Abstract
Background/Objectives:
Unintentional falls are a leading cause of injury for older adults, and evidence is needed to understand modifiable risk factors. We evaluated 1-year fall-related fracture risk and whether dispensing of medications with anticholinergic/sedating properties is temporally associated with an increased odds of these fractures.
Design:
A retrospective cohort study with nested self-controlled analyses conducted between January 1, 2014 and December 31, 2016.
Setting:
20% nationwide, random sample of US Medicare beneficiaries
Participants:
New users of medications with anticholinergic/sedating properties who were 66+ years old, had Medicare Parts A, B, and D coverage but no claims for medications with anticholinergic/sedating properties in the year prior to initiation were eligible.
Measurements:
We followed new users of medications with anticholinergic/sedating properties until first non-vertebral, fall-related fracture (primary outcome), Medicare disenrollment, death, or end of study data. We estimated the 1-year risk with corresponding 95% confidence intervals (CIs) of first fracture after new use. We applied the self-controlled case-crossover and case-time-control designs to estimate odds ratios (ORs) and 95% CIs by comparing anticholinergic and/or sedating medication exposure (any vs. none) during a 14-day hazard period preceding the fracture to exposure to these medications during an earlier 14-day control period.
Results:
A total of 1,097,989 Medicare beneficiaries initiated medications with anticholinergic/sedating properties in the study period. The 1-year cumulative incidence of fall-related fracture, accounting for death as a competing risk, was 5.0% (95% CI: 5.0%-5.0%). Using the case-crossover design (n=41,889), the adjusted OR for the association between anticholinergic/sedating medications and fractures was 1.03 (95% CI: 0.99, 1.08). Accounting for the noted temporal trend using the case-time-control design (n=209,395), the adjusted OR was 1.60 (95% CI: 1.52, 1.69).
Conclusion:
Use of anticholinergic/sedating medication was temporally associated with an increased odds of fall-related fractures. Patients and their healthcare providers should consider pharmacologic and non-pharmacologic treatments for the target condition that are safer.
Keywords: aging, cholinergic antagonists, hypnotics and sedatives, inappropriate prescribing, bone fracture
INTRODUCTION
Falls are one of the most common and burdensome adverse events for older adults (aged ≥65 years).1,2 One in four older adults experiences an unintentional fall annually, and fall-related injuries among older adults account for 2.8 million emergency department (ED) visits and 800,000 hospitalizations each year.1,3 For older adults residing in the US, fractures comprise 35% of non-fatal fall-related injuries and account for 61% of the non-fatal fall-related injury medical costs.4 Hip fractures, 95% of which are attributed to falls, frequently result in reduced long-term mobility and nursing home admission.2,5 Older adults who experience a hip fracture remain at an elevated risk for premature death for several years following the index fracture.6
While many studies have identified medications associated with increased fall risk, fall prevention interventions have mainly focused on exercise and vitamin D supplementation.7-12 Therefore, the US Preventive Services Task Force (USPSTF) has identified medication management interventions as a research need in its 2018 Recommendation Statement regarding interventions for fall prevention in community-dwelling older adults.13
The literature on the association between medication use and fall risk has been progressively synthesized descriptively (i.e., systematic reviews) and statistically (i.e., meta-analyses) since the 1990s.10-12,14,15 However, the dearth of sufficiently high quality observational studies of certain medication classes and groups makes it difficult to synthesize the literature conclusively.10-12 Specifically, there is a need for studies to (1) establish clear temporal relationships between medication exposure and health outcomes, (2) focus on chemical properties rather than broad pharmacologic groups (such as antihypertensives), (3) improve confounding control, and (4) expand medication ascertainment by incorporating longitudinal prescription data.10,11,16,17
To guide future medication management interventions, we sought to generate high-quality evidence regarding medication use and temporally associated fall-related fracture risk using a large, nationwide random sample of Medicare patients. We focused on older adults initiating use of medications with anticholinergic/sedating properties because of the high burden of polypharmacy among community-dwelling older adults and the associated downstream drug-related adverse events.18,19 This is also an identifiable population for whom fall-prevention interventions could feasibly be implemented. Among this population, our objectives were to (1) evaluate the 1-year risk of fall-related fractures and (2) examine the effect of transient exposure to medications with anticholinergic/sedating properties on fall-related fractures.
METHODS
Data Source and Study Population
We obtained a 20% nationwide, random sample of Medicare beneficiaries through a data use agreement between the Centers for Medicaid and Medicare Services (CMS) and the University of North Carolina at Chapel Hill (UNC-CH). We conducted a retrospective study of Medicare beneficiaries who initiated use of medications with anticholinergic and/or sedating properties during 2014 to 2016, where use was defined by claims for dispensed prescriptions. This time frame was chosen because Medicare Part D did not cover benzodiazepines (BZDs), a major class of medications with sedating properties, from 2006 to 2012.20 We required that each beneficiary have Medicare Parts A, B, and D coverage (with a 1 month grace period) and no Part D claims for medications with anticholinergic/sedating properties for at least one year prior to initiation. The index date was the date of first dispensing of a medication with anticholinergic/sedating properties. Patients were required to be ≥66 years at the index date because beneficiaries who receive coverage before age 65 are eligible based on disability or advanced disease. While patients could potentially meet the initiation definition more than once, we restricted the cohort to the first new use period and followed individuals from the index date until the first of the following events: first non-vertebral fracture (since vertebral fractures are more likely related to osteoporosis rather than to falls), disenrollment in Medicare Parts A, B, or D, death, or end of study data (December 31, 2016) (Supplementary Figure S1).
Outcome Assessment
Fall-related fractures were identified using diagnosis and procedure codes in Medicare Parts A and B claims using a modified Medicare-based algorithm for fracture identification.21 An orthopedic physical therapist and injury epidemiologist (YMG) helped modify the original algorithm to capture fall-related fractures by removing codes unlikely to be fall-related (e.g., related to motor vehicle crashes or violence). We required a fracture-related diagnosis code and a corresponding (i.e., same fracture site) procedure code (e.g., diagnostic imaging, cast application) up to seven days after the diagnosis code. Fractures having a motor vehicle crash diagnosis code on the same day as the diagnosis code were excluded. A forward-backward mapping approach was used to convert the ICD-9-CM codes in the original algorithm for the ICD-10-CM era.22 Codes are provided in the Supplementary Material.
Exposure Assessment
Two geriatric pharmacists (MJP and JCB) reviewed all unique medication generic names listed in the Part D claims and classified each as having anticholinergic or sedating effects, both, or neither, as previously described.23 For our primary exposure definition, we defined exposure to medications with anticholinergic/sedating properties dichotomously (any vs. none), and exposure was assessed by identifying continuous periods of use based on the days’ supply of the prescription. Overlapping prescriptions were shifted to account for forward stockpiling. Additional details are provided in the Supplementary Material.
Covariate Assessment
We assessed covariates during the 12 months prior to initiation using Medicare Part A, B, and D claim files. Demographic characteristics included age, race (Black, white, Asian, Hispanic, Native American, other, unknown), and sex (male, female). Dichotomous (any, none) proxy measures for socioeconomic status included: Partial Low-Income Subsidy, Full Low-Income Subsidy, and State Buy-In Parts A and B coverage during the 12 months prior to initiation. We estimated a proxy measure for frailty using the validated Faurot Medicare claims-based algorithm that incorporated predictors of activities of daily living dependency.24-26 Beneficiaries’ predicted probabilities of being frail were categorized as: low (0%-<10%), low/intermediate (10%-<20%), intermediate/high (20%-<50%), and high (≥50%).27 We estimated the Gagne Combined Comorbidity Score.28 We examined several healthcare utilization indicators including: number of unique dispensed medications (count by generic name), number of outpatient visits, number of ED visits, and number of hospital admissions. For time-varying confounder control during the control and hazard windows, we adjusted for dispensing of medication classes associated with increased fall risk (analgesics, antipsychotics, anticonvulsants, BZDs, antihypertensives, cardiac medications, antiarrhythmics, antidepressants, diuretics), as defined by the Agency for Healthcare Research and Quality (AHRQ).29 We did not adjust for use of AHRQ-defined medications with anticholinergic/sedating properties within the control and hazard windows since these medications were included in our primary exposure definition.
Statistical Analysis
New User Cohort
We described cohort characteristics using covariates assessed in the year prior to initiation and examined the most common anticholinergic/sedating medications initiated among these beneficiaries. We then estimated the 1-year first fracture rate and corresponding 95% confidence intervals (CIs), and the 1-year risk of first fracture following anticholinergic/sedating medication initiation with death as a competing event with bootstrapped 95% CIs (100 repetitions) using the Aalen-Johansen estimator.30
Nested Self-Controlled Analyses
To assess the transient effects of anticholinergic/sedating medication dispensing on fall-related fractures, we implemented two self-controlled study designs: the case-crossover and the case-time-control designs, nested within the new user cohort (Supplementary Figure S2). These designs control for time-fixed and slowly time-varying confounders since each beneficiary serves as their own control. Therefore, these designs help eliminate confounding by factors that do not change over a short time interval (e.g., prior history of falls/fractures). 31 The case-crossover design is restricted to individuals who experienced the event (i.e., cases) (Figure 1A).32 Each case’s person-time is subset into two windows (or periods): (1) a hazard period, or an “at risk” period, which immediately precedes the first event, and (2) a control period, which has the same length as the hazard period (to enable a matched analysis) but does not overlap with it.32,33 A washout period separates these two observation periods in which the exposure of interest is assessed. Only beneficiaries with discordant exposures (e.g., any exposure in the hazard period but no exposure in the control period or vice versa) contribute to the analysis. For an exposure to cause a transient increase in risk of the event, it ought to be more common in the hazard period than in the control period.
Figure 1.

Self-Controlled Study Design Schematics for the Main Analysis. (A) Case-Crossover Design, (B) Case-Time-Control Design
Both study designs were nested within our new user cohort. For the primary analyses, the control and hazard periods were each 14 days, and the washout period was 30 days. These exposure windows are biologically plausible since use of these medications is anticipated to elevate fall risk in the short term; fall-increasing medications whose use results in falls in the inpatient setting are typically prescribed between 24 hours to 7 days prior to the associated fall.34 Since this study was conducted among individuals in the non-acute setting (rather than an inpatient setting) and we studied a more severe outcome, we extended the control and hazard periods.
To estimate the matched (on case) odds ratio (OR) using the case-crossover design, we compared exposure frequencies during the control and hazard periods.32 This exposure OR, estimated using conditional logistic regression, estimates the incidence rate ratio that we would have obtained were we to study a full cohort.31 While time-fixed (and slow-varying) covariates (e.g., race, chronic conditions, exercise) were controlled for by design, we included dispensing (any vs. none) of each of the AHRQ-defined medication classes associated with fall risk (described above), measured during the control and hazard windows, in the adjusted models.
Since population-level temporal trends in exposure (e.g., increases in uptake of a new medication) and the existence of only certain exposure patterns in the population (persistent user bias) may bias case-crossover results, we also applied a case-time-control design, which has been demonstrated to account for these biases in simulations.35,36 This design had the same structure as the case-crossover, but also included matched controls (Figure 1B).37 Four controls were matched per case based on attained age (in days) and sex, where each control had at least the same amount of follow-up time as the case to which they were matched and did not become a case themselves subsequently.37 We compared the case-crossover results for the cases (i.e., an exposure OR in the cases) to the case-crossover results for the controls (i.e., an exposure OR in the controls) by dividing the cases’ exposure OR by the controls’ exposure OR to obtain the case-time-control OR.38
Sensitivity Analyses
We conducted sensitivity analyses to assess the robustness of our results. First, we examined both shorter (7-day) and longer (21-day) control and hazard windows. Second, since concurrent use of multiple prescriptions with anticholinergic/sedating properties may be indicated under certain circumstances, we used a less conservative approach that did not shift overlapping prescriptions but did account for days’ supply. Third, since patients who are hospitalized, in a nursing facility, or in hospice care may not use their Part D benefits, we restricted to patients not in these settings during the control and/or hazard windows. Fourth, to account for the potential for weakness induced by a recent in-patient stay, we adjusted for being in one of these settings during the control and/or hazard windows. Fifth, to better understand the drivers of the associations, we considered several alternative exposure definitions as sensitivity analyses: we defined exposure to (1) medications with sedating properties dichotomously (any vs. none) and (2) both properties (any vs. none). We also conducted analyses restricted to several of the most common prescription medications with sedating properties and both properties during the hazard and control windows.
The UNC-CH Institutional Review Board approved this research (Study #18-2999). Statistical analyses were conducted using SAS Statistical Software, version 9.3 (Cary, NC).
RESULTS
Patient Characteristics
The analysis population included 1,097,989 Medicare beneficiaries who initiated use of an anticholinergic/sedating medication during 2014 to 2016. The most commonly initiated medications tended to be sedatives only (initiated as a single drug): combination medications containing hydrocodone (24%), tramadol (11%), and combination medications containing oxycodone (7%)) (Table 2). Of the medications containing anticholinergic properties initiated as a single drug, meclizine was most common (4%). Furthermore, 6% of patients initiated multiple drugs simultaneously. Table 1 describes the population characteristics stratified by whether the patient initiated one drug with sedating only properties, one drug with both properties, or multiple drugs. Patients were 76 years of age on average, 56% identified as women, and 85% identified as white. Patients initiating a single drug tended to have more similar characteristics, whereas those initiating multiple drugs tended to be younger, have more healthcare utilization in acute settings, and higher frailty probability.
Table 2.
Most Common Anticholinergic/Sedating Medications Initiated Among Medicare Beneficiaries (2014-2016), Stratified by Medication Property
| A sedating drug only (n = 849,410) |
No. (%) | A drug with both properties only (n= 183,441) |
No. (%) |
Multiple drugs (n = 65,138) |
No. (%) |
Overall (n = 1,097,989) |
No. (%) |
|---|---|---|---|---|---|---|---|
| Combinationa with hydrocodone | 262,454 (31) |
Meclizine | 45,435 (25) |
Cyclobenzaprine, Combination with hydrocodone | 2,210 (3) |
Combination with hydrocodone | 262,545 (24) |
| Tramadol | 118,021 (14) |
Cyclobenzaprine | 18,735 (11) |
Combination with hydrocodone, tramadol | 1,888 (3) |
Tramadol | 118,021 (11) |
| Combination with oxycodone | 74,277 (9) |
Oxybutynin | 15,146 (8) |
Diazepam, Combination with hydrocodone | 1,823 (3) |
Combination with oxycodone | 74,277 (7) |
| Combination with codeine | 61,473 (7) |
Dicyclomine | 10,743 (6) |
Gabapentin, Combination with hydrocodone | 1,444 (2) |
Multiple drugs | 65,138 (6) |
| Gabapentin | 48,294 (6) |
Tizanidine | 9,893 (5) |
Gabapentin, Tramadol | 1,345 (2) |
Combination with codeine | 61,473 (6) |
| Alprazolam | 36,399 (4) |
Combination with atropine | 9,678 (5) |
Cyclobenzaprine, Tramadol | 1,203 (2) |
Gabapentin | 48,294 (4) |
| Lorazepam | 27,308 (3) |
Hydroxyzine | 9,101 (5) |
Combination with hydrocodone, promethazine | 978 (2) |
Meclizine | 45,435 (4) |
| Zolpidem | 22,149 (3) |
Prochlorperazine | 7,978 (4) |
Oxycodone, Tramadol | 925 (1) |
Alprazolam | 36,399 (3) |
| Oxycodone | 21,894 (3) |
Promethazine | 6,349 (4) |
Diazepam, Combination with oxycodone | 879 (1) |
Lorazepam | 27,308 (2) |
| Sertraline | 18,339 (2) |
Quetiapine | 5,422 (3) |
Lorazepam, Morphine | 837 (1) |
Zolpidem | 22,149 (2) |
Combination refers to combination medications (i.e., medications with more than one active ingredients).
Table 1.
Patient Characteristics of Anticholinergic and/or Sedating Medication Initiators Among Medicare Beneficiaries (2014-2016), stratified by medication initiated
| No. (%) | ||||
|---|---|---|---|---|
| Patient Characteristica |
A sedating drug only (n = 849,410) |
A drug with both properties only (n = 183,441) |
Multiple drugs (n = 65,138) |
Total (n = 1,097,989) |
| Age, mean (SD) | 75.9 (7.64) | 76.4 (7.71) | 75.0 (7.80) | 75.9 (7.67) |
| Sex, Male | 381,374 (44.9%) | 70,576 (38.5%) | 28,286 (43.4%) | 480,236 (43.7%) |
| Race | ||||
| White | 725,067 (85.4%) | 152,518 (83.1%) | 55,952 (85.9%) | 933,537 (85.0%) |
| Black | 63,364 (7.5%) | 14,338 (7.8%) | 4,670 (7.2%) | 82,372 (7.5%) |
| Other | 17,818 (2.1%) | 4,527 (2.5%) | 1,273 (2.0%) | 23,618 (2.2%) |
| Asian | 18,840 (2.2%) | 6,397 (3.5%) | 1,326 (2.0%) | 26,563 (2.4%) |
| Hispanic | 14,695 (1.7%) | 3,831 (2.1%) | 1,172 (1.8%) | 19,698 (1.8%) |
| Unknown | 9,626 (1.1%) | 1,830 (1.0%) | 745 (1.1%) | 12,201 (1.1%) |
| Healthcare Utilization | ||||
| Outpatient office visits, mean (SD) | 7.8 (6.30) | 7.9 (6.43) | 6.9 (6.18) | 7.8 (6.32) |
| Hospital adm, mean (SD) | 0.4 (0.92) | 0.3 (0.81) | 0.7 (1.32) | 0.4 (0.94) |
| ED visits, mean (SD) | 0.6 (1.16) | 0.5 (1.09) | 0.7 (1.39) | 0.6 (1.17) |
| Rx fills in last 30 days, mean (SD) | 1.5 (1.47) | 1.5 (1.47) | 1.2 (1.46) | 1.5 (1.47) |
| Polypharmacyb | 33,979 (4.0%) | 7,204 (3.9%) | 2,153 (3.3%) | 43,336 (3.9%) |
| SESc | ||||
| Any Parts & B State Buy-in | 123,503 (14.5%) | 29,618 (16.1%) | 12,040 (18.5%) | 165,161 (15.0%) |
| Any Low Income Subsidy | 24,284 (2.9%) | 5,260 (2.9%) | 2,093 (3.2%) | 31,637 (2.9%) |
| Health Status Indicators | ||||
| Frailty Probabilityd | ||||
| Low | 731,633 (86.1%) | 159,016 (86.7%) | 51,466 (79.0%) | 942,115 (85.8%) |
| Low/Middle | 59,281 (7.0%) | 12,511 (6.8%) | 5,319 (8.2%) | 77,111 (7.0%) |
| Middle/high | 38,999 (4.6%) | 7,893 (4.3%) | 4,986 (7.7%) | 51,878 (4.7%) |
| High | 19,497 (2.3%) | 4,021 (2.2%) | 3,367 (5.2%) | 26,885 (2.4%) |
| GCSe, mean (SD) | 1.4 (2.48) | 1.3 (2.44) | 1.8 (2.93) | 1.4 (2.51) |
Abbreviations: ED, emergency department
Assessed in the year prior to initiation of anticholinergic/sedating drugs
Assessed in the last 30 days and defined as (>=5 fill
SES, Socioeconomic status
Defined as Low (0%-<10%), Low/Middle (10%-<20%), Middle/high (20%-<50%), High (≥50%)
Gagne Comorbidity Score
Fall-Related Fractures and All-Cause Mortality
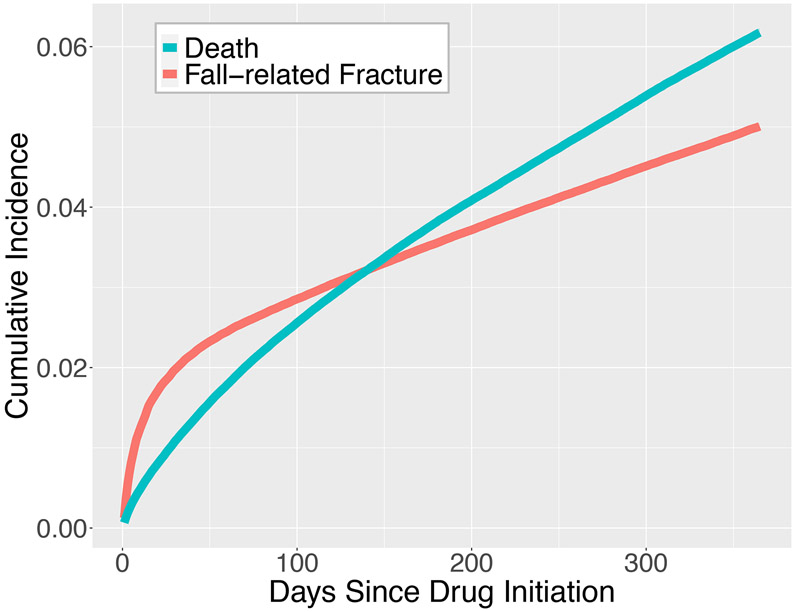
During the first year following medication initiation, 49,601 beneficiaries experienced a first, fall-related fracture (accounting for 51,893 fracture sites). The most common fracture sites were: radius (n = 10,922 (22.0%)), hip (n =9,266 (18.7%)), and humerus (n = 7,898 (15.9%)). The fall-related first fracture rate in the first year since medication initiation was 577 per 10,000 person-years (95% CI: 577-577). 59,004 of the beneficiaries died in the first year since medication initiation. The 1-year cumulative incidence of fall-related fracture, accounting for death as a competing risk, was 5.0% (95% CI: 5.0%, 5.0%) (Figure 2). While the 1-year cumulative incidence of mortality was 6.2% (95% CI: 6.1%, 6.2%).
Figure 2.
Cumulative Incidence of Fall-Related Fractures with Death as a Competing Risk
Transient Exposure to Medications with Anticholinergic/Sedating Properties and Fall-Related Fractures
The self-controlled, case-crossover 14-day period analysis included 41,889 beneficiaries who experienced a fall-related fracture following initiation of a medication with anticholinergic/sedating properties during 2014-2016. Beneficiaries experiencing a fall-related fracture in the first 58 days were excluded because there would not have been enough time to observe a 14-day control window, a 14-day hazard window, and a 30-day washout period between the two. We found that beneficiaries with any anticholinergic/sedating medication exposure (assessed by claims) during the hazard period did not have a notable increased odds of fall-related fracture, as compared to those with any exposure in the control period (OR: 1.04 (95% CI: 1.00, 1.09)). Further adjustment for exposure to known fall-increasing medications without anticholinergic/sedating properties had no relevant effect on the estimate (aOR: 1.03 (95% CI: 0.99, 1.08)) (Table 3).
Table 3.
Association of Anticholinergic/Sedating Medication Exposure and Fall-Related Fractures, Medicare Beneficiaries (2014-2016)
| Study Design |
Cases | Matched Controls | Crude Odds Ratio (95% CI) |
Adj. Odds Ratioc (95% CI) |
||||
|---|---|---|---|---|---|---|---|---|
| No. (%)a | Crude Exposure Odds Ratio (95% CI) |
No. (%) | Crude Exposure Odds Ratio (95% CI) |
|||||
| Hazard Period |
Control Period |
Hazard Period |
Control Period |
|||||
| Exposedb | Exposedb | |||||||
| (A) | (B) | (C) | (D) | (E) | (F) | (C)/(F) | (G) | |
| Primary Exposure Definition d | ||||||||
| Case-Crossover Study Designe | ||||||||
| 7-daye | 3,559 (8) |
3,759 (9) |
0.95 (0.90, 0.99) |
0.94 (0.90, 0.99) |
||||
| 14-day | 3,691 (9) |
3,540 (9) |
1.04 (1.00, 1.09) |
1.03 (0.99, 1.08) |
||||
| 21-day | 3,762 (9) |
3,360 (8) |
1.12 (1.07, 1.17) |
1.11 (1.06, 1.16) |
||||
| Case-Time Control Study Design | ||||||||
| 7-day | 3,559 (8) |
3,759 (9) |
0.95 (0.90, 0.99) |
8,538 (5) |
14,437 (8) |
0.59 (0.58, 0.61) |
1.60 (1.52, 1.69) |
1.60 (1.52, 1.69) |
| 14-day | 3,691 (9) |
3,540 (9) |
1.04 (1.00, 1.09) |
9,043 (5) |
13,942 (8) |
0.65 (0.63, 0.67) |
1.61 (1.52, 1.70) |
1.60 (1.52, 1.69) |
| 21-day | 3,762 (9) |
3,360 (8) |
1.12 (1.07, 1.17) |
9,476 (6) |
13,791 (9) |
0.69 (0.67, 0.71) |
1.63 (1.54, 1.72) |
1.62 (1.54, 1.71) |
We restricted to participants that could be observed in from the start of the control period to the end of the hazard period, hence, for the 7-day period analysis, the case-crossover n = 43,952 and the case-time control n = 219,710. For the 14-day period analysis, the case-crossover n = 41,889, and the case-time control n = 209,395. For the 21-day period analysis, the case-crossover n = 40,252 and the case-time control n = 201,210.
Refers to discordantly exposed patients (i.e., exposed in the hazard period and unexposed in the control period) who contribute to the analysis.
Adjusted for time-varying exposure of dispensing of medication classes associated with increased fall risk, as defined by the AHRQ.29 Fall risk medications with anticholinergic and/or sedating properties were excluded. Not adjusted for baseline covariates in Table 1.
The primary exposure definition defined a patient as exposed by accounting for continuous periods of use by incorporating the days supply of the prescription. It also shifted overlapping prescriptions to account for forward stockpiling.
Length of each of the control and hazard periods. The 14-day period was the main analysis.
The 14-day self-controlled, case-time-control analysis included the same 41,889 beneficiaries who experienced a fall-related fracture and 167,506 sex- and age-matched controls who did not experience a fall-related fracture during the study period. Among the controls, exposure to medications with anticholinergic/sedating properties was higher in the control period relative to the case period (OR: 0.65 (95% CI: 0.63, 0.67)), suggesting a temporal trend. Accordingly, compared to the case-crossover results, the case-time-control OR estimate was further from the null (matched aOR: 1.60 (95% CI: 1.52, 1.69)) and supported an elevated association between anticholinergic/sedating medication exposure and fall-related fractures.
Sensitivity Analyses
In both self-controlled analyses, when we varied the length of the control and hazard periods (examined both a shorter (7-day) and longer (21-day) period), the association was slightly attenuated (or unchanged) and then strengthened, respectively. For example, in the case-time-control analysis, the 7-day adjusted OR was 1.60 (95% CI: 1.52, 1.69) and the 21-day OR was 1.62 (95% CI: 1.54, 1.71) (Table 3). When we applied a less conservative exposure definition that did not shift overlapping prescriptions, the results were qualitatively the same (e.g., adjusted OR for case-time-control was 1.60 (95% CI: 1.51, 1.68)), relative to the primary exposure definition (Supplementary Table S3). Excluding patients in care settings where they may not be using their Part D benefits during the exposure assessment windows or adjusting for these stays in the windows did not affect the estimates meaningfully (Supplementary Tables S4-S5). Examining (1) medications with sedating properties dichotomously (any vs. none) and (2) medications both properties (any vs. none) separately, suggested that the combined results were being driven by the medications with sedating properties (Supplementary Tables S4-S5). Additionally, it was noted that use of specific common medications with sedating properties were more consistently associated with increased short-term odds of fall-related fractures, as compared to medications with both properties (Supplementary Tables S4-S5).
DISCUSSION
In this nationwide, random sample of Medicare beneficiaries, we found a positive association between anticholinergic/sedating medication use and increased short-term odds of fall-related fractures among beneficiaries who initiated use of medications with anticholinergic and/or sedating properties when using a case-time-control design. Fall-related fractures were more common shortly after medication initiation, accounting for the competing risk of death, and risk did not appear to mitigate substantially with time since initiation. The most common fracture sites were the radius, hip, and humerus. Initiators of medications with anticholinergic/sedating properties tended to initiate a single medication with only sedating properties, such as a combination medication containing hydrocodone or tramadol.
Given the substantial burden that falls and fall-related fractures impose on the individual and the healthcare system, the strong evidence we provide can help inform the medication management interventions called for by the USPSTF.13 While many of the medication classes often associated with fall risk (e.g., BZDs, antipsychotics, opioids, and antiepileptics) have anticholinergic/sedating properties,10-12,39 few studies have examined these two properties jointly in association with fractures.40 Our findings build on those of prior observational studies demonstrating positive associations between exposure to anticholinergic/sedating medications and falls41-44 and hip fractures.40 This study makes a novel contribution by focusing specifically on new use of medications with anticholinergic/sedating properties. Nesting analyses within a new user cohort helps minimize the bias induced by depletion of patients who are susceptible to medication-related adverse events that can arise when studying prevalent users.45
Our self-controlled analyses suggested that, as expected, the cases were more likely to have the discordant exposure pattern in which they were exposed in the hazard window but not in the control window when compared to the controls. However, we also noted a strong temporal trend among the controls, in which exposure to medications with anticholinergic/sedating properties was higher in the control period relative to the hazard period. This might be related to the most common drugs observed in the windows being pain-relief medications, some intended for shorter duration of use. Using a case-time-control design, we accounted for this temporal trend potentially biasing estimates generated from the case-crossover analyses.
Our study has several important strengths. First, self-controlled designs offer a clear advantage for slow and time-invariant confounding control, and have been implemented to examine certain medications with these properties (e.g., non-BZD hypnotics, BZDs) in association with hip fractures or fractures more broadly.46-49 Our study was the first to leverage this design to examine the association of these two medication properties jointly and separately with fall-related fractures. Second, we paired this design with medication claims data from a large, nationwide healthcare database, which provides rich longitudinal exposure and outcome data but can suffer from inadequate information on time-invariant or slow-varying patient characteristics (e.g., physical activity, smoking, diet), to innovatively address this research question.31 Third, we developed an updated fall-related fracture algorithm, based on an existing validated fracture identification Medicare-based algorithm.21 Fall-related fractures are a good proxy for severe falls; however, our definition excludes some very severe falls (such as those that result in traumatic brain injuries or other substantially disabling injuries).
One limitation of applying self-controlled study designs is that they precluded us from capturing early events since patients were required to have at minimum 58 days between medication initiation and the fall-related fractures. However, these results were only slightly attenuated when we shortened the hazard and control windows to 7 days each. Additionally, while we controlled for use of other prescription medications known to increase fall risk, the potential for residual confounding by time-varying factors not captured in the data, such as over-the-counter (OTC) medications and underlying clinical measures of physical function remains. There is also the potential for confounding by indication in the self-controlled analyses if acute conditions arose or there were changes in chronic condition severity during the 58 days prior to the fracture that affected subsequent dispensing. Next, while claims are audited and undergo validity checks, they do not capture OTC medications with these properties (e.g., some antihistamines) nor confirm that the patient was actually taking the prescription. We considered two exposure definitions, a more conservative definition that considered days’ supply and shifted overlapping prescriptions, and another that did not shift overlapping prescriptions, and both definitions yielded similar results. Nonetheless, nonadherence may lead to bias due to misclassification of exposure. Lastly, our study cannot determine the mechanism of fall-related fractures. These medications have complex effects including psychotropic effects on alertness and attention, and motor effects on movement coordination and balance responses.
Conclusions
Medication use is an important potentially modifiable risk factor for falls and fall-related fractures among older adults. In this study of Medicare beneficiaries, we observed a 5% cumulative risk of fall-related fracture within one year of initiating a medication with anticholinergic/sedating properties. Within this population, we also report an elevated odds of fall-related fracture associated with transient use of anticholinergic/sedating medications. Exploring effects of cumulative exposure would be an interesting future direction. Patients and their healthcare providers should carefully evaluate whether use of medications with anticholinergic/sedating properties is appropriate and whether safer alternatives exist. This work also supports close monitoring for falls and near falls among patients initiating these medications. Fall prevention interventions among older adults should consider the impact of combining exercise-based interventions, which are known to decrease risk13, and home visits to assess environmental hazards and recommend modifications (e.g., remove rugs/carpets, install grab bars)50, with effective medication management strategies.
Supplementary Material
SUPPLEMENTAL MATERIALS: Additional Details on the Methods and Sensitivity Analyses Results
Detailed Methods Description
Supplementary Figure S1. Study design schematic illustrating patients that were eligible for inclusion in the nested cohort.
Supplementary Figure S2. Study design schematic illustrating how the self-controlled case-crossover design was nested in the new user cohort (not to scale).
Supplementary Table S1. List of Medications with Anticholinergic and/or Sedating Properties Identified in Medicare Part D Claims (2013-2016)
Supplementary Table S2. Codes Used to Define Fall-related Fractures using Medicare Claims
Supplementary Table S3. Association of Anticholinergic/Sedating Medication Exposure and Fall-Related Fractures, Medicare Beneficiaries (2014-2016)
Supplementary Table S4. Additional Sensitivity Analyses on the Association of Anticholinergic/Sedating Medication Exposure and Fall-Related Fractures, Medicare Beneficiaries (2014-2016) Using a Case-Crossover Design
Supplementary Table S5. Additional Sensitivity Analyses on the Association of Anticholinergic/Sedating Medication Exposure and Fall-Related Fractures, Medicare Beneficiaries (2014-2016) Using a Case-Time-Control Design
Key Points.
Among older adults in the US, we report a short-term increase in fall-related fractures associated with anticholinergic/sedating medication use.
Why does this matter?
These results support efforts to promote safer pharmacologic and non-pharmacologic approaches to reduce fall risk among older adults.
ACKNOWLEDGEMENTS
We would like to thank Jessie K. Edwards, PhD who developed the code for cumulative incidence estimation and Charles E. Gaber, MPH who shared his adaptation of this code which allows for bootstrapping of the confidence intervals.
Funding:
This work was supported by the following sources. The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Medication Benefits and Harms in Older US Adults (GIL200811.0010); the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the CER Strategic Initiative of UNC’s Clinical and Translational Science Award (UL1TR002489); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine. SS received tuition and stipend support from the UNC Graduate School Dissertation Completion Fellowship during the conduct of the submitted work, and is currently supported by the PhRMA Foundation Postdoctoral Fellowship in Health Outcomes. DG is funded by the Australian National Health and Medical Research Council (NHMRC) Dementia Leadership Fellowship.
SS is a consultant for CERobs Consulting, LLC on projects unrelated to the submitted work. TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG056479) from the National Institute on Aging (NIA), and as Co-Investigator (R01 HL118255, R01MD011680), National Institutes of Health (NIH). He also receives salary support as Director of Comparative Effectiveness Research (CER), NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Takeda, AbbVie, Boehringer Ingelheim), from pharmaceutical companies (GSK, Amgen, AstraZeneca, Novo Nordisk), and from a generous contribution from Dr. Nancy A. Dreyer to the Department of Epidemiology, University of North Carolina at Chapel Hill. TS does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, and Novo Nordisk. JLL receives research support from the Center for Pharmacoepidemiology (members: GlaxoSmithKline, UCB BioSciences Inc, AbbVie, Boehringer Ingelheim, Takeda and Merck [past member]). JLL also receives salary support from AbbVie, Inc. for an unrelated research study. JLL’s spouse is a full-time, paid employee of and owns stock in GlaxoSmithKline.
Footnotes
CONFLICTS OF INTEREST: No potential conflicts of interest were disclosed by the other authors.
Presentations: This work was accepted for presentation at the International Society for Pharmaceopidemiology 2021 Annual Meeting and the Society for Epidemiologic Research 2021 Annual Meeting.
SPONSOR’S ROLE
The funder had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
REFERENCES
- 1.Huang L, Turner J, Brandt NJ. Interdisciplinary Collaboration in Medication-Related Falls Prevention in Older Adults. J Gerontol Nurs. 2018;44(4):11–15. doi: 10.3928/00989134-20180313-04 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Fatalities and injuries from falls among older adults--United States, 1993-2003 and 2001-2005. MMWR Morb Mortal Wkly Rep. 2006;55(45):1221–1224. http://www.ncbi.nlm.nih.gov/pubmed/17108890. Accessed May 19, 2018. [PubMed] [Google Scholar]
- 3.Bergen G, Stevens MR, Burns ER. Falls and Fall Injuries Among Adults Aged ≥65 Years — United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(37):993–998. doi: 10.15585/mmwr.mm6537a2 [DOI] [PubMed] [Google Scholar]
- 4.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12(5):290–295. doi: 10.1136/ip.2005.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khow KSF, Visvanathan R. Falls in the Aging Population. Clin Geriatr Med. 2017;33(3):357–368. doi: 10.1016/j.cger.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–1650. doi: 10.1007/s00198-009-0920-3 [DOI] [PubMed] [Google Scholar]
- 7.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47(7):850–853. http://www.ncbi.nlm.nih.gov/pubmed/10404930. Accessed July 19, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Final Recommendation Statement: Falls Prevention in Community-Dwelling Older Adults: Interventions. U.S. Preventative Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/falls-prevention-in-older-adults-interventions1. Published 2018. [Google Scholar]
- 9.Falisi G, Rastelli C, Panti F, Maglione H, Quezada Arcega R. Psychotropic drugs and bruxism. Expert Opin Drug Saf. 2014;13(10):1319–1326. doi: 10.1517/14740338.2014.947262 [DOI] [PubMed] [Google Scholar]
- 10.de Vries M, Seppala LJ, Daams JG, et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: I. Cardiovascular Drugs. J Am Med Dir Assoc. 2018;19(4):371.e1–371.e9. doi: 10.1016/j.jamda.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 11.Seppala LJ, Wermelink AMAT, de Vries M, et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: II. Psychotropics. J Am Med Dir Assoc. 2018;19(4):371.e11–371.e17. doi: 10.1016/j.jamda.2017.12.098 [DOI] [PubMed] [Google Scholar]
- 12.Seppala LJ, van de Glind EMM, Daams JG, et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19(4):372.e1–372.e8. doi: 10.1016/j.jamda.2017.12.099 [DOI] [PubMed] [Google Scholar]
- 13.Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults us Preventive Services Task Force recommendation statement. JAMA - J Am Med Assoc. 2018;319(16):1696–1704. doi: 10.1001/jama.2018.3097 [DOI] [PubMed] [Google Scholar]
- 14.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. doi: 10.1001/archinternmed.2009.357 [DOI] [PubMed] [Google Scholar]
- 15.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47(1):40–50. http://www.ncbi.nlm.nih.gov/pubmed/9920228. Accessed September 15, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62(10):1172–1181. http://www.ncbi.nlm.nih.gov/pubmed/17921433. Accessed September 15, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Seppala LJ, van der Velde N, Masud T, et al. EuGMS Task and Finish group on Fall-Risk-Increasing Drugs (FRIDs): Position on Knowledge Dissemination, Management, and Future Research. Drugs Aging. 2019;36(4):299–307. doi: 10.1007/s40266-018-0622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA - J Am Med Assoc. 2008;300(24):2867–2878. doi: 10.1001/jama.2008.892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: The process of deprescribing. JAMA Intern Med. 2015;175(5):827–834. doi: 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. Transition to Part D Coverage of Benzodiazepines and Barbiturates Beginning in 2013.; 2012. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/BenzoandBarbituratesin2013.pdf. Accessed October 10, 2018. [Google Scholar]
- 21.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–714. http://www.ncbi.nlm.nih.gov/pubmed/1619449. Accessed May 11, 2018. [DOI] [PubMed] [Google Scholar]
- 22.Fung KW, Richesson R, Smerek M, et al. Preparing for the ICD-10-CM Transition: Automated Methods for Translating ICD Codes in Clinical Phenotype Definitions. EGEMS (Washington, DC). 2016;4(1):1211. doi: 10.13063/2327-9214.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shmuel S, Pate V, Pepin MJ, et al. Quantifying cumulative anticholinergic and sedative drug load among US Medicare Beneficiaries. Pharmacoepidemiol Drug Saf. 2021;30(2):144–156. doi: 10.1002/pds.5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuthbertson CC, Kucharska-Newton A, Faurot KR, et al. Controlling for frailty in pharmacoepidemiologic studies of older adults. Epidemiology. April 2018:1. doi: 10.1097/EDE.0000000000000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59–66. doi: 10.1002/pds.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund JL, Sturmer T, Sanoff HK. Comparative effectiveness of postoperative chemotherapy among older patients with non-metastatic rectal cancer treated with preoperative chemoradiotherapy. J Geriatr Oncol. 2016;7(3):176–186. doi: 10.1016/j.jgo.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund JL, Sanoff HK, Peacock Hinton S, Muss HB, Pate V, Stürmer T. Potential Medication-Related Problems in Older Breast, Colon, and Lung Cancer Patients in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(1):41–49. doi: 10.1158/1055-9965.EPI-17-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun JW, Rogers JR, Her Q, et al. Adaptation and Validation of the Combined Comorbidity Score for ICD-10-CM. Med Care. 2017;55(12):1046–1051. doi: 10.1097/MLR.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 29.Quality A for HR and. Tool 3I: Medication Fall Risk Score and Evaluation Tools. Content last reviewed January 2013. http://www.ahrq.gov/professionals/systems/hospital/fallpxtoolkit/fallpxtk-tool3i.html. Published 2013. [Google Scholar]
- 30.Edwards JK, Hester LL, Gokhale M, Lesko CR. Methodologic Issues when Estimating Risks in Pharmacoepidemiology. Curr Epidemiol Reports. 2016;3(4):285–296. doi: 10.1007/s40471-016-0089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014;43(5):1645–1655. doi: 10.1093/ije/dyu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández-Díaz S, Hernán MA, Meyer K, Werler MM, Mitchell AA. Case-crossover and case-time-control designs in birth defects epidemiology. Am J Epidemiol. 2003;158(4):385–391. http://www.ncbi.nlm.nih.gov/pubmed/12915504. Accessed September 30, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Stürmer T, Maclure M. Case–crossover and case–time–control designs as alternatives in pharmacoepidemiologic research. Pharmacoepidemiol Drug Saf. 1997;6(S3):S51–S59. doi: [DOI] [PubMed] [Google Scholar]
- 34.Cashin RP, Yang M. Medications prescribed and occurrence of falls in general medicine inpatients. Can J Hosp Pharm. 2011;64(5):321–326. http://www.ncbi.nlm.nih.gov/pubmed/22479083. Accessed October 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallas J, Pottegård A, Wang S, Schneeweiss S, Gagne JJ. Persistent User Bias in Case-Crossover Studies in Pharmacoepidemiology. Am J Epidemiol. 2016;184(10):761–769. doi: 10.1093/aje/kww079 [DOI] [PubMed] [Google Scholar]
- 36.Bykov K, Wang SV, Hallas J, Pottegård A, Maclure M, Gagne JJ. Bias in case-crossover studies of medications due to persistent use: A simulation study. Pharmacoepidemiol Drug Saf. June 2020. doi: 10.1002/pds.5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61(12):e59. doi: 10.1136/oem.2004.014472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suissa S The case-time-control design. Epidemiology. 1995;6(3):248–253. doi: 10.1097/00001648-199505000-00010 [DOI] [PubMed] [Google Scholar]
- 39.Berdot S, Bertrand M, Dartigues J-F, et al. Inappropriate medication use and risk of falls--a prospective study in a large community-dwelling elderly cohort. BMC Geriatr. 2009;9:30. doi: 10.1186/1471-2318-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jamieson HA, Nishtala PS, Scrase R, et al. Drug Burden Index and Its Association With Hip Fracture Among Older Adults: A National Population-Based Study. Journals Gerontol Ser A. July 2018. doi: 10.1093/gerona/gly176 [DOI] [PubMed] [Google Scholar]
- 41.Byrne CJ, Walsh C, Cahir C, Bennett K. Impact of drug burden index on adverse health outcomes in Irish community-dwelling older people: a cohort study. BMC Geriatr. 2019;19(1):121. doi: 10.1186/s12877-019-1138-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamieson HA, Nishtala PS, Scrase R, et al. Drug Burden and its Association with Falls Among Older Adults in New Zealand: A National Population Cross-Sectional Study. Drugs Aging. 2018;35(1):73–81. doi: 10.1007/s40266-017-0511-5 [DOI] [PubMed] [Google Scholar]
- 43.Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23(7):753–758. doi: 10.1002/pds.3624 [DOI] [PubMed] [Google Scholar]
- 44.Blalock SJ, Renfro CP, Robinson JM, Farley JF, Busby-Whitehead J, Ferreri SP. Using the Drug Burden Index to identify older adults at highest risk for medication-related falls. BMC Geriatr. 2020;20(1). doi: 10.1186/s12877-020-01598-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lund JL, Richardson DB, Stürmer T. The Active Comparator, New User Study Design in Pharmacoepidemiology: Historical Foundations and Contemporary Application. Curr Epidemiol Reports. 2015;2(4):221–228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med. 2013;173(9):754–761. doi: 10.1001/jamainternmed.2013.3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dore DD, Zullo AR, Mor V, Lee Y, Berry SD. Age, Sex, and Dose Effects of Nonbenzodiazepine Hypnotics on Hip Fracture in Nursing Home Residents. J Am Med Dir Assoc. 2018;19(4):328–332.e2. doi: 10.1016/j.jamda.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang D-Y, Park S, Rhee C-W, et al. Zolpidem use and risk of fracture in elderly insomnia patients. J Prev Med Public Health. 2012;45(4):219–226. doi: 10.3961/jpmph.2012.45.4.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishtala PS, Chyou T yuan. Zopiclone Use and Risk of Fractures in Older People: Population-Based Study. J Am Med Dir Assoc. 2017;18(4):368.e1–368.e8. doi: 10.1016/j.jamda.2016.12.085 [DOI] [PubMed] [Google Scholar]
- 50.Nikolaus T, Bach M. Preventing falls in community-dwelling frail older people using a home intervention team (HIT): Results from the randomized falls-HIT trial. J Am Geriatr Soc. 2003;51(3):300–305. doi: 10.1046/j.1532-5415.2003.51102.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL MATERIALS: Additional Details on the Methods and Sensitivity Analyses Results
Detailed Methods Description
Supplementary Figure S1. Study design schematic illustrating patients that were eligible for inclusion in the nested cohort.
Supplementary Figure S2. Study design schematic illustrating how the self-controlled case-crossover design was nested in the new user cohort (not to scale).
Supplementary Table S1. List of Medications with Anticholinergic and/or Sedating Properties Identified in Medicare Part D Claims (2013-2016)
Supplementary Table S2. Codes Used to Define Fall-related Fractures using Medicare Claims
Supplementary Table S3. Association of Anticholinergic/Sedating Medication Exposure and Fall-Related Fractures, Medicare Beneficiaries (2014-2016)
Supplementary Table S4. Additional Sensitivity Analyses on the Association of Anticholinergic/Sedating Medication Exposure and Fall-Related Fractures, Medicare Beneficiaries (2014-2016) Using a Case-Crossover Design
Supplementary Table S5. Additional Sensitivity Analyses on the Association of Anticholinergic/Sedating Medication Exposure and Fall-Related Fractures, Medicare Beneficiaries (2014-2016) Using a Case-Time-Control Design