Abstract
Objectives
The aim of this study was to identify risk factors of in-hospital mortality among diabetic patients infected with COVID-19.
Study design
This is a retrospective cohort study.
Methods
Using logistic regression analysis, the independent association of potential prognostic factors and COVID-19 in-hospital mortality was investigated in three models. Model 1 included demographic data and patient history; model 2 consisted of model 1, plus vital signs and pulse oximetry measurements at hospital admission; and model 3 included model 2, plus laboratory test results at hospital admission. The odds ratios (ORs) and 95% confidence intervals (95% CIs) were reported for each predictor in the different models. Moreover, to examine the discriminatory powers of the models, a corrected area under the receiver-operating characteristic curve (AUC) was calculated.
Results
Among 560 patients with diabetes (men = 291) who were hospitalised for COVID-19, the mean age of the study population was 61.8 (standard deviation [SD] 13.4) years. During a median length of hospitalisation of 6 days, 165 deaths (men = 93) were recorded. In model 1, age and a history of cognitive impairment were associated with higher mortality; however, taking statins, oral antidiabetic drugs and beta-blockers was associated with a lower risk of mortality (AUC = 0.76). In model 2, adding the data for respiratory rate (OR 1.07 [95% CI 1.00–1.14]) and oxygen saturation (OR 0.95 [95% CI 0.92–0.98]) slightly increased the AUC to 0.80. In model 3, the data for platelet count (OR 0.99 [95% CI 0.99–1.00]), lactate dehydrogenase (OR 1.002 [95% CI 1.001–1.003]), potassium (OR 2.02 [95% CI 1.33–3.08]) and fasting plasma glucose (OR 1.04 [95% CI 1.02–1.07]) significantly improved the discriminatory power of the model to AUC 0.86 (95% CI 0.83–0.90).
Conclusions
Among patients with type 2 diabetes, a combination of past medical and drug history and pulse oximetry data, with four non-expensive laboratory measures, was significantly associated with in-hospital COVID-19 mortality.
Keywords: COVID-19, Diabetes, In-hospital mortality
Introduction
Diabetes is one of the most frequent comorbidities in patients who are hospitalised for coronavirus disease 2019 (COVID-19).1 Previous systematic reviews have demonstrated that diabetes is a risk factor for severe disease and is associated with an approximately 2–3 fold increased mortality rate from COVID-19 compared with patients without diabetes.2, 3, 4, 5 Results of studies among patients with diabetes have shown that some phenotypic characteristics, radiological and laboratory parameters have been associated with the severity of COVID-19;6 , 7 however, diabetes is a heterogeneous disease, and specific phenotypes associated with poorer outcome are inconsistent among studies. In addition, model development studies that predict outcomes among patients with diabetes are sparse due to insufficient sample sizes.8 , 9
In Iran, more than 4,580,000 confirmed cases of COVID-19 and 100,255 deaths had been reported (until 20 August 2021), according to the World Health Organisation (WHO) report.10 Furthermore, compared with other countries in the Middle East and North Africa (MENA) region, Iran has the highest total number of COVID-19 deaths (as of 20 August 2021).11 A multicentre, cross-sectional study conducted in 19 hospitals in Tehran, Iran, showed a case fatality rate (CFR) of 10.05% among 16,000 cases of COVID-19.12 In that study, the highest rate of mortality was observed in patients with diabetes. In another single-centre study including 2968 Iranian patients who were hospitalised with COVID-19, patients with diabetes had significantly higher rates of CFR compared with patients who had no comorbidities (9.73% vs 7.61%).13
Globally, in 2017, the MENA region had the second-highest prevalence of type 2 diabetes (10.8%), with an increasing trend of 1.5–2 times in the past three decades.14 Hence, it was expected that during the COVID-19 pandemic, patients with diabetes in this region would be greatly impacted.
As the burden of disease due to diabetes15 and COVID-1910 increases in Iran, the current study aims to: (1) describe the clinical and laboratory characteristics of patients with diabetes and COVID-19; (2) identify the risk factors of in-hospital mortality among these patients; and (3) develop a predictive model for in-hospital mortality among Iranian adult patients with type 2 diabetes who were hospitalised for COVID-19.
Methods
Study population and data collection
The study population included all adult patients (aged ≥18 years) with type 2 diabetes (n = 560) who were hospitalised for COVID-19, according to the algorithms suggested by the WHO,16 at a tertiary referral centre in Golestan province, Iran, between February and August 2020.
Two physicians extracted demographic data, medical and drug history, symptoms and signs, and laboratory parameters from electronic medical records. All inpatient medical records were then completed by telephone calls. Unfortunately, data were not complete for all patients. Details of missing data for each characteristic are shown in Table 1 .
Table 1.
Characteristics of diabetic patients hospitalised for COVID-19 infection (N = 560).
| Characteristics | Patients with available data | Total population | Patient outcome |
P-value | ||
|---|---|---|---|---|---|---|
| Deceased (n = 165) | Survived (n = 395) | |||||
| Demographic characteristics | ||||||
| Gender (male), n (%) | 560 | 291 (52%) | 93 (56.4%) | 198 (50.1%) | 0.19 | |
| Age (years), mean ± SD | 560 | 61.8 (13.4) | 64.3 (14.0) | 60.7 (13.1) | 0.004 | |
| BMI (kg/m2), mean ± SD | 479 | 29.2 (6.1) | 29.0 (6.5) | 29.3 (5.9) | 0.50 | |
| Marital status (married) n (%) | 560 | 551 (98.4%) | 162 (98.2%) | 389 (98.5%) | 0.65 | |
| Education, n (%) | 512 | 0.76 | ||||
| Illiterate/elementary | 317 (61.9%) | 98 (62.8%) | 219 (61.5%) | |||
| Below diploma/diploma | 124 (24.2%) | 39 (25%) | 85 (23.9%) | |||
| Higher than diploma | 71 (13.9%) | 19 (12.2%) | 52 (14.6%) | |||
| Area of residence, n (%) | 560 | 0.22 | ||||
| Rural | 157 (28.0%) | 40 (24.2%) | 117 (29.6%) | |||
| Urban | 403 (72.0%) | 125 (75.8%) | 278 (70.4%) | |||
| Duration of diabetes, mean ± SD | 469 | 8.01 (8.0) | 7.4 (8.9) | 8.3 (8.5) | 0.33 | |
| Comorbidities, n (%) | ||||||
| History of hypertension | 559 | 441 (78.9%) | 122 (73.9%) | 319 (81.0%) | 0.07 | |
| History of previous CAD | 515 | 220 (42.7%) | 63 (42.0%) | 157 (43.0%) | 0.84 | |
| History of stroke | 510 | 53 (10.4%) | 23 (15.4%) | 30 (8.3%) | 0.02 | |
| History of pulmonary disease | 506 | 94 (18.6%) | 35 (23.6%) | 59 (16.5%) | 0.08 | |
| History of diabetic foot | 485 | 76 (15.7%) | 50 (14.9%) | 26 (17.4%) | 0.50 | |
| Routine treatment before admission, n (%) | ||||||
| Antidiabetic drugs | 560 | 0.001 | ||||
| OADs | 215 (38.4%) | 45 (27.3%) | 170 (43%) | |||
| Insulin | 101 (18%) | 28 (17%) | 73 (18.5%) | |||
| Both OADs and insulin | 48 (8.6%) | 17 (10.3%) | 31 (7.8%) | |||
| Beta-blocker | 560 | 76 (13.6%) | 15 (9.1%) | 61 (15.4%) | 0.06 | |
| ARBs/ACE inhibitors | 560 | 249 (44.5%) | 66 (40.0%) | 183 (46.3%) | 0.20 | |
| Statins | 560 | 111 (19.8%) | 21 (12.7%) | 90 (22.8%) | 0.007 | |
| Antiplatelet drugs | 560 | 124 (22.1%) | 31 (18.8%) | 93 (23.5%) | 0.26 | |
| Chest CT imaging, n (%) | 423 | 0.31 | ||||
| Without involvement | 105 (24.8%) | 29 (24.4%) | 76 (25.0%) | |||
| Crazy paving + consolidation | 148 (35.0%) | 48 (40.3%) | 100 (32.9%) | |||
| Other | 170 (40.2%) | 42 (35.3%) | 128 (42.1%) | |||
| Clinical characteristics, n (%) | ||||||
| Dyspnoea | 548 | 338 (70.8%) | 108 (66.3%) | 280 (72.7%) | 0.15 | |
| Cough | 538 | 278 (51.7%) | 77 (48.4%) | 201 (53.0%) | 0.34 | |
| Fever | 540 | 275 (50.9%) | 90 (56.6%) | 185 (48.6%) | 9.09 | |
| Fatigue | 542 | 255 (47.0%) | 84 (52.5%) | 171 (44.8%) | 0.10 | |
| Gastrointestinal symptoms | 539 | 207 (38.4%) | 73 (45.9%) | 134 (35.3%) | 0.02 | |
| Cognitive impairment | 541 | 83 (15.3%) | 41 (25.8%) | 42 (11.0%) | <0.001 | |
| Anosmia/hyposmia/ageusia | 534 | 76 (14.2%) | 17 (10.8%) | 59 (15.7%) | 0.17 | |
| Vital signs on admission | ||||||
| SBP (mmHg), mean ± SD | 560 | 137.8 (25.7) | 132.2 (26.2) | 140.2 (25.2) | 0.001 | |
| DBP (mmHg), mean ± SD | 559 | 81.8 (14.8) | 78.8 (15.4) | 83.1 (14.3) | 0.002 | |
| Pulse (beats/min), mean ± SD | 558 | 99.06 (18.4) | 100.7 (19.1) | 98.4 (18.1) | 0.19 | |
| RR-breaths (per minute), median (IQR) | 559 | 20.0 (18.0–25) | 24.0 (20–28) | 20.0 (18–24) | <0.001 | |
| Temperature (°C), median (IQR) | 557 | 37.0 (37.0–37.5) | 37.2 (37–37.6) | 37.0 (37–37.5) | 0.15 | |
| SPO2 (%), median (IQR) | 560 | 91.0 (85–95) | 85.0 (72.5–91) | 93.0 (88–96) | <0.001 | |
| Admission plasma glucose (mg/dl), mean ± SD | 559 | 231.4 (114.6) | 231.8 (29.0) | 231.2 (108.1) | 0.95 | |
| White blood cell count (× 109/L), mean ± SD | 560 | 8.86 (4.8) | 9.82 (4.5) | 8.46 (4.8) | 0.002 | |
| Neutrophil count (%), median (IQR) | 499 | 79.0 (70–86) | 83.0 (76.5–88.0) | 77.0 (69.7–84) | <0.001 | |
| Lymphocyte count (%), median (IQR) | 499 | 16.0 (10–24) | 12.0 (8–19) | 18.0 (12–26) | <0.001 | |
| Haemoglobin (g/L), mean ± SD | 560 | 119.0 (19) | 118.0 (18.8) | 119.0 (19) | 0.34 | |
| Platelet count (× 109/L), mean ± SD | 560 | 214.0 (94.8) | 199.5 (90.0) | 220.1 (96.1) | 0.02 | |
| Prothrombin time (s), median (IQR) | 283 | 13.2 (13–14.7) | 13.7 (13–15.2) | 13.0 (13–14.0) | 0.77 | |
| Partial thromboplastin time (s), median (IQR) | 283 | 32.0 (27–39) | 32.0 (27.2–41) | 31.8 (27–37) | 0.82 | |
| HbA1c (%), median (IQR) | 70 | 9.4 (6.9–10.3) | 9.5 (6.4–10.3) | 9.4 (7–10.4) | 0.68 | |
| CRP (mg/L), mean ± SD | 496 | 1.2 (0.4) | 1.16 (0.37) | 1.18 (0.38) | 0.62 | |
| ESR (mm/h), median (IQR) | 330 | 66.0 (41–93) | 65.0 (41.5–94) | 66.0 (41–92.5) | 0.76 | |
| Albumin (g/dl), mean ± SD | 343 | 3.59 (0.5) | 3.39 (0.5) | 3.7 (0.5) | <0.001 | |
| LDH (U/L), median (IQR) | 528 | 563.5 (426.2–777.0) | 767.0 (537–995) | 512.0 (406–672) | <0.001 | |
| CPK, median (IQR) | 528 | 150.0 (88–297.0) | 201.5 (113.7–553.5) | 131.0 (81–222) | <0.001 | |
| Urea (mmol/L), median (IQR) | 559 | 1.43 (1.03–2.45) | 1.82 (1.25–3.14) | 1.32 (0.92–2.07) | <0.001 | |
| Creatinine (μmol/L), median (IQR) | 559 | 106.1 (88.4–141.4) | 114.9 (97.2–176.8) | 97.2 (88.4–132.6) | 0.001 | |
| eGFR (mL/min/1.73 m2), mean ± SD | 559 | 57.47 (26) | 51.0 (27.8) | 59.3 (24.8) | 0.001 | |
| Sodium (mEq/L), mean ± SD | 558 | 136.3 (5.7) | 136.2 (7.0) | 136.37 (5.0) | 0.73 | |
| Potassium (mEq/L), mean ± SD | 558 | 4.48 (0.7) | 4.6 (0.9) | 4.4 (0.7) | 0.01 | |
| AST (U/L), median (IQR) | 305 | 38.0 (27–65.5) | 48.0 (30–91) | 54.2 (23.7–58.2) | 0.001 | |
| ALT (U/L), median (IQR) | 305 | 34.0 (21–56.5) | 37.0 (22–73) | 30.5 (20–56) | 0.014 | |
| FPG (mg/dl), mean ± SD | 560 | 250.5 (114.7) | 282.2 (133.3) | 237.3 (103.3) | <0.001 | |
Abbreviations: SD, standard deviation; IQR, interquartile range; BMI, body mass index; OADs, oral antidiabetic drugs; ARB, angiotensin receptor blocker; ACE, angiotensin-converting enzyme; CAD, coronary artery disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; RR, respiratory rate; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; eGFR, estimated glomerular filtration rate (as calculated using the CKD Epidemiology Collaboration equation); AST, aspartate aminotransferase; ALT, alanine aminotransferase; FPG, fasting plasma glucose.
Clinical and laboratory measurements
Oropharyngeal swab specimens were collected and examined in predetermined laboratories across the province to detect SARS-CoV-2 viral nucleic acid using a real-time reverse transcription-polymerase chain reaction (RT-PCR) assay. Laboratory parameters, including white blood cells (WBC) count, neutrophils and lymphocytes, haemoglobin (Hb), blood urea nitrogen (BUN) concentration, creatinine, sodium, potassium, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), albumin, liver enzymes (including aspartate and alanine transaminases [AST and ALT]), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were collected for each patient. The primary outcome was in-hospital COVID-19 mortality.
Definition of terms
As suggested by the National Headquarters for the management and control of the novel coronavirus, we followed the WHO interim guidelines for diagnosing COVID-19 infection;17 thus, the case definition was based on both confirmed (i.e. positive PCR) and probable infected cases. Probable cases were defined as follows: first, either a febrile acute respiratory illness (ARI) with clinical, radiological or histopathological evidence of pulmonary parenchymal disease (e.g. pneumonia or acute respiratory distress syndrome [ARDS]), a direct epidemiological link to a laboratory-confirmed COVID-19 case, or testing for COVID-19 is unavailable or negative on a single inadequate specimen or shows inconclusive results; second, a febrile ARI that is not explained fully by any other aetiology, and the person resides or travelled in the Middle East, and testing for COVID-19 is inconclusive; third, an ARI of any severity, and a direct epidemiological link to a confirmed COVID-19 case, and testing for COVID-19 is inconclusive. Diabetes was defined by fasting plasma glucose (FPG) ≥7.0 mmol/L or random blood glucose ≥11.0 mmol/L or glycosylated haemoglobin A1c (HbA1c) ≥6.5% at admission or the patient is already receiving glucose-lowering medications.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height (m2). The estimated glomerular filtration rate (eGFR) was expressed in mL/min/1.73 m2 and was calculated using the chronic kidney diseases (CKD) Epidemiology Collaboration (CKD-EPI) equation,18 and CKD was defined as an eGFR of <60 mL/min per 1.73 m2.19 The education status was classified as illiterate/elementary school, below diploma/diploma and higher than a diploma.
Statistical analyses
The baseline characteristics were presented as mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables and frequencies (%) for categorical variables. Comparisons of baseline characteristics between patients who survived and those who died and between respondents (those with complete data on covariates) and non-respondents (those with missing data on some covariates at the baseline) were performed using Student’s t-test, Mann–Whitney test and Chi-squared test, as appropriate.
Using univariable logistic regression, the associations of different characteristics of patients at admission with in-hospital mortality were evaluated. Covariates with P-values <0.20 in univariable analysis were then entered in the multivariable models. Model 1 included demographic data, diabetes-related complications and drug history; model 2 included all significant variables in model 1, plus vital signs and pulse oximetry data; model 3 included significant variables in model 2, plus laboratory test data. Since the history of chronic insulin therapy (with or without oral antidiabetic drugs [OADs]) was an important indicator for the long duration of diabetes and a predictor of mortality among COVID-19 patients, this confounder was included in both models 2 and 3.
The odds ratios (ORs) and 95% confidence intervals (95% CIs) were reported for each predictor in different models.
The area under the receiver-operating characteristic curve (AUC) was used to assess the discrimination of models. According to the Hosmer et al. criteria, the AUC values were categorised as poor (≥0.5 to <0.7), acceptable (≥0.70 to <80), excellent (≥0.80 to <0.90) and outstanding (≥0.90) discriminations.20 A comparison of AUC values of different models with the same number of participants in the three models (i.e. n = 456) was performed using a non-parametric approach proposed by DeLong et al.21
In addition, to address overfitting, which mainly occurs in the model building process, the optimism-corrected AUC was estimated using 1000 bootstrap resamples for every underlying model. The difference between the original and the mean AUC of the 1000 replicates was used as a correction factor and subtracted from the original AUC. This bias-corrected AUC was used as a measure for internal validation.
To evaluate the calibration, which shows agreement between the observed (actual) outcomes and predictions, we used observed to predicted ratios, the Hosmer–Lemeshow goodness-of-fit test and a calibration plot. The calibration plot shows predicted in-hospital death probabilities (x-axis) against the observed outcomes (y-axis) in deciles of the predicted probabilities. Using the LOWESS (locally weighted scatter plot smoothing) line, we smoothed the calibration plot. Perfect predictions are on the 45° line (y = x). Validation of the goodness-of-fit of each underlying model was determined by the Hosmer–Lemeshow test in deciles based on the predicted risk. A non-significant test implied that the observed outcome did not differ significantly from the predicted mortality risk.
To encourage the integration of the prognostic model into everyday clinical situations, the mathematical formula of the prognostic algorithm obtained from logistic regression modelling was also incorporated into a nomogram. The nomogram developed herein serves as a graphical representation of our prognostic algorithm, incorporating significant prognostic factors as continuous variables to predict the risk of in-hospital mortality from COVID-19. Except for the variable selection, P < 0.05 was considered significant. Statistical analyses were performed with SPSS 22 (SPSS Inc., Chicago, IL, USA) and STATA 14 (StataCorp, college station, TX, USA).
Results
Comparison between respondents and non-respondents indicated no clinically important differences between these two groups, with the exception that respondents reported a higher frequency of angiotensin-converting enzyme (ACE)/angiotensin II receptor blockers (ARB) use and a lower prevalence of cough and gastrointestinal symptoms compared with non-respondents (see Supplementary Table S1).
The study sample included 560 patients with diabetes (men = 291). Among them, 364 (65%) were receiving glucose-lowering medication. The mean age of the total population was 61.8 (SD 13.4) years, and SARS-CoV-2 PCR testing was performed in 209 patients, with a positive result in 125 patients. The median duration of hospital stay was 6 (IQR 3–11) days, whereby 232 patients were admitted to the intensive care unit (ICU) for the median stay of 6 (IQR 3–11) days. In total, 165 in-hospital deaths were recorded (men = 93).
The baseline characteristics of patients who survived and those who died are compared in Table 1. The prevalence of overweight, obesity and CKD was 37.2%, 38.6% and 44.5%, respectively. A medical history of hypertension, coronary artery disease (CAD), stroke and pulmonary disease was observed in 78.9%, 42.7%, 10.4% and 18.6% of the participants, respectively. The mean level of plasma glucose at the time of hospital admission was 231.4 (114.6) mg/dl, and the level of HbA1c (only for 70 patients) was 9.4%. The most common glucose-lowering medications were metformin, followed by insulin, sulfonylurea and other oral glucose-lowering agents. Moreover, ACE inhibitors and/or ARBs, beta-blockers, statins and antiplatelet drugs were used by 44.5%, 13.6%, 19.8% and 22.1% of the participants, respectively.
The most common signs of COVID-19 on admission were dyspnoea, cough, fever, fatigue, gastrointestinal disorders, cognitive impairment and anosmia, hyposmia and ageusia. Thoracic computed tomography (CT) imaging was performed for all patients at hospital admission and did not reveal any abnormality in 25% of patients. Details of other results are shown in Table 1.
Patients who died compared with those who survived were older, more likely to have a history of stroke, and present with gastrointestinal symptoms and cognitive impairment. Moreover, they were less likely to be taking metformin and statins. In-hospital mortality was more likely in individuals who initially presented (i.e. at hospital admission) with significantly lower systolic blood pressure (SBP), diastolic blood pressure (DBP), oxygen saturation (SpO2) and lower levels of lymphocytes, platelet, albumin and eGFR, but higher levels of respiratory rate, WBC, neutrophils, LDH, CPK, creatinine, potassium, AST, ALT and FPG compared with patients who survived (all P-values were <0.05).
Table 2 shows multivariate prediction models for in-hospital mortality. In model 1, age (OR 1.02 [95% CI 1.00–1.04]) and a history of cognitive impairment (OR 3.17 [95% CI 1.77–5.68]) were associated with a significantly higher risk of in-hospital mortality. Moreover, prior use of OADs, beta-blockers and statins was associated with significant 55%, 51% and 49% lower risks of mortality, respectively. In model 2, age and history of cognitive impairment were independently associated with a higher risk of mortality, while the use of statins, beta-blockers and OADs, lower respiratory rate (OR 1.07 [95% CI 1.00–1.14]) and higher oxygen saturation (OR 0.95 [95% CI 0.92–0.98]) were associated with a significantly lower risk of mortality. Finally, in model 3, in addition to the significant predictors of model 2, the use of insulin (OR 0.42 [95% CI 0.19–0.94]), platelet count (OR 0.99 [95% CI 0.99–1.00]), LDH (OR 1.002 [95% CI 1.001–1.003]), potassium (OR 2.02 [95% CI 1.33–3.08]) and each 10 mg/dl increase in FPG (OR 1.04 [95% CI 1.04–1.07]) were found to be independently associated with the risk of death.
Table 2.
Multivariate prediction models of in-hospital mortality for patients with diabetes and COVID-19.
| Characteristics | Model 1 (N = 498) |
Model 2 (N = 534) |
Model 3 (N = 456) |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Prior to admission characteristics | ||||||
| Age | 1.02 (1.00–1.04) | 0.01 | 1.02 (1.00–1.04) | 0.01 | 1.03 (1.01–1.05) | 0.01 |
| Gender | 1.01 (0.65–1.56) | 0.97 | ||||
| Area of residence | 0.74 (0.45–1.19) | 0.19 | ||||
| Dyspnoea | 0.66 (0.42–1.04) | 0.07 | ||||
| History of fever | 1.53 (0.96–2.43) | 0.07 | ||||
| Cognitive impairment | 3.17 (1.77–5.68) | <0.001 | 2.06 (1.16–3.66) | 0.01 | 2.78 (1.35–5.71) | 0.006 |
| Fatigue | 1.34 (0.85–2.13) | 0.21 | ||||
| Gastrointestinal symptoms | 1.56 (0.97–2.51) | 0.06 | ||||
| Anosmia, hyposmia or ageusia | 0.54 (0.28–1.04) | 0.06 | ||||
| History of stroke | 1.22 (0.60–2.49) | 0.58 | ||||
| History of pulmonary disease | 1.51 (0.88–2.58) | 0.13 | ||||
| History of hypertension | 0.63 (0.36–1.12) | 0.12 | ||||
| Routine treatment before admission | ||||||
| OADs | 0.45 (0.26–0.75) | 0.003 | 0.55 (0.33–0.92) | 0.02 | 0.38 (0.12–0.73) | 0.04 |
| Insulin | 0.53 (0.28–1.01) | 0.055 | 0.83 (0.45–1.52) | 0.54 | 0.42 (0.19–0.94) | 0.03 |
| OADs and insulin | 0.97 (0.45–2.09) | 0.94 | 1.24 (0.57–2.71) | 0.58 | 1.01 (0.38–2.70) | 0.99 |
| Beta-blocker | 0.49 (0.24–0.99) | 0.05 | 0.43 (0.20–0.89) | 0.02 | 0.35 (0.14–0.88) | 0.02 |
| ARBs/ACE inhibitors | 0.98 (0.57–1.69) | 0.95 | ||||
| Statins | 0.51 (0.28–0.93) | 0.03 | 0.46 (0.24–0.86) | 0.01 | 0.37 (0.17–0.82) | 0.01 |
| Vital signs on admission | ||||||
| SBP | 0.99 (0.97–1.00) | 0.06 | ||||
| DBP | 1.007 (0.98–1.03) | 0.52 | ||||
| Pulse | 0.99 (0.98–1.00) | 0.16 | ||||
| RR | 1.07 (1.00–1.14) | 0.04 | 1.06 (0.97–1.15) | 0.18 | ||
| Temperature | 1.02 (0.80–1.45) | 0.62 | ||||
| SPO2 | 0.95 (0.92–0.98) | 0.001 | 0.95 (0.91–0.99) | 0.01 | ||
| Laboratory tests on admission | ||||||
| White blood cell count | 0.98 (0.93–1.03) | 0.48 | ||||
| Neutrophil count | 0.99 (0.90–1.08) | 0.80 | ||||
| Lymphocyte count | 0.97 (0.87–1.07) | 0.54 | ||||
| Platelet | 0.99 (0.99–1.00) | 0.002 | ||||
| LDH | 1.002 (1.001–1.003) | 0.00 | ||||
| Creatinine | 1.07 (0.78–1.47) | 0.68 | ||||
| eGFR | 1.00 (0.98–1.01) | 0.64 | ||||
| Potassium | 2.02 (1.33–3.08) | 0.001 | ||||
| FPG | 1.04 (1.02–1.07) | 0.001 | ||||
| AUC | 0.76 (0.70–0.80) | 0.80 (0.74–0.82) | 0.86 (0.83–0.90) | |||
Abbreviations: OADs, oral antidiabetic drugs; ARB, angiotensin receptor blocker; ACE, angiotensin-converting enzyme; SBP, systolic blood pressure; DBP, diastolic blood pressure; RR, respiratory rate; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate (as calculated using the CKD Epidemiology Collaboration equation); FPG, fasting plasma glucose; AUC, area under the receiver-operating characteristic curve; OR, odds ratio; CI, confidence interval.
The ORs correspond to each one-unit increase for continuous variables except for 10 mg/dL increase in FPG.
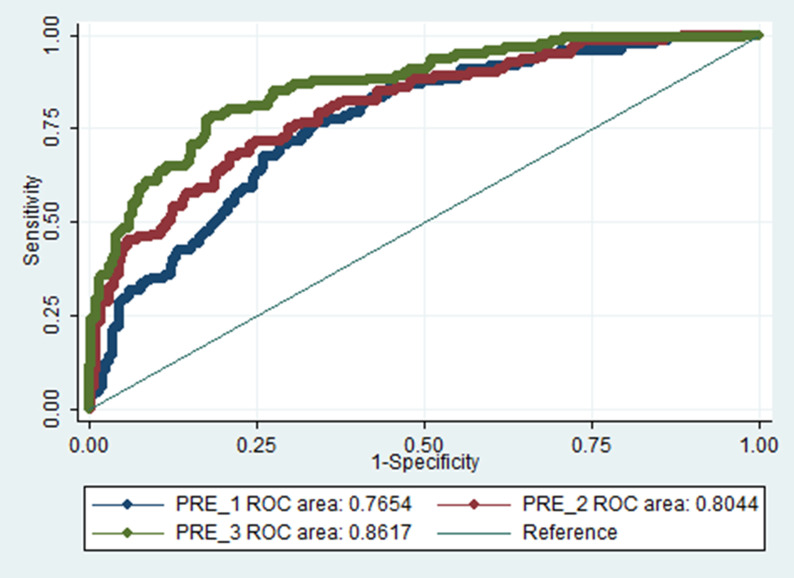
The values of discrimination power (AUC) of models were 0.75 (95% CI 0.70–0.80) for model 1, 0.80 (95% CI 0.74–0.82) for model 2, and 0.86 (95% CI 0.83–0.90) for model 3. The corrected AUC for model 3 was 0.82 (95% CI 0.79–0.89). Model 3 had the highest discrimination power (Fig. 1 ).
Fig. 1.
Receiver-operating characteristic curve for the three models. Blue curve, model 1 (age, history of cognitive impairment, use of statins, beta-blockers and oral antidiabetic drugs); red curve, model 2 (age, history of cognitive impairment, use of statins, beta-blockers and oral antidiabetic drugs, respiratory rate and oxygen saturation); and green curve, model 3 (age, history of cognitive impairment, use of statins, beta-blockers, oral antidiabetic drugs, insulin, oxygen saturation, platelet count, lactate dehydrogenase, potassium and fasting plasma glucose). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
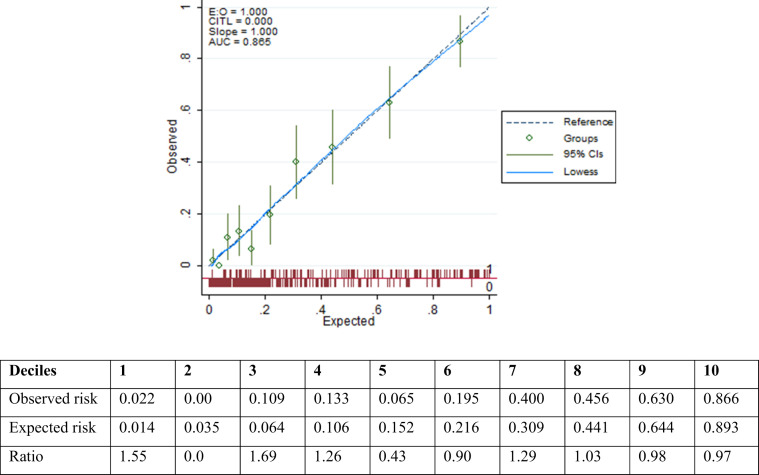
The calibration plot indicated good calibration for the risk prediction model within the data set (Fig. 2 ) and the Hosmer–Lemeshow goodness-of-fit test also showed good calibration (, P = 0.84).
Fig. 2.
Calibration curves for comparing actual and predicted in-hospital death probabilities according to model 3. The Hosmer–Lemeshow test for goodness-of-fit (, P = 0.84).
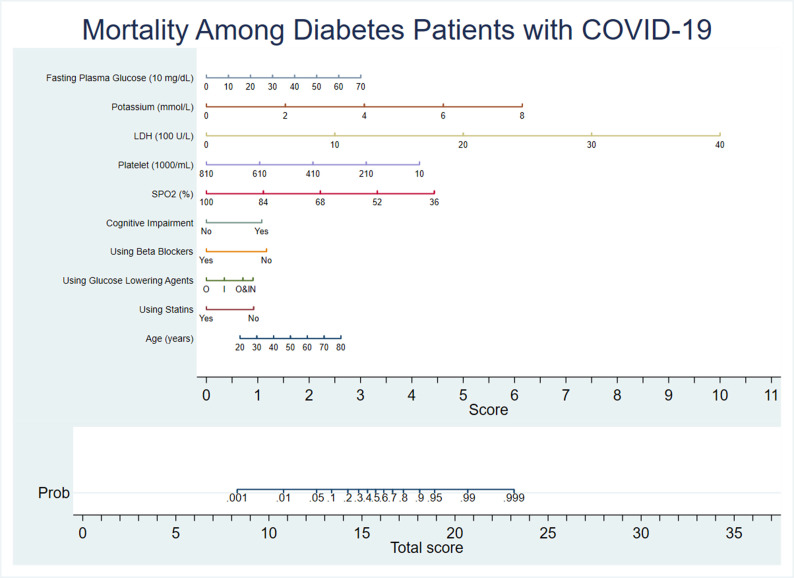
Fig. 3 shows the nomogram of the final model (model 3). According to the nomogram as an example, a 45-year-old patient with diabetes who presents at the emergency room with an SpO2 of 80%, FPG of 210 mg/dl, LDH of 800 U/L, potassium of 5 mEq/L and platelet count of 230 × 109/L, without a history of loss of consciousness and who is not receiving statin, beta-blocker or OAD treatment gets the score of (1.5 + 1 + 1 + 2 + 3.5 + 4 + 3 + 0 + 1 + 1.2 + 1 = 19.2) and will have a 95% probability of mortality.
Fig. 3.
Nomogram of covariates in model 3 for predicting in-hospital mortality among patients with diabetes who are hospitalised with COVID-19. Upon hospital admission, the risk of mortality for a patient with diabetes can be calculated by computing the corresponding score of points for each of the nine clinical characteristics and then adding them together. Looking at the bottom scale, the probability of in-hospital mortality corresponding to the calculated score can be calculated. LDH, lactate dehydrogenase; Prob, probability; O, oral antidiabetic drugs; I, insulin; O&I, oral antidiabetic drugs and insulin; N, no antidiabetic drugs.
Discussion
The current study was conducted in a large tertiary centre in the North East of Iran during the first half of 2020. Our findings, among 560 patients with diabetes who were hospitalised for COVID-19, showed a 30% in-hospital mortality rate following approximately one week of hospitalisation. Ageing, cognitive impairment and higher levels of LDH, potassium and FPG were found to be associated with an increased risk of death, while higher platelet levels and oxygen saturation, as well as taking oral glucose-lowering drugs, insulin, statins and beta-blockers, were significantly associated with a reduced risk of in-hospital mortality. We developed a simple model with its nomogram that showed an excellent discriminatory power for the prediction of mortality events among patients with diabetes who had been hospitalised with COVID-19.
Several studies6 , 8 , 22, 23, 24, 25 have assessed the characteristics and prognostic factors among the diabetic population with different results (Supplementary Table S2). Two studies specifically focused on patients with diabetes who were hospitalised for COVID-19. First, the CORONADO study7 showed a 10.6% risk of death and found several factors, including age, treated obstructive sleep apnoea and microvascular and macrovascular complications, to be independent predictors of death on day 7 (the current study observed a higher risk of mortality, and the only common predictor of death with our population was age). The other study among patients with diabetes who were hospitalised with COVID-19 was in the US6 and showed a mortality rate of 33.1%, which is comparable with the results obtained in our study. Furthermore, the US study showed that HbA1c was not associated with mortality events, while insulin treatment was a strong predictor of mortality. In the current study, we found that a high level of plasma glucose at hospital admission, as a proxy for the level of diabetes control,26 was significantly associated with an increased risk of mortality. Moreover, in contrast to the study of Agarwal et al., we demonstrated that a history of using OADs and insulin was significantly associated with a lower risk of in-hospital mortality. Importantly, according to a national study,27 despite some improvement in the knowledge and screening of diabetes in Iran, 24.7% of patients with diabetes were not aware of their disease. In addition, these newly diagnosed patients exhibited a coronary heart disease (CHD) risk comparable to patients without diabetes with a prior CHD event.28 Results of a systematic review and meta-analysis of 14 studies showed that male sex, age, history of cardiovascular disease (CVD), CKD, chronic obstructive pulmonary disease (COPD), high plasma glucose at hospital admission and chronic insulin use were associated with a high risk of death for patients with diabetes who also had COVID-19.25
Among patients with diabetes, a few studies found no association between statin use and poor outcome.7 , 22 , 25 , 29 Several studies30 , 31 with larger sample sizes among patients without diabetes showed that previous statin use in patients hospitalised with COVID-19 was associated with lower in-hospital mortality, which might be related to the immunomodulatory action or by preventing cardiovascular damage in addition to their lipid-lowering activity.32 These results are consistent with our study that also found a 63% lower risk of death among patients who used statins, despite the low number of lipid-lowering medication users in our study population. In another study, low statin use was also observed among patients with diabetes in Iran.33 It is interesting that the current study found that using beta-blockers was significantly associated with a lower risk of in-hospital mortality. The results of other studies among patients with diabetes who were infected with COVID-19 were inconsistent; one study showed a 19% higher risk, and another study found a 33% lower risk of mortality risk among beta-blocker users, although both associations were non-significant.7 , 22 According to different guidelines, using this group of medications for patients with diabetes is limited to those with acute coronary syndrome or who are experiencing heart failure (HF).34 Systematic reviews revealed that using beta-blockers was associated with improved outcomes among patients with HF, regardless of the diabetes status.35 Hence, in the current study population, with a prevalence of CVD >40%, we speculated that a large number of patients had some degree of existing HF and would benefit from beta-blockers. Unfortunately, echocardiography was not performed for most patients; hence, the ejection fraction data were not available.
To the best of our knowledge, no study has investigated the association between potassium level and COVID-19 mortality among patients with diabetes; however, in studies conducted in the general population, potassium was not associated with COVID-19 mortality risk36 or a higher risk37 of COVID-19 infection. In the current study, each 1 mEq/L higher potassium level was associated with a two-fold higher risk of in-hospital mortality. These findings are in line with previous studies among ICU patients showing that hyperkalaemia is an independent risk factor for death, even at a moderate increase above the normal levels.38 , 39 Importantly, the significant risk of a higher potassium level, which was found in the current study, was independent of several important confounding factors, including eGFR and ACE inhibitor/ARB use. However, pH value is a strong confounder for potassium level as metabolic acidosis can cause a potassium shift from the intracellular to the extracellular space; unfortunately, we did not have data on pH levels. The most important mechanism that is caused by hyperkalaemia is lowering of the resting membrane potential of the myocardium, resulting in decreased myocardial cell conduction velocity and increased rate of repolarisation.39
To date, only two studies conducted among Chinese and Australian populations have presented prediction models for mortality from COVID-19 among patients with diabetes.8 , 9 The study among the Chinese population9 developed a model containing predictor variables, including partial thromboplastin time (PTT), urea nitrogen, WBC count and LDH, with an AUC of 0.836. Moreover, the study in Australia8 showed that a score computed from age, arterial occlusive disease, eGFR, CRP and AST levels at hospital admission predicted in-hospital mortality with a C statistic of 0.89 and good calibration. Our prediction model with 11 variables, including four non-expensive laboratory measures, yielded similar excellent discriminatory power (0.86 [95% CI 0.83–0.90]) with acceptable calibration.
Strengths of the current study were the large sample size of patients who were hospitalised for COVID-19 and a considerable number of patients with diabetes, which, as discussed previously, imposes a great burden of disease in Iran.10 , 15
The current findings need to be interpreted in light of the study limitations. First, this study did not validate the model externally; however, the model showed a reasonable internal validity as examined by the bootstrapping method. Second, there were no data on HbA1c; however, we adjusted for plasma glucose as a surrogate of the HbA1c level.26 Third, a large number of patients were probable cases of COVID-19, and the cases were not confirmed with PCR. However, previous studies have shown that because of difficulty in obtaining reliable nasopharyngeal swab specimens, the timing of detection and limited detection capacity during the early stages of the outbreak, false-negative results are often seen in the PCR method.40 Moreover, CT imaging of the chest is a more reliable, feasible and rapid method to diagnose and assess COVID-19 compared with RT-PCR, especially in epidemic regions such as Iran.41, 42, 43 Fourth, we did not categorise patients as those with previous diabetes and those who had increased blood glucose due to COVID-19 infection, which might overestimate the number of patients with diabetes. Finally, data regarding in-hospital treatment were not included in our analysis.
In conclusion, approximately one-third of patients with diabetes who were hospitalised for COVID-19 in this large referral centre located in the North East of Iran died within 1 week of admission. A simple and non-expensive risk score consisting of 11 variables, including age, history of cognitive impairment, use of statins, OADs, insulin, beta-blockers, SpO2, platelet count, LDH, potassium and FPG levels, demonstrated excellent prediction for in-hospital mortality among patients with diabetes. This simple risk score may help physicians in emergency departments to assess the prognosis of patients with diabetes.
Author statements
Acknowledgements
The authors appreciate the efforts of healthcare workers and support staff at Sayyad Shirazi Hospital for providing patient care at considerable personal risk on the front lines of this pandemic. We would also like to express our solidarity with those who are or have been ill with COVID-19 and their families.
Ethics approval
This study was approved by the Ethics Committee of the Golestan University of Medical Science (Protocol no. IR.GOUMS.REC.1399.043) and was in accordance with the 1964 Declaration of Helsinki. We obtained verbal consent for publication from living patients and the relatives of deceased patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Competing interests
None declared.
Availability of data and materials
All data and materials are available upon request.
Author contributions
M.K, F.H and R.HT planned the study, researched the data and wrote the manuscript. M.H and MR.B analysed the data, reviewed and edited the manuscript. D.K, H.A and GR.R reviewed and edited the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2021.11.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Atkins J.L., Masoli J.A., Delgado J., Pilling L.C., Kuo C.-L.C., Kuchel G., et al. Preexisting comorbidities predicting severe COVID-19 in older adults in the UK biobank community cohort. medRxiv. 2020;75:2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia–a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr: Clin Res Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A., Byrne C.D., Zheng M.-H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metabol Cardiovasc Dis. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr: Clin Res Rev. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo L., Shi Z., Zhang Y., Wang C., Moreira N.C.D.V., Zuo H., et al. Comorbid diabetes and the risk of disease severity or death among 8807 COVID-19 patients in China: a meta-analysis. Diabetes Res Clin Pract. 2020;166:108346. doi: 10.1016/j.diabres.2020.108346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal S., Schechter C., Southern W., Crandall J.P., Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. 2020;43:2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020:1–16. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sourij H., Aziz F., Bräuer A., Ciardi C., Clodi M., Fasching P., et al. COVID-19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes Metabol. 2021;23:589–598. doi: 10.1111/dom.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su M., Yuan J., Peng J., Wu M., Yang Y., Peng Y.G. Clinical prediction model for mortality of adult diabetes inpatients with COVID-19 in Wuhan, China: a retrospective pilot study. J Clin Anesth. 2020;66:109927. doi: 10.1016/j.jclinane.2020.109927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.who.int/countries/irn/.
- 11.https://covid19.healthdata.org/iran-(islamic-republic-of)?view=total-deaths&tab=compare.
- 12.Zali A., Gholamzadeh S., Mohammadi G., Looha M.A., Akrami F., Zarean E., et al. Baseline characteristics and associated factors of mortality in COVID-19 Patients; an analysis of 16000 cases in Tehran, Iran. Arch Acad Emerg Med. 2020;8 [PMC free article] [PubMed] [Google Scholar]
- 13.Nikpouraghdam M., Farahani A.J., Alishiri G., Heydari S., Ebrahimnia M., Samadinia H., et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in Iran: a single center study. J Clin Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azizi F., Hadaegh F., Hosseinpanah F., Mirmiran P., Amouzegar A., Abdi H., et al. Metabolic health in the Middle East and north Africa. Lancet Diabetes Endocrinol. 2019;7:866–879. doi: 10.1016/S2213-8587(19)30179-2. [DOI] [PubMed] [Google Scholar]
- 15.Esteghamati A., Gouya M.M., Abbasi M., Delavari A., Alikhani S., Alaedini F., et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: national survey of risk factors for non-communicable diseases of Iran. Diabetes Care. 2008;31:96–98. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 16.Organization WH . WHO Regional Office for the Western Pacific; Manila: 2020. Algorithm for COVID-19 triage and referral: patient triage and referral for resource-limited settings during community transmission. [Google Scholar]
- 17.Organization WH . World Health Organization; 2020. Clinical management of severe acute respiratory infection when novel coronavirus ( nCoV) infection is suspected: interim guidance, 25 January 2020. [Google Scholar]
- 18.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro A.F., III, Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Eckardt K.-U., Tsukamoto Y., Levin A., Coresh J., Rossert J., et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 20.Hosmer D.W., Jr., Lemeshow S., Sturdivant R.X. John Wiley & Sons; 2013. Applied logistic regression. [Google Scholar]
- 21.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988:837–845. [PubMed] [Google Scholar]
- 22.Orioli L., Servais T., Belkhir L., Laterre P.-F., Thissen J.-P., Vandeleene B., et al. Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: a retrospective study from an academic center in Belgium. Diabetes Metab Syndr: Clin Res Rev. 2021;15:149–157. doi: 10.1016/j.dsx.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger S., Neuenschwander M., Lang A., Pafili K., Kuss O., Herder C., et al. 2020. Risk phenotypes of diabetes and association with COVID-19 severity and death–a living systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Association AD 6. Glycemic targets. Diabetes Care. 2015;38:S33–S40. doi: 10.2337/dc15-S009. [DOI] [PubMed] [Google Scholar]
- 27.Esteghamati A., Etemad K., Koohpayehzadeh J., Abbasi M., Meysamie A., Noshad S., et al. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract. 2014;103:319–327. doi: 10.1016/j.diabres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Hadaegh F., Fahimfar N., Khalili D., Sheikholeslami F., Azizi F. New and known type 2 diabetes as coronary heart disease equivalent: results from 7.6 year follow up in a Middle East population. Cardiovasc Diabetol. 2010;9:1–8. doi: 10.1186/1475-2840-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadini G.P., Morieri M.L., Boscari F., Fioretto P., Maran A., Busetto L., et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168:108374. doi: 10.1016/j.diabres.2020.108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12:1–9. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan W.Y., Young B.E., Lye D.C., Chew D.E., Dalan R. Statin use is associated with lower disease severity in COVID-19 infection. Sci Rep. 2020;10:1–7. doi: 10.1038/s41598-020-74492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castiglione V., Chiriacò M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother. 2020;6:258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahangiri-Noudeh Y., Akbarpour S., Lotfaliany M., Zafari N., Khalili D., Tohidi M., et al. Trends in cardiovascular disease risk factors in people with and without diabetes mellitus: a Middle Eastern cohort study. PLos One. 2014;9:e112639. doi: 10.1371/journal.pone.0112639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ettehad D., Emdin C.A., Kiran A., Anderson S.G., Callender T., Emberson J., et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 35.Shibata M.C., Flather M.D., Wang D. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail. 2001;3:351–357. doi: 10.1016/s1388-9842(01)00144-1. [DOI] [PubMed] [Google Scholar]
- 36.Mudatsir M., Fajar J.K., Wulandari L., Soegiarto G., Ilmawan M., Purnamasari Y., et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Research. 2020;9 doi: 10.12688/f1000research.26186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia P.D.W., Fumeaux T., Guerci P., Heuberger D.M., Montomoli J., Roche-Campo F., et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449. doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelhardt L.J., Balzer F., Müller M.C., Grunow J.J., Spies C.D., Christopher K.B., et al. Association between potassium concentrations, variability and supplementation, and in-hospital mortality in ICU patients: a retrospective analysis. Ann Intensive Care. 2019;9:1–11. doi: 10.1186/s13613-019-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahon G.M., Mendu M.L., Gibbons F.K., Christopher K.B. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834–1842. doi: 10.1007/s00134-012-2636-7. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Yao L., Li J., Chen L., Song Y., Cai Z., et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92:903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davarpanah A.H., Mahdavi A., Sabri A., Langroudi T.F., Kahkouee S., Haseli S., et al. Novel screening and triage strategy in Iran during deadly COVID-19 epidemic; value of humanitarian teleconsultation service. J Am Coll Radiol. 2020;17:734. doi: 10.1016/j.jacr.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rastad H., Karim H., Ejtahed H.-S., Tajbakhsh R., Noorisepehr M., Babaei M., et al. 2020. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials are available upon request.