Abstract
Background
Women who received a COVID-19 vaccination may display subclinical unilateral axillary lymphadenopathy on screening mammography, which can appear suspicious for malignancy, leading to additional diagnostic evaluation.
Purpose
To evaluate the prevalence of subclinical unilateral axillary lymphadenopathy (sLAD) on screening mammogram in women who received either the first or second dose of the Pfizer-BioNTech (Pfizer) or Moderna COVID-19 vaccines compared to women who have not.
Materials and Methods
In this IRB-approved, HIPAA complaint study from 12/14/2020 to 4/14/2021, 1027 patients presented for screening mammography and met study inclusion criteria. Patients with history of baseline lymphadenopathy or prior cancer diagnosis were excluded.
Results
: Of the 1027 women, 43 were recalled for unilateral sLAD. 34 women received a COVID-19 vaccination ipsilateral to the sLAD (Pfizer n=19, 44.2%; Moderna n=15, 34.9%), 9 did not (20.9%). Incidence of unilateral axillary sLAD was significantly higher (p-value<0.01) in those who received a COVID-19 vaccination within approximately 7 weeks preceding screening mammogram. 13.2% of patients who received the Pfizer vaccine and 9.5% of patients who received the Moderna vaccine developed sLAD. Moderna's vaccine elicited a more robust reaction in the elderly (Moderna 63.7 years vs. Pfizer 59.7 years). For both vaccines, sLAD resolved on average 46.5 days after the last COVID-19 vaccine (p=0.44).
Conclusion
Women who have received either mRNA COVID-19 vaccines may benefit from scheduling their screening mammogram before vaccination or consider delaying screening mammography 8 weeks. While Pfizer may have an overall more robust immune response, Moderna may elicit a stronger immune response in elderly women.
Summary
Women who received a COVID-19 vaccination before screening mammography were significantly more likely to present with subclinical axillary lymphadenopathy than women who did not receive the vaccine.
Key Results
13.2% of women who received a Pfizer-BioNTech vaccine exhibited subclinical axillary lymphadenopathy compared to 9.5% of those who received the Moderna vaccine. Only 1.2 % of those who did not receive a vaccine presented with subclinical unilateral axillary lymphadenopathy. The average time of resolution of the lymphadenopathy on diagnostic mammogram was 46.5 days overall, with Pfizer-BioNTech taking 50.7 days and Moderna 41.5 days.
Abbreviation: LAD, lymphadenopathy; sLAD, subclinical lymphadenopathy; DM, diagnostic mammogram; Pfizer, Pfizer-BioNTech
INTRODUCTION
This retrospective study aims to analyze the proportion of female screening mammography patients who develop subclinical unilateral axillary lymphadenopathy in response to receiving either the Pfizer-BioNTech (Pfizer) or Moderna COVID-19 vaccine, starting with the date the first COVID-19 vaccination was given in the United States, December 14, 2020, in order to determine if screening mammogram institutional guidelines should be updated to include COVID-19 vaccination status and timeline. This research will help guide clinical decision-making in regards to breast cancer screening in women who have received the COVID-19 vaccine in order to decrease the number of unnecessary diagnostic tests as more patients in the US receive these vaccines, or receive booster vaccinations in the future.
Unilateral axillary lymphadenopathy in the setting of uncertain etiology on a routine screening mammogram can be concerning for breast cancer, and warrants further diagnostic workup in the form of diagnostic mammography, ultrasound, biopsy, or a combination of these. However, this unilateral axillary lymphadenopathy can be a normal sign of an immune response in the setting of vaccination such as the flu vaccine, and more recently the two COVID-19 vaccines approved for use in the United States (1). Other benign etiologies include infection, inflammation, or trauma (1,2).
Women who have received either the Pfizer or Moderna COVID-19 vaccine have presented with unilateral axillary lymphadenopathy after both the first and second doses of these vaccines. A previous review study noted that this occurred most commonly in between 1-4 weeks post vaccination, and is usually subclinical in nature (3,4). If the lymphadenopathy is due to an immune response from the vaccine, and not malignancy, this represents additional cost for the follow up diagnostic studies as well as undue psychological distress for the patient (5). Further, it represents wasted healthcare resources in the form of primary care office visits, additional visits to referring physicians, and follow up. Updating institutional screening recommendations to suggest patients receive their mammograms either before vaccination or a number of weeks after the vaccine has been administered to allow resolution of the localized immune response.
Though it is known that vaccination is a possible cause of palpable axillary lymphadenopathy, it is not known what percentage of patients will develop subclinical unilateral axillary LAD after receiving either the Pfizer or Moderna COVID-19 vaccine. Given that the vaccines were designed to elicit a powerful immune response, we expect that the proportion of patients who will have unilateral axillary lymphadenopathy is much higher than for other vaccines. Therefore, there is a high likelihood that patients who may not have breast malignancy will be subjected to additional costly diagnostic tests, as well as additional radiation exposure, if institutional screening guidelines do not consider patient COVID-19 vaccination status. We hypothesize that the incidence of unilateral axillary lymphadenopathy will be higher in women who received a COVID-19 vaccination in the 4-6 weeks prior to performing mammography compared to women who were not vaccinated in this timeframe.
MATERIALS AND METHODS
In this IRB-approved, HIPAA complaint study, data was sourced from Penrad Mammography Tracking and Reporting software used by our institutions Breast Imaging department, its satellite centers, and online medical records. The IRB determined informed consent was not necessary for this study, as the study was purely retrospective, had no impact on patient treatment or standard of care, and all protected health information was de-identified prior to analysis. Starting with December 14, 2020 – the day the first COVID-19 vaccine was approved for use in the United States, the COVID-19 vaccination status of the patient, the date of vaccine administration, laterality of the administered vaccination, the type of vaccine received (Pfizer or Moderna), presence or absence of unilateral axillary lymphadenopathy on mammography, additional diagnostic studies, findings and recommendations of the interpreting physician after subclinical unilateral axillary lymphadenopathy was revealed were compiled. Data collection continued for all patients who presented for screening mammography until April 14, 2021. Prior imaging results were cross-referenced to ensure the patient did not have baseline axillary lymphadenopathy. All women who received a screening mammogram and met inclusion criteria since December 14, 2020 were included in the study, regardless of whether or not they had a COVID-19 vaccination. To qualify for the COVID-19 vaccinated cohort, the patient should have been administered a dose (first or second) of the Pfizer or Moderna vaccines within 8 weeks of screening mammogram. Exclusion criteria included male sex, receiving a mammogram before the aforementioned date, receiving a COVID-19 vaccine longer than 8 weeks away from screening mammogram, women who presented for diagnostic mammography including women with a palpable axillary lump or pain, or any woman with a prior history of breast cancer. For the purpose of this study, women who had baseline axillary lymphadenopathy at the time of their mammogram were considered to not have reactive lymphadenopathy so as not to artificially inflate the number of women with reactive lymphadenopathy.
Statistical Analysis
Continuous variables were presented as mean and standard deviation, while categorical variables as the frequencies and percentages of events. Differences between continuous data were compared by the Student's t-test, while the Pearson chi-square test used to compare differences in proportion between the groups. (Table 1 )
Table 1.
Descriptive Summary of Patient Characteristics and Outcomes by COVID-19 Vaccination Type and Status
| Moderna (N=158) |
Pfizer (N=144) | No Vaccine (N=725) | p-value | |
|---|---|---|---|---|
| Age (years) – mean± SD | 63.7 ± 12.7 | 59.7 ± 11.8 | 56.4 ± 10.7 | < 0.011 |
| Vaccine Arm | < 0.012 | |||
| Left | 100 (63.3%) | 103 (71.5%) | ||
| Right | 55 (34.8%) | 34 (23.6%) | ||
| Not reported | 3 (1.9%) | 7 (4.9%) | ||
| Lymphadenopathy | < 0.012 | |||
| No | 143 (90.5%) | 125 (86.8%) | 716 (98.8%) | |
| Yes | 15 (9.5%) | 19 (13.2%) | 9 (1.2%) | |
| Lymphadenopathy - ipsilateral to vaccine site | 14 (93.3%) | 19 (100%) | ||
| Lymphadenopathy - contralateral to vaccine site | 1 (6.7%) | 0 (0%) |
Student t-test
Pearson's Chi-squared test
RESULTS
There were 1027 women included in the study, 29.4% of women (n = 302) received a COVID-19 vaccine (Moderna, n = 158, 15.4%; Pfizer n = 144, 14.0%). Of these 1027 patients, 4.2% had unilateral axillary lymphadenopathy (n = 43), and 79.1% of these women received a COVID-19 vaccine (n = 34; Moderna, n = 15, 44.1%; Pfizer n = 19, 55.9%). Of the study population, only 0.9% presented with axillary LAD and did not receive a COVID-19 vaccine (n = 9), while 3.3% of the study population (n = 34) who received a COVID-19 vaccine presented with axillary LAD on screening mammogram (p < 0.01).
The incidence of unilateral axillary lymphadenopathy was significantly higher for those who received a Pfizer COVID-19 vaccination at 13.2% of patients compared to the Moderna vaccine at 9.5% of patients (Table 1). Incidence of unilateral sLAD in unvaccinated women was much lower at 1.2%, and in our study follow-up etiology was yet unknown, but these patients did not have malignancy (Table 1).
Of the 43 women recalled from screening mammography for sLAD, all women reported for initial diagnostic imaging evaluation and confirmed LAD identified on screening either utilizing mammography or a combination of mammography and sonography. Short-term follow-up imaging (BI-RADS 3) of 6-8 weeks was explicitly stated in the recommendation of these diagnostic evaluations. 88.4% of women (n = 38) returned for the recommended short-term diagnostic imaging. 11.6% of women were lost to follow-up (n = 5; Table 2 ). Vaccine related adenopathy was more likely to resolve on imaging follow-up compared to unvaccinated women, with 79.3% being returned to their previous screening schedule (n = 23; Table 2) compared to 66.7% of unvaccinated women (n = 6; Table 2).
Table 2.
Number of Patients with Resolved Subclinical LAD, Unresolved Subclinical LAD, and Lost to Follow-Up After 6-8 Weeks from Initial Screen/Diagnostic
| No vaccine | Moderna | Pfizer | Total | |
|---|---|---|---|---|
| LAD resolved on imaging follow-up - returned to previous screening schedule (BIRADS 2) | 6 | 12 | 11 | 29 |
| LAD not resolved - recommended continued imaging follow-up (presently BIRADS 3) | 3 | 2 | 4 | 9 |
| Lost to follow-up | 0 | 1 | 4 | 5 |
In women who had their sLAD resolve, the diagnostic imaging follow-up was on average 46.5 days since their last COVID-19 vaccine, with those who received the Pfizer vaccine taking 50.7 days to resolve, slightly longer on average than those who received Moderna at 41.5 days (Table 3 ). It is critical to note that the actual time to LAD resolution is likely sooner than the resolution on imaging, time to resolution is influenced by the follow-up interval, and patients were given a window of 6-8 weeks to follow up versus a precise interval of days, thus limiting the value of this follow-up data.
Table 3.
Distribution of Days to Subclinical LAD Resolution on Mammography in Vaccinated Women
| Total (N=29) | Moderna (N=14) | Pfizer (N=15) | p value | |
| LAD resolved - # days since last COVID-19 vaccine (Mean, SD) | 46.5 (29.6) | 41.5 (30.9) | 50.7 (29.0) | 0.44 |
DISCUSSION
Women who had an mRNA COVID-19 vaccine within a few weeks of mammography were more likely to present with subclinical unilateral axillary lymphadenopathy compared to those who had unilateral axillary sLAD but did not have a mRNA COVID-19 vaccine. According to the Centers for Disease Control and Prevention (CDC), 11.6% of recipients of the first dose of the Moderna COVID-19 vaccine developed unilateral axillary lymphadenopathy ipsilateral to the vaccination site (1). This percentage increased to 16% of those who received the second dose of the Moderna vaccine among those aged 18-641. This axillary swelling was the second most reported local reaction to the Moderna vaccine for both doses, second only to localized pain and tenderness around the injection site (6). The CDC did not report lymphadenopathy for the Pfizer vaccine, but noted both axillary and head and neck lymphadenopathy as an unsolicited adverse event that occurred more often in the treatment group than placebo, and considered it plausibly due to the vaccine (7).
Our results support the hypothesis that mRNA COVID-19 vaccinations result in a 267% increase in subclinical unilateral axillary lymphadenopathy compared to women who did not receive the vaccine. At the time of this study, these women with sLAD on screening required further imaging evaluation with diagnostic mammography or sonography. Please note since the time this study was conducted, there has been greater recognition of these vaccines’ ability to cause reactive lymphadenopathy, and thus some practices have adopted different follow-up recommendations. However, to date, no study has quantified the significance of sLAD in relation to screening mammography.
Evaluation of these women with suspected vaccine-related sLAD resulted in extensive wasted healthcare costs in the form of unnecessary appointments, procedures, and resources as well as having to endure emotional and financial burdens associated with an unnecessary workup. The CDC reports that in 2016 there were 17.3 million screening mammograms either ordered or performed, but does not report an average cost nationwide (8). Rim et. al. reports that the average cost of screening mammography in the United States in 2019 was $297, and diagnostic mammograms were even more expensive, costing $490 on average (9). Given the large number of screening mammograms performed in the United States annually, the additional studies recommended by the interpreting radiologist – at the hospitals in this study most commonly a combination of an ultrasound with a diagnostic mammogram—this represents a sizable additional cost to the U.S. healthcare system if guidelines are not updated. In this study alone, just the cost to the healthcare system of the initial additional unnecessary follow-up diagnostic mammograms alone is estimated to be in excess of $20,000, as no patients who presented with sLAD on screening resulted in a final diagnosis of malignancy upon further study. This cost does not include additional diagnostic mammograms that were performed some weeks later to ensure resolution, ultrasounds that were conducted in conjunction with the diagnostic mammograms, nor performance of biopsies and processing of tissue. It also does not take into account the burden these additional healthcare costs place on women of low socioeconomic status, who were not only disproportionately affected by the economic consequences of the pandemic, but are also more likely to die due to delays in screening (10).
Previous studies have recommended a broad range of recommendations for screening delay, around 4-12 weeks depending on the source (3,11,12). Their data showed that unilateral axillary sLAD can occur as early as one day after COVID-19 vaccination and persist well beyond one-month post-vaccine, but based their recommendations mostly off previous institutional guidelines (11). The Society of Breast Imaging recommends erring on the side of caution by postponing screening mammography in patients where it is feasible by 4-6 weeks so long as patient care is not compromised (12). In our study, the mean number of days it took for the reactive lymphadenopathy to resolve was 46.5 days from date of the most recent vaccination for both vaccine groups, or a little over 7 weeks (Table 3). It should be noted that this is time to resolution on follow-up imaging – the actual time to resolution of the sLAD is some time before the actual follow-up appointment. Further, there are additional reasons for lymphadenopathy on screening mammogram since a prior study, including administration of other vaccines, recent illness, inflammatory conditions, or other metastatic processes beyond the breast. Based upon these results, we recommend that patients and providers endeavor to either perform screening mammograms prior to COVID-19 vaccination, or postpone screening at least 8 weeks after most recent vaccination. This will not only reduce the number of costly and unnecessary diagnostic studies, but also help alleviate the psychological burden associated with an abnormal mammogram for patients. This may not be feasible or desirable for patients who are very behind on their regular screening schedule, and the best course of action for this patient population should be decided on a case-by-case basis. For those patients who do not have resolution of the lymphadenopathy on follow-up, additional studies such as biopsy are then recommended (12).
However, it must be impressed that the recommendation is to perform screening mammography within a reasonable timeframe, bearing in mind that older patients are at increased risk (5). It is estimated that there will be 2,500 additional deaths due to breast cancer because of delay in screening during the COVID-19 pandemic (13). Further, a patient in the vaccinated cohort in our study who received the Moderna vaccine on screening mammography had a mass without axillary lymphadenopathy, resulting in biopsy-proven malignancy. As she was asymptomatic, had her screening mammogram not been performed or significantly delayed, her breast cancer would have not been detected, likely resulting in increased morbidity or mortality due to a delay in diagnosis. Additionally, presence of reactive lymphadenopathy does not exclude the possibility of malignancy (4).
While a minority of patients in both cohorts are still in a BIRADS 3 (short-term imaging follow-up) algorithm, the trend is that patients with sLAD and short-term imaging follow-up, 79.3% returned to their previous screening schedule (BIRADS 2) in the vaccinated group compared to 66.7% of the unvaccinated group. This is to be expected, as the immunologic effect of the vaccine will eventually subside, and evidently resolve more quickly than unexplained axillary LAD due to other causes.
The age of the patients presenting with subclinical unilateral axillary LAD varied depending on vaccination as well as type of vaccine. Though the Pfizer vaccine in this study was more likely to cause subclinical axillary LAD, as a greater proportion of patients who received the vaccine developed sLAD (13.2% of all Pfizer vaccine recipients vs 9.5% of all Moderna recipients), the data shows a trend towards significance that Moderna may be more likely to cause lymphadenopathy in the elderly. The mean age of women presenting with unilateral axillary LAD who received the Moderna vaccine was 63.7 years compared to 59.7 years for the Pfizer vaccine and 56.4 years for the non-vaccinated group. Providers or patients who have a choice in deciding vaccine preference may consider that Pfizer tends to have an overall more robust immune response while Moderna may elicit a stronger immune response in elderly women specifically.
Furthermore, our study understates the true prevalence of vaccine-induced lymphadenopathy. As mentioned previously, the CDC's data shows that clinical lymphadenopathy occurs 11.6% of the time in those who got the first dose of the Moderna vaccine and 16% of the time with the second vaccine (1). The Pfizer vaccine is likely similar given it has a similar mechanism of action, though it was not reported by the CDC. Any woman who presents with clinical lymphadenopathy is immediately sent for a diagnostic mammogram, not a screening mammogram. Thus, as in our study the women who presented with sLAD were largely caught because they were due for their yearly screening mammogram and coincidentally had a COVID-19 within only a few weeks prior, and their subclinical axillary lymphadenopathy was an incidental finding. Thus, our data may understate the true incidence of lymphadenopathy within vaccinated individuals, as some women who would have been included did not meet the inclusion criteria as they received diagnostic mammograms.
Given that the SARS-CoV-2 virus continues to mutate, it is possible that receiving an COVID-19 vaccination booster will become an annual offering to patients – much like the seasonal influenza vaccine. Additionally, as time progresses, data may support that the mRNA vaccines will require boosters irrespective of mutations, which will represent additional exposure to the vaccine, and may be more likely to cause reactive lymphadenopathy due to immunogenic memory. Thus it is imperative that not only the date of vaccination is noted in patient records, but also injection site laterality. Awareness of the possibility of reactive lymphadenopathy due to the mRNA COVID-19 vaccines can improve scheduling screening mammograms around these vaccinations, decreasing unnecessary healthcare costs for both the patient and the healthcare system. Moreover, vaccine-induced adenopathy represents an important finding regardless of imaging modality, as reactive lymph nodes are also visualized on breast MRIs but can also be incidentally discovered on any image of the chest such as x-ray or CT (11). Thus, awareness of possible vaccine-induced axillary lymphadenopathy is crucial not just for the field of breast radiology, but the field of radiology as a whole.
Limitations of this study include regional bias, although there is some mitigation of this flaw as this is a multicenter study with different patient populations including socioeconomic class diversity. Patients born male were also not included in this study, and thus this data is not applicable to that population, although there is data that males also can develop lymphadenopathy after COVID vaccine administration. Due to sample size and rarity of sLAD, significance of all data endpoints was often challenging to attain, however, trends to significance were often appreciated.
We expect that as additional studies are performed and more data is collected, there will be more evidence nationally to support institutionally delaying screening mammograms for some time from last COVID-19 mRNA vaccine (anecdotally, we have suggested to referring providers and patients to try to delay screening 8 weeks from last vaccine dose, if feasible). Additional studies could also include the newer Johnson & Johnson vaccine, which none of the patients in this dataset received due to the timeframe included. We also recommend follow up studies with larger sample sizes in effort to determine the average timeframe of resolution of reactive lymphadenopathy to support more precise screening guidelines.
To conclude, women who received a COVID-19 vaccine within 8 weeks of their screening mammogram were significantly more likely to present with subclinical axillary lymphadenopathy compared to women who were not administered a COVID-19 vaccine, leading to unnecessary diagnostic evaluations. Providers should consider either delaying screening mammogram 8 weeks in patients who have received either the Pfizer-BioNTech or Moderna COVID-19 vaccines or boosters, or performing mammography before vaccination to avoid unnecessary, costly, and emotionally taxing follow-up. Fig. 1 , Fig. 2
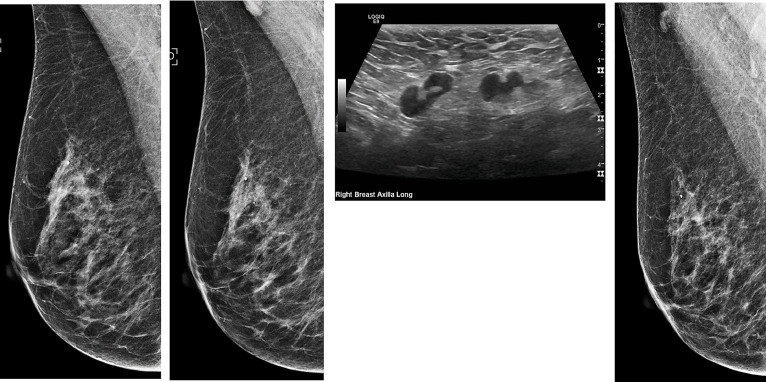
Fig 1.
55-year-old clinically asymptomatic woman with COVID-19 vaccine-induced lymphadenopathy. (1a) Screening mammogram mediolateral oblique view from 2020 demonstrating no right axillary lymphadenopathy. (1b) Screening mammogram mediolateral oblique view in 2021 – with patient reporting history of COVID-19 vaccination in the right deltoid 3 weeks before – reveals an enlarged, abnormal axillary lymph node. Otherwise, no suspicious mammographic abnormalities are identified in either breast. (1c) Patient returns for diagnostic ultrasound evaluation 5 days after abnormal screening mammogram. Sonogram shows multiple abnormally enlarged lymph nodes with prominent eccentric cortices. Of note, there is some preservation of the fatty hila. (1d) The patient returns in 5 weeks for short-term diagnostic mammogram follow-up which reveals a return of normal appearing axillary lymph nodes and a recommendation to return to normal screening mammography.
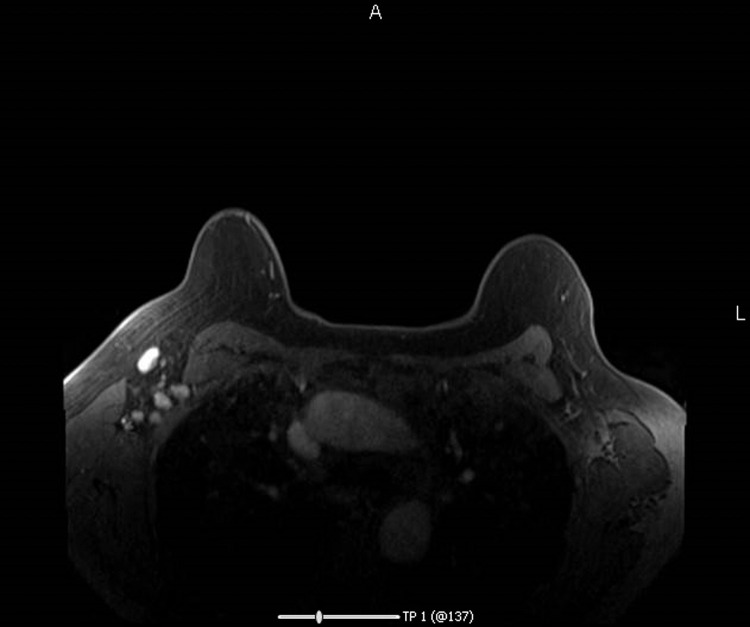
Fig 2.
38-year-old clinically asymptomatic woman presents for high-risk breast cancer screening Breast MRI with Contrast. Patient reports history of a COVID-19 vaccine administered 2.5 weeks before MRI. In the right axilla, there are multiple enlarged axillary lymph nodes. No suspicious breast MRI evidence of malignancy is otherwise identified in either breast.
Conflicts of Interest
No funds, grant or otherwise, were utilized to perform this study. No author of this study has a financial conflict of interest related to the contents of this study.
References
- 1.Mehta N, Sales RM, Babagbemi K, et al. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of COVID-19 Vaccination. Am J Roentgenol. 2021;217(4):831–834. doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 3.Keshavarz P, Yazdanpanah F, Rafiee F, et al. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28(8):1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faermann R, Nissan N, Halshtok-Neiman O, et al. COVID-19 vaccination induced lymphadenopathy in a specialized breast imaging clinic in israel: analysis of 163 cases. Acad Radiol. 2021;28(9):1191–1197. doi: 10.1016/j.acra.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garver K. Managing the risk of delayed breast cancer screening versus COVID-19 vaccination associated axillary lymphadenopathy. Acad Radiol. 2021;28(9):1198–1199. doi: 10.1016/j.acra.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Local reactions, systemic reactions, adverse events, and serious adverse events: Moderna COVID-19 vaccine. Centers for Disease Control and Prevention. 2020 www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html Published. [Google Scholar]
- 7.Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Centers for Disease Control and Prevention. 2020 www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html Published. [Google Scholar]
- 8.Rui P, Okeyode T. National ambulatory medical care survey: 2016 national summary tables. Centers for Disease Control and Prevention. 2021 https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf [Google Scholar]
- 9.Rim SH, Allaire BT, Ekwueme DU, et al. Cost-effectiveness of breast cancer screening in the national breast and cervical cancer early detection program. cancer causes control. 2019;30(8):819-826. doi:10.1007/s10552-019-01178-y [DOI] [PMC free article] [PubMed]
- 10.Momenimovahed Z, Salehiniya H. Delay in the diagnosis of breast cancer during coronavirus pandemic. EXCLI J 20Doc142 ISSN 1611-2156. 2021 doi: 10.17179/EXCLI2020-3318. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehman CD, D'Alessandro HA, Mendoza DP, et al. Unilateral lymphadenopathy After COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol. 2021;18(6):843–852. doi: 10.1016/j.jacr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm L.D, Stamatia Dogan Basak. SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination. 2021 https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf Published. [Google Scholar]
- 13.Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. JNCI J Natl Cancer Inst. 2021;113(11):1484–1494. doi: 10.1093/jnci/djab097. [DOI] [PMC free article] [PubMed] [Google Scholar]