Abstract
The present study reports the use of silver nanoparticles as a gene carrier, substituting gold microcarrier for biolistic gene delivery in Nicotiana tabacum L. Efficiency of biolistic transformation using silver nanoparticles (100 nm) was compared with that of gold microcarriers (0.6 micron) under varying helium pressure (450 psi, 650 psi, 900 psi and 1100 psi) and target distance (6 cm and 9 cm). Among the different concentrations (0.01–100 mgL−1) of silver nanoparticles tried, 10 mgL−1 produced the highest number of transient GUS expression (30) with statistical significance. Helium pressure of 650 and target distance of 9 cm, and 900 psi pressure and 6 cm distance resulted in the highest GUS expression with gold microcarriers and silver nanoparticles, respectively. Transformation efficiency was significantly higher with silver nanoparticles than gold microparticles as carriers resulting in a reduction up to 37.5-fold on the cost of consumables. Regeneration efficiencies of tissues bombarded with gold microcarriers and silver nanoparticles were 62.5% and 70.83%, respectively.
Keywords: Biolistics, Genetic transformation, β-Glucuronidase, Nicotiana tabacum L., pBI121, Silver nanoparticles
Introduction
Biolistic transformation is a highly efficient method of genetic modification for crop improvement (Kohli et al. 1998; Dai et al. 2001; Liu et al. 2019). High cost of the equipment and consumables such as gold microcarriers are the major limitations which prevents the widespread use of biolistic transformation (Dizaj et al. 2014). Cost-effective and efficient gene delivery system possessing high transformation efficiency and low cytotoxicity need to be developed.
Nanotechnology deals with materials of dimensions less than 100 nm, where the unique physical properties of such molecules make novel applications possible. Use of nanotechnology in genetic transformation has gained great momentum during the last decade. Tools of nanotechnology are being applied to biological sciences to serve as probes, sensors and vehicles for gene delivery (Torney et al. 2007; Sharma et al. 2019). Many nanoparticles have the ability to bind with DNA and protect DNA from enzymatic attack, and can aid in the delivery of DNA into the nucleus. Mesoporous silica nanoparticles, gold nanoparticles and zinc oxide nanoparticles have been used as carriers for gene transfer into microbes and plants (Torney et al. 2007; Chang et al. 2013; Wang et al. 2018).
Silver nanoparticles have anti-microbial property (Patel et al. 2015) and are able to increase the permeability of the bacterial membranes via the generation of many pits and gaps (Li et al. 2010). Nagamani et al. (2019) reported that silver nanoparticles can increase the efficiency of transformation in bacterial cells. The presence of cell wall in plant cells is a major hindrance for effective gene delivery. There are no reports yet on the use of silver nanoparticles as carriers for biolistic transformation of plants. Use of silver nanoparticles is supposed to aid in increased transformation efficiency of plant cells with comparatively reduced cost. Here we report efficacy of silver nanoparticles as carriers for gene delivery in Nicotiana tabacum L. using biolistic method.
Materials and methods
Plasmid
Plasmid pBI121, a binary Agrobacterium vector was used for plant transformation (Chen et al. 2003). The vector has a size of 14,758 bp and uidA (GUS) as reporter gene with the CaMV 35S promoter and kanamycin resistance gene as a selectable marker.
Biolistic transformation of Nicotiana tabacum L. with gold micro carriers
DNA mixture for bombardment was prepared by adding 10 µL of plasmid DNA (1 µg µL−1), 50 µL 2.5 M Calcium Chloride and 20 µL 0.1 M spermidine to 50 µL of gold microcarrier (0.6 micron). Nicotiana tabacum L. leaves were surface sterilized using sodium hypochlorite (4%) for 2 min and then washed twice with sterile distilled water. DNA mixture (20 µL) was coated on the macrocarrier and bombarded onto sterile leaf discs of Nicotiana tabacum placed on sterile basal MS medium in Petri plates. Bombardment was performed by PDS-1000 He gene gun (Bio-rad laboratories Inc., USA) using rupture disks of different psi viz., 450, 650, 900 and 1100 and with target distance of 6 and 9 cm to optimise the parameters of biolistic transformation for further comparison with silver nanoparticles. Gap distance of 6.35 mm and 28 inch Hg partial vacuum was maintained in the PDS-1000/He system. After bombardment the Petriplates were incubated in dark at 24 °C for 48 h.
Optimisation of parameters for biolistic bombardment of silver nanoparticles
The parameters viz., concentration of silver nanoparticles, helium pressure and target distance were varied to optimise them for the biolistic bombardment of Nicotiana tabacum L. using silver nanoparticles. DNA mixture was prepared with different concentrations (0.01, 0.05, 0.5, 1, 2, 10, 20 and 100 mgL−1) of silver nanoparticles (Sigma Aldrich) of size 100 nm. Bombardments were performed at 1100 psi, target distance of 6 cm, gap distance of 6.35 mm under 28 inch Hg partial vacuum in the PDS-1000/He system. The concentration which gave maximum transformation efficiency was used for further standardisation of helium pressure (450 psi, 650 psi, 900 psi, 1100 psi) and target distance (6 cm and 9 cm).
GUS histochemical assay
After two days of bombardment, the leaf discs were transferred to staining buffer (2 mM X-Gluc, 0.2% Triton X-100, 50 mM sodium phosphate buffer, pH 7.2, 2 mM potassium ferrocyanide and 2 mM potassium ferricyanide) and incubated overnight at 37 °C in dark. The leaves were de-stained in 70 percent ethanol until chlorophyll was removed. Blue spots were counted by observing under a stereo microscope at 100 × (Leica, Germany).
Selection of transformants
The leaf discs after two days of bombardment were screened for transformants on a selection medium supplemented with 1 mgL−1 NAA, 1 mgL−1 BA and 100 µg mL−1 kanamycin (Dixit et al. 2016). 3-week-old calli resistant to kanamycin and surviving in the selection medium were transferred to MS medium supplemented with 1 mgL−1 BA, 0.5 mgL−1 GA3, 0.25 mgL−1 IAA and 100 µg mL−1 kanamycin for the emergence of shoots (Dixit et al. 2016). The shoots that emerged from the calli were transferred to MS medium supplemented with 0.1 mgL−1 IAA and 100 µg mL−1 kanamycin for rooting (Dixit et al. 2016).
Confirmation of stable transgene integration using PCR
Leaves were collected from transformed plantlets and DNA was isolated using CTAB method (Doyle and Doyle 1990). Isolated DNA was subjected to PCR to confirm the transgene integration. GUS gene-specific primers (forward- 5′ACTCATTACGGCAAAGTGTGGGTCA3′ and reverse- 5′- TGGTGTAGAGCATTACGCTGCGAT3′) reported by Almasi et al. (2015) were used for the PCR amplification. DNA isolated from non-transformed plantlets served as control. Reaction mix of 25 μL was prepared with 2.5 μL PCR buffer mix, 0.5 mM each of dNTPs, 10 pmol each of forward (F) and reverse (R) primers, 1 U of Taq polymerase, and 300 ng DNA. PCR was performed with an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 1 min, annealing at 50 °C for 1 min, and extension at 72 °C for 1 min, followed by a final extension at 72 °C for 5 min. PCR products were electrophoresed on 1.5% agarose gel.
Statistical analysis
The numbers of GUS positive blue spots counted in the treatments were statistically compared for significance. The critical difference (CD) values at 5% level of significance were calculated using analysis of variance (ANOVA). For comparing the transformation efficiency of gold and silver nanoparticles as carriers, Student t-test was used at 5% level of significance. All the analyses were performed using the software Web Agri Stat Package (WASP), ICAR, India (http://www.ccari.res.in/waspnew.html).
Results and discussion
Biolistic transformation of Nicotiana tabacum with gold micro carriers
Non-bombarded leaf discs did not show any GUS activity, whereas blue spots were observed in all the bombarded tissues with gold microcarriers indicating transient expression of the gene (Fig. 1). Average number of GUS positive spots per bombardment is shown (Table 1). Helium pressure of 650 psi exhibited maximum transient expression with gold microcarriers (Table 1) which was statistically significant with other tested pressures. The highest transformation efficiency was earlier reported with 650 psi in nuclear transformation in tobacco leaves (Yasybaeva et al. 2017). Higher pressure of 1550 psi was demonstrated to cause damage to the target tissues in sorghum (Tadesse et al. 2003). There was a reduction in transient GUS expression in rice calli during bombardment at lower (450 psi) and higher helium pressures (1300 psi and 1550 psi) and the optimum was found to be 1100 psi (Zuraida et al. 2010). Reduced expression at lower pressure may be due to poor penetration capability of the microcarrier to the target tissues whereas higher-pressure bombardment may lead to injuries to target tissues.
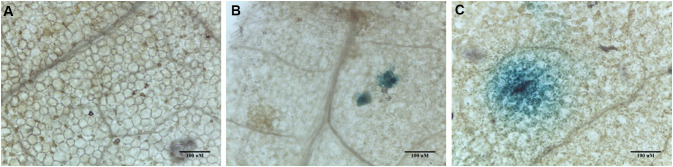
Fig. 1.
GUS stained leaves of Nicotiana tabacum L. following biolistic transformation with A pBI121, B pBI121 + gold microcarriers (0.6 micron) and C pBI121 + silver nanoparticles (100 nm) at optimized He pressure & target distance
Table 1.
Effect of different helium pressures and target distances in biolistic transformation of leaves of Nicotiana tabacum L. with gold microcarriers
| Helium pressure | Target distance | Mean* value for each He pressure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 cm | 9 cm | ||||||||
| R1 | R2 | R3 | Mean* | R1 | R2 | R3 | Mean* | ||
| 1100 psi | 5 | 9 | 8 | 7.3d | 9 | 14 | 12 | 11.7b | 9.5b |
| 900 psi | 15 | 18 | 13 | 15.3c | 8 | 16 | 13 | 12.3b | 13.8b |
| 650 psi | 30 | 28 | 30 | 29.3b | 65 | 71 | 67 | 67.7a | 48.5a |
| 450 psi | 46 | 54 | 49 | 49.7a | 17 | 11 | 13 | 13.7b | 31.7a |
| Mean* | 25.4** | 26.3** | |||||||
For different pressures, SEM = 1.21 and CD (0.05) = 3.643
For different distances, SEM = 0.85 and CD (0.05) are not significant
For the combination of pressure and distance, SEM = 1.70 and CD (0.05) = 5.2
SEM standard error (mean), CD critical difference
*Figures in a column followed by the same letter do not differ significantly (p ≥ 0.05)
**Mean values which do not differ significantly
Among the different target distances tried, a distance of 9 cm showed higher GUS gene expression than 6 cm (Table 1). However, the results were not statistically significant. Interaction analysis of the different parameters revealed that Helium pressure of 650 psi and target distance of 9 cm exhibited significantly higher transient expression of GUS (Table 1). Reduction in cell damage due to an increase in target distance may be a factor for increased expression at 9 cm observed in the present study. Similar results have been reported in wheat, castor, cowpea, mothbean, garden balsam (Impatiens balsamina) and olive (Kamble et al. 2003; Ikea et al. 2003; Sailaja et al. 2008; Perez-Barranco et al. 2009; Taha et al. 2009).
Optimisation of parameters for biolistic bombardment of silver nanoparticles
Bombardment using silver nanoparticles as DNA carrier into Nicotiana tabacum was successful and blue colour GUS expression was detected (Fig, 1). Among the different concentrations of silver nanoparticles tried for bombardment, 10 mgL−1 showed the maximum number of GUS positive blue spots and was statistically significant compared to other treatments (Table 2). Cellular uptake of nanoparticles is generally dependent on their size, charge and surface properties (Hillaireau and Couvreur 2009; Mailander and Landfester 2009). Bacterial cell viability was significantly reduced from 81 to 26% with an increase in concentration of silver nanoparticles from 4 to 33 ppm at 48 h exposure time (Jaiswal et al. 2015). Lower GUS expression at lower concentration (0.01, 0.05, 0.5 and 1 mgL−1) of silver nanoparticles observed in the present study may be due to an inadequate amount of silver nanoparticles to coat plasmid DNA.
Table 2.
Number of GUS positive blue spots per unit area of Nicotiana tabacum L. leaves bombarded with different concentrations of silver nanoparticles
| Treatment | Concentration of silver nanoparticles (mgL−1) | Average no. of blue spots* |
|---|---|---|
| T1 | 0.01 | 4.000cd |
| T2 | 0.05 | 0.667e |
| T3 | 0.5 | 1.333de |
| T4 | 1 | 2.667cde |
| T5 | 2 | 4.333c |
| T6 | 10 | 30.333a |
| T7 | 20 | 11.333b |
| T8 | 100 | 5.000c |
| Control | Sample buffer without silver nanoparticle | 0 |
*Figures in a column followed by the same letter do not differ significantly (p ≥ 0.05)
Difference in transient expression of GUS positive cells was observed under varying helium pressure and target distance with silver nanoparticles as carriers for bombardment. Among the different helium pressures tried, 900 psi exhibited a maximum number of GUS positive cells and the effect was statistically significant (Table 3). Variation in target distance also showed a significant difference in the number of GUS positive cells. Target distance of 6 cm showed significantly higher GUS gene expression than 9 cm (Table 3). Interaction effects of helium pressure and target distance using silver nanoparticles as carriers for biolistic bombardment revealed that the combination of 900 psi and 6 cm yielded significantly higher transient GUS expression among all the combinations tried (Table 3). Reduction in the size of carriers to nanoscale might have resulted in higher transient expression at higher helium pressure compared to gold microparticles. Bombardment of gold nanoparticles of size 50–100 nm was reported to be successful in transformation at 1100 psi helium pressure and 5 cm target distance in rice calli (Mortazavi and Zohrabi 2018). Bombardment of mesoporous silica nanoparticles was successful at 650 psi and 9 cm target distance in tobacco leaves and maize immature embryo (Torney et al. 2007). Nanoparticles like gold nanorods and gold capped with mesoporous silica nanoparticles are reported to exhibit significantly increased transient expression at higher pressures of 1350 and 1550 psi and lesser target distance of 4 cm in onion epidermis (Martin-Ortigosa et al. 2012).
Table 3.
Effect of different helium pressures and target distances in the biolistic transformation of leaves of Nicotiana tabacum L. with silver nanoparticles
| Helium pressure | Target distance | Mean* value foreach He pressure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 cm | 9 cm | ||||||||
| R1 | R2 | R3 | Mean* | R1 | R2 | R3 | Mean* | ||
| 1100 psi | 29 | 32 | 30 | 30.3b | 19 | 4 | 11 | 11.3** | 20.8b |
| 900 psi | 75 | 87 | 81 | 81.0a | 11 | 15 | 12 | 12.7** | 46.8a |
| 650 psi | 3 | 13 | 8 | 8.0 c | 5 | 3 | 3 | 3.7** | 5.8b |
| 450 psi | 37 | 12 | 18 | 22.3bc | 13 | 13 | 11 | 12.3** | 17.3b |
| Mean* | 35.4a | 10.0b | |||||||
For different pressures, SEM = 2.5 and CD (0.05) = 7.5
For different distances, SEM = 1.8 and CD (0.05) = 5.3
For the combination of pressure and distance, SEM = 3.5 and CD (0.05) = 10.6
SEM standard error (mean), CD critical difference
*Figures in a column followed by the same letter do not differ significantly (p ≥ 0.05)
**Mean values which do not differ significantly
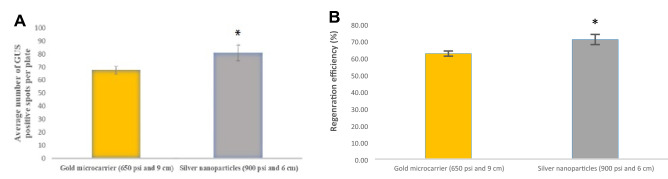
For a comparative analysis of gold microparticles with silver nanoparticles as carriers of DNA during biolistic bombardment, transformation efficiency of silver nanoparticles at its optimum conditions of 900 psi helium pressure and target distance of 6 cm were compared with that of gold microcarrier at its optimum of 650 psi and 9 cm. Silver nanoparticles as carrier exhibited significantly higher transient GUS expression than that for gold microparticles at optimum conditions. Student’s t-test identified the difference observed to be significant at 5% level of significance (Fig. 3A). One of the major drawbacks of biolistic transfection is cell/ tissue damage (O’Brien and Lummis 2006). One possible way to reduce tissue damage is to reduce the size of the carrier used for delivering DNA into host cell. The widely used micro-carriers are 0.6 and 1.0 μm diameter projectiles (O’Brien and Lummis 2006, 2011), but smaller-sized particles of less than 100 nm are also reported (Torney et al. 2007; O’Brien and Lummis 2011). O'Brien and Lummis (2011) compared the tissue damage between 1 µm gold microparticle and nanoparticle carriers and observed a cell damage of 9 ± 2% in nanoparticles and 22 ± 3% in microparticles. Use of nanometer sized particles has the potential advantage of increasing efficiency in transfection of smaller cells, organelles and specific cellular regions with reduced cell damage (O’Brien and Lummis 2011). In the present study, visible tissue damage was not observed in leaf tissues bombarded with silver nanoparticles and gold microcarriers. Lesser transient expression is reported when DNA coated with nano-sized gold and mesoporous silica nanoparticles were used for bombardment (Torney et al. 2007) and approximately 32 GFP-fluorescent foci per cotyledon were observed whereas gold microcarriers of size 0.6 μm produced approximately 73 GFP-fluorescent foci per cotyledon. Mortazavi and Zohrabi (2018) reported plasmid delivery through biolistic method by gold nanoparticles of size 50, 100, 600, and 1000 nm. Similar levels of transgene integration were observed with varying sizes of nanoparticles when used for the bombardment of rice embryogenic calli.
Fig. 3.
Comparison of gold microcarriers and silver nanoparticles as carriers for biolistic transformation of Nicotiana tabacum leaves with pBI121 at respective optimized conditions of helium pressure and target distance (*Significance was observed at p < 0.05)
Screening for transformed calli
The bombarded leaf discs were screened for transformants by transferring to MS medium supplemented with kanamycin as selection agent (Fig. 2). All the non-bombarded leaf discs started drying within one week of transfer to the selection medium whereas 62.5% of the leaf discs bombarded with DNA using gold microparticles and 70.83% of leaf discs bombarded with DNA using silver nanoparticles as carriers survived in the presence of kanamycin and exhibited callus induction and regeneration (Fig. 3B).
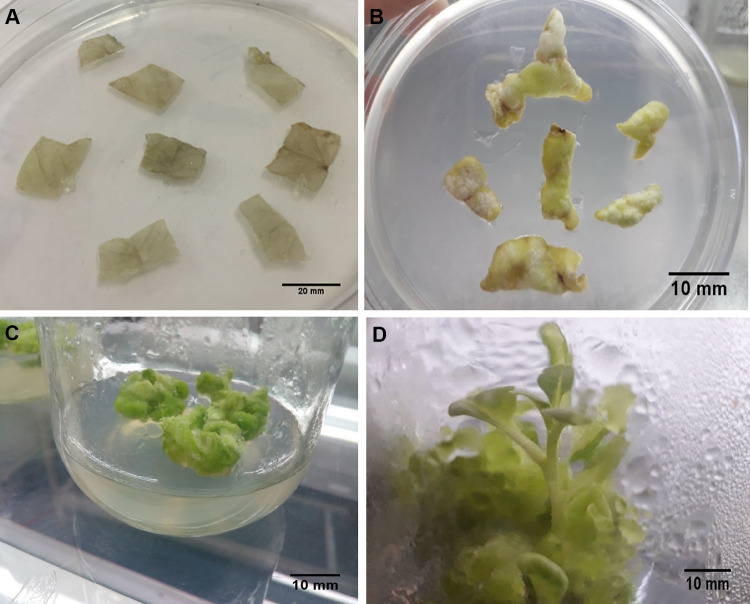
Fig. 2.
Regeneration of plantlets from Nicotiana tabacum L. leaves transformed with pBI121 by biolistic transformation with silver nanoparticles (100 nm) at optimized conditions (900 psi He pressure and 6 cm target distance). A Control leaves in callus induction and selection media 3 days after bombardment with silver nanoparticles without pBI121. B Leaves in callus induction and selection media 7 days after bombardment with pBI121 coated silver nanoparticles. C Callus induction from leaf disc bombarded with pBI121 coated silver nanoparticles after 3 weeks. D Regeneration of plantlets from callus
Confirmation of stable transgene integration using PCR
DNA from leaves of regenerated transformed and control plantlets when subjected to PCR to confirm the presence of GUS gene produced the expected amplicon of the size of 298 bp only in the former confirming the presence of GUS gene. There was no presence of GUS amplicon in the control (Fig. 4).
Fig. 4.
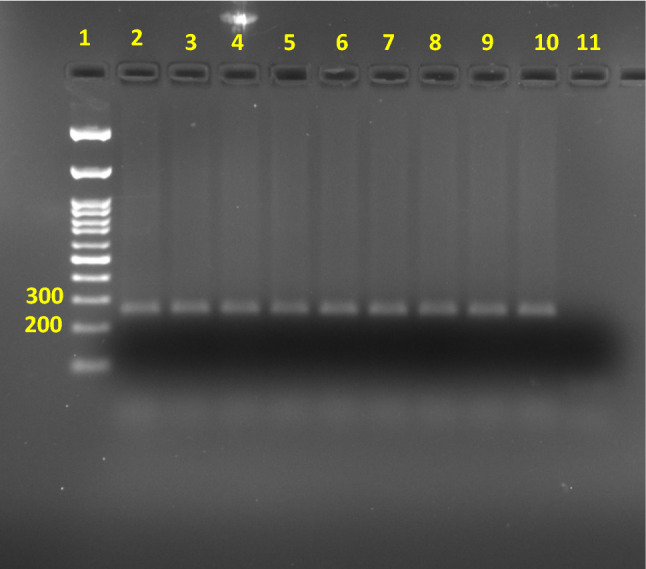
Stable transgene integration in regenerated plantlets checked by PCR using GUS gene-specific primers (Lane 1: 100 bp DNA marker; Lane 2: Positive control with pBI121 DNA used as PCR template; Lane 3–6: genomic DNA from regenerated plantlets transformed with gold microcarrier used as PCR template; Lane 7–10: genomic DNA from regenerated plantlets transformed with silver nanoparticles used as PCR template; Lane 11: non-template control
Conclusions
The present study found that silver nanoparticles (100 nm) at a concentration of 10 mgL−1 was successful in the efficient biolistic transformation of leaves of Nicotiana tabacum at a Helium pressure of 900 psi and target distance of 6 cm. Transformation efficiency was significantly higher using silver nanoparticles than gold microparticles as carriers with reduction in cost of consumables by 37.5 fold. This is the first report of the use of silver nanoparticles for biolistics based gene delivery in plant species. Other promising cost-effective nano-materials such as silica and zinc oxide could also be evaluated for their efficacy for gene delivery by biolistics.
Acknowledgements
Research facilities provided by Kerala Agricultural University is gratefully acknowledged
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Almasi MA, Aghapour-Ojaghkandi M, Bagheri K, Ghazvini M, Hosseyni-Dehabadi SM. Comparison and evaluation of two diagnostic methods for detection of npt II and GUS genes in Nicotiana tabacum. Appl Biochem Biotechnol. 2015;175(8):3599–3616. doi: 10.1007/s12010-015-1529-y. [DOI] [PubMed] [Google Scholar]
- Chang FP, Kuang LY, Huang CA, Jane WN, Hung Y, Yue-ie CH, Mou CY. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J Mater Chem B. 2013;1(39):5279–5287. doi: 10.1039/c3tb20529k. [DOI] [PubMed] [Google Scholar]
- Chen PY, Wang CK, To SSCKY. Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol Breeding. 2003;11(4):287–293. doi: 10.1023/A:1023475710642. [DOI] [Google Scholar]
- Dai S, Zheng P, Marmey P, Zhang S, Tian W, Chen S, Beachy RN, Fauquet C. Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol Breeding. 2001;7(1):25–33. doi: 10.1023/A:1009687511633. [DOI] [Google Scholar]
- Dixit S, Alam S, Sahoo S. In vitro selection and plant regeneration of CaCl2 & Mn-tolerant plants from leaf callus of Nicotiana tabacum L. Imp J Interdiscip Res. 2016;2(7):170–174. [Google Scholar]
- Dizaj SM, Jafari S, Khosroushahi AY. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res Lett. 2014;9(1):1–9. doi: 10.1186/1556-276X-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle J. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikea J, Ingelbrecht I, Uwaifo A, Thottappilly G. Stable gene transformation in cowpea (Vigna unguiculata L. Walp.) using particle gun method. Afr J Biotechnol. 2003;2(8):211–218. doi: 10.5897/AJB2003.000-1044. [DOI] [Google Scholar]
- Jaiswal S, Bhattacharya K, McHale P, Duffy B. Dual effects of β-cyclodextrin-stabilised silver nanoparticles: enhanced biofilm inhibition and reduced cytotoxicity. J Mater Sci Mater Med. 2015;26(1):52. doi: 10.1007/s10856-014-5367-1. [DOI] [PubMed] [Google Scholar]
- Kamble S, Misra HS, Mahajan SK, Eapen S. A protocol for efficient biolistic transformation of mothbean Vigna aconitifolia L. Jacq. Marechal. Plant Mol Biol Reporter. 2003;21(4):457–458. doi: 10.1007/BF02772595. [DOI] [Google Scholar]
- Kohli A, Leech M, Vain P, Laurie DA, Christou P. Transgene organization in rice engineered through direct DNA transfer supports a two-phase integration mechanism mediated by the establishment of integration hot spots. Proc Natl Acad Sci. 1998;95(12):7203–7208. doi: 10.1073/pnas.95.12.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WR, Xie XB, Shi QS, Zeng HY, You-Sheng OY, Chen YB. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85(4):1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Nannas NJ, Fu FF, Shi J, Aspinwall B, Parrott WA, Dawe RK. Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant Cell. 2019;31(2):368–383. doi: 10.1105/tpc.18.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailander V, Landfester K. Interaction of nanoparticles with cells. Biomacromol. 2009;10(9):2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- Martin-Ortigosa S, Valenstein JS, Sun W, Moeller L, Fang N, Trewyn BG, Lin VS, Wang K. Parameters affecting the efficient delivery of mesoporous silica nanoparticle materials and gold nanorods into plant tissues by the biolistic method. Small. 2012;8(3):413–422. doi: 10.1002/smll.201101294. [DOI] [PubMed] [Google Scholar]
- Mortazavi SE, Zohrabi Z. Biolistic co-transformation of rice using gold nanoparticles. Iran Agric Res. 2018;37(1):75–82. [Google Scholar]
- Nagamani G, Alex S, Soni KB, Anith KN, Viji MM, Kiran AG. A novel approach for increasing transformation efficiency in E. coli DH5α cells using silver nanoparticles. 3 Biotech. 2019;9(3):1–7. doi: 10.1007/s13205-019-1640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Lummis SC. Biolistic transfection of neuronal cultures using a hand-held gene gun. Nat Protoc. 2006;1(2):977. doi: 10.1038/nprot.2006.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Lummis SC. Nano-biolistics: a method of biolistic transfection of cells and tissues using a gene gun with novel nanometer-sized projectiles. BMC Biotechnol. 2011;11(1):1–6. doi: 10.1186/1472-6750-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Berthold D, Puranik P, Gantar M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep. 2015;5:112–119. doi: 10.1016/j.btre.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Barranco G, Torreblanca R, Padilla IM, Sánchez-Romero C, Pliego-Alfaro F, Mercado JA. Studies on genetic transformation of olive (Olea europaea L.) somatic embryos: I. Evaluation of different aminoglycoside antibiotics for nptII selection; II. Transient transformation via particle bombardment. Plant Cell Tissue Organ Culture. 2009;97(3):243–251. doi: 10.1007/s11240-009-9520-3. [DOI] [Google Scholar]
- Sailaja M, Tarakeswari M, Sujatha M. Stable genetic transformation of castor (Ricinus communis L.) via particle gun-mediated gene transfer using embryo axes from mature seeds. Plant Cell Rep. 2008;27(9):1509–1519. doi: 10.1007/s00299-008-0580-3. [DOI] [PubMed] [Google Scholar]
- Sharma S, Javed MN, Pottoo FH, Rabbani SA, Barkat M, Sarafroz M, Amir M. Bioresponse inspired nanomaterials for targeted drug and gene delivery. Pharmaceut Nanotechnol. 2019;7(3):220–233. doi: 10.2174/2211738507666190429103814. [DOI] [PubMed] [Google Scholar]
- Tadesse Y, Sagi L, Swennen R, Jacobs M. Optimisation of transformation conditions and production of transgenic sorghum (Sorghum bicolor) via microparticle bombardment. Plant Cell Tissue Organ Cult. 2003;75(1):1–8. doi: 10.1023/A:1024664817800. [DOI] [Google Scholar]
- Taha AM, Wagiran A, Ghazali H, Huyop F, Parveez GK. Optimization and transformation of garden balsam, Impatiens balsamina, mediated by microprojectile bombardment. Biotechnology. 2009;8(1):1–12. [Google Scholar]
- Torney F, Trewyn BG, Lin VS, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol. 2007;2(5):295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang F, Zhao J, Xu Y, Mao D, Zhu X, Luo Y, Alvarez PJ. Bacterial exposure to ZnO nanoparticles facilitates horizontal transfer of antibiotic resistance genes. NanoImpact. 2018;10(4):61–67. doi: 10.1016/j.impact.2017.11.006. [DOI] [Google Scholar]
- Yasybaeva G, Vershinina Z, Kuluev B, Mikhaylova E, Baymiev A, Chemeris A. Biolistic-mediated plasmid-free transformation for induction of hairy roots in tobacco plants. Plant Root. 2017;11:33–39. doi: 10.3117/plantroot.11.33. [DOI] [Google Scholar]
- Zuraida AR, RahinizaNurul KHMR, Roowi S, Zamri Z, Subramaniam S. Factors affecting delivery and transient expression of gusA gene in Malaysian indica rice MR 219 callus via biolistic gun system. Afr J Biotech. 2010;9(51):8810–8818. [Google Scholar]