis of pediatric rhabdomysarcoma. Cancer Res. 1999;59:1711s–1715s. [PubMed] [Google Scholar]
- 5.Beerli R R, Segal D J, Dreier B, Barbas C F., III Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc Natl Acad Sci USA. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendall A J, Ding J, Hu G, Shen M M, Abate-Shen C. Msx1 antagonizes the myogenic activity of Pax3 in migrating limb muscle precursors. Development. 1999;126:4965–4976. doi: 10.1242/dev.126.22.4965. [DOI] [PubMed] [Google Scholar]
- 7.Bennicelli J L, Advani S, Schafer B W, Barr F G. PAX3 and PAX7 exhibit conserved cis-acting transcription repression domains and utilize a common gain of function mechanism in alveolar rhabdomyosarcoma. Oncogene. 1999;18:4348–4356. doi: 10.1038/sj.onc.1202812. [DOI] [PubMed] [Google Scholar]
- 8.Bennicelli J L, Fredericks W J, Wilson R B, Rauscher III F J, Barr F G. Wild type PAX3 protein and the PAX3-FKHR fusion protein of alveolar rhabdomyosarcoma contain potent, structurally distinct transcriptional activation domains. Oncogene. 1995;11:119–130. [PubMed] [Google Scholar]
- 9.Bernasconi M, Remppis A, Fredericks W J, Rauscher III F J, Schafer B W. Induction of apoptosis in rhabdomyosarcoma cells through down-regulation of PAX proteins. Proc Natl Acad Sci USA. 1996;93:13164–13169. doi: 10.1073/pnas.93.23.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borycki A G, Li J, Jin F, Emerson C P, Epstein J A. Pax3 functions in cell survival and in pax7 regulation. Development. 1999;126:1665–1674. doi: 10.1242/dev.126.8.1665. [DOI] [PubMed] [Google Scholar]
- 11.Brand-Saberi B, Christ B. Genetic and epigenetic control of muscle development in vertebrates. Cell Tissue Res. 1999;296:199–212. doi: 10.1007/s004410051281. [DOI] [PubMed] [Google Scholar]
- 12.Chalepakis G, Goulding M, Read A, Strachan T, Gruss P. Molecular basis of splotch and Waardenburg Pax-3 mutations. Proc Natl Acad Sci USA. 1994;91:3685–3689. doi: 10.1073/pnas.91.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalepakis G, Jones F S, Edelman G M, Gruss P. Pax-3 contains domains for transcription activation and transcription inhibition. Proc Natl Acad Sci USA. 1994;91:12745–12749. doi: 10.1073/pnas.91.26.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalepakis G, Wijnholds J, Gruss P. Pax-3-DNA interaction: flexibility in the DNA binding and induction of DNA conformational changes by paired domains. Nucleic Acids Res. 1994;22:3131–3137. doi: 10.1093/nar/22.15.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobaleda C, Perez-Losada J, Sanchez-Garcia I. Chromosomal abnormalities and tumor development: from genes to therapeutic mechanisms. Bioessays. 1998;20:922–930. doi: 10.1002/(SICI)1521-1878(199811)20:11<922::AID-BIES7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Czerny T, Schaffner G, Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- 17.Dahl E, Koseki H, Balling R. Pax genes and organogenesis. Bioessays. 1997;19:755–765. doi: 10.1002/bies.950190905. [DOI] [PubMed] [Google Scholar]
- 18.Daston G, Lamar E, Olivier M, Goulding M. Pax-3 is necessary for migration but not differentiation of limb muscle precursors in the mouse. Development. 1996;122:1017–1027. doi: 10.1242/dev.122.3.1017. [DOI] [PubMed] [Google Scholar]
- 19.Datta S R, Brunet A, Greenberg M E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 20.Davis R J, D'Cruz C M, Lovell M A, Biegel J A, Barr F G. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 1994;54:2869–2872. [PubMed] [Google Scholar]
- 21.Dickman E D, Rogers R, Conway S J. Abnormal skeletogenesis occurs coincident with increased apoptosis in the Splotch (Sp2H) mutant: putative roles for Pax3 and PDGFRalpha in rib patterning. Anat Rec. 1999;255:353–361. doi: 10.1002/(SICI)1097-0185(19990701)255:3<353::AID-AR11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich S. Regulation of hypaxial muscle development. Cell Tissue Res. 1999;296:175–182. doi: 10.1007/s004410051278. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–1629. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
- 24.Douglass E C, Valentine M, Etcubanas E, Parham D, Webber B L, Houghton P J, Houghton J A, Green A A. A specific chromosomal abnormality in rhabdomyosarcoma. Cytogenet Cell Genet. 1987;45:148–155. doi: 10.1159/000132446. . (Erratum, 47:232, 1988.) [DOI] [PubMed] [Google Scholar]
- 25.Durham S K, Suwanichkul A, Scheimann A O, Yee D, Jackson J G, Barr F G, Powell D R. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology. 1999;140:3140–3146. doi: 10.1210/endo.140.7.6856. [DOI] [PubMed] [Google Scholar]
- 26.El-Badry O M, Minniti C, Kohn E C, Houghton P J, Daughaday W H, Helman L J. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1:325–331. [PubMed] [Google Scholar]
- 27.Epstein J A, Shapiro D N, Cheng J, Lam P Y, Maas R L. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci USA. 1996;93:4213–4218. doi: 10.1073/pnas.93.9.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein J A, Song B, Lakkis M, Wang C. Tumor-specific PAX3-FKHR transcription factor, but not PAX3, activates the platelet-derived growth factor alpha receptor. Mol Cell Biol. 1998;18:4118–4130. doi: 10.1128/mcb.18.7.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferracini R, Olivero M, Di Renzo M F, Martano M, De Giovanni C, Nanni P, Basso G, Scotlandi K, Lollini P L, Comoglio P M. Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene. 1996;12:1697–1705. [PubMed] [Google Scholar]
- 30.Ferry R J, Jr, Katz L E, Grimberg A, Cohen P, Weinzimer S A. Cellular actions of insulin-like growth factor binding proteins. Horm Metab Res. 1999;31:192–202. doi: 10.1055/s-2007-978719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredericks W J, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr F G, Rauscher F J., III The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcomas is a more potent transcriptional activator than PAX3. Mol Cell Biol. 1995;15:1522–1535. doi: 10.1128/mcb.15.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman J R, Fredericks W J, Jensen D E, Speicher D W, Huang X P, Neilson E G, Rauscher F J., III KAP-1, a novel co-repressor for the highly-conserved KRAB repression domain. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 33.Fukazawa H, Mizuno S, Uehara Y. A microplate assay for quantitation of anchorage-independent growth of transformed cells. Anal Biochem. 1995;228:83–90. doi: 10.1006/abio.1995.1318. [DOI] [PubMed] [Google Scholar]
- 34.Galibert M D, Yavuzer U, Dexter T J, Goding C R. Pax3 and regulation of the melanocyte-specific tyrosinase-related protein-1 promoter. J Biol Chem. 1999;274:26894–26900. doi: 10.1074/jbc.274.38.26894. [DOI] [PubMed] [Google Scholar]
- 35.Galili N, Davis R J, Fredericks W J, Mukhopadhyay S, Rauscher III F J, Emanuel B S, Rovera G, Barr F G. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 36.Genini M, Schwalbe P, Scholl F A, Schafer B W. Isolation of genes differentially expressed in human primary myoblasts and embryonal rhabdomyosarcoma. Int J Cancer. 1996;66:571–577. doi: 10.1002/(SICI)1097-0215(19960516)66:4<571::AID-IJC24>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Ginsberg J P, Davis R J, Bennicelli J L, Nauta L E, Barr F G. Upregulation of MET but not neural cell adhesion molecule expression by the PAX3-FKHR fusion protein in alveolar rhabdomyosarcoma. Cancer Res. 1998;58:3542–3546. [PubMed] [Google Scholar]
- 38.Goulding M D, Chalepakis G, Deutsch U, Erselius J R, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill A L, Phelan S A, Loeken M R. Reduced expression of pax-3 is associated with overexpression of cdc46 in the mouse embryo. Dev Genes Evol. 1998;208:128–134. doi: 10.1007/s004270050163. [DOI] [PubMed] [Google Scholar]
- 40.Hollenbach A D, Sublett J E, McPherson C J, Grosveld G. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J. 1999;18:3702–3711. doi: 10.1093/emboj/18.13.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inukai T, Inaba T, Yoshihara T, Look A T. Cell transformation mediated by homodimeric E2A-HLF transcription factors. Mol Cell Biol. 1997;17:1417–1424. doi: 10.1128/mcb.17.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inukai T, Inoue A, Kurosawa H, Goi K, Shinjyo T, Ozawa K, Mao M, Inaba T, Look A T. SLUG, a ces-1-related zinc finger transcription factor gene with antiapoptotic activity, is a downstream target of the E2A-HLF oncoprotein. Mol Cell. 1999;4:343–352. doi: 10.1016/s1097-2765(00)80336-6. [DOI] [PubMed] [Google Scholar]
- 43.Khan J, Bittner M L, Saal L H, Teichmann U, Azorsa D O, Gooden G C, Pavan W J, Trent J M, Meltzer P S. cDNA microarrays detect activation of a myogenic transcription program by the PAX3-FKHR fusion oncogene. Proc Natl Acad Sci USA. 1999;96:13264–13269. doi: 10.1073/pnas.96.23.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan J, Simon R, Bittner M, Chen Y, Leighton S B, Pohida T, Smith P D, Jiang Y, Gooden G C, Trent J M, Meltzer P S. Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res. 1998;58:5009–5013. [PubMed] [Google Scholar]
- 45.Kouraklis G, Triche T J, Wesley R, Tsokos M. Myc oncogene expression and nude mouse tumorigenicity and metastasis formation are higher in alveolar than embryonal rhabdomyosarcoma cell lines. Pediatr Res. 1999;45:552–558. doi: 10.1203/00006450-199904010-00015. [DOI] [PubMed] [Google Scholar]
- 46.Luckow B, Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Z Q, Tsai M J, Tsai S Y. Suppression of gene expression by tethering KRAB domain to promoter of ER target genes. J Steroid Biochem Mol Biol. 1999;69:155–163. doi: 10.1016/s0960-0760(98)00154-x. [DOI] [PubMed] [Google Scholar]
- 48.Magnaghi P, Roberts C, Lorain S, Lipinski M, Scambler P J. HIRA, a mammalian homologue of Saccharomyces cerevisiae transcriptional co-repressors, interacts with Pax3. Nat Genet. 1998;20:74–77. doi: 10.1038/1739. [DOI] [PubMed] [Google Scholar]
- 49.Mansouri A, Goudreau G, Gruss P. Pax genes and their role in organogenesis. Cancer Res. 1999;59:1707s–1710. [PubMed] [Google Scholar]
- 50.Mansouri A, Gruss P. Pax3 and Pax7 are expressed in commissural neurons and restrict ventral neuronal identity in the spinal cord. Mech Dev. 1998;78:171–178. doi: 10.1016/s0925-4773(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 51.Margolin J F, Friedman J R, Meyer W K, Vissing H, Thiesen H J, Rauscher F J., III Kruppel-associated boxes are potent transcriptional repression domains. Proc Natl Acad Sci USA. 1994;91:4509–4513. doi: 10.1073/pnas.91.10.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maroto M, Reshef R, Munsterberg A E, Koester S, Goulding M, Lassar A B. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- 53.May W A, Lessnick S L, Braun B S, Klemsz M, Lewis B C, Lunsford L B, Hromas R, Denny C T. The Ewing's sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol. 1993;13:7393–7398. doi: 10.1128/mcb.13.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mennerich D, Schafer K, Braun T. Pax-3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech Dev. 1998;73:147–158. doi: 10.1016/s0925-4773(98)00046-x. [DOI] [PubMed] [Google Scholar]
- 55.Merlino G, Helman L J. Rhabdomyosarcoma—working out the pathways. Oncogene. 1999;18:5340–5348. doi: 10.1038/sj.onc.1203038. [DOI] [PubMed] [Google Scholar]
- 56.Minniti C P, Luan D, O'Grady C, Rosenfeld R G, Oh Y, Helman L J. Insulin-like growth factor II overexpression in myoblasts induces phenotypic changes typical of the malignant phenotype. Cell Growth Differ. 1995;6:263–269. [PubMed] [Google Scholar]
- 57.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 58.Mu Z M, Le X F, Glassman A B, Chang K S. The biologic function of PML and its role in acute promyelocytic leukemia. Leuk Lymphoma. 1996;23:277–285. doi: 10.3109/10428199609054830. [DOI] [PubMed] [Google Scholar]
- 59.Peng H, Begg G E, Schultz D C, Friedman J R, Jensen D E, Speicher D W, Rauscher F J., III Reconstitution of the KRAB-KAP-1 repressor complex: a model system for defining the molecular anatomy of RING-B box-coiled-coil domain-mediated protein-protein interactions. J Mol Biol. 2000;295:1139–1162. doi: 10.1006/jmbi.1999.3402. [DOI] [PubMed] [Google Scholar]
- 60.Peso L, Gonzalez V M, Hernandez R, Barr F G, Nunez G. Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene. 1999;18:7328–7333. doi: 10.1038/sj.onc.1203159. [DOI] [PubMed] [Google Scholar]
- 61.Phelan S A, Ito M, Loeken M R. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–1197. doi: 10.2337/diab.46.7.1189. [DOI] [PubMed] [Google Scholar]
- 62.Phelan S A, Loeken M R. Identification of a new binding motif for the paired domain of Pax-3 and unusual characteristics of spacing of bipartite recognition elements on binding and transcription activation. J Biol Chem. 1998;273:19153–19159. doi: 10.1074/jbc.273.30.19153. [DOI] [PubMed] [Google Scholar]
- 63.Rauscher F J III, Vogt P, editors. Chromosomal translocations and oncogenic transcription factors. Vol. 220. Berlin, Germany: Springer-Verlag; 1997. [Google Scholar]
- 64.Ryan R F, Schultz D C, Ayyanathan K, Singh P B, Friedman J R, Fredericks W J, Rauscher F J., III KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol Cell Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez-Garcia I. Consequences of chromosomal abnormalities in tumor development. Annu Rev Genet. 1997;31:429–453. doi: 10.1146/annurev.genet.31.1.429. [DOI] [PubMed] [Google Scholar]
- 66.Schafer B W, Czerny T, Bernasconi M, Genini M, Busslinger M. Molecular cloning and characterization of a human PAX-7 cDNA expressed in normal and neoplastic myocytes. Nucleic Acids Res. 1994;22:4574–4582. doi: 10.1093/nar/22.22.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schafer K, Braun T. Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat Genet. 1999;23:213–216. doi: 10.1038/13843. [DOI] [PubMed] [Google Scholar]
- 68.Scheidler S, Fredericks W J, Rauscher III F J, Barr F G, Vogt P K. The hybrid PAX3-FKHR fusion protein of alveolar rhabdomyosarcoma transforms fibroblasts in culture. Proc Natl Acad Sci USA. 1996;93:9805–9809. doi: 10.1073/pnas.93.18.9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shapiro D N, Jones B G, Shapiro L H, Dias P, Houghton P J. Antisense-mediated reduction in insulin-like growth factor-I receptor expression suppresses the malignant phenotype of a human alveolar rhabdomyosarcoma. J Clin Investig. 1994;94:1235–1242. doi: 10.1172/JCI117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka T, Mitani K, Kurokawa M, Ogawa S, Tanaka K, Nishida J, Yazaki Y, Shibata Y, Hirai H. Dual functions of the AML1/Evi-1 chimeric protein in the mechanism of leukemogenesis in t(3;21) leukemias. Mol Cell Biol. 1995;15:2383–2392. doi: 10.1128/mcb.15.5.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tassabehji M, Newton V E, Leverton K, Turnbull K, Seemanova E, Kunze J, Sperling K, Strachan T, Read A P. PAX3 gene structure and mutations: close analogies between Waardenburg syndrome and the Splotch mouse. Hum Mol Genet. 1994;3:1069–1074. doi: 10.1093/hmg/3.7.1069. [DOI] [PubMed] [Google Scholar]
- 73.Wang W, Kumar P, Epstein J, Helman L, Moore J V, Kumar S. Insulin-like growth factor II and PAX3-FKHR cooperate in the oncogenesis of rhabdomyosarcoma. Cancer Res. 1998;58:4426–4433. [PubMed] [Google Scholar]
- 74.Watanabe A, Takeda K, Ploplis B, Tachibana M. Epistatic relationship between Waardenburg syndrome genes MITF and PAX3. Nat Genet. 1998;18:283–286. doi: 10.1038/ng0398-283. [DOI] [PubMed] [Google Scholar]
- 75.Wiggan O, Taniguchi-Sidle A, Hamel P A. Interaction of the pRB-family proteins with factors containing paired-like homeodomains. Oncogene. 1998;16:227–236. doi: 10.1038/sj.onc.1201534. [DOI] [PubMed] [Google Scholar]
- 76.Wilson D, Sheng G, Lecuit T, Dostatni N, Desplan C. Cooperative dimerization of paired class homeo domains on DNA. Genes Dev. 1993;7:2120–2134. doi: 10.1101/gad.7.11.2120. [DOI] [PubMed] [Google Scholar]