Abstract
Objective
We aimed to perform a meta-analysis to summarize the overall evidence from randomized controlled trials related to higher-intensity anticoagulation in hospitalized patients with COVID-19.
Methods
A systematic literature search was performed in electronic databases to identify randomized controlled trials comparing the clinical outcomes between intermediate/ therapeutic anticoagulation and prophylactic anticoagulation. Meta-analyses with random-effects models were used to estimate the pooled odds ratio (OR) for outcomes of interest at a 95% confidence interval (CI).
Results
Eight randomized controlled trials were included, with a total of 5405 hospitalized patients with COVID-19. The meta-analysis revealed no statistically significant difference in the odds of mortality (pooled OR = 0.92; 95% CI 0.71–1.19) but a statistically significant reduction in the odds of development of thrombotic events (pooled OR = 0.55; 95% CI 0.42–0.72), and significantly increased odds of development of major bleeding (pooled OR = 1.81; 95% CI 1.20–2.72) with the use of intermediate/therapeutic anticoagulation, relative to prophylactic anticoagulation. Subgroup analysis in patients with a severe course of COVID-19 observed a statistically significant reduction in the odds of development of thrombotic events (pooled OR = 0.66; 95% CI 0.45–0.98) but no significant difference in the odds of development of major bleeding events (pooled OR = 1.37; 95% CI 0.74–2.56), with the use of intermediate/therapeutic anticoagulation, relative to prophylactic anticoagulation.
Conclusion
There could be net clinical benefits with higher-intensity dosing of anticoagulation relative to prophylactic-dosing of anticoagulation among hospitalized patients with severe COVID-19.
Keywords: Anticoagulant, COVID-19, Enoxaparin, Heparin, Mortality, Thromboembolism
1. Introduction
The recognition of hypercoagulable state in patients with coronavirus disease 2019 (COVID-19), including elevated levels of factor VIII and fibrinogen as well as formation of neutrophil extracellular traps, has led to the utilization of venous thromboembolism (VTE) pharmacological prophylaxis. However, about 38% of hospitalized patients with a severe course of COVID-19 still developed VTE [1]. Therefore, there have been recommendations to use higher-intensity dosing (rather than prophylactic dosing) of anticoagulation to further reduce VTE risk in hospitalized patients with COVID-19, especially those with a severe course of illness. However, some clinicians are reluctant for higher-intensity dosing of anticoagulation due to the risk of bleeding events, which might outweigh the uncertain benefits. More clinical evidence is undoubtedly needed to resolve the dispute.
There have been attempts to trial the use of higher-intensity dosing of anticoagulation in hospitalized patients with COVID-19 relative to prophylactic dosing. However, a recent systematic review and meta-analysis [2] of 38 observational studies reported that patients who received prophylactic anticoagulation had significantly better survival (odds ratio = 0.65, 95% confidence interval 0.46 to 0.91) and significantly reduced risk of bleeding (odds ratio = 0.31, 95% confidence interval 0.21 to 0.45) compared to their counterparts treated with therapeutic anticoagulation, but with no significant difference in the risk of thromboembolism (odds ratio = 1.03, 95% confidence interval 0.70 to 1.49). While their findings seem to argue against the use of higher-intensity dosing of anticoagulation in patients with COVID-19, observational studies are notoriously vulnerable to the effect of confounding bias, and clinical evidence with higher quality is required. Randomized controlled trials are the gold standard for studying causal relationships between interventions and outcomes. Therefore, we aimed to perform a meta-analysis to summarize the overall evidence from the randomized controlled trials related to the use of higher-intensity dosing of anticoagulation in hospitalized patients with COVID-19.
2. Methods
This study was conducted according to the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [3]. A systematic literature search with no language restriction was performed in electronic databases, including PubMed, Google Scholar, Cochrane Central Register of Controlled Trials, and preprint servers (medRxiv, Research Square, SSRN), to identify eligible studies, published up to October 11, 2021. The search strategy was built based on the following keywords and their MeSH terms: “COVID-19”, “SARS-CoV-2”, “anticoagulant”, “anticoagulation”, “heparin”, and “randomized”. The clinical trial registries of the United States (clinicaltrials.gov) and the World Health Organization international (who.int/clinical-trials-registry-platform) were also searched to identify clinical trials with released findings. In addition, the reference lists of relevant articles were also reviewed to search for additional studies. Studies eligible for inclusion were randomized controlled trials comparing the clinical outcomes between higher-intensity dosing of anticoagulation (either intermediate or therapeutic anticoagulation) and prophylactic dosing of anticoagulation in hospitalized patients with COVID-19. We excluded studies with observational design, single-arm trials, non-randomized trials, and trials that did not report clinical outcomes of interest.
Two investigators (CSK and SSH) independently performed the literature screening, screened titles and abstracts, and thoroughly evaluated full-texts to identify potentially eligible studies. Any conflicts in the selection of studies were resolved through mutual discussion and consulting with the third investigator (DSR). The outcomes of interest were all-cause mortality, thrombotic events, and major bleeding events. Two investigators (CSK and SSH) independently evaluated each trial, who extracted the study characteristics using a pre-designed data abstraction form. Study characteristics extracted included the first author's surname, trial design, country, age of patients, the definition of severe course of illness, dosing regimen of intermediate/therapeutic anticoagulation, dosing regimen of prophylactic anticoagulation, all-cause mortality, VTE, and major bleeding events.
Two investigators (CSK and SSH) assessed the risk of bias of the trials included with Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) [4]. The overall risk of bias for each trial was categorized as 'low' if the risk of bias was low in all domains, 'some concerns' if the risk of bias was deemed to have some concerns in at least one domain and with no high risk of bias in any domain, or 'high' if the risk of bias was high in at least one domain, as per the RoB 2 tool. Disagreements were resolved through mutual discussion and consulting with the third investigator (DSR) if necessary.
Considering the potential heterogeneity across different studies, meta-analyses with the random-effects model were used to estimate the pooled odds ratio for outcomes of interest using intermediate/therapeutic anticoagulation relative to prophylactic anticoagulation, at 95% confidence intervals. Random-effects meta-analysis involves a process whereby the inverse variance weighting of the fixed model is reversed to a variable extent. We examined the heterogeneity between studies using the I2 statistics and the χ2 test, with significant heterogeneity being considered at 50% and p < 0.10, respectively. Sensitivity analysis was conducted to investigate the robustness of the results by including trials that examined the effect of therapeutic anticoagulation relative to prophylactic anticoagulation on the outcomes of interest. Publication bias was analyzed through visual inspection of funnel plot for asymmetry with the help of a triangle centred on a fixed effect summary estimate and extending 1.96 standard errors on either side. Subgroup analysis was conducted across trials that recruited only patients with a severe course of COVID-19. All analyses were performed using Meta XL, version 5.3 (EpiGear International, Queensland, Australia). The default option of MetaXL software, DerSimonian and Laird method, was used to run the random-effects model of meta-analysis.
3. Results
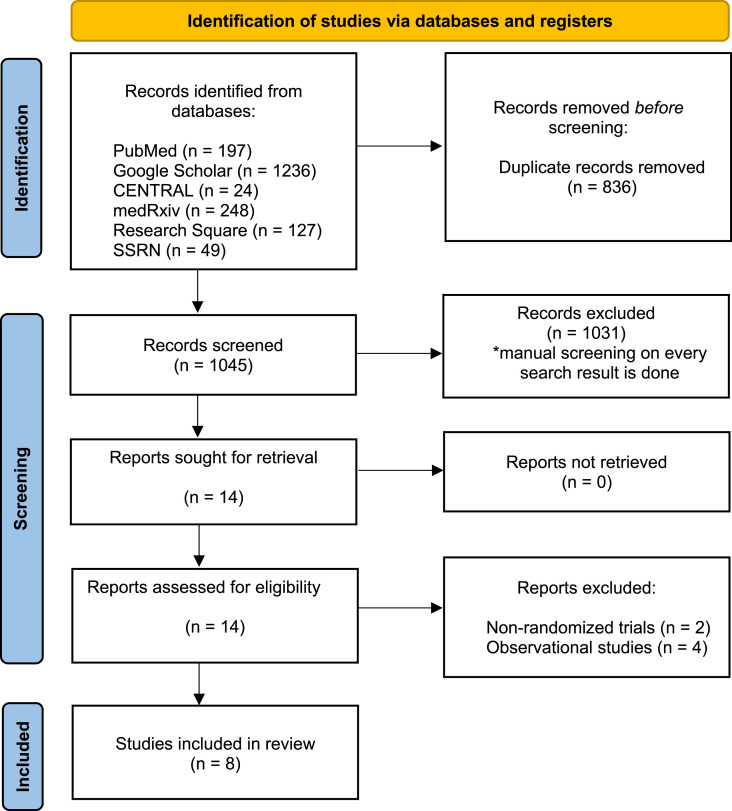
Our systematic literature search retrieved 1881 hits, of which 1045 were unique (titles retrieved after removing duplications). After screening against inclusion and exclusion criteria, eight randomized controlled trials [[5], [6], [7], [8], [9], [10], [11], [12]] were included (Fig. 1 ), with a total of 5405 patients (2746 patients randomized to the intermediate or therapeutic anticoagulation group, and 2659 patients randomized to the prophylactic anticoagulation group). Five of the included randomized trials in this systematic review and meta-analysis were from Iran (n = 1) [7], the United States (n = 2) [8,12], and Brazil (n = 2) [5,9], respectively, and one of the included randomized trial [11] was a global trial performed in six countries. In contrast, the remaining two randomized trials [6,10] were international multiplatform randomized controlled trials. Details of the included studies are shown in Table 1 . Six randomized trials [5,6,[9], [10], [11], [12]] compared therapeutic-dose anticoagulation against prophylactic-dose anticoagulation, while the other two trials [7,8] compared intermediate-dose anticoagulation against prophylactic-dose anticoagulation.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram of a process of study selection.
Table 1.
Study characteristics of included trials.
| Study | Study design | Country | Age (median/mean) | Definition of severe course of COVID-19 | Regimen of intermediate/therapeutic anticoagulation | Regimen of prophylactic anticoagulation | Mortality |
Thrombotic event |
Major bleeding event |
Risk of bias1 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intermediate/Therapeutic anticoagulation group (n/N; %) | Prophylactic anticoagulation group (n/N; %) | Intermediate/Therapeutic anticoagulation group (n/N; %) | Prophylactic anticoagulation group (n/N; %) | Intermediate/Therapeutic anticoagulation group (n/N; %) | Prophylactic anticoagulation group (n/N; %) | ||||||||
| Lemos et al [5] | Open label, randomized controlled trial | Brazil | Intermediate/Therapeutic anticoagulation group = 55 Prophylactic anticoagulation group = 58 |
Respiratory failure requiring mechanical ventilation | Subcutaneous enoxaparin; under 75 years-old with CrCl>50 mL/min: 1 mg/kg twice daily; with CrCl between 30 and 50 mL/min: 0.75 mg/kg twice daily; with CrCl between 10 and 30 mL/min: 1 mg/kg once daily; with CrCl<10 mL/min; older than 75 years with CrCl>50 mL/min: 0.75 mg/kg twice daily; with CrCl between 30 and 50 mL/min: 1 mg/kg once daily; with CrCl between 10 and 30 mL/min: 0.75 mg/kg once daily | Subcutaneous unfractionated heparin at a dose of 5000 IU three times daily (if weight <120 kg) and 7500 IU three times daily (if weight >120 kg) or enoxaparin at a dose of 40 mg once daily (if weight <120 kg) and 40 mg twice daily (if weight >120 kg) | 1/10; 10.0 | 3/10; 30.0 | 2/10; 20.0 | 2/10; 20.0 | 0/10; 0 | 0/10; 0 | Some concerns |
| The REMAP-CAP/ACTIV-4a/ATTACC trial (severe) [6] | Open label, adaptive, multiplatform, randomized controlled trial | Global | Intermediate/Therapeutic anticoagulation group = 60.2 Prophylactic anticoagulation group = 61.6 |
Intensive care unit-level respiratory or cardiovascular organ support (high flow nasal oxygen ≥20 L/min, non-invasive or invasive mechanical ventilation, extracorporeal life support, vasopressors, or inotropes) | Administered according to local site protocols for up to 14 days or recovery, which were not specified | Administered according to local practice, which was not specified | 189/529; 35.7 | 189/545; 34.7 | 27/471; 5.7 | 49/476; 10.3 | 15/482; 3.1 | 12/495; 2.4 | Some concerns |
| The REMAP-CAP/ACTIV-4a/ATTACC trial (moderate) [7] | Open label, adaptive, multiplatform, randomized controlled trial | Global | Intermediate/Therapeutic anticoagulation group = 60.2 Prophylactic anticoagulation group = 61.6 |
– | Administered according to local site protocols for up to 14 days or recovery which were not specified | Administered according to local practice which was not specified | 86/1171; 7.3 | 86/1048; 8.2 | 16/1180; 1.4 | 28/1046; 2.7 | 22/1180; 1.9 | 9/1047; 0.9 | Some concerns |
| The Inspiration trial [8] | Open label, randomized controlled trial | Iran | Intermediate/Therapeutic anticoagulation group = 60.2 Prophylactic anticoagulation group = 61.6 |
Admission into ICU | Subcutaneous enoxaparin; BMI <30 kg/m2 and CrCl ≥30 mL/min: 1 mg/kg once daily; BMI ≥30 kg/m2 and CrCl >30 mL/min: 0.6 mg/kg twice daily; BMI ≥30 kg/m2 and CrCl between 15 and 30 mL/min: 0.5 mg/kg twice daily Subcutaneous unfractionated heparin; CrCl≤15 mL/min: 10 000 units twice daily |
Subcutaneous enoxaparin; BMI <30 kg/m2 and CrCl≥30 mL/min: 40 mg once daily; BMI ≥30 kg/m2 and CrCl>30 mL/min: 40 mg twice daily; BMI ≥30 kg/m2 and CrCl between 15 and 30 mL/min: 30 mg once daily Subcutaneous unfractionated heparin; CrCl≤15 mL/min: 5000 units twice daily |
119/276; 43.1 | 117/286; 40.9 | 9/276; 3.3 | 10/286; 3.5 | 7/276; 2.5 | 4/286; 1.4 | Some concerns |
| Perepu et al. [9] | Open label, randomized controlled trial | United States | Intermediate/Therapeutic anticoagulation group = 65.0 Prophylactic anticoagulation group = 63.5 |
Admission into ICU and/or had a modified ISTH Overt DIC score ≥3 |
Subcutaneous enoxaparin; BMI <30 kg/m2: 1 mg/kg once daily; BMI ≥30 kg/m2: 0.5 mg/kg twice daily | Subcutaneous enoxaparin; BMI <30 kg/m2: 40 mg once daily; BMI ≥30 kg/m2: 30 mg twice daily; BMI ≥30 kg/m2 and admitted into ICU: 40 mg twice daily | 13/87; 14.9 | 18/86; 20.9 | 7/87; 8.0 | 6/86; 7.0 | 2/87; 2.2 | 2/86; 2.3 | Some concerns |
| Lopes et al [10] | Open label, randomized controlled trial | Brazil | Intermediate/Therapeutic anticoagulation group = 56.7 Prophylactic anticoagulation group = 56.5 |
– | Oral rivaroxaban; Clinically stable patients: 20 mg once daily; patients with CrCl of 30–49 mL/min or taking azithromycin: 15 mg once daily Subcutaneous enoxaparin; clinically unstable patients: 1 mg/kg twice daily Intravenous unfractionated heparin at a dose to achieve a target anti-Xa concentration (0·3–0·7 IU/mL) or a corresponding target activated partial thromboplastin time (1·5–2·5 times the mean normal value) |
Subcutaneous enoxaparin; BMI <40 kg/m2 and CrCl≥30 mL/min: 40 mg once daily; BMI ≥40 kg/m2 and CrCl >30 mL/min: 60 mg once daily or 40 mg twice daily Subcutaneous unfractionated heparin; BMI <40 kg/m2 and CrCl ≥30 mL/min: 5000 units twice or thrice daily; BMI ≥40 kg/m2 and CrCl >30 mL/min: 7500 units twice or thrice daily; BMI <40 kg/m2 and CrCl<30 mL/min: 5000 units twice or thrice daily; BMI ≥40 kg/m2 and CrCl <30 mL/min: 7500 units twice or thrice daily Subcutaneous fondaparinux; BMI <40 kg/m2: 2.5 mg once daily |
35/310; 11.3 | 23/304; 7.6 | 11/310; 3.5 | 18/304; 5.9 | 10/310; 3.2 | 4/304; 1.3 | Some concerns |
| Sholzberg et al. [11] | Open label, randomized controlled trial | Global | Intermediate/Therapeutic anticoagulation group = 60.4 Prophylactic anticoagulation group = 59.6 |
– | See appendix (Table S1) | See appendix (Table S2) | 4/228; 1.8 | 18/237; 7.6 | 2/228; 0.9 | 7/237; 3.0 | 2/228; 0.9 | 4/237; 1.7 | Some concerns |
| Spyropoulos et al. [12] | Double blind, randomized controlled trial | United States | Intermediate/Therapeutic anticoagulation group = 65.8 Prophylactic anticoagulation group = 67.7 |
– | Subcutaneous enoxaparin; CrCl≥30 mL/min: 1 mg/kg twice daily; CrCl 15–29 mL/min: 0.5 mg/kg twice daily | Subcutaneous unfractionated heparin; up to 22 500 units subcutaneously divided twice or thrice daily Subcutaneous enoxaparin; 30 mg or 40 mg once or twice daily Subcutaneous dalteparin; 2500 units or 5000 units daily |
25/129; 19.4 | 31/124; 25.0 | 14/129; 10.9 | 36/124; 29.0 | 6/129; 4.7 | 2/124; 1.6 | Low |
BMI: body mass index; CrCl: creatinine clearance; ICU: intensive care unit.
1Risk of bias was assessed using Version 2 of the Cochrane risk-of-bias tool for randomized trials.
The overall risk of bias assessed by RoB 2 is presented in Table 1. Except for the trial by Spyropoulos et al. [12], which had an overall low risk of bias, the remaining included trials had some concerns over the overall risk of bias. Both the trials by Lemos et al. [5] and Sholzberg et al. [11], respectively, had some concerns of bias for the domain of randomization due to a lack of information on allocation concealment and for both the domain of deviations from intervention and the domain of measurement of the outcome due to the open-label design of these two trials. The two Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP)/Accelerating Covid-19 Therapeutic Interventions and Vaccines-4 Antithrombotics Inpatient platform trial (ACTIV-4a)/Antithrombotic Therapy to Ameliorate Complications of Covid-19 (ATTACC) trials [6,10] which enrolled moderate and severe patients with COVID-19 respectively, had some concerns of bias for both the domain of deviations from intervention and the domain of measurement of the outcome due to the open-label design of these two trials and for the domain of missing outcome data since data was not available for some proportion of patients for the outcomes on mortality, thrombotic events, and major bleeding events. The Inspiration trial [7] had some concerns of bias for both the domain of deviations from intervention and the domain of measurement of the outcome due to the open-label design of the trial, and for the domain of missing outcome data since some proportion of patients in both groups were not included in the analysis due to escalation/de-escalation of assigned anticoagulation regimen. Both the trials by Perepu et al. [8] and Lopes et al. [9], respectively, had some concerns of bias for both the domain of deviations from intervention and the domain of measurement of the outcome due to the open-label design of these two trials. The aforementioned trials [[5], [6], [7], [8], [9], [10], [11], [12]] had a low risk of bias for other domains assessed.
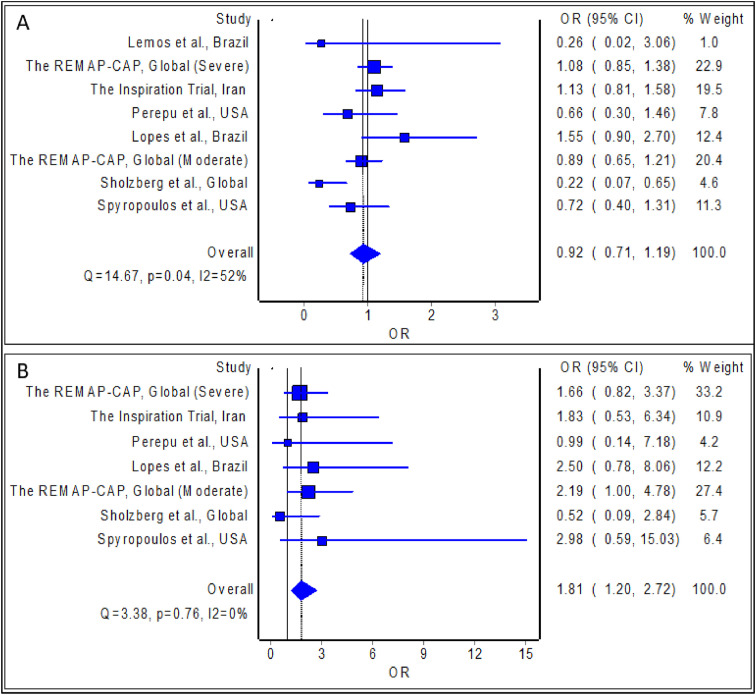
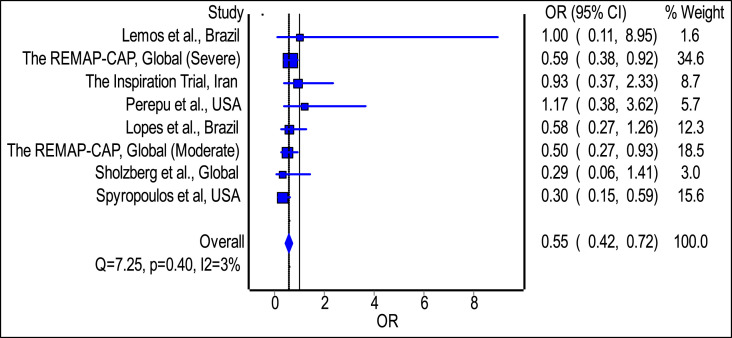
The meta-analysis revealed no statistically significant difference in the odds of mortality with the use of intermediate/therapeutic anticoagulation among hospitalized patients with COVID-19 relative to prophylactic anticoagulation; the estimated effect though indicated reduced mortality risk (Fig. 3(A); pooled odds ratio = 0.99; 95% confidence interval 0.85 to 1.15, n = 5404), but is with inadequate evidence against the null hypothesis of ‘no significant difference’, at the current sample size. In addition, the meta-analysis revealed a statistically significant reduction in the odds of development of thrombotic events with the use of intermediate/therapeutic anticoagulation among hospitalized patients with COVID-19 relative to prophylactic anticoagulation; the estimated effect indicated risk reduction (Fig. 2 ; pooled odds ratio = 0.55; 95% confidence interval 0.42 to 0.72, n = 5402), and with adequate evidence to refute the null hypothesis of ‘no significant difference’, at the current sample size. Finally, the meta-analysis revealed a statistically significant difference in the odds of development of major bleeding events with the use of intermediate/therapeutic anticoagulation among hospitalized patients with COVID-19 relative to prophylactic anticoagulation; the estimated effect indicated an increased risk of major bleeding (Fig. 3(B); pooled odds ratio = 1.81; 95% confidence interval 1.20 to 2.72, n = 5385), and with adequate evidence to refute the null hypothesis of ‘no significant difference’, at the current sample size.
Fig. 3.
Pooled odds ratio of mortality (A) and development of major bleeding (B) with the use of intermediate/therapeutic anticoagulation among hospitalized patients with COVID-19 relative to prophylactic anticoagulation.
Fig. 2.
Pooled odds ratio of development of thrombotic events with the use of intermediate or therapeutic anticoagulation among hospitalized patients with COVID-19 relative to prophylactic anticoagulation.
Sensitivity analysis of randomized trials [5,6,[9], [10], [11], [12]] which investigated the effect of therapeutic anticoagulation relative to prophylactic anticoagulation in hospitalized patients with COVID-19 revealed consistent findings with the main analysis; there was a statistically significant reduction in the odds of development of thrombotic events (Fig. S1; pooled odds ratio = 0.50; 95% confidence interval 0.37 to 0.66), and significantly increased odds in the development of major bleeding events (Fig. S1; pooled odds ratio = 1.86; 95% confidence interval 1.19 to 2.90), but with no statistically significant difference in the odds of mortality (Fig. S1; pooled odds ratio = 0.88; 95% confidence interval 0.62 to 1.25). Subgroup analysis of randomized trials [[5], [6], [7], [8]] which recruited patients exclusively with a severe course of COVID-19 observed statistically significant reduction in the odds of development of thrombotic events (pooled odds ratio = 0.66; 95% confidence interval 0.45 to 0.98) without a significant increase in the odds of mortality (pooled odds ratio = 1.04; 95% confidence interval 0.85 to 1.26) and the odds of development of major bleeding events (pooled odds ratio = 1.37; 95% confidence interval 0.74 to 2.56), with the use of intermediate/therapeutic anticoagulation among hospitalized patients with COVID-19 relative to prophylactic anticoagulation. The funnel plot to detect publication bias revealed reasonable symmetry, as only one study was located outside of the triangle (Fig. S2). It is evident that there are many studies with negative results are missing in the funnel plot.
4. Discussion
To the best of the authors’ knowledge, this is the first systematic review and meta-analysis to summarize the overall evidence from randomized controlled trials related to higher-intensity dosing of anticoagulation in hospitalized patients with COVID-19. The findings from our meta-analyses of randomized controlled trials indicate benefits with regard to the risk reduction of development of thrombotic events using higher-intensity dosing of anticoagulation in hospitalized patients with COVID-19, relative to prophylactic-dosing, which coincide with several real-world observations which adjusted for confounding bias. Recently, the retrospective study by Carallo et al. [13] (n = 42) reported significantly reduced hazard for the combined cardiovascular endpoint of deep venous thrombosis, myocardial infarction, pulmonary embolism, and cardiovascular death (hazard ratio = 0.21; 95% confidence interval 0.05 to 0.93) with higher-intensity dosing of anticoagulation compared to lower-intensity dosing of anticoagulation, after adjustment of covariates, among hospitalized patients with COVID-19. Likewise, Helms et al. [14] (n = 179) reported in their prospective study that the occurrences of pulmonary embolism and deep vein thrombosis were significantly lower with therapeutic anticoagulation (adjusted odds ratio = 0.19; 95% confidence interval 0.03 to 0.81 and adjusted odds ratio = 0.13; 95% confidence interval 0.01 to 0.89, respectively) compared to prophylactic anticoagulation, but without significant difference between the two anticoagulation modalities in the mortality rate.
The reduced risk of developing thrombotic events with higher-intensity dosing of anticoagulation might be related to the phenomenon of heparin resistance (the requirement for high doses of heparin to achieve therapeutic activity) in patients with a severe course of COVID-19, which had been reported in few observational studies [15,16]. Yet, the significantly increased risk of major bleeding detected from our meta-analysis could be a concern for the clinicians since it indicates that the harms of higher-intensity dosing of anticoagulation, especially major bleeding, probably outweigh its benefits, among hospitalized patients with COVID-19. However, with an assumed control risk of 0.062 (the frequency of development of thrombotic event in the control group was 62 per 1000), an estimated 37 patients would require administration of higher-intensity dosing of anticoagulation to prevent one thrombotic event. On the other hand, with an assumed control risk of 0.014 (the frequency of major bleeding in the control group was 14 per 1000), an estimated 90 patients would require administration of higher-intensity dosing of anticoagulation to experience one major bleeding event. Therefore, the benefits from the risk reduction of development of thrombotic events with higher-intensity dosing of anticoagulation have the potential to outweigh its risks of major bleeding, relative to prophylactic-dosing of anticoagulation, among hospitalized patients with COVID-19.
It should be noted that the baseline risks of bleeding of the included participants were not assessed in the included trials [[5], [6], [7], [8], [9], [10], [11], [12]]. Therefore, the acceptable baseline risk of bleeding, which would lead to net clinical benefit with higher-intensity-dosing anticoagulation, remains to be determined among hospitalized patients with COVID-19. Nevertheless, patients with a severe course of COVID-19 had no increased risk of major bleeding as per our subgroup analysis; this could be explained again by the phenomenon of heparin resistance, which may be more pronounced in patients with severe illness relative to mild-to-moderate illness. Hence, higher-intensity dosing of anticoagulation could be administered only in the subgroup of patients with severe illness (e.g., admission into intensive care units), with cognizant of the potentially favorable risk-benefit profile of higher-intensity dosing of anticoagulation among patients with low bleeding risks. On the other hand, an individualized anticoagulation approach should be adopted for patients with high bleeding risks (e.g., history of major bleeding) since this subgroup of patients had been excluded from the clinical trials.
The absence of mortality benefits based on our meta-analysis suggests that the initiation of higher-intensity dosing of anticoagulation when patients with COVID-19 progress to a severe course of illness might be too late to adequately contain the effect of established thrombo-inflammation; the use of anti-inflammatory therapies with established mortality benefits should still be the mainstay in this population of patients [17]. Again, it indicates that the use of higher-intensity dosing of anticoagulation in patients with a mild-to-moderate course of COVID-19, especially with high bleeding risk, might not be associated with a favorable risk-benefit ratio, particularly in the context of antiviral therapies such as remdesivir [18], which could retard the progression of the disease. However, more evidence from clinical trials is required. In addition, the development of thrombotic events is only part of the disease pathophysiology in patients with COVID-19 that leads to mortality, and much of the mortality has been associated with the cytokine storm syndrome and subsequent multiorgan failures [19,20].
Limitations of our systematic review and meta-analysis should be acknowledged; firstly, all the included trials [[5], [6], [7], [8], [9], [10], [11], [12]] had an open-label design, and thus bias in the ascertainment of outcomes, especially of thrombotic events, cannot be excluded, and future double-blind, randomized controlled trials should substantiate the current findings; secondly, the majority of the included trials [[5], [6], [7], [8], [9], [10], [11], [12]] enrolled a relatively small number of participants and with limited heterogeneity across the included trials, and thus, the possibility of a small mortality benefit cannot be confidently excluded, which should be confirmed in future larger-scale randomized controlled trials; thirdly, due to the availability of limited trials, we could not perform subgroup analysis across trials that administered intermediate-dosing of anticoagulation to the intervention group; lastly, we did not prepare and register our study protocol in PROSPERO which is typically recommended for systematic reviews and meta-analyses.
In conclusion, there were net clinical benefits with administering higher-intensity dosing of anticoagulation relative to prophylactic-dosing of anticoagulation among hospitalized patients with COVID-19, especially those with severe course of illness. Routine screening of bleeding risk may facilitate the clinical judgement on the intensity of anticoagulation to be administered in an individual patient hospitalized with COVID-19.
Funding
No external funding was used in the preparation of this manuscript.
Informed consent on studies with human and animal subjects
Not applicable.
Data availability
The data presented in this study are available in the manuscript.
Author and co-author contribution
Chia Siang Kow and Dinesh Sangarran Ramachandram were involved in study design, execution, analysis, manuscript drafting, and discussion. Syed Shahzad Hasan was involved in study design, analysis, and manuscript drafting.
Consent to publication
All authors consent for publication.
Declaration of competing interest
All authors declare that they have no potential conflicts of interest that might be relevant to the contents of this article.
Acknowledgement
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2021.11.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hasan S.S., Radford S., Kow C.S., Zaidi S.T.R. Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta-analysis. J Thromb Thrombolysis. 2020;50(4):814–821. doi: 10.1007/s11239-020-02235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsebaie M.A., Baral B., Shrivastava T., Tariq M.J., Kumi D., Elsebaie M. Clinical outcomes of prophylactic vs. Therapeutic doses of anticoagulation in COVID-19 patients: a systematic review and meta-analysis [abstract] Res Pract Thromb Haemost. 2021;5(Suppl 2) https://abstracts.isth.org/abstract/clinical-outcomes-of-prophylactic-vs-therapeutic-doses-of-anticoagulation-in-covid-19-patients-a-systematic-review-and-meta-analysis/ [Google Scholar]
- 3.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 5.Lemos A.C.B., do Espírito Santo D.A., Salvetti M.C., et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators. Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19 [published online ahead of print, 2021 Aug 4] N Engl J Med. 2021 doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B., Talasaz A.H., Rashidi F., et al. Intermediate vs standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to ICU: Ninety-day results from the INSPIRATION trial [published online ahead of print, 2021 Apr 17] Thromb Haemostasis. 2021 doi: 10.1055/a-1485-2372. [DOI] [Google Scholar]
- 8.Perepu U., Chambers I., Wahab A., Ten Eyck P., Wu C., Dayal S., et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomised controlled trial. J. Thromb. Haemost. 2021;19(9):2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes R.D., Silva P.G.M.B.E., Furtado R.H.M., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;S0140–6736(21) doi: 10.1016/S0140-6736(21)01203-4. 01203-01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators. Lawler P.R., Goligher E.C., Berger J.S., et al. Therapeutic anticoagulation with heparin in Noncritically ill patients with Covid-19 [published online ahead of print, 2021 Aug 4] N Engl J Med. 2021 doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sholzberg M., Tang G.H., Rahhal H., AlHamzah M., Kreuziger L.B., Áinle F.N., et al. Heparin for moderately ill patients with Covid-19. Preprint medRxiv. 2021:2021. 07.08.21259351. [Google Scholar]
- 12.Spyropoulos A.C., Goldin M., Giannis D., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial [published online ahead of print, 2021 Oct 7] JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carallo C., Pugliese F., Vettorato E., et al. Higher heparin dosages reduce thromboembolic complications in patients with COVID-19 pneumonia [published online ahead of print, 2021 Mar 23] J Invest Med. 2021:2020–20001628. doi: 10.1136/jim-2020-001628. [DOI] [PubMed] [Google Scholar]
- 14.Helms J., Severac F., Merdji H., et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: bi-center cohort study. Ann Intensive Care. 2021;11(1):14. doi: 10.1186/s13613-021-00809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White D., MacDonald S., Bull T., et al. Heparin resistance in COVID-19 patients in the intensive care unit [published correction appears in J Thromb Thrombolysis. 2020 Jun 22. J Thromb Thrombolysis. 2020;50(2):287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beun R., Kusadasi N., Sikma M., Westerink J., Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42(Suppl 1):19–20. doi: 10.1111/ijlh.13230. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J.A.C., Murthy S., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. J Am Med Assoc. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kow C.S., Aldeyab M., Hasan S.S. Effect of remdesivir on mortality in patients with COVID-19: a meta-analysis of randomized control trials. J Med Virol. 2021;93(4):1860–1861. doi: 10.1002/jmv.26638. [DOI] [PubMed] [Google Scholar]
- 19.Quirch M., Lee J., Rehman S. Hazards of the cytokine storm and cytokine-targeted Therapy in patients with COVID-19: review. J Med Internet Res. 2020;22(8) doi: 10.2196/20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Escobar L.G., Hoffman K.L., Choi J.J., et al. Cytokine signatures of end organ injury in COVID-19. Sci Rep. 2021;11(1):12606. doi: 10.1038/s41598-021-91859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the manuscript.