Abstract
Growing evidence has suggested that the gut-brain axis plays an important role in the pathogenesis of Parkinson’s disease (PD), and that this role is mediated by the interactions between bile acids (BAs) and intestinal microbiota. Given that cholecystectomy can lead to alterations in BAs and gut microbiota, we investigated whether cholecystectomy is linked to a higher risk of PD. We constructed a cohort of patients with an operation code of cholecystectomy from 2010 to 2015 (n = 161,838) and age- and sex-matched control subjects without cholecystectomy (n = 286,135) using the National Health Insurance Service database. Incident PD was traced over a maximum observation period of 7 years. We identified 1404 incident PD cases during 1,631,265 person-years of follow-up. The cholecystectomy group showed an elevated risk of PD compared to the control group, even after adjusting for potential confounding factors (adjusted hazard ratio [HR] 1.14, 95% confidence interval [CI] 1.02–1.27). When the data were split by sex, the risk elevation was significant in men (adjusted HR 1.22, 95% CI 1.06–1.41), but not in women (adjusted HR 1.03, 95% CI 0.88–1.22). Our results provide evidence that cholecystectomy is associated with an increased risk of developing PD. This association differed between men and women, suggesting sex-specific effects of cholecystectomy on the risk of PD.
Subject terms: Risk factors, Parkinson's disease
Introduction
It has been hypothesized that the gut–brain axis plays an important role in the development of Parkinson’s disease (PD)1. Evidence has shown that constipation can precede the onset of motor symptoms of PD by many years2. Aggregated α-synuclein, which is a pathologic hallmark of PD, has been observed in the enteric nervous system during the prodromal period3. This hypothesis is also supported by epidemiological data showing that truncal vagotomy, which interrupts the gut–brain connection through the vagus nerve, is related to a decreased risk of PD4,5. The suggested mechanisms underlying the gut–brain interaction in PD development include an altered gut microbiota, increased intestinal permeability, and intestinal and systemic inflammation6. These changes promote α-synuclein aggregation and microglial activation in the brain, resulting in nigrostriatal dopaminergic neurodegeneration7.
Cholecystectomy for various gallbladder disorders, including symptomatic gallstones, cholecystitis, and gallbladder tumors, is one of the most commonly performed surgical procedures worldwide8. The gallbladder acts as a reservoir and contractile pump of bile acids (BAs), and these compounds are known to interact bidirectionally with the intestinal microbiota9,10. Therefore, removal of the gallbladder modifies the enterohepatic circulation of BAs, and subsequently results in changes in microbiota composition and an increase in secondary BAs8,11. Recently, the potential role of BAs in various neurodegenerative disorders has been highlighted12,13. With respect to PD, previous data have shown that BA profiles are altered in animal models of PD14,15, and such changes were associated with gut microbiota dysbiosis in PD patients16. These observations are corroborated by genetic association studies, where mutations in the HSD3B7 gene, which catalyzes the second step of the classical BA synthesis pathway, were linked to PD17,18. Interestingly, BAs have shown neuroprotective properties in both animal models and clinical studies of PD19–21. In this context, cholecystectomy may contribute to the subsequent risk of PD mainly via changes in BA metabolism; however, the association of cholecystectomy with PD remains unknown.
In this population-based cohort study, we investigated the risk of PD among individuals with and without cholecystectomy. On the basis of previously published data, we hypothesized that cholecystectomy might be associated with a higher incidence of PD.
Results
Baseline characteristics
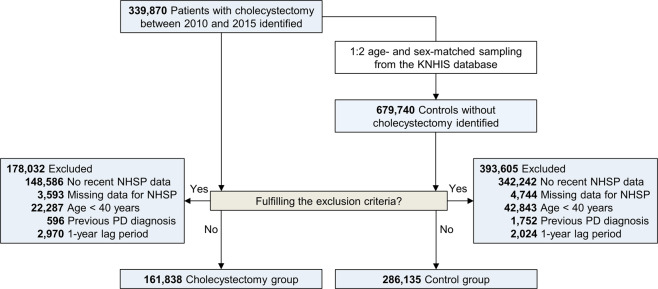
We extracted data on 339,870 patients who underwent cholecystectomy and 679,740 age- and sex-matched control subjects from the Korean National Health Insurance Service (KNHIS) database. After exclusion, a total of 161,838 patients and 286,135 control subjects were included in this study (Fig. 1). Table 1 shows the baseline characteristics of the study population according to the presence of cholecystectomy. The cholecystectomy group had a higher mean body mass index (BMI) and a higher prevalence of diabetes mellitus than the control group (absolute standardized mean difference [ASMD] > 0.1). Subgroups of either sex showed grossly similar baseline characteristics to those of the total cohort of participants (Table 1).
Fig. 1. Flow diagram of subject selection.
Abbreviations: KNHIS Korean National Health Insurance Service, NHSP National Health Screening Program, PD Parkinson’s disease.
Table 1.
Baseline characteristics of participants.
| Total | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Cholecystectomy (n = 161,838) | Non-cholecystectomy (n = 286,135) | ASMD | Cholecystectomy (n = 83,474) | Non-cholecystectomy (n = 147,020) | ASMD | Cholecystectomy (n = 78,364) | Non-cholecystectomy (n = 139,115) | ASMD |
| Age, years | 58.3 (11.2) | 58.7 (11.1) | 0.044 | 58.6 (11.1) | 59.2 (11.0) | 0.046 | 57.83 (11.2) | 58.3 (11.2) | 0.043 |
| Male sex | 83,474 (52%) | 147,020 (51%) | 0.004 | – | – | – | – | – | – |
| Smoking | 0.029 | 0.022 | 0.070 | ||||||
| Never smoker | 100,998 (62%) | 182,567 (64%) | 27,046 (32%) | 49,180 (33%) | 73,952 (94%) | 133,387 (96%) | |||
| Ex-smoker | 30,085 (19%) | 51,426 (18%) | 28,728 (34%) | 49,622 (34%) | 1,357 (2%) | 1,804 (1%) | |||
| Current smoker | 30,755 (19%) | 52,142 (18%) | 27,700 (33%) | 48,218 (33%) | 3,055 (4%) | 3,924 (3%) | |||
| Alcohola | 0.049 | 0.080 | 0.020 | ||||||
| Nondrinker | 102,732 (63%) | 175,047 (61%) | 37,443 (45%) | 60,107 (41%) | 65,289 (83%) | 114,940 (83%) | |||
| Moderate drinker | 48,583 (30%) | 92,335 (32%) | 36,092 (43%) | 69,118 (47%) | 12,491 (16%) | 23,217 (17%) | |||
| Heavy drinker | 10,523 (7%) | 18,753 (7%) | 9,939 (12%) | 17,795 (12%) | 584 (1%) | 958 (1%) | |||
| Regular exerciseb | 32,696 (20%) | 61,569 (22%) | 0.032 | 19,089 (23%) | 36,100 (25%) | 0.040 | 13,607 (17%) | 25,469 (18%) | 0.025 |
| Low income levelc | 33,281 (21%) | 59,898 (21%) | 0.009 | 15,163 (18%) | 27,027 (18%) | 0.006 | 18,118 (23%) | 32,871 (24%) | 0.012 |
| BMI, kg/m2 | 24.6 (3.2) | 24.0 (3.1) | 0.212 | 24.7 (3.0) | 24.1 (2.9) | 0.200 | 24.6 (3.5) | 23.8 (3.2) | 0.226 |
| Fasting blood glucose, mg/dl | 103.8 (27.2) | 101.7 (25.2) | 0.081 | 106.5 (29.2) | 104.5 (27.5) | 0.072 | 100.9 (24.7) | 98.7 (22.2) | 0.092 |
| Serum total cholesterol, mg/dl | 195.2 (38.5) | 197.1 (37.7) | 0.048 | 190.2 (37.6) | 192.9 (37.0) | 0.073 | 200.6 (38.8) | 201.4 (37.9) | 0.022 |
| History of diabetes mellitus | 30,909 (19%) | 42,200 (15%) | 0.116 | 18,574 (22%) | 25,782 (18%) | 0.118 | 12,335 (16%) | 16,418 (12%) | 0.115 |
| History of hypertension | 70,453 (44%) | 113,530 (40%) | 0.078 | 38,812 (47%) | 63,018 (43%) | 0.073 | 31,641 (40%) | 50,512 (36%) | 0.084 |
Data are n (%) and the mean (standard deviation).
aDaily amount of alcohol consumption was categorized as follows: heavy drinker, ≥30 g/day; moderate drinker, 1–30 g/day; and nondrinker.
bRegular exercise indicates ≥20 min of vigorous-intensity physical activity ≥3 days a week or ≥30 min of moderate-intensity physical activity ≥5 days a week.
cLower income level was defined at the lowest 20%.
Abbreviations: ASMD absolute standardized mean difference, BMI body mass index.
Impact of cholecystectomy on PD risk
We identified 1404 cases of incident PD during 1,631,265 person-years of follow-up (Table 2). The PD incidence rate was 0.93 per 1000 person-years among the cholecystectomy group and 0.82 per 1000 person-years in the control group. When the data were divided by sex, men had a higher incidence rate of PD than women. Furthermore, the difference in PD incidence according to cholecystectomy appeared to be greater in men (1.09 per 1000 person-years among the cholecystectomy group vs. 0.90 per 1000 person-years among the control group) than in women (0.77 per 1000 person-years among the cholecystectomy group vs. 0.74 per 1000 person-years among the control group) (Table 2).
Table 2.
IRs, HRs, and associated 95% CIs for Parkinson’s disease.
| Cholecystectomy | Total no. | Event | Person-years | IRa | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1b | Model 2c | Model 3d | P | ||||||
| Total | |||||||||
| Yes | 161,838 | 544 | 584,566 | 0.93 | 1.13 (1.02–1.26) | 1.17 (1.05–1.31) | 1.14 (1.02–1.27) | 0.019 | |
| No | 286,135 | 860 | 1,046,699 | 0.82 | 1 | 1 | 1 | ||
| Sex group | |||||||||
| Men | Yes | 83,474 | 323 | 297,274 | 1.09 | 1.21 (1.05–1.39) | 1.26 (1.09–1.45) | 1.22 (1.06–1.41) | 0.006 |
| No | 147,020 | 479 | 532,886 | 0.90 | 1 | 1 | 1 | ||
| Women | Yes | 78,364 | 221 | 287,292 | 0.77 | 1.04 (0.88–1.22) | 1.07 (0.91–1.26) | 1.03 (0.88–1.22) | 0.693 |
| No | 139,115 | 381 | 513,813 | 0.74 | 1 | 1 | 1 | ||
| Age group | |||||||||
| 40–49 | Yes | 42,148 | 16 | 155,297 | 0.10 | 1.37 (0.70–2.66) | 1.37 (0.70–2.66) | 1.39 (0.71–2.73) | 0.331 |
| No | 68,302 | 19 | 253,016 | 0.08 | 1 | 1 | 1 | ||
| 50–59 | Yes | 49,653 | 69 | 182,645 | 0.38 | 1.47 (1.07–2.02) | 1.47 (1.07–2.02) | 1.44 (1.04–1.98) | 0.027 |
| No | 86,460 | 83 | 322,469 | 0.26 | 1 | 1 | 1 | ||
| 60–69 | Yes | 40,497 | 193 | 147,809 | 1.31 | 1.29 (1.07–1.54) | 1.29 (1.07–1.55) | 1.25 (1.04–1.50) | 0.017 |
| No | 75,381 | 284 | 279,638 | 1.02 | 1 | 1 | 1 | ||
| ≥70 | Yes | 29,540 | 266 | 98,815 | 2.68 | 1.07 (0.93–1.24) | 1.07 (0.93–1.24) | 1.04 (0.90–1.20) | 0.618 |
| No | 55,992 | 474 | 191,576 | 2.48 | 1 | 1 | 1 | ||
aIRs were expressed as per 1000 person-years.
bModel 1 was not adjusted (crude).
cModel 2 was adjusted for age and sex.
dModel 3 was adjusted for age, sex, smoking status, alcohol consumption, regular exercise, income level, body mass index, total serum cholesterol, fasting blood glucose, and the presence of hypertension and diabetes mellitus.
Abbreviations: CI confidence interval, HR hazard ratio, IR incidence rate.
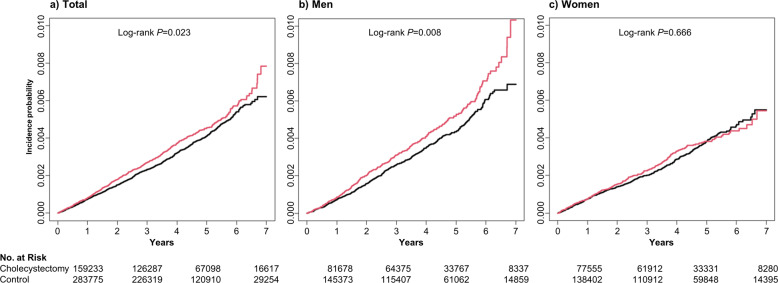
Figure 2 presents the Kaplan–Meier survival curves and log-rank tests for developing PD. In the total population, the cumulative incidence of PD in the cholecystectomy group was significantly higher than that in the control group (P = 0.023) (Fig. 2a). This significant difference was prominent in men (P = 0.008) (Fig. 2b), but not in women (P = 0.666) (Fig. 2c). As shown in Table 2, the crude HR of PD by cholecystectomy in the total population was 1.13 (95% [confidence interval [CI] 1.02–1.26) (Model 1). After adjusting for age, sex, and other covariates (Model 3), the hazard ratio (HR) was 1.14 (95% CI 1.02–1.27; P = 0.019) in the cholecystectomy group. Among men, this HR was 1.22 (95% CI 1.06–1.41; P = 0.006) and for women it was 1.03 (95% CI 0.88–1.22; P = 0.693).
Fig. 2. Kaplan–Meier estimates for the probability of incident Parkinson’s disease up to 7 years according to cholecystectomy.
a Total population; b Male population; c Female population.
When we divided the participants into four age groups (40–49 years, 50–59 years, 60–69 years, and ≥70 years), a significantly increased risk was observed in the 50–59 years (HR 1.44, 95% CI 1.04–1.98; P = 0.027) and 60–69 years group (HR 1.25, 95% CI 1.04–1.50; P = 0.017) (Table 2). However, we found no significant risk differences according to cholecystectomy in the 40–49 years and ≥70 years groups.
These results were consistent with those from additional matching (1:1 ratio) data analyses (Supplementary Table 1), as well as with those from analyses with a 2-year lag time (Supplementary Table 2).
Subgroup analysis
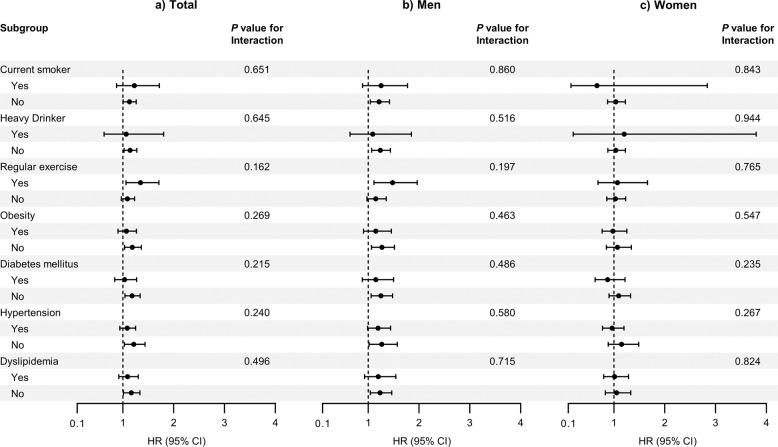
To assess the effect of modifiers on the association between cholecystectomy and PD risk, we conducted a stratified analysis using several factors including smoking status, alcohol consumption, physical activity, obesity, diabetes mellitus, hypertension, and dyslipidemia (Fig. 3). There was no significant difference in the risk of PD according to the above factors (P for interaction >0.05 for all).
Fig. 3. Subgroup analysis of Parkinson’s disease risk in patients with cholecystectomy.
a Total population; b Male population; c Female population. Abbreviations: CI confidence interval, HR hazard ratio.
Discussion
Using nationwide cohort data, we evaluated the impact of cholecystectomy on PD development. The present analyses showed that patients who underwent cholecystectomy had a significant elevation in the risk of PD development, and this tendency was prominent in men but not in women. In age-subgroup analyses, an increased risk of PD by cholecystectomy was particularly demonstrated in the 50–59 years and 60–69 years groups. These findings were consistent with the sensitivity analyses.
Previously, Marras et al.22 conducted a study in Canada to investigate the association between appendectomy and PD risk, where individuals who underwent cholecystectomy were included as a comparator group. In that study, no significant difference in PD risk was found between individuals with appendectomy and those with cholecystectomy. However, from this result, no conclusion could be drawn concerning the impact of cholecystectomy on PD development because they did not perform a direct comparison between individuals who underwent cholecystectomy and those who did not. In addition, results from observational studies on appendectomy and PD risk are inconsistent23. Nevertheless, their data showed that the incidence rate of PD was 40 events per 100,000 person-years in the cholecystectomy group, which was relatively higher than that in the appendectomy (31 events per 100,000 person-years) and control groups (21 events per 100,000 person-years).
While the underlying mechanisms by which cholecystectomy is linked to the risk of PD are not fully understood, an overview of the current knowledge regarding gallbladder and BA physiology may provide valuable insights. The gallbladder is an organ of the digestive system that primarily stores, concentrates, and releases BAs9. These actions play an important role in modulating the flow of BAs in enterohepatic circulation and regulating BA composition. A growing body of evidence has suggested a close relationship between BAs and the gut microbiota24. BAs can regulate the gut microbiota directly by exerting inhibitory effects or indirectly through the activation of intestinal barrier function, whereas some gut bacteria that produce bile salt hydrolase and 7α-dehydroxylase can modify the BA profile10.
Given that gut microbiota dysbiosis is regarded as a contributing element in the pathogenesis of PD7, one could speculate that cholecystectomy would increase PD risk by mediating postoperative changes in the intestinal microbiota. Previous reports have shown compositional alterations of gut microbiota following cholecystectomy, although there are some inconsistencies between the studies25–27. The cholecystectomy-related changes of gut microbiota shown previously include an increase in the relative abundance of Bifidobacterium and a decrease in Faecalibacterium, Roseburia, and Prevotella26,27, which are reported as a gut microbial composition associated with PD28,29. In particular, the latter species has also been linked to the progression of motor symptoms in PD29 and to isolated rapid eye movement sleep behavior disorder, which is the most potent prodromal marker of PD30.
Alternatively, evidence has shown that cholecystectomy leads to an increased proportion of secondary BAs31,32. The increase in production of secondary BAs can contribute to impaired metabolic functions mediated by systemic glucose and lipid homeostatic pathways33. Supportive studies have revealed a link between cholecystectomy and metabolic syndrome, independent of cholelithiasis8,11. Furthermore, patients with Alzheimer’s disease were shown to have significantly lower serum concentrations of a primary BA (cholic acid) and higher concentrations of the bacterially produced, secondary BA (deoxycholic acid), and an increased ratio of deoxycholic/cholic acid was shown to be associated with cognitive decline in these patients13. Considering that such metabolic changes could also contribute to the pathomechanisms of PD34,35, postoperative changes in secondary BAs may be at least partially responsible for PD development.
One might think that the risk factors for the possible conditions requiring cholecystectomy have altered the true association with the risk of PD. For example, gallstone disease is one of the most common biliary tract diseases, and cholecystectomy is regarded as the primary procedure for the treatment of symptomatic gallstones36. Gallstone disease and PD share some important risk factors including age, physical inactivity, obesity, diabetes mellitus, and rapid weight loss36,37. To minimize the impact of these confounding factors on our results, we performed further analyses by adding weight change as a covariate in individuals with additional health checkup data within 1 year after the index date. The analyses produced consistent results (Supplementary Table 3), confirming that our observations were not significantly affected by weight change as well as the other factors that were already controlled in the adjusted models. However, we cannot exclude the possibility that unmeasured or unknown confounding factors could have accounted for the relationships observed in this study.
An interesting finding of the present study is that the effect of cholecystectomy on PD risk was significant only in men. Although it could not be completely excluded that the higher incidence of PD in patients with cholecystectomy might be related to an increased contact with the healthcare system around the time of the surgery, this possibility cannot explain such sex-based differences. There are well-known sex differences in PD38. Epidemiological data have shown that men are at greater risk of PD than women39. The presentation and progression of PD can differ by sex38. Similarly, distinct profiles of gut microbiota and BAs have been reported in men and women, and estrogen has been consistently thought to be a biological factor explaining these disparities between the sexes40,41. Of note, it has been shown that estrogens directly impact the gut–brain axis42. Previous studies on PD have suggested a potential neuroprotective effect of estrogen against nigrostriatal dopaminergic damage via antioxidant, anti‐inflammatory, and anti‐apoptotic pathways38. In this framework, estrogen may attenuate the impact of cholecystectomy on PD risk, particularly in premenopausal women. However, this hypothesis is less likely to be significant, considering that the age of the female participants at baseline is mostly beyond the perimenopausal period. There has been no convincing association between other reproductive factors and PD development37, and therefore, whether these factors affect our results remains inconclusive. Further studies are necessary to reveal the exact mechanism of sex differences in the impact of cholecystectomy on PD risk.
The current data showed that a significantly elevated risk of PD by cholecystectomy was observed in individuals aged 50–69 years, but not in those aged ≥70 years. However, this observation may not be surprising because aging is the strongest risk factor for developing PD43. A nationwide epidemiological study in Korea showed that the incidence of PD increased steeply from age 60–69 years to 70–79 years, and peaked at age 80–89 years44. Accordingly, we assume that aging and possibly other environmental exposures for which information was not available in this dataset may contribute to the development of PD more than cholecystectomy in people aged ≥70 years. Our results also showed that there was no significant association between cholecystectomy and PD in individuals aged 40–49 years, which may be explained by the fact that PD rarely occurs before the age of 50 years44. In addition, genetic factors are more closely linked to young-onset PD45. Further research with a longer follow-up could help to clarify the impact of cholecystectomy on PD risk in this age group.
The major strengths of this study include a large sample size from a population-based cohort, extensive adjustment for potential confounders, and the presence of a matched comparison group. However, there are some limitations in this study. First, the KNHIS database depends on the clinician’s assignment of a diagnostic code for PD. PD diagnosis in clinical practice is not generally confirmed by pathologic confirmation, and thus, there is a possibility of misdiagnosis of PD. For example, atypical parkinsonian disorders such as the parkinsonian subtype of multiple system atrophy or progressive supranuclear palsy with predominant parkinsonism, might be included in a diagnostic code for PD. However, given that these disorders are relatively rare compared to PD, such bias is less likely to be significant in our results. Second, we cannot exclude the possibility that individuals with undiagnosed PD were included at baseline, which might bias the temporal association between cholecystectomy and PD risk. However, we used two different lag periods to reduce this potential bias, and the analyses revealed consistent results. Finally, the reasons for performing cholecystectomy were not available in this dataset, which limits the impact of underlying gallbladder disease on the development of PD.
In conclusion, the current nationwide cohort study revealed that cholecystectomy was associated with an increased risk of PD. This association differed between men and women, suggesting a potential sex difference in the impact of cholecystectomy on PD development. Further research should be performed to validate our findings in other datasets and to investigate the potential role of BAs and their interactions with the gut microbiota in the pathogenesis of PD.
Methods
Study design and participants
The KNHIS has launched a compulsory insurance program since 1997 that covers most of the healthcare services implemented in Korea. The KNHIS database stores information on all medical claims, including sociodemographic factors, healthcare utilization, diagnoses, and prescription drugs. Moreover, the KNHIS has been providing a National Health Screening Program (NHSP) at least once every 2 years for the entire population of Korean adults aged 40 years and older. The NHSP includes a self-reported questionnaire on health behaviors, measurements of blood pressure, height, and weight, and blood and urine tests. These data are also stored in the KNHIS database. Details of the standardized method for data collection by the KNHIS have been described elsewhere46.
Using the KNHIS database, we created a cohort of patients who underwent cholecystectomy between January 1, 2010 and December 31, 2015. The procedure code for cholecystectomy was gallbladder removal surgery (Q7380). We excluded the following patients: (1) those who had no NHSP data within 2 years before enrollment; (2) those who had missing data for at least one variable of NHSP; (3) those aged under 40 years; (4) those diagnosed with PD before enrollment; and (5) those who developed PD or died during the 1-year lag period. For the control population, age- and sex-matched individuals without cholecystectomy were selected randomly at a 1:2 ratio (two controls per one case) from the KNHIS database. Patients with cholecystectomy and their matched controls were assigned an index date identical to the date of cholecystectomy. The exclusion criteria applied to the control group were the same as those in the cholecystectomy group. All enrolled participants were followed until the date of death or until December 31, 2016.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB number: X-2003/601-904). The requirement for informed consent was waived because the study was based on routinely collected medical claims data.
Study endpoint
We identified incident PD using the ICD-10 code for PD (G20) in both outpatient visits and hospitalizations. To ensure the validity of PD diagnosis, we confirmed the diagnosis by matching it in the registry system for rare intractable diseases (RIDs) in the KNHIS. The Korean government has implemented a registration program for copayment reduction of up to 90% for this intractable disease, PD. Patients with newly diagnosed PD in Korea are registered to this system, and only neurologists or neurosurgeons can issue the required certification for PD diagnosis. Accordingly, we identified the development of PD using claims data with ICD-10 code G20, confirmed by the RID registration code V124. This method has also been used in many epidemiologic studies of PD in the Korean population47–49.
Covariates
Data regarding age, sex, smoking status, alcohol consumption, physical activity, income level, BMI, fasting blood glucose, total serum cholesterol, and medical history of diabetes mellitus and hypertension were obtained for all participants. All blood tests were performed in a fasting state. Regular exercise was defined as vigorous-intensity physical activity for ≥20 min on ≥3 days per week or moderate-intensity physical activity for ≥30 min on ≥5 days per week. Information on smoking status and alcohol consumption was obtained using a standardized self-administered questionnaire. Participants were classified by smoking status, as nonsmokers, ex-smokers, or current smokers, and by alcohol consumption as nondrinkers, moderate drinkers (less than 30 grams per day), or heavy drinkers (30 or more grams per day)50. Low-income level was defined as the lowest 20%.
Statistical analysis
All statistical analyses were performed using SAS 9.3 (Cary, NC). Baseline characteristics were compared using the ASMD, and a value more than 0.1 indicated a meaningful difference between the study groups51. Survival curves were constructed with Kaplan–Meier estimates and compared using the log-rank test. Cox proportional-hazards regression models were used to estimate HRs with 95% CIs for the risk of PD. Model 1 was an unadjusted model (crude); Model 2 was adjusted for age and sex; and Model 3 was further adjusted for smoking status, alcohol consumption, regular exercise, income level, BMI, total serum cholesterol, fasting blood glucose, and the presence of hypertension and diabetes mellitus. To enhance the reliability of the main results, we added two different sensitivity analyses to this study. First, because some participants were unmatched after applying the inclusion/exclusion criteria (Fig. 1), we performed a 1:1 propensity score matching according to age and sex in our dataset. For these matched data, we used stratified Cox proportional-hazards regression models. Second, we performed the same analytic procedure by setting a lag time of 2 years to account for reverse causation. All tests were two-sided and P values less than 0.05 were regarded as significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C1140). We thank the KNHIS for providing access to their data.
Author contributions
R.K., J-.Y.L., and C.M.S. contributed the conception and design of the study; S.P. and K.H. contributed to the acquisition and analysis of data; R.K., J-.Y.L., and C.M.S. contributed to the interpretation of results; R.K. drafted the manuscript; J-.Y.L., S.P., K.H., and C.M.S revised the manuscript. All authors read and approved the final version for publication.
Data availability
The datasets for this study are owned by the KNHIS. There are no current sharing agreements, and data are held under a data use contract with the KNHIS.
Code availability
No previously unreported custom computer code or algorithm was used to generate results in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jee-Young Lee, Email: wieber04@snu.ac.kr.
Cheol Min Shin, Email: scm6md@gmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-021-00245-z.
References
- 1.Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease—the gut–brain axis and environmental factors. Nat. Rev. Neurol. 2015;11:625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D. Advances in markers of prodromal Parkinson disease. Nat. Rev. Neurol. 2016;12:622–634. doi: 10.1038/nrneurol.2016.152. [DOI] [PubMed] [Google Scholar]
- 3.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 2016;79:940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 4.Svensson E, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 5.Liu B, et al. Vagotomy and Parkinson disease. A Swedish register–based matched-cohort study. Neurology. 2017;88:1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang HL, Lin CH. Altered gut microbiome and intestinal pathology in Parkinson’s disease. J. Mov. Disord. 2019;12:67–83. doi: 10.14802/jmd.18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Wu S, Tian Y. Cholecystectomy as a risk factor of metabolic syndrome: from epidemiologic clues to biochemical mechanisms. Lab. Investig. 2018;98:7–14. doi: 10.1038/labinvest.2017.95. [DOI] [PubMed] [Google Scholar]
- 9.Housset C, Chrétien Y, Debray D, Chignard N. Functions of the Gallbladder. Compr. Physiol. 2016;6:1549–1577. doi: 10.1002/cphy.c150050. [DOI] [PubMed] [Google Scholar]
- 10.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Ciaula A, Garruti G, Wang DQH, Portincasa P. Cholecystectomy and risk of metabolic syndrome. Eur. J. Intern. Med. 2018;53:3–11. doi: 10.1016/j.ejim.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant SM, DeMorrow S. Bile acid signaling in neurodegenerative and neurological disorders. Int. J. Mol. Sci. 2020;21:5982. doi: 10.3390/ijms21175982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MahmoudianDehkordi S, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimers Dement. 2019;15:76–92. doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham SF, et al. Biochemical profiling of the brain and blood metabolome in a mouse model of prodromal Parkinson’s disease reveals distinct metabolic profiles. J. Proteome Res. 2018;17:2460–2469. doi: 10.1021/acs.jproteome.8b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donovan SM, et al. Nigral overexpression of α-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol. Motil. 2020;32:e13726. doi: 10.1111/nmo.13726. [DOI] [PubMed] [Google Scholar]
- 16.Li P, et al. Gut microbiota dysbiosis is associated with elevated bile acids in Parkinson’s disease. Metabolites. 2021;11:29. doi: 10.3390/metabo11010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song GG, Lee YH. Pathway analysis of genome-wide association studies for Parkinson’s disease. Mol. Biol. Rep. 2013;40:2599–2607. doi: 10.1007/s11033-012-2346-9. [DOI] [PubMed] [Google Scholar]
- 18.Ghanbari M, et al. Genetic variants in microRNAs and their binding sites are associated with the risk of Parkinson disease. Hum. Mutat. 2016;37:292–300. doi: 10.1002/humu.22943. [DOI] [PubMed] [Google Scholar]
- 19.Rosa AI, et al. Tauroursodeoxycholic acid improves motor symptoms in a mouse model of Parkinson’s disease. Mol. Neurobiol. 2018;55:9139–9155. doi: 10.1007/s12035-018-1062-4. [DOI] [PubMed] [Google Scholar]
- 20.Mortiboys H, Aasly J, Bandmann O. Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain. 2013;136:3038–3050. doi: 10.1093/brain/awt224. [DOI] [PubMed] [Google Scholar]
- 21.Mortiboys H, et al. UDCA exerts beneficial effect on mitochondrial dysfunction in LRRK2(G2019S) carriers and in vivo. Neurology. 2015;85:846–852. doi: 10.1212/WNL.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marras C, Lang AE, Austin PC, Lau C, Urbach DR. Appendectomy in mid and later life and risk of Parkinson’s disease: a population-based study. Mov. Disord. 2016;31:1243–1247. doi: 10.1002/mds.26670. [DOI] [PubMed] [Google Scholar]
- 23.Lu HT, Shen QY, Xie D, Zhao QZ, Xu YM. Lack of association between appendectomy and Parkinson’s disease: a systematic review and meta-analysis. Aging Clin. Exp. Res. 2019;32:2201–2209. doi: 10.1007/s40520-019-01354-9. [DOI] [PubMed] [Google Scholar]
- 24.Wei B, et al. Alterations of gut microbiome in patients with type 2 diabetes mellitus who had undergone cholecystectomy. Am. J. Physiol. Endocrinol. Metab. 2021;320:E113–E121. doi: 10.1152/ajpendo.00471.2020. [DOI] [PubMed] [Google Scholar]
- 25.Yoon WJ, et al. The impact of cholecystectomy on the gut microbiota: a case-control study. J. Clin. Med. 2019;8:79. doi: 10.3390/jcm8010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, et al. Cholecystectomy damages aging-associated intestinal microbiota construction. Front. Microbiol. 2018;9:1402. doi: 10.3389/fmicb.2018.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren X, et al. Bacterial alterations in post-cholecystectomy patients are associated with colorectal cancer. Front. Oncol. 2020;10:1418. doi: 10.3389/fonc.2020.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boertien JM, Pereira PAB, Aho VTE, Scheperjans F. Increasing comparability and utility of gut microbiome studies in Parkinson’s disease: a systematic review. J. Parkinsons Dis. 2019;9:S297–S312. doi: 10.3233/JPD-191711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aho VTE, et al. Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707. doi: 10.1016/j.ebiom.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heintz-Buschart A, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malagelada JR, Go VLW, Summerskill WHJ, Gamble WS. Bile acid secretion and biliary bile acid composition altered by cholecystectomy. Am. J. Dig. Dis. 1973;18:455–459. doi: 10.1007/BF01076595. [DOI] [PubMed] [Google Scholar]
- 32.Roda E, et al. Enterohepatic circulation of bile acids after cholecystectomy. Gut. 1978;19:640–649. doi: 10.1136/gut.19.7.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang JYL. Bile acid metabolism and signaling. Compr. Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol. Med. 2013;19:176–186. doi: 10.1016/j.molmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Fanning S, Selkoe D, Dettmer U. Parkinson’s disease: proteinopathy or lipidopathy? NPJ Parkinsons Dis. 2020;6:3. doi: 10.1038/s41531-019-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammert F, et al. Gallstones. Nat. Rev. Dis. Prim. 2016;2:16024. doi: 10.1038/nrdp.2016.24. [DOI] [PubMed] [Google Scholar]
- 37.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 38.Meoni S, Macerollo A, Moro E. Sex differences in movement disorders. Nat. Rev. Neurol. 2020;16:84–96. doi: 10.1038/s41582-019-0294-x. [DOI] [PubMed] [Google Scholar]
- 39.Feigin VL, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jašarević E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across the lifespan. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016;371:20150122. doi: 10.1098/rstb.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phelps T, Snyder E, Rodriguez E, Child H, Harvey P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol. Sex. Differ. 2019;10:52. doi: 10.1186/s13293-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat. Rev. Neurosci. 2011;12:359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JH, et al. Trends in the incidence and prevalence of Parkinson’s disease in Korea: a nationwide, population-based study. BMC Geriatrics. 2019;19:320. doi: 10.1186/s12877-019-1332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Post B, et al. Young onset Parkinson’s disease: a modern and tailored approach. J. Parkinsons Dis. 2020;10:S29–S36. doi: 10.3233/JPD-202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seong SC, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int. J. Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong SM, et al. Body mass index, diabetes, and the risk of Parkinson’s disease. Mov. Disord. 2020;35:236–244. doi: 10.1002/mds.27922. [DOI] [PubMed] [Google Scholar]
- 48.Park JH, et al. Association of high-density lipoprotein cholesterol variability and the risk of developing Parkinson disease. Neurology. 2021;96:e1391–e1401. doi: 10.1212/WNL.0000000000011553. [DOI] [PubMed] [Google Scholar]
- 49.Kim R, Yoo D, Jung YJ, Han K, Lee JY. Sex differences in smoking, alcohol consumption, and risk of Parkinson’s disease: a nationwide cohort study. Park. Relat. Disord. 2020;71:60–65. doi: 10.1016/j.parkreldis.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Kim R, Yoo D, Jung YJ, Han K, Lee JY. Smoking cessation, weight change, and risk of Parkinson’s disease: analysis of national cohort data. J. Clin. Neurol. 2020;16:455–460. doi: 10.3988/jcn.2020.16.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this study are owned by the KNHIS. There are no current sharing agreements, and data are held under a data use contract with the KNHIS.
No previously unreported custom computer code or algorithm was used to generate results in this study.