Abstract
Background:
Despite evidence that Mycoplasma genitalium (MG) is a risk factor for adverse outcomes in pregnancy, screening in pregnant women is not currently recommended.
Methods:
Pregnant women between the ages of 13 and 29 years were recruited during their routine prenatal visits, screened for STIs and followed for one year. We compared women with MG to those with no STIs, excluding women with STIs other than MG (Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), or Trichomonas vaginalis (TV)) unless they were also co-diagnosed with MG. Adverse outcomes were extracted from participants’ medical records and compared between women with MG and those without STIs using exact or non-parametric approaches. Estimated differences were also adjusted for demographics using propensity scores with linear and logistic regression, where appropriate. We exclude women with MG and CT, NG, or TV diagnosis for primary analysis.
Results:
Of 281 participants enrolled from September 2015 until July 2019, 51 (18.1%) were diagnosed with MG. Of 51 women with MG, 12 (24%) were also diagnosed with CT, NG, or TV. All women with MG were offered treatment with azithromycin, however, only 28 (55%) were documented to receive treatment. Women with MG had similar outcomes to those with no STIs with a few exceptions. Average birth weight was lower among women with MG alone compared to women with no STIs when excluding co-infections (169 grams difference, 15 to 323).
Conclusions:
Our results indicate that MG is common in pregnant women and often presents as a co-infection. More research using population-based designs are needed to determine whether screening or treatment for women at risk for low birth weight or co-infections is warranted.
Keywords: STI, obstetrics, Mycoplasma genitalium, fetal growth, pregnancy, birth weight
Summary
Pregnant women with Mycoplasma Genitalium and their infants had similar outcomes to those without STIs, with the exception of birth weight, which was slightly lower among women with MG.
Introduction
Mycoplasma genitalium was first documented in the 1980s as a bacterial sexually transmitted infection (STI) related to infertility in women,1 and has become more common in the past 10-15 years.2 Prevalence studies conducted in general populations have indicated low overall prevalence at around 2%.3 However, specific populations have a burden as high as 7%. High-risk populations have been identified among younger women and racial/ethnic minorities with elevated STI risk. In particular, African American populations have been shown to have higher prevalence of MG, as high as 22%.4
Both women and men tend to be asymptomatic with MG infection, although urethritis has been associated with MG infection among men.5 Among women, MG infection has been associated with urethritis and cervicitis,6 PID and endometritis, as well as infertility.3 A detailed description of documented syndromes related to MG infection was recently published.3 In addition, a recent systematic review of prospective observational studies of MG identified associations of MG with reproductive tract diseases, including PID and cervicitis.7 Women with MG also may experience co-infection with other bacterial STIs such as Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) or with parasitic infections such as Trichomonas vaginalis (TV).8,9
Although some experience symptoms with MG infection, it is often asymptomatic7 and may go untreated, which has likely contributed to its persistence in the general population.10 Treatment is available for MG infection, but is often unsuccessful because MG infections can be resistant to treatment with macrolide antibiotics, with as many as 80% or more of infections having resistance-mediating mutations.11 MG has been treated with azithromycin and more recently with moxifloxacin due to the decreased efficacy of azithromycin.12 Azithromycin has not been associated with risk to fetuses and is not considered a risk for pregnant women or neonates,13 however the risks of moxifloxacin are not as well understood, and moxifloxacin may have teratogenic effects due to observed developmental toxicity in animals.14
Expectant mothers are a special population at risk for adverse sequelae of infections and are not exempt from acquiring STIs, including infection with MG.15 Despite the evidence that MG is a risk factor for adverse outcomes in pregnancy,7 the Centers for Diseases Control and Prevention (CDC) does not currently recommend screening for MG in asymptomatic women, pregnant or otherwise.16 The study of MG in pregnancy has been hampered by low prevalence and by low overall rates of adverse events such as miscarriage and preterm birth, and is likely to benefit from study in populations at high risk of MG infection.17 A recent systematic review of MG infection in pregnancy identified associations between MG and preterm birth and spontaneous abortion.7 There is less clarity in the literature about the effect of MG infection on the health of neonates,7 who may be vulnerable to infection while in utero. For example, in the presence of multiple infections, bacteria can be transported across fetal membranes.18 Infants may also be exposed to organisms during a vaginal birth.19 We aimed to examine the effect of MG infection in pregnant women and their newborns in a prospective cohort recruited in a population with high risk (expected 15%) of MG infection.15
Materials and Methods
Pregnant women between the ages of 13 and 29 years were recruited from a large, urban academic medical center on the east coast of the United States in a population with high STI prevalence during the time period from September 1 2015 until July 9 2019. Participants were recruited during visits for prenatal care where vaginal specimens were being collected for Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) screening per standard clinical protocol. All women with singleton pregnancies receiving prenatal care were eligible regardless of gestational age. Participants contributed specimens for MG and TV screening as well as general demographic information and whether they were experiencing symptoms at the time of the visit including vaginal discharge, bleeding, dysuria, dyspareunia or abdominal pain. Participants also answered questions relating to their risk for STIs using a standardized assessment tool, which allowed women’s risk for STIs to be scored from 0 to 10, with 10 indicating the highest risk and 0 the lowest.20
MG infection was assessed with the MG analyte specific Hologic/Gen-Probe transcription-mediated amplification assay.21 Diagnosis of TV infection was conducted with the Aptima TV assay in an academic research or clinical laboratory.22 Participant diagnoses of CT and NG were abstracted from participants’ electronic health record (EHR). Participants with MG infections indicated by screening were notified and offered treatment through the academic center’s Title X clinic, with decisions regarding treatment based on discussion between patients and their prenatal care providers. We determined whether or not patients were treated based on review of their EHR. Participants were followed for one year, and circumstances of their delivery were abstracted from the EHRs. Abstracted maternal variables included gestational age at delivery, spontaneous abortion, endometritis, pelvic inflammatory disease (PID), cervicitis, interim hospitalization, chorioamnionitis, hospital length of stay (days), and any diagnosis of maternal fever before or after delivery. Abstracted infant variables included birth weight, Apgar score at 1 and 5 minutes, sepsis diagnosis, and ICU admission. Preterm delivery was defined as gestational age less than 37 weeks and 0 days and low birth weight was defined as less than 2500 grams. The study protocol and all study activities were approved by the institutional review board of the Johns Hopkins School of Medicine (IRB00068584).
Statistical analysis
Descriptive analyses were used to summarize participant demographic and clinical data. We excluded participants diagnosed with CT, NG, or TV from all analysis, except for those who were co-diagnosed with MG. We focus on the comparison of women with MG diagnosis to women with no STIs. The non-parametric Wilcoxon rank sum was used to compare the gestational age and length of hospital stay for mothers, as well as birth weight and Apgar scores for infants. Other rates of adverse events during labor and delivery and for infants were compared across these groups with the non-parametric Fisher Exact test. Due to the potential for extreme confounding,23 we used linear (for averages) and logistic (for percentages) regression to adjust for age, past STI diagnosis, education, and race, with adjustment including weighting with propensity score for inverse likelihood of infection with MG.24 For propensity score estimates and regression adjustment, race was considered in two categories (Black or not Black), while age was continuous, and education was classified in four categories (high school or less, some college, college degree, or post graduate). Our enrollment target was based on the observed prevalence of MG (17%) in prior research among women with PID.25 We also repeated our analysis while excluding women with co-diagnosed CT, NG, or TV. In addition, as a sensitivity analysis, while excluding those with co-diagnosed CT, NG, or TV, we compared outcomes for mothers and infants for those with MG diagnosis without documented treatment with azithromycin to those with no STIs, although our study was not necessarily powered for this comparison.
Results
Three hundred seventy-three pregnant women were recruited into the study. Five had elective abortions (1.3%), 33 (9%) were lost to follow-up, 21 (5.6%) had incomplete or inconclusive STI testing information, and 33 (9%) had infections with CT, NG, and/or TV, but no MG diagnosis, leaving 281 women for analysis. Of 51 (18% of 281) women with MG diagnosis, 12 (24% of 51) were also diagnosed with CT, NG, or TV. Participants were predominately Black (82%), in their middle twenties (median 23 years, IQR 20 – 26) and with a high school diploma or lower attained education (68%). Most participants (73%) were asymptomatic, while 27% had at least one symptom. A summary of participant demographics, STI risk, and clinical presentation are shown in Table 1 overall and comparing those with MG to those with no STIs. Compared to women with no STIs, women with an MG infection were different in race (79% versus 98% Black, respectively, p < 0.001), and were slightly younger in age, but were otherwise similar in prior number of pregnancies, history of STI, scored risk for STI, education, and their presentation. Of all symptoms considered (discharge, bleeding, dysuria, irritation, dyspareunia, and abdominal pain), women with MG were not more likely to be symptomatic than women without STIs at 35% vs 24% (p=0.134). Of 51 women with positive MG screening, 28 (55%) had documented treatment with azithromycin, although rescreening for MG was not completed and so clearance could not be assessed.
Table 1.
Description of 281 pregnant women recruited from September 2015 until July 2019 and followed for 1 year.
| Overall n = 281 | Mycoplasma (potentially with CT, NG, or TV) n = 51 | No CT, NG, TV or MG n = 230 | p | |
|---|---|---|---|---|
| Age in years, mean (SD) | 23.3 (3.6) | 22.2 (3.2) | 23.5 (3.6) | 0.017 |
| Gestational age at enrollment (weeks), mean (SD) | 23.4 (10.6) | 24.1 (10.6) | 23.2 (10.7) | 0.580 |
| Prior pregnancies, mean (SD) | 1.5 (1.7) | 1.4 (1.7) | 1.5 (1.7) | 0.736 |
| History of STI, N (%) | 127 (45%) | 26 (51%) | 101 (44%) | 0.446 |
| STI Risk score (range 0 – 10), mean (SD)) | 4.0 (1.0) | 4.2 (0.9) | 3.9 (1.0) | 0.043 |
| Race, N (%) | < 0.001 | |||
| Black | 231 (82%) | 50 (98%) | 181 (79%) | |
| Other | 50 (18%) | 1 (2%) | 49 (21%) | |
| Education, N (%) | 0.216 | |||
| High school or less | 192 (68%) | 34 (67%) | 158 (69%) | |
| Some college | 66 (23%) | 16 (31%) | 50 (22%) | |
| College degree | 13 (5%) | 1 (2%) | 12 (5%) | |
| Post graduate | 10 (4%) | 0 (0%) | 10 (4%) | |
| Symptoms, N (%) | ||||
|
| ||||
| Vaginal discharge | 37 (13%) | 10 (20%) | 27 (12%) | 0.202 |
| Bleeding | 5 (2%) | 1 (2%) | 4 (2%) | 1.000 |
| Dysuria | 24 (9%) | 5 (10%) | 19 (8%) | 0.936 |
| Itching/irritation | 5 (2%) | 0 (0%) | 5 (2%) | 0.589 |
| Dyspareunia | 13 (5%) | 2 (4%) | 11 (5%) | 1.000 |
| Abdominal pain | 15 (5%) | 4 (8%) | 11 (5%) | 0.487 |
| At least one symptom | 73 (26%) | 18 (35%) | 55 (24%) | 0.134 |
SD – standard deviation; STI – sexually transmitted infection; CT – Chlamydia trachomatis; NG - Neisseria gonorrhoeae; TV – Trichomonas vaginalis.
Maternal outcomes
After one year of follow-up, maternal health data was summarized from participant medical records. We examined first the 281 women with a positive screen for MG (including those with MG as well as CT, NG, or TV diagnosis) or without any other STI. Interim hospitalization was the most common adverse event for these women at 24% (64 of 281), although preterm delivery and chorioamnionitis were also observed in 21 (8%) and 13 (5%) women, respectively (Table 2). There were 6 (2%) spontaneous abortions, although these were all among women who were negative for measured STIs. There was one case of PID and one case of cervicitis for women in the study, both of which were among women diagnosed with MG, although these differences were not statistically different (3% vs 0%, 95% CI 0.11 – infinity for PID, 3% vs 0%, 95% CI 0.11 – infinity for cervicitis). Preterm delivery was higher among women with MG compared to women without MG after adjusting for age, race, education, and risk of STIs (odds ratio 2.33, 95% CI 1.24 – 4.37). When excluding women with other STI co-diagnoses from those with MG, this association with preterm birth was not maintained (odds ratio 1.50, 0.73 – 3.10), as shown in Table 3.
Table 2.
Maternal and Infant outcomes among a cohort of 281 pregnant women and 273 infants recruited from September 2015 until July 2019 and followed for one year. Records were not available for eight infants. Women with an STI other than MG who were not co-diagnosed with MG are not shown (n=33). Statistically significant associations at 0.05 are in bold.
| Overall | Mycoplasma Genitalium (may include CT, NG, or TV) | No CT,NG,TV or MG | Unadjusted Difference (Odds Ratio or Mean Difference)† | Adjusted Difference (Odds Ratio or Mean Difference)†† | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Maternal | n=281 | n=51 | n=230 | Estimate | 95% CI | Estima | 95% CI |
| Preterm delivery | 21 (8%) | 5 (10%) | 16 (7%) | 1.49 | (0.52, 4.27) | 2.33 | (1.24, 4.37) |
| Spontaneous abortion | 6 (2%) | 0 (0%) | 6 (3%) | 0.00 | - | - | - |
| Endometritis | 2 (1%) | 1 (2%) | 1 (0%) | 4.50 | - | - | - |
| Pelvic inflammatory disease | 1 (0%) | 1 (2%) | 0 (0%) | Inf | (0.11, Inf) | - | - |
| Cervicitis | 1 (0%) | 1 (2%) | 0 (0%) | Inf | (0.11, Inf) | - | - |
| Interim hospitalization | 64 (24%) | 10 (20%) | 54 (24%) | 0.80 | (0.38, 1.71) | 0.74 | (0.47, 1.18) |
| Chorioamnionitis | 13 (5%) | 1 (2%) | 12 (5%) | 0.37 | (0.05, 2.88) | - | - |
| Maternal Fever | 6 (2%) | 1 (2%) | 5 (2%) | 0.93 | - | - | - |
| Length of Stay (days) | 2.6 (1.0) | 2.7 (1.1) | 2.6 (0.9) | 0.10 | (-0.20, 0.39) | 0.02 | (-0.23, 0.28) |
| Gestational age (weeks) | 38.5 (3.8) | 38.5 (2.2) | 38.5 (4.0) | 0.06 | (-1.09, 1.21) | 0.16 | (-0.71, 1.03) |
| Infant | n=273 | n=48 | n=225 | ||||
|
| |||||||
| Low birth weight (< 2500 g) | 22 (8%) | 7 (15%) | 15 (7%) | 2.37 | (0.91, 6.17) | - | - |
| Birth weight (grams) | 3174 (577) | 3012 (543) | 3209 (579) | -197 | (-376.7, -17.5) | -202 | (-345, -59) |
| Sepsis | 0 (0%) | 0 (0%) | 0 (0%) | - | - | - | - |
| ICU stay for infant | 24 (9%) | 4 (8%) | 20 (9%) | 0.93 | (0.30, 2.86) | - | - |
| Apgar (at 1 minute) | 7.6 (1.5) | 7.6 (1.1) | 7.5 (1.6) | 0.09 | (-0.39, 0.57) | 0.25 | (-0.09, 0.58) |
| Apgar (at 5 minutes) | 8.7 (1.1) | 8.6 (1.3) | 8.7 (1.1) | -0.08 | (-0.43, 0.27) | -0.04 | (-0.31, 0.22) |
Difference shown as MG – no STIs or MG relative to no STIs, estimated with the Student’s t test for continuous factors and the Chi-square test for categorical factors, except for factors with one or more category with a frequency below five, where Fisher’s exact test was used.
Adjusted for age, race, education, and risk of STIs using propensity scores. Comparisons with exact statistics are not adjusted.
ICU – Intensive care unit; CT – Chlamydia trachomatis; NG - Neisseria gonorrhoeae; TV – Trichomonas vaginalis; MG – Mycoplasma genitalium.
Table 3.
Excluding diagnoses of CT, NG, and TV, maternal and infant outcomes among a cohort of 269 pregnant women and 261 infants recruited from September 2015 until July 2019 and followed for one year. Records were not available for eight infants. Statistically significant associations at 0.05 are in bold.
| Overall | Mycoplasma Genitalium Alone | No CT,NG,TV or MG | Unadjusted Difference (Odds Ratio or Mean Difference)† | Adjusted Difference (Odds Ratio or Mean Difference)†† | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Maternal | n=269 | n=39 | n=230 | Estimate | 95% CI | Estimat | 95% CI |
| Preterm delivery | 18 (7%) | 2 (5%) | 16 (7%) | 0.74 | (0.16, 3.37) | 1.50 | (0.73, 3.10) |
| Spontaneous abortion | 6 (2%) | 0 (0%) | 6 (3%) | 0.00 | (0.00, 5.21) | - | - |
| Endometritis | 1 (0%) | 0 (0%) | 1 (0%) | 0.00 | (0.00, 225.21) | - | - |
| Pelvic inflammatory disease | 1 (0%) | 1 (3%) | 0 (0%) | Inf | (0.15, Inf) | - | - |
| Cervicitis | 1 (0%) | 1 (3%) | 0 (0%) | Inf | (0.15, Inf) | - | - |
| Interim hospitalization | 60 (23%) | 6 (16%) | 54 (24%) | 0.61 | (0.24, 1.53) | 0.48 | (0.28, 0.84) |
| Chorioamnionitis | 13 (5%) | 1 (3%) | 12 (5%) | 0.48 | (0.06, 3.84) | - | - |
| Maternal Fever | 5 (2%) | 0 (0%) | 5 (2%) | 0.00 | (0.00, 6.80) | - | - |
| Length of Stay (days) | 2.6 (1.0) | 2.6 (1.1) | 2.6 (0.9) | 0.07 | (-0.27, 0.41) | -0.04 | (-0.31, 0.22) |
| Gestational age (weeks.days) | 38.4 (3.6) | 38.5 (2.2) | 38.4 (4.0) | 0.14 | (-1.1, 1.4) | 0.30 | (-0.5, 1.2) |
| Infant | n=261 | n=36 | n=225 | ||||
|
| |||||||
| Low birth weight (< 2500 g) | 18 (7%) | 3 (8%) | 15 (7%) | 1.26 | (0.35, 4.59) | - | - |
| Birth weight (grams) | 3190 (575) | 3067 (544) | 3209 (579) | -142 | (-344, 60) | -169 | (-323, -15) |
| Sepsis | 0 (0%) | 0 (0%) | 0 (0%) | - | - | - | - |
| ICU stay for infant | 23 (9%) | 3 (8%) | 20 (9%) | 0.93 | (0.26, 3.31) | - | - |
| Apgar (at 1 minute) | 7.6 (1.5) | 7.6 (0.8) | 7.5 (1.6) | 0.10 | (-0.43, 0.64) | 0.20 | (-0.16, 0.55) |
| Apgar (at 5 minutes) | 8.7 (1.0) | 8.8 (0.4) | 8.7 (1.1) | 0.09 | (-0.26, 0.45) | 0.06 | (-0.15, 0.28) |
Difference shown as MG – no STIs or MG relative to no STIs, estimated with the Student’s t test for continuous factors and the Chi-square test for categorical factors, except for factors with one or more category with a frequency below five, where Fisher’s exact test was used.
Adjusted for age, race, education, and past STI diagnosis using propensity scores. Comparisons with exact statistics are not adjusted.
ICU – Intensive care unit; CT – Chlamydia trachomatis; NG - Neisseria gonorrhoeae; TV – Trichomonas vaginalis; MG – Mycoplasma genitalium.
Results for women with MG excluding 12 women with MG diagnosis and co-diagnosis of CT, NG, or TV are summarized in Table 3. Among these 269 women without CT, NG, or TV, interim hospitalization tended to be lower among women with MG compared to those with no STIs (adjusted odds ratio 0.48, 0.28 – 0.84). Hospital length of stay for mothers was similar for women with MG compared to those with no STIs. Gestational age at delivery also tended to be similar for women with MG diagnoses compared to women with no STIs, at 38 weeks and 5 days for women with MG, and 38 weeks and 4 days for women without MG. The difference adjusting for age, prior STI, education, and race was 3 days (95% CI −5 to 9). In a sensitivity analysis that excluded 21 (54% of 39) women with documented treatment for MG, there was no apparent association between untreated women diagnosed with MG alone and any adverse event considered (not shown) compared to the women with no STIs, including interim hospitalization.
Infant outcomes
Although electronic records were available for all 281 deliveries, there were 8 infants whose medical record could not be located, leaving 273 singleton infants with abstracted medical records. Adverse outcomes for infants are summarized in Table 2 for all infants with maternal MG diagnosis, including those with diagnoses of CT, NG, or TV, compared to infants with no maternal STIs. The most common adverse outcome for infants was being admitted to the ICU at 9% (24 of 281), although this was similar among infants born to women diagnosed with MG compared to those born to women without any STI (unadjusted 8% versus 9%, OR 0.93, 95% CI 0.30 – 2.86). Low birth weight was also common among these infants at 8% (22 of 281), which was not different for women with MG compared to women with no STIs after adjusting for race, age, education, and STI risk (odds ratio 2.37, 0.91 – 6.17). Average birth weight tended to be lower for women with MG than those with no STIs, with an average adjusted difference of 202g, 59 to 345g. Apgar score in the first minute tended to be similar for women with MG diagnosis compared to women without any STI, as shown in Table 2. The average differences in Apgar score for women with MG compared to infants with no maternal STIs, adjusted for age, mother’s past STI, education and were 0.3 points, −0.1 to 0.6, and 0.04 points, −0.2 to 0.3, at one and five minutes, respectively.
Differences for infants with maternal MG and those with no maternal STI were similar when excluding those with MG and co-diagnosis with CT, NG, or TV. When these 12 co-diagnoses were excluded, infants with maternal MG tended to weigh less than those with no maternal STI (average adjusted difference 169g, 95% CI 15 to 323, Table 3).
We also examined outcomes for infants depending on their mothers’ diagnosis of MG from among those without co-diagnoses while excluding 18 (50% of 36) infants with documented treatment for MG, leaving 18 infants with maternal MG compared to 225 infants with no maternal STI. The average adjusted reduction in birth weight for infants with maternal MG diagnosis compared to infants with no STI after excluding those with MG treatment was reduced from 169 to 34 grams (95% CI -118 to 186). Otherwise, associations between MG diagnosis and adverse events were qualitatively similar to those described above among all MG diagnoses compared to those with no STIs.
Discussion
We screened a cohort of pregnant women for MG infection and followed them for one year, examining the effects of MG on pregnant women, as well as the effects on infants. We did not find evidence that MG infections are related to gestational age, spontaneous abortion, Apgar score, or ICU stay for infants. We did find evidence that MG infections are related to a small reduction in birth weight, independent of related risk factors and other STIs.
Previous literature has examined whether neonates are more likely to be born with a low birth weight, in which no association between MG infection and SGA was detected.26 To our knowledge, there have not been other studies examining a change in average birth weight attributable to MG infection. The decrease we estimated here in birth weight at 169 grams is small and may not be clinically significant for most infants. More research is needed to determine the best recommendations for screening women and neonates who are at risk for low birth weight and MG.
We also found that women with MG were less likely to have interim hospitalization than women with no STIs, which has not been identified in other literature. This counterintuitive result may be due to a spurious association unlikely to be replicated elsewhere, or possibly to residual confounding by an unmeasured factor such as access to care. We consider these results unlikely to indicate an actual protective effect of MG. Interim hospitalizations among participants were for a variety of indications, including high blood pressure, chorioamnionitis, and preeclampsia.
We found evidence that MG infection is related to preterm birth, however, this association was not retained when excluding those with co-diagnosed STI. This is in contrast to a recent systematic review that identified an association between MG and preterm birth,7 however, not all of the studies included in this review were adjusted for co-diagnoses. This recent systematic review by Lis et al. also found that MG infection increased the likelihood of spontaneous abortion.7 The Lis review was likely able to detect smaller actual increases in preterm birth and spontaneous abortion due to an abundance of statistical power. It is also possible that studies in the Lis review were subject to confounding, where an association between MG and adverse outcomes may be real but not directly attributable to MG itself. Women with MG diagnoses tend to be different than women in the general population, including diagnosis with other STIs.4 Given that so few women have coinfections, it is difficult to determine the effects of specific infections among those with multiple diagnosed STI.
We repeated our analysis to compare women and infants with MG diagnosis to those with no STIs, while excluding those with documented treatment for MG. We did not observe any association between birthweight and MG diagnosis when women with treatment for MG were excluded. However, our power to detect differences in this sensitivity analysis was reduced relative to our primary analysis and likely excludes some women with active MG infections, since we were unable to assess clearance of MG. In addition, since we do not know why women chose to be treated for MG, we cannot extrapolate the expected benefit of treatment from this analysis. In practice, the decision about treatment is likely difficult for women and providers, given the mixed results in the literature and the lack of guidance from the CDC. If the effect of treatment for MG during pregnancy could be estimated, it could inform both clinical practice as well as the potential benefit of screening for MG.
Even though most women carry MG infection without acute symptoms,7 this cannot be taken for granted among those who are pregnant. In addition, fetuses are protected from many but not all bacteria in utero and the intrapartum period.27, 28 MG may not have serious sequelae in the short term for maternal or neonatal health and still have long term sequelae that warrant treatment post-partum (e.g. PID, infertility, etc).29 We have only studied a selection of specific measurable indicators, which does not necessarily preclude other morbidity with further assessment. For example, we did not examine whether preterm births were spontaneous or medically indicated, and we did not examine indications for interim hospitalization by type. Larger multi-site or population-based studies are likely needed to examine outcomes rarer than those presented here.
More research is needed to determine whether screening or screening and treatment for MG in pregnancy is beneficial.30 For MG screening to be most informative for treatment, resistance testing would likely be needed in accompaniment to general MG screening, given the high prevalence of resistant MG infections. Women with resistant infections may be unlikely to benefit from treatment with azithromycin, but would be ineligible for treatment with moxifloxacin due to the teratogenic risk. However, women may benefit from screening even in the event where treatment for MG is impossible. Providers could utilize knowledge of MG diagnosis to assist their patients in ways not related to direct treatment of MG, such as advising patients on measures to mitigate their risk of adverse events.31s Future research on MG will likely need to be at a larger scale than previous research to answer these questions. A multi-site clinical trial of MG screening (or treatment nested within screening) among high risk populations, for example, could increase both power and concerns about confounding. Ideally, future studies would also be able to follow women and infants prospectively over time and in heterogeneous settings.
Our study has limitations. We were unable to identify new versus persistent MG infections, although we expect that many of these MG infections were persistent.11 This would likely make it more difficult to detect differences between those with MG and those with no STIs, as women with treated and cleared MG infection may be less likely to have adverse events, but would be grouped with those having persistent MG. We also did not measure bacterial vaginosis among study participants, a known infectious driver of preterm birth.32s If bacterial vaginosis were more common among women with MG diagnosis, our results may be confounded by bacterial vaginosis, which would make it more likely that our analysis would find an effect of MG. We also were not able to follow all enrolled women until their delivery. Women who were lost had similar prevalence of MG compared with those who were retained in the study, with both groups having a prevalence of 18%.
Our study may also not have had adequate power to find a difference between women depending on their MG diagnosis. Power was further limited by the number of women recruited among our participants with MG infection without other STI diagnoses, which made adjustment for differences between those with MG infection and those without in some cases impossible. Rates of interim hospitalization were higher than expected, and lower than expected for endometritis and maternal fever. Low rates of adverse events would likely reduce power to detect associations between MG infections and outcomes in pregnancy.
Lastly, we were also limited by the timing of enrollment of women into our study, which was not restricted or controlled by gestational age. Given that participants were presenting for prenatal care, our results are not generalizable to women not seeking care, including women early on in their pregnancies. Adverse events occurring early on such as early pregnancy loss or ectopic pregnancy, which have both been associated with MG33s were not measured and could not be assessed from this study. Hence our results for spontaneous abortion may not be generalizable to all pregnant women. For example, we would not have identified an association between MG infection and spontaneous abortion if MG infection impacted early but not late pregnancy loss.
Our study also has strengths. We enrolled pregnant women from a population at high risk of MG infection and followed them prospectively over a one-year period to identify adverse outcomes occurring after the time of diagnosis. Those who are likely (or not) to benefit from screening and treatment of MG are similar to the study population represented here. Because we were able to recruit a relatively substantial number of women with MG infection, we were able to examine women having MG without co-diagnosed STI, which we anticipated could be confounders for adverse outcomes due to known risks of NG, CT, and TV for women and neonates.
Although it is difficult to rule out all negative impacts of MG infection for pregnant women and neonates, our results are inconsistent with a large impact. In the near term, screening for MG during pregnancy does not appear to be high priority for women and is likely to remain an informed decision for women and their providers. Future research is needed to determine whether the risks of MG infections during pregnancy warrant screening and treatment.
Supplementary Material
Figure 1.
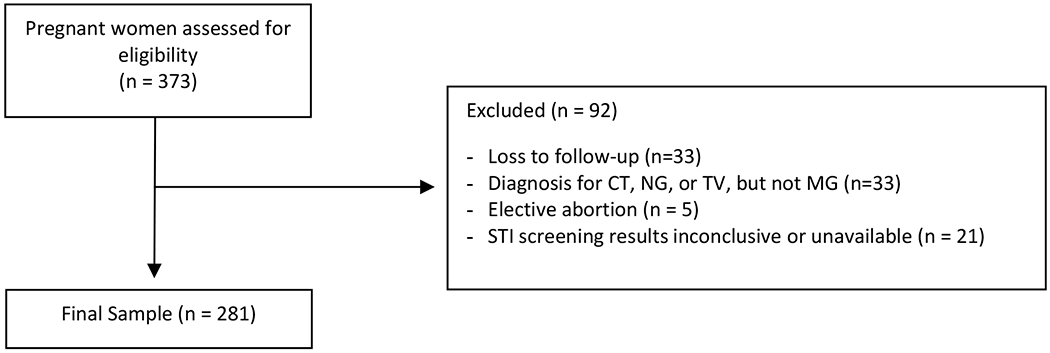
CONSORT diagram. MG- Mycoplasma Genitalium; CT – Chlamydia trachomatis; NG - Neisseria gonorrhoeae; TV – Trichomonas vaginalis.
Funding:
This work was generously supported by an unrestricted grant from Hologic, Inc to Johns Hopkins University and NICHD T32HD052459 [PI Trent, Funded Ronda]. Investigators Trent, Perin, and Gaydos were also supported by NINR 5R01NR013507.
Competing Interests
Aside from this grant, Dr. Trent also receives research support through a material transfer agreement with SpeeDx, LLC. through Johns Hopkins University and serves on the Trojan Sexual Health Advisory Council (Church & Dwight, Inc). Dr. Gaydos reports receiving research funding grants from Hologic and receiving speaker funding for educational lectures. The other co-authors declare that they have no competing interests.
References
- 1.Møller BR, Taylor-Robinson D, Furr PM, et al. Serological evidence that chlamydiae and mycoplasmas are involved in infertility of women. Reproduction. 1985;73(1):237–40. [DOI] [PubMed] [Google Scholar]
- 2.Getman D, Jiang A, O’Donnell M, et al. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54(9):2278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann L, Cina M, Egli-Gany D, Goutaki M, Halbeisen FS, Lohrer GR, Ali H, Scott P, Low N. Prevalence of Mycoplasma genitalium in different population groups: systematic review and meta-analysis. Sex Transm Infect. 2018. Jun 1;94(4):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korich F, Reddy NG, Trent M. Mycoplasma genitalium and Trichomonas vaginalis: addressing disparities and promoting public health control of two emerging sexually transmitted infections. Curr Opin Pediatr. 2020;32(4):482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Su X, Le W, Li S, Yang Z, Chaisson C, Madico G, Gong X, Reed GW, Wang B, Rice PA. Mycoplasma genitalium in symptomatic male urethritis: macrolide use is associated with increased resistance. Clin Infect Dis. 2020;70(5):805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: should we treat and how?. Clin Infect Dis. 2011;53(suppl_3):S129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis. 2015;61(3):418–26. [DOI] [PubMed] [Google Scholar]
- 8.Harrison SA, Olson KM, Ratliff AE, et al. Mycoplasma genitalium Coinfection in Women With Chlamydia trachomatis Infection. Sex Transm Dis. 2019;46(10):e101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upton A, Bissessor L, Lowe P, et al. Diagnosis of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis and Mycoplasma genitalium: an observational study of testing patterns, prevalence and co-infection rates in northern New Zealand. Sex Health. 2018;15(3):232–7. [DOI] [PubMed] [Google Scholar]
- 10.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24(3):498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosropour CM, Jensen JS, Soge OO, Leipertz G, Unutzer A, Pascual R, Barbee LA, Dombrowski JC, Golden MR, Manhart LE. High Prevalence of Vaginal and Rectal Mycoplasma genitalium Macrolide Resistance Among Female Sexually Transmitted Disease Clinic Patients in Seattle, Washington. Sex Transm Dis. 2020;47(5):321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stafford IA, Dunn JJ, Muldrew KL, et al. Trends in infection and antimicrobial resistance patterns of mycoplasma genitalium collected from pregnant women in Houston, TX. Am J Obstet Gynecol. 2019;221(6):697. [Google Scholar]
- 13.Sarkar M, Woodland C, Koren G, et al. Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth. 2006;6(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padberg S, Wacker E, Meister R, et al. Observational cohort study of pregnancy outcome after first-trimester exposure to fluoroquinolones. Antimicrob Agents Chemother. 2014;58(8):4392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trent M, Coleman JS, Hardick J, et al. Clinical and sexual risk correlates of Mycoplasma genitalium in urban pregnant and non-pregnant young women: cross-sectional outcomes using the baseline data from the Women’s BioHealth Study. Sex Transm Infect. 2018;94(6):411–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2021 STI Treatment Guidelines (CDC Web site; ). Available at: https://www.cdc.gov/std/treatment-guidelines/qa.htm. Accessed March 10, 2021. [Google Scholar]
- 17.Oakeshott P, Hay P, Taylor-Robinson D, et al. Prevalence of Mycoplasma genitalium in early pregnancy and relationship between its presence and pregnancy outcome. BJOG. 2004;111(12):1464–7. [DOI] [PubMed] [Google Scholar]
- 18.Thi Trung Thu T, Margarita V, Cocco AR, et al. Trichomonas vaginalis transports virulent mycoplasma hominis and transmits the infection to human cells after metronidazole treatment: a potential role in bacterial invasion of fetal membranes and amniotic fluid. J Pregnancy. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaser MJ, Dominguez-Bello MG. The human microbiome before birth. Cell Host Microbe. 2016;20(5):558–60. [DOI] [PubMed] [Google Scholar]
- 20.Gaydos CA, Jett-Goheen M, Barnes M, et al. Use of a risk quiz to predict infection for sexually transmitted infections: a retrospective analysis of acceptability and positivity. Sex Transm Infect. 2016;92(1):44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardick J, Giles J, Hardick A, et al. Performance of the Gen-Probe transcription-mediated [corrected] amplification research assay 279 compared to that of a multitarget real-time PCR for Mycoplasma genitalium 280 detection. J Clin Microbiol. 2006;44:1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaydos CA, Manhart LE, Taylor SN, et al. Molecular testing for Mycoplasma genitalium in the United States; results from the AMES† prospective multi-center clinical study. J Clin Microbiol, 2019; 57(11):e01125–19, p 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thoemmes FJ, Kim ES. A systematic review of propensity score methods in the social sciences. Multivariate Behav Res. 2011;46(1):90–118. [DOI] [PubMed] [Google Scholar]
- 24.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403. [DOI] [PubMed] [Google Scholar]
- 25.Trent M, Chung SE, Gaydos C, et al. Recruitment of Minority Adolescents and Young Adults into Randomised Clinical Trials: Testing the Design of the Technology Enhanced Community Health Nursing (TECH-N) Pelvic Inflammatory Disease Trial. Eur Med J Reprod Health. 2016;2(1):41–51. [PMC free article] [PubMed] [Google Scholar]
- 26.Labbe AC, Frost E, Deslandes S, et al. Mycoplasma genitalium is not associated with adverse outcomes of pregnancy in Guinea-Bissau. Sex Transm Infect. 2002;78(4):289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollier LM, Harstad TW, Sanchez PJ, et al. Fetal syphilis: clinical and laboratory characteristics. Obstet Gynecol. 2001;97(6):947–53. [DOI] [PubMed] [Google Scholar]
- 28.Morales WJ, Lim DV, Walsh AF. Prevention of neonatal group B streptococcal sepsis by the use of a rapid screening test and selective intrapartum chemoprophylaxis. Am J Obstet Gynecol. 1986. November 1;155(5):979–83. [DOI] [PubMed] [Google Scholar]
- 29.Svenstrup HF, Fedder J, Kristoffersen SE, et al. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil Steril. 2008;90(3):513–20. [DOI] [PubMed] [Google Scholar]
- 30.Donders GG, Ruban K, Bellen G, et al. Mycoplasma/Ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med. 2017;45(5):505–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


