Abstract
In the present work, Orange CD was chosen as an intriguing modifier for the electropolymerization on the surface of CPE by the CV technique. A novel, sensitive, and cost-effective poly (Orange CD) MCPE (PoOCD/MCPE) sensor was utilized for the selective detection of paracetamol (PA) in 0.2 M phosphate buffer solution (PBS) of pH 7.4. The oxidation peak current of PA was vastly enhanced at the sensor. The scan rate study is suggested that electro-oxidation of PA was adsorption-controlled. The pH study testifies the redox pathways transport with the same quantity of electrons and protons. The detection limit of PA is found to be 2.64 µM. DPV results show that substantial peak separation between PA, folic acid (FA), and dopamine (DA) could be facilitating their individual and simultaneous determination on the sensor. The decorated sensor demonstrates high sensitivity, stability, reproducibility, repeatability and has been successfully exploited for the detection of PA in a tablet with promising results.
Subject terms: Biochemistry, Chemistry, Physics
Introduction
Paracetamol (PA) is one of the most extensively used analgesics and antipyretic drugs in clinical practice1,2. It is a very effective agent recommended for mild to moderate pain alleviation such as flu-induced fever, migraine, arthritis, and extenuates pain (headache, toothache, joint, muscular, chronic, postoperative)3,4. PA relieves pain by inhibiting prostaglandin synthesis in the central nervous system, and it also relieves fever by sedating the hypothalamus heat-regulating center5. PA is easily degraded by glucuronidation and sulfation into inactive metabolites, which are excreted in the urine, with just 5% of PA remaining unaltered6. In general, PA is known to have an excellent safety profile at approved therapeutic doses. But, its toxic metabolite accumulation in case of overdosing and chronic use lead to harmful side effects such as liver problem, kidney damages, trembling, nervousness, seizures, insomnia and nausea and even death7–10. Therefore, developing a simple, fast response, economical, sensitive, accurate, and reliable detection method for the assessment of PA is highly demanded in the medical field. There are lots of methods like capillary electrophoresis11, titrimetry12, SEC, LC–MS, HPLC13–15, chemiluminescence16, spectrofluorimetric17, and spectrophotometry18–20 which have been availed for the assessment of PA. Among all these methods, the electrochemical method stands out with its simplicity, sensitivity, selectivity, modest and fast response.
Folic acid (FA) is a water-soluble vitamin B9 and also known as folacin that helps the growth of healthy new cells especially during pregnancy and controls the generation of ferrohaeme. FA is involved in a variety of biological tasks related to cell metabolism, including DNA replication, repair, and methylation, as well as the production of nucleotides, vitamins, and amino acids. Deficiency of FA causes anemia, leucopoenia, devolution of mentality, neurosis and also increases the chances of heart attack and stroke21–24. Dopamine (DA) is the neurotransmitter involved in the functioning of the central nervous system. DA is also utilized as an injectable medicine that stimulates the sympathetic nervous system, causing effects such as increased blood pressure and heart rate. Deficiency of DA may cause disorders like Parkinson’s disease, Schizophrenia, Alzheimer’s disease, and HIV infection25–28. When used for a long time, nonsteroidal anti-inflammatory agent like PA can prevent FA from being absorbed by the human being. The simultaneous measurement of PA and FA is particularly relevant since PA enhances the need for FA. The usage of PA protects dopaminergic neurons against oxidative stress damage produced by acute exposure to increased amounts of DA, according to in vitro studies. Furthermore, prolonged PA use in in vivo model has been shown to dramatically lower DA levels. Selective or simultaneous detection of PA, FA, and DA have been achieved by voltammetric method due to their electroactive natures29–32. In the electrochemical sensor field, electropolymerized MCPE has made a great contribution to the determination of biomolecules because of their good stability, homogeneity, strong adhesion of polymer film onto the electrode surface, more active sites, fine reproducibility, fine resolution voltammogram, low-cost, and easy preparation method33–36. As redox dyes are artificial electron donatives, they are effective to undergo electropolymerization and produce stable redox-active films37,38.
Present work explores, less studied Orange CD (Scheme 1) dye39 as a modifier for the electropolymerization on the CPE surface by CV technique. The performance of PoOCD/MCPE was assessed for the sensitive, selective determination of PA and simultaneous determination of PA, FA, and DA in biological pH 7.4. The sensor displayed higher electrocatalytic activity, as well as a low detection limit and large linear ranges for PA resolution. The practical applicability of the sensor has been tested by determining PA in tablets successfully. This work is intended to pave the way for the development of more efficient, dependable, and generally affordable sensors.
Scheme 1.

Structure of Orange CD.
Experimental
Materials and instrumentation
All analytical grade chemicals such as PA (Mwt = 151.16 gmol−1, purity 99%), FA (Mwt = 441.40 gmol−1, purity 99.5%), DA (Mwt = 189.64 gmol−1, purity 99%), graphite powder, Na2HPO4, and NaH2PO4·H2O was procured from Merck Chemicals (Mumbai, India) and Orange CD dye from Astik Dyestuff Pvt. Ltd (Gujarat, India). Stock solutions of orange CD, PA, FA, and DA with a concentration of 25 × 10–4 M were prepared in double-distilled water (DDW). The 0.2 M PBS was prepared by Na2HPO4 and NaH2PO4·H2O.
Voltammetric measurements were conducted in CHI-660c model (CH Instrument-660 electrochemical workstation, USA) analytical system. An electrochemical cell (25 ml) consisting of saturated Calomel electrode (Equip-tronics, Mumbai), platinum wire (Equip-tronics, Mumbai)) and bare CPE or PoOCD/MCPE, were acted as a reference, counter, and working electrodes respectively at room temperature.
Preparation of paracetamol tablet sample
In a mortar, a 500 mg of Calpol pill was acquired from local drug stores (Shivamogga, India) was finely pulverized. In a 100 ml flask, an adequate amount of homogenous white powder was dissolved in water. The solution was thoroughly agitated to get the appropriate concentration before being utilized in pharmaceutical sample analysis.
Working electrode construction
The bare CPE was prepared as described in the literature40. The PoOCD/MCPE was constructed by dipping bare CPE into 1 mM aqueous Orange CD with NaOH (0.1 M) as a supporting electrolyte. The electrochemical polymerization was performed at the potential between − 0.6 and 1.6 V with a scan rate (SR) of 100 mVs−1 using ten cycles. Then obtained electropolymerized electrode was rinsed in the DDW to eliminate unreacted molecules.
Results and discussion
Electrochemical polymerization of Orange CD on bare CPE
Figure 1 shows the CVs of electrochemical polymerization of 1 mM aqueous orange CD with NaOH (0.1 M) on the bare CPE surface in the potential cycling between − 0.6 and 1.6 V with SR of 100 mVs−1 using ten cycles. The examination of voltammograms by gradually increasing the progressing electropolymerization procedure reveals accumulation and growth of orange CD film on the surface of bare CPE41. The polymer film thickness affects the electrochemical response of the modified electrode. The film thickness was easily managed by regulating the number of voltammetric scans from 5 to 25 during electropolymerization. The experimental results analogous to it were obtained for the PA as shown in Fig. 2. As the current response achieves a maximum at ten multiple cycles, the optimum cycle number of ten was selected for the construction of PoOCD/MCPE and further voltammetric measurements.
Figure 1.

CVs of construction of PoOCD/MCPE with 0.1 M NaOH for ten cycles at SR of 100 mVs−1.
Figure 2.
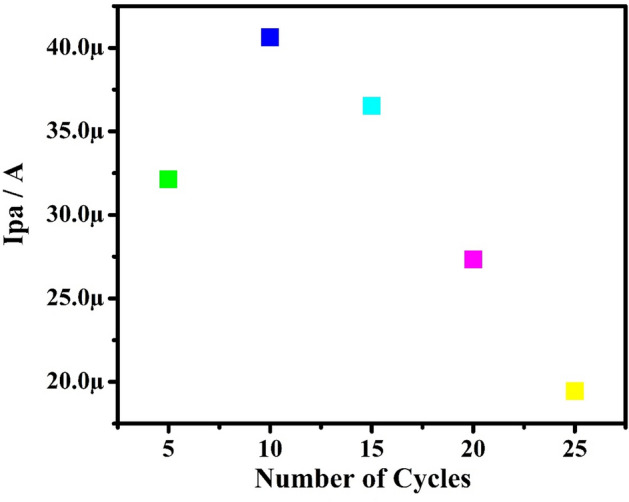
Graph of Ipa vs number of voltammetric scans.
Characterization of PoOCD/MCPE
For investigation of electrocatalytic activity of the MCPE, a potassium ferrocyanide system was used. Figure 3 displays the electrochemical activity of K4[Fe(CN)6] (freshly prepared) at bare CPE (A) and PoOCD/MCPE (B) containing 1 M KCI as supporting electrolyte obtained at an SR of 100 mVs−1 was recorded by CV method. The small redox peak current signal corresponds to bare CPE while PoOCD/MCPE shows enhanced peak current showing the dramatic increase in the rate of electron transfer33. According to Randles–Sevick’s Eq. (1), the electrocatalytic surface area of both bare CPE and MCPE was calculated42.
| 1 |
Figure 3.

CV results of K4 [Fe (CN)6] at bare CPE (A) and PoOCD/MCPE (B) at a SR 100 mVs−1.
The area of bare CPE (0.0295 cm2) is less than PoOCD/MCPE area (0.0499 cm2) which indicates that Orange CD acts as an effective modifier contributing a large surface and promotes the electron transfer between the electrode and the solution.
The surface morphological features of bare CPE and PoOCD/MCPE were characterized by SEM. The SEM of bare CPE (Fig. 4a) appears to be a rough surface with irregularly shaped and PoOCD/MCPE (Fig. 4b) appears to be a smooth with consistent ordering of the polymer film of Orange CD on the CPE surface. The remarkable distinction in the surface structure of both electrodes confirms the remarkable modification of the CPE surface by electropolymerized Orange CD.
Figure 4.
SEM of bare CPE (a) and PoOCD/MCPE (b).
Voltammetric measurements
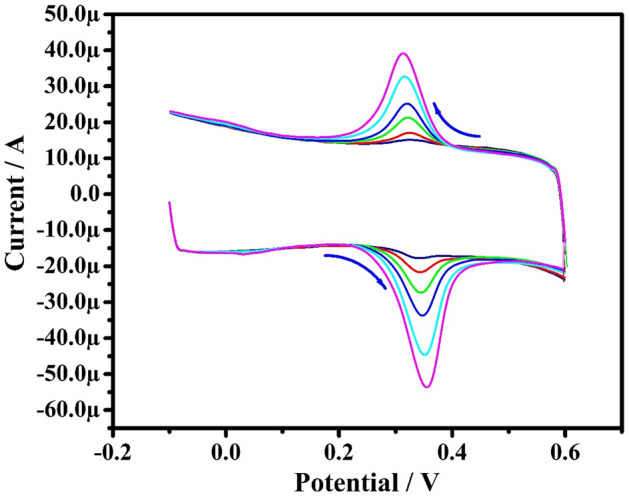
The electrochemical response of PA was studied on the bare CPE (A) and PoOCD/MCPE (B) in 0.2 M PBS (pH 7.4) at an SR 100 mVs−1 by CV method as displayed in Fig. 5. An irreversible voltammogram was obtained at bare CPE for PA with an anodic peak potential of 0.357 V indicating the poor response as well as the occurrence of only oxidation. But at the same condition, PoOCD/MCPE exhibited a significant increase in the current signals giving a sharp reversible voltammogram. The anodic and cathodic peak potential for PA were found to be 0.349 V and 0.320 V respectively reveals the occurrence of both oxidation and reduction at proposed PoOCD/MCPE.
Figure 5.
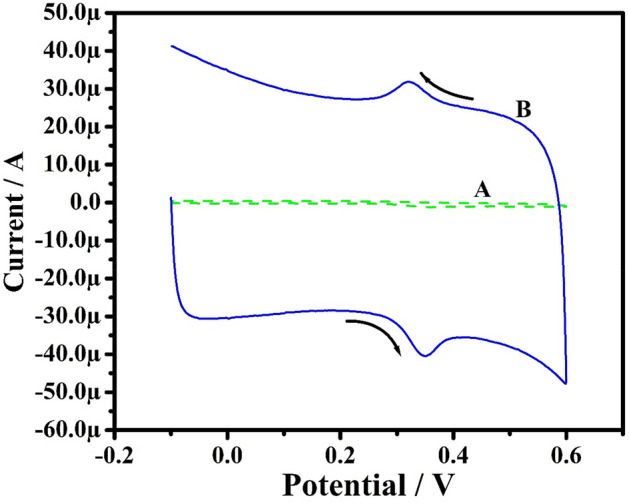
CVs for PA (0.1 mM) in 0.2 M PBS (pH 7.4) at bare CPE (A) and PoOCD/MCPE (B) at SR 100 mVs−1.
The impact of potential scan rate (SR) for the electrochemical studies of 0.1 mM PA in 0.2 M PBS (pH 7.4) from 50 to 500 mVs−1 was investigated by the CV method at PoOCD/MCPE as depicted in Fig. 6. It is found that the redox peak currents rise with rising scan rates. The electrode phenomenon is controlled by adsorption at PoOCD/MCPE for PA as deduced from the good linearity with regression equations Ipa (µA) = 0.34 ʋ (mV/s) + 5.56 (µA) (R2 = 0.9998), Ipa (µA) = 1.02 ʋ (mV/s) − 6.39 (µA) (R2 = 0.9909) and Ipa (µA) = 0.90 log ʋ (V/s) − 6.21 (µA) (R2 = 0.9998) of the Ipa vs SR (Fig. 7), Ipa vs square root of SR (Fig. 8) and log Ipa vs log SR (Fig. 9) plots respectively43,44. The heterogeneous rate constant (k0) were estimated for such voltammograms whose ∆Ep (experimental peak potential difference) values are greater than 10 mV using the Eq. (2)45 and the results were incorporated in Table 1.
| 2 |
Figure 6.
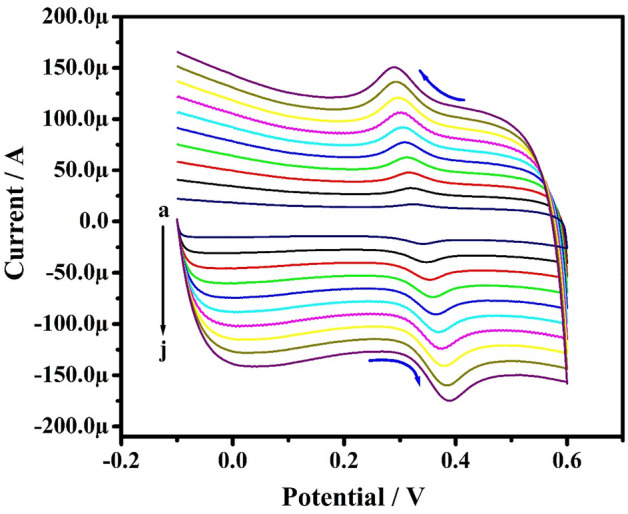
CVs obtained for PA (0.1 mM) at PoOCD/MCPE with various SR (50–500) mVs−1 in PBS (0.2 M, pH 7.4).
Figure 7.
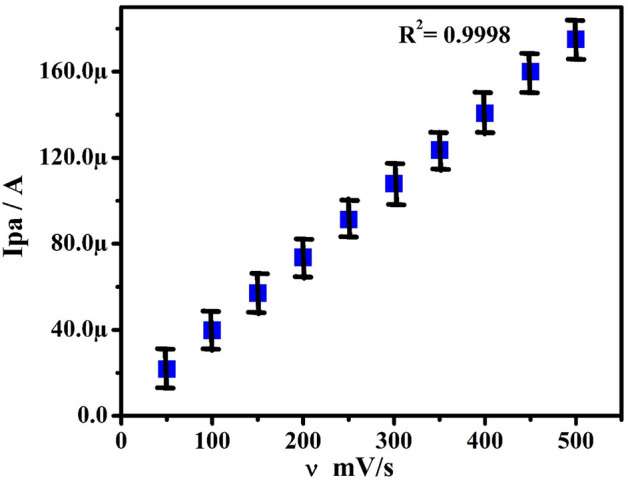
Graph of Ipa vs SR of PA (0.1 mM) in PBS (0.2 M, pH 7.4).
Figure 8.
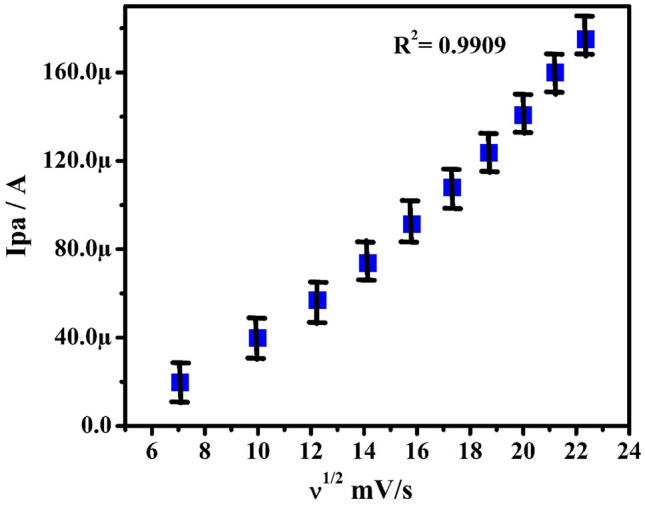
Graph of Ipa vs square root of SR of PA (0.1 mM) in PBS (0.2 M, pH 7.4).
Figure 9.

Graph of log Ipa vs log SR of PA (0.1 mM) in PBS (0.2 M, pH 7.4).
Table 1.
Variation of the voltammetric parameters for PA at different scan rates.
| Scan rate (mVs−1) | ΔEp (mV) | k0 (s−1) |
|---|---|---|
| 50 | 19 | 1.276 |
| 100 | 29 | 2.277 |
| 150 | 37 | 3.118 |
| 200 | 48 | 3.666 |
| 250 | 51 | 4.428 |
| 300 | 64 | 4.579 |
| 350 | 72 | 4.876 |
| 400 | 83 | 4.914 |
| 450 | 90 | 5.103 |
| 500 | 101 | 5.000 |
The effect of PA concentration on redox behavior was studied at PoOCD/MCPE. Figure 10 depicts the CVs of 10–60 µM PA at PoOCD/MCPE in PBS (pH 7.4) at the SR of 50 mVs−1. By increasing PA concentration, the redox peak current gradually increased. Ipa vs PA concentration (Fig. 11) plot shows good linearity with regression equation Ipa (µA) = 0.7 (µM) + 6.38 (µA) (R2 = 0.9990). LOD and LOQ were calculated according to the Eqs. (3) and (4)6,46 for PA were found to be 2.64 µM and 8.81 µM respectively. The LOD of this modified electrode for the estimation of PA in comparison to other reported electrodes is given in Table 2
| 3 |
| 4 |
where S is the standard deviation, M is the slope.
Figure 10.
CVs for PA at different concentrations (10–60 µM) in PBS (0.2 M, pH 7.4) at PoOCD/MCPE.
Figure 11.

Graph of Ipa vs PA concentration.
Table 2.
Comparisons of the LOD of PoOCD/MCPE with other modified electrode reported.
| Sl. no. | Electrode | Limit of detection (μM) | Method | References |
|---|---|---|---|---|
| 1 | Poly-NA-MCPE | 7.2 | CV | 2 |
| 2 | Diacerein/MCPE | 3.8 | DPV | 47 |
| 3 | N-DHPB-MWNT/CPE | 10.0 | DPV | 48 |
| 4 | Pd/Al | 50.0 | DPV | 49 |
| 5 | C60/GCE | 50.0 | DPV | 50 |
| 6 | Cu-poly-TTC | 5.0 | CV | 51 |
| 7 | PVA-Fe3O4/MGCE | 8.0 | DPV | 52 |
| 8 | GrRAC sensor | 8.36 | DPV | 53 |
| 9 | TiO2 nanoparticle MCPE | 5.25 | CV | 54 |
| 10 | PoOCD/MCPE | 2.64 | CV | This work |
The pH plays a remarkable role in asses the number of participating electrons and protons in the oxidation mechanisms of the PA. The increase of pH (6.2–7.8) over PA (10 µM) oxidation at PoOCD/MCPE shifts Epa towards a more negative direction as analyzed by CV are shown in Fig. 12. Figure 13 illustrates the Epa vs pH values of PA graph that are linear with a slope of 0.0601 V/pH (R2 = 0.995). This suggests that during the oxidation of the PA, the same number of protons and electrons are participated3,47 and the possible electrooxidation was shown in Scheme 2.
Figure 12.
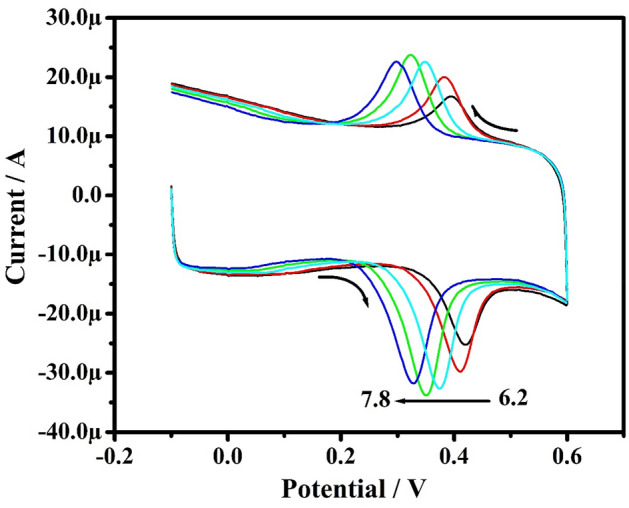
CVs for PA with varied pH at PoOCD/MCPE.
Figure 13.
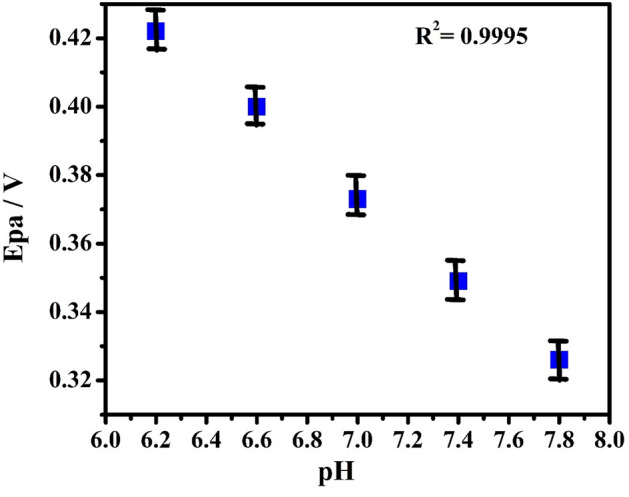
Graph of Epa vs varied pH for PA.
Scheme 2.
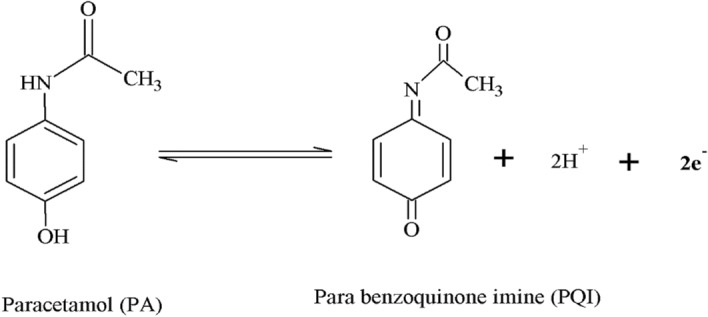
Oxidation mechanism of PA.
Simultaneous resolution of analytes PA, FA and DA
This study aimed to utilize the developed sensor for the selective and sensitive estimation of PA in the existence of FA and DA. Figure 14 illustrates the CVs recorded for the equimolar mixture (0.1 mM) of analytes PA, FA, and DA in 0.2 M PBS (pH 7.4) at SR 50 mVs−1 at bare CPE (A) and PoOCD/MCPE (B). At bare CPE, a low current signal with poor sensitivity was observed. However, in the same condition, the PoOCD/MCPE has shown a higher current signal with improved sensitivity for oxidation of DA, PA, and FA at 0.134 V, 0.408 V, and 0.695 V respectively. Hence, the developed PoOCD/MCPE serves as an excellent sensor for the PA.
Figure 14.
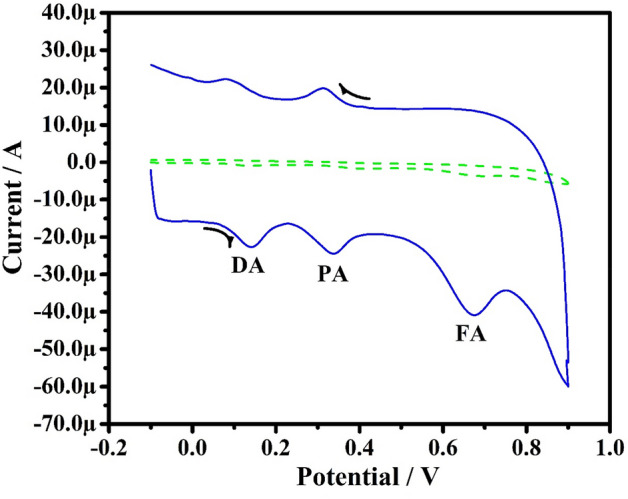
CV obtained for simultaneous studies of PA (0.1 M), FA (0.1 M) and DA (0.1 M) on bare CPE (A) and PoOCD/MCPE (B).
Interference studies
Studies were conducted by the DPV method in the solution mixture containing PA, FA, and DA at PoOCD/MCPE. The concentration of one analyte was varied, whereas the others were kept constant. Figure 15 illustrates the DPVs of PA by increasing the concentration of PA from 10 to 60 µM when holding the concentration of FA and DA constant. The oxidation peak current of PA increased linearly with increasing PA concentration from 10 to 60 µM and anodic peak current for FA and DA remaining constant. Similarly, it was also observed that the peak potentials remain unaltered with any enhancement in the peak current for the other two analytes. Figures 16 and 17 self illustrates the DVPs of FA (from 10 to 50 µM) and DA (from 10 to 60 µM) by keeping the other two analytes constant. These observations reveal that the oxidation of PA, FA, and DA has negligible influence on the variation of the other analytes. Therefore, PoOCD/MCPE showed good selectivity and sensitivity for the resolution of PA, FA, and DA.
Figure 15.
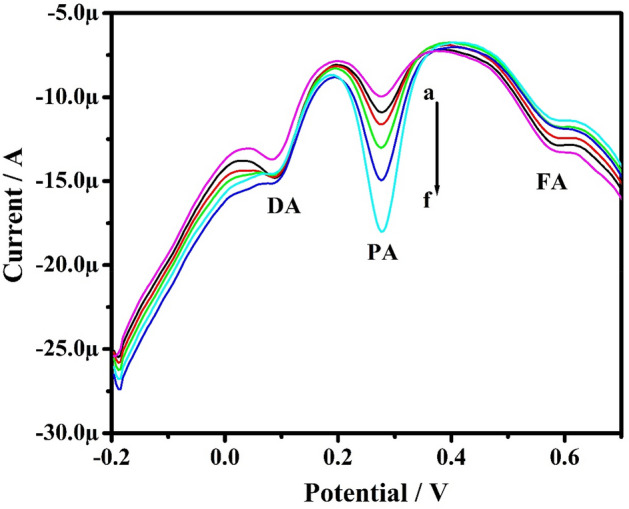
DPVs for PA at different concentrations (10–60 µM) in PBS (0.2 M, pH 7.4) at SR 50 mVs−1 at PoOCD/MCPE.
Figure 16.
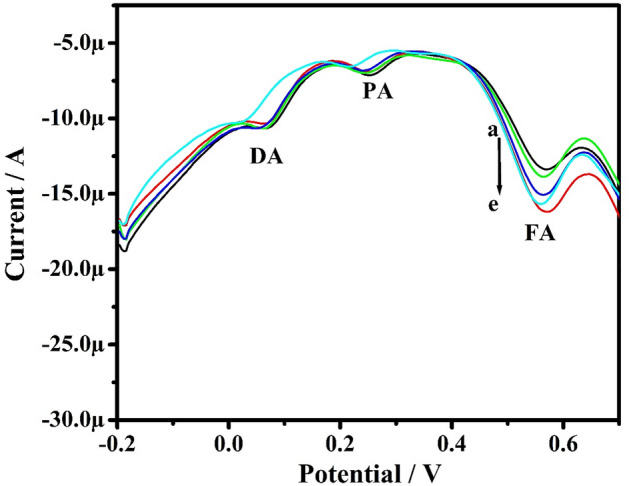
DPVs for FA at different concentrations (10–50 µM) in PBS (0.2 M, pH 7.4) at SR 50 mVs−1 at PoOCD/MCPE.
Figure 17.
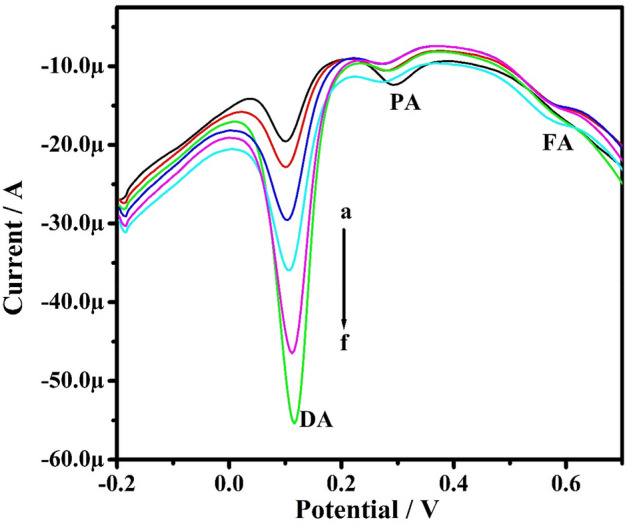
DPVs for DA at different concentrations (10–60 µM) in PBS (0.2 M, pH 7.4) at SR 50 mVs−1 at PoOCD/MCPE.
Repeatability, reproducibility and stability study
The repeatability of the PoOCD/MCPE for 0.1 mM PA in 0.2 M PBS (pH 7.4) was examined through five successive measurements and the RSD value of 2.3% demonstrates the superior repeatability of the MCPE. The reproducibility of the MCPE was investigated by fabricating five different MCPE under the same conditions. The RSD value obtained to be 4.8% confirms the good reproducibility. The stability of the MCPE was studied by 15 successive cycles (data not shown) remained 98% of its original current response for PA even after 15 cycles shows the good stability of the PoOCD/MCPE.
Determination of PA in tablet sample
To evaluate the efficacy of PoOCD/MCPE in practical analysis, PA was successfully determined in tablet (Calpol 500 mg) by the CV method. The recovery test was done using the standard addition technique and the obtained results for four consecutive PA concentrations in the range from 10 to 40 μM were tabulated in Table 3. The acceptable percentage recoveries in the range of 98.28 ± 0.985 to 99.81 ± 0.545 obtained specify that the proposed sensor might be enough for practical application and can be employed for the determination of PA in pharmaceutical formulations.
Table 3.
Evaluation of PA in tablet using PoOCD/MCPE.
| Content | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| 500 mg paracetamol tablet | 10 | 9.9394 | 99.39 ± 0.125 |
| 20 | 19.6563 | 98.28 ± 0.985 | |
| 30 | 29.8751 | 99.58 ± 0.315 | |
| 40 | 39.9242 | 99.81 ± 0.545 |
Conclusion
This article reports the fabrication of novel, simple, sensitive and less cost sensor PoOCD/MCPE for voltammetric resolution of PA. The sensor shows high sensitivity, selectivity, and anti- interference capability for the electrochemical oxidation of PA. The developed PoOCD/MCPE displayed well separated and resolved peaks for the electro-oxidation of PA, FA, and DA. The sensor can be used for determining the PA individually and simultaneously in the existence of FA and DA. The capability of the sensor was studied by estimating PA in the tablet. The developed sensor can also be applied to estimate some other biomolecules in the pharmaceutical industry.
Author contributions
S.D.S.: Electrochemical Sensors experiments, Formal analysis, Writing—original draft. B.E.K.S.: Conceptualization, Supervision, Writing—review and editing. J.K.S.: Writing—review and editing. S.C.S.: Writing—review and editing. S.A.H.: Writing—review and editing.
Funding
This study was funded by Jain University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
B. E. Kumara Swamy, Email: bek@kuvempu.ac.in.
S. C. Sharma, Email: scsharma.bangalore@gmail.com
References
- 1.Beitollahi H, Sheikhshoaie I. Novel nanostructure-based electrochemical sensor for simultaneous determination of dopamine and acetaminophen. Mater. Sci. Eng. C. 2012;32:375–380. [Google Scholar]
- 2.Teradale AB, Ganesh PS, KumaraSwamy BE, Das SN. Application of poly (nicotinamide) modified carbon paste electrode sensor for the electrocatalytic determination of acetaminophen and folic acid. Anal. Bioanal. Electrochem. 2018;10:203–219. [Google Scholar]
- 3.Tanuja SB, KumaraSwamy BE, Pai KV. Electrochemical determination of paracetamol in presence of folic acid at nevirapine modified carbon paste electrode: A cyclic voltammetric study. J. Electroanal. Chem. 2017;798:17–23. [Google Scholar]
- 4.Song Y, Zhang Y, Li J, Tan C, Li Y. Preparation of poly ionic liquid-mesoporous carbon nanospheres and its application in simultaneous determination of hydroquinone and catechol, and detection of paracetamol. J. Electroanal. Chem. 2020;865:114157. [Google Scholar]
- 5.Mahmoud BG, Khairy M, Rashwan FA, Banks CE. Simultaneous voltammetric determination of acetaminophen and isoniazid (hepatotoxicity-related drugs) utilizing bismuth oxide nanorod modified screen-printed electrochemical sensing platforms. Anal. Chem. 2017;89:2170–2178. doi: 10.1021/acs.analchem.6b05130. [DOI] [PubMed] [Google Scholar]
- 6.Teradale AB, Lamani SD, Ganesh PS, KumaraSwamy BE, Das SN. Electrochemical sensor for the determination of paracetamol at carbamazepine film coated carbon paste electrode. Z. Phys. Chem. 2018;3:345–358. [Google Scholar]
- 7.Tefera M, Geto A, Tessema M, Admassie S. Simultaneous determination of caffeine and paracetamol by square wave voltammetry at poly (4-amino-3-hydroxy naphthalene sulfonic acid)—Modified glassy carbon electrode. Food Chem. 2016;210:156–162. doi: 10.1016/j.foodchem.2016.04.106. [DOI] [PubMed] [Google Scholar]
- 8.Amare M, Teklay W. Voltammetric determination of paracetamol in pharmaceutical tablet samples using anthraquinone modified carbon paste electrode. Cogent Chem. 2019;5:1576349. [Google Scholar]
- 9.Fernandez C, Heger Z, Kizek R, Ramakrishnappa T, Boruń A, Faisal NH. Pharmaceutical electrochemistry: The electrochemical oxidation of paracetamol and its voltammetric sensing in biological samples based on screen printed graphene electrodes. Int. J. Electrochem. Sci. 2015;10:7440–7452. [Google Scholar]
- 10.Gilmartin MAT, Hart JP. Rapid detection of paracetamol using a disposable, surface-modified screen-printed carbon electrod. Analyst. 1994;119:2431–2437. doi: 10.1039/an9941900833. [DOI] [PubMed] [Google Scholar]
- 11.Lecoeur M, Rabenirina G, Schifano N, Odou P, Ethgen S, Lebuffe G, Foulon G. Determination of acetaminophen and its main metabolites in urine by capillary electrophoresis hyphenated to mass spectrometry. Talanta. 2019;205:120108. doi: 10.1016/j.talanta.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho RM, Freire RS, Rath S, Kubota LT. Effects of EDTA on signal stability during electrochemical detection of acetaminophen. J. of Pharma. Biomed. Anal. 2004;34:871–878. doi: 10.1016/j.jpba.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Youssef SH, Mohamed D, Hegazy MAM, Badawey A. Analytical methods for the determination of paracetamol, pseudoephedrine and brompheniramine in Comtrex tablets. BMC Chem. 2019;13:78. doi: 10.1186/s13065-019-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad NR, Omar FK. HPLC method for determination of paracetamol in pharmaceutical formulations and environmental water samples. World J. Pharma. Res. 2018;7:15. [Google Scholar]
- 15.Palur K, Archakam SC, Koganti B. Chemometric assisted UV spectrophotometric and RP-HPLC methods for simultaneous determination of paracetamol, diphenhydramine, caffeine and phenylephrine in tablet dosage form. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;243:118801. doi: 10.1016/j.saa.2020.118801. [DOI] [PubMed] [Google Scholar]
- 16.Iranifam M, Khodaei S, Saadati M. Chemiluminescence reaction of graphene oxide—Luminol—Dissolved oxygen and its application for determination of isoniazid and paracetamol. Microchem. J. 2019;146:850–855. [Google Scholar]
- 17.Tavallali H, Hamid Y. Spectrofluorometric determination of paracetamol in pharmaceutical formulations. Asian J. Biochem. Pharma. Res. 2011;1:2. [Google Scholar]
- 18.Pasha C. Determination of paracetamol in pharmaceutical samples by spectrophotometric method. Ecletica Quim J. 2020;45:37–46. [Google Scholar]
- 19.Chefirat B, Zergui A, Belmessabih MN, Rahmani C, Rezk-kallah H. Validation of a spectrophotometric method for the determination of paracetamol in plasma applicable for toxicological emergencies in laboratories with limited resources. Toxicol. Anal. Clin. 2020;32:266–277. [Google Scholar]
- 20.Doğan B, Elik A, Altunay N. Determination of paracetamol in synthetic urea and pharmaceutical samples by shaker-assisted deep eutectic solvent micro extraction and Spectrophotometry. Microchem. J. 2020;154:104645. [Google Scholar]
- 21.Ganesh PS, KumaraSwamy BE. Poly (Patton and Reeder’s) modified carbon paste electrode sensor for folic acid. J. Biosens. Bioelectron. 2016;7:1. [Google Scholar]
- 22.Unnikrishnan B, Yang YL, Chen SM. Amperometric determination of folic acid at multi-walled carbon nanotube-polyvinyl sulfonic acid composite film modified glassy carbon electrode. Int. J. Electrochem. Sci. 2011;6:3224–3237. [Google Scholar]
- 23.Kumar M, KumaraSwamy BE, Reddy S, Zhao W, Chethana S, Kumar VG. ZnO/functionalized MWCNT and Ag/functionalized MWCNT modified carbon paste electrodes for the determination of dopamine, paracetamol and folic acid. J. Electroanal. Chem. 2019;835:96–105. [Google Scholar]
- 24.Wei S, Zhao F, Xu Z, Zeng B. Voltammetric determination of folic acid with a multi-walled carbon nanotube-modified gold electrode. Microchim Acta. 2006;152:285–290. [Google Scholar]
- 25.Demirkan B, Bozkurt S, Cellat K, Arıkan K, Yılmaz M, Şavk A, Çalımlı MH, Nas MS, Atalar MN, Alma MH, Sen F. Palladium supported on polypyrrole/reduced graphene oxide nanoparticles for simultaneous biosensing application of ascorbic acid, dopamine, and uric acid. Sci. Rep. 2020;10:2946. doi: 10.1038/s41598-020-59935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Immanuel S, Aparna TK, Sivasubramanian R. A facile preparation of Au-SiO2 nanocomposite for simultaneous electrochemical detection of dopamine and uric acid. Surf. Interfaces. 2019;14:82–91. [Google Scholar]
- 27.Schindler S, Bechtold T. Mechanistic insights into the electrochemical oxidation of dopamine by cyclic voltammetry. J. Electroanal. Chem. 2019;836:94–101. [Google Scholar]
- 28.Kumar M, KumaraSwamy BE, Rekha DR. Electrosensitive determination of dopamine, ascorbic acid and uric acid using poly (benzamide) film modified carbon paste electrode. Sci. Lett. J. 2015;4:211. [Google Scholar]
- 29.Kuskur CM, KumaraSwamy BE, Jayadevappa H. Electrochemical behaviour of norepinephrine in the presence of paracetamol and folic acid at poly (congo red) modified carbon paste electrode. Anal. Bioanal. Electrochem. 2018;10:658–674. [Google Scholar]
- 30.Kannan A, Sevvel R. A highly selective and simultaneous determination of paracetamol and dopamine using poly-4-amino-6-hydroxy-2-mercaptopyrimidine (Poly-AHMP) film modified glassy carbon electrode. J. Electroanal. Chem. 2017;791:8–16. [Google Scholar]
- 31.Ashoka NB, KumaraSwamy BE, Jayadevappa H, Sharma SC. Simultaneous electroanalysis of dopamine, paracetamol and folic acid using TiO2–WO3 nanoparticle modified carbon paste electrode. J. Electroanal. Chem. 2020;859:113819. [Google Scholar]
- 32.Beitollahi H, Mohadesi A, Mohammadi S, Pahlavan A, Maleh HK, Akbari A. New voltammetric strategy for determination of dopamine in the presence of high concentrations of acetaminophen, folic acid and N-acetylcysteine. J. Mol. Liq. 2012;169:130–135. [Google Scholar]
- 33.Chetankumar K, KumaraSwamy BE, Sharma SC. Poly (benzoguanamine) modified sensor for catechol in presence of hydroquinone: A voltammetric study. J. Electroanal. Chem. 2019;849:113365. [Google Scholar]
- 34.Volkov A, Tourillon G, Lacaze P, Dubois J. Electrochemical polymerization of aromatic amines IR, XPS and PMT study of thin film formation on a Pt electrode. J. Electroanal. Chem. 1980;115:279–291. [Google Scholar]
- 35.Hareesha N, Manjunatha JG. Electro-oxidation of formoterol fumarate on the surface of novel poly (thiazole yellow-G) layered multi-walled carbon nanotube paste electrode. Sci. Rep. 2021;11:12797. doi: 10.1038/s41598-021-92099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shashikumara JK, KumaraSwamy BE, Sharma SC, Hariprasad SA, Mohanty K. Poly (red DSBR)/Al-ZnO modified carbon paste electrode sensor for dopamine: a voltammetric Study. Sci. Rep. 2021;11:14310. doi: 10.1038/s41598-021-93723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chitravathi S, KumaraSwamy BE, Mamatha GP, Sherigara BS. Electrochemical behavior of poly(naphthol green B)-film modified carbon paste electrode and its application for the determination of dopamine and uric acid. J. Electroanal. Chem. 2012;667:66–75. [Google Scholar]
- 38.Kuskur CM, KumaraSwamy BE, Shivakumar K, Jayadevappa H, Sharma SC. Poly (sunset yellow) sensor for dopamine: A voltammetric study. J. Electroanal. Chem. 2019;840:52–59. [Google Scholar]
- 39.Shashikumara JK, KumaraSwamy BE, ChetanKumar K. Sensitive and selective sensor for 3, 4-dihydroxyphenethylamine and uric acid at poly (Orange CD) modified carbon paste electrode. Chem. Data Coll. 2021;32:100661. [Google Scholar]
- 40.Chetankumar K, KumaraSwamy BE, Sharma SC, Hariprasad SA. An efficient electrochemical sensing of hazardous catechol and hydroquinone at direct green 6 decorated carbon paste electrode. Sci. Rep. 2021;11:15064. doi: 10.1038/s41598-021-93749-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin Q, Bai X, Hu Z. Electropolymerization of a conductive β-cyclodextrin polymer on reduced graphene oxide modified screen-printed electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2016;782:50–58. [Google Scholar]
- 42.Sainz R, Pozo M, Vilas-Varela M, Castro-Esteban J, Corral MP, Vázquez L, Blanco E, Peña D, Martin-Gago JA, Ellis GJ, Petit-Dominguez MD, Quintana C, Casero E. Chemically synthesized chevron-like graphene nanoribbons for electrochemical sensors development: Determination of epinephrine. Sci. Rep. 2020;10:14614. doi: 10.1038/s41598-020-71554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goyal RN, Gupta VK, Chatterjee S. Voltammetric biosensors for the determination of paracetamol at carbonnanotube modified pyrolytic graphite electrode. Sens. Actuat. B. 2010;149:252–258. [Google Scholar]
- 44.Harisha KV, Swamy BEK, Ganesh PS, Jayadevappa H. An electrochemical sensor for the determination of 5-amino salicylic acid at poly (alanine) modified carbon paste electrode: A cyclic voltammetric study. J. Anal. Bioanal. Tech. 2018;10:1273–1287. [Google Scholar]
- 45.Hareesha N, Manjunatha JG. Fast and enhanced electrochemical sensing of dopamine at cost-effective poly (dl-phenylalanine) based graphite electrode. J. Electroanal. Chem. 2020;878:114533. [Google Scholar]
- 46.Reddy YVM, Rao VP, Reddy AVB, Lavanya M, Venu M, Madhavi G. Determination of dopamine in the presence of ascorbic acid and uric acid using poly (Spands Reagent) modified carbon paste electrode. Mater. Sci. Eng. C. 2015;57:378–386. doi: 10.1016/j.msec.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Sathisha A, KumaraSwamy BE. Simultaneous electrochemical determination of paracetamol, dopamine and diclofenac at diacerein modified carbon paste electrode: A voltammetric study. Anal. Bioanal. Electrochem. 2018;10:1437–1448. [Google Scholar]
- 48.Ensafi AA, Karimi-Maleh H, Mallakpour S, Hatami M. Determination of N-acetylcysteine and acetaminophen by voltammetric method using N-(3,4-dihydroxyphenethyl)-3, 5dinitrobenzamide modified multiwall carbon nanotube paste electrode. Sens. Actuat. B. 2011;155:464–472. [Google Scholar]
- 49.Pournaghi-Azar MH, Saadatirad A. Simultaneous determination of paracetamol, ascorbic acid and codeine by differential pulse voltammetry on the aluminum electrode modified by thin layer of palladium. Electroanalysis. 2010;22:1592–1598. [Google Scholar]
- 50.Goyal RN, Singh SP. Voltammetric determination of paracetamol at C60-modified glassy carbon electrode. Electrochim. Acta. 2006;51:3008–3012. [Google Scholar]
- 51.Boopathi M, Won M, Shima Y. A sensor for acetaminophen in a blood medium using a Cu (II)-conducting polymer complex modified electrode. Anal. Chim. Acta. 2004;12:191–197. [Google Scholar]
- 52.Andawiyah R, Mulyasuryani A, Sulistyarti H. Voltammetric determination of paracetamol using polyvinyl alcohol (PVA)-Fe3O4 modified glassy carbon electrode. Mater. Sci. Eng. 2020;833:012059. [Google Scholar]
- 53.Monteiro MKS, Santos ECMM, Silva DR, Martínez-Huitle CA, dos Santos EV. Simultaneous determination of paracetamol and caffeine in pharmaceutical formulations and synthetic urine using cork-modified graphite electrodes. J. Solid State Electrochem. 2020;24:1789–1800. [Google Scholar]
- 54.Manjunatha KG, KumaraSwamy BE, Madhuchandra HD, Vishnumurthy KA. Synthesis, characterization and electrochemical studies of titanium oxide nanoparticle modified carbon paste electrode for the determination of paracetamol in presence of adrenaline. Chem. Data Coll. 2021;31:100604. [Google Scholar]



