Abstract
Hypothesis:
The prevalence of monocyte-derived macrophages within cochlear vessels may increase following cochlear implantation.
Background:
Recently, we reported an increase in the number of Iba1-positive macrophages in selected cochlear sites such as the osseous spiral lamina and Rosenthal’s canal following cochlear implantation. Activation of the immune system induces the recruitment of monocyte-derived macrophages. The prevalence of monocyte-derived macrophages within cochlear vessels may increase following cochlear implantation. However, the delivery system of macrophages to the human cochlea is incompletely understood.
Methods:
The prevalence of macrophages and monocytes within cochlear blood vessels in 10 human subjects who had undergone unilateral cochlear implantation was studied by light microscopy using anti-Iba1 immunostaining. The densities of Iba1-positve monocytes per area of lumen of cochlear vessels in the sections near the round window in implanted ears were compared with the contralateral unimplanted ears. The correlation between the densities of Iba1-positve monocytes and the duration (months after the cochlear implantation) was also evaluated.
Results:
The prevalence of Iba1-positive macrophages/monocytes in vessels near the round window in implanted ears (mean 26%, median 21%) was greater than in opposite unimplanted ears (mean 5.2%, median 2.5%: p<0.01). The density of Iba1-positive monocytes in implanted ears (mean 32, median 16 cells/105μm2) tended to be greater than that in unimplanted ears (mean 6.6, median 0.93 cells/105μm2: p=0.08). The density of Iba1-positive monocytes was significantly correlated with duration of implantation but not in the unimplanted ears.
Conclusion:
An increase in prevalence of Iba1-positive macrophages/monocytes within cochlear blood vessels after cochlear implantation was demonstrated. These findings suggest a delivery system of Iba1-positive macrophages through cochlear vessels in human that is ongoing for long duration.
Keywords: Cochlear implant, Human, Macrophage, Monocyte, Iba1, Temporal bone pathology
Introduction
Macrophages contribute to the important role of immune-activity such as repair and fibrosis (1). These macrophages are classified as resident or recruited macrophages based on their origin (2). Resident macrophages are derived from the yolk sac and fetal liver during embryogenesis (3). On the other hand, recruited macrophages are derived from monocytes from the bone marrow (4, 5). In the human inner ear, existence of macrophages was confirmed in normal ears (6). However, there is little evidence describing the process of recruitment of macrophages to the human inner ear.
Recently, we reported an increase in density of Iba1-positive macrophages in human cochleae after cochlear implantation compared to contralateral unimplanted ears (7). Because migration of monocyte-derived macrophages through blood vessels into the cochlea occurs in mice induced by inflammation following acoustic trauma (8), we speculated that bone marrow-derived monocytes may be recruited into the human inner ear during chronic inflammation (9, 10) or a foreign body response (11) secondary to cochlear implantation and may result in an increase of Iba1-positive macrophages. If macrophages are delivered through the blood vessels, we would expect more Iba1-positve macrophages and monocytes within the blood vessels in the cochlear implanted ears than in contralateral unimplanated ears. However, little evidence has been obtained from human temporal bone studies in this respect. In order to clarify our hypothesis, this otopathologic study examined the prevalence of Iba1-positive cells within cochlear blood vessels following cochlear implantation.
Materials and Methods
Selection of Cases
This study was approved by the institutional review board of the Massachusetts Eye and Ear (exemption #4). From the collection of the Otopathology Laboratory of the Massachusetts Eye and Ear, 20 temporal bones from 10 patients who in life had undergone unilateral cochlear implantation were used as our previous study (7, 12). Our criteria for the cases chosen in this study was excellent preservation with few postmortem artifacts. The demographic and clinical data for these 10 cases are shown in Table 1.
Table1.
Demographics of cases studied.
| Case | Age at Death/Sex |
Etiology of Hearing Loss | Cochlear Implant-Ear | Approach | Age at Onset of Deafness |
Age at Implantation |
Duration of Implantation |
|---|---|---|---|---|---|---|---|
| (years) | (years) | (months) | |||||
| 1 | 84/F | Presbycusis | Nucleus 22-R | RWE | 71 | 72 | 151 |
| 2 | 76/M | Unknown | Nucleus 22-L | RWE | 45 | 59 | 210 |
| 3 | 89/M | Unknown | AB Hi Res 90K-R | RWE | 85 | 86 | 33 |
| 4 | 78/M | Unknown/Noise exposure | Nucleus 24-L | RWE | 74 | 74 | 83 |
| 5 | 89/F | Genetic (suspected) | Nucleus 24-L | Cochl | 72 | 79 | 127 |
| 6 | 96/ M | Genetic (suspected)/ Noise exposure | Nucleus Freedom-R | Cochl | 90 | 91 | 61 |
| 7 | 93/F | Unknown | AB Hi Res 90K-L | RWE | 82 | 85 | 110 |
| 8 | 81/ M | Unknown/Noise exposure | AB Hi Res Advantage 1J-R | RWE | 73 | 80 | 12 |
| 9 | 70/M | Unknown/Noise exposure | Nucleus hybrid S8-L | Cochl | 63 | 63 | 75 |
| 10 | 94/F | Genetic (suspected) | AB CII positioner-R | RWE | 82 | 82 | 143 |
| Average | 85 | 74 | 77 | 101 |
F: female; M: male; L: left; R: right; AB: Advanced Bionics; Cochl: cochleostomy; RWE: cochleostomy by round window enlargement approach
Histologic Preparation
Temporal bones were removed after death, fixed in 10% buffered formalin, decalcified in EDTA, dehydrated in graded alcohols, and embedded in celloidin. Serial sections with a thickness of 20 μm were cut in the horizontal (axial) plane. Every tenth section was stained with hematoxylin and eosin (H&E) and mounted on glass slides by a method described by Merchant and Nadol (13).
Immunohistochemistry methods
For immunostaining, a total of 20 sections across the round window from 10 patients were selected. An antibody against ionized calcium-binding adaptor molecule 1 (Iba1), made in rabbit, (Wako chemicals USA, Inc, Richmond, VA) at a dilution of 1:200 to 1:2000 was used to label macrophages in the same manner as previously published (6, 7, 12).
Quantitative assessment of Iba1-positive macrophages/monocytes within cochlear blood vessels
Inner and Middle ear
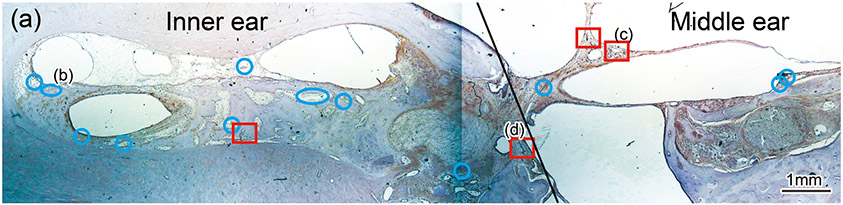
Blood vessels were identified within the sections through the round window (Fig. 1(a)-(m)). In the inner ear of both implanted and unimplanted cases, several vessels are commonly seen within scala tympani and scala vestibuli using light microscopy at 40×. Some vessels were identified within the stria vascularis and spiral ligament.
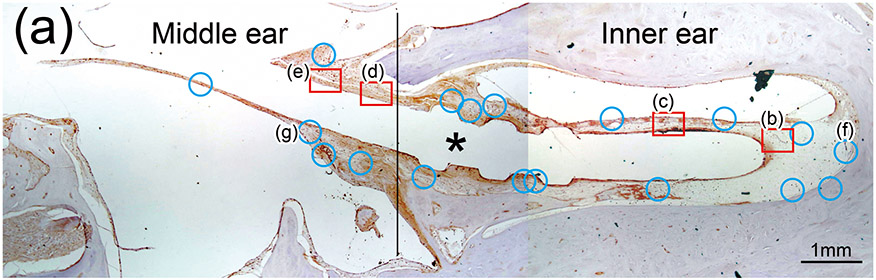
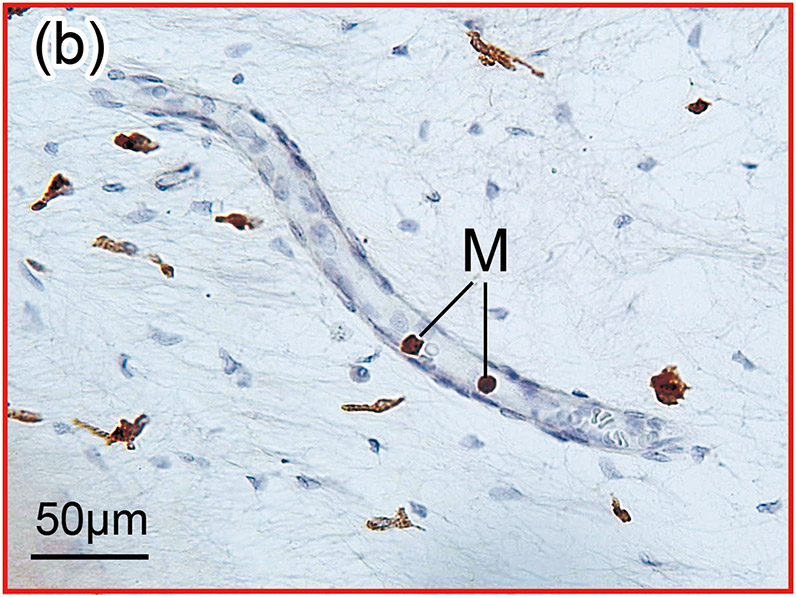
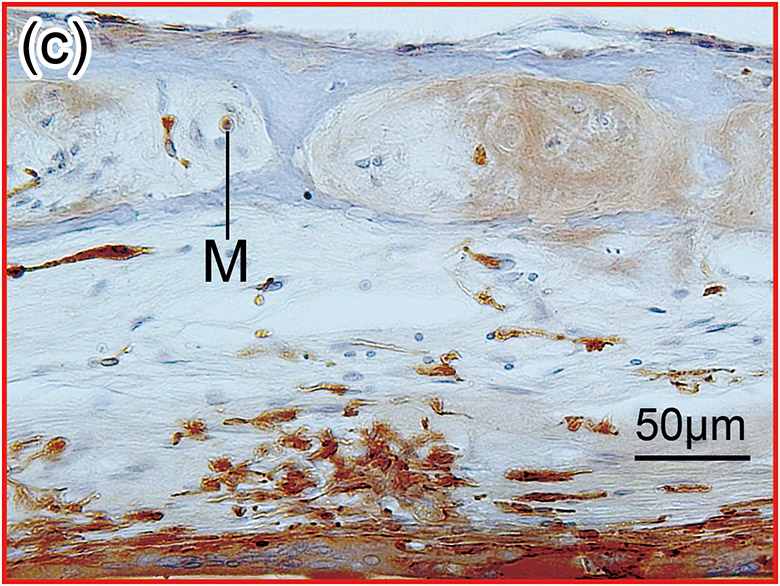
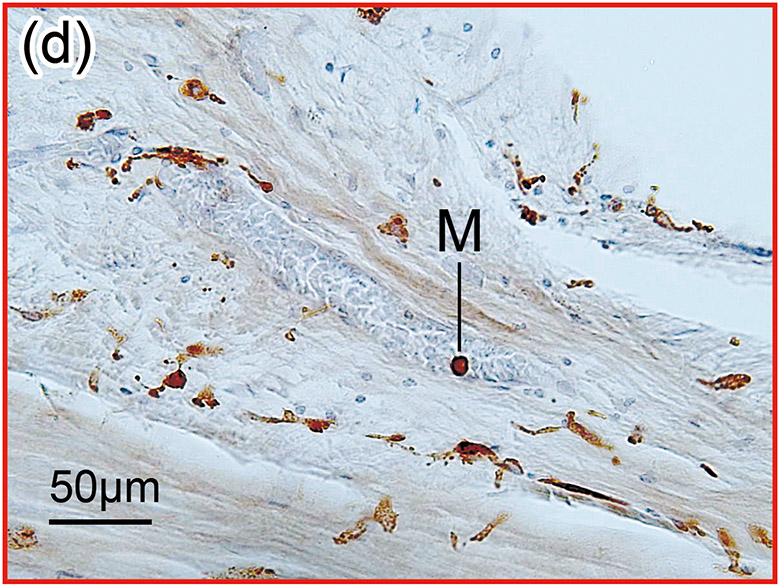
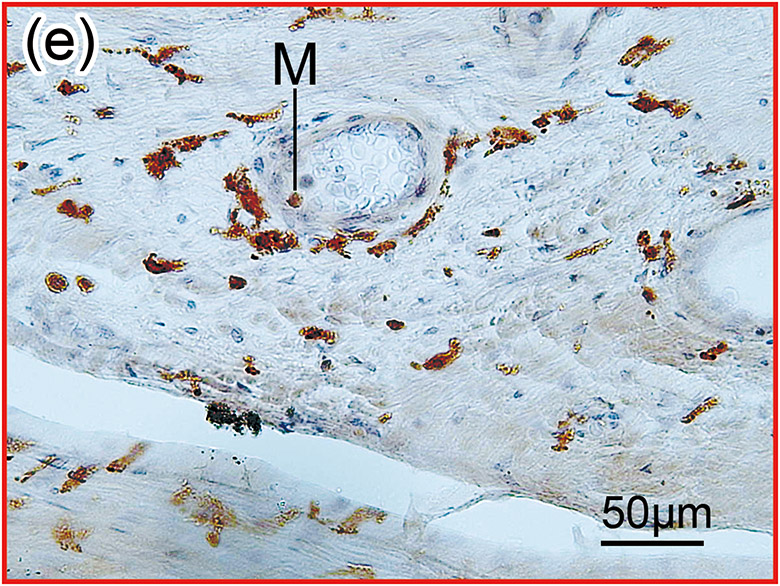
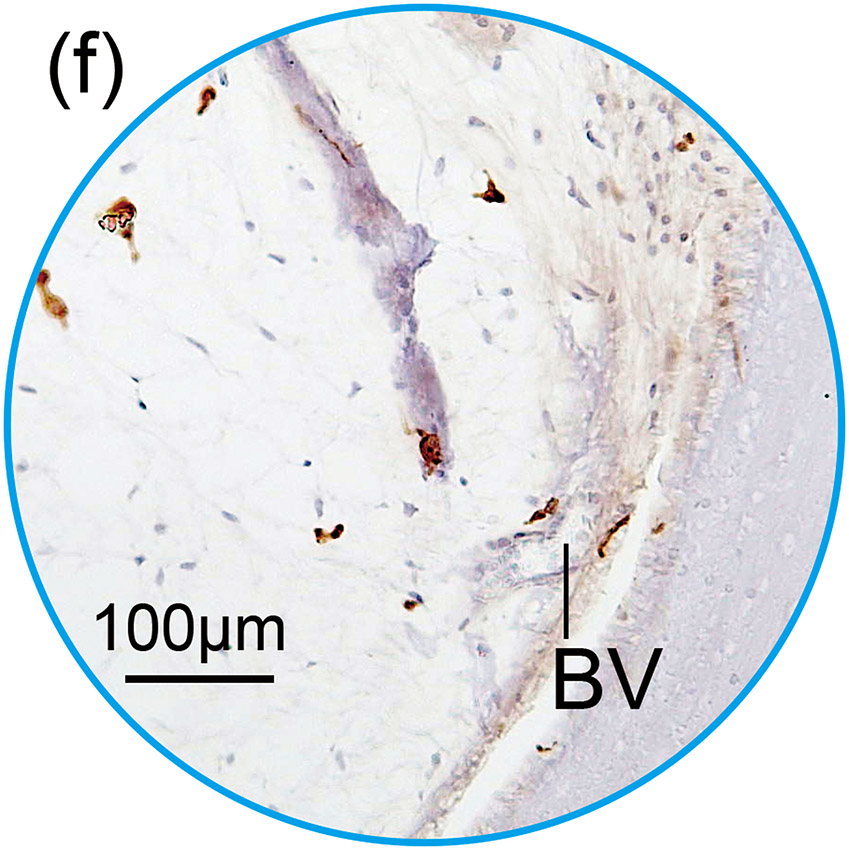
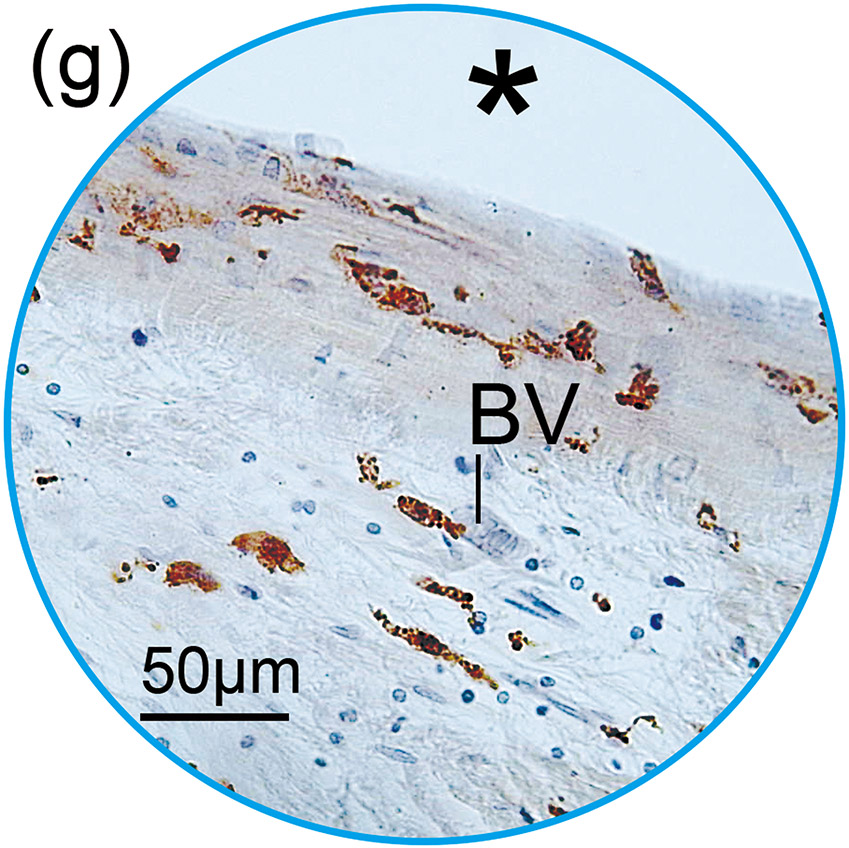
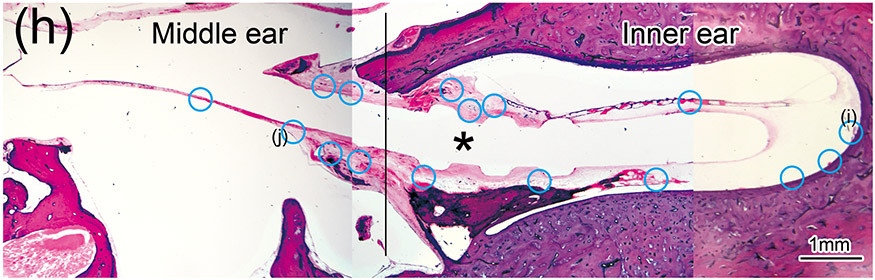
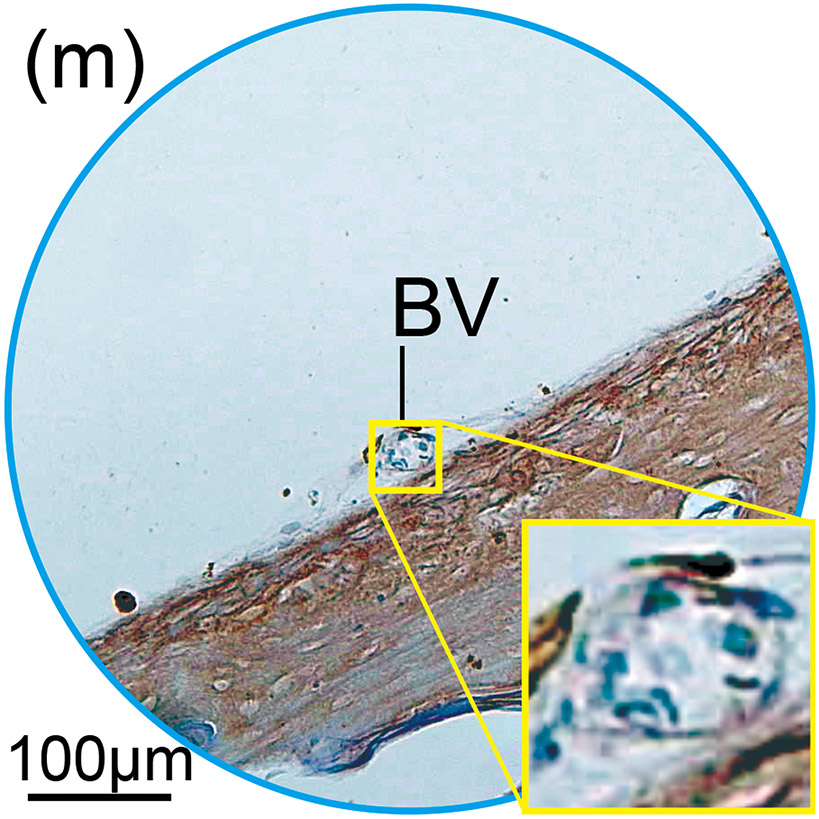
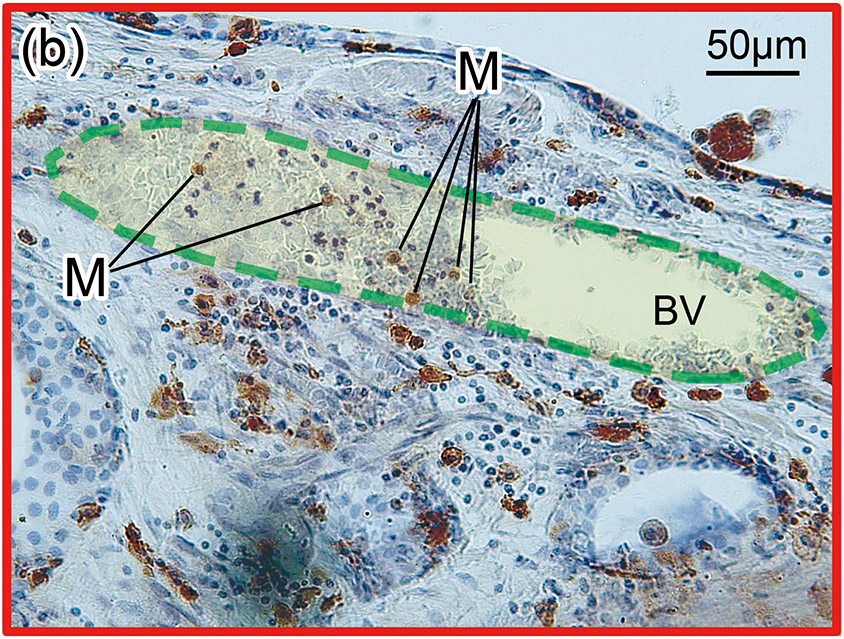
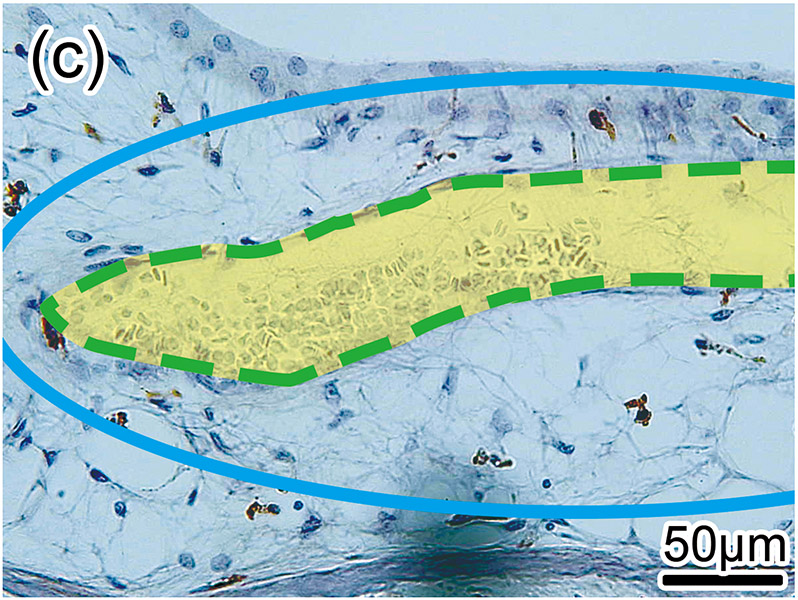
Figure 1. The prevalence of blood vessel and macrophage/monocyte in Iba1-immunostaining ofcochlea in implanted (a)-(g) and H&E stained (h)-(j) and unimplanted (k)-(m) ears and graph (n).
(a) Round window section of left implanted cochlea of case 4 with Iba1 immunostaining. The prevalence of blood vessels (BVs) was evaluated within the inner ear and middle ear where fibrous sheath surrounded the electrode track (*). There were 15 BVs in the inner ear and 7 BVs in the middle ear in this case. Red boxes and blue circles demonstrate positive and negative BVs which containing Iba1-positive macrophages/monocytes within a lumen, respectively. There were two positive BVs in both inner and middle ear that resulted in 13.3% and 28.3% of positive rate, respectively (Table 2).
(b) and (c): A higher magnification of the red boxed area in the inner ear of scala tympani and osseous spiral lamina. There were Iba1-positive monocytes (M) within the BVs.
(d) and (e): A higher magnification of the red boxed area in the middle ear. There were Iba1-positive monocytes (M) and erythrocytes within the blood vessel.
(f) and (g): A higher magnification of the blue circled area in inner and middle ear. Those BVs were negative for Iba1-positive macrophages/monocytes within the lumen.
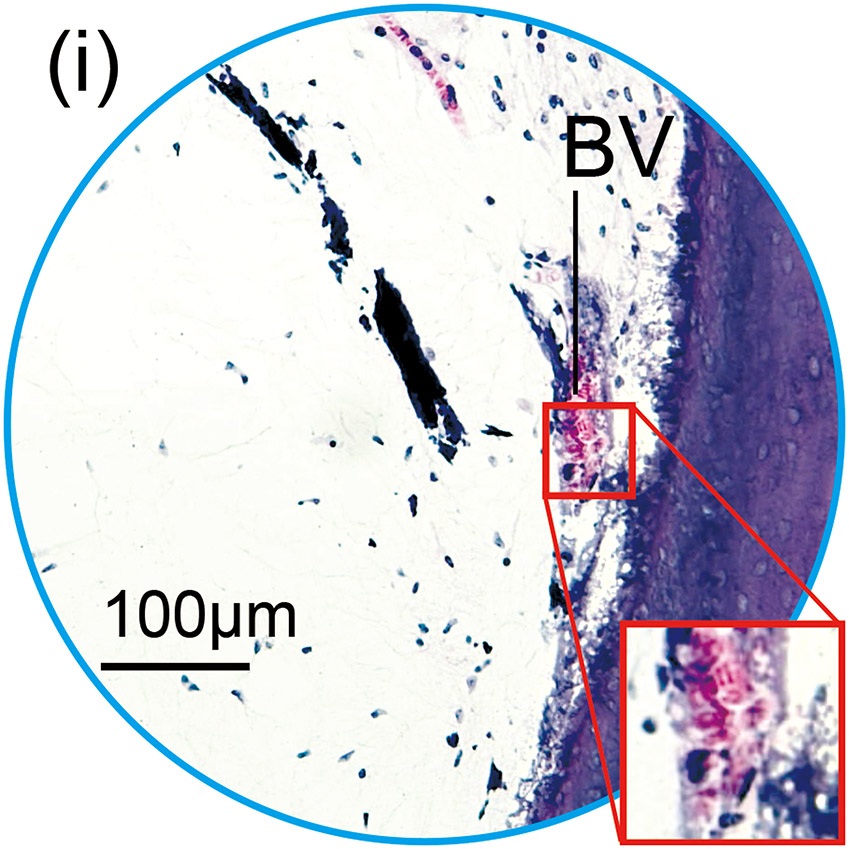
(h) An adjacent section to that shown in Fig. 1(a) with H&E staining. The presence of BVs in the section stained with Iba1 was confirmed using an adjacent section with H&E staining.
(i) and (j): A higher magnification of the blue circled area in inner and middle ear. BVs containing red erythrocytes within the lumen were identified. These BVs confirmed as BV seen in Fig. 1(f) and (g) with immunostaining.
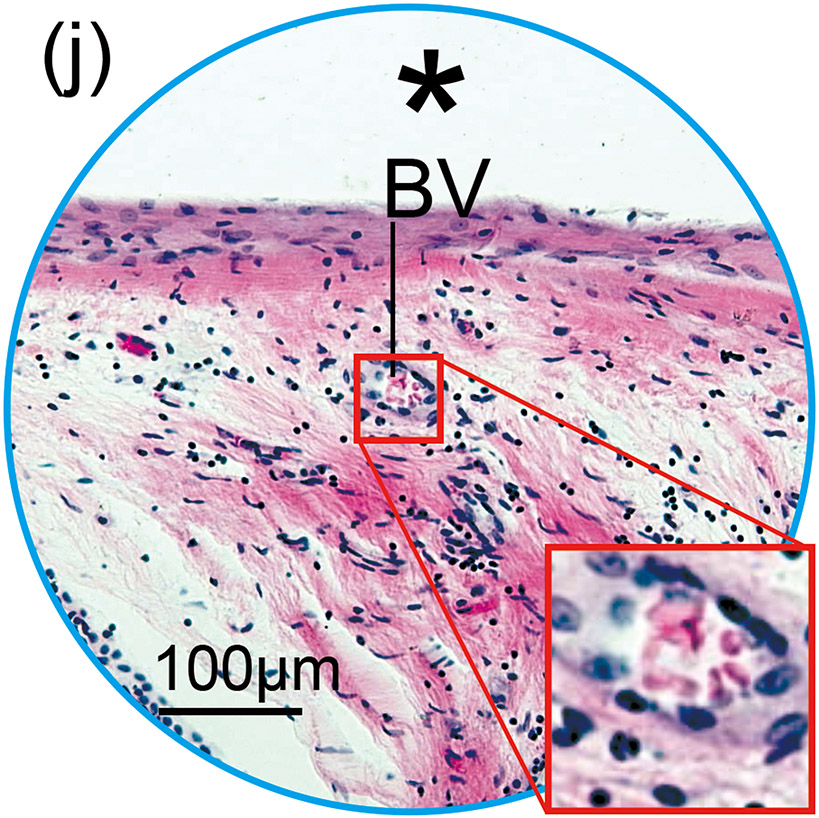
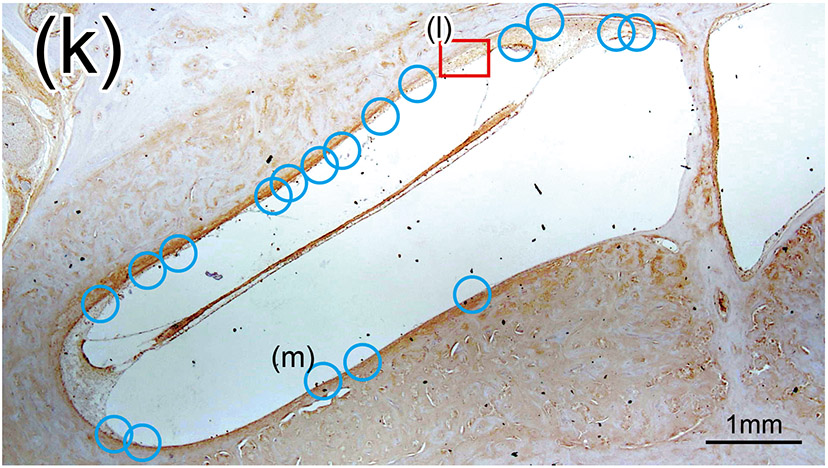
(k) Round window section of right unimplanted cochlea of case 4 with Iba1 immunostaining. The prevalence and positive rate of BVs was evaluated within the inner ear. There were 19 cross sections of BVs and one positive BV in unplanted ear, which resulted in 5.3% of positive rate.
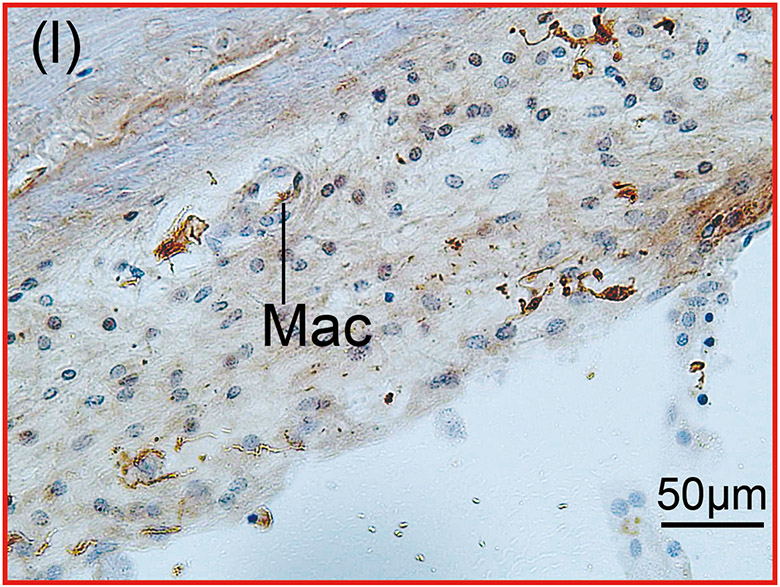
(l) One positive BV contained Iba1-positive macrophage (Mac).
(m) A negative BV of inner ear in unplanted ear.
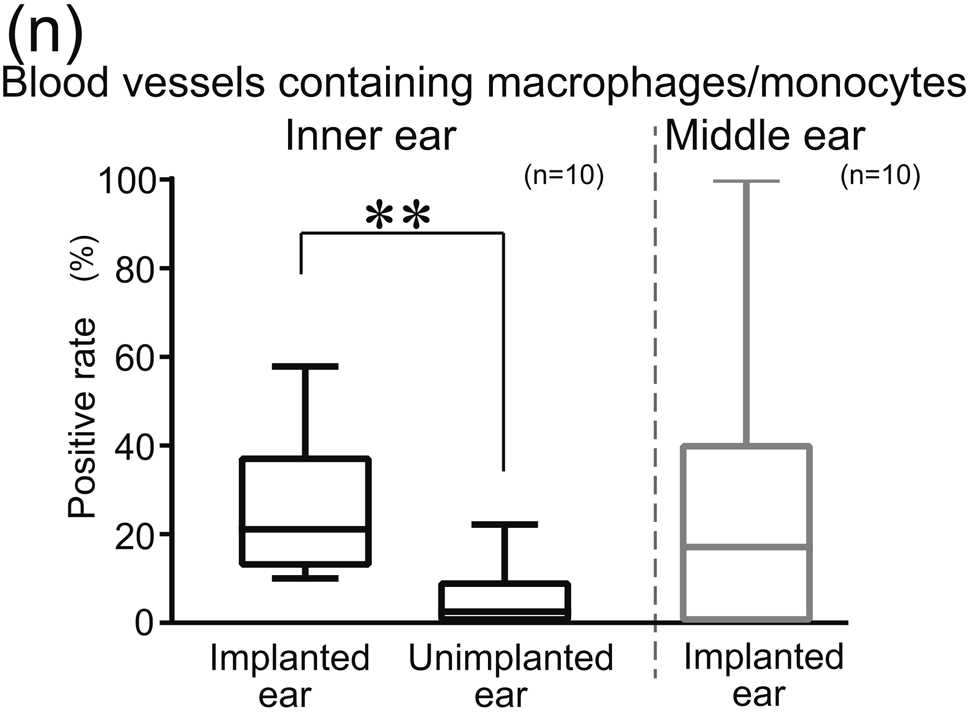
(n) Positive rates of Iba1-positive macrophages/monocytes in inner ear were compared between implant and unimplanted ears. Box-whisker plot of the positive rates of BVs containing Iba1-positive macrophages/monocytes in inner ear of implanted and unimplanted ears and in the middle ear of implanted ears. Wilcoxon matched-pairs signed rank test was used for statistical analysis between implanted and unimplanted ears. The asterisks (*) indicate the statistical significance (**: p < 0.01).
In middle ears of the patients with cochlear implantation, electrodes were surrounded by fibrotic sheaths outside of the round window (Fig. 1(a), (h) and 2(a)). There were some blood vessels within fibrotic sheaths. Iba1-positive macrophages/monocytes within blood vessels of only the implanted ears were also investigated quantitatively.
Figure 2. Quantitative assessment of density of Iba1-positive monocytes within cochlear blood vessels (a)-(d) and graph (e).
(a) Round window section of right implanted cochlea of case 3 with Iba1 immunostaining. There was new bone formation within the scala tympani of basal turn.
(b) Typical positive BV with vasodilatation in the middle ear near the round window was shown. The luminal area colored by light yellow was measured. There were six Iba1-positive monocytes (M) within the lumen outlined by green dash line.
(c) Negative BV with vasodilatation in the inner ear was shown. The density of monocytes per luminal area colored by light yellow was calculated.
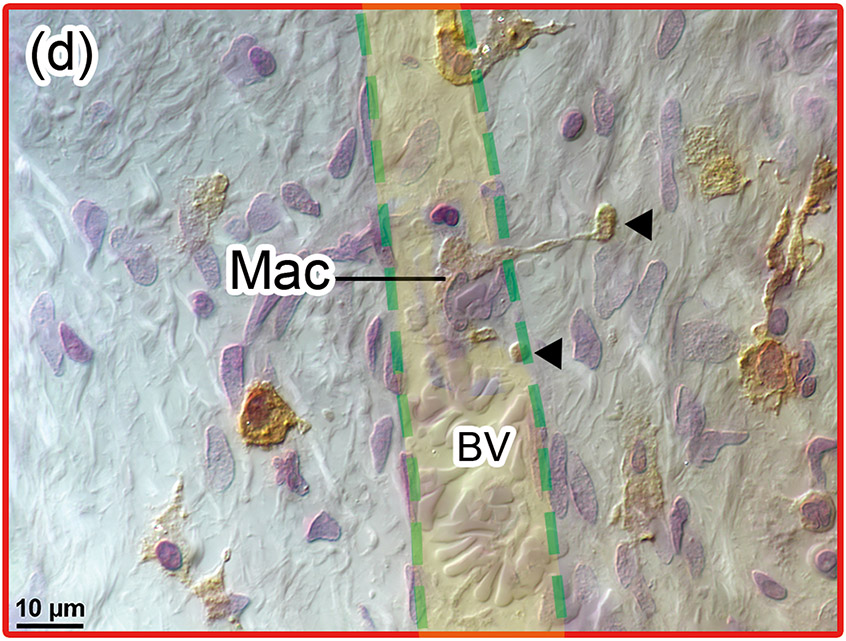
(d) Another positive BV within the red box near the round window was shown by Normarski (differential interference contrast) microscopy. Interestingly, long processes of a ramified macrophage (Mac) penetrated the vessel wall (arrowheads), consistent with a macrophage passing through the vessel wall.
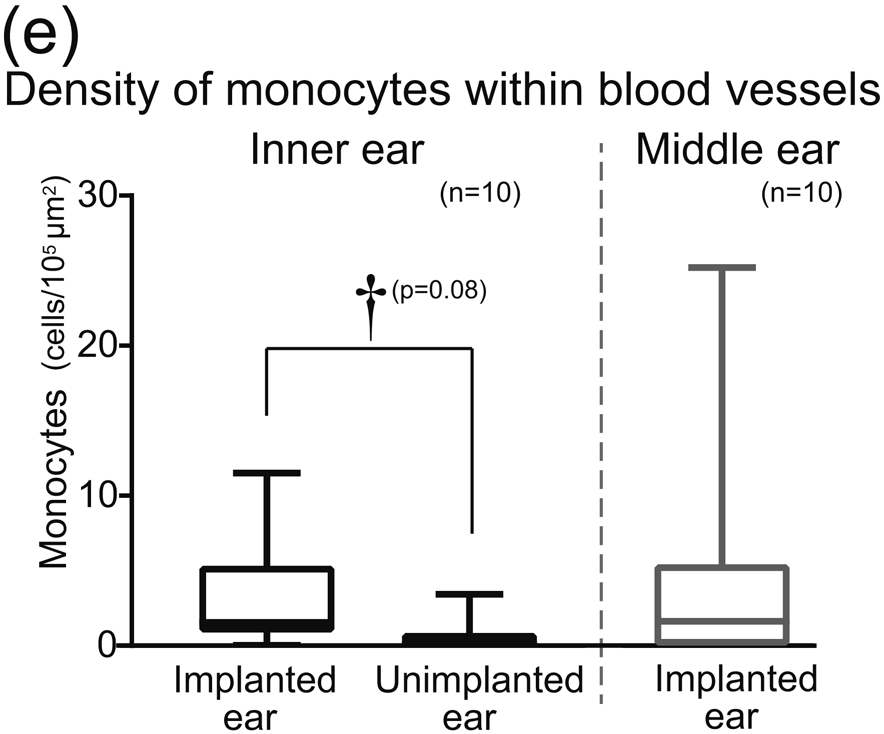
(e) The density of monocytes within blood vessels in inner ears of implanted and unimplanted ears and in the middle ear of implanted ears are shown by box-whisker plot. Wilcoxon matched-pairs signed rank test was used for statistical analysis between implanted and unimplanted ears. The daggers (†) indicate a significant tendency (†: 0.05 < p < 0.1).
1. Positive rate of blood vessels
First, the prevalence of the vessels in inner and middle ears were counted using an Excelis HD camera & monitoring system (AccuScope, Inc., Commack, NY), coupled to an Olympus BX51 light microscope. The prevalence of vessels containing the Iba1-positive cells (Fig. 1(b)-(g) and (k)-(m)) was calculated. The vessels within the bony labyrinth were excluded from this study. The images at 40× (1920 × 1080 pixels) were captured using imaging software CaptaVision (Capta Vision: AccuScope Inc.) and displayed on a LED monitor (VH27, Hewlett-Packard). The presence of red blood cells within the lumen was used to confirm the identification of the blood vessels using the adjacent H&E section (Fig. 1(h)-(j)). The Iba1-positive rates of the vessels containing macrophages/monocytes within the lumen were compared between the implanted and the unimplanted ears (Fig. 1(n)).
2. Density of Iba1-positive monocytes within blood vessels
Second, these Iba1-positive macrophages within the vessels were distinguished from monocytes based on their appearance. Monocytes are round in shape with a small cytoplasm and no processes (Fig 1(b)-(e) and Fig. 2(b)). Macrophages have various shapes and a larger cytoplasm with processes (Fig 1(i) and Fig. 2(d)). The density of the Iba1-positive monocytes within the vessels (number of monocyte/ luminal area) was calculated. The luminal area of each vessel was measured using imaging software Image J (1.52a) (National Institutes of Health, USA) (Fig. 2(b)-(d)). The density of Iba1-positive monocytes within the vessels was calculated in both implanted and unimplanted ears and was expressed as the number of monocytes per 100,000 μm2 (Fig. 2(e)).
3. Correlations between Density of Iba1-positive monocytes and Duration of implantation
In order to study the effect of duration following cochlear implantation on the density of monocytes, the correlations between the obtained density of Iba1-positive monocytes within blood vessels and duration of implantation (months after implantation) were calculated.
Statistical Analysis
Statistical comparison between the implanted and the contralateral unimplanted ears was done by Wilcoxon matched-pairs signed rank test. p<0.05 is considered significant.
To evaluate the correlation of duration of implantation (months after implantation) with the density of Iba1-positive monocyte within the vessels, Spearman’s coefficients of correlation test was used. p<0.05 is considered significant. All statistical analyses were performed using GraphPad software (GraphPad Prism version 7.02; GraphPad Software, Inc., LaJolla, CA).
Results
1. Prevalence of vessels containing Iba1-positive macrophages/monocytes
There were many cochlear blood vessels within the section through the round window (Fig. 1(a), (h) and 2(a)). To specify which is artery or vein was beyond the limit of this study. The prevalence of vessels with Iba1-positive macrophages/monocytes were shown in Table 2. Most Iba1-positive cells were classified monocytes in both implanted and unimplanted ears. The positive rate of blood vessels containing Iba1-positive macrophages/monocytes in implanted ears (mean 26%, median 21%) was greater than in unimplanted ears (mean 5.2%, median 2.5%: p<0.01) (Fig. 1(n)).
Table2.
Prevalence of cochlear blood vessels and Iba1-positive macrophages/monocytes within the vessels.
| Inner ear |
Middle ear |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Implanted ear |
Unimplanted ear |
Implanted ear |
|||||||||||||
| Number of BV | Number of Iba1-positive cells |
Number of BV | Number of Iba1-positive cells |
Number of BV | Number of Iba1-positive cells |
||||||||||
|
|
|
|
|||||||||||||
| Case | Containing Iba1-positive cells |
Total | Positive rate (%) |
Mac | Mono | Containing Iba1-positive cells |
Total | Positive rate (%) |
Mac | Mono | Containing Iba1-positive cells |
Total | Positive rate (%) |
Mac | Mono |
|
|
|
||||||||||||||
| 1 | 6 | 13 | 46.2 | 0 | 31 | 2 | 9 | 22.2 | 0 | 2 | 2 | 4 | 50.0 | 0 | 2 |
| 2 | 11 | 19 | 57.9 | 5 | 58 | 0 | 9 | 0 | 0 | 0 | 2 | 2 | 100 | 0 | 2 |
| 3 | 2 | 11 | 18.2 | 1 | 1 | 0 | 12 | 0 | 0 | 0 | 1 | 5 | 20.0 | 0 | 7 |
| 4 | 2 | 15 | 13.3 | 0 | 3 | 1 | 19 | 5.3 | 1 | 0 | 2 | 7 | 28.6 | 0 | 3 |
| 5 | 4 | 14 | 28.6 | 0 | 6 | 0 | 21 | 0 | 0 | 0 | 3 | 8 | 37.5 | 0 | 5 |
| 6 | 3 | 30 | 10.0 | 1 | 3 | 1 | 19 | 5.3 | 0 | 1 | 1 | 7 | 14.3 | 0 | 1 |
| 7 | 4 | 39 | 10.3 | 2 | 2 | 2 | 21 | 9.5 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 8 | 3 | 15 | 20.0 | 0 | 6 | 1 | 10 | 10.0 | 0 | 1 | 1 | 21 | 4.8 | 0 | 1 |
| 9 | 4 | 38 | 10.5 | 0 | 5 | 0 | 21 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 10 | 7 | 20 | 35.0 | 2 | 6 | 1 | 20 | 5.0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
BV: Blood vessel; Mac: Macrophage; Mono: Monocyte
2. Density of Iba1-positive monocytes within blood vessels
Because some cochlear vessels showed vasodilatation caused by inflammation following cochlear implantation (Fig. 2(b) and (c)), the total area might be larger in implanted ears than in unimplanted ears. Therefore, we also evaluated the number of Iba1-positive monocytes per luminal area of the vessel. The density of Iba1-positive monocytes in implanted ears (mean 32, median 16 cells/105μm2) tended to be greater than in unimplanted ears (mean 6.6, median 0.93 cells/105μm2: p=0.08) (Fig. 2(e)).
3. Correlations between Density of Iba1-positive monocytes and Duration of implantation
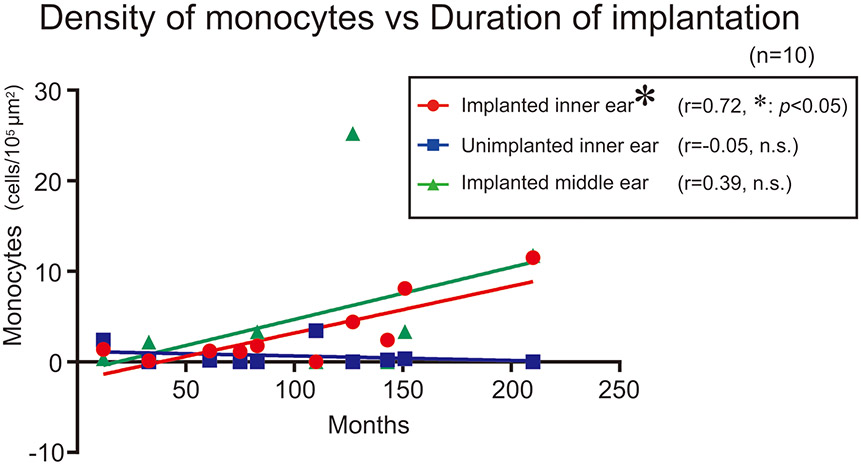
Numbers of Iba1-positive monocytes within vessels per area of lumen in implanted ears were significantly correlated with duration (months after implantation) but not in unimplanted ears nor middle ears (Fig. 3).
Figure 3. Correlation between densities of monocytes within blood vessels and duration after implantation.
Scatter plot of densities of monocytes versus duration (months after cochlear implantation) are shown. Red circles and blue squares indicate implanted and unimplanted inner ears, respectively. Green triangles indicate implanted middle ears. The asterisks (*) indicate the statistical significance (*: p < 0.05) (Spearman’s coefficients of correlation test).
Discussion
This temporal bone study is the first report focused on the delivery system of macrophages to the cochlea following cochlear implantation in the human. Histopathological findings in Fig. 2(d) show a macrophage extending a longer process outside of a blood vessel wall. This figure may demonstrate evidence that macrophages are delivered through cochlear vessels.
Our investigation demonstrated a significantly greater prevalence of cochlear vessels containing macrophage/monocyte in implanted ears compared to unimplanted ears. The density of Iba1-positive monocytes within cochlear vessels tended to be greater in implanted ears. These results support our hypothesis that an increase of macrophages in the cochleae of implanted ears results in recruitment of macrophages derived from Iba1-positive monocytes and delivery through the vessels.
Density of Iba1-positive monocytes in the inner ear was significantly correlated with duration (months after implantation) but not in the middle ear nor the inner ear in unimplanted ears. It is suggested that immune responses involving Iba1-positive monocytes in implanted ears are active and ongoing for long periods following implantation. If this duration dependent increase in macrophages were mainly pro-inflammatory, we might expect the foreign-body response following cochlear implantation to better correlate with duration of implantation. However, Seyyedi and Nadol (14) reported no correlation between the degree of foreign-body response and the duration after cochlear implantation. In the mouse retina, contribution of monocyte-derived macrophages to both anti-inflammatory and neuroprotective effects was reported (15). In a study of human cochleae using super resolution microscopy, the connection between ramified Iba1-positive cells and nerve fibers, as well as spiral ganglion soma, was observed. It was suggested that a linked network may exist between macrophages and neurons that may survey the environment and protect spiral ganglion neurons (16). Considering these results, it is suggested that the duration dependent increase in monocyte-derived macrophages is not associated with the foreign-body response, which may imply that the main role of monocyte-derived macrophages in the cochlea is anti-inflammatory rather than pro-inflammatory. To prove our assumption, further immunologic studies using specific markers for anti-inflammatory macrophages are needed.
Acknowledgments
The authors thank Diane Jones, Barbara Burgess, and Meng Yu Zhu for their expert preparation of temporal bone specimens, and Garyfallia Pagonis for helpful technical assistance in creating digitized images of the temporal bone sections.
(source of support)
Grants #U24DC013983 and R01DC000152-34, National Institute on Deafness and Other Communication Disorders (NIDCD)
Abbreviation list:
- Iba1
ionized calcium-binding adaptor molecule 1
- H&E
hematoxylin and eosin
Footnotes
(disclosure)
All authors declare no conflicts of interests related to this manuscript.
References
- 1.Wynn TA, and Barron L Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis 2010; 30: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016; 44:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeffel G, Chen J, Lavin Y, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015; 42: 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, Ito J. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008; 86:1758–67. [DOI] [PubMed] [Google Scholar]
- 5.Hirose K, Rutherford MA, Warchol ME. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hear Res. 2017; 352:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Malley JT, Nadol JB, McKenna MJ. Anti CD163+, Iba1+, and CD68+ Cells in the adult human inner ear: normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol Neurotol 2016; 37:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okayasu T, Quesnel AM, O'Malley JT, Kamakura T, Nadol JB Jr. The Distribution and Prevalence of Macrophages in the Cochlea Following Cochlear Implantation in the Human: An Immunohistochemical Study Using Anti-Iba1 Antibody. Otol Neurotol. 2020; 41:e304–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol 2005;489:180–94. [DOI] [PubMed] [Google Scholar]
- 9.Nadol JB, O’Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res 2014;318:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamakura T, O'Malley JT, Nadol JB Jr. Preservation of Cells of the Organ of Corti and Innervating Dendritic Processes Following Cochlear Implantation in the Human: An Immunohistochemical Study. Otol Neurotol. 2018; 39:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Malley JT, Burgess BJ, Galler D, Nadol JB. Foreign body response to silicone in cochlear implant electrodes in the human. Otol Neurotol 2017;38:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okayasu T, O’Malley JT, Nadol JB Jr. Density of macrophages immunostained with anti-Iba1 antibody in the vestibular endorgans after cochlear implantation in the human. Otol Neurotol 2019; 40:e774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merchant S, Nadol JB Jr. Schuknecht’s Pathology of the Ear, 3rd ed. Shelton: People’s Medical Publishing House; 2010. 34–40. [Google Scholar]
- 14.Seyyedi M, Nadol JB Jr. Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol. Neurotol 2014; 35:1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.London A, Itskovich E, Benhar I et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med 2011; 208: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Molnar M, Garnham C, Benav H, Rask-Andersen H. Macrophages in the Human Cochlea: Saviors or Predators-A Study Using Super-Resolution Immunohistochemistry. Front Immunol. 2018; 9: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]