Abstract
Bacterial infections of the cornea, or bacterial keratitis (BK), are notorious for causing rapidly fulminant disease and permanent vision loss, even among treated patients. In the last sixty years, dramatic upward trajectories in the frequency of BK have been observed internationally, driven in large part by the commercialization of hydrogel contact lenses in the late 1960s. Despite this worsening burden of disease, current evidence-based therapies for BK – including broad-spectrum topical antibiotics and, if indicated, topical corticosteroids – fail to salvage vision in a substantial proportion of affected patients. Amid growing concerns of rapidly diminishing antibiotic utility, there has been renewed interest in urgently needed novel treatments that may improve clinical outcomes on an individual and public health level. Bridging the translational gap in the care of BK requires the identification of new therapeutic targets and rational treatment design, but neither of these aims can be achieved without understanding the complex biological processes that determine how bacterial corneal infections arise, progress, and resolve. In this chapter, we synthesize the current wealth of human and animal experimental data that now inform our understanding of basic BK pathophysiology, in context with modern concepts in ocular immunology and microbiology. By identifying the key molecular determinants of clinical disease, we explore how novel treatments can be developed and translated into routine patient care.
Keywords: Bacterial keratitis, microbial keratitis, corneal infections, immunology, innate immunity, adaptive immunity, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae
INTRODUCTION
Bacterial corneal infections, or bacterial keratitis (BK), are sight-threatening emergencies that can lead to rapid vision loss. In the canonical System of Ophthalmology (1965), Sir Duke-Elder observed that BK caused by Pseudomonas aeruginosa – now regarded among the most common and destructive of corneal pathogens – was “relatively rare”, with only 100 reported cases in the literature at that time (Duke-Elder, 1965). Today, BK ranks among the leading causes of global ocular morbidity (Ung et al., 2019a), with steep upward trends in disease frequency driven by the widescale adoption of hydrogel contact lenses in the late 1960s (Galentine et al., 1984; Golden et al., 1971; Stapleton et al., 2008), and a rapidly growing world population (Bourne et al., 2021). Most treatment approaches to BK involve the collection of corneal cultures followed by broad-spectrum topical antibiotics, with surgical interventions usually reserved to treat complications such as worsening infection and/or stromal thinning and perforation (Jones, 1980; Jones, 1981a; Lin et al., 2019). Though most patients treated with the current standard of care will achieve microbiologic resolution of their infection, clinical outcomes in BK leave much to be desired. In tertiary care settings, a significant proportion (~30%) of patients develop long-term moderate-to-severe monocular vision loss, defined as a best-corrected visual acuity of < 20/60 (McClintic et al., 2014; Prajna et al., 2019), and outcomes in resource-poor settings are even more grim (Arunga et al., 2019a; Burton et al., 2011). Poor vision is commonly attributed to infections that progress despite intensive treatment, and chronic sequelae such as visually-significant stromal scarring, cataract and glaucoma (Lotti and Dart, 1992). Though mostly unilateral in nature, BK is associated with profound and underappreciated human costs, including diminished quality of life (Arunga et al., 2019b; Li et al., 2014; Rose-Nussbaumer et al., 2016), reduced work productivity (O’Brien et al., 2015), and substantial economic losses (Collier et al., 2014; Cope et al., 2018).
Despite a growing burden of disease, therapeutic options for treating BK remain sparse. Broad-spectrum topical antibiotics (Hanet et al., 2012; McDonald et al., 2014) and corticosteroids (Herretes et al., 2014; Srinivasan et al., 2012) are the only evidence-based treatments that have been shown to salvage vision, and antibiotics are fast losing their utility due to the emergence of antimicrobial resistance (AMR) among corneal pathogens (Asbell et al., 2015; Thomas et al., 2019). In clinical medicine, bridging translational gaps in the care of any condition hinges on the identification of new therapeutic targets and rational treatment design. In BK, neither of these aims can be realized without a comprehensive understanding of disease pathophysiology, which can be distilled into three main pillars: (a) mechanisms of natural resistance against disease; (b) the processes by which pathogens breach host defenses to establish infection; and (c) host-pathogen dynamics responsible for the acute and chronic manifestations of disease. Though increasingly sophisticated experimental models of BK incorporating “omics” research have shed unprecedented light on the pathogenesis of these infections, most paradigms of understanding converge on a pathway typical of classic innate immunity (Chidambaram et al., 2017; Karthikeyan et al., 2013). BK invariably begins with altered ocular surface homeostasis – most commonly seen in contact lens wear, trauma, and ocular surface disease – that permits bacterial corneal invasion. The rapid accumulation of bacterial toxins, exoproducts, and cellular debris provokes a florid host inflammatory response orchestrated by the corneal epithelium, stromal keratocytes, resident immune cells, and infiltrating leukocytes dominated by polymorphonuclear cells (PMNs) (Wilhelmus and Dan, 1996). Unfortunately, the activation of the local inflammasome (Martinon et al., 2002) is double-edged; indiscriminate release of reactive oxygen species, lysosomal enzymes, and autolytic proteins may result in bacterial eradication, but their collateral toxicity to the surrounding stroma may lead to permanent structural modifications that impair vision (Kessler et al., 1977b; Steuhl et al., 1987). This clinically-oriented chapter will draw on foundational concepts in ocular immunology and microbiology to dissect the cellular mechanisms that underlie the pathogenesis of BK, and in doing so explore elusive therapies that may offer hope for patients afflicted by this devastating infection.
1. THE OCULAR SURFACE IN HEALTH: INTRINSIC HOST DEFENSES
i. Anatomical protections
Under healthy conditions, the ocular surface is endowed with complex defense mechanisms that are choreographed to prevent prolonged exposure to environmental pathogens, antigens, and allergens (Gipson, 2007; Stern et al., 2004). These mechanisms are crucial in maintaining the integrity of the transparent cornea and its posterior structures, to which even the slightest damage can result in suboptimal visual function. The outermost protections of the ocular surface – including the eyelids, eyelashes, lacrimal system, lipid-secretory glands (of Meibom, Moll, and Zeis), and the conjunctival mucosa – work in concert to trap particles and neutralize foreign antigens. The initiation of the blink reflex irrigates the ocular surface with a renewed tear film as particles are washed into the lacrimal puncta, a process aided by persistent desquamation of conjunctival and corneal epithelial cells (Knop and Knop, 2007). Furthermore, the conjunctival mucosa is endowed with rich swathes of eye-associated lymphoid tissue that modulate immune responses to noxious environmental stimuli (Chodosh et al., 1998). Among many other functions, ocular surface lymphoid tissues are responsible for the production of secretory immunoglobulin A (sIgA) and priming of ocular surface immune cells, the actions of which are finely equilibrated to avoid overexuberant and potentially harmful immune responses to common environmental antigens. Intact ocular surface defenses are remarkably effective in preventing corneal infection; extreme concentrations of P. aeruginosa and S. aureus in the order of 1011 colony forming units (CFU/mL) do not lead to appreciable adhesion, colonization, nor penetration of intact mouse corneas, even when enucleated eyes are placed directly into the inoculum (Alarcon et al., 2011; Augustin et al., 2011; Wan et al., 2018). The importance of functioning ocular surface defenses is underscored by the observation that individual corneal epithelial cells cultured in vitro are highly susceptible to infection by P. aeruginosa (Fleiszig et al., 1995). Except for the rare pathogens that penetrate intact corneal epithelium, such as Neisseria gonorrhea, Listeria monocytogenes, and Shigella spp. (O’Brien, 2005; Perez-Santonja et al., 2009; Tjia et al., 1988), BK is rare in the absence of trauma or conditions that disturb ocular surface homeostasis (Gerke and Magliocco, 1971).
ii. The trilaminar tear film
The precorneal tear film – composed of external lipid, aqueous, and innermost mucus layers – is a biologically dynamic substrate that provides lubrication, nutrition, oxygenation, and protection for the ocular surface (Figure 1A). The intrinsic antimicrobial properties of tears were first described by Scottish physician-microbiologist Alexander Fleming in 1922, who conducted a classic study describing the role of a tear enzyme (named “lysozyme”) that inhibited in vitro growth of Gram-positive cocci (Fleming and Allison, 1922). The study was widely derided at the time of publication because its findings challenged scientific dogma that attributed host defenses exclusively to the role of mechanical protections, including blinking, epithelial shedding, and tear washout (Fleming, 1932; Fleming and Allison, 1922). The tear film is now understood to have a broad spectrum of activity against bacteria (Fleiszig et al., 2003; Kwong et al., 2007; McNamara et al., 2005), viruses (Little et al., 1969; Selinger et al., 1979; Waarts et al., 2005), fungi (Davidson and Kuonen, 2004; Sack et al., 2001), and protozoa (Alizadeh et al., 2001; Carnt and Stapleton, 2016; Leher et al., 1998). Remarkably, modern proteomic analyses have now identified over 1500 proteins in tears (Zhou et al., 2012), which are grouped as either classical (80–90%) and non-classical proteins (10–20%) (McDermott, 2013) (Figure 1B). Classical proteins with known antimicrobial activity include lysozyme (Aho et al., 1996; Fleming, 1922; Fleming, 1932; Lee-Huang et al., 1999; Regan, 1950), lactoferrin (Broekhuyse, 1974; Flanagan and Willcox, 2009; Kijlstra, 1990; Stuchell et al., 1981), lipocalin (Dartt, 2011; Redl, 2000), secretory IgA (sIgA) (Franklin and Remus, 1984; Vinding et al., 1987; Wieczorek et al., 1988), and active complement (Sack et al., 1996; Yamamoto and Allansmith, 1979). Nonclassical proteins include secretory phospholipase A (sPLA2) (Buckland et al., 2000; Qu and Lehrer, 1998; Turner et al., 2007), secretory leukocyte protease inhibitor (SLPI) (Doumas et al., 2005; Hiemstra et al., 1996; Tomee et al., 1997), glycocalyx-forming precorneal mucins (Gipson and Argueso, 2003; Gipson et al., 2014; Hori, 2018), surfactant (Brauer et al., 2007; Ni et al., 2005), antimicrobial peptides (Garreis et al., 2011; Kalmodia et al., 2019; You et al., 2010), and lacritin (McKown et al., 2014). In addition to direct antimicrobial effects, tears also likely increase cellular resistance to bacterial invasion by upregulating host innate defenses at the transcriptional level (Mun et al., 2013; Mun et al., 2011).
FIGURE 1. The ocular surface in health.
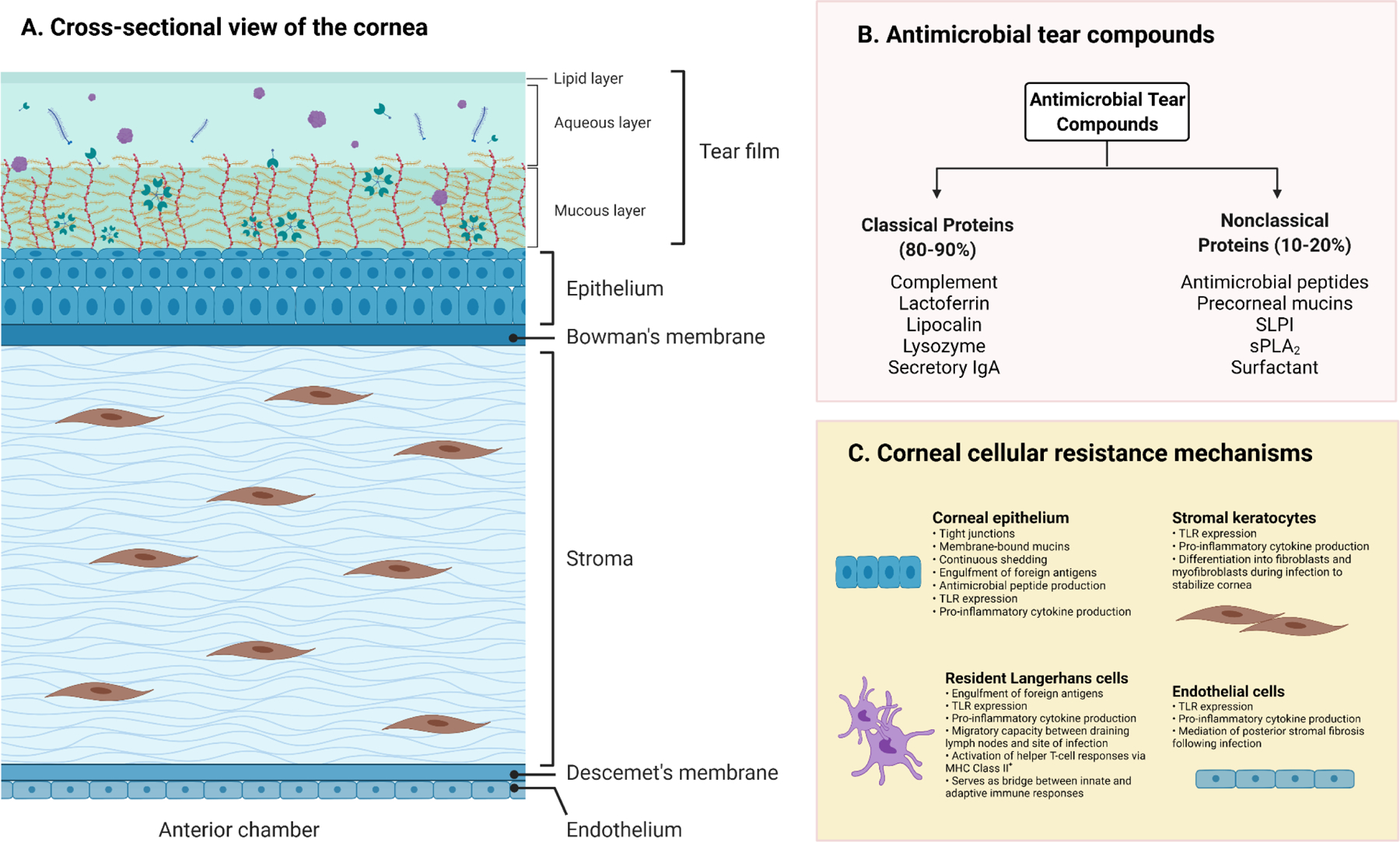
(A) Cross sectional view of the cornea and pre-corneal tear film. The trilaminar tear film consists of outermost lipid, middle aqueous, and innermost mucous layers that trap foreign particles and are highly concentrated with protective antimicrobial compounds. (B) Summary of antimicrobial tear compounds, divided into classical and nonclassical proteins. (C) Summary of major resistance mechanisms against infection in corneal cells. Note that Langerhans cell (LC) populations in the cornea are highly diverse; two major populations include mature LCs that express MHC Class II+ reside in the peripheral corneal epithelium, and another population of MHC Class II− LCs that reside in the central cornea, and which require stimulation (e.g., with PAMPs) to mature. Key – IgA: immunoglobulin A; MHC: major histocompatibility complex; SLPI: secretory leucocyte protease inhibitor; sPLA2: secretory phospholipase A; TLR: toll-like receptor. Created with Biorender.com under a standard academic license.
Moreover, the composition of classical and nonclassical proteins in tears is subject to diurnal changes that are not well-understood (McNamara et al., 2005; Sack et al., 2005). Open-eye environments are rich with lacrimal gland-derived lysozyme, lactoferrin, and lipocalin. In contrast, tears in closed-eye environments are typically concentrated with pro-inflammatory cytokines, reactive oxygen species (ROS), proteases, and complement derived from local PMNS, which are possibly signatures of subclinical inflammatory responses mounted against entrapped organisms during sleep (Sack et al., 1992; Tan et al., 1993; Willcox et al., 1997). Harnessing the naturally-occurring and non-toxic components of tears may therefore present an important innovation in expanding current treatment strategies in BK.
iii. Corneal epithelium
The corneal epithelium boasts an impressive suite of physical protections, including desmosome-dependent tight junctions (Sugrue and Zieske, 1997), membrane-bound mucins (Argüeso et al, 2006; Gipson, 2004; Govindarajan and Gipson, 2010), continuous epithelial sloughing and replacement (Fleiszig, 2006), and the barrier effect of the epithelial basal lamina (Figure 1A and 1C) (Abrams et al., 2000; Alarcon et al., 2009; Forte et al., 2010; Torricelli et al., 2013b). However, the corneal epithelium is also a sentinel participant in initiating host innate immune defenses against foreign antigens. For example, corneal epithelial cells have been shown to secrete local antimicrobial peptides, including human α– and β–defensins, cathelicidin (LL-37), keratin 6A antimicrobial peptides (KAMPs), and surfactant protein-D (Gordon et al., 2005; Hazlett and Wu, 2011; Huang et al., 2007; Kumar et al., 2007; Lee et al., 2016; Mohammed et al., 2017; Ni et al., 2008; Tam et al., 2012). Corneal epithelial cells also have the ability to “phagocytose” or vacuolize invading pathogens (Angus et al., 2008; Fleiszig et al., 1995; Niederkorn et al., 1989).
Corneal epithelial cells also contain a host of recently discovered pattern recognition receptors (PRRs) that are evolutionarily conserved to induce innate immune responses against microbial ligands known as pathogen-associated molecular patterns (PAMPS) (Li et al., 2008; Pearlman et al., 2008; Willcox, 2007) (Figure 2). Well-recognized bacterial PAMPs include virulence mechanisms and/or cellular signatures of invading pathogens, including bacterial DNA, cell wall products (e.g., lipoteichoic acid or LTA, and lipopolysaccharide or LPS, in Gram-positive and negative bacteria, respectively), cell appendages (e.g., pili and flagella), and toxins (Li et al., 2008; Medzhitov, 2001). The most well-studied PRRs within ocular tissues are membrane-bound surface PRRs known as toll-like receptors (TLR) (Deguine and Barton, 2014), which are considered part of the human TLR/IL-1R (TIR) family responsible for upregulating pro-inflammatory pathways following detection of infectious stimuli (Martin and Wesche, 2002) (Table 1). TLRs 1 through 10, with the exception of TLR8, are found in abundance on corneal epithelial cells (Jin et al., 2007; Ueta, 2008; Wu et al., 2007) and resident dendritic cells (Kaisho and Akira, 2001). Though less studied in ocular surface cells, a family of intracytoplasmic PRRs comprised of the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) NOD1 and NOD2, are now also considered important detectors of microbial ligands such as peptidoglycans and LPS (Clarke et al., 2010; Inohara et al., 2001; Strober et al., 2006), and have recently been found on corneal epithelial cells (Oh et al., 2017; Scurrell et al., 2009). PRRs confer a level of specificity once thought to be absent from the innate immune system, and may play a crucial role in the host’s discrimination of self- from non-self-antigens (Chang et al., 2006).
FIGURE 2: The putative role of toll-like receptors (TLRs) in the cornea.
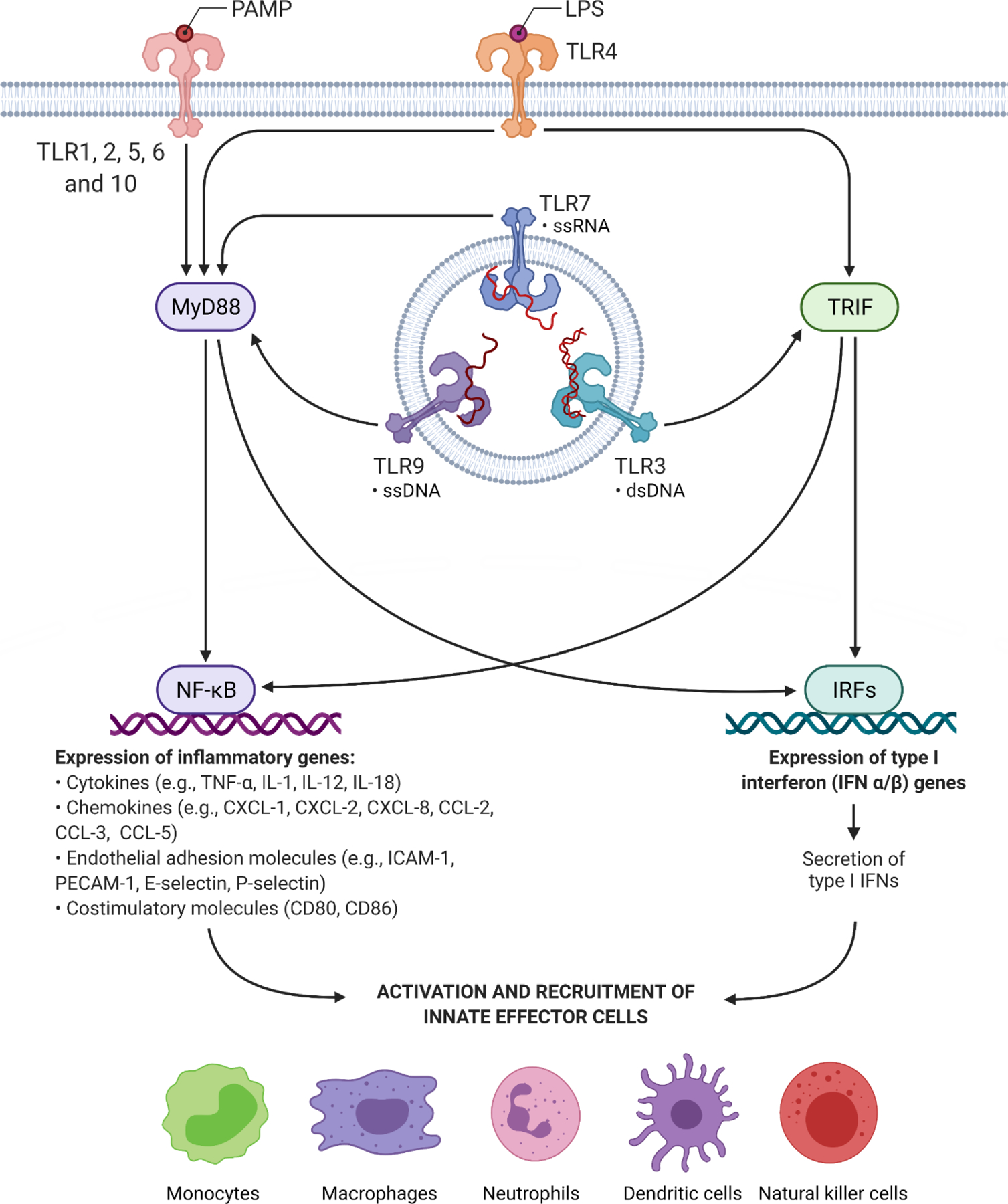
TLRs are evolutionarily conserved membrane-bound pattern recognition receptors (PRRs) that initiate host innate immune responses to pathogen-associated molecular patterns (PAMPs). TLRs are differentially localized within cells, either on the cell surface (TLR1, 2, 4, 5, 6, 10) or within intracellular endosomes (TLR 3, 7, and 9). TLR8 is not known to exist in the cornea. With the exception of TLR3, TLRs activate the myeloid differentiation primary response protein 88 (MyD88), a master adaptor protein that upregulates NF-κB and MAPK-dependent pathways, leading to the expression of pro-inflammatory cytokines, chemokines, adhesion molecules, and co-stimulatory molecules that are essential for the recruitment of innate effector cells. On the other hand, TLR3 (and also TLR4) stimulate alternative adaptor proteins, such as toll-interleukin receptor domain-containing adapter-inducing interferon-β TRIF), leadin to the upre ulation of interferon regulatory factor (IRF) pathways that lead to the production of type I interferons IFN-α and IFN-β While IFNs have been historically associated with viral infections, recent evidence suggests that IFNs are also potent inducers of innate immune effectors in the setting of bacterial infection (Monroe et al., 2010). For further details regarding PAMP recognition among TLRs, refer to Table 1. Created with Biorender.com under a standard academic license.
TABLE 1.
Putative location and ligands of toll-like receptors in the human cornea, based on in vivo animal and in vitro human cell culture studies.
| TLRα | Putative Location in Cornea | Mice or Human? | Putative Location in Cell | Ligands | Activation of NF-kB or TRIF (IFN-3) pathways? | References |
|---|---|---|---|---|---|---|
| 1 | Epithelium Stroma | Both | Surface | LTA and PGN (Gram-positives) LPS (Gram-negatives) |
NF-κB | (Kumar et al., 2004) |
| 2δ | Epithelium Stroma Endothelium | Both | Surface | Fungal zymosan and β-glycan (fungi) LTA and PGN (Gram-positives) LPS (Gram-negatives) Phospholipomannan Envelope glycopeptides (viruses) |
NF-κB | (Adhikary et al., 2008; Choi et al., 2011;Ebihara et al., 2007; Jin et al., 2009;Johnson et al., 2005; Kot et al., 2019; Kumar et al., 2004, 2006a; Tullos et al., 2013; Zhao and Wu, 2008) |
| 3 | Epithelium Stroma | Both | Endosomal | dsRNA | TRIF (IFN-3) | (Kumar et al., 2006b; Liu et al., 2008; Ueta et al., 2005) |
| 4 | Epithelium Stroma Endothelium | Both | Surface | LPS (Gram-negatives) Glycoinositol phospholipids (protozoa) Envelope glycopeptides (viruses) |
TRIF (IFN-3) and NF-kB | (Choi et al., 2011; Jin et al., 2009; Johnson et al., 2005; Kot et al., 2019; Kumagai et al., 2005; Kumar et al., 2006a; Zhao and Wu, 2008) |
| 5 | Epithelium Stroma | Both | Surface | Flagellin | NF-κB | (Hayashi et al., 2001; Zhang et al., 2003) |
| 6 | Epithelium Stroma | Both | Surface | LTA and PGN (Gram-positives) LPS (Gram-negatives) |
NF-κB | (Redfern et al., 2011) |
| 7 | Epithelium Stroma | Both | Endosomal | Viral ssRNA | NF-κB | (Chang et al., 2006; Redfern et al., 2011) |
| 8 | No known corneal presence | Both | Surface | Viral ssRNA | NF-κB | (Heil et al., 2004; Redfern et al., 2011) |
| 9 | Epithelium Stroma Endothelium | Both | Endosomal | Bacterial CpG DNA motifs Viral CpG DNA motifs | NF-κB | (Ebihara et al., 2007; Inoue, 2014; Johnson et al., 2005;Takeda et al., 2011) |
| 10 | Epithelium Stroma | Human | Surface | Unknown† | NF-κB | (Hasan et al., 2005; Redfern et al., 2011) |
Key–dsRNA: double stranded ribonucleic acid; IFN: interferon; LPS: lipopolysaccharide; LTA: lipoteichoic acid; NF-κB: nuclear factor κB; PGN: peptidoglycans; ssRNA: single-stranded ribonucleic acid; TRIF: TIR-domain-containing adapter-inducing interferon-β
In total, 13 TLRs have been discovered among mice and humans; only those found in humans are listed in this table.
TLR10 homodimerizes with TLR1 and TLR2, su estin they may have similar li ands (Hasan et al., 2005).
TLR2 dimerizes with TLR1 to recognize tri-acylated lipoproteins; TLR2 dimerizes with TLR6 to recognize di-acylated lipoproteins (Pearlman et al., 2008).
In corneal epithelial cells, downstream signaling following PRR activation occurs through myeloid differentiation primary response protein 88 (MyD88)-dependent (Deguine and Barton, 2014; Sun et al., 2006; Sun et al., 2010) and MyD88-independent pathways (Takeda et al., 2003) (Figure 2). MyD88 is regarded as the master adaptor protein for most TLRs, with the exception of TLR3, and its activation triggers a potent pro-inflammatory cascade involving transcription factors such as nuclear factor-κB (NF-κB) and the mitogen-associated protein kinases (MAPKs) (Janssens and Beyaert, 2002). During infection, MyD88 activation leads to the massive release of pro-inflammatory chemokines, rapid recruitment of phagocytes into the cornea (Huang et al., 2006; Yu and Hazlett, 2006), increased local antimicrobial peptide production (Gao et al., 2013), and increased corneal epithelial resistance to bacterial adhesion and traversal (Tam et al., 2011). Non-MyD88 pathways involve a host of adaptor proteins including TIR-domain-containing adaptor molecule 1 (TICAM-1, also known as TIR-domain-containing adaptor inducing IFN-β or TRIF, responsible for TLR3/TL4 signaling), and TIR-domain containing adaptor molecule 2 (TICAM-2 or TRAM, responsible for TL4-signaling). Non-MyD88 adaptor proteins have been implicated in the phosphorylation of transcription factors such as interferon regulatory factor 3 (IRF3), leading to the production of the Type I interferons IFN-α and IFN-β (Kumar and Yu, 2006). Beyond the formidable protections provided by the precorneal tear film, the corneal epithelium harbors a vast array of immune protections designed to shield the underlying stroma from harmful environmental insults.
iv. Corneal stroma
The clinical manifestations of most corneal infections arise from acute and chronic inflammatory changes that occur in the stroma, which can lead to long-term structural alterations that cause vision loss. However, it is still commonly believed that the corneal stroma is an immunologically inert tissue, with native cell populations that are unable to mount immune responses to antigenic challenge. Mannis and Smolin, for instance, boldly declared that “immune mechanisms available in the avascular cornea are limited” (Mannis and Smolin, 1996). This misconception may have been in part due to the historical evolution in our understanding of corneal immune privilege, where scientific consensus once viewed the stroma largely as an immunologic bystander, devoid of effector cells including resident leukocyte populations such as antigen presenting cells (APCs) (Streilein, 1987; Streilein et al., 1979). Even when the first discoveries of resident corneal Langerhans cells (LCs) were made in the early 1980s, such populations appeared confined to the peripheral corneal epithelium (Gillette et al., 1982; Rodrigues et al., 1981). This observation lent support to the idea that the activation of immune effector mechanisms in the cornea was the remit of cells that did not necessarily reside within the stroma, even when the stroma was the focus of inflammation. Other persistent sources of confusion include flawed models of corneal infection that, despite the absence of experimental validation, have gained wide acceptance and are still widely taught. For instance, the distinguished ophthalmologist Barrie Jones often referred to the stroma as a passive “immunological blotter”, suggesting that stromal keratocytes did not contribute significantly to the pathogenesis of viral keratitis (Jones, 1958). This hypothesis is now wholly inconsistent with our current body of evidence in infectious ocular diseases (Jonas et al., 2020; Rajaiya et al., 2015).
Efforts to characterize innate and adaptive immune responses in the corneal stroma now reveal a vastly different picture. Stromal keratocytes are nestled in a densely-packed, collagen-laden extracellular matrix (ECM) co-inhabited by bone marrow-derived APCs, macrophages, and monocytes, with occasional transit of other white cells (Brissette-Storkus et al., 2002; Hamrah et al., 2003c; Liu et al., 2002). These cell populations have essential and dynamic roles in normal immune function of the cornea and the preservation of normal vision. Current paradigms in ocular immunology advance the notion that ocular tissues are engaged in a perpetual state of what preeminent ocular immunopathologist Streilein described as a “dangerous compromise” (Streilein, 1987). Streilein proposed that immunoregulatory activities in the eye, particularly ocular surface structures that interface with the external environment, must delicately balance two opposing immunologic needs in order to maintain vision. The first included the need to clear infectious agents, principally via mechanisms that allow for appropriate discrimination of self- and non-self-antigens. The second was the requirement for the intensity of inflammatory responses to be commensurate to the threat posed by the noxious stimulant, in order to minimize the likelihood of those very same responses inflicting blinding structural damage on the host (Streilein, 2003). Indeed, there is now an abundance of experimental data to support the role of the stroma in effecting host immune responses that, under conditions of severe stress, override the competing need to preserve immune privilege. This is the basis on which many inflammatory eye diseases are now understood.
BK (Figure 3) is a classic example of a disease where immune equipoise is tilted dramatically towards an overzealous and protracted inflammatory response launched by the corneal epithelium as described above, resident keratocytes (Cendra et al., 2017; Ebihara et al., 2007; Kumagai et al., 2005; Marino et al., 2015; Nishida, 2010; Wilson, 2020a), and bone-marrow derived resident LCs and macrophages (Matsumoto et al., 2005) (Figure 4). This will be discussed in greater detail in subsequent sections, but in brief the initial activation of PRRs in response to bacterial PAMPs activates inflammatory pathways that lead to the rapid infiltration of PMNs to the site of infection (Girgis et al., 2003; Hume et al., 2001). This is followed by a procession of migratory LCs (Hazlett et al., 2002a; Hazlett et al., 1986), natural killer cells (Lighvani et al., 2005), and CD4+ and CD8+ T-lymphocytes (Hazlett et al., 2000; Kwon and Hazlett, 1997) that enter the cornea. The pro-inflammatory storm responsible for leucocyte recruitment includes a host of cytokines, chemokines, and adhesion molecules with overlapping autocrine-paracrine functions. Pro-inflammatory cytokines that inundate the cornea include tumor necrosis factor-α (TNF-α) (Kernacki et al., 1998a), interleukin-1 (IL-1) (Fukuda et al., 2017; Rudner et al., 2000; Thakur et al., 2004), IL-8 (Jimenez-Martinez et al., 2013; Kernacki et al., 2000; Kumagai et al., 2005; Xue et al., 2003a), IL-12 (Hazlett et al., 2002b), IL-18 (Huang et al., 2002), interferons IFN-β and IFN-γ (Hazlett et al., 2002b; Huang and Hazlett, 2003), and macrophage migration inhibitory factor (MIF) (Thakur et al., 2001). Chemokines include chemokine ligand 1 (CXCL-1, also known as GRO1 or KC) (Gadjeva et al., 2010; Lin et al., 2007), CXCL-2 (Bryant-Hudson and Carr, 2012), CXCL-5 (Bryant-Hudson and Carr, 2012), CCL-2 or monocyte chemoattractant protein 1 (MCP-1) (Kimura et al., 2012; Tran et al., 1996), CCL-3 or macrophage inflammatory protein (MIP-1α) (Kernacki et al., 1998b), and CCL-5, also known as the Regulated upon activation, normal T cell expressed and presumably secreted (RANTES) (Kimura et al., 2012). Prominent adhesion molecules include intracellular adhesion molecule-1 (ICAM-1) (Hobden et al., 1999; Kumagai et al., 2005) and platelet endothelial cell adhesion molecule (PECAM-1) (Khatri et al., 2002). Together, these pro-inflammatory mediators quickly overwhelm anti-inflammatory mediators such as IL-4 (Cole et al., 2007), IL-6 (Hume et al., 2006), and IL-10 (Cole et al., 2003). Furthermore, APC populations in the cornea are now thought to play a critical role in both innate and adaptive arms of the immune response (Hamrah and Dana, 2007). In addition to expressing TLRs so critical in provoking acute inflammatory responses, LCs serve as a bridge to adaptive immune responses by stimulating CD4+ helper T-cell responses that arbitrate the persistence of local PMNs in the cornea during the subacute phase of infection, a finding that has been associated with severe BK phenotypes (e.g., perforation) (Hazlett, 2002). In sum, the wealth of experimental data should lay to rest any lingering doubt as to whether the corneal stroma actively participates in host immune responses. Indeed, understanding how distortions of stromal immunoregulation occur in inflammatory ocular diseases may be the key to understanding the clinical manifestations of conditions such as BK, and help inform therapeutic strategies to prevent chronic stromal remodeling and vision loss.
FIGURE 3. Clinical slit-lamp photography of sight-threatening bacterial keratitis caused by common pathogens.
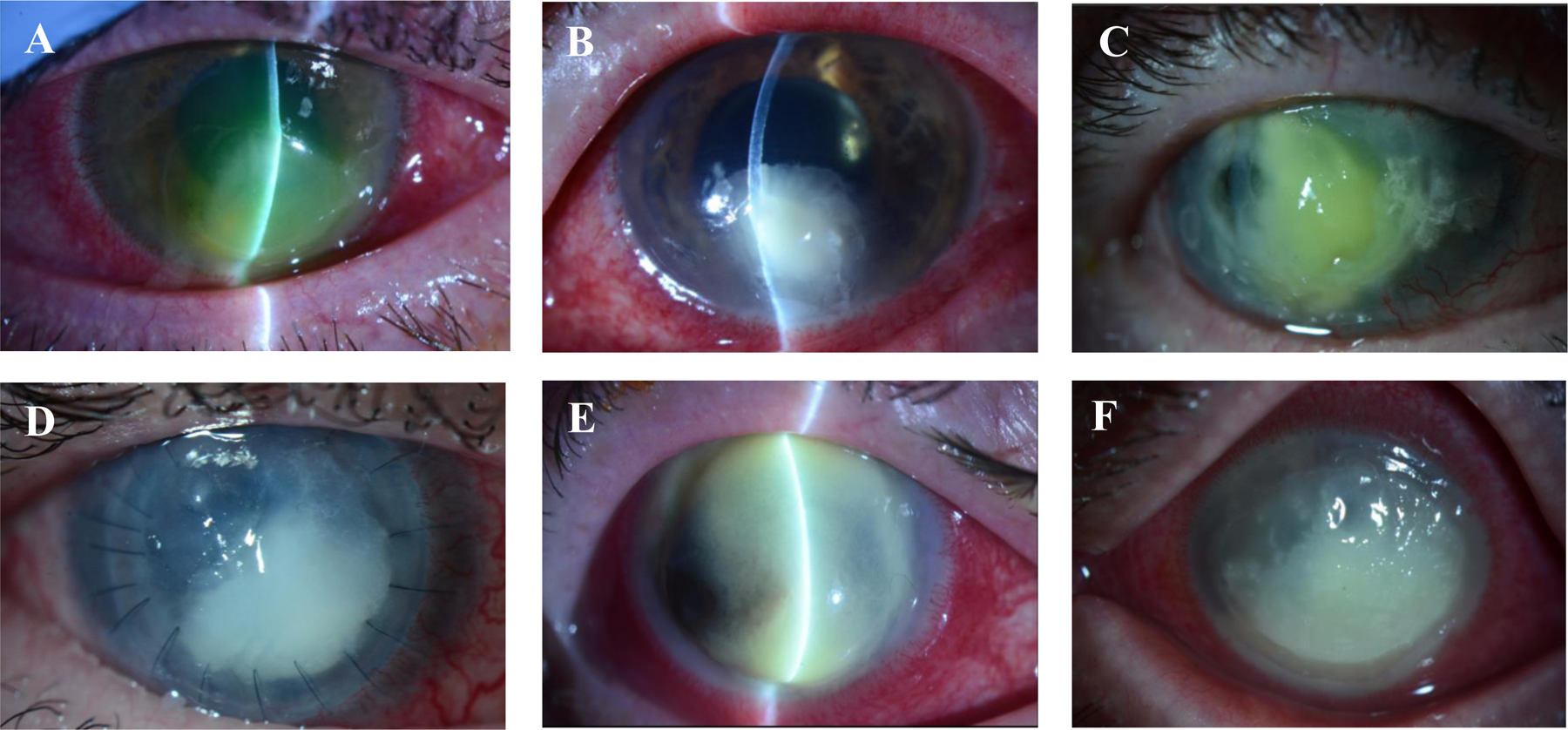
(A) A large, inferior P. aeruginosa corneal ulcer in a soft contact lens user, requiring therapeutic penetrating keratoplasty following perforation; (B) severe methicillin-sensitive S. aureus ulcer arising in the setting of previous herpetic eye disease; (C) large, diffuse, and necrotic S. pneumoniae ulcer arising in an old penetrating keratoplasty, crossing the graft-host junction; (D) M. catarrhalis ulcer causing graft failure in a penetrating keratoplasty; (E) S. marcescens ulcer in a patient with possible underlying herpetic eye disease; and (F) polymicrobial infection (caused by Streptococcus mitis and Enterococcus faecalis).
FIGURE 4: Schematic overview of host innate responses to bacterial corneal pathogens, which involve the loss of corneal immune privilege.
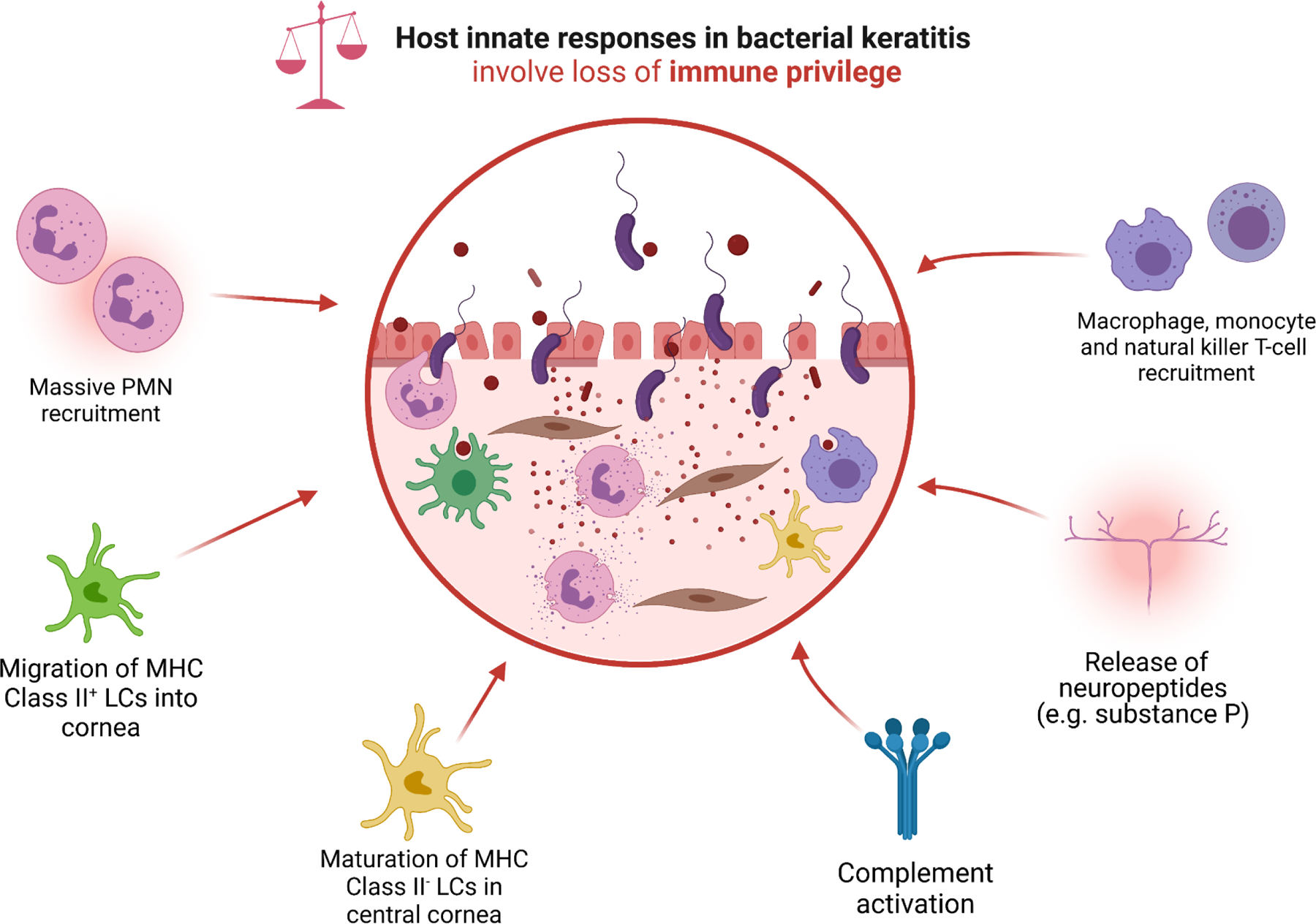
Pattern recognition receptors (PRRs), predominantly toll-like receptors (TLRs), ligate with pathogen-associated molecular patterns (PAMPs) including bacterial lipoteichoic acid and lipopolysaccharides. PRR-binding leads to the upregulation of pro-inflammatory transcription factors such as NF-κB, with subsequent cellular release of pro-inflammatory cytokines (TNF-α, IL-1, IL-6, IL-8, IL12, IL-18, IFNs and MIF), chemokines (CXCL-1, CXCL-2, CXCL-5, CCL-4, CCL-5, IL-18, MCP-1), endothelial adhesion molecules (ICAM-1, MAC-1, PECAM-1, VCAM-1, E-selectin, and P-selectin). Several cell types (e.g., corneal epithelial cells) also inappropriately upregulate the production and release of digestive MMPs, including MMP-1, MMP-2, MMP-3, and MMP-9. This pro-inflammatory storm of cytokines induces massive neutrophil recruitment; migration of MHC Class II+ LCs from the corneal limbus, conjunctiva, and surrounding vascular beds into the stroma; maturation of resident stromal MHC Class II− LCs; recruitment of macrophages and monocytes; activation of complement; and release of neuropeptides such as substance P. The activation of LCs serves as a bridge to the adaptive immune response, which is characterized by recruitment of Th1-predominant helper-T cells that preside over the persistence of PMNs, and are associated with severe disease phenotypes (e.g., corneal perforation).
v. Corneal endothelium
The corneal endothelium is a monolayered sheet of flat squamous epithelial cells that reside on Descemet’s membrane. While most research has focused on the highly specialized role of the corneal endothelium in maintaining stromal hydration and optical transparency through the action of multiple ion pumps (e.g., basolateral Na+/K+-ATPase and Na+/HCO3− transporters), there is emerging evidence that suggests that endothelial cells also play a role in inducing host immune responses to foreign antigens. This should not be surprising, given the range of immune-mediated disorders that affect the endothelium, including endothelial “disciform”) herpetic keratitis, endothelial allograft rejection, and some forms of anterior uveitis. When provoked by antigenic challenge such as viral infection, human corneal endothelial cells are quite capable of releasing pro-inflammatory cytokines including IL-1α, IL-1β, CCL-2 (MCP-1) and CXCL-8 (IL-8) (Miyazaki et al., 2017; Wilson et al., 1994; Yamagami et al., 2003). Not surprisingly, TLRs 2, 3 and 9 that respond to an array of infectious antigens, including LPS, LTA, and CpG motifs on bacterial DNA, have been found on the surface of human corneal endothelial cells (Inoue, 2014; Kot et al., 2019; Takeda et al., 2011). Furthermore, there is now evidence from animal models that injury to Descemet’s membrane (e.g., in corneal perforation) may be associated with fibrosis of the posterior corneal stroma, with the differentiation of keratocytes into fibroblasts and myofibroblasts possibly mediated in part by mediators secreted by the endothelium (Marino et al., 2017; Medeiros et al., 2019). Therefore, although the corneal endothelium has not been widely studied in the setting of BK, it would seem inconsistent with our understanding of corneal immunology for these cells to remain completely submissive to infectious processes. Taken together, the ocular surface and entire cornea harbor a remarkable immune apparatus with many protections that have evolved to conserve vision. The complications of infection, including perforation and chronic stromal modeling, seem to occur when residual inflammation abrogates corneal immune privilege over a protracted period, which under healthy conditions requires tight regulation of corneal homeostasis.
2. THE OCULAR SURFACE IN DISEASE
i. Contact lenses and altered ocular surface homeostasis
In the economically developed world, contact lens wear is the single most important modifiable risk factor in the pathogenesis of BK. Conservative and outdated estimates now suggest that there are at least 140 million contact lens wearers globally (Nichols et al., 2013; Stapleton et al., 2007) with over 45 million in the United States alone (Cope et al., 2016; Konne et al., 2019). Population-based cohort studies estimate that the annual incidence of BK among wearers of daily hydrogel and silicone hydrogel contact lenses ranges from 1.9 to 6.9 and 4.9 to 11.9 cases per 10,000 persons, respectively, with far higher estimates of 9.3 to 96.4 and 18.0 to 25.4 cases per 10,000 persons, respectively, for extended wear lenses (Cheng et al., 1999; Jeng et al., 2010; Morgan et al., 2005; Nilsson and Montan, 1994; Poggio et al., 1989; Schein et al., 1989; Seal et al., 1999; Stapleton et al., 2008). This translates to nearly 1 million annual physician encounters, and 250,000 hours of clinical care due to some form of keratitis in the US, with the majority of these cases likely due to BK (Collier et al., 2014). This scale of contact lens infections is perhaps not surprising, given nearly all wearers (>99%) have been shown in large population surveys to have at least one contact lens risk behavior that predisposes to infection (Cope et al., 2015).
Despite decades of research, the exact mechanisms by which contact lenses predispose to infection are not entirely clear. It is true that the most likely sources of pathogens in BK include bacterial contamination of contact lens cases (Willcox et al., 2010; Wu et al., 2010) and poor hygiene practices such as inadequate disinfection, sharing of contact lenses, topping off storage solutions, and inappropriate overnight wear (Dart et al., 1991; Zimmerman et al., 2017). However, as shown earlier, the finding that large microbial boluses do not cause infection when applied to intact corneas suggests that other factors are necessary to precipitate disease. For this reason, contact lens-related BK has been aptly described as a multifactorial condition, with underlying pathophysiological processes most likely related to changes within the ocular surface microenvironment (Fleiszig et al., 2020; Liesegang, 1997; Robertson and Cavanagh, 2008). Key factors that have been identified include: (a) reduced tear exchange and volume (McNamara et al., 1999; Paugh et al., 2001; Wei et al., 2014); (b) adsorption of tear proteins onto the surface of lenses, thereby limiting their availability and/or activation (Baines et al., 1990; Willcox et al., 2001); (c) decreased shedding of the ocular surface epithelium (Cavanagh et al., 2002; Ladage et al., 2001); (d) promotion of bacterial biofilms, particularly on the posterior lens interface with the apical corneal epithelium (Elder et al., 1995; Slusher et al., 1987; Stapleton and Dart, 1995; Tam et al., 2010); (e) reduced production of important ocular surface antimicrobial peptides in corneal epithelial cells, includin human β-defensin 2 and LL-37 (Maltseva et al., 2007); (f) enhanced pathogen adherence to corneal epithelium (Fleiszig et al., 1992); (g) lipid-raft formations within epithelial cells that internalize bacterial pathogens (Yamamoto et al., 2006a; Yamamoto et al., 2005; Yamamoto et al., 2006b); (h) induction of ocular surface dysbiosis (Sankaridurg et al., 2000; Stapleton et al., 1995b); and (i) low grade ocular surface inflammation (Alghamdi et al., 2020; Gad et al., 2019; Metruccio et al., 2019). Alterations of ocular surface immunity are particular fascinating; for instance, extended contact lens wear has been associated with centripetal migration of LCs into the cornea, which in susceptible C57BL/6 mice has been shown to be involved in activating Th1 CD4+-dependent pathways that are associated with severe stromal thinning and perforation (Hazlett et al., 1999; Kwon and Hazlett, 1997). In short, contact lens wearers are susceptible to infection due to ocular surface changes that result in an unstable tear film, reduced ocular surface turnover, increased opportunity for bacterial adaptation to corneal epithelium, and dysregulation of ocular surface immunity. These factors may help explain the explosive emergence of P. aeruginosa, an opportunistic pathogen that does not otherwise infect the intact ocular surface, as the dominant cause of BK following the introduction of contact lenses over 50 years ago (Mondino et al., 1986; Ormerod and Smith, 1986).
Although significant advances have been made in our understanding of contact lens-related infections, several uncertainties remain. For example, it has been traditionally taught that lens-induced hypoxia is a risk factor for BK (Liesegang, 1997). Early studies of ocular surface physiology, including the landmark Gothenburg study conducted by Holden and colleagues (1985), showed that extended wear conventional hydrogels resulted in thinning of the corneal epithelium and stroma, suppressed aerobic metabolism, and increased bacterial adherence to corneal epithelium (Holden et al., 1985; Ladage et al., 2001). Several animal models have since corroborated these data by showing that lens-induced hypoxia may be associated with impaired corneal epithelial wound healing (Madigan et al., 1987), loss of tight junctions within corneal epithelium (Mauger and Hill, 1992), and attenuated TLR-dependent innate immunity (Hara et al., 2009), which together contribute to the increased likelihood of infection among contact lens wearers (Imayasu et al., 1994; Zaidi et al., 2004; Zhang et al., 2008). However, epidemiologic data suggest that the population-level consequences of contact lens-induced hypoxia may be overstated. Despite high hopes, the introduction of high oxygen-permeable silicone hydrogel contact lenses in the late 1990s has not reduced the incidence of contact lens-related infections (Robertson, 2013; Stapleton et al., 2013), suggesting there are mechanisms by which contact lenses alter ocular homeostasis that are independent of hypoxia-driven pathways. Addressing these persistent uncertainties in the relationship between contact lens and BK will be critical in designing innovations that may reduce risk of infection, including contact lenses that promote tear exchange, reduce biofilm formation, elute antimicrobial compounds, or even have inbuilt means of auto-decontamination (Seggio et al., 2019; Xiao et al., 2018).
ii. Ocular surface disease
A vast range of conditions render the ocular surface susceptible to bacterial infection. The mechanisms by which this occurs include: (a) mechanical and chemical trauma that expose the corneal stroma, e.g., foreign bodies, corneal abrasions, chemical and thermal burns, corneal transplantation and photorefractive procedures (Vajpayee et al., 2007); (b) anatomical abnormalities e.g., lid defects including ectropion, entropion, floppy eyelid syndrome, eyelash misdirection and trichiasis, and exposure keratopathy (Ezra et al., 2005); (c) altered function, activation, volume and/or composition of the tear film, e.g., severe dry eye disease (Baudouin et al., 2016); (d) loss of ocular surface integrity, e.g., conditions confined to the cornea, including bullous keratopathy (Luchs et al., 1997) and neurotrophic ulceration, or conditions involving the entire ocular surface, including atopic keratoconjunctivitis, ocular cicatricial pemphigoid (OCP) and Stevens-Johnson syndrome (SJS); (e) impaired immune function of the cornea, e.g., diabetes mellitus, immunodeficiencies including HIV infection (Jeng et al., 2010), malignancy treated with chemotherapy (Hazlett et al., 1977), bone marrow transplantation (Balaram et al., 2005), graft-versus-host disease (Franklin et al., 1983), severe malnutrition (Keenan and McLeod, 2013), and/or among critically-ill patients (Parkin et al., 1997); (f) medication toxicity, e.g., misuse of topical corticosteroids and anesthetic agents; and (g) microbiome-associated changes, as described in more detail below (Cavuoto et al., 2019; Zilliox et al., 2020).
The mechanisms listed above offer only a simple categorization of how altered ocular surface homeostasis may predispose to infection, and many conditions do not fit neatly into any one grouping. For example, corneal allograft transplantation is associated with surgical incision(s), surgical instrumentation, application of easily-contaminated sutures, and the use of topical corticosteroids that alter ocular surface immunity in the post-surgical period. Complex multifactorial diseases such as OCP and SJS are characterized by chronic cicatricial changes associated with severe anatomical abnormalities (e.g., fornix shortening, symblepharon formation, and chronic ocular surface exposure), tear film instability owing to destruction of lacrimal gland ducts, conjunctival goblet cell loss, and Meibomian gland atrophy, and altered corneal epithelial cell integrity due to persistent corneal epithelial defects, limbal stem cell deficiency, and ocular surface keratinization (Miserocchi et al., 2014; Sotozono et al., 2007). Critically-ill patients who develop exposure keratopathy owing to impaired blink reflexes and common lid abnormalities are prone to corneal epithelial breakdown and subsequent ulceration. Therefore, any patient who is treated for BK requires careful management of the risk factors that predisposed the patient to infection in the first instance.
iii. The ocular surface microbiome
The increasing availability and decreasing cost of next-generation sequencing (NGS) now provide unprecedented opportunities to explore host-microbiome interactions in a multitude of body sites, including the ocular surface (Ung et al., 2020b; Zegans and Van Gelder, 2014). Most studies of the ocular surface to date have sampled the conjunctiva (e.g., with swabbing or with washings), which is now widely regarded as paucimicrobial when compared to microbiome-rich sites such as the gastrointestinal tract (Doan et al., 2016; Ozkan et al., 2018). Although studying the corneal microbiome has been historically more challenging, recent murine models have shown that commensal populations on the corneal surface are small to negligible, and far less diverse than the conjunctiva (Wan et al., 2018). The overall low diversity and volume of the conjunctival and corneal microbiomes may be due to the antimicrobial properties of the tear fluid, as well as MyD88/IL-IR-dependent mechanisms that serve housekeeping immune functions along a healthy ocular surface. Indeed, the ocular surfaces of MyD88/IL-1R knockout mice appear to permit essentially normal growth of commensal organisms. Taken together, these findings have led some researchers to postulate that there is no “core” corneal microbiome under healthy conditions (Fleiszig et al., 2020). While this may be true, however, these observations in themselves do not exclude the possibility of microbial populations – however small and transitory – having some role in the pathogenesis of a broad spectrum of ocular surface diseases, including BK (Dong et al., 2011; Okonkwo et al., 2020).
The challenge for human microbiome studies is to establish functional and clinicopathologic correlations with taxa observed within sequenced samples. In the gastrointestinal tract, for example, reduced commensal abundance and diversity increase susceptibility to disease caused by opportunistic pathogens such as Clostridium difficile (Ng et al., 2013), which can be treated upon reconstitution of the gut microflora (e.g., with fecal transplantation) (Cammarota et al., 2014). While there is no current parallel in ocular surface immunology, there is emerging evidence that suggests that the presence of ocular surface microbiota may be vital to prime innate defense mechanisms against infection. For example, in one model of P. aeruginosa keratitis, germ-free Swiss Webster mice were far more susceptible to infection than mice that had been mono-colonized with coagualase negative staphylococci, and differences in susceptibility were reportedly due to elevations found in the key pro-inflammatory cytokine IL-1β (Kugadas et al., 2016). Similarly, one murine model of S. aureus keratitis showed that the application of an antibody directed against the β-1-6-linked poly-N-acetylglucosamine (PNAG) surface polysaccharide of S. aureus was only effective in treating keratitis among conventional mice with an intact microbiome; germfree C57BL/6 mice could not mount an effective immune response because neutrophils could not be recruited to the site of infection (Zaidi et al., 2014). These studies suggest that the ocular surface microbiota may be an important source of foreign antigens that are involved in conditioning mucosal immunity, and that neutrophil maturation may depend on the presence of inflammatory mediators such as IFN (Kugadas and Gadjeva, 2016) and IL-6 (Kugadas and Gadjeva, 2015). On the other hand, it is not known whether observed changes in microbiome composition among contact lens wearers may predispose to infection simply due to higher densities of contaminating bacteria, or whether altered microbial composition promotes immune dysregulation. Previous studies have observed a shift towards higher proportions of Pseudomonas spp. and Acinetobacter spp. among contact lens wearers, with a concurrent reduction in flora more typical of healthy ocular surfaces, including Staphylococci spp. and Corynebacterium spp. (Shin et al., 2016). The significance of such findings is yet to be determined.
3. CLINICAL EPIDEMIOLOGY OF BACTERIAL KERATITIS
i. Clinicopathologic features of bacterial keratitis
The hallmark features of BK include corneal stromal infiltration(s) with overlying epithelial defect(s), anterior chamber inflammation, and conjunctival injection and chemosis (Figure 3). The patient will often complain of severe pain, decreased vision, and photophobia. These signs and symptoms are the physical manifestations of acute inflammation within anterior segment structures, which is characterized by leucocyte migration, increased vascular dilatation and permeability, and edema. Extravasation of centripetally recruited leucocytes, predominantly PMNs, from the perilimbal and conjunctival vascular beds to the cornea occurs within four hours of the inoculation event, and eventually manifests clinically as stromal infiltration (Hyndiuk, 1981). Exudation of serum proteins and leucocytes into the anterior chamber from engorged and leaky iris vascular tufts is observed as flare and cells, respectively, and with cellular settling due to gravity sometimes forming a hypopyon. Reduced or distorted vision in the acute phase can be attributed to stromal infiltration and surrounding corneal edema, which disturbs the collagenous lamellae and causes scattering of light. Progressive ulceration, stromal thinning, and perforation are the result of progressive release of collagenases, proteinases and other autolytic enzymes from the ensuing inflammatory process. The most severe cases of tissue destruction result in liquefactive tissue necrosis, often seen in mucopurulent infections caused by P. aeruginosa and S. pneumoniae (Figure 3A and C). When assessing patients with suspected BK, it is important to be mindful that by the time stromal infiltrates become clinically visible, the infection is well-established. The incubation period is highly variable across corneal pathogens and between individuals. This means that the absence of a stromal infiltrate does not preclude the possibility of bacterial infection, for instance among contact lens wearers who might present with non-traumatic epithelial defects that often misdiagnosed as a contact lens-associated “corneal abrasion” In some cases, the first sign of BK may be granular elevations of the corneal epithelium, which even in the absence of stromal infiltration should be treated as infected when it is observed in a contact lens wearer (Rosenfeld et al., 1990).
ii. The causes of bacterial keratitis
Virtually any bacteria can colonize and invade the cornea in the setting of ocular surface compromise. Etiological studies suggest that the bacterial pathogens involved in BK are similar worldwide, though such studies must be viewed in light of biases related to differential selection of patients who undergo culture, non-standardized approaches to culture interpretation, and poor overall sensitivity of culture-based microbiology; for a full review, see (Ung et al., 2019b). Nonetheless, dominant organisms include Staphylococcus spp. (particularly S. aureus), Streptococcus spp. (including S. pneumoniae and the viridans group), Pseudomonas spp. (P. aeruginosa is the dominant species), Enterobacteriaceae (including Serratia spp., Klebsiella spp., Citrobacter spp., and Proteus spp.), and Gram-negative cocci (including H. influenzae and M. catarrhalis) (Teweldemedhin et al., 2017). The composition of causative agents varies with geography, climate, humidity and temperature, and the underlying risk factors of the affected population. For instance, S. pneumoniae, Nocardia spp. and P. aeruginosa consistently rank among the top few bacteria isolated from bacterial corneal ulcers in locations such as India, where risk factors include trauma sustained in agricultural settings, use of traditional eye medicines, and over-the-counter availability of topical corticosteroids (Lalitha et al., 2017; Srinivasan et al., 2012). In well-resourced countries where contact lens use is common, P. aeruginosa, S. aureus, Serratia spp. are the most common organisms (Stapleton et al., 1995a; Ung et al., 2020c). Polymicrobial infections are not uncommon and have been reported to account for nearly a quarter of culture-positive cases in some academic medical centers (Jones, 1981b).
Less common etiologies are often, but not always, associated with specific patient risk factors. For example, Bacillus spp., coagulase negative staphylococci (e.g., S. epidermidis), Corynebacterium spp., and Propionibacterium spp., are in healthy persons not usually considered pathogenic. Growth of these organisms in corneal cultures should warrant suspicion for contamination, unless such growth is consistent (e.g., on at least two rows of ‘c’ streaks on solid agar, or if growth is recorded on at least one row of ‘c’ streaks and is accompanied by the appropriate morphology on stain microscopy) (Ung et al., 2020c). However, one should be mindful that even sparse growth of common contaminants can be considered important in the setting of severe immunosuppression, e.g., in cancer patients, poorly-controlled diabetics, patients with cicatrizing ocular surface disease (e.g., OCP and SJS), and in the context of chronic ocular corticosteroid use. Post-laser in-situ keratomileusis (LASIK) corneal infections are typically flap-associated and are among the most common risk factors for nontuberculous mycobacterial (e.g., Mycobacterium chelonae complex) infection (Freitas et al., 2003). Atypical organisms with unusual patterns of antimicrobial susceptibility, characteristic of hospital-associated infections, may be isolated from critically ill and exposure-prone patients receiving ventilatory support or care for major injuries such as burns (O’Brien, 2003). Though history and examination findings are critical to make a provisional diagnosis of BK, definitive identification of the causative agent is ultimately only achieved with the collection of corneal tissue (e.g., corneal scrapings) for microscopy and culture-based microbiology (Dahlgren et al., 2007). The advent of rapid and sensitive molecular diagnostic strategies may in the future provide a more complete picture of the spectrum of bacteria capable of causing corneal infection.
iii. Antimicrobial resistance in bacterial keratitis
The pace at which antimicrobial resistance (AMR) is being acquired among infectious agents, predominantly bacteria, is now a cause of global concern. Though the resistance genes that encode AMR are ancient and predated the antibiotic era D’Costa et al., 2011), bacteria are subject to accelerated selective pressures that have been widely attributed to the indiscriminate use of antibiotics in human and veterinary medicine, as well livestock husbandry and agriculture (Bispo et al., 2020a). AMR is often thought of as a mutation-dependent process, but perhaps even more important is the role of genetic promiscuity within and between bacterial species, which offers diverse paths to obtain novel gene content such as horizontal (lateral) gene transfer, transduction via phage, and conjugation of AMR-encoding plasmids (Tenover, 2006). In ophthalmology, the growing prevalence of multi-drug resistance among important bacterial pathogens that infect the eye has cast doubt on the long-term utility of common antibiotics. It is widely believed that novel resistant strains can arise as a direct result of selective pressures that drive microbial adaptation, and/or by the process of microbial reconstitution, where resistant strains simply replace empty anatomical niches created following a course of broad-spectrum antibiotics. Ocular prostheses and contact lenses that function as a microbial reservoir (e.g., within biofilms) may also have a role in the transfer of resistance genes among ocular flora. However, quantifying the scale of AMR in ophthalmology is difficult, in part because descriptive thresholds to interpret mean inhibitory concentrations (MICs) are still widely determined on the basis of systemic antibiotic administration, rather than the unique pharmacokinetics of topical antibiotics when applied to the ocular surface (Kaye et al., 2010).
Worldwide, the first line of BK treatment is broad-spectrum empiric antibiotics. Unfortunately, targeted therapy is often not possible because corneal cultures are unrevealing in over half of all cases, an internationally consistent finding (Ung et al., 2019b). Without antimicrobial susceptibility testing, clinical care and antimicrobial stewardship practices are severely limited. As a result, concerning trends towards increased community multi-drug resistance among common ocular isolates has been observed in the last thirty years. For instance, the most recent results of the US national Antimicrobial Resistance Monitoring of Ocular Microorganisms (ARMOR) surveillance system found that over 30% of corneal S. aureus and coagulase-negative staphylococci isolates are now resistant to the 8-methoxyfluoroquinolones moxifloxacin and gatifloxacin, which were introduced as topical monotherapies for BK only recently in the early 2000s (Asbell et al., 2020). Astonishingly, the prevalence of resistance to these fourth-generation fluoroquinolones appears to have occurred over a period of little over 10 years, with the first cases reported in 2006–2007 (Jhanji et al., 2007; Moshirfar et al., 2006). Indeed, the time-scale for the emergence of AMR need not be a matter of years: a secondary analysis of the Steroids for Corneal Ulcers Trial (SCUT) showed that patients who had been treated with a fluoroquinolone prior to enrollment had moxifloxacin MICs that were up to 3.48 times higher than those who were fluoroquinolone treatment-naïve (Ray et al., 2013). Taken together, these observations explain the lower than expected commercial success of earlier-generation fluoroquinolones such as ofloxacin and ciprofloxacin, which are no longer considered first line therapies for BK. Fortunately, overall resistance observed among P. aeruginosa and S. pneumoniae for fluoroquinolones, cephalosporins, glycopeptides (e.g., vancomycin) and aminoglycosides (e.g., tobramycin) remain low (<10%) (Asbell et al., 2020), though case reports of multi-drug resistant P. aeruginosa requiring last-resort topical colistin and carbapenems are clearly a worrying development (Chatterjee and Agrawal, 2016; Vazirani et al., 2015). The development of new therapies that may circumvent considerations of AMR characterizes a critical unmet need in the care of all infections, including BK.
4. A UNIFIED FRAMEWORK OF BACTERIAL KERATITIS PATHOGENESIS
i. General principles and models of bacterial keratitis pathogenesis
Fundamentally, the pathophysiology of BK comprises a sequence of host-pathogen interactions that can be summarized as follows: (a) bacterial adherence, colonization, and invasion of host corneal tissue, a process governed by the intrinsic virulence of the pathogen and its ability to evade or counteract host immune defenses (Jones, 1978; Reichert and Stern, 1984); (b) the induction of host inflammatory responses consisting of an initial burst of innate immune activity, typically within minutes to hours, followed by the adaptive immune response that ensues over a period of days, weeks, and months; and (c) the long-term visual impact of corneal remodeling, in large part contingent on the extent of visual axis involvement O’Brien and Hazlett, 1996). There is now a wealth of experimental data from animal models that have utilized varying modes of infection to study the different components of BK pathogenesis. Traditional animal models of BK have typically used infection strategies such as direct stromal contamination after corneal abrasion (“scratch” or scarification models), intrastromal injection, and passage of contaminated sutures through the cornea (Callegan et al., 1992; Hessburg et al., 1963; Kernacki et al., 1999; O’Callaghan et al., 1999). Although such models are useful in the study of host-pathogen dynamics that occur in the stroma, they offer little insight into how the protective mechanisms of the corneal epithelium are breached in the setting of infection. For this reason, approaches that have used more superficial forms of ocular surface manipulation, including tissue blotting (Alarcon et al., 2011), scratch-and-heal (Lee et al., 2003), and the placement of contaminated lenses (Metruccio et al., 2019; Rhem et al., 2000; Szliter et al., 2002; Tam et al., 2010; Zhang et al., 2008) have been helpful in exploring how corneal defenses are compromised with respect to common disturbances of ocular surface homeostasis (e.g., contact lens wear). In this section, we offer a conceptual framework using the aforementioned principles, applying current paradigms in ocular surface immunology and microbiology to understand how corneal infections arise, progress, and resolve. The goal is not to recapitulate the depth of excellent reviews that have been published (Fleiszig et al., 2020; Hazlett, 2004; O’Callaghan, 2018), but to instead offer a parsimonious and hypothesis-generating overview that explicitly links the determinants of BK pathophysiology to its clinical manifestations. Importantly, this may aid in identifying novel therapeutics that may help achieve improved patient outcomes.
ii. Pseudomonas aeruginosa keratitis
P. aeruginosa, of the family Pseudomonadaceae, is an encapsulated Gram-negative rod that is by far the most well-studied of all corneal bacterial pathogens. P. aeruginosa is found ubiquitously in the environment, including soil, water, vegetative matter, sinks, pools, and toilet surfaces. Up to 25% of individuals are thought to be carriers (Berthelot et al., 2001). P. aeruginosa is most frequently described as an opportunistic pathogen because it rarely infects healthy body sites (Stover et al., 2000). Instead, P. aeruginosa is most frequently associated with hospital-acquired infections that arise from contaminated medical devices (e.g., ventilators, peripheral and central vascular lines, and indwelling urinary catheters), dehisced or contaminated wounds (e.g., surgical incisions and diabetic foot ulcers), and within vulnerable immunosuppressed patient populations (e.g., the critically-ill, patients with cystic fibrosis, and those affected by severe burns) (Kerr and Snelling, 2009). Shortly after the commercialization of contact lenses in the 1960s, P. aeruginosa gained notoriety among ophthalmologists as a particularly virulent pathogen capable of causing perforation and blindness within 24 hours of symptom onset (Dixon et al., 1966). It should not be surprising that P. aeruginosa quickly found a niche among contact lens wearers, given the bacteria’s expansive array of virulence factors, broad cellular tropisms, low nutritional requirements, and astonishing versatility in forming tenacious biofilms on organic and inorganic surfaces. It has been estimated that only ~50 CFUs of P. aeruginosa are required to infect the cornea (Kupferman and Leibowitz, 1979).
Each P. aeruginosa bacterium consists of a large genome (~6Mbp) (Stover et al., 2000), with a cell surface composed of lipopolysaccharide (LPS), hair-like pili (fimbriae), alginate, type III secretion systems (T3SS), and a single motile flagellum (Argueso, 2021; Fletcher et al., 1993; Gupta et al., 1994; Hauser, 2009; Lyczak et al., 2000; Zaidi and Pier, 2008). Models of P. aeruginosa keratitis have shown that disease does not occur without areas of denuded stroma (Ramphal et al., 1981; Stern et al., 1985); superficial fluorescein-staining epithelial injuries allow for bacterial adhesion, but such injuries are insufficient to allow for bacterial translocation through the epithelium (Alarcon et al., 2011). In the presence of a compromised ocular surface and exposed stroma, however, scratch-injury or scarification animal models demonstrate appreciable infiltration of P. aeruginosa into the corneal stroma within minutes (Dart and Seal, 1988; Tazawa, 1990). Given the opportunity, ocular strains of P. aeruginosa express highly sophisticated virulence factors to overcome the external defenses of the ocular surface. Adherence and subsequent entry into corneal epithelial cells are mediated by factors including: (a) attachment of LPS to receptors such as the cystic fibrosis transmembrane-conductance regulator (CFTR) (Zaidi et al., 1999), galectin (Gupta et al., 1997), and galactose- or mannose-containing glycoproteins (Hazlett et al., 1987); (b) pili (fimbriae), which are hairy processes that attach to a variety of receptors, including sialic acid residues (Gupta et al., 1994; Rudner et al., 1992); (c) CFTR-mediated lipid rafts (Zaidi et al., 2008), and (d) the production of a polysaccharide-rich biofilm (or glycocalyx) that shields microcolonies from the hostile tear film and local opsonophagocytosis (Hyndiuk, 1981; Saraswathi and Beuerman, 2015; Thanabalasuriar et al., 2019) (Table 2).
TABLE 2.
Summary of key epidemiologic and virulence factors for ocular strains of P. aeruginosa, S. aureus, and S pneumoniae.
| Pathogen | P. aeruginosa | S. aureus | S. pneumoniae |
|---|---|---|---|
| Key epidemiologic features |
|
|
|
| Minimum infectious dose in cornea (CFUs) | ~50 | ~100 | Unknown |
| Cell surface features | Lipopolysaccharide (LPS) Pili (fimbriae) Flagella Alginate Glycocalyx (biofilm)-forming peptides Type III secretion systems |
Lipoprotein Lipoteichoic acid (LTA) MSCRAMMs including collagen, elastin, fibronectin, fibrinogen, and laminin-binding proteins, as well as clumping factor Peptidoglycans Protein A |
Lipoteichoic acid (LTA) Peptidoglycans Polysaccharide capsule Pneumococcal adherence and virulence factors A and B (PavA and PavB) Pneumococcal choline-binding protein Pneumococcal surface antigen A (PsaA) Pneumococcal surface protein A (PspA) Pneumococcal surface protein C (PspC) Protein A |
| Secreted virulence factors | Exotoxin A Hemolysins Large exoprotease Leucocidin Metalloproteinases (Las A protease, Las B protease, alkaline protease, modified protease) Phospholipases Protease IV P. aeruginosa small protease (PASP) |
α-toxin Catalase Coagulase DNAse Hemolysins (α-, β-, δ-, and γ- toxins) Hyaluronidase Leucocidin Staphylokinase Superantigens (enterotoxins A – D) |
Autolysin Pneumolysin Reactive oxygen species (e.g., hydrogen peroxide) Secretory IgA protease |
| Antimicrobial resistance in ocular strains (in the US) | Low; <10% of ocular isolates resistant to fluoroquinolones and aminoglycosides (tobramycin). However, multi-drug resistant strains requiring last-line carbapenems and colistin are becoming of increasing concern. | High and rapidly accumulating:
|
Low; <10% of ocular isolates resistant to fluoroquinolones. |
T3SS are considered among the most important virulence mechanisms for Gram-negative pathogens, and can be likened to a series of fine molecular needles that allow invading bacteria to inject effector proteins and/or toxins into host cells (Czechowska et al., 2014; Kroken et al., 2018). Ocular strains of P. aeruginosa are now commonly identified as cytotoxic, invasive, or neither, on the basis of their T3SS (Evans et al., 2007; Fleiszig et al., 1996; Fleiszig et al., 1994; Lomholt et al., 2001). Cytotoxic isolates, which swarm around host cells but do not invade, encode the exoenzyme ExoU, a deployable intracellular phospholipase that induces rapid death of target corneal epithelial cells and leucocytes (Fleiszig et al., 1996; Fleiszig et al., 1997; Sato and Frank, 2004). By contrast, invasive isolates are internalized by host cells, and encode the exoenzyme ExoS, a dual-function N-terminal GTPase activating protein and C-terminal Ras-ADP-ribosyltransferase (ADPR). The putative role of ExoS is to subvert the innate immune response by inducing apoptosis in infiltrating PMNs (Karthikeyan et al., 2013), reduce the production of reactive oxygen species among those that survive (Vareechon et al., 2017), and allow bacteria to resist vacuolar acidification (Heimer et al., 2013). Two other T3SS effector proteins, ExoT and ExoY, have been less studied, but may be involved in the disruption of the actin cytoskeleton of host cells (Cowell et al., 2005; Garrity-Ryan et al., 2000) and induction of immune cell apoptosis through the action of ADPR (Sun et al., 2012). The genomic epidemiology of ocular P. aeruginosa suggests that multiple population structures are highly adapted to the corneal epithelium. For example, ExoU-containing cytotoxic isolates appear to be more common among contact lens-users (Lomholt et al., 2001; Stewart et al., 2011; Winstanley et al., 2005), while ExoS-containing invasive isolates predominate in lower-income settings where contact lens use is less frequent (Borkar et al., 2014; Borkar et al., 2013).
P. aeruginosa T3SS are augmented by powerful exotoxins such as exotoxin A, phospholipases, hemolysins, which have direct cytopathic effects and may also activate endogenous corneal proteases (Johnson and Allen, 1978; Twining et al., 1993). Furthermore, ocular P. aeruginosa strains express a wide array of proteases, including the metalloproteinases (Las A protease, las B protease, alkaline protease, and modified protease), protease IV, large exoprotease (Lep A), and P. aeruginosa small protease (PASP) (Heck et al., 1986; Iglewski et al., 1977; Kessler et al., 1977a; Marquart et al., 2005; O’Callaghan et al., 2019; Twining et al, 1986; Twining et al, 1993). Collectively, and perhaps synergistically, these products are involved in either digesting the components of the corneal ECM (composed of collagen, laminin, proteoglycans), and/or inactivating or degrading important host immune enzymes found within the local tear film and cornea. As such, P. aeruginosa has the capacity to subvert host responses by reducing the impact of important proteins and mediators, including IgA and IgG (Engel et al., 1998), pro-inflammatory interleukins (McClellan et al., 2006), complement (e.g., C1q, C2, C3) (Hong and Ghebrehiwet, 1992; Laarman et al., 2012), and IFN-γ (Horvat and Parmely, 1988). Remarkably, the expression of P. aeruginosa virulence factors may be dictated by the phenomenon of quorum sensing, where gene transcription is dictated by local bacterial population densities and composition (Pesci et al., 1997; Whiteley et al., 1999; Willcox et al., 2008). Quorum sensing has been hypothesized to constitute a form of immune escape, where bacteria evade host immune responses until they reach a critical population density needed to begin actively invading nearby tissues.
iii. Staphylococcus aureus keratitis
S. aureus, of the family Staphylococcaceae, is a ubiquitous Gram-positive coccus consistently identified as a leading cause of corneal infections. One of the most impressive, albeit concerning, features of S. aureus is the speed at which some ocular strains appear to acquire novel mechanisms of antimicrobial resistance, easily outpacing P. aeruginosa and S. pneumoniae. For example, approximately 30% of all corneal S. aureus (and coagulase negative staphylococci) isolates in the US are now resistant to all fluoroquinolones, including fourth-generation moxifloxacin and gatifloxacin (Asbell et al., 2020). This percentage exceeds 80% among S. aureus isolates that are already methicillin-resistant, an observation that forms the rationale behind the preference for fortified glycopeptides (e.g., vancomycin) to provide Gram-positive coverage in empiric treatment regimens. At our institution, MRSA accounts for roughly 5% of all culture-positive cases, most of which are considered community rather than hospital-acquired (Ung et al., 2020c), with nearly all isolates in our center are resistant to all fluoroquinolones (Bispo et al., 2020b). S. aureus corneal infections are commonly associated with trauma, ophthalmic surgery (including contaminated sutures and ocular prostheses), and contact lens use. Unlike P. aeruginosa, however, S. aureus also commonly colonizes structures adjacent to or in close proximity to the cornea, including the conjunctival mucosa, eyelids and surrounding skin, and in the nasal epithelium. For this reason, it is generally thought that S. aureus ocular infections among non-contact lens wearers arise from these endogenous sources, which also predisposes to recurrent infection (Somerville et al., 2020). Unsurprisingly, the minimum infectious dose of S. aureus in the cornea in the order of only 100 CFUs, similar to figures reported for P. aeruginosa (Kupferman and Leibowitz, 1976).
The universal success of S. aureus as a pathogen is in large part due to redundancy in its broad array of virulence factors. S. aureus contains a heterogenous collection of cell surface adhesins that facilitate attachment to the ocular surface epithelium and surrounding extracellular matrix, including collagen, elastin, fibronectin, fibrinogen, and laminin-binding proteins (Foster and Hook, 1998; Liesegang, 2005). These proteins are collectively known as Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMMs) (Foster and Hook, 1998; Patti et al., 1994), which are essential for biofilm formation. Colonization and subsequent invasion into the corneal epithelium are assisted by the expression of virulence factors that are mostly governed by the quorum-sensing accessory gene regulator (agr) (Novick, 2003; Peng et al., 1988; Traber et al., 2008). Important virulence factors include: (a) anti-phagocytic capsular polysaccharides (Que and Moreillon, 2010); (b) pore-forming cytotoxins including the α– , β–, δ–, and γ– toxins (hemolysins) (Callegan et al., 1994; Girgis et al., 2005; O’Callaghan et al., 1997; Putra et al., 2019); (c) secreted polypeptides (superantigens) such as enterotoxins A – D that may be involved in anesthetizing innate immune responses during early infection (Fraser and Proft, 2008; Salgado-Pabon et al., 2013); and (d) release of a host of extracellular enzymes, including coagulase, hyaluronidase, DNAse, staphopain, and other proteases (Cheung et al., 2002; Hume et al., 2020; Jett and Gilmore, 2002a, b; Moreau et al., 1997; Novick, 2003).
The most important extracellular enzymes produced by S. aureus include those that neutralize host antimicrobial compounds, assist abscess formation, and degrade ECM. For example, the enzyme catalase inhibits PMN-derived ROS; coagulase degrades fibrinogen into fibrin, allowing for abscess capsule formation; and hyaluronidase decomposes endogenous corneal glycosaminoglycans, thereby allowing S. aureus to establish microplanes for bacterial spread. One curious feature of the S. aureus virulon is that the timing of virulence factor expression is largely dependent on the phase in which the bacteria are growing (Ziebandt et al., 2004). For instance, fibronectin-binding and clumping factor proteins that assist in bacterial adhesion and possibly invasion are only expressed during the exponential phase of growth (McGavin et al., 1997), suggesting a critical CFU population size or density must be reached prior to bacterial attachment and colonization. However, such growth may also be facilitated by the formation of protective biofilms that anchor bacterial colonies on the ocular surface through MSCRAMM proteins such as protein A and fibronectin-binding protein A (Hall et al., 2003; Hou et al., 2012; Walsh et al., 2008).
iv. Streptococcus pneumoniae keratitis
Though considered the most common cause of BK in many low-income countries (Bharathi et al., 2002), S. pneumoniae (or the pneumococcus), of the family Streptococcaceae, is perhaps the most poorly understood of the major corneal bacterial pathogens. A Gram-positive facultative anaerobe visualized as single cocci, lance-shaped diplococci, or as short chains, S. pneumoniae colonizes the nasopharynx in over 60% of children from infancy, and remains part of the normal respiratory flora into adulthood, albeit at a lower frequency (Faden et al., 1997; Ung et al., 2020a; Weiser et al., 2018). There are now over 100 serotypes of S. pneumoniae that have been identified, grouped according to surface proteins found on the famed anionic polysaccharide capsule. The pneumococcal polysaccharide capsule is the most well-studied of all its virulence factors, and is thought to camouflage the bacterium from complement-mediated opsonophagocytosis, electrostatically repel leukocytes, reduce attachment of host immunoglobulins, and facilitate extracellular proliferation (Geno et al., 2015; Hyams et al., 2010). Distinct from rogue unencapsulated S. pneumoniae clades that cause outbreaks of epidemic conjunctivitis (Crum et al., 2004; Valentino et al., 2014), it is believed that most strains associated with BK are encapsulated and from classically invasive pneumococcal serotypes (Bispo P., personal communication) (Norcross et al., 2010). However, the exact composition of encapsulated variants among ocular strains of S. pneumoniae are not known because typing is rarely pursued in a clinical setting, and therefore has not been subject to systematic study. Encapsulated and unencapsulated strains both induce florid keratitis in animal models (Guzek et al., 1998; Moore et al., 2009; Reed et al., 2005), suggesting that there are mechanisms that allow the pneumococcus to infect the cornea independent of capsular virulence factors.
The S. pneumoniae virulon is replete with adhesins that facilitate attachment to the corneal epithelium, including cell wall products (e.g., pneumococcal surface antigen A, PsaA), cell-surface proteins that bind to choline residues (e.g., pneumococcal surface proteins A and C, PspA and PspC, and pneumococcal choline-binding protein) and also peptides that bind to ECM with high affinity (e.g., the multidomain pneumococcal adherence and virulence factors A and B, PavA and PavB) (Crain et al., 1990; Hammerschmidt, 2006; Holmes et al., 2001; Jensch et al., 2010; Jinno et al., 2020; Paterson and Orihuela, 2010). Although S. pneumoniae is regarded largely as an extracellular pathogen, it expresses several adhesins that can mediate internalization of bacterium into corneal epithelial cells. For example, the choline-binding protein PspC is thought to be involved in binding to polymeric immunoglobulin receptors on epithelial cells, allowing for subsequent endocytosis into the host cell cytoplasm (Brock et al., 2002; Zhang et al., 2000). S. pneumoniae, unlike P. aeruginosa and S. aureus, is not known to be a prolific toxin-producer. However, the main toxin in its repertoire, pneumolysin (formerly α-hemolysin), is highly conserved among invasive isolates (Karthikeyan et al., 2013), and exhibits direct cytotoxic effects on corneal epithelium by inducing pore formation in cholesterol-containing cell membranes (Johnson et al., 1990; Taylor et al., 2013). Pneumolysin is highly immunogenic, and causes severe inflammation by activating inflammatory pathways that are mediated by classical complement, IL-1, IL-8, and TNF-α (Lawrence et al., 2015; Marquart et al., 2007). This may explain why some pneumococcal ulcers undergo rapid liquefactive necrosis in a large proportion of cases, even among patients who are promptly treated (Figure 3C).
v. Induction of the host innate response
Evidently, bacteria have evolved many mechanisms to evade ocular surface defenses and establish infection in compromised corneas. Nonetheless, the host innate response to infection in BK is understood to be remarkably uniform (Wilhelmus and Dan, 1996). Murine models have shown that PMN-depleted mice treated with a corneal intrastromal bolus of P. aeruginosa succumb to sepsis within 48 hours (Hazlett et al., 1977), demonstrating that the innate host response is essential for survival. In the setting of infection, the pro-inflammatory cascade in BK is less an orderly sequence of events than a storm of concomitant cytokine production and release, inflammatory cell infiltration into the cornea and surrounding ocular surface tissues, and intersecting autocrine feedback loops that potentiate the initial host response. To synthesize the current literature, the key drivers of the innate immune system in the setting of corneal infection include:
Rapid recognition of bacterial ligands (PAMPs) by PRRs, including TLRs and NLRs, which are found on or within corneal epithelial cells, resident LCs, macrophages, and stromal keratocytes (Cendra et al., 2017; Chinnery et al., 2008; Ebihara et al., 2007; Kumagai et al., 2005; Marino et al., 2015; Matsumoto et al., 2005; Nishida, 2010; Redfern et al., 2011; Wilson, 2020a) (Figure 2 and Table 1). The most important TLRs that have been implicated in Gram-positive BK include TLR1 and TLR2, which recognize cell surface proteins such as lipoteichoic acids and peptidoglycans (Kumar et al., 2004; Tullos et al., 2013), while important TLRs in Gram-negative BK include TLR4 and TLR5 for the recognition of LPS and flagellin, respectively (Hayashi et al., 2001; Kumagai et al., 2005; Zhang et al., 2003). TLR9 is involved in the recognition of bacterial CpG DNA (Ebihara et al., 2007; Johnson et al., 2005). For further details, see Table 1.
TLRs 1–9, with the exception of TLR3, activate MyD88-dependent pathways (Deguine and Barton, 2014; Johnson et al., 2005; Sun et al., 2006; Sun et al., 2010). TLR3 and also TLR-4 activate MyD88-independent pathways through alternative adaptor proteins such as TRIF (TICAM-1) and TICAM-2 (Kumar and Yu, 2006; Takeda et al., 2003) (Figure 2 and Table 1).
MyD88-dependent pathways lead to the upregulation of transcription factors NF-κB and MAPK (in particular, p42/p44, JNK and p38) (Adhikary et al., 2008; Zhang et al., 2005), which mediate the release of pro-inflammatory cytokines (e.g., TNF-α, IL-1, IL-2, IL-6, IL-8, IL-12) (Jimenez-Martinez et al., 2013; Kernacki et al., 1998a; Kumagai et al., 2005; Rudner et al., 2000; Thakur et al., 2004), pro-inflammatory chemokines (CCL-3, CCL-4, CCL-5, CXCL-1, IL-18 and MCP-1) (Gadjeva et al., 2010; Kernacki et al., 1998b; Kimura et al., 2012; Lin et al., 2007), adhesion molecules (ICAM-1, MAC-1, PECAM-1, VCAM-1, E-selectin and P-selectin) (Goldberg et al., 1994; Hazlett, 2007; Hobden et al., 1995; Khatri et al., 2002; Philipp and Gottinger, 1993), immune peptides (hβD-2, LL-37, KAMPs) (Chan et al., 2017; Maltseva et al., 2007; McDermott et al., 2003), and MMPs (e.g., MMP-1, MMP-2, MMP-3 and MMP-9) (Li et al., 2001; Sakimoto et al., 2003).
Non-MyD88-dependent pathways lead to activation of the adaptor protein TRIF, which upregulates the production of Type I IFNs, first IFN-β and subsequently IFN-α (Kumar and Yu, 2006; Pearlman et al., 2008). Though IFNs have been historically associated with viral immune responses, they are now understood to play an important role in innate immune cell chemotaxis in bacterial infections (Decker et al., 2005).
Activation of the corneal neuroimmune axis results in the release of inflammatory neuropeptides, including tachykinins such as Substance P, from corneal nerves and infiltrating macrophages, dendritic cells, and lymphocytes (McClellan et al., 2008; O’Connor et al., 2004). Substance P is anti-apoptotic among leukocytes (Zhou et al., 2008), and may regulate the production of IFNs that are important in effecting adaptive T-cell responses (Lighvani et al., 2005).
Activation of host complement pathways, including C3-mediated activation of classic, lectin, and alternative cascades, which are essential in mobilizing PMNs into the site of infection and in enhancing the efficiency of opsonophagocytosis (Cleveland et al., 1983; Hazlett and Berk, 1984; Osthoff et al., 2014).
Migration of ocular surface MHC Class II+ LCs from the conjunctival mucosa and corneal limbus to the site of infection (Yamagami et al., 2005), and concomitant maturation of central corneal MHC Class II− LCs with exposure to microbial ligands (Chang et al., 2006; Hendricks et al., 1992; Kaisho and Akira, 2001; Kaisho et al., 2001).
Pro-inflammatory mediators that flood the ocular surface in BK lead to pronounced ocular surface vascular dilatation, increased endothelial permeability, and sequestration of immune cells, serum proteins, and pro-inflammatory cytokines in the cornea. It has long been appreciated that neutrophils are the most abundant acute-phase cellular effectors, although monocytes and macrophages also play important supporting role. In addition to releasing inflammatory cytokines, PMNs create a highly acidic corneal phagolysosome, engulfing foreign antigens, degranulating lysosomal enzymes, and producing nitric oxides. Furthermore, PMNs undergo oxidative burst to liberate reactive oxygen species (ROS), such as superoxide anions and hypochlorous acids, that are directly toxic to bacteria (Ando et al., 1990; Hazlett, 2004). More recently, neutrophils responding to various PAMPs have also been associated with the formation of neutrophil extracellular traps (NETs), which consist of chromatin-protease-antimicrobial peptide complexes that ensnare bacteria to prevent their further spread into the cornea (Geddes-McAlister et al., 2019; Papayannopoulos et al., 2010; Thanabalasuriar et al., 2019). Although these mechanisms of first defense are critical in the clearance of invading bacteria, unfortunately PMN products are also highly toxic to the corneal stroma, digesting collagen lamellae and a corneal ground substance laden with proteoglycans, laminins, and elastin. This damage is amplified by the inappropriate upregulation and release of autodigestive proteins among host corneal cells, including MMPs such as MMP-2 (a gelatinase) and MMP-9 (a proteinase) (Li et al., 2001; Sakimoto et al., 2003). Therefore, while the PMN-predominant innate response is clearly vital in achieving bacterial eradication, it is the persistent dysregulation of innate responses – in essence a prolonged deviation of corneal “immune privilege” – that are in part responsible for the acute manifestations of BK.
vi. Induction of the adaptive immune system
While most models of BK have traditionally focused on the role of innate immunity, it has become increasingly clear that functional adaptive immune responses serve a critically important role in the pathogenesis, clinical manifestations, and outcomes of BK, particularly for severe disease phenotypes characterized by progressive stromal thinning, melt, and perforation (Hazlett, 2002). Clonal expansion of antigen-specific T and B-lymphocytes are the hallmark of the adaptive immune response, which is designed to purge residual microbes and their associated antigens following the early phase of infection. Although the cornea was once believed to be devoid of resident immune cells, it is now widely recognized that the cornea is home to diverse populations of bone-marrow derived APCs, mostly LCs, that straddle the innate and acquired arms of the immune system. LCs have a prominent role in the pathogenesis of a broad range of ocular inflammatory disorders, with a variety of characteristics and putative functions including the ability to internalize apoptotic or dead microbes and their associated antigens, the expression of TLRs that respond to PAMPs, the production of soluble pro-inflammatory cytokines including IFNs, TNF-α and IL-1β, and the ability to migrate to and from local draining lymph nodes for antigen presentation and priming of naïve lymphocytes (Hamrah et al., 2003a; Kelsall et al., 2002; Rescigno and Borrow, 2001).
Corneal LCs are characterized by differential expression of a number of important cell surface markers associated with varying stages of cell maturation, including major histocompatibility complex (MHC) and the B7 family of co-stimulatory molecules. For example, the corneal limbus is inhabited by mature or “professional” LCs that constitutively express MHC Class II+ (Gillette et al., 1982; Hazlett et al., 1999; Niederkorn et al., 1989), while central regions of the cornea are home to universally immature MHC Class II− LCs that do not express canonical costimulatory molecules such as CD80, and CD86 (Dana, 2004; Hamrah et al., 2003b; Hamrah et al., 2003c). It is well established that inflammatory stimuli prompt centripetal migration of peripheral MHC Class II+ LCs into the central corneal epithelium, thereby initiating T-cell-mediated inflammation that predominates in the subacute phase of infection (Dana et al., 1998; Dekaris et al., 1999; Hamrah et al., 2002; Hazlett et al., 2002a; Jager et al., 1995; Yamagami et al., 2005). For example, MHC Class II+ LCs migrate into the central cornea within 24 hours following challenge with heat-killed S. aureus, and somewhat astonishingly, persist within the cornea for up to 6 weeks (Niederkorn et al., 1989). On the other hand, the maturation of MHC Class II− LCs in the central cornea is thought to occur upon exposure to a broad range of stimuli, including ligation of TLRs with a range of exogenous (e.g., PAMPs such as LPS and LTA) (Hertz et al., 2001; Kaisho and Akira, 2001; Lopez et al., 2004), and endogenous products (e.g., antimicrobial peptides such as β-defensin-2) (Biragyn et al., 2002).
The spatial distribution of main effector cells of the acquired immune system remain far from clear in the setting of BK. However, Hazlett and colleagues have shown in C57BL/6 (perforation-susceptible) and BALB/c (perforation-resistant) inbred mouse models of P. aeruginosa keratitis that Th1 CD4+ T-lymphocytes, possibly sourced from the conjunctiva and local draining lymph nodes, infiltrate the cornea at 5 days post-infection (Kernacki et al., 2001). Th1 CD4+ chemotaxis to the cornea is possibly regulated by lymphocyte chemoattractant MIP-1α (Kernacki et al., 2001) and downstream TLR signals (Biragyn et al., 2002). Once present, Th1 CD4+ cells mediate the persistence of local PMNs within the corneal stroma (Kwon and Hazlett, 1997). The continued recruitment of PMNs to the cornea in BK – even if the pathogen has been eradicated – is hypothesized to lead to progressive stromal lysis in the acute setting, and contribute to corneal scarring over the longer term. Indeed, continued PMN recruitment in C57BL/6 mice appears to be dependent on LC–Th1 CD4+ interactions that are stimulated with B7/CD28 ligation, and are sustained by upregulated pro-inflammatory mediators such as IL-12 and IFN-γ (Hazlett, 2004; Hazlett et al., 2001). On the other hand, infection in IL-18 dependent and Th2-responsive BALB/c has been associated with a dampening of the inflammatory response, more efficient clearance of PMNs, and therefore decreased severity of infection (Hazlett et al., 2000). Remarkably, BALB/c mice can be rendered susceptible (i.e., prone to perforation) in response to P. aeruginosa challenge if B7.1-expressing LCs are induced into the central cornea prior to the infectious challenge. Although the exact mechanisms by which LCs stimulate T-cell recruitment have not been fully elucidated, this is thought to involve the constitutive expression of important chemokines among LCs, including MIP-1α, RANTES, and CCR-5 (Haelens et al., 1996; Yamagami et al., 2005). Immune modulators, including antagonists of the CCR5 system, have therefore been proposed as a potential target to halt stromal thinning in inflammatory processes such as BK.
vii. Corneal wound healing
The cornea is an organ that can be described as having poor functional reserve. This is because even the slightest disturbances in its precise architecture – usually precipitated by trauma, inflammation, and or infection – can have a profound impact on corneal transparency, topography, refractive power, tensile strength, and avascularity. Under healthy conditions, keratocytes are responsible for maintaining the structural integrity and optical transparency of the stroma, laying down collagen fibrils (mostly collagen types I, V, VI, and XII) in carefully arranged lamellae, producing a ground substance rich with proteoglycans such as sulfate-containing proteins keratan, dermatan, and chondroitin, and also secreting local collagenases to decompose aged matrices that require renewal (Bron, 2001; Pei et al., 2004; Wilson et al., 2012). This highly-regulated system is subverted in the injured cornea, leading to the formation of corneal opacities that cause vision loss by obstructing the visual axis, and/or inducing irregular astigmatism upon focal stromal thinning. Although corneal scarring is the most common cause of vision loss in BK, much of our understanding of post-infectious repair pathways have been drawn from animal models of corneal wounding that have typically used mechanical or chemical injuries, for instance abrasions, photorefractive procedures, and chemical burns (Linna and Tervo, 1997; Lorenzo-Martin et al., 2019). Nonetheless, it is thought that cellular and biochemical responses are generalizable across a broad range of corneal injuries, though this may be subject to revision as new data from alternative models emerge.
Corneal repair pathways are activated upon injury and infection of corneal epithelial cells, which lead to the constitutive production of soluble mediators that are both pro-inflammatory (e.g., IL-1α and IL-1β) and pro-fibrogenic (e.g., platelet derived growth factor or PDGF, insulin-like growth factor or ILGF, and transforming growth factors-β1 and β2, henceforth TGF-β) (Wilson, 2020b). If the epithelial basement membrane is breached, these mediators soak freely into the underlying stroma, where they mix with the onslaught of bone marrow-derived inflammatory cells that infiltrate the cornea from the perilimbal vasculature and tear film. Under these conditions, some keratocytes undergo apoptosis, while others transition from their quiescent to active repair phenotypes. Keratocytes that transform into corneal fibroblasts gain motility, secrete IL-1α, release MMPs, and produce collagenous ECM (West-Mays and Dwivedi, 2006; Yu et al., 2010), a process vital in providing makeshift structural support to the stroma in an attempt to prevent descemetocele formation and frank perforation. While advantageous in the early phases of infection, the persistence of pro-fibrogenic mediators eventually favors the terminal differentiation of fibroblasts into contractile myofibroblasts which coalesce around corneal wounds, express α-smooth muscle actin α-SMA) (Jester et al., 1996), vimentin, and desmin (Chaurasia et al., 2009), secrete ECM proteins such as fibronectin (Wilson, 2012), and lay down highly disorganized and opaque matrix (Santhanam et al., 2017). A separate lineage of myofibroblast progenitors, bone-marrow derived fibrocytes, have also been shown to be involved in disordered collagen deposition and angiogenesis (de Oliveira and Wilson, 2020; Lassance et al., 2018).
The regeneration of the epithelial basement membrane is critical in halting the flow of fibrogenic mediators into the stroma (Marino et al., 2017), and also the reciprocal flow of fibroblast-derived cross-talk molecules such as keratocyte-derived hepatocyte and keratinocyte growth factors (HGF and KGF, respectively) (Wilson et al., 1999). Without TGF-β, PDGF, and other stimulatory molecules, myofibroblasts undergo apoptosis and the scarring process gradually diminishes, although many months may elapse before the scar reaches full maturity (Torricelli et al., 2013a). This process highlights the clinical importance of achieving early re-epithelialization in affected patients, and also suggests that there are key rate-dependent steps in the genesis of post-infectious opacities that may serve as potential therapeutic targets (Jester et al., 1997; Singh et al., 2014). Clearly, as with the pendular nature of most inflammatory cascades involved in BK, the need to activate mechanisms that lead to fibrosis in the short term (to prevent potentially catastrophic stromal thinning) must be tempered by mechanisms that promote a prompt return to stromal homeostasis after the pathogen has been cleared (e.g., by reducing myofibroblast activity when it is unneeded). Further details of corneal wound healing are found in several excellent reviews (Ljubimov and Saghizadeh, 2015; Wilson, 2020b).
5. NOVEL THERAPEUTICS FOR BACTERIAL KERATITIS
i. Current standards of care
As mentioned earlier, the only evidence-based treatments for BK are broad spectrum topical antibiotics, and in select cases, topical corticosteroids and surgical intervention. Common antimicrobial regimens include either topical monotherapy with a fourth-generation fluoroquinolone, or a combination of fortified antibiotics with appropriate broad-spectrum coverage. Fortified antibiotics typically include a cephalosporin or vancomycin for Gram-positive organisms, combined an aminoglycoside such as tobramycin or gentamicin for Gram-negative organisms (Lin et al., 2019). While the necessity of antimicrobial therapy has never come under serious question, the role of corticosteroids has in the past courted significant controversy among ophthalmologists. Heated debates that raged from the mid-1950s – when corticosteroids first became available in topical formulations – hinged on whether immunosuppressive therapy could exacerbate disease by dampening essential host inflammatory responses during acute infection, or whether a therapeutic benefit could be achieved by suppressing the destructive stromal lysis that seemed to accompany leucocyte infiltration into the cornea (Gordon, 2000; Laibson, 1973; Thygeson et al., 1960; Wilhelmus, 2002).
The ocular steroid dilemma in BK was not adequately addressed until the conduct and publication of the National Institutes of Health (NIH)-sponsored Steroids for Corneal Ulcers Trial (SCUT), a moderately-sized (n = 500), double-blinded randomized controlled trial that evaluated the therapeutic benefit of a relatively lightly-dosed course of topical 1% prednisolone sodium phosphate, compared to placebo, among culture-proven cases treated at baseline with standard topical 0.5% moxifloxacin (Srinivasan et al., 2012). Although no difference was found between treatment and placebo groups for the primary endpoint of best spectacle-corrected visual acuity (BSCVA) at three months, primary and secondary analyses demonstrated that corticosteroid-treated patients with central, deep and severe ulcers not caused by Nocardia achieved a 3- and 12-month BSCVA of at least 1-line better than compared to the placebo arm within these subgroups (Ray et al., 2014; Srinivasan et al., 2014). Though SCUT did not have the required power to detect differences in visual outcome by bacterial etiology, its conclusions provide the clinical rationale for the cautious application of topical corticosteroids in severe, culture-proven non-Nocardia BK after at least 48-hours of improvement while on antimicrobial treatment.
The indications for surgical intervention in BK vary in large part according to the phase of infection. In the acute setting, severe lesions that undergo progressive stromal thinning, descemetocele formation, and local perforation may require the application of a corneal tissue adhesive to provide globe closure and tectonic support for a short period. However, commonly-used adhesives such as cyanoacrylate are limited by their direct toxic effects on corneal cells, propensity to worsen corneal inflammation, and induction of pro-angiogenic pathways that lead to corneal neovascularization (Yin et al., 2019). A less common alternative is amniotic membrane transplantation (AMT), which has purported anti-inflammatory properties that may lead to accelerated ocular surface healing (Kim et al., 2001), though data on effectiveness remain mixed. In unsalvageable, rapidly progressing lesions and in large corneal perforations, the only prospect for visual recovery (and globe preservation) is to replace the diseased corneal tissue with a penetrating therapeutic corneal graft. While successful in many cases, corneal grafts that are performed in an infected eye are associated with an increased likelihood of disease recurrence, high-grade post-operative inflammation, and/or early graft failure, when compared to grafts performed in non-inflamed eyes (Tan et al., 2008). For long-term blinding corneal opacities, the only effective treatment is usually the placement of a corneal graft or keratoprosthesis; both require life-long ophthalmic care and cannot guarantee a return to the patient’s pre-infection vision. Furthermore, because the health and socioeconomic burdens of BK are heavily skewed towards low-income countries, optical rehabilitation through surgery is simply not an option for the many patients, estimated to number in the millions, who experience blindness from infectious corneal ulcers each year (Ung et al., 2019a; Whitcher and Srinivasan, 1997).
ii. A new paradigm in BK treatment
The controversies that once surrounded topical corticosteroids illustrate the lack of therapeutic precision offered by historical and current standards of evidence-based care in BK. For example, because topical corticosteroids are agnostic to inflammatory pathways, their use as blanket immunosuppressants must be timed and titrated carefully to achieve their desired effect. Similarly, the use of broad-spectrum empiric antimicrobials, commonly through the entire duration of a patient’s infection, represents an unnuanced “scorched earth” approach to achieve pathogen eradication (O’Brien, 2003). The future of BK care will require treatments that target the many dimensions of BK pathophysiology, specifically those that are most strongly associated with severity of disease and clinical outcomes. Novel treatments must be safe, minimally toxic, made widely available, and ideally would have multimodal mechanisms of action. This could include: (a) enhancing natural ocular surface defenses through the application of naturally-occurring and potentially non-toxic antimicrobial tear products; (b) optimizing the health of compromised ocular surfaces, including among contact-lens wearers; (c) preventing the adhesion, colonization, and invasion of bacterial pathogens on the corneal epithelium; (d) amplifying the antibacterial effects of the host innate response, while attenuating the persistent and destructive nature of residual inflammation within the cornea; (e) reducing the impact of disordered corneal remodeling in the post-infectious phase. As shown earlier, the pathogenesis of BK – while complex and incompletely understood – is replete with opportunities for therapeutic interventions that may improve patient care.
iii. New treatments on the horizon
Rich evidence from animal models of BK suggests there are many candidate therapies that merit further investigation. Precise molecular treatments are often applied in experimental studies to delineate the functional significance of varying inflammatory mediators or pathogen virulence factors in disease pathways. The need to interrogate new potential treatments is urgent: only one class of treatments – comprising the fourth-generation fluoroquinolones moxifloxacin, besifloxacin, and gatifloxacin – has been approved for use in bacterial ocular surface infections in the last 20 years (Food and Drug Administration, 2003, 2010; Haas et al., 2011). Novel antimicrobial compounds that may not be subject to the same concerns surrounding AMR include antimicrobial peptides and other bacteriostatic compounds in tear fluid (Fleiszig et al., 2003; Kwong et al., 2007; Mannis and Smolin, 1996), as well as synthetic host defense peptides (Clemens et al., 2017). Monoclonal antibodies directed against important cell wall constituents on bacteria may also reduce the virulence of invading bacteria. Those that have been tested in ocular models of P. aeruginosa keratitis include alginate (Zaidi and Pier, 2008), H2 and F (porin) proteins (Moon et al., 1988), LPS (Welsh et al., 1984), and T3SS (Le et al., 2019). Exotoxin-inhibitors that target ExoU (Foulkes et al., 2021; Phillips et al., 2003) and ExoS (Aiello et al., 2010; Sharma et al., 2020; Uusitalo et al., 2017) have also shown promise as antimicrobial compounds. Furthermore, bacterial adhesion and internalization may be reduced by competitive binding of host receptors such as gangliotetraosylceramide (asialo GM1) and pilus-binding protein (Hazlett et al., 1993; Hobden et al., 1996), and as well as the inhibition of lipid raft-dependent endocytosis (Zaidi et al., 2008).
Immune modulators may attenuate the damage associated with residual host inflammatory responses. Clinically, a number of available medications are already used for this purpose. For example, anticollagenases such as the tetracycline class of antibiotics are believed to reduce stromal lysis, and are used widely by ophthalmologists even though their effect in preventing corneal perforation has not been studied in the setting of a randomized controlled trial (Golub et al., 1983; Seedor et al., 1987). In research settings, attempts to temper the host inflammatory response have involved blocking TLR-dependent pathways. This has been achieved using TLR-specific antibodies (Huang et al., 2005), and through small molecule inhibitors, e.g., glycyrrhizin, which blocks the binding of an endogenous “danger signal”, high mobility group box-1 (HMGB1), to TLRs (Ekanayaka et al., 2018; Ekanayaka et al., 2016). Monoclonal antibodies that target important inflammatory cytokines, including IL-1β (Karicherla and Hobden, 2010; Xue et al., 2003b) and IL-17 (Zaidi et al., 2012) have also been associated with decreased severity of disease. Alternatively, vaccination with microbe-derived products is an intriguing method that may dull host inflammatory responses in the cornea. Vaccination with heat-killed P. aeruginosa (Masinick et al., 1997), LPS endotoxins (Kreger et al., 1986), flagellin (Kumar et al., 2008), staphylococcal α-toxin toxoid (Hume et al., 2000), and pneumococcal pneumolysin toxoid (Norcross et al., 2011) have all been demonstrated in animal models to reduce BK severity. With some imagination, ophthalmologists may be one day be possible to safely administer prophylactic topical vaccinations among those with a high baseline risk of developing BK (e.g., severe ocular surface disease and/or in corneal transplants). Therapies that could conceivably reduce post-infectious corneal scarring could include anti-TGF-β and anti-PDGF agents that could augment the effect of corticosteroids that are prescribed for this indication (Chouhan et al., 2019; Esmaeili et al., 2016; Hill et al., 2018; Jester et al., 1997; Singh et al., 2014). Novel methods of drug delivery – including the use of nanoparticles, drug-eluting contact lenses, and microemulsions – could further improve drug bioavailability on the ocular surface (Dutta et al., 2016; Sharma and Taniguchi, 2017; Ustundag-Okur et al., 2015).
CONCLUSIONS
It is a sobering reality of ophthalmic care that evidence-based strategies to prevent and treat BK have remained largely stagnant in the past few decades. Amid growing concerns of diminishing antibiotic utility in treating common ocular pathogens, attention has naturally turned to the development of medical innovations that may help overcome our therapeutic stasis. A simple framework that reconstructs the complex biology and host-pathogen interactions that determine the clinical course of disease is helpful to identify precise targets for therapeutic intervention and future research. Indeed, our wealth of experimental animal and human data suggests that our current standard of care could be augmented by treatments that bolster host defenses on the ocular surface, reduce the likelihood of bacterial corneal invasion, modulate the destructive components of the host immune response, and optimize corneal wound healing in the post-infectious phase of disease. As the global burden of BK grows, the next frontier of clinical management will lie in exploring how such treatments can be developed and translated into routine patient care.
Highlights.
Bacterial corneal infections can cause rapidly fulminant disease and permanent vision loss.
Topical antimicrobials and corticosteroids fail to salvage vision in many patients.
The pathogenesis of bacterial corneal infections involves loss of corneal immune privilege.
Innate immune responses are orchestrated by the corneal epithelium, stromal keratocytes, and resident immune cells.
Novel treatments targeting the molecular bases of infection may improve patient outcomes.
DISCLOSURES
LU has no disclosures, and is funded in part by the Dozoretz Family Private Foundation. JC is a consultant for the US Food and Drug Administration, and holds research grants from the National Institutes of Health, National Eye Institute (RO1 EY 013124-17 and R01 EY021558-07). JC is co-editor-in-chief for the British Journal of Ophthalmology.
ABBREVIATIONS
- α-SMA
α-smooth muscle actin
- AMR
antimicrobial resistance
- AMT
amniotic membrane transplantation
- APC
antigen presenting cell
- ARMOR
Antimicrobial Resistance Monitoring of Ocular Microorganisms
- BSCVA
best spectacle-corrected visual acuity
- BK
bacterial keratitis
- C3
complement component 3
- CCL
chemokine (C-C motif) ligand
- CFU
colony forming unit
- CTFR
cystic fibrosis transmembrane-conductance regulator
- CXCL
chemokine ligand
- ECM
extracellular matrix
- hβD-2
human β-defensin 2
- HGF
hepatocyte growth factor
- HMGB-1
high mobility group box-1
- HIV
human immunodeficiency virus
- ICAM-1
intracellular adhesion molecule 1
- IFN
interferon
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IL
interleukin
- ILGF
insulin-like growth factor
- IRF3
interferon regulatory factor 3
- JNK
c-Jun N-terminal kinases
- KAMP
keratin-derived antimicrobial peptide
- KGF
keratinocyte growth factor
- LASIK
laser-assisted in-situ keratomileusis
- LC
Langerhans cell
- LL-37
cathelicidin
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- MAC1
macrophage-1 antigen
- MAPK
mitogen-activated protein kinases
- MHC
major histocompatibility complex
- MIC
mean inhibitory concentration
- MIF
macrophage migration inhibitory factor
- MIP
macrophage inflammatory protein
- MMP
matrix metalloproteinase
- MSCRAMMs
microbial surface components recognizing adhesive matrix
- MyD88
myeloid differentiation primary response protein 88
- NETS
neutrophil extracellular trap
- NF-κB
nuclear factor κB
- NIH
National Institutes of Health
- NGS
next generation sequencing
- NLR
nucleotide-binding oligomerization domain-like receptors
- NOD
nucleotide-binding oligomerization domain
- OCP
ocular cicatricial pemphigoid
- PAMP
pathogen-associated molecular pattern
- PavA
pneumococcal adherence and virulence factor A
- PavB
pneumococcal adherence and virulence factor B
- PECAM-1
platelet endothelial cell adhesion molecule
- PDGF
platelet-derived growth factor
- PMN
polymorphonuclear cell
- PNAG
poly-N-acetylglucosamine
- PRR
pathogen recognition receptors
- PsaA
pneumococcal surface antigen A
- PspA
pneumococcal surface protein A
- PspC
pneumococcal surface protein C
- P. aeruginosa
Pseudomonas aeruginosa
- RANTES
regulated upon activation, normal T-cell expressed and presumably secreted
- ROS
reactive oxygen species
- SCUT
Steroids for Corneal Ulcers Trial
- S. aureus
Staphylococcus aureus
- sIgA
secretory immunoglobulin A
- SJS
Stevens-Johnson syndrome
- SLPI
secretory leukocyte protease inhibitor
- sPLA2
secretory phospholipase A
- ssRNA
single stranded RNA
- S. pneumoniae
Streptococcus pneumoniae
- T3SS
type III secretion systems
- TGF
transforming growth factor
- Th1
type 1 T-helper cell
- Th2
type 2 T-helper cell
- TICAM-1
toll/interleukin-1 receptor-domain containing adaptor molecule 1
- TICAM-2
toll/interleukin-1 receptor-domain containing adaptor molecule 2
- TIR
toll/interleukin-1 receptor
- TIRAP
toll/interleukin-1 receptor-domain containing adaptor protein
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor – α
- TRAM
toll/interleukin-1 receptor-domain containing adaptor molecule 2
- VCAM-1
vascular cell adhesion protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None to declare.
REFERENCES
- Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ, 2000. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 299, 39–46. [DOI] [PubMed] [Google Scholar]
- Adhikary G, Sun Y, Pearlman E, 2008. C-Jun NH2 terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J. Leukoc. Biol 83, 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ, 1996. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest. Ophthalmol. Vis. Sci 37, 1826–1832. [PubMed] [Google Scholar]
- Aiello D, Williams JD, Majgier-Baranowska H, Patel I, Peet NP, Huang J, Lory S, Bowlin TL, Moir DT, 2010. Discovery and Characterization of Inhibitors of Pseudomonas aeruginosa Type III Secretion. Antimicrob. Agents Chemother 54, 1988–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon I, Kwan L, Yu C, Evans DJ, Fleiszig SM, 2009. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect. Immun 77, 3264–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon I, Tam C, Mun JJ, LeDue J, Evans DJ, Fleiszig SM, 2011. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest. Ophthalmol. Vis. Sci 52, 1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi W, Markoulli M, Papas E, 2020. The relationship between tear film MMP-9 and meibomian gland changes during soft contact lens wear. Cont Lens Anterior Eye 43, 154–158. [DOI] [PubMed] [Google Scholar]
- Alizadeh H, Apte S, El-Agha MS, Li L, Hurt M, Howard K, Cavanagh HD, McCulley JP, Niederkorn JY, 2001. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20, 622–627. [DOI] [PubMed] [Google Scholar]
- Ando E, Ando Y, Inoue M, Morino Y, Kamata R, Okamura R, 1990. Inhibition of corneal inflammation by an acylated superoxide dismutase derivative. Invest. Ophthalmol. Vis. Sci 31, 1963–1967. [PubMed] [Google Scholar]
- Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ, Fleiszig SM, 2008. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect. Immun 76, 1992–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, 2021. Galectins as Regulators of Corneal Inflammation. Curr Opin Physiol 19, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argüeso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK, 2006. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest. Ophthalmol. Vis. Sci 47, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunga S, Kintoki GM, Gichuhi S, Onyango J, Newton R, Leck A, Macleod D, Hu VH, Burton MJ, 2019a. Delay Along the Care Seeking Journey of Patients with Microbial Keratitis in Uganda. Ophthalmic epidemiology 26, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunga S, Wiafe G, Habtamu E, Onyango J, Gichuhi S, Leck A, Macleod D, Hu V, Burton M, 2019b. The impact of microbial keratitis on quality of life in Uganda. BMJ Open Ophthalmol 4, e000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW, 2015. Antibiotic Resistance Among Ocular Pathogens in the United States: Five-Year Results From the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol 133, 1445–1454. [DOI] [PubMed] [Google Scholar]
- Asbell PA, Sanfilippo CM, Sahm DF, DeCory HH, 2020. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA ophthalmology 138, 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin DK, Heimer SR, Tam C, Li WY, Le Due JM, Evans DJ, Fleiszig SM, 2011. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect. Immun 79, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines MG, Cai F, Backman HA, 1990. Adsorption and removal of protein bound to hydrogel contact lenses. Optom. Vis. Sci 67, 807–810. [DOI] [PubMed] [Google Scholar]
- Balaram M, Rashid S, Dana R, 2005. Chronic ocular surface disease after allogeneic bone marrow transplantation. Ocul Surf 3, 203–211. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Messmer EM, Aragona P, Geerling G, Akova YA, Benitez-del-Castillo J, Boboridis KG, Merayo-Lloves J, Rolando M, Labetoulle M, 2016. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol 100, 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot P, Grattard F, Mahul P, Pain P, Jospe R, Venet C, Carricajo A, Aubert G, Ros A, Dumont A, 2001. Prospective study of nosocomial colonization and infection due to Pseudomonas aeruginosa in mechanically ventilated patients. Intensive Care Med. 27, 503–512. [DOI] [PubMed] [Google Scholar]
- Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi, Palaniappan R, 2002. Aetiological diagnosis of microbial keratitis in South India - a study of 1618 cases. Indian J. Med. Microbiol 20, 19–24. [PubMed] [Google Scholar]
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW, 2002. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298, 1025–1029. [DOI] [PubMed] [Google Scholar]
- Bispo PJM, Ung L, Chodosh J, Gilmore MS, 2020a. The Challenge of Antibiotic Resistance in Corneal Infection, in: Colby K, Dana MR (Eds.), Foundations of Corneal Disease. Springer, Cham, Switzerland, pp. 277–288. [Google Scholar]
- Bispo PJM, Ung L, Chodosh J, Gilmore MS, 2020b. Hospital-Associated Multidrug-Resistant MRSA Lineages Are Trophic to the Ocular Surface and Cause Severe Microbial Keratitis. Front Public Health 8, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkar DS, Acharya NR, Leong C, Lalitha P, Srinivasan M, Oldenburg CE, Cevallos V, Lietman TM, Evans DJ, Fleiszig SM, 2014. Cytotoxic clinical isolates of Pseudomonas aeruginosa identified during the Steroids for Corneal Ulcers Trial show elevated resistance to fluoroquinolones. BMC ophthalmology 14, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkar DS, Fleiszig SM, Leong C, Lalitha P, Srinivasan M, Ghanekar AA, Tam C, Li WY, Zegans ME, McLeod SD, Lietman TM, Acharya NR, 2013. Association between cytotoxic and invasive Pseudomonas aeruginosa and clinical outcomes in bacterial keratitis. JAMA Ophthalmol 131, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne R, Steinmetz JD, Flaxman S, Briant PS, Taylor HR, Resnikoff S, Casson RJ, Abdoli A, Abu-Gharbieh E, Afshin A, Ahmadieh H, Akalu Y, Alamneh AA, Alemayehu W, Alfaar AS, Alipour V, Anbesu EW, Androudi S, Arabloo J, Arditi A, Asaad M, Bagli E, Baig AA, Bärnighausen TW, Battaglia Parodi M, Bhagavathula AS, Bhardwaj N, Bhardwaj P, Bhattacharyya K, Bijani A, Bikbov M, Bottone M, Braithwaite T, Bron AM, Butt ZA, Cheng C-Y, Chu D-T, Cicinelli MV, Coelho JM, Dagnew B, Dai X, Dana R, Dandona L, Dandona R, Del Monte MA, Deva JP, Diaz D, Djalalinia S, Dreer LE, Ehrlich JR, Ellwein LB, Emamian MH, Fernandes AG, Fischer F, Friedman DS, Furtado JM, Gaidhane AM, Gaidhane S, Gazzard G, Gebremichael B, George R, Ghashghaee A, Golechha M, Hamidi S, Hammond BR, Hartnett MER, Hartono RK, Hay SI, Heidari G, Ho HC, Hoang CL, Househ M, Ibitoye SE, Ilic IM, Ilic MD, Ingram AD, Irvani SSN, Jha RP, Kahloun R, Kandel H, Kasa AS, Kempen JH, Keramati M, Khairallah M, Khan EA, Khanna RC, Khatib MN, Kim JE, Kim YJ, Kisa S, Kisa A, Koyanagi A, Kurmi OP, Lansingh VC, Leasher JL, Leveziel N, Limburg H, Majdan M, Manafi N, Mansouri K, McAlinden C, Mohammadi SF, Mohammadian-Hafshejani A, Mohammadpourhodki R, Mokdad AH, Moosavi D, Morse AR, Naderi M, Naidoo KS, Nangia V, Nguyen CT, Nguyen HLT, Ogundimu K, Olagunju AT, Ostroff SM, Panda-Jonas S, Pesudovs K, Peto T, Quazi Syed Z, Rahman MHU, Ramulu PY, Rawaf S, Rawaf DL, Reinig N, Robin AL, Rossetti L, Safi S, Sahebkar A, Samy AM, Saxena D, Serle JB, Shaikh MA, Shen TT, Shibuya K, Shin JI, Silva JC, Silvester A, Singh JA, Singhal D, Sitorus RS, Skiadaresi E, Skirbekk V, Soheili A, Sousa RARC, Spurlock EE, Stambolian D, Taddele BW, Tadesse EG, Tahhan N, Tareque MI, Topouzis F, Tran BX, Travillian RS, Tsilimbaris MK, Varma R, Virgili G, Wang YX, Wang N, West SK, Wong TY, Zaidi Z, Zewdie KA, Jonas JB, Vos T, 2021. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. The Lancet Global Health 9, e130–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer L, Kindler C, Jager K, Sel S, Nolle B, Pleyer U, Ochs M, Paulsen FP, 2007. Detection of surfactant proteins A and D in human tear fluid and the human lacrimal system. Invest. Ophthalmol. Vis. Sci 48, 3945–3953. [DOI] [PubMed] [Google Scholar]
- Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL, 2002. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest. Ophthalmol. Vis. Sci 43, 2264–2271. [PMC free article] [PubMed] [Google Scholar]
- Brock SC, McGraw PA, Wright PF, Crowe JE Jr., 2002. The human polymeric immunoglobulin receptor facilitates invasion of epithelial cells by Streptococcus pneumoniae in a strain-specific and cell type-specific manner. Infect. Immun 70, 5091–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse RM, 1974. Tear lactoferrin: a bacteriostatic and complexing protein. Investigative ophthalmology 13, 550–554. [PubMed] [Google Scholar]
- Bron AJ, 2001. The architecture of the corneal stroma. Br. J. Ophthalmol 85, 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant-Hudson KM, Carr DJ, 2012. CXCL1-deficient mice are highly sensitive to pseudomonas aeruginosa but not herpes simplex virus type 1 corneal infection. Invest. Ophthalmol. Vis. Sci 53, 6785–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland AG, Heeley EL, Wilton DC, 2000. Bacterial cell membrane hydrolysis by secreted phospholipases A(2): a major physiological role of human group IIa sPLA(2) involving both bacterial cell wall penetration and interfacial catalysis. Biochim. Biophys. Acta 1484, 195–206. [DOI] [PubMed] [Google Scholar]
- Burton MJ, Pithuwa J, Okello E, Afwamba I, Onyango JJ, Oates F, Chevallier C, Hall AB, 2011. Microbial keratitis in East Africa: why are the outcomes so poor? Ophthalmic epidemiology 18, 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Engel LS, Hill JM, O’Callaghan RJ, 1994. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect. Immun 62, 2478–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Hobden JA, Hill JM, Insler MS, O’Callaghan RJ, 1992. Topical antibiotic therapy for the treatment of experimental Staphylococcus aureus keratitis. Invest. Ophthalmol. Vis. Sci 33, 3017–3023. [PubMed] [Google Scholar]
- Cammarota G, Ianiro G, Gasbarrini A, 2014. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J. Clin. Gastroenterol 48, 693–702. [DOI] [PubMed] [Google Scholar]
- Carnt N, Stapleton F, 2016. Strategies for the prevention of contact lens-related Acanthamoeba keratitis: a review. Ophthalmic Physiol. Opt 36, 77–92. [DOI] [PubMed] [Google Scholar]
- Cavanagh HD, Ladage PM, Li SL, Yamamoto K, Molai M, Ren DH, Petroll WM, Jester JV, 2002. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium: a 13-month clinical trial. Ophthalmology 109, 1957–1969. [DOI] [PubMed] [Google Scholar]
- Cavuoto KM, Banerjee S, Galor A, 2019. Relationship between the microbiome and ocular health. Ocul Surf 17, 384–392. [DOI] [PubMed] [Google Scholar]
- Cendra MDM, Christodoulides M, Hossain P, 2017. Signaling Mediated by Toll-Like Receptor 5 Sensing of Pseudomonas aeruginosa Flagellin Influences IL-1beta and IL-18 Production by Primary Fibroblasts Derived from the Human Cornea. Front Cell Infect Microbiol 7, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JKL, Yuen D, Too PH-M, Sun Y, Willard B, Man D, Tam C, 2017. Keratin 6a reorganization for ubiquitin–proteasomal processing is a direct antimicrobial response. J. Cell Biol 217, 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, McCluskey PJ, Wakefield D, 2006. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol 90, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Agrawal D, 2016. Multi-drug resistant Pseudomonas aeruginosa keratitis and its effective treatment with topical colistimethate. Indian journal of ophthalmology 64, 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, de Medeiros FW, Smith SD, Wilson SE, 2009. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res 89, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG, Geerards AJ, Kijlstra A, 1999. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 354, 181–185. [DOI] [PubMed] [Google Scholar]
- Cheung AL, Projan SJ, Gresham H, 2002. The Genomic Aspect of Virulence, Sepsis, and Resistance to Killing Mechanisms in Staphylococcus aureus. Curr. Infect. Dis. Rep 4, 400–410. [DOI] [PubMed] [Google Scholar]
- Chidambaram JD, Kannambath S, Srikanthi P, Shah M, Lalitha P, Elakkiya S, Bauer J, Prajna NV, Holland MJ, Burton MJ, 2017. Persistence of Innate Immune Pathways in Late Stage Human Bacterial and Fungal Keratitis: Results from a Comparative Transcriptome Analysis. Front Cell Infect Microbiol 7, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery H, Sun Y, Carlson E, McMenamin P, Pearlman E, 2008. TLR ligand-induced keratitis is partially reconstituted in TLR−/−chimeric mice by donor TLR+ bone marrow-derived cells in the corneal stroma. Invest. Ophthalmol. Vis. Sci 49, 498–498. [Google Scholar]
- Chodosh J, Nordquist RE, Kennedy RC, 1998. Comparative anatomy of mammalian conjunctival lymphoid tissue: a putative mucosal immune site. Dev. Comp. Immunol 22, 621–630. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Kim MK, Ko JH, Lee HJ, Jeong HJ, Wee WR, Seong SY, Akira S, 2011. Effect of Toll-like receptor 2 and 4 of corneal fibroblasts on cytokine expression with co-cultured antigen presenting cells. Cytokine 56, 265–271. [DOI] [PubMed] [Google Scholar]
- Chouhan G, Moakes RJA, Esmaeili M, Hill LJ, deCogan F, Hardwicke J, Rauz S, Logan A, Grover LM, 2019. A self-healing hydrogel eye drop for the sustained delivery of decorin to prevent corneal scarring. Biomaterials 210, 41–50. [DOI] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN, 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med 16, 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens LE, Jaynes J, Lim E, Kolar SS, Reins RY, Baidouri H, Hanlon S, McDermott AM, Woodburn KW, 2017. Designed Host Defense Peptides for the Treatment of Bacterial Keratitis. Invest. Ophthalmol. Vis. Sci 58, 6273–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland RP, Hazlett LD, Leon MA, Berk RS, 1983. Role of complement in murine corneal infection caused by Pseudomonas aeruginosa. Invest. Ophthalmol. Vis. Sci 24, 237–242. [PubMed] [Google Scholar]
- Cole N, Hume EB, Khan S, Garthwaite L, Schubert T, Reeve V, Willcox MD, 2007. The corneal response to infection with Staphylococcus aureus in the absence of interleukin-4. Immunol. Cell Biol 85, 333–337. [DOI] [PubMed] [Google Scholar]
- Cole N, Krockenberger M, Stapleton F, Khan S, Hume E, Husband AJ, Willcox M, 2003. Experimental Pseudomonas aeruginosa keratitis in interleukin-10 gene knockout mice. Infect. Immun 71, 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder JS, Beach MJ, 2014. Estimated burden of keratitis—United States, 2010. MMWR. Morbidity and mortality weekly report 63, 1027–1030. [PMC free article] [PubMed] [Google Scholar]
- Cope JR, Collier SA, Rao MM, Chalmers R, Mitchell GL, Richdale K, Wagner H, Kinoshita BT, Lam DY, Sorbara L, Zimmerman A, Yoder JS, Beach MJ, 2015. Contact Lens Wearer Demographics and Risk Behaviors for Contact Lens-Related Eye Infections--United States, 2014. MMWR Morb. Mortal. Wkly. Rep 64, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope JR, Collier SA, Srinivasan K, Abliz E, Myers A, Millin CJ, Miller A, Tarver ME, 2016. Contact Lens-Related Corneal Infections - United States, 2005–2015. MMWR Morb. Mortal. Wkly. Rep 65, 817–820. [DOI] [PubMed] [Google Scholar]
- Cope JR, Konne NM, Jacobs DS, Dhaliwal DK, Rhee MK, Yin J, Steinemann TL, 2018. Corneal Infections Associated with Sleeping in Contact Lenses - Six Cases, United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep 67, 877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell BA, Evans DJ, Fleiszig SM, 2005. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol. Lett 250, 71–76. [DOI] [PubMed] [Google Scholar]
- Crain M, Waltman W, Turner J, Yother J, Talkington D, McDaniel L, Gray B, Briles D, 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun 58, 3293–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum NF, Barrozo CP, Chapman FA, Ryan MA, Russell KL, 2004. An outbreak of conjunctivitis due to a novel unencapsulated Streptococcus pneumoniae among military trainees. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 39, 1148–1154. [DOI] [PubMed] [Google Scholar]
- Czechowska K, McKeithen-Mead S, Al Moussawi K, Kazmierczak BI, 2014. Cheating by type 3 secretion system-negative Pseudomonas aeruginosa during pulmonary infection. Proceedings of the National Academy of Sciences of the United States of America 111, 7801–7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, 2011. Antibiotic resistance is ancient. Nature 477, 457–461. [DOI] [PubMed] [Google Scholar]
- Dahlgren MA, Lingappan A, Wilhelmus KR, 2007. The clinical diagnosis of microbial keratitis. Am. J. Ophthalmol 143, 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana MR, 2004. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest. Ophthalmol. Vis. Sci 45, 722–727; 721. [DOI] [PubMed] [Google Scholar]
- Dana MR, Dai R, Zhu S, Yamada J, Streilein JW, 1998. Interleukin-1 receptor antagonist suppresses Langerhans cell activity and promotes ocular immune privilege. Invest. Ophthalmol. Vis. Sci 39, 70–77. [PubMed] [Google Scholar]
- Dart JK, Seal DV, 1988. Pathogenesis and therapy of Pseudomonas aeruginosa keratitis. Eye (Lond.) 2 Suppl, S46–55. [DOI] [PubMed] [Google Scholar]
- Dart JK, Stapleton F, Minassian D, 1991. Contact lenses and other risk factors in microbial keratitis. Lancet 338, 650–653. [DOI] [PubMed] [Google Scholar]
- Dartt DA, 2011. Tear lipocalin: structure and function. Ocul Surf 9, 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson HJ, Kuonen VJ, 2004. The tear film and ocular mucins. Vet. Ophthalmol 7, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira RC, Wilson SE, 2020. Fibrocytes, Wound Healing, and Corneal Fibrosis. Invest. Ophthalmol. Vis. Sci 61, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Muller M, Stockinger S, 2005. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol 5, 675–687. [DOI] [PubMed] [Google Scholar]
- Deguine J, Barton GM, 2014. MyD88: a central player in innate immune signaling. F1000Prime Rep 6, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaris I, Zhu SN, Dana MR, 1999. TNF-alpha regulates corneal Langerhans cell migration. J. Immunol 162, 4235–4239. [PubMed] [Google Scholar]
- Dixon JM, Young CA Jr., Baldone JA, Halberg GP, Sampson W, Stone W Jr., 1966. Complications associated with the wearing of contact lenses. JAMA 195, 901–903. [PubMed] [Google Scholar]
- Doan T, Akileswaran L, Andersen D, Johnson B, Ko N, Shrestha A, Shestopalov V, Lee CS, Lee AY, Van Gelder RN, 2016. Paucibacterial Microbiome and Resident DNA Virome of the Healthy Conjunctiva. Invest. Ophthalmol. Vis. Sci 57, 5116–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Brulc JM, Iovieno A, Bates B, Garoutte A, Miller D, Revanna KV, Gao X, Antonopoulos DA, Slepak VZ, 2011. Diversity of bacteria at healthy human conjunctiva. Invest. Ophthalmol. Vis. Sci 52, 5408–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas S, Kolokotronis A, Stefanopoulos P, 2005. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect. Immun 73, 1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke-Elder S, 1965. System of Ophthalmology: Diseases of the Outer Eye, Part I. C.V. Mosby company, St. Louis. [Google Scholar]
- Dutta D, Vijay AK, Kumar N, Willcox MDP, 2016. Melimine-Coated Antimicrobial Contact Lenses Reduce Microbial Keratitis in an Animal Model. Invest. Ophthalmol. Vis. Sci 57, 5616–5624. [DOI] [PubMed] [Google Scholar]
- Ebihara N, Yamagami S, Chen L, Tokura T, Iwatsu M, Ushio H, Murakami A, 2007. Expression and function of toll-like receptor-3 and −9 in human corneal myofibroblasts. Invest. Ophthalmol. Vis. Sci 48, 3069–3076. [DOI] [PubMed] [Google Scholar]
- Ekanayaka SA, McClellan SA, Barrett RP, Hazlett LD, 2018. Topical Glycyrrhizin Is Therapeutic for Pseudomonas aeruginosa Keratitis. J Ocul Pharmacol Ther 34, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayaka SA, McClellan SA, Barrett RP, Kharotia S, Hazlett LD, 2016. Glycyrrhizin Reduces HMGB1 and Bacterial Load in Pseudomonas aeruginosa Keratitis. Invest. Ophthalmol. Vis. Sci 57, 5799–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder MJ, Stapleton F, Evans E, Dart JK, 1995. Biofilm-related infections in ophthalmology. Eye (Lond.) 9 (Pt 1), 102–109. [DOI] [PubMed] [Google Scholar]
- Engel LS, Hill JM, Caballero AR, Green LC, O’Callaghan RJ, 1998. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J. Biol. Chem 273, 16792–16797. [DOI] [PubMed] [Google Scholar]
- Esmaeili M, Ahmed Z, Rauz S, Grover LM, Logan A, 2016. The Therapeutic Utility of Decorin Eye Drops for the Prevention of Corneal Scarring. Invest. Ophthalmol. Vis. Sci 57, 1291–1291. [Google Scholar]
- Evans DJ, McNamara NA, Fleiszig SM, 2007. Life at the front: dissecting bacterial-host interactions at the ocular surface. Ocul Surf 5, 213–227. [DOI] [PubMed] [Google Scholar]
- Ezra DG, Lewis G, Healy M, Coombes A, 2005. Preventing exposure keratopathy in the critically ill: a prospective study comparing eye care regimes. Br. J. Ophthalmol 89, 1068–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y, 1997. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J. Infect. Dis 175, 1440–1445. [DOI] [PubMed] [Google Scholar]
- Flanagan JL, Willcox MD, 2009. Role of lactoferrin in the tear film. Biochimie 91, 35–43. [DOI] [PubMed] [Google Scholar]
- Fleiszig S, Zaidi TS, Pier GB, 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun 63, 4072–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig S, Zaidi TS, Preston MJ, Grout M, Evans DJ, Pier GB, 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun 64, 2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, 2006. The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom. Vis. Sci 83, 866–873. [DOI] [PubMed] [Google Scholar]
- Fleiszig SM, Efron N, Pier GB, 1992. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Invest. Ophthalmol. Vis. Sci 33, 2908–2916. [PubMed] [Google Scholar]
- Fleiszig SM, Evans DJ, Do N, Vallas V, Shin S, Mostov KE, 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun 65, 2861–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Kwong MS, Evans DJ, 2003. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun 71, 3866–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Zaidi TS, Fletcher EL, Preston MJ, Pier GB, 1994. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect. Immun 62, 3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SMJ, Kroken AR, Nieto V, Grosser MR, Wan SJ, Metruccio MME, Evans DJ, 2020. Contact lens-related corneal infection: Intrinsic resistance and its compromise. Progress in retinal and eye research 76, 100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, 1922. On a remarkable bacteriolytic element found in tissues and secretions. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character 93, 306–317. [Google Scholar]
- Fleming A, 1932. Lysozyme: President’s Address. Proc. R. Soc. Med 26, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Allison V, 1922. Observations on a bacteriolytic substance “lysozyme”) found in secretions and tissues. Br. J. Exp. Pathol 3, 252. [Google Scholar]
- Fletcher EL, Fleiszig SM, Brennan NA, 1993. Lipopolysaccharide in adherence of Pseudomonas aeruginosa to the cornea and contact lenses. Invest. Ophthalmol. Vis. Sci 34, 1930–1936. [PubMed] [Google Scholar]
- Food and Drug Administration, 2003. Vigamox (Monofloxacin hydrochloride) Opthalmic Solution. US FDA, Bethesda, MD, USA. [Google Scholar]
- Food and Drug Administration, 2010. Zymaxid (gatifloxacin ophthalmic solution) 0.5%. US FDA, Bethesda, MD, USA. [Google Scholar]
- Forte R, Cennamo G, Del Prete S, Cesarano I, Del Prete A, 2010. Scanning electron microscopy of corneal epithelium in soft contact lens wearers. Cornea 29, 732–736. [DOI] [PubMed] [Google Scholar]
- Foster TJ, Hook M, 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6, 484–488. [DOI] [PubMed] [Google Scholar]
- Foulkes DM, McLean K, Zheng Y, Sarsby J, Haneef AS, Fernig DG, Winstanley C, Berry N, Kaye SB, 2021. A pipeline to evaluate inhibitors of the Pseudomonas aeruginosa exotoxin U. Biochem. J 478, 647–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RM, Kenyon KR, Tutschka PJ, Saral R, Green WR, Santos GW, 1983. Ocular manifestations of graft-vs-host disease. Ophthalmology 90, 4–13. [DOI] [PubMed] [Google Scholar]
- Franklin RM, Remus LE, 1984. Conjunctival-associated lymphoid tissue: evidence for a role in the secretory immune system. Invest. Ophthalmol. Vis. Sci 25, 181–187. [PubMed] [Google Scholar]
- Fraser JD, Proft T, 2008. The bacterial superantigen and superantigen-like proteins. Immunol. Rev 225, 226–243. [DOI] [PubMed] [Google Scholar]
- Freitas D, Alvarenga L, Sampaio J, Mannis M, Sato E, Sousa L, Vieira L, Yu MC, Martins MC, Hoffling-Lima A, Belfort R Jr., 2003. An outbreak of Mycobacterium chelonae infection after LASIK. Ophthalmology 110, 276–285. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Ishida W, Fukushima A, Nishida T, 2017. Corneal Fibroblasts as Sentinel Cells and Local Immune Modulators in Infectious Keratitis. Int. J. Mol. Sci 18, 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad A, Vingrys AJ, Wong CY, Jackson DC, Downie LE, 2019. Tear film inflammatory cytokine upregulation in contact lens discomfort. Ocul Surf 17, 89–97. [DOI] [PubMed] [Google Scholar]
- Gadjeva M, Nagashima J, Zaidi T, Mitchell RA, Pier GB, 2010. Inhibition of macrophage migration inhibitory factor ameliorates ocular Pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 6, e1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galentine PG, Cohen EJ, Laibson PR, Adams CP, Michaud R, Arentsen JJ, 1984. Corneal ulcers associated with contact lens wear. Archives of ophthalmology (Chicago, Ill. : 1960) 102, 891–894. [DOI] [PubMed] [Google Scholar]
- Gao N, Sang Yoon G, Liu X, Mi X, Chen W, Standiford TJ, Yu FS, 2013. Genome-wide transcriptional analysis of differentially expressed genes in flagellin-pretreated mouse corneal epithelial cells in response to Pseudomonas aeruginosa: involvement of S100A8/A9. Mucosal Immunol. 6, 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreis F, Gottschalt M, Schlorf T, Glaser R, Harder J, Worlitzsch D, Paulsen FP, 2011. Expression and regulation of antimicrobial peptide psoriasin (S100A7) at the ocular surface and in the lacrimal apparatus. Invest. Ophthalmol. Vis. Sci 52, 4914–4922. [DOI] [PubMed] [Google Scholar]
- Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel J, 2000. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization ofPseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun 68, 7100–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes-McAlister J, Kugadas A, Gadjeva M, 2019. Tasked with a challenging objective: Why do neutrophils fail to battle Pseudomonas aeruginosa biofilms. Pathogens 8, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH, 2015. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev 28, 871–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke JR, Magliocco MV, 1971. Experimental Pseudomonas aeruginosa Infection of the Mouse Cornea. Infect. Immun 3, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette TE, Chandler JW, Greiner JV, 1982. Langerhans cells of the ocular surface. Ophthalmology 89, 700–711. [DOI] [PubMed] [Google Scholar]
- Gipson IK, 2004. Distribution of mucins at the ocular surface. Exp. Eye Res 78, 379–388. [DOI] [PubMed] [Google Scholar]
- Gipson IK, 2007. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci 48, 4390; 4391–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Argueso P, 2003. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol 231, 1–49. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB, 2014. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One 9, e100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis DO, Sloop GD, Reed JM, O’Callaghan RJ, 2003. A new topical model of Staphylococcus corneal infection in the mouse. Invest. Ophthalmol. Vis. Sci 44, 1591–1597. [DOI] [PubMed] [Google Scholar]
- Girgis DO, Sloop GD, Reed JM, O’Callaghan RJ, 2005. Effects of toxin production in a murine model of Staphylococcus aureus keratitis. Invest. Ophthalmol. Vis. Sci 46, 2064–2070. [DOI] [PubMed] [Google Scholar]
- Goldberg MF, Ferguson TA, Pepose JS, 1994. Detection of cellular adhesion molecules in inflamed human corneas. Ophthalmology 101, 161–168. [DOI] [PubMed] [Google Scholar]
- Golden B, Fingerman LH, Allen HF, 1971. Pseudomonas corneal ulcers in contact lens wearers. Epidemiology and treatment. Archives of ophthalmology (Chicago, Ill. : 1960) 85, 543–547. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, Ramamurthy NS, 1983. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J. Periodontal Res 18, 516–526. [DOI] [PubMed] [Google Scholar]
- Gordon YJ, 2000. The evolution of antiviral therapy for external ocular viral infections over twenty-five years. Cornea 19, 673–680. [DOI] [PubMed] [Google Scholar]
- Gordon YJ, Huang LC, Romanowski EG, Yates KA, Proske RJ, McDermott AM, 2005. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res 30, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan B, Gipson IK, 2010. Membrane-tethered mucins have multiple functions on the ocular surface. Exp. Eye Res 90, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Berk RS, Masinick S, Hazlett LD, 1994. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect. Immun 62, 4572–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Masinick S, Garrett M, Hazlett LD, 1997. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect. Immun 65, 2747–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzek JP, Cline DJ, Row PK, Wessels IF, Beeve S, Ispirescu S, Aprecio RM, Kettering JD, Gano DL, Nelson GM, 1998. Rabbit Streptococcus pneumoniae keratitis model and topical therapy. Invest. Ophthalmol. Vis. Sci 39, 2012–2017. [PubMed] [Google Scholar]
- Haas W, Gearinger LS, Usner DW, Decory HH, Morris TW, 2011. Integrated analysis of three bacterial conjunctivitis trials of besifloxacin ophthalmic suspension, 0.6%: etiology of bacterial conjunctivitis and antibacterial susceptibility profile. Clin. Ophthalmol 5, 1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haelens A, Wuyts A, Proost P, Struyf S, Opdenakker G, van Damme J, 1996. Leukocyte migration and activation by murine chemokines. Immunobiology 195, 499–521. [DOI] [PubMed] [Google Scholar]
- Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, Johnson MA, Ross JM, Hutchins JT, Patti JM, 2003. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect. Immun 71, 6864–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt S, 2006. Adherence molecules of pathogenic pneumococci. Curr. Opin. Microbiol 9, 12–20. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Chen L, Zhang Q, Dana MR, 2003a. Novel expression of vascular endothelial growth factor receptor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am. J. Pathol 163, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Dana MR, 2007. Corneal antigen-presenting cells. Chem. Immunol. Allergy 92, 58–70. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR, 2003b. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol 74, 172–178. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Liu Y, Zhang Q, Dana MR, 2003c. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest. Ophthalmol. Vis. Sci 44, 581–589. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Zhang Q, Liu Y, Dana MR, 2002. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest. Ophthalmol. Vis. Sci 43, 639–646. [PubMed] [Google Scholar]
- Hanet MS, Jamart J, Chaves AP, 2012. Fluoroquinolones or fortified antibiotics for treating bacterial keratitis: systematic review and meta-analysis of comparative studies. Can. J. Ophthalmol 47, 493–499. [DOI] [PubMed] [Google Scholar]
- Hara Y, Shiraishi A, Ohashi Y, 2009. Hypoxia-altered signaling pathways of toll-like receptor 4 (TLR4) in human corneal epithelial cells. Mol. Vis 15, 2515–2520. [PMC free article] [PubMed] [Google Scholar]
- Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Brière F, Vlach J, Lebecque S, 2005. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. The Journal of Immunology 174, 2942–2950. [DOI] [PubMed] [Google Scholar]
- Hauser AR, 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol 7, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A, 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. [DOI] [PubMed] [Google Scholar]
- Hazlett L, Wu M, 2011. Defensins in innate immunity. Cell Tissue Res. 343, 175–188. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, 2002. Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell Biol. 21, 383–390. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, 2004. Corneal response to Pseudomonas aeruginosa infection. Progress in retinal and eye research 23, 1–30. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, 2007. Bacterial infections of the cornea (Pseudomonas aeruginosa). Chem. Immunol. Allergy 92, 185–194. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, Berk RS, 1984. Effect of C3 depletion on experimental Pseudomonas aeruginosa ocular infection: histopathological analysis. Infect. Immun 43, 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett LD, Masinick S, Barrett R, Rosol K, 1993. Evidence for asialo GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosa adhesion. Infect. Immun 61, 5164–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett LD, McClellan S, Barrett R, Rudner X, 2001. B7/CD28 costimulation is critical in susceptibility to Pseudomonas aeruginosa corneal infection: a comparative study using monoclonal antibody blockade and CD28-deficient mice. J. Immunol 166, 1292–1299. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, McClellan S, Kwon B, Barrett R, 2000. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest. Ophthalmol. Vis. Sci 41, 805–810. [PubMed] [Google Scholar]
- Hazlett LD, McClellan SA, Rudner XL, Barrett RP, 2002a. The role of Langerhans cells in Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci 43, 189–197. [PubMed] [Google Scholar]
- Hazlett LD, McClellan SM, Hume EB, Dajcs JJ, O’Callaghan RJ, Willcox MD, 1999. Extended wear contact lens usage induces Langerhans cell migration into cornea. Exp. Eye Res 69, 575–577. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, Moon MM, Dawisha S, Berk RS, 1986. Age alters ADPase positive dendritic (Langerhans) cell response to P. aeruginosa ocular challenge. Curr. Eye Res 5, 343–355. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, Moon MM, Strejc M, Berk RS, 1987. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest. Ophthalmol. Vis. Sci 28, 1978–1985. [PubMed] [Google Scholar]
- Hazlett LD, Rosen DD, Berk RS, 1977. Pseudomonas eye infections in cyclophosphamide-treated mice. Invest. Ophthalmol. Vis. Sci 16, 649–652. [PubMed] [Google Scholar]
- Hazlett LD, Rudner XL, McClellan SA, Barrett RP, Lighvani S, 2002b. Role of IL-12 and IFN-gamma in Pseudomonas aeruginosa corneal infection. Invest. Ophthalmol. Vis. Sci 43, 419–424. [PubMed] [Google Scholar]
- Heck LW, Morihara K, Abrahamson DR, 1986. Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect. Immun 54, 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S, 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529. [DOI] [PubMed] [Google Scholar]
- Heimer SR, Evans DJ, Stern ME, Barbieri JT, Yahr T, Fleiszig SMJ, 2013. Pseudomonas aeruginosa Utilizes the Type III Secreted Toxin ExoS to Avoid Acidified Compartments within Epithelial Cells. PLoS One 8, e73111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks R, Janowicz M, Tumpey T, 1992. Critical role of corneal Langerhans cells in the CD4-but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. The Journal of Immunology 148, 2522–2529. [PubMed] [Google Scholar]
- Herretes S, Wang X, Reyes JM, 2014. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database Syst. Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL, 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol 166, 2444–2450. [DOI] [PubMed] [Google Scholar]
- Hessburg PC, Truant JP, Penn WP, 1963. Pseudomonas infections of the cornea in rabbits: an in vivo comparison of polymixin B and colistin sulfate, Proceedings of the 2nd Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, pp. 131–139. [Google Scholar]
- Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH, 1996. Antibacterial activity of antileukoprotease. Infect. Immun 64, 4520–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LJ, Moakes RJA, Vareechon C, Butt G, Ng A, Brock K, Chouhan G, Vincent RC, Abbondante S, Williams RL, Barnes NM, Pearlman E, Wallace GR, Rauz S, Logan A, Grover LM, 2018. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. NPJ Regen Med 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobden JA, Gupta SK, Masinick SA, Wu X, Kernacki KA, Berk RS, Hazlett LD, 1996. Anti-receptor antibodies inhibit Pseudomonas aeruginosa binding to the cornea and prevent corneal perforation. Immunol. Cell Biol 74, 258–264. [DOI] [PubMed] [Google Scholar]
- Hobden JA, Masinick-McClellan S, Barrett RP, Bark KS, Hazlett LD, 1999. Pseudomonas aeruginosa keratitis in knockout mice deficient in intercellular adhesion molecule 1. Infect. Immun 67, 972–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobden JA, Masinick SA, Barrett RP, Hazlett LD, 1995. Aged mice fail to upregulate ICAM-1 after Pseudomonas aeruginosa corneal infection. Invest. Ophthalmol. Vis. Sci 36, 1107–1114. [PubMed] [Google Scholar]
- Holden BA, Sweeney DF, Vannas A, Nilsson KT, Efron N, 1985. Effects of long-term extended contact lens wear on the human cornea. Invest. Ophthalmol. Vis. Sci 26, 1489–1501. [PubMed] [Google Scholar]
- Holmes AR, McNab R, Millsap KW, Rohde M, Hammerschmidt S, Mawdsley JL, Jenkinson HF, 2001. The pavA gene of Streptococcus pneumoniae encodes a fibronectin- binding protein that is essential for virulence. Mol. Microbiol 41, 1395–1408. [DOI] [PubMed] [Google Scholar]
- Hong YQ, Ghebrehiwet B, 1992. Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin. Immunol. Immunopathol 62, 133–138. [DOI] [PubMed] [Google Scholar]
- Hori Y, 2018. Secreted Mucins on the Ocular Surface. Invest. Ophthalmol. Vis. Sci 59, DES151–DES156. [DOI] [PubMed] [Google Scholar]
- Horvat RT, Parmely MJ, 1988. Pseudomonas aeruginosa alkaline protease degrades human gamma interferon and inhibits its bioactivity. Infect. Immun 56, 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Sun X, Wang Z, Zhang Y, 2012. Biofilm-forming capacity of Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa from ocular infections. Invest. Ophthalmol. Vis. Sci 53, 5624–5631. [DOI] [PubMed] [Google Scholar]
- Huang LC, Jean D, Proske RJ, Reins RY, McDermott AM, 2007. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr. Eye Res 32, 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Barrett RP, McClellan SA, Hazlett LD, 2005. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci 46, 4209–4216. [DOI] [PubMed] [Google Scholar]
- Huang X, Hazlett LD, 2003. Analysis of Pseudomonas aeruginosa corneal infection using an oligonucleotide microarray. Invest. Ophthalmol. Vis. Sci 44, 3409–3416. [DOI] [PubMed] [Google Scholar]
- Huang X, Hazlett LD, Du W, Barrett RP, 2006. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J. Immunol 177, 548–556. [DOI] [PubMed] [Google Scholar]
- Huang X, McClellan SA, Barrett RP, Hazlett LD, 2002. IL-18 contributes to host resistance against infection with Pseudomonas aeruginosa through induction of IFN-γ production The Journal of Immunology 168, 5756–5763. [DOI] [PubMed] [Google Scholar]
- Hume EB, Cole N, Garthwaite LL, Khan S, Willcox MD, 2006. A protective role for IL-6 in staphylococcal microbial keratitis. Invest. Ophthalmol. Vis. Sci 47, 4926–4930. [DOI] [PubMed] [Google Scholar]
- Hume EB, Cole N, Khan S, Walsh BJ, Willcox MD, 2020. The role of staphopain a in Staphylococcus aureus keratitis. Exp. Eye Res 193, 107994. [DOI] [PubMed] [Google Scholar]
- Hume EB, Dajcs JJ, Moreau JM, O’Callaghan RJ, 2000. Immunization with alpha-toxin toxoid protects the cornea against tissue damage during experimental Staphylococcus aureus keratitis. Infect. Immun 68, 6052–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume EB, Dajcs JJ, Moreau JM, Sloop GD, Willcox MD, O’Callaghan RJ, 2001. Staphylococcus corneal virulence in a new topical model of infection. Invest. Ophthalmol. Vis. Sci 42, 2904–2908. [PubMed] [Google Scholar]
- Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS, 2010. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun 78, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndiuk RA, 1981. Experimental Pseudomonas keratitis. Transactions of the American Ophthalmological Society 79, 541–624. [PMC free article] [PubMed] [Google Scholar]
- Iglewski BH, Burns RP, Gipson IK, 1977. Pathogenesis of corneal damage from pseudomonas exotoxin A. Invest. Ophthalmol. Vis. Sci 16, 73–76. [PubMed] [Google Scholar]
- Imayasu M, Petroll WM, Jester JV, Patel SK, Ohashi J, Cavanagh HD, 1994. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology 101, 371–388. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ogura Y, Chen FF, Muto A, Nunez G, 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem 276, 2551–2554. [DOI] [PubMed] [Google Scholar]
- Inoue Y, 2014. Review of clinical and basic approaches to corneal endotheliitis. Cornea 33 Suppl 11, S3–8. [DOI] [PubMed] [Google Scholar]
- Jager MJ, Gregerson DS, Streilein JW, 1995. Regulators of immunological responses in the cornea and the anterior chamber of the eye. Eye (Lond.) 9 (Pt 2), 241–246. [DOI] [PubMed] [Google Scholar]
- Janssens S, Beyaert R, 2002. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci 27, 474–482. [DOI] [PubMed] [Google Scholar]
- Jeng BH, Gritz DC, Kumar AB, Holsclaw DS, Porco TC, Smith SD, Whitcher JP, Margolis TP, Wong IG, 2010. Epidemiology of ulcerative keratitis in Northern California. Archives of ophthalmology (Chicago, Ill. : 1960) 128, 1022–1028. [DOI] [PubMed] [Google Scholar]
- Jensch I, Gámez G, Rothe M, Ebert S, Fulde M, Somplatzki D, Bergmann S, Petruschka L, Rohde M, Nau R, 2010. PavB is a surface- exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol. Microbiol 77, 22–43. [DOI] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM, 1996. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea 15, 505–516. [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD, 1997. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea 16, 177–187. [PubMed] [Google Scholar]
- Jett BD, Gilmore MS, 2002a. Host-parasite interactions in Staphylococcus aureus keratitis. DNA Cell Biol. 21, 397–404. [DOI] [PubMed] [Google Scholar]
- Jett BD, Gilmore MS, 2002b. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect. Immun 70, 4697–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanji V, Sharma N, Satpathy G, Titiyal J, 2007. Fourth-generation fluoroquinolone-resistant bacterial keratitis. J. Cataract Refract. Surg 33, 1488–1489. [DOI] [PubMed] [Google Scholar]
- Jimenez-Martinez MC, Santacruz C, Estrada-Garcia I I, Perez-Tapia M, Garfias Y, 2013. Clinical and immunological profile of patients with corneal ulcers due to microbial keratitis (P3157). The Journal of Immunology 190, 43.40–43.40. [Google Scholar]
- Jin X, Qin Q, Chen W, Qu J, 2007. Expression of toll-like receptors in the healthy and herpes simplex virus-infected cornea. Cornea 26, 847–852. [DOI] [PubMed] [Google Scholar]
- Jin X, Qin Q, Tu L, Qu J, 2009. Glucocorticoids inhibit the innate immune system of human corneal fibroblast through their suppression of toll-like receptors. Mol. Vis 15, 2435–2441. [PMC free article] [PubMed] [Google Scholar]
- Jinno A, Hayashida A, Jenkinson HF, Park PW, 2020. Syndecan-1 Promotes Streptococcus pneumoniae Corneal Infection by Facilitating the Assembly of Adhesive Fibronectin Fibrils. mBio 11, e01907–01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E, 2005. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest. Ophthalmol. Vis. Sci 46, 589–595. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Allen JH, 1978. The role of hemolysin in corneal infections with Pseudomonas aeruginosa. Invest. Ophthalmol. Vis. Sci 17, 480–483. [PubMed] [Google Scholar]
- Johnson MK, Hobden JA, Hagenah M, O’Callaghan RJ, Hill JM, Chen S, 1990. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr. Eye Res 9, 1107–1114. [DOI] [PubMed] [Google Scholar]
- Jonas RA, Ung L, Rajaiya J, Chodosh J, 2020. Mystery eye: Human adenovirus and the enigma of epidemic keratoconjunctivitis. Progress in retinal and eye research 76, 100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BR, 1958. The clinical features of viral keratitis and a concept of their pathogenesis. Proc. R. Soc. Med 51, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, 1980. Strategy for the initial management of suspected microbial keratitis, Symposium on medical and surgical diseases of the cornea. Transactions of the New Orleans Academy of Ophthalmology. CV Mosby, St. Louis, Mo, pp. 86–119. [Google Scholar]
- Jones DB, 1978. Pathogenesis of bacterial and fungal keratitis. Trans Ophthalmol Soc U K 98, 367–371. [PubMed] [Google Scholar]
- Jones DB, 1981a. Decision-making in the management of microbial keratitis. Ophthalmology 88, 814–820. [DOI] [PubMed] [Google Scholar]
- Jones DB, 1981b. Polymicrobial keratitis. Transactions of the American Ophthalmological Society 79, 153–167. [PMC free article] [PubMed] [Google Scholar]
- Kaisho T, Akira S, 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22, 78–83. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S, 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol 166, 5688–5694. [DOI] [PubMed] [Google Scholar]
- Kalmodia S, Son KN, Cao D, Lee BS, Surenkhuu B, Shah D, Ali M, Balasubramaniam A, Jain S, Aakalu VK, 2019. Presence of Histatin-1 in Human Tears and Association with Aqueous Deficient Dry Eye Diagnosis: A Preliminary Study. Scientific reports 9, 10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karicherla P, Hobden JA, 2010. Nona-D-arginine amide for prophylaxis and treatment of experimental Pseudomonas aeruginosa keratitis. Curr. Eye Res 35, 220–224. [DOI] [PubMed] [Google Scholar]
- Karthikeyan RS, Priya JL, Leal SM Jr., Toska J, Rietsch A, Prajna V, Pearlman E, Lalitha P, 2013. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS One 8, e64867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye S, Tuft S, Neal T, Tole D, Leeming J, Figueiredo F, Armstrong M, McDonnell P, Tullo A, Parry C, 2010. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Invest. Ophthalmol. Vis. Sci 51, 362–368. [DOI] [PubMed] [Google Scholar]
- Keenan JD, McLeod SD, 2013. Bacterial keratitis. Ophthalmology: Expert Consult: Online and Print, 216–224. [Google Scholar]
- Kelsall BL, Biron CA, Sharma O, Kaye PM, 2002. Dendritic cells at the host-pathogen interface. Nat. Immunol 3, 699–702. [DOI] [PubMed] [Google Scholar]
- Kernacki KA, Barrett RP, Hobden JA, Hazlett LD, 2000. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. The Journal of Immunology 164, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Kernacki KA, Barrett RP, McClellan S, Hazlett LD, 2001. MIP-1alpha regulates CD4+ T cell chemotaxis and indirectly enhances PMN persistence in Pseudomonas aeruginosa corneal infection. J. Leukoc. Biol 70, 911–919. [PubMed] [Google Scholar]
- Kernacki KA, Goebel DJ, Poosch MS, Hazlett LD, 1998a. Early cytokine and chemokine gene expression during Pseudomonas aeruginosa corneal infection in mice. Infect. Immun 66, 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernacki KA, Goebel DJ, Poosch MS, Hazlett LD, 1998b. Early cytokine and chemokine gene expression duringPseudomonas aeruginosa corneal infection in mice. Infect. Immun 66, 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernacki KA, Hobden JA, Hazlett LD, 1999. Murine Model of Bacterial Keratitis, in: Zak O, Sande MA (Eds.), Handbook of Animal Models of Infection. Academic Press, London, pp. 361–366. [Google Scholar]
- Kerr KG, Snelling AM, 2009. Pseudomonas aeruginosa: a formidable and ever-present adversary. J. Hosp. Infect 73, 338–344. [DOI] [PubMed] [Google Scholar]
- Kessler E, Kennah HE, Brown SI, 1977a. Pseudomonas protease. Purification, partial characterization, and its effect on collagen, proteoglycan, and rabbit corneas. Invest. Ophthalmol. Vis. Sci 16, 488–497. [PubMed] [Google Scholar]
- Kessler E, Mondino BJ, Brown SI, 1977b. The corneal response to Pseudomonas aeruginosa: histopathological and enzymatic characterization. Invest. Ophthalmol. Vis. Sci 16, 116–125. [PubMed] [Google Scholar]
- Khatri S, Lass JH, Heinzel FP, Petroll WM, Gomez J, Diaconu E, Kalsow CM, Pearlman E, 2002. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Invest. Ophthalmol. Vis. Sci 43, 2278–2284. [PubMed] [Google Scholar]
- Kijlstra A, 1990. The role of lactoferrin in the nonspecific immune response on the ocular surface. Reg. Immunol 3, 193–197. [PubMed] [Google Scholar]
- Kim JS, Kim JC, Hahn TW, Park WC, 2001. Amniotic membrane transplantation in infectious corneal ulcer. Cornea 20, 720–726. [DOI] [PubMed] [Google Scholar]
- Kimura K, Orita T, Nomi N, Fujitsu Y, Nishida T, Sonoda KH, 2012. Identification of common secreted factors in human corneal fibroblasts exposed to LPS, poly(I:C), or zymosan. Exp. Eye Res 96, 157–162. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N, 2007. Anatomy and immunology of the ocular surface. Chem. Immunol. Allergy 92, 36–49. [DOI] [PubMed] [Google Scholar]
- Konne NM, Collier SA, Spangler J, Cope JR, 2019. Healthy Contact Lens Behaviors Communicated by Eye Care Providers and Recalled by Patients - United States, 2018. MMWR Morb. Mortal. Wkly. Rep 68, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot K, Kosik-Bogacka D, Lanocha-Arendarczyk N, Wojtkowiak-Giera A, Kolasa-Wolosiuk A, 2019. Expression of Toll-Like Receptors (TLR2 and TLR4) in the Eyes of Mice with Disseminated Acanthamoebiasis. Biomed Res Int 2019, 1401894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger AS, Lyerly DM, Hazlett LD, Berk RS, 1986. Immunization against experimental Pseudomonas aeruginosa and Serratia marcescens keratitis. Vaccination with lipopolysaccharide endotoxins and proteases. Invest. Ophthalmol. Vis. Sci 27, 932–939. [PubMed] [Google Scholar]
- Kroken AR, Chen CK, Evans DJ, Yahr TL, Fleiszig SMJ, 2018. The Impact of ExoS on Pseudomonas aeruginosa Internalization by Epithelial Cells Is Independent of fleQ and Correlates with Bistability of Type Three Secretion System Gene Expression. mBio 9, e00668–00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugadas A, Christiansen SH, Sankaranarayanan S, Surana NK, Gauguet S, Kunz R, Fichorova R, Vorup-Jensen T, Gadjeva M, 2016. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis. PLoS Pathog. 12, e1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugadas A, Gadjeva M, 2015. Impact of microbiota on ocular immunity (MUC9P.745). The Journal of Immunology 194, 205.209–205.209. [Google Scholar]
- Kugadas A, Gadjeva M, 2016. The presence of microbiota protects against Pseudomonas aeruginosa induced keratitis. The Journal of Immunology 196, 67.16–67.16. [Google Scholar]
- Kumagai N, Fukuda K, Fujitsu Y, Lu Y, Chikamoto N, Nishida T, 2005. Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci 46, 114–120. [DOI] [PubMed] [Google Scholar]
- Kumar A, Hazlett LD, Yu FS, 2008. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect. Immun 76, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yin J, Zhang J, Yu FS, 2007. Modulation of corneal epithelial innate immune response to pseudomonas infection by flagellin pretreatment. Invest. Ophthalmol. Vis. Sci 48, 4664–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yu FS, 2006. Toll-like receptors and corneal innate immunity. Curr. Mol. Med 6, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu FS, 2004. Innate immune response of corneal epithelial cells to Staphylococcus aureus infection: role of peptidoglycan in stimulating proinflammatory cytokine secretion. Invest. Ophthalmol. Vis. Sci 45, 3513–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu FS, 2006a. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes and infection 8, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu FS, 2006b. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology 117, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferman A, Leibowitz HM, 1976. Quantitation of bacterial infection and antibiotic effect in the cornea. Archives of ophthalmology (Chicago, Ill. : 1960) 94, 1981–1984. [DOI] [PubMed] [Google Scholar]
- Kupferman A, Leibowitz HM, 1979. Topical antibiotic therapy of Pseudomonas aeruginosa keratitis. Archives of ophthalmology (Chicago, Ill. : 1960) 97, 1699–1702. [DOI] [PubMed] [Google Scholar]
- Kwon B, Hazlett LD, 1997. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J. Immunol 159, 6283–6290. [PubMed] [Google Scholar]
- Kwong MS, Evans DJ, Ni M, Cowell BA, Fleiszig SM, 2007. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect. Immun 75, 2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laarman AJ, Bardoel BW, Ruyken M, Fernie J, Milder FJ, van Strijp JA, Rooijakkers SH, 2012. Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J. Immunol 188, 386–393. [DOI] [PubMed] [Google Scholar]
- Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Petroll WM, Cavanagh HD, 2001. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology 108, 1279–1288. [DOI] [PubMed] [Google Scholar]
- Laibson PR, 1973. Current therapy of herpes simplex virus infection of the cornea. International ophthalmology clinics 13, 39–52. [PubMed] [Google Scholar]
- Lalitha P, Manoharan G, Karpagam R, Prajna NV, Srinivasan M, Mascarenhas J, Das M, Porco TC, Lietman TM, Cevallos V, Keenan JD, 2017. Trends in antibiotic resistance in bacterial keratitis isolates from South India. Br. J. Ophthalmol 101, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassance L, Marino GK, Medeiros CS, Thangavadivel S, Wilson SE, 2018. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp. Eye Res 170, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SL, Feil SC, Morton CJ, Farrand AJ, Mulhern TD, Gorman MA, Wade KR, Tweten RK, Parker MW, 2015. Crystal structure of Streptococcus pneumoniae pneumolysin provides key insights into early steps of pore formation. Sci. Rep 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HN, Tran VG, Vu TTT, Gras E, Le VTM, Pinheiro MG, Aguiar-Alves F, Schneider-Smith E, Carter HC, Sellman BR, Stover CK, DiGiandomenico A, Diep BA, 2019. Treatment Efficacy of MEDI3902 in Pseudomonas aeruginosa Bloodstream Infection and Acute Pneumonia Rabbit Models. Antimicrob. Agents Chemother 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Huang S, Huang PL, Sun Y, Huang PL, Kung HF, Blithe DL, Chen HC, 1999. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proceedings of the National Academy of Sciences of the United States of America 96, 2678–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Evans DJ, Fleiszig SM, 2003. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest. Ophthalmol. Vis. Sci 44, 5220–5227. [DOI] [PubMed] [Google Scholar]
- Lee JTY, Wang G, Tam YT, Tam C, 2016. Membrane-Active Epithelial Keratin 6A Fragments (KAMPs) Are Unique Human Antimicrobial Peptides with a Non-αβ Structure Frontiers in Microbiolo y 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leher H, Kinoshita K, Alizadeh H, Zaragoza FL, He Y, Niederkorn J, 1998. Impact of oral immunization with Acanthamoeba antigens on parasite adhesion and corneal infection. Invest. Ophthalmol. Vis. Sci 39, 2337–2343. [PubMed] [Google Scholar]
- Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC, 2001. Regulation of MMP-9 production by human corneal epithelial cells. Exp. Eye Res 73, 449–459. [DOI] [PubMed] [Google Scholar]
- Li Q, Kumar A, Gui J-F, Yu F, 2008. Staphylococcus aureus lipoproteins trigger human corneal epithelial innate response through toll-like receptor-2. Microbial pathogenesis 44, 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hong J, Wei A, Wang X, Chen Y, Cui X, Sun X, Liu Z, Xu J, 2014. Vision-related quality of life in patients with infectious keratitis. Optom. Vis. Sci 91, 278–283. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ, 1997. Contact lens-related microbial keratitis: Part II: Pathophysiology. Cornea 16, 265–273. [PubMed] [Google Scholar]
- Liesegang TJ, 2005. Gram-positive Cocci, in: Tasman W, Jaeger EA (Eds.), Duane’s Foundations of Clinical Ophthalmology. Lippincott Williams & Williams, Philadelphia, pp. 1–15. [Google Scholar]
- Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD, 2005. Substance P regulates natural killer cell interferon-gamma production and resistance to Pseudomonas aeruginosa infection. Eur. J. Immunol 35, 1567–1575. [DOI] [PubMed] [Google Scholar]
- Lin A, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Varu DM, Musch DC, Dunn SP, Mah FS, American Academy of Ophthalmology Preferred Practice Pattern, C., External Disease, P., 2019. Bacterial Keratitis Preferred Practice Pattern(R). Ophthalmology 126, P1–P55. [DOI] [PubMed] [Google Scholar]
- Lin M, Carlson E, Diaconu E, Pearlman E, 2007. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J. Leukoc. Biol 81, 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linna T, Tervo T, 1997. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr. Eye Res 16, 640–649. [DOI] [PubMed] [Google Scholar]
- Little JM, Centifanto YM, Kaufman HE, 1969. Immunoglobulins in human tears. Am. J. Ophthalmol 68, 898–905. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR, 2002. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. The Journal of experimental medicine 195, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kimura K, Yanai R, Chikama T, Nishida T, 2008. Cytokine, chemokine, and adhesion molecule expression mediated by MAPKs in human corneal fibroblasts exposed to poly(I:C). Invest. Ophthalmol. Vis. Sci 49, 3336–3344. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, 2015. Progress in corneal wound healing. Progress in retinal and eye research 49, 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomholt JA, Poulsen K, Kilian M, 2001. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect. Immun 69, 6284–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, Moran TM, 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol 173, 6882–6889. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Martin E, Gallego-Munoz P, Mar S, Fernandez I, Cidad P, Martinez-Garcia MC, 2019. Dynamic changes of the extracellular matrix during corneal wound healing. Exp. Eye Res 186, 107704. [DOI] [PubMed] [Google Scholar]
- Lotti R, Dart JK, 1992. Cataract as a complication of severe microbial keratitis. Eye (Lond.) 6 (Pt 4), 400–403. [DOI] [PubMed] [Google Scholar]
- Luchs JI, Cohen EJ, Rapuano CJ, Laibson PR, 1997. Ulcerative keratitis in bullous keratopathy. Ophthalmology 104, 816–822. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB, 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes and infection 2, 1051–1060. [DOI] [PubMed] [Google Scholar]
- Madigan MC, Holden BA, Kwok LS, 1987. Extended wear of contact lenses can compromise corneal epithelial adhesion. Curr. Eye Res 6, 1257–1260. [DOI] [PubMed] [Google Scholar]
- Maltseva IA, Fleiszig SM, Evans DJ, Kerr S, Sidhu SS, McNamara NA, Basbaum C, 2007. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp. Eye Res 85, 142–153. [DOI] [PubMed] [Google Scholar]
- Mannis M, Smolin G, 1996. Natural defense mechanisms of the ocular surface. Ocular infection and immunity. Mosby, St. Louis, Mo, 185–190. [Google Scholar]
- Marino A, Pergolizzi S, Lauriano ER, Santoro G, Spataro F, Cimino F, Speciale A, Nostro A, Bisignano G, 2015. TLR 2 activation in corneal stromal cells by Staphylococcus aureus- induced keratitis. APMIS 123, 163–168. [DOI] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Bose K, Tam KP, Wilson SE, 2017. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp. Eye Res 161, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart ME, Caballero AR, Chomnawan M, Thibodeaux BA, Twining SS, O’Callaghan RJ, 2005. Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions. Invest. Ophthalmol. Vis. Sci 46, 3761–3768. [DOI] [PubMed] [Google Scholar]
- Marquart ME, Monds KS, McCormick CC, Dixon SN, Sanders ME, Reed JM, McDaniel LS, Caballero AR, O’Callaghan RJ, 2007. Cholesterol as treatment for pneumococcal keratitis: cholesterol-specific inhibition of pneumolysin in the cornea. Invest. Ophthalmol. Vis. Sci 48, 2661–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MU, Wesche H, 2002. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta 1592, 265–280. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J, 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- Masinick SA, Montgomery CP, Montgomery PC, Hazlett LD, 1997. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Invest. Ophthalmol. Vis. Sci 38, 910–918. [PubMed] [Google Scholar]
- Matsumoto K, Ikema K, Tanihara H, 2005. Role of cytokines and chemokines in pseudomonal keratitis. Cornea 24, S43–S49. [DOI] [PubMed] [Google Scholar]
- Mauger TF, Hill RM, 1992. Corneal epithelial healing under contact lenses. Quantitative analysis in the rabbit. Acta ophthalmologica 70, 361–365. [DOI] [PubMed] [Google Scholar]
- McClellan SA, Huang X, Barrett RP, Lighvani S, Zhang Y, Richiert D, Hazlett LD, 2006. Matrix metalloproteinase-9 amplifies the immune response to Pseudomonas aeruginosa corneal infection. Invest. Ophthalmol. Vis. Sci 47, 256–264. [DOI] [PubMed] [Google Scholar]
- McClellan SA, Zhang Y, Barrett RP, Hazlett LD, 2008. Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators downregulated. Invest. Ophthalmol. Vis. Sci 49, 1502–1511. [DOI] [PubMed] [Google Scholar]
- McClintic SM, Prajna NV, Srinivasan M, Mascarenhas J, Lalitha P, Rajaraman R, Oldenburg CE, O’Brien KS, Ray KJ, Acharya NR, Lietman TM, Keenan JD, 2014. Visual outcomes in treated bacterial keratitis: four years of prospective follow-up. Invest. Ophthalmol. Vis. Sci 55, 2935–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, 2013. Antimicrobial compounds in tears. Exp. Eye Res 117, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ, 2003. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci 44, 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald EM, Ram FS, Patel DV, McGhee CN, 2014. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomised controlled trials. Br. J. Ophthalmol 98, 1470–1477. [DOI] [PubMed] [Google Scholar]
- McGavin MJ, Zahradka C, Rice K, Scott JE, 1997. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect. Immun 65, 2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown RL, Coleman Frazier EV, Zadrozny KK, Deleault AM, Raab RW, Ryan DS, Sia RK, Lee JK, Laurie GW, 2014. A Cleavage-potentiated Fragment of Tear Lacritin Is Bactericidal. J. Biol. Chem 289, 22172–22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara NA, Andika R, Kwong M, Sack RA, Fleiszig SM, 2005. Interaction of Pseudomonas aeruginosa with human tear fluid components. Curr. Eye Res 30, 517–525. [DOI] [PubMed] [Google Scholar]
- McNamara NA, Polse KA, Brand RJ, Graham AD, Chan JS, McKenney CD, 1999. Tear mixing under a soft contact lens: effects of lens diameter. Am. J. Ophthalmol 127, 659–665. [DOI] [PubMed] [Google Scholar]
- Medeiros CS, Saikia P, de Oliveira RC, Lassance L, Santhiago MR, Wilson SE, 2019. Descemet’s Membrane Modulation of Posterior Corneal Fibrosis. Invest. Ophthalmol. Vis. Sci 60, 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol 1, 135–145. [DOI] [PubMed] [Google Scholar]
- Metruccio MME, Wan SJ, Horneman H, Kroken AR, Sullivan AB, Truong TN, Mun JJ, Tam CKP, Frith R, Welsh L, George MD, Morris CA, Evans DJ, Fleiszig SMJ, 2019. A novel murine model for contact lens wear reveals clandestine IL-1R dependent corneal parainflammation and susceptibility to microbial keratitis upon inoculation with Pseudomonas aeruginosa. Ocul Surf 17, 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserocchi E, Iuliano L, Berchicci L, Bandello F, Modorati G, 2014. Tear film osmolarity in ocular mucous membrane pemphigoid. Cornea 33, 668–672. [DOI] [PubMed] [Google Scholar]
- Miyazaki D, Uotani R, Inoue M, Haruki T, Shimizu Y, Yakura K, Yamagami S, Suzutani T, Hosogai M, Isomura H, Inoue Y, 2017. Corneal endothelial cells activate innate and acquired arm of anti-viral responses after cytomegalovirus infection. Exp. Eye Res 161, 143–152. [DOI] [PubMed] [Google Scholar]
- Mohammed I, Said DG, Dua HS, 2017. Human antimicrobial peptides in ocular surface defense. Progress in retinal and eye research 61, 1–22. [DOI] [PubMed] [Google Scholar]
- Mondino BJ, Weissman BA, Farb MD, Pettit TH, 1986. Corneal ulcers associated with daily-wear and extended-wear contact lenses. Am. J. Ophthalmol 102, 58–65. [DOI] [PubMed] [Google Scholar]
- Monroe KM, McWhirter SM, Vance RE, 2010. Induction of type I interferons by bacteria. Cell. Microbiol 12, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon MM, Hazlett LD, Hancock RE, Berk RS, Barrett R, 1988. Monoclonal antibodies provide protection against ocular Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci 29, 1277–1284. [PubMed] [Google Scholar]
- Moore QC 3rd, McCormick CC, Norcross EW, Onwubiko C, Sanders ME, Fratkin J, McDaniel LS, O’Callaghan RJ, Marquart ME, 2009. Development of a Streptococcus pneumoniae keratitis model in mice. Ophthalmic Res. 42, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JM, Sloop GD, Engel LS, Hill JM, O’Callaghan RJ, 1997. Histopathological studies of staphylococcal alpha-toxin: effects on rabbit corneas. Curr. Eye Res 16, 1221–1228. [DOI] [PubMed] [Google Scholar]
- Morgan P, Efron N, Hill E, Raynor M, Whiting M, Tullo A, 2005. Incidence of keratitis of varying severity among contact lens wearers. British Journal of Ophthalmology 89, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshirfar M, Mirzaian G, Feiz V, Kang PC, 2006. Fourth-generation fluoroquinolone-resistant bacterial keratitis after refractive surgery. J. Cataract Refract. Surg 32, 515–518. [DOI] [PubMed] [Google Scholar]
- Mun J, Tam C, Chan G, Kim JH, Evans D, Fleiszig S, 2013. MicroRNA-762 Is Upregulated in Human Corneal Epithelial Cells in Response to Tear Fluid and Pseudomonas aeruginosa Antigens and Negatively Regulates the Expression of Host Defense Genes Encoding RNase7 and ST2. PLoS One 8, e57850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun JJ, Tam C, Evans DJ, Fleiszig SMJ, 2011. Modulation of epithelial immunity by mucosal fluid. Sci. Rep 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, Sonnenburg JL, 2013. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SM, 2005. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect. Immun 73, 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Tam C, Verma A, Ramphal R, Hawgood S, Evans DJ, Fleiszig SM, 2008. Expression of surfactant protein D in human corneal epithelial cells is upregulated by Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol 54, 177–184. [DOI] [PubMed] [Google Scholar]
- Nichols JJ, Willcox MD, Bron AJ, Belmonte C, Ciolino JB, Craig JP, Dogru M, Foulks GN, Jones L, Nelson JD, Nichols KK, Purslow C, Schaumberg DA, Stapleton F, Sullivan DA, members of the, T.I.W.o.C.L.D., 2013. The TFOS International Workshop on Contact Lens Discomfort: executive summary. Invest. Ophthalmol. Vis. Sci 54, TFOS7–TFOS13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J, Peeler J, Mellon J, 1989. Phagocytosis of particulate antigens by corneal epithelial cells stimulates interleukin-1 secretion and migration of Langerhans cells into the central cornea. Reg. Immunol 2, 83–90. [PubMed] [Google Scholar]
- Nilsson SE, Montan PG, 1994. The annualized incidence of contact lens induced keratitis in Sweden and its relation to lens type and wear schedule: results of a 3-month prospective study. CLAO J. 20, 225–230. [DOI] [PubMed] [Google Scholar]
- Nishida T, 2010. Commanding roles of keratocytes in health and disease. Cornea 29 Suppl 1, S3–6. [DOI] [PubMed] [Google Scholar]
- Norcross EW, Sanders ME, Moore QC 3rd, Taylor SD, Tullos NA, Caston RR, Dixon SN, Nahm MH, Burton RL, Thompson H, McDaniel LS, Marquart ME, 2011. Active Immunization with Pneumolysin versus 23-Valent Polysaccharide Vaccine for Streptococcus pneumoniae Keratitis. Invest. Ophthalmol. Vis. Sci 52, 9232–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross EW, Tullos NA, Taylor SD, Sanders ME, Marquart ME, 2010. Assessment of Streptococcus pneumoniae capsule in conjunctivitis and keratitis in vivo neuraminidase activity increases in nonencapsulated pneumococci following conjunctival infection. Curr. Eye Res 35, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol 48, 1429–1449. [DOI] [PubMed] [Google Scholar]
- O’Brien KS, Lietman TM, Keenan JD, Whitcher JP, 2015. Microbial keratitis: a community eye health approach. Community Eye Health 28, 1–2. [PMC free article] [PubMed] [Google Scholar]
- O’Brien TP, 2003. Management of bacterial keratitis: beyond exorcism towards consideration of organism and host factors. Eye (Lond.) 17, 957–974. [DOI] [PubMed] [Google Scholar]
- O’Brien TP, 2005. Bacterial Keratitis, in: Foster CS, Azar DT, Dohlman C (Eds.), Smolin and Thoft’s The Cornea: scientific Foundations and Clinical Practice, Fourth ed. Lippincott Williams & Wilkins, pp. 235–288. [Google Scholar]
- O’Callaghan R, Engel L, Hill J, 1999. The rabbit intrastromal injection model of bacterial keratitis, Handbook of animal models of infection. Elsevier, pp. 367–374. [Google Scholar]
- O’Callaghan RJ, 2018. The Pathogenesis of Staphylococcus aureus Eye Infections. Pathogens 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, Hartford OM, Engel LS, Hill JM, 1997. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect. Immun 65, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F, 2004. The role of substance P in inflammatory disease. J. Cell. Physiol 201, 167–180. [DOI] [PubMed] [Google Scholar]
- O’Brien T, Hazlett L, 1996. Patho enesis of ocular infection Ocular infection and immunity Mosby Year Book, St. Louis, Mo, 200–214. [Google Scholar]
- O’Callaghan R, Caballero A, Tan A, Bierdeman M, 2019. Pseudomonas aeru inosa Keratitis: Protease IV and PASP as Corneal Virulence Mediators. Microorganisms 7, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Ko JH, Ryu JS, Lee HJ, Kim MK, Wee WR, 2017. Transcription Profiling of NOD-like Receptors in the Human Cornea with Disease. Ocul. Immunol. Inflamm 25, 364–369. [DOI] [PubMed] [Google Scholar]
- Okonkwo A, Rimmer V, Walkden A, Brahma A, Carley F, McBain AJ, Radhakrishnan H, 2020. Next-Generation Sequencing of the Ocular Surface Microbiome: In Health, Contact Lens Wear, Diabetes, Trachoma, and Dry Eye. Eye Contact Lens 46, 254–261. [DOI] [PubMed] [Google Scholar]
- Ormerod LD, Smith RE, 1986. Contact lens-associated microbial keratitis. Archives of ophthalmology (Chicago, Ill. : 1960) 104, 79–83. [DOI] [PubMed] [Google Scholar]
- Osthoff M, Brown KD, Kong DC, Daniell M, Eisen DP, 2014. Activation of the lectin pathway of complement in experimental human keratitis with Pseudomonas aeruginosa. Mol. Vis 20, 38–45. [PMC free article] [PubMed] [Google Scholar]
- Ozkan J, Coroneo M, Willcox M, Wemheuer B, Thomas T, 2018. Identification and Visualization of a Distinct Microbiome in Ocular Surface Conjunctival Tissue. Invest. Ophthalmol. Vis. Sci 59, 4268–4276. [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A, 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol 191, 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin B, Turner A, Moore E, Cook S, 1997. Bacterial keratitis in the critically ill. Br. J. Ophthalmol 81, 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson GK, Orihuela CJ, 2010. Pneumococcal microbial surface components recognizing adhesive matrix molecules targeting of the extracellular matrix. Mol. Microbiol 77, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti JM, Allen BL, McGavin MJ, Hook M, 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol 48, 585–617. [DOI] [PubMed] [Google Scholar]
- Paugh JR, Stapleton F, Keay L, Ho A, 2001. Tear exchange under hydrogel contact lenses: methodological considerations. Invest. Ophthalmol. Vis. Sci 42, 2813–2820. [PubMed] [Google Scholar]
- Pearlman E, Johnson A, Adhikary G, Sun Y, Chinnery HR, Fox T, Kester M, McMenamin PG, 2008. Toll-like receptors at the ocular surface. Ocul Surf 6, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Sherry DM, McDermott AM, 2004. Thy-1 distinguishes human corneal fibroblasts and myofibroblasts from keratocytes. Exp. Eye Res 79, 705–712. [DOI] [PubMed] [Google Scholar]
- Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P, 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol 170, 4365–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Santonja JJ, Artola A, Javaloy J, Alio JL, Abad JL, 2009. Microbial keratitis after corneal collagen crosslinking. J. Cataract Refract. Surg 35, 1138–1140. [DOI] [PubMed] [Google Scholar]
- Pesci EC, Pearson JP, Seed PC, Iglewski BH, 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol 179, 3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp W, Gottinger W, 1993. Leukocyte adhesion molecules in diseased corneas. Invest. Ophthalmol. Vis. Sci 34, 2449–2459. [PubMed] [Google Scholar]
- Phillips RM, Six DA, Dennis EA, Ghosh P, 2003. In Vivo Phospholipase Activity of the Pseudomonas aeruginosa Cytotoxin ExoU and Protection of Mammalian Cells with Phospholipase A2 Inhibitors*. J. Biol. Chem 278, 41326–41332. [DOI] [PubMed] [Google Scholar]
- Poggio EC, Glynn RJ, Schein OD, Seddon JM, Shannon MJ, Scardino VA, Kenyon KR, 1989. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. The New England journal of medicine 321, 779–783. [DOI] [PubMed] [Google Scholar]
- Prajna NV, Srinivasan M, Mascarenhas J, Lalitha P, Rajaraman R, McClintic SM, O’Brien KS, Ray KJ, Acharya NR, Lietman TM, Keenan JD, 2019. Visual Impairment in Fungal Versus Bacterial Corneal Ulcers 4 Years After Successful Antimicrobial Treatment. Am. J. Ophthalmol 204, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putra I, Rabiee B, Anwar KN, Gidfar S, Shen X, Babalooee M, Ghassemi M, Afsharkhamseh N, Bakhsh S, Missiakas D, Nezamabadi A, Milani B, Eslani M, Djalilian AR, 2019. Staphylococcus aureus alpha-hemolysin impairs corneal epithelial wound healing and promotes intracellular bacterial invasion. Exp. Eye Res 181, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu XD, Lehrer RI, 1998. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect. Immun 66, 2791–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que Y-A, Moreillon P, 2010. Staphylococcus aureus (including staphylococcal toxic shock), in: Mandell GL, Bennett JE, Dolin R (Eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Churchill Livingstone Elsevier, Philadelphia, USA, pp. 2543–2578. [Google Scholar]
- Rajaiya J, Zhou X, Barequet I, Gilmore MS, Chodosh J, 2015. Novel model of innate immunity in corneal infection. In Vitro Cell. Dev. Biol. Anim 51, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R, McNiece MT, Polack FM, 1981. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Annals of ophthalmology 13, 421–425. [PubMed] [Google Scholar]
- Ray KJ, Prajna L, Srinivasan M, Geetha M, Karpagam R, Glidden D, Oldenburg CE, Sun CQ, McLeod SD, Acharya NR, Lietman TM, 2013. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol 131, 310–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KJ, Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Glidden DV, Oldenburg CE, Sun CQ, Zegans ME, McLeod SD, Acharya NR, Lietman TM, 2014. Early addition of topical corticosteroids in the treatment of bacterial keratitis. JAMA Ophthalmol 132, 737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern RL, Reins RY, McDermott AM, 2011. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp. Eye Res 92, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redl B, 2000. Human tear lipocalin. Biochim. Biophys. Acta 1482, 241–248. [DOI] [PubMed] [Google Scholar]
- Reed JM, O’Callaghan RJ, Girgis DO, McCormick CC, Caballero AR, Marquart ME, 2005. Ocular virulence of capsule-deficient streptococcus pneumoniae in a rabbit keratitis model. Invest. Ophthalmol. Vis. Sci 46, 604–608. [DOI] [PubMed] [Google Scholar]
- Regan E, 1950. The lysozyme content of tears. Am. J. Ophthalmol 33, 600–605. [Google Scholar]
- Reichert R, Stern G, 1984. Quantitative adherence of bacteria to human corneal epithelial cells. Archives of ophthalmology (Chicago, Ill. : 1960) 102, 1394–1395. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Borrow P, 2001. The host-pathogen interaction: new themes from dendritic cell biology. Cell 106, 267–270. [DOI] [PubMed] [Google Scholar]
- Rhem MN, Lech EM, Patti JM, McDevitt D, Höök M, Jones DB, Wilhelmus KR, 2000. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect. Immun 68, 3776–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, 2013. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye Contact Lens 39, 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Cavanagh HD, 2008. The Clinical and Cellular Basis of Contact Lens-related Corneal Infections: A Review. Clin. Ophthalmol 2, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MM, Rowden G, Hackett J, Bakos I, 1981. Langerhans cells in the normal conjunctiva and peripheral cornea of selected species. Invest. Ophthalmol. Vis. Sci 21, 759–765. [PubMed] [Google Scholar]
- Rose-Nussbaumer J, Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Srinivasan M, Raghavan A, Oldenburg CE, O’Brien KS, Ray KJ, Porco TC, McLeod SD, Acharya NR, Keenan JD, Lietman TM, Mycotic Ulcer Treatment Trial, G., 2016. Risk factors for low vision related functioning in the Mycotic Ulcer Treatment Trial: a randomised trial comparing natamycin with voriconazole. Br. J. Ophthalmol 100, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld SI, Mandelbaum S, Corrent GF, Pflugfelder SC, Culbertson WW, 1990. Granular epithelial keratopathy as an unusual manifestation of Pseudomonas keratitis associated with extended-wear soft contact lenses. Am. J. Ophthalmol 109, 17–22. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Kernacki KA, Barrett RP, Hazlett LD, 2000. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J. Immunol 164, 6576–6582. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Zheng Z, Berk RS, Irvin RT, Hazlett LD, 1992. Corneal epithelial glycoproteins exhibit Pseudomonas aeruginosa pilus binding activity. Invest. Ophthalmol. Vis. Sci 33, 2185–2193. [PubMed] [Google Scholar]
- Sack RA, Conradi L, Krumholz D, Beaton A, Sathe S, Morris C, 2005. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest. Ophthalmol. Vis. Sci 46, 1228–1238. [DOI] [PubMed] [Google Scholar]
- Sack RA, Nunes I, Beaton A, Morris C, 2001. Host-defense mechanism of the ocular surfaces. Bioscience reports 21, 463–480. [DOI] [PubMed] [Google Scholar]
- Sack RA, Sathe S, Hackworth LA, Willcox MD, Holden BA, Morris CA, 1996. The effect of eye closure on protein and complement deposition on Group IV hydrogel contact lenses: relationship to tear flow dynamics. Curr. Eye Res 15, 1092–1100. [DOI] [PubMed] [Google Scholar]
- Sack RA, Tan KO, Tan A, 1992. Diurnal tear cycle: evidence for a nocturnal inflammatory constitutive tear fluid. Invest. Ophthalmol. Vis. Sci 33, 626–640. [PubMed] [Google Scholar]
- Sakimoto T, Shoji J, Sawa M, 2003. Active form of gelatinases in tear fluidin patients with corneal ulcer or ocular burn. Jpn. J. Ophthalmol 47, 423–426. [DOI] [PubMed] [Google Scholar]
- Salgado-Pabon W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, Peterson ML, Schlievert PM, 2013. Superantigens are critical for Staphylococcus aureus Infective endocarditis, sepsis, and acute kidney injury. mBio 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaridurg PR, Sharma S, Willcox M, Naduvilath TJ, Sweeney DF, Holden BA, Rao GN, 2000. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J. Clin. Microbiol 38, 4420–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Marino GK, Torricelli AA, Wilson SE, 2017. EBM regeneration and changes in EBM component mRNA expression in stromal cells after corneal injury. Mol. Vis 23, 39–51. [PMC free article] [PubMed] [Google Scholar]
- Saraswathi P, Beuerman RW, 2015. Corneal Biofilms: From Planktonic to Microcolony Formation in an Experimental Keratitis Infection with Pseudomonas Aeruginosa. Ocul Surf 13, 331–345. [DOI] [PubMed] [Google Scholar]
- Sato H, Frank DW, 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol 53, 1279–1290. [DOI] [PubMed] [Google Scholar]
- Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR, Group MKS, 1989. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med 321, 773–778. [DOI] [PubMed] [Google Scholar]
- Scurrell E, Stanley R, Schoniger S, 2009. Immunohistochemical detection of NOD1 and NOD2 in the healthy murine and canine eye. Vet. Ophthalmol 12, 269–275. [DOI] [PubMed] [Google Scholar]
- Seal DV, Kirkness CM, Bennett HG, Peterson M, Keratitis Study G, 1999. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye 22, 49–57. [DOI] [PubMed] [Google Scholar]
- Seedor JA, Perry HD, McNamara TF, Golub LM, Buxton DF, Guthrie DS, 1987. Systemic tetracycline treatment of alkali-induced corneal ulceration in rabbits. Archives of ophthalmology (Chicago, Ill. : 1960) 105, 268–271. [DOI] [PubMed] [Google Scholar]
- Seggio M, Nostro A, Ginestra G, Quaglia F, Sortino S, 2019. Contact Lenses Delivering Nitric Oxide under Daylight for Reduction of Bacterial Contamination. Int. J. Mol. Sci 20, 3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger DS, Selinger RC, Reed WP, 1979. Resistance to infection of the external eye: the role of tears. Surv. Ophthalmol 24, 33–38. [DOI] [PubMed] [Google Scholar]
- Sharma A, Taniguchi J, 2017. Review: Emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis. Ocul Surf 15, 670–679. [DOI] [PubMed] [Google Scholar]
- Sharma P, Elofsson M, Roy S, 2020. Attenuation of Pseudomonas aeruginosa infection by INP0341, a salicylidene acylhydrazide, in a murine model of keratitis. Virulence 11, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Price K, Albert L, Dodick J, Park L, Dominguez-Bello MG, 2016. Changes in the Eye Microbiota Associated with Contact Lens Wearing. mBio 7, e00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Jaini R, Torricelli AA, Santhiago MR, Singh N, Ambati BK, Wilson SE, 2014. TGFbeta and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp. Eye Res 121, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusher MM, Myrvik QN, Lewis JC, Gristina AG, 1987. Extended-wear lenses, biofilm, and bacterial adhesion. Archives of ophthalmology (Chicago, Ill. : 1960) 105, 110–115. [DOI] [PubMed] [Google Scholar]
- Somerville TF, Shankar J, Aldwinckle S, Sueke H, Neal T, Horsburgh MJ, Kaye SB, 2020. Recurrent microbial keratitis and endogenous site Staphylococcus aureus colonisation. Scientific reports 10, 18559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotozono C, Ang LP, Koizumi N, Higashihara H, Ueta M, Inatomi T, Yokoi N, Kaido M, Dogru M, Shimazaki J, Tsubota K, Yamada M, Kinoshita S, 2007. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology 114, 1294–1302. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Glidden DV, Ray KJ, Hong KC, Oldenburg CE, Lee SM, Zegans ME, McLeod SD, Lietman TM, Acharya NR, Steroids for Corneal Ulcers Trial, G., 2012. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Archives of ophthalmology (Chicago, Ill. : 1960) 130, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, O’Brien KS, Glidden DV, Ray KJ, Oldenburg CE, Zegans ME, Whitcher JP, McLeod SD, Porco TC, Lietman TM, Acharya NR, Steroids for Corneal Ulcers Trial, G., 2014. The steroids for corneal ulcers trial (SCUT): secondary 12-month clinical outcomes of a randomized controlled trial. Am. J. Ophthalmol 157, 327–333 e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Dart J, 1995. Pseudomonas keratitis associated with biofilm formation on a disposable soft contact lens. Br. J. Ophthalmol 79, 864–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Dart JK, Seal DV, Matheson M, 1995a. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol. Infect 114, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Keay L, Edwards K, Holden B, 2013. The epidemiology of microbial keratitis with silicone hydrogel contact lenses. Eye Contact Lens 39, 79–85. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Keay L, Edwards K, Naduvilath T, Dart JK, Brian G, Holden BA, 2008. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 115, 1655–1662. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Keay L, Jalbert I, Cole N, 2007. The epidemiology of contact lens related infiltrates. Optom. Vis. Sci 84, 257–272. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Willcox MD, Fleming CM, Hickson S, Sweeney DF, Holden BA, 1995b. Changes to the ocular biota with time in extended- and daily-wear disposable contact lens use. Infect. Immun 63, 4501–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern GA, Lubniewski A, Allen C, 1985. The interaction between Pseudomonas aeruginosa and the corneal epithelium: An electron microscopic study. Arch. Ophthalmol 103, 1221–1225. [DOI] [PubMed] [Google Scholar]
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC, 2004. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res 78, 409–416. [DOI] [PubMed] [Google Scholar]
- Steuhl KP, Doring G, Henni A, Thiel HJ, Botzenhart K, 1987. Relevance of host-derived and bacterial factors in Pseudomonas aeruginosa corneal infections. Invest. Ophthalmol. Vis. Sci 28, 1559–1568. [PubMed] [Google Scholar]
- Stewart RMK, Wiehlmann L, Ashelford KE, Preston SJ, Frimmersdorf E, Campbell BJ, Neal TJ, Hall N, Tuft S, Kaye SB, Winstanley C, 2011. Genetic Characterization Indicates that a Specific Subpopulation of Pseudomonas aeruginosa is Associated with Keratitis Infections. J. Clin. Microbiol 49, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV, 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406, 959–964. [DOI] [PubMed] [Google Scholar]
- Streilein JW, 1987. Immune regulation and the eye: a dangerous compromise. FASEB J. 1, 199–208. [PubMed] [Google Scholar]
- Streilein JW, 2003. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol 3, 879–889. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Toews GB, Bergstresser PR, 1979. Corneal allografts fail to express Ia antigens. Nature 282, 326–327. [DOI] [PubMed] [Google Scholar]
- Strober W, Murray PJ, Kitani A, Watanabe T, 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol 6, 9–20. [DOI] [PubMed] [Google Scholar]
- Stuchell RN, Farris RL, Mandel ID, 1981. Basal and reflex human tear analysis. II. Chemical analysis: lactoferrin and lysozyme. Ophthalmology 88, 858–861. [DOI] [PubMed] [Google Scholar]
- Sugrue SP, Zieske JD, 1997. ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp. Eye Res 64, 11–20. [DOI] [PubMed] [Google Scholar]
- Sun Y, Hise AG, Kalsow CM, Pearlman E, 2006. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect. Immun 74, 5325–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, 2010. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and-independent pathways. The Journal of Immunology 185, 4272–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Karmakar M, Taylor PR, Rietsch A, Pearlman E, 2012. ExoS and ExoT ADP Ribosyltransferase Activities Mediate Pseudomonas aeruginosa Keratitis by Promoting Neutrophil Apoptosis and Bacterial Survival. The Journal of Immunology 188, 1884–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szliter EA, Morris CA, Carney F, Gabriel MM, Hazlett LD, 2002. Development of a new extended-wear contact lens model in the rat. CLAO J. 28, 119–123. [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S, 2003. Toll-like receptors. Annu. Rev. Immunol 21, 335–376. [DOI] [PubMed] [Google Scholar]
- Takeda S, Miyazaki D, Sasaki S, Yamamoto Y, Terasaka Y, Yakura K, Yamagami S, Ebihara N, Inoue Y, 2011. Roles played by toll-like receptor-9 in corneal endothelial cells after herpes simplex virus type 1 infection. Invest. Ophthalmol. Vis. Sci 52, 6729–6736. [DOI] [PubMed] [Google Scholar]
- Tam C, LeDue J, Mun JJ, Herzmark P, Robey EA, Evans DJ, Fleiszig SMJ, 2011. 3D Quantitative Imaging of Unprocessed Live Tissue Reveals Epithelial Defense against Bacterial Adhesion and Subsequent Traversal Requires MyD88. PLoS One 6, e24008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C, Mun JJ, Evans DJ, Fleiszig SM, 2010. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest. Ophthalmol. Vis. Sci 51, 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C, Mun JJ, Evans DJ, Fleiszig SM, 2012. Cytokeratins mediate epithelial innate defense through their antimicrobial properties. The Journal of clinical investigation 122, 3665–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DT, Janardhanan P, Zhou H, Chan YH, Htoon HM, Ang LP, Lim LS, 2008. Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology 115, 975–982 e971. [DOI] [PubMed] [Google Scholar]
- Tan KO, Sack RA, Holden BA, Swarbrick HA, 1993. Temporal sequence of changes in tear film composition during sleep. Curr. Eye Res 12, 1001–1007. [DOI] [PubMed] [Google Scholar]
- Taylor SD, Sanders ME, Tullos NA, Stray SJ, Norcross EW, McDaniel LS, Marquart ME, 2013. The cholesterol-dependent cytolysin pneumolysin from Streptococcus pneumoniae binds to lipid raft microdomains in human corneal epithelial cells. PLoS One 8, e61300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa H, 1990. [Adherence of Pseudomonas aeruginosa to the rabbit corneal epithelium]. Nippon Ganka Gakkai Zasshi 94, 269–276. [PubMed] [Google Scholar]
- Tenover FC, 2006. Mechanisms of antimicrobial resistance in bacteria. The American journal of medicine 119, S3–S10. [DOI] [PubMed] [Google Scholar]
- Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M, 2017. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 17, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Barrett RP, McClellan S, Hazlett LD, 2004. Regulation of Pseudomonas aeruginosa corneal infection in IL-1 beta converting enzyme (ICE, caspase-1) deficient mice. Curr. Eye Res 29, 225–233. [DOI] [PubMed] [Google Scholar]
- Thakur A, Xue ML, Wang W, Lloyd A, Wakefield D, Willcox MD, 2001. Expression of macrophage migration inhibitory factor during Pseudomonas keratitis. Clinical & experimental ophthalmology 29, 179–182. [DOI] [PubMed] [Google Scholar]
- Thanabalasuriar A, Scott BNV, Peiseler M, Willson ME, Zeng Z, Warrener P, Keller AE, Surewaard BGJ, Dozier EA, Korhonen JT, Cheng LI, Gadjeva M, Stover CK, DiGiandomenico A, Kubes P, 2019. Neutrophil Extracellular Traps Confine Pseudomonas aeruginosa Ocular Biofilms and Restrict Brain Invasion. Cell Host Microbe 25, 526–536 e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RK, Melton R, Asbell PA, 2019. Antibiotic resistance among ocular pathogens: current trends from the ARMOR surveillance study (2009–2016). Clin Optom (Auckl) 11, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygeson P, Hogan MJ, Kimura SJ, 1960. The unfavorable effect of topical steroid therapy on herpetic keratitis. Transactions of the American Ophthalmological Society 58, 245–262. [PMC free article] [PubMed] [Google Scholar]
- Tjia K, van Putten J, Pels E, Zanen H, 1988. The interaction between Neisseria gonorrhoeae and the human cornea in organ culture. Graefe’s archive for clinical and experimental ophthalmology 226, 341–345. [DOI] [PubMed] [Google Scholar]
- Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF, 1997. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J. Infect. Dis 176, 740–747. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Agrawal V, Santhiago MR, Wilson SE, 2013a. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest. Ophthalmol. Vis. Sci 54, 4026–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE, 2013b. The corneal epithelial basement membrane: structure, function, and disease. Invest. Ophthalmol. Vis. Sci 54, 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP, 2008. agr function in clinical Staphylococcus aureus isolates. Microbiology (Reading) 154, 2265–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MT, Tellaetxe-Isusi M, Elner V, Strieter RM, Lausch RN, Oakes JE, 1996. Proinflammatory cytokines induce RANTES and MCP-1 synthesis in human corneal keratocytes but not in corneal epithelial cells. Beta-chemokine synthesis in corneal cells. Invest. Ophthalmol. Vis. Sci 37, 987–996. [PubMed] [Google Scholar]
- Tullos NA, Thompson HW, Taylor SD, Sanders M, Norcross EW, Tolo I, Moore Q, Marquart ME, 2013. Modulation of immune signaling, bacterial clearance, and corneal integrity by toll-like receptors during streptococcus pneumoniae keratitis. Curr. Eye Res 38, 1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner HC, Budak MT, Akinci MA, Wolosin JM, 2007. Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Invest. Ophthalmol. Vis. Sci 48, 2050–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining SS, Davis SD, Hyndiuk RA, 1986. Relationship between proteases and descemetocele formation in experimental Pseudomonas keratitis. Curr. Eye Res 5, 503–510. [DOI] [PubMed] [Google Scholar]
- Twining SS, Kirschner SE, Mahnke LA, Frank DW, 1993. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Invest. Ophthalmol. Vis. Sci 34, 2699–2712. [PubMed] [Google Scholar]
- Ueta M, 2008. Innate immunity of the ocular surface and ocular surface inflammatory disorders. Cornea 27 Suppl 1, S31–40. [DOI] [PubMed] [Google Scholar]
- Ueta M, Hamuro J, Kiyono H, Kinoshita S, 2005. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem. Biophys. Res. Commun 331, 285–294. [DOI] [PubMed] [Google Scholar]
- Ung L, Acharya NR, Agarwal T, Alfonso EC, Bagga B, Bispo PJ, Burton MJ, Dart JK, Doan T, Fleiszig SM, Garg P, Gilmore MS, Gritz DC, Hazlett LD, Iovieno A, Jhanji V, Kempen JH, Lee CS, Lietman TM, Margolis TP, McLeod SD, Mehta JS, Miller D, Pearlman E, Prajna L, Prajna NV, Seitzman GD, Shanbhag SS, Sharma N, Sharma S, Srinivasan M, Stapleton F, Tan DT, Tandon R, Taylor HR, Tu EY, Tuli SS, Vajpayee RB, Van Gelder RN, Watson SL, Zegans ME, Chodosh J, 2019a. Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull. World Health Organ 97, 854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung L, Bispo PJM, Bryan NC, Andre C, Chodosh J, Gilmore MS, 2020a. The Best of All Worlds: Streptococcus pneumoniae Conjunctivitis through the Lens of Community Ecology and Microbial Biogeography. Microorganisms 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung L, Bispo PJM, Doan T, Van Gelder RN, Gilmore MS, Lietman T, Margolis TP, Zegans ME, Lee CS, Chodosh J, 2020b. Clinical metagenomics for infectious corneal ulcers: Rags to riches? Ocul Surf 18, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J, 2019b. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol 64, 255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung L, Wang Y, Vangel M, Davies EC, Gardiner M, Bispo PJM, Gilmore MS, Chodosh J, 2020c. Validation of a Comprehensive Clinical Algorithm for the Assessment and Treatment of Microbial Keratitis. Am. J. Ophthalmol 214, 97–109. [DOI] [PubMed] [Google Scholar]
- Ustundag-Okur N, Gokce EH, Bozbiyik DI, Egrilmez S, Ertan G, Ozer O, 2015. Novel nanostructured lipid carrier-based inserts for controlled ocular drug delivery: evaluation of corneal bioavailability and treatment efficacy in bacterial keratitis. Expert Opin Drug Deliv 12, 1791–1807. [DOI] [PubMed] [Google Scholar]
- Uusitalo P, Hägglund U, Rhöös E, Scherman Norberg H, Elofsson M, Sundin C, 2017. The salicylidene acylhydrazide INP0341 attenuates Pseudomonas aeruginosa virulence in vitro and in vivo. The Journal of Antibiotics 70, 937–943. [DOI] [PubMed] [Google Scholar]
- Vajpayee RB, Sharma N, Sinha R, Agarwal T, Singhvi A, 2007. Infectious keratitis following keratoplasty. Surv. Ophthalmol 52, 1–12. [DOI] [PubMed] [Google Scholar]
- Valentino MD, McGuire AM, Rosch JW, Bispo PJ, Burnham C, Sanfilippo CM, Carter RA, Zegans ME, Beall B, Earl AM, Tuomanen EI, Morris TW, Haas W, Gilmore MS, 2014. Unencapsulated Streptococcus pneumoniae from conjunctivitis encode variant traits and belong to a distinct phylogenetic cluster. Nat Commun 5, 5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareechon C, Zmina SE, Karmakar M, Pearlman E, Rietsch A, 2017. Pseudomonas aeruginosa Effector ExoS Inhibits ROS Production in Human Neutrophils. Cell Host Microbe 21, 611–618 e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazirani J, Wurity S, Ali MH, 2015. Multidrug-Resistant Pseudomonas aeruginosa Keratitis: Risk Factors, Clinical Characteristics, and Outcomes. Ophthalmology 122, 2110–2114. [DOI] [PubMed] [Google Scholar]
- Vinding T, Eriksen JS, Nielsen NV, 1987. The concentration of lysozyme and secretory IgA in tears from healthy persons with and without contact lens use. Acta ophthalmologica 65, 23–26. [DOI] [PubMed] [Google Scholar]
- Waarts BL, Aneke OJ, Smit JM, Kimata K, Bittman R, Meijer DK, Wilschut J, 2005. Antiviral activity of human lactoferrin: inhibition of alphavirus interaction with heparan sulfate. Virology 333, 284–292. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, Miajlovic H, Gorkun OV, Foster TJ, 2008. Identification of the Staphylococcus aureus MSCRAMM clumping factor B (ClfB) binding site in the alphaC-domain of human fibrinogen. Microbiology (Reading) 154, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan SJ, Sullivan AB, Shieh P, Metruccio MME, Evans DJ, Bertozzi CR, Fleiszig SMJ, 2018. IL-1R and MyD88 Contribute to the Absence of a Bacterial Microbiome on the Healthy Murine Cornea. Front. Microbiol 9, 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Zhu M, Petroll WM, Robertson DM, 2014. Pseudomonas aeruginosa infectious keratitis in a high oxygen transmissible rigid contact lens rabbit model. Invest. Ophthalmol. Vis. Sci 55, 5890–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser JN, Ferreira DM, Paton JC, 2018. Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol 16, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh NH, Rauch AJ, Gaffin SL, 1984. Topical immunotherapy for pseudomonas keratitis in rabbits: use of antilipopolysaccharide plasma. Br. J. Ophthalmol 68, 828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ, 2006. The keratocyte: corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol 38, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcher JP, Srinivasan M, 1997. Corneal ulceration in the developing world--a silent epidemic. Br. J. Ophthalmol 81, 622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M, Lee KM, Greenberg EP, 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America 96, 13904–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek R, Jakobiec FA, Sacks EH, Knowles DM, 1988. The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions. Ophthalmology 95, 100–109. [DOI] [PubMed] [Google Scholar]
- Wilhelmus KR, 2002. Indecision about corticosteroids for bacterial keratitis: an evidence-based update. Ophthalmology 109, 835–842. [DOI] [PubMed] [Google Scholar]
- Wilhelmus R, Dan BJ, 1996. Bacterial keratitis, in Pepose JS, Wilhelmus KR (eds): Ocular Infection and Immunity. St. Citeseer. [Google Scholar]
- Willcox MD, 2007. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom. Vis. Sci 84, 273–278. [DOI] [PubMed] [Google Scholar]
- Willcox MD, Carnt N, Diec J, Naduvilath T, Evans V, Stapleton F, Iskandar S, Harmis N, de la Jara PL, Holden BA, 2010. Contact lens case contamination during daily wear of silicone hydrogels. Optom. Vis. Sci 87, 456–464. [DOI] [PubMed] [Google Scholar]
- Willcox MD, Harmis N, Cowell, Holden Williams, T., 2001. Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials 22, 3235–3247. [DOI] [PubMed] [Google Scholar]
- Willcox MD, Morris CA, Thakur A, Sack RA, Wickson J, Boey W, 1997. Complement and complement regulatory proteins in human tears. Invest. Ophthalmol. Vis. Sci 38, 1–8. [PubMed] [Google Scholar]
- Willcox MDP, Zhu H, Conibear TCR, Hume EBH, Givskov M, Kjelleberg S, Rice SA, 2008. Role of quorum sensing by Pseudomonas aeruginosa in microbial keratitis and cystic fibrosis. Microbiology (Reading) 154, 2184–2194. [DOI] [PubMed] [Google Scholar]
- Wilson SE, 2012. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp. Eye Res 99, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020a. Corneal myofibroblasts and fibrosis. Exp. Eye Res 201, 108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020b. Corneal wound healing. Exp. Eye Res 197, 108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR, 1999. Stromal-epithelial interactions in the cornea. Progress in retinal and eye research 18, 293–309. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He YG, 1994. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp. Eye Res 59, 63–71. [DOI] [PubMed] [Google Scholar]
- Wilson SL, El Haj AJ, Yang Y, 2012. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. J Funct Biomater 3, 642–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C, Kaye SB, Neal TJ, Chilton HJ, Miksch S, Hart CA, And The Microbiology Ophthalmic G., 2005. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J. Med. Microbiol 54, 519–526. [DOI] [PubMed] [Google Scholar]
- Wu XY, Gao JL, Ren MY, 2007. Expression profiles and function of Toll-like receptors in human corneal epithelia. Chin. Med. J. (Engl.) 120, 893–897. [PubMed] [Google Scholar]
- Wu YT, Zhu H, Harmis NY, Iskandar SY, Willcox M, Stapleton F, 2010. Profile and frequency of microbial contamination of contact lens cases. Optom. Vis. Sci 87, E152–158. [DOI] [PubMed] [Google Scholar]
- Xiao A, Dhand C, Leung CM, Beuerman RW, Ramakrishna S, Lakshminarayanan R, 2018. Strategies to design antimicrobial contact lenses and contact lens cases. J Mater Chem B 6, 2171–2186. [DOI] [PubMed] [Google Scholar]
- Xue ML, Thakur A, Willcox MD, Zhu H, Lloyd AR, Wakefield D, 2003a. Role and regulation of CXC-chemokines in acute experimental keratitis. Exp. Eye Res 76, 221–231. [DOI] [PubMed] [Google Scholar]
- Xue ML, Wakefield D, Willcox MD, Lloyd AR, Di Girolamo N, Cole N, Thakur A, 2003b. Regulation of MMPs and TIMPs by IL-1beta during corneal ulceration and infection. Invest. Ophthalmol. Vis. Sci 44, 2020–2025. [DOI] [PubMed] [Google Scholar]
- Yamagami H, Yamagami S, Inoki T, Amano S, Miyata K, 2003. The effects of proinflammatory cytokines on cytokine-chemokine gene expression profiles in the human corneal endothelium. Invest. Ophthalmol. Vis. Sci 44, 514–520. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Hamrah P, Miyamoto K, Miyazaki D, Dekaris I, Dawson T, Lu B, Gerard C, Dana MR, 2005. CCR5 chemokine receptor mediates recruitment of MHC class II-positive Langerhans cells in the mouse corneal epithelium. Invest. Ophthalmol. Vis. Sci 46, 1201–1207. [DOI] [PubMed] [Google Scholar]
- Yamamoto GK, Allansmith MR, 1979. Complement in tears from normal humans. Am. J. Ophthalmol 88, 758–763. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Yamamoto N, Jester JV, Petroll WM, Cavanagh HD, 2006a. Prolonged hypoxia induces lipid raft formation and increases Pseudomonas internalization in vivo after contact lens wear and lid closure. Eye Contact Lens 32, 114–120. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Yamamoto N, Petroll MW, Cavanagh HD, Jester JV, 2005. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens–wearing rabbit and cultured human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci 46, 1348–1355. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Yamamoto N, Petroll MW, Jester JV, Cavanagh HD, 2006b. Regulation of Pseudomonas aeruginosa internalization after contact lens wear in vivo and in serum-free culture by ocular surface cells. Invest. Ophthalmol. Vis. Sci 47, 3430–3440. [DOI] [PubMed] [Google Scholar]
- Yin J, Singh RB, Al Karmi R, Yung A, Yu M, Dana R, 2019. Outcomes of Cyanoacrylate Tissue Adhesive Application in Corneal Thinning and Perforation. Cornea 38, 668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Fitzgerald A, Cozzi PJ, Zhao Z, Graham P, Russell PJ, Walsh BJ, Willcox M, Zhong L, Wasinger V, Li Y, 2010. Post-translation modification of proteins in tears. Electrophoresis 31, 1853–1861. [DOI] [PubMed] [Google Scholar]
- Yu F, Hazlett LD, 2006. Toll-like receptors and the eye. Invest. Ophthalmol. Vis. Sci 47, 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Yin J, Xu K, Huang J, 2010. Growth factors and corneal epithelial wound healing. Brain Res. Bull 81, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi T, Bajmoczi M, Zaidi T, Golan DE, Pier GB, 2008. Disruption of CFTR-dependent lipid rafts reduces bacterial levels and corneal disease in a murine model of Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci 49, 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi T, Mowrey-McKee M, Pier GB, 2004. Hypoxia increases corneal cell expression of CFTR leading to increased Pseudomonas aeruginosa binding, internalization, and initiation of inflammation. Invest. Ophthalmol. Vis. Sci 45, 4066–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi T, Pier GB, 2008. Prophylactic and therapeutic efficacy of a fully human immunoglobulin G1 monoclonal antibody to Pseudomonas aeruginosa alginate in murine keratitis infection. Infect. Immun 76, 4720–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi T, Zaidi T, Cywes-Bentley C, Lu R, Priebe GP, Pier GB, 2014. Microbiota-driven immune cellular maturation is essential for antibody-mediated adaptive immunity to Staphylococcus aureus infection in the eye. Infect. Immun 82, 3483–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi TS, Lyczak J, Preston M, Pier GB, 1999. Cystic Fibrosis Transmembrane Conductance Regulator-Mediated Corneal Epithelial Cell Ingestion of Pseudomonas aeruginosaIs a Key Component in the Pathogenesis of Experimental Murine Keratitis. Infect. Immun 67, 1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi TS, Zaidi T, Pier GB, Priebe GP, 2012. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect. Immun 80, 3706–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegans ME, Van Gelder RN, 2014. Considerations in understanding the ocular surface microbiome. Am. J. Ophthalmol 158, 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu XY, Yu FS, 2005. Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr. Eye Res 30, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu K, Ambati B, Yu FS, 2003. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest. Ophthalmol. Vis. Sci 44, 4247–4254. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Mostov KE, Lamm ME, Nanno M, Shimida S, Ohwaki M, Tuomanen E, 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102, 827–837. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gabriel MM, Mowrey-McKee MF, Barrett RP, McClellan S, Hazlett LD, 2008. Rat Silicone Hydrogel Contact Lens Model: Effects of High vs. Low Dk Lens Wear. Eye & contact lens 34, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wu XY, 2008. Aspergillus fumigatus antigens activate immortalized human corneal epithelial cells via toll-like receptors 2 and 4. Curr. Eye Res 33, 447–454. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW, 2012. In-depth analysis of the human tear proteome. J. Proteomics 75, 3877–3885. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Barrett RP, McClellan SA, Zhang Y, Szliter EA, van Rooijen N, Hazlett LD, 2008. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci 49, 4458–4467. [DOI] [PubMed] [Google Scholar]
- Ziebandt AK, Becher D, Ohlsen K, Hacker J, Hecker M, Engelmann S, 2004. The influence of agr and sigmaB in growth phase dependent regulation of virulence factors in Staphylococcus aureus. Proteomics 4, 3034–3047. [DOI] [PubMed] [Google Scholar]
- Zilliox MJ, Gange WS, Kuffel G, Mores CR, Joyce C, de Bustros P, Bouchard CS, 2020. Assessing the ocular surface microbiome in severe ocular surface diseases. Ocul Surf 18, 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AB, Richdale K, Mitchell GL, Kinoshita BT, Lam DY, Wagner H, Sorbara L, Chalmers RL, Collier SA, Cope JR, Rao MM, Beach MJ, Yoder JS, 2017. Water Exposure is a Common Risk Behavior Among Soft and Gas-Permeable Contact Lens Wearers. Cornea 36, 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]


