Abstract
Objectives:
The creation and maintenance of durable hemodialysis access is critically important for reducing patient morbidity and controlling overall costs within health systems. Our objective was to quantify the costs associated with hemodialysis access creation and its maintenance over time within a rate-controlled health system where charges equate to payments.
Methods:
The Maryland Health Services Cost Review Commission administrative claims database was used to identify patients who underwent first-time access creation from 2012–2020. Patients were identified using CPT codes for access creation, and costs were accrued for the initial encounter and all subsequent outpatient access-related encounters. T-tests and Wilcoxon tests were used to compare reinterventions and access-related costs ($USD) between arteriovenous fistulae (AVF) and arteriovenous grafts (AVG). Multivariable modeling was used to quantify the association of access type with charge variation.
Results:
Overall, 12,716 patients underwent first-time access creation (69.3% AVF vs. 30.7% AVG). There was no difference in freedom from reintervention between the two access types at any point following creation (HR: 1.03, 95%CI: 0.97–1.10); however, AVF were associated with a lower number of cumulative reinterventions (1.50 vs. 2.24) compared to AVG (P<0.0001). AVF was associated with lower overall costs in the year of creation ($9,388 vs. $13,539, P<0.0001), a difference that remained significant over the subsequent 3 years. The lower costs associated with AVF were present both in the costs associated with creation and subsequent maintenance. On multivariable analysis, AVF was associated with a $3,557 reduction in total access-related costs versus AVG (95%CI -$3828, -3287).
Conclusion:
AVF require fewer interventions and are associated with lower costs at placement and over the first three years of maintenance compared to AVG. The use of AVF for first-time hemodialysis access represents an opportunity for healthcare savings in appropriately selected patients with a high preoperative likelihood of AVF maturation.
Introduction
As the number of patients with end stage kidney disease (ESKD) in the United States increases, establishing durable hemodialysis access for this patient population has become a focal point for reducing patient morbidity and controlling overall costs within health systems1–3. From 2010 to 2018, the number of hemodialysis access maintenance procedures billed to Medicare grew by 25%4. Despite the increasing burden of hemodialysis access on the healthcare system, the individual costs associated with the creation and subsequent upkeep of permanent hemodialysis access are complex and have proved difficult to quantify.
While implementation of the Fistula First Breakthrough Initiative (FFBI) increased the proportion of autogenous access among hemodialysis patients5, some experts have pointed out that arteriovenous fistulae (AVF) are not always the best option for all patients from a cost or outcomes standpoint6, particularly if multiple reinterventions are required7. Current guidelines recommend autogenous access when feasible due to its superior patency8, but some sources suggest that select subpopulations would benefit from an individualized approach to access selection when considering cost6, particularly with respect to ESKD patients of advanced age9.
As part of the Medicare’s Quality Payment Program (QPP) and Merit Based Incentive Payments System (MIPS) the Centers for Medicare and Medicaid Services have begun to implement episode-based cost measures, establishing pre-determined thresholds for certain procedures and their associated postoperative care10,11. These set payments are designed to promote high-value care12. Episode-based cost measures have previously been demonstrated to reduce overall procedural costs when studied in the context of endovascular aortic repair13, and it is postulated that similar measures will soon be developed for commercial payer populations14. In order to establish effective and realistic reimbursement thresholds for care episodes, granular cost data is needed for a number of procedures, including the creation of hemodialysis access15.
The aim of our study was to quantify the costs associated with first time hemodialysis access creation and its maintenance over time within a rate-controlled health system where charges equate to payments. We also aimed to compare costs and maintenance interventions for AVF versus arteriovenous grafts (AVG).
Methods
Study Design
This is a retrospective cohort study utilizing data from the Maryland Health Services Cost Review Commission (HSCRC) database16. The HSCRC is an administrative claims database unique to the state of Maryland that captures data from all healthcare delivery settings in the state, capturing more than 6 million patient encounters annually. This database encompasses both the inpatient and outpatient setting, including procedures performed in acute care hospitals, ambulatory surgery centers, and office-based laboratories. For each encounter, payer source and total charges associated with the encounter are recorded. Since 1977, Maryland has implemented a unique all-payer model in which the rates for procedures are pre-determined by the state regardless of whether the payer is Medicare or a third party17,18. The standardization established by this system reduces disparities between charges rendered and eventual cost to the payer18. Because charges equal payments in Maryland, the charges in the HSCRC database equate to costs,
The primary study cohort was defined by identifying all patients who underwent first time hemodialysis access creation captured in the HSCRC between January 2012 and June 2020. For the purposes of this study, encounters were limited to outpatient visits only as the majority of hemodialysis access creation and interventions occur in the outpatient setting. While some creation and revision of access does take place on an inpatient basis, these generally occur as ancillary procedures in the setting of acute illness, making it challenging to isolate the costs only related to hemodialysis access creation and maintenance using administrative codes. Patients were excluded if they had prior permanent hemodialysis access creation, were lost to follow up immediately following the operation (i.e., did not follow up within 90 days), or died/received a kidney transplant within 90 days of their access creation.
Current procedural terminology (CPT) codes were used to identify procedures associated with creation of permanent hemodialysis access, including both AVF and AVG. Maintenance of hemodialysis access was defined as all invasive procedures and surveillance imaging studies related to the AVG or AVF. Relevant outpatient encounters were identified with CPT codes and all costs associated with the relevant encounter were included in the accrual of total costs (Supplemental Table 1). Surgical and endovascular interventions were defined separately. Invasive diagnostic procedures (i.e., fistulograms) without an associated endovascular intervention were classified as surveillance imaging rather than an intervention. Comorbidities were identified using the International Classification of Diseases (ICD) Version 9 and 10 codes. The Institutional Review Board of the Johns Hopkins University School of Medicine approved this study and waived informed consent requirements given that this was a retrospective analysis of a de-identified data source.
Study Outcomes
The primary outcome of this study was total costs ($USD) associated with outpatient first-time hemodialysis access creation and its subsequent maintenance, including endovascular and open surgical revision. Secondary outcomes were time to first reintervention (days), average number of reinterventions (both surgical and endovascular), and duration of usable access following creation (days). The latter outcome was defined as the number of days from initial creation to creation of a second hemodialysis access or placement of a hemodialysis catheter, both of which were classified as failure. Kidney transplant, death or loss to follow up (defined as 90 days with no billing activity of any kind recorded in HSCRC) were defined as censoring criteria. All costs were adjusted for inflation using the 2020 Consumer Price Indices from the U.S. Bureau of Labor Statistics19.
Statistical Analysis
We described the study cohort, comorbidities, and payer data using descriptive statistics. For the primary analysis, mean costs were calculated overall and compared by access type using univariate analysis (Student’s t and Mann-Whitney tests). Multivariable logistic regression was performed to examine factors associated with variation in total costs, both overall and stratified by access type. All variables with P<0.01 on univariate analysis were included in the multivariable model. We also performed a sensitivity analysis for the primary outcome among adults ≥80 years of age.
For the secondary analysis, Kaplan Meier survival curves and Cox proportional hazard models stratified by access type were generated to examine freedom from reintervention and duration of usable access over time. All statistical analyses were performed using Stata Version 14.1 (StataCorp LP, College Station TX). P-values were reported as statistically significant at a level of α≤0.05.
Results
Study cohort
Overall, 12,716 patients undergoing first time hemodialysis access creation met criteria for inclusion (Table 1). Of these, 8,818 (69.3%) received an AVF and 3,898 (30.7%) received an AVG. AVG (vs. AVF) use decreased from 32.5% at the start of the study period to 26.4% at the conclusion of the study period. Patients receiving AVG were older, more frequently female (50.9% vs. 39.9%), non-white (72.5% vs. 60.3%) and had Medicare insurance (68.0% vs 60.1%) compared to patients with AVF (all P<0.0001). There were some statistically different but small magnitude (<2.5%) differences with respect to medical comorbidities between groups, including hypertension and diabetes (Table 1). A larger percentage of AVG patients had ESKD at the time of access creation (86.4% vs. 83.9%, P<0.0001).
Table 1.
Demographics and comorbidities of patients undergoing first-time hemodialysis access creation by access type; AVF, arteriovenous fistula; AVG, arteriovenous graft; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ESKD, end stage kidney disease
| Total | AVF | AVG | P value | |
|---|---|---|---|---|
| n | 12716 | 8818 | 3898 | |
| Age | ||||
| <40 | 900 | 673 (8.0%) | 227 (6.1%) | <0.0001 |
| 40–64 | 4966 | 3515 (41.7%) | 1451 (38.8%) | |
| 65–79 | 4780 | 3273 (38.8%) | 1507 (40.3%) | |
| ≥80 | 1528 | 976 (11.6%) | 552 (14.8%) | |
| Sex | ||||
| Male | 7210 | 5295 (60.1%) | 1915 (49.1%) | <0.0001 |
| Female | 5505 | 3522 (39.9%) | 1983 (50.9%) | |
| Race | ||||
| White | 4570 | 3497 (39.6%) | 1073 (27.5%) | <0.0001 |
| Black | 6763 | 4319 (50.0%) | 2444 (62.7%) | |
| Other | 1383 | 1002 (11.4%) | 381 (9.8%) | |
| Payer | ||||
| Medicare | 7955 | 6304 (60.1%) | 2651 (68.0%) | <0.0001 |
| Medicaid | 1602 | 1134 (12.9%) | 468 (12.0%) | |
| Commercial | 2833 | 2165 (24.6%) | 668 (17.1%) | |
| Self-pay | 92 | 70 (0.8%) | 22 (0.6%) | |
| Other | 234 | 145 (0.2%) | 89 (2.3%) | |
| Comorbidities | ||||
| Hypertension | 11093 | 7741 (87.9%) | 3352 (86.0%) | 0.005 |
| Diabetes mellitus | 6774 | 4767 (54.1%) | 2007(51.5%) | 0.007 |
| CAD | 2955 | 2046 (23.2%) | 909 (23.3%) | 0.885 |
| CHF | 2335 | 1603 (18.2%) | 732 (18.8%) | 0.420 |
| COPD | 1239 | 836 (9.5%) | 403 (10.3%) | 0.133 |
| ESKD | 10760 | 7394 (83.9%) | 3366 (86.4%) | <0.0001 |
Reinterventions
The mean length of follow-up for the cohort overall was 653 days (95% CI 657–660) (Table 2). Patients with an AVG had a significantly shorter mean time to first reintervention when compared with AVF patients (80 days vs. 181 days, P<0.001) and required a greater number of total reinterventions during the access lifetime (2.24 vs 1.50 reinterventions, P<0.001). The difference in number of reinterventions was significantly higher for AVG vs. AVF for both the year of creation (4.08 vs. 2.52) as well as the subsequent 5 years (Table 2; P<0.01). The overall difference in number of cumulative reinterventions for AVG vs. AVF was significant when stratified by surgical (0.57 vs. 0.34) and endovascular reinterventions (1.95 vs. 1.23; both P<0.0001). The interventions billed in the first year were most frequently angioplasty (14% AVG vs. 11% AVF) and surgical thrombectomy (5% AVG vs 1% AVF).
Table 2.
Outcomes of first-time hemodialysis access creation by access type; AVF, arteriovenous fistula;AVG, arteriovenous graft
| Total | 95% CI | AVF | 95% CI | AVG | 95% CI | P value | |
|---|---|---|---|---|---|---|---|
| n | 12716 | 8818 | 3898 | ||||
| Mean length of follow-up (days) | 653 | 657–660 | 637 | 628–645 | 684 | 673–695 | <0.0001 |
| Average time to first reintervention (days) | 148 | 136–160 | 181 | 167–195 | 80 | 58–102 | <0.0001 |
| Average number of surgical reinterventions | 0.42 | 0.41–0.43 | 0.34 | 0.33–0.35 | 0.57 | 0.55–0.59 | <0.0001 |
| Average number of endovascular reinterventions | 1.48 | 1.46–1.51 | 1.23 | 1.20–1.25 | 1.95 | 1.90–2.00 | <0.0001 |
| Average number of reinterventions overall | 1.76 | 1.74–1.79 | 1.50 | 1.47–1.53 | 2.24 | 2.18–2.30 | <0.0001 |
| Year 0 | 3.00 | 2.84–3.15 | 2.52 | 2.38–2.67 | 4.08 | 3.70–4.45 | <0.0001 |
| Year 1 | 1.81 | 1.62–1.99 | 1.41 | 1.23–1.58 | 2.65 | 2.23–3.07 | <0.0001 |
| Year 2 | 1.85 | 1.61–2.10 | 1.42 | 1.16–1.67 | 2.82 | 2.26–3.37 | <0.0001 |
| Year 3 | 1.87 | 1.57–2.17 | 1.48 | 1.17–1.79 | 2.81 | 2.11–3.52 | 0.0001 |
| Year 4 | 1.57 | 1.18–1.97 | 1.19 | 0.91–1.48 | 2.50 | 1.34–3.67 | 0.0031 |
| Year 5 | 1.44 | 0.99–1.88 | 1.07 | 0.75–1.39 | 2.37 | 1.02–3.71 | 0.0100 |
| Freedom from reintervention | 0.3647 | ||||||
| Year 1 | 0.69 | 0.70–0.71 | 0.69 | 0.66–0.68 | 0.69 | 0.75–0.77 | |
| Year 2 | 0.62 | 0.60–0.61 | 0.62 | 0.58–0.59 | 0.61 | 0.63–0.65 | |
| Year 3 | 0.58 | 0.54–0.55 | 0.59 | 0.53–0.54 | 0.57 | 0.56–0.57 | |
| Year 4 | 0.55 | 0.49–0.50 | 0.56 | 0.48–0.50 | 0.53 | 0.49–0.52 | |
| Year 5 | 0.52 | 0.45–0.46 | 0.53 | 0.45–0.46 | 0.52 | 0.44–0.47 |
There was no significant difference in the proportion of patients with AVG vs. AVF who remained free from reintervention over time (log-rank P=0.36; Figure 1A). Patients who received an AVG had a longer period of documented usable access compared to patients who received an AVF (mean 412 days vs. 283 days, P<0.001), which was largely attributable to early AVF failure (Figure 1B).
Figure 1.
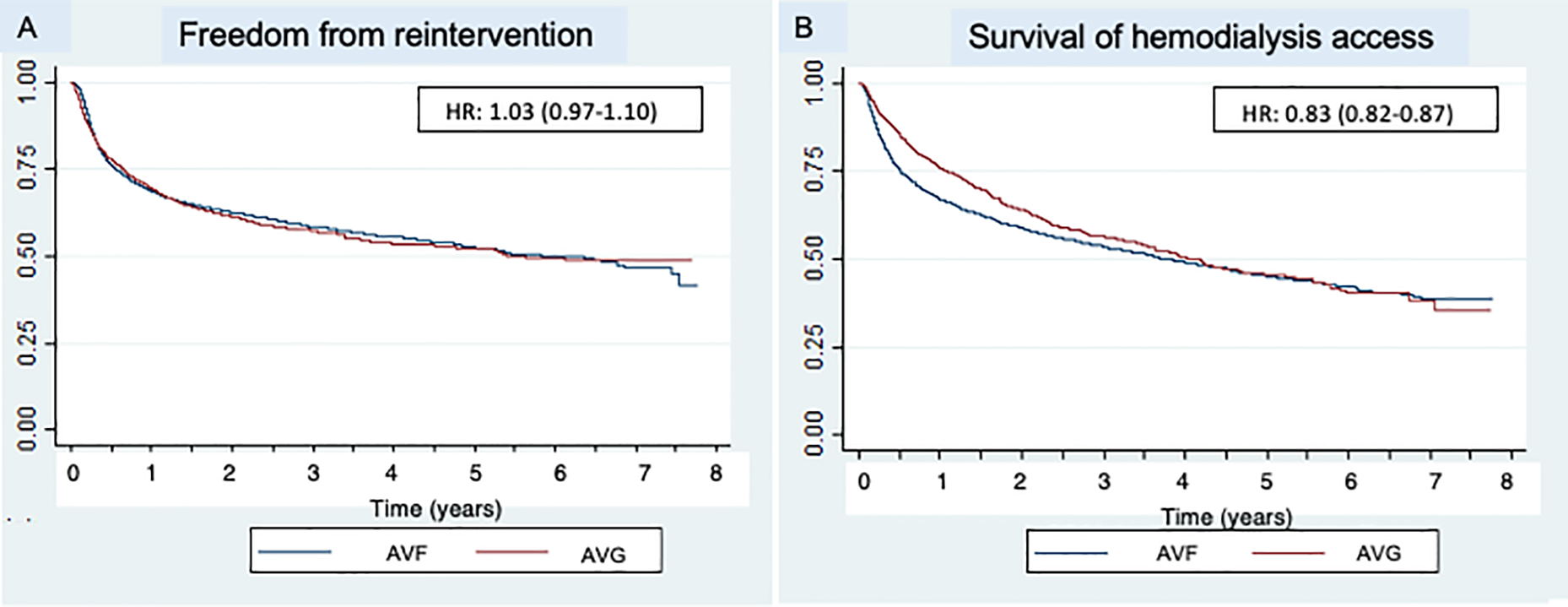
Kaplan Meier curves describing outcomes of first-time hemodialysis access creation stratified by access type, including freedom from reintervention (panel A) and survival of the hemodialysis access (panel B)
Estimated costs associated with AVG vs. AVF
Total costs associated with hemodialysis access creation and maintenance in the initial year were $10,658 (95%CI: $10,510–10,805) (Table 3). Costs were significantly higher for AVG ($13,539, 95%CI: $13,223–13,856) vs. AVF ($9,388, 95% CI: $9,236–9,541) during the year of creation. Costs remained significantly higher for AVG vs. AVF for the subsequent 3 years of follow-up (all, P<0.001), but were not significant at years 4 and 5 (Table 3). AVG was associated with significantly higher costs compared to AVF in all aspects of care during the first year, including initial access creation ($9,213 vs $6,504), overall access maintenance ($4,535 vs. $3,001) and cumulative reintervention costs ($4,118 vs. $2,737) (all P<0.0001; Figure 2). In the first year, the costs associated with AVG vs. AVF reinterventions were significantly higher for both surgical ($1,410 vs. $893) and endovascular reinterventions overall ($3,259 vs. $1,989) (both P<0.0001).
Table 3.
Mean costs associated with first-time hemodialysis access creation by access type, reported in United States dollars ($USD) and adjusted for inflation; AVF, arteriovenous fistula; AVG, arteriovenous graft
| Total | 95% CI | AVF | 95% CI | AVG | 95% CI | P value | |
|---|---|---|---|---|---|---|---|
| Total costs ($USD) | |||||||
| Year 0 | 10,658 | 10,510–10,805 | 9,388 | 9,236–9,541 | 13,539 | 13,223–13,856 | <0.0001 |
| Year 1 | 2,019 | 1,873–2,165 | 1,603 | 1,463–1,743 | 2,894 | 2,554–3.234 | <0.0001 |
| Year 2 | 1,685 | 1,517–1,854 | 1,321 | 1,152–1,491 | 2,487 | 2,101–2,874 | <0.0001 |
| Year 3 | 1,547 | 1,340–1,755 | 1,244 | 1,036–1,452 | 2,2 0 | 1,7 7–2,763 | <0.0001 |
| Year 4 | 1,261 | 1,039–1,482 | 1,144 | 902–1,387 | 1,5 49 | 1,068–2,030 | 0.1051 |
| Year 5 | 1,329 | 1,036–1,623 | 1,206 | 878–1,534 | 1,643 | 1,022–2,263 | 0.1892 |
| Mean costs for creation | 7,332 | 7271–7393 | 6,504 | 6,443–6,564 | 9,213 | 9,088–9,337 | <0.0001 |
| Mean costs for maintenance | |||||||
| Year 0 | 3,470 | 3,345–3,596 | 3,001 | 2,870–3,132 | 4,535 | 4,256–4,815 | <0.0001 |
| Year 1 | 2,019 | 1,873–2,165 | 1,603 | 1,463–1,743 | 2,894 | 2,554–3,234 | <0.0001 |
| Year 2 | 1,685 | 1.517–1,854 | 1,321 | 1,152–1,491 | 2,487 | 2,101–2,874 | <0.0001 |
| Year 3 | 1,547 | 1,340–1,755 | 1,244 | 1,036–1,452 | 2,270 | 1,777–2,763 | <0.0001 |
| Year 4 | 1,261 | 1,039–1,482 | 1,144 | 902–1,387 | 1,549 | 1,068–2,030 | 0.1051 |
| Year 5 | 1,329 | 1,036–1,623 | 1,206 | 878–1,534 | 1,643 | 1,022–2,263 | 0.1892 |
| Mean costs for any reintervention | |||||||
| Year 0 | 3,159 | 3,040–3.279 | 2,737 | 2,612–2,863 | 4,118 | 3,852–4,384 | <0.0001 |
| Year 1 | 1,877 | 1,735–2,018 | 1,488 | 1,353–1,623 | 2,695 | 2,362–3,028 | <0.0001 |
| Year 2 | 1,591 | 1,428–1,755 | 1,249 | 1,086–1,412 | 2,344 | 1.966–2,722 | <0.0001 |
| Year 3 | 1,449 | 1,247–1,650 | 1,160 | 957–1,363 | 2,136 | 1,658–2,615 | <0.0001 |
| Year 4 | 1,151 | 948–1,355 | 1,040 | 824–1,256 | 1,428 | 965–1,890 | 0.0907 |
| Year 5 | 1,273 | 983–1,564 | 1,148 | 826–1,471 | 1,591 | 970–2,211 | 0.1793 |
| Mean costs for surgical reinterventions | |||||||
| Year 0 | 1,051 | 988–1,115 | 893 | 831–955 | 1,410 | 1,258–1,562 | <0.0001 |
| Year 1 | 500 | 421–579 | 351 | 289–414 | 812 | 605–1,019 | <0.0001 |
| Year 2 | 447 | 360–534 | 332 | 239–425 | 701 | 512–890 | 0.0001 |
| Year 3 | 438 | 336–539 | 347 | 246–449 | 653 | 410–897 | 0.007 |
| Year 4 | 414 | 292–537 | 384 | 252–517 | 489 | 219–758 | 0.4485 |
| Year 5 | 479 | 327–631 | 452 | 274–630 | 549 | 257–841 | 0.5728 |
| Mean costs for endovascular reinterventions | |||||||
| Year 0 | 2,377 | 2,273–2,481 | 1,989 | 1,881–2,097 | 3,259 | 3,025–3,492 | <0.0001 |
| Year 1 | 1,560 | 1,434–1,685 | 1,220 | 1,099–1,340 | 2,275 | 1,982–2,568 | <0.0001 |
| Year 2 | 1,342 | 1,195–1,490 | 1,031 | 889–1,173 | 2,028 | 1,676–2,381 | <0.0001 |
| Year 3 | 1,143 | 970–1,315 | 887 | 720–1,054 | 1,753 | 1,329–2,177 | <0.0001 |
| Year 4 | 826 | 664–987 | 713 | 546–880 | 1,104 | 725–1,482 | 0.0317 |
| Year 5 | 843 | 596–1,088 | 756 | 480–1,033 | 1,059 | 544–1,575 | 0.0001 |
Figure 2.
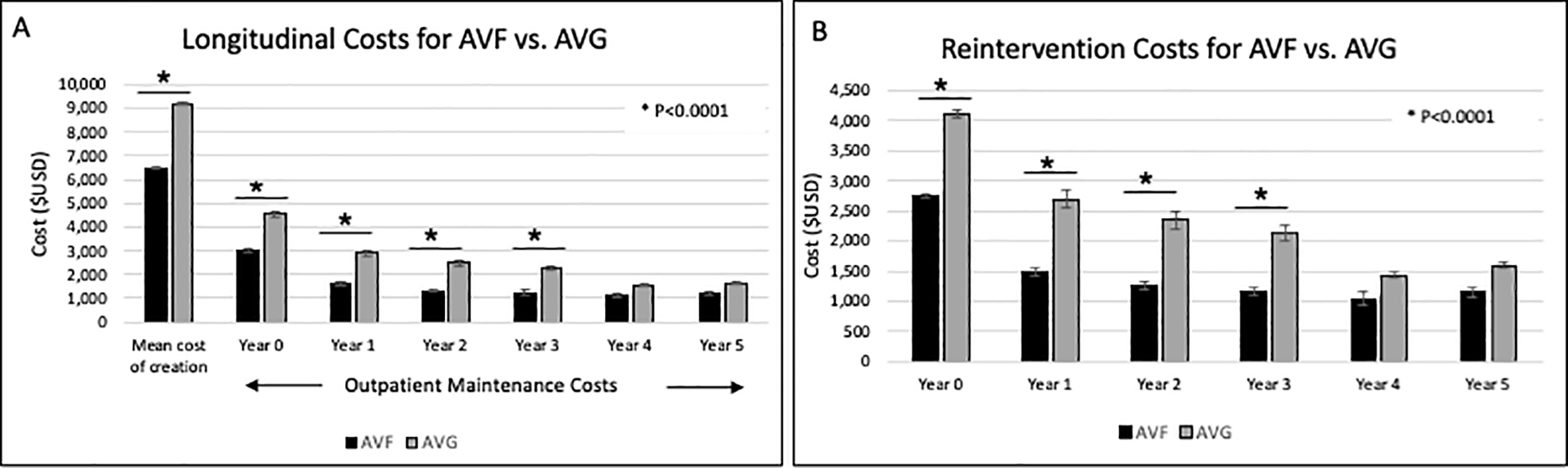
Bar graphs depicting longitudinal trends of first-time hemodialysis access creation stratified by access type, including overall costs associated with access creation and maintenance (panel A) and combined surgical and endovascular reintervention costs (panel B); all costs expressed in $USD and adjusted for inflation
After adjusting for patient demographics and comorbidities, the risk-adjusted lifetime costs associated with initial hemodialysis access creation and maintenance varied significantly by access type (Table 4); AVF was associated with a -$3,557 (95% CI -$3,287, -3,828) reduction in overall costs compared with AVG. In a sensitivity analysis limited to patients ≥80 years of age, AVF was persistently associated with lower overall costs compared to AVG (adjusted mean difference -$2,373, 95%CI -$2,969, -$1,777).
Table 4.
Risk-adjusted mean lifetime costs for first-time hemodialysis access creation & maintenance; AVF, arteriovenous fistula; AVG, arteriovenous graft; $USD, United States dollars, adjusted for inflation
| Predictors | All | ≥80 years of age | ||
|---|---|---|---|---|
| Adjusted mean cost difference, $USD | 95% CI | Adjusted mean cost difference, $USD | 95% CI | |
| AVF (vs. AVG) | −3557 | −3828, −3287 | −2373 | −2969,−1777 |
| Age | ||||
| <40 | Ref | - | - | - |
| 40–64 | 34 | −459–527 | - | - |
| 65–79 | −568 | −1099,−36 | - | - |
| ≥80 | −1110 | −1723,−498 | - | - |
| Female sex (vs. male) | 458 | 208–708 | 61 | −116–736 |
| Race | ||||
| White | Ref | - | Ref | - |
| Black | 1036 | 285–1153 | 1174 | 549–1798 |
| Other | 37 | −394–468 | 576 | −396–1548 |
| Payer | ||||
| Medicare | Ref | - | Ref | - |
| Medicaid | 719 | 285–1153 | −338 | −4780–4104 |
| Commercial | −865 | −1204,−525 | −816 | −2,236–603 |
| Self-pay | −665 | −2022–690 | −75 | −4509–4358 |
| Other | 1111 | 208–2014 | 2710 | −215–5635 |
| Hypertension (vs. none) | −1017 | −1346,−688 | −1180 | −1940,−419 |
| Diabetes mellitus (vs. none) | −8 | −281–265 | 268 | −344–882 |
| ESKD (vs. none) | 304 | −5–614 | 489 | −204–1183 |
Discussion
Due to wide variation in the ESKD patient population and the healthcare system at large, defining the most cost-effective access option for patients requiring hemodialysis in the United States has proved challenging. While AVF have long been regarded as the superior choice for hemodialysis access when anatomically feasible8, the economic advantages of this strategy have been called into question in some subpopulations with worse AVF outcomes and shorter lifespans6,9. In this work, we define costs associated with outpatient creation and maintenance of a first-time hemodialysis circuit stratified by access type in an effort to define a realistic cost threshold for episode-based hemodialysis access care. Overall, these data demonstrate that AVG are more costly to place and to maintain than AVF, a result that remains significant on multivariable analysis and within an elderly (≥80 years of age) subgroup. These results suggest that in appropriately selected patients, AVF represent an opportunity for healthcare savings due to decreased costs associated with creation and maintenance over time.
With respect to episode-based costs, we found in this study that, in the first year, the mean cost of permanent first-time hemodialysis access placement and the subsequent outpatient maintenance was $10,658. We found that this varied significantly by access type, with AVF associated with decreased overall costs compared with AVG. Previous research in this regard has been mixed, but largely supports our findings20–23. Two large retrospective examinations of Medicare patients demonstrated that AVF was associated with significantly reduced access-related costs when compared with AVG ($16,864 vs $20,961)20 and ($14,444 vs. $16,111)23. One study utilizing 2014 data did report outpatient only access-related claims and demonstrated a difference of -$270 per dialysis month in favor of AVF, which is comparable to our results when averaged over the course of a year ($3,240 vs. $4,151)23. In contrast to these prior attempts to quantify hemodialysis access-related costs, the present results are unique for two main reasons. First, the majority of the charge data included in the prior studies is not within the last five years, with the most recent data included from 201420–23. The present study captures data up to and including the year 2020, incorporating the implementation of bundled CPT codes for hemodialysis maintenance procedures in 201724 that significantly reduced access related payments4. Due to the unique Maryland rate-controlled system, our study is also able to incorporate non-Medicare patients and control potential confounding between amounts charged to the payer versus the eventual cost paid. This enhances the generalizability of our results overall and their relevance as a comparison point for episode-based cost setting moving forward12.
While the costs associated with AVF creation and maintenance in this study are less than those associated with AVG creation at all phases of care, the costs associated with failed AVF should not be overlooked. When examining overall patency of the two access types, AVG tended to have a longer duration of usable access when compared with AVF (Figure 1B). While it is impossible to distinguish which AVF failed to mature and which AVF failed after maturation on the basis of administrative coding, these findings likely represent the subset of AVF which failed to mature, shifting the overall mean duration of access usability to a lower value. Previous research examining the costs associated with hemodialysis access have demonstrated AVF failure to mature as a significant driver of access-related costs25. Our findings emphasize the importance of individualized decision making for patients undergoing hemodialysis access creation and identifying those at risk for maturational AVF failure, as this subset of patients does not fully realize the benefits of autogenous access from a cost or quality of life perspective21. In our cohort only 7.4% of patients overall underwent formal preoperative vein mapping, which may represent an opportunity for improvement to identify patients with anatomy amenable to an AVF26.
Despite the risk of AVF maturation failure, previous work suggests that once AVF are usable, they require fewer interventions to maintain functionality27. Our results support this notion; while Kaplan Meier analysis did not demonstrate a difference in freedom from reintervention between the two access types (Figure 1A), the number of interventions required to maintain the access was significantly higher for AVG when compared to AVF in the year of creation and the subsequent 5 years of use, translating into persistently higher costs for AVG (Figure 2). Cost-effectiveness analyses have demonstrated that while repeated interventions to salvage existing access are costly, they tend to be less costly than creation of new hemodialysis access7. The cost of repeated interventions should be kept in mind when considering AVF vs. AVG creation in patients who may be a candidate for both. Reintervention costs should also be considered and balanced with cost of new hemodialysis access creation when making the decision to abandon questionably functioning existing access.
The optimal access type in patients ≥80 years of age has been an area of controversy from a healthcare utilization standpoint. While AVF in the elderly have been shown to require more interventions to become functional, a longer period of catheter dependence compared with AVG, and a lower maturation rate compared with the general population29, AVF require fewer interventions once the access has been used successfully29. Our results demonstrate that AVF are associated with reduced costs (-$2,373,) compared with AVG in a subgroup analysis of ≥80 year-old patients. Our findings support those of previous studies, which have demonstrated that, despite challenges associated with fistula maturation in the elderly population, AVF represent a cost-effective option for most adults with amenable anatomy and a life expectancy >2 years9.
It is important to note that the current analysis focuses on defining the costs of creating and maintaining first-time permanent dialysis access for the purpose of defining standard episode-based care costs. Our study was designed to inform physicians about cost considerations of an isolated episode of hemodialysis access creation, particularly as guidelines have moved away from a fistula-first focus toward a more patient-centered approach30,31. We do not quantify total costs associated with creating and maintaining permanent dialysis access over the lifetime of a dialysis patient, all of which are important considerations when selecting appropriate access type for individual patients. Future studies quantifying the lifetime costs of maintaining permanent hemodialysis access will be important to contextualize our findings moving forward.
Limitations of this study are those inherent to its retrospective nature as well as the use of an administrative database, including the possibility of coding inaccuracy and the inability to distinguish AVF that fail to mature from those that fail after maturation. Hemodialysis access maintenance performed on an inpatient basis was not included in this analysis due to challenges with separating hemodialysis access costs with possibly unrelated medical costs; however, inpatient dialysis creation and maintenance also represents a critically ill subpopulation of ESKD patients and does not necessarily represent expected costs associated with standard hemodialysis access creation or maintenance. It should also be noted that some planned procedures, such as second stage superficialization of a basilic vein transposition, do not have unique billing codes and likely represent a small subset of the revisions recorded here. Our results are also restricted to first-time hemodialysis access patients and may not apply to the uniquely challenging situation of patients requiring redo access30. Strengths of this work include its large sample size, contemporary administrative data source, capture of a variety of outpatient procedural care settings, and use of longitudinal charge data. Use of the Maryland HSCRC database minimizes discrepancy between charges rendered and costs paid and captures claims billed to all payers, increasing the capture of eventual Medicare patients who may have claims billed to private insurance prior to initiating dialysis.
Conclusions
In this study, the mean costs associated with permanent first-time hemodialysis access are $10,658 in the first year. These costs vary significantly by access type, with AVF associated with significantly decreased costs in the year of creation as well as in the first three years of maintenance when compared with AVG. The lower costs associated with AVF compared to AVG is largely attributable to the increased number of interventions required to maintain an AVG, generating a significant difference in risk-adjusted lifetime costs by access type. The difference in costs for AVF compared to AVG remained significant for patients 80 years of age and older, suggesting that AVF creation may represent an opportunity for savings in patients with a high probability of AVF maturation.
Supplementary Material
Supplemental Table 1. CPT codes for hemodialysis access creation, maintenance and surveillance
Funding:
Dr. Hicks is supported by a grant from the NIH/NIDDK (1K23DK124515).
Footnotes
Author conflicts of interest: The authors declare that they have no conflicts of interest or relevant financial relationships to disclose.
Presented virtually as an oral presentation at the 2021 Vascular and Endovascular Surgical Society Winter Annual Meeting
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Renal Data System. “End stage renal disease, Ch3: Vascular Access.” USRDS 2020 Annual Data Report. Available at: https://adr.usrds.org/2020. Accessed 1 Feb 2021.
- 2.Erickson KF, Qureschi S, & Winkelmayer WC. “The role of big data in the development and evaluation of US dialysis care.” Am J Kidney Dis. 2018. Oct;72(4):560–8. [DOI] [PubMed] [Google Scholar]
- 3.Amedia CA, Bolton WK, Cordray T, Hakim R, Howard R, Jackson J, et al. “Vascular access for HD: aligning payment with quality.” Semin Dial. Jan-Feb 2011;24(1):37–40. [DOI] [PubMed] [Google Scholar]
- 4.Lindquester WS, Dhangana R, & Warhadpande S. “Bundled Medicare payments: trends in utilization and physician payments for dialysis arteriovenous fistula and graft maintenance procedures from 2010 to 2018.” AJR Am J Roentgenol. 2020. Oct;2015(4):785–9. [DOI] [PubMed] [Google Scholar]
- 5.Lynch JR, Mohan S, & McClellan WM. “Achieving the goal: results from the Fistula First Breakthrough Initiative.” Curr Opin Nephrol Hypertens. 2011. Nov;20(6):583–92. [DOI] [PubMed] [Google Scholar]
- 6.Allon M. “Vascular access for hemodialysis patients: new data should guide decisionmaking.” Clin J Am Soc Nephrol. 2019. Jun;14(6):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooke BS, Griffin CL, Kraiss LW, Kim J, & Nelson R. “Cost-effectiveness of repeated interventions on failing arteriovenous fistulas.” J Vasc Surg. 2019. Nov;70(5):1620–8. [DOI] [PubMed] [Google Scholar]
- 8.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, et al. “KDOQI clinical practice guideline for vascular access: 2019 update.” Am J Kidney Dis. 2020. Apr;75(4):S1–164. [DOI] [PubMed] [Google Scholar]
- 9.Hall RK, Myers ER, Rosas SE, O’Hare AM, & Colon-Emeric CS. “Choice of hemodialysis access in older adults: a cost-effectiveness analysis.” Clin J Am Soc Nephrol. 2017. Jun;12(6):947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quality Payment Program. “2020 MIPS: Summary of Cost Measures.” Available at: https://www.cms.gov/files/document/2020-mips-summary-cost-measures.pdf. Accessed 29 Nov 2020.
- 11.Shireman PK, Woo K & Lipsitz EC. “Hemodialysis access creation episode-based cost measure.” J Vasc Surg. 2019. Apr;69(4):1322. [DOI] [PubMed] [Google Scholar]
- 12.Hudson ME. “Preparing for budget-based payment methodologies: global payment and episode-based payment.” Curr Opin Anaesthesiol. 2015. Oct;28(5):610–4. [DOI] [PubMed] [Google Scholar]
- 13.Itoga NK, Tang N, Patterson D, Ohkuma R, Lew R, Mell MW, et al. “Episode-based cost reduction for endovascular aneurysm repair.” J Vasc Surg. 2019. Jan;69(1):219–225.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddique SM & Mehta SJ. “Holding gastroenterologists accountable for colonoscopy through MACRA episode-based cost measure.” Clin Gastroenterol Hepatol. 2019. May;17(6):1015–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quality Payment Program. “Merit-Based Incentive Payment System (MIPS): Hemodialysis access creation measure.” Available at: https://qpp.cms.gov/docs/cost_specifications/2019-12-17-mif-ebcm-hd-access.pdf. Accessed 30 Nov 2020.
- 16.Maryland Heath Services Cost Review Commission. “Clinical Public Use Data Requests.” Available at: https://hscrc.maryland.gov/Pages/hsp-data-request.aspx. Accessed 23 Nov 2020.
- 17.Centers for Medicare and Medicaid Services. “Innovation Models: Maryland All-Payer Model.” Updated Mar 2020. Available at: https://innovation.cms.gov/innovation-models/maryland-all-payer-model. Accessed 24 Nov 2020.
- 18.Cohen H. “Maryland’s All-Payor Hospital Payment System.” 2005. Available at: https://hscrc.maryland.gov/documents/pdr/GeneralInformation/MarylandAll-PayorHospitalSystem.pdf. Accessed 24 Nov 2020.
- 19.Bureau of Labor Statistics. CPI Inflation Calculator. Updated Mar. 2020. https://www.bls.gov/data/inflation_calculator.htm. Accessed Jul 2020.
- 20.Wagner JK, Fish L, Weisbord SD & Yuo TH. “Hemodialysis access cost comparisons among incident tunneled catheter patients.” J Vasc Access. 2020. May;21(3):308–13. [DOI] [PubMed] [Google Scholar]
- 21.Xue H, Lacson E Jr, Wang W, Curhan GC & Brunelli SM. “Choice of vascular access among incident hemodialysis patients: a decision and cost-utility analysis.” Clin J Am Soc Nephrol. 2010. Dec;5(12):2289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, et al. “Establishment and maintenance of vascular access in incident hemodialysis patients: a prospective cost analysis.” J Am Soc Nephrol. 2005. Jan;16(1):201–9. [DOI] [PubMed] [Google Scholar]
- 23.Nordyke RJ, Reichert H, Bylsma LC, Jackson JJ, Gage SM, Fryzek J, et al. “Costs attributable to arteriovenous fistula and arteriovenous graft placements in hemodialysis patients with Medicare coverage.” Am J Nephrol. 2019;50(4):320–8. [DOI] [PubMed] [Google Scholar]
- 24.Roddy SP & Lerner BM. “New bundled CPT codes for dialysis circuit interventions.” J Vasc Surg. 2017. Jun;65(6):1859–61. [DOI] [PubMed] [Google Scholar]
- 25.Al-Balas A, Lee T, Young CJ, Kepes JA, Barker-Finkel J & Allon M. “The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter.” J Am Soc Nephrol. 2017. Dec;28(12):3679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dageforde LA, Harms KA, Feurer ID & Shaffer D. “Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency.” J Vasc Surg. 2015. Jan;61(1):170–6. [DOI] [PubMed] [Google Scholar]
- 27.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, et al. “Cumulative patency of contemporary fistulas versus grafts (2000–2010).” Clin J Am Soc Nephrol. 2013. May;8(5):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hicks CW, Wang P, Kernodle A, Lum YW, Black JH III, & Makary MA. “Assessment of use of arteriovenous graft vs arteriovenous fistula for first-time permanent hemodialysis access.” JAMA Surg. 2019. Sep;154(9):844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee T, Qian J, Thamer M & Allon M. “Tradeoffs in vascular access selection in elderly patients initiating hemodialysis with a catheter.” Am J Kidney Dis. 2018. Oct;72(4):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Balas A, Lee T, Young CJ & Allon M. “Choice of a second vascular access in hemodialysis patients whose initial arteriovenous fistula failed to mature.” J Vasc Surg. 2018. Dec;68(8):1858–64. [DOI] [PubMed] [Google Scholar]
- 31.Kalloo, Blake PG & Wish. “A patient-centered approach to hemodialysis vascular acess in the era of Fistula First.” Semin Dial. Mar-Apr 2016;29(2):148–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. CPT codes for hemodialysis access creation, maintenance and surveillance


