Abstract
Background:
The purpose of this study was to examine the incidence of acute kidney injury and chronic renal impairment following branched endovascular aneurysm repair (BEVAR) of complex thoracoabdominal aortic aneurysms (TAAA) using the Medtronic Valiant Thoracoabdominal Aortic Aneurysm stent graft system (MVM), the physician-modified Visceral Manifold, and Unitary Manifold stent graft systems. The objective was to report the acute and chronic renal function changes in patients following complex TAAA aneurysm repair.
Methods:
This is an analysis of 139 patients undergoing branched endovascular repair for complex TAAAs between 2012 and 2020. Patient renal function was evaluated using serum creatinine and estimated glomerular filtration rate at baseline, 48 hr, discharge, 1 month, 6 months, and annually to 2 years. Patients on dialysis prior to the procedure were excluded from data analysis.
Results:
A total of 139 patients (mean age 71.13; 64.7% male) treated for TAAA with BEVAR met inclusion criteria and were evaluated. A total of 530 visceral vessels were stented. A majority of patients (n = 131, 94.2%) underwent a single procedure while 8 required staged procedures. Thirty-day, 1-year and 2-year all-cause mortality rates were 5.8%, 25.2%, and 32.4%, respectively. Primary and secondary patency rates at a median follow-up of 26.9 months (95% CI; 21.1 – 32.7) were 96.2% and 97.5% for all vessels and 95.4% and 96.9% for renal arteries, respectively. Postoperative acute kidney injury (AKI) was identified in 22 (15.8%) patients. At discharge, 16 patients (11.6%) had an increase in CKD stage with 3 requiring permanent dialysis. Five additional patients required permanent dialysis over the 2-year follow-up period for a total of 8 (5.8%). Increasing age (HR = 1.0327, P = 0.0477), hemoglobin < 7 prior to procedure (HR = 2.4812, P = 0.0093), increasing maximum aortic diameter (HR = 1.0189, P = 0.0084), presence of AKI (HR = 2.0757, P = 0.0182), and increase in CKD stage (HR = 1.3520, P = 0.002) at discharge were significantly associated with decreased patient survival.
Conclusions:
Postoperative AKI and a chronic decline in renal function continue to be problematic in endovascular repair of complex aortic aneurysms. This study found that BEVAR using the manifold configuration resulted in immediate and mid-term renal function that is comparable to similar analyses of branched and/or fenestrated grafts.
INTRODUCTION
Open surgical repair has historically been the standard of care in the management of thoracoabdominal aortic aneurysms (TAAA). First described in 2001, endovascular TAAA repair is less invasive and may be offered to patients who would previously not have been candidates for open repair due to underlying comorbidities.1,2 Recent studies comparing open and endovascular repair for complex aneurysm have revealed similar outcomes in regard to perioperative mortality, permanent dialysis, cerebrovascular accident, and permanent paraplegia.3,4 The continued development and refinement of endovascular repair options for complex aneurysms is encouraging, however, postoperative acute kidney injury (AKI) and long-term decline in renal function remain problematic. Renal complications have been associated with significant increases in major postoperative complications, length of hospital stays, and mortality, yet the pathophysiology of decreased renal function following repair remains incompletely understood.4
Patency of the renal arteries is a concern present in both open and endovascular complex aneurysm repairs. Small renal artery diameter (<4.0 mm)5 invites complications of poor patency. Another factor is the complicating flow dynamics of the renal arteries, as they arise relatively perpendicular from the aorta. This alters the natural physiologic flow diversion with any intervention of the proximal renal arteries.6,7 These factors, and perhaps others, contribute to making repair of aortic aneurysms with involvement of the renal vessels especially difficult. Current options for endovascular repair of TAAA are the use of fenestrated (FEVAR) versus branched (branched endovascular aneurysm repair, BEVAR) grafts.
The Medtronic Valiant Thoracoabdominal Aortic Aneurysm multibranch system (MVM; Medtronic, Dublin, Ireland), physician-modified Visceral Manifold (pmMVM),8 and Unitary Manifold (UVM)9 stent graft systems for patients with TAAA have been under investigation since 2012. The objective of this study is to analyze the incidence of AKI and chronic kidney disease (CKD) following complex branch aneurysm repair and other factors associated with a decline in renal function comparing patients at high and normal risk for renal dysfunction.
MATERIALS AND METHODS
Patient Selection
Consecutive patients from 2012–2020 were treated for TAAA with 127 enrolled in 5 early feasibility, prospectively maintained, non-randomized, physician-sponsored investigational device exemption (PS-IDE) studies. Four centers evaluated only the Medtronic MVM while 1 center evaluated the Medtronic MVM, physician-modified MVM (pmMVM), and physician-modified UVM. An additional 25 patients were treated with the pmMVM prior to the initiation of a PS-IDE with their data collected retrospectively. Study sites included Sanford Health (Sioux Falls, SD), University of South Florida (Tampa, FL), The Christ Hospital (Cincinnati, OH), NYU Langone Medical Center (New York, NY), and Vanderbilt University Medical Center (Nashville, TN). The studies were approved by the Institutional Review Board at each institution requiring informed consent of participants. Patients who were on dialysis at the time of procedure, intentionally did not have their renal arteries stented, or whose procedures were staged but not completed at the time of this analysis were excluded (n = 13).
Patients with Crawford type I–V, paravisceral, pararenal, and short-neck infrarenal aneurysms as well as dissection were treated. Patients were not excluded for urgent or emergent presentation. Patients were treated with either the MVM or UVM stent graft system based on their anatomy if they did not meet the instructions for use of a commercially available device.
Patient Monitoring
Aneurysm morphology was assessed pre- and postoperatively with computed tomography angiography when renal function allowed. For patients with poor renal function, computed tomography without contrast in combination with duplex ultrasound was used. Patients were observed at 1 month, 6 months, 12 months, and annually for 5 years. Postoperative assessment included freedom from major adverse events and technical success (freedom from aneurysm enlargement, aneurysm rupture, conversion to open repair, secondary intervention for type I and III endoleaks, patency-related events, and device integrity failure).
Devices and Procedures
Devices used for TAAA repair in this study include the physician-modified MVM (n = 26), Medtronic MVM (n = 51), and physician-modified UVM (n = 62). The MVM was designed for the treatment of Crawford extent I, II, III and V thoracoabdominal aneurysms, and the UVM was designed for repair of Crawford type IV, juxtarenal, pararenal, and short-neck infrarenal aneurysms (<10 mm) (Table 1).8 The MVM and UVM configurations are non-anatomic designs and have similar near-wall hemodynamics based on Computational Fluid Dynamics simulations, which demonstrate the establishment of laminar flow before entering the visceral vessels.10 Therefore, data was not categorized based on device configuration.
Table I.
Overall characteristics of all patients (N = 139)
| Characteristics | All patients (N = 139) |
|---|---|
|
| |
| Age | 71.13 (8.49) |
| Male | 90 (64.7) |
| CHF | 35 (25.2) |
| Hypertension | 123 (88.5) |
| Smoking | 123 (88.5) |
| COPD | 66 (47.5) |
| Diabetes | 26 (18.7) |
| History of Stroke | 37 (26.6) |
| Baseline Egfr | 64.00 [52.50, 82.50] |
| Baseline Creatinine | 1.05 [0.83, 1.24] |
| Baseline CKD Stage 3/4/5 | 24 (17.3) |
| Symptomatic | 44 (31.7) |
| Active or Contained Rupture | 5 (3.6) |
| Aortic Angulation | |
| Thoracic n = 129 | 35.00 [19.80, 56.10] |
| Diaphragm n = 132 | 30.00 [20.00, 45.00] |
| Renal n = 132 | 24.75 [12.05, 42.60] |
| ASA Score III/IV/V | 139 (100.0) |
| Hemoglobin < 7 | 12 (8.6) |
| Total Contrast (mL) | 91.00 [55.50, 145.00] |
| Total Intervention time (min) | 273.00 [200.50, 406.00] |
| Total Fluoroscopy time (min) | 82.40 [66.30, 113.60] |
| Accessory renal sacrifice (n = 112) | 9 (8.0) |
| Previous interventions | |
| Endovascular | 38 (27.3) |
| Open | 19 (13.7) |
| Both Endovascular and Open | 7 (5.0) |
| Disease type | |
| Crawford I–III Aneurysm | 56 (40.3) |
| Crawford IV Aneurysm | 39 (28.1) |
| Crawford V Aneurysm | 2 (1.4) |
| Para/juxtarenal Aneurysm | 31 (22.3) |
| Retrograde Type B Aortic Dissection | 11 (7.9) |
| Maximum Aortic diameter (mm) | 61.00 [55.00, 70.00] |
ASA, American Society of Anesthesiologists; CHF, Congestive Heart Failure; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease.
Categorical variables are presented as number (%). Continuous variables are presented as mean (standard deviation) or median [interquartile range].
The device used was chosen based on the extent of the aneurysm or aortic dissection. Patients were treated despite degree of aortic tortuosity, branch occlusions, rupture, dissection, or previous stent placement. Sizing of the thoracic bifurcation or unitary device was based on the diameter of the proximal seal zone. Consideration was given to the diameter below the celiac artery and SMA due to potential crowding. An aortic diameter of less than 21 mm has been prohibitive without sacrificing the celiac artery. Of the 112 patients for whom the presence or absence of accessory renal arteries was recorded, only the dominant branch determined intraoperatively by angiography was stented, sacrificing the accessory branch. Therefore, a maximum of 4 arteries were stented for each patient. During the index procedure, a combination of iodinated contrast and CO2 gas was utilized to minimize the amount of iodinated contrast exposure to the patient, especially in those presenting with underlying renal insufficiency. Staged procedures involved 1 of the 3 protocol defined methods: controlled endoleak, thoracic stenting, or iliac limb technique. The controlled endoleak technique refers to leaving a portion of the covered bridging stent between the visceral vessel and the manifold limb open for a short segment using a bare metal stent, thus creating a controlled Type IV endoleak and allowing sac perfusion, which is thought to reduce the risk of spinal cord ischemia. Graft development and further procedural techniques are described extensively in prior publications.8,9
Analysis of Renal Function
AKI is defined by the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) classification, which includes an increase of serum creatinine (SCr) ≥ 0.3mg/dl (≥26.5μmol/l) within 48 hr or increase in SCr ≥ 1.5 times baseline within 7 days.11 Stages of chronic renal decline are defined by the National Kidney Foundation clinical practice guidelines.12,13 Estimated glomerular filtration rate (eGFR) is calculated using the Modification of Diet in Renal Disease formula.14 SCr was obtained for each patient preoperatively, 48 hr postoperatively, just prior to discharge, at 1 month, 6 months, and annually thereafter.
For analysis of CKD stage changes, patients were divided into either a high-risk for postoperative dialysis group or a normal risk for postoperative dialysis group. High-risk patients are defined by a presence of a single kidney, creatinine ≥ 2.0 mg/dL, or history of dialysis. All patients were monitored for AKI and CKD stage changes at the above intervals.
Statistical Analysis
Assessment of normality of all continuous characteristics showed creatinine and eGFR measures as not normally distributed. Therefore, median and interquartile ranges are reported. The nonparametric Mann-Whitney U test was used to analyze differences between groups. Patient age was normally distributed and consequently analyzed using Welch’s t-test and reported as mean and standard deviation. Discrete variables are reported as counts and percentages and analyzed using Fisher’s exact test or Chi-squared tests. Fisher’s exact test was used for comparison of eGFR classification between groups. Paired comparison of non-normally distributed data was analyzed using the Wilcoxon signed-rank test. The association between occlusions and patient characteristics, including stent type, was assessed using Welch’s t-test or Fisher’s exact test. Survival analysis was performed using Kaplan-Meier estimates and log rank tests to compare groups. Statistical significance was defined by P< 0.05. Data were analyzed using R version 4.0.015 utilizing tableone,16 survival,17,18 and survminer.19
RESULTS
Of 152 patients who were treated for TAAA with either the MVM or UVM from 2012–2020, 139 were included in this analysis. Exclusion criteria is included in the methods section. Eight patients had staged procedures while 131 were completed in a single procedure. Six patients (4.0%) were treated emergently, and 10 (7.2%) were treated urgently. Of the 16 patients treated emergently or urgently, 5 (31.3%) presented with either an active or contained rupture. Two of the 5 patients with ruptures ended up on permanent dialysis (40.0%) however, there was no statistical significance between post-operative renal function and rupture. 530 of 534 vessels were stented, including 132 celiac arteries, 139 SMA arteries, and 259 renal arteries. Further patient characteristics can be found in Table I. Thirty-five patients (25.2%) had prior infrarenal repair resulting in the potential for jailed visceral vessels due to suprarenal fixation. Of these, renal artery access was possible for 33 patients, while 2 patients (2/35, 5.7%) had suprarenal fixation from previously placed Cook Zenith Flex infrarenal bifurcated device plus added cuff, preventing access to 3 of the 4 renal arteries. Over the course of the study, these are the only renal arteries that were unable to be accessed due to suprarenal fixation.
Renal Function Analysis
AKI was identified postoperatively in 22 (15.8%) of 139 patients. There was no significant difference in baseline SCr (P= 0.5199) for AKI versus non-AKI patients or baseline eGFR (P= 0.6348) for AKI versus non-AKI patients. Interestingly, there was also no significant difference between pre- and 48-hr postoperative SCr or eGFR (P= 0.7219 and P= 0.7392, respectively) (Table II). Median SCr levels at discharge were significantly lower than preoperative levels (P< 0.0001), and discharge eGFR levels were significantly higher (P= 0.0013). Though there was no identified clinical detriment, SCr values become statistically higher, and eGFR values become statistically lower than preoperative levels at 1-month follow-up and remained consistent through 2 years of follow-up (Table II). Stage changes per patient are represented in Supplemental Figure 1. Risk factors significantly associated with greater incidence of AKI were intervention time (P= 0.0023) and fluoroscopy time (P= 0.0041). No significance was found for age, gender, history of renal failure, type of aneurysm treated, amount of contrast used, moderate-severe CKD stage before intervention, sacrifice of accessory renal arteries, aortic angulation, active or contained rupture, presence of symptoms, or other patient characteristics (Table III).
Table II.
Creatinine and eGFR changes from baseline
| N | Pre | Post | P value | ||
|---|---|---|---|---|---|
|
| |||||
| Creatinine (median [IQR]) | |||||
| Pre-Op/Screening | 48 Hr | 139 | 1.05 [0.83, 1.24] | 1.04 [0.81, 1.37] | 0.7219 |
| Pre-Op/Screening | Pre-Discharge | 138 | 1.06 [0.83, 1.24] | 0.90 [0.72, 1.29] | <0.0001 |
| Pre-Op/Screening | 1 Month | 101 | 1.05 [0.82, 1.24] | 1.13 [0.84, 1.47] | 0.0474 |
| Pre-Op/Screening | 6 Months | 76 | 1.00 [0.82, 1.22] | 1.15 [0.90, 1.48] | 0.0011 |
| Pre-Op/Screening | 12 Months | 70 | 1.14 [0.84, 1.35] | 1.27 [0.92, 1.66] | 0.0023 |
| Pre-Op/Screening | 24 Months | 35 | 1.07 [0.82, 1.29] | 1.28 [0.96, 1.60] | 0.0002 |
| eGFR (median [IQR]) | |||||
| Pre-Op/Screening | 48 Hr | 139 | 64.00 [52.50, 82.50] | 66.00 [46.00, 88.50] | 0.7392 |
| Pre-Op/Screening | Pre-Discharge | 138 | 64.00 [53.00, 82.75] | 73.50 [48.25, 90.00] | 0.0013 |
| Pre-Op/Screening | 1 Month | 101 | 66.00 [52.00, 86.00] | 60.00 [44.00, 81.00] | 0.0126 |
| Pre-Op/Screening | 6 Months | 76 | 66.50 [55.25, 86.25] | 58.00 [40.50, 73.00] | <0.0001 |
| Pre-Op/Screening | 12 Months | 70 | 60.50 [50.25, 74.75] | 51.50 [37.25, 68.25] | 0.0003 |
| Pre-Op/Screening | 24 Months | 35 | 64.00 [52.00, 85.00] | 54.00 [39.50, 63.50] | 0.0002 |
Table III.
Comparison of AKI and non-AKI patients
| Characteristics | AKI (n = 22) | Non-AKI (n = 117) | P value |
|---|---|---|---|
|
| |||
| Age | 69.47 (6.65) | 71.44 (8.79) | 0.2354 |
| Male | 13 (59.1) | 77 (65.8) | 0.5449 |
| Congestive Heart Failure | 4 (18.2) | 31 (26.5) | 0.5932 |
| Hypertension | 19 (86.4) | 104 (88.9) | 0.7193 |
| Smoking | 22 (100.0) | 101 (86.3) | 0.0752 |
| COPD | 10 (45.5) | 56 (47.9) | 1.0000 |
| Diabetes | 4 (18.2) | 22 (18.8) | 1.0000 |
| History of Stroke | 4 (18.2) | 33 (28.2) | 0.4346 |
| Baseline eGFR | 65.50 [58.25, 81.50] | 63.00 [52.00, 85.00] | 0.6348 |
| Baseline Creatinine | 0.92 [0.82, 1.24] | 1.06 [0.83, 1.24] | 0.5199 |
| Baseline CKD Stage 3/4/5 | 3 (13.6) | 21 (17.9) | 0.7658 |
| Symptomatic | 8 (36.4) | 36 (30.8) | 0.6229 |
| Active or Contained Rupture | 1 (4.5) | 4 (3.4) | 0.5833 |
| Aortic Angulation | |||
| Thoracic | 48.45 [22.50, 73.38] | 30.00 [15.00, 52.10] | 0.0654 |
| Diaphragm | 42.70 [25.75, 100.00] | 30.00 [20.00, 36.67] | 0.0227 |
| Renal | 31.35 [16.25, 77.50] | 20.90 [11.00, 38.83] | 0.0951 |
| Hemoglobin < 7 | 4 (18.2) | 8 (6.8) | 0.0984 |
| ASA Score III/IV/V | 22 (100.0) | 117 (100.0) | NA |
| Contrast Volume (mL) | 78.00 [54.75, 173.50] | 91.00 [57.00, 140.00] | 0.9563 |
| Intervention Time (min) | 398.50 [275.25, 511.00] | 254.00 [197.00, 375.00] | 0.0023 |
| Fluoroscopy Time (min) | 117.00 [87.30, 157.30] | 77.10 [65.40, 108.70] | 0.0041 |
| Accessory renal sacrifice | 1 (6.7) | 8 (8.2) | 1.0000 |
| Aneurysm type | 0.2655 | ||
| Crawford I-III Aneurysm | 13 (59.1) | 43 (36.8) | |
| Crawford IV Aneurysm | 6 (27.3) | 33 (28.2) | |
| Crawford V Aneurysm | 0 (0.0) | 2 (1.7) | |
| Para/juxtarenal Aneurysm | 3 (13.6) | 28 (23.9) | |
| Retrograde Type B Aortic Dissection | 0 (0.0) | 11 (9.4) | |
CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease.
Categorical variables are presented as number (%). Continuous variables are presented as mean (standard deviation) or median [interquartile range].
One patient died within the first 48 hr postoperatively; thus, post-discharge data is only available for 138 patients. At the time of discharge, 122 of 138 (88.4%) patients had their CKD stage remain the same or improve. Sixteen of 138 (11.6%) experienced an increase in CKD stage with 3 patients requiring permanent dialysis at discharge. Over the first year of follow-up, 5 additional patients required permanent dialysis. Currently, 33 patients have reached 2 years of follow-up with only 4 (12.1%) experiencing an increase in CKD stage, though no additional patients required permanent dialysis. The remaining 29 (87.9%) patients experienced either sustained or improved renal function (Table IV). No clinically significant difference in renal function was observed in patients who underwent staged procedures (8 of 139, 5.8%) or those who had an accessory renal artery sacrificed (9 of 112, 8.0%). Patient characteristics were not significantly different for patients who underwent staged procedures compared to those that did not.
Table IV.
Chronic kidney disease stage change by time point
| Baseline to Discharge n = 138a | Discharge to 1 Month n = 124 | 1 Month to 6 Months n = 88b | 6 Months to 12 Month n = 69b | 12 Months to 24 Months n = 33c | |
|---|---|---|---|---|---|
|
| |||||
| Better | 20 (14.5) | 5 (4.0) | 5 (5.7) | 7 (10.1) | 4 (12.1) |
| Same | 102 (73.9) | 96 (77.4) | 71 (80.7) | 53 (76.8) | 25 (75.8) |
| Worse | 16 (11.6) | 23 (18.5) | 12 (13.6) | 9 (13.0) | 4 (12.1) |
| Permanent Dialysisd | 3 (2.2) | 1 (0.8) | 1 (1.1) | 3 (4.3) | 0 (0.0) |
One patient did not have discharge data
One patient did not have eGFR data front the previous time point
Two patients did not have eGFR data from the previous time point
Dialysis patients are not included in the n, as they are a subset of patients with worsened renal function.
Risk Stratification
Patients stratified into the high-risk category (30/139, 21.6%) had an in-hospital dialysis rate of 16.7% (5/30) with 3 of the 5 requiring only temporary dialysis. The normal risk group (109/139, 78.4%) had an in-hospital dialysis rate of 1.8% (2/109) with 1 of the 2 requiring temporary dialysis. Following discharge, dialysis was initiated in 9 additional patients (9/139, 6.5%) during the first year with 4 being considered high-risk for dialysis at the time of procedure.
Of the 5 patients for whom dialysis was initiated after discharge in the normal risk group, 2 required temporary dialysis, while the remaining 3 required permanent dialysis. While all 3 patients requiring permanent dialysis after discharge presented with renal arteries measuring 4 mm or less, only 1 required dialysis due to a 4-vessel occlusion that occurred on post-op day twelve. Following lytic therapy and thrombectomy, all 4 vessels were patent. Unfortunately, a retroperitoneal bleed from the left renal artery resulted in the embolization of the left renal artery. The remaining 2 patients experienced an overall decline in health at 6 months and 1 year resulting in the need for permanent dialysis.
Two patients in the high-risk category required permanent dialysis at 1 year due to progression of kidney disease while 2 required temporary dialysis following device occlusion, which resolved with successful re-intervention. No new patients required dialysis at 2 years in either group. Altogether, 8 total patients (8/139, 5.8%) required permanent dialysis over the 2-year follow-up period.
A greater number of high-risk patients (60.0 vs. 5.5%) had only 3 vessels stented (94.5% of normal risk had 4 vessels) with 17/30 (56.7%) having 1 patent renal artery. There was significantly less iodinated contrast used during the index procedure for high-risk patients (P= 0.0305) due to the use of CO2 contrast to minimize the risk of kidney injury in those patients. High-risk patients also had more Crawford types I-III aneurysms (66.7 vs. 33.0%) and greater proportions of patients with CHF (P= 0.0038) and Hgb < 7 (P= 0.0022) prior to intervention In concordance with criteria for “high-risk” designation, these patients had significantly higher SCr (P< 0.0001), lower eGFR (P< 0.0001), and more patients with CKD stage of 3 or greater (P< 0.0001) prior to intervention (Table V).
Table V.
Comparison of high risk and normal risk patients
| Characteristics | High risk (n = 30) | Normal risk (n = 109) | P value |
|---|---|---|---|
|
| |||
| Age | 73.34 (8.77) | 70.52 (8.35) | 0.1227 |
| Male | 20 (66.7) | 70 (64.2) | 0.8038 |
| Congestive Heart Failure | 14 (46.7) | 21 (19.3) | 0.0039 |
| Hypertension | 29 (96.7) | 94 (86.2) | 0.1933 |
| Smoking | 27 (90.0) | 96 (88.1) | 1.0000 |
| COPD | 15 (50.0) | 51 (46.8) | 0.8375 |
| Diabetes | 9 (30.0) | 17 (15.6) | 0.1096 |
| History of Stroke | 10 (33.3) | 27 (24.8) | 0.3582 |
| History of Renal Failure | 3 (10.0) | 0 (0.0) | 0.0093 |
| Baseline eGFR | 47.00 [31.25, 60.50] | 68.00 [59.00, 87.00] | <0.0001 |
| Baseline Creatinine | 1.39 [1.14, 2.08] | 0.97 [0.81, 1.18] | <0.0001 |
| CKD Stage 3/4/5 | 14 (46.7) | 10 (9.2) | <0.0001 |
| Symptomatic | 12 (40.0) | 32 (29.4) | 0.2760 |
| Active or Contained Rupture | 1 (3.3) | 4 (3.7) | 1.0000 |
| Aortic Angulation | |||
| Thoracic | 45.00 [20.00, 70.00] | 30.00 [18.00, 50.50] | 0.1355 |
| Diaphragm | 30.00 [20.00, 45.00] | 30.00 [20.00, 42.50] | 0.5696 |
| Renal | 30.00 [15.00, 45.00] | 21.00 [10.00, 37.65] | 0.1665 |
| Hemoglobin < 7 | 6 (20.0) | 6 (5.5) | 0.0222 |
| ASA Score III/IV/V | 30 (100.0) | 109 (100.0) | NA |
| Contrast volume (mL) | 64.00 [44.00, 109.75] | 97.00 [67.00, 150.00] | 0.0305 |
| Intervention time (min) | 271.00 [199.50, 459.25] | 273.00 [202.00, 396.00] | 0.7567 |
| Fluoroscopy time (min) | 80.65 [68.00, 137.40] | 82.40 [65.70, 111.20] | 0.4245 |
| Accessory renal sacrifice | 0 (0.0) | 9 (10.5) | 0.1139 |
| Disease type | 0.0247 | ||
| Crawford I-III Aneurysm | 20 (66.7) | 36 (33.0) | |
| Crawford IV Aneurysm | 4 (13.3) | 35 (32.1) | |
| Crawford V Aneurysm | 0 (0.0) | 2 (1.8) | |
| Para/juxtarenal Aneurysm | 5 (16.7) | 26 (23.9) | |
| Type B Aortic Dissection | 1 (3.3) | 10 (9.2) | |
| Number of vessels stented | <0.0001 | ||
| 2 | 1 (3.3) | 0 (0.0) | |
| 3 | 18 (60.0) | 6 (5.5) | |
| 4 | 11 (36.7) | 103 (94.5) | |
ASA, American Society of Anesthesiologists; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease.
Categorical variables are presented as number (%); Continuous variables are presented as mean (standard deviation) or median [interquartile range].
Complications and Re-interventions
Postoperative complications for any visceral vessel including occlusions, dissection, type I or III endoleaks, or loss of device integrity occurred in 13 of 139 patients (9.4%). Seven of 139 patients (5.0%) required re-interventions for any branch complications with patency achieved in 12/12 (100%) total vessels. Primary and secondary patency rates at a median follow-up of 26.9 months (95% CI; 21.1–32.7) for all vessels are 510/530 vessels (96.2%) and 517/530 vessels (97.5%), respectively.
Any renal artery occlusion was noted in 10 of 139 patients (7.2%). Seven patients (5.0%) developed renal artery occlusion within 1 year of operation. Three additional patients (2.1%) developed silent renal artery occlusion (no elevation in SCr) at 2 years postoperatively and did not require re-intervention. Three patients (2.2%) required re-intervention for renal vessel occlusion with patency achieved in 4/4 (100%) renal arteries treated. Primary and secondary renal artery patency at a median follow-up of 26.9months (95% CI; 21.1–32.7) are 95.4% (247 of 259 renal arteries) and 96.9% (251 of 259 renal arteries), respectively. No significant predictors of any arterial occlusion were identified on bivariate or logistic regression analysis of patient characteristics, choice of bridging stent, or number of bridging stents.
Patient Survival
Thirty-day, 1-year and 2-year all-cause mortality rates were 5.8%, 25.2%, and 32.4%, respectively. Major adverse events within the first thirty days can be found in Table VI. Patient characteristics including greater age (P= 0.0477), Hgb < 7 prior to procedure (P= 0.0093), and greater maximum aortic diameter (P= 0.0084) were significantly associated with decreased patient survival (Table VII). The impacts of postoperative AKI and increase in CKD stage at discharge on survival are highlighted in Figures 1 and 2, respectively. Both presence of AKI (P= 0.0182) and increase in CKD stage at discharge (P= 0.002), including patients requiring postoperative dialysis, were significantly associated with decreased patient survival. No significant difference in survival was identified for procedural staging, or other patient/aneurysm characteristics or complications.
Table VI.
Major adverse events in first 30 days
| Overall | |
|---|---|
| N | 139 |
|
| |
| All-Cause Mortality (within 30 days) (n (%)) | 8 (5.8) |
| Bowel Ischemia (n (%)) | 2 (1.4) |
| MI (n (%)) | 2 (1.4) |
| Paraplegia (n (%)) | 5 (3.6) |
| Renal Failure (n (%)) | 5 (3.6) |
| Respiratory Failure (n (%)) | 19 (13.7) |
| Stroke (n (%)) | 3 (2.2) |
Table VII.
Hazard ratio and 95% confidence intervals (CI) of all-cause mortality by patient characteristics
| Characteristics | Hazard ratio | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|
|
| ||||
| Age | 1.0327 | 1.0003 | 1.0662 | 0.0477 |
| Sex (reference = Male) | 1.1627 | 0.6675 | 2.0252 | 0.5945 |
| ASA Score III/IV/V | – | – | – | – |
| Congestive Heart Failure | 1.2965 | 0.7119 | 2.3614 | 0.3959 |
| Hypertension | 1.0065 | 0.4305 | 2.3529 | 0.9881 |
| Smoking | 1.6864 | 0.6096 | 4.6653 | 0.3141 |
| COPD | 1.4707 | 0.8657 | 2.4983 | 0.1537 |
| Diabetes | 1.7220 | 0.9488 | 3.1251 | 0.0739 |
| History of Stroke | 0.8828 | 0.4728 | 1.6482 | 0.6955 |
| History of Renal Failure | 1.4766 | 0.3567 | 6.1135 | 0.5908 |
| CKD Stage 3/4/5 | 1.3520 | 0.7130 | 2.5636 | 0.3556 |
| Baseline eGFR | 0.9884 | 0.9756 | 1.0015 | 0.0823 |
| Baseline Creatinine | 1.2564 | 0.8376 | 1.8846 | 0.2699 |
| Symptomatic | 1.1939 | 0.6874 | 2.0736 | 0.5292 |
| Active or Contained Rupture | 1.9141 | 0.4604 | 7.9581 | 0.3718 |
| Aortic Angulation | ||||
| Thoracic | 1.0074 | 0.9994 | 1.0155 | 0.0697 |
| Diaphragm | 0.9988 | 0.9919 | 1.0057 | 0.7259 |
| Renal | 1.0050 | 0.9976 | 1.0124 | 0.1881 |
| Hemoglobin < 7 | 2.4812 | 1.2513 | 4.9200 | 0.0093 |
| Contrast volume (mL) | 0.9996 | 0.9955 | 1.0037 | 0.8481 |
| Intervention time (min) | 1.0007 | 0.9991 | 1.0023 | 0.3834 |
| Fluoroscopy time (min) | 1.0000 | 0.9955 | 1.0046 | 0.9961 |
| Accessory renal sacrifice | 0.0000 | 0.0000 | +inf | 0.9968 |
| Previous interventions (reference = Both) | ||||
| Endovascular | 0.4941 | 0.1646 | 1.4828 | 0.2086 |
| Neither | 0.4088 | 0.1420 | 1.1770 | 0.0973 |
| Open | 0.4593 | 0.1397 | 1.5104 | 0.2002 |
| Disease type (reference = Type B Aortic Dissection) | ||||
| Crawford I-III Aneurysm | 2.4124 | 0.7310 | 7.9610 | 0.1483 |
| Crawford IV Aneurysm | 1.4938 | 0.4245 | 5.2560 | 0.5318 |
| Crawford V Aneurysm | 1.3008 | 0.1351 | 12.5224 | 0.8199 |
| Para/juxtarenal Aneurysm | 2.0819 | 0.5509 | 7.8677 | 0.2797 |
| Maximum Aortic diameter (mm) | 1.0189 | 1.0048 | 1.0332 | 0.0084 |
| Number of vessels stented (Reference = 2) | ||||
| 3 | 0.2623 | 0.0335 | 2.0525 | 0.2023 |
| 4 | 0.1451 | 0.0194 | 1.0870 | 0.0603 |
| AKI | 2.0757 | 1.1320 | 3.8062 | 0.0182 |
| Staged | 0.3162 | 0.0436 | 2.2910 | 0.2545 |
| High Risk | 1.7035 | 0.9490 | 3.0580 | 0.0743 |
ASA, American Society of Anesthesiologists; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease.
Categorical variables are presented as number (%); Continuous variables are presented as mean (standard deviation) or median [interquartile range].
Fig. 1.
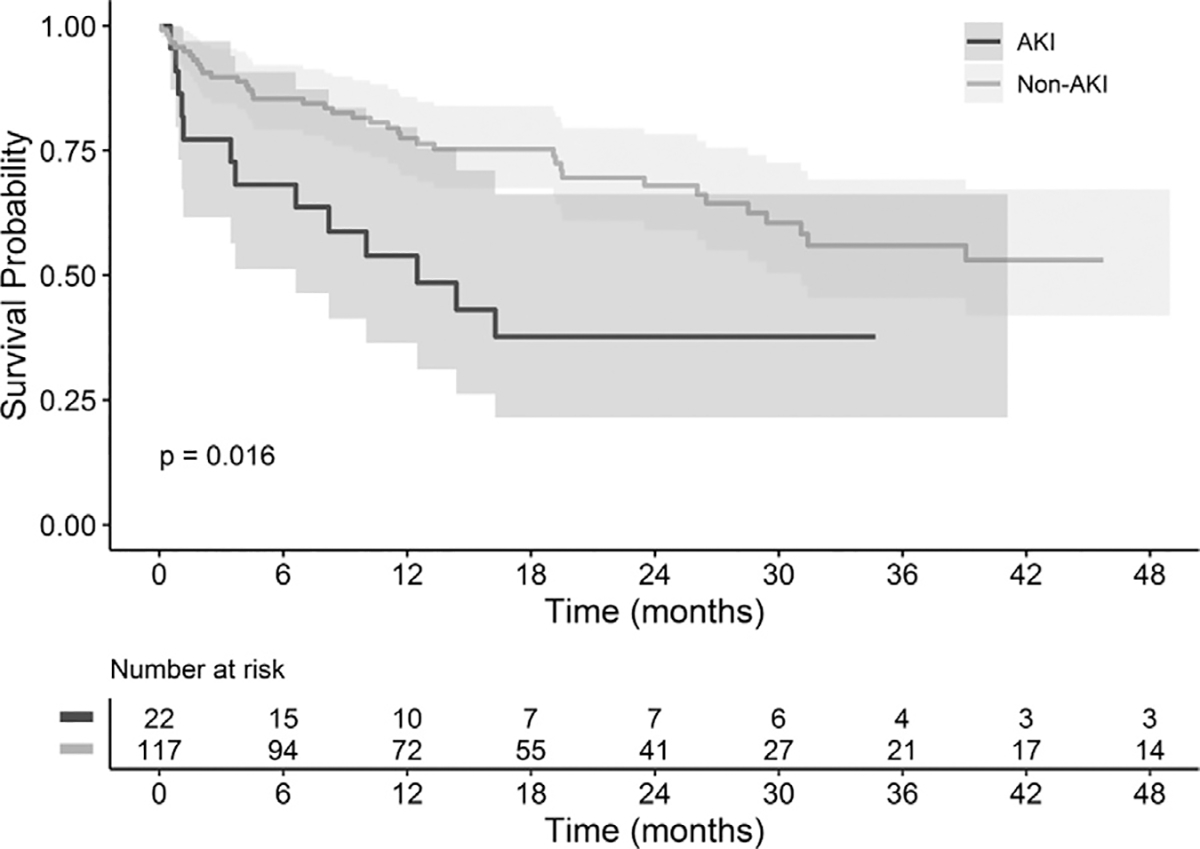
Comparison of survival between AKI and non-AKI patients. Kaplan-Meier and log rank testes were utilized to compare the survival of patients with and without post-operative AKI. Survival of patients with post-operative AKI is represented in black while patients without AKI is represented in gray.
Fig. 2.
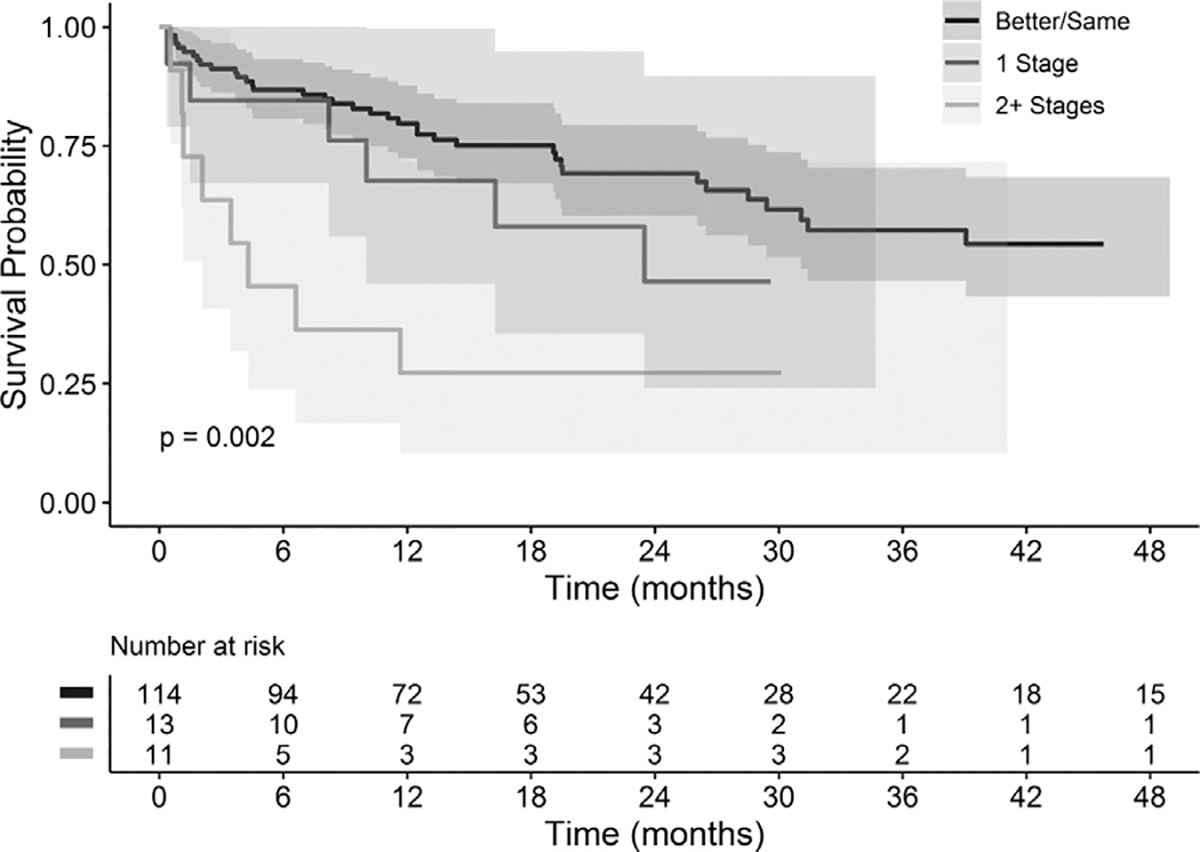
Comparison of survival by CKD stage change from baseline to discharge. Kaplan-Meier and log rank tests were utilized to compare the survival of patients with CKD stage changes from baseline to discharge. Survival of patients shoes stage remained the same or got better are represented in black, patients whose stage increased by one is represented in dark gray, and patients whose stage increased by two or more stages is represented in light gray.
DISCUSSION
In this study, we investigated early- to mid-term changes in renal function following BEVAR for complex aortic aneurysm repair. Acute and chronic declines in renal function following TAAA repair are well-established complications of both open and endovascular aneurysm repair.3 Proposed mechanisms for renal injury are likely multifactorial and include intra- and postoperative contrast loads, renal micro embolization, shaggy aorta or thrombus burden, renal ischemia, and/or improper deployment of grafts.20,21 In this study, patient and procedural risk factors associated with greater rates of AKI included total intervention time (P= 0.0023) and active fluoroscopy time (P= 0.0041). Complex anatomy including angulation at the diaphragm (P= 0.0227) was found to be significant and likely directly related to increased intervention and fluoroscopy time.
Interestingly, no significant correlations were found between postoperative renal function and amount of iodinated contrast used during intervention or for patients who required sacrifice of accessory renal vessels, as might have been expected, though there remains much debate in the current literature whether modern iodinated contrast use truly increases risk of AKI and long-term renal function.22 Preprocedural CKD stage of 3 or greater and aneurysm type were also not significantly associated with risk of AKI.
Recent publications have compared the effects of BEVAR versus FEVAR on renal branch patency, renal function, and short- and long-term patient survival. Due to anatomical challenges, a vast majority of patients treated with FEVAR have pararenal, or para-visceral aneurysms while BEVAR is more applicable to true type IV and types I-III TAAA. Greenberg et al.23 demonstrated that patients with type I–III TAAA are a population with inherently higher rates of renal failure, spinal cord ischemia and death. Therefore, comparing outcomes for aneurysms treated with BEVAR versus FEVAR may not be entirely justified due to the difference in population of patients treated and the near wall hemodynamic differences between anatomic and non-anatomically based configurations. Our previous computational flow dynamic studies, along with the percentage of patients experiencing sustained or improved CKD, lead us to believe that the flow dynamics of the UVM and MVM provide favorable end organ perfusion. With these devices being in early investigation under PS-IDEs, the intent is to provide treatment to patients regardless of anatomical limitations. A benefit of this technology is that there is the potential to stage a procedure at any point if there are complications. To this point, we have been able to accommodate every patient’s anatomy that has presented without clinically significant crowding of the visceral limbs. This includes dissections with small true lumens. Further long-term studies will be needed to investigate patency as additional experience is gained with these devices.10
Unfortunately, studies evaluating renal function following TAAA repair remain heterogenous. Current rates of AKI after FEVAR range from 22.7% to as high as 43%.24,25 Current literature reports permanent dialysis rates following FEVAR ranging from 0.44–1.8%.26,27 Marzelle et al.,28 however, reported a permanent dialysis rate of 5.6% in their analysis of patients undergoing both FEVAR and BEVAR with their FEVAR-predominant group requiring permanent dialysis at a rate of 4.3%. Cucuruz et al.29 recently reported a 35% significant decline in renal function and a 14% permanent dialysis rate following BEVAR. These prior series are compared to a 15.8% rate of AKI and 5.8% permanent dialysis rate in this study, though this rate drops to 1.8% of patients who were not deemed high-risk for renal failure preoperatively. Further, 2-year primary renal vessel patency rates in this study (95.4%) were within the range of prior series for both BEVAR (90.4–96.5%) and FEVAR (93–97.7%), which vary in length of follow-up.30–33 As we know, CKD is a chronic progressive disease and is common in the population of patients with TAAA. Regardless of procedural success or device design, there will likely remain a population of patients for whom renal function will continue to decline. For this reason, we first analyzed renal function and outcomes for all patients and then separated the patients into high and normal risk before reevaluating the outcomes.
In this analysis, 37.4% (52/139) of patients had pre-existing stage 3 or greater CKD and 62.6% (87/159) had a baseline eGFR > 60. We saw that a large percentage of patients retained their preoperative renal function at the time of discharge with 88.4% (122/138) either remaining the same or improving their CKD stage. The same can be said for 1-year (60/69, 86.9%) and 2-year (29/33, 87.9%) data. Interestingly, only 30.8% (4 of 13) of the patients who had a decrease in CKD stage at 1 and 2 years were categorized as high-risk based on pre-procedural comorbidities. It should be noted that the significantly greater proportion of patients with CKD stage 3 or greater (P< 0.0001) in the high-risk group would be expected given patients with SCr ≤2.0 or those with history of dialysis were designated as “high-risk.”
Of factors analyzed, increased patient age, increased aortic diameter, preoperative Hgb < 7, postoperative AKI, and CKD stage increase were found to significantly impact survival of the patients in this study. Increased patient age, increased aortic diameter, and anemia have been identified as risk factors for perioperative and long-term mortality following aortic aneurysm repair in prior series.34,35 Interestingly, anemia has also been noted to be independently associated with greater aneurysm size.35 These factors may represent opportunities for risk stratification and targets for reduction in postoperative mortality. As in prior studies, patients affected by AKI postoperatively were also found to have significantly lower survival (Fig. 1). Patient survival was also noted to decrease significantly as CKD stage increased from baseline (Fig. 2). This knowledge presents an opportunity to potentially reduce patient mortality with concentration on avoiding intra- and postoperative factors that can contribute to decreasing renal function such as maintaining adequate intravascular volume. It should be noted, however, that the lower AKI rates identified in our data were not correlated with significantly greater survival compared to prior surveys. The population of patients with complex aortic aneurysms, unfortunately, have comorbidities at rates notably greater than the general population, which may confound these results. Further large, randomized, controlled trials determining ideal management of these patients with long-term follow-up are needed to analyze these relationships more accurately.
When treating complex thoracoabdominal aneurysms, it is important to consider whether we are providing true clinical benefit to the patient, and that can often be very difficult to discern. Given this population’s age and comorbidities, renal function for many will decline with or without intervention. It is interesting that renal function in the immediate postoperative period was found to be significantly improved from baseline at discharge but then declines progressively at the 1-month follow-up visit and beyond (Table II). Potential explanations for this include the increased fluid load associated with protocol for spinal cord injury prevention as well as the near-wall hemodynamics of the graft providing laminar flow prior to reaching the ostium, potentially avoiding microembolization into the renal vessels. While compiled permanent dialysis rates were higher than the pooled average from FEVAR series, a majority of these patients were deemed high-risk for renal failure preoperatively. These early results indicate renal function and vessel patency following BEVAR with the manifold endovascular system is comparable to available FEVAR grafts despite the potential for treating a greater range of aneurysm anatomy.
There are several important limitations of this study. First, the status of this study remains investigational, thus only patients for whom an existing FDA-approved device was not a viable option for management based on aneurysm and anatomical characteristics were selected for repair. Second, it is difficult to assess the clinical significance of changes in CKD stages in the acute setting such as at discharge, as some patients may still be in the acute phase of kidney injury, making a definitive stage change difficult to identify. Third, while this study has several participating sites, a majority of these patients were treated at a single center. Finally, many patients have not yet reached the 2-year follow-up period, so longer-term outcomes have yet to be fully assessed.
CONCLUSION
This study set out to examine the incidence of acute kidney injury and chronic renal dysfunction following branched endovascular repair of complex aortic aneurysms with parallel branch endograft systems. Rates of permanent dialysis are comparable to prior series, and rates of AKI are lower, though this has not translated to a significant survival benefit. Postoperative AKI and increase in CKD stage at discharge were found to be associated with significantly lower survival. Lower rates of AKI in this study may be attributed at least partially to the near-wall hemodynamics of the non-anatomical manifold configuration. While more data is needed, this study indicates that BEVAR using the manifold configuration is a viable option as an off-the shelf stent graft system to repair a wide range of complex thoracoabdominal aneurysms.
Supplementary Material
Funding:
A portion of this research was supported by Medtronic (Grant number 20141960).
Footnotes
Conflicts of Interest: PK has received payments related to license and royalty interest with Medtronic; KP has received payments related to license and royalty interest with Medtronic; AV has received payments related to license and royalty interest with Medtronic; GA is a paid consultant for Medtronic; MS is on advisory boards and is a speaker for Medtronic and Gore and is a speaker for Cook; ML, JV, VB, TM and TN have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.avsg.2021.04.045.
REFERENCES
- 1.Chuter TA, Gordon RL, Reilly LM, et al. An endovascular system for thoracoabdominal aortic aneurysm repair. J Endovasc Ther 2001;8:25–33. doi: 10.1177/152660280100800104. [DOI] [PubMed] [Google Scholar]
- 2.Mastracci TM, Greenberg RK, Hernandez AV, et al. Defining high risk in endovascular aneurysm repair. 2010;51:1088–95.e1. 10.1016/j.jvs.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Rocha RV, Lindsay TF, Friedrich JO, et al. Systematic review of contemporary outcomes of endovascular and open thoracoabdominal aortic aneurysm repair. J Vasc Surg 2020;71:1396–412 e12. doi: 10.1016/j.jvs.2019.06.216. [DOI] [PubMed] [Google Scholar]
- 4.Piffaretti G, Mariscalco G, Bonardelli S, et al. Predictors and outcomes of acute kidney injury after thoracic aortic endograft repair. J Vasc Surg 2012;56:1527–34. doi: 10.1016/j.jvs.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 5.Karkkainen JM, Tenorio ER, Pather K, et al. Outcomes of small renal artery targets in patients treated by fenestrated-branched endovascular aortic repair. Eur J Vasc Endovasc Surg 2020;59:910–17. doi: 10.1016/j.ejvs.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Suess T, Anderson J, Danielson L, et al. Examination of near-wall hemodynamic parameters in the renal bridging stent of various stent graft configurations for repairing visceral branched aortic aneurysms. 2015. doi: 10.1016/j.jvs.2015.04.421. [DOI] [PubMed]
- 7.Albert S, Balaban RS, Neufeld EB, et al. Influence of the renal artery ostium flow diverter on hemodynamics and atherogenesis. 2014;47:1594–602. doi: 10.1016/j.jbiomech.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson J, Nykamp M, Danielson L, et al. A novel endovascular debranching technique using physician-assembled endografts for repair of thoracoabdominal aneurysms. J Vasc Surg 2014;60:1177–84. doi: 10.1016/j.jvs.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen B, Malek M, Vandenhull A, et al. A novel physician-assembled endograft for the repair of pararenal, paravisceral, Crawford type IV thoracoabdominal aortic aneurysms and aneurysms requiring treatment after prior repair. J Vasc Surg 2020. doi: 10.1016/j.jvs.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Suess T, Anderson J, Sherman A, et al. Shear accumulation as a means for evaluating risk of thromboembolic events in novel endovascular stent graft designs. J Vasc Surg 2017;65:1813–19. doi: 10.1016/j.jvs.2016.07.108. [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney inter 2012;2:1–138 Suppl.. [Google Scholar]
- 12.National kidney foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47 %m 12859163. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 16.Yoshida K. Tableone: Create ‘Table 1’ to Describe Baseline Characteristics. R package version 0.11.1; 2020. https://CRAN.R-project.org/package=tableone.
- 17.Therneau T. A package for survival analysis in R. 2020.
- 18.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model, xiii. New York: Springer; 2000. p. 350. [Google Scholar]
- 19.Kassambara A, Kosinski M, Biecek P. Survminer: Drawing Survival Curves using ‘ggplot2. R package version 0.4.9; 2020. https://CRAN.R-project.org/package=survminer.
- 20.Saratzis AN, Goodyear S, Sur H, et al. Acute kidney injury after endovascular repair of abdominal aortic aneurysm. J Endovasc Ther 2013;20:315–30. doi: 10.1583/12-4104mr2.1. [DOI] [PubMed] [Google Scholar]
- 21.Walsh SR, Tang TY, Boyle JR. Renal consequences of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2008;15:73–82. doi: 10.1583/07-2299.1. [DOI] [PubMed] [Google Scholar]
- 22.McDonald JS, McDonald RJ, Comin J, et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology 2013;267:119–28. doi: 10.1148/radiol.12121460. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair. Circulation 2008;118:808–17. doi: 10.1161/circulationaha.108.769695. [DOI] [PubMed] [Google Scholar]
- 24.Tran K, Fajardo A, Ullery BW, et al. Renal function changes after fenestrated endovascular aneurysm repair. 2016;64:273–80. 10.1016/j.jvs.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Haddad F, Greenberg RK, Walker E, et al. Fenestrated endovascular grafting: the renal side of the story. J Vasc Surg 2005;41:181–90. doi: 10.1016/j.jvs.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Katsargyris A, Oikonomou K, Klonaris C, et al. Comparison of outcomes with open, fenestrated, and chimney graft repair of juxtarenal aneurysms: are we ready for a paradigm shift? J Endovasc Ther 2013;20:159–69. doi: 10.1583/1545-1550-20.2.159. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Gonzalez T, Pinçon C, Maurel B, et al. Renal outcomes following fenestrated and branched endografting. Eur J Vasc Endovasc Surg 2015;50:420–30. doi: 10.1016/j.ejvs.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Marzelle J, Presles E, Becquemin JP. Results and factors affecting early outcome of fenestrated and/or branched stent grafts for aortic aneurysms. Ann Surg 2015;261:197–206. doi: 10.1097/sla.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 29.Cucuruz B, Kasprzak PM, Gallis K, et al. Midterm outcome of renal function after branched thoracoabdominal aortic aneurysm repair. J Vasc Surg 2020;71:1119–27. doi: 10.1016/j.jvs.2019.06.200. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Gonzalez T, Mastracci T, Carrell T, et al. Midterm outcomes of renal branches versus renal fenestrations for thoraco-abdominal aneurysm repair. 2016;52:141–8. 10.1016/j.ejvs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Eagleton MJ, Follansbee M, Wolski K, et al. Fenestrated and branched endovascular aneurysm repair outcomes for type II and III thoracoabdominal aortic aneurysms. J Vasc Surg 2016;63:930–42. doi: 10.1016/j.jvs.2015.10.095. [DOI] [PubMed] [Google Scholar]
- 32.Panuccio G, Bisdas T, Berekoven B, et al. Performance of bridging stent grafts in fenestrated and branched aortic endografting. Eur J Vasc Endovasc Surg 2015;50:60–70. doi: 10.1016/j.ejvs.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Silingardi R, Gennai S, Leone N, et al. Standard “off-the-shelf” multibranched thoracoabdominal endograft in urgent and elective patients with single and staged procedures in a multicenter experience. J Vasc Surg 2018;67:1005–16. doi: 10.1016/j.jvs.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 34.Coselli JS, Lemaire SA, Miller CC, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg 2000;69:409–14. doi: 10.1016/s0003-4975(99)01478-2. [DOI] [PubMed] [Google Scholar]
- 35.Diehm N, Benenati JF, Becker GJ, et al. Anemia is associated with abdominal aortic aneurysm (AAA) size and decreased long-term survival after endovascular AAA repair. J Vasc Surg 2007;46:676–81. doi: 10.1016/j.jvs.2007.06.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


