Abstract
The effects of packaging materials, package shape, and secondary packaging on the inactivation of indigenous mesophilic aerobic bacteria in Korean steamed rice cakes using in-package atmospheric dielectric barrier discharge cold plasma (ADCP) treatment were investigated. Inactivation of indigenous mesophilic aerobic bacteria by ADCP treatment (21 kV, 3 min) was significantly increased by 0.6 and 0.8 log CFU/g (p < 0.05) from 0.7 ± 0.1 and 0.5 ± 0.1 CFU/g, respectively, when polypropylene (PP) and low-density polyethylene (LDPE) were laminated with nylon, respectively. Secondary packaging lowered the inactivation level by 0.7–0.8 log CFU/g from 1.1 to 1.3 log CFU/g. In-package ADCP treatment did not alter the water vapor permeability, oxygen transmission rate, and tensile properties of PP, LDPE, nylon/PP, and nylon/LDPE. Thus, the results demonstrated that lamination of PP or LDPE with nylon and treatment before secondary packaging may be effective strategies for microbial inactivation by in-package ADCP treatment.
Keywords: Cold plasma, Plastic package, Rice cake, Microbial decontamination, Package properties
Introduction
Steamed rice cake (SRC) is a processed rice product with a sleek, white surface produced by steaming and compressing rice powder after adding salt and oil (Kim et al., 2017; Ku et al., 2018). It is a home meal replacement widely consumed in various countries, such as Korea, China, and Japan (Park and Yoon, 2019). Sanitary management is important to minimize microbial contamination during the processing of rice cakes because microorganisms can rapidly proliferate during the storage and distribution processes due to high water activity of the product (Jeong et al., 2012; Meng and Kim, 2020). Korean SRC is washed with a sodium hypochlorite solution or sprayed with alcohol to inactivate microorganisms during the production process. However, sodium hypochlorite solutions may generate carcinogenic residual chlorine-based chemicals and ethyl alcohol may leave a unacceptable odor on the product (Jung et al., 2019). Therefore, a novel microbial disinfection process has been sought for Korean SRC.
In-package atmospheric dielectric barrier discharge cold plasma (ADCP) treatment applies electric energy to a package positioned between two electrodes to generate microdischarges and produce plasma within the package in the atmosphere (Pankaj et al., 2015). Cold plasma (CP) contains ultraviolet (UV) photons; excited molecules; charged particles; reactive oxygen species, including hydroxyl radicals, ozone, hydrogen peroxide, and atomic oxygen; and reactive nitrogen species, including nitric oxide and nitrogen dioxide radicals (Li et al., 2016; Misra et al., 2014). The various reactive species formed within CP can non-thermally inactivate microbial contaminants in food. The reactive species in CP can diffuse into the microbial cells and damage the cells by reducing pH, damaging DNA, and oxidizing lipids, proteins, and nucleic acids (Han et al., 2016; Huang et al., 2020). Charges can be accumulated at the outer surface of cell membranes, which can generate electrostatic force that can irreversibly damage the cell structure (Pignata et al., 2017). In addition, during CP treatment, microorganisms are exposed to intense bombardment by the radicals, which provoke surface lesions that the living cell cannot repair (known as “etching”) (Pignata et al., 2017). Furthermore, the exposure of cells to UV photons can cause the dimerization of thymine bases in their DNA strands (Huang et al., 2020). Kang et al (2021) reported that ADCP treatment at 25 kV for 3 min inactivated Salmonella contaminated in SRC by 2.3 log CFU/g, without altering the color and firmness of the rice cake.
ADCP achieves in-package treatment by forming plasma within a sealed package (Lee et al., 2020), thus leading to the prevention of post-processing contamination that can occur between the time of microbial inactivation and packaging as well as recontamination during packaging (Feizollahi et al., 2021). ADCP is an economical technology that uses air as a plasma-forming gas, and thus it has garnered considerable research attention for its possible application in the food production industry (Kim et al., 2019; Min et al., 2017; Pignata et al., 2017). The in-package plasma technology uses plastic packages as dielectric layers (Misra et al., 2019). Nonetheless, studies on the appropriate packaging requirements for ADCP, such as those pertaining to the package type, package shape, and volume ratio of the headspace and sample, are lacking. To ensure the quality and safety of in-package ADCP-treated food, it is important to assess changes in the packaging material following ADCP treatment (Misra et al., 2019). Thus, the objectives of this study were to investigate the effect of packaging parameters, including the packaging material type, packaging shape, and secondary packaging, on the inactivation of indigenous mesophilic aerobic microorganisms in SRC using in-package ADCP treatment and to examine the effect of ADCP treatment on the water vapor permeability, oxygen transmission rate, and tensile properties of the packaging materials.
Materials and methods
Materials
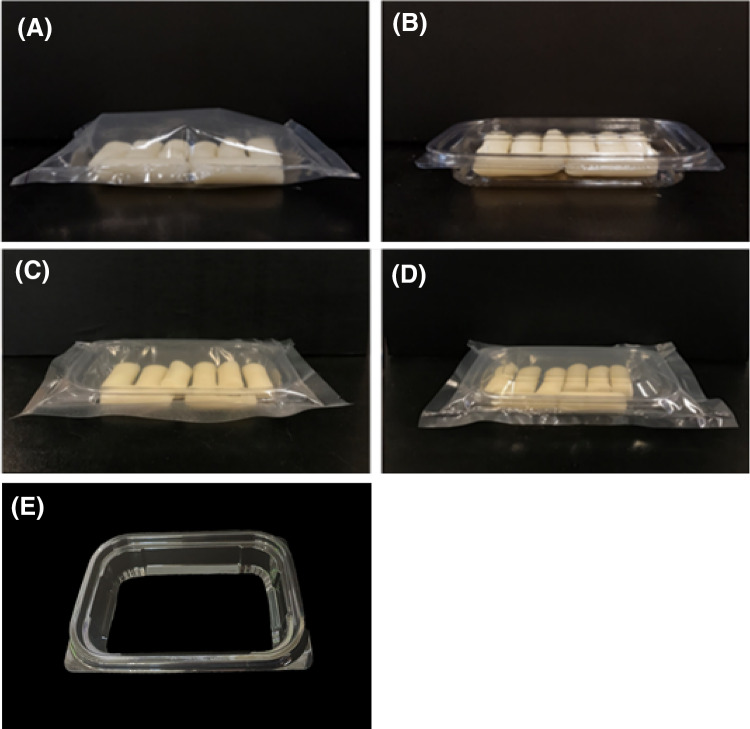
Commercial Korean SRC were used in this study (Duksan Food, Goesan, Korea), which were composed of 99% rice and < 1% refined salt. Each SRC was cylindrical in shape (1.5 cm in diameter and 4 cm in height) and weighed 8.0 ± 0.3 g. The SRC, stored in a refrigerator within 5 days of production, were purchased from a grocery store and then stored at 4 °C within two days of purchase until use in the experiments. A polyethylene terephthalate container (PET; length: 14 cm, width: 10 cm, height: 3 cm, volume: 235 mL, thickness: 0.30 ± 0.01 mm; Dongyang D&P, Chilgok, Korea) was used to form the frame for producing semi-rigid containers (Fig. 1E). Seventy micrometer-thick polypropylene (PP; Makepack, Pocheon, Korea), low-density polyethylene (LDPE; Boltmall1, Goyang, Korea), nylon/PP (Wonchang, Seoul, Korea), and nylon/LDPE (Hanmipojang, Busan, Korea) films were used to prepare flexible pouches and semi-rigid containers.
Fig. 1.
Images of a steamed rice cake-containing nylon/low-density polyethylene (LDPE) pouch package (A), a polyethylene terephthalate (PET) semi-rigid package (B), a nylon/LDPE semi-rigid package with a PET frame (C), a PET semi-rigid package placed in a nylon/LDPE pouch (secondary packaging) (D), and the PET frame used to structure the semi-rigid package (E)
ADCP treatment system and treatments
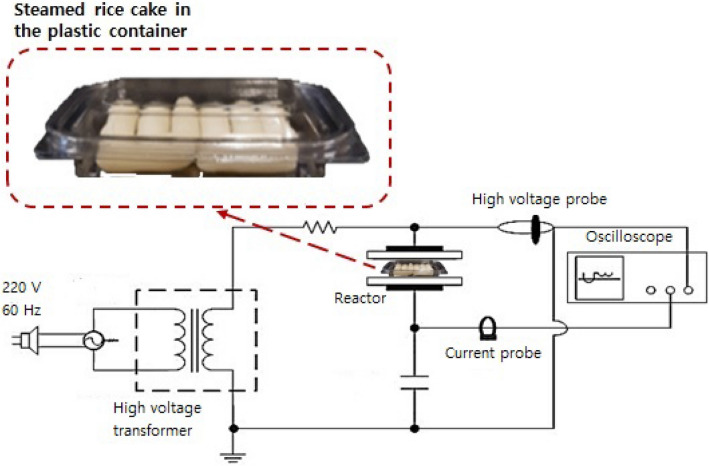
In this study, the in-package ADCP treatment system introduced by Kim et al. (2019) was used. The treatment system consists of a high-voltage transformer that can deliver a high voltage of up to 50 kV, a treatment reactor comprising two aluminum electrodes (20 × 16 cm each; Kwang Lim Co., Ltd., Hwasung, Korea) and borosilicate (29 × 25 × 0.4 cm), and measurement devices, including a high-voltage probe (EP-50; Pulse Electronic Engineering Co., Ltd., Noda, Japan) and an oscilloscope (TDS-3012b Oscilloscope; Tektronix, Beaverton, OR, USA) (Fig. 2). A pouch or container containing eight SRC pieces (64 g) was placed between the two electrodes. The SRC pieces were directly treated with CP at 21 kV, which is the maximum voltage that does not generate dielectric breakdown in the package, for 3 min with shaking at 300 rpm. Treatment durations longer than 3 min for food decontamination are considered too long (Roh et al., 2020). The distance between borosilicate and the upper electrode was 3.5 cm.
Fig. 2.
Schematic diagram of the in-package atmospheric dielectric barrier discharge cold plasma treatment system used in the current study
In the experiments conducted to investigate the effect of packaging material on the inactivation of indigenous mesophilic aerobic microorganisms in SRC and examine the changes in the physical properties of packaging materials following ADCP treatment, eight SRC pieces were placed in a pouch composed of PP, LDPE, nylon/PP, or nylon/LDPE (volume: 235 mL, length: 14 cm, width: 10 cm) and the pouch was filled with air using an air compressor and then sealed using a heat-sealing machine (PFS-400; Wenzhou Huazhen Machinery Co., Ltd., Wenzhou, China). The packaged samples were treated with CP. The PP and LDPE layers of the nylon/PP and nylon/LDPE pouches were in contact with the SRC, respectively. To investigate the effect of package shape on the microbial inactivation by ADCP treatment, pouches (Fig. 1A) and semi-rigid containers (Fig. 1C) with the identical volume (235 mL) were prepared. The semi-rigid containers were built on a supporting frame (Fig. 1E), prepared by removing the lid and cutting the side and bottom areas of the commercially available PET container using a sterile razor. The packaging material used for the study of the package shape was the nylon/LDPE film. To study the effect of secondary packaging on the inactivation of microorganisms in SRC by ADCP treatment, SRC pieces were packaged in the PET container and packaged again in a nylon/LDPE pouch (volume: 550 mL, length: 18 cm, width: 13 cm) as exhibited in Fig. 1D. To investigate the effect of the empty space between the first and second packaging materials on the inactivation of indigenous microorganisms in SRC, a sample in which the upper surface of the PET container was pasted to the bottom surface of the secondary packaging material using a laminate adhesive (Loctite 401; Henkel, Dusseldorf, Germany) and a sample in which the two were not pasted to one another were prepared.
SRC-containing PP, LDPE, or nylon/LDPE packages were treated with CP and then sampled from areas that did not contact the SRC to determine the water vapor permeability, oxygen transmission rate, and tensile properties of the packaging materials.
Microbial analysis
Eight treated or untreated SRC pieces and 80 mL of 0.1% sterilized peptone water (Difco™, Franklin Lakes, NJ, USA) were placed in a sterilized bag (710 mL; Whirl–Pak® Write-On Bags; Nasco Co., Fort Atkinson, WI, USA) and pummeled for 3 min at 300 rpm by a stomacher blender (Stomacher Lab Blender Model 400, Seward Medical, London U.K.). The suspension was diluted in 0.1% sterilized peptone water (Difco™). The diluted sample was dispensed into aerobic plate count Petrifilm (3 M, St. Paul, MN, USA) and incubated at 37 °C for 48 h prior to enumeration.
Water vapor permeability measurement
The water vapor permeability of the packaging materials was determined using the gravimetric modified cup method (Coelho et al., 2020) based on the American Society of Testing and Materials (ASTM) E96–92 standard methods (ASTM, 1990). The measurement was performed inside a cabinet with anhydrous calcium sulfate to equilibrate the relative humidity (RH) to 2.5 ± 0.5%. The temperature inside the cabinet was maintained at 23 ± 2 °C. To ensure uniform RH throughout the cabinet, a fan was installed inside the cabinet with its speed set to obtain an air velocity of 152 m/min. A cylindrical cup made of polymethylmethacrylate (Plexiglas™, Darmstadt, Germany) was used as the test cup. The RH inside the cabinet was measured using a data logger (Testo 174H; Lenzkirch, Germany).
Oxygen transmission rate determination
The oxygen transmission rate of the packaging materials was measured using an Oxysense 5250i (Oxysense, Inc., Dallas, USA) (Nanni et al., 2019; Tedeschi et al., 2018). The temperature and RH inside the chamber were maintained at 23 ± 2 °C and 52 ± 2%, respectively. A film was cut into 6.5 × 6.5 cm pieces to prepare test samples. The oxygen concentration inside the chamber was lowered to 0.02 ± 0.02% by introducing nitrogen gas before measuring the oxygen transmission rate. An oxy-dot, a sensor inside the chamber, was used to measure oxygen absorption over time at 20-min intervals to obtain the oxygen transmission rate values. The resulting oxygen transmission rate graph flattened over time as the oxygen transmission rate stabilized, and the value at a flat point on the graph was used as the oxygen transmission rate of the film (Nanni et al., 2019; Tedeschi et al., 2018).
Tensile property determination
The tensile strength, percentage elongation at break, and elastic modulus, which are the tensile properties of packaging materials, were determined using the D882-01 standard method (ASTM, 2001; Garcia et al., 2020). The packaging materials (8 × 50 mm) were stored in a humid box at a temperature of 23 ± 2 °C and RH of 52 ± 2% for 48 h before measurement. A saturated solution of magnesium nitrate (Thermo Fisher Scientific, Waltham, MA, USA) was used to equilibrate the films into an environment (52 ± 2% RH). A tensile property tester (Withlab Co., Ltd., Anyang, Korea) was used for the measurements. The distance between the grips and crosshead speed before the experiment were 50 mm and 50 mm/min, respectively.
Statistical analysis
All experiments were repeated three times. Two treated and untreated samples (packages contained eight SRC pieces) were prepared for each repeat experiment of microbial inactivation. To assess the water vapor permeability, oxygen transmission rate, and tensile properties of the packaging materials, three package samples were prepared for each treatment (ADCP treatment or no ADCP treatment) in each repeat experiment. To determine the tensile properties, six measurement specimens were prepared per package sample. Two measurement specimens were prepared per package sample to measure the water permeability and oxygen transmission rate. The differences in average values were analyzed using a one-way analysis of variance in SPSS (ver. 24, SPSS Inc., Chicago., IL, USA). Tukey’s range test was conducted for cases showing significant differences (α = 0.05).
Results and discussion
Effects of packaging material type and package shape on microbial inactivation
Table 1 shows the levels of inactivation of indigenous mesophilic aerobic microorganisms in SRC following ADCP treatment of different packaging materials. The initial microbial concentration before ADCP treatment was 7.6 ± 0.2 log CFU/g. No significant difference in microbial inactivation was found between the PP and LDPE pouches (p > 0.05; Table 1). In addition, microbial inactivation efficiency did not differ between nylon/PP and nylon/LDPE (p > 0.05). However, significant differences were found between PP and nylon/PP and between LDPE and nylon/LDPE (p < 0.05), indicating that the additional nylon layer in the film increased the level of microbial inactivation. This result can be explained by the dielectric permittivity of the packaging material. While PE and PP have similar dielectric permittivity values (approximately 2.3) (Afsar, 1987), nylon has a higher dielectric permittivity value ranging from 4.0 to 6.0 (Lampman, 2003). The higher the dielectric permittivity is, the higher are the polarization and charge accumulation upon receiving electric energy from an external source (Kim et al., 2009). It appears that the accumulation of a large number of charges on the packaging material promotes electric discharges, and the subsequent formation of a large number of reactive species within the plasma increases the level of microbial inactivation (Kim et al., 2019). The lack of difference in the level of microbial inactivation between PE and PP may be attributed to their similar dielectric permittivities. The results suggest that packaging materials with high dielectric permittivity values would be effective as packaging for foods to be decontaminated by in-package ADCP treatment.
Table 1.
Effects of packaging material type on the inactivation of total mesophilic aerobic bacteria in steamed rice cakes using in-package atmospheric dielectric barrier discharge cold plasma treatment
| Packaging materials | Microbial reduction (log CFU/g) |
|---|---|
| Low-density polyethylene | 0.5 ± 0.1b |
| Polypropylene | 0.7 ± 0.1b |
| Nylon/low-density polyethylene | 1.3 ± 0.2a |
| Nylon/casted polypropylene | 1.3 ± 0.2a |
Microbial count of untreated rice cakes: 7.6 ± 0.2 log CFU/g
The results are expressed as the mean ± standard deviation (n = 6). Values followed by different letters in the same column are significantly different (p < 0.05)
The ADCP treatment reduced the concentration of indigenous mesophilic aerobic microorganisms by 1.3 ± 0.2 and 1.1 ± 0.4 log CFU/g in the SRC samples packaged in a pouch and PET semi-rigid container, respectively. No significant difference in microbial inactivation was observed with respect to package shape (p > 0.05). Compared to the pouch, the PET semi-rigid container has more uniform contact with the borosilicate on its bottom surface and has a uniform height difference from the upper electrode. Thus, it can cause electric discharges to occur over a relatively large area, producing a greater amount of plasma reactive species within the packaging material and achieving greater microbial inactivation (Kim et al., 2019). The lack of difference in the inactivation of indigenous mesophilic aerobic microorganisms in SRC according to package shape in this study can be attributed to sample shaking applied during treatment, which evenly distributed reactive species throughout the packages in both pouches and the containers, thereby minimizing potential differences in the chances of reactive species reacting with microorganisms induced by the package shape (Kim et al., 2019; Roh et al., 2019).
Effects of the use of secondary packaging on microbial inactivation
ADCP treatment reduced the counts of indigenous microorganisms in SRC by 1.3 ± 0.2 and 1.1 ± 0.4 log CFU/g for the SRC pieces packaged in a pouch and semi-rigid container, respectively. The treatment inactivated the microorganisms by only 0.4 ± 0.1 and 0.5 ± 0.2 log CFU/g in the SRC sample that was first packaged in a semi-rigid container and then secondly packaged using a pouch with and without an empty space between the two packaging materials, respectively. Regardless of whether there was an empty space between the container and pouch, ADCP treatment achieved significantly lower microbial inactivation for these samples than the sample that was packaged in a pouch or semi-rigid container only (p < 0.05). The dielectric permittivity of the package can be changed by wrapping it in nylon/LDPE (Kim et al., 2019), which can negatively affect the efficacy of ADCP treatment on microbial inactivation. Furthermore, when an empty space exists between the primary packaging (PET container) and secondary packaging (nylon/LDPE pouch), there is a loss of electrical energy that is required to generate microdischarges inside the container. However, the results demonstrated that the empty space (315 mL) (Fig. 3) was not substantial to reduce the microdischarges and efficacy of ADCP treatment on microbial inactivation. Thus, it is suggested that samples should be packaged with a single packaging material only for in-package ADCP treatment and later packaged with a second material after the treatment. In cases where ADCP treatment must be performed on products with secondary packaging because of the nature of the production line, the space between the first and second packaging materials must be minimized, and the proper type of dielectric material for ADCP treatment must be selected while considering the dielectric permittivity of both packaging materials.
Fig. 3.
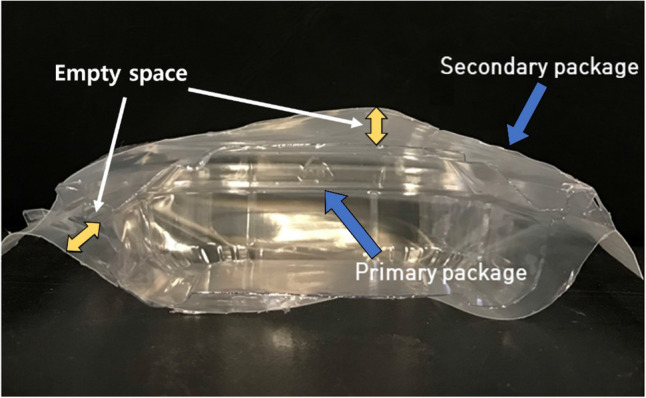
Cross-sectional view of the secondary package demonstrating its structure, including an empty space between the primary and secondary packages
Effects of ADCP treatment on packaging material properties
The physical properties of a packaging material are an important index for determining whether the packaging material can maintain its functioning (Pankaj et al., 2015). Table 2 summarizes the changes in the physical properties of the different packaging materials following in-package ADCP treatment. No significant differences in water vapor permeability, oxygen transmission rate, and tensile properties were observed between the ADCP-treated and untreated packaging materials (Table 2; p > 0.05). A previous study reported that energetic plasma particles generated during ADCP treatment destroy the bonds between polymer chains on the surface of a plastic film, increase the surface roughness of the film through etching, and increase the hydrophilicity of the film surface by producing carboxylic acids or ethyl groups (Chen et al., 2020; Pankaj et al., 2015; Song et al., 2016). However, no significant differences in the properties were observed between the ADCP-treated and untreated packaging materials (Table 2; p > 0.05). This may be due to the fact that CP only manages to act on the surface layer of the plastic film (Song et al., 2016), failing to affect its tensile properties and permeabilities. These results are in agreement with those of previous studies. Puligundla et al. (2016) reported that the tensile force and strength of PE, PP, nylon, and paper foil packaging materials did not change following 2, 4, 6, 8, and 10 min of ADCP at 10 kV. Pankaj et al. (2015) reported that while the contact angle and surface roughness of a polymeric film changed after 5 min of ADCP at 80 kV, its water vapor permeability and oxygen transmission rate did not significantly change compared to the untreated film. The results of this study suggest that in-package ADCP treatment can inactivate microorganisms in packaged SRC without affecting the food packaging functions induced by its tensile and barrier properties.
Table 2.
Effects of in-package atmospheric dielectric barrier discharge cold plasma (ADCP) treatment on the water vapor permeability, oxygen transmission rate, tensile strength, percentage (%) elongation at break, and elastic modulus of polypropylene (PP), low-density polyethylene (LDPE), nylon/PP, and nylon/LDPE films
| Packaging materials | Treatments | Water vapor permeability (g·mm/k·Pa·h·m2) | Oxygen transmission rate (mL/m2·day·atm) | Tensile strength (MPa) | % Elongation at break (%) | Elastic modulus (MPa) |
|---|---|---|---|---|---|---|
| PP | Untreated | 0.014 ± 0.001a | 718.27 ± 175.07a | 14.79 ± 0.59a | 425.08 ± 1.54a | 615.82 ± 13.34a |
| ADCP-treated | 0.013 ± 0.003a | 727.81 ± 44.58a | 13.21 ± 1.43a | 425.97 ± 2.54a | 613.55 ± 3.72a | |
| LDPE | Untreated | 0.016 ± 0.002a | 1201.78 ± 278.83a | 18.71 ± 1.07a | 425.51 ± 1.49a | 211.03 ± 21.08a |
| ADCP-treated | 0.016 ± 0.001a | 1225.46 ± 265.75a | 18.31 ± 0.50a | 427.34 ± 3.09a | 209.30 ± 32.12a | |
| Nylon/PP | Untreated | 0.016 ± 0.002a | 168.58 ± 39.73a | 29.35 ± 2.10a | 87.99 ± 18.72a | 1063.70 ± 12.05a |
| ADCP-treated | 0.015 ± 0.006a | 163.65 ± 27.32a | 29.81 ± 2.33a | 87.67 ± 10.71a | 1076.75 ± 20.01a | |
| Nylon/LDPE | Untreated | 0.013 ± 0.001a | 257.46 ± 28.58a | 30.93 ± 8.18a | 83.03 ± 8.53a | 641.18 ± 5.27a |
| ADCP-treated | 0.012 ± 0.001a | 239.80 ± 18.88a | 30.77 ± 3.34a | 83.80 ± 9.08a | 645.10 ± 36.71a |
Film thickness: 70 µm
The results are expressed as the mean ± standard deviation (n = 9). Values followed by the same letter in the same column are not significantly different (p > 0.05)
Packaging SRC in a flexible pouch containing nylon increased the inactivation of indigenous microorganisms compared to packaging them in a pouch that did not contain nylon. Shaking during ADCP treatment was thought to improve the efficacy of in-package ADCP treatment in decontaminating the SRC in pouches. Nonetheless, the shape of the packaging (pouch or semi-rigid container) did not affect microbial inactivation by CP. Secondary packaging reduced microbial inactivation by ADCP treatment regardless of whether there was a space between the first and second packaging materials. Because in-package ADCP treatment did not affect the water vapor permeability, oxygen transmission rate, and tensile properties of PP, LDPE, nylon/PP, and nylon/LDPE, ADCP treatment was expected not to alter the functions of food packaging. Thus, this study recommends choosing packaging materials while considering their dielectric permittivities and applying secondary packaging after ADCP treatment for effective in-package ADCP treatment. Although this study demonstrates that in-package ADCP treatment does not negatively affect barrier and tensile properties, further research on confirming whether packaging materials release potentially hazardous molecules following ADCP treatment is needed for the industrial application of in-package ADCP treatment.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2B5B01069364).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joo Hyun Kang, Email: as03226@naver.com.
Ye Jeong Jeon, Email: dpwjd7510@naver.com.
Sea Cheol Min, Email: smin@swu.ac.kr.
References
- Afsar MN. Precision millimeter-wave measurements of complex refracive index, complex dielectric permittivity, and loss tangent of common polymers. IEEE Transactions on Instrumentation and Measurement. 36: 530-536 (1987)
- ASTM. E96–92. Standard test methods for water vapor transmission of materials. American Society for Testing and Materials, Philadelphia, PA, USA (1990)
- ASTM. D882–01. Standard test method for tensile properties of thin plastic sheeting. American Society for Testing and Materials, Philadelphia, PA, USA (2001)
- Chen G, Chen Y, Jin N, Li J, Dong S, Li S, Zhenya Z, Chen Y. Zein films with porous polylactic acid coatings via cold plasma pre-treatment. Industrial Crops and Products. 150: 112382 (2020)
- Coelho CCS, Silva RBS, Carvalho CWP, Rossi AL, Teixeira J A, Freitas-Silva O, Cabral LMC. Cellulose nanocrystals from grape pomace and their use for the development of starch-based nanocomposite films. International Journal of Biological Macromolecules. 159: 1048-1061 (2020) [DOI] [PubMed]
- Feizollahi E, Misra NN, Roopesh MS. Factors influencing the antimicrobial efficacy of dielectric barrier discharge (DBD) atmospheric cold plasma (ACP) in food processing applications. Critical Reviews in Food Science and Nutrition. 61: 666-689 (2021) [DOI] [PubMed]
- Garcia-Garcia D, Carbonell-Verdu A, Arrieta MP, López-Martínez J, Samper MD. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polymer Degradation and Stability. 179: 109259 (2020)
- Han L, Patil S, Boehm D, Milosavljević V, Cullen PJ, Bourke P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Applied and Environmental Microbiology. 82: 450-458 (2016) [DOI] [PMC free article] [PubMed]
- Huang M, Zhuang H, Zhao J, Wang J, Yan W, Zhang J. Differences in cellular damage induced by dielectric barrier discharge plasma between Salmonella Typhimurium and Staphylococcus aureus. Bioelectrochemistry. 132: 107445 (2020) [DOI] [PubMed]
- Jeong SH, Choi SY, Cho JI, Lee SH, Hwang IG, Na HJ, Oh DH, Bahk GJ, Ha SD. Microbiological contamination levels in the processing of Korean rice cakes. Journal of Food Hygiene and Safety. 27: 161-168 (2012)
- Jung H, Yuk HG, Yoon WB. Effect of LED light on the inactivation of Bacillus cereus for extending shelf-life of extruded rice cake and simulation of the patterns of LED irradiation by various arrays of LEDs. Journal of Applied Biological Chemistry. 62: 181-186 (2019)
- Kang JH, Bai J, Min SC. Inactivation of indigenous microorganisms and Salmonella in Korean rice cakes by in-package cold plasma treatment. International Journal of Environmental Research and Public Health. 18: 3360 (2021) [DOI] [PMC free article] [PubMed]
- Kim B, Kim YM, Cho IS. Evaluation and determination of air void for asphalt concrete using a dielectric constant measurement. Korean Society of Road Engineers. 11: 95-104 (2009)
- Kim MJ, Oh SG, Chung HJ. Impact of heat-moisture treatment applied to brown rice flour on the quality and digestibility characteristics of Korean rice cake. Food Science and Biotechnology. 26: 1579-1586 (2017) [DOI] [PMC free article] [PubMed]
- Kim SY, Bang IH, Min SC. Effects of packaging parameters on the inactivation of Salmonella contaminating mixed vegetables in plastic packages using atmospheric dielectric barrier discharge cold plasma treatment. Journal of Food Engineering. 242: 55-67 (2019)
- Ku SK, Hong JS, Choi HD, Park JD, Kim YB, Choi HW, Kim HW, Kim TK, Choi YS. A study of the quality characteristics of frozen Korean rice cake by using different thawing methods. Food Science and Biotechnology. 27: 1343-1351 (2018) [DOI] [PMC free article] [PubMed]
- Lampman, S. Characterization and failure analysis of plastics. ASM International, Materials Park, OH, USA. pp. 87-181 (2003)
- Lee ES, Cheigh CI, Kang JH, Lee SY, Min SC. Evaluation of in-package atmospheric dielectric barrier discharge cold plasma treatment as an intervention technology for decontaminating bulk ready-to-eat chicken breast cubes in plastic containers. Applied Sciences. 10: 6301 (2020)
- Li R, Liu Y, Cheng W, Zhang W, Xue G, Ognier S. Study on remediation of phenanthrene contaminated soil by pulsed dielectric barrier discharge plasma: The role of active species. Chemical Engineering Journal. 296: 132-140 (2016)
- Meng LW, Kim SM. Effects of different carbohydrases on the physicochemical properties of rice flour, and the quality characteristics of fermented rice cake. Food Science and Biotechnology. 29: 503-512 (2020) [DOI] [PMC free article] [PubMed]
- Min SC, Roh SH, Niemira BA, Boyd G, Sites JE, Uknalis J, Fan X. In-package inhibition of E. coli O157: H7 on bulk romaine lettuce using cold plasma. Food Microbiology. 65: 1-6 (2017) [DOI] [PubMed]
- Misra NN, Pankaj SK, Walsh T, O’ Regan F, Bourke P, Cullen PJ. In-package nonthermal plasma degradation of pesticides on fresh produce. Journal of Hazardous Materials. 271: 33-40 (2014) [DOI] [PubMed]
- Misra NN, Yepez X, Xu L, Keener K. In-package cold plasma technologies. Journal of Food Engineering. 244: 21-31 (2019)
- Nanni G, Heredia-Guerrero JA, Paul UC, Dante S, Caputo G, Canale C, Athanassiou A, Fragouli D, Bayer IS. Poly (furfuryl alcohol)-Polycaprolactone blends. Polymers. 11: 1069 (2019) [DOI] [PMC free article] [PubMed]
- Pankaj SK, Bueno-Feeror C, Misra NN, O’ Neill L, Tiwari BK, Bourke P, Cullen PJ. Characterization of dielectric barrier discharge atmospheric air cold plasma treated gelatin films. Food Packaging and Shelf Life. 6: 61-67 (2015)
- Park HW, Yoon WB. A quantitative microbiological exposure assessment model for Bacillus cereus in pasteurized rice cakes using computational fluid dynamics and Monte Carlo simulation. Food Research International. 125: 108562 (2019) [DOI] [PubMed]
- Pignata C, D'angelo D, Fea E, Gilli G. A review on microbiological decontamination of fresh produce with nonthermal plasma. Journal of Applied Microbiology. 122: 1438-1455 (2017) [DOI] [PubMed]
- Puligundla P, Lee T, Mok C. Inactivation effect of dielectric barrier discharge plasma against foodborne pathogens on the surfaces of different packaging materials. Innovative Food Science and Emerging Technologies. 36: 221-227 (2016)
- Roh SH, Lee SY, Park HH, Lee ES, Min SC. Effects of the treatment parameters on the efficacy of the inactivation of Salmonella contaminating boiled chicken breast by in-package atmospheric cold plasma treatment. International Journal of Food Microbiology. 293: 24-33 (2019) [DOI] [PubMed]
- Roh SH, Oh YJ, Lee SY, Kang JH, Min SC. Inactivation of Escherichia coli O157: H7, Salmonella, Listeria monocytogenes, and Tulane virus in processed chicken breast via atmospheric in-package cold plasma treatment. LWT - Food Science and Technology. 127: 109429 (2020)
- Song AY, Oh YA, Roh SH, Kim JH, Min, SC. Cold oxygen plasma treatments for the improvement of the physicochemical and biodegradable properties of polylactic acid films for food packaging. Journal of Food Science. 81: E86-E96 (2016) [DOI] [PubMed]
- Tedeschi G, Guzman Puyol S, Paul UC, Barthel M, Goldoni L, Caputo G, Ceseracciu G, Athanassiou A, Heredia Guerrero JA. Thermoplastic cellulose acetate oleate films with high barrier properties and ductile behavior. Chemical Engineering Journal. 348: 840-849 (2018)