Abstract
Anorexia nervosa is complicated by low bone mineral density (BMD) and increased fracture risk associated with low bone formation and high bone resorption. The lumbar spine is most severely affected. Low bone formation is associated with relative insulin-like growth factor 1 (IGF-1) deficiency. Our objective was to determine whether bone anabolic therapy with recombinant human (rh)IGF-1 used off-label followed by antiresorptive therapy with risedronate would increase BMD more than risedronate or placebo in women with anorexia nervosa. We conducted a 12-month, randomized, placebo-controlled study of 90 ambulatory women with anorexia nervosa and low areal BMD (aBMD). Participants were randomized to 3 groups: 6 months of rhIGF-1 followed by 6 months of risedronate (“rhIGF-1/Risedronate”)(n=33), 12 months of risedronate (“Risedronate”)(n=33), or double placebo (“Placebo”)(n=16). Outcome measures were lumbar spine [1° endpoint: postero-anterior (PA) spine], hip, and radius aBMD by dual-energy x-ray absorptiometry (DXA) and vertebral, tibial, and radial volumetric (v)BMD and estimated strength by high-resolution peripheral quantitative computed tomography (HR-pCT)(for extremity measurements) and multi-detector computed tomography (for vertebral measurements). At baseline, mean age, body mass index (BMI), aBMD and vBMD were similar among groups. At 12 months, mean PA lumbar spine aBMD was higher in the rhIGF-1/Risedronate (p=0.03) group, and trended towards being higher in the Risedronate group, than Placebo. Mean lateral lumbar spine aBMD was higher in the rhIGF-1/Risedronate than the Risedronate or Placebo groups (p<0.05). Vertebral vBMD was higher, and estimated strength trended toward being higher, in the rhIGF-1/Risedronate than Placebo group (p<0.05). Neither hip or radial aBMD or vBMD, nor radial or tibial estimated strength, differed among groups. rhIGF-1 was well tolerated. Therefore, sequential therapy with rhIGF-1 followed by risedronate increased lateral lumbar spine aBMD more than risedronate or placebo. Strategies that are anabolic and antiresorptive to bone may be effective at increasing BMD in women with anorexia nervosa.
Keywords: nutrition, DXA, anabolics, antiresorptives
Introduction
Anorexia nervosa is a prevalent disease of young women leading to low bone mineral density (BMD) at multiple skeletal sites. Characterized by persistent food restriction and difficulty recognizing the seriousness of the illness, anorexia nervosa is a chronic condition that persists despite psychiatric and nutritional counseling in the majority of patients(2). Low bone mass and fractures are prevalent and severe medical complications of anorexia nervosa(3–6). We have reported an areal BMD (aBMD) Z-score of ≤−1 at at least one skeletal site in 92% of women with anorexia nervosa, and an aBMD Z-score of ≤−2.5 at at least one skeletal site in 38% of women with this diagnosis(7). The lumbar spine is most severely affected. Previous data have also suggested that women with anorexia nervosa have impaired estimated bone strength at the lumbar spine(8,9), as well as impaired cortical and trabecular bone microarchitecture and estimated bone strength at the radius and tibia(10–13). Impaired skeletal integrity is associated with a 1.8-fold increased risk of fractures in women with anorexia nervosa compared to healthy women of a similar age(14), including an increased risk of debilitating spinal fractures(5).
Currently, no therapies are FDA-approved for low BMD in anorexia nervosa. This is a critical unmet need because low BMD often persists as a comorbid medical condition even with weight restoration and resumption of menstrual periods(15–18). Uncoupling of bone formation and resorption, such that bone formation is decreased and bone resorption is increased, may be a key mechanism responsible for the impaired skeletal integrity in this disorder(19). Therapies that target bone resorption alone have fallen short of normalizing aBMD, perhaps due to a failure to address the deficit in bone formation, relative resistance to antiresorptive therapy, or other factors. For instance, we previously demonstrated that risedronate increased postero-anterior (PA) lumbar spine aBMD by mean 3.2% compared to placebo over 12 months, but risedronate’s maximal effect occurred after 6 months, with no further increase between 6 and 12 months(20).
Relative insulin-like growth factor 1 (IGF-1) deficiency is a key etiologic factor underlying low bone formation and low bone mass in anorexia nervosa. Women with anorexia nervosa have relative IGF-1 deficiency due to starvation-induced growth hormone (GH) resistance(21), and both in vivo data in experimental animal models and clinical studies have demonstrated the importance of systemic IGF-1 levels to bone mass(22). In women with anorexia nervosa, serum IGF-1 level is a significant predictor of bone formation marker levels, and short-term administration of recombinant human IGF-1 (rhIGF-1) stimulates bone formation(19). We previously demonstrated that administration of rhIGF-1 plus oral contraceptives increased PA lumbar spine aBMD by mean 1.8% (vs 0.3% in rhIGF-1 alone vs −1.0% in placebo) over 9 months(23).
We hypothesized that sequential therapy with rhIGF-1 used off-label for 6 months followed by risedronate for 6 months would improve BMD (primary endpoint: PA lumbar spine aBMD at 12 months), bone microarchitecture, and estimated bone strength more than risedronate alone for 12 months or placebo in women with anorexia nervosa.
Material and Methods
Study participants
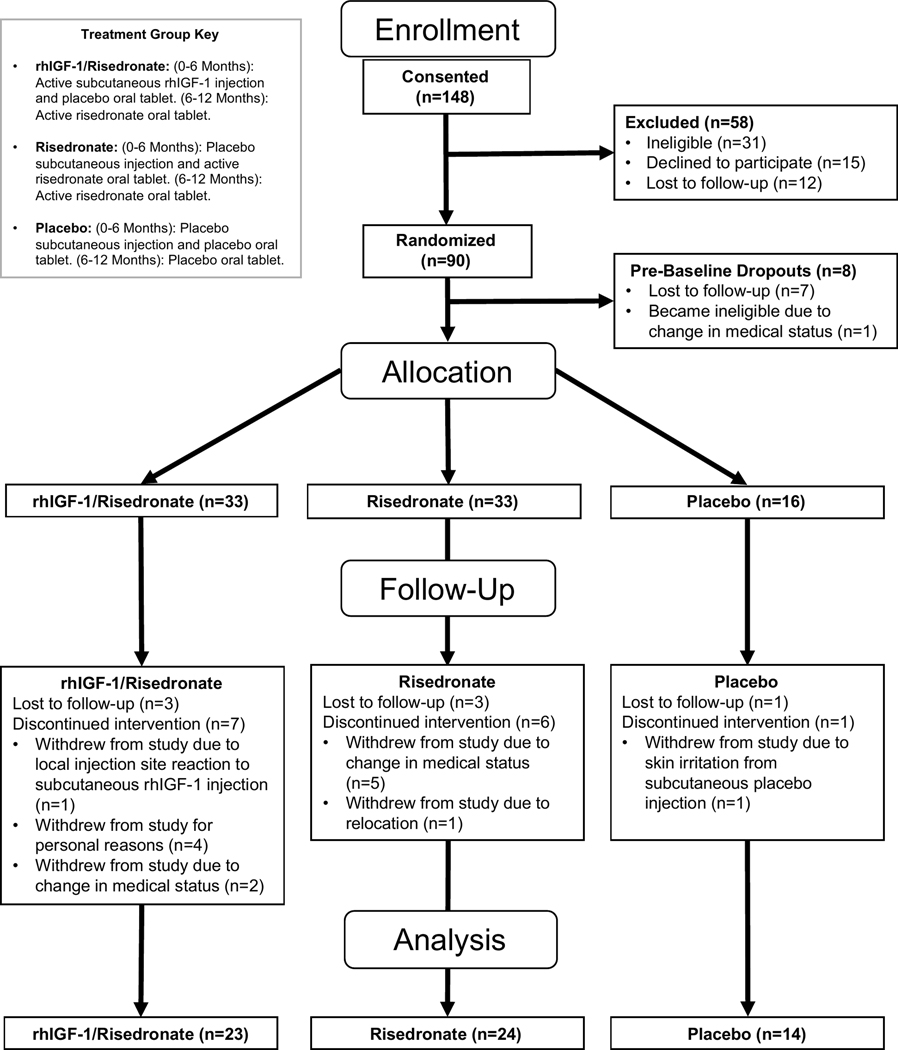
Ninety women with anorexia nervosa, aged 18 to 45 years, were randomized, and 82 were included in the analysis (Figure 1). All subjects fulfilled criteria for anorexia nervosa or atypical anorexia nervosa as assessed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 5th edition(2). Women with low-weight anorexia nervosa [body mass index (BMI) < 18.5 kg/m2] and atypical anorexia nervosa (individuals who meet psychological criteria for anorexia nervosa but are not low weight, defined as BMI ≥ 18.5 kg/m2), were included. Note we elected to broaden the sample of women with anorexia nervosa to include those with typical and atypical presentations in order to increase generalizability of findings as those with atypical presentations also frequently have low bone mineral density(24). Subjects were required to have at least one aBMD Z-score or T-score <−1.0 at the lumbar spine, hip, or wrist measured within 6 months of the screening visit. To participate in the study, all subjects had to be estrogen replete (i.e. menses within the previous 3 months) or be taking systemic estrogen therapy at the time of the baseline visit (based on data from our prior study suggesting that rhIGF-1 is more effective in the setting of systemic estrogen use than rhIGF-1 alone(23)). Subjects were additionally required to have normal thyroid function tests, and normal serum 25OH vitamin D (≥ 20 ng/mL) and calcium levels. All subjects were required to be followed by a treatment team, to use a medically accepted means of contraception for the duration of the study, and to have had a dental check-up within the past year. Exclusion criteria included: contraindications to risedronate use; binge-eating/purging subtype of anorexia nervosa with regular vomiting and significant periodontal disease; invasive dental procedure within the previous 3 months or planned within the next year; any other disorder known to affect bone; any medication known to affect bone metabolism within the previous 3 months excluding exogenous estrogen (bisphosphonates must have been discontinued for at least 1 year); serum potassium <3.0 meq/L, alanine aminotransferase (ALT) >3x upper limit of normal, or estimated glomerular filtration rate (eGFR) <30 ml/min; pregnant or breastfeeding; diabetes mellitus; active substance abuse, including alcohol; suicidality; or history of malignancy or thromboembolic disorders. Baseline clinical characteristics have been previously reported on a subset of these subjects(8,9,13,24), but no longitudinal data have been previously published.
Figure 1.
Consort diagram.
Study design
The study was approved by the Partners Human Research Committee institutional review board, and was registered on ClinicalTrials.gov as NCT01724489. All subjects gave informed consent before study participation. Study subjects were recruited from 2011 to 2018 through collaborating healthcare providers and through advertisements. All procedures were performed at the Massachusetts General Hospital (MGH). At the screening visit, a medical history and physical examination were performed. Nutritional evaluation, including weight in a gown and height, were measured, and BMI and percent “ideal body weight” (using 1983 Metropolitan Life Insurance Tables(25)) were calculated. A blood draw for a comprehensive metabolic panel, complete blood count, thyroid stimulating hormone (TSH), and 25OH vitamin D, and a urine pregnancy test were performed. Dual-energy x-ray absorptiometry (DXA) scans of the lumbar spine, hip, radius, and total body were completed if the subject had not had a DXA scan within the previous 6 months. Eligible subjects returned for a baseline visit, in which medical history, physical exam, nutritional evaluation, and a urine pregnancy test were repeated. Blood was drawn for serum IGF-1, glucose, ALT, and potassium, as well as bone metabolism markers in a subset (n=74). DXA scans of the lumbar spine, hip, radius, and total body were completed if a DXA had not been performed at the screening visit or had not taken place within 1 month of the baseline visit. High-resolution peripheral quantitative computed tomography (HR-pQCT) and finite element analysis (FEA) of the radius and tibia and multi-detector computed tomography (MDCT) of the L1 vertebral body were performed to determine structural characteristics of the radius and tibia, and spine, respectively. Eating disorder psychopathology was measured by the Eating Disorder Examination-Questionnaire (EDE-Q)(26) and the Eating Disorder Inventory-2 (EDI-2)(27). Physical activity was measured by the Paffenbarger Exercise Questionnaire(28).
At the baseline visit, participants were randomly assigned by the research pharmacy to 1 of 3 groups in a 2:2:1 fashion in this double-blind, randomized, placebo-controlled trial. Placebos were identical to the active drugs in appearance. In addition, all participants received calcium 1200mg and vitamin D 800 IU daily.
“rhIGF-1/Risedronate” group:
0–6 Months: Active subcutaneous rhIGF-1 injection (30 mcg/kg subcutaneously twice daily, Ipsen Pharmaceuticals, Cambridge, MA) and placebo oral tablet.
6–12 Months: Active risedronate oral tablet (35mg PO weekly).
“Risedronate” group:
0–6 Months: Placebo subcutaneous injection and active risedronate oral tablet (35mg PO weekly).
6–12 Months: Active risedronate oral tablet (35mg PO weekly).
“Placebo” group:
0–6 Months: Placebo subcutaneous injection and placebo oral tablet.
6–12 Months: Placebo oral tablet.
After the baseline visit, follow-up occurred at months 1, 3, 4.5, 6, 7.5, 9, 10.5, and 12. Serum IGF-1 levels were measured at each visit during the first 6 months of the study. A healthcare professional not involved with the study monitored serum IGF-1 levels and titrated rhIGF-1 doses to maintain IGF-1 levels within the age-adjusted normal range for each participant. To maintain blinding of participants and investigators, the monitor sham dose-adjusted a subject assigned to placebo injections concurrently with each participant receiving active rhIGF-1. A clinical evaluation, nutritional evaluation, and urine pregnancy test were performed at each visit. DXA scans of the lumbar spine, hip, radius, and total body, and HR-pQCT scans of the radius and tibia, were performed at months 6 and 12. MDCT scans of the L1 vertebral body were performed at month 12 in a subset of participants (MDCT scans: n=15 in rhIGF-1/Risedronate group, n=16 in Risedronate group, n=12 in Placebo group). Safety labs, namely potassium, ALT, and glucose, were measured at months 1, 3, 4.5, 6, 9 and 12. Blood was drawn for bone metabolism markers at months 6 (n=44) and 12 (n=41) in a subset.
Laboratory methods
Serum IGF-1 was measured by high-resolution liquid chromatography/mass spectrometry (LC/MS) with analytical measurement range of 16–2,000 ng/mL and coefficient of variation of ≤ 5% (Quest Diagnostics, San Juan Capistrano, CA). Serum 25OH vitamin D was measured by liquid chromatography/tandem mass spectrometry (LC/MS-MS) using assays certified by the National Institutes of Health Vitamin D Standardization Certification. Comprehensive metabolic panel, complete blood count, and TSH were measured using standardized clinical methods. Serum N-terminal propeptide of type I procollagen (PINP) was measured by chemiluminescence with a sensitivity of <1.0 ng/mL, interassay variability of 5% and intra-assay variability of 3% (Immunodiagnostic Systems, Tyne and Wear, UK). Serum C-terminal telopeptides of type I collagen (CTX) was measured by chemiluminescence with sensitivity of 0.023 ng/mL, interassay variability of 6% and intra-assay variability of 3% (Immunodiagnostic Systems, Tyne and Wear, UK).
DXA
Areal BMD (aBMD) at the PA and lateral lumbar spine, total hip, femoral neck, and total radius, as well as body composition, were assessed in all participants by DXA (Hologic Discovery A or Horizon A, Hologic, Inc., Waltham, MA) with precision of 0.02 g/cm2 at the lumbar spine for aBMD(29) and <2% for lean mass(30). Precision for aBMD measurements at the L2-L4 lateral lumbar spine has been reported to be 1.2% using Hologic scanners (Hologic QDR-200, Hologic, Inc., Waltham, MA)(31). Standard cross-calibration procedures were performed between the Discovery A and Horizon A scanners as recommended by a 2005 International Society of Clinical Densitometry position statement(32). All DXA scans were reviewed by at least two investigators. If vertebral deformities were present or if the ribs or pelvis interfered with the lateral spine image, individual vertebrae (or the entire PA or lateral lumbar spine DXA if necessary) were excluded from all timepoints for that participant. For participants 20 years of age or younger, PA lumbar spine and hip BMD reference data were from the Bone Mineral Density in Childhood Study(33). For participants 20 years of age or older, PA lumbar spine BMD reference data were from Hologic(34), total hip BMD reference data were from National Health and Nutrition Examination Survey (NHANES) phase II dataset(35), and BMD reference data for all femoral regions except total hip were from NHANES phase I dataset(36). For the lateral lumbar spine and radius BMD we used non-published proprietary reference data from Hologic. Body composition reference data were from the 1999–2004 NHANES dataset(37).
HR-pQCT
HR-pQCT was used to measure volumetric BMD (vBMD) at the ultradistal tibia and radius (Xtreme CT; Scanco Medical AG, Brüttisellen, Switzerland) with an isotropic voxel size of 82 μm3 and precision of 0.7–1.5% per previously published methods(38). The non-dominant arm or leg was scanned unless there was a prior fracture at that region, in which case the contralateral side was scanned. Finite element analysis was used to estimate the biomechanical properties of the bone in the setting of simulated axial compression per previously published methods(39). Failure load (N) was estimated by scaling the resultant load from a 1% apparent compressive strain until 2% of all elements reached an effective strain >7000 μstrain.
Multi-detector computed tomography (MDCT)
Trabecular and cortical vBMD and estimated strength by FEA of the L1 vertebral body were assessed using an MDCT scan obtained using Definition Flash™ (Siemens Medical Solutions, Erlangen, Germany), an FDA-approved clinical scanner with an estimated precision of 1.2%. The following scan protocol was used: Tube voltage = 120kVp, Tube Current = 350mA, Rotation speed = 1 sec, Pitch = 0.85, Scan length = 6cm (approximately), and Field-of-view = 300 mm. Tomographic slice data at multiple slice thicknesses were reconstructed using bone and soft-tissue kernels. Biomechanical Computed Tomography (BCT) analysis was performed by O.N. Diagnostics, LLC (Berkeley, CA) using their FDA-approved Finite Element Analysis package. The following variables were estimated: (1) strength (N) of the vertebral body under compressive loading conditions, (2) total vBMD (mg/cm3) of the entire vertebral body, (3) vBMD (mg/cm3) of the trabecular compartment, and (4) vBMD (mg/cm3) of the peripheral compartment, i.e. the peripheral 2mm layer of bone which includes the cortical shell.
Statistical analysis
JMP Statistical Discoveries (version 14PRO, SAS Institute, Cary, NC) was used for statistical analyses of baseline clinical characteristics, within-group changes from baseline to 12 months, and side effects; variables were assessed for normality using the Shapiro-Wilk test and, if non-normal, were log-transformed. R version 4.5 was used for other analyses. The primary analysis was a shared baseline repeated measures analysis of variance with the primary endpoint being the differences between each of the groups at 12 months. This analysis properly accounts for missing data by including subjects who are missing their 12 month visit (by including their partial data appropriately), as well as properly accounting for the baseline value of the outcome variable(40). The primary analysis was intention-to-treat, with the primary endpoint PA lumbar spine aBMD at 12 months, with the following comparisons at 12 months considered secondary: lateral lumbar spine, total hip, femoral neck, and total radius aBMD by DXA; total, trabecular, and peripheral vertebral vBMD by MDCT; and radial and tibial total vBMD and failure load by HR-pQCT. The comparison of sequential rhIGF-1 and risedronate therapy to risedronate alone and the comparison of each of these to double placebo were separate scientific questions, and therefore primary endpoint analyses were not corrected for multiple comparisons(41). With 90 evaluable subjects, the chance of detecting a difference in the rate of PA lumbar spine aBMD increase of 2.5% between the sequential rhIGF-1 and risedronate therapy and risedronate alone groups at a two-sided significance level of 0.05 was >80%. This was based on the assumption that the SD of the response variable (PA lumbar spine aBMD) is 2.69%(23), and on the 2.5% difference we found in our preliminary data from an open-label study of sequential rhIGF-1 followed by risedronate compared to risedronate alone at 12 months. A secondary analysis of change in aBMD and vBMD within groups was performed using paired t-tests. Statistical significance was defined as a two-tailed P value of ≤ 0.05. Baseline data are reported as mean ± SD. Endpoint data are reported as least squares mean (LS mean) corrected for baseline ± SEM. Figures of the raw data are displayed as box and whisker plots with boxes representing the median and interquartile range, and whiskers representing the minimum and maximum values.
Results
Of 148 women who were assessed for eligibility, 90 were eligible and randomized (Figure 1). Eight women were excluded prior to baseline because they were lost to follow-up or experienced a change in medical status making them ineligible for participation. Of the 33 participants randomized to receive rhIGF-1 followed by risedronate (“rhIGF-1/Risedronate” group), 10 were excluded from follow-up analysis because they were lost to follow-up (n=3) or discontinued the intervention (n=7). Of the 33 participants randomized to receive risedronate alone (“Risedronate” group), 9 were excluded from follow-up analysis because they were lost to follow-up (n=3) or discontinued the intervention (n=6) for the reasons cited on Figure 1. Of the 16 participants randomized to receive placebo (“Placebo” group), 2 were excluded from follow-up analysis because they were lost to follow-up (n=1) or discontinued the intervention (n=1) for the reason cited on Figure 1.
Clinical characteristics
Clinical characteristics of the study participants are shown in Table 1. There were no significant differences among the three groups in the variables tested, including pre-treatment aBMD and vBMD at all sites, except that the mean length of time the participants had had anorexia nervosa (“duration of anorexia nervosa”) was longer in the rhIGF-1/Risedronate group compared to the Placebo group. Overall, 49% had low-weight anorexia nervosa, 51% of subjects had atypical anorexia nervosa (BMI ≥ 18.5 kg/m2) and 32% were amenorrheic (and thus started on systemic estrogen therapy); the prevalence of atypical anorexia nervosa and amenorrhea was similar among the groups.
Table 1.
Baseline characteristics
| rhIGF-1/risedronate (n=33) | Risedronate (n=33) | Placebo (n=16) | Overall P-value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 28 ± 6 | 28 ± 7 | 25 ± 6 | 0.32 |
| Caucasian, n (%) | 31 (94) | 32 (97) | 15 (94) | 1.00 |
| BMI, kg/m² | 18.4 ± 1.5 | 18.4 ± 2.2 | 18.6 ± 1.7 | 0.88 |
| Lowest past BMI, kg/m² | 15.1 ± 1.6 | 14.7 ± 2.1 | 15.8 ± 1.7 | 0.16 |
| Duration of anorexia nervosa, y | 12.2 ± 6.1a | 12.6 ± 8.9ab | 7.7 ± 6.4b | 0.04 |
| Physical activity, hrs/wk | 3.9 ± 4.3 | 4.4 ± 4.4 | 3.5 ± 4.1 | 0.70 |
| Amenorrheic, n (%) | 10 (30%) | 12 (36%) | 4 (25%) | 0.82 |
| Body composition | ||||
| Lean mass, kg | 36.7 ± 4.6 | 35.0 ± 5.5 | 36.6 ± 3.2 | 0.33 |
| Laboratory measurements | ||||
| IGF-1, ng/mL | 195 ± 69 | 177 ± 70 | 187 ± 85 | 0.60 |
| IGF-1, Z-score | −0.0 ± 0.8 | −0.2 ± 1.0 | −0.4 ± 0.9 | 0.38 |
| PINP, ng/mL | 68 ± 34 | 71 ± 72 | 65 ± 25 | 0.59 |
| CTX, ng/mL | 0.39 ± 0.21 | 0.34 ± 0.30 | 0.39 ± 0.27 | 0.30 |
| Areal BMD | ||||
| Postero-anterior spine, g/cm2 | 0.88 ± 0.12 | 0.86 ± 0.12 | 0.88 ± 0.12 | 0.80 |
| Postero-anterior spine, Z-score | −1.4 ± 1.1 | −1.6 ± 1.0 | −1.3 ± 1.0 | 0.65 |
| Lateral spine, g/cm2 | 0.67 ± 0.09 | 0.66 ± 0.09 | 0.67 ± 0.07 | 0.88 |
| Lateral spine, Z-score | −1.6 ± 1.1 | −1.7 ± 1.1 | −1.6 ± 0.9 | 0.89 |
| Total hip, g/cm2 | 0.84 ± 0.11 | 0.79 ± 0.12 | 0.87 ± 0.11 | 0.08 |
| Total hip, Z-score | −0.8 ± 0.9 | −1.2 ± 1.0 | −0.6 ± 0.9 | 0.07 |
| Femoral neck, g/cm2 | 0.72 ± 0.10 | 0.68 ± 0.13 | 0.75 ± 0.11 | 0.11 |
| Femoral neck, Z-score | −1.1 ± 0.9 | −1.4 ± 1.1 | −0.8 ± 1.0 | 0.12 |
| Total radius, g/cm2 | 0.55 ± 0.05 | 0.53 ± 0.05 | 0.55 ± 0.05 | 0.15 |
| Total radius, Z-score | −0.3 ± 0.9 | −0.8 ± 0.9 | −0.5 ± 0.9 | 0.14 |
| HR-pQCT | ||||
| Radial failure load, N | 3415 ± 619 | 3185 ± 487 | 3292 ± 618 | 0.28 |
| Radial total BMD, mg/cm3 | 314.5 ± 68.5 | 303.4 ± 61.7 | 315.5 ± 61.7 | 0.74 |
| Tibial failure load, N | 8971 ± 1398 | 8722 ± 1759 | 9095 ± 1281 | 0.69 |
| Tibial total BMD, mg/cm3 | 282.9 ± 61.5 | 285.3 ± 61.9 | 287.6 ± 49.2 | 0.97 |
| MDCT | ||||
| Vertebral strength, N | 5843 ± 1250 | 5664 ± 1457 | 5674 ± 1345 | 0.88 |
| Vertebral total BMD, mg/cm3 | 197.3 ± 34.0 | 193.0 ± 37.1 | 196.0 ± 37.5 | 0.92 |
| Vertebral trabecular BMD, mg/cm3 | 155.3 ± 31.1 | 147.8 ± 33.4 | 151.1 ± 39.0 | 0.75 |
| Vertebral peripheral BMD, mg/cm3 | 277.1 ± 45.2 | 279.7 ± 49.0 | 282.2 ± 38.4 | 0.94 |
Values reported as mean ± SD.
Groups with different superscripts differ significantly at p<0.05.
Abbreviations- BMI: body mass index. IGF-1: insulin-like growth factor-1. PINP: amino-terminal propeptide of type I procollagen. CTX: C-terminal telopeptides of type I collagen. HR-pQCT: high resolution peripheral quantitative computed tomography. MDCT: multi-detector computed tomography. ab - Groups with different superscripts differ significantly at p<0.05.
RhIGF-1 dosing and levels
At 6 months, the mean rhIGF-1 dose in the rhIGF-1/Risedronate group was 1.1 ± 0.5 mg (21.0 ± 9.4 mcg/kg) twice daily. By study design, after 6 months of rhIGF-1 administration, mean serum IGF-1 levels were significantly higher in the rhIGF-1/Risedronate group (324 ± 109 ng/mL, Z-score 1.3 ± 0.9) compared to the Risedronate (190 ± 49 ng/mL, Z-score 0.3 ± 0.7) and Placebo groups (177 ± 50 ng/mL, Z-score −0.4 ± 0.6) (p<0.0001). As expected, mean serum IGF-1 levels were similar among the groups at 12 months (196 ± 78 vs 194 ± 66 vs 183 ± 52 ng/mL; Z-scores 0.2 ± 1.0 vs 0.2 ± 0.7 vs −0.2 ± 0.6; p=0.86).
Areal BMD
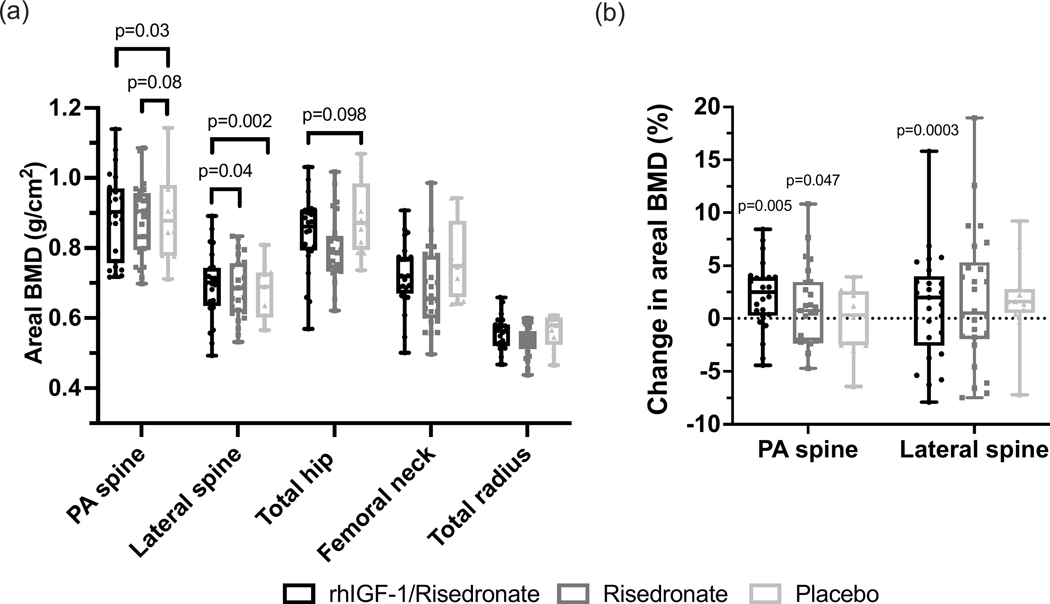
Pre-treatment mean PA lumbar spine aBMD did not differ among the groups (Table 1). At 12 months, the LS mean PA lumbar spine aBMD in the rhIGF-1/Risedronate group was significantly higher compared to the Placebo group (0.89 ± 0.01 vs 0.87 ± 0.02 g/cm2) (p=0.03), and was statistically similar compared to the Risedronate group (0.89 ± 0.01 g/cm2) (p=0.61) (primary a priori comparison) (Figure 2a). LS mean PA lumbar spine aBMD did not differ between the Risedronate and Placebo groups (p=0.08) (primary a priori comparison) (Figure 2a). Mean PA lumbar spine aBMD increased by 1.9 ± 0.6% in the rhIGF-1/Risedronate group, 1.7 ± 0.8% in the Risedronate group, and decreased by 0.3 ± 0.8% in the Placebo group over the 12-month study period (p<0.05 for within group difference from baseline to 12 months in the rhIGF-1/Risedronate and Risedronate groups) (Figure 2b). Mean PA lumbar spine aBMD Z-scores at 12 months were −1.2 ± 0.2, −1.5 ± 0.2 in the Risedronate group, and −1.3 ± 0.3 in the Placebo group.
Figure 2.
(a) Areal BMD values by DXA at 12 months at the postero-anterior (PA) lumbar spine, lateral lumbar spine, total hip, femoral neck, and total radius. (b) Percent change in areal BMD at the PA and lateral lumbar spine from baseline to 12 months. Raw data presented as box and whisker plots with boxes representing median and interquartile range and whiskers representing minimum and maximum values. P-values in figure 2a represent differences between groups when analyzed according to a shared baseline repeated measures analysis of variance. P-values in figure 2b represent differences within groups from baseline to 12 months using paired t-tests.
Pre-treatment lateral lumbar spine aBMD did not differ among the groups (Table 1). At 12 months, LS mean lateral lumbar spine aBMD in the rhIGF-1/Risedronate group was significantly higher compared to both the Risedronate group (p=0.04) and the Placebo group (p=0.002) (0.69 ± 0.01 vs 0.68 ± 0.01 vs 0.66 ± 0.01 g/cm2) (Figure 2a). Mean lateral lumbar spine aBMD increased by 4.2 ± 1.0% in the rhIGF-1/Risedronate group, 1.7 ± 1.0% in the Risedronate group, and decreased by 1.1 ± 1.3% in the Placebo group over the 12-month study period (p<0.05 for within group difference from baseline to 12 months in the rhIGF-1/Risedronate group) (Figure 2b). Mean lateral lumbar spine aBMD Z-scores at 12 months were −1.2 ± 0.2 in the rhIGF-1/Risedronate group, −1.5 ± 0.2 in the Risedronate group, and −1.6 ± 0.3 in the Placebo group.
Pre-treatment total hip aBMD did not differ among the groups (Table 1). At 12 months, LS mean total hip aBMD did not differ among the groups (Figure 2a). Mean total hip aBMD increased by 1.4 ± 0.6% in the IGF-1/Risedronate group, 1.3 ± 0.8% in the Risedronate group, and decreased by 1.0 ± 1.0% in the Placebo group over the 12-month study period (p=NS).
Pre-treatment femoral neck and total wrist aBMD did not differ among the groups (Table 1). At 12 months, LS mean femoral neck and total wrist aBMD did not differ among the groups (Figure 2a). Mean femoral neck aBMD increased by 1.2 ± 0.8% in the IGF-1/Risedronate group, 0.9 ± 1.0% in the Risedronate group, and decreased by 0.9 ± 1.2% in the Placebo group, while mean total radius aBMD increased by 0.45 ± 0.4% in the IGF-1/Risedronate group, 0.8 ± 0.4% in the Risedronate group, and 0.1 ± 0.4% in the Placebo group, over the 12-month study period (p=NS).
L1 vertebral volumetric BMD and estimated strength by MDCT
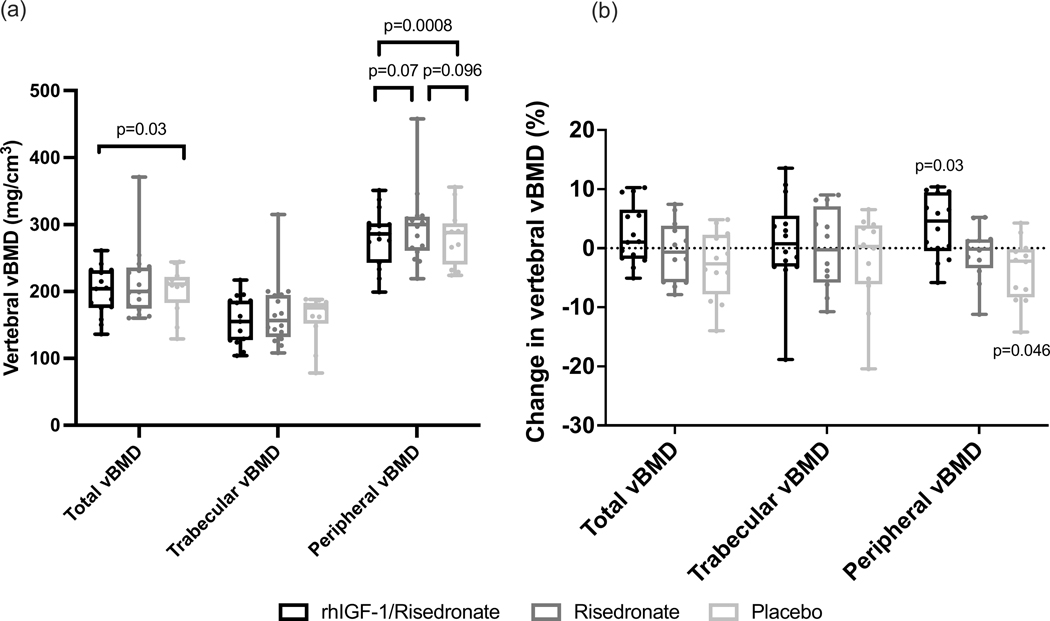
Pre-treatment vertebral vBMD of the entire vertebral body, the trabecular compartment, and the peripheral compartment (i.e. the peripheral 2mm layer of bone which includes the cortical shell) by MDCT did not differ among the groups (Table 1). At 12 months, LS mean vertebral total vBMD in the rhIGF-1/Risedronate group was significantly higher compared to the Placebo group (203.6 ± 5.8 vs 193.8 ± 6.1 mg/cm3) (p=0.03), and was similar compared to the Risedronate group (198.8 ± 5.8 mg/cm3) (p=0.25) (Figure 3a). LS mean vertebral total vBMD was similar between the Risedronate and Placebo groups (p=0.28) (Figure 3a). At 12 months, LS mean vertebral trabecular vBMD was similar among the groups (Figure 3a). At 12 months, LS mean vertebral peripheral vBMD in the rhIGF-1/Risedronate group was higher compared to the Placebo group (293.1 ± 6.9 vs 272.3 ± 7.4 mg/cm3) (p=0.0008), and did not differ from the Risedronate group (282.7 ± 6.9 mg/cm3) (p=0.07) (Figure 3a). LS mean vertebral peripheral vBMD did not differ between the Risedronate and Placebo groups (p=0.096) (Figure 3a). From baseline to 12 months, mean vertebral total and trabecular vBMD by MDCT did not change within any group, but mean vertebral peripheral vBMD increased by 3.8 ± 1.4% in the rhIGF-1/Risedronate group and decreased by 3.9 ± 1.7% in the Placebo group (p<0.05 for within group difference from baseline to 12 months in the rhIGF-1/Risedronate and Placebo groups) (Figure 3b).
Figure 3.
(a) Vertebral total, trabecular, and peripheral volumetric BMD (vBMD) values by MDCT at 12 months. (b) Percent change in vertebral total, trabecular, and peripheral vBMD values from baseline to 12 months. Raw data presented as box and whisker plots with boxes representing median and interquartile range and whiskers representing minimum and maximum values. P-values in figure 3a represent differences between groups when analyzed according to a shared baseline repeated measures analysis of variance. P-values in figure 3b represent differences within groups from baseline to 12 months using paired t-tests.
Pre-treatment vertebral estimated strength by MDCT did not differ among the groups (Table 1). At 12 months, LS mean vertebral estimated strength did not differ among the groups.
Radial and tibial volumetric BMD and estimated strength by HR-pQCT
Pre-treatment radial and tibial bone vBMD and estimated strength by HR-pQCT did not differ among the groups (Table 1). There was no difference in radial or tibial vBMD or estimated strength among the groups at 12 months (data not shown).
Markers of bone metabolism
In the subset of participants who were assessed for serum PINP and CTX levels, pre-treatment levels did not differ among groups (rhIGF/Risedronate n=38, Risedronate n=28, Placebo n=16) (Table 1). At 6 months (the end of the rhIGF-1 treatment period), LS mean serum PINP and CTX levels were significantly higher in the rhIGF-1/Risedronate group (n=18) compared to the Risedronate group (n=15), and similar to the Placebo group (n=11) (PINP 63 ± 6 vs 34 ± 7 vs 55 ± 8 ng/mL, respectively, p=0.0004 for the comparison rhIGF-1/Risedronate vs Risedronate; CTX 0.33 ± 0.04 vs 0.16 ± 0.05 vs 0.31 ± 0.05 ng/mL, respectively, p=0.005 for the comparsion rhIGF-1/Risedronate vs Risedronate). LS mean serum PINP and CTX levels were significantly lower in the Risedronate group compared to the Placebo group (PINP p=0.02; CTX p=0.04).
At 12 months, LS mean serum PINP and CTX levels were significantly lower in both the rhIGF-1/Risedronate (n=13) and Risedronate groups (n=17) compared to the Placebo group (n=11) (PINP 37 ± 7 vs 34 ± 7 vs 62 ± 8 ng/mL, respectively, p≤0.01 for both comparisons; CTX 0.19 ± 0.04 vs 0.19 ± 0.04 vs 0.33 ± 0.04 ng/mL, respectively, p<0.05 for both comparisons). LS mean serum PINP and CTX levels were similar between the rhIGF-1/Risedronate and the Risedronate groups (PINP p=0.74; CTX p=0.96).
Muscle mass
Pre-treatment lean mass did not differ among groups (Table 1). At 6 months (the end of the rhIGF-1 treatment period), LS mean lean mass was significantly higher in the IGF-1/Risedronate group (36.83 ± 0.61 kg) compared to the Risedronate group (35.76 ± 0.61 kg, p=0.03), and did not differ from the Placebo group (36.30 ± 0.84 kg, p=0.09). LS mean lean mass was similar between the Risedronate and Placebo groups (p=0.84). Mean lean mass increased by 2.1 ± 1.0% in the rhIGF-1/Risedronate group, decreased by 0.3 ± 1.0% in the Risedronate group, and decreased by 0.6 ± 1.2% in the Placebo group over 6 months. At 12 months, there was no difference in lean mass among the 3 groups.
Predictors of change in BMD
In the entire cohort, change in lean mass from baseline to 12 months was positively associated with change in lateral lumbar spine aBMD by DXA over 12 months (R=0.30, p=0.02). Change in serum IGF-1 from baseline to 6 months was positively associated with change in vertebral total vBMD (R=0.36, p=0.03) and vertebral peripheral vBMD (R=0.51, p=0.001) over 12 months.
Adverse events and compliance
rhIGF-1 and risedronate were generally well tolerated with no significant differences among groups in frequency of self-reported side effects (Table 2). Injection site irritation occurred in 34% of subjects, with a similar frequency between active medication and placebo groups. One subject in the rhIGF-1/Risedronate group discontinued the intervention due to a local injection site reaction to rhIGF-1 that resolved within days of study drug discontinuation, and one subject in the Placebo group discontinued due to skin irritation from placebo injections.
Table 2.
Subject-reported adverse events
| IGF-1/risedronate (n=33) | Risedronate (n=33) | Placebo (n=16) | Overall P-value | |
|---|---|---|---|---|
| Acid reflux | 4 (12) | 3 (9) | 2 (13) | 1.0 |
| Constipation | 0 (0) | 3 (9) | 0 (0) | 0.22 |
| Diarrhea | 0 (0) | 2 (6) | 0 (0) | 0.67 |
| Nausea | 1 (3) | 5 (15) | 1 (6) | 0.27 |
| Upset stomach | 3 (9) | 1 (3) | 0 (0) | 0.52 |
| Achiness | 1 (3) | 4 (12) | 0 (0) | 0.26 |
| Dizziness | 0 (0) | 0 (0) | 1 (6) | 0.20 |
| Headache | 1 (3) | 1 (3) | 2 (13) | 0.32 |
| Injection site irritation and/or bruising | 15 (45) | 11 (33) | 2 (13) | 0.07 |
| Local injection site reaction | 1 (3) | 0 (0) | 0 (0) | 1.0 |
Data are n (%)
In the rhIGF-1/Risedronate group, the mean number of missed doses of rhIGF-1 was 11 ± 13 out of 360 doses over 6 months, and of risedronate was 1.1 ± 1.6 out of 24 doses over 6 months, per self-report. In the Risedronate group, the mean number of missed doses of risedronate was 1.9 ± 2.5 out of 48 doses over 12 months per self-report.
Discussion
We have demonstrated that sequential therapy of 6 months of rhIGF-1 followed by 6 months of risedronate increased lateral lumbar spine aBMD more than risedronate alone or placebo in women with anorexia nervosa. This is important as the lumbar spine is the primary site of bone loss in women with anorexia nervosa. However, neither hip or radial aBMD, nor radial or tibial vBMD, improved with sequential therapy. These data suggest that strategies that are anabolic and antiresorptive to bone and address the underlying bone metabolism dysregulation (low bone formation and high resorption) may be effective at increasing BMD in women with anorexia nervosa.
Low BMD and fractures are prevalent among women with anorexia nervosa(3–6). Uncoupling of bone formation and resorption, resulting in low bone formation and high bone resorption, is a key mechanism of low bone mass in this disease. Therapies that have aimed to increase bone formation, e.g. rhIGF-1, or reduce bone resorption, e.g. risedronate, alone have increased lumbar spine aBMD by 1.8 to 3.2% over 9 or 12 months, respectively(20,23). In a study of women with postmenopausal osteoporosis, risedronate increased lumbar spine aBMD by 3.4% over 12 months(42). Large, randomized trials in postmenopausal women with osteoporosis, which is characterized by both increased bone resorption and formation (due to coupling), have demonstrated that sequential anabolic therapy followed by antiresorptive therapy is more effective than antiresorptive therapy alone(43,44), and this strategy may be particularly important when bone formation is reduced as in anorexia nervosa.
We have demonstrated that sequential anabolic therapy with rhIGF-1 followed by antiresorptive therapy with risedronate significantly increased aBMD at the lumbar spine, which is the primary site of bone loss in anorexia nervosa. The effect of sequential therapy with rhIGF-1 and risedronate was especially pronounced at the lateral lumbar spine aBMD site by DXA, which measures aBMD of the vertebral bodies without contribution from the posterior vertebral elements(45). A significant difference was not observed between sequential therapy with rhIGF-1 followed by risedronate and risedronate alone on PA lumbar spine aBMD, perhaps because the spinous processes obscured the effects of sequential therapy on the vertebral bodies. The MDCT data suggest that sequential therapy with rhIGF-1 and risedronate had a significant effect on L1 vertebral peripheral vBMD compared to double placebo. The different regions of interest for L2-L4 lateral lumbar spine aBMD by DXA versus L1 lumbar spine vBMD by MDCT, as well as the small number of participants with MDCT data, may explain the differential results between the two modalities. It may be that sequential therapy with rhIGF-1 and risedronate acts on the cortical shell of the vertebral bodies, and that we would have seen a significant difference between sequential therapy with rhIGF-1 followed by risedronate compared to risedronate alone if more vertebral bodies were included in the MDCT analysis or a larger number of participants were studied. We did not detect a difference with sequential rhIGF-1 and risedronate therapy in aBMD or vBMD at the hip, radius, and tibia.
The changes in markers of bone formation and resorption were largely what one would expect with anabolic or antiresorptive therapy. As indicated above, anorexia nervosa is associated with uncoupling of bone formation and resorption such that bone formation is low, but bone resorption is high. Recombinant human IGF-1 increased bone formation (and thus also bone resorption) compared to risedronate, while risedronate suppressed bone resorption (and thus also bone formation) compared to placebo.
We also demonstrated that rhIGF-1 was anabolic to muscle in women with anorexia nervosa, which is in agreement with our previous reports(23). This is salient given it is well known that muscle is a positive determinant of aBMD, which it was in our cohort, and that muscle loss is an unavoidable consequence of caloric restriction in anorexia nervosa. However, the effects of rhIGF-1 on muscle mass reversed once therapy was stopped, as all groups had similar muscle mass at 12 months.
One limitation of this study is the dropout rate, but it should be noted that the dropout rate was similar to prior studies in women with anorexia nervosa, which typically have low accrual and high drop-out rates(46). In addition, the statistical analysis included data from interim study visits from subjects who dropped out before the end of the study. The results of our study are not generalizable to adolescents with anorexia nervosa, as adolescence is normally a state of high bone turnover and rapid bone accrual, and bone metabolism in adolescents with the disease is characterized by both low bone formation and resorption. A strength of the study is the inclusion of a broad sample of restricting type anorexia nervosa including both low-weight and atypical anorexia nervosa, but the results may not be generalizable to women with the binge/purge subtype of anorexia nervosa. Although lateral lumbar spine aBMD is not recommended for use in clinical practice by the International Society of Clinical Densitometry, its use in clinical investigation may be informative since lateral lumbar spine aBMD measures BMD of the vertebral bodies without contribution from the posterior vertebral elements. Whether other more potent sequential anabolic and antiresorptive therapies in adults, such as teriparatide(47) and denosumab, could yield similar or better results to rhIGF-1 and risedronate warrants further investigation. In addition, because effects of growth hormone therapy on bone are general not detectable for a year or more after the initiation of therapy(48–50), a longer duration of rhIGF-1 treatment might result in greater increases in BMD is this population; further studies are necessary to determine whether this might be the case.
In conclusion, six months of rhIGF-1 followed by 6 months of risedronate increased lateral lumbar spine aBMD, a primary site of bone loss in women with anorexia nervosa, more than risedronate alone or double placebo in women with anorexia nervosa and low aBMD. This study suggests that sequential bone anabolic followed by antiresorptive therapy—addressing the dysregulated bone metabolism characterized by low bone formation and high bone resorption in women with anorexia nervosa—may be an effective strategy to improve low BMD in anorexia nervosa. However, sequential therapy did not improve hip or radial aBMD nor radial or tibial vBMD. Although the gain in lateral lumbar spine aBMD compared to risedronate alone may not justify the cost and effort associated with this regimen, and rhIGF-1 is not FDA approved for the treatment of low BMD in women with anorexia nervosa, this study adds to the literature on relative IGF-1 deficiency and the pathophysiology of bone loss in this disorder. Since our study was small and therefore not definitive, and only positive for a secondary outcome, longer-term, randomized studies of this and other sequential bone anabolic and antiresorptive therapies are needed. The treatment of low BMD in women with anorexia nervosa is a critical unmet need given the high prevalence of low BMD and fractures in this disease, both in women in whom anorexia nervosa is a chronic condition and in women who recover but in whom low BMD remains a long-term medical complication.
Acknowledgments
Funding
The project described was supported by National Institutes of Health Grant Numbers R01 DK052625; K23 DK115903; K24 HL092902; (1)1UL1TR001102; 1UL1TR002541-01; 1 UL1 RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources; and 8 UL1 TR000170, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science.
Ipsen Biopharmaceuticals in Cambridge, MA, provided the study medication at no cost. Ipsen had no input into the study design, analysis, or interpretation of the results. Ipsen provided a courtesy review of the manuscript. The review was for scientific accuracy only, and the authors retained full editorial control of the content.
Footnotes
Disclosure Page
MSH, AKimball, EM, KNB, KS, KTE, VS, SE, ED, TW, LC, RJK, SG, DM, MAB, COT, RG, DS, and AKlibanski have nothing to disclose. MM has served on the scientific advisory board for Abbvie and Ipsen and has received consulting fees from Sanofi. KKM has received study medication from Pfizer and an investigator-initiated research grant from Amgen, and has had equity in Bristol-Myers Squibb, General Electric, Boston Scientific, Amgen and Becton Dickinson.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Hudson JI, Hiripi E, Pope HG Jr., Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. February 1 2007;61(3):348–58. Epub 2006/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association AP. Diagnostic and statistical manual of mental disorders: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Ayers JW, Gidwani GP, Schmidt IM, Gross M. Osteopenia in hypoestrogenic young women with anorexia nervosa. Fertil Steril. February 1984;41(2):224–8. Epub 1984/02/01. [DOI] [PubMed] [Google Scholar]
- 4.Brotman AW, Stern TA. Osteoporosis and pathologic fractures in anorexia nervosa. The American journal of psychiatry. April 1985;142(4):495–6. Epub 1985/04/01. [DOI] [PubMed] [Google Scholar]
- 5.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA. March 6 1991;265(9):1133–8. Epub 1991/03/06. [PubMed] [Google Scholar]
- 6.Szmukler GI, Brown SW, Parsons V, Darby A. Premature loss of bone in chronic anorexia nervosa. Br Med J (Clin Res Ed). January 5 1985;290(6461):26–7. Epub 1985/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Annals of internal medicine. November 21 2000;133(10):790–4. Epub 2000/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann KN, Schorr M, Bruno AG, Bredella MA, Lawson EA, Gill CM, et al. Vertebral Volumetric Bone Density and Strength Are Impaired in Women With Low-Weight and Atypical Anorexia Nervosa. The Journal of clinical endocrinology and metabolism. January 1 2017;102(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann KN, Bruno AG, Bredella MA, Schorr M, Lawson EA, Gill CM, et al. Vertebral Strength and Estimated Fracture Risk Across the BMI Spectrum in Women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. February 2016;31(2):281–8. Epub 2015/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazeli PK, Faje AT, Cross EJ, Lee H, Rosen CJ, Bouxsein ML, et al. Serum FGF-21 levels are associated with worsened radial trabecular bone microarchitecture and decreased radial bone strength in women with anorexia nervosa. Bone. August 2015;77:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frolich J, Hansen S, Winkler LA, Andresen AK, Hermann AP, Stoving RK. The Role of Body Weight on Bone in Anorexia Nervosa: A HR-pQCT Study. Calcif Tissue Int. July 2017;101(1):24–33. Epub 2017/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milos G, Hauselmann HJ, Krieg MA, Ruegsegger P, Gallo LM. Are patterns of bone loss in anorexic and postmenopausal women similar? Preliminary results using high resolution peripheral computed tomography. Bone. January 2014;58:146–50. Epub 2013/10/03. [DOI] [PubMed] [Google Scholar]
- 13.Schorr M, Fazeli PK, Bachmann KN, Faje AT, Meenaghan E, Kimball A, et al. Differences in Trabecular Plate and Rod Structure in Premenopausal Women Across the Weight Spectrum. The Journal of clinical endocrinology and metabolism. October 1 2019;104(10):4501–10. Epub 2019/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solmi M, Veronese N, Correll CU, Favaro A, Santonastaso P, Caregaro L, et al. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta psychiatrica Scandinavica. May 2016;133(5):341–51. Epub 2016/01/15. [DOI] [PubMed] [Google Scholar]
- 15.Bachrach LK, Katzman DK, Litt IF, Guido D, Marcus R. Recovery from osteopenia in adolescent girls with anorexia nervosa. The Journal of clinical endocrinology and metabolism. March 1991;72(3):602–6. Epub 1991/03/01. [DOI] [PubMed] [Google Scholar]
- 16.Brooks ER, Ogden BW, Cavalier DS. Compromised bone density 11.4 years after diagnosis of anorexia nervosa. Journal of women’s health. June 1998;7(5):567–74. Epub 1998/07/03. [DOI] [PubMed] [Google Scholar]
- 17.Hartman D, Crisp A, Rooney B, Rackow C, Atkinson R, Patel S. Bone density of women who have recovered from anorexia nervosa. The International journal of eating disorders. July 2000;28(1):107–12. Epub 2000/05/09. [DOI] [PubMed] [Google Scholar]
- 18.Mueller SM, Immoos M, Anliker E, Drobnjak S, Boutellier U, Toigo M. Reduced Bone Strength and Muscle Force in Women 27 Years After Anorexia Nervosa. The Journal of clinical endocrinology and metabolism. August 2015;100(8):2927–33. Epub 2015/06/19. [DOI] [PubMed] [Google Scholar]
- 19.Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. November 1996;81(11):3864–70. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 20.Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, et al. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. The Journal of clinical endocrinology and metabolism. July 2011;96(7):2081–8. Epub 2011/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, et al. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. The Journal of clinical endocrinology and metabolism. June 1999;84(6):2056–63. Epub 1999/06/18. [DOI] [PubMed] [Google Scholar]
- 22.Isgaard J, Nilsson A, Lindahl A, Jansson JO, Isaksson OG. Effects of local administration of GH and IGF-1 on longitudinal bone growth in rats. Am J Physiol. April 1986;250(4 Pt 1):E367–72. Epub 1986/04/01. [DOI] [PubMed] [Google Scholar]
- 23.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. The Journal of clinical endocrinology and metabolism. June 2002;87(6):2883–91. Epub 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 24.Schorr M, Thomas JJ, Eddy KT, Dichtel LE, Lawson EA, Meenaghan E, et al. Bone density, body composition, and psychopathology of anorexia nervosa spectrum disorders in DSM-IV vs DSM-5. The International journal of eating disorders. April 2017;50(4):343–51. Epub 2016/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foundation ML. New weight standards for men and women. Statistical Bulletin Metropolitan Life Insurance Company. 1959;40:1–4. [Google Scholar]
- 26.Fairburn CG BS. Appendix B: Eating disorder examination questionnaire. In: CG F, editor. Cognitive Behavior Therapy and Eating Disorders. New York, NY: Guildford Press; 2008. p. 209–314. [Google Scholar]
- 27.Fairburn CG CZ. Eating Disorder Inventory-2. Professional Manual. Odessa: Psychological Assessment Research, Inc.; 1991. [Google Scholar]
- 28.Paffenbarger RS Jr., Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Medicine and science in sports and exercise. January 1993;25(1):60–70. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 29.Whittaker LG, McNamara EA, Vath S, Shaw E, Malabanan AO, Parker RA, et al. Direct Comparison of the Precision of the New Hologic Horizon Model With the Old Discovery Model. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. Oct - Dec 2018;21(4):524–8. Epub 2017/12/20. [DOI] [PubMed] [Google Scholar]
- 30.Nowitz M, Monahan P. Short term in vivo precision of whole body composition measurements on the Horizon A densitometer. J Med Imaging Radiat Oncol. April 2018;62(2):179–82. Epub 2017/07/30. [DOI] [PubMed] [Google Scholar]
- 31.Blake GM, Jagathesan T, Herd RJ, Fogelman I. Dual X-ray absorptiometry of the lumbar spine: the precision of paired anteroposterior/lateral studies. Br J Radiol. July 1994;67(799):624–30. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd JA, Lu Y, Wilson K, Fuerst T, Genant H, Hangartner TN, et al. Cross-calibration and minimum precision standards for dual-energy X-ray absorptiometry: the 2005 ISCD Official Positions. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. Jan-Mar 2006;9(1):31–6. Epub 2006/05/30. [DOI] [PubMed] [Google Scholar]
- 33.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. The Journal of clinical endocrinology and metabolism. October 2011;96(10):3160–9. Epub 2011/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly T. Bone mineral density reference databases for American men and women. Journal of Bone and Mineral Research. 1990;5:S249. [Google Scholar]
- 35.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1998;8(5):468–89. Epub 1998/12/16. [DOI] [PubMed] [Google Scholar]
- 36.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Proximal femur bone mineral levels of US adults. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1995;5(5):389–409. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 37.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PloS one. September 15 2009;4(9):e7038. Epub 2009/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. The Journal of clinical endocrinology and metabolism. December 2005;90(12):6508–15. Epub 2005/09/29. [DOI] [PubMed] [Google Scholar]
- 39.Pistoia W, van Rietbergen B, Laib A, Ruegsegger P. High-resolution three-dimensional-pQCT images can be an adequate basis for in-vivo microFE analysis of bone. J Biomech Eng. April 2001;123(2):176–83. Epub 2001/05/09. [DOI] [PubMed] [Google Scholar]
- 40.Trials. NRCPoHMDiC. The Prevention and Treatment of Missing Data in Clinical Trials. Washington (DC): National Academies Press (US); 2010. [PubMed] [Google Scholar]
- 41.KJ R. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. . [PubMed] [Google Scholar]
- 42.McClung MR, Miller PD, Brown JP, Zanchetta J, Bolognese MA, Benhamou CL, et al. Efficacy and safety of a novel delayed-release risedronate 35 mg once-a-week tablet. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. January 2012;23(1):267–76. Epub 2011/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. August 11 2005;353(6):555–65. Epub 2005/08/12. [DOI] [PubMed] [Google Scholar]
- 44.Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. September 19 2015;386(9999):1147–55. Epub 2015/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkelstein JS, Cleary RL, Butler JP, Antonelli R, Mitlak BH, Deraska DJ, et al. A comparison of lateral versus anterior-posterior spine dual energy x-ray absorptiometry for the diagnosis of osteopenia. The Journal of clinical endocrinology and metabolism. March 1994;78(3):724–30. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 46.Halmi KA, Agras WS, Crow S, Mitchell J, Wilson GT, Bryson SW, et al. Predictors of treatment acceptance and completion in anorexia nervosa: implications for future study designs. Arch Gen Psychiatry. July 2005;62(7):776–81. Epub 2005/07/06. [DOI] [PubMed] [Google Scholar]
- 47.Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, et al. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. April 2014;99(4):1322–9. Epub 2014/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillberg P, Mallmin H, Petren-Mallmin M, Ljunghall S, Nilsson AG. Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. The Journal of clinical endocrinology and metabolism. November 2002;87(11):4900–6. Epub 2002/11/05. [DOI] [PubMed] [Google Scholar]
- 49.Saaf M, Hilding A, Thoren M, Troell S, Hall K. Growth hormone treatment of osteoporotic postmenopausal women - a one-year placebo-controlled study. European journal of endocrinology. May 1999;140(5):390–9. Epub 1999/05/07. [DOI] [PubMed] [Google Scholar]
- 50.Landin-Wilhelmsen K, Nilsson A, Bosaeus I, Bengtsson BA. Growth hormone increases bone mineral content in postmenopausal osteoporosis: a randomized placebo-controlled trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. March 2003;18(3):393–405. Epub 2003/03/07. [DOI] [PubMed] [Google Scholar]