Abstract
IMPORTANCE:
Adapting one’s gait speed to external circumstances is critical for safe ambulation. Dopamine (DA), critical for adapting to increased task demands, predicts usual gait speed and may exert a greater role in complex tasks like rapid gait speed.
OBJECTIVE:
We hypothesized that a genotypic proxy indicator of greater prefrontal DA signaling would predict significantly faster rapid gait.
DESIGN:
Longitudinal cohort study over 8 years
SETTING:
Community-dwelling adults with no baseline mobility disability
PARTICIPANTS:
N = 2,353 participants from the Health ABC Study
MEASUREMENTS:
Repeated measures of walking speed (meters/sec) were obtained in response to: “walk as fast as possible… (rapid gait) or “walk at your usual pace (usual gait).” Catechol-O-methyltransferase (COMT) val158met polymorphism indicated DA signaling (val/val=higher metabolism, lower DA signaling; met/met=lower metabolism, higher DA signaling).
RESULTS:
Participants declined in rapid gait from 1.55 (SD=.33) to 1.35 m/s (SD=0.34). Across the full follow-up period, the met/met genotype was associated with significantly greater rapid gait slowing. In mixed effect models, between-group differences were independent of covariates, and remained similar after adjustment for sensorimotor function, cognition, depressive symptoms, and energy. Follow-up analyses indicated the met/met genotype had a significantly faster rapid gait speed compared to the val/val genotype for the first 3 years (p < .01) but not years 4–8 (p > .05).
CONCLUSION:
Greater prefrontal DA measured with COMT polymorphism may facilitate short-term adaptation to rapid walking demands that are lost over time. Studies should examine whether these effects are long-term and the underlying mechanistic pathways.
Keywords: COMT, rapid gait speed, trajectory, motor task adaptation
Introduction
Gait slowing predicts adverse health, including greater disability 1 and mortality 2. Usual gait is commonly assessed 3, but rapid gait, or walking “as fast as possible”, appears more sensitive to age-related decline 4–6. Rapid gait outperforms usual gait in predicting older adult health metrics, including cognitive decline 7, 8 and may be more relevant for safe ambulation 9. The ideal usual gait speed is ≥ 1.0 m/s 10, but certain everyday activities may require faster speeds (e.g., speed of 1.34 m/s to safely maneuver a crosswalk 11).
The ability to increase gait to varying circumstances 12 may reflect a successful adaptive response. The central nervous system (CNS) may play an important role in regulating rapid gait. Surprisingly, little is known about CNS contributions to rapid gait, but evidence suggests that complex gait tasks-including rapid gait- may have greater prefrontal cognitive involvement compared to simpler, usual gait speed task conditions 13. For example, lower cognitive function 14 and greater fatigue/fatigability 15 are associated with slower rapid gait, but the neurobiological basis of these associations have only recently been examined 16. Because of the increased reliance of the prefrontal cortex in complex walking conditions, it is feasible that polymorphisms implicated in both motor and cognitive actions would contribute to one’s ability to maintain rapid gait across time.
Genetic variation in the catechol-O-methyltransferase (COMT) val158met polymorphism can approximate prefrontal DA availability 17. COMT encodes monoamine-metabolizing enzymes in the brain with an important effect in the prefrontal cortex (PFC). The COMT val158met polymorphism determines the COMT enzyme level, which mediates synaptic DA levels. The val allele is associated with higher COMT (i.e., more DA-metabolizing enzymes) and results in faster DA clearance and lower tonic DA levels; the met allele is associated with lower COMT enzyme activity causing slower DA clearance and resulting in higher prefrontal tonic DA levels 17. Homozygosity for either genotype is associated with a slower usual gait speed both cross-sectionally 18 and longitudinally 19. Whether this is evident in complex tasks like rapid gait speed is unknown.
The current study examines whether COMT gene polymorphism is associated with rapid gait speed, cross-sectionally and longitudinally. Further, we explore whether such association would be modified after adjustment for potential explanatory pathways, specifically: lower sensorimotor and cognitive function and depressive symptoms. We hypothesized the COMT-rapid gait speed association was robust to health-related confounders but would be attenuated (i.e., weakened) by these possible explanatory DA sub-pathways.
Methods
Participants
The current study used data from the Health, Aging, and Body Composition (Health ABC) study which included 3,075 community-dwelling White and Black older adults 70 – 79 years living in Memphis, TN, or Pittsburgh, PA. Exclusion criteria for the parent study were reported difficulty walking ¼ mile, climb 10 stairs, or inability to perform activities of daily living. Those requiring assistive devices or had life-threatening cancers were also excluded. For the current analyses, our sample included participants with available data for the COMT gene polymorphism, rapid pace gait speed at Year 2 (first year of assessment) and at least one additional follow-up, resulting in 2,353 participants (n = 722 missing ≥1 of the inclusion criteria). Unless stated, the baseline value for a given variable was an individual’s Year 2 (1998–1999) value. Participants were followed across eight years (mean follow-up time = 7.10, SD = 2.35 years).
Measures
Outcome. Rapid gait speed.
Participants were asked to walk down a 20-meter hallway as fast as they could and timed with a stopwatch once per visit. Higher scores reflected faster gait in meters per second.
Predictor. COMT.
The Val158Met polymorphism (rs4680) of COMT was measured from genomic DNA extracted from ethylenediaminetetraacetic acid (EDTA) anticoagulated whole blood by standard methods (Gentra Systems, Minneapolis, MN) and polymerase chain reaction (PCR)-based COMT genotyping 20.
Covariates.
All covariates were identified as possible risk or protective factors for maintaining rapid gait speed based on their association with either usual or rapid gait speed. Variables indicating potential explanatory pathways linking COMT polymorphism with rapid gait speed includes measures of function in sensorimotor, cognitive, depressive symptoms, and energy domains.
Usual gait speed.
Participants walked down a 20-meter hallway at their usual pace and timed with a stopwatch. To account for overall change in usual gait speed, we calculated person-specific gait speed slopes by a linear mixed model with random intercepts and random slopes similarly to previously published data 7, 21. All available usual gait speed values from a given individual informed the person-specific slope. Negative values indicated declining and positive values indicated increasing usual gait speed over time, respectively.
Demographics.
Demographics included self-reported age, race (Black or White), and gender.
Health factors.
Body mass index (BMI) was calculated from Year 1 (1997–1998) height and weight. Ankle-brachial index was obtained in Year 1 (1997–1998) from systolic blood pressure in both the arm and ankle measured twice then averaged. Lower index values indicated peripheral arterial disease. The Bailey-Lovie distance visual acuity test indicated whether a participant had corrected vision better (0) or worse (1) than 20/50. Self-reported presence or absence of diabetes, coronary heart disease, congestive heart failure, and cerebrovascular disease were obtained via self-report/medical records at the Year 1 (1997–1998) assessment.
Sensorimotor function.
For the standing balance test, we summed the time each position (semi-tandem, full-tandem, and single leg stand, for a maximum time of 30 seconds each) was held without any support 22. Times were converted to standardized scores by dividing the results by the maximal performance achievable in cohorts of older adults comparable to our study population (i.e., 90 seconds for standing balance). This procedure converted the balance score to a ratio ranging from 0 (worst performance) to 1 (best performance achievable by a population of this age and condition). Speed in meters/second on the 400m walk test from Year 1 was used to indicate functional fitness 23; participants were asked to walk 10 laps that were ¼ mile each as quickly and comfortably as possible without running.
Knee pain.
The self-reported absence (0) or presence (1) of knee pain with activities was obtained in Year 3.
Exercise and Walking.
Self-reported time in the prior week engaging in exercise and walking was converted in kcal/kg/week.
Cognitive function.
Cognitive status was assessed using the Teng Modified Mini-Mental State Examination (3MS) 24. Higher scores indicated better cognitive function. Cognitive processing speed was measured by the Digit Symbol Substitution Task (DSST) 25. Participants were instructed to write symbols that correspond to numbers as quickly as possible in 90 seconds. Higher scores indicated more correctly copied symbols. For both cognitive domains, person-specific slopes were calculated using cognitive data from all available waves. Positive values reflected better performance and negative values reflected worse performance across time.
Depressive symptoms.
Depressive symptoms were measured using the Center for Epidemiological Studies- Depression (CES-D) scale 26. A person-specific slope was calculated using all available CES-D assessments.
Energy.
Self-reported energy was a single-item question asking about a participant’s usual energy level in the previous month. Scores ranged from 0 (no energy) to 10 (most energy they have ever had).
Analytic Approach
Bivariate associations of covariates with baseline rapid gait speed were analyzed with Spearman correlations or independent samples t-tests to assess group differences. We also examined whether there were significant between-genotype differences in average rapid gait speed with a separate ANOVA for each year; ANOVAs were not adjusted for any covariates. This was done to identify whether there were potential critical periods where there between-group differences, as mixed effects models examine overall change over time but do not identify between-group differences within a particular timepoint.
To calculate the unadjusted usual gait speed, cognition, and depressive symptoms slopes, we applied longitudinal mixed effect models and used random intercept and random slope of years for each participant to control for repeated measures and varying slopes across individuals. Linear mixed effects models were used to examine whether there was a significant COMT*time interaction after controlling for time, time2, and usual gait speed slope. Models were adjusted for demographics and other health factors. To address collinearity, separate models were adjusted for those variables indicating potential explanatory pathways: sensorimotor, cognition, depressive symptoms, and energy. A final parsimonious model retaining COMT and all significant covariates and explanatory pathways (p < .05, uncorrected for multiple comparisons) was determined using backward selection procedures.
Analyses were completed in SAS, Version 9.4 (SAS Institute, Cary, NC), and two-tailed significance was set at p < .05.
Results
The analytic sample (N = 2,353) had an average age of 74.60 years (SD = 2.84) at baseline; a majority of the sample was White (n = 1,467, 62.3%), there were slightly more women (n = 1,223, 52.0%), and the sample was generally healthy (Table 1). The average baseline rapid gait speed was 1.55 m/s (SD = 0.33), comparable to other healthy older adult samples 4, 27, 28. Over an average follow-up time of 7.10 (SD = 2.35) years, rapid gait declined by 13% to 1.35 m/s (SD = 0.34) at the study’s end.
Table 1.
Analytic sample (N = 2,353) characteristics and bivariate association with baseline rapid gait speed.
| N (%) or Mean (SD) | Spearman’s rho with baseline rapid gait speed or group mean differences (SE) | p-value | ||
|---|---|---|---|---|
| Rapid Gait Speed (m/s) | 1.55 (0.33) | -- | -- | |
| COMT Genotype, n (%) | Val/Val: | 769 (32.7%) | −.06 (.02) | .001 |
| Met/Val: | 1,137 (48.3%) | −.03 (.18) | .055 | |
| Met/Met (REF): | 447 (19.0%) | -- | -- | |
| Demographics. | ||||
| Age, years | 74.60 (2.84) | −.15 | <.001 | |
| White, n (%) | 1,467 (62.3%) | .21 (.01; White > Black) | <.001 | |
| Women, n (%) | 1,223 (52.0%) | −.21 (.01; men > women) | <.001 | |
| Usual Gait Speed (m/s) | 1.14 (0.21) | .76 | <.001 | |
| Other health factors. | ||||
| Body mass index (kg/m2) | 27.31 (4.80) | −.21 | <.001 | |
| Ankle-Brachial Index (mm Hg) | 1.07 (.18) | .20 | <.001 | |
| Visual Acuity ≤20/50, n Yes(%) (Y3) | 101 (4.5%) | −.20 (.03; better > worse acuity) | <.001 | |
| Diabetes, n Yes (%) (Y1) | 121 (5.1%) | −.09 (.03; no > yes) | .004 | |
| Coronary Heart Disease, n Yes(%) (Y1) | 404 (17.2%) | .02 (.02; yes > no) | .205 | |
| Congestive Heart Failure, n Yes(%) (Y1) | 55 (2.3%) | −.18 (.05; no > yes) | .002 | |
| Cerebrovascular Disease, n Yes(%) (Y1) | 152 (6.5%) | −.16 (.03; no > yes) | <.001 | |
| Sensorimotor. | ||||
| EPESE score, Balance (Y1) | 3.75 (.75) | .22 | <.001 | |
| 400m Walk Speed (m/s) (Y1) | 1.26 (.21) | .71 | <.001 | |
| Subjective Knee Pain. | ||||
| Knee Pain with Activity, n Yes(%) (Y3) | 538 (23.1%) | −.15 (.02; no pain > pain) | <.001 | |
| Exercise and Walking. | ||||
| Weekly exercise/walking (kcal/kg/week) | 3.46 (6.87) | .344 | <.001 | |
| Cognition. | ||||
| 3MS, points | 90.79 (7.77) | .26 | <.001 | |
| DSST (n correct, Y1) | 36.92 (14.26) | .31 | <.001 | |
| Depressive Symptoms. | ||||
| CES-D score (Y1) | 4.59 (5.24) | −.13 | <.001 | |
| Energy. | ||||
| Self-Reported Energy, points | 6.69 (1.75) | .18 | <.001 | |
Note. SD = standard deviation. EPESE = Establishing Population for the Epidemiological Study of the Elderly. DSST = Digit Symbol Substitution Test. 3MS = Teng Modified Mini-Mental State Examination. CES-D = Center for Epidemiologic Studies-Depression scale. Y = year from Health ABC dataset (e.g., Y3 = Year 3). If not indicated, data are from Year 2.
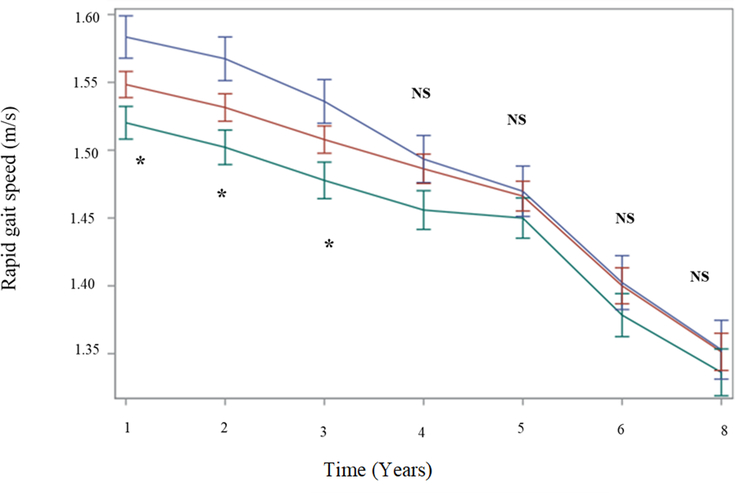
At baseline, there was a marked advantage in rapid gait for met carriers compared to val/val. The met/met genotype was significantly faster than the val/val genotype and marginally faster than the met/val genotype (Table 1). These between-group differences were maintained at each year of follow-up (Figure 1), although they were statistically significant at baseline through year three and not in years four through eight. (Figure 1).
Figure 1. Estimated unadjusted rapid gait speed (m/s) across time by COMT genotype.
Note. Year = 1 indicates the analytic baseline. Blue = met/met; Red = met/val; Green = val/val. * denotes significant between-group differences among homozygous val compared to homozygous met genotype, p < .05. NS = nonsignificant differences among three COMT genotypes.
In LME models with a COMT*time interaction, adjusted for usual gait speed slope, met/val (est. = 0.004, SE = 0.001, p = .005) and val/val genotypes (est. = 0.003, SE = 0.002, p = 0.498) were both associated with significantly less decline in rapid gait compared to the met/met genotype across the full study period (Table 2, Model 1). Results remained similar after the inclusion of demographics and health factors (Table 2, Models 2–3). Associations remained similar also after adjustment for variables indicating potential explanatory pathways (Table 2, Models 4–7). In the final parsimonious model retaining only the significant covariates from each block, both met/val (est. = 0.004, SE = 0.001, p = .015) and val/val (est. = 0.003, SE = 0.002, p = .048) genotypes were associated with significantly less decline compared to the met/met genotype. In this model, less decline in rapid gait was associated with: less decline in usual gait speed, younger age, male gender White race, better balance, no knee pain with activities, no cerebrovascular disease, lower BMI, less decline in Teng 3MS and DSST, and improvement in depressive symptoms.
Table 2.
Results of linear mixed effects models predicting rapid gait speed.
| Unstandardized estimate (SE), p-value | ||
|---|---|---|
| Time*Met/Val | Time*Val/Val | |
| Model 1: Usual gait speed | .004 (.001), p=.015 | .003 (.002), p=.050 |
| Model 1 + Demographic (Model 2) | .004 (.001), p=.005 | .003 (.002), p=.056 |
| Model 1 + Health Factors (Model 3) | .004 (.001), p=.004 | .004 (.002), p=.067 |
| Model 1 + Sensorimotor Function (Model 4) | .005 (.002), p=.005 | .004 (.002), p=.042 |
| Model 1 + Knee Pain (Model 5) | .004 (.001), p=.005 | .003 (.002), p=.049 |
| Model 1 + Exercise & Walking (Model 6) | .005 (.001), p= .002 | .003 (.001), p=.042 |
| Model 1 + Cognition (Model 7) | .004 (.001), p=.015 | .003 (.002), p=.035 |
| Model 1 + Depressive Symptoms (Model 8) | .004 (.0001), p=.006 | .003 (.002), p=.035 |
| Model 1 + Energy (Model 9) | .004 (.001), p=.015 | .003 (.002), p=.050 |
Note. Time*Met/Met was the reference group. Model 1 was additionally adjusted for the main effects of COMT genotype and time. SE = Standardized error.
Discussion
In this sample of community-dwelling older adults, COMT polymorphism was associated with rapid gait cross-sectionally and over time, independent of usual gait speed, health factors and other potential explanatory factors. Specifically, those with the met/met genotype, a proxy marker of greater tonic prefrontal DA, experienced significantly greater decline in rapid gait speed across the follow-up period compared to those with at least one val allele. Follow-up analyses indicated that for the first three years, met/met carriers were significantly faster than val/val carriers. In comparison, there were no significant differences in rapid gait speed by COMT polymorphism for the later years of follow-up. Taken together, these results suggest that while greater prefrontal DA may be associated with greater slowing over time, this was only partly attributable to their higher baseline rapid gait.
Participants increased their gait speed in response to the instructions “Walk as fast as you can, until I tell you to stop,” as shown by the difference of 0.41 m/s between gait conditions. This increase may indicate motor adaptation, which requires an integrated contribution of multiple domains in addition to sensorimotor, including attention, motivation, and adequate energy. The dopaminergic network is critical in regulating these domains; thus, it is possible the relationship between COMT polymorphism and changes in rapid gait may have occurred through several pathways. Accumulating evidence implicates the COMT val158met polymorphism as important for motor adaptation 18, 29–32. Our data appear to support this evidence; we found that in the first three years rapid gait was significantly slower for val/val than for the met/met genotype. COMT also influences motivation responsivity, which in turn can influence the performance changes in response to external stimuli. The COMT met/met allele is associated with more activation during reward anticipation, greater motivation, and greater reward sensitivity compared to the val/val allele 33. Thus, those with the met/met genotype were possibly more motivated to perform well on the task. Our study did not have specific measures of motor adaptation or motivation to test these pathways; the study’s measures of depressive symptoms, energy, and fitness may only capture some facets of these domains. Future studies with more tailored motivation and motor adaptation tasks should further explore this pathway.
Since COMT is one indicator of prefrontal DA 34 , our results suggest that decline in rapid gait may be modulated, at least in part, by DA signaling effects in the prefrontal cortex 30. Increasing one’s walking speed and maintaining that rapid gait in response to external stimuli requires attention, a cognitive ability that is regulated by DA in the prefrontal cortex. It is also possible that COMT polymorphism is associated with rapid gait through its association with memory efficacy (i.e., ability to learn, recall, adapt to memory tasks) 35, which allows individuals to learn and adapt to motor tasks more quickly 29, 36. Although memory efficacy was not assessed, we adjusted for two cognitive tests reflecting some aspects of these domains, the Teng 3MS and DSST. While changes in both cognitive domains were positively associated with better rapid gait, they did not fully attenuate the COMT*time interaction. Cognitive-related processes - at least as determined by our measures - do not fully attenuate the association between prefrontal DA and changes in rapid gait. Domains not measured by these assessments may, however, attenuate this relationship and should be considered in future work.
Our multivariable results replicated findings that older age, being a woman, and higher weight status were associated with slower rapid gait 4, 37 but do not significantly influence the role of COMT on changes in rapid gait. While we controlled for sensorimotor function, it is possible that rapid gait is influenced by peripheral mechanisms we were unable to account for, e.g., peripheral COMT or other diseases that impair gait.
Rapid gait is multifactorial, and we found no evidence that one variable alone could fully predict rapid gait changes. In conjunction with prior research, our study suggests that demographic, peripheral, and CNS function uniquely contribute to one’s ability to maintain the capacity to increase their gait speed. We also partially replicated prior work that rapid gait was associated with demographic 4 and peripheral locomotor measures 4. Up to 1/3 of the variability in rapid gait performance may be driven by genetics 38, 39, and our findings indicate the COMT polymorphism at least partially explains these group differences.
Our findings have important implications for future research. Those with the val/val genotype could be targeted in an effort to prevent or delay the onset of mobility limitations, as those with poorer baseline function or greatest risk of mobility decline tend to receive the greatest benefits from behavioral intervention efforts 40. Targeting those with the val/val genotype prior to major declines in rapid gait can ensure their ability to maintain safe community ambulation. Providing targeted prevention/intervention programs for those at risk of poorest rapid gait may effectively allocate treatment efforts to those likely to receive the greatest benefits.
Our findings suggest that a frontal cortical relatively higher dopaminergic state – illustrated by the met/met COMT genotype - in older adults enhances the ability of the brain to adapt to rapid speed gait in the short-term but may lose its influence on rapid gait over time. Furthermore, these results suggest the COMT-rapid gait relationship may not follow the U-shaped function seen in usual gait 18 but rather follow a dose-dependent pattern whereby greater tonic DA availability is associated with faster rapid gait. That is, there is less evidence that COMT heterozygosity is optimal for rapid compared to usual gait. The current results also failed to replicate gender-specific differences of COMT genotype on gait as previously demonstrated 18, 41. Prior work from our group found evidence of a significant U-shaped association between COMT genotype and usual gait where those with val/val and met/met genotypes had more gait slowing over 10 years compared to those with the met/val genotype among Black older adults 19. In the current analyses, there were too few Black older adults to confidently draw conclusions based on subgroup analysis. Future replication is warranted, but there is weak evidence that demographic characteristics moderate the COMT-rapid gait association.
The average loss of gait speed over the 8-year period in our study participants demonstrated a biphasic curve where an initial, less prominent decline in rapid gait speed may be modulated by frontal DA functions but then converged to a steeper decline without specific advantages of the COMT genotype. Such a biphasic curve suggest that effects of prefrontal DA compensation assessed by the val158met polymorphism only provide temporary protection. These results fail to support the resource modulation hypothesis which states that genetic variability may exert a greater influence when brain resources become limited like that seen in normative aging 42. It is important to note, however, that DA changes in non-prefrontal regions, other non-DA aging factors, or a complex combination of genes may become the driving forces of age-associated decline in rapid gait. For example, brain-derived neurotrophic factor (BDNF) and dopamine D3 receptor (DRD3) had a stronger association with timed walking among the oldest-old men but not women 41. Therefore, findings could be related to multi-gene profiling effects where interactions between COMT and DRD3 may play important but less predictable roles. Future research is warranted to examine the changing influence of multiple SNPs on complex gait as individuals age.
Declining rapid gait speed has consequences for safe mobility and should be a public health priority. DA-related genetic polymorphisms may exert short-term influence in one’s capacity to adapt to increasing motor demands, even among high-functioning older adults. Future examinations should consider whether more circulating DA or DA concentrated in other pathways 29 are associated with greater maintenance of rapid gait. Such studies would further inform if dopaminergic drugs in targeted older persons with hypodopaminergic states may be a promising approach to maintain older adult mobility.
KEY POINTS.
Across eight years, those with the genotype indicative of greater dopamine signaling experienced significantly greater decline in rapid gait speed, even after adjusting for demographic and health confounders. Follow-up analyses indicated that those with greater dopamine signaling, however, were significantly faster for the first three years but then performed similarly to the other genotypes for the remaining five years.
A proxy indicator of greater prefrontal dopaminergic signaling offers short-term protection against gait slowing among healthy older adults, but eventually this advantage is lost.
WHY DOES THIS PAPER MATTER?
The ability to adapt one’s gait is important for safe everyday mobility, and this article is the first to identify the role of genetic variability on one’s ability to maintain this skill.
FUNDING:
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. BNS was also supported by a National Institute on Aging Kirschstein Institutional National Research Service Award (T32-AG055381) awarded to the University of Pittsburgh (PIs: Drs. Mary Ganguli and Caterina Rosano).
Sponsor’s role: The sponsors had no role in the design of the study, in data collection or analyses, or in the decision to submit the study for publication.
Footnotes
Conflicts of interest: None of the authors have any real or perceived conflicts of interest to disclose.
References
- 1.Hong S, Kim S, Yoo J, et al. Slower gait speed predicts decline in Instrumental Activities of Daily Living in community-dwelling elderly: 3-year prospective finding from Living Profiles of Older People Survey in Korea Journal of Clinical Gerontology and Geriatrics. 2016;7(4):141–145. doi: 10.1016/j.jcgg.2016.05.002 [DOI] [Google Scholar]
- 2.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Journal of the American Medical Association. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornyak V, VanSwearingen JM, Brach JS. Measurement of gait speed. Topics in Geriatric Rehabilitation. 2012;28(1):27–32. doi: 10.1097/TGR.0b013e318233e75b [DOI] [Google Scholar]
- 4.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: Reference values and determinants. Age and Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15 [DOI] [PubMed] [Google Scholar]
- 5.Artaud F, Singh-Manoux A, Dugravot A, Tzourio C, Elbaz A. Decline in fast gait speed as a predictor of disability in older adults. Journal of the American Geriatrics Society. 2015;63(6):1129–1136. doi: 10.1111/jgs.13442 [DOI] [PubMed] [Google Scholar]
- 6.Ko S-U, Hausdorff JM, Ferrucci L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: Results from the Baltimore Longitudinal Study of Ageing. Age and Ageing. 2016;39(6):688–694. doi: 10.1093/ageing/afq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosso AL, Metti AL, Faulkner K, et al. Complex walking tasks and risk for cognitive decline in high functioning older adults. Journal of Alzheimer’s Disease. 2019;71(s1):S65–S73. doi: 10.3233/JAD-181140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: A prospective study. Age and Ageing. 2009;38(5):509–514. doi: 10.1093/ageing/afp093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillel I, Gazit E, Nieuwboer A, et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. European Review of Aging and Physical Activity. 2019;16:6. doi: 10.1186/s11556-019-0214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middleton A, Fritz SL, Lusardi M. Walking speed: The functional vital sign. Journal of Aging and Physical Activity. 2015;23(2):314–322. doi: 10.1123/japa.2013-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salbach NM, O’Brien K, Brooks D, et al. Speed and distance requirements for community ambulation: A systematic review. Archives of Physical Medicine and Rehabilitation. 2014;95(1):117–128.e11. doi: 10.1016/j.apmr.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 12.Montufar J, Arango J, Porter M, Nakagawa S. Pedestrians’ normal walking speed and speed when crossing a street. Transportation Research Record. 2007;2002:90–97. doi: 10.3141/2002-12 [DOI] [Google Scholar]
- 13.Fitzpatrick AL, Buchanan CK, Nahin RL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2007;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244 [DOI] [PubMed] [Google Scholar]
- 14.Callisaya ML, Launay CP, Srikanth VK, Verghese J, Allali G, Beauchet O. Cognitive status, fast walking speed and walking speed reserve- The Gait and Alzheimer Interactions Tracking (GAIT) study. GeroScience. 2017;39(2):231–239. doi: 10.1007/s11357-017-9973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonsick EM, Glynn MW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: Perceived fatigability as a marker of impending decline in mobility-intact older adults. Journal of the American Geriatrics Society. 2016;64(6):1287–1292. doi: 10.1111/jgs.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N, Rosano C, Karim HT, Studenski SA, Rosso AL. Regional gray matter density associated with fast-paced walking in older adults: A voxel-based morphometry study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. in press;doi: 10.1093/gerona/glaa091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. American Journal of Human Genetics. 2004;75(5):807–821. doi: 10.1086/425589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiology of Aging. 2010;31(3):523–531. doi: 10.1016/j.neurobiolaging.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metti AL, Rosano C, Boudreau R, et al. Catechol-O-Methyltransferase genotype and gait speed changes over 10 years in older adults. Journal of the American Geriatrics Society. 2017;65(9):2016–2022. doi: 10.1111/jgs.14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biological Psychiatry. 1999;46(4):557–567. doi: 10.1016/s0006-3223(99)00098-0 [DOI] [PubMed] [Google Scholar]
- 21.Rosano C, Aizenstein HJ, Newman AB, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage. 2012;62(1):307–313. doi: 10.1016/j.neuroimage.2012.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 23.Lange-Maia BS, Newman AB, Strotmeyer ES, Harris TB, Caserotti P, Glynn NW. Performance on fast and usual-paced 400m walk tests in older adults: Are they comparable? Aging, Clinical, and Experimental Research. 2015;27(3):309–314. doi: 10.1007/s40520-014-0287-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of Clinical Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 25.Wechsler D Manual for the Wechsler Adult Intelligence Scale, Revised. Psychological Corporation; 1981. [Google Scholar]
- 26.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D. American Journal of Preventive Medicine. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 27.Lusardi MM, Pellecchia GL, Schulman M. Functional performance in community living older adults. Journal of Geriatric Physical Therapy. 2003;26(3):14–22. doi: 10.1519/00139143-200312000-00003 [DOI] [Google Scholar]
- 28.Kyrönlahti SM, Stenholm S, Raitanen J, Neupane S, Koskinen S, Tiainen K. Educational differences in decline in maximum gait speed in olderr adults over an 11-year follow-up. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2021;76(4):703–709. doi: 10.1093/gerona/glaa196 [DOI] [PubMed] [Google Scholar]
- 29.Noohi F, Boyden NB, Kwak Y, et al. Association of COMT val158met and DRD2 G>T genetic polymorphisms with individual differences in motor learning and performance in female young adults Journal of Neurophysiology. 2014;111(3):628–640. doi: 10.1152/jn.00457.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogueira NGdH, Bacelar MFB, de Paula Ferreira B, Parma JO, Lange GM. Association between the catechol-O-methyltransferase (COMT) Val158Met polymorphism and motor behavior in healthy adults: A study review. Brain Research Bulletin. 2019;144:223–232. doi: 10.1016/j.brainresbull.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 31.Noohi F, Boyden NB, Kwak Y, et al. Interactive effects of age and multi-gene profile on motor learning and sensorimotor adaptation. Neuropsychologia. 2016;84:222–234. doi: 10.1016/j.neuropsychologia.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baetu I, Burns NR, Urry K, Barbante GG, Pitcher JB. Commonly-occurring polymorphisms in the COMT, DRD1 and DRD2 genes influence different aspects of motor sequence learning in humans Neurobiology of Learning and Memory. 2015;125:176–188. doi: 10.1016/j.nlm.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 33.Dreher J-C, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witte AV, Flöel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Research Bulletin. 2012;88(5):418–428. doi: 10.1016/j.brainresbull.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 35.Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. The American Journal of Psychiatry. 2002;159(4):652–654. doi: 10.1176/appi.ajp.159.4.652 [DOI] [PubMed] [Google Scholar]
- 36.Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. Journal of Cognitive Neuroscience. 2009;22(9):1917–1930. doi: 10.1162/jocn.2009.21351 [DOI] [PubMed] [Google Scholar]
- 37.Wennman H, Jerome GJ, Simonsick EM, et al. Adiposity markers as predictors of 11-year decline in maximal walking speed in late midlife. Journal of Applied Gerontology. in press;doi: 10.1177/0733464820911542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pajala S, Era P, Koskenvuo M, et al. Contribution of genetic and environmental factors to individual differences in maximal walking speed with and without second task in older women. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2005;60A(10):1299–1303. doi: 10.1093/gerona/60.10.1299 [DOI] [PubMed] [Google Scholar]
- 39.Tiainen K, Pajala S, Sipilä S, et al. Genetic effects in common on maximal walking speed and muscle performance in older women Scandinavian Journal of Medicine & Science in Sports. 2007;17(3):274–280. doi: 10.1111/j.1600-0838.2006.00553.x [DOI] [PubMed] [Google Scholar]
- 40.Smith-Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D. Impact of cognitive training on balance and gait in older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013;70:357–366. doi: 10.1093/geronb/gbt097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hupfeld KE, Vaillancourt DE, Seidler RD. Genetic markers of dopaminergic transmission predict performance for older males but not females. Neurobiology of Aging. 2018;66(180):180.e11–180.e21. doi: 10.1016/j.neurobiolaging.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindenberger U, Nagel IE, Chicherio C, Li S-C, Heekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Frontiers in Neuroscience. 2008;2(2):234–244. doi: 10.3389/neuro.01.039.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]