Coronavirus disease 2019 (COVID-19) is rampant worldwide and has affected more than 215 countries and regions. According to the World Health Organization’s report, there were 226,018,919 COVID-19 cases and 4,654,898 deaths as of March 14, 2021, although 300,002,228 vaccines have been administered globally. COVID-19 patients have different clinical phenotypes and can be divided into asymptomatic, normal, mild, severe, and critical patients. However, the factors that lead to severe/critical cases remain controversial, and microecological dysfunction is one of the potential causes (Saleh et al., 2020). Previous studies have reported that the gut microbiome affects the severity of COVID-19 (Zuo et al., 2020; Lv et al., 2021), but the correlations between the respiratory microbiome (RM) and COVID-19 severity have seldom been detected. Since the RM is closely related to the occurrence of COVID-19 (Wypych et al., 2019), we propose to explore the RM characteristics in COVID-19 patients with different severities and elucidate the RM changes along with the clinical treatment.
In this study, we recruited 39 COVID-19 patients from The First Affiliated Hospital of Guangzhou Medical University and Guangzhou Eighth People’s Hospital, including 12 asymptomatic (Group 1), 15 normal/mild (Group 2), and 12 severe/critical (Group 3) patients (Supplementary Methods; Table S1). After clinical examinations in all COVID-19 patients (Table S1), we compared the clinical indices between the three groups and discovered a deteriorated clinical situation in the older patients (Fig. S1). Compared with asymptomatic patients, COVID-19 patients with normal/mild symptoms had significantly increased blood pressure and decreased leukocytes (adjusted P < 0.05; Fig. S1), which was clinical evidence of viral infection. Among the severe/critical COVID-19 patients, we observed disordered clinical indices, such as decreased heartbeats and lymphocytes and increased blood pH and body temperature (adjusted P < 0.05; Fig. S1). Generally, the findings are in accordance with those of previous studies, and the worsened vital signals in older patients suggest a high risk of developing severe/critical cases (Liu et al., 2020; Zhou et al., 2020).
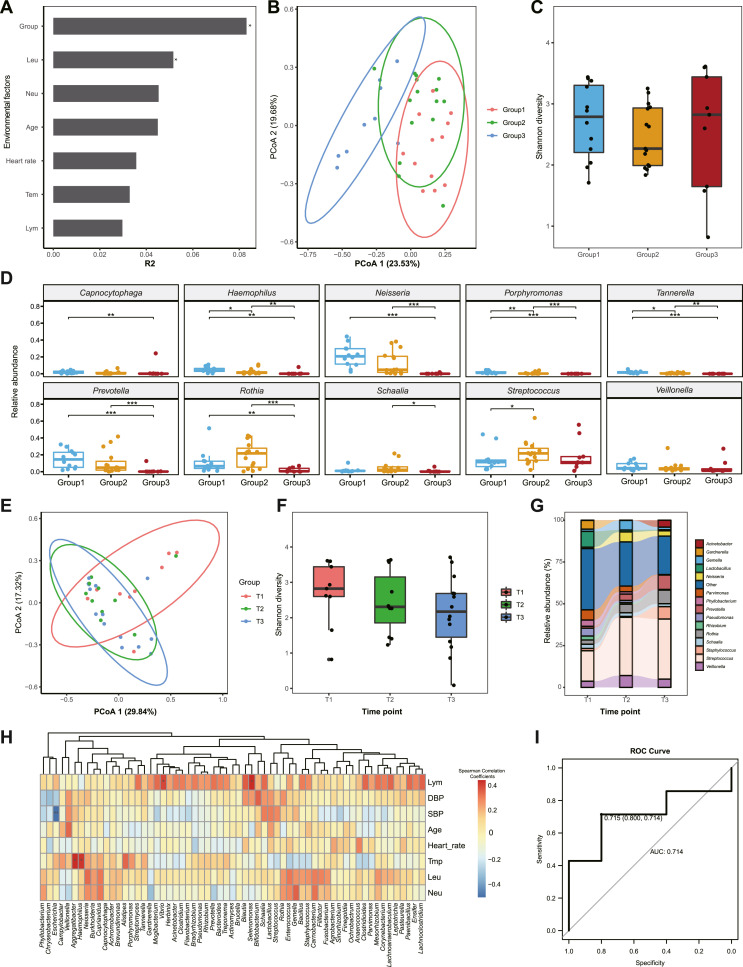
To investigate potential bacterial infections, we collected sputum samples from the COVID-19 patients and detected their RMs with the Oxford Nanopore Technology sequencing platform (Supplementary Methods). A total of 4,692,525 clean reads, which were longer than 100 bp, were generated with an average of 120,316.1 ± 148,799.4 per sample. After taxonomical annotation, we discovered that the reads were assigned to 1157 genera in all samples. Based on the clinical indices and the Bray-Curtis distance matrix obtained from RM compositions, we performed the permutational analysis of variance (PERMANOVA; Table S2) and discovered that disease severity (R2 = 0.083, P < 0.01) contributed significantly to the RM differences (Fig. 1 A). Additionally, principal coordinate analysis (PCoA) revealed the overlap of Group 1 and Group 2 and the separation of Group 3 (Fig. 1B). These results suggested that COVID-19 patients with different severities had different RM characteristics, and the severe/critical patients exhibited more distinct RM features as compared with the asymptomatic and normal/mild patients. However, we did not observe significantly different bacterial diversity among the three groups, which might have been caused by the insufficient sample size (Fig. 1C). To detect the common RM alterations in patients, we selected the top 10 abundant genera from Group 1, Group 2, and Group 3, respectively, and found distinct RM compositions between the groups. The severe/critical patients exhibited significantly decreased abundances of Neisseria, Rothia, and Prevotella (adjusted P < 0.05; Fig. 1D), which are generally abundant in healthy people (Schenck et al., 2016; Man et al., 2017), partially consistent with previous discoveries. Moreover, the distinct RM characteristics in Group 3 were confirmed when we performed sample clustering based on the top 10 abundant genera (Fig. S2). To further confirm the altered species in severe/critical patients, we selected the top 10 abundant species from the three groups and discovered the significantly decreased Neisseria mucosa, N. subflava, Rothia mucilaginosa, Prevotella intermedia, and P. jejuni (adjusted P < 0.05; Fig. S3). Since individual RM differences were detected among patients in Group 3, we analyzed their RM and discovered individually enriched pathogens, such as abnormally abundant Parvimonas (40.93% in patient 8752), Flavobacterium (12.91% in patient 7336), Staphylococcus (9.35% in patient 4706), and Gardnerella (46.76% in patient 8015; Table S3). We deduced that the asymptomatic/normal/mild COVID-19 patients possessed RM with characteristics similar to those of healthy people. Conversely, the severe/critical patients contained individually distinct RM features, which differentially infecting pathogens may cause (Man et al., 2017; Wu and Segal, 2018).
Fig. 1.
RM characteristics of COVID-19 patients with different severities and their longitudinal changes during clinical treatment. A: The impacts of environmental factors on the RM. We used the R2 value from PERMANOVA to evaluate the influences of the environmental factors. B: PCoA of the RM features at the genus level. The distribution of the samples from the three groups is marked with circles with 95% confidence intervals. C: Comparison of bacterial diversity among the three groups. Shannon diversity was applied to represent bacterial diversity. D: Comparisons of the genus abundances between the three groups. We selected the top 10 genera from the three groups and compared their abundances between the groups. The blue, orange, and red boxes represent Group 1, Group 2, and Group 3, respectively. E: PCoA of the RM alterations during treatment. The distribution of the samples from the three groups is marked with circles with 95% confidence intervals. The samples from the T1, T2, and T3 groups are colored pink, green, and blue, respectively. F: Longitudinal alterations of RM bacterial diversity during treatment. Shannon diversity was calculated at the genus level. G: Longitudinal changes in RM composition during treatment. The top 15 genera are colored by different colors on the right of the plot. H: The correlations between clinical indices and RM components. In the heatmap, the positive and negative relations are represented by blue and red squares, respectively. I: Random Forest Model used to predict the severity of COVID-19. Based on three-fold cross-validation, the AUC value of the model was 0.714. ∗, adjusted P < 0.05; ∗∗, adjusted P < 0.01; ∗∗∗, adjusted P < 0.001. DBP, diastolic blood pressure; SBP, systolic blood pressure; Tmp, temperature; Leu, leukocyte; Lym, lymphocyte.
To further detect the longitudinal alteration of the RM in association with COVID-19 during clinical treatment, we collected sputum from the severe/critical patients the day after enrolment (T1), two weeks after treatment (T2), and one day before discharge from the hospital (T3). Notably, PCoA revealed a high coincidence of samples from the T2 and T3 groups after clinical treatment, distinct from the T1 group, suggesting significant impacts of treatment on the RM (Fig. 1E). Since we have applied antibiotics to severe/critical patients, we speculated that the results were caused by antibiotic application (Table S1). In addition, we further performed β-diversity to detect the RM differences among the T1, T2, and T3 groups and confirmed the significantly altered RM in T2 and T3 patients after clinical treatment (Fig. S4). However, the RM diversity in the patients did not change statistically during clinical treatment, although decreased Shannon diversity was observed (Fig. 1F). During treatment, we discovered continually increased Rothia and Prevotella, suggesting the recovery of RM balance in the patients (Figs. 1G and S5). We also found decreased pathogens, such as Gardnerella (from 46.76% to in patient 8015) and Parvimonas (from 40.93% to in patient 8752), hinting at eliminating pathogens and reducing bacterial infection risks (Table S4). However, Lactobacillus, which is recognized as a probiotic, also decreased. Therefore, we speculated that the phenomena were caused by the indiscriminate elimination of bacteria by antibiotics (Schwartz et al., 2020), and we should remain cautious during antibiotic application.
Applying Spearman correlation analysis to the RM and clinical indices, we found positive correlations between body temperature and Aggregatibacter (R = 0.433, adjusted P < 0.05) and Haemophilus (R = 0.408, adjusted P < 0.05), which implied the potential relationships between the increased pathogens and the aggravated inflammations in patients (Fig. 1H; Table S5). Meanwhile, lymphocytes positively correlated to Vibrio (R = 0.376, P < 0.05) and Selenomonas (R = 0.426, adjusted P < 0.05). In contrast, systolic blood pressure is negatively associated with the abundance of Escherichia in the respiratory tract (R = ˗0.525, adjusted P < 0.05). To discriminate COVID-19 patients with different severities, we further constructed a Random Forest model using the RM compositions. In the model, the area under the curve (AUC) value reached 0.714 with 3-fold validation (Fig. 1I). Facilitated by respiratory biomarkers for distinguishing patients with different severities (Fig. S6), the COVID-19-risk model could predict the clinical severity of the enrolled COVID-19 patients.
From previous reports, we have learned that the gut microbiome involves the severity of COVID-19 through the host’s immune system (van der Lelie and Taghavi, 2020; Yeoh et al., 2021) and the characteristics of the RM in association with COVID-19 with different severities remain unexplored. This study explored the RM in COVID-19 patients with different severities, but there were several shortcomings. First, we lacked RM controls from healthy people, enabling us to detect the magnitude of RM alterations in COVID-19 patients. Second, limited by the small sample size, the accuracy of the COVID-19 risk model needs to be improved with samples from other countries. Last but not least, we should perform the gut metagenomic and immunologic examinations in the patients, helping us gain a deeper insight into the roles of the microbiome on clinical severities.
In conclusion, our study revealed differences in RM characteristics in COVID-19 patients with different severities, emphasized bacterial coinfection in severe/critical patients, and established a COVID-19-risk model for predicting the coinfection or clinical severity of COVID-19 patients.
Data availability
The clinical information for the patients and the taxonomic information of the respiratory microbiome can be accessed in the Supplementary data. All other information is available from the authors upon reasonable request.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was funded by the Guangzhou Institute of Respiratory Health Open Project (Funds provided by the China Evergrande Group) (2020GIRHHMS14); Zhongnanshan Medical Foundation of Guangdong Province (ZNSA-2020003); the Department of Science and Technology of Guangdong Province (2020B1111340004); the Traditional Chinese Medicine Bureau of Guangdong Province (2020ZYYJ05). Furthermore, we would like to thank the patients, along with the nurses and clinical staff who provided patient care; the staff in the hospital respiratory medicine departments; the staff in the hospital clinical laboratories; the technical staff of the State Key Laboratory of Respiratory Disease for their excellent assistance; and the staff at the Guangdong Centers for Disease Control for the diagnosis of SARS-CoV-2 infection. Furthermore, we would also like to thank the AJE team for polishing the English language of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgg.2021.11.002.
Supplementary data
The following are the supplementary data to this article:
References
- Liu W., Guan W.J., Zhong N.S. Strategies and advances in combating COVID-19 in China. Engineering. 2020;6:1076–1084. doi: 10.1016/j.eng.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L., Jiang H., Chen Y., Gu S., Xia J., Zhang H., Lu Y., Yan R., Li L. The faecal metabolome in COVID-19 patients is altered and associated with clinical features and gut microbes. Anal. Chim. Acta. 2021;1152:338267. doi: 10.1016/j.aca.2021.338267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man W.H., de Steenhuijsen Piters W.A., Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh J., Peyssonnaux C., Singh K.K., Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck L.P., Surette M.G., Bowdish D.M. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590:3705–3720. doi: 10.1002/1873-3468.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.J., Langdon A.E., Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020;12:82. doi: 10.1186/s13073-020-00782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelie D., Taghavi S. COVID-19 and the gut microbiome: more than a gut feeling. mSystems. 2020:5. doi: 10.1128/mSystems.00453-20. e00453-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B.G., Segal L.N. The lung microbiome and its role in pneumonia. Clin. Chest Med. 2018;39:677–689. doi: 10.1016/j.ccm.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019;20:1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., Chung A.C., Cheung C.P., Tso E.Y., Fung K.S., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A.C.K., Cheung C.P., Chen N., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical information for the patients and the taxonomic information of the respiratory microbiome can be accessed in the Supplementary data. All other information is available from the authors upon reasonable request.