Abstract
Accumulation of bisretinoids such as A2E and its isomer iso-A2E is thought to mediate blue light-induced oxidative damage associated with age-related macular degeneration (AMD) and autosomal recessive Stargardt disease (STGD1). We hypothesize that increasing dietary intake of the macular carotenoids lutein and zeaxanthin in individuals at risk of AMD and STGD1 can inhibit the formation of bisretinoids A2E and iso-A2E, which can potentially ameliorate macular degenerative diseases. To study the beneficial effect of macular carotenoids in a retinal degenerative diseases model, we used ATP-binding cassette, sub-family A member 4 (Abca4−/−/) /β,β-carotene-9′,10’-oxygenase 2 (Bco2−/−) double knockout (KO) mice that accumulate elevated levels of A2E and iso-A2E in the retinal pigment epithelium (RPE) and macular carotenoids in the retina. Abca4−/−/Bco2−/− and Abca4−/− mice were fed a lutein-supplemented chow, zeaxanthin-supplemented chow or placebo chow (~2.6 mg of carotenoid/mouse/day) for three months. Visual function and electroretinography (ERG) were measured after one month and three months of carotenoid supplementation. The lutein and zeaxanthin supplemented Abca4−/−/Bco2−/− mice had significantly lower levels of RPE/choroid A2E and iso-A2E compared to control mice fed with placebo chow and improved visual performance. Carotenoid supplementation in Abca4−/− mice minimally raised retinal carotenoid levels and did not show much difference in bisretinoid levels or visual function compared to the control diet group. There was a statistically significant inverse correlation between carotenoid levels in the retina and A2E and iso-A2E levels in the RPE/choroid. Supplementation with retinal carotenoids, especially zeaxanthin, effectively inhibits bisretinoid formation in a mouse model of STGD1 genetically enhanced to accumulate carotenoids in the retina. These results provide further impetus to pursue oral carotenoids as therapeutic interventions for STGD1 and AMD.
Keywords: Abca4, Bco2, bisretinoids, carotenoids, knockout mice, retina, RPE, STGD1
Introduction
Lipofuscin, a fluorescent by-product of the visual cycle, accumulates with age in the retinal pigment epithelium (RPE)—(Sparrow et al., 2012-, Sparrow, 2016)-. The pyridinium bisretinoid A2E and its cis-trans isomers (iso-A2E) form from chemical reactions of retinaldehyde and are prominent fluorophores of RPE lipofuscin (Parish et al., 1998). Bisretinoids such as A2E contribute to drusen formation in dry AMD and present as autofluorescent flecks in autosomal recessive Stargardt disease (STGD1) (Sparrow et al., 2015). A2E and iso-A2E are initially formed in photoreceptor outer segments by the condensation of all-trans-retinal or 11-cis-retinal molecules with phosphatidylethanolamine to form the Schiff-base N-retinylidene phosphatidylethanolamine (NRPE). NRPE then reacts with one more molecule of retinal to form a phosphatidylethanolamine-bisretinoid, A2-PE, a precursor of A2E and iso-A2E. Hydrolysis of A2-PE in the RPE leads to formation of A2E and iso-A2E (Liu et al., 2000, Kiser et al., 2014). Translocation of N-retinyl-phosphatidylethanolamine across photoreceptor outer segment disk membranes is carried out by the ATP-binding cassette, subfamily A, member 4 (ABCA4) transporter protein under normal healthy conditions. Absence or dysfunction of ABCA4 in STGD1 patients leads to excessive accumulation of A2E and other bisretinoids in the RPE, resulting in retinal cell damage and vision loss (Sears et al., 2017, Gao et al., 2018). Levels of A2E and its isomers are elevated with aging, dry AMD, and in STGD1 (Delori et al., 1995, Mata et al., 2000, Kim et al., 2004, Burke et al., 2014).
A2E is an amphiphilic molecule with a detergent-like structure that may influence membrane properties (De and Sakmar, 2002) and inhibit lysosomal functions (Finnemann et al., 2002). In the presence of blue light and oxygen, A2E serves as a photosensitizer that generates reactive singlet oxygen and undergoes photo-oxidation and photodegradation resulting in retinal damage and apoptosis of photoreceptor and RPE cells (Rózanowska et al., 1995, Gaillard et al., 1995, Wu et al., 2010, Yamamoto et al., 2012, Ueda et al., 2016a). A2E photo-oxidation products can also activate the complement system and cause inflammation (Zhou et al., 2006) and DNA damage in RPE cells (Sparrow et al., 2003). A2E and its photo-oxidized products including methylglyoxal impair mitochondrial dynamics and function, leading to apoptosis of RPE cells (Wu et al., 2010, Alaimo et al., 2019). Photosensitization of A2E accelerates the production of inflammatory cytokines, which leads to DNA damage and telomere deletion and triggers RPE senescence (Wang et al., 2018). Photo-oxidation of bisretinoids such as A2E also elevates lipid peroxidation (Zhou et al., 2005) and up-regulates the expression of oxidative stress and complement activation genes and down-regulates protective complement regulatory proteins in a STGD1 mouse model (Radu et al., 2011). Thus, STGD1 and AMD may both be caused by oxidative stress that triggers chronic inflammation of the RPE (Radu et al., 2011). Oxidative stress is an important causative factor for retinal degenerative diseases, especially since the protective effects of antioxidant enzymes decrease with age. Supplementation with macular carotenoids may protect the retina from photo-oxidation and oxidative stress.
Lutein and zeaxanthin are dietary xanthophyll carotenoids that are selectively accumulated at high concentrations in the human macular region where they protect the eye from oxidative damage and enhance visual performance (Bernstein et al., 2016). In vitro studies have shown that lutein and zeaxanthin protect against A2E/A2-PE-mediated photo-oxidation by filtering high-energy, short-wavelength blue light and/or by their antioxidant activities (Kim et al., 2006). In vivo studies in Japanese quail (Coturnix japonica) have shown that lutein and zeaxanthin can attenuate the formation of A2E and its isomers in the RPE. Supplementation with lutein and zeaxanthin in Japanese quail led to lower levels of A2E relative to an unsupplemented control group, and zeaxanthin was more effective than lutein (Bhosale et al., 2009). Human donor eye studies have likewise shown that levels of A2E in the human macula and periphery are inversely correlated with retinal carotenoid levels (Bhosale et al., 2009).
The effects of carotenoid supplementation on A2E and iso-A2E formation have not been studied previously in mice due to the lack of a suitable rodent model that accumulates carotenoids in the retina because wild type (WT) mice have a very active β,β-carotene oxygenase 2 (Bco2) enzyme that efficiently cleaves orally administered lutein and zeaxanthin (Li et al., 2014, Thomas et al., 2020). To study the beneficial effects of macular carotenoids in a relevant disease model, we crossbred Abca4−/− mice that accrue large amounts of RPE bisretinoids as they age (Weng et al., 1999, Mata et al., 2000, Kim et al., 2004) with Bco2−/− “macular pigment mice” that readily accumulate lutein and zeaxanthin in their retinas in response to oral supplementation (Li et al., 2017). We report inverse correlations between bisretinoid and macular carotenoids levels in these Abca4−/−/Bco2−/− double knockout (KO) mice and study their visual performance in response to carotenoid supplementation.
Materials and Methods
Materials
Lutein and zeaxanthin HPLC standards (≥ 98% pure) were provided by Kemin Health (Des Moines, IA, USA) and DSM Nutritional Products, Ltd. (Kaiseraugst, Switzerland), respectively. Standards of A2E and iso-A2E were synthesized from all-trans-retinal and ethanolamine as previously described (Parish et al., 1998). Lutein, zeaxanthin, and placebo Actilease® beadlets were supplied by DSM Nutritional Products, Ltd. (Kaiseraugst, Switzerland). Lutein and zeaxanthin Actilease® beadlets were mixed with previously reported base diet (Testdiet®, Richmond, IN) at a dosage of 1 g/kg of the base diet (~2.6 mg per mouse per day) (Lindshield et al., 2008). All organic solvents were of HPLC grade and were purchased from Thermo Fisher (Waltham, MA, USA).
Animal handling and carotenoid feeding experiment
Abca4−/− founders on a C57BL/6 background purchased from Jackson Laboratories (Farmington, CT, USA) and Bco2−/− founders on a C57BL/6 background provided by Prof. Johannes von Lintig (Case Western Reserve University) were used to generate Abca4−/−/Bco2−/− mice at the University of Utah vivarium. RPE65 variant sequencing was based on a previously published protocol (Kim et al., 2004). In brief, DNA derived from mouse tail by standard procedures was PCR-amplified with the forward 5′-ACCAGAAATTTGGAGGGAAAC-3′ and reverse 5′-CCCTTCCATTCAGAGCTTCA-3′ primers. The resulting 545 bp PCR product was analyzed on 2% agarose gel. DNA was eluted from the gel using a QIAquick gel extraction kit (Qiagen, Germantown, MD). The eluted samples along with the forward primer were submitted to a University of Utah core facility for DNA sequencing. Mice were housed in the vivarium under controlled conditions (26 °C, 12-h light/dark cycle) with ad libitum access to food and water. Three-month-old Abca4−/− and Abca4−/−/Bco2−/− mice (n=12 mice/group) were fed with lutein or zeaxanthin beadlets for three months. The control group received placebo beadlets mixed with the base diet. The mice were studied by electroretinography (ERG), optokinetic response (OKR), and optical coherence tomography (OCT) after one and three months of feeding. At the end of the study, the mice were anesthetized with isoflurane (2%), blood was collected by cardiac puncture, and tissues such as eyes, liver, and brain were collected. The eyes were dissected under a stereo zoom microscope (Motic, SMZ-168) to separate the retina from the RPE/choroid. The blood was centrifuged at 5,000 rpm @ 4 °C to separate serum. All collected materials were labeled and stored at −80 °C until analyzed. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Utah and conformed to the standards in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. A schematic representation of the experimental design is shown in Figure 1.
Figure 1.
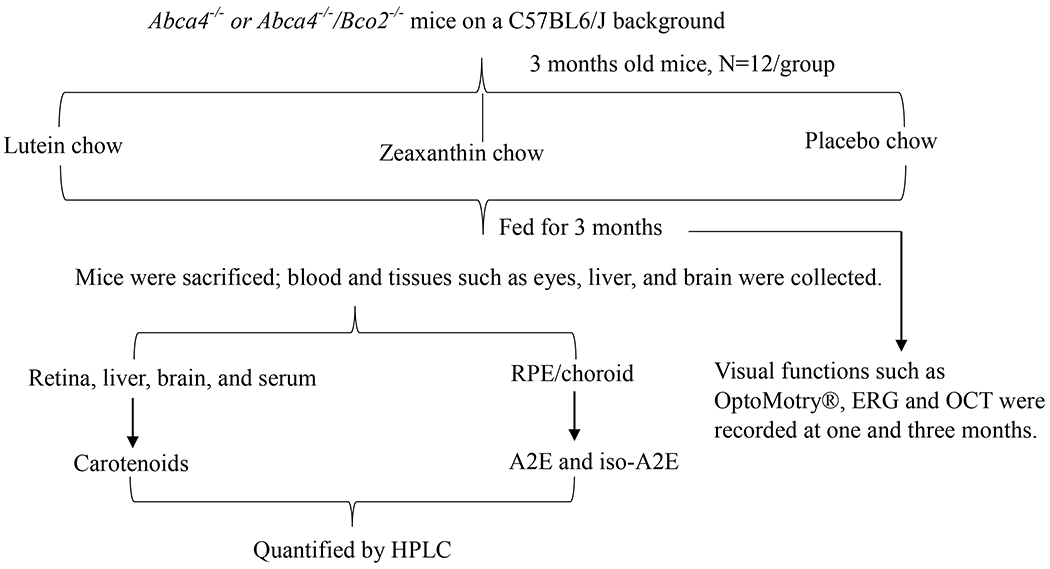
Schematic representation of animal experimental design showing different treatment groups with their respective timelines and endpoint measurements.
Bisretinoid extraction and analysis by HPLC
A2E and its isomers were extracted and isolated from RPE/choroid according to a previously described protocol with slight modifications (Gutierrez et al., 2010). One ml of chloroform: methanol (1:1 v/v) and 0.25 ml of 0.01 M phosphate-buffered saline (1x PBS) were added to pooled RPE/choroid samples (n=2-3 pairs) and were homogenized in a Mini Bead Beater (BioSpec Products Bartlesville, OK, USA) for 15 sec and then bath sonicated (Fisher FS60H) on ice for 15 min. The mixture was centrifuged at 5,000 rpm for 10 min to collect the organic layer into a separate tube. The leftover aqueous layer was re-extracted with 0.5 ml of chloroform, and the above-mentioned steps of homogenization, sonication, and centrifugation were repeated twice. The collected organic layer was evaporated to dryness under nitrogen gas and then re-suspended with 0.5 ml of chloroform and 0.5 ml of water and centrifuged at 5,000 rpm for 5 min. The organic layer was separated, evaporated, and re-dissolved in 100 μl methanol with 0.1% trifluoroacetic acid (TFA). The vials were centrifuged at 10,000 rpm for 10 min to remove the small amounts of insoluble solid particles prior to analysis. A gradient of 84-100% acetonitrile (A) with 0.05% TFA in H2O (B) over 35 minutes was used to separate A2E at a flow rate of 0.5 ml min−1 on an Atlantis dC18 column (4.6 × 150 mm, 3 μm, Waters Corporation, Milford, MA). The column was maintained at room temperature, and the HPLC photodiode array (PDA) detector was monitored at 440 nm. A2E and iso-A2E peaks were confirmed by respective retention times, PDA spectra, and co-elution with HPLC standards (Wu et al., 2009).
Lutein and zeaxanthin extraction and analysis by HPLC
Lutein and zeaxanthin in the tissues such as liver, brain, eyes, and serum of mice were extracted and analyzed according to a previously reported method (Khachik et al., 2002). Briefly, pooled retinas (n=2-3 pairs) were homogenized using a Bead Beater, and the carotenoids were extracted three times using tetrahydrofuran (THF) containing 0.1% butylated hydroxytoluene (BHT) and bath sonicated each time at 4-10 °C for 30 min. The extracts were centrifuged at 5,000 rpm for 5 min, and the organic supernatant was collected and evaporated to dryness under nitrogen gas. The liver and brain samples were homogenized with a mechanical homogenizer (PRO Scientific, Oxford, CT, USA), and carotenoids were extracted from 0.3 to 0.5 g tissue homogenates using the above method for ocular tissues. To extract the carotenoids from serum samples, ethanol containing 0.1% BHT was added to precipitate the proteins, and then ethyl acetate was added to extract the carotenoids. The sample was centrifuged at 5,000 rpm for 5 min at 4 °C, and the organic supernatant layer was collected. Then the aqueous layer was extracted twice with ethyl acetate and extracted once with hexane. The pooled organic layers were dried under nitrogen gas. The evaporated sample was re-dissolved in HPLC mobile phase and centrifuged at 10,000 rpm for 10 min, and the clear supernatant phase was injected into the HPLC system. HPLC separations were performed on a silica-based nitrile bonded column (25 cm length × 4.6 mm internal diameter: 5-μm spherical particle (Regis Chemical, Morton Grove, IL)). The eluent consisted of an isocratic mixture of hexanes (75%), dichloromethane (25%), methanol (0.3%), and N, N-di-isopropylethylamine (0.1%). The column flow rate was 1 ml/min. The column temperature was maintained at 25 °C, and the monitoring wavelength was 445 nm. Lutein and zeaxanthin peaks were confirmed by respective retention times, PDA spectra, and co-elution with HPLC standards. RPE/choroid carotenoid levels were not quantified in order to have adequate amounts of tissues for bisretinoid analysis. Previous studies from our laboratory have shown that RPE/choroid lutein and zeaxanthin levels in Bco2−/− mice are consistently ~4.5-times higher than the overlying retina (Li et al., 2017, Li et al., 2018).
Retinoids extraction and analysis by ultra-performance liquid chromatography (UPLC)
For retinoid quantification, mice were dark-adapted for 18 h, and frozen mouse eyecups (1 eye/sample) were homogenized and derivatized using O-ethylhydroxylamine (Kane et al., 2008) on ice under dim red light. Retinal O-ethyloxime and other retinoids (all-trans-retinol and all-trans-retinyl palmitate) were extracted with hexane and resuspended in acetonitrile. Samples were eluted on a Waters Acquity UPLC system using a CSH C18 column (1.7 μm, 2.1 × 100 mm; Waters, Milford, MA) and gradients of water (A) and acetonitrile (B) containing 0.1% of formic acid as follows: 0-5 min, 60% B; 5-60 min, 60-70% B; 60-70 min, 70-100% B; 70-90 min, 100% B min (flow rate of 0.3 mL/min). Retinal (O-ethyl) oximes (11-cis-retinal and all-trans-retinal) were monitored at 360 nm, and all-trans-retinol and all-trans-retinyl palmitate were monitored at 320 nm. UV absorbance peaks were identified by comparison with external standards of synthesized retinoids (Lima de Carvalho et al., 2020).
Optokinetic response (OKR) testing
Abca4−/− and Abca4−/−/Bco2−/− mice supplemented with and without carotenoids (n = 10/group) for three months had their visual acuity assessed via OKR using OptoMotry® (Cerebral Mechanics, Lethbridge, AB, Canada) after one month and three months of feeding. The OptoMotry® system displays a virtual rotating cylinder with a vertical black-and-white sinusoidal grating pattern. Each mouse was placed separately on a 2-inch elevated platform at the center surrounded by four inward-facing computer screens, and their movements were supervised by a video camera. The epicenter of the virtual rotating cylinder is maintained at the position of the mouse head. During the analysis of spatial frequency and contrast sensitivity, the researcher carefully observes the movement of the mice on the elevated platform and marks the score on the software. Rotation speed (12 degrees/s) and contrast (100%) were kept constant during the analysis of the spatial frequency threshold. Spatial frequency (0.19 cycle/degree) was kept constant during the analysis of contrast sensitivity. Photopic measurements were conducted with the mice adapted to room light (165 lux). Scotopic measurements were conducted under infrared light on mice dark-adapted for at least 8 hours using liquid crystal displays (LCDs) covered by neutral density filters (ND16- Lee299). Mice fed with placebo chow devoid of carotenoids acted as a control group. The observer was blind to the genotype and dietary group of the mice (Li et al., 2018) .
Electroretinography (ERG)
Ketamine (Ketalar, 100 mg/mL, 80 mg/kg body weight) and xylazine (AnaSed, 20 mg/mL, 10 mg/kg body weight) anesthetics were purchased from the University of Utah pharmacy. Sterile water was used to dilute up to 10 ml working stock of ketamine/xylazine anesthetic mixture. Mice were anesthetized through intraperitoneal injection of a ketamine/xylazine mixture 0.1 ml/10 g body weight. Mouse eyes were dilated with a few drops of tropicamide ophthalmic solution, USP 1%. Subsequently, mice were placed onto a 37°C thermostatic heating pad in a resting position, and one drop of artificial tears (Refresh Liquigel, Alcon, Fort Worth, TX, USA) was added to each eye to maintain hydration and to prevent corneal desiccation. For scotopic measurements, mice were placed in a dark chamber for at least 8 hours with ad libitum access to food and water (Barabas et al., 2013).
Full-field ERG potentials were measured as described (Barabas et al., 2013) using a UTAS E-3000 (LKC Technologies, Gaithersburg, MD). Mice were anesthetized with the ketamine/xylazine mixture and placed on a controlled warming pad. ERGs were measured between a gold corneal and a stainless-steel scalp electrode with a 0.3- to 500-Hz band-pass filter. Scotopic and photopic ERGs were recorded by using increasing flash intensities from 0.00025 to 79 cd·s/m2 (scotopic) and from 2.5 to 79 cd·s/m2 (photopic) using 5-7 mice per group. Two to twelve a- and b-wave traces were averaged for every stimulus intensity, and the mean values at each stimulus were compared with an unpaired two-tailed t-test.
Optical coherence tomography (OCT)
OCT images were captured using a Phoenix Micron IV image-guided OCT system (Pleasanton, CA). In each eye, four OCT images were averaged to generate mean retinal thickness and outer nuclear layer (ONL) thickness values. InSight software was used for sectioning average ONL thicknesses with n=6/group.
Protein Isolation and Western Blot Analysis
Cells and tissues were homogenized on ice in RIPA buffer (140 mM NaCl, 1 mM EDTA, 10 mM Tris HC1 pH 8.0, 0.1% Sodium Deoxycholate, 1mM PMSF, 1% Triton X-100 and cOmplete™, EDTA-free Protease Inhibitor Cocktail). Bradford assay (Thermo Fisher Scientific) was carried out, and 20 μg protein was resolved by 4-15% Mini-PROTEAN® TGX Precast Protein Gel. Transfer to a 0.45-μm nitrocellulose membrane was done using a Trans-Blot SD semidry transfer cell (Bio-Rad) at 20 V for 1 h. Membranes were subsequently washed in TBS with 0.1% Tween-20 and then blocked using 5% Blotting-Grade Blocker (Bio-Rad) containing 0.1% Tween-20 for 1 h. Primary antibodies were diluted in the 10% blocking buffer, and the membranes were incubated overnight at 4 °C. The antibodies used and their dilutions were as follows: 1:1000 dilution of anti-RPE65 antibody (Proteintech®), and 1:1000 dilution of anti-β-actin (A2066, Sigma-Aldrich). Proteins were visualized using Amersham ECL Prime Western Blotting Detection Reagent on an iBright™ CL750 Imaging System following incubation with HRP-conjugated secondary antibodies at 1:500 dilutions for 1 h at room temperature (Shyam et al., 2017)
Statistical analysis
Data are presented as mean ± SEM. The statistical analyses were performed using one-way and two-way analysis of variance (ANOVA) and 2-sided Student t-tests and Sidak‘s multiple comparison test on Prism software (GraphPad Software Inc., La Jolla, CA, USA) with p<0.05 considered as significant.
Results
Abca4−/−/Bco2−/− and Abca4−/− mice were supplemented with lutein or zeaxanthin beadlets for three months, and their serum and tissue distributions of lutein and zeaxanthin were analyzed and compared to animals fed placebo beadlets (Figure 2). Abca4−/−/Bco2−/− mice fed with zeaxanthin had significantly increased accumulation of zeaxanthin in serum (4.3-fold), retina (11.4-fold), liver (1.6-fold), and brain (1.6-fold), relative to zeaxanthin-supplemented Abca4−/− mice. Similarly, lutein supplemented Abca4−/−/Bco2−/− mice had significantly increased accumulation of lutein in serum (2.7-fold), retina (8.6-fold), liver (1.4-fold), and brain (1.5-fold), compared to the lutein supplemented Abca4−/− mice. Lutein and zeaxanthin levels were below the limits of quantification in all animals fed placebo chow.
Figure 2.
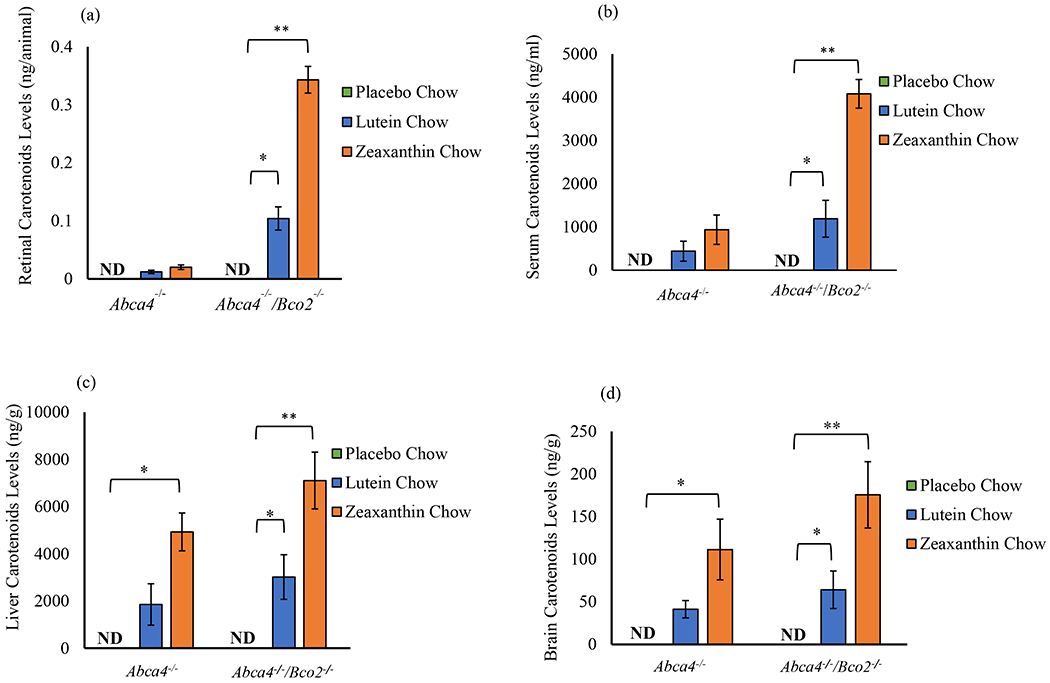
Effect of lutein and zeaxanthin supplementation on serum and tissue distributions in Abca4−/−/Bco2−/− and Abca4−/− mice supplemented for three months. The carotenoid distribution in (a) retina, (b) serum, (c) liver, and (d) brain shows significantly elevated levels of lutein and zeaxanthin in Abca4−/−/Bco2−/− mice. Mice fed lutein had exclusively lutein in all tissues. Mice fed zeaxanthin had exclusively zeaxanthin in all tissues. Data presented as mean ± SEM (n=12 mice/group, p values: **p<0.01, *p<0.05; N.D.: not detected).
The three-month effects of carotenoid supplementation on Abca4−/−/Bco2−/− and Abca4−/− mice on A2E and iso-A2E levels are shown in Figure 3. Compared to the placebo-fed group, A2E and iso-A2E levels decreased significantly in the zeaxanthin-supplemented Abca4−/−/Bco2−/− mice by 63.2% and 71.3%, respectively. Likewise, the lutein supplemented Abca4−/−/Bco2−/− group’s A2E and iso-A2E levels significantly decreased by 33.0% and 43.3%, respectively, when compared to the placebo-fed group. A2E and iso-A2E levels decrease slightly in response to carotenoid supplementation in the Abca4−/− mice but not as significant as observed in Abca4−/− /Bco2−/− mice. A2E and iso-A2E levels in RPE/choroid were inversely proportional to retinal carotenoid levels (Figure 4), with zeaxanthin consistently more bioavailable to the retina and more effective at suppressing A2E and iso-A2E levels in the RPE/choroid.
Figure 3.
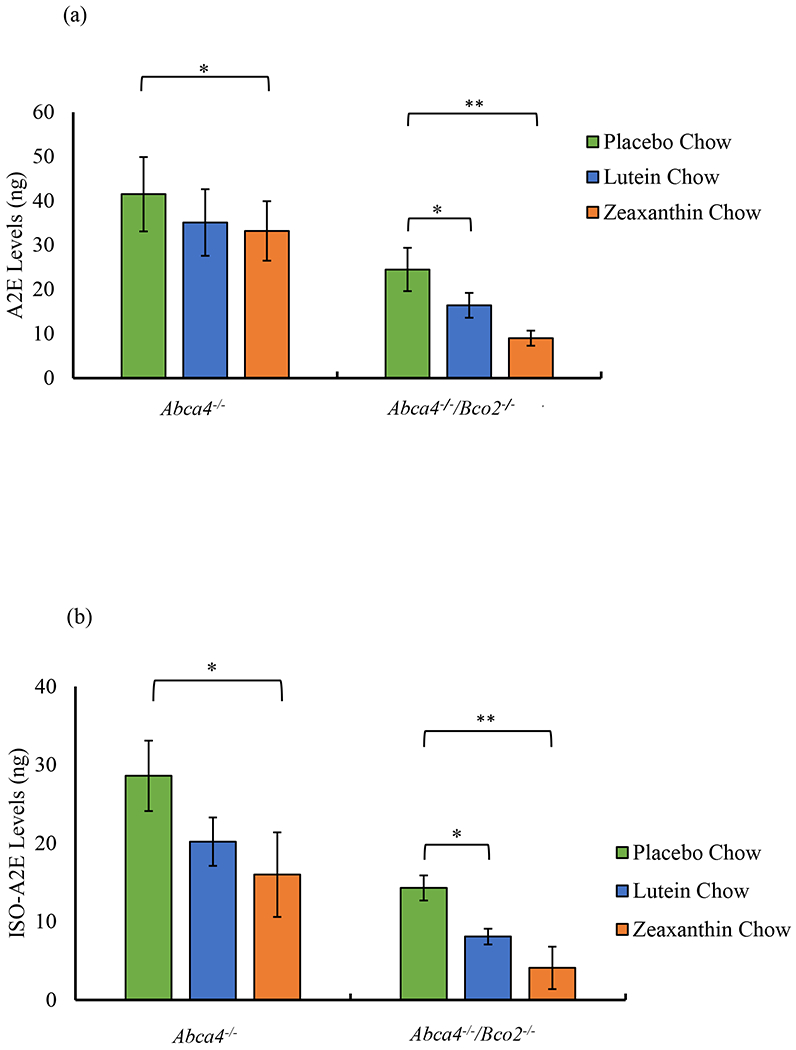
Effect of lutein and zeaxanthin supplementation on RPE/choroid A2E and iso-A2E levels in Abca4−/−/Bco2−/− and Abca4−/− mice. (a) Presents A2E levels, and (b) presents iso-A2E levels in Abca4−/−/Bco2−/− and Abca4−/− mice fed with the indicated carotenoids for three months. A placebo group supplemented carotenoid-free beadlets serves as the control. Data presented as mean ± SEM (n=12 mice/group, p values: **p<0.01, *p<0.05; N.D.: not detected).
Figure 4.
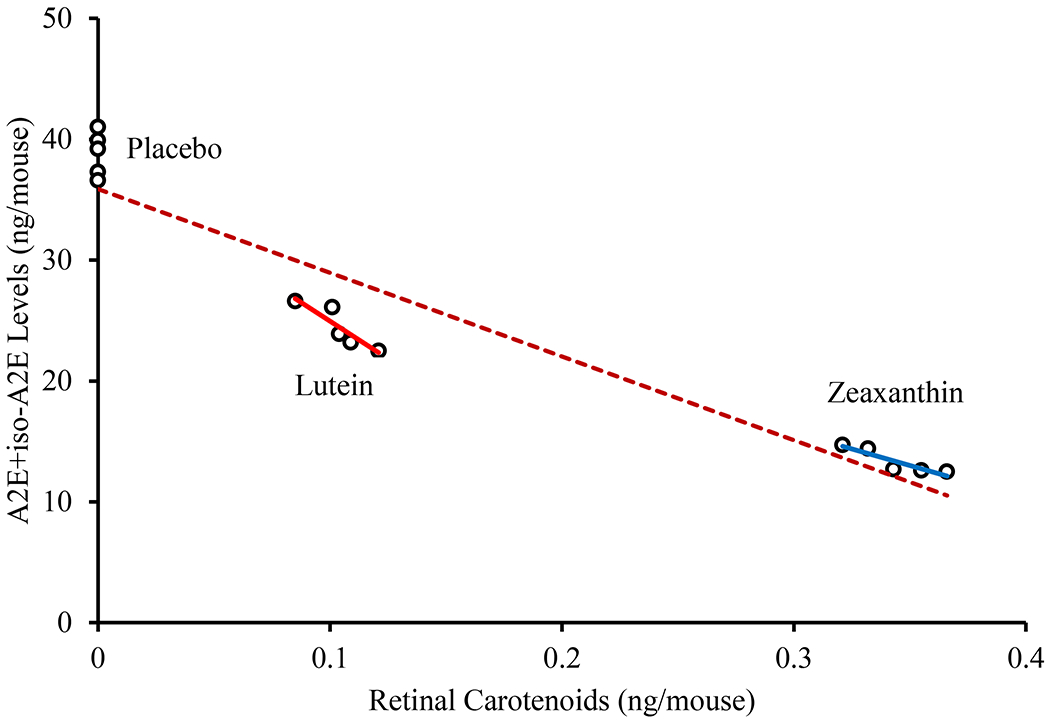
Distribution of RPE/choroid A2E + iso-A2E levels in relation to retinal carotenoids in Abca4−/−/Bco2−/− mice. There was a statistically significant inverse correlation between retinal carotenoids and A2E and iso-A2E levels in RPE/choroid (all p values < 0.05). Red line refers to lutein with R2 = 0.815, blue line refers to zeaxanthin with R2 = 0.825 and the brown dotted line refers to combined regression plot of lutein, zeaxanthin and placebo group with R2 = 0.904.
In Figure 3, we noted that knockout of Bco2 partially suppresses A2E and iso-A2E with or without carotenoid supplementation. To determine whether deficiency in Bco2 alters retinoid content in the mouse eye, we measured retinoids in dark-adapted Abca4−/−/Bco2−/− mice as compared with Abca4−/− mice (Figure S1). UPLC measurement of retinoids revealed that in the Abca4−/− mice, levels of retinoids (11-cis-retinal, all-trans-retinal, all-trans-retinol and alltrans-retinyl ester) were not significantly different relative to the Abca4−/−/Bco2−/− mice (P > 0.05, two-way ANOVA and Sidak’s multiple comparison test). Similarly, there were no differences in RPE65 expression levels between Abca4−/− and Abca4−/−/Bco2−/− mice (Figure S2).
To analyze the effects of lutein and zeaxanthin on visual performance of mice after 1 and 3 months of carotenoid feeding, Abca4−/− and Abca4−/−/Bco2−/− mice were tested for scotopic and photopic visual acuity and contrast sensitivity using OptoMotry®. The results of visual performance measured after 1 month (Figure 5) and 3 months of supplementation (Figure 6) follow the same pattern, with the Abca4−/−/Bco2−/− zeaxanthin supplementation group showing significantly better scotopic and photopic visual acuity and contrast sensitivity relative to the lutein and placebo groups. Compared to the placebo group, the rod and cone spatial frequencies of the zeaxanthin-supplemented Ahca4−/−/Bco2−/− “ mice increased by 16% and 13%, respectively, after 3 months of supplementation, while the rod and cone contrast sensitivities improved by 37% and 22%. Rod and cone spatial frequencies of the lutein supplemented Abca4−/−/Bco2−/− mice increased 11% and 9%, and the rod and cone contrast sensitivities improved by 25% and 13%, when compared to the placebo group. Carotenoid supplementation in Abca4−/− mice did not improve visual function relative to the placebo group.
Figure 5.
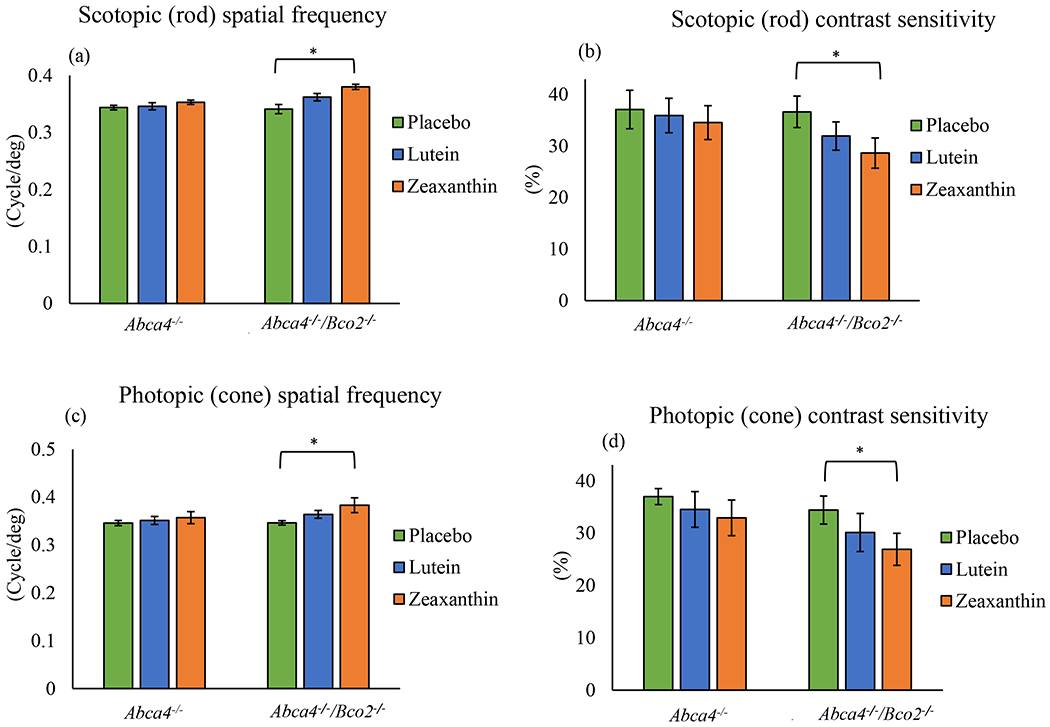
Visual performance measured by optokinetic response (OKR) using OptoMotry® in Abca4−/−/Bco2−/− and Abca4−/− mice after one month of carotenoid supplementation. (a) Scotopic spatial frequency; (b) Scotopic contrast sensitivity; (c) Photopic spatial frequency; (d) Photopic contrast sensitivity. Values indicate means ± SD; 10 mice were used in each group; **p<0.01, *p<0.05.
Figure 6.
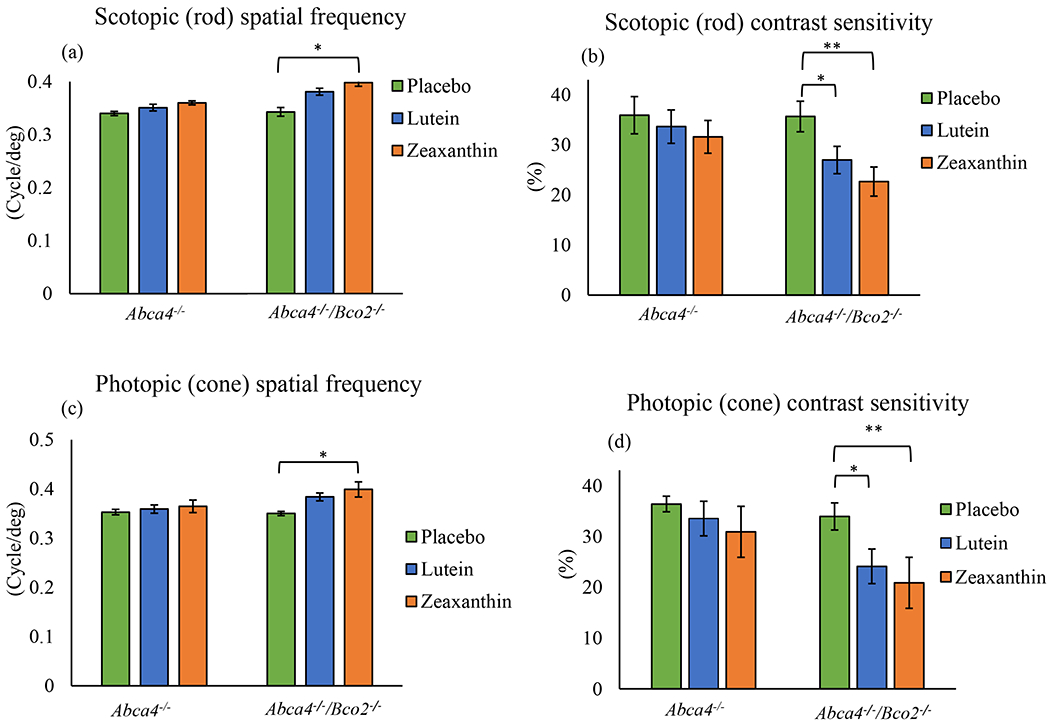
Visual performance measured by optokinetic response (OKR) using OptoMotry in Abca4−/−/Bco2−/− and Abca4−/− mice after three months of carotenoid supplementation. (a) Scotopic spatial frequency; (b) Scotopic contrast sensitivity; (c) Photopic spatial frequency; (d) Photopic contrast sensitivity. Values indicate means ± SD; 10 mice were used in each group; **p<0.01, *p<0.05.
Rod and cone photoreceptor responses were measured by scotopic and photopic flash ERG full-field potentials (Weymouth and Vingrys, 2008). After one and three months of carotenoid or placebo supplementation in Abca4−/−, and Abca4−/−/Bco2−/− mice, scotopic and photopic ERG responses were measured relative to WT mice of the same age (4-6-months-old). We found no significant a-wave or b-wave differences relative to age-matched WT mice under any feeding conditions in either mouse line at one month (Figure 7) or at three months (Figure 8).
Figure 7.
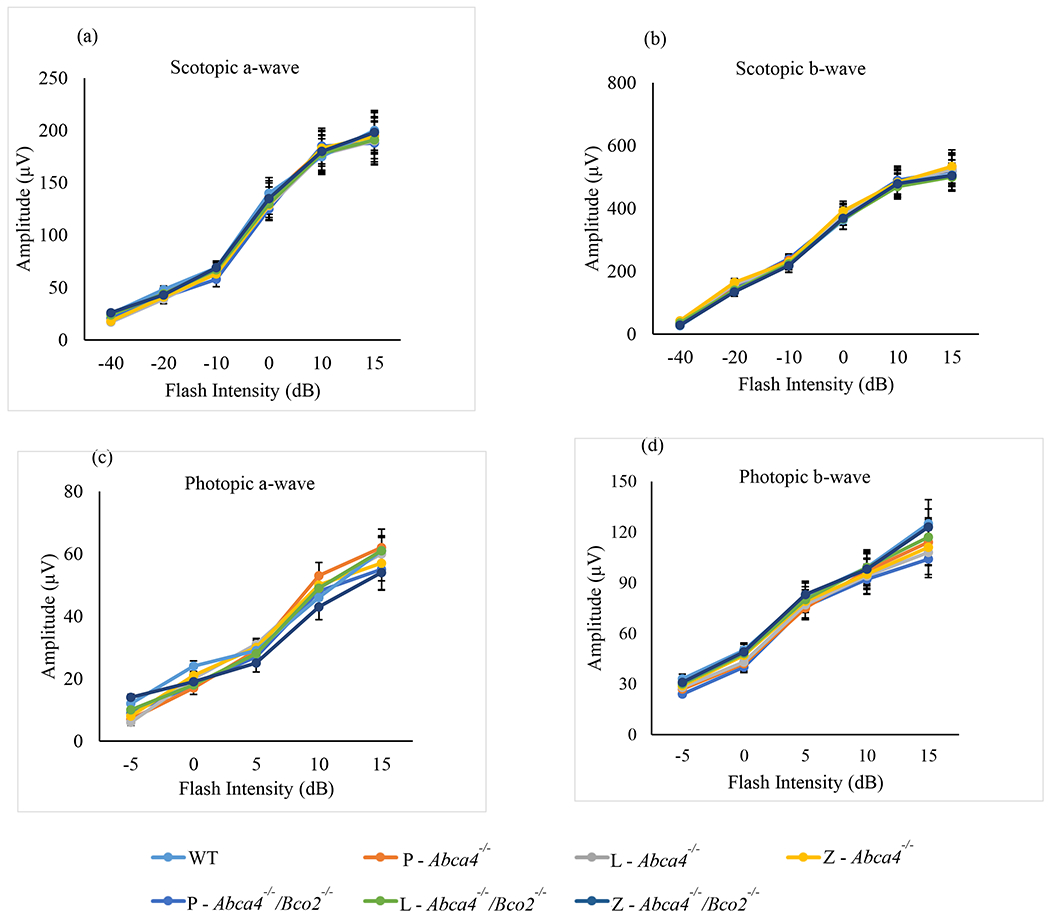
ERG responses in Abca4−/−/Bco2−/− and Abca4−/− mice after one month of carotenoid supplementation. (a) Scotopic a-wave amplitudes. (b) Scotopic b-wave amplitudes. (c) Photopic a-wave amplitudes. (d) Photopic b-wave amplitudes. Mice were dark-adapted, and their scotopic and photopic ERG response were recorded (n=6 mice/group). L-lutein, Z-zeaxanthin and P-placebo chow. Data presented as mean ± SEM. The differences were not significant.
Figure 8.
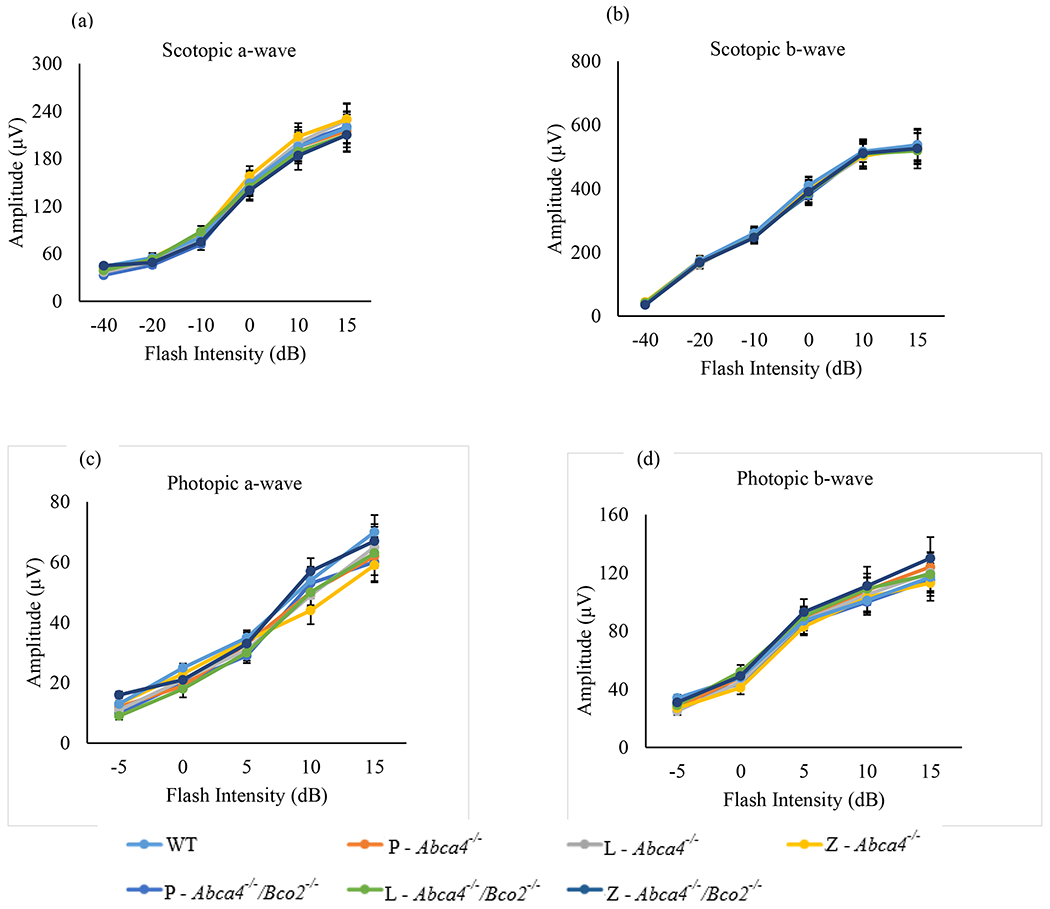
ERG response in Abca4−/−/Bco2−/− and Abca4−/− mice after three months of carotenoid supplementation. (a) Scotopic a-wave amplitudes. (b) Scotopic b-wave amplitudes. (c) Photopic a-wave amplitudes. (d) Photopic b-wave amplitudes. Mice were dark-adapted, and their scotopic and photopic ERG response were recorded (n=6 mice/group). L-lutein, Z-zeaxanthin and P-placebo chow. Data presented as mean ± SEM. The differences were not significant.
Photoreceptor cell viability was assessed by measuring ONL thickness by OCT in Abca4−/− and Abca4−/−/Bco2−/− after three months of carotenoid or placebo supplementation (Figure 9). WT mice of the same age (six-months-old) were used as controls. There were no significant differences relative to WT mice in either mouse line, irrespective of their supplementation status.
Figure 9.
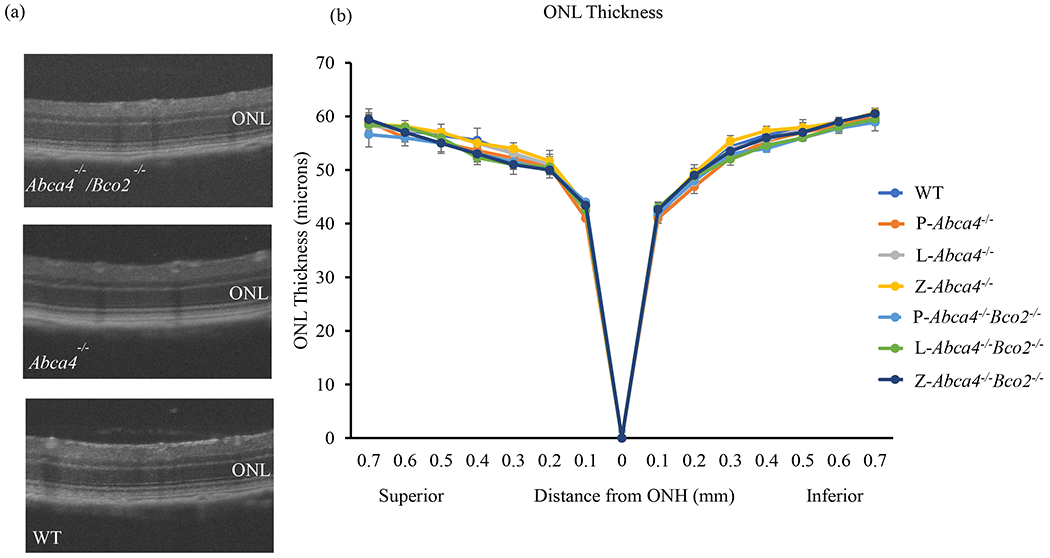
Quantification of outer nuclear layer (ONL) thickness in Abca4−/−/Bco2−/− and Abca4−/− mice after three month of carotenoid supplementation at the age of 6 months. (a) OCT images of Abca4−/−/Bco2−/−, Anca4−/−, and WT mice of the same age. (b) ONL thickness measurements are plotted as a function of distance from the optic nerve head (ONH) in the inferior and superior hemispheres. Data presented as mean ± SEM (n=6 mice/group); L-lutein, Z-zeaxanthin and P-placebo chow. The differences were not significant.
Discussion
Retinal lipofuscin is a complex aggregate of bisretinoid fluorophores, with A2E and iso-A2E being prominent members of this group and key contributors in the pathogenesis of retinal degeneration such as AMD (Winkler et al., 1999) and ABCA4-associated Stargardt disease (STGD1) (Weng et al., 1999, Sparrow, 2016, Sears et al., 2017). Lipofuscin accumulation in the RPE increases with age and in STGD1 and AMD (Delori et al., 2001, Burke et al., 2014). Abca4−/− mice exhibit elevated levels of RPE lipofuscin components such as A2E and iso-A2E (Weng et al., 1999, Kim et al., 2004). Abca4−/− mice are the principal animal models available for studying ABCA4-related retinopathy (Wu et al., 2010, Wu et al., 2014, Cremers et al., 2020). Several studies have shown that photo-oxidation of bisretinoids by blue light results in the generation of singlet oxygen and other reactive oxygen species which can lead to photo-oxidation and photodegradation, with the release of carbonyls and aldehyde-bearing molecule fragments leading to retinal cell death (Sparrow et al., 2003, Wielgus et al., 2010, Ueda et al., 2016b). A2E negatively affects lysosome and proteosome function owing to its detergent-like properties (Sparrow et al., 2014) and triggers complement pathways (Zhou et al., 2006) and inflammation (Parmar et al., 2018). When measured in cadaver human eyes, the fluorescence distribution associated with RPE lipofuscin increases from the periphery to the posterior pole of the eye, with a consistent dip at the fovea (Weiter et al., 1986).
The macular carotenoids are exclusively obtained from the diet in humans. Lutein, zeaxanthin, and lutein’s metabolite meso-zeaxanthin present as a yellow, oval spot at the center of the retina called the macula lutea. They are thought to protect the retina from photo-oxidation by filtering highly phototoxic blue light and by acting as antioxidants that scavenge free radicals and highly reactive singlet oxygen molecules (Krinsky et al., 2003, Bernstein et al., 2016). Zeaxanthin and meso-zeaxanthin are present at high concentrations at the center of the fovea distributed from the inner limiting membrane to the outer limiting membrane and then extending more peripherally in the macula’s inner and outer plexiform layers, while lutein is more diffusely distributed at much lower concentrations (Li et al., 2020). These macular carotenoids are also present in lower concentrations in the RPE (Bernstein et al., 2001). where they may protect against A2E-mediated photo-oxidation associated with inherited and age-related retinal degenerations (Bian et al., 2012). Macular pigment optical density in STGD1 patients is significantly lower than in healthy human subjects (Zhao et al., 2003, Aleman et al., 2007), and human cadaver eyes have significantly lower levels of A2E in the macular region where carotenoid levels are high compared to the peripheral retina where A2E levels are high and carotenoid levels are low. Moreover, prospective carotenoid supplementation studies in Japanese quail demonstrated nearly complete inhibition of A2E formation and oxidation (Bhosale et al., 2009).
While bird studies of the interrelationships of macular carotenoids and A2E are important, avian and mammalian eyes differ substantially in their handling of carotenoids because birds have a diverse array of xanthophyll carotenoids present as fatty acid esters in discrete photoreceptor oil droplets, while mammals do not. Therefore, a small laboratory mammal model would be useful to further study carotenoid/bisretinoid interactions, especially in Abca4 KO mice which accumulate high levels of A2E and iso-A2E with age, but no non-primate mammals naturally concentrate more than trace amounts of lutein and zeaxanthin in their retinas even with massive oral supplementation. We have solved this problem by knocking out the mouse carotenoid cleavage enzyme Bco2 and then crossing these “macular pigment mice” with Abcci4 KO mice.
Macular carotenoid feeding experiments in Abca4−/−/Bco2−/− mice led to elevated levels of lutein and zeaxanthin in the retina. Lutein and zeaxanthin levels in Abca4−/−/Bco2−/− mice were higher by 8.6 and 11.4-fold higher compared to Abca4−/− mice. Our results show that zeaxanthin was especially effective at reducing A2E and iso-A2E levels in Abca4−/−/Bco2−/− mice relative to lutein or placebo supplementation. Although this finding could be due to zeaxanthin’s superior bioavailability in our studies, it has been previously reported that dietary supplementation with zeaxanthin shows a better photo-protective effect than lutein, α-tocopherol, or γ-tocopherol against light-induced photoreceptor damage in Japanese quail (Thomson et al., 2002a, Thomson et al., 2002b).
Knockout of Bco2 partially suppresses RPE/choroid A2E and iso-A2E levels with or without carotenoid supplementation (Figure 3). This could have been due to differences in RPE65 activity or ocular carotenoid levels associated with Bco2 knockout. Our Abca4−/− and Abca4−/−/Bco2−/− mice are both from pigmented backgrounds, and all have an ATG at codon 450 (methionine) of RPE65 as opposed to a CTG at codon 450 (leucine) typically seen in BALB/c mice. RPE65 protein expression profiling by western blot showed that both Abca4−/− and Abca4−/−/Bco2−/− mice had identical RPE65 protein expression levels (Figure S2). Similarly, there were no differences in ocular retinoid levels in mice with or without functional Bco2 (Figure S1). These results indicate that the decreases in A2E and iso-A2E levels in carotenoid supplemented Abca4−/− and Abc4−/−/Bco2−/− mice that we observed were due to carotenoid bioactivity, not an RPE65 Leu450Met polymorphism or Bco2-related alterations in retinoid levels.
In vitro studies have also shown that zeaxanthin is more effective than lutein and α-tocopherol in suppressing A2E and A2-PE photo-oxidation (Kim et al., 2006) and that it is highly efficient at quenching singlet oxygen relative to lutein (Różanowski et al., 2008), possibly due to zeaxanthin’s more extensive double-bond conjugation (Stahl et al., 1997, Li et al., 2010). Since Bco2−/− mice accumulate ~4.5-fold higher levels of lutein and zeaxanthin in their RPE/choroids relative to their retinas in response to supplementation (Li et al., 2017, Li et al., 2018), our experiments cannot distinguish a light screening mechanism due to carotenoid deposition in the retina versus a direct chemical inhibition of bisretinoid formation in the retina and/or RPE. The mechanisms best known to suppress bisretinoid formation involve the visual cycle and can include competitive inhibition of RPE65 or reduced availability of vitamin A (Sparrow, 2016). Whether Bco2 deficiency and the accumulation of macular carotenoids in retina interferes with vitamin A access to RPE is a possibility that deserves investigation.
Apart from their photo-protective effects in the eye, the macular carotenoids can also improve visual acuity and contrast sensitivity in humans and mice. In our study, zeaxanthin supplementation especially enhanced rod and cone visual performance relative to placebo in Abca4−/−/Bco2−/− mice, while lutein supplementation had more modest effects. These findings are consistent with previously published reports of carotenoid-mediated enhancement of visual performance in Bco2−/− mice (Li et al., 2018) and in human clinical trials (Stringham et al., 2011), (Nolan et al., 2016).
Scotopic and photopic a- and b-wave ERG amplitudes and ONL thickness in Abca4−/−/Bco2−/− and Abca4−/− mice displayed no difference when compared to WT mice of the same age. Abca4−/− mice on a pigmented background reflect the early stages of human STGD1, with accumulation of lipofuscin and bisretinoids, but they do not show any signs of retinal degeneration up to 18 months of age (Issa et al., 2013). Melanin itself has antioxidant activity, and the melanin in melanolipofuscin granules would attenuate light and the fluorescence intensity of bisretinoids (Paavo et al., 2018). Further, the formation of melanolipofuscin granules may protect the RPE from oxidative stress and damage caused by lipofuscin (Różanowski et al., 2008, Issa et al., 2013),. Since Abca4−/− mice on a non-pigmented background exhibit degeneration with age, it will be interesting to repeat these experiments in non-pigmented Abca4−/−/Bco2−/− mice to see if zeaxanthin and lutein can protect against retinal degeneration as well. For now, the elevated levels of bisretinoids such as A2E and iso-A2E in pigmented mice with Abca4 defects continue to be used as biomarkers to monitor the efficacy of therapeutic intervention drugs and nutraceuticals, and our studies reported here provide further evidence that attenuation of bisretinoid formation using zeaxanthin and/or lutein alone or in combination with other antioxidants could be an effective therapeutic intervention against STGD1 and AMD.
Supplementary Material
Highlights.
Bisretinoids such as A2E mediate oxidative damage leading to STGD1 and AMD.
Abca4−/−/Bco2−/− mice accumulate A2E in RPE and macular carotenoids in retina.
Macular carotenoids significantly lower A2E levels in Abca4−/−/Bco2−/− mice.
Statistically significant inverse correlation between retinal carotenoids and A2E.
Acknowledgments
We would like to thank Dr. Johannes von Lintig from Case Western Reserve University for generously providing the Bco2−/− founder mice. This work was supported by NIH grants EY-11600, EY-14800, and by unrestricted departmental funds from Research to Prevent Blindness, New York City, NY.
Abbreviations
- ABCA4
ATP-binding cassette transporter subfamily A4
- AMD
age-related macular degeneration
- A2E
pyridinium bisretinoids
- A2-PE
phosphatidyl-pyridinium bisretinoid
- BCO2
β-carotene-oxygenase 2
- BHT
butylated hydroxytoluene
- ERG
electroretinography
- HPLC
high performance liquid chromatography
- KO
knockout
- LCD
liquid crystal display
- OCT
optical coherence tomography
- OKR
optokinetic response
- ONL
outer nuclear layer
- PBS
phosphate-buffered saline
- PDA
photodiode array
- RPE
retinal pigment epithelium
- STGD1
Stargardt disease type 1
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- UPLC
ultra-performance liquid chromatography
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No authors have any conflicts of interest.
References
- Alaimo A, Liñares GG, Bujjamer JM, Gorojod RM, Alcon SP, Martínez JH, Baldessari A, Grecco HE, Kotler ML, 2019. Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: implications for age-related macular degeneration. Arch Toxicol 93, 1401–1415. 10.1007/s00204-019-02409-6 [DOI] [PubMed] [Google Scholar]
- Aleman TS, Cideciyan AV, Windsor EAM, Schwartz SB, Swider M, Chico JD, Sumaroka A, Pantelyat AY, Duncan KG, Gardner LM, Emmons JM, Steinberg JD, Stone EM, Jacobson SG, 2007. Macular Pigment and Lutein Supplementation in ABCA4-associated Retinal Degenerations. Invest Ophthalmol Vis Sci 48, 1319–1329. 10.1167/iovs.06-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas P, Liu A, Xing W, Chen C-K, Tong Z, Watt CB, Jones BW, Bernstein PS, Križaj D, 2013. Role of ELOVL4 and very long-chain polyunsaturated fatty acids in mouse models of Stargardt type 3 retinal degeneration. Proc Natl Acad Sci U S A 110, 5181–5186. 10.1073/pnas.1214707110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB, 2001. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp. Eye Res 72, 215–223. 10.1006/exer.2000.0954 [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM, 2016. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Progress in Retinal and Eye Research 50, 34–66. 10.1016/j.preteyeres.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale P, Serban B, Bernstein PS, 2009. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch. Biochem. Biophys 483, 175–181. 10.1016/j.abb.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Q, Gao S, Zhou J, Qin J, Taylor A, Johnson EJ, Tang G, Sparrow JR, Gierhart D, Shang F, 2012. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic Biol Med 53, 1298–1307. 10.1016/j.freeradbiomed.2012.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TR, Duncker T, Woods RL, Greenberg JP, Zernant J, Tsang SH, Smith RT, Allikmets R, Sparrow JR, Delori FC, 2014. Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci 55, 2841–2852. 10.1167/iovs.13-13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers FPM, Lee W, Collin RWJ, Allikmets R, 2020. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Progress in Retinal and Eye Research 100861. 10.1016/j.preteyeres.2020.100861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Sakmar TP, 2002. Interaction of A2E with Model Membranes. Implications to the Pathogenesis of Age-related Macular Degeneration. J Gen Physiol 120, 147–157. 10.1085/jgp.20028566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delori FC, Goger DG, Dorey CK, 2001. Age-Related Accumulation and Spatial Distribution of Lipofuscin in RPE of Normal Subjects. Invest. Ophthalmol. Vis. Sci 42, 1855–1866. [PubMed] [Google Scholar]
- Finnemann SC, Leung LW, Rodriguez-Boulan E, 2002. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. PNAS 99, 3842–3847. 10.1073/pnas.052025899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard ER, Atherton SJ, Eldred G, Dillon J, 1995. Photophysical Studies on Human Retinal Lipofuscin. Photochemistry and Photobiology 61, 448–453. 10.1111/j.1751-1097.1995.tb02343.x [DOI] [PubMed] [Google Scholar]
- Gao Z, Liao Y, Chen C, Liao C, He D, Chen J, Ma J, Liu Z, Wu Y, 2018. Conversion of all-trans-retinal into all-trans-retinal dimer reflects an alternative metabolic/antidotal pathway of all-trans-retinal in the retina. J. Biol. Chem 293, 14507–14519. 10.1074/jbc.RA118.002447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez DB, Blakeley L, Goletz PW, Schey KL, Hanneken A, Koutalos Y, Crouch RK, Ablonczy Z, 2010. Mass Spectrometry Provides Accurate and Sensitive Quantitation of A2E. Photochem Photobiol Sci 9, 1513–1519. 10.1039/c0pp00230e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa PC, Barnard AR, Singh MS, Carter E, Jiang Z, Radu RA, Schraermeyer U, MacLaren RE, 2013. Fundus Autofluorescence in the Abca4−/− Mouse Model of Stargardt Disease—Correlation With Accumulation of A2E, Retinal Function, and Histology. Invest. Ophthalmol. Vis. Sci 54, 5602–5612. 10.1167/iovs.13-11688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Napoli JL, 2008. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem 378, 71–79. 10.1016/j.ab.2008.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachik F, Moura F.F. de, Zhao D-Y, Aebischer C-P, Bernstein PS, 2002. Transformations of Selected Carotenoids in Plasma, Liver, and Ocular Tissues of Humans and in Nonprimate Animal Models. Invest. Ophthalmol. Vis. Sci 43, 3383–3392. [PubMed] [Google Scholar]
- Kim SR, Fishkin N, Kong J, Nakanishi K, Allikmets R, Sparrow JR, 2004. Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E. PNAS 101, 11668–11672. 10.1073/pnas.0403499101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Nakanishi K, Itagaki Y, Sparrow JR, 2006. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Experimental Eye Research 82, 828–839. 10.1016/j.exer.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Kiser PD, Golczak M, Palczewski K, 2014. Chemistry of the Retinoid (Visual) Cycle. Chem. Rev 114, 194–232. 10.1021/cr400107q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA, 2003. Biologic Mechanisms of the Protective Role of Lutein and Zeaxanthin in the Eye. Annual Review of Nutrition 23, 171–201. 10.1146/annurev.nutr.23.011702.073307 [DOI] [PubMed] [Google Scholar]
- Li B, Ahmed F, Bernstein PS, 2010. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Archives of Biochemistry and Biophysics, Carotenoids 504, 56–60. 10.1016/j.abb.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, George EW, Rognon GT, Gorusupudi A, Ranganathan A, Chang F-Y, Shi L, Frederick JM, Bernstein PS, 2020. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. PNAS. 10.1073/pnas.1922793117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Rognon GT, Mattinson T, Vachali PP, Gorusupudi A, Chang F-Y, Ranganathan A, Nelson K, George EW, Frederick JM, Bernstein PS, 2018. Supplementation with Macular Carotenoids Improves Visual Performance of Transgenic Mice. Arch Biochem Biophys 649, 22–28. 10.1016/j.abb.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS, 2014. Inactivity of human β,β-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. PNAS 111, 10173–10178. 10.1073/pnas.1402526111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Vachali PP, Shen Z, Gorusupudi A, Nelson K, Besch BM, Bartschi A, Longo S, Mattinson T, Shihab S, Polyakov NE, Suntsova LP, Dushkin AV, Bernstein PS, 2017. Retinal accumulation of zeaxanthin, lutein, and β-carotene in mice deficient in carotenoid cleavage enzymes. Exp. Eye Res 159, 123–131. 10.1016/j.exer.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima de Carvalho JR, Kim HJ, Ueda K, Zhao J, Owji AP, Yang T, Tsang SH, Sparrow JR, 2020. Effects of deficiency in the RLBP1-encoded visual cycle protein CRALBP on visual dysfunction in humans and mice. J Biol Chem 295, 6767–6780. 10.1074/jbc.RA120.012695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindshield BL, King JL, Wyss A, Goralczyk R, Lu C-H, Ford NA, Erdman JW, 2008. Lycopene Biodistribution Is Altered in 15,15′-Carotenoid Monooxygenase Knockout Mice. The Journal of Nutrition 138, 2367–2371. 10.3945/jn.108.099663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR, 2000. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem 275, 29354–29360. 10.1074/jbc.M910191199 [DOI] [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH, 2000. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A 97, 7154–7159. 10.1073/pnas.130110497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JM, Power R, Stringham J, Dennison J, Stack J, Kelly D, Moran R, Akuffo KO, Corcoran L, Beatty S, 2016. Enrichment of Macular Pigment Enhances Contrast Sensitivity in Subjects Free of Retinal Disease: Central Retinal Enrichment Supplementation Trials – Report 1. Invest. Ophthalmol. Vis. Sci 57, 3429–3439. 10.1167/iovs.16-19520 [DOI] [PubMed] [Google Scholar]
- Paavo M, Zhao J, Kim HJ, Lee W, Zernant J, Cai C, Allikmets R, Tsang SH, Sparrow JR, 2018. Mutations in GPR143/OA1 and ABCA4 Inform Interpretations of Short-Wavelength and Near-Infrared Fundus Autofluorescence. Invest. Ophthalmol. Vis. Sci 59, 2459–2469. 10.1167/iovs.18-24213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J, 1998. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci U S A 95, 14609–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar VM, Parmar T, Arai E, Perusek L, Maeda A, 2018. A2E-associated cell death and inflammation in retinal pigmented epithelial cells from human induced pluripotent stem cells. Stem Cell Research 27, 95–104. 10.1016/j.scr.2018.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, Hu J, Yuan Q, Welch DL, Makshanoff J, Lloyd M, McMullen S, Travis GH, Bok D, 2011. Complement System Dysregulation and Inflammation in the Retinal Pigment Epithelium of a Mouse Model for Stargardt Macular Degeneration. J Biol Chem 286, 18593–18601. 10.1074/jbc.M110.191866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rózanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sama T, 1995. Blue Light-induced Reactivity of Retinal Age Pigment: IN VITRO GENERATION OF OXYGEN-REACTIVE SPECIES (*). Journal of Biological Chemistry 270, 18825–18830. 10.1074/jbc.270.32.18825 [DOI] [PubMed] [Google Scholar]
- Różanowski B, Cuenco J, Davies S, Shamsi F, Zadlo A, Dayhaw-Barker P, Rozanowska M, Sarna T, Boulton M, 2008. The Phototoxicity of Aged Human Retinal Melanosomes. Photochemistry and photobiology 84, 650–7. 10.1111/j.1751-1097.2007.00259.x [DOI] [PubMed] [Google Scholar]
- Sears AE, Bernstein PS, Cideciyan AV, Hoyng C, Issa PC, Palczewski K, Rosenfeld PJ, Sadda S, Schraermeyer U, Sparrow JR, Washington I, Scholl HPN, 2017. Towards Treatment of Stargardt Disease: Workshop Organized and Sponsored by the Foundation Fighting Blindness. Trans. Vis. Sci. Tech 6, 6–6. 10.1167/tvst.6.5.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS, 2017. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc. Natl. Acad. Sci. U.S.A 114, 10882–10887. 10.1073/pnas.1706332114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow J, Zhou J, Cai B, 2003. DNA is a target of the photodynamic effects elicited in A2E-laden RPE by blue-light illumination. Investigative ophthalmology & visual science 44, 2245–51. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, 2016. Vitamin A-aldehyde adducts: AMD risk and targeted therapeutics. PNAS 113, 4564–4569. 10.1073/pnas.1600474113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J, 2012. The bisretinoids of retinal pigment epithelium. Progress in Retinal and Eye Research 31, 121–135. 10.1016/j.preteyeres.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Marsiglia M, Allikmets R, Tsang S, Lee W, Duncker T, Zernant J, 2015. Flecks in Recessive Stargardt Disease: Short-Wavelength Autofluorescence, Near-Infrared Autofluorescence, and Optical Coherence Tomography. Invest Ophthalmol Vis Sci 56, 5029–5039. 10.1167/iovs.15-16763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Zhou J, Ghosh SK, Liu Z, 2014. Bisretinoid Degradation and the Ubiquitin-Proteasome System. Adv Exp Med Biol 801, 593–600. 10.1007/978-1-4614-3209-8_75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, Peters M, Martin HD, Sies H, 1997. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 18, 89–92. 10.1093/carcin/18.1.89 [DOI] [PubMed] [Google Scholar]
- Stringham JM, Garcia PV, Smith PA, McLin LN, Foutch BK, 2011. Macular pigment and visual performance in glare: benefits for photostress recovery, disability glare, and visual discomfort. Invest. Ophthalmol. Vis. Sci 52, 7406–7415. 10.1167/iovs.10-6699 [DOI] [PubMed] [Google Scholar]
- Thomas LD, Bandara S, Parmar VM, Srinivasagan R, Khadka N, Golczak M, Kiser PD, von Lintig J, 2020. The human mitochondrial enzyme BCO2 exhibits catalytic activity toward carotenoids and apocarotenoids. J. Biol. Chem 295, 15553–15565. 10.1074/jbc.RA120.015515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson LR, Toyoda Y, Delon FC, Garnett KM, Wong Z-Y, Nichols CR, Cheng KM, Craft NE, Kathleen Dorey C, 2002a. Long Term Dietary Supplementation with Zeaxanthin Reduces Photoreceptor Death in Light-damaged Japanese Quail. Experimental Eye Research 75, 529–542. 10.1006/exer.2002.2050 [DOI] [PubMed] [Google Scholar]
- Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK, 2002b. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest. Ophthalmol. Vis. Sci 43, 3538–3549. [PubMed] [Google Scholar]
- Ueda K, Zhao J, Kim HJ, Sparrow JR, 2016a. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. PNAS 113, 6904–6909. 10.1073/pnas.1524774113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Zhao J, Kim HJ, Sparrow JR, 2016b. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc Natl Acad Sci U S A 113, 6904–6909. 10.1073/pnas.1524774113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Feng Y, Han P, Wang F, Luo X, Liang J, Sun Xiangjun, Ye J, Lu Y, Sun Xiaodong, 2018. Photosensitization of A2E triggers telomere dysfunction and accelerates retinal pigment epithelium senescence. Cell Death Dis 9. 10.1038/s41419-017-0200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiter JJ, Delori FC, Wing GL, Fitch KA, 1986. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest. Ophthalmol. Vis. Sci 27, 145–152. [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH, 1999. Insights into the Function of Rim Protein in Photoreceptors and Etiology of Stargardt’s Disease from the Phenotype in abcr Knockout Mice. Cell 98, 13–23. 10.1016/S0092-8674(00)80602-9 [DOI] [PubMed] [Google Scholar]
- Weymouth AE, Vingrys AJ, 2008. Rodent electroretinography: Methods for extraction and interpretation of rod and cone responses. Progress in Retinal and Eye Research 27, 1–44. 10.1016/j.preteyeres.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Wielgus A, Collier R, Martin E, Lih F, Tomer K, Chignell C, Roberts J, 2010. Blue light induced A2E oxidation in rat eyes—Experimental animal model of dry AMD. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 9, 1505–12. 10.1039/c0pp00133c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P, 1999. Oxidative damage and age-related macular degeneration. Mol Vis 5, 32. [PMC free article] [PubMed] [Google Scholar]
- Wu L, Nagasaki T, Sparrow JR, 2010. Photoreceptor cell degeneration in Abcr (−/−) mice. Adv Exp Med Biol 664, 533–539. 10.1007/978-1-4419-1399-9_61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Ueda K, Nagasaki T, Sparrow JR, 2014. Light Damage in Abca4 and Rpe65rd12 Mice. Invest Ophthalmol Vis Sci 55, 1910–1918. 10.1167/iovs.14-13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR, 2009. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J Biol Chem 284, 20155–20166. 10.1074/jbc.M109.021345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR, 2010. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. PNAS 107, 7275–7280. 10.1073/pnas.0913112107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Zhou J, Hunter JJ, Williams DR, Sparrow JR, 2012. Toward an Understanding of Bisretinoid Autofluorescence Bleaching and Recovery. Invest. Ophthalmol. Vis. Sci 53, 3536–3544. 10.1167/iovs.12-9535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D-Y, 2003. Resonance Raman Measurement of Macular Carotenoids in Retinal, Choroidal, and Macular Dystrophies. Arch Ophthalmol 121, 967. 10.1001/archopht.121.7.967 [DOI] [PubMed] [Google Scholar]
- Zhou J, Cai B, Jang YP, Pachydaki S, Schmidt AM, Sparrow JR, 2005. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp Eye Res 80, 567–580. 10.1016/j.exer.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Zhou J, Jang YP, Kim SR, Sparrow JR, 2006. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. PNAS 103, 16182–16187. 10.1073/pnas.0604255103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


