Abstract
Background:
The purpose was to examine whether longitudinal changes in self-reported energy predict incident mobility disability and mortality. We further explored whether changes in energy-related behaviors (physical activity, appetite, or sleep quality) would explain these associations.
Methods:
N = 2,021 participants from the Health, Aging and Body Composition Study free from mobility disability and with at least three energy assessments from year 2 to year 10.
Measurements:
The outcomes were time to first self-reported inability to walk ¼ mile (mobility disability) and death. Self-reported energy level (SEL) was a single-item indicator over the prior month, ranging from 0 to 10; person-specific slopes measured whether individuals increased or decreased in SEL across the total follow-up time (mean 7.09 years, + 1.72, range 2–8 years). Potential energy-related mediators were baseline and change in: self-reported physical activity, appetite, and sleep quality. Covariates were baseline levels and change in: demographics, health characteristics and behaviors, tiredness, cognition, mood, and gait speed.
Results:
A total of 947 developed disability and 567 died over the study follow-up. A one-point change in SEL over the follow-up (or an average 0.125 points/year) was inversely associated with a 35% risk of incident mobility disability (hazard ratio = 0.65, 95% CI = 0.55, 0.76, p < .001) and 33% risk of death (hazard ratio = 0.67, 95% CI = 0.42, 0.87, p = .003), independent of covariates. Potential energy-related mediators did not attenuate this association.
Conclusions:
In this longitudinal analysis of community dwelling older adults, energy decline was common and a significant independent predictor of disability risk and mortality
Keywords: mobility disability, mortality, energy, vitality
Introduction
Age-related mobility disability predisposes older adults to adverse health events and mortality 1. Therefore, it is critical to develop diagnostic tools to identify high-risk individuals before disability clinically manifests. Although there are myriad contributors to mobility disability 2, diagnostic tools that are easily administered in primary clinical care settings are lacking. There are numerous protocols to assess indicators of mobility impairment and disability, all of which require specialized equipment, training, or are otherwise burdensome to administer in care and research settings 3.
Reduced energy is a common complaint among older adults 4, easy to assess, and is emerging as an early indicator of suboptimal aging. It is not clear whether energy and fatigue are two unipolar moods or the opposite ends of the same continuum. While prior literature treated reduced energy as synonymous with increased fatigue or tiredness, newer research suggests that energy and fatigue are distinct unipolar states rather than each other’s inverse 5–7. Recent cross-sectional work from our research group 8 indicates that there is a weak association between self-reported fatigue (binary tiredness over prior month) and energy. Moreover, energy and fatigue have distinct neurobiological substrates 5. Thus, we focused on the role of energy as an indicator of health independent of fatigue. Furthermore, both energy loss and increased exhaustion are criteria for phenotypic frailty 9, indicating that repeated assessments may reflect future risk of mobility disability. The unique contribution of energy loss to disability, however, remains obscured as phenotypic frailty typically evaluates both fatigue and energy.
Declining energy may correspond to disengagement in healthful behaviors that promote independent physical function and survival. For example, decreased energy may be associated with higher risk of physical disability due to reduction of healthful but high-energy activities such as exercise 10–12. Humans are predisposed to conserve energy 13, 14, so they may adapt their behavior to conserve their limited energy. In particular, older adults may replenish lost energy through physical activity, diet, or sleep 15, 16. Because health behaviors influence vitality 17, 18, it is important to control for these when examining the diagnostic utility of self-reported energy. However, most research relied on scales conflating feelings of fatigue, vitality, energy, and other similar domains, instead of considering them individually.
The aim of the current study was to measure decline in self-reported energy levels (SEL) and its predictive value on incident mobility disability and mortality while controlling for tiredness. We also explored the potential explanatory role of energy-related behaviors, namely physical activity, appetite, and sleep.
Methods
Study Design and Sample
The Health, Aging and Body Composition (Health ABC) cohort was a longitudinal cohort study of 3,075 older adults designed to examine how changes in body composition contribute to mobility disability and other functional declines. Older adults aged 70 – 79 years were recruited from research sites in Memphis and Pittsburgh beginning in 1997 – 1998 and followed annually in person and via phone follow-up until 2010 – 2011. Participants were eligible for Health ABC if they reported no baseline difficulty walking ¼ mile or climbing 10 steps. Because self-reported energy was not assessed until Year 2 (1998 – 1999), Year 2 was treated as the analytic baseline assessment for these analyses unless otherwise stated. Data for the current analyses were collected from baseline to Year 10 (2006 – 2007); participants selected for the current analyses required a valid baseline self-reported energy score (n = 1 out of bound range; n = 30 with missing energy at Year 2) and at least two follow-up energy assessments. Individuals who reported an inability to walk ¼ mile at either Years 1 (an exclusion criteria of the parent Health ABC cohort) or 2 were also excluded from the current analysis, yielding a final sample for these analyses of N = 2,021 older adults with a mean and standard deviation follow-up time of 7.09 (1.72) years.
Variables
Self-reported energy level (SEL).
Participants reported their usual level of energy over the prior month ranging from zero (no energy) to 10 (most energy they’ve ever had). Person-specific SEL slopes were calculated by a linear mixed model with random intercepts and random slopes similarly to previously published Health ABC data 19, 20. All available self-reported energy scores from a given individual informed the person-specific slope for mortality, and all available energy scores until reported mobility disability were used to calculate the energy slope mobility disability. Negative values indicated declines in SEL over time, and positive values indicated increased SEL over time. Additional detail on calculating slopes can be found in the Analytic Approach. SEL was assessed annually from Years 2 (1998 – 1999) to 10 (2006 – 2007).
Incident mobility disability and mortality.
Every six months, participants self-reported if they experienced difficulty walking a ¼ mile due to a health or physical problem 21. Mobility disability onset was defined as the number of years from the baseline assessment to the first occurrence of self-reported walking difficulty. Likewise, survival time was calculated as the number of years from the baseline interview until mortality or the last known date of contact. Deaths were confirmed by death certificates provided by family members or the state office of vital records through Year 10 (2006 – 2007).
Covariates.
All covariates were a priori identified as possible risk or protective factors for incident mobility disability or mortality. Demographic covariates included self-reported age, race (Black or White), gender, and years of education. The number of baseline health conditions were summed to indicate baseline comorbidities. The specific health conditions included self-reported diabetes, coronary heart disease, congestive heart failure, cerebrovascular disease, high blood pressure, and cancer. To account for new cases of health conditions, we also created a health conditions slope such that positive numbers indicate an increase in the number of health conditions from Year 1 (1997 – 1998) to Year 10 (2006 – 2007). Ankle-brachial index, a measure of lower extremity peripheral artery disease, was obtained in Year 1 (1997 – 1998) from systolic blood pressure in both the arm and ankle measured twice then averaged. Lower index values indicate the presence of peripheral arterial disease. Smoking status was coded as (1) ever or (0) never smoked. Body mass index (BMI) was calculated using a person’s recorded height and weight.
Tiredness was based on a participant’s response to the prompt, “In the past month, on average, have you been feeling unusually tired during the day?” Scores indicated whether the participant reported (1) yes or (0) no at baseline.
Cognitive status was assessed using the Teng Modified Mini-Mental State Examination (Teng 3MS) 22. Higher scores indicated better general cognitive function. Cognitive processing speed was not assessed at Year 2, so the Year 1 (1997 −1998) scores on the Digit Symbol Substitution Task 23 were used to reflect baseline performance. Participants were instructed to write symbols that correspond to numbers as quickly as possible in 90 seconds. Higher scores indicated more correctly copied symbols. Year 1 (1997 – 1998) depressive symptoms were assessed using the Center for Epidemiological Studies-Depression questionnaire 24; a modified version of the CES-D without the two questions pertaining to energy was computed and used in sensitivity analyses. Usual and rapid gait speed were assessed on a 20-meter walkway once per visit. Participants were instructed to walk at their usual pace or as quickly as possible, respectively. Scores reflected one’s speed in meters per second. Slopes for cognition, depressive symptoms, and gait were calculated to adjust for concurrent changes in dementia status, processing speed, mood, and walking speed.
Potential mediators.
Participants were asked to report on their engagement in walking-related physical activity in a usual week. Activities were converted to reflect kilocalories burned per kilogram per week in walking. Physical activity slopes were calculated from Years 1 (1997–1998) through 6 (2002–2003) and reflect the unadjusted slope of weekly kilocalories used per week walking and exercising. Notably, the physical activity slope is calculated over a shorter timespan compared to SEL (i.e., 6 compared to 8 years).
Year 2 appetite was assessed using the question, “In the past month, would you say that your appetite or desire to eat has been…?” The response options (very good, good, moderate, poor, very poor, don’t know) were read by an interviewer and shown on a flash card. Because few participants reported poor appetite, those experiencing “very good” or “good” appetite were coded as having a good appetite (1), and those experiencing “moderate,” “poor,” and “very poor” appetite were coded as having a poor appetite (0).
Participants were asked the following about their nighttime sleep quality at Year 2: During a typical month how often do you have: “trouble falling asleep?”; “Wake up during the night and have difficulty getting back to sleep?”; and “Wake up too early in the morning and are unable to get back to sleep?” Response options to these questions were: never (0), ≤ once a month (1), 2 to 4 times a month (2), 5 to 15 times a month (3), and > 15 times a month (4). Variables were summed to reflect a 0 – 12 score, with higher values indicating poorer nighttime sleep quality.
Analytic Approach
The unadjusted person-specific slopes for SEL were computed using longitudinal mixed effect models with a random intercept and slope of years for each participant to control for repeated measures and varying slopes across individuals. For example, unadjusted slopes were calculated with all available energy levels until reported mortality, study dropout, or study completion. Parametric assumptions of normality were evaluated using thresholds of −/+2 for skewness and kurtosis. The same approach was used to computed slopes of potential mediators and covariates of interest that had repeated measures: physical activity, comorbid health conditions, cognition, gait, and depressive symptoms. Bivariate associations with SEL were analyzed with Spearman correlations or independent samples t-tests to assess group differences.
Cox regression models examined whether SEL slopes predicted risk of incident mobility disability and mortality, controlling first for baseline SEL, then for significant covariates and lastly for potential mediators. To examine how the inclusion of various covariates affected the estimates of SEL predicting the outcomes, we introduced covariates in a blockwise fashion: (1) demographics, (2) health conditions, (3) peripheral health, (4) tiredness, (5) cognition, (6) depressive symptoms, (7) gait, (8) and then for those variables indicating potential explanatory pathways (physical activity, appetite, sleep quality). Because mobility limitations predict all-cause mortality 27, any experience of mobility disability across the study period was dummy-coded and included in the mortality analyses as an additional covariate. A final parsimonious model retaining SEL slopes and significant covariates (p < .05) was determined using backward selection procedures. This was done to examine whether SEL slopes remained robust even after accounting for the multiple demographic and health contributions to mobility disability and mortality. Variables with variance inflation factors > 2 were also removed to address multicollinearity. To aid with interpretability, SEL slopes were scaled to reflect a one-unit change across the eight-year follow-up period (i.e., instead of a one-unit change in energy slope per year, it reflects a one-unit change across eight years). Analyses were completed in SAS, Version 9.4 (SAS Institute, Cary, NC). Two-tailed significance was set at p < .05.
Results
The analytic sample of 2,021 older adults had an average baseline age of 74.5 years (SD = 2.8); a majority of the sample was White (n = 1,320, 65.3%), and there were slightly more women (n = 1,014 50.2%) (Table 1). The sample reported having generally high baseline energy (average of 6.9 out of 10, corresponding to high energy) similar to that in other Health ABC studies 8, 25. Of the 2,021 participants, 947 (46.9%) developed mobility disability and 348 (17.2%) died over the study period. On average, the sample had a 7% annual decline in energy, corresponding to an annual loss of .07 points. SEL slope was normally distributed (skewness = −0.22, kurtosis = 1.54). Baseline tiredness but not baseline SEL was significantly associated with greater SEL decline, suggesting that change over time was largely independent of one’s initial energy level. SEL decline was significantly greater in relation to White race, older age, an overall worse health profile, depressive symptoms, and worse cognitive and physical function, both at baseline and over time (Supplemental Table 1). Greater increases in physical activity over time were associated with faster declines in SEL slope (Supplemental Table 1).
Table 1:
Analytic sample characteristics (N = 2,021).
| Characteristic | M (SD) or n (%) |
|---|---|
| Self-reported energy (Y2) | 6.90 (1.69) |
| Self-reported energy slope (annual change in pts.) | −0.07 (0.05) |
| Mobility Disability, n (%) yes | 947 (46.9%) |
| Mortality, n (%) yes | 348 (17.2%) |
| Demographics | |
| White race | 1320 (65.3%) |
| Women | 1014 (50.2%) |
| Age (Y2) | 74.49 (2.84) |
| Education Less than high school High school Postsecondary (Ref) |
407 (20.2%) 651 (32.3%) 959 (47.5%) |
| Health-related factors | |
| Comorbid health conditions (# of health conditions, Y2) | 1.12 (0.96) |
| Comorbidity slope (annual change in # of conditions) | 0.05 (0.03) |
| Ankle-arm index (Y1) | 1.09 (0.17) |
| Behavioral-related factors | |
| Smoking history (Y2), n(%) ever yes | 1084 (53.7%) |
| BMI (Y2) | 26.92 (4.51) |
| Self-reported tiredness (Y2), n(%) yes | 422 (20.9%) |
| Teng 3MS (Y1) | 91.28 (7.39) |
| Teng 3MS slope (annual change in pts.) | −0.43 (0.67) |
| Digit Symbol Substitution Test (Y1) | 37.91 (14.04) |
| Digit Symbol Substitution Test slope (annual change in pts.) | −0.65 (0.50) |
| CES-D (Y1) | 4.31 (4.96) |
| CES-D slope (annual change in pts.) | 0.41 (0.27) |
| Physical performance | |
| Usual gait speed (Y2) | 1.18 (0.20) |
| Usual gait speed slope (annual change in m/s) | −0.04 (0.01) |
| Rapid gait speed (Y2) | 1.61 (0.31) |
| Rapid gait speed slope (annual change in m/s) | −0.04 (0.02) |
| Weekly walking and exercise (Y2), (kcal/kg/week) | 4.45 (8.32) |
| Weekly walking and exercise slope | −0.03 (0.14) |
| Appetite (Y2), n(%) good appetite | 1896 (93.9%) |
| Sleep Quality (Y2) | 3.24 (2.74) |
Note. Self-reported energy levels (SEL) slope reflects the unadjusted slope from baseline until death or study completion. Y = year of data collection (e.g., Y2 = Year 2); M = mean; SD = standard deviation; BMI = body mass index; Teng 3MS = Teng Modified Mini-Mental State Examination; CES-D = Center of Epidemiological Studies-Depression scale; kCal = kilocalories; kg = kilograms.
Incident Mobility Disability
Figure 1a presents the unadjusted energy levels comparing those who developed incident mobility disability with those who did not; those who did not develop incident mobility disability reported higher energy levels at all timepoints. After accounting for baseline energy, there was an inverse association between SEL slope and risk of incident mobility disability (hazard ratio = 0.44, 95% CI = 0.38, 0.50, p < .001). That is, increasing energy by one point across eight years (annual increase of .125 points) was associated with a 56% decreased risk of incident mobility disability (unadjusted hazard ratio = 0.44, 95% CI = 0.39, 0.51, p <.001). This association was largely stable after adjustment for demographic and health covariates (hazard ratio range: 0.43 – 0.56). Adding the hypothesized energy-related mediators to the model did not significantly modify these results (hazard ratio range: 0.45 – 0.47). In the final parsimonious model, a one-point change in SEL over the entire follow-up time was inversely associated with a 35% risk of incident mobility disability (hazard ratio = 0.65, 95% CI = 0.55, 0.76, p < .001). In this model, other independent predictors of higher risk of mobility disability were: lower baseline SEL and physical performance (i.e., usual and rapid gait speed), and higher levels of comorbidities, BMI, tiredness, depression and ankle-arm index. Less physical activity change over time was positively associated with an increased risk of incident mobility disability (Supplemental Table 2). Compared to other candidate predictors of mobility disability, SEL slopes appeared to have similar predictive ability to CES-D slope.
Figure 1.
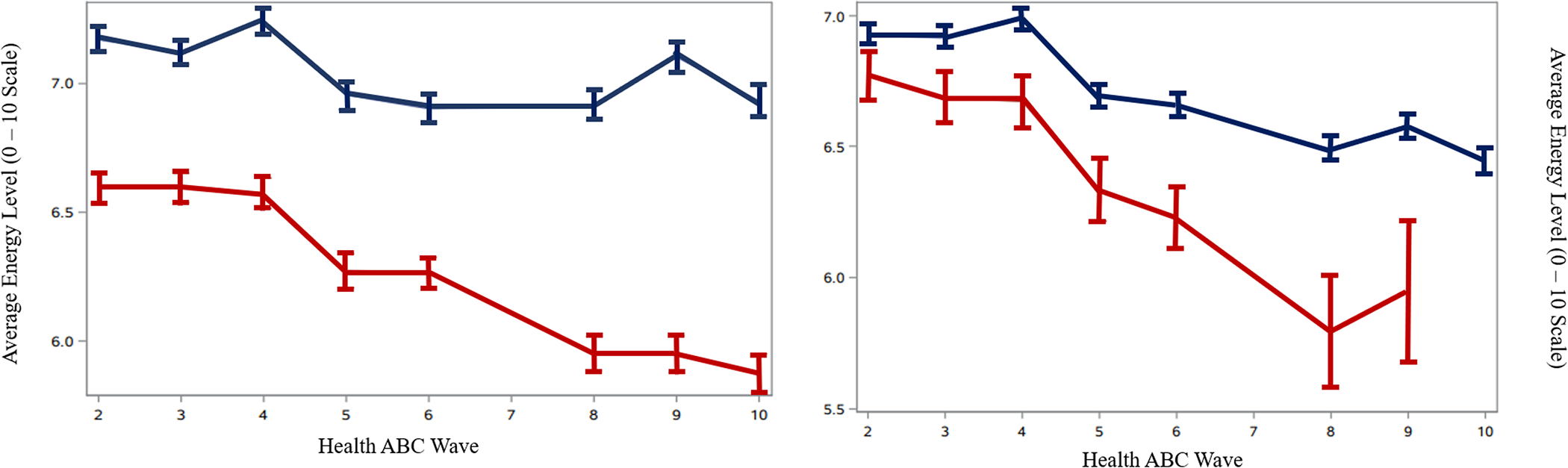
Mean energy level across time for those who did (red line) and did not (blue line) develop (A) mobility disability or (B) die across the follow-up period.
Note. The red line indicates the average energy level of individuals who developed (A) mobility disability or (B) died, and the blue line indicates average energy level of individuals who did not (A) develop mobility disability or (B) die over the follow-up period. Energy levels could range from a score of 0 – 10. The time interval for each Health ABC Wave corresponds to a year.
Incident All-Cause Mortality
Figure 1b presents the unadjusted energy levels comparing those who died over the follow-up period with those who survived; those who did survived reported higher energy levels at all timepoints. After accounting for baseline energy, there was an inverse association between change in SEL over time and risk of mortality (hazard ratio = 0.67, 95% CI = 0.54, 0.85, p = .001). That is, increasing energy by one point across eight years (or an annual increase of .125 points) was associated with a 33% decreased risk of death. This association was largely stable across demographic and health covariate blocks (hazard ratio range: 0.62 – 0.79). As with mobility disability, including gait and mobility did not eliminate the statistical significance of this association. The hypothesized energy-related mediators did not significantly attenuate the risk of death (hard ratio range: 0.67 – 0.68). Other independent predictors of higher mortality risk were: lower baseline SEL, lower baseline digit symbol substitution score, greater loss in usual gait speed, and not experiencing incident mobility disability. Men were at a greater risk of death compared to women (Supplemental Table 2). Compared to other candidate predictors of mortality, SEL slopes appeared to have similar predictive ability to reported mobility disability before death.
Discussion
Individuals who reported greater energy loss across eight years were at an increased risk of incident mobility disability and mortality. Associations were robust to baseline SEL, demographic and health covariates, as well as self-reported tiredness, and volitional energy-related behaviors such as physical activity, appetite, or sleep. Our findings lend credence to the notion that SEL may be an early indicator of suboptimal aging 8, 25–27 and are the first to identify that SEL decline is associated with prospective clinically-relevant health outcomes. The current study further highlights the value of subjective perceptions to predict clinically-relevant outcomes 8, 25, 27.
Why would SEL decline predict physical disability? One way to answer this question is to examine the factors contributing to both SEL decline and disability, and then assess whether such factors explain the SEL-related effects on disability. SEL slopes were weakly associated with those energy-related behaviors which we hypothesized would also be the main mediators: physical activity, appetite, and sleep quality. The bivariate association that was present with energy-related factors was not directionally consistent with our hypothesis; faster increases in physical activity were associated with greater decline in energy. Notably, there was an inverse association between physical activity slope with mobility disability risk as well. The association with physical activity is inconsistent with prior work by our research team in a subsample of this cohort 25 that found lower SEL was cross-sectionally associated with lower energy expenditure. It is possible that these relationships are spurious, as we were unable to calculate a physical activity slope across the same span of time as SEL. Complicating this is the likely bidirectional association between SEL with physical activity; chronic physical activity was associated with increased SEL in one study 11, but a predisposition to high SEL was associated with increased physical activity in another 28. Future work should first examine concurrent energy-exercise associations prior to making strong assertions about their longitudinal association or temporal precedence.
While SEL did not appear to reflect physical activity, appetite, or sleep, it was significantly associated with markers of overall health (e.g., comorbid health conditions), and mental and physical function and well-being. This is in line with a prior theory that posits subjective vitality (which included subjective energy) reflects psychological, biological, and environmental processes 18. The association with depressive symptoms persisted even after removing the energy/tiredness related questions in the CES-D. It has previously been suggested SEL is an indicator of psychological well-being, and energy scales have been included in prior vitality research 29. These results suggest that while depressive symptoms and energy are related, however, energy loss reflected in depressive symptom questionnaires is not the same as energy loss measured by SEL.
Notably, there was no significant association between SEL and cognitive function as measured in this study. This is inconsistent with prior work that found an association between SEL and cognition assessed via Trailmaking Task B 28. It is possible that SEL is related to cognitive domains that were not measured in our current cognitive battery (e.g., executive function with Trailmaking Task B) and warrants future research.
SEL slope was associated with self-reported tiredness, although there was no evidence that these scores were collinearly associated. This replicates prior work that found energy and fatigue (or tiredness in this study) are not values along one continuum but likely reflect separate states 5–7. Albeit frequently reported together and used as interchangeable terms, fatigue and energy are different constructs 5, 6, 30 with distinct neurobiological substrates 5. That is, an individual experiencing high fatigue may not necessarily experience low energy at the same time. Further evidence that energy and tiredness may impact health through different processes was present in the model building procedure. That is, energy significantly predicted both health outcomes, whereas tiredness was only associated with incident mobility disability (but removed in the final parsimonious model). Even when controlling for the percent of assessments participants reported feeling tired (e.g., someone reporting feeling tired for 3 of 5 assessments would report feeling tired 60% of the time), associations between energy with both outcomes remained unchanged (data not shown). This suggests that energy loss partially reflects changes in health, psychological, and physical factors. However, energy is not fully explained by these factors and is independently associated with physical disability and mortality.
The current results should be interpreted cautiously due to some limitations in our analysis. Notably, we were unable to examine how concurrent longitudinal changes in all of the hypothesized energy-related mediators impacted the SEL slope-mobility/mortality relationships. Extending this work to incorporate confounder slopes is important as changes in energy behaviors may signal homeostatic disruption and be more important in impacting self-reported mobility and mortality trajectories rather than a single assessment of energy behaviors. Additionally, our data are representative of community-dwelling older adults who reported high baseline energy and may not reflect clinical or less healthy samples. It is possible that energy loss among these subgroups is dissimilar and warrants future exploration, particularly with conditions that likely influence energy levels such as arthritis. Furthermore, how energy changes after mobility disability onset remains a possibility for future inquiry.
Despite this, our study had several strengths. First, we were able to use a well-characterized longitudinal cohort to control for several important health domains as well as possible contributors to one’s SEL. Secondly, the inclusion of participants with at least three energy assessments (baseline + ≥ two follow-up) ensures that there are enough follow-up assessments to confidently predict an individual’s slope. Over 50% of the sample had data available for all waves, so it is possible that in studies with fewer assessments or a shorter period of time between assessments there would not be enough change in energy. Lastly, we treated energy and fatigue as distinct constructs and found that declining SEL, rather than those who are tired, are particularly vulnerable to disability.
SEL is a promising upstream factor that may serve as a diagnostic indicator and possible therapeutic target for promoting older adult health. Our SEL assessment was not collinear with tiredness or other energy-related factors, further suggesting that SEL is a distinct construct that uniquely contributes to health. Physical disability is common and costly 1, with a paucity of effective prevention or intervention strategies. Not only can SEL changes be a promising indicator of disability, but promoting high SEL may be intrinsically motivating for older adults to engage in health behaviors 17, 18.
Supplementary Material
Supplemental Table 1. Spearman’s ρ or group mean differences between demographic and health characteristics with SEL slope
Supplemental Table 2. Parsimonious Cox regression model of energy predicting incident mobility disability and mortality.
KEY POINTS.
Do changes in self-reported energy levels predict incident mobility disability and mortality among community-dwelling older adults?
In this longitudinal cohort study of 2,021 older adults free from disability, followed for 8 years, a one-point decline in a 0–10 energy scale predicted a 35% and 33% greater risk of incident mobility disability and death.
Changes in perceptions of energy could be helpful diagnostic tools to identify those at greater risk of adverse health events.
WHY DOES THIS PAPER MATTER?
This is the first study to demonstrate the predictive utility of changes in a single- item energy measure to clinically-relevant health outcomes independent of health and energy-related behaviors.
ACKNOWLDGMENTS:
Funding: This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. BNS was also supported by a National Institute on Aging Kirschstein Institutional National Research Service Award (T32-AG055381) awarded to the University of Pittsburgh (PIs: Drs. Mary Ganguli and Caterina Rosano). All coauthors have no conflicts of interest to disclose.
Sponsor’s Role: The sponsors did not play a role in the design, methods, data collection, analysis or preparation of this paper.
References
- 1.Musich S, Wang SS, Ruis J, Hawkins K, Wicker E. The impact of mobility limitations on health outcomes among older adults. Geriatric Nursing. 2018;39(2):162–169. doi: 10.1016/j.gerinurse.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 2.Yeom H-A, Feury J, Keller C. Risk factors for mobility limitation in community-dwelling older adults: A social ecological perspective. Geriatric Nursing. 2008;29(2):133–140. doi: 10.1016/j.gerinurse.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. November 2013. 2013;68(11):1379–1386. doi: 10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H, Gurland BJ, Maurer MS. Self-reported lack of energy (anergia) among elders in a multiethnic community. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2008;63(7):707–714. doi: 10.1093/gerona/63.7.707 [DOI] [PubMed] [Google Scholar]
- 5.Loy BD, Cameron MH, O’Connor PJ. Perceived fatigue and energy are independent unipolar states: Supporting evidence. Medical Hypotheses. 2018;113:46–51. doi: 10.1016/j.mehy.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boolani A, O’Connor PJ, Reid J, Ma S, Mondal S. Predictors of feelings of energy differ from predictors of fatigue Fatigue: Biomedicine, Health & Behavior. 2018;7(1):12–28. doi: 10.1080/21641846.2018.1558733 [DOI] [Google Scholar]
- 7.Deng N, Guyer R, Ware JE Jr.. Energy, fatigue, or both? A bifactor modeling approach to the conceptualization and measurement of vitality. Quality of Life Research. 2015;24(1):81–93. doi: 10.1007/s11136-014-0839-9 [DOI] [PubMed] [Google Scholar]
- 8.Ehrenkranz RC, Rosso AL, Sprague BN, et al. Functional correlates of self-reported energy levels in the Health, Aging, and Body Composition Study. Journal of Aging Clinical and Experimental Research. in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 10.Egerton T, Chastin SFM, Stensvold D, Helbostad JL. Fatigue may contribute to reduced physical activity among older people: An observational study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2016;71(5):670–676. doi: 10.1093/gerona/glv150 [DOI] [PubMed] [Google Scholar]
- 11.Puetz TW. Physical activity and feelings of energy and fatigue: Epidemiological evidence. Sports Medicine. 2006;36(9):767–780. doi: 10.2165/00007256-200636090-00004 [DOI] [PubMed] [Google Scholar]
- 12.Ellingson LD, Kuffel AE, Vack NJ, Cook DB. Active and sedentary behaviors influence feelings of energy and fatigue in women. Medical and science in Sports and Exercise. 2014;46(1):192–200. doi: 10.1249/MSS.0b013e3182a036ab [DOI] [PubMed] [Google Scholar]
- 13.Christie ST, Schrater P. Cognitive cost as dynamic allocation of energetic resources Frontiers in Neuroscience. 2015;9:289. doi: 10.3389/fnins.2015.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noakes TD. Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis Frontiers in Physiology. 2012;3:82. doi: 10.3389/fphys.2012.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the childhood cancer survivor study Cancer. 2011;117(11):2559–2568. doi: 10.1002/cncr.25797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho Sampaio RA, Sewo Sampaio PY, Yamada M, Tsuboyama T, Arai H. Self-reported quality of sleep is associated with bodily pain, vitality and cognitive impairment in Japanese older adults. Geriatrics & Gerontology International. 2014;14(3):628–635. doi: 10.1111/ggi.12149 [DOI] [PubMed] [Google Scholar]
- 17.Ryan RM, Frederick C. On energy, personality, and health: Subjective vitality as a dynamic reflection of well-being. Journal of Personality. 1997;65(3):529–565. doi: 10.1111/j.1467-6494.1997.tb00326.x [DOI] [PubMed] [Google Scholar]
- 18.Lavrusheva O The concept of vitality. Review of vitality-related research domain. New Ideas in Psychology. 2020;56:100752. doi: 10.1016/j.newideapsych.2019.100752 [DOI] [Google Scholar]
- 19.Rosso AL, Metti AL, Faulkner K, et al. Complex walking tasks and risk for cognitive decline in high functioning older adults. Journal of Alzheimer’s Disease. 2019;71(s1):S65–S73. doi: 10.3233/JAD-181140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosano C, Aizenstein HJ, Newman AB, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage. 2012;62(1):307–313. doi: 10.1016/j.neuroimage.2012.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of Clinical Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 23.Wechsler D Manual for the Wechsler Adult Intelligence Scale, Revised. Psychological Corporation; 1981. [Google Scholar]
- 24.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 25.Tian Q, Glynn NW, Ehrenkranz RC, Sprague BN, Rosso AL, Rosano C. Perception of energy and objective measures of physical activity in older adults. Journal of the American Geriatrics Society. in press;doi: 10.1111/jgs.16577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avlund K Fatigue in older adults: An early indicator of the aging process? Aging Clinical and Experimental Research. 2010;22(2):100–115. doi: 10.1007/BF03324782 [DOI] [PubMed] [Google Scholar]
- 27.Renner SW, Cauley JA, Brown PJ, et al. Higher fatigue prospectively increases the risk of falls in older men. Innovation in Aging. in press;doi: 10.1093/geroni/igaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boolani A, Manierre M. An exploratory multivariate study examining correlates of trait mental and physical fatigue and energy. Fatigue: Biomedicine, Health & Behavior. 2019;7(1):29–40. doi: 10.1080/21641846.2019.1573790 [DOI] [Google Scholar]
- 29.Rouse PC, Veldhuijzen Van Zanten JJJCS, Ntoumanis N, et al. Measuring the positive psychological well-being of people with rheumatoid arthritis: A cross-sectional validation of the subjective vitality scale. Arthritis Research & Therapy. 2015;17:312. doi: 10.1186/s13075-015-0827-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerdal A, Kottorp A, Gay CL, Lee KA. Lee Fatigue and Energy Scales: Exploring aspects of validity in a sample of women with HIV using an application of a Rasch model. Psychiatry Research. 2013;205(3):241–246. doi: 10.1016/j.psychres.2012.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Spearman’s ρ or group mean differences between demographic and health characteristics with SEL slope
Supplemental Table 2. Parsimonious Cox regression model of energy predicting incident mobility disability and mortality.


