Abstract
Background/Objective:
As pharmacists work to ensure reimbursement for chronic disease management services on the national level, evidence of their impact on important health metrics, such as medication adherence, is needed. However, summative evidence is lacking on the effectiveness of pharmacists to improve medication adherence in older adults. The objective was to assess the effectiveness of pharmacist-led interventions on medication adherence in older adults (65+ years).
Design/Setting/Participants:
Using a systematic review and meta-analytic approach, a comprehensive search of publications in PubMed, Scopus, CINAHL, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Google Scholar was conducted through April 2, 2020 for randomized clinical trials of pharmacist-led interventions to improve medication adherence in older adults. A standardized mean difference effect size (Cohen’s d) was calculated for medication adherence in each study. Study effect sizes were pooled using a random-effects model, with effect sizes weighted by inverse of its total variance.
Measurements:
Medication adherence using any method of measurement.
Results:
Among 40 unique randomized trials of pharmacist-led interventions with data from 8,822 unique patients (mean age, range: 65 to 85 years), the mean effect size was 0.57 (k=40; 95% Confidence Interval [CI]: 0.38–0.76). When two outlier studies were excluded from analysis, the mean effect size reduced to 0.41 (k=38; 95% CI: 0.27–0.54). A sensitivity analysis of medication adherence outcome by time point resulted in a mean effect size of 0.64 at three months (k=12; 95% CI: 0.32–0.97), 0.30 at six months (k=13; 95% CI: 0.11–0.48), 0.22 at 12 months (k=12; 95% CI: 0.08–0.37), and 0.36 for outcome time points beyond 12 months (k=5; 95% CI: 0.02–0.70).
Conclusion:
This meta-analysis found a significant improvement in medication adherence among older adults receiving pharmacist-led interventions. Implementation of pharmacist-led interventions supported by Medicare reimbursement could ensure older adults’ access to effective medication adherence support.
Keywords: adherence, clinical pharmacy, evidence-based medicine, meta-analysis, pharmaceutical care
INTRODUCTION
Medication non-adherence is a common and costly global health care problem.1,2 In the United States alone, it is estimated that medication non-adherence causes nearly 125,000 deaths per year.3 Older adults take more medications than their younger counterparts and consequently are at high risk of medication non-adherence.4 Ten percent of hospitalizations and 23% of nursing home admissions are linked to non-adherence, and medication non-adherence directly costs the United States health care system over $100 billion annually.3 Moreover, older adults face unique barriers to medication adherence, including cognitive impairment, age-related sensory deficits, and polypharmacy resulting from the presence of multiple chronic conditions.5,6 Thus, improving medication adherence in older adults is a priority of clinicians and policy makers, as well as patients and their caregivers.
Pharmacists are the most accessible healthcare provider. Among approximately 680,000 active Medicare beneficiaries in 2016, the median number of visits to community pharmacies was more than twice as high as encounters with primary care physicians (13 vs 7).7 Given the frequency of contact with older adults and advanced clinical training, pharmacists are well-positioned to deliver chronic disease management services, including identifying barriers to and improving medication adherence.8 However, pharmacists’ ability to improve older adult health is hindered because Medicare does not recognize them as health care providers or provide them reimbursement for such services.9 Medicare Part D pays pharmacies for an annual medication review to reduce drug costs, but this falls short of ongoing chronic disease management. As pharmacists work to ensure reimbursement for chronic disease management services on the national (e.g., Medicare) and state levels, robust evidence of their impact on important health metrics, such as medication adherence, is needed. Previous systematic reviews in general adult samples have reported beneficial effects of pharmacist-led interventions on medication adherence.10–12 However, to the best of our knowledge, the literature on pharmacist-led interventions to improve medication adherence in older adults across health conditions has not yet been systematically examined.
To summarize published literature and estimate the treatment effect, we conducted a comprehensive and contemporary systematic review and meta-analysis to assess the effectiveness of pharmacist-led interventions on medication adherence in older adults (65+ years). We also sought to better understand potential mechanisms for any beneficial effects through moderator analyses of key intervention characteristics.
METHODS
Standard systematic review and meta-analysis methods were used to conduct and report this project in accordance with PRISMA guidelines.13 The protocol was registered with PROSPERO (CRD42020175323).
Information sources and search
An experienced reference librarian was consulted to conduct searches. Searches were run using both controlled vocabulary (i.e., MeSH terms) and keywords in the title or abstract fields. The following databases were searched: PubMed, Scopus, CINAHL, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Google Scholar. Databases were searched from inception through April 2, 2020. No limitations were put on the search in terms of language, date of publication, or geography. A reproducible search strategy can be found in Supplementary Methods.
Eligibility criteria and study selection
We included reports of published studies testing pharmacist-led interventions to improve older adults’ (mean age 65+ years) adherence to prescribed medications. We excluded samples focusing on persons with major psychiatric or substance abuse problems, as well as incarcerated/institutionalized persons. These special populations often have unique barriers to adherence or are not directly responsible for managing their medication.
Diverse adherence interventions were eligible for inclusion. Studies with varied measures of adherence (e.g., electronic cap devices, pharmacy refills) were included. We did not exclude studies based on the type of adherence measure used. If multiple measures of adherence were reported, we coded all measures of adherence. We did not exclude studies based on the type of medication for which adherence was measured. Studies reported in English were included. We only included those studies using a randomized controlled design and those reporting medication adherence data from which we could calculate an effect size.
Outcomes
The primary outcome was medication adherence, reported as the standardized mean difference effect size. For studies in which multiple measures of adherence were reported, we prioritized objective over subjective measures for analysis. Secondary outcomes (when reported) included: health condition-specific measures of disease control (e.g., blood pressure, hemoglobin A1c), hospital admission/readmission, mortality, and quality of life. The effect measures for secondary outcomes varied depending on outcomes reported.
Data extraction
Initial search results were screened by two members of the research team. In this screening, titles and abstracts were read to evaluate whether the study included a pharmacist-led intervention to improve medication adherence. Any possibly eligible citations were marked for full text retrieval. The full texts of possibly eligible studies were then reviewed, and the reasons for ineligibility were noted in the study tracking database (Covidence).
We developed a data extraction codebook for this project. The coding frame was used to record results of primary studies, as well as characteristics of sources, primary study participants, research methods, and interventions. To establish that data were coded reliably, two trained coders independently extracted data, which was then compared between coders to achieve 100% agreement. Any disagreements were resolved via a third person. Data were coded at a micro level to enhance validity.
Electronic coding forms and databases were used to reduce errors and facilitate data comparison to check for accuracy.
The codebook included study year, mean age, sex, race/ethnicity, geographic location, selective inclusion of subjects with adherence problems, presence of cognitive impairment, health characteristics, and number of prescribed medications. Research design characteristics included adherence measures, nature of control groups, and use of intention-to-treat (ITT) analyses. We coded details about interventions, including intervention delivery mechanisms, dose, and location/setting. Intervention content characteristics (e.g., barriers management, prompts) were extensively coded.
In addition to the main codebook, each included study was evaluated using the pharmacist patient care services intervention reporting (PaCIR) checklist.14 PaCIR is designed to guide authors to include sufficient intervention details so as to improve the consistency in medical literature reporting of pharmacists’ patient care interventions. There are 9 critical elements included in PaCIR that are evaluated: replicability, patient population, patient/other data sources, environment, delivery, frequency and duration, pharmacist role/responsibility, attribution, and unique attributes. For each element, coders indicated if it is “applicable/present”, “applicable/absent”, or “not applicable.” PaCIR is designed to supplement (not replace) the primary reporting guideline (e.g., PRISMA).
Risk of bias assessment
Study quality was addressed in two ways. First, two members of the research team independently evaluated included studies using the Cochrane risk of bias tool.15 Risk of bias was assessed for the primary outcome – medication adherence. Second, we considered methodological quality as an empirical question, coding data about aspects of study quality and potential risks for bias, and then analyzed that data in moderator analyses to see whether potential risks for bias were significantly related to study effect size.
Statistical analyses
Study characteristics were described using descriptive statistics. Meta-analyses were conducted using Comprehenive Meta-Analysis software (Biostat, Inc., Englewood, NJ, USA). For each study, a standardized mean difference effect size (Cohen’s d) was calculated for continuous variable outcomes (medication adherence, quality of life, blood pressure), and a relative risk was calculated for dichotomous outcomes (readmission, mortality).
Study effect sizes were pooled using a random-effects model. The random-effects model was chosen a priori due to the expected heterogeneity in samples and interventions common in health behavior intervention research.16 Each study’s effect size was weighted by the inverse of its total variance (sampling variance plus the calculated between-study variance, or T2). Studies with significant standardized residuals were examined as potential outliers. Heterogeneity was assessed using the heterogeneity statistic, Q, as well as I2, which indicates the proportion of unexplained between-study heterogeneity in the meta-analysis. Publication bias was assessed visually using funnel plots and statistically with Egger’s test.17
Moderator analyses were conducted using subgroup analyses for categorical variables and meta-regression for continuous variables. Subgroup analyses required a minimum of five studies per group.18
RESULTS
Our literature searches resulted in 4,708 citations. After removing 994 duplicates, the titles and abstracts of 3,714 studies were screened, yielding 529 potentially eligible studies for full-text review. Of these, 489 were excluded, resulting in a sample of 40 eligible studies (k) for analysis, reporting outcome data for 8,822 participants (n). The study screening process, with reasons for exclusion, is outlined in Figure 1.
Figure 1.
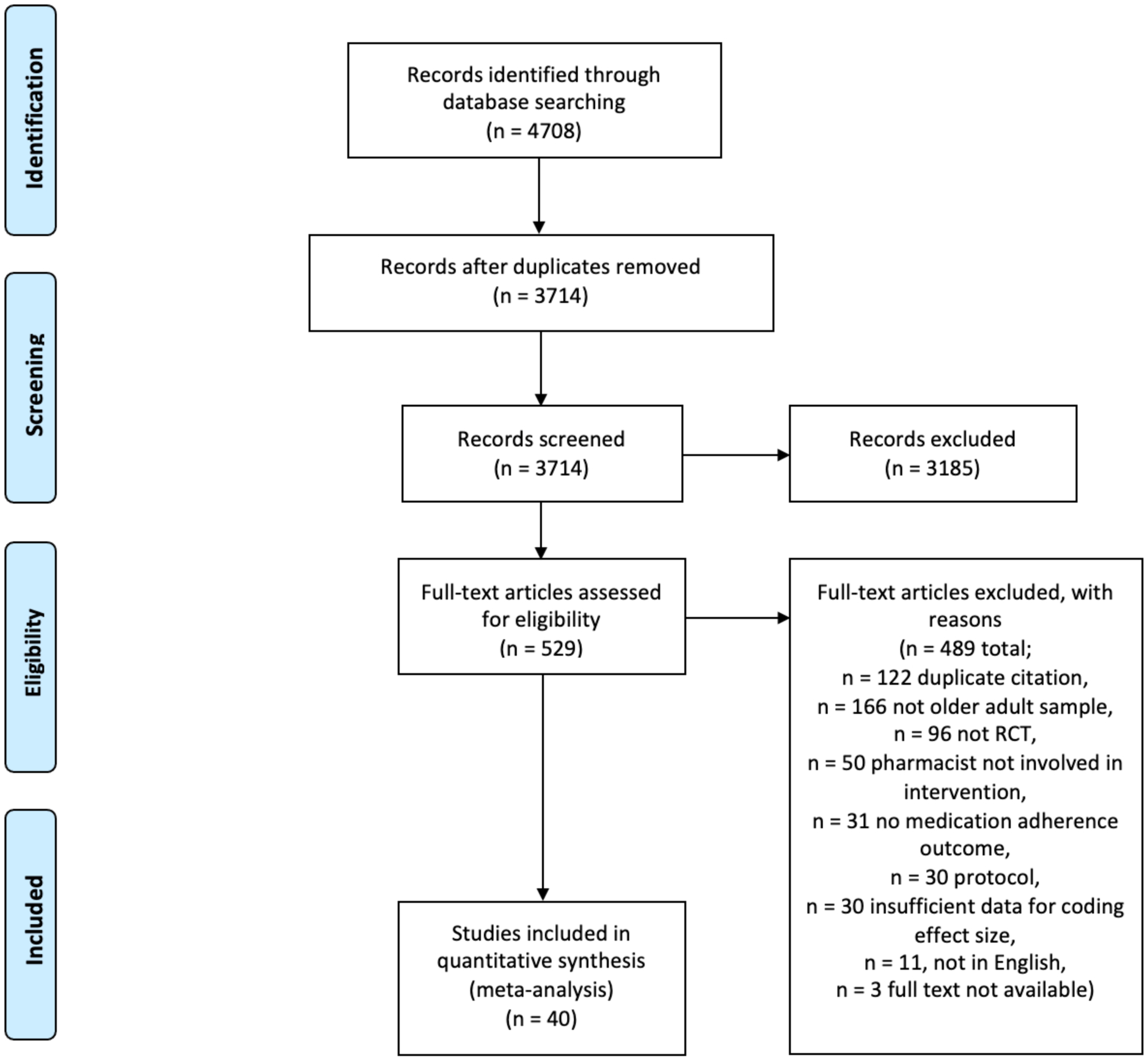
PRISMA Flow Diagram
Primary Study Characteristics
The 40 eligible studies were published between 1994 and 2019. Mean sample ages ranged from 65 to 85 years. Nearly half of the studies were conducted in either the USA (k=10) or the UK (k=9). The median percentage of women in the samples was 52.2 (range: 0–100). Race and ethnicity were seldom reported. Individual study characteristics can be found in Supplementary Table 1.
Overall Effect Sizes
For the most distal medication adherence outcome in each study, the mean effect size was 0.57 (k=40; 95% Confidence Interval [CI]: 0.38–0.76) (Figure 2). However, two studies were statistical outliers, with significant standardized residuals.19,20 When these studies were excluded from the analysis, the mean effect size reduced to 0.41 (k=38; 95% CI: 0.27–0.54). Secondary outcome effect sizes for quality of life (Figure 3), blood pressure, mortality, and hospitalizations/readmissions are reported in Table 1 (Supplementary Figures 1–3). Pharmacist-led interventions had statistically significant beneficial effects on quality of life (k=11; mean effect size: 0.29; 95% CI: 0.04–0.55) as well as systolic (k=8; mean effect size: 0.42; 95% CI: 0.10–0.73) and diastolic blood pressure (k=8; mean effect size: 0.35; 95% CI: 0.03–0.68). Pharmacist-led interventions were associated with reduced hospitalizations/readmissions and mortality, although these effects were not statistically significant. Hemoglobin A1c was not reported in enough studies for analysis.
Figure 2. Forest Plot of Medication Adherence Outcomes from Pharmacist-led Interventions.
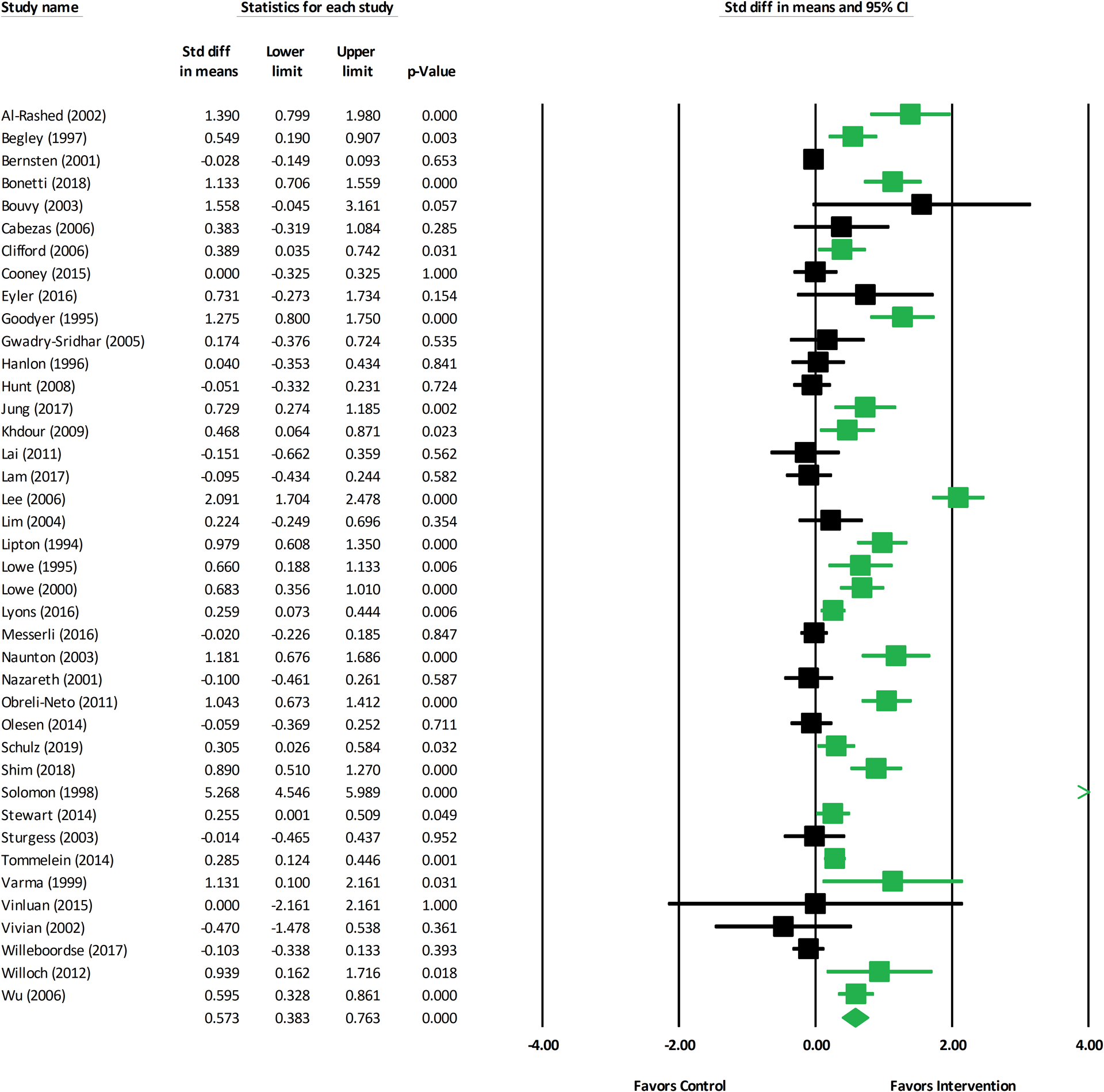
Abbreviations: CI: confidence interval; Std diff: standardized difference
Figure 3. Forest Plot of Quality of Life Outcomes from Pharmacist-led Interventions.
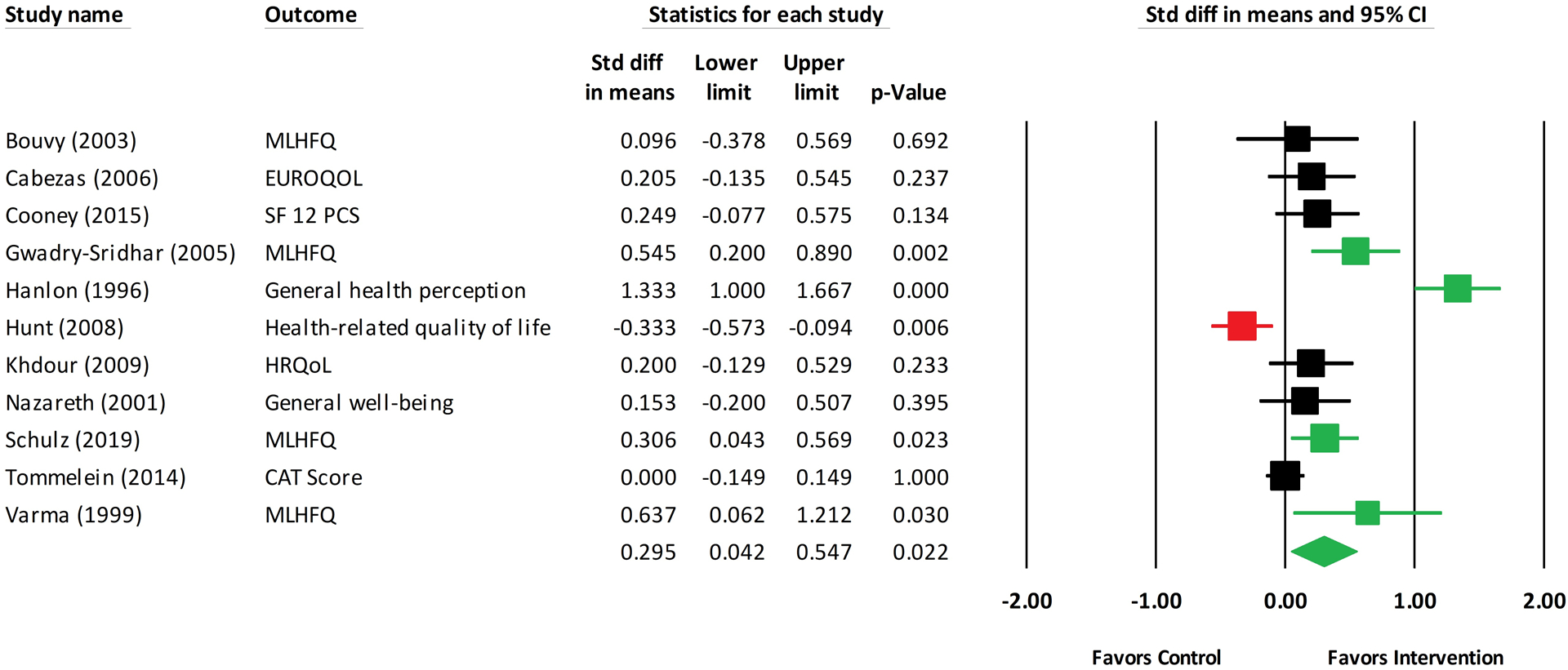
Abbreviations: CAT: COPD Assessment Test; CI: confidence interval; EUROQOL: European Quality of Life scale; HRQoL: health-related quality of life; MLHFQ: Minnesota Living with Health Failure Questionnaire; SF 12 PCS: Short Form 12 physical component score; Std diff: standardized difference
Table 1.
Medication Adherence Outcomes and Secondary Outcomes
| Comparisons | k | Mean ES | 95% CI | Q | I 2 |
|---|---|---|---|---|---|
| Medication adherence, all eligible studies | 40 | 0.57*** | 0.38, 0.76 | 466.33*** | 91.64 |
| Medication adherence, outliers removed | 38 | 0.41*** | 0.27, 0.54 | 199.78*** | 81.48 |
| Quality of life | 11 | 0.29* | 0.04, 0.55 | 77.18*** | 87.04 |
| Systolic blood pressure | 8 | 0.42** | 0.10, 0.73 | 55.40*** | 87.36 |
| Diastolic blood pressure | 8 | 0.35* | 0.03, 0.68 | 61.24*** | 88.57 |
| Mortality | 9 | 0.79 | 0.61, 1.01 | 11.32 | 29.30 |
| Hospitalizations/readmissions | 7 | 0.82 | 0.67, 1.01 | 8.25 | 27.25 |
k = number of comparisons
ES = effect size (Cohen’s d, except for mortality and hospitalizations [relative risk])
Q = heterogeneity statistic (weighted squared deviations from summary effect)
I2 = index of heterogeneity beyond within-study sampling error
p < .05,
p < .01,
p < .001
Because the effects of health behavior interventions tend to wane with time, we performed a sensitivity analysis of medication adherence outcome by time point (Table 2). Mean effect size was 0.64 at three months (k=12; 95% CI: 0.32–0.97), 0.30 at six months (k=13; 95% CI: 0.11–0.48), 0.22 at twelve months (k=12; 95% CI: 0.08–0.37), and 0.36 for outcome time points beyond twelve months (k=5; 95% CI: 0.02–0.70). Since not all studies reported outcomes at each time point, the number of studies varied by time point.
Table 2.
Adherence Outcomes by Time Point
| Comparisons | k | d | 95% CI | Q | I 2 |
|---|---|---|---|---|---|
| Medication adherence, 3-month outcome | 12 | 0.64*** | 0.32, 0.97 | 93.14*** | 88.19 |
| Medication adherence, 6-month outcome | 13 | 0.30** | 0.11, 0.48 | 52.99*** | 77.36 |
| Medication adherence, 12-month outcome | 12 | 0.22** | 0.08, 0.37 | 22.15* | 50.33 |
| Medication adherence, beyond 12 months | 5 | 0.36* | 0.02, 0.70 | 42.92*** | 90.68 |
k = number of comparisons
d = standardized mean difference effect size
ES = estimated mean of true effect sizes (d index)
Q = heterogeneity statistic (weighted squared deviations from summary effect)
I2 = index of heterogeneity beyond within-study sampling error
p < .05,
p < .01,
p < .001
Moderator Analyses for Medication Adherence Outcomes
Medication adherence effect sizes did not vary based on year of publication (β=−0.013, p=0.13, k=38), mean age of the sample (β=0.013, p=0.32, k=33), the percent of women in the sample (β=0.004, p=0.28, k=31), the number of intervention sessions (β=0.016, p=0.74, k=9), the reported minutes per session (β=−0.002, p=0.56, k=9), or the overall duration of the intervention (β=0.000, p=0.94, k=9).
Subgroup analyses of intervention characteristics showed mixed findings (Supplementary Table 2). Interventions that included medication education had a larger mean effect size than did interventions without medication education (0.50 vs. 0.19, p = 0.02). However, pharmacist-led interventions that included disease education had lower effect sizes than did interventions without disease education (0.15 vs. 0.50, p < 0.01). Effect size differences were also found based on the location where interventions were delivered (p =0.01). Interventions delivered in the home (d=0.48, k=7) or in multiple sites (d=0.64, k=12) had larger effect sizes than interventions delivered in the clinic (d=0.20, k=8) or pharmacy (d=0.13, k=7). The mean effect size of studies where the intervention was delivered at least partly in a community pharmacy had smaller effect sizes than interventions not delivered in a community pharmacy, such as in a hospital prior to discharge (0.20 vs. 0.49, p=0.02). Significant differences were not found for the other intervention characteristics (e.g., medication counseling, medication calendars, patient self-monitoring of medication-taking, medication regimen review, packaging interventions, dose modification).
We also examined effect sizes grouped by type of adherence outcome measure. Differences were not statistically significant (p = 0.13), but objective measures had larger effect sizes than did self-reported adherence measures. The four studies that used pharmacy refill data had a mean effect size of 0.41, and the eight studies using pill counts had a mean effect size of 0.89. The 19 studies using self-report measures had a mean effect size of 0.34. Only one study used electronic monitoring, and one used a combination of measures.
Potential Biases
Only one risk of bias moderator was found to have significant differences in subgroups. Studies that reported an ITT analysis had a lower effect size than did studies where an ITT analysis was not used or where use of ITT was unclear (0.20 vs. 0.53, p=0.01). Statistics for the other risk of bias moderator analyses are reported in Supplementary Table 3, and the risk of bias ratings are reported in Supplementary Table 4. Pharmacist patient care services intervention reporting (PaCIR) checklist ratings are reported in Supplementary Table 5. The most frequently reported domains were patient/other data sources, environment, and delivery of the intervention, with the patient population and frequency and duration of the intervention being adequately reported less frequently.
While publication bias is more difficult to determine with smaller numbers of studies, we detected likely publication bias from the funnel plot of the eligible studies (Supplementary Figure 4). This was further confirmed statistically with an Egger’s test intercept of 2.22 (p < 0.01).
DISCUSSION
Across 40 randomized trials19–58, we found a beneficial effect of pharmacist-led interventions to improve medication adherence in older adults, in the presence of high heterogeneity and suggestion of publication bias. Excluding two outlier studies from the analysis, the mean effect size reduced but remained beneficial. A sensitivity analysis of medication adherence outcome by time point showed that the effect tended to decrease over follow up time. Interventions including medication education had a larger effect size than did interventions without medication education, and interventions delivered in the home or across multiple sites had particularly large effect sizes. The type of adherence outcome measure was not statistically significantly associated with effect size; however, studies using an ITT analysis had a lower effect size than did studies without use of an ITT analysis. In addition, pharmacist-led interventions had statistically significant beneficial effects on quality of life and blood pressure values in older adults. Overall, our results add to a growing literature on the positive impact of pharmacists on health outcomes.59–63
When implementing interventions, it is important to understand the components driving any beneficial effect on health outcomes. A previous meta-analysis of interventions to improve medication adherence in older adults showed larger effect sizes for interventions employing special medication packaging, dose modification, patient self-monitoring of medication-taking, and written instructions.64 However, we did not find significant differences in effect sizes from these intervention components in our review. It is important to note that intervention content and behavior change mechanisms are often poorly described, thus making it difficult to harmonize and analyze intervention moderators across studies. In addition, moderator analyses only consider a single component at a time, and beneficial effects of pharmacist-led interventions might come from a combination of components. In fact, medication adherence experts emphasize the multifactorial nature of medication non-adherence, calling for development and scaling of comprehensive adherence-promoting programs.65 Moreover, a recent Cochrane review found low-quality evidence that pharmacist-led mixed educational and behavioral interventions may improve medication adherence in older adults when measured dichotomously (n=8 studies) or continuously (n=2 studies).66 Taken together, it is likely that medication education alone is a necessary but not sufficient component to improve medication adherence in older adults.
Our main finding of a beneficial effect of pharmacist-led interventions on improving medication adherence in older adults has potential policy implications. Despite recommendations from public health experts that recognizing pharmacists as health care providers would improve patient access and health outcomes in a cost-effective manner67, Medicare does not recognize pharmacists as providers and thus does not reimburse them for their work. As a result, individual states have varying approaches to allow for direct reimbursement of pharmacist-provided health services.68 Establishing federal statutory recognition of pharmacists as healthcare providers under Medicare Part B would significantly improve older adults’ access to needed health care while incentivizing and compensating the providers delivering the care.69 Pharmacists currently provide Medication Therapy Management (MTM) services under Medicare Part D, but stand-alone prescription drug plans do not have a strong incentive to invest in these services because they are not at risk for medical costs (covered via Part B).70 Simply put, there is often a misalignment of financial incentives between Medicare Part B and Part D policies, with pharmacists limited in their potential clinical impact. The Part D Enhanced MTM model is testing whether providing Part D sponsors with additional payment incentives and more regulatory flexibility drives improvements in therapeutic outcomes with reduction in net Medicare expenditures.71 Six Part D sponsors are participating in this five-year innovation program, with an end date of December 31, 2021. This dual approach of lobbying for pharmacist provider status under Medicare Part B and also testing novel models under Part D is promising to advance pharmacy practice for healthcare delivery to older adults.
Our study has important limitations. First, due to the small number of comparisons included in many moderator categories, robust associations between intervention moderators and medication adherence outcomes were difficult to draw. Moderator analyses are only exploratory, and should be considered hypothesis-generating, providing suggestions and support for future studies. Second, risk of bias moderators show that results of medication adherence interventions must be interpreted carefully, with attention to potential risks for bias in each study. Third, high heterogeneity was detected, which is not surprising for health behavior interventions. Fourth, we detected possible publication bias so results should be interpreted in that context. Fifth, despite using rigorous searching and screening methods, some eligible studies may have been missed. Sixth, we did not search for any cost-effectiveness outcomes. Finally, our search strategy did not include persistence or non-persistence. However, medication persistence is linked to the MeSH heading medication adherence so that studies using medication persistence as an entry term would show up in the search results for medication adherence.
In this systematic review and meta-analysis, we report that pharmacist-led interventions were found to have a moderate beneficial effect on improving medication adherence in older adults that tended to decrease over longer durations of follow up.
Interventions utilizing medication education and delivered in the patient’s home or across multiple settings were found to be the most successful.
Supplementary Material
Supplementary Methods. Search Strategy
Supplementary Table 1 Description of Included Randomized Controlled Trials
Supplementary Table 2. Intervention Moderators
Supplementary Table 3. Risk of Bias Moderators
Supplementary Table 4. Risk of Bias Assessments
Supplementary Table 5. Pharmacist Patient Care Services Intervention Reporting (PaCIR) Assessments
Supplementary Figure 1. Forest Plot of Blood Pressure Outcomes
Supplementary Figure 2. Forest Plot of Mortality Outcomes
Supplementary Figure 3. Forest Plot of Hospitalization/Readmission Outcomes
Supplementary Figure 4. Funnel Plot
KEY POINTS
Pharmacist-led interventions were found to improve medication adherence in older adults.
WHY DOES THIS MATTER?
This research is important because reimbursement of pharmacist-led interventions would facilitate the implementation of effective interventions to improve medication adherence in older adults.
ACKNOWLEDGEMENTS
Funding:
ZA Marcum was supported by the National Institute on Aging of the National Institutes of Health (K76AG059929). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sponsor’s Role:
The sponsor had no role in the design, methods, data collection, analysis, or preparation of this paper.
Footnotes
Conflict of Interest Statement: The authors have no conflicts to report.
Presentation of Work: This work has not been previously presented. We submitted an abstract based on this work to the Gerontological Society of America Annual Meeting, which will take place November 10–14, 2021.
REFERENCES
- 1.Sabaté E, World Health Organization. Adherence to Long-Term Therapies : Evidence for Action. World Health Organization; 2003. [Google Scholar]
- 2.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;2014(11):CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–665. [DOI] [PubMed] [Google Scholar]
- 4.Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.” JAMA. 2010;304(14):1592–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcum ZA, Gellad WF. Medication adherence to multidrug regimens. Clin Geriatr Med. 2012;28(2):287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berenbrok LA, Gabriel N, Coley KC, Hernandez I. Evaluation of frequency of encounters with primary care physicians vs visits to community pharmacies among Medicare beneficiaries. JAMA Netw Open. 2020;3(7):e209132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Patient Care Process for Delivering Comprehensive Medication Management (CMM): Optimizing Medication Use in Patient-Centered, Team-Based Care Settings. CMM in Primary Care Research Team. July 2018. Available at: http://www.accp.com/cmm_care_process [Google Scholar]
- 9.Isasi F, Krofah E. The Expanding Role of Pharmacists in a Transformed Health Care System. Washington, DC: National Governors Association for Best Practices. January 2015. Available at: https://www.nga.org/wp-content/uploads/2019/08/1501TheExpandingRoleOfPharmacists.pdf. [Google Scholar]
- 10.Chisholm-Burns MA, Lee JK, Spivey CA, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48(10):923–933. [DOI] [PubMed] [Google Scholar]
- 11.Mes MA, Katzer CB, Chan AHY, Wileman V, Taylor SJC, Horne R. Pharmacists and medication adherence in asthma: a systematic review and meta-analysis. Eur Respir J. 2018;52(2):1800485. [DOI] [PubMed] [Google Scholar]
- 12.Readdean KC, Heuer AJ, Parrott JS. Effect of pharmacist intervention on improving antidepressant medication adherence and depression symptomology: A systematic review and meta-analysis. Res Social Adm Pharm. 2018;14(4):321–331. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clay PG, Burns AL, Isetts BJ, Hirsch JD, Kliethermes MA, Planas LG. PaCIR: A tool to enhance pharmacist patient care intervention reporting. J Am Pharm Assoc (2003). 2019;59(5):615–623. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruppar T Meta-analysis: How to quantify and explain heterogeneity? Eur J Cardiovasc Nurs. 2020;19(7):646–652. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA(editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 19.Solomon DK, Portner TS, Bass GE, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash). 1998;38(5):574–585. [DOI] [PubMed] [Google Scholar]
- 20.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296(21):2563–2571. [DOI] [PubMed] [Google Scholar]
- 21.Al-Rashed SA, Wright DJ, Roebuck N, Sunter W, Chrystyn H. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begley S, Livingstone C, Hodges N, Williamson V. Impact of domiciliary pharmacy visits on medication management in an elderly population. Int J Pharm Pract. 1997;5(3):111–121. [Google Scholar]
- 23.Bernsten C, Björkman I, Caramona M, et al. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs Aging. 2001;18(1):63–77. [DOI] [PubMed] [Google Scholar]
- 24.Bonetti AF, Bagatim BQ, Mendes AM, et al. Impact of discharge medication counseling in the cardiology unit of a tertiary hospital in Brazil: A randomized controlled trial. Clinics (Sao Paulo). 2018;73:e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoe AW, Leufkens HGM. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail. 2003;9(5):404–411. [DOI] [PubMed] [Google Scholar]
- 26.Clifford S, Barber N, Elliott R, Hartley E, Horne R. Patient-centred advice is effective in improving adherence to medicines. Pharm World and Sci. 2006;28(3):165–170. [DOI] [PubMed] [Google Scholar]
- 27.Cooney D, Moon H, Liu Y, et al. A pharmacist based intervention to improve the care of patients with CKD: A pragmatic, randomized, controlled trial. BMC Nephrol. 2015;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyler R, Shvets K, Blakely ML. Motivational interviewing to increase postdischarge antibiotic adherence in older adults with pneumonia. Consult Pharm. 2016;31(1):38–43. [DOI] [PubMed] [Google Scholar]
- 29.Goodyer LI, Miskelly F, Milligan P. Does encouraging good compliance increase patients’ clinical condition in heart failure? Br J Clin Pract. 1995;49(4):173–176. [PubMed] [Google Scholar]
- 30.Gwadry-Sridhar FH, Arnold JMO, Zhang Y, Brown JE, Marchiori G, Guyatt G. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. [DOI] [PubMed] [Google Scholar]
- 31.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–437. [DOI] [PubMed] [Google Scholar]
- 32.Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23(12):1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung SH, Lee OS, Kim HS, et al. Medication adherence improvement by using administration timing simplification protocol (ATSP) in cardiovascular disease patients. J Atheroscler Thromb. 2017;24(8):841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol. 2009;68(4):588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai PSM, Chua SS, Chew YY, Chan SP. Effects of pharmaceutical care on adherence and persistence to bisphosphonates in postmenopausal osteoporotic women. J Clin Pharm Ther. 2011;36(5):557–567. [DOI] [PubMed] [Google Scholar]
- 36.Lam AY, Nguyen JK, Parks JJ, Morisky DE, Berry DL, Wolpin SE. Addressing low health literacy with “Talking Pill Bottles”: A pilot study in a community pharmacy setting. J Am Pharm Assoc (2003). 2017;57(1):20–29.e3. [DOI] [PubMed] [Google Scholar]
- 37.Lim WS, Low HN, Chan SP, Chen HN, Ding YY, Tan TL. Impact of a pharmacist consult clinic on a hospital-based geriatric outpatient clinic in Singapore. Ann Acad Med Singap. 2004;33(2):220–227. [PubMed] [Google Scholar]
- 38.Lipton HL, Bird JA. The impact of clinical pharmacists’ consultations on geriatric patients’ compliance and medical care use: a randomized controlled trial. Gerontologist. 1994;34(3):307–315. [DOI] [PubMed] [Google Scholar]
- 39.López Cabezas A, Falces Salvador C, Cubí Quadrada D, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failiure. Farm Hosp. 2006;30(6):328–342. [DOI] [PubMed] [Google Scholar]
- 40.Lowe CJ, Courtney EA, Purvis J. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310:1229–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effect of a medicine review and education programme for older people in general practice. Br J Clin Pharmacol. 2000;50(2):172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyons I, Barber N, Raynor DK, Wei L. The Medicines Advice Service Evaluation (MASE): a randomised controlled trial of a pharmacist-led telephone based intervention designed to improve medication adherence. BMJ Qual Saf. 2016;25(10):759–769. [DOI] [PubMed] [Google Scholar]
- 43.Messerli M, Blozik E, Vriends N, Hersberger KE. Impact of a community pharmacist-led medication review on medicines use in patients on polypharmacy--a prospective randomised controlled trial. BMC Health Serv Res. 2016;16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naunton M, Peterson GM. Evaluation of home-based follow-up of high-risk elderly patients discharged from hospital. J Pharm Pract Res. 2003;33:176–182. [Google Scholar]
- 45.Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H. A pharmacy discharge plan for hospitalized elderly patients--randomized controlled trial. Age Ageing. 2001;30(1):33–40. [DOI] [PubMed] [Google Scholar]
- 46.Obreli-Neto PR, Guidoni CM, de Oliveira Baldoni A, et al. Effect of a 36-month pharmaceutical care program on pharmacotherapy adherence in elderly diabetic and hypertensive patients. Int J Clin Pharm. 2011;33(4):642–649. [DOI] [PubMed] [Google Scholar]
- 47.Olesen C, Harbig P, Buus KM, Barat I, Damsgaard EM. Impact of pharmaceutical care on adherence, hospitalisations and mortality in elderly patients. Int J Clin Pharm. 2014;36(1):163–171. [DOI] [PubMed] [Google Scholar]
- 48.Schulz M, Griese-Mammen N, Anker SD, et al. Pharmacy-based interdisciplinary intervention for patients with chronic heart failure: results of the PHARM-CHF randomized controlled trial. Eur J of Heart Fail. 2019;21(8):1012–1021. [DOI] [PubMed] [Google Scholar]
- 49.Shim YW, Chua SS, Wong HC, Alwi S. Collaborative intervention between pharmacists and physicians on elderly patients: a randomized controlled trial. Ther Clin Risk Manag. 2018;14:1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solomon DK, Portner TS, Bass GE, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash). 1998;38(5):574–585. [DOI] [PubMed] [Google Scholar]
- 51.Stewart K, George J, Mc Namara KP, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial). J Clin Pharm Ther. 2014;39(5):527–534. [DOI] [PubMed] [Google Scholar]
- 52.Sturgess IK, McElnay JC, Hughes CM, Crealey G. Community pharmacy based provision of pharmaceutical care to older patients. Pharm World Sci. 2003;25(5):218–226. [DOI] [PubMed] [Google Scholar]
- 53.Tommelein E, Mehuys E, van Hees T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): A randomized controlled trial. Br J Clin Pharmacol. 2014;77(5):756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: interventions and outcomes. Pharmacotherapy. 1999;19(7):860–869. [DOI] [PubMed] [Google Scholar]
- 55.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy. 2002;22(12):1533–1540. [DOI] [PubMed] [Google Scholar]
- 56.Willeboordse F, Schellevis FG, Chau SH, Hugtenburg JG, Elders PJM. The effectiveness of optimised clinical medication reviews for geriatric patients: Opti- Med a cluster randomised controlled trial. Fam Pract. 2017;34(4):437–445. [DOI] [PubMed] [Google Scholar]
- 57.Willoch K, Blix HS, Pedersen-Bjergaard AM, Eek AK, Reikvam A. Handling drug-related problems in rehabilitation patients: a randomized study. Int J Clin Pharm. 2012;34(2):382–388. [DOI] [PubMed] [Google Scholar]
- 58.Wu JYF, Leung WYS, Chang S, et al. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ. 2006;333(7567):522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bosnic-Anticevich SZ. Asthma management in primary care: caring, sharing and working together. Eur Respir J. 2016;47(4):1043–1046. [DOI] [PubMed] [Google Scholar]
- 60.Yaghoubi M, Mansell K, Vatanparastc H, Steeves M, Zeng W, Farag M. Effects of pharmacy-based interventions on the control and management of diabetes in adults: a systematic review and meta-analysis. Can J Diabetes. 2017;41(6):628–641. [DOI] [PubMed] [Google Scholar]
- 61.Manzoor BS, Cheng WH, Lee JC, Uppuluri EM, Nutescu EA. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: a systematic review. Ann Pharmacother. 2017;51(12):1122–1137. [DOI] [PubMed] [Google Scholar]
- 62.Lin G, Huang R, Zhang J, Li G, Chen L, Xi X. Clinical and economic outcomes of hospital pharmaceutical care: a systematic review and meta-analysis. BMC Health Services Research. 2020;20(1):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2014;3(2):e000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conn VS, Hafdahl AR, Cooper PS, Ruppar TM, Mehr DR, Russell CL. Interventions to improve medication adherence among older adults: meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist. 2009;49(4):447–462. [DOI] [PubMed] [Google Scholar]
- 65.Zullig LL, Peterson ED, Bosworth HB. Ingredients of successful interventions to improve medication adherence. JAMA. 2013;310(24):2611–2612. [DOI] [PubMed] [Google Scholar]
- 66.Cross AJ, Elliott RA, Petrie K, Kuruvilla L, George J. Interventions for improving medication‐taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev. 2020;5(5):CD012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giberson S, Yoder S, Lee MP. Improving Patient and Health System Outcomes through Advanced Pharmacy Practice. A Report to the U.S. Surgeon General. Office of the Chief Pharmacist. U.S. Public Health Service. December 2011. [Google Scholar]
- 68.Nguyen E, Walker K, Adams JL, Wadsworth T, Robinson R. Reimbursement for pharmacist-provided health care services: a multistate review. J Am Pharm Assoc (2003). 2021;61(1):27–32. [DOI] [PubMed] [Google Scholar]
- 69.Avalere Health LLC. Developing Trends in Delivery and Reimbursement of Pharmacist Services. November 2015. Available at: https://naspa.us/wp-content/uploads/2015/11/103015_Avalere_NACDS_WhitePaper_LP_Final.pdf.
- 70.Gray C, Cooke CE, Brandt N. Evolution of the Medicare Part D Medication Therapy Management program from inception in 2006 to the present. Am Health Drug Benefits 2019;12(5):243–251. [PMC free article] [PubMed] [Google Scholar]
- 71.Part D Enhanced Medication Therapy Management Model. May 2021. Available at: https://innovation.cms.gov/innovation-models/enhancedmtm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Search Strategy
Supplementary Table 1 Description of Included Randomized Controlled Trials
Supplementary Table 2. Intervention Moderators
Supplementary Table 3. Risk of Bias Moderators
Supplementary Table 4. Risk of Bias Assessments
Supplementary Table 5. Pharmacist Patient Care Services Intervention Reporting (PaCIR) Assessments
Supplementary Figure 1. Forest Plot of Blood Pressure Outcomes
Supplementary Figure 2. Forest Plot of Mortality Outcomes
Supplementary Figure 3. Forest Plot of Hospitalization/Readmission Outcomes
Supplementary Figure 4. Funnel Plot


